Chapter 18
The Somatosensory System II: Nociception, Thermal Sense, and Touch
Cutaneous Nociceptors and Primary Neurons
Transient Receptor Potential Channels and Thermal Sensations
Peripheral Sensitization and Primary Hyperalgesia
Central Sensitization and Secondary Hyperalgesia
Pain Receptors in Muscles, Joints, and Viscera
Central Pathways from the Body
Spinal Trigeminal Pathway: Anterior Trigeminothalamic Tract
Central Pathways from the Face and Oral Cavity
One crucial role of the somatosensory system is to supply the brain with information related to insults that cause tissue damage. These signals ascend the neuraxis in a fiber bundle called the anterolateral system (ALS). Anyone who has used a hammer or hot skillet has had experience with this system. Hit your thumb with a hammer and, if you are lucky, only high-threshold mechanoreceptors that signal excess skin deformation will be activated. If you are unlucky, tissue is damaged and the result is pain (nociception). Specifically, mechanonociceptors have been stimulated. One common response is to gently rub the damaged area. This activates central nervous system (CNS) pathways that decrease the transmission of nociceptive signals and alter the perception of pain.
After the hammer has done its damage, tissues release chemicals that activate another type of pain receptor, chemonociceptors. These receptors may contribute to the mechanism underlying long-term pain and tenderness (hyperalgesia). Similarly, the temperature of a skillet is detected by thermoreceptors in the skin and transmitted through the ALS. If a burn is produced, the tissue damage is signaled by high-frequency firing of thermonociceptors. ALS activation can lead to a variety of responses, including withdrawal reflexes, the conscious perception of pain, emotional effects such as suffering, and behavioral changes aimed at avoiding the cause of the pain.
OVERVIEW
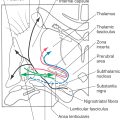
Nondiscriminative (poorly localized) touch, innocuous thermal, and nociceptive (mechanical, chemical, and thermal) sensations (from the body and back of the head) are conveyed by bundles of fibers that collectively make up the ALS. This system transmits signals originating in peripheral receptors to spinal cord and brainstem neurons (Fig. 18-1). These signals are then relayed to lateral and medial thalamic nuclei and from there to the trunk and extremity representations in the primary and secondary somatosensory cortex and limbic cortex. The anterior trigeminothalamic pathway (Fig. 18-1; see also Fig. 18-11) carries similar signals that originate from receptors in the face, oral cavity, teeth, and front of the head. These are relayed through brainstem and thalamic nuclei to face areas of the sensory cortex. The touch fibers of the ALS differ from those described for the posterior column–medial lemniscal system (PCMLS) (see Chapter 17) in several ways: (1) they yield a generalized feeling of being touched but do not give precise localization, (2) their receptive fields are larger, and (3) they are smaller in diameter and more slowly conducting. Disruption of the ALS can produce symptoms ranging from reduced sensibility (hypesthesia), to numbness, tingling, and prickling (paresthesia), to a complete loss of sensibility (anesthesia).
ANTEROLATERAL SYSTEM
Fibers within the lateral spinothalamic tract were considered to carry only pain and thermal information, whereas the anterior spinothalamic tract was thought to be concerned only with nondiscriminative touch. This older view of separate tracts conveying separate modalities of sensory information is not used in this chapter. Current thinking holds that all parts of the ALS carry all modalities (pain, temperature, and touch) but that there are direct and indirect pathways for this information to reach the brain. The direct pathway is the neospinothalamic pathway (spinal cord → lateral thalamus → somatosensory cortices), and the indirect pathway is the polysynaptic paleospinothalamic pathway (spinal cord → reticular formation → medial thalamus → cingulate, frontal + limbic cortices). Both of these pathways, plus other ascending fibers as defined later, collectively form the ALS.
The ALS is a composite bundle that includes spinothalamic, spinomesencephalic, spinoreticular, spinobulbar, and spinohypothalamic fibers. Spinothalamic fibers project directly from the spinal cord to the ventral posterior nuclei (ventral posterolateral [VPL] and ventral posterior inferior [VPI] nuclei) and the posterior nuclear group (including the ventral medial nucleus VMpo) of the thalamus. Collaterals to the reticular formation arise from some of these axons. Spinomesencephalic axons project to the periaqueductal gray (PAG) and to the tectum; the latter are spinotectal fibers. Although spinoreticular fibers project to the reticular formation of the medulla, pons, and midbrain, collaterals may ascend as reticulothalamic fibers to other targets, such as the intralaminar and dorsomedial nuclei of the thalamus. Spinohypothalamic fibers terminate in hypothalamic areas and nuclei, including some that give rise to hypothalamospinal axons. Projections of less relevance to the somatosensory system, such as spinoolivary fibers, are grouped under the category of spinobulbar fibers.
Cutaneous Nociceptors and Primary Neurons
The receptors for nondiscriminative touch, innocuous thermal stimuli, and nociceptive stimuli are distributed in glabrous and hairy skin as well as in deep tissues, including muscles, joints, blood vessels, and viscera (Table 18-1). Morphologically, these receptors are all free nerve endings (see Fig. 17-1); that is, they lack specialized receptor cells or encapsulations. Because of this lack of specialization, the basis for their submodality specificity is unclear. Nonetheless, these submodalities are transduced by activation of peripheral branches of either thinly myelinated Aδ (A-delta) fibers or unmyelinated C fibers. The density of free nerve endings and the corresponding size of receptive fields vary over the body surface in the same way as for other cutaneous receptors (see Fig. 17-3), being highest on the hands and in the perioral area. Regardless of size or location, however, each field is exquisitely sensitive to thermal, chemical, or mechanical stimuli.
Table 18-1 Classification of Cutaneous Mechanical, Thermal, and Nociceptive Receptors by Small-Diameter Fibers and Their Adequate Stimuli
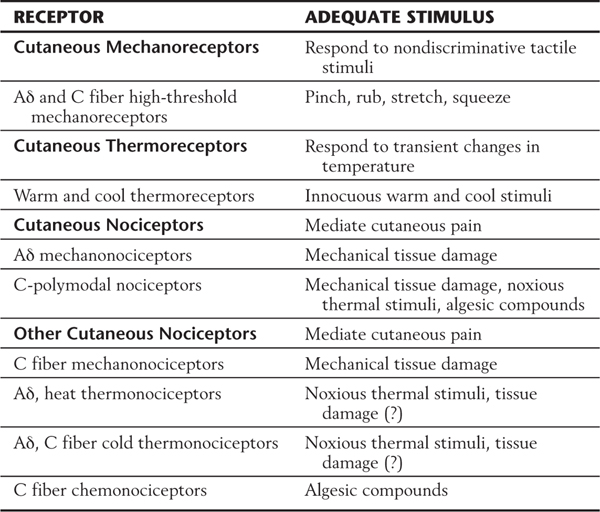
Nondiscriminative touch results from the stimulation of free nerve endings that act as nonnoxious high-threshold mechanoreceptors (Table 18-1). These receptors respond to any rough stimulus, including tapping, squeezing, rubbing, and stretching of the skin, that does not result in tissue damage (Fig. 18-2A). Nerve fibers associated with these receptors generally have no background activity when they are unstimulated; when they are stimulated, they respond with a sustained discharge that signals stimulus duration (Fig. 18-2A).
Nonnociceptive thermoreceptors fall into two classes: those activated by warmth (35° C to 45° C) and those activated by cooling (17° C to 35° C). They show a graded response to changes in ambient temperature (Fig. 18-2B). With repeated stimulation, these receptors become sensitized and have a decreased activation threshold and a larger response to the application of a stimulus.
Nociceptors (pain receptors) are found in cutaneous as well as in deep structures. Two major classes of cutaneous pain receptors have been identified. They are the Aδ mechanical nociceptors and C-polymodal nociceptors. These receptors are found at the end of peripheral processes of thinly myelinated (Aδ) or nonmyelinated (C) fibers (Table 18-1). The cutaneous receptive field of an Aδ nociceptor consists of a number of small sensitive spots (2 to 30) scattered over an area of skin. Each spot ranges from 50 to 180 µm in diameter. Aδ mechanical nociceptors respond to mechanical injury accompanied by tissue damage. C-polymodal nociceptors respond to mechanical, thermal, and chemical stimuli. The cutaneous receptive field of a C-polymodal nociceptor usually consists of one or two sensitive spots, with each spot covering an area of skin 1 to 2 mm2. For a comparable region of skin, the C fiber spots are larger but fewer in number than the Aδ spots, which are smaller but more numerous.
Other cutaneous receptors that respond to high-threshold or noxious stimuli have been identified. They include receptors that respond to extreme temperature changes (thermonociceptors) (Table 18-1) and receptors that respond to chemicals, irritants, or algesic (pain-producing) compounds (chemonociceptors) (Table 18-1). Extreme heat (>45° C) or cold (<17° C) that burns or freezes the skin produces high-frequency firing in both Aδ and C thermonociceptors (Fig. 18-2B). C fiber chemonociceptors (Table 18-1) are activated by the release of endogenous substances associated with tissue damage and inflammation, such as bradykinin and hydrogen ions, and foreign irritants such as insect venoms.
Transient Receptor Potential Channels and Thermal Sensations
Specialized surface membrane receptors called transient receptor potential (TRP) channels have recently been identified in cutaneous Aδ and C thermonociceptors. Several of these channels respond to application of either noxious heat or noxious cold. The most common of these receptors, the capsaicin receptor TRPV1, is activated by capsaicin (the active ingredient of chili peppers), noxious temperatures (>43° C), and protons. A second channel, the TRPV2 receptor, is activated by high intensities of noxious heat (>52° C). The cold receptor TRPA1 responds to very low temperatures (<16° C) and some chemical substances. When activated, these receptors can signal thermal pain.
Nonnociceptive thermal stimuli activate TRP channels in Aδ and C thermoreceptors. Warm thermoreceptors express TRP3 (>33° C) and TRP4 (24° C to 34° C) receptors. Cold thermoreceptors express TRPM8 (<23° C) receptors, which respond to cooling and menthol. Activation of these receptors can signal the range of innocuous thermal sensations.
Peripheral Sensitization and Primary Hyperalgesia
Pain receptors, unlike Meissner corpuscles or Merkel cells, demonstrate a unique phenomenon called sensitization. After an insult, these receptors become more sensitive (lower pain activation threshold) and thus more responsive (increases in firing rate) to noxious stimulation within their receptive fields. Although the mechanisms responsible for receptor sensitization are not completely known, irritating chemicals (capsaicin), inflammatory mediators (bradykinin, prostaglandins), and neurotransmitters (serotonin, histamine, norepinephrine) released from damaged skin or byproducts from plasma, or both, are thought to contribute to this phenomenon. As a result of this heightened sensitivity, the affected area is exquisitely sensitive to painful stimuli, and patients experience a sensory disturbance called hyperalgesia (exaggerated response to a painful stimulus). This condition can be differentiated into primary hyperalgesia and secondary hyperalgesia. Primary hyperalgesia occurs in the region of damaged skin and is probably the result of receptor sensitization. Secondary hyperalgesia occurs in the skin immediately bordering the damaged tissue. Although receptor sensitization may contribute to secondary hyperalgesia, there is likely to be a central (e.g., spinal) component as well.
Central Sensitization and Secondary Hyperalgesia
Sensitization of peripheral nociceptors causes an increase in spontaneous activity in the Aδ and C fibers. The central processes of these fibers enter the posterior horn of the spinal cord, where they activate posterior horn neurons in laminae I to V. Ongoing inputs from these injured peripheral nociceptors evoke a number of changes in the central processing of sensory information by the posterior horn neurons. These changes include a marked increase in the receptive field size of the posterior horn neurons (to include skin areas not involved in the initial injury), an increased response of the cells to the application of suprathreshold stimuli, a decreased threshold to stimulus application in the receptive field, and the activation of the cell by novel inputs (e.g., a light breeze). This phenomenon is known as central sensitization, and it represents a potentiated state in which the system has been shifted from one functional level (normal) to another (sensitized). In most patients, an innocuous stimulus, such as a gentle breeze or a light touch, can evoke pain sensation in the skin bordering the damaged tissue. The perception of an innocuous stimulus as painful is referred to as allodynia and can be the result of central sensitization.
Pain Receptors in Muscles, Joints, and Viscera
In addition to cutaneous pain receptors, pain receptors in muscles, joints, blood vessels, and viscera have also been identified (Table 18-2). Muscle pain is mediated by receptors of both group III (thinly myelinated) and group IV (nonmyelinated) afferent fibers. Excessive stretch or contraction after strenuous exertion may activate the muscle pain receptors of group III fibers. Receptors of group IV fibers may be activated in response to the release of algesic compounds after muscle injury or ischemia. Joint pain, including arthritis, may be caused by inflammation. This pain is mediated by receptors associated with group III and group IV fibers. Visceral pain is often described as being diffuse and difficult to localize and is frequently referred to an overlying somatic body location. In addition, visceral pain usually involves autonomic reflexes. Visceral pain receptors located in the heart, respiratory structures, gastrointestinal tract, and urogenital tract are poorly identified (Table 18-2). These receptors can be activated by intense mechanical stimuli including overdistention or traction, ischemia, and endogenous compounds, including bradykinin, prostaglandins, hydrogen ions, and potassium ions. Activation of these receptors produces pain.
Table 18-2 Classification of Deep and Visceral Nociceptors and Their Adequate Stimuli
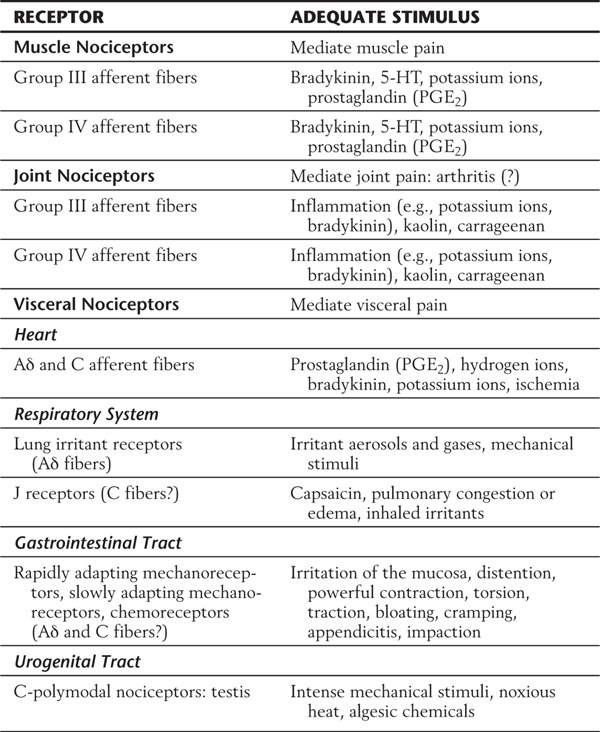
5-HT, 5-hydroxytryptamine.
Of the two primary afferent fiber types carrying nociceptive sensations, Aδ fibers have a slightly faster conduction velocity (5 to 30 m/s) than that of C fibers. They carry well-localized acute sensations that do not evoke an affective component to the sensory experience. A pinprick, used clinically to test ALS function, is one stimulus that activates Aδ fibers. On the other hand, C fibers are smaller and conduct more slowly (0.5 to 2 m/s). They transmit poorly localized diffuse sensations that produce a noticeable affective response. For example, the dull, persistent ache that follows a muscle pull results from activation of C fibers. Both Aδ and C fibers are considerably smaller and conduct more slowly than fibers of the PCMLS. A nerve block or anoxia preferentially affects large-diameter, heavily myelinated fibers and thus usually results in loss of discriminative tactile, vibratory, and postural sensations to varying degrees. Local anesthetics, such as lidocaine and bupivacaine, preferentially affect small-diameter Aδ and C fibers by blocking sodium channels and thus result in loss of nociception (analgesia).
Cell bodies of C and Aδ fibers are generally small compared with other pseudounipolar neurons in the posterior root ganglion. The central processes of these cells enter the spinal cord via the lateral division of the posterior root (see Fig. 17-5). Many smaller fibers contain excitatory amino acids such as glutamate as well as peptides such as substance P and calcitonin gene–related peptide that may serve as neurotransmitters. The central processes of these fibers also contain surface membrane receptors. They include a γ-aminobutyric acid (GABA) receptor, a serotonin (5-hydroxytryptamine) receptor, and a mu opioid receptor. Pharmacologic therapies as well as descending fibers (see Figs. 18-20 and 18-21) can act at these presynaptic sites and suppress the initiation of the pain signal or block transmitter release from primary afferent terminals. In addition to their normal trajectory into the posterior horn, a small number of C fibers enter the spinal cord through the anterior root of a spinal nerve. It is possible that these fibers provide a basis for the return of pain after posterior rhizotomy, a procedure in which posterior roots are sectioned in an attempt to alleviate intractable pain.
