CHAPTER 14 The Role of Arthroscopy in Midcarpal Instability
Introduction
The concept of midcarpal instability (MCI) has evolved slowly since it was first described in 1934. Many investigators have contributed to our understanding of this condition over the years, which led to the consolidated classification by the senior author (DML) (Table 14.1).1 It appears that MCI represents several distinct clinical entities differing in the cause and direction of subluxation but sharing the common characteristic of abnormal force transmission at the midcarpal joint. The following discussion centers on intrinsic MCI. Extrinsic MCI due to a dorsally mal-united distal radius fracture is treated by a distal radius osteotomy and hence falls outside the scope of this discussion.
| Intrinsic | Extrinsic |
|---|---|
| A. Palmar | A. Distal radius mal-union |
| B. Dorsal | |
| C. Combined |
Pathomechanics
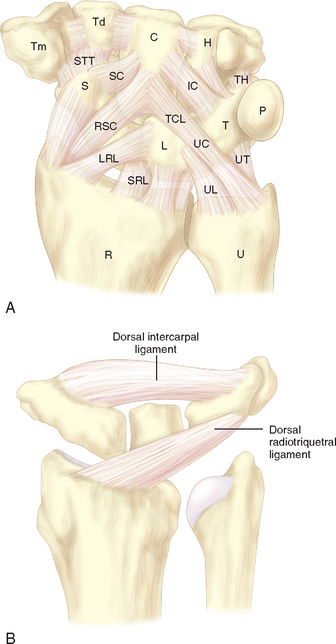
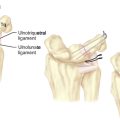
The mechanism of the clunk in palmar midcarpal instability (PMCI) has been described in detail by Lichtman et al.2 The palmar arcuate ligament complex is comprised of a radial arm that is confluent with and distal to the radioscaphocapitate (RSC) ligament and an ulnar arm or the triquetrohamate-capitate ligament (TCL) (Figure 14.1). In the normal situation, the proximal carpal row moves smoothly from a flexed position when the wrist is in radial deviation to extension when the wrist is in ulnar deviation. This is due to the progressive tightening effect of the arcuate ligament as it stretches out to length (which incrementally pulls the midcarpal row into extension) and to the carpal bone geometry, which causes the triquetrum to translate dorsally along the helicoidal facet of the hamate. When the arcuate ligament is attenuated, this synchronous motion is lost.
Studies by Trumble et al.3 and Viegas and coauthors4 have shown that sectioning either the TCL or the dorsal radiocarpal (i.e., dorsal radiotriquetral ligament) can produce a volar intercalated segmental instability (VISI) deformity and simulate PMCI. More recently, Lichtman showed in vivo that tightening the DRCL alone can stabilize the proximal carpal row and eliminate the clunk of PMCI—emphasizing the potential importance of dorsal ligament laxity in the pathogenesis of this disorder.5 The senior author now believes that PMCI is caused by laxity of both the TCL and the DRCL, which allows an excessive palmar sag of the heads of the capitate and hamate at the midcarpal joint. This produces a VISI pattern of the proximal row in the nonstressed wrist. This sag results in a loss of joint contact across the midcarpal joint, which manifests clinically as a loss of the smooth transition of the proximal row from flexion to extension as the wrist deviates ulnarward.
The proximal carpal row thus stays in a flexed position until the terminal extent of ulnar deviation, when the helicoidal shape of the hamate facet suddenly forces the triquetrum dorsally. This snaps the lunate and subsequently the scaphoid into extension, causing a sudden reversal of the VISI. This sudden proximal row extension is responsible for the painful and rapid catch-up clunk that occurs. As the wrist moves back to neutral, the triquetrum translates down the hamate facet—which allows the proximal row to drop back into VISI while the distal row again settles palmarly into its slightly subluxated starting point (Figure 14.2a and b).
