Chapter 5 The respiratory system
This chapter deals with common respiratory symptoms, and the examination of the respiratory system.
The respiratory history
Presenting symptoms (Table 5.1)
Cough and sputum
| Major symptoms |
| Cough |
| Sputum |
| Haemoptysis |
| Dyspnoea (acute, progressive or paroxysmal) |
| Wheeze |
| Chest pain |
| Fever |
| Hoarseness |
| Night sweats |
A differential diagnosis of cough based on its character is shown in Table 5.2 and on its duration is shown in Table 5.3.
TABLE 5.2 Differential diagnosis of cough based on its character
| Origin | Character | Causes |
| Naso-pharynx/larynx | Throat clearing, chronic | Postnasal drip, acid reflux |
| Larynx | Barking, painful, acute or persistent | Laryngitis, pertussis (whooping cough), croup |
| Trachea | Acute, painful | Tracheitis |
| Bronchi | Intermittent, sometimes productive, worse at night | Asthma |
| Worse in morning | Chronic obstructive pulmonary disease (COPD) | |
| With blood | Bronchial malignancy | |
| Lung parenchyma | Dry then productive | Pneumonia |
| Chronic, very productive | Bronchiectasis | |
| Productive, with blood | Tuberculosis | |
| Irritating and dry, persistent | Interstitial lung disease | |
| Worse on lying down, sometimes with frothy sputum | Pulmonary oedema | |
| ACE inhibitors | Dry, scratchy, persistent | Medication-induced |
TABLE 5.3 Differential diagnosis of cough based on its duration
| Acute cough (<3 weeks duration): differential diagnosis |
ACE = angiotensin-converting enzyme.
COPD = chronic obstructive pulmonary disease.
PND = paroxysmal nocturnal dyspnoea.
Patients’ descriptions of their cough may be helpful. In children, a cough associated with inflammation of the epiglottis may have a muffled quality and cough related to viral croup is often described as ‘barking’. Cough caused by tracheal compression by a tumour may be loud and brassy. Cough associated with recurrent laryngeal nerve palsy has a hollow sound because the vocal cords are unable to close completely; this has been described as a bovine cough. A cough that is worse at night is suggestive of asthma or heart failure, while coughing that comes on immediately after eating or drinking may be due to incoordinate swallowing or oesophageal reflux or, rarely, a tracheo-oesophageal fistula.
Haemoptysis
Haemoptysis (coughing up of blood) can be a sinister sign of lung disease (Table 5.4) and must always be investigated. It must be distinguished from haematemesis (vomiting of blood) and from nasopharyngeal bleeding (Table 5.5).
TABLE 5.4 Causes (differential diagnosis) of haemoptysis and typical histories
| Respiratory | |
| Bronchitis | Small amounts of blood with sputum |
| Bronchial carcinoma | Frank blood, history of smoking, hoarseness |
| Bronchiectasis | Large amounts of sputum with blood |
| Pneumonia | Fever, recent onset of symptoms, dyspnoea |
| (The above four account for about 80% of cases) | |
| Pulmonary infarction | Pleuritic chest pain, dyspnoea |
| Cystic fibrosis | Recurrent infections |
| Lung abscess | Fever, purulent sputum |
| Tuberculosis (TB) | Previous TB, contact with TB, HIV-positive status |
| Foreign body | History of inhalation, cough, stridor |
| Goodpasture’s* syndrome | Pulmonary haemorrhage, glomerulonephritis, antibody to basement membrane antigens |
| Wegener’s granulomatosis | History of sinusitis, saddle-nose deformity |
| Systemic lupus erythematosus | Pulmonary haemorrhage, multi-system involvement |
| Rupture of a mucosal blood vessel after vigorous coughing | |
| Cardiovascular | |
| Mitral stenosis (severe) | |
| Acute left ventricular failure | |
| Bleeding diatheses | |
Note: Exclude spurious causes, such as nasal bleeding or haematemesis.
* Ernest W Goodpasture (1886–1960), pathologist at Johns Hopkins, Baltimore. He described this syndrome in 1919.
TABLE 5.5 Features distinguishing haemoptysis from haematemesis and nasopharyngeal bleeding
| Favours haemoptysis | Favours haematemesis | Favours nasopharyngeal bleeding |
| Mixed with sputum | Follows nausea | Blood appears in mouth |
| Occurs immediately after coughing | Mixed with vomitus; follows dry retching |
Ask how much blood has been produced. Mild haemoptysis usually means less than 20 mL in 24 hours. It appears as streaks of blood discolouring sputum. Massive haemoptysis is more than 250 mL of blood in 24 hours and represents a medical emergency. Its most common causes are carcinoma, cystic fibrosis, bronchiectasis and tuberculosis.
Breathlessness (dyspnoea) (Table 5.6)
The awareness that an abnormal amount of effort is required for breathing is called dyspnoea. It can be due to respiratory or cardiac disease, or lack of physical fitness. Careful questioning about the timing of onset, severity and pattern of dyspnoea is helpful in making the diagnosis (Questions box 5.2 and Table 5.7).1 The patient may be aware of this
| Respiratory |
| 1 Airways disease |
Questions box 5.2
Questions to ask the breathless patient
! denotes symptoms for the possible diagnosis of an urgent or dangerous problem.
TABLE 5.7 Differential diagnosis of dyspnoea based on time course of onset
| Seconds to minutes—favours: |
| Hours or days—favours: |
| Weeks or longer—favours: |
only on heavy exertion or have much more limited exercise tolerance. Dyspnoea can be graded from I to IV based on the New York Heart Association classification:
It is more useful, however, to determine the amount of exertion that actually causes dyspnoea—that is, the distance walked or the number of steps climbed.
The association of dyspnoea with wheeze suggests airways disease, which may be due to asthma or chronic obstructive pulmonary disease (COPD) (Table 5.8). The duration and variability of the dyspnoea are important. Dyspnoea that worsens progressively over a period of weeks, months or years may be due to interstitial lung disease (ILD). Dyspnoea of more rapid onset may be due to an acute respiratory infection (including bronchopneumonia or lobar pneumonia) or to pneumonitis (which may be infective or secondary to a hypersensitivity reaction). Dyspnoea that varies from day to day or even from hour to hour suggests a diagnosis of asthma. Dyspnoea of very rapid onset associated with sharp chest pain suggests a pneumothorax (Table 5.9). Dyspnoea that is described by the patient as inability to take a breath big enough to fill the lungs and associated with sighing suggests anxiety. Dyspnoea that occurs on moderate exertion may be due to the combination of obesity and a lack of physical fitness (a not uncommon occurrence).
TABLE 5.8 Characteristics of chronic obstructive pulmonary disease (COPD)
| History |
| Examination |
TABLE 5.9 Differential diagnosis of dyspnoea of sudden onset based on other features
| Presence of pleuritic chest pain—favours: |
| Absence of chest pain—favours: |
| Presence of central chest pain—favours: |
| Presence of cough and wheeze—favours: |
Chest pain
Chest pain due to respiratory disease is usually different from that associated with myocardial ischaemia (page 35). The pleura and central airways have pain fibres and may be the source of respiratory pain. Pleural pain is characteristically pleuritic in nature: sharp and made worse by deep inspiration and coughing. It is typically localised to one area of the chest. It may be of sudden onset in patients with lobar pneumonia, pulmonary embolism and infarction or pneumothorax, and is often associated with dyspnoea. The sudden onset of pleuritic chest pain and dyspnoea is an urgent diagnostic problem, as all three of these conditions may be life-threatening if not treated promptly.
Other presenting symptoms
Sleep apnoea is an abnormal increase in the periodic cessation of breathing during sleep. Patients with obstructive sleep apnoea (OSA) (where airflow stops during sleep for periods of at least 10 seconds and sometimes for over 2 minutes, despite persistent respiratory efforts) typically present with daytime somnolence, chronic fatigue, morning headaches and personality disturbances. Very loud snoring may be reported by anyone within earshot. These patients are often obese and hypertensive. The Epworth sleepiness scale is a way of quantifying the severity of sleep apnoea (Table 5.10).
| ‘How easily would you fall asleep in the following circumstances?’* |
* A normal score is between 0 and 9. Severe sleep apnoea scores from 11 to 20.