The strip of skin that is innervated by the peripheral cutaneous branches of a given spinal nerve is called a dermatome (Figs. 18-3 and 18-4). The central processes of these nerves that convey cutaneous input terminate in the posterior horn. It is clinically useful to examine dermatomes that have relationships to landmarks on the body: for example, C7 for the index finger, the T4 to T5 border at the nipples, T10 at the navel, L1 along the pelvic rim, L5 for the big toe, and S4 and S5 for the genitalia and anus (Fig. 18-4). There is overlap between the peripheral and central distribution of adjacent spinal nerves and consequently between their dermatomes (Fig. 18-3). This overlap reduces the effects of injury to a single spinal root.
Figure 18-3. The dermatomes formed by the peripheral processes of adjacent spinal nerves overlap on the body surface (see Fig. 18-4). The central processes of these fibers also overlap in their spinal distribution.
Shingles (herpes zoster) is a disease of viral etiology that is noteworthy for its dermatomal distribution (Fig. 18-5). Subsequent to a bout of chickenpox, viral DNA may infect and become latent in trigeminal and posterior root ganglion cells. The virus may reactivate periodically, producing infectious virions that travel down the peripheral processes of the neurons to produce a painful skin irritation in the dermatomal distribution of the ganglion (Fig. 18-5). When an injury or disease process affects a series of nerve roots, the result is diminished sensibility (hypesthesia) over the dermatomes served by those roots. The borders of the hypesthetic region correspond to dermatomal boundaries. However, the most debilitating aspect of this disease is a poorly understood recurrence of pain. Known as postherpetic neuralgia, this condition falls under the category of neuropathic pain.
Clinically, it is important to test patients for intact ALS and PCMLS function. In testing of the ALS, a single pinpoint applied to the skin should evoke a response (perception of pain) from the patient. ALS modalities are also evaluated by comparing pinprick of one side of the face with the other side or by comparing more distal portions of the extremities (toes and fingers) with more proximal portions (knees and elbows). Patients with diabetes mellitus may experience a sensory loss, including pinprick, that starts distally and proceeds proximally; this is a stocking-glove sensory loss because it initially affects the feet and hands first. Function of the intact PCMLS is tested by the simultaneous application of two points spaced at measured intervals. As the points are moved closer, the ability to identify them as separate stimuli (two-point discrimination) decreases and eventually disappears. The PCMLS is also tested by applying a 128-Hz tuning fork (vibratory sense) to a bone prominence or the tip of a finger or toe. The patient perceives this as a buzzing sensation. It is common to test both pain/thermal sense and discriminative touch/vibratory sense on both sides of the face and body to see if there are asymmetries.
Central Pathways from the Body
Aδ and C fibers enter the spinal cord via the lateral division of the posterior root entry zone. The fibers travel in the posterolateral fasciculus (Lissauer tract) and bifurcate into ascending and descending branches (see Fig. 17-5). Some fiber collaterals terminate on interneurons in the spinal gray matter. These connections participate in the circuits that mediate spinal reflexes, such as the flexor withdrawal reflex (see Chapter 9).
The central target of nociceptive primary afferent fibers includes laminae I, II, and V of the posterior horn (Fig. 18-6). Aδ fibers target laminae I and V. Rexed lamina I, the posteromarginal nucleus (or zone), receives mainly input from Aδ fibers (Fig. 18-7A). Neurons in this nucleus (or zone) project to other spinal cord laminae, the brainstem reticular formation, and the thalamus (Fig. 18-7B). Lamina II, the substantia gelatinosa, is divided into outer (IIo) and inner (IIi) layers. Input to IIo and IIi is derived primarily from C fiber primary afferents, and IIi also receives input from collaterals of nonnociceptive afferent fibers. Lamina II contains excitatory and inhibitory interneurons that project to other laminae of the posterior horn. In addition, some neurons in IIo are involved in relaying sensory information to supraspinal sites, including the thalamus (Fig. 18-7B).
Figure 18-7. Summary of posterior horn laminae and their major sensory inputs (A) and major outputs (B).
Neurons in laminae III and IV, the nucleus proprius (posterior proper sensory nucleus), receive nonnoxious inputs from the periphery. Cells in these laminae project to deeper laminae of the spinal cord, to the posterior column nuclei, and to other supraspinal relay centers including the medulla, pons, midbrain, thalamus, and hypothalamus (Fig. 18-7B).
Lamina V neurons receive both noxious and nonnoxious (nociceptive and nonnociceptive) inputs and project to the medullary and mesencephalic reticular formation, thalamus, and hypothalamus (Fig. 18-7B). Neurons in deeper laminae of the spinal gray receive (directly and indirectly) noxious and nonnoxious inputs and connect with neurons in other spinal cord levels (propriospinal connections).
Posterior horn neurons exhibit numerous membrane receptors. These include a GABA receptor, a serotonin receptor, a mu opioid receptor, and the AMPA and NMDA receptors for glutamate. Descending fibers from the raphe nuclei and locus ceruleus can act on postsynaptic sites and can effectively block forward transmission of the pain signal.
The functional properties of posterior horn neurons reflect the type of primary afferent fiber input received. These neurons are classified as low threshold (nonnociceptive), nociceptive specific (noxious), wide dynamic range (nonnociceptive and noxious), or deep on the basis of their responses to different stimulus modalities.
The fibers of the ALS participate in both direct and indirect spinothalamic pathways (Fig. 18-1). Most Aδ fibers contribute to the direct (neospinothalamic) pathway, which carries nondiscriminative tactile, innocuous thermal, and nociceptive signals. When these Aδ fibers enter the posterolateral fasciculus and bifurcate, their branches travel rostrocaudally for three to five spinal levels. The descending branches terminate on interneurons within the spinal gray and contribute to the execution of segmental spinal reflexes. The ascending branches terminate on second-order neurons (tract cells) in lamina I of the posterior horn (Fig. 18-7A). These tract cells, in turn, project to the lateral region of the thalamus. The great majority of their axons cross the midline of the spinal cord obliquely via the anterior (ventral) white commissure and ascend in the contralateral ALS. A few ascend in the ipsilateral ALS. The thalamic (third-order) neurons of these pathways are located mainly in the VPL, the VPI, and the posterior group including VMpo. This laterally organized, direct pathway underlies rapid and precise localization and discrimination of nociceptive stimuli.
The polysynaptic indirect (paleospinothalamic) component of the ALS relays noxious and innocuous mechanical and thermal information to the medial brainstem reticular formation. The input to this pathway originates chiefly from C fibers. Branches of these fibers ascend and descend one or two levels in the posterolateral fasciculus to synapse on interneurons in laminae II and III (Fig. 18-7B). These interneurons influence tract cells in laminae V to VIII, which send axons that cross obliquely through the anterior white commissure (over a distance of one to three segments) to join the contralateral ALS. These spinoreticular fibers terminate in the brainstem reticular formation, which in turn projects to the medial thalamic nuclei. This medially organized indirect pathway contributes to the perception of dull pain sensations as well as to behavioral and motivational changes associated with nociceptive stimulation.
Fibers in the ALS are arranged somatotopically in the spinal cord. Axons from lower levels (coccygeal and sacral) of the body are found posterolaterally, whereas those from more rostral levels of the cord are added in an orderly anteromedial sequence (Figs. 18-8 and 18-9). The deficits seen in patients with certain types of spinal cord lesions reflect this somatotopic pattern (Fig. 18-8). For example, an intramedullary tumor located on one side of the cord that expands laterally results in a loss of pain and thermal sensations that initially begins in cervical levels on the contralateral side and proceeds caudally as the lesion enlarges. On the other hand, an extramedullary tumor that expands medially, causing cord compression, results in a loss of these same sensations that first begins in lumbosacral levels on the contralateral side and proceeds rostrally as the lesion expands (Fig. 18-8).
 Figure 18-9. The anterolateral system in its entirety and blood supply to these fibers in the spinal cord and medulla.
Figure 18-9. The anterolateral system in its entirety and blood supply to these fibers in the spinal cord and medulla.
Because of its location, the ALS does not receive blood supply from a single vessel. Instead, its blood supply originates from the arterial vasocorona and via sulcal branches of the anterior spinal artery (Fig. 18-8). Consequently, occlusion of either of these vessels results in a patchy loss of nociceptive, thermal, and touch sensations over the contralateral side of the body beginning about two spinal segments below the lesion. In contrast, a complete loss of these sensations is seen in patients who have had an anterolateral cordotomy for relief of intractable pain.
The ALS may be involved in trauma or diseases of the spinal cord. For example, a hemisection of the spinal cord (as in the Brown-Séquard syndrome) results in a combination of sensory and motor losses. Sensory deficits include (1) contralateral loss of nociceptive and thermal sensations over the body below the level of the lesion (ALS damage) and (2) ipsilateral loss of discriminative tactile, vibratory, and position sense over the body below the level of the lesion (posterior column damage) (Fig. 18-10 A). The motor loss is manifested as an ipsilateral paralysis of the leg or leg and arm, depending on the level of the hemisection (see Chapter 24).
Syringomyelia, a condition in which there is cystic cavitation of central regions of the spinal gray matter, may impinge on the anterior white commissure and decussating ALS fibers (Fig. 18-10B). When it is located at the C4 to C5 levels of the spinal cord, this lesion produces bilateral loss of nondiscriminative tactile, nociceptive, and thermal sensations beginning several segments below the level where the fibers are interrupted. The symptoms present as sensory losses in the configuration of a cape draped over the shoulders and extending down to nipple level.
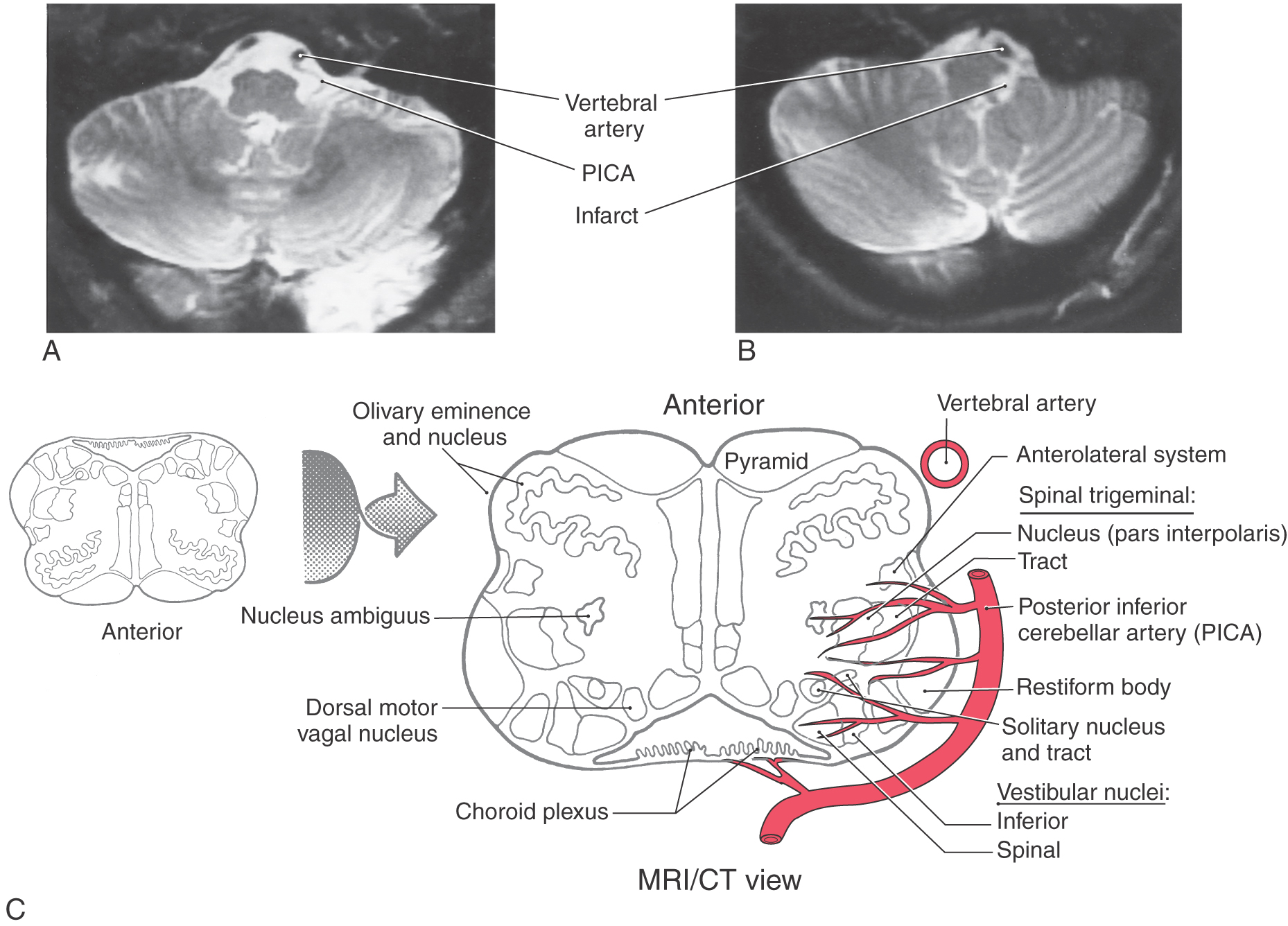
In the medulla, ALS fibers retain their position near the anterolateral surface. They are located anterior to the spinal trigeminal nucleus and posterolateral to the inferior olive and remain separated from the PCMLS as both course through the medulla and pons (Figs. 18-9 and 18-11). Therefore vascular lesions or tumors in the lower brainstem can affect discriminative touch and nociception differentially. For example, a lesion in medial portions of the medulla may result in a contralateral loss of discriminative touch and vibratory sense but not of pain and thermal sensation; this is a dissociated sensory loss (one modality absent but not another). At the pontomedullary junction, the medial lemniscus begins to rotate to a mediolateral orientation (see Fig. 12-13). By midpontine levels, the ALS is adjacent to the lateral extreme of the medial lemniscus (Figs. 18-9 and 18-11); this is the portion of the medial lemniscus containing fibers relaying information from the contralateral lower extremity.
Within the midbrain, the close relationship between the ALS and medial lemniscus is maintained (Figs. 18-9 and 18-11) even though the latter structure has been shifted somewhat posterolaterally by the decussating fibers of the superior cerebellar peduncle and the red nucleus. In this juxtaposition, small lesions or vascular insults may involve one or portions of both the ALS and medial lemniscus (Fig. 18-12); they are now in a single vascular territory. The patient in Figure 18-12B has a small lesion of the lateral midbrain involving the brachium of the inferior colliculus and the immediately adjacent ALS on the left. He experienced a loss of pain and thermal sense on the right side of the body only, sparing the head. The patient in Figure 18-12C has a lesion involving the brachium of the inferior colliculus, the ALS, and the lateral portions of the medial lemniscus on the right. She had a loss of pain and thermal sense on the left side of the body (sparing the head) and a loss of proprioception and vibratory sense on the left lower extremity (upper extremity is spared). In these patients, there was no discernible hearing loss. After traversing the midbrain, the ALS and medial lemniscus enter and terminate in the thalamus (Figs. 18-9 and 18-11).
As the ALS ascends through the medulla, it decreases in size because of the departure of the spinoreticular axons, which originate in laminae V to VIII and terminate in the reticular formation. The medial reticular formation also receives collaterals from lamina I spinothalamic axons (Fig. 18-9). Several other pathways ascend in the ALS. For example, spinomesencephalic axons may terminate in the PAG or, as spinotectal fibers, in deep layers of the superior colliculus and anterior pretectum. Many tract cells with axons in the ALS project via collaterals to multiple targets as the primary axon ascends through the brainstem.
