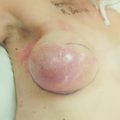
The role of imaging in breast diagnosis including screening and excision of impalpable lesions
Introduction
Breast cancer is a major health problem. Worldwide it has an increasing incidence, with over 1 million newly diagnosed cases each year, and is the commonest cancer to affect women and the commonest cause of cancer death in women. Breast cancer mortality in the UK is among the highest in the world, with approximately 28 deaths per 100 000 women per annum. This equates to around 48 000 new breast cancers diagnosed and 11 500 deaths attributable to breast cancer each year. Approximately 1 in 9 women in the UK will develop breast cancer at some time during their life.1
Early detection and improvements in treatment have led to a 30% reduction in breast cancer mortality in the UK in all age groups over the past 30 years.2
Imaging in symptomatic breast practice
Imaging is required at all three stages of this process, and mammography and ultrasound have a pivotal role to play. About 60% of breast cancer is diagnosed in symptomatic breast referral clinics. These clinics follow protocols that define the triple test, the combination of clinical assessment, imaging (mammography and ultrasound) and core biopsy or needle cytology, as the required standard.4 ‘One-stop’ clinics are recommended at which all the necessary tests required to make a diagnosis, including needle biopsy, are performed at one clinic visit. In order to achieve the earliest possible diagnosis of symptomatic breast cancer, women are encouraged through a variety of health promotion methods to present to these clinics as soon as they develop any change in their breasts. The clinical and imaging assessments in these clinics should be performed by appropriately trained and experienced staff. All such staff do not need to be doctors but they need to be able to give an independent opinion and be trained to an appropriate level.
Breast imaging techniques
X-ray mammography has been the basis of breast imaging for more than 30 years. The sensitivity of mammography for breast cancer is age dependent. The denser the breast, the less effective this method is for detecting early signs of breast cancer. Breast density tends to be higher in younger women and increased density obscures early signs of breast cancer. The sensitivity of mammography for breast cancer in women over 60 years of age approaches 95%, while mammography can be expected to detect less than 50% of breast cancers in women under 40 years of age.5
Most mammography in the UK is now carried out using digital image acquisition.6–9 There are major benefits from acquiring mammograms in direct digital format.9 Compared with conventional film/screen mammography, the benefits of full-field digital mammography include better imaging of the dense breast, the application of computer-aided detection and a number of logistical advantages providing potential for more efficient mammography services.10,11 The much wider dynamic range of digital mammography means that visualisation of the entire breast density range on a single image is easily achievable. In the clinical setting, comparative studies have shown that digital mammography performs in general as well as film/screen mammography but is better in younger women and in women with dense breasts.6,7
Ultrasound
High-frequency (≥10 MHz) ultrasound is a very effective diagnostic tool for the investigation of focal breast symptoms.12 Ultrasound does not involve ionising radiation and is a very safe imaging technique. It has a high sensitivity for breast pathology and also a very high negative predictive value.13
Ultrasound is the technique of choice for the further investigation of focal symptomatic breast problems at all ages. Under 40 years of age, when the risk of breast cancer is very low, it is usually the only imaging technique required. Over 40, when the risk of breast cancer begins to increase, it is often used in conjunction with mammography. Ultrasound is less sensitive than mammography for the early signs of breast cancer and is therefore not used for population screening. However, ultrasound does increase the detection of small breast cancers in women who have a dense background pattern on mammography.5 In the screening setting there is clear evidence that the addition of ultrasound improves small cancer detection rates, particularly in women with dense breasts, but there is currently insufficient evidence of any mortality benefit and insufficient resources to allow for routine ultrasound screening of women with dense breasts on mammography. Adding ultrasound to mammography or magnetic resonance imaging (MRI) screening does increase cancer detection but also significantly increases the false-positive rate. Ultrasound is the technique of first choice for biopsy of both palpable and impalpable breast lesions visible on scanning.
Ultrasound is now used routinely to assess the axilla in women with breast cancer in most units. Axillary nodes that show abnormal morphology can be sampled accurately by fine-needle aspiration (FNA) or needle core biopsy.16,17 Discussion of the role of axillary ultrasound and FNA and core biopsy can be found in Chapter 7.
Magnetic resonance mammography (MRM)
MRM is now widely available. In order to image the breast the patient is scanned prone and injection of intravenous contrast is required. MRM is the most sensitive technique for detection of breast cancer, approaching 100% for invasive cancer and up to 92% for ductal carcinoma in situ (DCIS), but it has a high false-positive rate.18,19,20 Rapid acquisition of images facilitates assessment of signal enhancement curves that can be helpful in distinguishing benign from malignant disease. Significant overlap in the enhancement patterns is seen, so needle sampling of the lesions detected is often required. Magnetic resonance-guided breast biopsy is available in a few centres but most breast lesions seen on MRM that are larger than 5 mm can be seen on ultrasound if they are clinically significant.