The dorsal pattern of MCI has not been studied as extensively. It appears that laxity of the radial arm of the palmar arcuate ligament permits the capitate and hamate to translate dorsally to an excessive degree, especially with ulnar deviation of the wrist.6,7 It is of note that in both the palmar and dorsal patterns the proximal row always moves into extension and the distal row translates dorsally with ulnar deviation. It is the timing and force of this movement that differentiate the two patterns.
Diagnosis
Clinical Findings
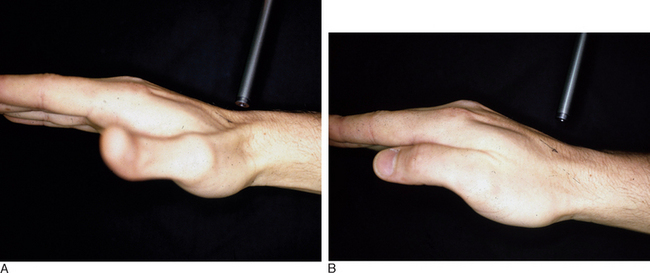
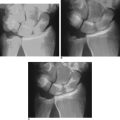
In PMCI the patient presents with a history of clunking of the wrist. Patients can often reproduce the clunk on both sides because generalized ligamentous laxity frequently coexists. However, the patient may have a trivial injury that accentuates this normal laxity—resulting in a painful clunk. Upon physical examination, close inspection will reveal a sag of the midcarpal joint with the wrist in radial deviation—which is reduced with active or passive ulnar deviation (Figure 14.3a and b). The clunk may be reproduced by performing the midcarpal shift test.2 This test is performed by placing the patient’s wrist in neutral with the forearm in pronation. A palmar force is then applied to the hand at the level of the distal capitate. The wrist is simultaneously loaded and deviated ulnarly. The test result is positive if a painful clunk occurs that reproduces the patient’s symptoms.
Imaging
Static X-rays are typically normal, but occasionally reveal a mild VISI pattern with the wrist in the neutral position. Arthrograms are normal, unless there are associated intracarpal or triangular fibrocartilage (TFC) tears. MRI findings are nonspecific. Videofluoroscopy provides the hallmarks for diagnosis of this condition. With normal wrist kinematics, the proximal carpal row rotates synchronously from flexion to extension as ulnar deviation of the wrist is achieved. With PMCI, the proximal row maintains a volar flexed position until terminal ulnar deviation is reached—at which point it suddenly snaps into extension.
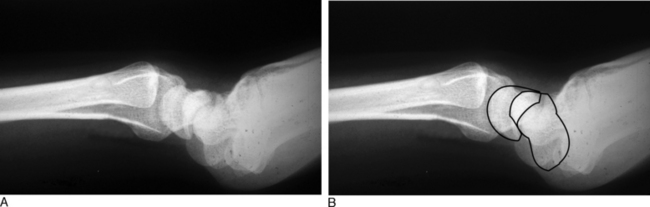
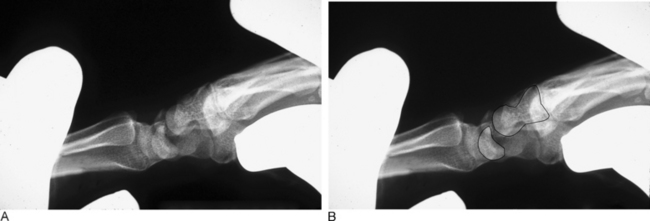
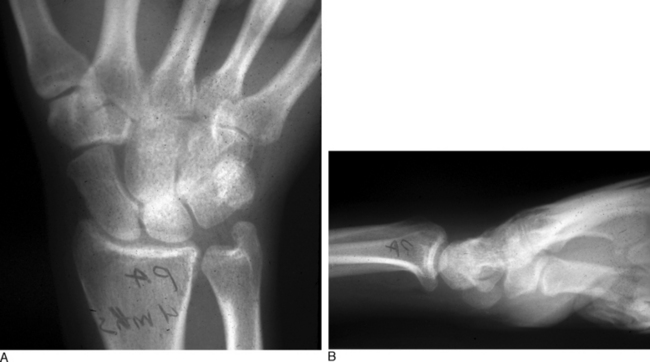
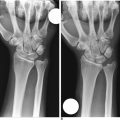
