The role of adjuvant systemic therapy in patients with operable breast cancer
Introduction
The mortality from breast cancer has fallen by over 15% in the UK over the last 15 years, despite a rising incidence. The improvement in survival coincides with the widespread uptake of adjuvant systemic therapy and increasing evidence of its survival benefit. The rationale for this treatment is that over half of women with operable breast cancer who receive local regional treatment alone will die from metastatic disease, indicating the presence of micrometastases at the time of initial clinical presentation. Traditionally, the major risk factors for recurrence have been the involvement of axillary nodes, poor histological grade, large tumour size and histological evidence of lymphovascular invasion around the tumour site. The absence of oestrogen and progestogen receptor and the overexpression of human epidermal growth factor receptor 2 (HER-2) also carry an adverse prognosis. The only way to improve survival for these women is to administer effective systemic medical treatment, using endocrine therapy, chemotherapy and targeted biological therapies, along with surgery and radiotherapy. It is now recognised that breast cancer comprises a number of subtypes, each with a distinct biological behaviour and prognosis, and increasingly molecular factors rather than classical histopathological features are being used to determine the degree of residual risk after breast cancer surgery, and all the judicious use of potentially toxic treatments.1 Gene expression profiling has emerged as a new determinant of recurrence risk and a major current challenge is to assimilate this new technology into treatment planning.
Adjuvant endocrine therapy
Approximately 75% of invasive breast cancer patients present with hormone-receptor positive disease.2 As the oestrogen receptor (ER) pathway is key to the growth of these cancers, modulation of ER activation is an essential component of treatment for these women. Since the observation by Beatson more than 100 years ago that oophorectomy could induce regression of advanced breast cancer,3 endocrine treatment has proved one of the most valuables therapies in cancer medicine.
Tamoxifen
Until recently tamoxifen was the standard adjuvant endocrine therapy. The results of the most recent overview of tamoxifen trials involving around 21 000 women carried out by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) have shown that tamoxifen given for about 5 years reduces the risk of death by around one-third (relative risk (RR) = 0.71 ± 0.07).4 The proportional reduction is not significantly affected by age, nodal status or use of chemotherapy; the absolute benefit of course relates to the absolute risk. The reduction in the risk of recurrence is seen both during the 5 years of treatment (RR = 0.53 ± 0.03) and extends into years 5–9 (RR = 0.70 ± 0.06), but there was no further reduction in risk beyond 10 years. The benefits were similar and highly significant in both ER-positive/progesterone receptor (PgR)-positive and ER-positive/PgR-negative disease. The reduction was greater in strongly positive ER disease (RR = 0.51 ± 0.07) than in marginally ER-positive disease (RR = 0.65 ± 0.07).
Tamoxifen duration
The overview data indicate that 5 years of tamoxifen is more effective than shorter durations. Until very recently, there was no convincing evidence that more than 5 years of tamoxifen had a further advantage. Indeed, the largest published trial so far of tamoxifen for more than 5 years (National Surgical Adjuvant Breast and Bowel Project (NSABP) B14) showed that tamoxifen for more than 5 years had an unexpected adverse influence on disease-free survival (DFS) (78% vs. 82% with placebo, P = 0.03) and was also associated with higher rates of endometrial cancer, ischaemic heart disease and cerebral vascular disease.5 Recently a much larger international trial involving 11 500 patients, the Adjuvant Tamoxifen Longer Against Shorter (ATLAS) trial, has addressed the question of long-term tamoxifen duration. Results have so far been presented only in abstract form, but showed a small but significant reduction in recurrence comparing 5 years with more than 5 years treatment (hazard ratio (HR) = 0.88).6 Preliminary results from a similar UK trial (aTTom: adjuvant Tamoxifen – To offer more?) involving 8000 patients are reported to be consistent with those of ATLAS, but mature data are awaited.7
Aromatase inhibitors
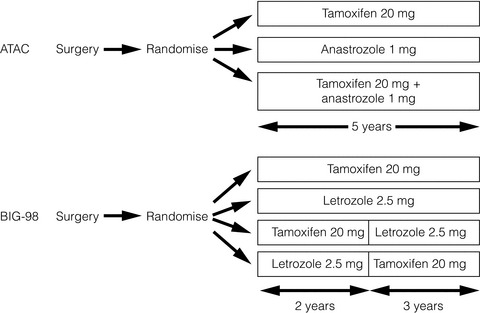
In the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial involving over 9000 women, anastrozole was compared with tamoxifen and with a combination of the two drugs, and was shown to be superior to both in terms of DFS. With a median follow-up of 120 months, a 5-year DFS benefit of 4.3% (HR = 0.86) emerged in hormone receptor-positive patients.10
In the BIG1-98 trial involving more than 8000 women, letrozole was compared with tamoxifen in a four-arm trial as follows: letrozole monotherapy; tamoxifen monotherapy; sequential tamoxifen then letrozole; sequential letrozole then tamoxifen; all for a total of 5 years. With a median follow-up period of 8.7 years, letrozole was significantly better than tamoxifen in terms of both DFS (HR = 0.82, 95% confidence interval (CI) 074–0.92) and overall survival (OS) (HR = 0.79, CI 0.69–0.90).11 This is in contrast to the ATAC trial, where no overall survival benefit was seen for anastrozole over tamoxifen. The results of these two trials are summarised in Table 12.1.
Table 12.1
Results from the ATAC and BIG1-98 trials
| ATAC* | BIG1-98* | |
| No. of patients | 6241 | 8010 |
| Median follow-up (years) | 10 | 8.7 |
| DFS (hazard ratio) | 0.91 | 0.82 |
| 5-year DFS difference (%)# | 2.7 | 3.1 |
| OS (hazard ratio) | 0.97† | 0.79 |
First-line aromatase inhibitors: bad prognosis subgroups: ER-positive, PgR-negative breast cancers are recognised as having a poorer outcome.12 In the EBCTCG analysis, patients with PgR poor tumours had a worse prognosis but nevertheless had a similar proportional benefit to adjuvant tamoxifen compared with control.4
The relative gain with anastrozole and letrozole respectively over tamoxifen is similar in both the ATAC and the BIG 1-98 trials,13 but in both instances the absolute gain is greater in patients with PgR-negative cancers, because of the greater risk of recurrence.
HER-2-positive tumours have a worse prognosis than HER-2-negative cancers and in a neoadjuvant endocrine therapy trial comparing letrozole with tamoxifen, a large and highly significant benefit was seen for letrozole over tamoxifen in terms of clinical response in this small subgroup (88 vs. 21%, P = 0.0004).14 In a similar neoadjuvant trial comparing anastrozole with tamoxifen, the IMmediate Preoperative Arimidex Compared with Tamoxifen (IMPACT) trial, a numerical although non-significant difference was again seen in favour of the aromatase inhibitor for HER-2-positive tumours (58% vs. 22%, P = 0.09).15
These results were not, however, confirmed in the equivalent adjuvant trials. In the ATAC trial there was no evidence of a proportionately greater benefit for anastrozole over tamoxifen in HER-2-positive tumours compared with other subtypes.13 A similar finding was true when letrozole was compared with tamoxifen in the BIG1-98 trial.16 Invasive lobular cancers appear to benefit more from letrozole than tamoxifen.17
Comparative toxicities of front-line anastrozole/letrozole and tamoxifen: The ATAC and BIG1-98 trials have both shown that tamoxifen is associated with a small but significant increase in the incidence of hot flushes compared with anastrozole or letrozole (4.5–5% increase), vaginal bleeding (3.3–3.7% increase), vaginal discharge (8.6% increase), endometrial carcinoma (0.2–0.4% increase) and venous thromboembolism (1.4–2% increase). The ATAC trial has likewise shown a small but significant increase in ischaemic cerebral vascular disease (1.1% increase) with tamoxifen compared with anastrozole, but this has not been confirmed in the BIG1-98 trial with letrozole. In contrast, anastrozole and letrozole have been shown to be associated with a statistically significant increase in the incidence of musculoskeletal problems (6.5–8% increase) and fractures (1.7–2.2% increase).