Breast cancer screening
The mortality benefit of screening is greatest in women aged 55–70 years.27,28 The mortality benefit of screening women aged between 40 and 55 is approximately 20%. Screening women under the age of 40 has not been shown to provide any mortality benefit.27,28
Population screening
Breast screening has been introduced in many countries over the past 25 years. In most countries, screening is recommended in all women aged 40 and over but in countries that provide population-based screening, women of 50 and over are specifically targeted. Breast cancer screening was introduced in the UK in 1987 and provides screening by invitation, free at the point of delivery, to all women between the ages of 50 and 70.1 Women over 70 can attend but are not invited. Over 70% of the invited population need to attend for a significant overall mortality benefit to be achieved. Women under the age of 50 are not offered screening in the UK unless they are at increased risk. A randomised trial of extending the screening invitation to the age range 47–73 years was started in 2010. The results of this trial will not be available until after 2020. From 2010 screening with annual MRI in addition to mammography has been offered to women at very high risk of breast cancer, including BRCA1 and BRCA2 gene carrriers.
Method and frequency
Women aged 50–70 in the UK are invited for mammography every 3 years. There has been some concern that this screening interval is too long. Mammography can be expected to detect breast cancer approximately 2 years before it becomes clinically apparent. The frequency of mammographic screening is determined by the lead-time of breast cancer. Based on the average growth time of breast cancer in different ages, this means that mammographic screening should ideally be carried out yearly in women aged 40–50, every 2 years in women aged 50–60 and every 3 years thereafter. However, the UK breast screening frequency trial completed in 1995 did not show any predicted benefit for women aged 50–64 screened every year compared with those screened every 3 years.33 Screening once every 3 years can be expected to detect approximately two-thirds of all breast cancers that will arise during the 3-year screening interval.
Factors affecting the effectiveness of screening
HRT increases breast density and in a proportion of women this reduces the sensitivity of mammography for breast cancer.9,32–40 Up to 25% of women taking combined oestrogen/progestogen preparations continuously show increased density on mammography. This effect is significantly less with other HRT preparations. As well as reducing sensitivity HRT also reduces the specificity of mammographic screening. HRT also increases the risk of developing breast cancer.34
Quality assurance
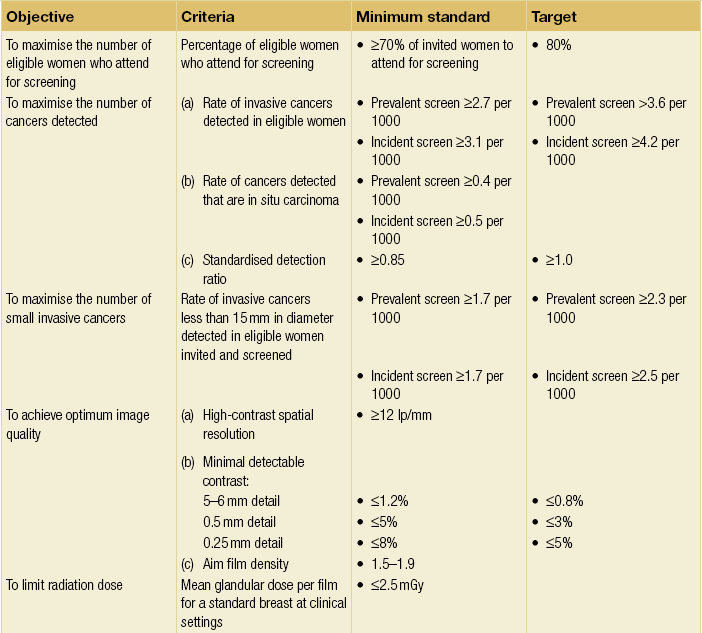
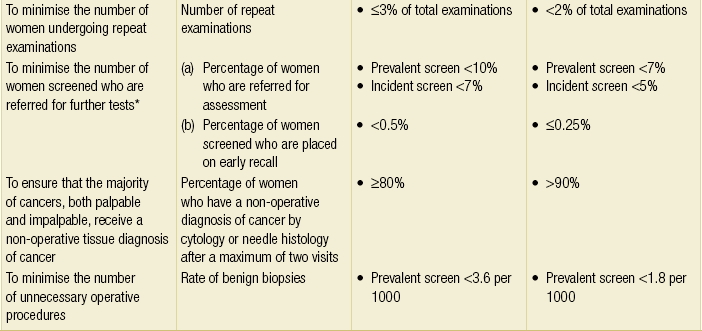
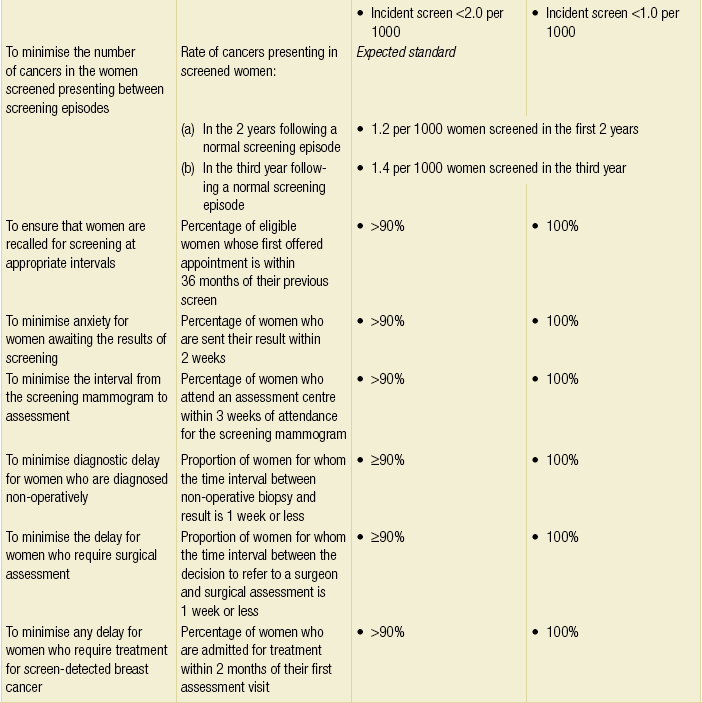
Breast screening programmes should have inbuilt quality assurance; the UK NHS Breast Screening Programme is subject to comprehensive quality assessment and all 100 screening units across the country have to comply with nationally defined standard guidelines. There are national targets for screening set by a central Department of Health Advisory Committee (Table 1.1).