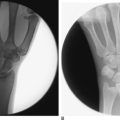
In dynamic dorsal MCI, X-rays are usually normal. In chronic cases, X-rays often show a volar intercalated segmental instability (VISI) pattern (Figure 14.4a and b). The capitolunate displacement test shows dorsal subluxation of the proximal carpal row in addition to dorsal subluxation of the capitate from the lunate (Figure 14.5a and b).8 This led Louis and colleagues to coin the term capitolunate instability pattern (CLIP) wrist.9
Arthroscopic Findings
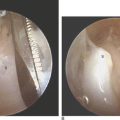
There are no arthroscopic findings that are diagnostic of MCI. Inspection of the radiocarpal joint may reveal a nonspecific synovitis. One of us has seen an associated tear of the dorsal radiocarpal ligament (DJS). In this case, an arthroscopic DRCL repair failed to correct the MCI.10 Inspection of the midcarpal row may demonstrate erosive lesions along the matching surfaces of the triquetrum and the hamate. Laxity of the lunotriquetral ligament may be seen, although this is not invariable. Midcarpal arthroscopy may reveal laxity of the triquetrohamate-capitate ligament, but this is difficult to gauge. More commonly, synovitis obscures the view of the ligament.
Nonoperative Treatment
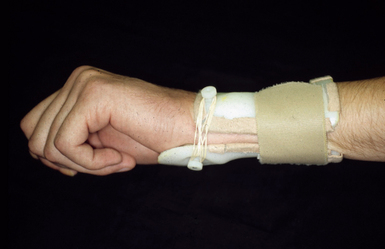
Nonsurgical treatment consists of activity modification, NSAIDs, and splinting.11 Various pisiform support splints have been described. They work based on the observation that applying dorsally directed pressure under the pisiform reduces the carpal sag along with the VISI position of the carpal row. Applying this principle, a three-point dynamic splint may maintain the reduction while permitting wrist motion in milder cases (Figure 14.6). The splint may be worn full time for six to eight weeks to reduce the midcarpal synovitis, and then be worn as needed.
The senior author has observed that active co-contraction of the extensor carpi ulnaris, flexor carpi ulnaris, and hypothenar muscles can reduce the sagging of the midcarpal joint. In fact, some patients can eliminate the catch-up clunk by contracting these muscles before ulnar deviation of the wrist. Patients are taught this isometric muscle contraction as part of the therapy program. Definitive treatment of this condition, however, ultimately requires surgical treatment.
