Chapter 17
The Somatosensory System I: Tactile Discrimination and Position Sense
Posterior Column–Medial Lemniscal System
Primary Somatosensory (SI) Cortex
Additional Cortical Somatosensory Regions
Anterior and Posterior Trigeminothalamic Tracts
Receptive Field Properties of Cortical Neurons
Neuroimaging and Functional Localization
Plasticity and Reorganization in the Primary Somatosensory Cortex
Posterior Spinocerebellar Tract
When you reach into your pocket to determine the types of coins present, you are gathering information through the activation of specialized receptors of the somatosensory system. Specifically, the size of a coin is determined by noting the joint angles when the coin is held between the forefinger and thumb. “Heads and tails” may be identified with the use of slowly adapting receptors sensitive to stimuli that indent the skin. Dimes can be distinguished from pennies by stroking their edges with the fingertips and activating rapidly adapting receptors. This information is transmitted to the cerebral cortex by a multisynaptic pathway called the posterior column–medial lemniscal system. At the same time, much of this information, along with information concerning muscle tension and length, is also transmitted to the cerebellar cortex, where it is used to regulate muscle activity that allows manipulation of the coins. The spinocerebellar pathways are among those that subserve these nonconscious somatosensory functions.
OVERVIEW
In general, the somatosensory system transmits and analyzes touch or tactile information from external and internal locations on the body and head. The result of these processes leads to the appreciation of somatic sensations, which can be subdivided into the submodalities discriminative touch, flutter-vibration, proprioception (position sense), crude (nondiscriminative) touch, thermal (hot and cold) sensation, and nociception (pain). The following anatomically and functionally discrete pathways transmit these signals: (1) the posterior column–medial lemniscal pathway, (2) the trigeminothalamic pathways, (3) the spinocerebellar pathways, and (4) the anterolateral system.
This chapter describes pathways that transmit discriminative touch, flutter-vibration, and proprioceptive information. These pathways are the posterior column–medial lemniscal pathway, portions of the trigeminothalamic pathways originating in the principal trigeminal sensory nucleus, and the spinocerebellar pathways. The pathways subserving the submodalities of nociception (commonly referred to as pain), thermal sense, and crude touch, itch, and tickle comprise the anterolateral system. These and portions of the trigeminothalamic pathways are described in Chapter 18.
POSTERIOR COLUMN–MEDIAL LEMNISCAL SYSTEM
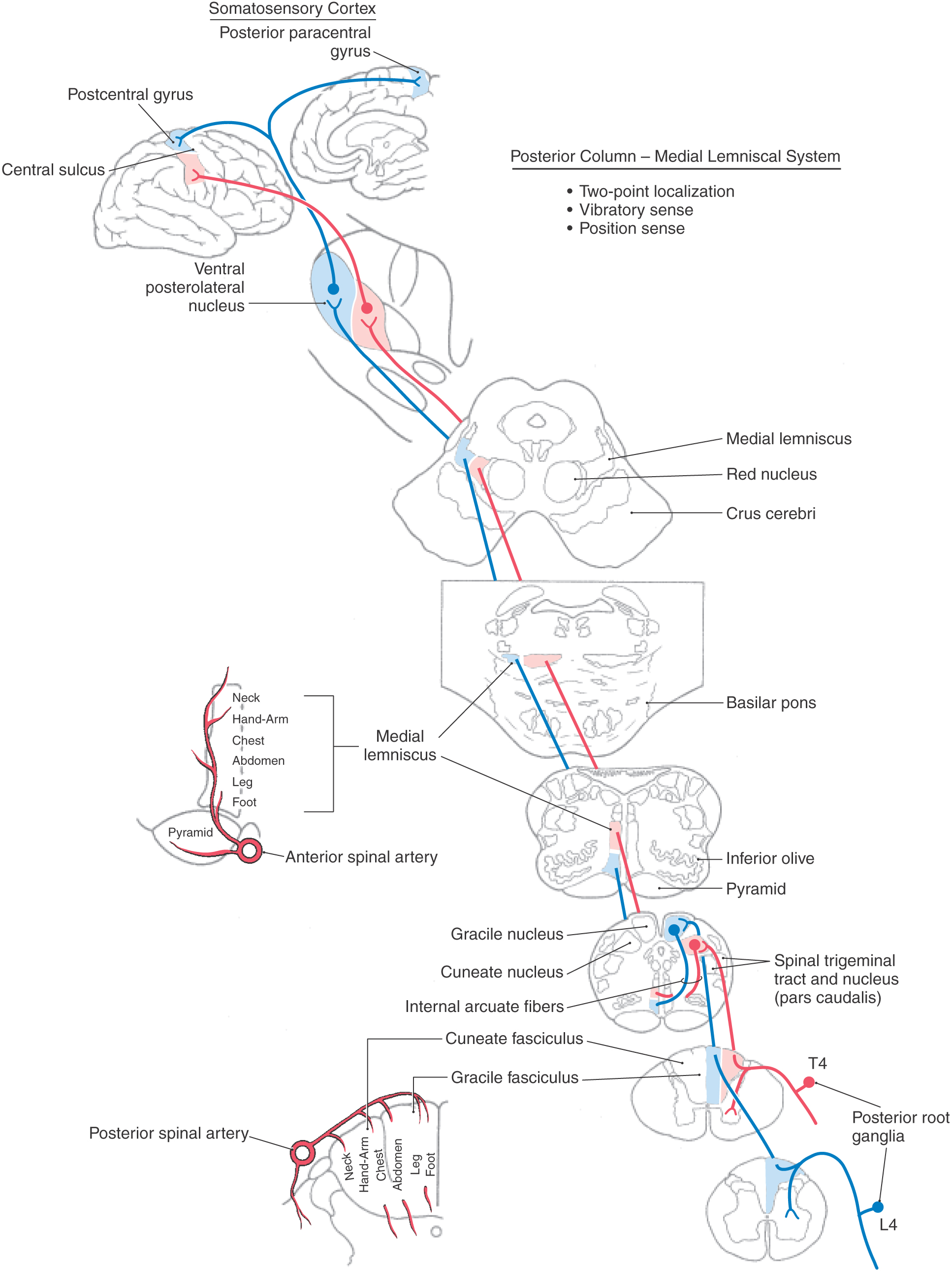
The posterior column–medial lemniscal system (PCMLS), shown later in Figures 17-7 and 17-8, is involved with the perception and appreciation of mechanical stimuli. It underlies the capacity for fine form (size and shape) and texture discrimination, form recognition of three-dimensional shape (stereognosis), and motion detection. This pathway is also involved in transmitting information related to conscious awareness of body position (proprioception) and limb movement (kinesthesia) in space.
Characteristic features of the PCMLS include transmission on somatic afferent (SA) fibers that have fast conduction velocities, a limited number of synaptic relays in which processing of the signal occurs, and a precise somatotopic organization. These features provide the basis for the accurate localization of the body region touched. There is only limited convergence along the pathway; consequently, the signal is transmitted with high fidelity and a high degree of spatial and temporal resolution. This pathway signals somatic sensations by use of frequency and population codes. In frequency coding, a cell’s firing rate signals stimulus intensity or temporal aspects of the tactile stimulus. In population coding, the distribution in time and space of activated cells in the central nervous system signals location of the stimulus as well as its motion or direction, if any.
The high degree of resolution in the PCMLS is the result of inhibitory mechanisms such as feed-forward, feedback, and lateral (surround) inhibition. This mechanism is a feature found initially within the posterior column nuclei and is present through all the relays of the PCML pathway. It sharpens and enhances the discrimination between separate points on the skin and is critical for two-point discrimination. The ability to discriminate between two points simultaneously applied varies widely over different parts of the body.
Peripheral Mechanoreceptors
The first step in evoking somatic sensations is the activation of peripheral mechanoreceptors. Mechanical pressure, such as skin deformation, is transduced into an electrical signal in the peripheral process of a primary afferent neuron (see Chapter 3). This leads to a depolarizing graded membrane potential across the membrane of the neuron. If this potential depolarizes the trigger zone, located at the first myelin segment of the axon, to threshold, an action potential is produced (see Chapter 3). In most receptors, transduction occurs between the mechanoreceptor and the subjacent primary afferent membrane. However, in some cases (i.e., Merkel cells), the nonneural cells of the receptor complex may influence their associated primary afferent axon by vesicular release of a transmitter substance.
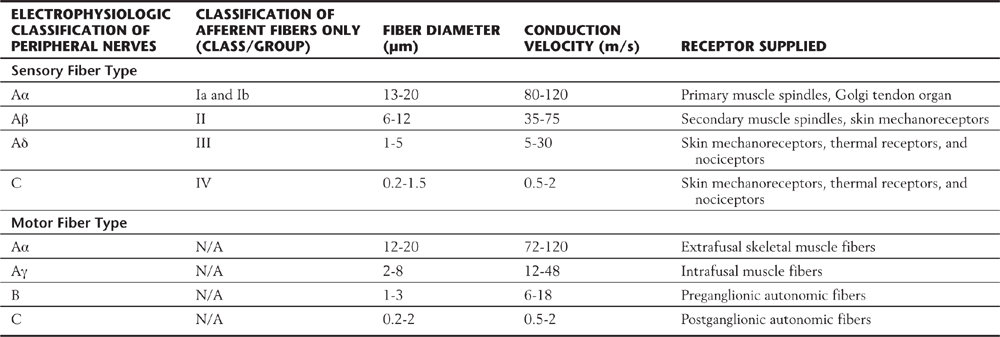
Each morphologic type of mechanoreceptor responds to different tactile stimuli. Cutaneous tactile receptors (Table 17-1; Fig. 17-1) are located in the basal epidermis and dermis of glabrous (palms, soles, lips) and hairy skin. These low-threshold mechanoreceptors may be encapsulated, such as Meissner, Pacinian, and Ruffini corpuscles, or unencapsulated, such as Merkel cell–neurite complexes (commonly referred to as Merkel cells) and hair follicle receptors. Meissner corpuscles, some hair follicle receptors, and Pacinian corpuscles respond to transient, phasic, or vibratory stimuli. These receptors respond to each initial application or removal of a stimulus but fail to respond during maintained stimulation. Consequently, they are rapidly adapting (RA) receptors (Fig. 17-2A). Hair follicle receptors are also capable of signaling motion, its direction or orientation, and its velocity.
Table 17-1 Cutaneous Mechanoreceptors and Their Associated Fiber Types and Sensations
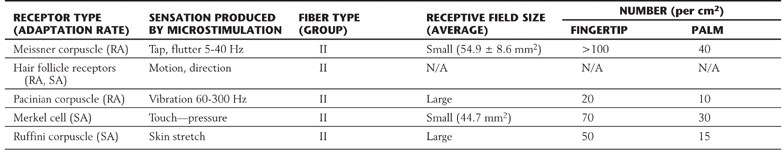
RA, rapidly adapting; SA, slowly adapting.
Merkel cells, Ruffini corpuscles, and some hair follicle receptors signal tonic events such as discrete small indentations in the skin. They provide input related to both the displacement and velocity of a stimulus. They are also capable of encoding stimulus intensity or duration because they are slowly adapting (SA) and are active so long as the stimulus is present (Fig. 17-2A). For example, Merkel cells are crucial to reading of Braille.
Deep tactile mechanoreceptors are found within the dermis of the skin, in the fascia surrounding muscles and bone, and in the periodontium. These receptors include Pacinian corpuscles, Ruffini corpuscles, and other encapsulated nerve endings located in the periosteum, the deep fascia, and the mesenteries. The receptors of this group respond to pressure, vibration (Fig. 17-2B and Table 17-1), skin stretch and distention, or tooth displacement.
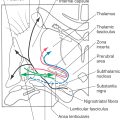
Proprioceptive receptors (Table 17-2; Fig. 17-1) are located in muscles, tendons, and joint capsules. These receptors include muscle spindles and their associated nuclear bag and chain muscle fibers that are innervated by Ia and II afferent nerve fibers. The Golgi tendon organs and their group Ib fibers and the encapsulated Ruffini-type joint receptors also function in this capacity. They respond to static limb and joint position or to the dynamic movement of the limb (kinesthesia) and are important sources of information for balance, posture, and limb movement.