The screening process
The common types of mammographic abnormality and their positive predictive value for cancer are shown in Table 1.2. Well-defined masses are almost always benign and do not require recall, whereas ill-defined masses and spiculated lesions always require further assessment (Figs 1.1 and 1.2). Clustered microcalcifications account for a high proportion of recalls that result in needle biopsy. More than 20% of screen-detected breast cancer is DCIS, mostly high or intermediate grade, and most of this type of cancer is detected by the presence of clustered microcalcifications (Fig. 1.3). Invasive cancer is usually represented on mammography by either an ill-defined or spiculated mass. It is essential to detect these lesions at small size as they more commonly represent grade 2 or 3 invasive cancer.
Table 1.2
Positive predictive value (PPV) for malignancy of mammographic signs
| Sign | PPV (%) |
| Well-defined mass | <1 |
| Ill-defined mass | 35–50 |
| Spiculated mass | 50–90 |
| Architectural distortion | 20–40 |
| Asymmetric density | <2 |
| Clustered microcalcifications | 15 |
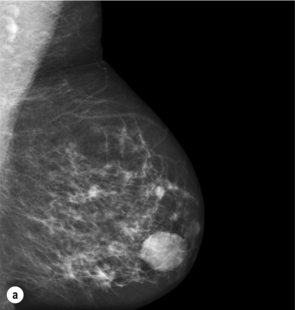
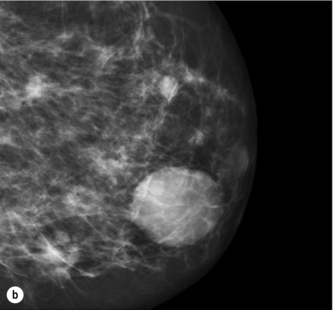
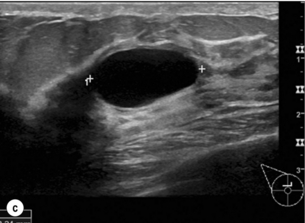
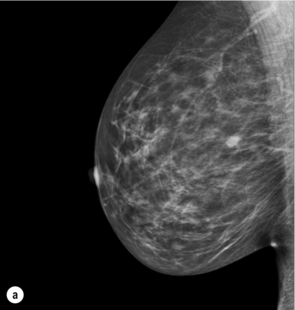
Figure 1.1 (a,b) Digital mammograms showing multiple well-defined masses, the typical appearance of simple breast cysts. (c) Ultrasound image showing typical features of a simple cyst, i.e. a well-circumscribed anechoic mass with distal acoustic enhancement.
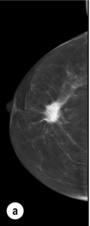
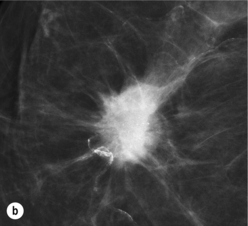
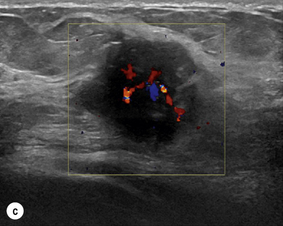
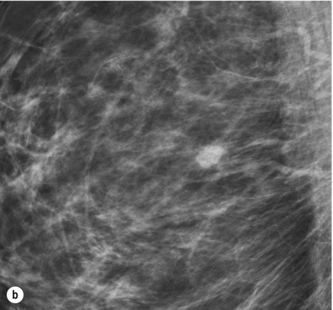
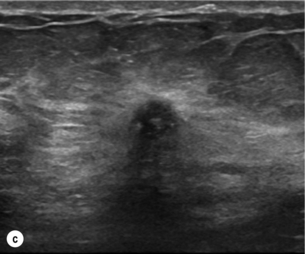
Figure 1.2 (a,b) Digital mammograms showing a spiculated mass in the right breast. The appearances are typical of an invasive carcinoma. The mass contains microcalcifications and there is evidence of skin tether. (c) Doppler ultrasound image showing the typical features of an invasive carcinoma with an irregular mass containing abnormal central vessels.
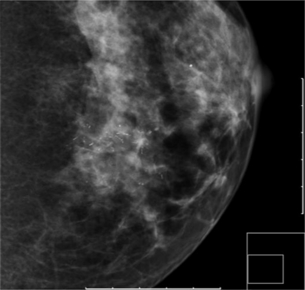
Figure 1.3 Digital mammograms showing a cluster of casting microcalcifications in the left breast. The appearances are typical of high-grade ductal carcinoma in situ.
