Chapter 9
The Spinal Cord
Contemporary View of the Functional Components of Spinal Nerves
Sensory Components of the Spinal Nerve
Neurotransmitters of Primary Sensory Neurons
Deafferentation Pain and the Posterior Root Entry Zone Procedure
Motor Components of the Spinal Nerve
Neurotransmitters of Spinal Motor Neurons and Myasthenia Gravis
Pathways and Tracts of the Spinal Cord
Functions of Descending Tracts
Although small in diameter, the spinal cord is the most important conduit between the body and the brain. It conveys sensory input from the arms, trunk, legs, and most of the viscera and contains fibers and cells that control the motor elements found in these structures. Consequently, injury to the spinal cord, especially at cervical levels, may cause permanent and catastrophic deficits or death.
OVERVIEW
The spinal cord participates in four essential functions. First, it receives primary sensory input from receptors in skin, skeletal muscles, and tendons (somatosensory fibers) and from receptors in thoracic, abdominal, and pelvic viscera (viscerosensory fibers). This sensory input may participate in spinal reflexes, be conveyed to higher levels of the neuraxis, or both.
Second, the spinal cord contains somatic motor neurons that innervate skeletal muscles and visceral motor neurons that, after synapsing in peripheral ganglia, influence smooth and cardiac muscle and glandular epithelium. Any disease process that damages the somatic motor neuron (as in poliomyelitis), compromises its ability to elicit a response in the skeletal muscle (as in myasthenia gravis), or affects both descending fibers and lower motor neurons (as in amyotrophic lateral sclerosis, also called Lou Gehrig disease) will result in weakness or paralysis.
Third, somatosensory fibers enter the spinal cord and influence anterior horn motor neurons either directly or indirectly through interneurons. These activated motor neurons, in turn, produce rapid involuntary contractions of skeletal muscles. The sensory fiber, the associated motor neuron, and the resultant involuntary muscle contraction constitute the circuit of the spinal reflex. Reflexes are essential to normal function and can be used as diagnostic tools to assess the functional integrity of the spinal cord.
Fourth, the spinal cord contains descending fibers that influence the activity of spinal neurons. These fibers originate in the cerebral cortex and brainstem, and damage to them adversely influences the activity of spinal motor and sensory neurons. In many cases the position of a lesion in the brainstem or spinal cord may give rise to a predictable or characteristic series of deficits, such as in decorticate rigidity or an alternating hemianesthesia.
In addition, injury to peripheral nerves will result in motor or sensory deficits distal to the lesion. These are most noticeable in the extremities and may be manifested as motor deficits (flaccid paralysis), a significant decrease or loss of essential spinal reflexes (hyperreflexia, hyporeflexia, areflexia), a loss of sensation (anesthesia), or abnormal sensations (paresthesia).
DEVELOPMENT
Neural Plate
As discussed in Chapter 5, the spinal cord arises from the caudal portion of the embryonic neural plate and from the caudal eminence. The neural plate gives rise to the cervical, thoracic, and lumbar levels, whereas the caudal eminence gives rise to the sacral and coccygeal levels. The neural plate appears as a specialized area of ectoderm (neuroectoderm or neuroepithelial cells) posterior to the notochord at about 18 days (Fig. 9-1A, B). By 20 days of gestation, the neural plate is an oblong structure that is larger at its rostral area (future brain) and tapered caudally (future spinal cord).
Beginning on day 21, the edges of the neural plate (neural folds) enlarge posteromedially to meet on the midline (Fig. 9-1B, C). The initial apposition of the neural folds to form the neural tube takes place at what will become, in the adult, cervical levels of the spinal cord. This closure simultaneously proceeds in rostral and caudal directions, ultimately creating small openings at either end between the cavity of the neural tube and the surrounding amniotic cavity. These openings are the anterior and posterior neuropores; they close at 24 and 26 days of gestation, respectively.
Neural Tube
The portion of the neural tube that will differentiate into the spinal cord initially consists of the ventricular zone and the marginal zone (see Chapter 5). The ventricular zone contains cells, neuroblasts, and glioblasts that are undergoing mitosis and the precursor cells that will form the ependymal cell layer lining the central canal. The marginal zone is initially narrow and contains the processes of cells of the ventricular zone but does not contain their cell bodies or nuclei.
After their final cell division, postmitotic neurons and glial cells migrate outward to form the intermediate zone. This establishes the basic three-layered structure—ventricular zone, intermediate zone, and marginal zone—of the neural tube. As development progresses, the intermediate zone enlarges, and the processes arising from the maturing neurons of this zone enter the marginal zone. The neural crests detach from the lateral edge of the neural plate and assume a location anterolateral to the neural tube.
Structures of the developing neural tube can be correlated with their adult counterparts (Fig. 9-1C). Neural crest cells differentiate into cells of the posterior root ganglia, among other structures. The intermediate zone (the term preferred instead of mantle zone) contains columns of maturing neurons, forming the paired alar plates posteriorly and the paired basal plates anteriorly. The alar and basal plates are separated from each other by the sulcus limitans. Maturing neurons of the alar plate differentiate into the tract neurons and interneurons of the posterior horn of the adult, and those of the basal plate become motor neurons and interneurons of the anterior horn. The intermediate gray (Fig. 9-1D, white area between blue and pink), an important region of the spinal cord insinuated between the posterior and anterior horns in the adult, originates from portions of both alar and basal plates.
The marginal zone is invaded by processes of maturing neurons located in the ventricular zone (Fig. 9-1C) and by the descending axons of neuroblasts found in the developing brainstem and cerebral cortex. These axons, most of which become myelinated, form the various tracts of the white matter of the adult spinal cord.
Neural Tube Defects
A variety of defects result from the failure of the neural tube to close properly (Fig. 9-1). Rachischisis occurs when the neural folds do not join at the midline and the undifferentiated neuroectoderm remains exposed. In its extreme form, rachischisis totalis (or holorachischisis), the entire spinal cord remains open. Rachischisis partialis (or merorachischisis) is the situation in which the spinal cord is partially closed and partially flayed open.
A failure of the anterior neuropore to close results in a failure of the skull and the underlying brain to properly develop. Although it is commonly called anencephaly (meaning without the brain), the term meroanencephaly (meaning without part of the brain) is actually more accurate because the brainstem may be fairly intact but the forebrain and cerebellum are largely absent. Rachischisis and meroanencephaly are catastrophic developmental defects that in most cases (particularly the latter) are not compatible with life.
In other cases, the neural tube may develop normally but the surrounding vertebrae may not form properly, resulting in spina bifida occulta or spina bifida cystica. Spina bifida occulta is characterized by partially missing vertebral arches; the area of the defect may be indicated by a patch of dark hairs. Spina bifida cystica is seen as enlargements that may contain only meninges and cerebrospinal fluid (meningocele) or meninges, cerebrospinal fluid, and portions of the spinal cord (meningomyelocele). These are described in greater detail in Chapter 5.
SPINAL CORD STRUCTURE
The adult spinal cord is composed of a butterfly-shaped central area of neuron cell bodies, the gray matter, and a surround of myelinated and unmyelinated fibers, the white matter. Although the cavity of the neural tube was prominent during development, this space is a small ependyma-lined central canal in the adult spinal cord (Fig. 9-1C).
Surface Features
The human spinal cord extends from the foramen magnum to the level of the first or second lumbar vertebra. It consists of 8 cervical, 12 thoracic, 5 lumbar, and 5 sacral levels plus 1 coccygeal level (Fig. 9-2). Although it is generally cylindric, the cord has cervical (C4 to T1) and lumbosacral (L1 to S2) enlargements, which serve, respectively, the upper and lower extremities.
There are eight cervical roots (and spinal cord levels) but only seven cervical vertebrae. So how do the roots relate to their corresponding vertebrae? The C1 root is located between the base of the skull and the C1 vertebra (Fig. 9-2). Therefore roots C1 through C7 are located above (rostral to) their respectively numbered vertebrae, and the C8 root is located between the C7 and T1 vertebrae. Beginning with the T1 vertebra and extending caudally, all roots are located caudal to their respectively numbered vertebrae (Fig. 9-2). It is also important to remember that each level or segment of the spinal cord is specified by the intervertebral foramen through which the posterior and anterior roots originating from that cord level exit (Fig. 9-2).
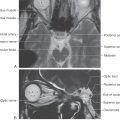
There are few superficial markings on the spinal cord (Fig. 9-3). The posterior median sulcus separates the posterior portion of the cord into halves and contains a delicate layer of pia, the posterior median septum. The posterolateral sulcus, which runs the full length of the cord, represents the entry point of posterior root (sensory) fibers. This area is frequently called the posterior (dorsal) root entry zone. In cervical and upper thoracic regions, a posterior intermediate sulcus and septum are found between the posterolateral and posterior median sulci. This sulcus and septum are insinuated between the medially located gracile fasciculus and the laterally located cuneate fasciculus (Figs. 9-3 and Fig. 9-12). Because of the organization of the gracile and cuneate fasciculi, the posterior intermediate septum is present only in upper thoracic and cervical cord levels.
On the anterolateral surface of the spinal cord, the anterolateral sulcus is the exit point for anterior root (motor) fibers (Fig. 9-3). Because the anterior roots exit in a somewhat irregular pattern, this sulcus is not as distinct as the posterolateral sulcus.
The anterior median fissure is a prominent space dividing the anterior part of the cord into halves (Fig. 9-3). This fissure contains delicate strands of pia and, more important, the sulcal branches of the anterior spinal artery.
Spinal Meninges
The tubular dural sac that encloses the spinal cord is attached cranially to the rim of the foramen magnum, and its closed caudal end is anchored to the coccyx by the filum terminale externum (Figs. 9-2 and 9-3). This dural sac is separated from the vertebrae by the epidural space. The spinal cord, in turn, is attached to the dural sac by the laterally placed denticulate ligaments and by the filum terminale internum. This latter structure extends caudally from the end of the spinal cord, the conus medullaris, and terminates in the attenuated (closed) portion of the dural sac, which is located adjacent to the S2 vertebra. The filum terminale externum extends caudally from the closed dural sac to its attachment on the inner aspect of the coccyx (Fig. 9-2).
The arachnoid mater adheres to the inner surface of the dura mater, and the pia mater is intimately attached to the surface of the cord. The subarachnoid space between these layers is continuous with the subarachnoid space around the brain and is likewise filled with cerebrospinal fluid. In adults the conus medullaris is located at the level of the L1 or L2 vertebral body. Extending caudally from this point to the end of the dural sac is an enlarged part of the spinal subarachnoid space, the lumbar cistern (Fig. 9-2). This cistern contains the posterior and anterior roots from spinal segments L2 to Coc1 as they sweep caudally. Collectively, these roots form the cauda equina. The method of choice to obtain a sample of cerebrospinal fluid for diagnostic purposes is the lumbar puncture (spinal tap), in which a large-bore needle is introduced between the L3 and L4 or L4 and L5 vertebral arches into the lumbar cistern (Fig. 9-2).
White Matter
The white matter of the spinal cord is divided into three large regions, each of which is composed of individual tracts or fasciculi. The posterior funiculus is located between the posterior median septum and the medial edge of the horn (Fig. 9-3). At cervical levels, this area consists of the gracile and cuneate fasciculi; collectively, these are commonly referred to as the posterior columns.
The lateral funiculus is the area of white matter located between the posterolateral and anterolateral sulci (Fig. 9-3). This region of the cord contains clinically important ascending and descending tracts, the locations of which are shown in Figure 9-12. Those most important in diagnosis of the neurologically impaired patient are the lateral corticospinal tract and the anterolateral system (ALS).
Located between the anterolateral sulcus and the ventral median fissure is a comparatively small region, the anterior funiculus (Fig. 9-3). This area contains reticulospinal and vestibulospinal fibers, portions of the ALS, the anterior corticospinal tract, and a composite bundle called the medial longitudinal fasciculus (MLF).
Two small but important components of the white matter are the anterior white commissure and the posterolateral (dorsolateral) tract (Fig. 9-3). The anterior white commissure is located on the anterior midline and is separated from the central canal by a narrow band of small cells. The posterolateral tract, the tract of Lissauer, is a small bundle of lightly myelinated and unmyelinated fibers capping the posterior horn.
Gray Matter
The gray matter of the spinal cord is composed of neuron cell bodies, their dendrites and the initial part of the axon, the axon terminals of fibers synapsing in this area, and glial cells. Because this area has few myelinated fibers, it appears distinctly light and has a characteristic shape in myelin-stained sections (Figs. 9-3 and Fig. 9-5).