Table 17-2 Muscle and Joint Proprioceptors and Their Associated Fiber Types and Sensations
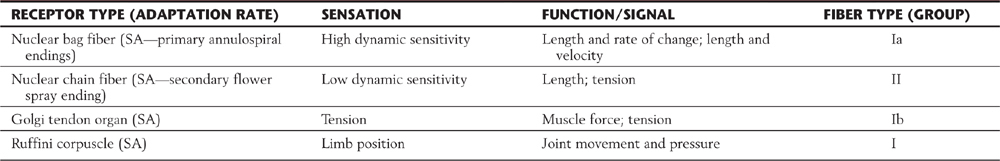
SA, slowly adapting.
The accuracy with which a tactile stimulus is localized depends on the density of receptors and the size of their receptive fields (Fig. 17-3). The greatest density of cutaneous tactile receptors is found on the tips of the glabrous digits and in the perioral region. Other regions, like the back, have much lower density, thus creating a receptor density gradient between various body parts. The receptive field is the area of skin innervated by branches of an SA fiber, the stimulation of which activates its receptors (Fig. 17-3). Small receptive fields are found in areas such as the fingertips, where receptor density is high and each receptor serves an extremely small area of skin. In such regions, the individual is able to discriminate small variations in a variety of sensory inputs. In other regions, receptor density is low and each receptor serves an expansive area of skin, creating large receptive fields with resultant reduction in discriminative ability.
At all levels of the tactile pathway, densely innervated body parts are represented by greater numbers of neurons and take up a disproportionately large part of the somatosensory system’s body representation. In this respect, there is an inverse relationship between the size of the receptive field and the representation of that body part in the somatosensory cortex. For example, the trunk, with its large receptive fields, has a small representation in the somatosensory cortex, whereas the fingers, with their small receptive fields, have a large representation in the somatosensory cortex (compare Fig. 17-3 with Fig. 17-10). As a result, the fingertips and lips provide the central nervous system with the most specific and detailed information about a tactile stimulus.
Primary Afferent Fibers
As initially described in Chapter 9, primary afferent SA fibers consist of (1) a peripheral process extending from the posterior root ganglion either to contact peripheral mechanoreceptors or to end as free nerve endings, (2) a central process extending from the posterior root ganglion into the central nervous system, and (3) a pseudounipolar cell body in the posterior root ganglion. The peripheral distribution of the afferent nerves arising from each spinal level delineates the segmental pattern of dermatomes. In clinical testing, these ribbon-like strips of skin are associated primarily with fibers and pathways that convey pain and thermal information; they are considered in Chapter 18.
Peripheral nerves are classified by two schemes. One is based on their contribution to a compound action potential (A, B, and C waves) recorded from an entire mixed peripheral nerve (e.g., sciatic nerve) after electrical stimulation of that nerve. The other scheme specific to cutaneous fibers (e.g., lateral antebrachial cutaneous nerve, sural nerve) is based on fiber diameter, myelin thickness, and conduction velocity (classes I, II, III, and IV) (Table 17-3; Fig. 17-4). The two schemes are related because conduction velocity determines a fiber’s contribution to the compound action potential. Discriminative touch, vibratory sense, and position sense are transmitted by group Ia, Ib, and II fibers (Tables 17-1 and 17-2).
Table 17-3 Peripheral Sensory and Motor Fibers: Groups, Diameters, and Conduction Velocities
Spinal Cord and Brainstem
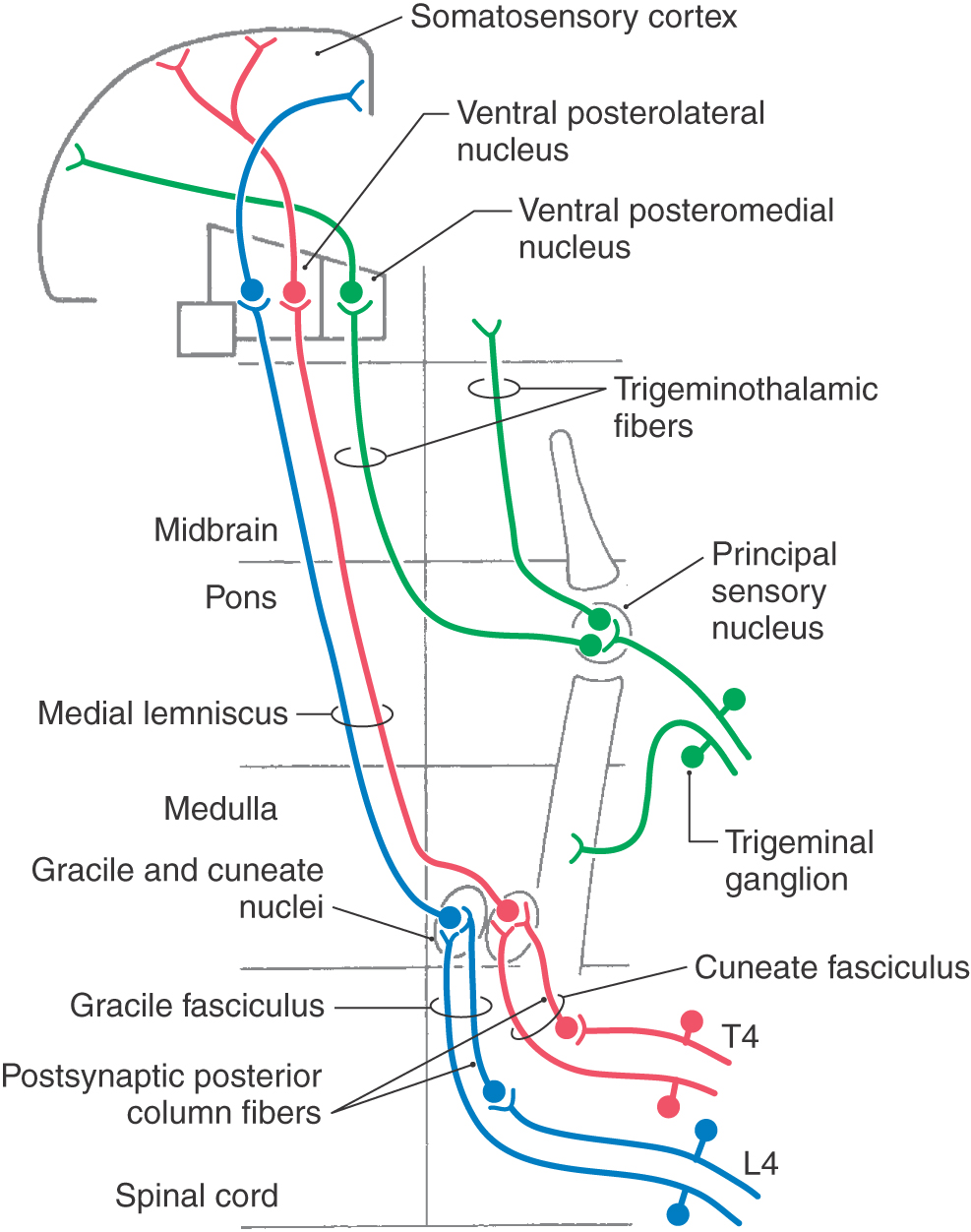
On the basis of cell size and fiber diameter, primary sensory fibers are categorized as large and small. Large-diameter fibers subserve discriminative touch, flutter-vibration, and proprioception (groups Ia, Ib, II, and Aβ; Tables 17-1 and 17-2). They enter the spinal cord via the medial division of the posterior root (see Chapter 9) and then branch (Fig. 17-5). One set of branches terminates on second-order neurons in the spinal cord gray matter at, above, and below the level of entry. These branches contribute to a variety of spinal reflexes and to ascending projections such as postsynaptic posterior column fibers. The largest set of branches ascends cranially and contributes to the formation of the gracile and cuneate fasciculi. These fiber bundles are collectively termed the posterior columns owing to their position in the spinal cord (Figs. 17-5 to 17-7).
Within the posterior columns, fibers from different dermatomes are organized topographically. Sacral level fibers assume a medial position, and fibers from progressively more rostral levels (up to thoracic level T6) are added laterally to form the gracile fasciculus (Figs. 17-5 and 17-6). Thoracic fibers from above T6 and cervical fibers form the laterally placed cuneate fasciculus in the same manner. Thus the lower extremity is represented medially and the upper extremity is represented laterally within the posterior columns (Figs. 17-5 and 17-6). Compromise of blood flow in the posterior spinal artery, which supplies the posterior funiculus, or mechanical injury to the posterior columns (as in Brown-Séquard syndrome) results in an ipsilateral reduction or loss of discriminative, positional, and vibratory tactile sensations at and below the segmental level of the injury. Symptoms indicative of damage to fibers of the posterior columns are also seen in tabes dorsalis (progressive locomotor ataxia). This disease is caused by infection with Treponema pallidum and is associated with neurosyphilis. The fibers of the posterior columns degenerate, and the patient has ataxia (related to the lack of sensory input, clinically referred to as sensory ataxia), loss of muscle stretch (tendon) reflexes, and proprioceptive losses from the extremities. In sensory ataxia, the patient may also have a wide-based stance and may place the feet to the floor with force in an effort to create the missing proprioceptive input.
The posterior column nuclei, the gracile and cuneate nuclei, are found in the posterior medulla at the rostral end of their respective fasciculi. They are supplied by the posterior spinal artery (Fig. 17-7). The cell bodies of the gracile and cuneate nuclei are the second-order neurons in the PCMLS. They receive input from first-order neurons having cell bodies in the ipsilateral posterior root ganglia (Figs. 17-7 and 17-8). The gracile nucleus receives input from sacral, lumbar, and lower thoracic levels via the gracile fasciculus; the cuneate nucleus receives input from upper thoracic and cervical levels through the cuneate fasciculus.
In addition to the somatotopic organization of projections to the posterior column nuclei, there is a submodality segregation of tactile inputs within these nuclei. The second-order relay neurons are arranged into a core “clusters” region surrounded by a covering “shell” region that allows submodality segregation of the excitatory primary afferent input. Rapidly adapting and slowly adapting inputs terminate centrally within the core. Muscle spindle and joint inputs project preferentially to the rostral shell region. Pacinian corpuscle input is restricted to the caudal shell region.
The posterior column nuclei have an inner core region containing large projection neurons surrounded by a diffuse shell of small fusiform and radiating cells. The shell area contains interneurons responsible for feedback inhibition in the posterior column nuclei. This feedback alters activity of projection neurons of the inner core. The posterior column nuclei also receive descending axons from the contralateral primary somatosensory cortex and from the medullary reticular formation (nucleus reticularis gigantocellularis). The presence of non–posterior column inputs to these projection cells suggests that information received by the posterior column nuclei is not simply relayed but undergoes signal processing.