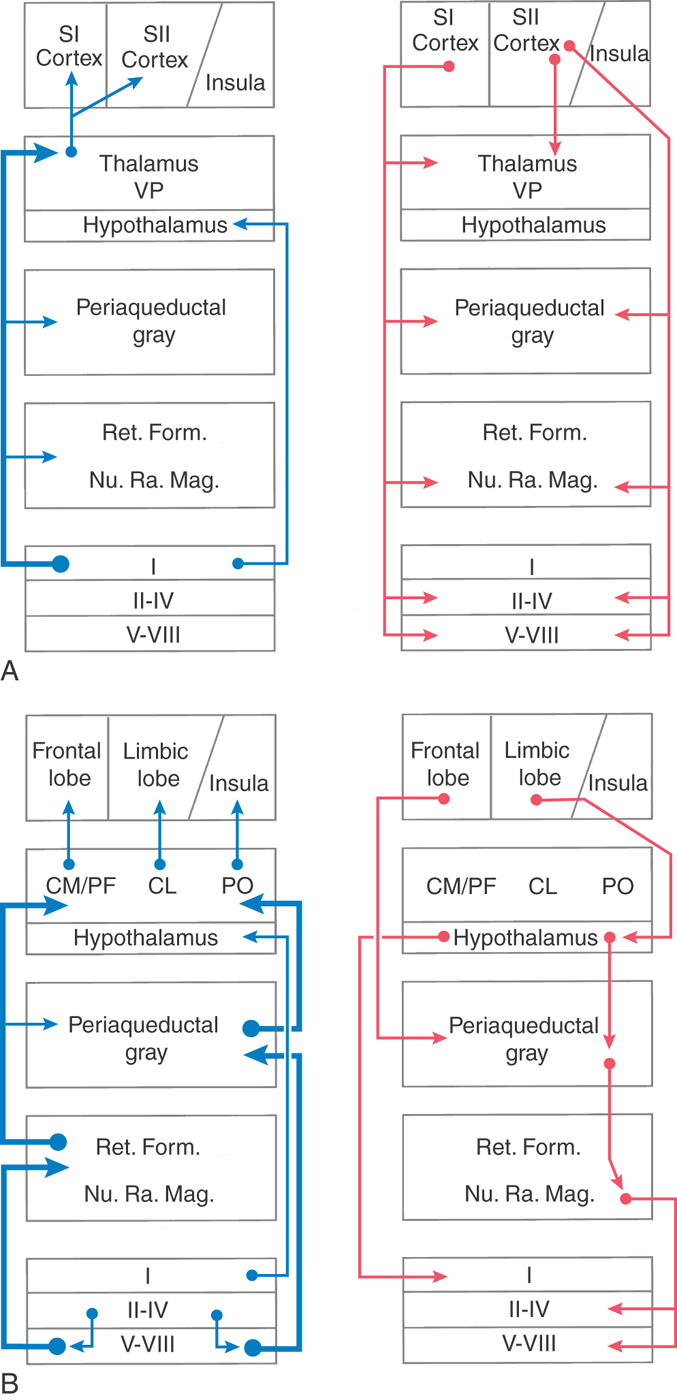
In addition to direct spinothalamic fibers, spinal cord neurons also project to brainstem targets that indirectly influence thalamic nuclei. Most notably, the reticular formation, which receives spinoreticular fibers, projects via reticulothalamic fibers to the intralaminar nuclei and posterior group (Fig. 18-9). The intralaminar nuclei project to the striatum, anterior cingulate cortex, amygdala, and hypothalamus and subserve the alerting response to painful stimuli. Nuclei of the posterior thalamic group, which includes VMpo, project to the secondary somatosensory (SII) cortex and to the retroinsular cortex. These polysynaptic pathways may underlie the dull, poorly localized, but persistent painful sensations that are perceived with localized thalamic lesions.
Somatosensory information, including nociceptive input from posterior horn cells, also ascends directly to the hypothalamus via the spinohypothalamic fibers of the ALS. In addition, spinal input is indirectly conveyed to the hypothalamus by way of synaptic relays in the reticular formation and in the PAG (Fig. 18-19). Through these ascending pathways, nociceptive information is transmitted to brain centers, such as the limbic system, that underlie emotional and autonomic responses to nociceptive stimuli. Motivational-affective behaviors including arousal and attention along with the suffering component of the pain experience are believed to be mediated by projections to these regions.
Input to the VPL is somatotopically organized such that lower body areas are represented laterally and upper body regions (exclusive of the head) are represented medially (Figs. 18-10 and 18-13). Within the VPL, fibers of the ALS terminate on clusters of cells located in the periphery of the nucleus. Most of these cells are different from the ones targeted by PCML axons. However, some VPL neurons, called multimodal cells, receive input from both ALS and PCML pathways. The functional classes of cells found in the VPL reflect the peripheral input received by tract cells of the spinal cord. These classes include, as in the spinal cord, nociceptive-specific, wide dynamic range, low-threshold nonnociceptive, and deep neurons.
Thalamocortical axons carrying nondiscriminative tactile, nociceptive, and thermal signals project via the posterior limb of the internal capsule to the somatosensory cortices (Figs. 18-9 and 18-11). Fibers originating in the VPL project mainly to the SI cortex (areas 3 and 1), whereas those from the posterior nucleus and VMpo terminate principally in the SII and insular cortices. Fibers from medial thalamic nuclei, including intralaminar and dorsomedial nuclei, project to cingulate, frontal, and limbic cortices. The somatotopy observed in the VPL is reflected in the cortex. Thalamocortical fibers from lateral areas of the VPL project to the posterior paracentral gyrus (thigh, leg, and foot), whereas progressively more medial parts of the VPL project in an orderly manner to sequentially more lateral areas of the postcentral gyrus (Figs. 18-9, 18-11, and 18-13). These thalamocortical fibers terminate primarily at the 3b/1 border on specific physiologic classes of SI neurons: low-threshold nonnociceptive, nociceptive-specific, and wide dynamic range cells. Loss of nociceptive and thermal sensations over the contralateral body and face can result from vascular compromise of either the middle (for trunk, upper extremity, and face) or the anterior (for the lower extremity) cerebral artery (Fig. 18-13). Not only the sensation but also the ability to localize is lost.
Not all nociceptive information reaches the thalamus via the ALS. The spinocervicothalamic pathway is a supplemental multimodal pathway that carries discriminative innocuous tactile information as well as nociceptive signals. This pathway begins with afferent fibers that terminate on second-order cells in laminae III and IV of the posterior horn. The axons of these second-order cells travel in the ipsilateral lateral funiculus to spinal levels C1 and C2, where they terminate on third-order neurons in the lateral cervical nucleus. The axons of these cells decussate at the level of the spinomedullary junction and ascend in the medial lemniscus. Like PCML axons, these cervicothalamic axons terminate in the VPL nucleus. This pathway is not essential for pain perception and is not especially prominent in humans. However, these fibers and the uncrossed axons in the ALS may be the basis for the retention of some nociceptive function after lesions involving the ALS or for the return of pain perception after anterolateral cordotomy.
SPINAL TRIGEMINAL PATHWAY: ANTERIOR TRIGEMINOTHALAMIC TRACT
Primary Neurons
Cranial nerves V, VII, IX, and X serve the cutaneous receptors of the face, the oral cavity, and the dorsum of the head except for the area served by the cervical nerves (Figs. 18-14 and 18-15). In addition to cutaneous structures, the trigeminal nerve also innervates deep tissues, including the temporomandibular joint, the meninges, tooth pulp, and the periodontium. The primary sensory fibers of these nerves have their cell bodies in the trigeminal ganglion, the geniculate ganglion of cranial nerve VII, and the superior ganglia of cranial nerves IX and X. Aδ and C fiber nociceptors are found throughout the face and oral cavity, and they are particularly prominent in the tooth pulp. Some of these fibers extend into the dentinal tubules, and carious lesions of the tooth expose these and other pulpal nerves to stimuli that result in dental pain. It is probable that the dull aching pain caused by pulp inflammation is the product of C fiber activity. Dental hypersensitivity, often characterized by sharp sensation, represents Aδ fiber activity. The cornea also receives a large number of nociceptive fibers. This corneal innervation forms the afferent limb of the corneal (blink) reflex. The meninges are also supplied by fibers of the trigeminal ganglion cells that terminate in the spinal trigeminal nucleus. These fibers are thought to be involved in the pain of migraine headaches.
Figure 18-14. A, Peripheral distribution of the trigeminal nerve and the inverted somatotopic arrangement of the hemiface within the spinal trigeminal nucleus pars caudalis. B, Functional onion skin pattern of facial pain is superimposed along the caudal to rostral axis of the pars caudalis. CNs, cranial nerves. Compare with Figure 8-15.
Figure 18-15. A, The somatotopic organization of the spinal trigeminal tract and nucleus when viewed in a clinical (axial MRI and CT) orientation; in this orientation, the face is represented right-side up. B, The posteroanterior arrangement of the facial dermatomes (V1, V2, V3) in the spinal trigeminal tract and nucleus and the respective rostrocaudal termination patterns of spinal trigeminal tract fibers within the spinal trigeminal nucleus in this orientation. This illustrates, in a three-dimensional view, the anatomic basis for the onion skin pattern of sensory representation and corresponding deficits. As seen by the numbers, perioral regions of the face are represented rostrally in the pars caudalis and progressively more posterior facial regions in progressively more caudal regions of the pars caudalis and in upper cervical cord levels. Compare with Figure 18-14.
The central processes of small and large trigeminal ganglion cells are part of the trigeminal sensory root, which attaches to the pons (Figs. 18-16 and 18-17 [see p. 255]). The bifurcating small-diameter axons course posteromedially into the pontine tegmentum, sending an ascending branch to the principal sensory nucleus. The descending branch of these fibers joins with numerous other unbranched small-diameter fibers to form a prominent fiber bundle in the posterolateral brainstem, the spinal trigeminal tract (Fig. 18-16). Through the caudal pons and the rostral medulla, this tract is internal to the restiform body. However, in the lower medulla caudal to the obex, it forms a superficial landmark lateral to the cuneate tubercle, known as the trigeminal tubercle (tuberculum cinereum). This landmark served as a useful reference point for surgeons, who discovered that sectioning the spinal tract of the trigeminal nerve at this level (tractotomy) provides substantial relief from facial pain on the operated side.
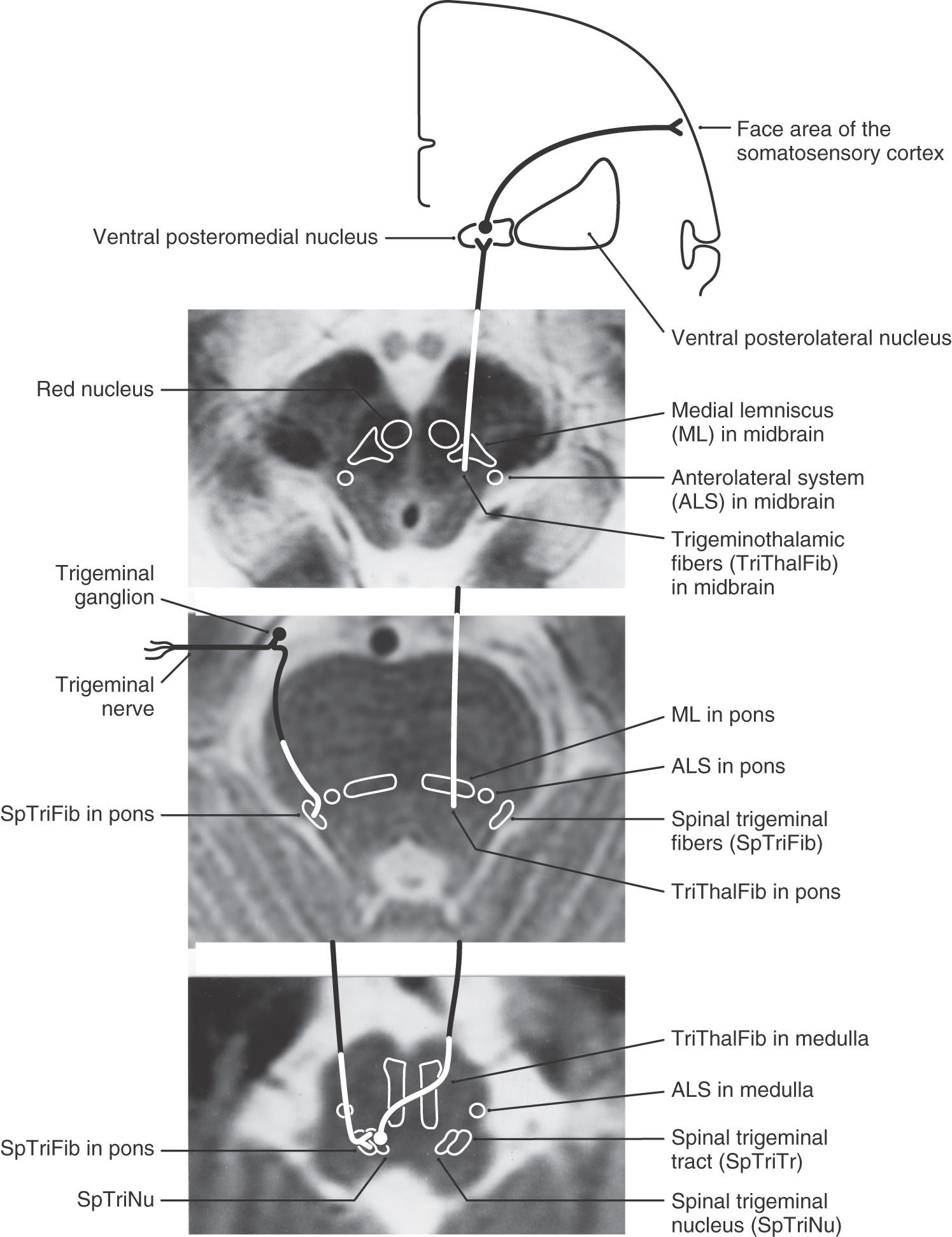
Figure 18-17. The location of the spinal trigeminal tract and nucleus and anterior trigeminothalamic fibers in magnetic resonance images at representative levels of the medulla, pons, and midbrain. This illustrates the location of these fibers when viewed in images routinely used in the clinical setting. It is essential to remember that the face is represented right-side up when the spinal trigeminal tract and nucleus are viewed in the clinical orientation. Compare with Figure 18-16.
The spinal trigeminal tract extends from the middle pons to the second or third cervical spinal cord segment, where its fibers interdigitate with those of the posterolateral fasciculus (Lissauer tract) (Figs. 18-6 and 18-16). In addition to the large contributions from the trigeminal nerve, small numbers of fibers conveying somatic afferent information from the ear on cranial nerves VII, IX, and X also enter the spinal trigeminal tract and terminate in the spinal trigeminal nucleus. The primary afferent neurons associated with cranial nerves VII, IX, and X have cell bodies in their respective ganglia, enter the medulla, and take a position adjacent to those of the mandibular division in the spinal trigeminal tract.
When the medulla is viewed in an anatomic orientation, the face is represented upside down in the spinal trigeminal tract and nucleus. (Fig. 18-14). In other words, the hemiface representation is inverted from its normal anatomic position (Fig. 18-14). The ophthalmic representation is located inferiorly in the tract and nucleus, and the mandibular representation is located superiorly. In this orientation, commonly illustrated in basic and clinical science texts, posterior (i.e., posterior column nuclei, fourth ventricle) is up and anterior (i.e., pyramids) is down in the image of the medulla (Fig. 18-14). When the medulla is viewed in a clinical orientation (as seen in MRI or CT), the face is represented right-side up (in its normal anatomic orientation) in the spinal trigeminal tract and nucleus (Fig. 18-15). When the medulla is viewed in the clinical setting, the pyramid is up in the image, the fourth ventricle is down, and within the spinal tract and nucleus the ophthalmic representation is superior and the mandibular representation is inferior (Fig. 18-15). Understanding of pathways in the clinical orientation is essential in dealing with the neurologically compromised patient.
The peripheral distribution of the branches of the trigeminal nerve (V1, V2, and V3) delineates the facial dermatomes (Figs. 18-14 and 18-15). Unlike the spinal segmental dermatomes, which partially overlap, the boundaries between adjacent facial dermatomes are sharply defined. This segregation of trigeminal branches is maintained by their central processes in the spinal trigeminal tract. An unfortunate clinical condition that illustrates the divisional pattern of the trigeminal system is herpes zoster, or shingles. Patients with shingles have a characteristic rash that outlines the affected dermatome or spinal cord segment; the ophthalmic or maxillary division is usually affected, and the rash is unilateral (Fig. 18-5).
Injury to trigeminal nerve fibers produces a paresthesia restricted to specific regions of the face. The pain of tic douloureux (trigeminal neuralgia) produces episodic “paroxysmal” pain usually restricted to the peripheral distribution of the maxillary or mandibular division on one side. Trigeminal neuralgia is further characterized by the presence of “trigger zones,” which on the most gentle stimulation (such as a light breeze or a brush with a wisp of cotton) produce stabbing pain on one side of the face. The precise etiology of this condition remains enigmatic, but vascular compression of the trigeminal nerve root and the presence of microneuromas are likely causes.