The spinal gray matter is divided into a posterior (dorsal) horn, an anterior (ventral) horn, and the region where these meet, commonly called the intermediate zone (or intermediate gray). On the basis of the shape, size, and distribution of neurons located in these areas, the gray matter is divided into laminae (Rexed laminae) I to IX and an area X around the central canal (Fig. 9-3). These laminae are also characterized by the input they receive and the trajectory of axons originating from each lamina.
The posterior horn is composed of laminae I to VI (Fig. 9-3). The most distinct structure in the posterior horn, the substantia gelatinosa (lamina II), is capped by cells of the posteromarginal nucleus (lamina I). Laminae III to VI are located sequentially below the substantia gelatinosa. Laminae III and IV may also be called the nucleus proprius (posterior or dorsal proper sensory nucleus); their cells have elaborate dendrites that extend into lamina II. Laminae V and VI, which form the base of the posterior horn, are usually divided into medial and lateral portions.
The intermediate zone, lamina VII, extends from the area of the central canal to the lateral edge of the spinal gray and varies in shape at different levels of the cord. Particularly characteristic of lamina VII at thoracic levels are the posterior thoracic nucleus (dorsal nucleus of Clarke) and the lateral horn, which contains the intermediolateral nucleus, frequently called the intermediolateral cell column.
The anterior horn is made up of laminae VIII and IX (Fig. 9-3). Lamina VIII contains a population of smaller cells that are interneurons and tract cells. Lamina IX consists of several distinct clusters of large motor neurons whose axons directly innervate skeletal muscle.
Blood Supply
The blood supply to the spinal cord is derived from the anterior and posterior spinal arteries and from branches of segmental arteries (Fig. 9-4). The segmental branches that serve the posterior and anterior roots and the posterior root ganglia are the radicular arteries, and branches that largely bypass the roots to supplement the blood supply to the cord are the spinal medullary arteries. One especially large spinal medullary artery, the artery of Adamkiewicz, is most often seen at L2 on the left. This vessel is an important source of blood supply to the cord and must be preserved during surgery in this area. At each level, terminal branches of the spinal medullary arteries join to form an arterial network, the arterial vasocorona, on the surface of the spinal cord (Fig. 9-4).
The posterior columns and peripheral parts of the lateral and anterior funiculi are served by the posterior spinal arteries and arterial vasocorona. Most of the gray matter and the adjacent parts of the white matter are served by the central branches of the anterior spinal artery (Fig. 9-4). These central branches tend to alternate: one serves the left side of the cord, the next serves the right side.
Trauma, as in hyperextension of the cervical spine or mechanical injury to the cord, may cause occlusion or spasm of the anterior spinal artery. The result is bilateral damage to the cervical cord (central cervical cord syndrome). The characteristic features of this syndrome are bilateral weakness of the extremities, primarily evident in the arms, forearms, and hands; a patchy loss of sensation below the lesion; and urinary retention.
REGIONAL CHARACTERISTICS
Although all spinal levels have posterior, lateral, and anterior funiculi and posterior and anterior horns, their shapes and proportions vary between major spinal regions (Fig. 9-5). For example, cervical (C4 to T1) and lumbosacral (L1 to S2) cord levels have prominent posterior and anterior horns because of the extensive sensory input from and motor outflow to the upper and lower extremities. In contrast, the posterior and anterior horns at thoracic levels are small, sensory input is less dense, and there is no appendicular musculature at these levels.
When viewing the spinal cord in the clinical setting, as in magnetic resonance imaging (MRI) or computed tomography (CT), it is important to note that anterior and posterior are reversed compared with the orientation commonly used in the anatomic setting (Fig. 9-5). It is absolutely essential to remember the orientation and consequent location of tracts in the spinal cord in CT and MRI because these images are used in the evaluation of the neurologically compromised patient. In short, anterior is up in the image and posterior is down (Fig. 9-5). In addition, the right and left sides of the patient are standardized; the observer’s right is the patient’s left and the observer’s left is the patient’s right.
Cervical Levels
The cervical cord is round to oval and proportionately larger than at other spinal levels (Fig. 9-5). There is a large amount of white matter because a full complement of ascending and descending fiber tracts is present. The gracile and cuneate fasciculi are especially obvious structures at cervical levels.
At cervical levels C1 to C3, the posterior and anterior horns are comparatively small, which results in a more round than oval shape of the upper cervical spinal cord. The horns at C3 to C4 are becoming larger as part of the cervical enlargement; thus the shape of the spinal cord is transitioning from more round to more oval. At cervical levels C4 to C8, the posterior and anterior horns are large, reflecting the sensory input from and motor innervation to the upper extremity. At these levels, the spinal cord is distinctly oval (Fig. 9-5). The oval shape of the spinal cord is also obvious in a myelogram at lower cervical levels (Fig. 9-5).
Thoracic Levels
In general, the thoracic cord is round and the posterior and anterior horns are small (Fig. 9-5). From upper to lower thoracic levels, there is a progressive decrease in the amount of white matter. Although both the gracile and cuneate fasciculi are present at upper thoracic levels (above T6), only the gracile fasciculus is present at lower thoracic levels (below T6). However, the small size of the posterior and anterior horns makes the white matter in thoracic levels appear proportionately large. As seen in a myelogram at about midthoracic levels, there is more space around the spinal cord than at lower cervical levels (Fig. 9-5).
Two structures especially obvious in the gray matter of lamina VII at thoracic levels are the posterior thoracic nucleus (dorsal nucleus of Clarke) and the lateral horn (Fig. 9-5). The posterior thoracic nucleus, a prominent medial cell group, contains neurons whose axons project to the cerebellum. The lateral horn is a protrusion into the lateral funiculus formed by the underlying intermediolateral cell column. These cells are preganglionic sympathetic neurons whose axons will terminate in either paravertebral or prevertebral ganglia.
Lumbar Levels
At lumbar levels the cord is also round (Fig. 9-5). The posterior and anterior horns are large, and there is considerably less white matter than at higher levels. Therefore, the posterior and anterior horns appear proportionately large, the reverse of the situation at thoracic levels. In parallel with the situation at cervical levels, the proportionately large size of the posterior and anterior horns at lumbar levels accommodates, respectively, the significant sensory input from and motor outflow to the lower extremity. The posterior thoracic nucleus (dorsal nucleus of Clarke) is usually obvious at L1 and possibly L2 levels.
As the posterior and anterior roots descend in the more caudal portions of the dural sac, they form fascicles around the lower lumbar and sacral levels of the spinal cord. Myelograms at these levels clearly illustrate the position of the roots in relation to the spinal cord, and as they descend, they coalesce to form the cauda equina within the lumbar cistern (Figs. 9-2 and 9-5).
Sacral Levels
At sacral levels, the spinal cord is round and smaller than at lumbar levels (Fig. 9-5). It consists mainly of gray matter; the white matter forms a relatively thin shell. The intermediate gray matter at levels S2, S3, and S4 contains preganglionic parasympathetic cell bodies (the sacral visceromotor nucleus). The substantia gelatinosa (lamina II) is especially obvious at sacral levels.
At sacral levels the cord is small and surrounded by the posterior and anterior roots that are descending in the dural sac (Fig. 9-5). At this point, the roots are passing caudal to form the cauda equina. The subarachnoid space around the lumbar, sacral, and coccygeal cord is continuous with the space of the lumbar cistern; a myelogram through the lumbar cistern reveals the roots forming the cauda equina but no spinal cord (Fig. 9-5).
SPINAL NERVES
The spinal nerves are formed by the junction of the posterior and anterior roots of the spinal cord (Fig. 9-6). As there are 31 spinal cord levels (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, 1 coccygeal), so are there 31 corresponding pairs of spinal nerves. Each spinal nerve contains afferent fibers that convey sensory input from the periphery and efferent fibers arising from spinal motor neurons. These fibers, plus circuits in the spinal gray, are the structural basis for the spinal reflexes routinely tested in the neurologic examination.
The spinal nerve may contain up to four types of fibers. Two of these are sensory and have their cell bodies in the posterior root ganglion, and two are motor and have their cell bodies in the anterior horn of the spinal cord gray matter (Figs. 9-6 and 9-7).
Contemporary View of the Functional Components of Spinal Nerves
The sensory fibers of the spinal nerve have their cell bodies in the posterior root ganglia; they convey sensation from the body wall as broadly defined and from visceral structures that are made up of smooth muscle, cardiac muscle, or glandular epithelium (or a combination of these tissues). In the traditional view, these afferent cell bodies and axons may be assigned the functional components of general somatic afferent (GSA) and general visceral afferent (GVA), respectively. The motor components of the spinal nerve have their cell bodies in the spinal gray matter and directly innervate skeletal muscle (general somatic efferent, GSE) or indirectly innervate visceral structures after a synapse in a motor (parasympathetic or sympathetic) ganglion (general visceral efferent, GVE). A more contemporary view takes into account advances in embryology and favors eliminating the designation general and stipulating that these functional components are somatic afferent (SA), visceral afferent (VA), somatic efferent (SE), and visceral efferent (VE). Whereas the contemporary and simplified version is used here, either the contemporary or traditional version can be used in a teaching environment to describe the sensory and motor components of spinal nerves.
Sensory Components of the Spinal Nerve
Sensory information is brought to the spinal cord by neuronal processes whose cell bodies reside in the posterior root ganglia. The central processes of these neurons penetrate the spinal cord; their peripheral processes arise from sensory receptors. Sensory input originates from (1) the body surface; (2) deep structures such as muscles, tendons, and joints; and (3) internal organs. Fibers conveying input from the first two areas are classified as somatic afferent (SA), whereas sensory fibers from visceral structures are classified as visceral afferent (VA). The SA fibers are further classified as either exteroceptive or proprioceptive (Figs. 9-6 and 9-7).
Exteroceptive SA fibers arise from (1) receptors that are sensitive to mechanical, thermal, or chemical stimuli that may cause tissue damage or (2) receptors sensitive to discriminative touch or vibratory stimuli. The former fibers (Aδ and C) are slowly conducting (0.5 to 30 m/s) and unmyelinated, or lightly myelinated, and they enter the cord via the lateral division of the posterior root (Fig. 9-6). These fibers ascend or descend (or both) in the posterolateral tract (tract of Lissauer) before entering the posterior horn to terminate primarily in laminae I to V. The latter fibers (Aβ) are rapidly conducting (30 to 70 m/s) and heavily myelinated, and they enter the cord through the medial division of the posterior root (Fig. 9-6). After entering the posterior funiculus, these fibers give rise to ascending or descending collaterals.
Proprioceptive SA fibers originate from receptors located in muscles, tendons, or joints that are sensitive to stretch or pressure; some vibratory sense is also conveyed by these fibers (Figs. 9-6 and 9-7). These are rapidly conducting (70 to 120 m/s; Ia, Ib, Aα, and Aβ), heavily myelinated fibers that also enter the medial division of the posterior root. The central processes of these proprioceptive fibers (and of the heavily myelinated exteroceptive fibers) directly enter and ascend in the posterior columns, or they branch into the spinal gray to synapse in relay nuclei (such as the posterior nucleus of Clarke) or on cells in the anterior horn that participate in spinal reflexes.
Spinal nerves also convey sensory information from thoracic, abdominal, and pelvic viscera. This interoceptive VA input originates primarily from receptors that are sensitive to nociceptive stimuli (Figs. 9-6 and 9-7). These fibers travel through (for example) the splanchnic nerves and traverse the sympathetic chain and white communicating ramus to enter the spinal nerve. Their central processes enter the lateral division of the posterior root and terminate in laminae I and V to VII. These VA fibers are also lightly myelinated and slowly conducting (1 to 20 m/s).
Neurotransmitters of Primary Sensory Neurons
Although several neuroactive substances have been implicated as transmitters in primary afferent fibers, those having an important role are substance P (SP), calcitonin gene–related peptide (CGRP), and glutamate. Small-diameter (Aδ and C) fibers arising from visceral and somatic structures—that is, VA and small-diameter SA fibers—use one or more of these three neurotransmitters, and it is probable that some large-diameter, heavily myelinated SA fibers use glutamate. Specifically, SP and CGRP can be found in small-diameter VA and SA fibers, and these peptides plus glutamate are also found in the smaller cell bodies of the posterior root ganglion, from which these fibers arise. Centrally, fibers and terminals containing these three neurotransmitters can be found in laminae I, II, and V, where the small-diameter axons synapse with cells that relay the information to higher levels of the neuraxis. Conversely, some of the larger cell bodies in the posterior root ganglia, which give rise to large-diameter SA fibers, contain glutamate. This transmitter is also found in the posterior columns in large-diameter, heavily myelinated fibers, indicating that this projection may function in the relay of proprioceptive information.