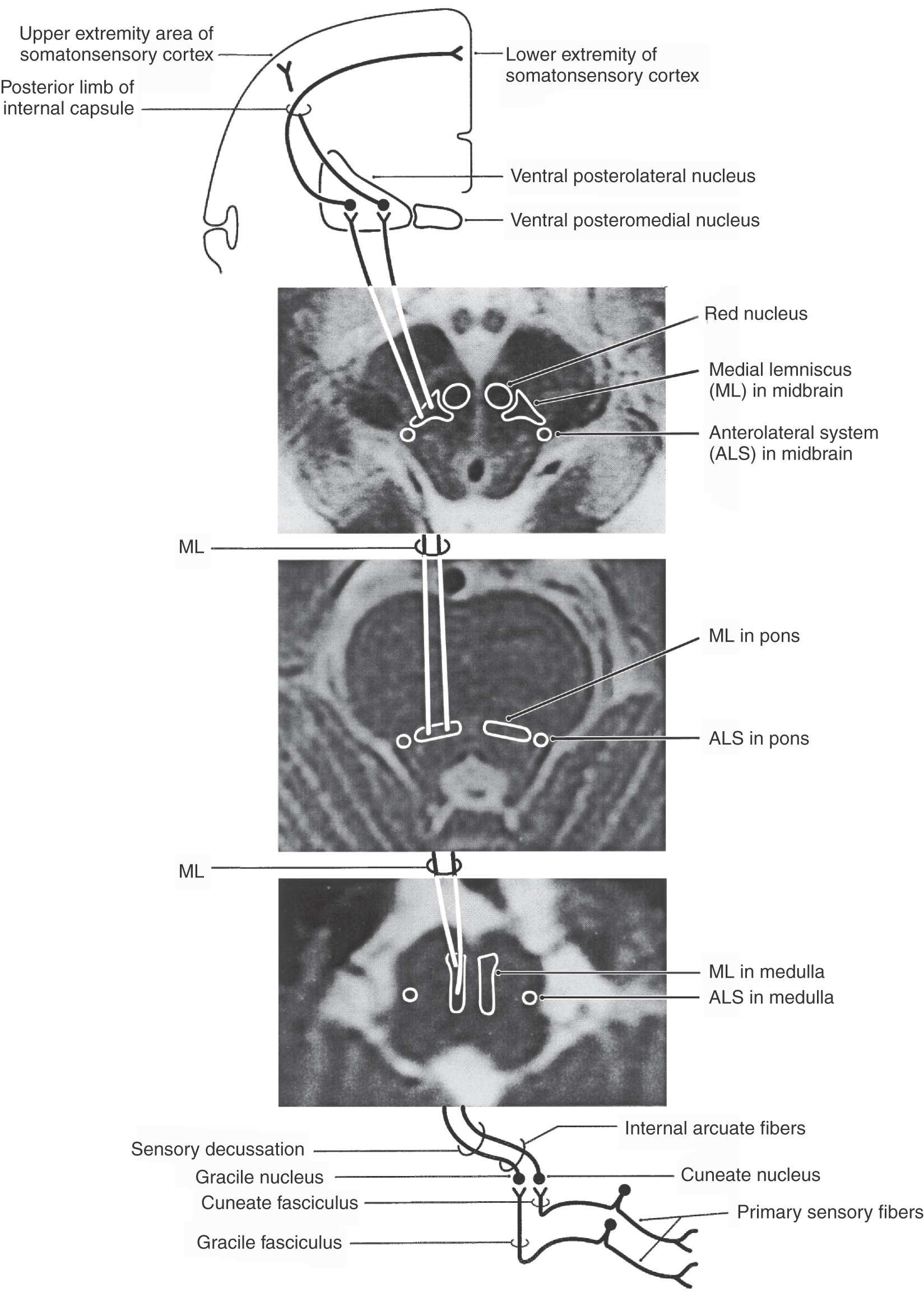
The second-order cells in the core region of the posterior column nuclei send their axons to the contralateral thalamus (Figs. 17-7 and 17-8). In the medulla, the internal arcuate fibers, axons of cells in the posterior column nuclei, arc anteromedially toward the midline, decussate, and ascend as the medial lemniscus on the opposite side. Fibers in the medial lemniscus that arise in the cuneate nucleus are located in superior portions of the medial lemniscus (and convey information from the upper extremity), and those from the gracile nucleus are located in its inferior parts (and relay data from the lower extremity) (Figs. 17-6 and 17-8). The anterior spinal artery supplies the medial lemniscus in the medulla, and penetrating branches of the basilar artery (paramedian and short circumferential) supply it in the pons. Vascular damage at these brainstem levels leads to deficits in discriminative touch, vibratory, and positional sensibilities over the contralateral side of the body. As the medial lemniscus moves rostrally through the brainstem, it rotates laterally so that the upper extremity representation comes to lie medially and the lower extremity laterally in the pons (Figs. 17-7 and 17-8). As the medial lemniscus traverses the midbrain, it is shifted laterally and posteriorly by the appearance of medial structures such as the red nucleus (Figs. 17-7 and 17-8). The midbrain lesion in Figure 17-9 compromised only the medial lemniscus on the right side and resulted in a loss of discriminative touch and proprioception on the patient’s left side. This patient did not experience the loss of any other modality. This somatotopic organization is generally maintained as the medial lemniscus terminates on cells in the ventral posterolateral nucleus (VPL) of the thalamus.
The postsynaptic posterior column pathway, a small supplemental pathway in humans that relays nondiscriminative tactile signals to supraspinal levels, consists of non–primary afferent axons carrying tactile signals in the posterior columns (see Fig. 17-14). The cells of origin of this pathway are located in laminae III and IV of the posterior horn. Axons of the second-order postsynaptic posterior column pathway travel in the posterior columns and together with other tactile primary afferent fibers terminate in the posterior column nuclei. Cells of these nuclei relay this postsynaptic posterior column input to the contralateral thalamus via the medial lemniscus. Although this pathway is small, it may provide the morphologic basis for the return of some tactile sensation after vascular lesions involving the PCMLS.
Ventral Posterior Nucleus
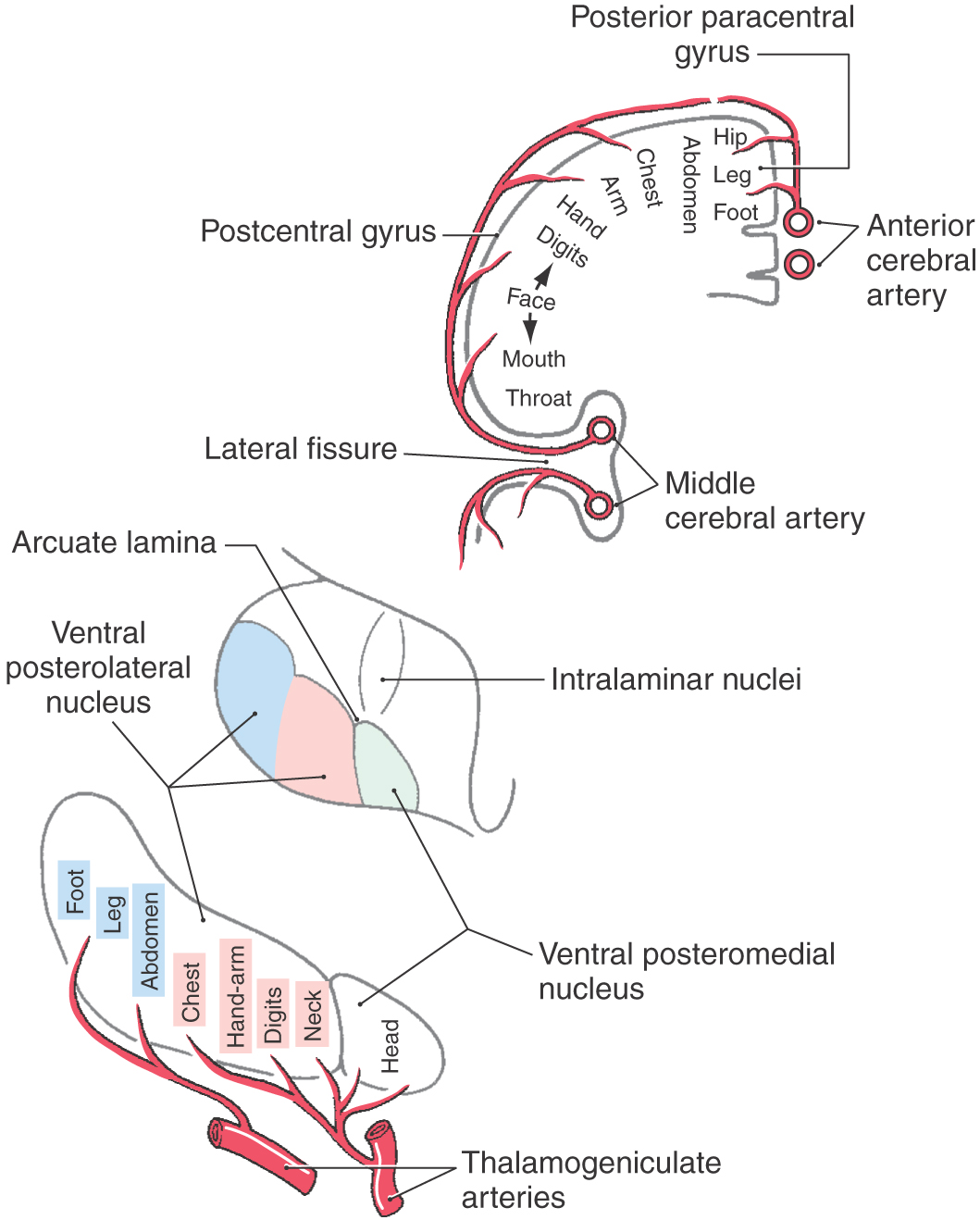
The ventral posterior nucleus, sometimes called the ventrobasal complex, is a wedge-shaped cell group located caudally in the thalamus. Its lateral border abuts the internal capsule, and ventrally it borders on the external medullary lamina. The ventral posterior nucleus is composed of the laterally located ventral posterolateral nucleus (VPL) and the medially located ventral posteromedial nucleus (VPM). Although these nuclei have also been termed the ventralis caudalis externus and ventralis caudalis internus in humans, the more widely used and recognized terms VPL and VPM are used in this book. The VPL is separated from the VPM by fibers of the arcuate lamina. The ventral posterior nucleus (VPM and VPL) is supplied by thalamogeniculate branches of the posterior cerebral artery, and compromise of these vessels can result in loss of all tactile sensation over the contralateral body and head (Fig. 17-10).
The VPL receives ascending input from the medial lemniscus, and input to the VPM is from the trigeminothalamic tracts. Within the VPL, medial lemniscal fibers from the contralateral cuneate nucleus terminate medial to those from the gracile nucleus. As a result, the representation of the lower extremity is lateral and that of the upper extremity is medial in the VPL (Fig. 17-10). The representation of an individual body part is organized as a C-shaped lamina. Tactile signals are also represented in other thalamic nuclei receiving lemniscal input, including the ventral posterior inferior nucleus and the pulvinar and lateral posterior group.
In addition to their somatotopic organization, the medial lemniscal fibers that terminate in the ventral posterior nucleus are segregated on the basis of their functional properties. Rapidly and slowly adapting inputs terminate on different cell groups within the core region of the VPL. Pacinian inputs and inputs arising from joints and muscles are confined to a shell region on the posterior, rostral, and anterior edges of the nucleus. Individual lemniscal axons arborize in the sagittal plane to terminate on longitudinal cell clusters, called rods, in the VPL. This arrangement of inputs and target cells creates isorepresentations consisting of neurons with similar receptive fields and submodalities arranged along a rostrocaudal axis.
The VPL for the trunk and extremities (and VPM for the head) contains two populations of identified neurons. The first consists of large-diameter multipolar cells that give rise to axons that traverse the posterior limb of the internal capsule and terminate mainly in the primary (SI) and secondary (SII) somatosensory cortices. These thalamocortical cells and fibers are the third-order neurons in the PCMLS that provide excitatory (glutaminergic) input to the cortex. The second population consists of inhibitory (GABAergic) local circuit interneurons, which receive excitatory corticothalamic inputs and influence the firing rates of thalamocortical cells. In addition, these thalamocortical cells are also influenced by GABAergic input from the thalamic reticular nucleus and by excitatory (glutaminergic) corticothalamic fibers that arise in layer VI of the primary and secondary somatosensory cortices.