Surgical Treatment
Initial efforts by the senior author were directed at soft-tissue reconstruction by rerouting of the extensor carpi ulnaris to stabilize the triquetrohamate joint.2 The long-term results were disappointing. This evolved to a direct advancement and tightening of the arcuate ligament complex. Reefing of the DRCL is now preferred. This was based on the observation that temporarily reefing the DRCL by plicating it with a clamp significantly reduced the clunking during the midcarpal shift test. In severe cases, midcarpal arthrodesis is still preferred over soft-tissue procedures (Figure 14.7a and b).12–14
Dorsal Reefing of the DRCL (DML)
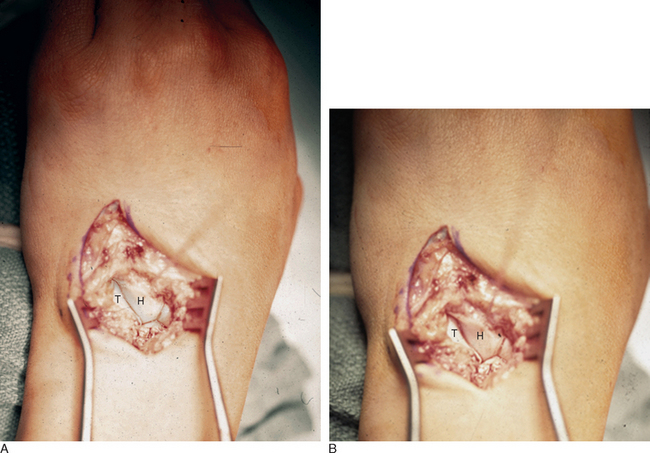
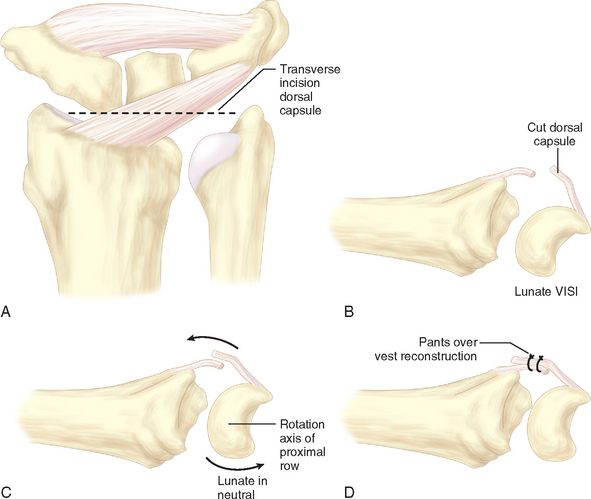
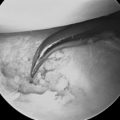
The patient is positioned in the supine position, with the arm abducted and lying on an arm board. The procedure is performed under tourniquet control after limb exsanguination. An 8-cm dorsal longitudinal incision is made centered over Lister’s tubercle. The skin is elevated from the extensor retinaculum, and then the extensor pollicis longus is elevated from its groove and retracted radially. The fourth extensor compartment is elevated along with the finger extensor tendons and retracted ulnarward to expose the dorsal capsule. The DRCL is identified as it courses from its origin just ulnar to Lister’s tubercle to its insertion on the dorsal aspect of the triquetrum. The DRCL is divided by making a 3-cm transverse incision in the dorsal capsule approximately 1 cm distal to the end of the radius, with the wrist distracted (Figure 14.8a through d).
Arthroscopic Capsular Shrinkage (RWC)
Thermal capsular shrinkage has not enjoyed great success in the shoulder. However, its role in the treatment of wrist disorders remains promising.15,16 Thermal energy unwinds the collagen triple helix in capsular and ligamentous structures, with subsequent healing in a shortened or tightened position. The biomechanical properties of the tissue do not appear to be detrimentally altered if shrinkage is limited and if ablation or excess focal treatment is avoided.17 This concept has led to the use of these techniques as a treatment option for midcarpal instability of the wrist.
A midcarpal radial portal is then established. The scapholunate and lunotriquetral joints are inspected and probed for laxity. The TCL is identified as it runs obliquely from the triquetrum, across the proximal corner of the hamate, to the palmar neck of the capitate. A midcarpal ulnar portal is established and used for introduction of the thermal probe. The TCL is then shrunk while again adjusting the tension with correction of any VISI deformity.
Results
In 1993, Lichtman et al. published a review of 13 patients who underwent 15 surgical procedures for MCI, at an average follow-up of 48 months.12 Nine patients had one of several soft-tissue procedures. The first patient had a triquetrohamate ligament reconstruction using the extensor carpi ulnaris tendon, two patients had a palmar capsular reefing, one patient had a dorsal radiocarpal capsulodesis, and five patients underwent a distal advancement of the ulnar arm of the arcuate ligament. Two patients required repeat surgery due to failure. Six patients underwent a limited midcarpal arthrodesis. It was clear that midcarpal arthrodesis was the most reliable procedure. Only two of the patients with an arcuate ligament advancement and the patient with the dorsal capsulodesis had a satisfactory outcome compared to all six of the midcarpal fusions.