Patients with central sleep apnoea (where there is cessation of inspiratory muscle activity) may also present with somnolence but do not snore excessively (Table 5.11).
TABLE 5.11 Abnormal patterns of breathing
| Type of breathing | Cause(s) |
| 1 Sleep apnoea—cessation of airflow for more than 10 seconds more than 10 times a night during sleep | Obstructive (e.g. obesity with upper airway narrowing, enlarged tonsils, pharyngeal soft tissue changes in acromegaly or hypothyroidism) |
| 2 Cheyne-Stokes* breathing—periods of apnoea (associated with reduced level of consciousness) alternate with periods of hyperpnoea (lasts 30 s on average and is associated with agitation). This is due to a delay in the medullary chemoreceptor response to blood gas changes | |
| 3 Kussmaul’s breathing (air hunger)— deep, rapid respiration due to stimulation of the respiratory centre | Metabolic acidosis (e.g. diabetes mellitus, chronic renal failure) |
| 4 Hyperventilation, which results in alkalosis and tetany | Anxiety |
| 5 Ataxic (Biot†) breathing—irregular in timing and depth | Brainstem damage |
| 6 Apneustic breathing—a post-inspiratory pause in breathing | Brain (pontine) damage |
| 7 Paradoxical respiration—the abdomen sucks inwards with inspiration (it normally pouches outwards due to diaphragmatic descent) | Diaphragmatic paralysis |
* John Cheyne (1777–1836), Scottish physician who worked in Dublin, described this in 1818. William Stokes (1804–1878), Irish physician, described it in 1854.
Treatment
It is important to find out what drugs the patient is using (Table 5.12), how often they are taken and whether they are inhaled or swallowed. The patient’s previous and current medications may give a clue to the current diagnosis. Bronchodilators and inhaled steroids are prescribed for COPD and asthma. A patient’s increased use of bronchodilators suggests poor control of asthma and the need for review of treatment. Chronic respiratory disease, including sarcoidosis, hypersensitivity pneumonias and asthma, may have been treated with oral steroids. Oral steroid use may predispose to tuberculosis or pneumocystis pneumonia. Patients with chronic lung conditions like cystic fibrosis or bronchiectasis will often be very knowledgeable about their treatment and can describe the various forms of physiotherapy that are essential for keeping their airways clear.
| Cough |
Past history
One should always ask about previous respiratory illness, including pneumonia, tuberculosis or chronic bronchitis, or abnormalities of the chest X-ray that have previously been reported to the patient. Many previous respiratory investigations may have been memorable, such as bronchoscopy, lung biopsy and video-assisted thoracoscopy. Spirometry, with or without challenge testing for asthma, may have been performed. Many severe asthmatics perform their own regular peak flow testing (page 128). Ask about the results of any of these investigations. Patients with the acquired immunodeficiency syndrome (AIDS) have a high risk of developing Pneumocystis jiroveci (carinii) pneumonia and indeed other chest infections, including tuberculosis.
Occupational history
In no system are the patient’s present and previous occupations of more importance (Table 5.13).2 A detailed occupational history is essential. The occupational lung diseases or pneumoconioses cause interstitial lung disease by damaging the alveoli and small airways. Prolonged exposure to substances whose use is now heavily restricted is usually required. Cigarette smoking has an additive effect for these patients. These occupational conditions are now rare, and the most common occupational lung disease is asthma.
| Substance | Disease |
| Coal | Coal worker’s pneumoconiosis |
| Silica | Silicosis |
| Asbestos | Asbestosis |
| Talc | Talcosis |
One must ask about exposure to dusts in mining industries and factories (e.g. asbestos, coal, silica, iron oxide, tin oxide, cotton, beryllium, titanium oxide, silver, nitrogen dioxide, anhydrides). Heavy exposure to asbestos can lead to asbestosis (Table 5.14), but even trivial exposure can result in pleural plaques or mesothelioma (malignant disease of the pleura). The patient may be unaware that his or her occupation involved exposure to dangerous substances; for example, factories making insulating cables and boards very often used asbestos until 25 years ago. Asbestos exposure can result in the development of asbestosis, mesothelioma or carcinoma of the lung up to 30 years later. Relatives of people working with asbestos may be exposed when handling work clothes.
TABLE 5.14 Possible occupational exposure to asbestos
| Asbestos mining, including relatives of miners |
| Naval dockyard workers and sailors—lagging of pipes |
| Builders—asbestos in fibreboard (particles are released during cutting or drilling) |
| Factory workers—manufacture of fibro-sheets, brake linings, some textiles |
| Building maintenance workers—asbestos insulation |
| Building demolition workers |
| Home renovation |
Exposure to organic dusts can cause a local immune response to organic antigens and result in allergic alveolitis. Within a few hours of exposure, patients develop flu-like symptoms. These often include fever, headache, muscle pains, dyspnoea without wheeze and dry cough. The culprit antigens may come from mouldy hay, humidifiers or air conditioners, among others (Table 5.15).
TABLE 5.15 Allergic alveolitis—sources
| Bird fancier’s lung | Bird feathers and excreta |
| Farmer’s lung | Mouldy hay or straw (Aspergillus fumigatus) |
| Byssinosis | Cotton or hemp dust |
| Cheese worker’s lung | Mouldy cheese (Aspergillus clavatus) |
| Malt worker’s lung | Mouldy malt (Aspergillus clavatus) |
| Humidifier fever | Air-conditioning (thermophilic Actinomycetes) |
Social history
A smoking history must be routine, as it is the major cause of COPD and lung cancer (see Table 1.2, page 6). It also increases the risk of spontaneous pneumothorax and of Goodpasture’s syndrome. It is necessary to ask how many packets of cigarettes a day a patient has smoked and how many years the patient has smoked. An estimate should be made of the number of packet-years of smoking. Remember that this is based on 20-cigarette packets and that packets of cigarettes are getting larger; curiously, most manufacturers now make packets of 30 or 35. More recently, giant packets of 50 have appeared. These are too large to fit into pockets and must be carried in the hands as a constant reminder to the patient of his or her addiction. Occupation may further affect cigarette smokers; for example, asbestos workers who smoke are at an especially high risk of lung cancer. Passive smoking is now regarded as a significant risk for lung disease and the patient should be asked about exposure to other people’s cigarette smoke at home and at work.
Many respiratory conditions are chronic, and may interfere with the ability to work and exercise and interfere with normal family life. In some cases involving occupational lung disease there may be compensation matters affecting the patient. Ask about these problems and whether the patient has been involved in a pulmonary rehabilitation programme. Housing conditions may be inappropriate for a person with a limited exercise tolerance or an infectious disease. An inquiry about the patient’s alcohol consumption is important. The drinking of large amounts of alcohol in binges can sometimes result in aspiration pneumonia, and alcoholics are more likely to develop pneumococcal or Klebsiella pneumonia. Intravenous drug users are at risk of lung abscess and drug-related pulmonary oedema. Sexual orientation or history of intravenous drug use may be related to an increased risk of HIV infection and susceptibility to infection. Such information may influence the decision about whether to advise treatment at home or in hospital.