Primary Somatosensory (SI) Cortex
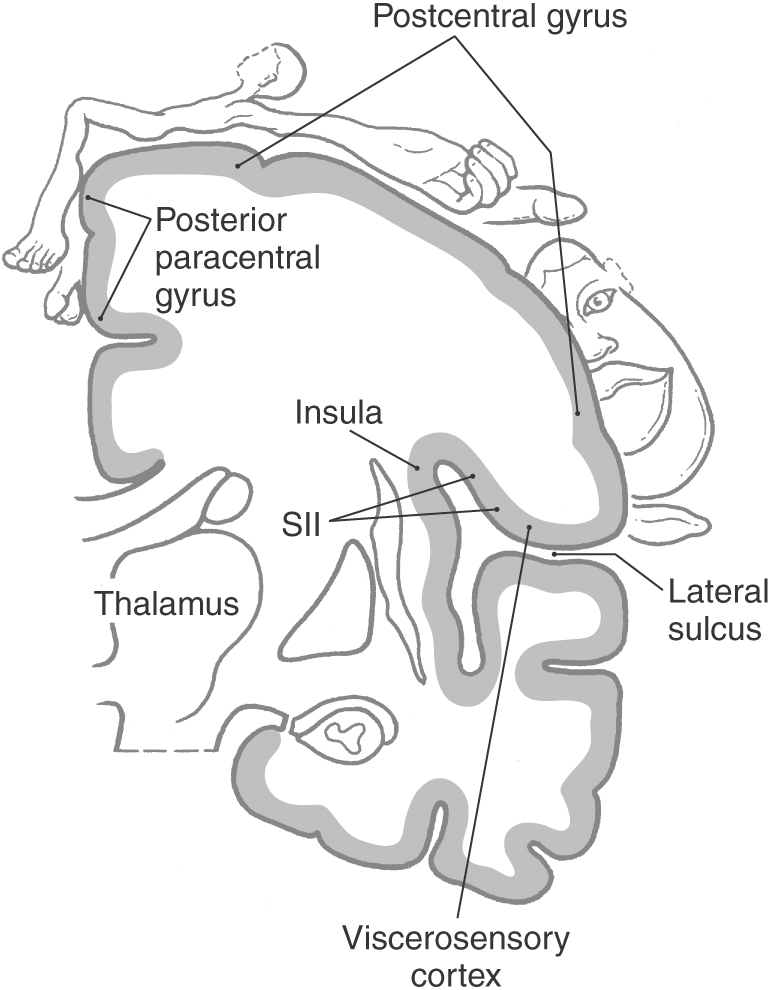
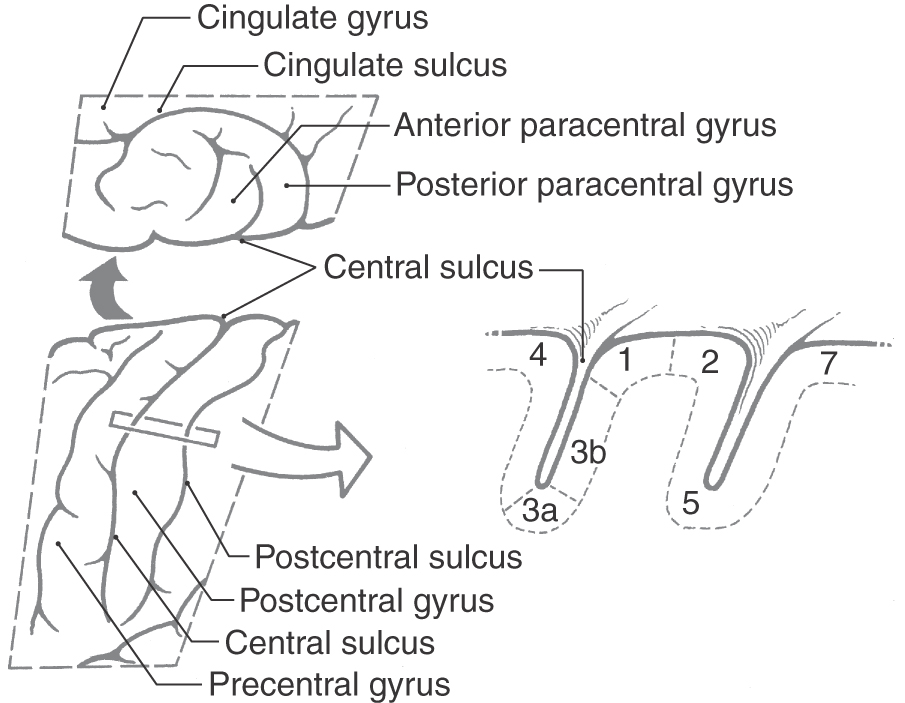
Axons from third-order thalamic neurons terminate in the primary somatosensory (SI) cortex (Figs. 17-7, 17-10, and 17-11). This cortical region is bordered anteriorly by the central sulcus and posteriorly by the postcentral sulcus and comprises the postcentral gyrus and the posterior paracentral gyrus (Fig. 17-12). The cortex contains a somatotopic representation of the body surface (a homunculus, or “little man”), which is laid out in a “foot to tongue” pattern along the medial to lateral axis (Fig. 17-11). Body regions with a high density of receptors, such as the hand and the lips, have a disproportionately large amount of cortical tissue dedicated to their central representation. In contrast, regions with low receptor density, such as the back, have small cortical representations (Figs. 17-3 and 17-11). Blood supply to the SI cortical areas is provided by the anterior and middle cerebral arteries. Vascular lesions involving the middle cerebral artery produce tactile loss over the contralateral upper body and face, and those involving the anterior cerebral artery affect the contralateral lower limb.
Figure 17-12. The primary somatosensory (SI) cortex of the parietal lobe. The views on the left show the location of SI in the postcentral gyrus (lateral view, bottom) and as it extends medially into the posterior paracentral gyrus (top). See Figure 17-11 for an overview of the entire hemisphere. The cross section at right shows the subdivisions of the SI cortex into the four cytoarchitecturally distinct areas 3a, 3b, 1, and 2. Rostral to these is motor cortex (area 4), and caudal to them are association areas 5 and 7.
On histologic examination, the primary somatosensory cortex is subdivided into four distinct areas; from anterior to posterior, these are Brodmann areas 3a, 3b, 1, and 2 (Fig. 17-12). Area 3a is located in the depths of the central sulcus and abuts area 4 (primary motor cortex). Areas 3b and 1 extend up the bank of the sulcus onto the shoulder of the postcentral gyrus, whereas area 2 lies on the gyral surface and abuts area 5 (somatosensory association cortex).
Each of these four cytoarchitectural areas of the SI cortex receives submodality-specific inputs. Areas 3a and 2 are primarily targeted by neurons in the shell region of the VPL. They receive proprioceptive inputs arising from muscle spindle afferents (mainly area 3a), Golgi tendon organs, and joint afferents (mainly area 2). These two areas are capable of processing kinesthetic information related to muscle length and tension as well as static and transient joint position. Areas 3b and 1 are mainly targeted by neurons in the core region of the VPL. They receive cutaneous afferents from receptors such as Meissner corpuscles (RA) and Merkel cells (SA). In addition to receiving input that originates from cutaneous touch receptors such as Meissner and Merkel endings, areas 3b and 1 also receive input from cutaneous receptors that transmit information concerned with pain and thermal sensations. The anterolateral system, the pain pathway, is described in Chapter 18.
Small lesions in various parts of the somatosensory cortex may result in characteristic types of sensory losses. Lesions involving area 1 produce a deficit in texture discrimination, whereas damage to area 2 results in loss of size and shape discrimination (astereognosis). Injury to area 3b has a more profound effect than does damage to either area 1 or 2 alone, producing deficits in both texture and size and shape discrimination. This difference suggests that there is hierarchical processing of tactile information in the SI cortex, with area 3b performing the initial processing and distributing the information to areas 1 and 2. However, lesions involving the somatosensory cortex usually include larger areas and frequently result in more global deficits, such as a loss of proprioception, position sense, vibratory sense, and pain and thermal sensations on the contralateral side of the body.
Additional Cortical Somatosensory Regions
The secondary somatosensory (SII) cortex lies deep in the inner face of the upper bank of the lateral sulcus (Fig. 17-11). It, too, contains a somatotopically organized representation of the body surface. Inputs to SII cortex arise from the ipsilateral SI cortex as well as from the ventral posterior inferior nucleus (VPI) of the thalamus, a triangle-shaped nucleus lying anterior (ventral) to VPL and VPM. This cortical area is also supplied primarily by the middle cerebral artery, so it cannot substitute functionally for SI after vascular compromise of this artery (Fig. 17-10).
Posterior to area 2, additional parietal cortical regions also receive tactile inputs. These regions include area 5 and lateral portions of area 7 (7b). The anterior pulvinar and lateral posterior group, which receive some medial lemniscal input, project to areas 5 and 7 (Fig. 17-12). In addition, they also receive input from the primary somatosensory cortex. Lesions in the parietal association area can produce agnosia, in which contralateral body parts are lost from the personal body map. Sensation is not radically altered, but the limb is not dressed and is not recognized as part of the patient’s own body.
TRIGEMINAL SYSTEM
Most of the somatosensory information from craniofacial structures, including the oral and nasal cavities, is transmitted to the brainstem over the trigeminal nerve. The relay and central processing of input from trigeminal primary afferent neurons occur in a column of brainstem neurons that begins rostrally in the middle pons and extends caudally to overlap with the posterior horn of the upper cervical spinal cord. The primary afferent neurons of the trigeminal nerve, the brainstem nuclei, and pathways described in the following discussion are referred to as the trigeminal system. Like spinal cord somatosensory pathways, trigeminal pathways can be subdivided into those for tactile discrimination, flutter-vibration, and proprioception (as described in this chapter) and those for pain and thermal sensations (as described in Chapter 18).
Trigeminal Nerve
As its name implies, the trigeminal nerve has three peripheral divisions: ophthalmic (V1), maxillary (V2), and mandibular (V3). A few nerve fibers in the facial (cranial nerve VII), the glossopharyngeal (cranial nerve IX), and the vagus (cranial nerve X) nerves transmit SA innervation from a small cutaneous area around the ear. The peripheral distribution of these nerves delineates the facial dermatomes (see Chapter 18).
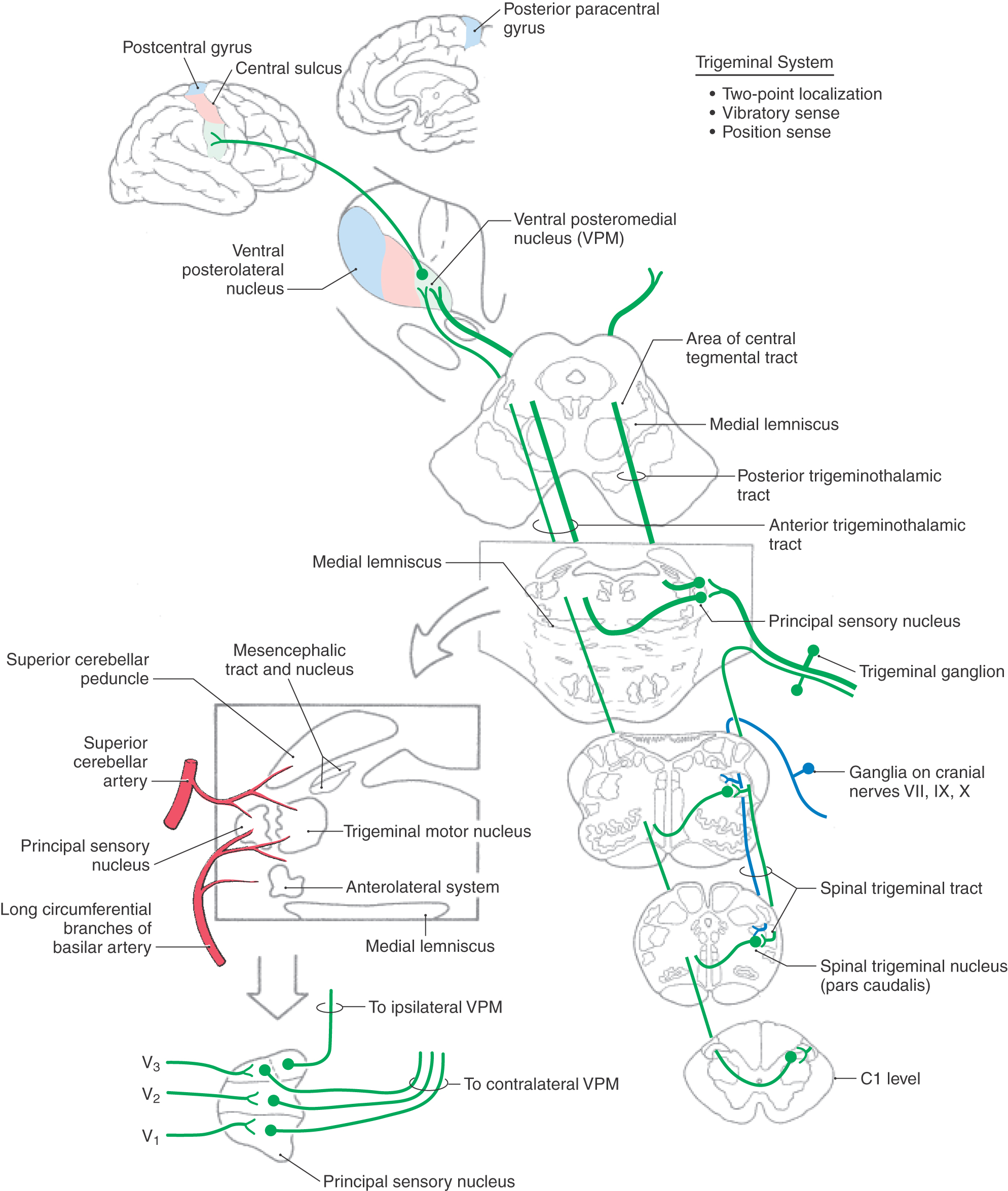
The cell bodies of trigeminal primary afferent neurons are located in the trigeminal (gasserian or semilunar) ganglion (Figs. 17-13 and 17-14) and in the mesencephalic trigeminal nucleus. The central processes of trigeminal ganglion cells form the large sensory root (portio major) of the trigeminal nerve as they enter the lateral aspect of the pons. Within the brainstem, central processes of most trigeminal ganglion cells bifurcate into ascending and descending branches before terminating on second-order neurons in the brainstem trigeminal sensory nuclei. The ascending branches terminate in the principal sensory nucleus, located in the pons, and the descending branches coalesce to form the spinal (descending) tract of the trigeminal nerve. The axons of this tract terminate throughout the rostrocaudal extent of the spinal nucleus of the trigeminal nerve, which lies just medial to the tract. Discussion in this chapter focuses on the more rostral components of the trigeminal system, including the principal sensory nucleus and the trigeminal mesencephalic nucleus. These nuclei and their connections are primarily involved in tactile discrimination, proprioception, and kinesthesia from the head. The role of more caudal components of the trigeminal system, which serve a primary role in pain and thermal sensations, is considered in Chapter 18.