The performance of the NHS Breast Screening Programme in 2010 is shown in Table 1.3. The screening programme was predicted to produce a 25% reduction in mortality (1750 cancers per year) directly attributable to early detection through screening by the year 2010.
Table 1.3
NHS Breast Screening Programme: results 2010
| 2008/2009 | 2009/2010 | |
| Total number of women invited (50–70) | 2 642 511 | 2 662 298 |
| Acceptance rate | 73.8% | 73.5% |
| Number of women screened (invited) | 1947.424 | 1 954 815 |
| Number of women screened (self-referral) | 44,070 | 43 410 |
| Total number of women screened | 1 991 494 | 1 998 225 |
| Number of women recalled for assessment | 87,400 | 82 650 |
| Percentage of women recalled for assessment | 4.4% | 4.1% |
| Number of benign surgical biopsies | 1644 | 1519 |
| Number of cancers detected | 15 673 | 15 517 |
| Number of in situ cancers detected | 3253 | 3064 |
| Number of cancers less than 15 mm | 6460 | 6544 |
| Standardised detection ratio (invited only) | 1.45 | 1.44 |
From NHS Breast Screening Programme Annual Review 2011. Available at http://www.cancerscreening.nhs.uk.
Screening women at increased risk
Methods of screening young women at increased risk: mammography
BRCA1-related breast cancer is usually high grade and often has a ‘pushing’ margin. It rarely presents with associated DCIS. The mammographic features are therefore usually of a mass lesion with no associated microcalcification and no architectural distortion (Fig. 1.4a). Such cancers often present symptomatically as interval cases. BRCA2-related cancers are more similar to sporadic cases and may be more likely to be detected by mammography. Ultrasound screening significantly improves sensitivity when there is a dense mammographic background pattern but has a lower positive predictive value and has not been shown to be a useful screening modality. Ultrasound features of BRCA1 cancers are often benign or indeterminate (Fig. 1.4b). If mammographic screening is performed, it should be repeated annually in women under age 50.
Methods of screening young women at high risk: MRI
MRI is the most sensitive method of imaging young women but has significant resource implications.44,48 The specificity of MRI has been a concern, although with second-look recall (after which many potentially abnormal findings may resolve), targeted ultrasound and the slowly increasing availability of MRI-guided biopsy, this may be less of a problem than initially thought. The MARIBS study evaluating MRI in addition to mammography and several other studies have shown that MRI is the most sensitive screening test for young high-risk women, but it is arguable whether the cancers detected are sufficient to change the outcome of these young women.49 On the basis of its better performance compared with mammography in increasing sensitivity, NICE has recommended annual MRI surveillance for women aged 30–39 years with a 10-year risk of >8% and women aged 40–49 years with a 10-year risk >20%.43 MRI is also recommended for high-risk women with a dense mammographic background pattern.
Image-guided breast biopsy
Needle biopsy is highly accurate in determining the nature of most breast lesions.50–53 Patients with benign conditions avoid unnecessary surgery; carrying out open surgical biopsy for diagnosis should be regarded as a failure of the diagnostic process. For patients who are proven to have breast cancer, needle biopsy provides accurate understanding of the type and extent of disease so ensuring that patients, and the doctors treating them, are able to make informed treatment choices. Needle biopsy not only provides accurate information on the nature of malignant disease, such as histological type and grade, but also facilitates pretreatment assessment of tumour biology.
Which biopsy technique?
FNA versus needle core biopsy
There has been much debate about the comparative benefits of FNA and core biopsy,50–53 but 14 G 22-mm automated core biopsy provides significantly greater sensitivity, specificity and positive predictive value than FNA. Results with core biopsy are particularly superior to FNA in stereotactic biopsy of microcalcifications and architectural distortions.
The overall better performance achievable with core biopsy compared with FNA is illustrated in the performance of the NHS Breast Screening Programme in the UK. In 1994, using FNA as the primary diagnostic technique, fewer than 10% of 90 units were able to achieve the target of 70% preoperative diagnosis rate for cancer. By 2003, most units had converted to automated core biopsy and all units achieved the minimum standard and the majority exceeded the expected standard of 90% preoperative diagnosis rate.1
Vacuum-assisted biopsy (VAB)
The predominant reasons for not achieving an accurate diagnosis by needle biopsy are sampling error (missing the target) and failure to retrieve sufficient representative material. These problems have been largely addressed by the development of larger directional core techniques that yield significantly greater volumes of tissue.54–56
VAB is a very successful method for improving the diagnostic accuracy of borderline breast lesions and lesions at sites in the breast difficult to biopsy using other techniques. VAB has been shown to understage both in situ and invasive cancer approximately half as often as conventional core biopsy (typically 10% vs. 20%).57,58 The VAB technique has a higher sensitivity because it allows sampling of lesions at sites that are difficult to biopsy using either FNA or core biopsy and because the amount of tissue harvested is at least five times greater per core specimen.
The indications for VAB include:
• failed ‘conventional’ core biopsy;
• small clusters of microcalcifications;
• papillary and mucocele-like lesions;
Core biopsy and VAB are now the recommended techniques for sampling calcifications and mammographic architectural distortions.59,60 For calcifications it is imperative that there is proof of representative sampling with specimen radiography. If calcification is not demonstrated on the specimen radiograph and the histology is benign, then management cannot be based on this result as there is a high risk of sampling error; the procedure must either be repeated or open surgical biopsy carried out.