Of note, tamoxifen is associated with a significant increase in gynaecological surgery compared with either of the aromatase inhibitors. In the ATAC trial 5.1% of women had hysterectomies compared with 1.3% on anastrozole.18 In the BIG1-98 trial 288 women (9.1%) have required endometrial biopsies compared with 77 (2.3%) with letrozole.9
Sequential therapy with aromatase inhibitors after tamoxifen
Until recently, there was considerable interest in trials assessing the benefit of sequential adjuvant aromatase inhibitors given 2–3 years after tamoxifen. For example, in the Intergroup Exemestane Study (IES), 4274 patients who had already been on tamoxifen for around 2 years were randomised double-blind to continuing on tamoxifen or switching to exemestane to complete 5 years of treatment. Updated results with a median follow-up of 91 months have shown a significant reduction in the risk of relapse and an improvement in OS (HR = 0.86, 95% CI 0.75–0.99) with the switch.19 Three other sequential trials involving anastrozole have shown similar results.20–22
Two trials have addressed this issue directly, however, and recently reported results. Two of the arms in BIG1-98 compared tamoxifen for 2 years followed by a switch to letrozole with letrozole alone for 5 years and found no significant benefit of the switch compared with letrozole up front (8-year DFS 85.9% vs. 87.5%).11 Likewise, in the TEAM (Tamoxifen Exemestane Adjuvant Multinational) trial, 9779 patients were randomised to tamoxifen for 2–3 years followed by exemestane to complete 5 years or to exemestane up front for 5 years. No significant difference was found in DFS (85% vs. 86%) with a median follow-up of 5.1 years.23
Extended adjuvant therapy with aromatase inhibitors
The risk of recurrence of early breast cancer continues for at least 10 years after diagnosis and is greater in patients with hormone receptor-positive cancers.24 In the EBCTCG overview analysis more than half of breast cancer recurrences occur after the 5-year mark.4
Against this background, the results of the MA17 trial evaluating the benefit of extended adjuvant therapy with letrozole in women still in remission after 5 years of tamoxifen were of great importance in that they demonstrated a significant DFS benefit in favour of letrozole.25 This benefit has continued, and indeed increased with time, with an initial HR of 0.52 (95% CI 0.40–0.64) 12 months after randomisation, increasing to 0.19 (0.04–0.34) after 48 months. Recently a similar and perhaps even greater benefit has been reported for the subgroup of 889 younger women < 50 and premenopausal at the time of diagnosis but who subsequently became postmenopausal during their 5 years of tamoxifen, with an absolute gain in 4-year DFS of 10%.26 This represents a risk reduction of 75% with extended adjuvant endocrine therapy compared with 33% in the much larger group who were postmenopausal from the outset.
In two similar but smaller trials, extended adjuvant anastrozole (ABCSG-6a) and extended adjuvant exemestane (NSABP-B33), both after 5 years of tamoxifen, showed that extended therapy with an aromatase inhibitor reduces the risk of recurrence significantly.27,28
Other aromatase inhibitor issues
Aromatase inhibitors are contraindicated in premenopausal women. Likewise, caution must be observed in their use in younger women following chemotherapy-induced amenorrhoea. In an audit carried out at the Royal Marsden Hospital 12 of 45 younger women (27%), median age 47, treated with an aromatase inhibitor following chemotherapy-induced amenorrhoea (27%) developed clinical or biochemical return of ovarian function (including up to the age of 53 years).29 Aromatase inhibitors should therefore be used with great caution in this group of women and ideally serum oestradiol should be monitored using a high sensitivity assay.
Vaginal dryness, atrophy and dyspareunia are significant issues in women on aromatase inhibitors. In a small study, six of seven women given vaginal oestradiol (Vagifem®) while on an aromatase inhibitor developed a significant rise in serum oestradiol from less than 5 pmol/L to a mean of 72 pmo/L (maximum 219 pmol/L) at 2 weeks.30
Endocrine therapy in premenopausal women
A key question is whether ovarian suppression in addition to tamoxifen (and chemotherapy where appropriate) is superior to tamoxifen alone in the management of premenopausal breast cancer. In the INT-101 trial, the addition of goserelin and tamoxifen to standard adjuvant therapy with CAF (cyclophosphmide, adriamycin and fluorouracil) significantly improved DFS; 9-year DFS rates were 57% for CAF, 60% for CAF plus goserelin, and 68% for CAF plus goserelin and tamoxifen.31 An unplanned retrospective analysis of these data suggested that the addition of goserelin to CAF was most beneficial in those women under the age of 40. A prospective trial, SOFT (Suppression of Ovarian Function), is currently addressing this question and has recruited 3000 premenopausal women with hormone receptor-positive disease randomised to tamoxifen alone for 5 years or ovarian suppression with either tamoxifen or exemestane for 5 years in women post-chemotherapy who are still menstruating, or who have not received chemotherapy. It is currently in active follow-up. In a randomised trial involving 927 premenopausal women, no significant differences in risk reduction were seen after 12 years of follow-up between tamoxifen alone (27%) or a combination of tamoxifen with the luteinising hormone-releasing hormone (LHRH) analogue goserelin (24%).32
The SOFT trial also addresses the important question of whether an aromatase inhibitor is superior to tamoxifen in premenopausal patients who have undergone ovarian suppression. The only trial to present data on this so far is ABCSG-12 and the Austrian Group reported no significant difference in outcome for 1803 women randomised to tamoxifen or anastrozole, both given with goserelin, with a median 62 months follow-up.33
Obesity and adjuvant endocrine therapy
An increase in body mass index is associated with an increased risk of breast cancer recurrence34,35 and this was recently confirmed in patients in the ATAC trial.36 Moreover, the benefit of anastrozole over tamoxifen in terms of distant recurrence was lost in patients with a body mass index of 25 kg/m2 or greater, and a similar trend was seen for all recurrences. The Austrian ABCSG-6 and -6a trials in which patients who had maintained a continued remission on tamoxifen for 5 years were randomised to a further 3 years of anastrozole or not have reported similar findings. Outcome was not influenced by body weight during tamoxifen therapy, but during extended adjuvant therapy an exploratory analysis found that women with normal body weight randomised to anastrozole had a significant gain in DFS (HR = 0.46, P = 0.02) and OS (HR = 0.37, P = 0.02), whereas no gain was seen in patients with a body mass index of greater than 25 kg/m2.37 This raises the intriguing possibility that anastrozole, a relatively weak aromatase inhibitor, is unable to inhibit fully the excess aromatase associated with adiposity. In contrast, in the BIG 1-98 trial, the benefit of letrozole, a much more potent aromatase inhibitor than anastrozole, over tamoxifen was maintained whatever the body mass index.38 Further data are required, but these results suggest that anastrozole may not be the optimal aromatase inhibitor in women with higher body mass indices.
See Table 12.3 for a summary of recommendations for adjuvant endocrine therapy.
Table 12.3
Summary of recommendations for adjuvant endocrine therapy
| Menopausal status* | Recommendation |
| Premenopausal | Tamoxifen 5 years |
| Postmenopausal† | Anastrazole 5 years or Letrozole 5 years |
| Women who are menopausal after 5 years of tamoxifen | Consider: Anastrazole Letrozole Exemestane in high-risk patients |
| Women who have completed 5 years of aromatase inhibitor | Currently no data Consider option of continuing in high-risk patients |
*Based on pre-chemotherapy menopausal status.
†Caution in women under the age of 50; return of ovarian function on aromatase inhibitor is possible.