Central Pathways from the Face and Oral Cavity
The spinal trigeminal nucleus, located medial to the spinal tract, is the site of termination for fibers of the spinal trigeminal tract (Figs. 18-16 and 18-17). On the basis of cytoarchitecture, this nucleus is divided into a pars caudalis, a pars interpolaris, and a pars oralis. The caudal subnucleus (pars caudalis) (Figs. 18-14 and 18-15) extends from C2 or C3 rostrally to the level of the obex. This part of the spinal nucleus shares many cytoarchitectural similarities with the posterior horn. For this reason, it has been termed the medullary posterior horn and has been divided into layers that correspond to Rexed spinal cord laminae (Fig. 18-6). The substantia gelatinosa is largely continuous with lamina II of the spinal cord, and the magnocellular region is continuous with laminae III and IV. The pars caudalis and the posterior horn also show homology in the distribution of neurotransmitters. For example, substance P and calcitonin gene–related peptide are localized in nociceptive C fibers that terminate in both of these areas.
The pars caudalis plays an important role in the transmission of nondiscriminative touch, nociceptive, and thermal sensations from the face and oral cavity. This role is reflected by the fact that central processes of Aδ and C fibers terminate somatotopically in this subnucleus. In addition to the somatotopy within the pars caudalis, an onion skin pattern (also called onion-peel sensory loss) of facial pain representation is oriented along the rostrocaudal axis of the subnucleus (Figs. 18-14B and 18-15). The nociceptive fibers that innervate circumoral and intraoral zones (teeth, gums, and lips) terminate rostrally, close to the obex at the interface of the pars interpolaris with the pars caudalis. Fibers innervating progressively more caudal and lateral regions of the face terminate in progressively more caudal regions of the spinal trigeminal nucleus, pars caudalis (Fig. 18-15). Many second-order neurons in the subnucleus caudalis receive convergent input from small-diameter fibers that innervate cutaneous and deep tissues (jaw muscles and the temporomandibular joint). Convergence of information from different regions is thought to contribute to the referral of pain and may be involved in the manifestation of less well understood clinical problems such as temporomandibular joint disorders and atypical facial pain.
At medullary levels, the posterior inferior cerebellar artery supplies the territory of the ALS fibers as well as the spinal trigeminal nucleus and tract (Figs. 18-9 and 18-16). Vascular lesions involving this vessel produce characteristic sensory symptoms collectively known as the lateral medullary (Wallenberg) syndrome or the posterior inferior cerebellar artery syndrome. The sensory symptoms of this syndrome may include a contralateral loss of pain (hemianalgesia) and temperature (hemithermoanesthesia) sensibility over the body and ipsilateral loss of these modalities over the face. However, the extent of damage after posterior inferior cerebellar artery lesions shows remarkable variation, and the combination of symptoms is representative of the structures served by this artery (Fig. 18-18).
The interpolar subnucleus (pars interpolaris) is located between the level of the obex and the rostral pole of the hypoglossal (XII) nucleus. The most rostral subdivision is the oral subnucleus (pars oralis), which extends from the level of the rostral pole of the hypoglossal nucleus to the caudal end of the trigeminal motor nucleus (Figs. 18-14 and 18-16). Some neurons in the pars interpolaris and the pars oralis contribute to ascending somatosensory pathways, whereas others project to the cerebellum. In addition to projection neurons, the spinal trigeminal nucleus, particularly the subnucleus oralis, contains many local circuit neurons involved in brainstem reflexes.
The axons of second-order trigeminothalamic neurons in the spinal trigeminal nucleus decussate, then coalesce to form the anterior trigeminothalamic tract, and ascend through the brainstem just posterior to the medial lemniscus (Figs. 18-16 and 18-17). These fibers terminate in the ventral posteromedial (VPM), the posterior, and the intralaminar nuclei of the thalamus. As noted in Chapter 17, this pathway also carries crossed fibers from the principal trigeminal nucleus. The principal nucleus fibers terminate in the core of VPM, whereas spinal nucleus fibers terminate in its periphery. At the pontomesencephalic junction, anterior trigeminothalamic fibers are adjacent to ALS fibers at the lateral margin of the medial lemniscus (Figs. 18-9 and 18-13). Like ALS fibers, ascending anterior trigeminothalamic axons terminate in or give rise to collaterals that supply the reticular formation.
A particularly prominent target of some of these collaterals is the parabrachial nuclear complex. Located adjacent to the superior cerebellar peduncle (brachium conjunctivum), the parabrachial nuclei serve as an important relay for spinal and trigeminal pain fibers as well as for ascending axons carrying visceral sensory information. In addition to regulating oral and facial reflexes, projections from the reticular formation terminate in the dorsal thalamus in the intralaminar nuclei and the medial region of the posterior nucleus. The intralaminar nuclei project widely to the striatum and cortex, especially the frontal and somatosensory cortex. The medial region of the posterior nucleus projects to the head representation in the secondary somatosensory cortex.
PAIN PERCEPTION
Vascular compromise of middle or anterior cerebral arteries (Fig. 18-13) produces a loss of sensibility (discriminative, nondiscriminative, thermal, and nociceptive) over contralateral regions of the body, head, and face. Over time, however, appreciation of sensation may return (partially or totally). Pain sensations are first to return, followed by nondiscriminative tactile and thermal sensations. Discriminative tactile, vibratory, and proprioceptive sensations lag far behind and often fail to return to normal levels. If occlusion of the middle cerebral artery affects most of the postcentral gyrus, sensation begins to return first on the face and oral regions, then on the neck and trunk, and finally on the extremities and the distal parts of the limbs. This return of function indicates that other cortical areas may partially take over the appreciation of somatosensory stimuli, using the input they receive through nonlemniscal, non-ALS pathways.
At least some forms of somatosensory stimuli can be perceived at subcortical levels. In fact, electrical stimulation of the primary somatosensory cortex does not result in a complaint of pain, whereas thalamic stimulation may elicit paresthesia and sensations of dull pain and pressure. Furthermore, painful stimuli can be recognized and produce suffering without the presence of primary and secondary cortices, leading to the concept that pain is perceived at subcortical levels. However, damage to specific cortical regions eliminates the ability to precisely localize pain, suggesting that such localization is a function of the somatosensory cortex and its lemniscal inputs (Fig. 18-15A).
A second dissociation can occur in pain pathways. Pain perception and its affective component, suffering, are served by separate brain regions. The neospinothalamic pathway to the primary somatosensory cortex is involved in the localization of painful stimuli (Fig. 18-19A). Paleospinothalamic pathways that access the hypothalamus and limbic system via the reticular formation and PAG are involved in the suffering component of the pain experience (Fig. 18-19B). This dissociation can be regulated pharmacologically as some drugs eliminate suffering without affecting pain perception. For example, patients taking benzodiazepines (e.g., Valium) report that the pain is still present but that its unpleasant nature is diminished.
CHRONIC AND NEUROPATHIC PAIN AND THE THALAMUS
Results of stereotaxic surgery for treatment of chronic pain or movement disorders have provided tremendous insights into the role of the thalamus in pain perception. Before the performance of such surgical procedures, physiologic identification of the desired target is undertaken in these patients. Single-neuron recording and microstimulation have demonstrated that neurons within the human VPL/VPM (collectively called ventrocaudal [Vc] nuclei by some neurosurgeons) are involved with processing of tactile, thermal, and pain signals. Patients report that microstimulation of Vc evokes sensations of touch, warmth, coolness, tingling, burning, or pain localized to specific body areas. These recordings also reveal that a population of thalamic cells activated by innocuous tactile stimuli are mixed with other neurons activated by mechanical and thermal stimuli in the painful range. Single-neuron recording has demonstrated that microstimulation in VPL/VPM (Vc) evokes the sensation of angina, suggesting that these nuclei play a role in pain localization regardless of its origin, that is, cutaneous or visceral.
The human VPL/VPM (Vc) can undergo changes (i.e., it exhibits plasticity) after deafferentation, which can occur directly as a result of damage to ascending pathways or secondarily as a result of removal of sensory inputs (e.g., amputation). These changes may contribute to chronic pain or phantom limb pain. They involve the upregulation and downregulation of neurochemicals within the nucleus, changes in local circuitry, and changes in the functional state of Vc neurons. For example, in patients who have undergone leg amputation, single-neuron recordings reveal that the thalamic region formerly receiving input from the lower leg and foot responded to stimulation of the stump (thigh). These patients also described the presence of nonpainful tingling over the stump in response to microstimulation in this same area. This type of functional plasticity can also be observed after cerebral stroke.
In an attempt to bring about relief for chronic or neuropathic pain, two nonpharmacologic therapies have been used: thalamic lesioning and deep brain stimulation. Lesions have been centered in either the lateral thalamus or the medial thalamus. Lateral thalamic lesions involve the somatosensory thalamus (VPL/VPM). Although producing some transient relief for pain, these lesions have unwanted side effects, including loss of cutaneous and position sense in the affected limb as well as impaired motor function. Lesions in the medial thalamus involve the centromedian-parafascicular complex as well as the central lateral nucleus and the dorsomedial nucleus. Medial thalamic lesions produce transient relief from intractable pain but fail to produce loss of pain and thermal sensations. These lesions do not produce the unwanted sensory loss seen with lateral thalamic lesions.
Deep brain electrical stimulation has been used for more than several decades in patients suffering from chronic pain or deafferentation pain. Stimulating electrodes centered in the somatosensory thalamus, the centromedian-parafascicular complex, or the periventricular gray (PVG)–PAG activate neurons within their vicinity and thus may contribute to stimulus-induced analgesia. Cortical stimulation has also been shown to produce relief of chronic pain of neuropathic origin. An explanation for this effect has yet to be determined. An evaluation of different brain regions that may contribute to the stimulus-induced analgesia was carried out with positron emission tomography (PET). After thalamic stimulation, increased regional cortical blood flow was noted in the rostral insula, a region activated in studies of experimental pain, neuropathic pain, and warm and cool innocuous stimuli, as well as in the anterior insular cortex. These results suggest that stimulation of the somatosensory thalamus may activate a pain modulation circuit that involves thalamocortical thermal pathways.
Central or thalamic pain is a poorly understood sequela of natural or surgical lesions of structures involved in somatic sensibility. Central pain was originally observed with thalamic lesions, but it can occur with lesions of the ALS below the level of the thalamus, including brainstem or spinal cord.
Central pain syndrome can also result from vascular lesions. Patients surviving the Wallenberg syndrome (Fig. 18-18) may ultimately develop central pain, suggesting some sparing of alternative or parallel pain pathways. In central pain syndrome, the analgesia that initially results from the lesion is replaced after a period of weeks, months, or years by spontaneous paresthesia, dysesthesia, or unusual painful responses. Allodynia and hyperalgesia are common neurologic signs associated with the central pain syndrome. Patients often characterize central pain as burning, aching, pricking, or lacerating and as occurring in paroxysms that vary in intensity and are poorly localized. A further example of the functional plasticity that occurs after injury to the brain is the fact that central pain can be exacerbated by unrelated sensations, including visual, auditory, and olfactory stimuli.
Central pain may last for years and is intractable to current analgesics. Pharmacologic agents, such as antidepressants and antiepileptic drugs, have been used with varying degrees of success to treat central pain. Although the etiology of this condition has not been elucidated, it is possible that this type of pain represents a deafferentation phenomenon, that is, it results from removal of primary afferent influence on central neurons. The time course and symptoms of central pain suggest that it may be due to the sprouting of inappropriate connections of nonnociceptive or nociceptive fibers, to increased excitability of central pain neurons, or to removal of inhibitory influences on pain neurons.
Patients experiencing central pain may obtain temporary relief from transcutaneous electrical nerve stimulation (TENS) (electrical stimulation of nerves through the skin), from electrical stimulation of the posterior columns, or from chronic stimulation of the PAG or PVG regions by stereotaxically positioned electrodes (deep brain stimulation). Neuroablative surgical procedures that have been used in the treatment of central pain include anterolateral cordotomy, trigeminal tractotomy, lesions of the posterior root entry zone, thalamotomies, and cortical ablation. Unfortunately, none of these procedures is successful in the long term.
Novel Itch Pathway
Microneurographic recordings in human single-nerve fibers have identified a unique histamine-selective C fiber population that conducts itch sensations. Itch receptors display large receptive fields (85 mm2 on the lower leg) and the fibers are slowly conducting (0.5 m/s). A distinct population of histamine-responsive second-order cells in lamina I of the posterior horn was identified as part of the central neural substrate for itch perception. These second-order neurons project to the lateral thalamus (VPI and VPL). Whereas PET studies of itching behavior showed no activation in the thalamus or the secondary somatosensory (SII) cortex, brain regions that responded to pain—bilateral activation of areas 3 and 6, increased activity in the anterior cingulate gyrus (area 8), area 40 and superior temporal gyrus (area 22), and medial frontal gyrus (areas 45-46)—were noted.
A functional magnetic resonance image (fMRI) study directly comparing cortical activation patterns of itch versus pain showed that the neural responses in posterior cingulate cortex and posterior insula to itching were significantly higher than those to pain. Neural activity in the thalamus associated with pain was significantly higher than that associated with itching. SII did not show a significant difference between itching and pain. No observed significant difference in activity between itching and pain in the pre–supplementary motor area, the bilateral anterior insula, the anterior cingulate cortex, or the basal ganglia was noted. Thus there are significant differences in the central processing of information related to the sensations of itch and pain.
CONTROL OF PAIN TRANSMISSION
The relaying of information from the spinal cord to supraspinal centers is an important event in the higher order processing of nociceptive sensory signals. On the basis of the localization of putative neurotransmitters and secondary messengers in the posterior horn, several candidates, such as peptides (calcitonin gene–related peptide, substance P), glutamate, and nitric oxide, may be involved in this process. These and other chemical agents underlie the central pharmacology of nociceptive transmission and are responsible for the varied qualities associated with central pain pathways. Pain can be classified as acute or chronic, fast or slow, dull or sharp, and burning, throbbing, or aching. Because pain is such a complex sensory experience, reduction or elimination of nociceptive sensations is of obvious clinical importance. Effective clinical approaches that can be used for the purpose of controlling pain include pharmacologic intervention and stimulation-produced analgesia.