The respiratory examination
Examination anatomy
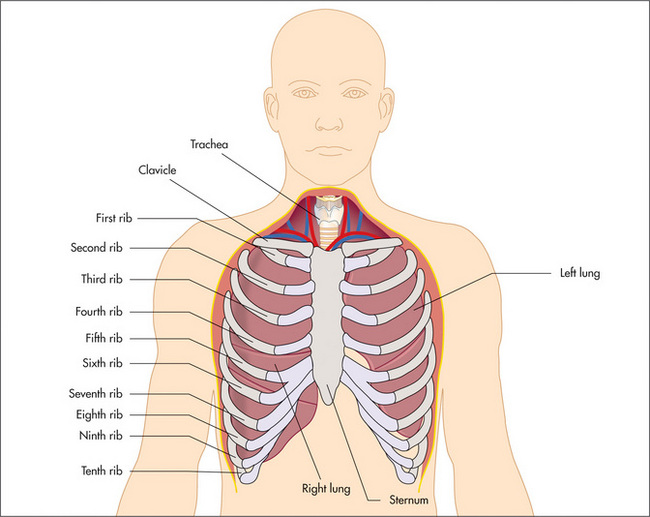
The position of the heart with its apex pointing to the left means that the left lung is smaller than the right and has only two lobes, which are separated by the oblique fissure. The right lung has both horizontal (upper) and oblique (lower) fissures dividing it into three lobes (Figure 5.1).
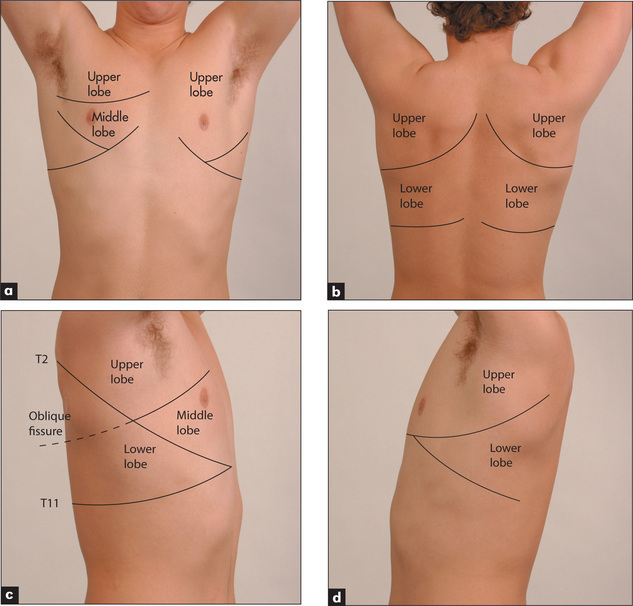
Figure 5.1 Lobes of the lung
(a) Anterior. (b) Posterior. (c) Lobes of the right lung. (d) Lobes of the left lung. Refer to Figure 5.15, page 137, for a list of the segments in each lobe.
The muscles of respiration are the diaphragm upon which the bases of the lungs rest and the intercostal muscles. During inspiration the diaphragm flattens and the intercostal muscles contract to elevate the ribs. Intrathoracic pressure falls as air is forced under atmospheric pressure into the lungs. Expiration is a passive process resulting from elastic recoil of the muscles. Abnormalities of lung function or structure may change the normal anatomy and physiology of respiration, for example as a result of over-inflation of the lungs (COPD, page 133). Muscle and neurological diseases can also affect muscle function adversely, and abnormalities of the control of breathing in the respiratory centres of the brain in the pons and medulla can interfere with normal breathing patterns.
During the respiratory examination, keep in mind the surface anatomy (Figure 5.1) of the lungs and try to decide which lobes are affected.
Positioning the patient
The patient should be undressed to the waist.3 Women should wear a gown or have a towel or some clothing to cover their breasts when the front of the chest is not being examined. If the patient is not acutely ill, the examination is easiest to perform with him or her sitting over the edge of the bed or on a chair.
General appearance
Characteristic signs of chronic obstructive pulmonary disease (COPD)a
Look to see whether the accessory muscles of respiration are being used. This is a sign of an increase in the work of breathing, and COPD is an important cause. These muscles include the sternomastoids, the platysma and the strap muscles of the neck. Characteristically the accessory muscles cause elevation of the shoulders with inspiration, and aid respiration by increasing chest expansion. Contraction of the abdominal muscles may occur in expiration in patients with obstructed airways. Patients with severe COPD often have indrawing of the intercostal and supraclavicular spaces during inspiration. This is due to a delayed increase in lung volume despite the generation of large negative pleural pressures.
In some cases, the pattern of breathing is diagnostically helpful (Table 5.11). Look for pursed-lips breathing, which is characteristic of patients with severe COPD. This manoeuvre reduces the patient’s breathlessness, possibly by providing continuous positive airways pressure and helping to prevent airways collapse during expiration. Patients with severe COPD may feel more comfortable leaning forward with their arms on their knees. This position compresses the abdomen and pushes the diaphragm upwards. This partly restores its normal domed shape and improves its effectiveness during inspiration. Increased diaphragmatic movements may cause downward displacement of the trachea during inspiration—tracheal tug.
Sputum
Sputum should be inspected. Careful study of the sputum is an essential part of the physical examination. The colour, volume and type (purulent, mucoid or mucopurulent), and the presence or absence of blood, should be recorded.
Stridor
Obstruction of the larynx or trachea (the extra-thoracic airways) may cause stridor, a rasping or croaking noise loudest on inspiration. This can be due to a foreign body, a tumour, infection (e.g. epiglottitis) or inflammation (Table 5.16). It is a sign that requires urgent attention.
| Sudden onset (minutes) |
The hands
As usual, examination in detail begins with the hands.
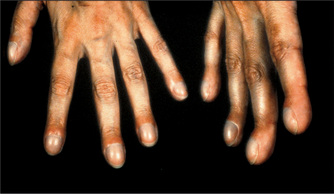
Clubbing
Look for clubbing, which is due to respiratory disease in up to 80% of cases (Figure 5.3, Table 4.9 on page 51). An uncommon but important association with clubbing is hypertrophic pulmonary osteoarthropathy (HPO). HPO is characterised by the presence of periosteal inflammation at the distal ends of long bones, the wrists, the ankles, the metacarpal and the metatarsal bones. There is swelling and tenderness over the wrists and other involved areas. Rarely HPO may occur without clubbing. The causes of HPO include primary lung carcinoma and pleural fibromas. Remember, chronic bronchitis and emphysema do not cause clubbing. It is important to note that clubbing does not occur as a result of COPD.
Flapping tremor (asterixis)
Ask the patient to dorsiflex the wrists with the arms outstretched and to spread out the fingers. A flapping tremor with a 2- to 3-second cycle may occur with severe carbon dioxide retention, usually due to severe COPD.4 The problem is an inability to maintain a posture. Asterixisb can also be demonstrated by asking the patient to protrude the tongue or lift the leg and keep the foot dorsiflexed. However, this is a late and unreliable sign and can also occur in patients with liver or renal failure. Patients with severe carbon dioxide retention may be confused, and typically have warm peripheries and a bounding pulse.
The face
Sinusitis is suggested by tenderness over the sinuses on palpation. If acute sinusitis is suspected, a torch can be used to transilluminate the frontal and maxillary sinuses.5 A torch is placed in the patient’s mouth and the sinuses examined in a dark room. Normal transillumination generally excludes sinusitis. Complete opacification suggests sinusitis but partial opacification is less helpful. The torch should then be used to look for purulent discharge in the pharynx.
Look at the patient’s face for the red, leathery, wrinkled skin of the smoker.6 There may be facial plethora or cyanosis if superior vena caval obstruction is present. Look for the characteristics of obstructive sleep apnoea (see above).
Inspect the eyes for evidence of the rare Horner’sc syndrome (a constricted pupil, partial ptosis and loss of sweating), which can be due to an apical lung carcinoma (Pancoastd tumour) compressing the sympathetic nerves in the neck. There may be skin changes on the face that suggest scleroderma or connective tissue disease.
The trachea
The position of the trachea is most important, and time should be spent establishing it accurately. From in front of the patient the forefinger of the right hand is pushed up and backwards from the suprasternal notch until the trachea is felt (Figure 5.4). If the trachea is displaced to one side, its edge rather than its middle will be felt and a larger space will be present on one side than the other. Slight displacement to the right is fairly common in normal people. This examination is uncomfortable for the patient, so you must be gentle.
Significant displacement of the trachea suggests, but is not specific for, disease of the upper lobes of the lung (Table 5.17).
| 1 Towards the side of the lung lesion |
The chest
The chest should be examined anteriorly and posteriorly by inspection, palpation, percussion and auscultation.3 Compare the right and left sides during each part of the examination.