Adjuvant chemotherapy
Age and chemotherapy
In general, the absolute gain from chemotherapy is higher for younger than older women.39 It is likely, however, that this difference relates mainly to the biological characteristics of breast cancer being more favourable to chemotherapy response (ER negativity, for example) in younger women, rather than an intrinsic adverse interaction between age and chemotherapy efficacy.41
Elderly women with breast cancer have been under-represented in clinical trials to date, but this is changing. The Cancer and Leukaemia Group B (CALGB) 49907 trial demonstrated that standard adjuvant chemotherapy was superior to single-agent oral chemotherapy with capecitabine in women over the age of 65, and suggested that the benefit was more pronounced in women with hormone receptor-negative tumours.42 However, it is also clear that older women experience significantly greater toxicity with adjuvant cytotoxic treatment,43–46 and there are a number of trials under way that aim to define those elderly patients for whom chemotherapy is most appropriate.
Nodal status
Initially adjuvant chemotherapy tended to be reserved for patients with axillary node involvement on the basis of higher risk. It is now clear that the proportional reduction in the risk of recurrence is similar for those with node-negative as for node-positive disease.39 Nevertheless, since the absolute risk is greater with nodal involvement, so is the absolute benefit. Although nodal involvement carries a worse prognosis, this does not necessarily imply chemotherapy benefit and we are now in an era when molecular markers are at least as important as nodal status in determining chemotherapy benefit (see below).
ER status
There has been considerable controversy over the years as to whether patients with ER-positive disease gain as much from adjuvant chemotherapy as those whose tumours are ER negative. The 2011 Oxford Overview data indicate that the proportional benefits are very similar, both in older and younger women.39
Anthracycline-based chemotherapy
Anthracyclines have been used widely for the last decade or more, and have largely replaced older CMF (cyclophosphamide/methotrexate/fluorouracil) regimens. The 2005 Overview data (including trials involving a total of around 40 000 women) established clearly the efficacy of anthracycline-based adjuvant regimens in early breast cancer, and indicated an additional proportional risk of recurrence of around 11% and a proportional reduction in mortality of around 16%.47
Dose of anthracyclines
The two main anthracyclines in current use are adriamycin (doxorubicin) and epirubicin. The Cancer and Leukaemia Group B (CALGB) 9344 trial randomised women with node-positive breast cancer to receive four courses of anthracycline chemotherapy to one of three different adriamycin dose levels (60, 75 or 90 mg/m2), followed by four cycles of paclitaxel or not.52 This important dose escalation trial showed no benefit for adriamycin doses above 60 mg/m2 and this dose should now be considered standard.
The French Adjuvant Study Group (FASG)-05 trial randomised lymph node-positive women with poor prognosis and found a dose effect in favour of six cycles of FEC100 (epirubicin 100 mg/m2) over six cycles of FEC50 (epirubicin 50 mg/m2).53 A significant improvement in the DFS (66.3 months vs. 54.8 months) and 5-year OS (77.4% vs. 65.3%) was seen in the FEC100 group but there were significantly more side-effects in the FEC100 group. These included neutropenia, anaemia, nausea and vomiting, stomatitis, alopecia and grade 3 infections. It is important to note that this trial does not determine that an epirubicin dose of 100 mg/m2 is optimal. All that can be concluded is that 50 mg/m2 is suboptimal and that a dose between the two is likely to achieve the best balance between efficacy and toxicity.
In the 2011 Oxford meta-analysis, four cycles of anthracycline chemotherapy appeared equivalent to six courses of standard CMF, but there was a clear improvement in recurrence and mortality when regimens with a cumulative anthracycline dosage of more than 240 mg/m2 adriamycin or 360 mg/m2 epirubicin (for example, fluorouracil/adriamycin/cyclophosphamide (FAC) or FEC) were compared with CMF (risk ratio 0.89 and 0.84 for recurrence and mortality respectively).39
Higher doses of anthracylines are related to long-term complications such as an increased incidence of acute myeloid leukaemia (AML)/myelodysplasia. In the EBC-1/MA.5 study by the NCIC CTG, which used the very high epirubicin dose of 120 mg/m2, a disturbingly high incidence of AML/myelodysplasia was reported (2% at 10-year follow-up).50 Likewise, in a study that analysed the toxicity of adjuvant chemotherapy treatment in elderly patients, there was a linear increase in the incidence of AML/terms of recurrence-free survival (P < 0.001) with a reported incidence of 1.8% for the group of age > 65 years.43
Cardiotoxicity is a further concern with the anthracyclines. Symptomatic congestive heart failure (CHF) is a rare but very serious complication in patients receiving an anthracycline-based chemotherapy regimen with an incidence that relates to the cumulative dose received.54,55 As with secondary AML, there is an association between the risk of cardiotoxicity and increasing age. Recent long-term data on cardiac safety in more than 40 000 early breast cancer patients of an older age treated with adjuvant anthracycline regimens have shown an increased risk of cardiotoxicity compared with non-anthracycline chemotherapy treatment. This was statistically significant in the group of patients aged 66–70 with a 26% increased risk of developing CHF. This difference in rates of CHF continued to increase through more than 10 years of follow-up.45
Anthracyclines and HER-2-positive disease
The CALGB 8541 trial reported in 397 node-positive patients that high expression of HER-2 was associated with benefit from standard doses of doxorubicin (60 mg/m2) but not from lower doses of anthracyclines.56 In contrast, this dose–response effect was not seen in the majority whose tumours did not overexpress HER-2. An additional cohort of 595 patients showed an even stronger correlation between HER-2 overexpression and CAF dose efficacy with further follow-up.57 No significant interaction was observed in the CALGB 9344 study between HER-2 status and the use of doses of doxorubicin > 60 mg/m2.58
In a retrospective study of 639 formalin-fixed paraffin-embedded specimens obtained from 710 premenopausal women with node-positive breast cancer who had received either cyclophosphamide, epirubicin and fluorouracil (CEF) or CMF, HER2 amplification or overexpression was associated with a poor prognosis regardless of the type of treatment. In patients whose tumours showed amplification of HER2, CEF was superior to CMF in terms of recurrence-free survival (RFS) (HR = 0.52, 95% CI 0.34–0.80; P = 0.003) and OS (HR = 0.65, 95% CI 0.42–1.02; P = 0.06).59 Similarly, a retrospective evaluation of patients in the Southwest Oncology Group study (SWOG) 8814 trial, which randomised postmenopausal patients with node-positive hormone receptor-positive tumours between tamoxifen and tamoxifen plus CAF chemotherapy, showed that CAF offered a substantial advantage for patients with HER-2-positive cancers but little, if any, advantage for those with HER-2-negative tumours.60
Recently, the Breast Cancer International Research Group (BCIRG)-006 trial published results of a non-anthracycline regimen combined with trastuzumab in patients with HER-2-positive early breast cancer.61 This prospective study randomised 3222 women to one of three treatment arms: doxorubicin and cyclophosphamide followed by docetaxel (AC-T), the same regimen plus 52 weeks of trastuzumab (AC-TH), or docetaxel and carboplatin plus 52 weeks of trastuzumab (TCH). Predictably, both trastuzumab-containing regimens improved DFS and OS significantly compared to the AC arm, but there were no significant differences between AC-T and TCH in these outcome measures (DFS at 5 years 75%, 84% and 81% for AC, AC-T and TCH respectively, and OS 87%, 92% and 91% respectively). Anthracycline-based treatments resulted in significantly higher rates of cardiotoxicity and leukaemia, and TCH was better tolerated overall.