ANTERIOR AND POSTERIOR TRIGEMINOTHALAMIC TRACTS
Peripheral Receptors
Tactile sensations originating in the head are transduced into nerve impulses by the same types of nerve terminals and specialized sensory receptor organs found in other parts of the body (Fig. 17-1). However, owing to their association with structures unique to this region, some of these sensory endings serve specialized functions. For example, receptors in the periodontal ligament (the primarily collagenous connective tissue surrounding each tooth) are exquisitely sensitive to tooth displacement and bite force. A large number of encapsulated receptors, particularly Meissner corpuscles, are found beneath the surface of the lips and perioral skin. The precision of two-point tactile discrimination on the lips and perioral regions is comparable with that on the fingertips. Most of the primary afferent neurons concerned with perception of discriminative sensation from the face and oral cavity have large-diameter (e.g., Aβ) axons. Some of these ascend without branching, whereas others bifurcate before terminating in the principal sensory nucleus.
The principal (chief) sensory nucleus is situated in the middle pons at the rostral pole of the spinal trigeminal nucleus (Figs. 17-13 and 17-14). The principal sensory nucleus can be divided into dorsomedial and ventrolateral regions. The dorsomedial division receives most of its primary afferent input from the oral cavity, and the ventrolateral division receives input from all three components of the trigeminal nerve. Thus the somatotopic representation within the principal sensory nucleus is inverted, with V1 (the ophthalmic division) being anterior, V3 (the mandibular division) being posterior, and V2 (the maxillary division) sandwiched in between (Fig. 17-13). This certainly is the case when the brain is viewed in the anatomic orientation; anterior/ventral is down and posterior/dorsal is up. However, when the brain is viewed in the clinical orientation, as in magnetic resonance imaging or computed tomography, the somatotopic representation of the face is upright. This point is discussed further and illustrated in Chapter 18.
Second-order neurons in the principal sensory nucleus have relatively small receptive fields. In addition, they are subject to the same types of intranuclear modulations as are cells in the posterior column nuclei (e.g., feed-forward, feedback, lateral inhibition, and descending modulation from the SI cortex), which serves to sharpen the contrast between adjacent receptive fields. Neurons in the principal sensory nucleus relay discriminative tactile information from the head to the ventral posteromedial nucleus (VPM). Neurons in the ventrolateral part of the principal sensory nucleus give rise to axons that project to the contralateral VPM, along with fibers that originate in the more caudally located spinal trigeminal nucleus. This combined ascending projection forms the trigeminal lemniscus, or anterior (ventral) trigeminothalamic tract (Figs. 17-13 and 17-14), which courses close to the medial lemniscus. Neurons in the dorsomedial division of the principal sensory nucleus project to the ipsilateral VPM by way of the posterior (dorsal) trigeminothalamic tract (Fig. 17-13). This pathway ascends in the pontine tegmentum lateral to the periaqueductal gray in close association with the central tegmental tract. The afferent projections from the principal sensory nucleus terminate somatotopically within the VPM so that the oral cavity is represented medially and the external facial structures are represented more laterally (Figs. 17-10 and 17-13). Third-order thalamocortical neurons in the VPM project via the posterior limb of the internal capsule to the laterally placed face area of SI in the postcentral gyrus (Figs. 17-10 and 17-11). Perioral regions have the highest peripheral innervation density, and consequently the largest representation, along the postcentral gyrus (Fig. 17-11).
Proprioceptive endings (muscle spindles) in muscles of mastication and some periodontal ligament receptors (modified Ruffini endings) are innervated by primary afferent neurons located in the trigeminal mesencephalic nucleus. This brainstem nucleus consists of a slender column of pseudounipolar cells of neural crest origin that remain within the neural tube during development. Cells of the mesencephalic nucleus extend from the rostral pons to upper midbrain levels, where they form a thin band of neurons along the lateral edge of the periaqueductal gray. An important difference between the cell bodies of the mesencephalic nucleus and typical ganglion cells is that the former receive synaptic inputs from peptidergic and monoaminergic neurons in the brainstem. This synaptic influence on the neurons of the trigeminal mesencephalic nucleus provides a unique form of presynaptic modulation before central relay of the primary afferent information.
The processes of cells in the trigeminal mesencephalic nucleus form the mesencephalic tract of the trigeminal nerve. This tract is located directly adjacent to the mesencephalic nucleus (Figs. 17-13 and 17-17) and also extends rostrally, where it borders the midbrain aqueductal gray. The central processes of the trigeminal mesencephalic neurons generally branch in the area posterior (dorsal) to the trigeminal motor nucleus to innervate cells of the motor nucleus. This input to the motor nucleus forms the afferent limb of the myotatic jaw jerk reflex. This clinically useful reflex consists of the processes of trigeminal mesencephalic nucleus neurons that innervate muscle spindles in jaw closing muscles and terminate monosynaptically on trigeminal motor neurons. In turn, the axons of trigeminal motor neurons innervate muscles (i.e., temporalis) that elevate the jaw. A gentle tap on the jaw activates the afferent fibers of this reflex and initiates a contraction of the homonymous muscle (e.g., temporalis) as well as its synergists (e.g., masseter). Trigeminal mesencephalic afferents from the periodontal ligament provide feedback to jaw muscle motor neurons during mastication to regulate bite force, although this input is not monosynaptic. In addition to providing collaterals to the trigeminal motor nucleus, a bundle of descending branches of mesencephalic tract fibers distributes to the reticular formation, the spinal trigeminal nucleus, and the cerebellum. The central connections of the trigeminal mesencephalic nucleus are consistent with their broad participation in the coordination of oral motility patterns, including mastication, swallowing, and speech.
Proprioceptive input from the mesencephalic nucleus is also provided to the principal sensory nucleus and the spinal trigeminal nucleus. Some proprioceptive receptors are innervated by trigeminal ganglion cells, such as those with receptors in the temporomandibular joint, the extraocular muscles, and some periodontal ligaments. Because most trigeminal ganglion axons bifurcate when they enter the brainstem, both the principal sensory and the spinal trigeminal nucleus receive proprioceptive input. Proprioceptive input to the spinal trigeminal nucleus is relayed to the cerebellum, the spinal cord, and the thalamus. However, the principal sensory nucleus receives a disproportionate share of large-diameter, heavily myelinated fibers and may be considered the trigeminal homologue of the posterior column nuclei. These pathways provide the substrate for cortical processing that permits the full hedonic appreciation of foods with different textural properties (oral stereognosis).
RECEPTIVE FIELD PROPERTIES OF CORTICAL NEURONS
Neurons located in cortical areas representing the body and head are organized into functional units called cortical columns (see Fig. 32-9). These are distributed from the pial surface to the cortical white matter. Each column contains neurons responsive to one submodality, and the cells in a column all have similar peripheral receptive field loci. Thalamocortical inputs terminate on stellate cells in layer IV and lower parts of layer III of the SI cortex. Axons of the stellate cells distribute information vertically to the pyramidal cells within individual columns.
The receptive field properties of cortical neurons are more complex than those at subcortical levels. Cortical neurons respond to a specific stimulus orientation (edges) and to specific textures. They are also capable of coding the velocity, speed, and direction of moving stimuli. At least three distinct populations of neurons receive proprioceptive inputs. The first consists of simple neurons that receive input from a single joint or muscle group. These rapidly adapting cells signal movement. The second group consists of postural neurons that signal the final position of a joint once the movement is completed. The third is made of neurons that receive inputs from several joints and muscle groups (multijoint) and signal complex joint-muscle interactions.
The functional properties of cortical neurons reflect the processing and integration of sensory information as it ascends from the posterior column and ventral posterior nuclei to the final processing station in the cortical columns. This sensory signal processing can include (1) convergence of afferent input, which increases receptive field size while decreasing resolution; (2) divergence of output signal, which allows relay cells to amplify the sensory signal and supply it to multiple targets; (3) facilitation; and (4) inhibition. These processes act in concert to enhance the signal-to-noise ratio in terms of both space and time.
In general, larger receptive fields and more complex inhibitory surrounds are displayed by cortical neurons than by their subcortical inputs. For example, a tactile stimulus in the center of a receptive field results in amplification of the sensory signal and increased activity in a restricted population of cortical cells. Conversely, stimulation at the edge of the receptive field suppresses the activity in these neurons. This mechanism provides the circuits active in two-point discrimination.
NEUROIMAGING AND FUNCTIONAL LOCALIZATION
Neuroimaging techniques including functional magnetic resonance imaging (fMRI), regional cerebral blood flow (rCBF) studies, positron emission tomography (PET), and magnetoencephalography (MEG) have been used in clinical studies of the somatosensory pathway in humans. These techniques have elegantly demonstrated the functional organization of somatosensory areas activated by application of various tactile stimuli. For example, PET studies have identified cortical areas 3b and 1 as participating in the discrimination of moving stimuli, whereas cortical area 2 is activated when subjects palpate objects focusing on shape and curvature. Functional MRI studies have identified two areas of increased blood flow, suggesting a concomitant increase in cerebral cortical activity in response to air puff stimulation applied to various loci on the upper limb. One cortical area, located in the depth of the central sulcus, corresponds to area 3b. The other cortical locus of increased activity identified in these studies is posterior and lateral and corresponds to area 1. PET studies have also provided evidence that a tactile stimulus activates both primary (SI) and secondary (SII) somatosensory cortices.
Plasticity and Reorganization in the Primary Somatosensory Cortex
Brain injury, whether resulting from birth or other trauma, tumors, or stroke, can affect anyone and can be devastating. However, on closer examination, some individuals appear to “recover” lost functions, whereas others remain relatively unchanged. In general, the younger the person with the trauma, the more “recovery” is noted.
To understand possible mechanisms for this apparent recovery, we must first look at brain development. The brain of a child is malleable or plastic. It forms multiple, redundant neural connections linking various brain areas. Many of these connections will be retained through usage and experience, whereas others will be “pruned” by programmed cell death (apoptosis) and other cellular mechanisms. These processes will continue for a finite time (a critical period), thus giving many brain regions the potential to function in a variety of ways. Children with brain trauma due to a birth injury may appear to be normal with respect to sensory, motor, and cognitive abilities. It is only after inspection of a brain scan (e.g., from MRI) that the abnormal brain anatomy resulting from the injury is appreciated. The developing brain possesses the ability to reassign brain functions to other brain regions. This is commonly referred to as plasticity.