Guidance techniques for breast needle biopsy
Ultrasound provides real-time visualisation of the biopsy procedure and visual confirmation of adequate sampling. Between 80% and 90% of breast abnormalities will be clearly visible on ultrasound and amenable to biopsy using this technique. For impalpable abnormalities not visible on ultrasound, stereotactic X-ray-guided biopsy is required. A few lesions are only visible on MRI and require magnetic resonance-guided biopsy.61 A number of different approaches have been developed for this procedure using both closed and open magnets. VAB is the biopsy technique recommended for magnetic resonance-guided sampling.
Number of samples
A simple rule for satisfactory sampling using needle techniques is to obtain sufficient material to achieve a diagnosis.59,60,62 For ultrasound-guided core biopsy, a diagnosis may be possible on a single core. Showing on ultrasound that the needle has passed through the centre of the abnormality and by examining the sample with the naked eye, it is usually, but not always, possible to confirm whether a satisfactory sample has been obtained. Because of this and the knowledge that some lesions are heterogeneous, more extensive sampling of a lesion increases sensitivity. The number of core specimens obtained should reflect the nature of the abnormality being sampled. For ultrasound-guided biopsy where there is a suspicion of carcinoma, it is recommended that multiple core specimens are obtained.
Biopsy results
It is important that the results of FNA and/or core needle breast biopsy are always correlated with the clinical and imaging findings before clinical management is discussed with the patient. This is best achieved by reviewing each case prior to any surgical or other therapeutic procedure at multidisciplinary meetings.50
Surgery for clinically occult breast lesions
The number of impalpable, clinically occult breast lesions detected by screening is increasing. Accurate localisation techniques are required to facilitate their surgical excision. The hooked wire is the most commonly employed technique and has proved very reliable but does have inherent associated problems. There are various designs of localisation wire in common use. All have some form of anchoring device such as a hook with a splayed or barbed tip. The wire is deployed under stereotactic or ultrasound guidance within a rigid over-sheath cannula, which is then removed once positioning is satisfactory (Fig. 1.5). The patient is then transferred to the operating theatre with the wire in situ. Most wires are very flexible and when the cannula is removed the wire may assume a quite circuitous course, especially after stereotactic insertion when the breast is released from compression. In a very fatty breast in which there is no solid lesion or the wire has not transfixed the lesion, care must be taken to avoid displacing the wire. A cosmetically considered incision is placed near to the tip of the wire and an excision performed. Accurate wire placement is essential and ideally the shortest possible length of wire should be within the breast. In recent practice this has been greatly facilitated by the use of radio-opaque and ultrasound visible markers placed at the time of initial stereotactic biopsy such that wire localisation can be performed under ultrasound guidance (Fig. 1.6). In addition, for superficial lesions a skin marker may be more appropriate.
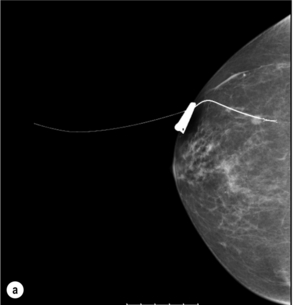
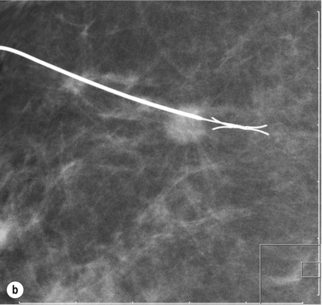
Figure 1.5 Digital mammogram showing a Reidy localisation wire placed under ultrasound guidance transfixing and marking an impalpable mass to facilitate surgical wide local excision (as shown in Fig. 1.4).
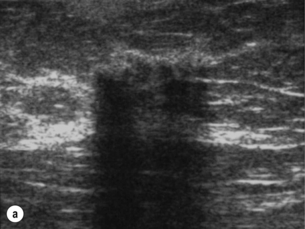
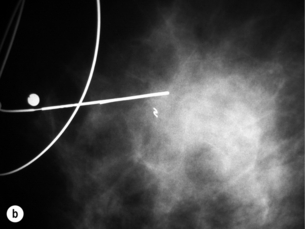
Figure 1.6 (a) Ultrasound showing a cluster of gel pellets placed at the site of a previous stereotactic biopsy. The clear visibility of the pellets facilitates ultrasound localisation for surgery of abnormalities that would normally require X-ray localisation. (b) Mammogram after ultrasound localisation in the same case showing accurate placement of the marker wire.
Radioisotope occult lesion localisation
Radioisotope occult lesion localisation (ROLL) has been advocated as an alternative to the hooked-wire technique.63 ROLL was first described by the Milan group using 99mTc-labelled human macroaggregate albumin, using scintigraphy and a hand-held gamma probe to guide surgical excision. The Nottingham method has modified the Milan technique and uses radio-opaque contrast injected with the radiolabel and immediate check mammography (Fig. 1.7). Subsequently some centres have combined ROLL with sentinel node biopsy. ROLL uses essentially the same equipment as sentinel node biopsy. It has been described using macroaggregate (which does not migrate from the injection site) or low-molecular-weight colloid (which does migrate and is normally used for sentinel node biopsy). In both situations it is radiolabelled with 99mTc and injected directly into the lesion. The threshold of the signal processor on the gamma detector is then adjusted so that an audible signal is heard only when the probe is directly over the lesion. The probe then directs excision intraoperatively.