This trial raises the critical question of whether adjuvant chemotherapy regimens should always include an anthracycline. There has been much interest in defining the biological mechanism that underlies the anthracycline sensitivity of HER-2-positive breast cancers, and a number of studies have implicated alterations in topoisomerase II-α (TOP2A) in this process. The TOP2A gene regulates DNA replication and RNA transcription and is considered a target of anthracyclines. It is located on chromosome 17, in close proximity to the HER2 gene, and the two are frequently co-amplified. However, despite a number of pre-clinical and clinical studies, (including a sub-study of the BCIRG-006 trial described above) suggesting a predictive role for TOP2A amplification and benefit from anthracyclines, other studies disagree, so at present there is no indication to look for TOP2A amplification when considering treatment selection.62,63
Taxanes
Paclitaxel (Taxol) and docetaxel (Taxotere) have emerged as two of the most active cytotoxic agents against breast cancer. In the metastatic setting, these compounds have been shown to be active in anthracyline-resistant breast cancers.64 Several randomised trials have evaluated the benefit of taxanes combined with anthracyclines in the adjuvant treatment of early breast cancer,52,65–69 but their exact role remains controversial. The majority have shown a DFS benefit, but some have failed to show a benefit in OS65,70 and in endocrine receptor-positive tumours.52,70 A meta-analysis of 13 randomised trials involving more than 22 000 patients assessing the addition of a taxane to an anthracycline-based regimen71 showed an absolute improvement at 5 years of approximately 5% for recurrence and 3% for death. This benefit is present irrespective of the number of lymph nodes involved (N1–3 vs. N4 +), ER status (ER positive vs. ER negative) or age/menopausal status (≤ 50 years/premenopausal vs. > 50 years/postmenopausal). The most recent Oxford meta-analysis of polychemotherapy included data from 44 000 women in 33 taxane studies.39 A significant reduction in breast cancer mortality (15–20%) was found when trials that added four separate cycles of a taxane to anthracycline chemotherapy (thereby prolonging adjuvant chemotherapy duration) were compared with anthracycline chemotherapy alone, but this benefit was much smaller (though still significant) when studies in which the number of anthracycline cycles was increased to balance treatment duration were analysed. The results of this meta-analysis suggest that the benefit from taxanes is independent of age, nodal status or hormone receptor status. It should also be noted, however, that results from the largest adjuvant taxane trial, the UK Taxotere as Adjuvant Chemotherapy Trial (TACT), involving 4162 patients, did not show a significant benefit for the addition of docetaxel to standard anthracycline chemotherapy.70
Adjuvant docetaxel has also been tested instead of an anthracycline in patients with early breast cancer. In a prospective US Oncology phase III trial, a total of 1106 patients were randomised to received either four cycles of standard AC (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2) or four cycles of TC (docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2) as adjuvant treatment for early breast cancer.72 Treatment with TC achieved a significant improvement in 5-year DFS compared with AC (86% vs. 80% respectively, HR = 0.67; P = 0.015). With further follow-up a significant overall survival benefit has also emerged.73 There was significantly more nausea and vomiting in patients receiving AC compared with TC, whereas patients receiving docetaxel experienced more oedema, myalgia, arthralgia and a higher rate of fever and neutropenia compared with AC (5% vs. 2.5%; P = 0.07).
Which taxane and which schedule?
The optimal schedule is determined by the type of taxane selected, as demonstrated by the pivotal ECOG 1199 trial.74 Nearly 5000 women with node-positive or high-risk node-negative disease were enrolled and received standard AC chemotherapy for four cycles, followed by paclitaxel or docetaxel, either given every 3 weeks for four cycles or weekly for 12 cycles. Progression-free survival was superior in those treated with 3-weekly docetaxel (HR = 1.23), or weekly paclitaxel (HR = 1.27), when compared with the standard treatment of paclitaxel given 3-weekly. A survival gain was demonstrated in patients treated with weekly compared with 3-weekly paclitaxel (HR = 1.32). On the basis of these results, weekly paclitaxel or 3-weekly docetaxel are considered standards of care in adjuvant breast cancer treatment.
Duration of chemotherapy
The optimum duration of chemotherapy remains uncertain. The 1998 EBCTG meta-analysis assessed five CMF-based trials and found no survival benefit for more than 6 months treatment,75 but the most recent data suggest that regimens utilising chemotherapy regimens longer than four cycles of AC (more cycles or higher cumulative dose) are more effective.39 A French FASG-01 trial showed a significant benefit in DFS of six cycles of FEC50 over three cycles of FEC50 or 75, and improved OS with six cycles of FEC50 over three cycles.76
Preliminary results of the CALGB 40101 trial were presented recently in abstract form77 and indicate that for women with early breast cancer and no or limited lymph node disease (0–3), four cycles of 3-weekly AC or weekly paclitaxel were equivalent in efficacy to six cycles of either. Further trials of chemotherapy duration are needed.
Dose density
Recently interest has developed in accelerated (also called dose-dense) chemotherapy in which treatment is given at 2-week rather than 3-week intervals with G-CSF (granulocyte colony-stimulating factor) support to overcome the risk of neutropenic sepsis. The CALGB 9741 trial has shown that accelerated 2-weekly AC × 4 followed by accelerated paclitaxel × 4 improved efficacy over the same eight courses given conventionally at 3-weekly intervals in women with node-positive breast cancer, with 4-year DFS of 82% and 75% respectively.78 In addition, the accelerated arm was associated with less neutropenic sepsis. Likewise, an Italian trial, so far presented only in abstract form, has shown a similar increase in efficacy with reduced risk of neutropenic sepsis when six courses of FEC chemotherapy were given in accelerated fashion compared with the conventional approach.79
A recent systematic review and meta-analysis of dose-dense chemotherapy for early or locally advanced breast cancer reported improved outcomes with this approach, but included a number of trials with heterogeneous study designs and treatments, and for this reason did not provide meaningful conclusions.80
Which patients really benefit from adjuvant chemotherapy? The role of molecular markers
The EBCTCG Overview shows that, overall, the survival of patients with hormone receptor-positive disease is significantly improved by chemotherapy over and above tamoxifen, with an HR of 0.66.39 The important question, however, is to identify those women for whom the gain is large enough to be of real clinical benefit when balanced against toxicity. Various guidelines for chemotherapy decision-making have been proposed; one of the best recognised is the St Gallen Consensus. In the most recent update, the 2011 St Gallen panel suggested that subtypes of breast cancer can be defined by gene array profiles, and that each subtype differs in its epidemiological risk factors, natural history, and response to systemic and local therapies.1 Surrogate immunohistochemical markers of gene expression array information allow an approximate and simplified classification system of intrinsic subtypes (see Table 12.4). This latest consensus demonstrates a paradigm shift from the use of traditional clinico-pathological features to determine the risk of recurrence, towards an assessment of the underlying biology of the tumour. This is also evidenced by the fact that most of the panel supported further research into the role of molecular profiling techniques (discussed below) as prognostic and predictive tools in early breast cancer.
Table 12.4
Intrinsic subtypes of breast cancer and approximation by immunohistochemistry (St Gallen 2011)
| Intrinsic subtype | Clinico-pathological definition |
| Luminal A | ER positive HER-2 negative Ki67 low (< 14%)* |
| Luminal B | ER positive HER-2 negative Ki67 high |
| HER-2 overexpression | HER-2 overexpressed ER absent |
| Basal-like | ‘Triple negative’ ER, PgR absent HER-2 negative |
*Definition of Ki67 low established by comparison with PAM50 intrinsic subtyping.119
Further insight into this issue comes from an analysis of the SWOG 8814 trial, in which postmenopausal women with node-positive hormone receptor-positive tumours were randomised to tamoxifen alone or tamoxifen with anthracycline-containing chemotherapy (cyclophosphamide, adriamycin and 5-fluorouracil).81 Overall, there was a significant benefit in favour of those receiving chemotherapy concurrently with tamoxifen but in a retrospective subset analysis, patients with a high ER score (Allred score 7 or 8) showed no benefit from the addition of chemotherapy even in the presence of involved nodes. Likewise, women whose tumours were HER-2 negative showed no benefit from the addition of chemotherapy unless they had four or more nodes involved. This analysis should be considered by hypothesis generation rather than being definitive, but emphasises the need to identify molecular markers to predict which patients really benefit from chemotherapy.