Deafferentation Pain and the Posterior Root Entry Zone Procedure
In general, the phenomenon of deafferentation pain occurs when the anatomic pathways for pain perception—that is, intact nerve rootlets, tracts, and nerves themselves—are partially or entirely disrupted. This condition may develop, for example, after amputation (traumatic or otherwise), peripheral nerve injury, lesions of central tracts resulting in hemiplegia or quadriplegia or paraplegia, or damage to the posterior rootlets at the rootlet-cord interface. Deafferentation pain may be perceived as dull and aching, pins-and-needles (sharp pain), searing, or burning sensations. The mechanism for the pain is likely due to a combination of an increased sensitivity of the central (disconnected or damaged) neurons (central sensitization), plasticity changes in the damaged cell groups, a decrease in descending inhibition, or an increase in facilitation at the lesion site.
An especially instructive example of cause, treatment, and potential complication is seen in avulsion of the posterior rootlets. This injury, commonly seen in accidents involving motorcycles, is the forceful separation (avulsion, a pulling or tearing out) of the posterior roots from the spinal cord, more often in the brachial plexus. The pain is in the distribution of the damaged posterior roots.
One treatment for this intractable pain is the DREZ (for dorsal root entry zone) procedure, although PREZ (for posterior root entry zone) procedure has a nice ring. In this procedure, a small electrode is placed into the posterior horn at the entry zone (hence the name of the procedure) and radiofrequency lesions are made at the levels of the avulsed roots. Significant or total relief from pain is seen in 80% to 90% of these patients. Interestingly enough, complications from this procedure include deficits related to the laterally adjacent corticospinal tract or the medially adjacent cuneate fasciculus. These are, respectively, a weakness of the upper or lower extremity on the same side and the loss of proprioceptive and vibratory sensations on the ipsilateral upper extremity. Some patients will describe the proprioceptive problem as a buzzing sensation on the upper extremity on the side of the procedure.
Motor Components of the Spinal Nerve
The spinal cord gives rise to two types of motor fibers: (1) those that directly innervate skeletal (striated) muscle; and (2) visceromotor (autonomic) fibers, preganglionic fibers that synapse on neuron cell bodies located in a peripheral visceromotor ganglion. Postganglionic fibers arise from these ganglia and innervate smooth muscle, cardiac muscle, or glandular epithelium (Figs. 9-6 and 9-7).
The motor cells that innervate skeletal muscle are located in the anterior horn; these cells and their peripheral processes are classified as somatic efferent (SE) (Fig. 9-7). These SE cells in the anterior horn also supply motor innervation to the specialized intrafusal muscle fibers of the muscle spindles (neuromuscular spindles), sensory structures in muscles that detect muscle length and various aspects of contraction dynamics. Large motor neurons in the anterior horn are organized in two general but overlapping patterns (Fig. 9-8). First, cells innervating proximal muscles are located medially, and cells innervating more distal muscles are located progressively more laterally. This explains why the anterior horn is smaller and narrower at thoracic than at cervical and lumbar levels. At thoracic levels, the anterior horn contains motor neurons innervating the axial muscles of the trunk, whereas at cervical and lumbar levels, it also contains the more lateral groups of motor neurons that innervate the limbs. Second, within the anterior horn at C4 to T1 and L1 to S2, motor neurons innervating extensors tend to be more anteriorly located in the horn, whereas those innervating flexors tend to be more posteriorly located.
 Figure 9-8. Representation of the general organization of motor neurons in the anterior horn.
Figure 9-8. Representation of the general organization of motor neurons in the anterior horn.
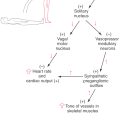
The visceromotor (autonomic) motor neurons of the spinal cord are classified as visceral efferent (VE) and have their cell bodies in lamina VII (Figs. 9-6 and 9-7). At cord levels T1 to L2, these cells belong to the intermediolateral cell column (sympathetic cells); at sacral levels S2 to S4, they belong to the parasympathetic system and form the sacral visceromotor nucleus located in the lateral part of lamina VII. Unlike the single-neuron SE projection, visceromotor pathways consist of two neurons in series (Fig. 9-6). The spinal cord neuron projects to a visceromotor ganglion and is therefore classified as VE preganglionic. In the ganglion, it synapses with a VE postganglionic neuron, which innervates the target structure.
Motor fibers (SE and VE) exit in the anterior root and pass into the spinal nerve (Fig. 9-6). SE fibers continue through the spinal nerve and are conveyed by the progressive branching of peripheral nerves to the skeletal muscles of the body. In contrast, VE preganglionic fibers leave the spinal nerve to join the sympathetic trunk via the white communicating ramus (Fig. 9-6). Once they have entered the sympathetic trunk, these preganglionic fibers follow any of several routes, which are considered in detail in Chapter 29. VE postganglionic fibers from cells of the sympathetic chain ganglia rejoin the spinal nerves via the gray communicating ramus, whereas those of the prevertebral ganglia distribute only to the gut. Also, VE preganglionic parasympathetic neurons are present at S2 to S4 levels. Their axons leave the spinal cord in the anterior roots to eventually join branches of the ventral primary rami that form the pelvic nerve.
Neurotransmitters of Spinal Motor Neurons and Myasthenia Gravis
The three populations of spinal motor neurons are (1) large anterior horn cells (alpha motor neurons) that innervate extrafusal skeletal muscle cells; (2) smaller cells (gamma motor neurons) that innervate only the intrafusal fibers of the muscle spindles; and (3) cells that give rise to preganglionic sympathetic (T1 to L1) or parasympathetic (S2 to S4) fibers, which terminate in peripheral visceromotor (autonomic) ganglia. All three of these cell populations use acetylcholine as their neurotransmitter. Consequently, acetylcholine is abundant in axon terminals at the neuromuscular junction, and numerous nicotinic acetylcholine receptors are present on the postsynaptic junctional folds of the muscle membrane.
Myasthenia gravis, a neurologic disease characterized by moderate to profound muscle weakness, is closely correlated with the presence of circulating antibodies directed against nicotinic receptor sites on the postsynaptic membrane. The result is a blockage of transmission at the neuromuscular junction. A characteristic of this disease is muscle fatigability; as the day progresses, muscle fatigue becomes progressively worse.
This disease is most frequently seen in patients between 20 and 40 years of age, although younger patients may exhibit symptoms. There are three characteristics of myasthenia gravis. First, muscle weakness may wax and wane for periods of minutes or hours, one day, or several days or weeks. Second, muscles controlling eye movement are frequently involved first (in about 40% of patients), resulting in diplopia and ptosis, and are ultimately involved in about 85% of all patients. Muscles of the pharynx or larynx, face, and extremities may eventually be involved, but almost always together with ocular muscles. These patients exhibit dysarthria and dysphagia. Third, the weakness responds to the administration of drugs that enhance cholinergic transmission.
SPINAL REFLEXES
Afferent fibers in spinal nerves may synapse on tract cells that relay information to higher levels of the neuraxis, or they may terminate on motor neurons or interneurons, both of which may participate in reflex circuits. Reflexes require an afferent fiber, interneurons or motor neurons, and a target tissue, usually skeletal muscle. Reflexes may be relatively simple and confined to a single cord level (intrasegmental) or complex, involving multiple cord segments (intersegmental). Certain disease or central nervous system lesions can affect spinal reflexes, resulting in reflexes that are greatly exaggerated (hyperreflexia), diminished (hyporeflexia), or absent (areflexia). Numerous reflexes are part of the standard neurologic examination (see Chapter 33); only a few examples are given here.
Muscle Stretch Reflex
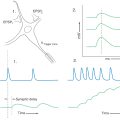
Although this is sometimes called a tendon reflex or deep tendon reflex, it is more correctly called a muscle stretch reflex because the stimulus is stretch of a muscle spindle located within the muscle. This reflex may be elicited by tapping any large tendon; a common example is the knee jerk or quadriceps stretch reflex (Fig. 9-9, left side). A brisk tap on the patellar tendon stretches the primary sensory endings in muscle spindles located in the quadriceps femoris muscle, sending an impulse toward the posterior root ganglion via heavily myelinated, rapidly conducting group Ia fibers. The central processes of these afferent axons synapse on and excite motor neurons in the anterior horn that innervate the quadriceps femoris muscle. The result is a sudden contraction of these muscles and extension (dorsiflexion) of the leg at the knee. Because this reflex requires only one synapse and is a response to muscle stretch, it also may be called a monosynaptic stretch reflex or a myotatic reflex.
An extension of the simple stretch reflex is seen in reciprocal inhibition and autogenic inhibition (also called the inverse myotatic reflex). In reciprocal inhibition, one group of muscles is excited and the antagonistic group is inhibited (Fig. 9-9, left side). In this situation, the muscle spindle is stretched by a tap on the patellar tendon, and the impulse enters the spinal cord via a group Ia primary sensory fiber. This fiber branches and has excitatory terminations on quadriceps femoris motor neurons and on group Ia inhibitory (glycinergic) interneurons. As a result, the quadriceps (extensor) contracts, whereas the interneurons inhibit spinal motor neurons innervating the hamstring (flexor) muscles, which remain passive. This action enhances the effectiveness of the reflex.
The receptor involved in autogenic inhibition is the Golgi tendon organ (Fig. 9-9, right side). This receptor responds to relatively high tension (higher than that needed to activate the muscle spindles). Activation causes an increase in the rate of firing of the group Ib sensory fibers that arise from this receptor. In the spinal cord, these fibers terminate on group Ib inhibitory (glycinergic) interneurons, which inhibit motor neurons that innervate the muscle attached to the tendon from which the afferent volley originated.
Flexor Reflex
A further level of complexity in spinal reflexes is seen in the flexor reflex (withdrawal reflex or nociceptive reflex) (Fig. 9-10). This type of reflex is initiated by cutaneous input, is frequently a response to nociceptive stimuli, and represents an attempt to protect a body part by extricating it from the source of injury. Lightly myelinated or unmyelinated primary sensory fibers (Aδ or C fibers) conveying nociceptive input enter the posterolateral tract (of Lissauer), where they may branch and ascend or descend for short distances. Many of these fibers enter the spinal gray, where they have excitatory synaptic contacts with ascending tract cells and with both excitatory and inhibitory interneurons (Fig. 9-10). Whereas tract neurons relay this nociceptive information to higher levels of the neuraxis, the excitatory glutaminergic interneurons synapse on flexor motor neurons, resulting in activation of the ipsilateral flexor muscles of the thigh (iliopsoas), leg (hamstring muscles), and foot (tibialis anterior) and withdrawal of the extremity. This action is enhanced by the synapse of inhibitory interneurons on extensor (antagonistic) motor neurons and the resultant decreased activity (inhibition) of extensor muscles, for example, the quadriceps femoris muscles. The flexor reflex, considering its afferent and efferent limbs, involves several spinal segments.
Crossed Extension Reflex
The crossed extension reflex builds on the basic circuits of the flexor reflex but also involves musculature of the contralateral side of the body (Fig. 9-11). By way of interneurons, nociceptive input on Aδ or C fibers excites ipsilateral leg flexor motor neurons and inhibits ipsilateral leg extensor motor neurons. Consequently, the flexors contract, the extensors relax, and the extremity is withdrawn from the painful stimulus. If the reflex occurs during standing or walking, however, the opposite leg must participate in the response to keep the person from falling. The same nociceptive input that resulted in withdrawal on the ipsilateral side is conveyed to interneurons that project to the contralateral anterior horn (Fig. 9-11). These fibers excite motor neurons polysynaptically, innervating contralateral extensor muscles and inhibiting motor neurons that innervate contralateral flexor muscles. Thus, there is an ipsilateral flexion and withdrawal from the stimuli accompanied by an extension of the contralateral leg to support the body.
PERIPHERAL NERVE LESIONS
The posterior and anterior roots from one level of the spinal cord join to form a single spinal nerve, which in turn combines with other spinal nerves to form the peripheral nerves of the body. Whereas most peripheral nerves are mixed (containing motor and sensory fibers), a few may contain only motor or sensory fibers. Consequently, damage to peripheral nerves may give rise to motor deficits, sensory deficits, or a combination of deficits. The etiology and types of peripheral nerve disease are numerous, and only general examples are discussed here.