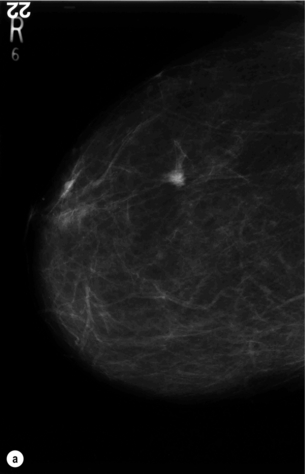
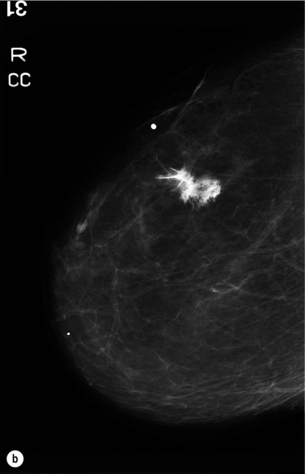
Figure 1.7 (a) Mammogram of the right breast showing a small impalpable cancer. (b) Mammogram after injection of radionuclide mixed with X-ray contrast confirming satisfactory localisation (ROLL).
In a randomised trial of ROLL versus wire localisation, 2% of ROLL patients had a failed technique due to intraductal injection of radiolabelled colloid and dye that gave a ductogram appearance on check mammography in both cases.64,65 As the radio-opaque dye is absorbed rapidly, both cases were successfully converted to wire localisation. The main differences between ROLL and wire guidance were that both surgeons and radiologists found ROLL easier to perform overall and patients found ROLL less painful. There has been no significant difference in accuracy of marking, operating time, mean specimen weight, intraoperative re-excision or second therapeutic operation in the majority of reports, although a recent European trial reported greater volumes of tissue were excised using ROLL than standard wire localisation. Some studies have suggested that obtaining clear margins may be significantly easier with ROLL. In reality there is little to choose between ROLL and wire localisation. ROLL may be a more suitable technique in the localisation of non-mass lesions (e.g. DCIS), although even for these lesions when multiple wires are placed any advantage is small.
There are various methods described for combining ROLL with sentinel node biopsy.66–68 Low-molecular-weight colloid can be injected at a different site, at the same site with a different radiolabel, or into the tumour. With intratumoral injection of 99mTc nanocolloid, only one injection is required and high success rates have been reported. Combined with radio-opaque contrast, this modification of the Nottingham method has proved simple and successful.
References
1. Office of National Statistics. Available from: http://www.ons.gov.uk/ons/rel/cancer-unit/cancer-incidence-and-mortality/2008-2010/stb-cancer-incidence-and-mortality-in-the-united-kindom–2008-2010.html, 2012.
2. Blanks, R.G., Moss, S.M., McGahan, C.E., et al, Effects of NHS breast screening programme on mortality from breast cancer in England and Wales, 1990–8: comparison of observed with predicted mortality. Br Med J 2000; 321:665–669. 10987769
3. National Institute for Health and Clinical Excellence NICE Clinical Guideline 80: Early and locally advanced breast cancer. 2009 Available from. http://guidance.nice.org.uk/CG80/NICEGuidance/pdf/English [[accessed 23.07.12]].
4. Willett, A.M., Michell, M.J., Lee, M.J.R. Available from. Best Practice Guideline for Patients Presenting with Breast Symptoms. 2010. [accessed 23.07.12]. http://www.associationofbreastsurgery.org.uk/media/4585/best_practice_diagnostic_guidelines_for_patients_presenting_with_breast_symptoms.pdf
5. Kolb, T.M., Lichy, J., Newhouse, J.H., Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 2002; 225:165–175. 12355001
6. Skaane, P., Young, K., Skjennald, A., Comparison of film-screen mammography and full-field mammography with soft-copy reading in a population-based screening program: the Oslo II study. Radiology 2002; 225:267. 12115272
7. Pisano, E.D., Gatsonsis, C., Hendrick, E., et al, Diagnostic performance of digital versus film mammography for breast cancer screening. N Engl J Med 2005; 353:1773–1783. 16169887
8. James, J.J., The current status of digital mammography. Clin Radiol 2004; 59:1–10. 14697370
9. Committee on Technologies for the Early Detection of Breast CancerMammography and beyond: developing technologies for the early detection of breast cancer. Washington, DC: National Academy Press, 2001.
10. Legood, R., Gray, A. NHSBSP Equipment Report 0403A cost comparison of full-field digital mammography with film-screen mammography in breast cancer screening. Sheffield: NHS Breast Screening Programme Publications, 2004.