Multiple gene expression assays including Oncotype DX
DNA micro array analysis has classified breast cancers according to gene expression signatures, to quantify more accurately the likelihood of breast cancer recurrence and predict the magnitude of chemotherapy benefit. Currently, the most widely used of these is a 21-gene assay now offered as a commercial reference laboratory test (Oncotype DX Genomic Health Inc.). This is based on formalin-fixed material from which the level of gene expression is used to determine a recurrence score predicting the likelihood of distant recurrence.82 The Oncotype DX assay has been applied to a subset of patients in the NSABP B-20 trial, randomising women with node-negative disease to tamoxifen and chemotherapy (CMF or MF) versus tamoxifen alone. It was found that women with a low recurrence score had no significant benefit from chemotherapy, whereas those with a high recurrence score had a major and significant benefit with an absolute decrease in the 10-year rate of distant recurrence of 28% (88% vs. 60% free of distant recurrence).83 Patients with an intermediate recurrence score had a relatively small benefit and such patients are now being included in a trial randomising women with cancers with intermediate scores to chemotherapy or not in addition to endocrine therapy (TAILORx). Oncotype DX has also been validated in ER-positive patients in the ATAC trial84 and in node-positive patients in SWOG 8814;85 the key message from these data is that many patients, even those with node-positive disease, may not benefit from chemotherapy. The question with regard to Oncotype DX is its additional benefit over standard immunohistochemistry. This was addressed in a study where proliferation as measured by Ki67, were combined with ER, PgR and HER-2 to form the IHC4 score.86 The score appeared to further risk-stratify those patients deemed intermediate risk by the Adjuvant Online and Nottingham Prognostic Index (NPI) and correlated closely with Oncotype DX. The main issue with the IHC4 is quality control; measuring Ki67 in a reproducible manner continues to be a problem in many laboratories.87 Similarly, a 70-gene signature has also shown strong correlation with outcome88,89 and identifies a good and a poor prognosis group. A second trial, MINDACT, is assessing the value of this signature, in predicting which patients with hormone receptor-positive tumours might also benefit from chemotherapy.
Bisphosphonates
Two of three early trials indicated a benefit for the use of oral clodronate compared with placebo in the adjuvant setting in early breast cancer.90–93 Both positive trials observed a reduction in bone metastases and improvement in overall survival. The NSABP B-34 study is the largest trial to compare clodronate with placebo in addition to adjuvant chemo- or hormone therapy and results were presented recently. The trial’s primary end-point of superior disease-free survival was not met, but there appeared to be distinct benefits of clodronate for women over the age of 50, including a trend toward improved overall survival.94
The results of two large trials of a much more potent bisphosphonate, zoledronic acid, have been published recently. The Austrian Breast and Colorectal Cancer Study Group trial-12 (ABSCG-12) randomised premenopausal women with hormone receptor-positive early breast cancer to anastrazole or tamoxifen, with or without zoledronic acid.33 All patients received goserelin for ovarian suppression. The investigators reported that disease-free survival was improved with the addition of zoledronic acid (HR = 0.68), although zoledronic acid did not significantly affect overall survival. More recently, the AZURE trial randomising pre- and postmenopausal women to receive standard adjuvant systemic therapy with or without zoledronic acid produced complex results.95 Overall, no difference in disease-free survival was observed between these two groups, but in a pre-planned analysis of AZURE, postmenopausal patients (similar to the premenopausal population of ABSCG-12 who were rendered ‘postmenopausal’ with goserelin) had a small but significant disease-free survival advantage, which was apparent early after diagnosis. The results of ABSCG-12 and AZURE suggest that there may be an interaction between menopausal status and effect of bisphosphonates. This hypothesis was supported by the results of two further studies of adjuvant bisphosphonates presented in late 2011. In an unplanned analysis of the ZO-FAST study, disease-free survival and overall survival were improved by the addition of zoledronic acid to adjuvant endocrine therapy in women who were established to be postmenopausal,96 while the GAIN (German Adjuvant Intergroup Node-Positive) study,97 although negative overall, suggested a beneficial effect of bisphosphonates in older women.
Trastuzumab (Herceptin)
Trastuzumab is a recombinant humanised monoclonal antibody specific to the human HER-2 receptor. HER-2 is amplified in 15–20% of breast cancers. It plays a critical role in tumour development, and is an independent marker of survival, with amplification/ overexpression carrying an adverse prognosis.98,99 Trastuzumab was developed as targeted therapy against HER-2100 and has established efficacy, including a significantly improved survival benefit in metastatic breast cancer.101,102
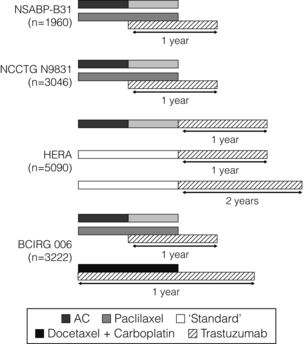
Four large, multicentre randomised adjuvant trials involving more than 12 000 women have assessed whether trastuzumab given concurrently with a taxane after anthracycline chemotherapy (adriamycin/cyclophosphamide, AC) (NSABP B-31; Intergroup N9831; BCIRG 006)61,103 or concurrently with a non-anthracycline regimen of taxotere and carboplatin (BCIRG 006),61 or sequentially after any standard chemotherapy schedule (Herceptin in Adjuvant Breast Cancer (HERA) trial),104 or sequentially after AC and a taxane (Intergroup N9831)105 can improve disease-free survival and overall survival (Table 12.4). In all these trials trastuzumab was given for 1 year; in the HERA trial a third arm is also evaluating treatment for 2 years (Fig. 12.2).
Chemotherapy and trastuzumab: concurrent or sequential?
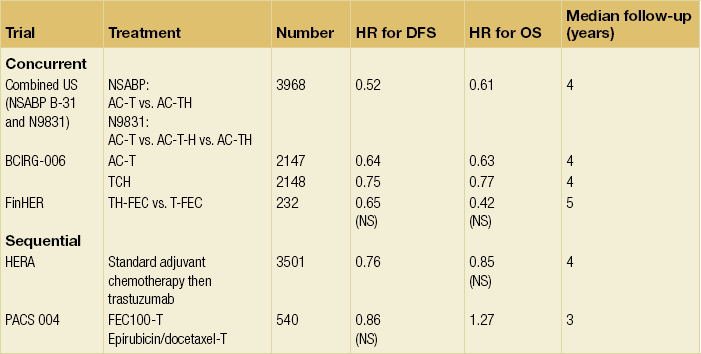
Indirect comparisons of these trials suggest improved benefit when trastuzumab is given concurrently with chemotherapy (NSABP B-31; Intergroup N9831; BCIRG 006; FinHer) rather than when it is administered sequentially (HERA) (Table 12.5). Likewise, a much smaller French PACS 004 trial involving 540 women also assessed trastuzumab given sequentially after chemotherapy and so far this is the only negative trial.108 The inferior results of the PACS trial raises the important issue of whether trastuzumab given sequentially after chemotherapy may be inferior to concurrent administration. The definitive answer to this question comes from the N9831 trial, in which patients were randomised to control (AC followed by weekly paclitaxel, arm A), versus AC followed by weekly paclitaxel and thereafter trastuzumab sequentially (arm B), versus AC followed by weekly paclitaxel with concurrent trastuzumab (arm C).