Inspection
Shape and symmetry of chest
When the anteroposterior (AP) diameter is increased compared with the lateral diameter, the chest is described as barrel-shaped (Figure 5.5). An increase in the AP diameter compared with the lateral diameter (the thoracic ratio) beyond 0.9 is abnormal and is seen often in patients with severe asthma or emphysema. It is not always a reliable guide to the severity of the underlying lung disease and may be present in normal elderly people. Severe kyphoscoliosis is a cause of asymmetrical chest deformity.
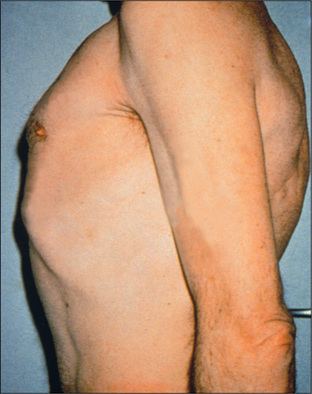
Figure 5.5 Barrel chest
From McDonald FS, ed., Mayo Clinic images in internal medicine, with permission. © Mayo Clinic Scientific Press and CRC Press.
A pigeon chest (pectus carinatum) is a localised prominence (an outward bowing of the sternum and costal cartilages)(Figure 5.6). It may be a manifestation of chronic childhood respiratory illness, in which case it is thought to result from repeated strong contractions of the diaphragm while the thorax is still pliable. It also occurs in rickets.
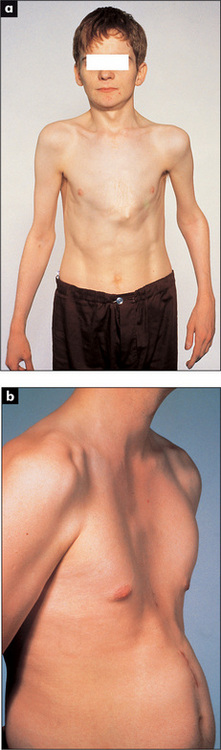
Figure 5.6 (a) Funnel chest (pectus excavatum); (b) Pigeon chest (pectus carinatum)
From Mir MA, Atlas of Clinical Diagnosis, 2nd edn. Edinburgh: Saunders, 2003, with permission.
A funnel chest (pectus excavatum) is a developmental defect involving a localised depression of the lower end of the sternum (Figure 5.6). The problem is usually an aesthetic one, but in severe cases lung capacity may be restricted.
Harrison’s sulcuse is a linear depression of the lower ribs just above the costal margins at the site of attachment of the diaphragm. It can result from severe asthma in childhood, or rickets.
Lesions of the chest wall may be obvious. Look for scars from previous thoracic operations, or from chest drains for a previous pneumothorax or pleural effusion. Surgical removal of a lung (pneumonectomy) or of the lobe of a lung (lobectomy) leaves a long diagonal posterior scar on the thorax. The presence of three 2–3 cm scars suggests previous video-assisted thoracoscopic surgery, which can be performed to biopsy lymph nodes or carry out lung reduction surgery or pleurodesis. Thoracoplasty causes severe chest deformity; this operation was performed for tuberculosis and involved removal of a large number of ribs on one side of the chest to achieve permanent collapse of the affected lung. It is no longer performed because of the availability of effective antituberculous chemotherapy.f Radiotherapy may cause erythema and thickening of the skin over the irradiated area. There is sharp demarcation between abnormal and normal skin. There may be small tattoo marks indicating the limits of the irradiated area. Signs of radiotherapy usually indicate that the patient has been treated for carcinoma of the lung, breast or, less often, for lymphoma.
Prominent veins may be seen in patients with superior vena caval obstruction. It is important to determine the direction of blood flow (page 166).
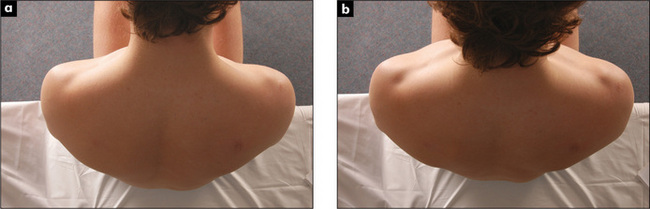
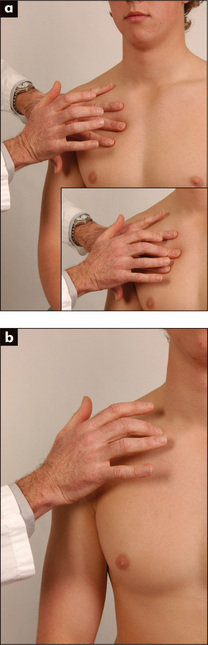
Movement of the chest wall should be noted. Look for asymmetry of chest wall movement anteriorly and posteriorly. Assessment of expansion of the upper lobes is best achieved by inspection from behind the patient, looking down at the clavicles during moderate respiration (Figure 5.7). Diminished movement indicates underlying lung disease. The affected side will show delayed or decreased movement. For assessment of lower lobe expansion, the chest should be inspected posteriorly.
Reduced chest wall movement on one side may be due to localised lung fibrosis, consolidation, collapse, pleural effusion or pneumothorax. Bilateral reduction of chest wall movement indicates a diffuse abnormality such as COPD or diffuse interstitial lung disease. Unilateral reduced chest excursion or splinting may be present when patients have pleuritic chest pain or injuries such as rib fractures.
Palpation
Chest expansion
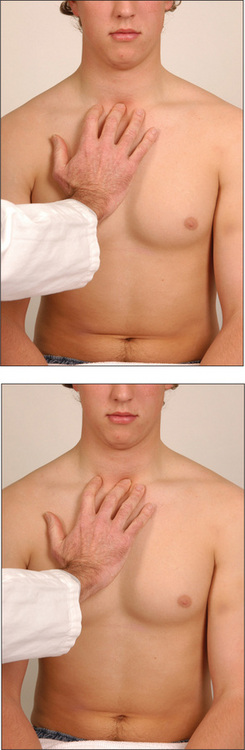
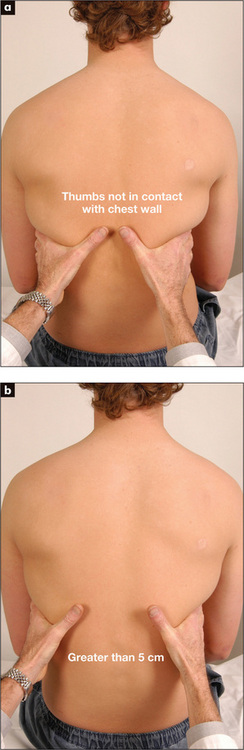
Place the hands firmly on the chest wall with the fingers extending around the sides of the chest. The thumbs should almost meet in the middle line and should be lifted slightly off the chest so that they are free to move with respiration (Figure 5.8). As the patient takes a big breath in, the thumbs should move symmetrically apart at least 5 cm. Reduced expansion on one side indicates a lesion on that side. The causes have been discussed above.
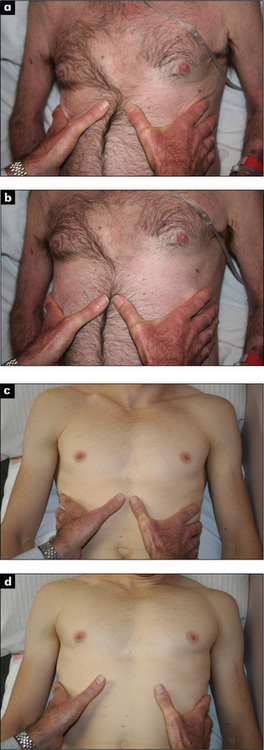
If COPD is suspected, Hoover’s signg may be sought (Figure 5.9). The examiner places the hands along the costal margins with the thumbs close to the xiphisternum. Normally inspiration causes them to separate, but the overinflated chest of the COPD patient cannot expand in this way and the diaphragm pulls the ribs and the examiner’s thumbs closer together7 (positive LR 4.2).8
Apex beat
When the patient is lying down, establishing the position of the apex beat may be helpful (page 60), as displacement towards the side of the lesion can be caused by collapse of the lower lobe or by localised interstitial lung disease (ILD). Movement of the apex beat away from the side of the lung lesion can be caused by pleural effusion or tension pneumothorax. The apex beat is often impalpable in a chest which is hyperexpanded secondary to chronic obstructive pulmonary disease.