Radiculopathy
Radiculopathy (radix is Latin for “root”) is the result of damage to a nerve root. The most common cause is spondylolysis or intervertebral disk disease with resultant damage to one or more nerve roots. Because of the overlap of dermatomes on the body, compression of a single root may not cause a significant sensory loss. However, the main symptom experienced by these patients is the perception of a sharp, burning pain (patients will frequently describe these as “shooting pains”) in the dermatomal distribution of the damaged spinal nerve. Cervical disk disease may result in pain in the base of the neck, over the shoulder, or down the upper extremity; lumbar disk problems may result in low back pain or in pain radiating down the lower extremity, as in sciatica.
Mononeuropathy
The most common cause of mononeuropathy (deficits reflecting the distribution of a single anatomically defined peripheral nerve) is trauma. Other causes include entrapment and compression syndromes. Characteristic examples of traumatic mononeuropathy deficits and the damaged nerve are as follows: deviation of the tongue on protrusion/hypoglossal nerve; loss of flexion-adduction and extension of the fingers/ulnar nerve; loss of dorsiflexion of the foot and toes/deep peroneal nerve; loss of pronation of the forearm and movements of the fingers/median nerve; and loss of flexion of the toes/tibial nerve.
One of the more common entrapment mononeuropathies is the carpal tunnel syndrome. Basically, the median nerve is compressed by fluid accumulation in the synovial sheaths of the carpal tunnel, creating a largely sensory deficit (although weakness of some finger muscles may occur). The symptoms are numbness, tingling, and pain from the thumb, index finger, and middle finger; one treatment is to section the transverse carpal ligament to relieve the pressure on the median nerve.
Polyneuropathy
As its name implies, a polyneuropathy includes motor and sensory deficits that reflect damage to multiple peripheral nerves. Although there are a variety of diseases that may affect multiple peripheral nerves, one of the most common is diabetes mellitus. In diabetes, the more distal portions of the fibers are affected first (distal axonopathy), starting in the lower extremity and then progressing to the upper extremity. The small-diameter myelinated and unmyelinated fibers are affected first, followed by larger diameter fibers as the disease progresses.
Patients may experience numbness and a loss of pain and thermal sensations in the feet (affecting the longest fibers first), progressing up to about the knees; then the same deficits are perceived in the hands, progressing up the forearms. As the disease progresses, larger diameter fibers become involved, and the vibratory and position sensations are diminished or lost. Because this sensory loss starts with the feet and legs and jumps to the hand and forearm, it is common to describe this pattern as a stocking-glove sensory loss. Although it is seen as primarily a sensory loss, these patients may also exhibit weakness of distal portions of the extremities and have hyporeflexia.
Two other examples of lesions that result in loss of function related to peripheral nerves are sensory neuronopathy and motor neuronopathy. Sensory neuronopathy is a loss of cell bodies in the posterior root ganglion that results in a sensory loss involving both distal and proximal portions of an extremity and may include most or all sensory modalities. Motor neuronopathy is seen in a loss of anterior horn motor neurons with resultant flaccid weakness, muscle fasciculations, and eventual muscle atrophy.
PATHWAYS AND TRACTS OF THE SPINAL CORD
The spinal cord white matter consists of (1) long ascending and descending fibers or tracts, which link the spinal cord to higher levels of the neuraxis, and (2) propriospinal fibers that project from one spinal level to another (Fig. 9-12; Table 9-1). Ascending fibers convey information to higher levels of the neuraxis. Some descending fibers modulate the transmission of nociceptive information in the posterior horn; others influence the activity of motor neurons. Ascending and descending propriospinal fibers form the basis for a wide variety of intraspinal reflexes.
Table 9-1 Synopsis of the Principal Tracts and Fibers Located in the Funiculi, Their Laterality in the Cord, Origin, and Termination
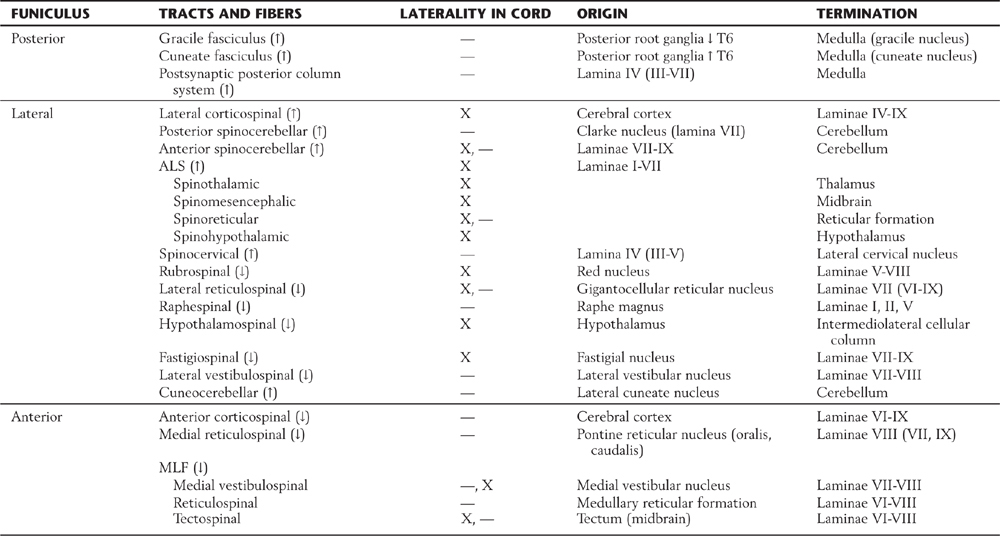
ALS, anterolateral system; MLF, medial longitudinal fasciculus; ↓, descending; ↑, ascending.
Many tracts or fibers in the nervous system are named according to the location of their cell body of origin and the area where their axons terminate. For example, the corticospinal fibers originate in the cerebral cortex (cortico-) and end in the spinal cord (-spinal). These are descending fibers because the cortex is a more rostral part of the neuraxis than is the spinal cord. Likewise, the term spinothalamic fiber indicates that the cell of origin is in the spinal cord and the termination is in the thalamus; these are ascending fibers. In many situations the name of the tract or group of fibers indicates three important facts about that fiber population: (1) whether they are ascending or descending (corticospinal versus spinocerebellar); (2) the location of the cell body of origin (cortex versus spinal cord); and (3) the place where the axons in the tract terminate (spinal cord versus cerebellum). Keeping these basic principles in mind will expedite learning the many tracts and pathways of the spinal cord.
Functions of Ascending Tracts
The gracile and cuneate fasciculi, collectively called the posterior columns, are composed of the central processes of heavily myelinated primary sensory fibers that convey proprioceptive, tactile, and vibratory information from the ipsilateral side of the body (Fig. 9-12; Table 9-1). Fibers in the gracile fasciculus originate from sacral, lumbar, and lower thoracic (below T6) levels; those in the cuneate fasciculus originate from upper thoracic (above T6) and cervical levels. Injury to the posterior columns on one side results in a loss of proprioception, discriminative touch, and vibratory sense below the level of the lesion on the same side. However, there is clinical evidence that pain signals may also be transmitted via the posterior columns, especially the gracile fasciculus, from the pelvic viscera to the thalamus. Comparable information may also ascend through the cuneate fasciculus. This information is most likely processed through the postsynaptic posterior column system.
The posterior (dorsal) spinocerebellar and anterior (ventral) spinocerebellar tracts are located on the lateral surface of the cord, meeting at approximately the level of the denticulate ligament (Figs. 9-3 and 9-12; Table 9-1). The fibers of the posterior (dorsal) spinocerebellar tract arise from the posterior thoracic nucleus (of Clarke) in lamina VII at T1 to L2, and the fibers of the anterior (ventral) spinocerebellar tract arise primarily from cells of laminae V to VIII and from large ventral horn neurons called spinal border cells, both at lumbosacral levels. The information conveyed on spinocerebellar fibers, through synaptic relays in the cerebellum, thalamus, and motor cortex, influences the efficiency of motor activity.
In the anterolateral area of the spinal cord, there is a large composite bundle called the anterolateral system (ALS) (Fig. 9-12; Table 9-1). This system encompasses those regions of the white matter that were classically divided into anterior and lateral spinothalamic tracts. The ALS contains spinothalamic, spinomesencephalic (spinotectal, spinoperiaqueductal), spinohypothalamic, and spinoreticular fibers. The majority of fibers that coalesce to form the ALS ascend about two spinal levels as they cross the midline in the anterior white commissure.
In general, fibers of the ALS convey nociceptive, thermal, and poorly localized (crude) touch information to higher levels of the neuraxis. Consequently, injury to the spinal cord that involves ALS fibers will result in a loss of pain, temperature, and crude touch sensations on the contralateral side of the body beginning about two segments below the lesion. Damage to these fibers as they cross in the anterior white commissure results in bilateral loss of pain and thermal perception at about the level of the lesion, with sparing of these modalities at lower levels. The ALS is somatotopically organized; this means that in the spinal cord, lower portions of the body are represented more posterolaterally and upper levels are represented anteromedially (Fig. 9-12).
Nociceptive input and some discriminative touch are also carried by postsynaptic posterior column fibers and by the spinocervicothalamic tract. The postsynaptic posterior column fibers originate from laminae III to VIII (mainly IV) and ascend ipsilaterally in the dorsal columns. The spinocervicothalamic fibers arise from the same laminae but ascend as a diffuse population in the posterior part of the lateral funiculus to end in the lateral cervical nucleus at levels C1 to C3. The existence of these minor fiber populations in humans may explain the recurrence of pain perception in some patients who have had an anterolateral cordotomy for intractable pain.
Other, more diffusely arranged ascending fibers include spinoolivary, spinovestibular, and spinoreticular fibers. These are discussed in later chapters in relation to functional systems.
Functions of Descending Tracts
The lateral funiculus (Figs. 9-3 and 9-12; Table 9-1) contains the lateral corticospinal and rubrospinal tracts as well as other more diffusely organized fiber populations (reticulospinal, fastigiospinal, raphespinal, hypothalamospinal). Corticospinal fibers arise from the cerebral cortex and descend through the brainstem. At the medulla–spinal cord junction, most fibers cross to form the lateral corticospinal tract, but some remain uncrossed as the anterior corticospinal tract. Lateral corticospinal fibers are somatotopically arranged; fibers that originate from lower extremity areas of the cerebral cortex and project to lumbosacral levels are lateral, whereas those traveling to cervical levels from upper extremity areas of the cortex are medial (Fig. 9-12). One important function of this tract is to influence spinal motor neurons, especially those controlling fine movements of the distal musculature. Consequently, lesions of lateral corticospinal fibers on one side of the cervical cord result in ipsilateral paralysis of the upper and lower extremities (hemiplegia). In contrast, a lesion of corticospinal fibers above (rostral to) the spinal cord–medulla junction and therefore above the decussation of these fibers will result in hemiplegia on the opposite (contralateral) side of the body.
Rubrospinal fibers arise from the red nucleus of the midbrain, cross at that level, and descend in the spinal cord with lateral corticospinal fibers (Fig. 9-12). In general, rubrospinal fibers excite flexor motor neurons and inhibit extensor motor neurons.
Although diffusely arranged, other descending fibers in the lateral funiculus serve important functions (Fig. 9-12; Table 9-1). Reticulospinal fibers in this area originate from the medullary reticular formation, and fastigiospinal fibers originate from the fastigial nucleus of the cerebellum. At spinal levels, the reticulospinal fibers are uncrossed and the fastigiospinal fibers are crossed. Because their function is to help maintain posture, these fibers tend to excite extensor motor neurons and inhibit flexor motor neurons. Raphespinal fibers originate mainly from the nucleus raphe magnus of the brainstem, descend bilaterally in posterior areas of the lateral funiculus, and function to modulate the transmission of nociceptive information at spinal levels. The activity of VE motor neurons of the intermediolateral cell column is influenced by hypothalamospinal fibers, which descend through lateral areas of the brainstem and spinal cord. Lesions in the brainstem or cervical spinal cord that interrupt these fibers result in ipsilateral ptosis, miosis, anhidrosis, and enophthalmos (Horner syndrome).