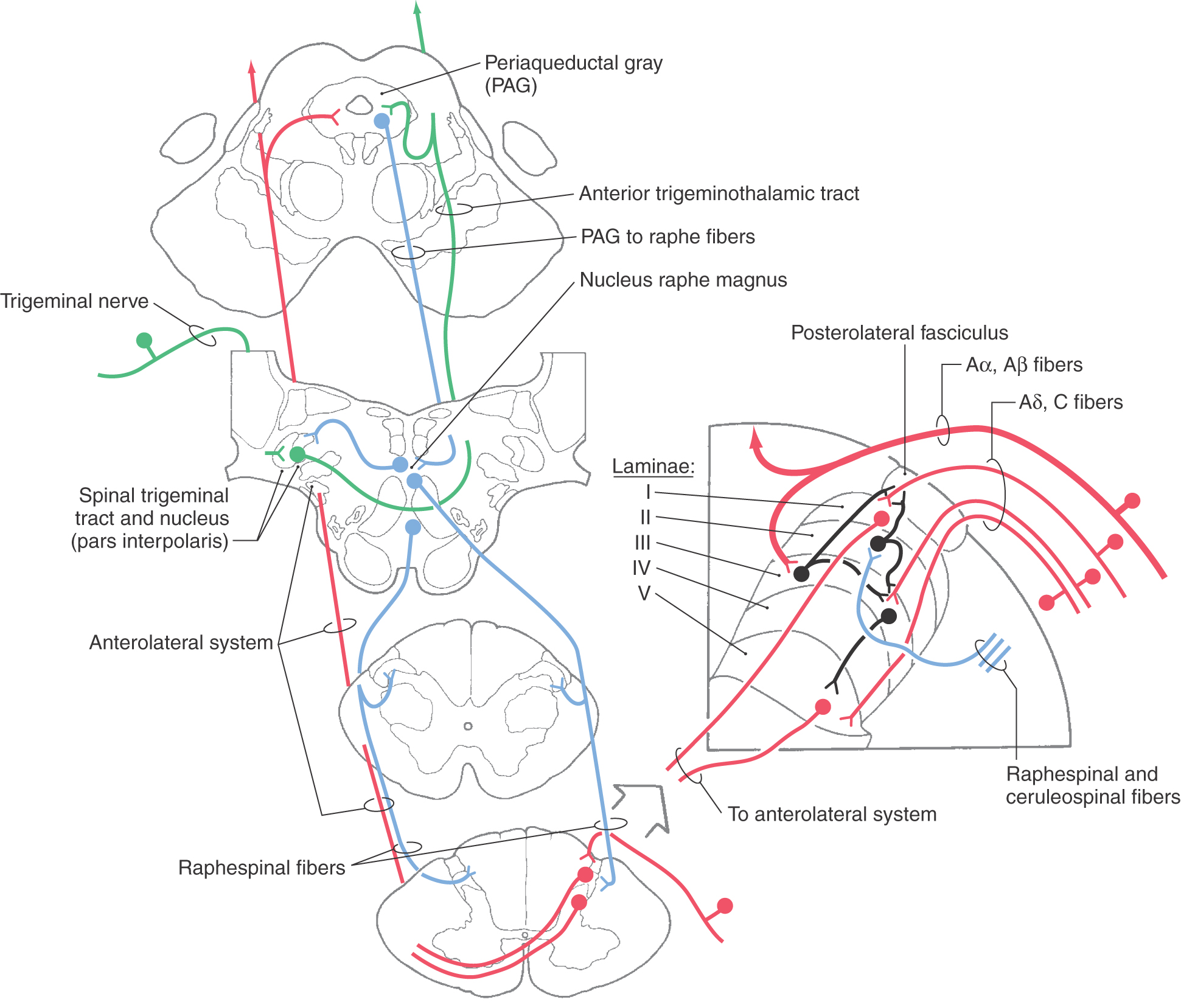
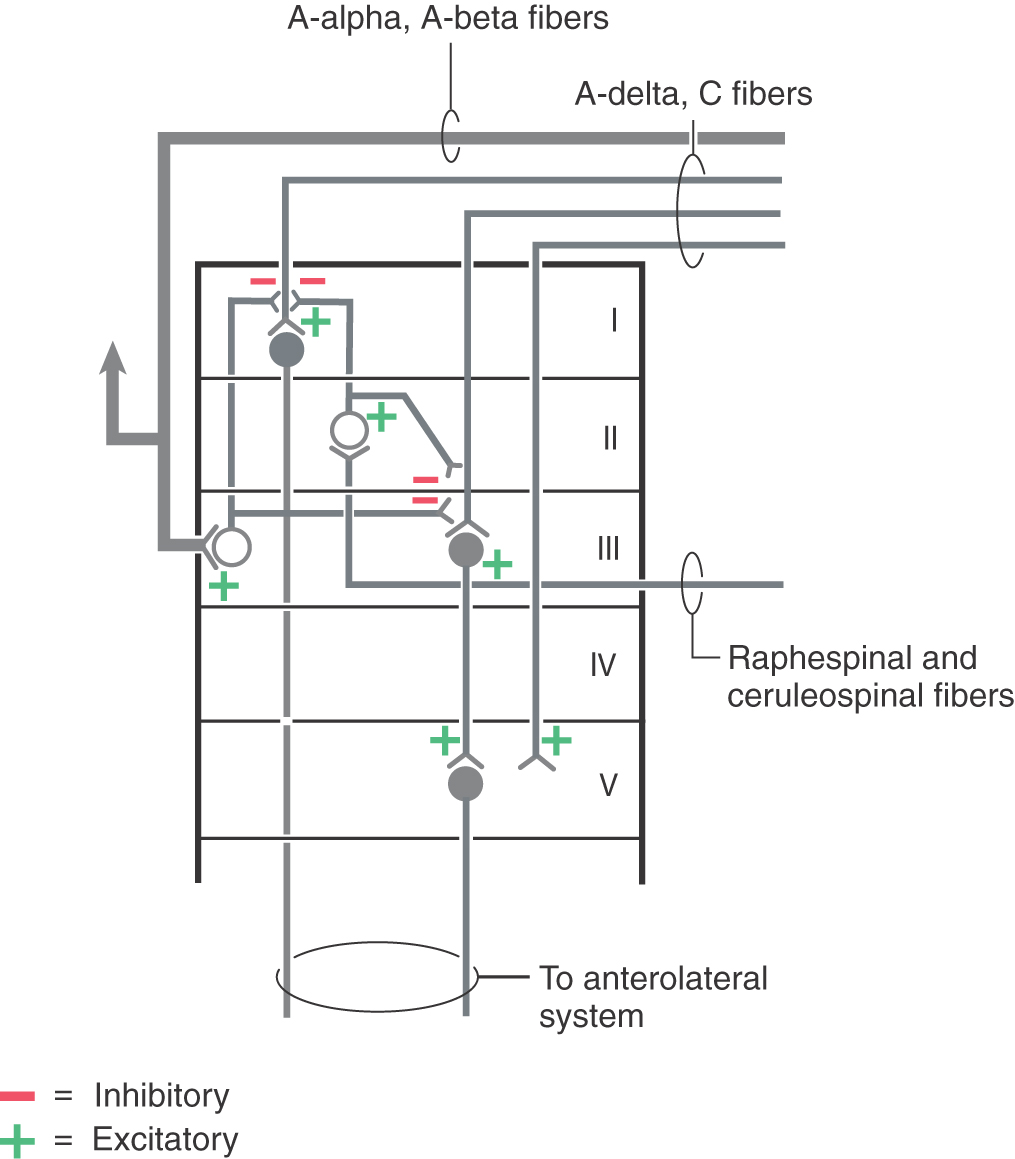
The CNS has circuits designed to modulate pain transmission (Figs. 18-19 to 18-21). These circuits have components at all levels of the neuraxis. They are capable of controlling nociceptive neuron firing and are sensitive to opiates. Central structures implicated in the descending control of nociceptive transmission include (1) the somatosensory, frontal, and limbic cortices, (2) the paraventricular nucleus of the hypothalamus, (3) the PAG, (4) the midbrain locus ceruleus, and (5) the raphe nuclei and adjacent medullary reticular formation. Descending pathways originating in these structures are activated by ascending afferent pain signals. For example, ascending nociceptive signals reach the PVG and activate PAG neurons via an enkephalinergic pathway. Descending PAG fibers, in turn, exert an excitatory influence on serotoninergic neurons in the medullary nucleus raphe magnus (Fig. 18-20) or noradrenergic neurons in the locus ceruleus. Raphespinal and ceruleospinal neurons project to the posterior horn of the spinal cord and pars caudalis of the trigeminal nucleus, where they terminate on enkephalinergic interneurons in laminae II and III (Figs. 18-20 and 18-21). Enkephalin acts both presynaptically by inhibiting incoming pain fibers and postsynaptically by inhibiting neuronal cell bodies that contribute to the ALS (Figs. 18-20 and 18-21). In addition, the hypothalamus (via hypothalamospinal fibers) projects directly to medullary and spinal cord posterior horn neurons that act on incoming nociceptive signals (Fig. 18-19). Hypothalamospinal fibers also project to the intermediolateral cell column of the upper thoracic cord levels.
Stimulation-produced analgesia relies on electrical stimulation of CNS structures to induce the release of endogenous chemicals, such as enkephalin, from cells in pain control circuits. As noted previously, endogenous opiates such as enkephalin inhibit pain transmission. Stimulation of the PVG, the PAG, or the nucleus raphe magnus results in the release of enkephalin or monoamines (serotonin or norepinephrine), producing analgesia. Systemic administration of pharmacologic opiates, such as morphine, excites periventricular and periaqueductal neurons, supplementing their natural activity. This increase in activity suppresses neurons in the spinal and medullary posterior horns that transmit painful information, also producing analgesia. The direct delivery of opioids to the spinal cord (epidural anesthetic techniques) also is used to produce a powerful analgesia for surgical procedures and deliveries.
Current therapies for the control of pain transmission include transcutaneous electrical nerve stimulation and chronic stimulation of the posterior columns by implanted electrodes. Posterior column stimulation activates large-diameter myelinated fibers. Antidromic activation of these fibers discharges collaterals in the posterior horn (Fig. 18-21). These collaterals stimulate the enkephalinergic interneurons in the posterior horn that inhibit the transmission of pain signals. This stimulation also provides long-term diminution of pain for reasons that are poorly understood. Acupuncture-like stimulation also may produce local analgesia by stimulating these fibers.