Duration of trastuzumab
The optimal duration of adjuvant trastuzumab therapy is unknown. Results of the 2-year treatment arm from the HERA trial showed no benefit over 1 year. The preliminary results of the FinHer randomised study suggested similar results with only 9 weeks of trastuzumab treatment combined with nonanthracycline chemotherapy,107 with an increase in 3-year RFS compared with those receiving chemotherapy alone (89% vs. 78%, HR = 0.32; P = 0.02).78 This effect lost statistical significance with longer follow-up, but results may have been influenced by crossover in the control arm once the first results were announced.109 A confirmatory trial, the Synergism or Long Duration (SOLD) study, is currently randomising 3000 patients with HER-2-positive early breast cancer to 9 or 52 weeks of adjuvant trastuzumab in an attempt to clarify this issue.
Small HER-2-positive breast cancers
It is becoming clear that small (less than 10 mm) HER-2-positive cancers have a worse prognosis than similarly small HER-2-negative tumours.110–112 The adjuvant trials of trastuzumab largely excluded patients with tumours of this size, but in the HERA trial patients with small (1.1–2 cm) node-negative breast cancers had a very similar benefit to those with larger tumours from the addition of trastuzumab (HR = 0.53),113 and it is reasonable to expect that this group would derive a similar reduction in risk from adjuvant chemotherapy and trastuzumab. The issue of whether to give a modified, shorter chemotherapy regimen with trastuzumab in this situation remains controversial: a US group has recently carried out a non-randomised phase II study of single agent paclitaxel with trastuzumab in 400 patients with small HER-2-positive breast cancer and results are awaited (clinical trials NCT 00542451).
Cardiotoxicity with trastuzumab
The only significant toxicity associated with trastuzumab (and one that was quite unexpected from preliminary experimental studies) is cardiotoxicity, particularly when given concurrently with or after anthracyclines. Updated cardiac safety data from three of the adjuvant trastuzumab trials were presented in 2010. Independent retrospective review of the NSAPB B-31 and N9831 trials reported that the risk of symptomatic CHF from trastuzumab was low, but that it increased from 0.45% for patients treated with chemotherapy alone to 2% when trastuzumab was added to chemotherapy.114 The majority of patients (86.1%) experienced complete or partial recovery. A second, similar analysis of the HERA trial confirmed a low incidence of cardiac end-points; severe CHF occurred in 0.8% versus 0% and significant decreases in left ventricular ejection fraction (LVEF) occurred in 3.6% versus 0.6% in the trastuzumab and control arms respectively.115 Approximately 80% of patients who suffered a cardiac event achieved ‘acute recovery’, defined as two or more sequential LVEF measurements of 50% or more, after the initial low ejection fraction.
Triple-negative breast cancer
Triple-negative breast cancers are defined as lacking expression of the ER, PgR, and HER-2 receptors.116 They are usually associated with a high histological grade117 and are recognised to have a more aggressive natural history than other breast cancer subtypes. Although considered as one group, they consist of basal cancers, metaplastic cancers and a heterogeneous mixture of other true tumour types. Some triple negatives have low levels of expression of ER rather than being true ER zero. Standard adjuvant anthracycline chemotherapy results in poorer outcomes for triple-negative patients,118 and retrospective data from CALGB 9344 suggest that triple-negative breast cancers specifically benefit from adjuvant taxanes.58 New therapies for this subtype, including the angiogenesis inhibitor bevacizumab, are also being investigated. The value of bevacizumab in breast cancer is, however, far from clear and its use outside clinical trials cannot be justified.
References
1. Goldhirsch, A., Wood, W.C., Coates, A.S., et al, Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–1747. 21709140
2. Li, C.I., Daling, J.R., Malone, K.E., Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21(1):28–34. 12506166
3. Beatson, G. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet. 1896; ii:104–107.
4. Davies, C., Godwin, J., Gray, R., et al, Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. 21802721 The updated meta-analysis of adjuvant tamoxifen confirms that 5 years of adjuvant tamoxifen confers a mortality benefit to women with hormone receptor-positive breast cancer, regardless of age, nodal status and use of adjuvant chemotherapy.
5. Fisher, B., Jeong, J.H., Bryant, J., et al, Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364(9437):858–868. 15351193
6. Peto, R., Davies, C., on Behalf of the ATLAS Collaboration, ATLAS (Adjuvant Tamoxifen, Longer Against Shorter). International randomized trial of 10 versus 5 years of adjuvant tamoxifen among 11 500 women: preliminary results. on Behalf of the. Breast Cancer Res Treat. 2007; 106(Suppl. 1):48. [abstract].
7. Gray, R.G., et al. aTTom (adjuvant Tamoxifen – To offer more?): randomized trial of 10 versus 5 years of adjuvant tamoxifen among 6,934 women with estrogen receptor-positive (ER +) or ER untested breast cancer – preliminary results. J Clin Oncol. 26, 2008. [(May 2 – Suppl; abstract 513)].
8. Baum, M., Budzar, A.U., Cuzick, J., et al, Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–2139. 12090977
9. Thurlimann, B., Keshaviah, A., Coates, A.S., et al, A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747–2757. 16382061
10. Cuzick, J., Sestak, I., Baum, M., et al, Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135–1141. 21087898
11. Regan, M.M., Neven, P., Giobbie-Hurder, A., et al, Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol. 2011;12(12):1101–1108. 22018631 The ATAC and BIG1-98 trials (Refs 8–11) established upfront aromatase inhibitors as superior treatment over tamoxifen for postmenopausal women with hormone receptor-positive breast cancer.
12. Arpino, G., Weiss, H.L., Clark, G.M., et al, Hormone receptor status of a contralateral breast cancer is independent of the receptor status of the first primary in patients not receiving adjuvant tamoxifen. J Clin Oncol. 2005;23(21):4687–4694. 15837971
13. Dowsett, M., Allred, C., and on Behalf of the TransATAC Investigators, Relationship between quantitative ER and PgR expression and HER2 status with recurrence in the ATAC trial and on Behalf of the. San Antonio Breast Cancer Symp 2006; 48 [abstract].
14. Ellis, M.J., Coop, A., Singh, B., et al, Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 2001;19(18):3808–3816. 11559718
15. Smith, I.E., Dowsett, M., Yap, Y.S., et al, Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23(22):5108–5116. 15998903
16. Viale, G., Regan, M., Dell’Orto, P., et al. Central review of ER, PgR and HER-2 in BIG 1-98 evaluating letrozole vs. tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive breast cancer. San Antonio Breast Cancer Symp. 44, 2005. [abstract].
17. Metzger, O., Giobbie-Hurder, A., Mallon, E., et al. Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1-98 trial. Cancer Res. 2012; 72(24 suppl):S1. [S1].
18. Duffy, S., Jackson, T.L., Lansdown, M., et al, The ATAC (‘Arimidex’, Tamoxifen, Alone or in Combination) adjuvant breast cancer trial: first results of the endometrial sub-protocol following 2 years of treatment. Hum Reprod. 2006;21(2):545–553. 16210385
19. Bliss, J.M., Kilburn, L.S., Coleman, R.E., et al, Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol. 2012;30(7):709–717. 22042946
20. Jonat, W., Gnant, M., Boccardo, F., et al, Effectiveness of switching from adjuvant tamoxifen to anastrozole in postmenopausal women with hormone-sensitive early-stage breast cancer: a meta-analysis. Lancet Oncol. 2006;7(12):991–996. 17138220
21. Boccardo, F., Rubagotti, A., Puntoni, M., et al, Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol. 2005;23(22):5138–5147. 16009955
22. Jakesz, R., Jonat, W., Gnant, M., et al, Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366(9484):455–462. 16084253
23. van de Velde, C.J., Rea, D., Seynaeve, C., et al, Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet. 2011;377(9762):321–331. 21247627
24. Saphner, T., Tormey, D.C., Gray, R., Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–2746. 8874335
25. Goss, P.E., Ingle, J.N., Martino, S., et al, A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802. 14551341
26. Goss, P., et al. Outcomes of women who were premenopausal at diagnosis of early stage breast cancer in the NCIC CTG MA17 trial. Cancer Res. 69(Suppl. 3; 24), 2009.