The anterior funiculus (Fig. 9-12; Table 9-1) contains reticulospinal and vestibulospinal fibers, the anterior corticospinal tract, and the medial longitudinal fasciculus (MLF). Reticulospinal fibers in this area arise in the pontine reticular formation of the brainstem, whereas vestibulospinal fibers originate from the vestibular nuclei. Lateral vestibulospinal fibers arise from the lateral vestibular nucleus, and medial vestibulospinal fibers originate primarily from the medial vestibular nucleus. Reticulospinal and vestibulospinal fibers of the anterior funiculus function in postural mechanisms through their general excitation of extensor motor neurons and inhibition of flexor motor neurons. Fibers of the anterior corticospinal tract are uncrossed, but most of these fibers cross in the ventral white commissure before terminating on medial motor neurons that innervate axial muscles.
The MLF, although small, is generally regarded as a composite bundle containing medial vestibulospinal fibers (from the medial vestibular nucleus), tectospinal fibers (from the superior colliculus), interstitiospinal fibers (from the interstitial nucleus), and some reticulospinal fibers (Fig. 9-12; Table 9-1). Tectospinal and vestibulospinal fibers are found only at cervical levels; the other fibers extend to lower cord levels. These fibers terminate primarily in laminae VII and VIII but ultimately influence motor neurons innervating primarily axial and neck musculature.
The comparatively simple structure of the spinal cord significantly misrepresents its functional importance. Although the cord is smaller in diameter than the little finger, descending motor control of the body below the neck and all sensory input from the same areas must traverse it. Consequently, small lesions in the spinal cord that would be considered of little consequence in larger parts of the brain may cause global deficits or death. As the cord merges into the brainstem, the organization and function of the central nervous system become progressively more complex.
DEFICITS CHARACTERISTIC OF SPINAL CORD LESIONS
The functional and clinical characteristics of ascending and descending tracts of the spinal cord are described in later chapters. It is appropriate at this point to touch on some general features that correlate with the structure of the spinal cord.
Syringomyelia
Cavitation of the central regions of the spinal cord, as in a small syringomyelia, will frequently damage fibers crossing in the anterior white commissure (Fig. 9-3). This bundle conveys fibers from the posterior horn across the midline to enter the ALS on the opposite side (Fig. 9-6). Consequently, a lesion of this structure will damage fibers coursing in both directions, resulting in a bilateral loss of pain and thermal sensations that correlate with the damaged levels of the spinal cord. For example, if the lesion is in mid to low cervical levels, the pain and thermal sensory deficits will fall over the shoulders and arm in a “cape distribution.” A large syrinx that involves the anterior white commissure and extends into the anterior horn results in a bilateral sensory loss, as noted earlier, and weakness of the corresponding extremity. Because these lesions are usually in the cervical levels, extension of the syrinx into one anterior horn results in an ipsilateral weakness of the upper extremity; if both anterior horns are involved, the weakness is bilateral. In syringomyelia, the cavity that develops in central areas of the spinal cord does not have a lining of ependymal cells and therefore is not an enlargement of the central canal. This is sometimes called a noncommunicating syringomyelia to differentiate it from a cystic structure that may connect with the central canal (communicating syringomyelia). On the other hand, a cavitation of the central canal is called a hydromyelia (or hydrosyringomyelia).
Brown-Séquard Syndrome
A functional hemisection of the spinal cord results in a clinical picture that reflects damage to the lateral corticospinal tract, the ALS, and the posterior columns (a Brown-Séquard syndrome). A lesion on the right at C4 to C5 will result in muscle weakness or paralysis (hemiparesis, hemiplegia) on the right side (corticospinal damage); loss of pain and thermal sensations on the left side (ALS damage—these fibers cross in the anterior white commissure); and loss of proprioception, vibratory sense, and discriminative touch on the right (gracile and cuneate fasciculi injury). These lesions are frequently called functional hemisections in recognition of the fact that the cord is not perfectly cut halfway across but may be injured or deformed by, for example, pieces of a damaged vertebra. The net result is a loss of function on half of the spinal cord.
High Cervical Cord Lesion
Injury to high cervical levels of the spinal cord is, in general, a catastrophic event. In addition to the potential for a total loss of sensation for the body below the lesion and of voluntary motor control below the lesion, there is another important complicating factor. The phrenic nucleus is located in the central regions of the anterior horn at levels C3 to C7. This cell group innervates the diaphragm and in high cervical lesions is disconnected from the centers of the medulla that control breathing. Consequently, in patients with high cervical lesions, preservation of the ability to breathe becomes a major factor in care.
Acute Central Cervical Spinal Cord Syndrome
The acute central cervical spinal cord syndrome, commonly called the central cord syndrome, is an incomplete spinal cord injury. This may result from hyperextension of the neck (sometimes in a patient with bone spurs on the vertebrae) that momentarily occludes blood supply to the cord via the anterior spinal artery. Consequently, the deficits reflect the territory served by the branches of this vessel. The results are bilateral weakness of the extremities (more so of the upper than of the lower), varying degrees and patterns of pain and thermal sensation loss, and bladder dysfunction. Many of these patients recover most or all of their function within 4 to 6 days. In general, function of the lower extremities returns first, bladder function next, and function of the upper extremities last. Pain and thermal sensations may return at any time, and posterior column sensations are not affected in these patients.
Variations on these main themes may occur. For example, a spinal cord hemisection at T8 would affect the body below that level but would spare the upper trunk and upper extremity. A lesion involving the posterior columns bilaterally would result in proprioceptive and discriminative touch losses below the level of the lesion but would spare pain and thermal sensations. In our study of systems neurobiology, we shall explore these and other examples of dysfunction resulting from spinal cord lesions.
Sources and Additional Reading
Brown AG. Organization in the Spinal Cord: The Anatomy and Physiology of Identified Neurons. Berlin: Springer-Verlag; 1981.
Rexed B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954;100:297–379.

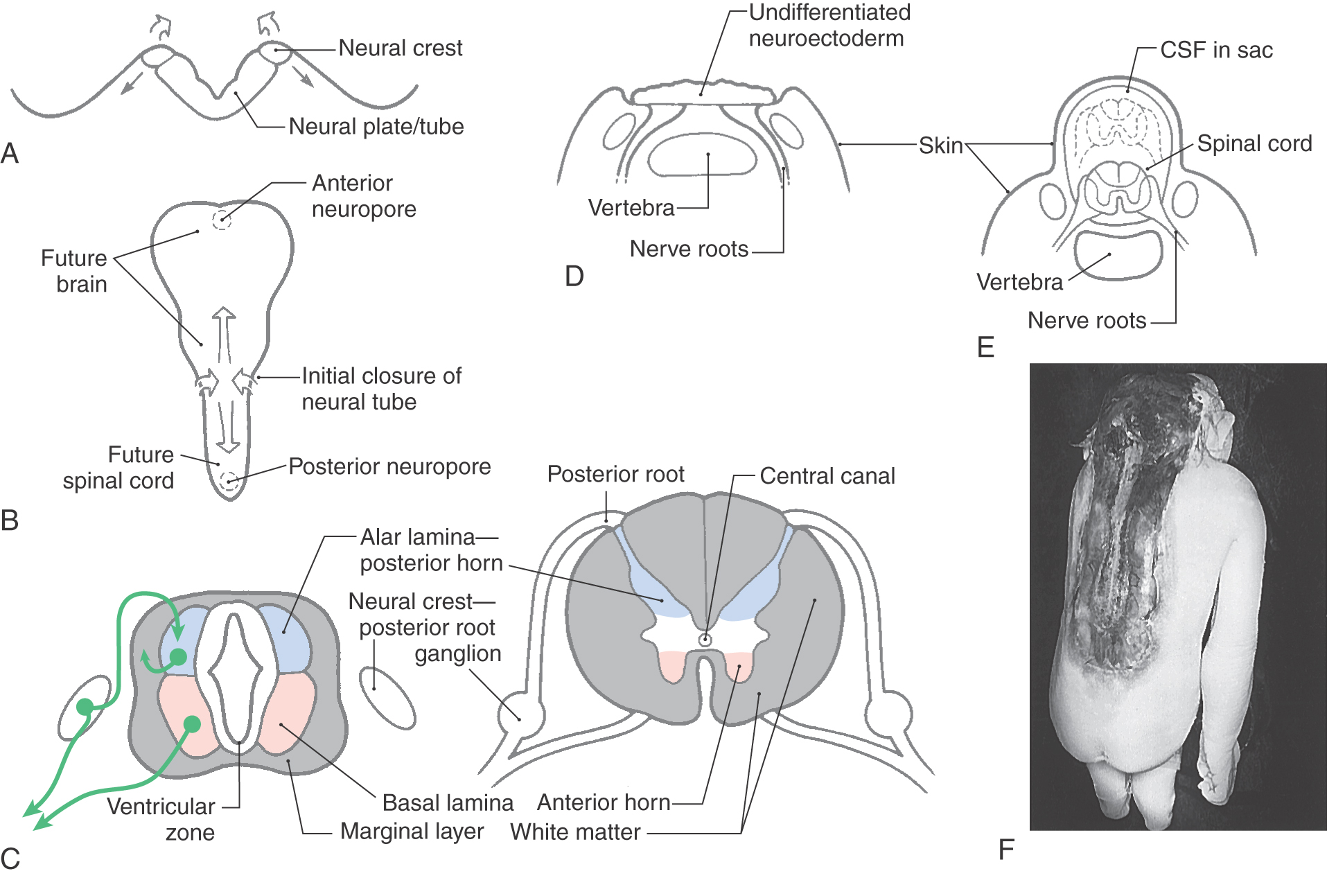
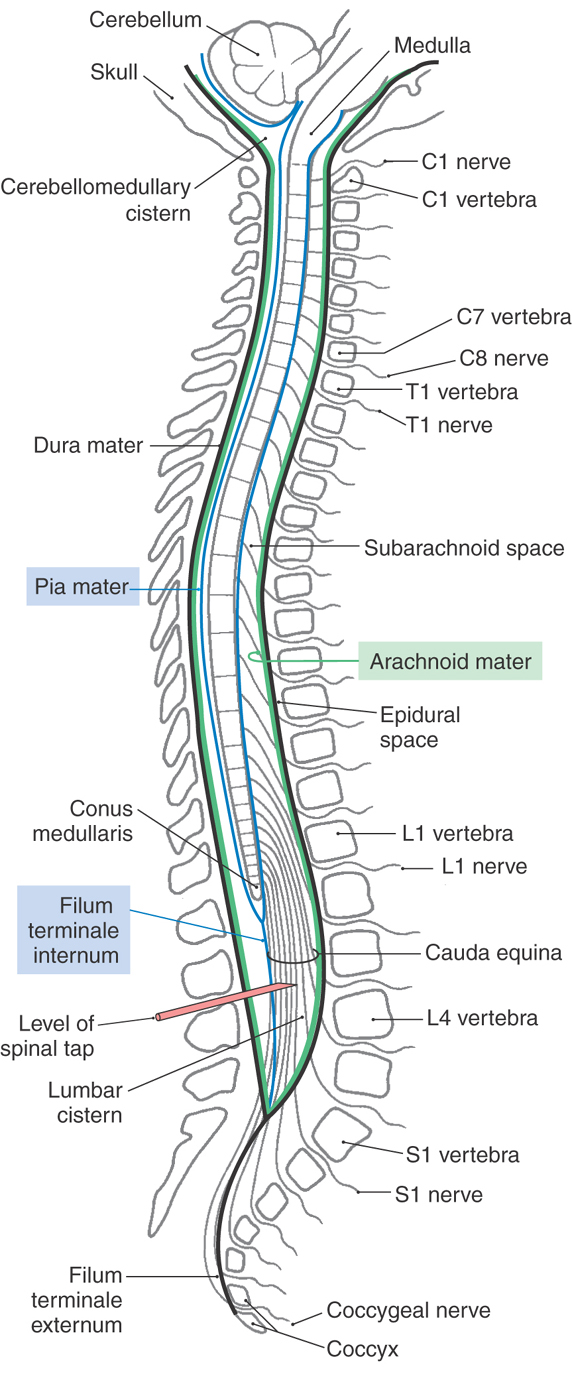
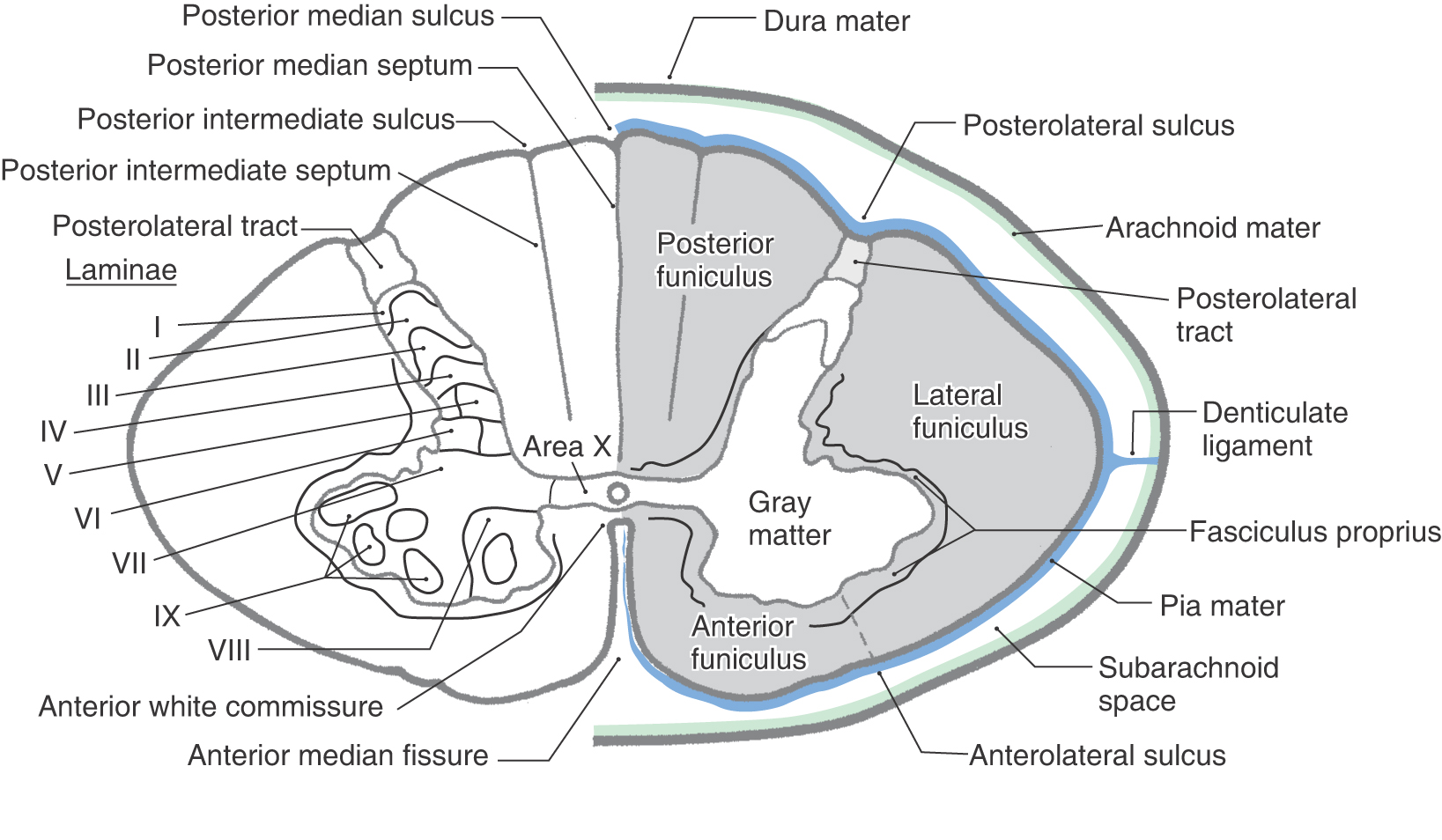
 Figure 9-3.