Vocal (tactile) fremitus
Palpate the chest wall with the palm of the hand while the patient repeats ‘ninety-nine’. The front and back of the chest are each palpated in two comparable positions, with the palm of one hand on each side of the chest. In this way differences in vibration on the chest wall can be detected. This can be a difficult sign to interpret, with considerable inter-observer variability, and it is no longer a routine part of the examination. It depends on the recognition of changes in vibration conducted to the examiner’s hands while the patient speaks. Practice is needed to appreciate the difference between normal and abnormal. Vocal fremitus is more obvious in men because of their lower-pitched voices. It may be absent in normal people (high-pitched voice or thick chest wall). It is only abnormal if different on one side from the other. The causes of change in vocal fremitus are the same as those for vocal resonance (page 127).
Ribs
Gently compress the chest wall anteroposteriorly and laterally. Localised pain suggests a rib fracture, which may be secondary to trauma or may be spontaneous as a result of tumour deposition, bone disease or sometimes the result of severe and prolonged coughing. Tenderness over the costochondral junctions suggests the diagnosis of costochondritis as the cause of chest pain.
Percussion
Percussion of symmetrical areas of the anterior, posterior and axillary regions is necessary (Figure 5.10). Percussion in the supraclavicular fossa over the apex of the lung and direct percussion of the clavicle with the percussing finger are a traditional part of the examination. For percussion posteriorly, the scapulae should be moved out of the way by asking the patient to move the elbows forward across the front of the chest; this rotates the scapulae anteriorly.
The feel of the percussion note is as important as its sound. The note is affected by the thickness of the chest wall, as well as by underlying structures. Percussion over a solid structure, such as the liver or a consolidated or collapsed area of lung, produces a dull note. Percussion over a fluid-filled area, such as a pleural effusion, produces an extremely dull (stony dull) note. Percussion over the normal lung produces a resonant note and percussion over hollow structures, such as the bowel or a pneumothorax, produces a hyperresonant note (Good signs guide 5.1).
| Sign | Positive LR | Negative LR |
| Dullness—pneumonia | 3.0 | NS |
| Hyperresonance—COPD | 5.1 | NS |
NS = not significant.
COPD = chronic obstructive pulmonary disease.
From McGee S, Evidence-based physical diagnosis, 2nd edn. St Louis: Saunders, 2007.
Auscultation
Breath sounds
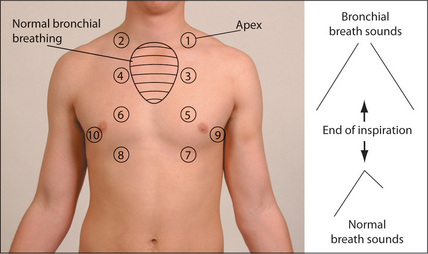
Using the diaphragm of the stethoscope, one should listen to the breath sounds in the areas shown in Figure 5.11.9–11 It is important to compare each side with the other. Remember to listen high up into the axillae and, using the bell of the stethoscope applied above the clavicles, to listen to the lung apices. A number of observations must be made while auscultating and, as with auscultation of the heart, different parts of the cycle must be considered. Listen for the quality of the breath sounds, the intensity of the breath sounds, and the presence of additional (adventitious) sounds.
Quality of breath sounds
Bronchial breath sounds are present when turbulence in the large airways is heard without being filtered by the alveoli, producing a different quality. Bronchial breath sounds have a hollow, blowing quality. They are audible throughout expiration and there is often a gap between inspiration and expiration. The expiratory sound has a higher intensity and pitch than the inspiratory sound. Bronchial breath sounds are more easily remembered than described. They are audible in normal people, posteriorly over the right upper chest where the trachea is contiguous with the right upper bronchus. They are heard over areas of consolidation, as solid lung conducts the sound of turbulence in main airways to peripheral areas without filtering. Causes of bronchial breath sounds are shown in Table 5.18.
| Common |
Note: The large airways must be patent.
Occasionally breath sounds over a large cavity have an exaggerated bronchial quality. This very hollow or amphoric sound has been likened to that heard when air passes over the top of a hollow jar (Greek amphoreus).
Added (adventitious) sounds
There are two types of added sounds—continuous (wheezes) and interrupted (crackles).
Wheezes must be distinguished from stridor (page 118), which sounds very similar to wheeze but is louder over the trachea and is always inspiratory (wheezes usually occur in expiration—the majority—but can occur in both inspiration and expiration).
Interrupted non-musical sounds are best called crackles.12,13 There is a lot of confusion about the naming of these sounds, perhaps as a result of mistranslations of Laënnec. Some authors describe low-pitched crackles as rales and high-pitched ones as crepitations, but others do not make this distinction. The simplest approach is to call all these sounds crackles, but also to describe their timing and pitch. Crackles are sometimes present in normal people but these crackles will always clear with coughing.
The timing of crackles is of great importance. Early inspiratory crackles (cease before the middle of inspiration) suggest disease of the small airways, and are characteristic of COPD.12 The crackles are heard only in early inspiration and are of medium coarseness. They are different from those heard in left ventricular failure, which occur later in the respiratory cycle.
Late or pan-inspiratory crackles suggest disease confined to the alveoli. They may be fine, medium or coarse in quality. Fine crackles have been likened to the sound of hair rubbed between the fingers, or to the sound Velcro makes when pulled apart—they are typically caused by interstitial lung disease (pulmonary fibrosis). Characteristically, more crackles are heard in each inspiration when they are due to fibrosis—up to 14 compared with 1 to 4 for COPD and 4 to 9 for cardiac failure. As ILD becomes more severe the crackles extend earlier into inspiration and are heard further up the chest.hMedium crackles are usually due to left ventricular failure. Here the presence of alveolar fluid disrupts the function of the normally secreted surfactant. Coarse crackles are characteristic of pools of retained secretions and have an unpleasant gurgling quality. They tend to change with coughing, which also has an unpleasant gurgling quality. Bronchiectasis is a common cause, but any disease that leads to retention of secretions may produce these features. (See Good signs guide 5.2.)
| Sign | Positive LR | Negative LR |
| Crackles | ||
| Pulmonary fibrosis in asbestos workers | 5.9 | 0.2 |
| Pneumonia patients with cough and fever | 2.0 | 0.8 |
| Early inspiratory crackles | ||
| Detecting COPD | 14.6 | NS |
| Severe COPD | 20.8 | 0.1 |
| Unforced (audible during breathing at rest) wheezing | ||
| Detecting COPD | 6.0 | NS |
NS = not significant. COPD = chronic obstructive pulmonary disease.
From McGee S, Evidence-based physical diagnosis, 2nd edn. St Louis: Saunders, 2007.
Vocal resonance
Auscultation over the chest while a patient speaks gives further information about the lungs’ ability to transmit sounds. Over normal lung, the low-pitched components of speech are heard with a booming quality and high-pitched components are attenuated. Consolidated lung, however, tends to transmit high frequencies so that speech heard through the stethoscope takes on a bleating quality (called aegophony by Laënnec14—from Greek aix ‘goat’, phone ‘voice’). When a patient with aegophony says ‘e’ as in ‘bee’ it sounds like ‘a’ as in ‘bay’.