27. Jakesz, R., et al. Extended adjuvant treatment with anastrozole: results from the Austrian Breast and Colorectal Cancer Study Group Trial 6a (ABCSG-6a). Proc Am Soc Clin Oncol. 527, 2005. [abstract].
28. Mamounas, E.P., Jeong, J.H., Wickerham, D.L., et al, Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol. 2008;26(12):1965–1971. 18332472
29. Smith, I.E., Dowsett, M., Yap, Y.S., et al, Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol. 2006;24(16):2444–2447. 16735701
30. Kendall, A., Dowsett, M., Folkerd, E., et al, Caution: Vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol. 2006;17(4):584–587. 16443612
31. Davidson, N.E., O’Neill, A.M., Vukov, A.M., et al, Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101 (E5188). J Clin Oncol. 2005;23(25):5973–5982. 16087950
32. Sverrisdottir, A., Johansson, H., Johansson, U., et al, Interaction between goserelin and tamoxifen in a prospective randomised clinical trial of adjuvant endocrine therapy in premenopausal breast cancer. Breast Cancer Res Treat. 2011;128(3):755–763. 21625929
33. Gnant, M., Mlineritsch, B., Stoeger, H., et al, Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12(7):631–641. 21641868
34. Reeves, G.K., Pirie, K., Beral, V., et al, Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. Br Med J. 2007;335(7630):1134. 17986716
35. Loi, S., Milne, R.L., Friedlander, M.L., et al, Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1686–1691. 16030102
36. Sestak, I., Distler, W., Forbes, J.F., et al, Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28(21):3411–3415. 20547990
37. Pfeiler, G., et al. Impact of body mass index (BMI) on the efficacy of endocrine therapy in postmenopausal breast cancer patients – an analysis of the ABCSC 6 and 6a trial. Cancer Res. 70(24), 2010. [Suppl 2, abstract PD09-05].
38. Ewertz, M., Gray, K.P., Regan, M.M., et al, Obesity and risk of recurrence or death after adjuvant endcrine therapy with letrozole or tamoxifen in the breast international group 1-98 trial. J Cin Oncol. 2012;30(32):3967–3975. 23045588
39. Early Breast Cancer Trialists’ Collaborative Group, Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. 22152853 The 2011 Oxford Overview demonstrates that adjuvant chemotherapy produces a proportional reduction in recurrence and mortality, independently of age, nodal status and hormone receptor status. It also confirms that modern chemotherapy regimens are more effective than older ones.
40. Dowsett, M., Goldhirsch, A., Hayes, D.F., et al, International Web-based consultation on priorities for translational breast cancer research. Breast Cancer Res. 2007;9(6):R81. 18034879
41. van der Hage, J.A., Mieog, J.S., van de Vijver, M.J., et al, Efficacy of adjuvant chemotherapy according to hormone receptor status in young patients with breast cancer: a pooled analysis. Breast Cancer Res. 2007;9(5):R70. 17931406
42. Muss, H.B., Berry, D.A., Cirrincione, C.T., et al, Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–2065. 19439741
43. Muss, H.B., Berry, D.A., Cirrincione, C.T., et al, Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25(24):3699–3704. 17704418
44. Muss, H.B., Woolf, S., Berry, D., et al, Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293(9):1073–1081. 15741529
45. Pinder, M.C., Duan, Z., Goodwin, J.S., et al, Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25(25):3808–3815. 17664460
46. Patt, D.A., Duan, Z., Fang, S., et al, Acute myeloid leukemia after adjuvant breast cancer therapy in older women: understanding risk. J Clin Oncol. 2007;25(25):3871–3876. 17664457
47. Early Breast Cancer Trialists’ Collaborative Group, Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. 15894097
48. Bergh, J., Wiklund, T., Erikstein, B., et al, Tailored fluorouracil, epirubicin, and cyclophosphamide compared with marrow-supported high-dose chemotherapy as adjuvant treatment for high-risk breast cancer: a randomised trial. Scandinavian Breast Group 9401 study. Lancet. 2000;356(9239):1384–1391. 11052580
49. Hutchins, L.F., Green, S.J., Ravdin, P.M., et al, Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. J Clin Oncol. 2005;23(33):8313–8321. 16293862
50. Levine, M.N., Bramwell, V.H., Pritchard, K.I., et al, Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1998;16(8):2651–2658. 9704715
51. Poole, C.J., Earl, H.M., Hiller, L., et al, Epirubicin and cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy for early breast cancer. N Engl J Med. 2006;355(18):1851–1862. 17079759 A number of trials, including the United Kingdom’s NEAT trial, confirm the greater efficacy of adjuvant anthracycline-based chemotherapy regimens in early breast cancer.
52. Henderson, I.C., Berry, D.A., Demetri, G.D., et al, Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–983. 12637460
53. French Adjuvant Study Group, Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol. 2001;19(3):602–611. 11157009
54. Perez, E.A., Suman, V.J., Davidson, N.E., et al, Effect of doxorubicin plus cyclophosphamide on left ventricular ejection fraction in patients with breast cancer in the North Central Cancer Treatment Group N9831 Intergroup Adjuvant Trial. J Clin Oncol. 2004;22(18):3700–3704. 15365066
55. Swain, S.M., Whaley, F.S., Ewer, M.S., Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. 12767102
56. Wood, W.C., Budman, D.R., Korzun, A.H., et al, Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330(18):1253–1259. 8080512
57. Thor, A.D., Berry, D.A., Budman, D.R., et al, erbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancer. J Natl Cancer Inst. 1998;90(18):1346–1360. 9747866
58. Hayes, D.F., Thor, A.D., Dressler, L.G., et al, HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357(15):1496–1506. 17928597
59. Pritchard, K.I., Shepherd, L.E., O’Malley, F.P., et al, HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354(20):2103–2111. 16707747
60. Ravdin, P.M., Green, S., Albain, K.S., et al. Initial report of the SWOG biological correlative study of c-erbB-2 expression as a predictor of outcome in a trial comparing adjuvant CAF T with tamoxifen alone. Proc Am Soc Clin Oncol. 17(97), 1998. [abstract].
61. Slamon, D., Eiermann, W., Robert, N., et al, Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. 21991949
62. Press, M.F., Sauter, G., Buyse, M., et al, Alteration of topoisomerase II-alpha gene in human breast cancer: association with responsiveness to anthracycline-based chemotherapy. J Clin Oncol. 2011;29(7):859–867. 21189395
63. Gianni, L., Norton, L., Wolmark, N., et al, Role of anthracyclines in the treatment of early breast cancer. J Clin Oncol. 2009;27(28):4798–4808. 19687331
64. Ghersi, D., Wilcken, N., Simes, R.J., A systematic review of taxane-containing regimens for metastatic breast cancer. Br J Cancer. 2005;93(3):293–301. 16052223
65. Mamounas, E.P., Bryant, J., Lembersky, B., et al, Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23(16):3686–3696. 15897552
66. Fountzilas, G., Skarlos, D., Dafni, U., et al, Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high-risk operable breast cancer: a randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol. 2005;16(11):1762–1771. 16148021
67. Martin, M., Pienkowski, T., Mackey, J., et al, Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352(22):2302–2313. 15930421
68. Evans, T.R., Yellowlees, A., Foster, E., et al, Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: an Anglo-Celtic cooperative oncology group study. J Clin Oncol. 2005;23(13):2988–2995. 15860854
69. Roche, H., Fumoleau, P., Spielmann, M., et al, Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24(36):5664–5671. 17116941
70. Ellis, P., Barrett-Lee, P., Johnson, L., et al, Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, phase III, randomised controlled trial. Lancet. 2009;373(9676):1681–1692. 19447249
71. De Laurentiis, M., Cancello, G., D’Agostino, D., et al, Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26(1):44–53. 18165639
72. Jones, S.E., Savin, M.A., Holmes, F.A., et al, Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24(34):5381–5387. 17135639
73. Jones, S.E., Holmes, F., O’Shaughnessy, J., et al. Extended follow-up and analysis by age of the US Oncology Adjuvant trial 9735: docetaxel/cyclophosphamide is associated with an overall survival benefit compared to doxorubicin/cyclophosphamide and is well-tolerated in women 65 or older. San Antonio Breast Cancer Symp. 12, 2007. [abstract].