Figure 9-3. 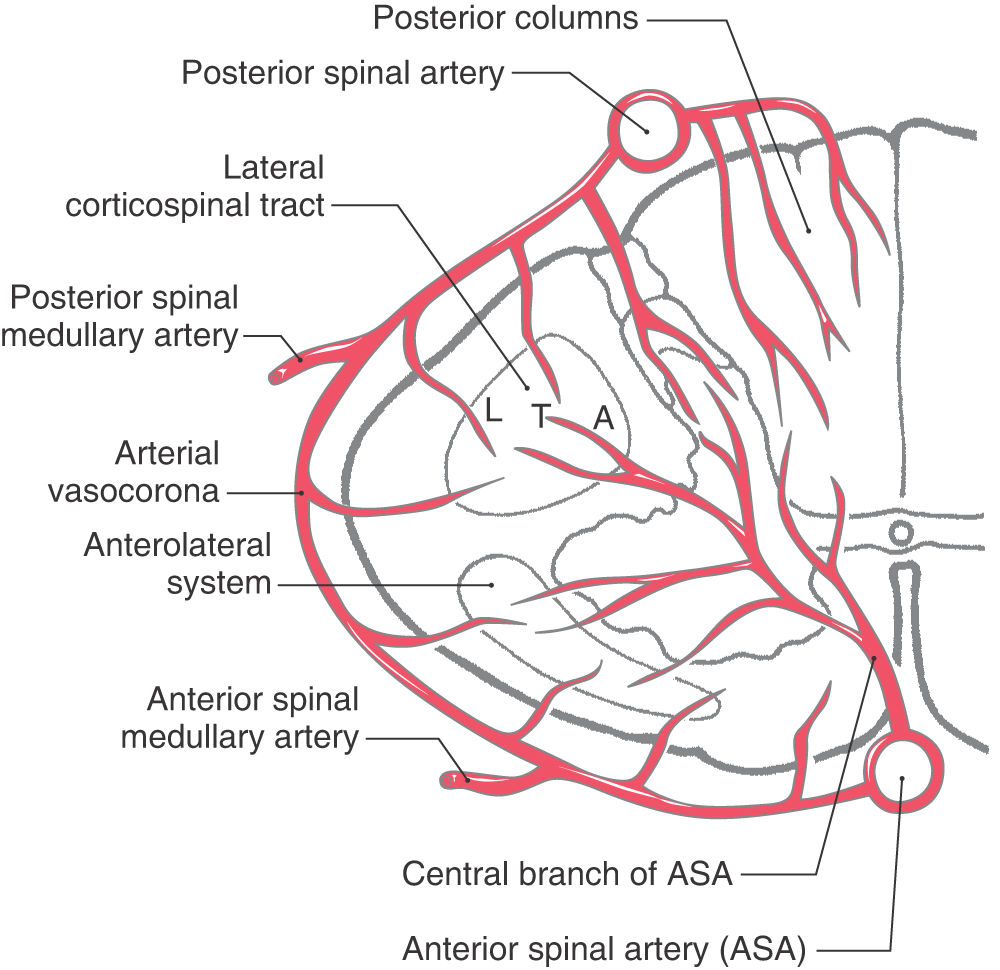
 Figure 9-4.
Figure 9-4. 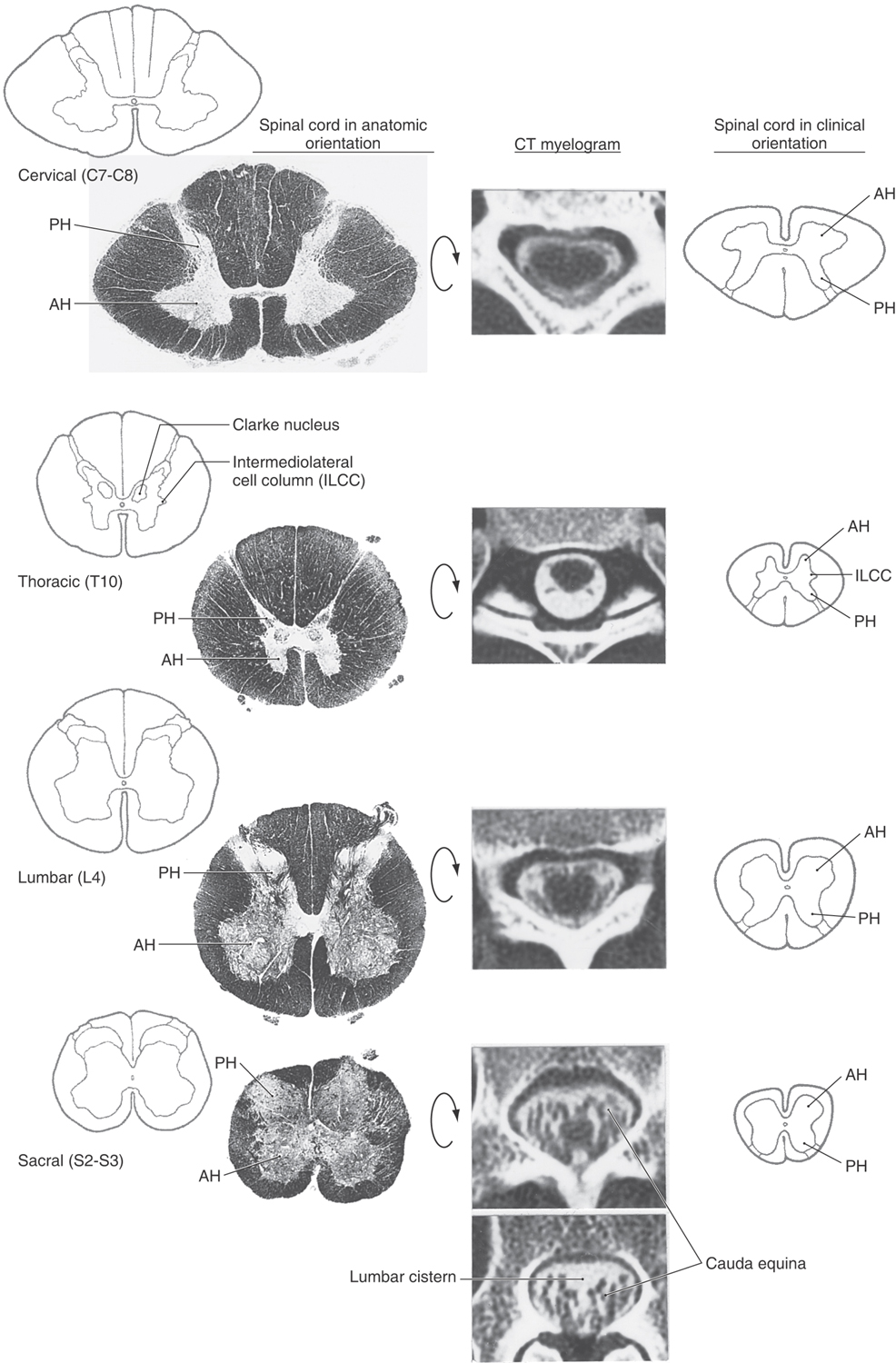
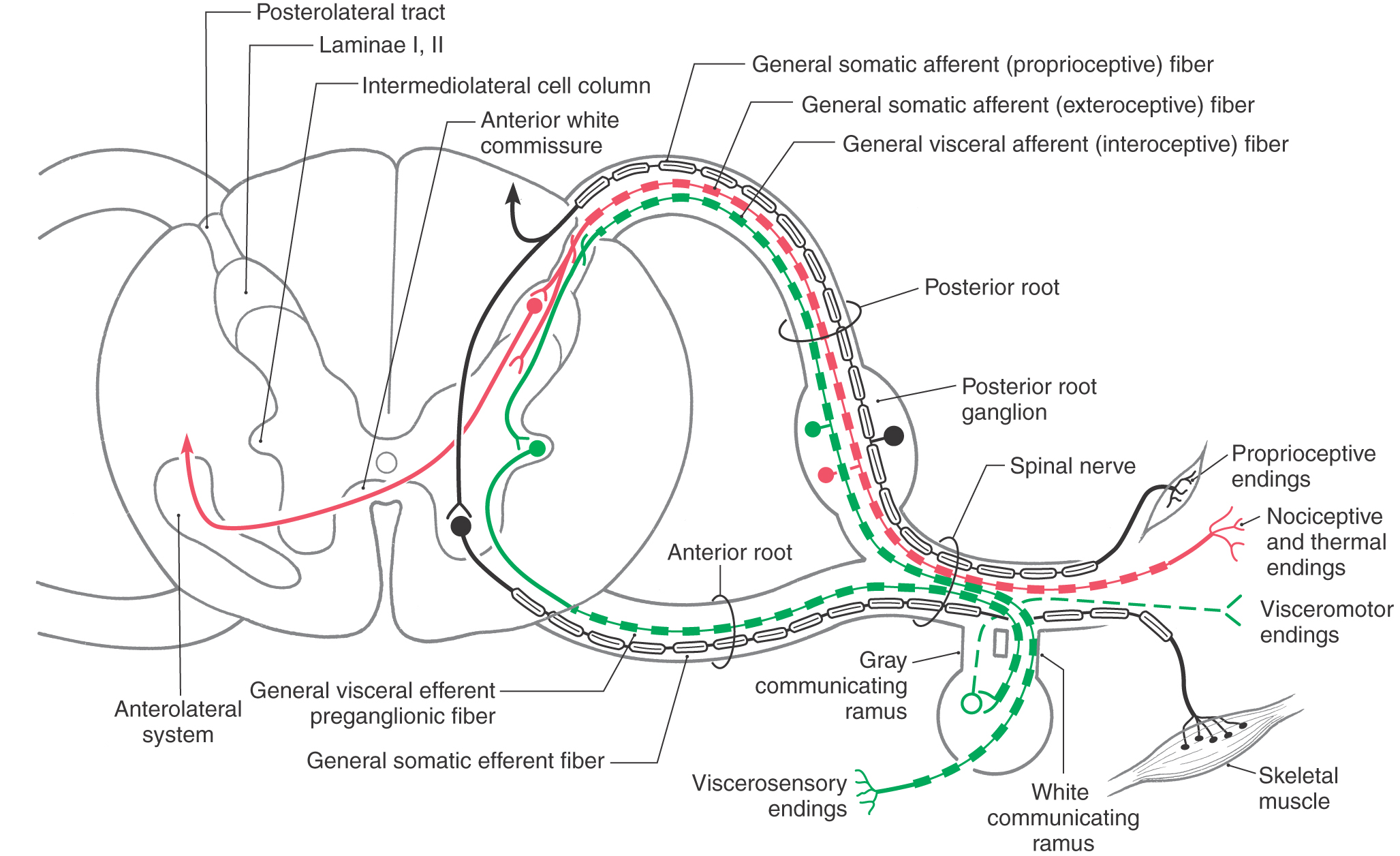