Increased vocal resonance is a helpful sign in confirming consolidation but may not be necessary as a routine. Ask the patient to say ‘ninety-nine’ while you listen over each part of the chest. Over consolidated lung the numbers will become clearly audible, while over normal lung the sound is muffled. If vocal resonance is present, bronchial breathing is likely to be heard (Table 5.18). Sometimes vocal resonance is increased to such an extent that whispered speech is distinctly heard; this is called whispering pectoriloquy.
If a very localised abnormality is found at auscultation, try to determine the lobe and approximately which segment or segments are involved (Figure 5.1, page 116).
The heart
Lay the patient down at 45 degrees and measure the jugular venous pressure for evidence of right heart failure (page 58). Next examine the praecordium. It is important to pay close attention to the pulmonary component of the second heart sound (P2). This is best heard at the second intercostal space on the left. It should not be louder than the aortic component, best heard at the right second inter-costal space. If the P2 is louder (and especially if it is palpable), pulmonary hypertension should be strongly suspected. There may be signs of right ventricular failure or hypertension. Pulmonary hypertensive heart disease (cor pulmonale) may be due to COPD, ILD, pulmonary thromboembolism, marked obesity, sleep apnoea or severe kyphoscoliosis.
The abdomen
Palpate the liver for ptosis,i due to emphysema, or for enlargement from secondary deposits of tumour in cases of lung carcinoma.
Other
Pemberton’sj sign
Ask the patient to lift the arms over the head and wait for one minute.15 Note the development of facial plethora, cyanosis, inspiratory stridor and non-pulsatile elevation of the jugular venous pressure. This occurs in superior vena caval obstruction.
Bedside assessment of lung function
Forced expiratory time
Physical examination can be complemented with an estimate of the forced expiratory time (FET).16 Measure the time taken by a patient to exhale forcefully and completely through the open mouth after taking a maximum inspiration. The normal forced expiratory time is 3 seconds or less. Note any audible wheeze or cough. An increased FET indicates airways obstruction. The combination of a significant smoking history and an FET of 9 seconds or more is predictive of COPD (positive LR 9.6).8 A peak flow meter or spirometer, however, will provide a more accurate measurement of lung function.
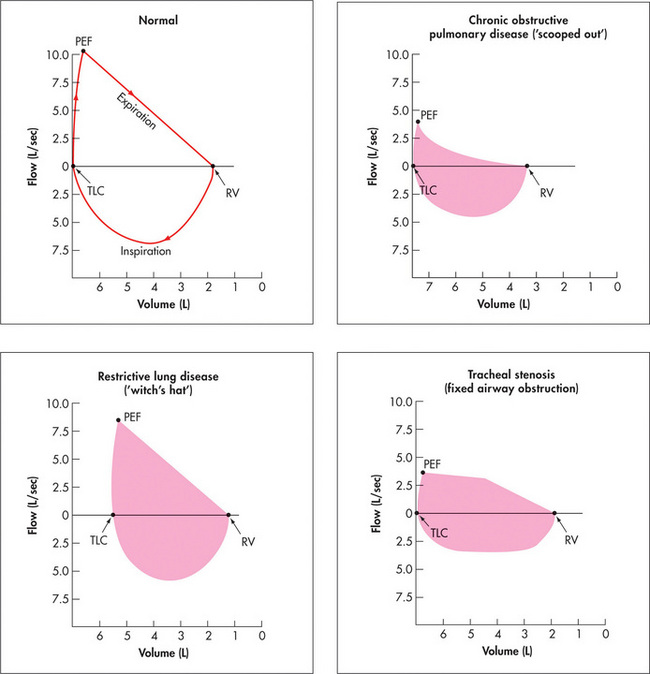
Flow volume curve
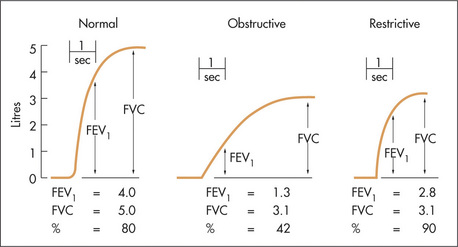
As a part of spirometric assessment, the flow volume curve may be measured using a portable electronic device. This measures expiratory and inspiratory flow as a function of exhaled volume rather than against time. It is a simple and reproducible test easily performed in the respiratory laboratory or at the bedside. The FVC, FEV1 and various flow measurements (e.g. peak flow) can be calculated from the curve (Figure 5.13).
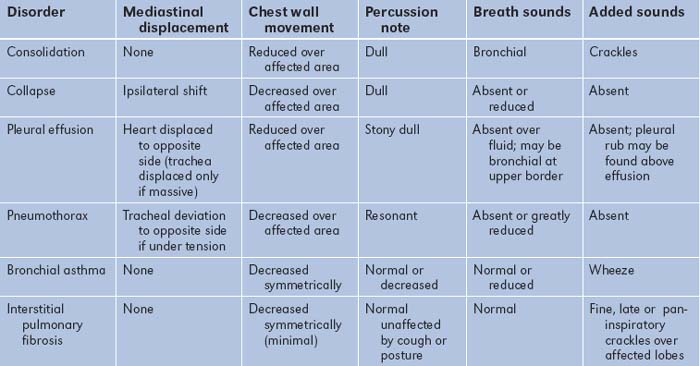
Correlation of physical signs and respiratory disease (Table 5.19)
Consolidation (lobar pneumonia)
Pneumonia is defined as inflammation of the lung which is characterised by exudation into the alveoli. X-ray changes of new shadowing in one or more lung segments (lobes) are present. Pneumonia is now classified as:
This classification allows prediction of the likely pathogens and assists in the choice of antibiotics for treatment. The signs of lobar pneumonia are characteristic and are referred to clinically as consolidation.17
Signs
| Sign | Positive LR | Negative LR |
| General appearance | ||
| Cachexia | 4.0 | NS |
| Abnormal mental state | 2.2 | NS |
| Vital signs | ||
| Temperature >37.8°C | 2.2 | 0.7 |
| Respiratory rate >28/minute | 2.2 | 0.8 |
| Heart rate >100 beats/minute | 1.6 | 0.7 |
| Lung findings | ||
| Percussion dullness | 3.0 | NS |
| Reduced breath sounds | 2.3 | 0.8 |
| Bronchial breath sounds | 3.3 | NS |
| Aegophony | 4.1 | NS |
| Crackles | 2.0 | 0.8 |
| Wheezes | NS | NS |
NS = not significant.
From McGee S, Evidence-based physical diagnosis, 2nd edn. St Louis: Saunders, 2007.
Atelectasis (Collapse)
Pleural effusion
This is a collection of fluid in the pleural space. Note that pleural collections consisting of blood (haemothorax), chyle (chylothorax) or pus (empyema) have specific names, and are not called pleural effusions although the physical signs are similar.
Signs
Causes
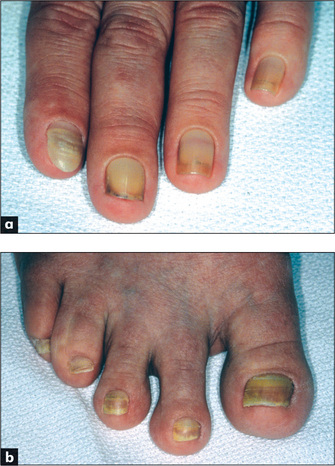
Yellow nail syndrome
This is a rare condition which is caused by hypoplasia of the lymphatic system. The nails are thickened and yellow (Figure 5.14) and there is separation of the distal nail plate from the nail bed (onycholysis). It may be associated with a pleural effusion and bronchiectasis, and usually with lymphoedema of the legs.
Pneumothorax
Leakage of air from the lung or a chest wall puncture into the pleural space causes a pneumothorax.
Causes
Bronchiectasis
Signs
Most likely during an exacerbation of the condition.