74. Sparano, J.A., Wang, M., Martino, S., et al, Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358(16):1663–1671. 18420499
75. Early Breast Cancer Trialists’ Collaborative Group, Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352(9132):930–942. 9752815
76. Fumoleau, P., Kerbrat, P., Romestaing, P., et al, Randomized trial comparing six versus three cycles of epirubicin-based adjuvant chemotherapy in premenopausal, node-positive breast cancer patients: 10-year follow-up results of the French Adjuvant Study Group 01 trial. J Clin Oncol. 2003;21(2):298–305. 12525522
77. Shulman, L.N., et al. Four vs 6 cycles of doxorubicin and cyclophosphamide or paclitaxel as adjuvant therapy for breast cancer in women with 0–3 positive axillary nodes: CALGB 40101 – a 2 × 2 factorail phase III trial: first results comparing 4 vs 6 cycles of therapy. Cancer Res. 70(24), 2010. [Suppl 2, abstract S6-3].
78. Citron, M.L., Berry, D.A., Cirrincione, C., et al, Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431–1439. 12668651
79. Venturini, M., Del Mastro, L., Aitini, E., et al, Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. J Natl Cancer Inst. 2005;97(23):1724–1733. 16333028
80. Bonilla, L., Ben-Aharon, I., Vidal, L., et al, Dose-dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst. 2010;102(24):1845–1854. 21098761
81. Albain, K.S., Barlow, W.E., Ravdin, P.M., et al, Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9707):2055–2063. 20004966
82. Paik, S., Shak, S., Tang, G., et al, A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. 15591335
83. Paik, S., Tang, G., Shak, S., et al, Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734. 16720680
84. Dowsett, M., Cuzick, J., Wale, C., et al, Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829–1834. 20212256
85. Albain, K.S., Barlow, W.E., Shak, S., et al, Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65. 20005174
86. Barton, S., Zabaglo, L., A’Hern, R., et al, Assessment of the contribution of the IHC4+C score to decision making in clinical practice in early breast cancer. Br J Cancer. 2012;106(11):1760–1765. 22531639
87. Dowsett, M., Nielsen, T.O., A’Hern, R., et al, Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2011;103(22):1656–1664. 21960707
88. van de Vijver, M.J., He, Y.D., van’t Veer, L.J., et al, A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. 12490681
89. van’t Veer, L.J., Dai, H., van de Vijver, M.J., et al, Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. 11823860
90. Diel, I.J., Jaschke, A., Solomayer, E.F., et al, Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol. 2008;19(12):2007–2011. 18664560
91. Diel, I.J., Solomayer, E.F., Costa, S.D., et al, Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339(6):357–363. 9691101
92. Powles, T., Paterson, S., Kanis, J.A., et al, Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol. 2002;20(15):3219–3224. 12149294
93. Saarto, T., Blomqvist, C., Virkkunen, P., et al, Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-year results of a randomized controlled trial. J Clin Oncol. 2001;19(1):10–17. 11134190
94. Paterson, A.H.G., et al. NSABP protocol B-34: a clinical trial comparing adjuvant clodronate vs placebo in early stage breast cancer patients receiving systemic chemotherapy and/or tamoxifen or no therapy – final analysis. Cancer Res. 71(24), 2011. [Suppl, abstract S2-3].
95. Coleman, R.E., Marshall, H., Cameron, D., et al, Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365(15):1396–1405. 21995387
96. de Boer, R., et al. Long-term survival outcomes among postmenopausal women with hormone receptor-positive early breast cancer receiving adjuvant letrozole and zoledronic acid: 5-year follow-up of ZO-FAST. Cancer Res. 71(24), 2011. [Suppl, abstract S1-3].
97. Mobus, V., et al. GAIN (German Adjuvant Intergroup Node Positive) study: a phase III multicenter trial to compare dose dense, dose intense ETC vs EC-TX and ibandronate vs observation in patients with node-positive primary breast cancer – first interim efficacy analysis. Cancer Res. 71(24), 2011. [Suppl, abstract S2-4].
98. Slamon, D.J., Clark, G.M., Wong, S.G., et al, Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. 3798106
99. Slamon, D.J., Godolphin, W., Jones, L.A., et al, Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. 2470152
100. Finn, R.S., Slamon, D.J., Monoclonal antibody therapy for breast cancer: herceptin. Cancer Chemother Biol Response Modif 2003; 21:223–233. 15338747
101. Slamon, D.J., Leyland-Jones, B., Shak, S., et al, Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. 11248153
102. Marty, M., Cognetti, F., Maraninchi, D., et al, Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–4274. 15911866
103. Perez, E.A., Romond, E.H., Suman, V.J., et al, Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–3373. 21768458
104. Gianni, L., Dafni, U., Gelber, R.D., et al, Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12(3):236–244. 21354370
105. Perez, E.A., Suman, V.J., Davidson, N.E., et al, Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol. 2011;29(34):4491–4497. 22042958 These three randomised trials (along with Ref. 60) of adjuvant concurrent trastuzumab with chemotherapy demonstrate a reduction in recurrence and death in women with HER-2-positive breast cancer. All trials published updated results in 2011, confirming that these benefits are maintained with longer follow-up.
106. Joensuu, H., Kellokumpu-Lehtinen, P.L., Bono, P., et al, Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809–820. 16495393
107. Goldhirsch, A., Piccart-Gebhart, M.J., Procter, M., et al. HERA Trial: 2 years versus 1 year of trastuzumab after adjuvant chemotherapy in women with HER2-positive early breast cancer at 8 years of median follow up. Cancer Res. 2012; 72(24 suppl):S5. [S2].
108. Spielmann, M., Roché, H., Delozier, T., et al, Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27(36):6129–6134. 19917839
109. Joensuu, H., et al. FinXX final 5-year analysis: results of the randomised, open-label, phase III trial in medium-to-high risk early breast cancer. Cancer Res. 70(24), 2010. [Suppl 2, abstract S4-1].
110. Curigliano, G., Viale, G., Bagnardi, V., et al, Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27(34):5693–5699. 19884553
111. Park, Y.H., Kim, S.T., Cho, E.Y., et al, A risk stratification by hormonal receptors (ER, PgR) and HER-2 status in small (< or = 1 cm) invasive breast cancer: who might be possible candidates for adjuvant treatment? Breast Cancer Res Treat. 2009;119(3):653–661. 19957028
112. Chavez-MacGregor, M., Gonzalez-Angulo, A.M., HER2-neu positivity in patients with small and node-negative breast cancer (pT1a,b,N0,M0): a high risk group? Clin Adv Hematol Oncol. 2009;7(9):591–598. 20020671
113. Untch, M., Gelber, R.D., Jackisch, C., et al, Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann Oncol. 2008;19(6):1090–1096. 18296421
114. Russell, S.D., Blackwell, K.L., Lawrence, J., et al, Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28(21):3416–3421. 20530275
115. Procter, M., Suter, T.M., de Azambuja, E., et al, Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28(21):3422–3428. 20530280
116. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363(20):1938–48.
117. Reis-Filho, J.S., Tutt, A.N., Triple negative tumours: a critical review. Histopathology. 2008;52(1):108–118. 18171422
118. Tan, D.S., Marchió, C., Jones, R.L., et al, Triple negative breast cancer: molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res Treat. 2008;111(1):27–44. 17922188
119. Cheang, M.C., Chia, S.K., Voduc, D., et al, Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–750. 19436038