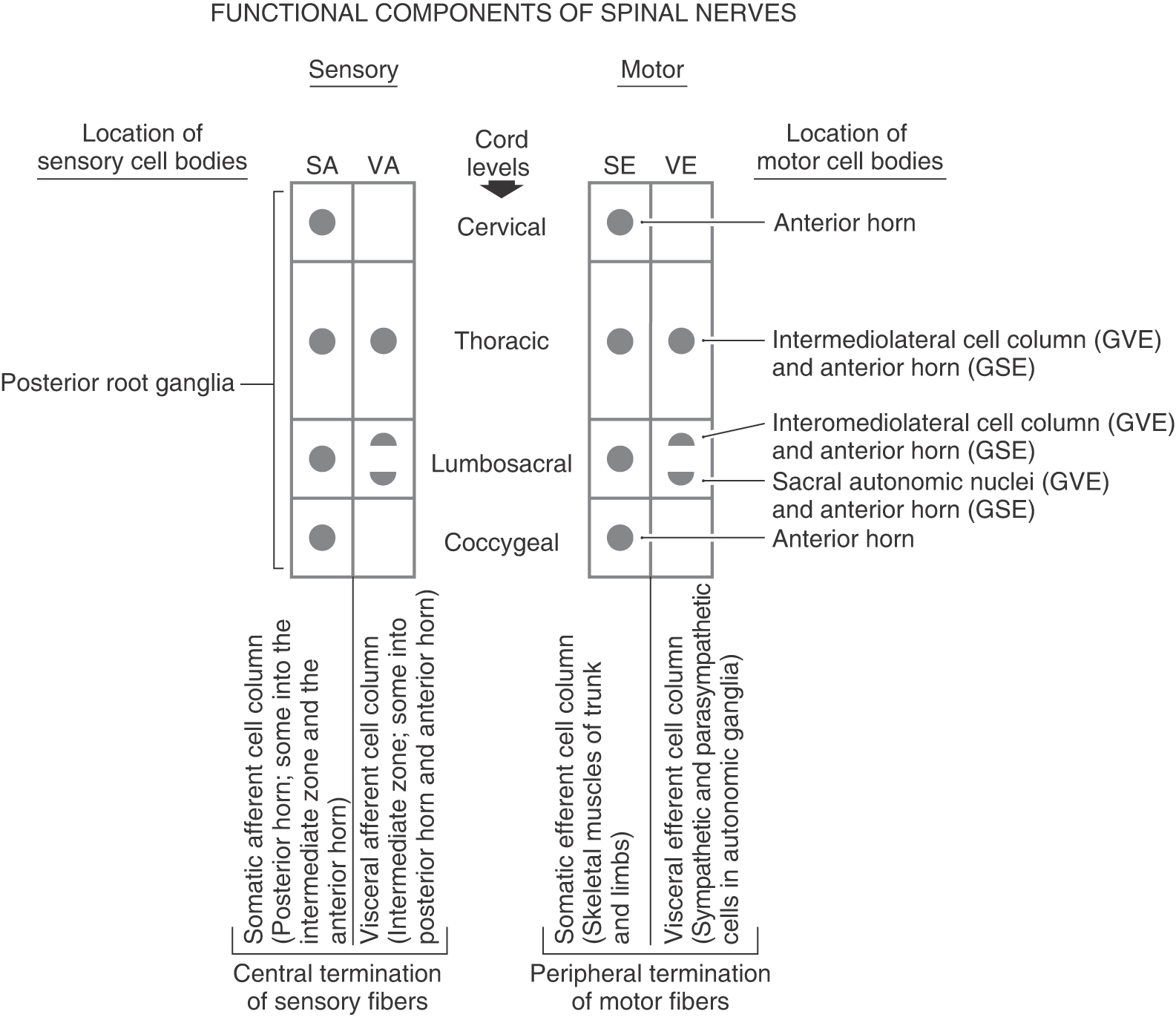
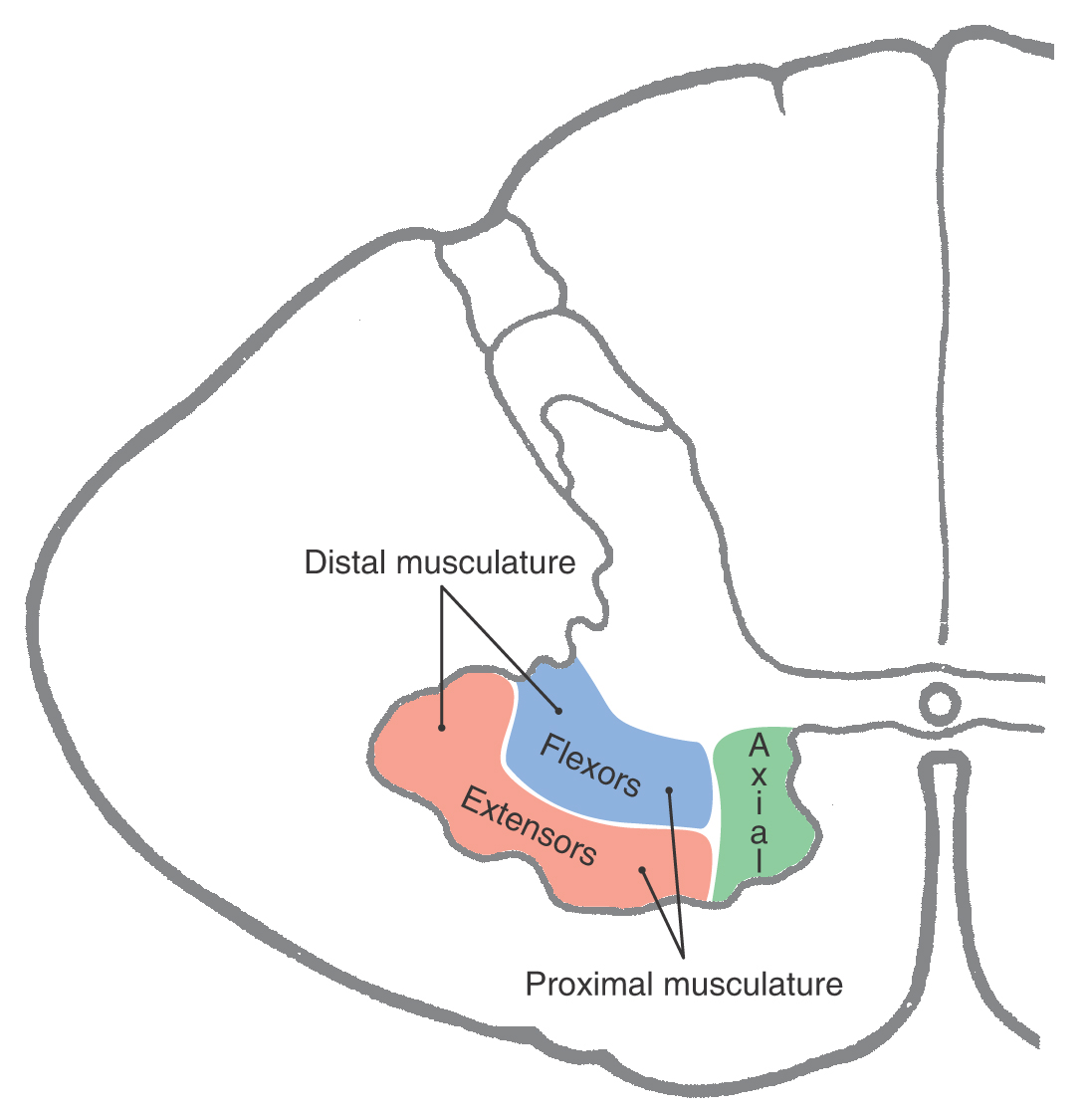
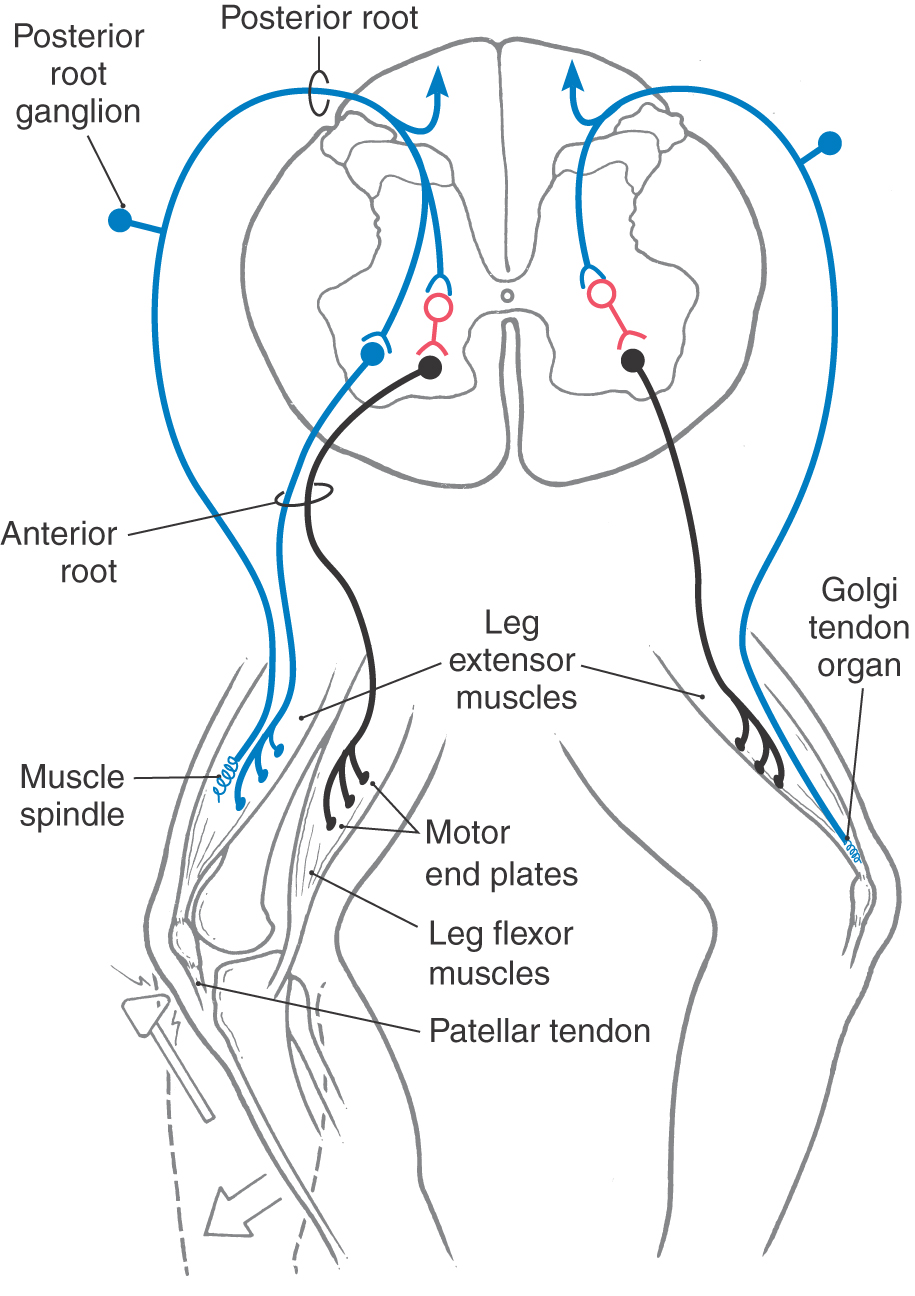