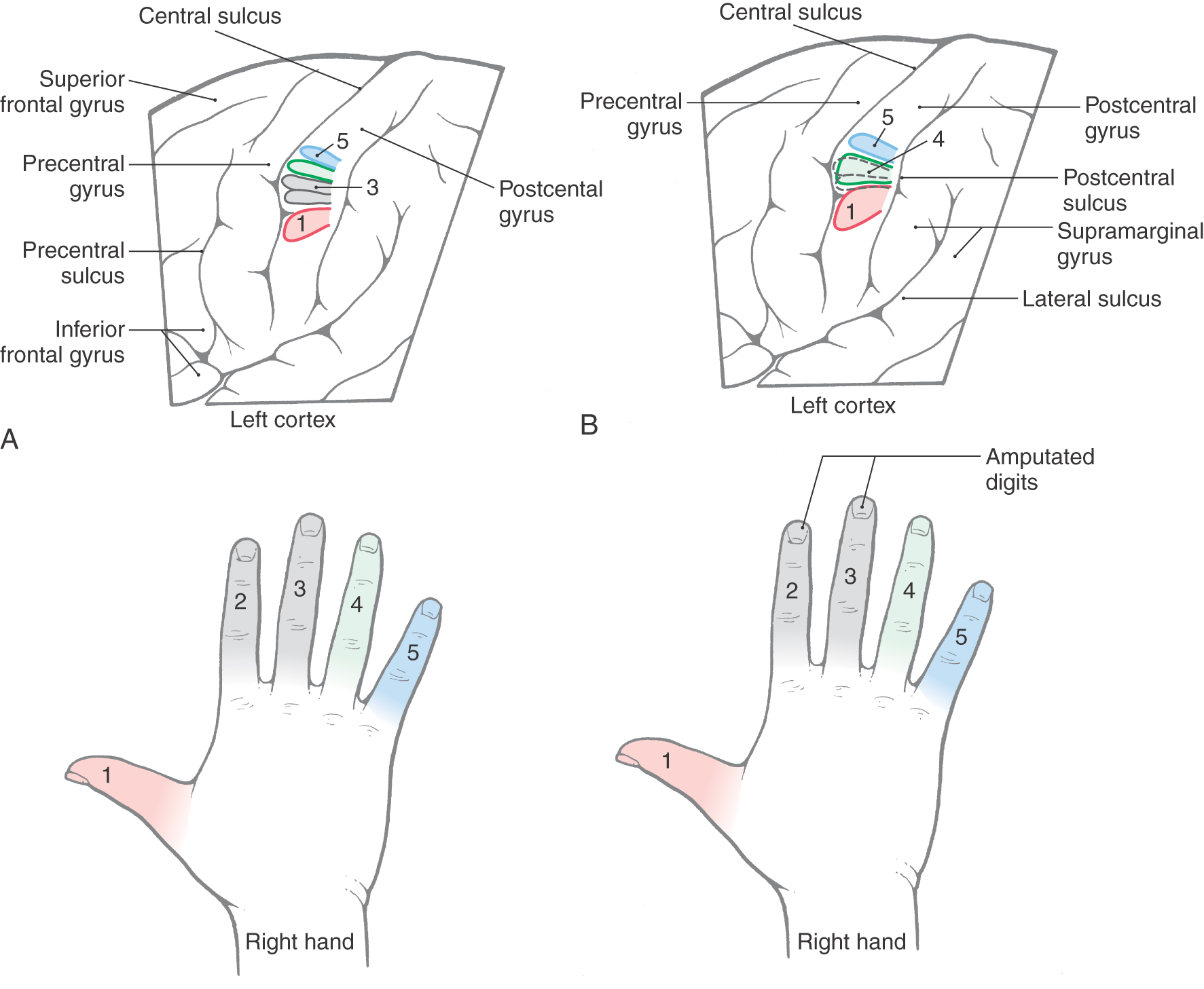
In contrast to that in children, the nervous system in the adult has passed beyond the critical periods of brain development and has become relatively nonmalleable. It has been a common view that most neural connections in the adult are stable and have lost much of their capacity to form new synapses. Evidence suggests, however, that the somatosensory cortex can undergo reorganization. An example of this phenomenon is the changes in the cortical map after limb or digit amputation. Normally (Fig. 17-15A), each digit has a sequential representation in the somatosensory cortex. When digits are amputated, there is a loss of input to the corresponding areas of the somatosensory cortex from the missing digits. There appears to be an expansion of cortical representation of body parts flanking the amputated digits into those cortical regions that previously had the map of the now-missing body parts (Fig. 17-15B). The cortical neurons or areas representing the missing body part now respond when skin regions adjacent to the amputated body part are stimulated. Although many of these cortical changes are subtle, one study suggests that the time course of this reorganization can be rapid, beginning within 10 days after amputation. Thus it appears that the adult brain can exhibit plastic changes and undergo reorganization in response to specific peripheral perturbations. Similar phenomena have been described by molecular biologic methods within hours after experimental induction by appropriate stimuli.
A similar mechanism is also probably at work in older patients who have a stroke. In these patients, there may initially be a complete loss of function followed by a partial recovery that may extend over many months. In contrast to young patients, who may experience a complete (or almost complete) recovery, older patients may experience less than full recovery. In other words, the older the brain, the less plasticity it seems to have.
AFFERENT CEREBELLAR PATHWAYS
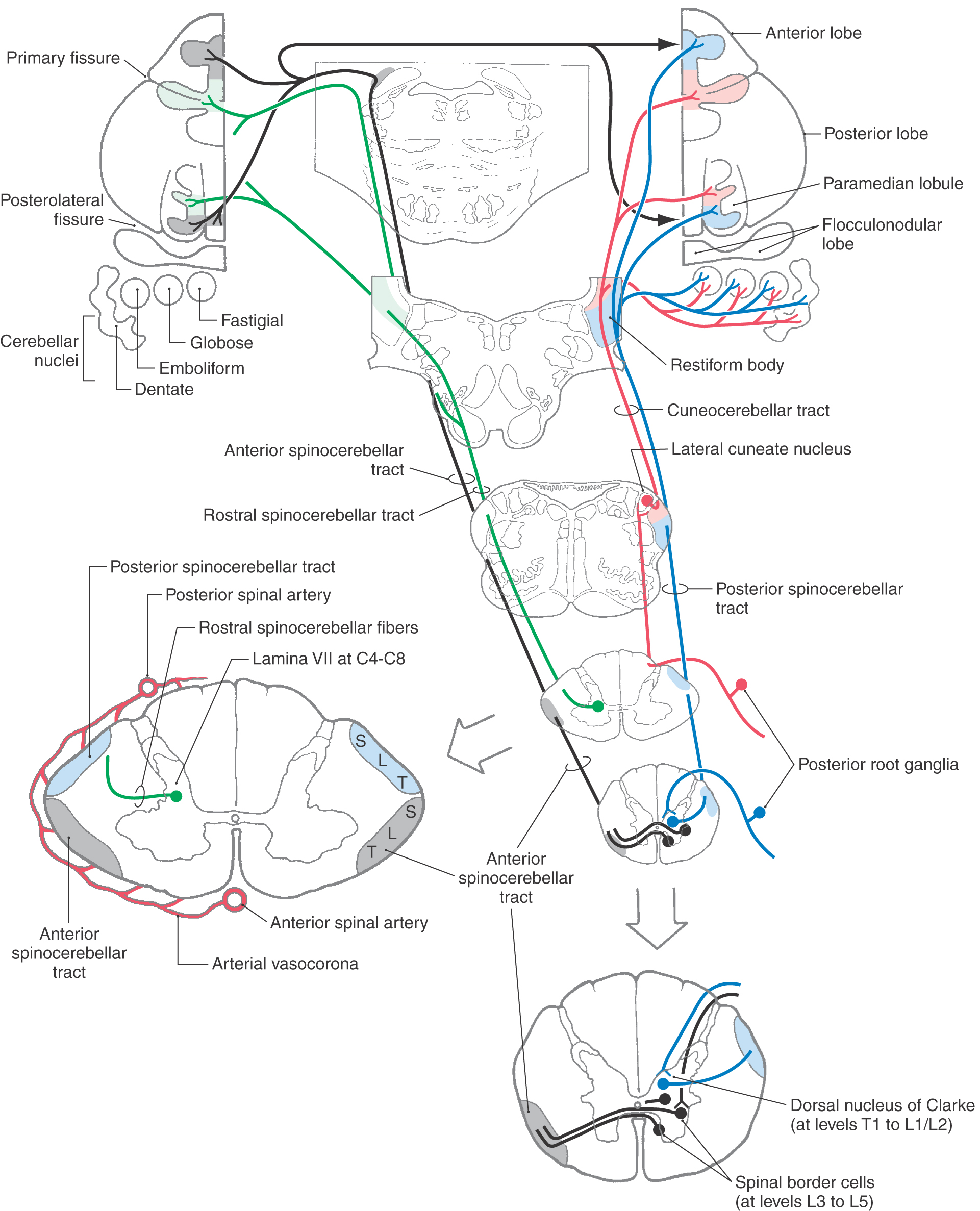
Four spinocerebellar pathways transmit proprioceptive information and limited cutaneous (exteroceptive) signals from cutaneous mechanoreceptors to the cerebellum (Fig. 17-16). These sensory signals include information about limb position, joint angles, and muscle tension and length. Input to the cerebellum plays an integral role in guiding cerebellar control of body muscle tone, movement, and posture.
 Figure 17-16. Organization of posterior, anterior, and rostral spinocerebellar tracts and of the cuneocerebellar tract.
Figure 17-16. Organization of posterior, anterior, and rostral spinocerebellar tracts and of the cuneocerebellar tract.
Spinocerebellar tract axons terminate in the cerebellar nuclei and, as mossy fibers, in the vermis and paravermal region of the cerebellum. These areas are sometimes collectively called the spinocerebellum. This afferent input to the cerebellum forms a pair of somatotopic representations of the body surface in the anterior lobe and the paravermis of the posterior lobe (Fig. 17-16). Degeneration of the major spinocerebellar tracts occurs in diseases such as Friedreich ataxia. The result is cerebellar ataxia—lack of coordination during walking and other movements that occurs because the cerebellum is not receiving the sensory feedback necessary to regulate movement.
Posterior Spinocerebellar Tract
Proprioceptive afferents and a limited number of cutaneous afferents from the lower limb and lower trunk travel in the posterior spinocerebellar tract to reach the ipsilateral cerebellar cortex (Fig. 17-16). Posterior root fibers from the trunk and lower limb terminate on cells in the dorsal nucleus of Clarke, which is located in lamina VII of the intermediate zone in spinal segments T1 to L2. Primary afferent fibers from the spinal cord levels caudal to L2 ascend in the posterior funiculus to reach this nucleus.
Group I muscle spindle and Golgi tendon organ afferents monosynaptically activate cells in the Clarke nucleus (thoracic nucleus). The discharge rate of these posterior spinocerebellar tract cells shows a linear relationship to muscle length; therefore their firing rate can encode muscle length as a frequency code. Group II and group III tactile fibers also terminate on other spinocerebellar cells in the Clarke nucleus. Axons from cells in the dorsal nucleus of Clarke traverse the ipsilateral lateral funiculus and collect on the surface of the spinal cord lateral to the corticospinal tract. These fibers ascend to reach the cerebellum via the restiform body.
Cuneocerebellar Tract
The cuneocerebellar tract is the upper limb equivalent of the posterior spinocerebellar tract (Fig. 17-16). Posterior root fibers in spinal segments C2 to T4 carry muscle spindle and exteroceptive information in the ipsilateral cuneate fasciculus to the cuneate nucleus. In the lower medulla, proprioceptive primary afferent fibers terminate somatotopically in the lateral cuneate nucleus. Cells of the lateral cuneate nucleus project as cuneocerebellar fibers to the cerebellum via the restiform body. Exteroceptive input arising from the rostral end of the cuneate nucleus also ascends to the cerebellar cortex to terminate in the folia of the anterior lobe in lobule V.