IMAGING OF THE PAIN PATHWAY
Electroencephalography (EEG), PET, fMRI, and magnetoencephalography (MEG) have provided insight into the localization of brain regions responsible for the processing of pain signals. EEG recordings show patterns of increased brain activity after the application of painful stimuli. This increased activity is especially prevalent in the somatosensory cortex and the frontal cortex.
When PET is used to identify changes in regional cerebral blood flow, painful stimuli are found to activate SI and SII cortices as well as the anterior cingulate cortex, the anterior insula, the supplemental motor area of motor cortex, and different thalamic nuclei. The cingulate cortex and the anterior insula are connected with nontraditional somatosensory brain regions, including the limbic cortex. This widespread cortical activation may provide a morphologic basis for integrating the location of painful stimuli with memory and emotion. PET has also been used to study possible gender differences in pain perception to noxious heat stimuli. Similar increases in cortical activation in the posterior insula and anterior cingulate cortex as well as in the cerebellar vermis have been described for both sexes. However, increases in the contralateral prefrontal cortex activity, contralateral insula, and thalamus noted in female subjects suggest that pain perception and processing may be different in males and females.
fMRI techniques reveal increased thalamic and cortical activation in response to application of innocuous (tactile, cool, and warm) and nociceptive (cold and hot) stimuli. Regions of increased activity in response to innocuous stimuli included the contralateral thalamus (VPL), posterior insula, and bilateral SII cortex. Application of innocuous thermal stimuli fails to activate SII. Painful thermal stimuli, however, activate the anterior insular cortex as well as the contralateral SII cortex.
With use of a carbon dioxide laser to activate Aδ and C nociceptors, MEG revealed that both the contralateral SI and bilateral SII cortices are involved in the processing of pain stimuli. These results support the view that SI provides a mechanism to code spatial, temporal, and intensity qualities of a painful stimulus. These various methods are providing a more complete identification of structures included in pain pathways and responsible for the processing of painful stimuli.
Sources and Additional Reading
Brodal A. Neurological Anatomy in Relation to Clinical Medicine. 3rd ed New York: Oxford University Press; 1981.
Wall PD, Melzack R. Textbook of Pain. ed 4 Edinburgh: Churchill Livingstone; 1999.

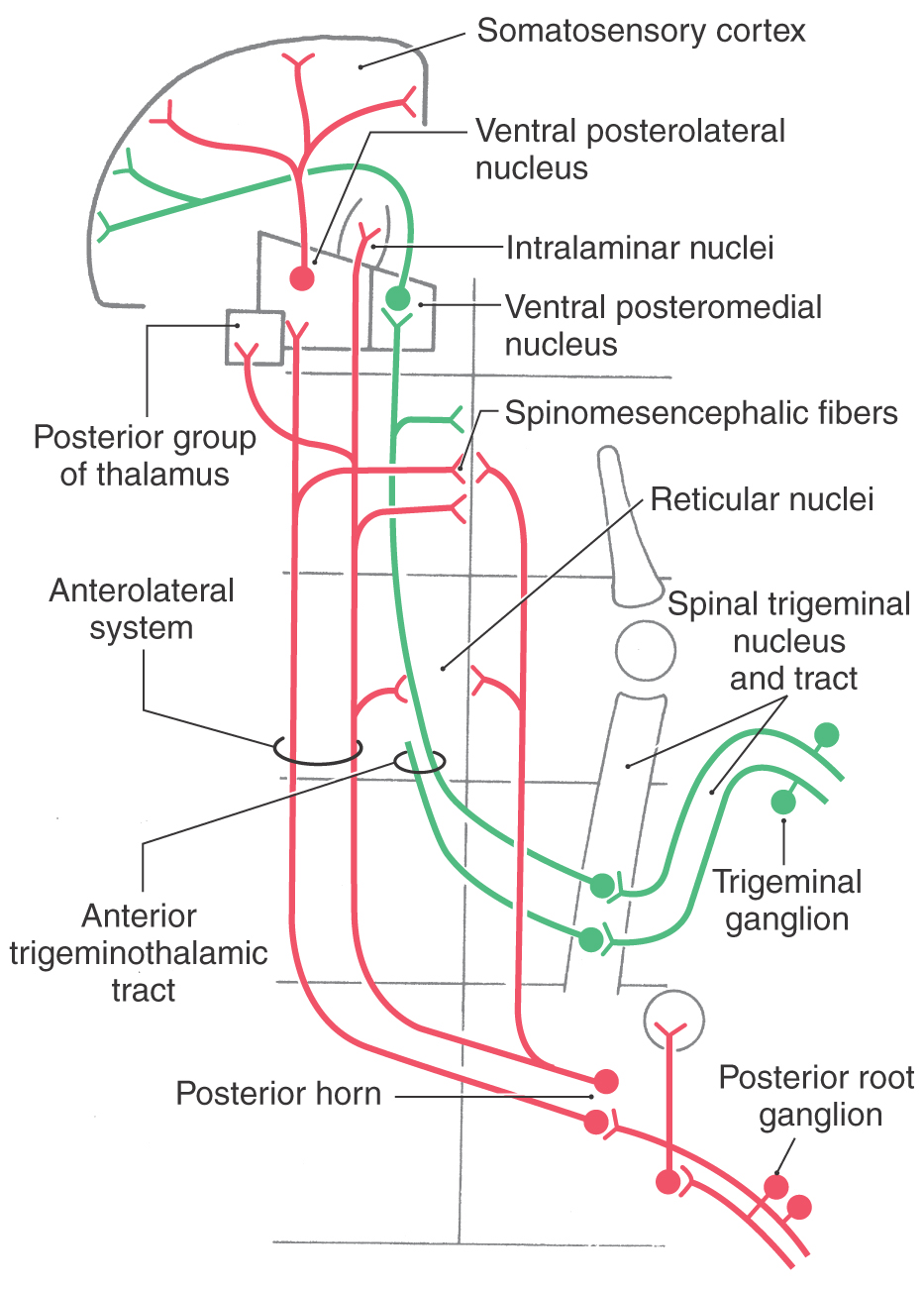
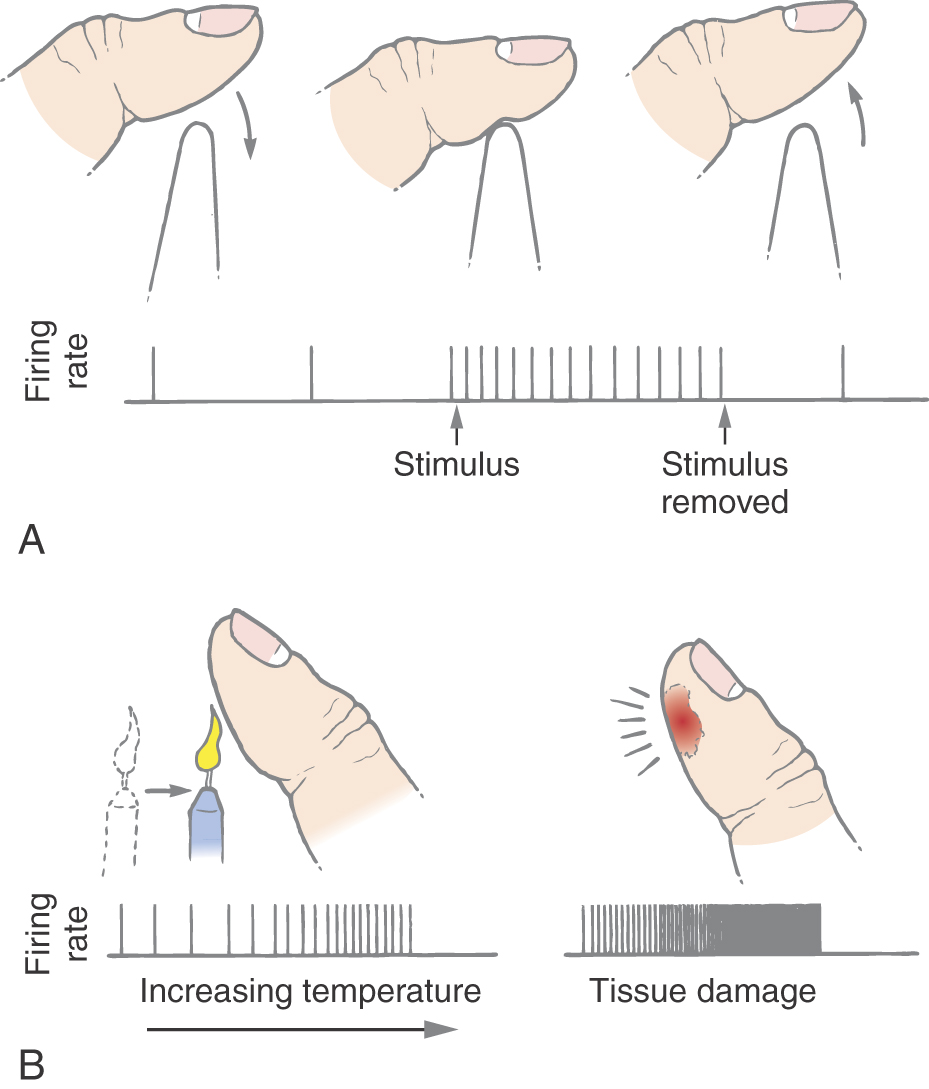
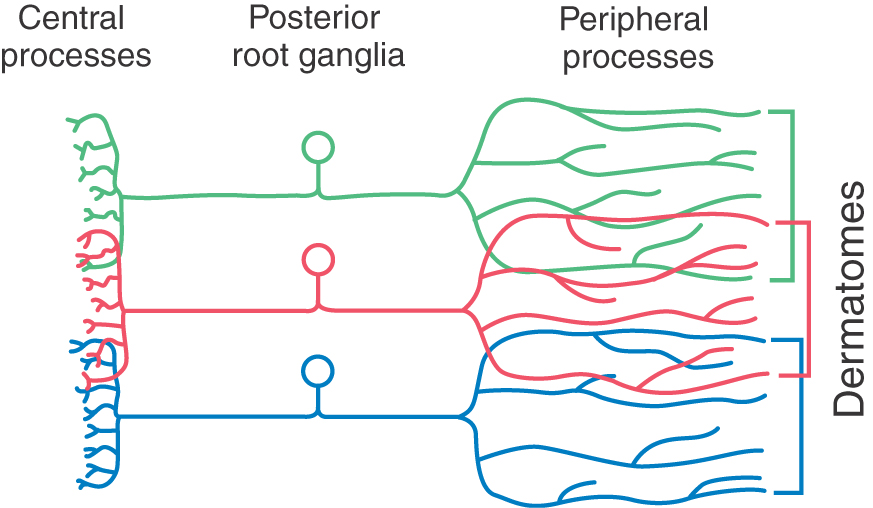
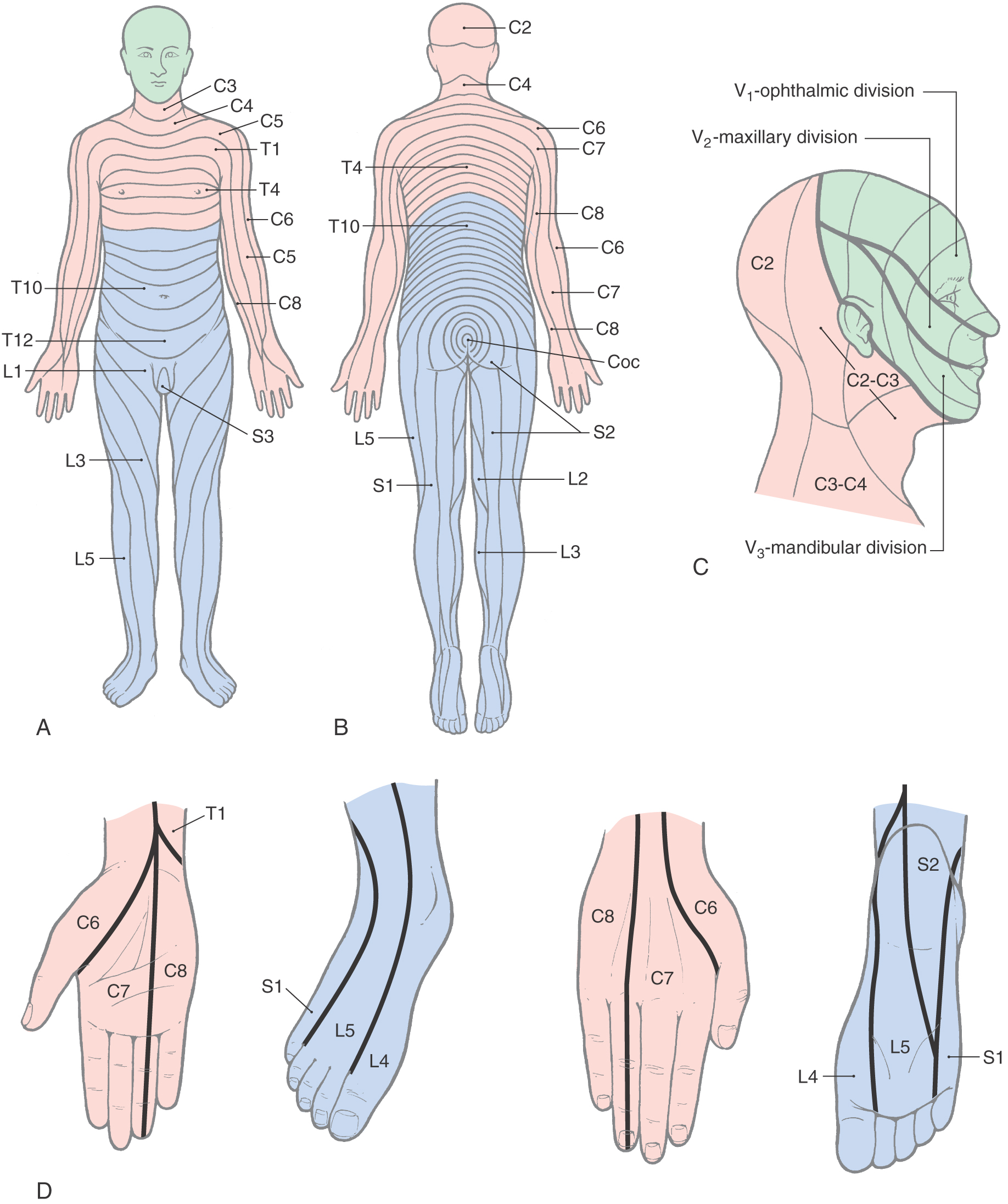
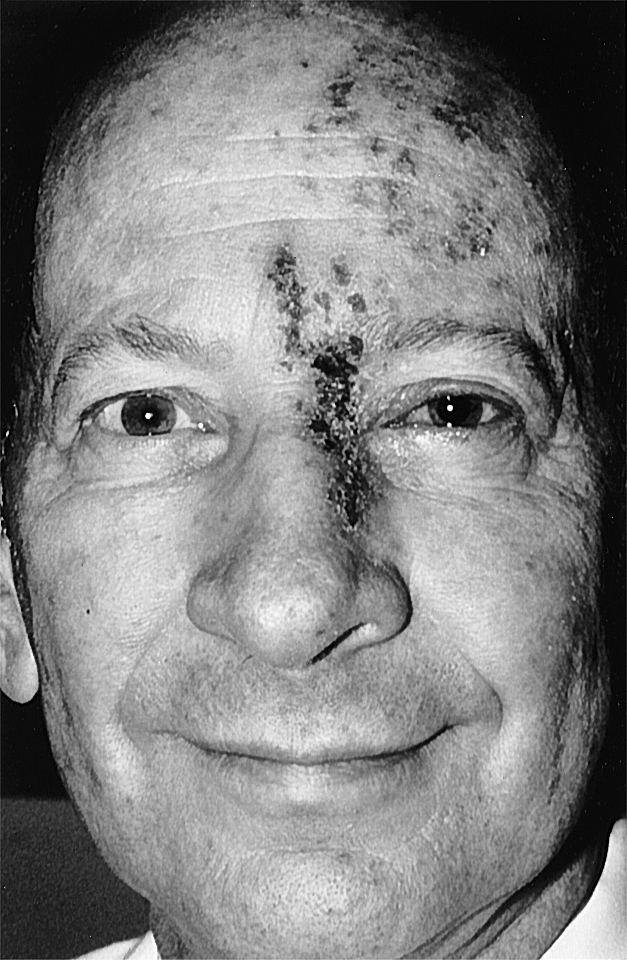
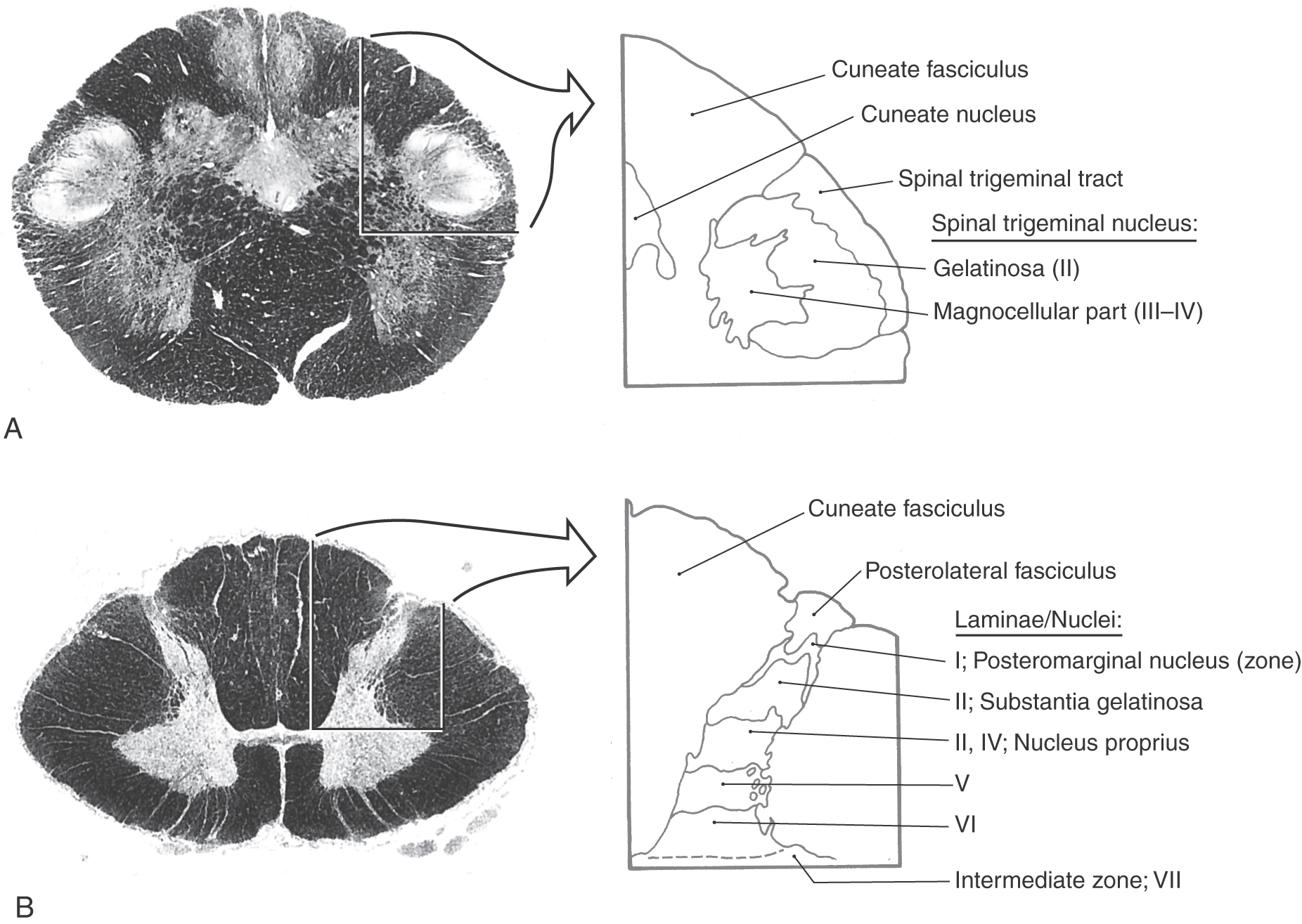
 Figure 18-6.
Figure 18-6. 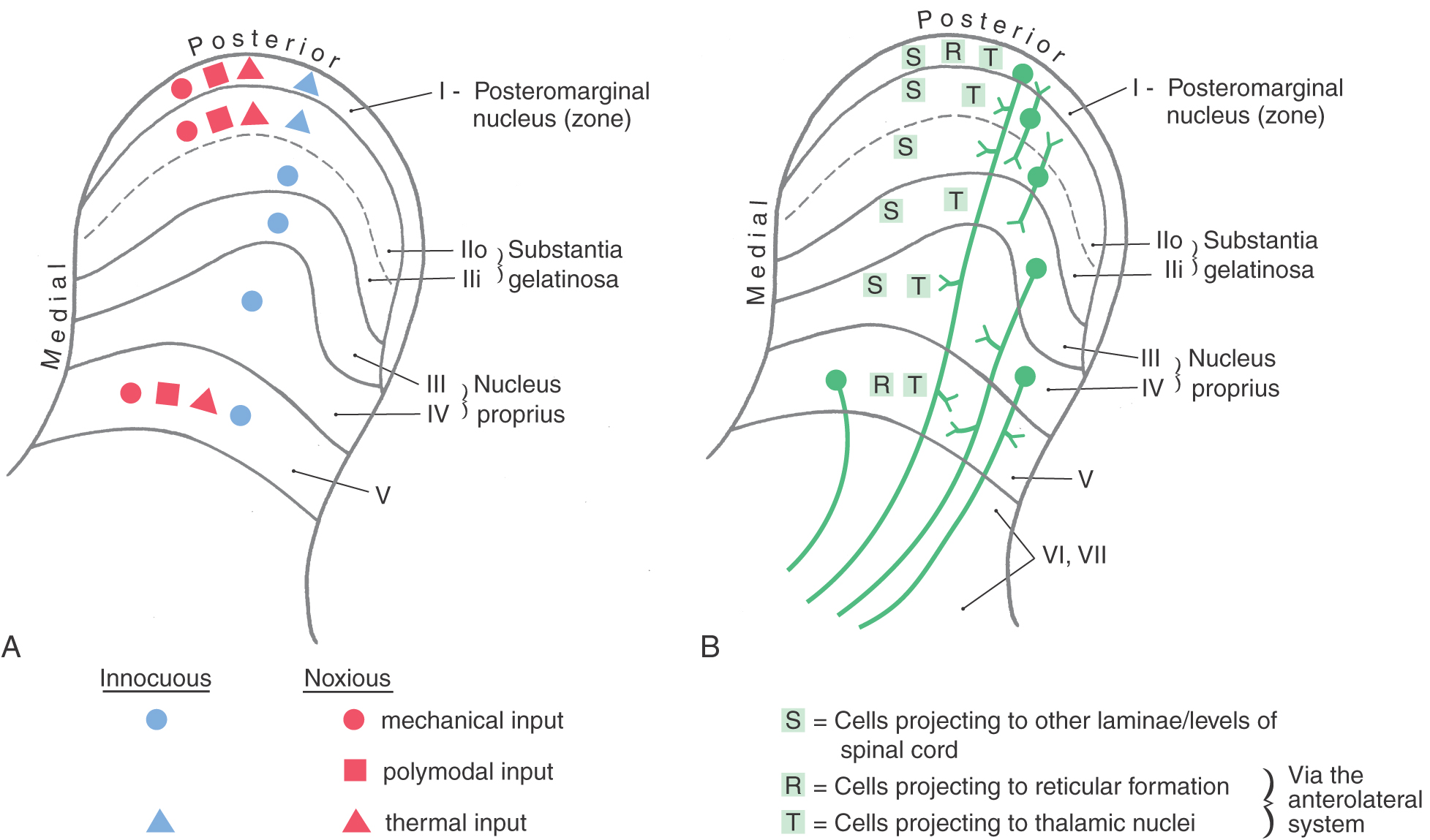
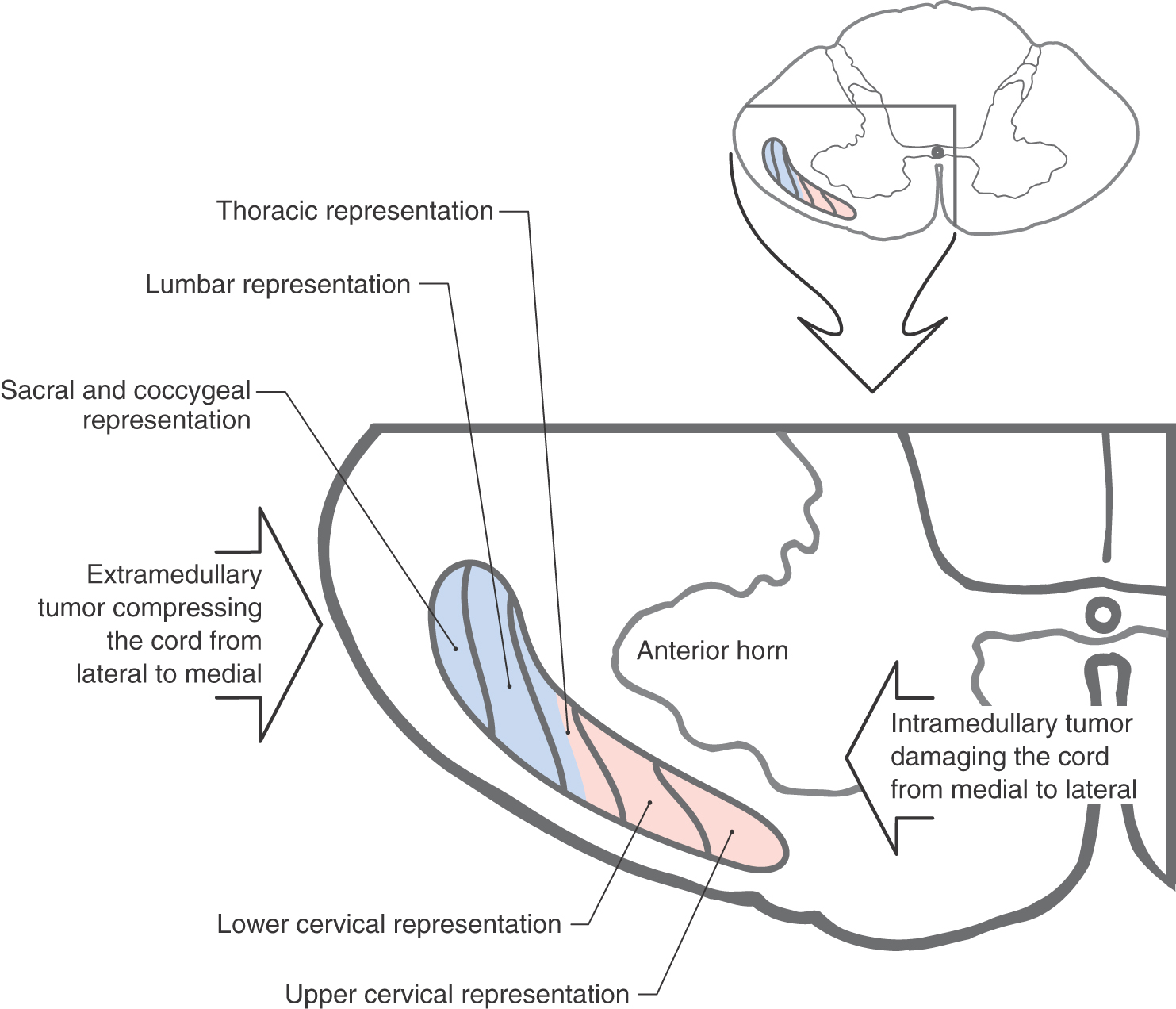
 Figure 18-8.