Bronchial asthma
Signs
Chronic obstructive pulmonary disease (COPD, chronic airflow limitation)
This represents a spectrum of abnormalities: from predominantly emphysema, where there is pathologically an increase beyond normal in the size of the air spaces distal to the terminal bronchioles, to chronic bronchitis, where there is mucous gland hypertrophy, increased numbers of goblet cells and hypersecretion of mucus in the bronchial tree resulting in a chronic cough and sputum. Chronic obstructive pulmonary disease limitation does not cause clubbing or haemoptysis. Fifty per cent of patients with chronic bronchitis have emphysema, so there is often considerable overlapping of signs.18
The diagnosis can often be made on the basis of three findings:
If two or three of these are present, the positive LR of COPD is 25.7.
Signs
| Sign | Positive LR | Negative LR |
| Hoover’s sign | 4.2 | 0.5 |
| Absent cardiac dullness, left sternal border | 11.8 | NS |
| Early inspiratory crackles | 14.6 | NS |
| Unforced wheeze | 2.8 | 0.8 |
| Greatly reduced breath sounds | 10.2 | — |
| Forced expiratory time: | ||
| <3s | 0.2 | — |
| 3–9s | NS | — |
| >9s | 4.1 | — |
From McGee, S, Evidence-based physical diagnosis, 2nd edn. St Louis: Saunders, 2007.
Chronic bronchitis
Signs
The signs are the result of bronchial hypersecretion and airways obstruction.
Interstitial lung disease (ILD)
Diffuse fibrosis of the lung parenchyma impairs gas transfer and causes ventilation–perfusion mismatching. This fibrosis may be the result of inflammation (alveolitis and interstitial inflammation) or granulomatous disease (Table 5.20). It has often no known cause (idiopathic interstitial fibrosis) or is secondary to a disease of unknown aetiology (e.g. sarcoidosis, connective tissue disease). It can result from inhalation of mineral dusts (focal fibrosis), replacement of lung tissue following disease which damages the lungs (e.g. aspiration pneumonia, tuberculosis). Collagen diseases and vasculitis are important causes.
| Secondary to alveolitis (previously called fibrosing alveolitis) |
| Unknown cause |
SLE = systemic lupus erythematosus.
Signs
Causes
Tuberculosis
Primary tuberculosis
A Ghonn focus with hilar lymphadenopathy occurs usually in children.
Usually no abnormal chest signs are found, but segmental collapse, due to bronchial obstruction by the hilar lymph nodes, occasionally occurs. Erythema nodosum (page 191) is an important associated sign, but is rare.
Mediastinal compression
Signs
Carcinoma of the lung
Respiratory and chest signs
Apical (Pancoast) tumour
Horner’s syndrome, recurrent laryngeal nerve palsy (hoarseness), C8/T1 nerve root lesion.
Non-metastatic extrapulmonary manifestations
Sarcoidosis
Pulmonary embolism (PE)
Embolism to the lungs often occurs without symptoms or signs. One should always entertain this diagnosis if there has been sudden and unexplained dyspnoea when a patient has risk factors for embolism (Table 5.21). Pleuritic chest pain and haemoptysis occur only when there is infarction. Syncope or the sudden onset of severe substernal pain can occur with massive embolism.
| 1 Previous PE |
| 2 Immobilisation (long aeroplane flight or especially after surgery) |
| 3 Known clotting-factor abnormalities |
| 4 Known malignancy |
Note: A firm diagnosis cannot be made on the symptoms and signs alone.
The chest X-ray
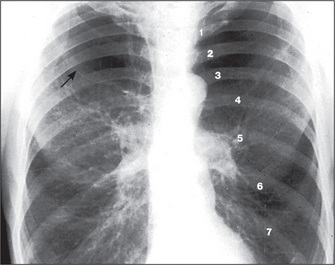
The radiological appearance of a normal lung, with the lung segments labelled, is shown in Figure 5.15.
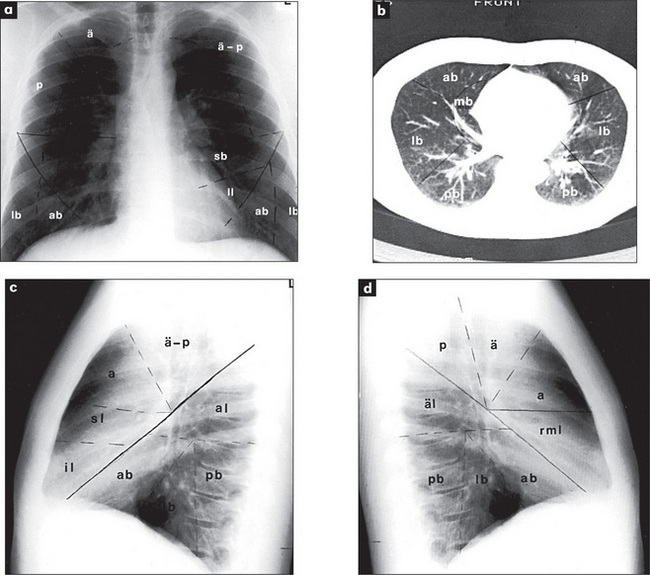
Figure 5.15 Lung segments
Right upper lobe: ä = apical segment; a = anterior segment, p = posterior segment.
Right middle lobe (rml): m = medial segment, l = lateral segment.
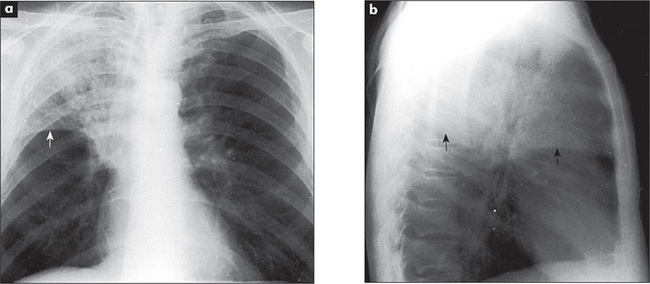
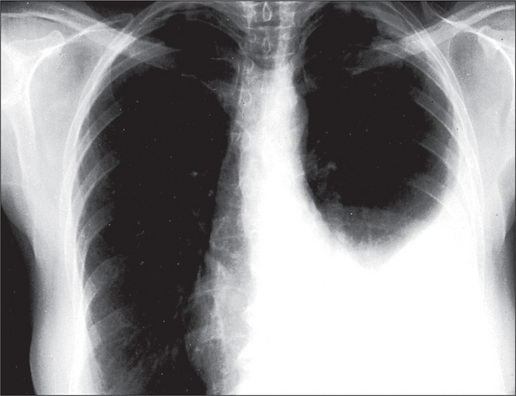
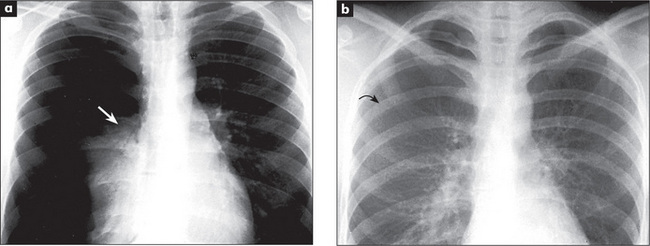
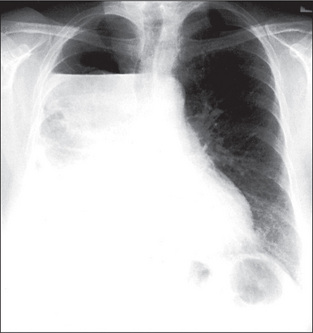
The radiological changes of consolidation, pleural effusion, pneumothorax and hydropneumothorax are shown in Figures 5.16 to 5.19.
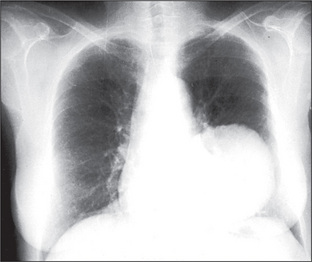
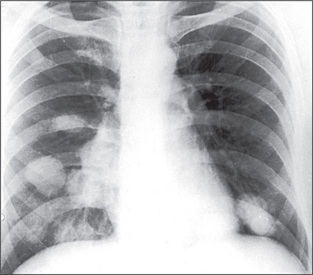
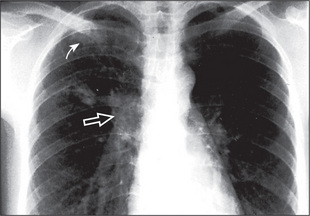
A pulmonary mass is obvious in Figure 5.20, while multiple metastases are seen in Figure 5.21. Primary tuberculosis is shown in Figure 5.22, and Figure 5.23 illustrates the features of emphysema.