Anterior Spinocerebellar Tract
This pathway relays information from group I afferents arising in the lower limb. The cells of origin of this pathway are located in lumbar segments L3 to L5. They are located in the lateral part of Rexed laminae V to VII as well as along the anterolateral border of the anterior horn, where they are called spinal border cells (Fig. 17-16). The axons of anterior spinocerebellar tract (ASCT) cells immediately cross the midline in the anterior white commissure and ascend in the lateral funiculus anterior to the posterior spinocerebellar tract. In the pons, these fibers turn posterolateral to enter the cerebellum via the superior cerebellar peduncle. Most fibers recross to terminate in the cerebellum ipsilateral to their side of origin. These ASCT fibers are distributed more laterally in the cerebellum than are those of the posterior tract. Cells giving rise to ASCT fibers are strongly influenced by descending projections of the reticulospinal and corticospinal pathways. Reticulospinal input inhibits ASCT cells, and the corticospinal input facilitates ASCT cells. Vestibulospinal and rubrospinal projections also monosynaptically excite ASCT cells.
Rostral Spinocerebellar Tract
This tract, the upper limb equivalent of the ASCT, arises from cell bodies located in lamina VII of the cervical enlargement (C4 to C8) (Fig. 17-16). The efferent projections from these neurons ascend uncrossed in the lateral funiculus of the spinal cord. Although most of these axons enter the cerebellum via the restiform body, some travel in the superior cerebellar peduncle. The rostral spinocerebellar tract from the upper limb and the anterior spinocerebellar tract from the lower limb relay cutaneous tactile information from Meissner, Merkel, and Pacinian mechanoreceptors (group II and group III afferents) to the cerebellum.