Figure 18-8. 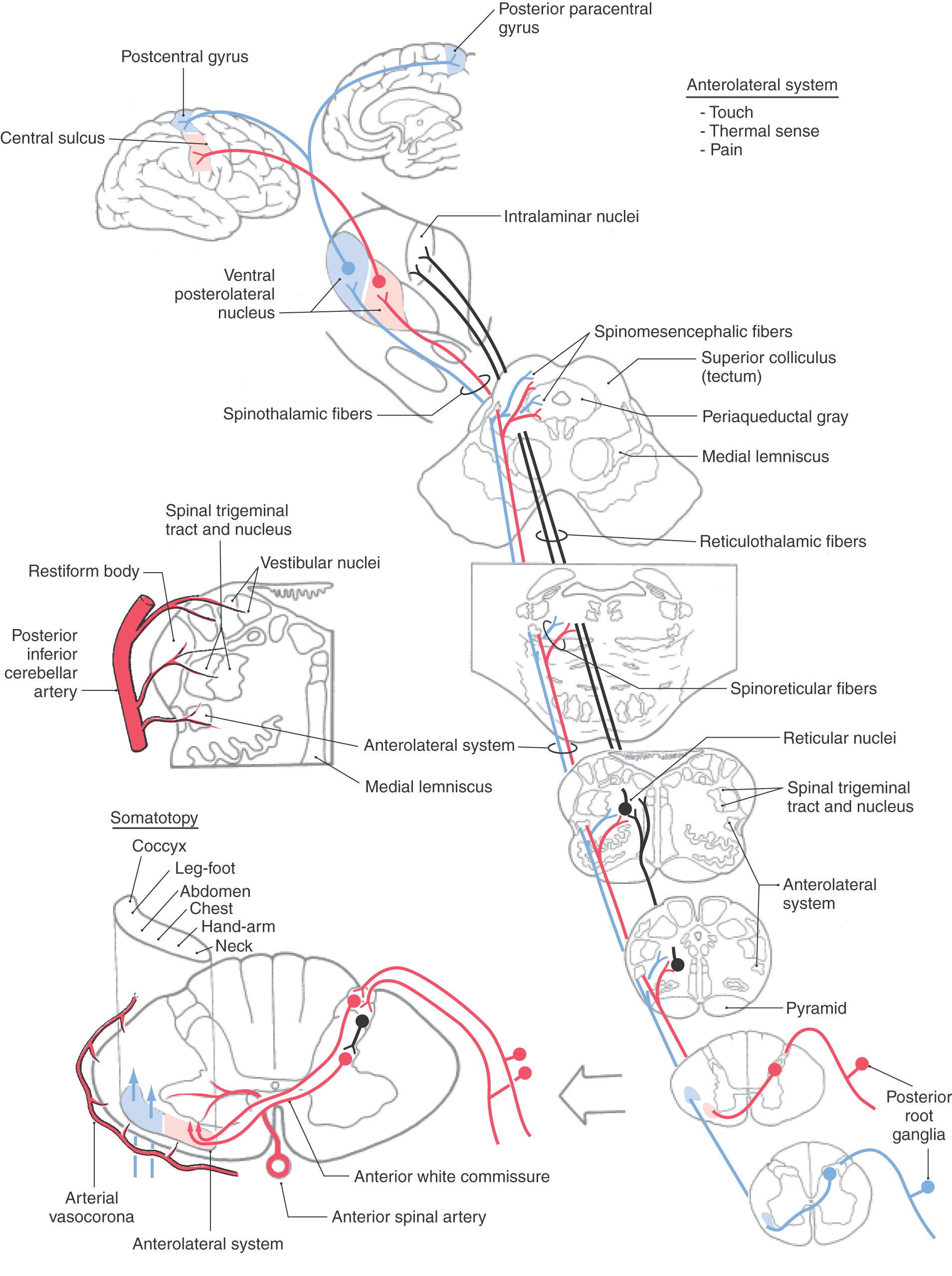
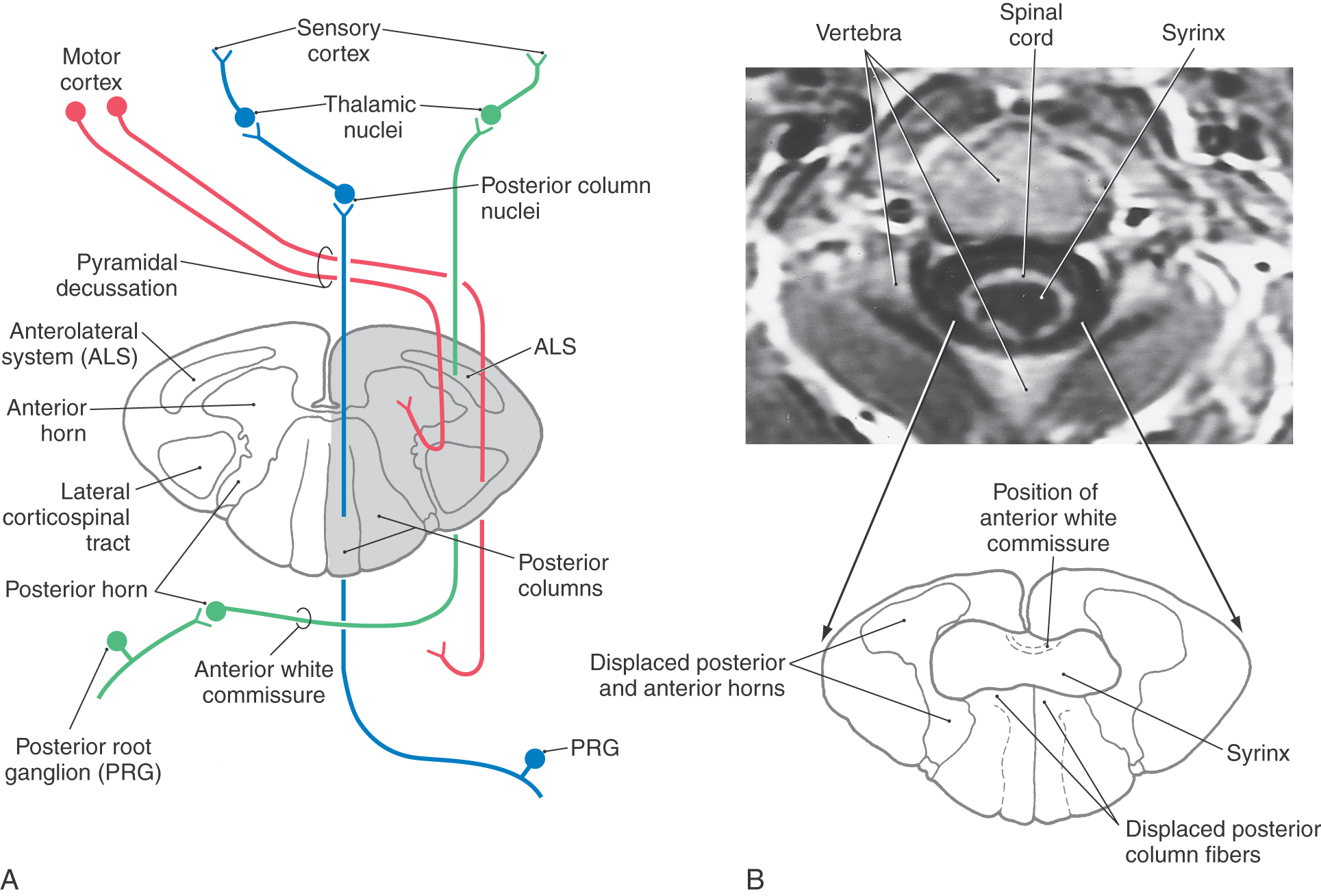
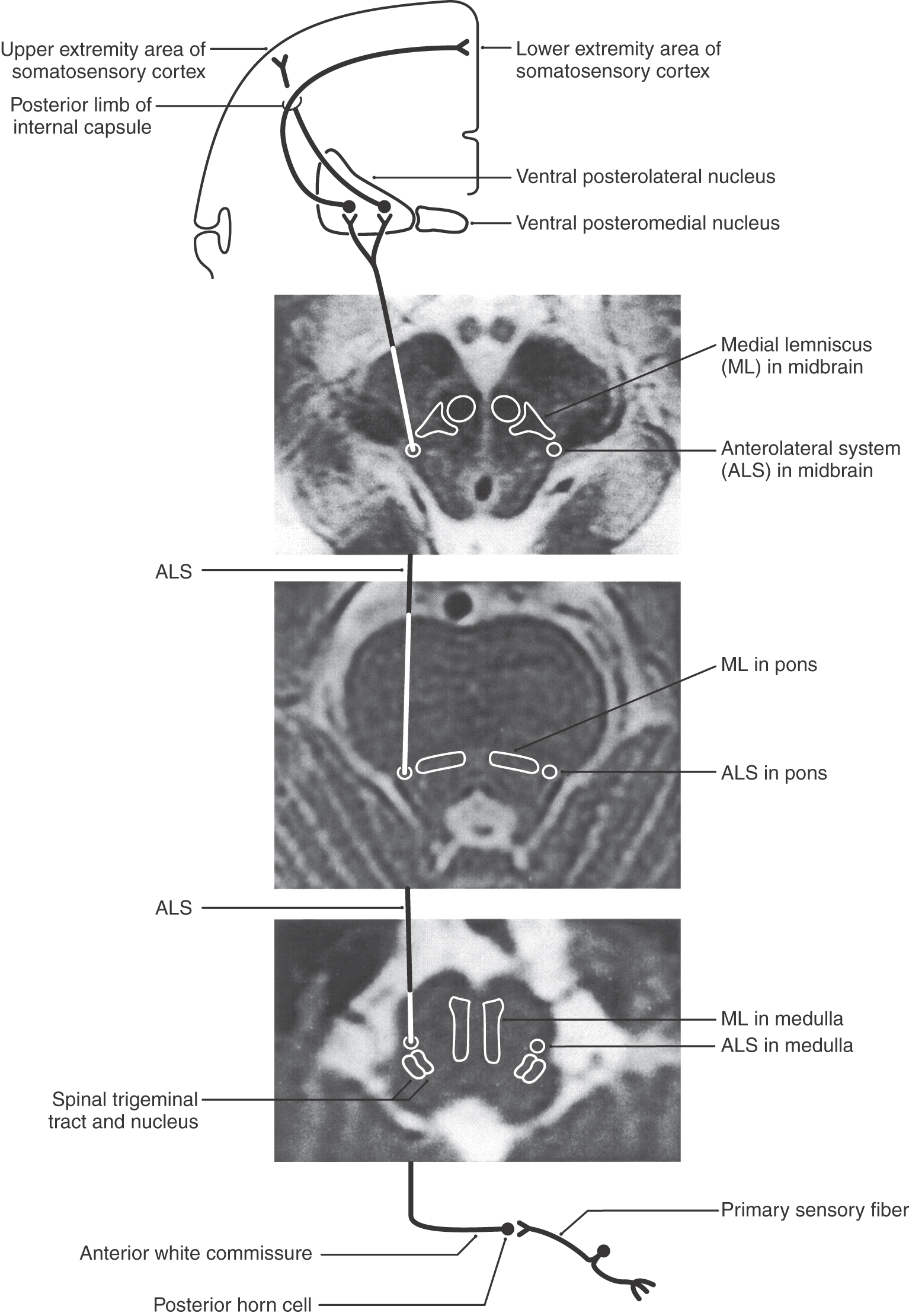
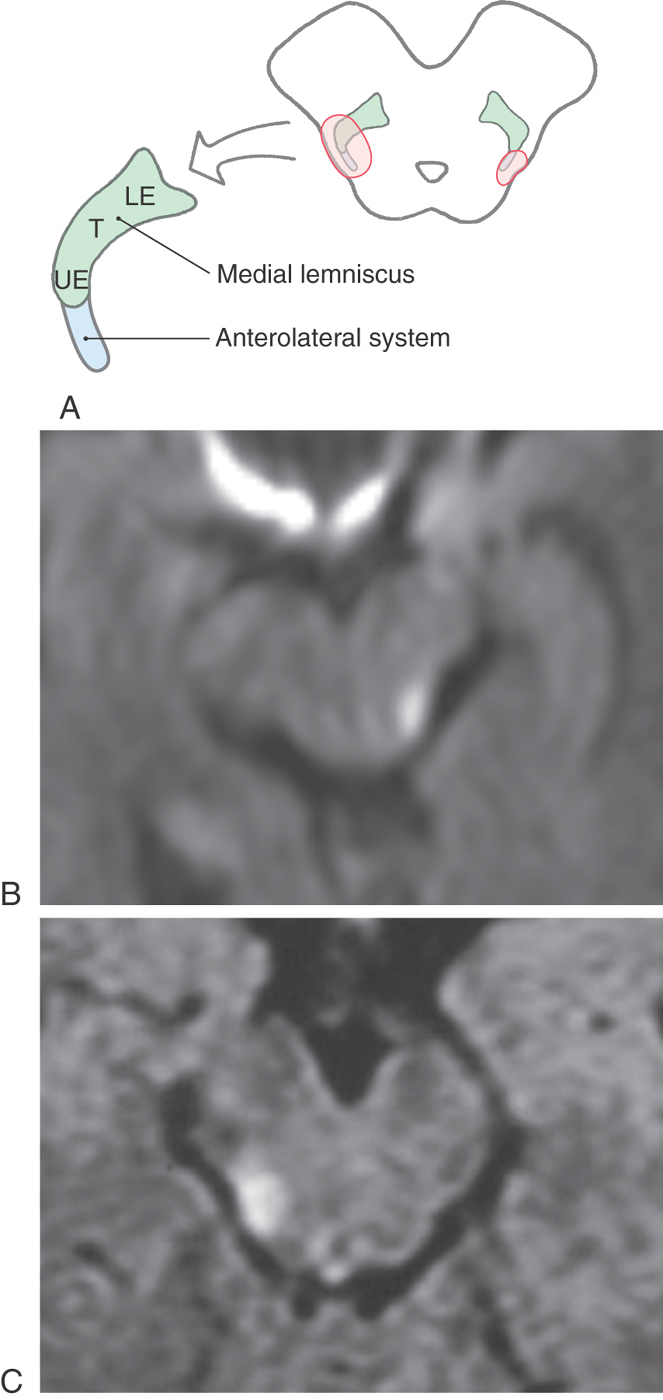