11. Gur, D., Sumkin, J.H., Rockette, H.E., et al, Changes in breast cancer detection and mammography recall rates after the introduction of a computer-aided detection system. J Natl Cancer Inst 2004; 96:185–190. 14759985
12. Wilson, A.R.M., Teh, W. Mini symposium: Imaging of the breast. Ultrasound of the breast. Imaging. 1998; 9:169–185.
13. Lister, D., Evans, A.J., Burrell, H.C., et al, The accuracy of breast ultrasound in the evaluation of clinically benign discrete breast lumps. Clin Radiol 1998; 53:490–492. 9714387
14. Berg, W.A., Blume, J.D., Cormack, J.D., ACRIN 6666 Investigators, Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008; 299:2151–2163. 18477782
15. Berg, W.A., Zhang, Z., Lehrer, D., et al, Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012; 307:1394–1404. 22474203
16. Damera, A., Evans, A.J., Cornford, E.J., et al, Diagnosis of axillary nodal metastases by ultrasound guided core biopsy in primary operable breast cancer. Br J Cancer 2003; 89:1310–1313. 14520465
17. Houssami, N., Ciatto, S., Turner, R., et al, Preoperative ultrasound-needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg 2011; 254:243–251. 21597359
18. Kuhl, C., The current status of breast MR imaging. Part 2. Clinical applications. Radiology 2007; 244:672–691. 17709824
19. Sardanelli, F., Boetes, C., Borisch, B., et al, Magnetic resonance imaging of the breast: recommendations of the EUSOMA working group. Eur J Cancer 2010; 46:1296–1316. 20304629
20. Kuhl, C.K., Schrading, S., Bieling, H.B., et al, MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet 2007; 370:485–492. 17693177
21. Kuhl, C.K., Schmutzler, R.K., Leutner, C.C., et al, Mammography, breast ultrasound and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005; 23:8469–8476. 16293877
22. Warner, E., Plewes, D.B., Shumak, R.S., et al, Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer. J Clin Oncol 2001; 19:3524–3531. 11481359
23. Stoutjesdijk, M.J., Boetes, C., Jager, G.J., et al, Magnetic resonance imaging and mammography in women with a hereditary risk of breast cancer. J Natl Cancer Inst 2001; 93:1095–1102. 11459871
24. Brekelmans, C.T.M., Seynaeve, C., Bartels, C.C.M., et al, Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol 2001; 19:924–930. 11181654
25. Olsen, O., Gotzsche, P.C., Cochrane review on screening for breast cancer with mammography. Lancet 2001; 358:1340–1342. 11684218
26. Olsen O, Gotzsche PC. Systematic review of screening for breast cancer with mammography. Available at http://image.thelancet.com/extras/fullreport.pdf
27. . WHO handbook on cancer prevention. 7th ed, Lyons: IARC Press; 2002. A comprehensive review of all the available data on the effectiveness of breast cancer screening in reducing breast cancer mortality.
28. Nystrom, L., Andersson, I., Bjurstam, N., et al, Long-term effects of mammographic screening: update overview of the Swedish randomised trials. Lancet 2002; 359:909–919. 11918907 Long-term follow-up of the combined Swedish trials showing significant mortality benefit after more than 20 years.
29. Tabar, L., Vitak, B., Tony, H.H., et al, Beyond randomised controlled trials: organised mammographic screening substantially reduces breast carcinoma mortality. Cancer 2001; 91:1724–1731. 11335897
30. Duffy, S., Tabar, L., Chen, H.H., et al, The impact of organised mammographic screening on breast carcinoma mortality in seven Swedish counties. Cancer 2002; 95:458–469. 12209737
31. Hackshaw, A.K., Paul, E.A., Breast self-examination and death from breast cancer: a meta-analysis. Br J Cancer 2003; 88:1047–1053. 12671703
32. Smith, R.A., Saslow, D., Sawyer, K.A., et al, American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin 2003; 53:141–169. 12809408
33. Breast Screening Frequency Trial Group, The frequency of breast cancer screening: results from the UKCCCR randomized trial. Eur J Cancer 2002; 38:1458–1464. 12110490
34. Million Women Study Collaborators, Breast cancer and hormone replacement therapy in the Million Women Study. Lancet 2003; 362:419–427. 12927427 Report of significantly increased risk of breast cancer in women taking HRT in the UK.
35. Perrson, I., Thurfjell, E., Holmberg, I., Effect of estrogen and estrogen–progestin replacement regimes on mammographic breast parenchymal density. J Clin Oncol 1997; 15:3201–3207. 9336356
36. Sendag, F., Cosan Terek, M., Ozsener, S., et al, mammographic density changes during different postmenopausal hormone replacement therapies. Fertil Steril 2001; 76:445–450. 11532462
37. Evans, A., Hormone replacement therapy and mammographic screening. Clin Radiol 2002; 57:563–564. 12096852
38. Litherland, J.C., Stallard, S., Hole, D., et al, The effect of hormone replacement therapy on the sensitivity of screening mammograms. Clin Radiol 1999; 54:285–288. 10362232
39. Kavanagh, A.M., Mitchell, H., Giles, G.G., Hormone replacement therapy and accuracy of mammographic screening. Lancet 2000; 355:270–274. 10675074
40. Litherland, J.C., Evans, A.J., Wilson, A.R.M., The effect of hormone replacement therapy on recall rate in the National Health Breast Screening Programme. Clin Radiol 1997; 52:276–279. 9112944
41. Evans, A.J., Pinder, S.E., Ellis, I.O., et al, Screen detected ductal carcinoma in situ (DCIS): over-diagnosis or obligate precursor of invasive disease? J Med Screen 2001; 8:149–151. 11678555
42. Evans, A.J., Burrell, H.E., Pinder, S.E., et al, Detecting which invasive cancers at mammographic screening saves lives? J Med Screen 2001; 8:86–90. 11480449
43. National Institute for Clinical Excellence Guidance on cancer services. Familial breast cancer. National Institute for Clinical Excellence, London, 2006. Available from. http://www.nice.org.uk [[accessed 30.10.12]].