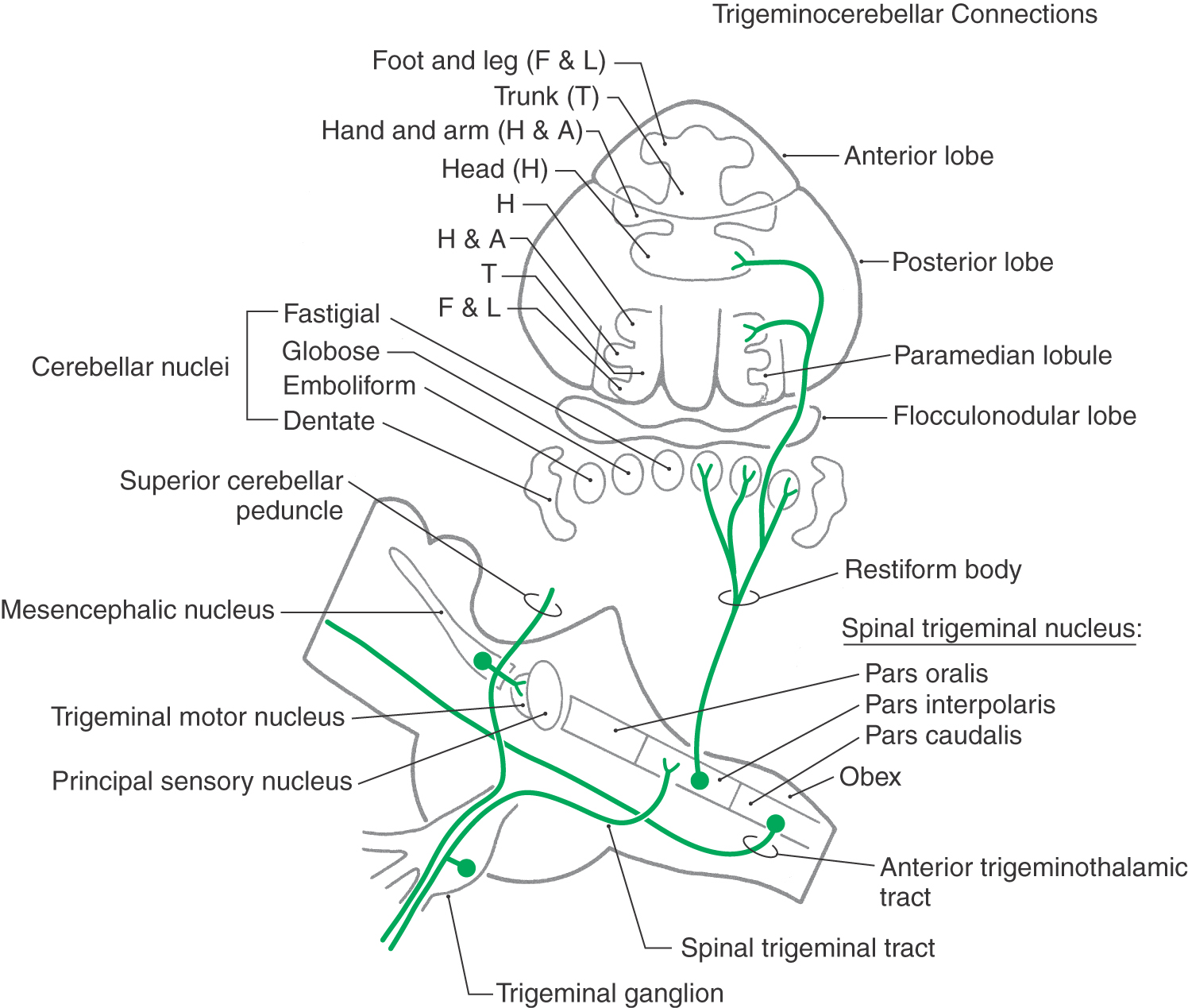
Trigeminocerebellar Connections
The oral motor system requires continual feedback during mastication. As food is chewed, its texture and consistency are altered, changing the demands on jaw muscles. In addition, adaptation is required for long-range functional changes. For example, there are modifications in jaw motility patterns during the transition from suckling to chewing in the newborn and from natural dentition to the use of dentures. It is probable that proprioceptive information reaching the cerebellum from jaw muscle spindles, periodontal afferents, and the temporomandibular joint is involved in these processes.
Branches of the central processes of the mesencephalic trigeminal neurons are distributed to the cerebellar hemispheres and nuclei via the superior cerebellar peduncle (Fig. 17-17). Additional proprioceptive signals from the spinal trigeminal nucleus pars interpolaris and pars caudalis enter the cerebellum by way of the restiform body. They contribute a head representation to the two somatotopic maps in the cerebellar cortex.
Sources and Additional Reading
Bodegard A, Geyer S, Naito E, Zilles K, Roland PE. Somatosensory areas in man activated by moving stimuli: Cytoarchitectural mapping and PET. Neuroreport. 2000;11:187–191.
Parent A. Carpenter’s Human Neuroanatomy. ed 9 Baltimore: Williams & Wilkins; 1995.

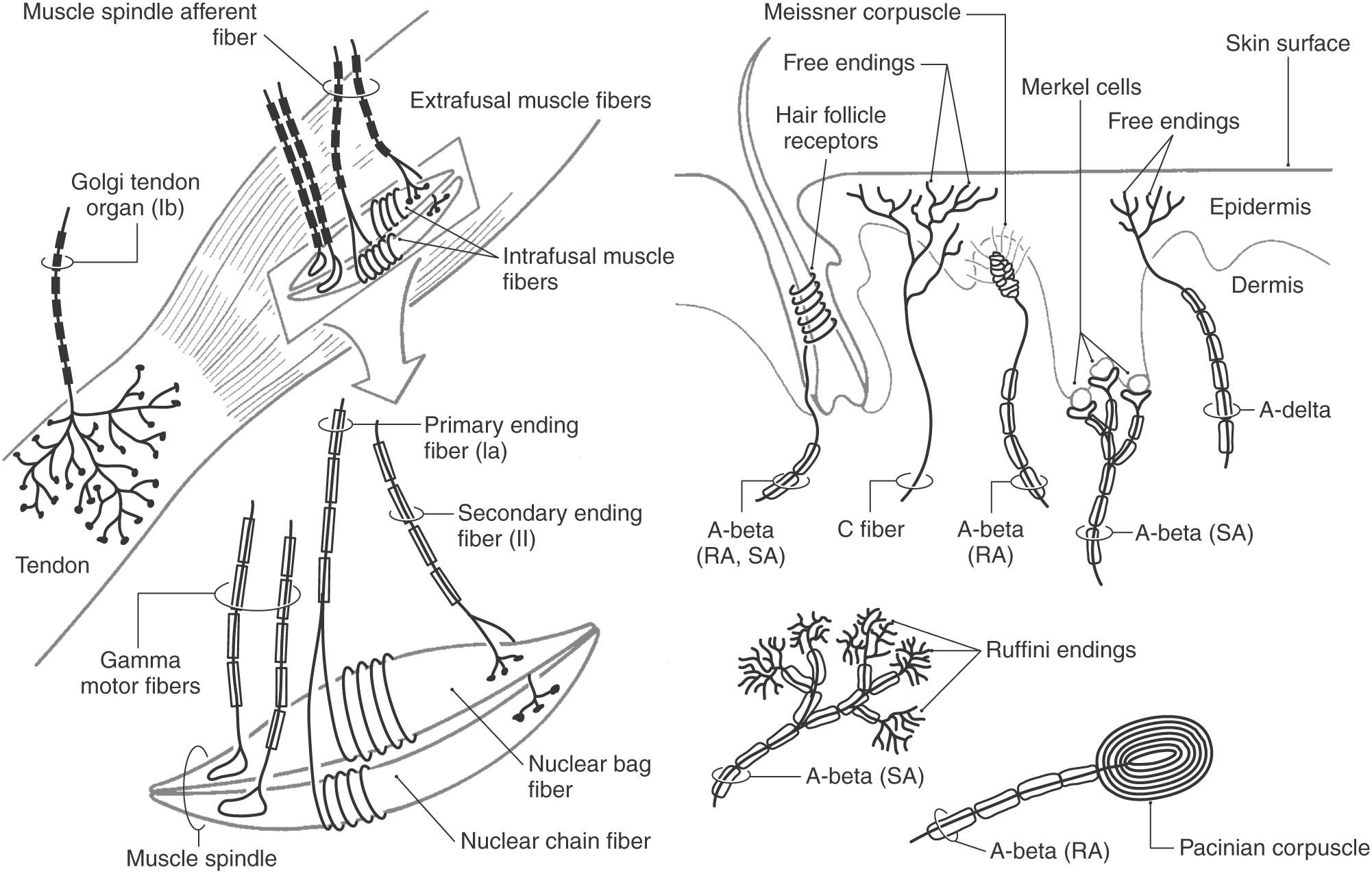
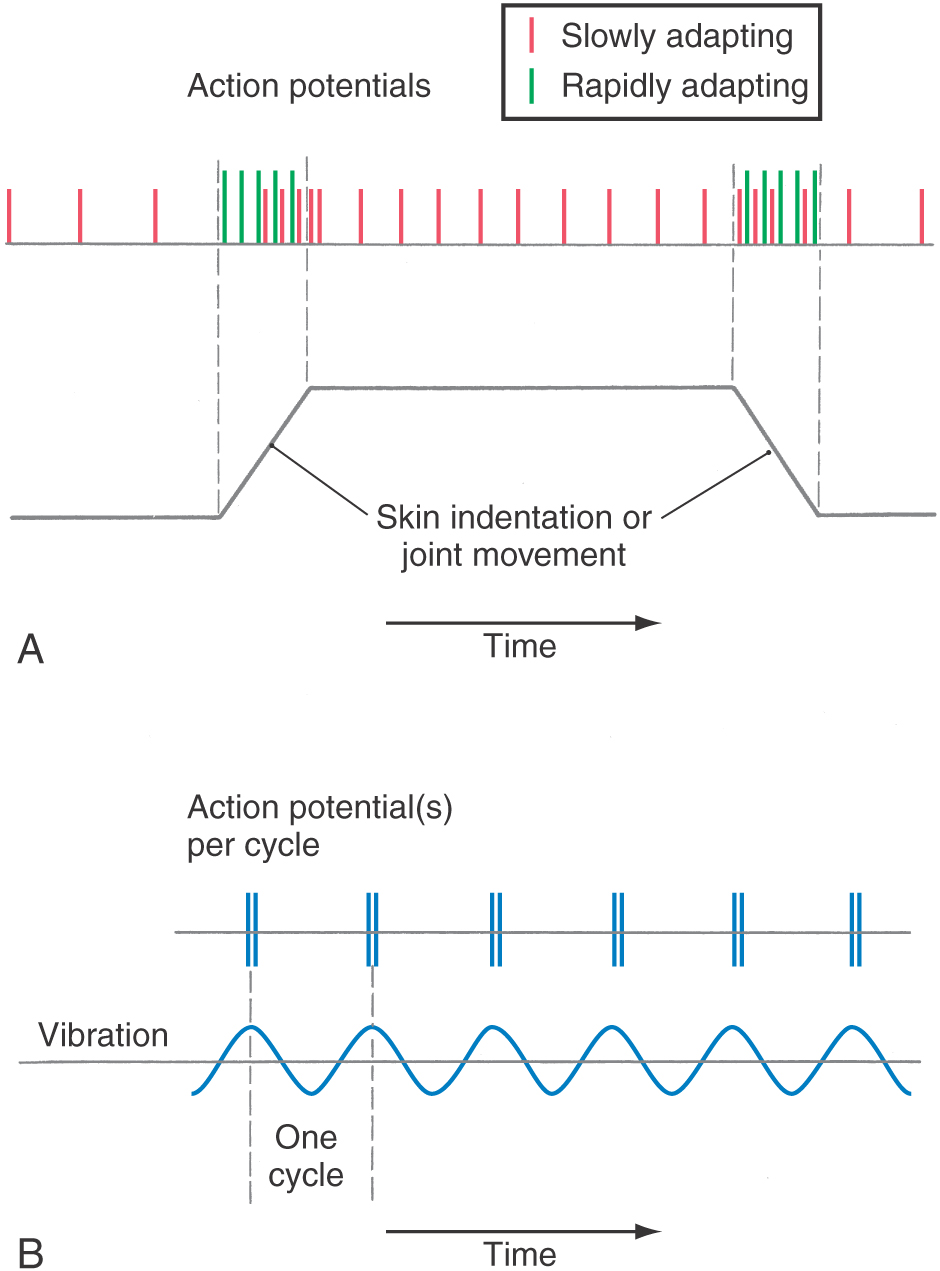
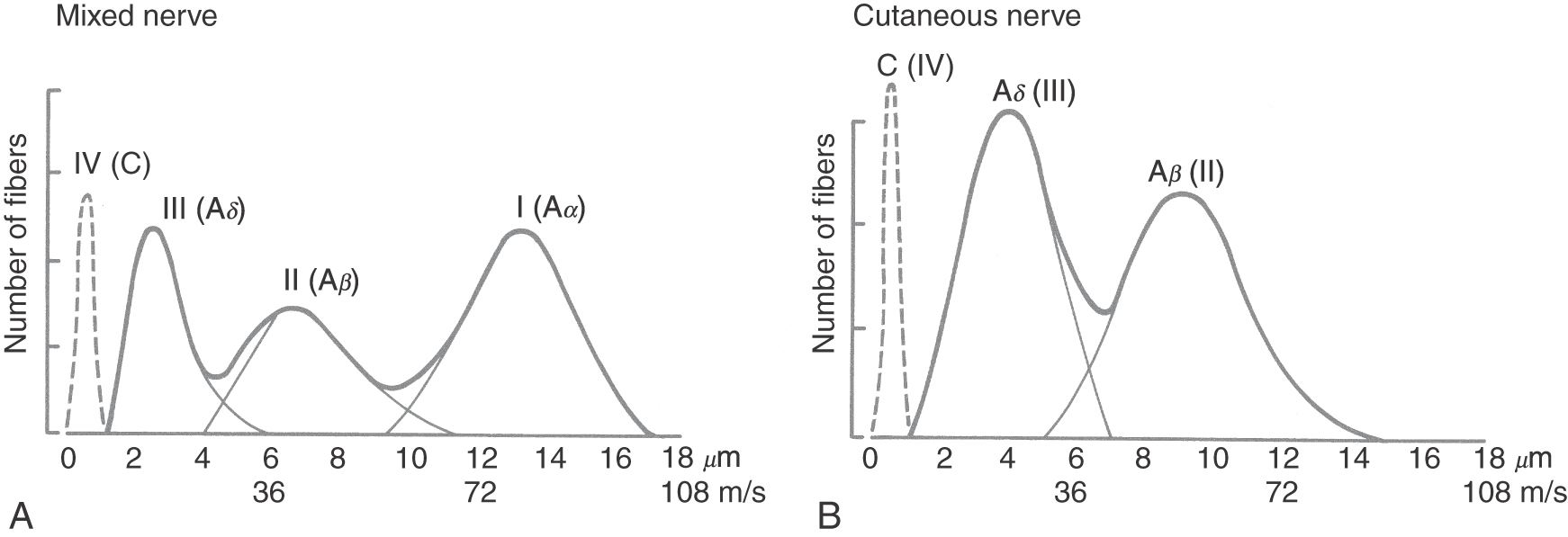
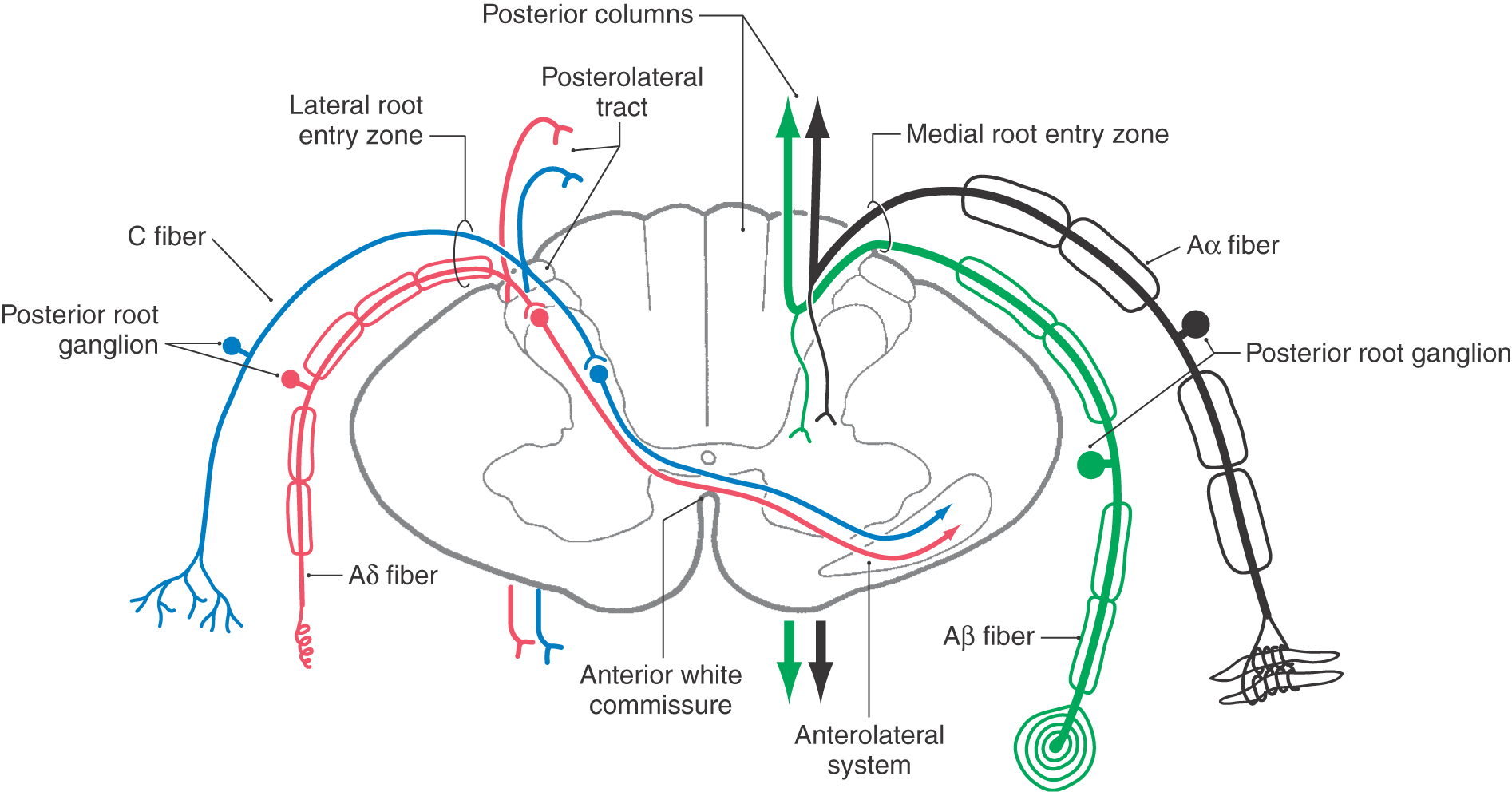
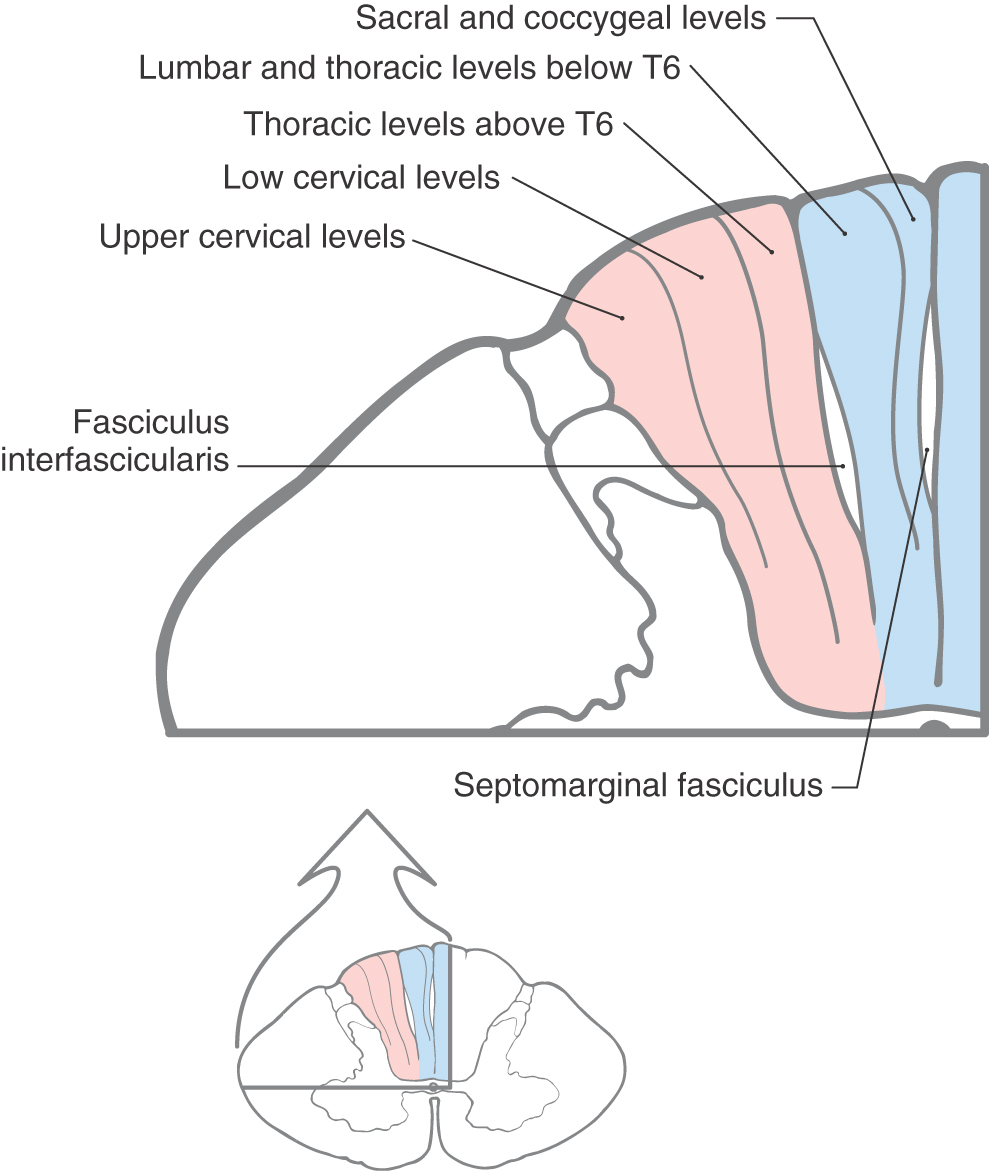
 Figure 17-6.
Figure 17-6.