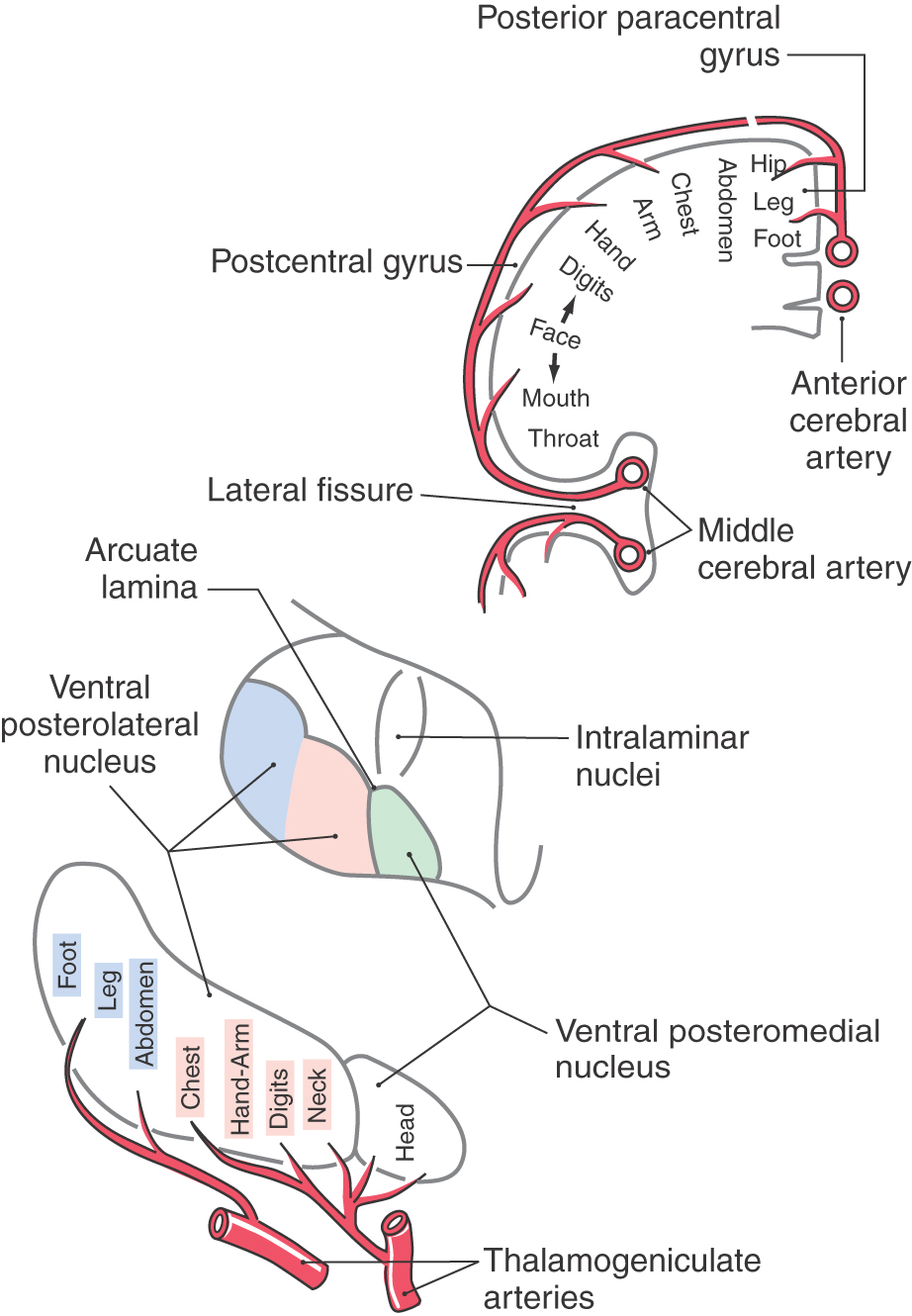
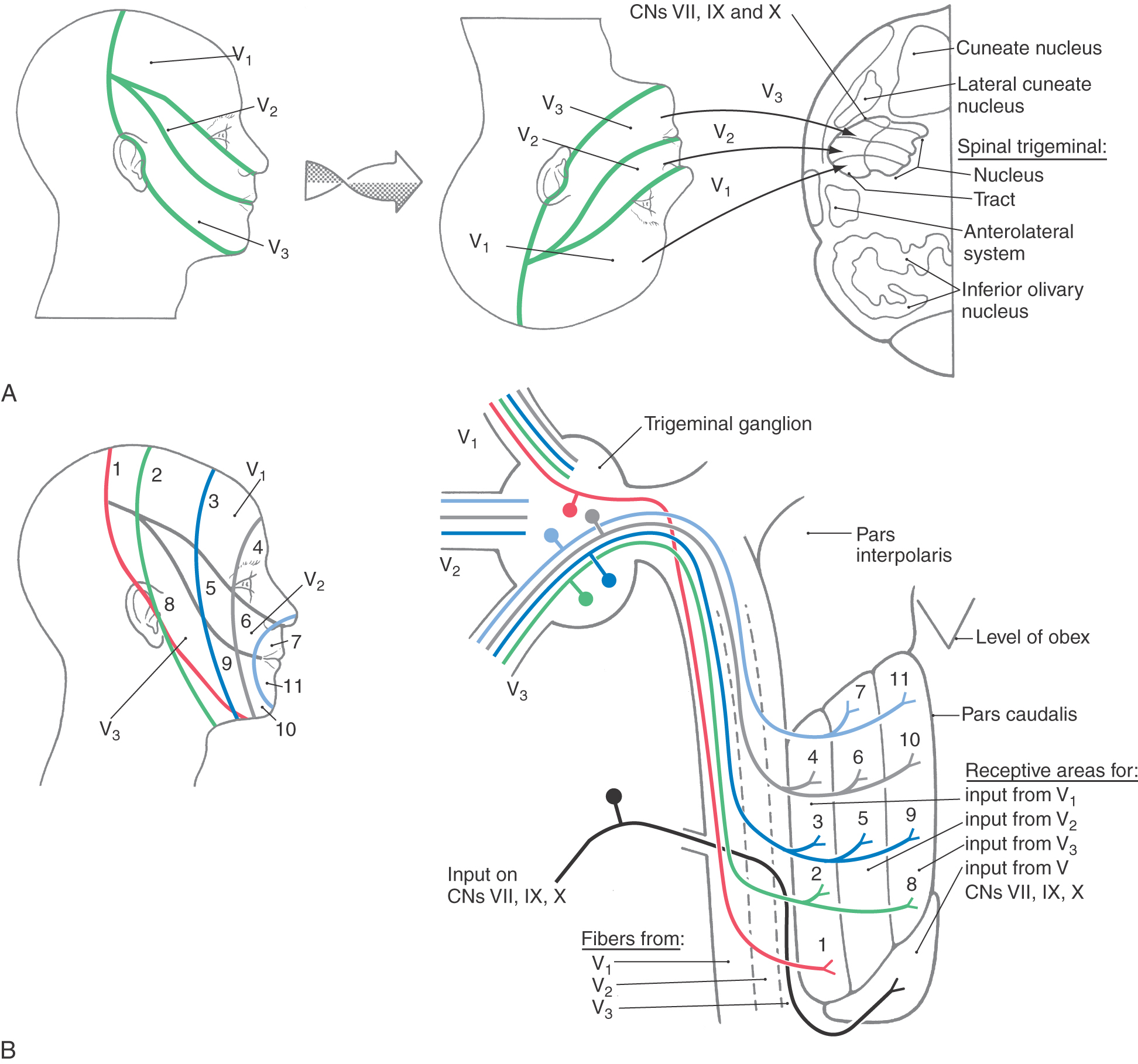
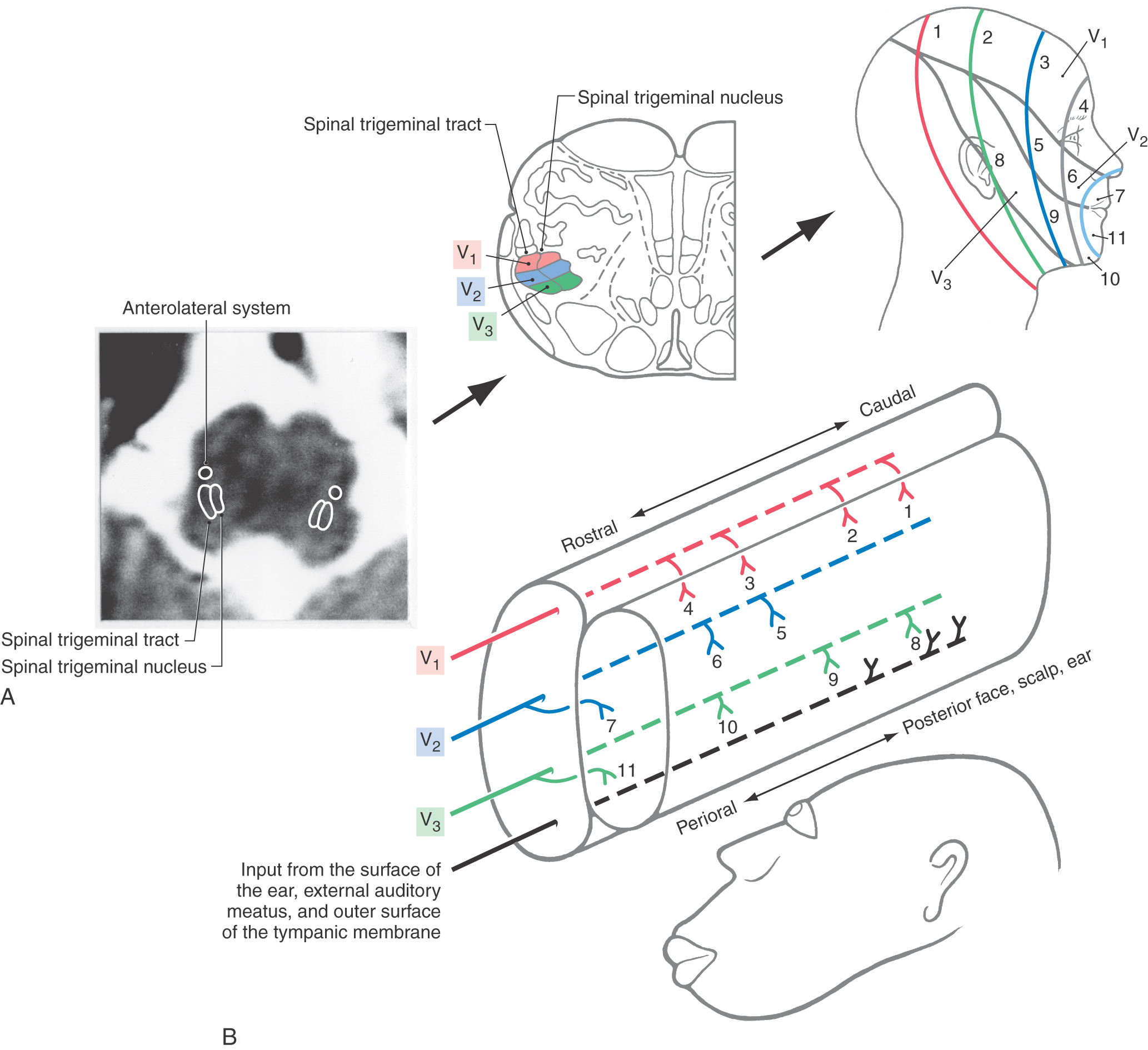
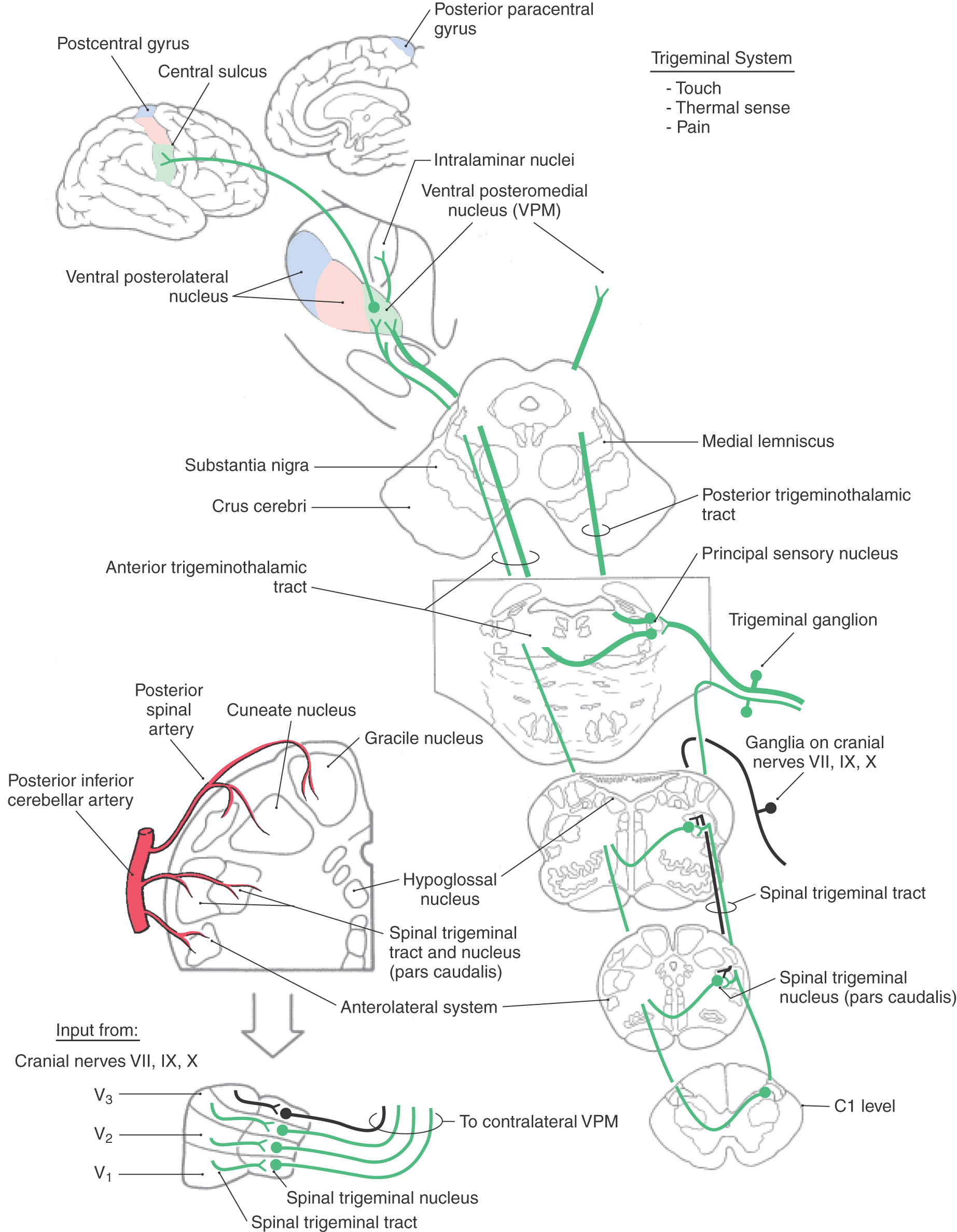
 Figure 18-16.
Figure 18-16.