Summary
The respiratory examination: a suggested method (Figure 5.24)
Ask the patient to undress to the waist (provide women with a gown), and to sit over the side of the bed. In the clinic or surgery the examination can often be performed with the patient sitting on a chair. While standing back to make your usual inspection (does the patient appear breathless while walking into the room or undressing?), ask if sputum is available for inspection. Purulent sputum always indicates respiratory infection, and a large volume of purulent sputum is an important clue to bronchiectasis. Haemoptysis is also an important sign. Look for dyspnoea at rest and count the respiratory rate. Note any paradoxical inward motion of the abdomen during inspiration (diaphragmatic paralysis). Look for use of the accessory muscles of respiration, and any intercostal indrawing of the lower ribs anteriorly (a sign of emphysema). General cachexia should also be noted.
Figure 5.24 Respiratory system
Return to the front of the chest. Inspect again for chest deformity, distended veins, radiotherapy changes and scars. Palpate the supraclavicular nodes carefully. Then proceed with percussion and auscultation as before. Listen high up in the axillae too. Before leaving the chest feel the axillary nodes and examine the breasts (Chapter 14).
1. Schmitt BP, Kushner MS, Wiener SL. The diagnostic usefulness of the history of the patient with dyspnea. J Gen Intern Med. 1986;1:386-393. History alone was correct three out of four times when deciding the cause of dyspnoea in defined circumstances
2. Anonymous. Obtaining an exposure history. Agency for Toxic Substances and Disease Registry. United States Department of Health and Human Services, Public Health Service, Atlanta, Georgia. Am Fam Phys. 1993;48:483-491.
3. Mulrow CD, Dolmatch BL, Delong ER, et al. Observer variability in the pulmonary examination. J Gen Intern Med. 1986;1:364-367. Documents the poor reliability of many respiratory signs
4. Conn HO. Asterixis: Its occurrence in chronic obstructive pulmonary disease, with a. commentary on its general mechanism. N Engl J Med. 1958;259:564-569.
5. Williams JWJnr, Simel DL, Roberts LR, Samsa GP. Clinical evaluation for sinusitis; making the diagnosis by history and physical examination. Ann Intern Med. 1992;117:705-710. The doctor’s impression of the likelihood of sinusitis was superior to findings of a purulent nasal discharge, history of maxillary toothache, poor response to nasal decongestants and abnormal transillumination
6. Model D. Smokers’ face: an underrated clinical sign? BMJ. 1985;291:1760-1762. A red face with leathery skin and excessive wrinkling, associated with a gaunt look, may help identify up to half of chronic smokers.
7. Garcia-Pachon E. Paradoxical movement of the lateral rib margin (Hoover sign) for detecting obstructive airway disease. Chest. 2002;12:651-655.
8. McGee S. Evidence-based physical diagnosis, 2nd edn. St Louis: Saunders; 2007.
9. Kraman SS. Lung sounds for the clinician. Arch Intern Med. 1986;146:411-412. Describes the physiological evidence for many auscultatory findings
10. Earis J. Lung sounds. Thorax. 1992;47:671-672.
11. Forgacs P. The functional basis of lung sounds. Chest. 1978;73:399-405.
12. Nath AR, Caple LH. Respiratory crackles: early and late. Thorax. 1974;29:223-227. Severe airways obstruction causes crackles in the first half of inspiration. In contrast, late crackles are not associated with airways obstruction
13. Walshaw MJ, Nissa M, Pearson MG, et al. Expiratory lung crackles in patients with fibrosing alveolitis. Chest. 1990;97:407-409. Inspiratory crackles are usually considered more important, but this report suggests that expiratory crackles occur intermittently in fibrosing alveolitis, usually in mid-expiration
14. Shapira JD. About egophony. Chest. 1995;108:865-867.
15. Wallace C, Siminoski K. The Pemberton sign. Ann Intern Med. 1996;125:568-569. Discusses the mechanism of this useful sign, which is present when a large retrosternal goitre compresses the thoracic inlet
16. Schapira RM, Schapira MM, et al. The value of the forced expiratory time in the physical diagnosis of obstructive airways disease. JAMA. 1993;270:731-736. In patients with chronic obstructive airways who have a low pretest probability, an appropriate low-end cut-off is required (e.g. 3 seconds)
17. Metlay JP, Kappor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. Jama. 1997;278:1440-1445. Normal vital signs and normal chest auscultation substantially reduce the likelihood of pneumonia, but a chest X-ray is required for a firm diagnosis
18. Holloman DR, Simmel DL, Goldberg JS. Diagnosis of obstructive airways disease from the clinical examination. J Gen Intern Med. 1993;8:63-68. A history of smoking, self-reported wheezing and wheezing detected at auscultation combined had a high predictive value for chronic obstructive airways disease. The forced expiratory time added little additional information to these predictors
Bourke SJ. Lecture notes on respiratory disease, 6th edn. Oxford: Blackwell; 2003.
Forgacs P. The functional basis of pulmonary sounds. Chest. 1978;73:399-405.
Hansen-Flaschen J, Nordberg J. Clubbing and hypertrophic osteoarthropathy. Clin Chest Med. 1987;8:287-298.
James DG, Studdy PR. Color atlas of respiratory diseases, 2nd edn. Chicago: Mosby-Year Book; 1992.
Light RW. Pleural effusion. N Eng J Med. 2002;346:1971-1977.
a This condition has undergone many changes in nomenclature, and it is pleasing to think that chest physicians have something to keep them occupied. The term COPD encompasses emphysema, chronic bronchitis, chronic obstructive lung disease (COLD) and chronic airflow limitation (CAL). It now seems quite firmly established. The diagnosis of COPD depends on clinical, radiographic and lung function assessment. There may be components of what used to be called chronic bronchitis and emphysema.
b The word is derived from the Greek word sterigma which means to support, and refers to a flapping tremor.
c Johann Horner (1831–1886), professor of ophthalmology in Zurich, described this syndrome in 1869.
d Henry Khunrath Pancoast (1875–1939), professor of roentgenology, University of Pennsylvania, described this in 1932.
e Edward Harrison (1766–1838), British general practitioner in Lincolnshire, described this deformity in rickets in 1798. The sign has also been ascribed to Edwin Harrison (1779–1847), a London physician.
f It also probably didn’t help!
g Charles Hoover (1865–1927), professor of medicine in Cleveland from 1907. He also described Hoover’s test for non-organic limb weakness.
h Expiratory crackles may also occur with lung fibrosis.13
i From the Greek word for falling, this was once mostly applied to the eyelid but now seems accepted as a description of the displacement of any organ.
j Hugh Pemberton (1891–1956), physician, Liverpool, UK.
k Joe Vincent Meigs (1892–1963), Professor of Gynaecology at Harvard, described this in 1937.
l The formal definition of an exudate is that the fluid has at least one of the following (Light’s) criteria; 1. fluid protein/serum protein >0.5, 2. pleural fluid LDH/serum LDH >0.6, 3. pleural fluid LDH >2/3 normal upper limit of LDH in serum. The fluid is otherwise a transudate.
m Henrik Samuel Conrad Sjögren (1899–1986), Stockholm ophthalmologist. He described the syndrome in 1933.
n Anton Ghon (1866–1936), Austrian pathologist and Professor of Anatomical Pathology in Prague. He described the lesion in 1912.
o First described by William Hunter (1718–83; brother of John Hunter) in a patient with a syphilitic aortic aneurysm.
p ML Eaton, 20th century American physician, and EH Lambert (b. 1915), American neurologist.