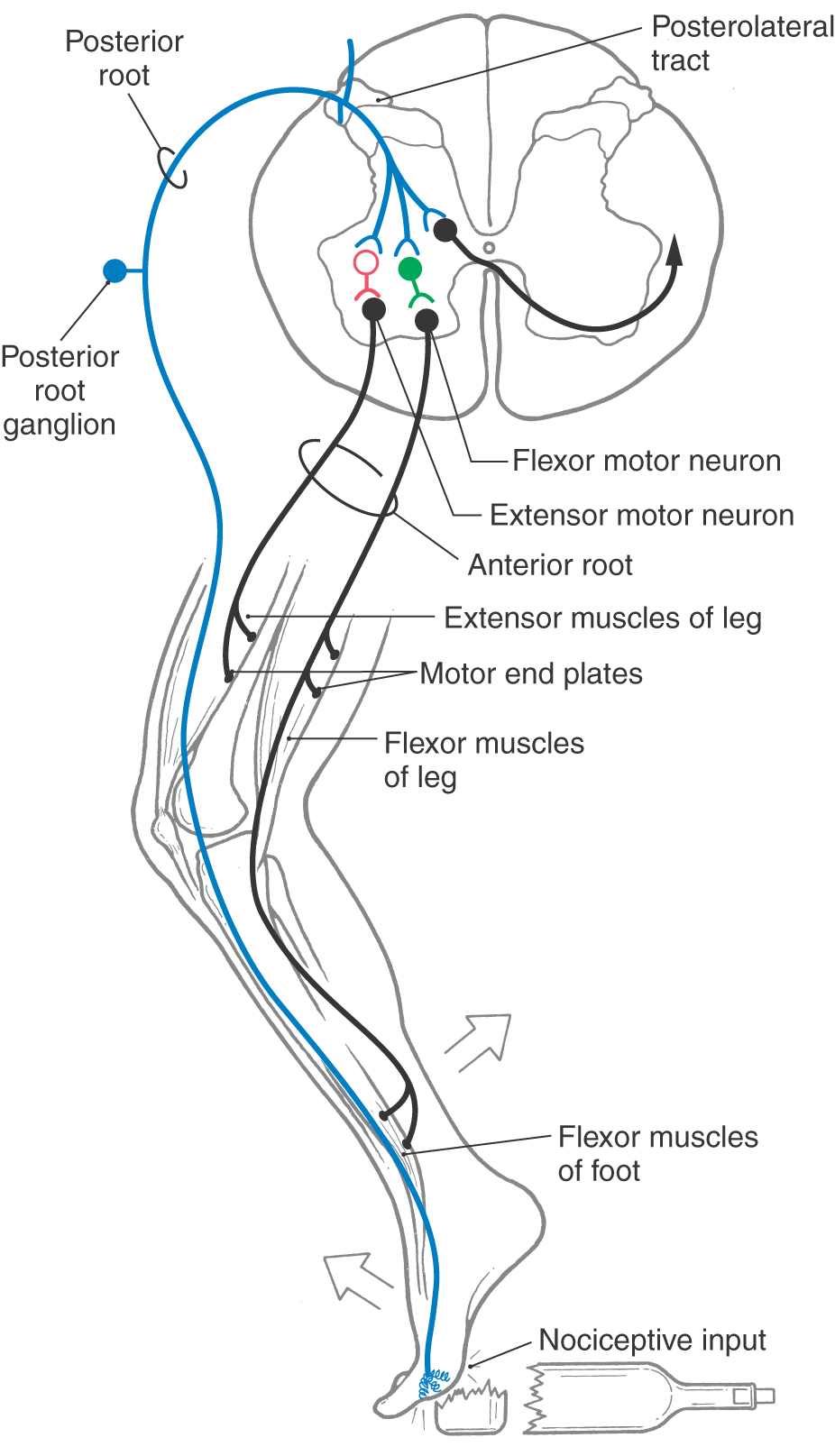
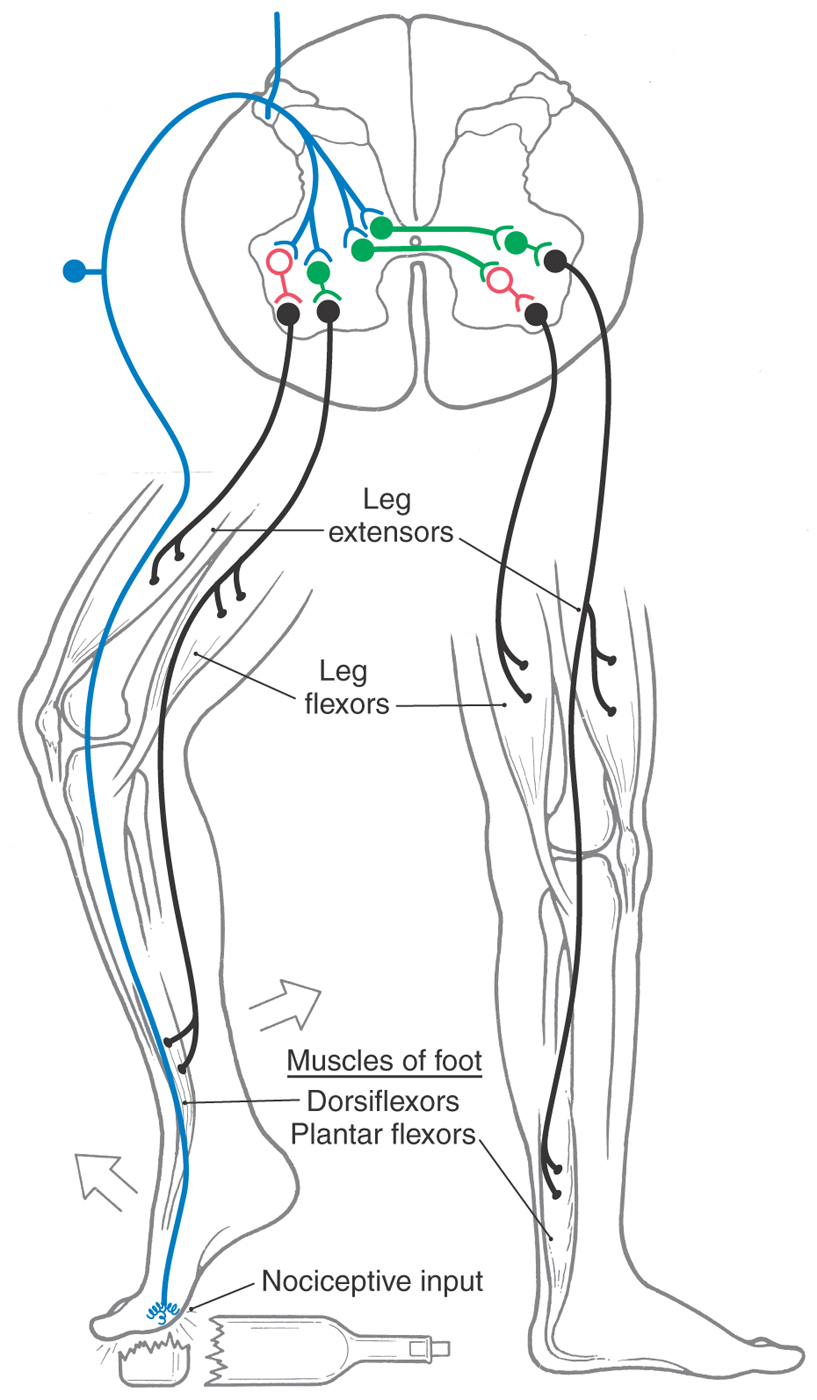
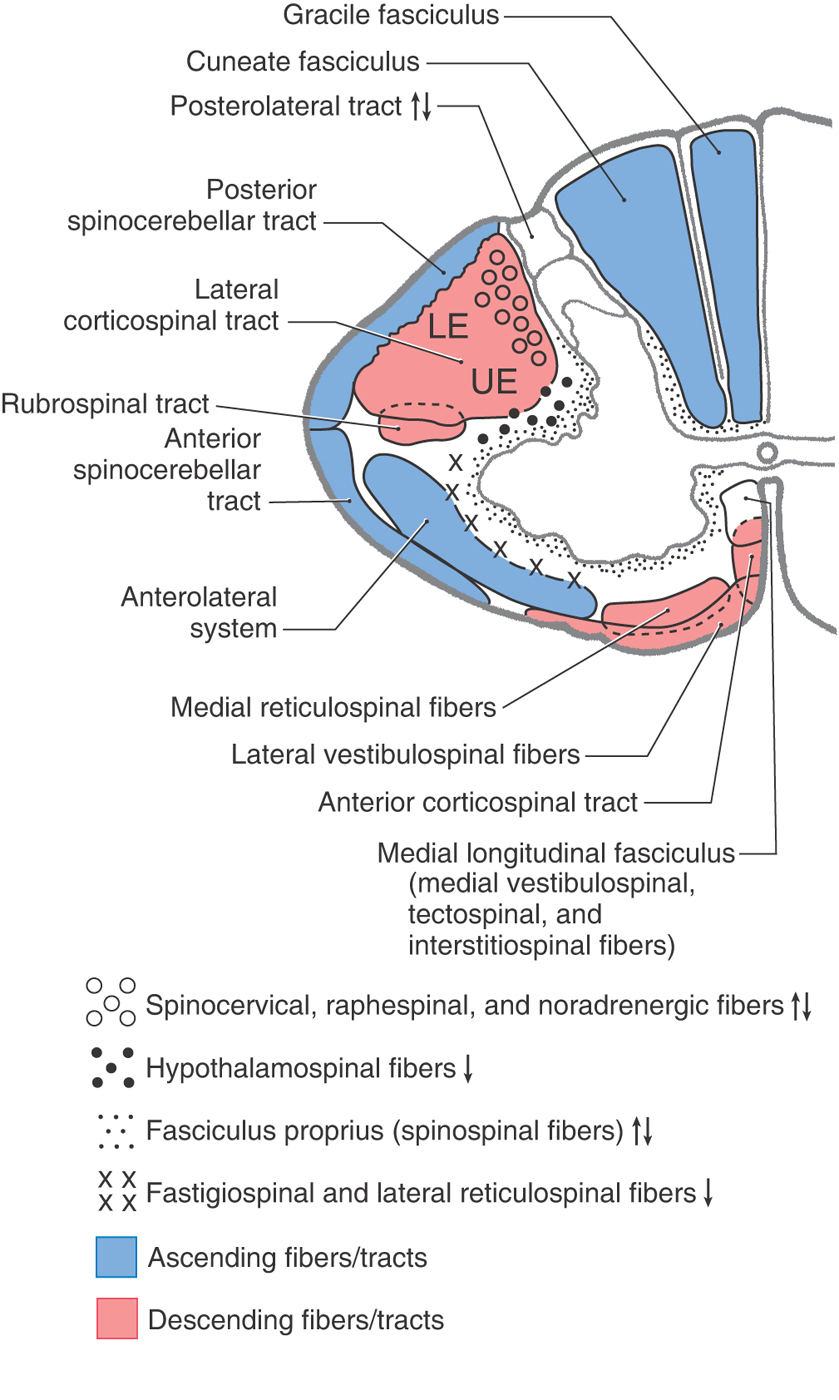
 Figure 9-12.
Figure 9-12.