44. Robson, M., Breast cancer surveillance in women with hereditary risk due to BRCA1 or BRCA2 mutations. Clin Breast Cancer 2004; 5:260–268. 15507170
45. Tilanus-Linthorst, M., Verhoog, L., Obdeijn, I.-M., et al, A BRCA1/2 mutation, high breast density and prominent pushing margins of a tumour independently contribute to a frequent false-negative mammography. Int J Cancer 2002; 102:91–95. 12353239
46. Warner, E., Plewes, D.B., Hill, K.A., et al, Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 2004; 292:1317–1325. 15367553
47. Hamilton, L.J., Evans, A.J., Wilson, A.R.M., et al, Breast imaging findings in women with BRCA1- and BRCA2-associated breast cancer. Clin Radiol 2004; 59:895–902. 15451348
48. Kriege, M., Brekelmans, C.T.M., Boetes, C., et al, Efficacy of MRI and mammography for breast cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004; 351:427–437. 15282350
49. Leach, M.O., Boggis, C.R., Dixon, A.K., et al, Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 2005; 365:1769–1778. 15910949
50. Teh, W., Wilson, A.R.M., Definitive non-surgical breast diagnosis: the role of the radiologist. Clin Radiol 1998; 53:81–84. 9502082
51. Britton, P.D. Fine needle aspiration or core biopsy. Breast. 1999; 8:1–4.
52. Britton, P.D., McCann, J. Needle biopsy in the NHS Breast Screening Programme 1996/7: how much and how accurate? Breast. 1999; 8:5–11.
53. Vargas, H.I., Agbunag, R.V., Khaikhali, I., State of the art of minimally invasive breast biopsy: principles and practice. Breast Cancer 2000; 7:370–379. 11114867
54. Heywang-Kobrunner, S.H., Schaumloffel, U., Viehweg, P., et al, Minimally invasive stereotaxic vacuum core breast biopsy. Eur Radiol 1998; 8:377–385. 9510569
55. Brem, R.F., Schoonjans, J.M., Sanow, L., et al, Reliability of histologic diagnosis of breast cancer with stereotactic vacuum-assisted biopsy. Am Surg 2001; 67:388–392. 11308011
56. Parker, S.H., Klaus, A.J., McWey, P.J., et al, Sonographically guided directional vacuum-assisted breast biopsy using a handheld device. AJR Am J Roentgenol 2001; 177:405–408. 11461871
57. Kettritz, U., Rotter, K., Murauer, M., et al, Stereotactic vacuum biopsy in 2874 patients: a multicenter study. Cancer 2004; 100:245–251. 14716757
58. Brenner, R.J., Bassett, L.W., Fajardo, L.L., et al, Stereotactic core needle breast biopsy: a multi-institutional prospective trial. Radiology 2001; 218:866–872. 11230668
59. Wilson, A.R.M. Therapeutic applications of vacuum biopsy. Breast Cancer Res. 2008; 4:31.
60. Wilson, R., Kavia, S., Comparison of large-core vacuum-assisted breast biopsy and excision systems. Recent Results Cancer Res 2009; 173:23–41. 19763447
61. Kuhl, C.K., Morakkabati, N., Leutner, C.C., et al, MR imaging-guided large-core (14-gauge) needle biopsy of small lesions visible at breast MR imaging alone. Radiology 2001; 220:31–39. 11425969
62. Fishman, J.E., Milikowski, C., Ramsinghani, R., et al, US-guided core-needle biopsy of the breast: how many specimens are necessary? Radiology 2003; 226:779–782. 12601206
63. Luini, A., Zurrida, S., Paganelli, G., et al, Comparison of radioguided excision with wire localisation of occult breast lesions. Br J Surg 1999; 86:522–525. 10215829
64. Rampaul, R.S., Bagnall, M., Burrell, H., et al, Radioisotope for occult lesion localisation: results from a prospective randomised trial of ROLL versus wire guidance in occult lesions of the breast. Br J Surg 2004; 91:1575–1577. 15505875
65. Rampaul, R.S., Macmillan, R.D., Evans, A.J., Intraductal injection of the breast: a potential pitfall of radioisotope occult lesion localisation. Br J Radiol 2003; 76:425–426. 12814931
66. Patel, A., Pain, S.J., Britton, P., et al, Radioguided occult lesion localisation (ROLL) and sentinel node biopsy for impalpable invasive breast cancer. Eur J Surg Oncol 2004; 30:918–923. 15498634
67. Tanis, P.J., Deurloo, E.E., Valdes Olmos, R.A., Single intralesional tracer dose for radio-guided excision of clinically occult breast cancer and sentinel node. Ann Surg Oncol 2001; 8:850–855. 11776502
68. Gray, R.J., Giuliano, R., Dauway, E.L., et al, Radioguidance for nonpalpable primary lesions and sentinel lymph node(s). Am J Surg 2001; 182:404–406. 11720680