In 2003, Culp et al. reported their experience of five patients who underwent an arthroscopic capsular shrinkage.5 Eight patients to date have now undergone this procedure. The average age was 33 (range 29 to 57) years. Follow-up has averaged 9 months (range 3 to 18) months. The midcarpal clunk has resolved in six of the eight patients with pain resolution. Range of motion has decreased 20% in the flexion/extension plane, and grip strengths have increased by an average of 15%. Long-term follow-up is still needed to assess the efficacy of this procedure.
1 Lichtman DM, Wroten ES. Understanding midcarpal instability. J Hand Surg [Am]. 2006;31:491-498.
2 Lichtman DM, Schneider JR, Swafford AR, Mack GR. Ulnar midcarpal instability: Clinical and laboratory analysis. J Hand Surg [Am]. 1981;6:515-523.
3 Trumble TE, Bour CJ, Smith RJ, Glisson RR. Kinematics of the ulnar carpus related to the volar intercalated segment instability pattern. J Hand Surg [Am]. 1990;15:384-392.
4 Viegas SF, Patterson RM, Peterson PD, et al. Ulnar-sided perilunate instability: An anatomic and biomechanic study. J Hand Surg [Am]. 1990;15:268-278.
5 Lichtman DM, Culp RW, Joshi A. Palmar midcarpal instability. In: [last name] EM, editor. Operative Arthroscopy. Third Edition. Philadelphia: Lippincott Williams & Wilkins; 2003:737-742.
6 Johnson RP, Carrera GF. Chronic capitolunate instability. J Bone Joint Surg Am. 1986;68:1164-1176.
7 Apergis EP. The unstable capitolunate and radiolunate joints as a source of wrist pain in young women. J Hand Surg [Br]. 1996;21:501-506.
8 White SJ, Louis DS, Braunstein EM, Hankin FM, Greene TL. Capitate-lunate instability: Recognition by manipulation under fluoroscopy. AJR Am J Roentgenol. 1984;143:361-364.
9 Louis DS, Hankin FM, Greene TL. Chronic capitolunate instability. J Bone Joint Surg Am. 1987;69:950-951.
10 Slutsky D. Arthroscopic repair of dorsoradiocarpal ligament tears. The Journal of Arthroscopic and Related Surgery. 2005;21:1486e1. 1486e8
11 Lichtman DM, Pollock GR. Midcarpal and proximal carpal instabilities. In: Lichtman DM, Alexander AH, editors. The Wrist and Its Disorders. Philadelphia: W.B. Saunders; 1997:316-328.
12 Lichtman DM, Bruckner JD, Culp RW, Alexander CE. Palmar midcarpal instability: Results of surgical reconstruction. J Hand Surg [Am]. 1993;18:307-315.
13 Goldfarb CA, Stern PJ, Kiefhaber TR. Palmar midcarpal instability: The results of treatment with 4-corner arthrodesis. J Hand Surg [Am]. 2004;29:258-263.
14 Rao SB, Culver JE. Triquetrohamate arthrodesis for midcarpal instability. J Hand Surg [Am]. 1995;20:583-589.
15 D’Alessandro DF, Bradley JP, Fleischli JE, Connor PM. Prospective evaluation of thermal capsulorrhaphy for shoulder instability: Indications and results, two- to five-year follow-up. Am J Sports Med. 2004;32:21-33.
16 Hayashi K, Markel MD. Thermal capsulorrhaphy treatment of shoulder instability: Basic science. Clin Orthop Relat Res. 2001;390:59-72.
17 Medvecky MJ, Ong BC, Rokito AS, Sherman OH. Thermal capsular shrinkage: Basic science and clinical applications. Arthroscopy. 2001;17:624-635.