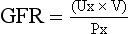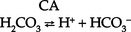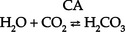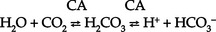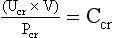The Renal System
SYSTEMWIDE ELEMENTS
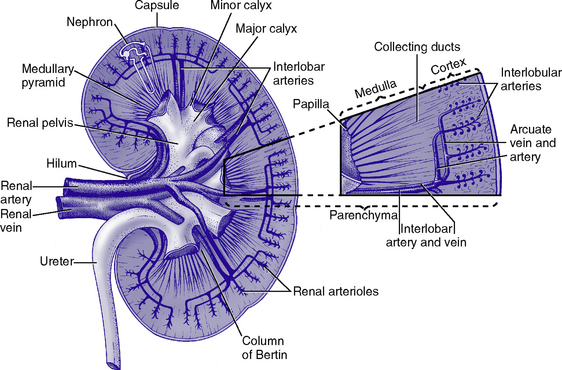
a. Anatomic structures of the kidney: Most humans are born with two kidneys; a small number are born with one. The kidneys are located in the retroperitoneal space above the waist (Figure 5-1).
(a) Metabolically active portion of the kidney, where aerobic metabolism occurs and where ammonia and glucose are formed
(b) Metabolic needs more than satisfactorily met by an abundant oxygen supply
(c) Contains all glomeruli and portions of the proximal and distal tubules
(a) Region of active glycolytic metabolism; supplies energy for active transport
(b) Metabolism demands high oxygen consumption, yet oxygen supply limited
(c) Plays role in concentration of urine
(d) Composed of 6 to 10 renal pyramids, formed by collecting ducts and extending into the renal pelvis
(e) Site of the deepest part of the long loops of Henle and the collecting ducts of the nephron
iii. Renal sinus, pelvis, and collecting system
(a) Papillae: Rounded projections of renal tissue located at the apical ends of the renal pyramids positioned with the base facing the cortex and the apices facing the renal pelvis; the apical portion opens into the minor calices
(b) Corticomedullary junction: Point of division between the cortex and the medulla formed by the base of the pyramids
(c) Renal lobe: Composed of a pyramid plus the surrounding cortical tissue
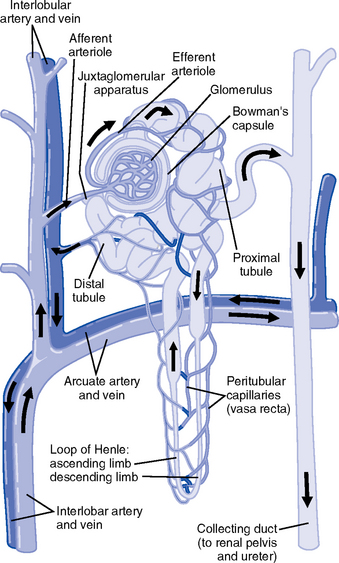
iv. Nephron: Anatomic microscopic structure (Figure 5-2)
(a) Structural and functional unit of the kidney
(b) Approximately 1 million in each kidney
(c) Compensates for a significant degree of nephron destruction by
(d) Types of nephrons, based on location and function
(1) Cortical nephrons located in the outer region of the cortex; contain short loops of Henle with a low capacity for sodium reabsorption
(2) Juxtamedullary nephrons located in the inner cortex adjacent to the medulla; have long loops of Henle that penetrate deep into the medulla and have a greater capacity for concentration of urine because they are sodium-retaining nephrons
(e) Functional segments of the nephron
a) Bowman’s capsule: Specialized portion of the proximal tubule that supports the glomerulus
b) Glomerulus: Capillary bed with semipermeable membrane
1) Normally permeable to water, electrolytes, nutrients, wastes; relatively impermeable to large protein molecules, albumin, erythrocytes
2) Composed of three cellular layers: Fenestrated endothelial layer, basement membrane, and epithelium podocyte cells that contribute to characteristic semipermeability of this membrane
3) Characteristics of cellular layers: Endothelial cells contain fenestrations 50 to 100 nm wide, favoring the movement of water and solute; remaining layers are less porous, with openings 1500 nm thick, which may explain the impedance of macromolecules
i. Glomerular ultrafiltration is the first step in the formation of urine
(a) Characteristics of glomerular filtrate
(1) Normal: Protein-free and red blood cell (RBC)–free, plasmalike substance with a specific gravity (SG) of 1.010. Filtrate contains water, electrolytes, glucose, amino acids, acid-base components, wastes, and other solutes. Pharmaceutical agents can also be included in filtrate.
(2) Small and middle-sized molecules (up to 60 to 75 kd): Pass freely through the glomerular membrane (i.e., inulin 5 kd, albumin >60 kd)
(3) Abnormal: Increased permeability of the glomerular membrane allows erythrocytes and protein to be filtered into urine. SG of urine may artificially increase because of the presence of protein or glucose.
(4) Increased osmotically active substances (glucose, urea): Can cause diuresis
(b) Filtration is determined by the glomerular pressure and presence of a normal semipermeable glomerular membrane
(1) Glomerular hydrostatic pressure is 50 mm Hg and favors filtration; this capillary hydrostatic pressure reflects cardiac output
(2) Colloid osmotic pressure of 25 mm Hg and Bowman’s capsule hydrostatic pressure of 10 mm Hg oppose hydrostatic pressure and thus oppose filtration
a) Colloid osmotic pressure results from oncotic pressure of plasma proteins in the glomerular blood supply
b) Bowman’s capsule pressure reflects renal interstitial pressure
(3) Net filtration pressure is derived using the following formula:
| Glomerular hydrostatic pressure (facilitates): | +50 mm Hg |
| Colloid osmotic pressure (opposes): | −25 mm Hg |
| Bowman’s capsule pressure (opposes): | −10 mm Hg |
| Net pressure favoring filtration: | +15 mm Hg |
(c) Glomerular filtration rate (GFR)
(1) Clinical assessment tool to determine renal function
(2) Definition: Volume of plasma cleared of a given substance per minute (may be determined by using endogenous creatinine)
(4) Normal adult GFR: 125 ml/min or 180 L/day
(5) Normal adult urine volume: 1 to 2 L/day, reflecting greater than 99% reabsorption of filtrate
a) Changes in glomerular hydrostatic pressure
1) Secondary to changes in systemic blood pressure (BP)
2) Caused by variation in afferent or efferent arteriolar tone; increased afferent arteriole resistance decreases GFR; increased efferent arteriole tone increases GFR
b) Alterations in oncotic pressure due to dehydration, hypoproteinemia, or hyperproteinemia
c) Alterations in Bowman’s capsule pressure due to urinary tract or nephron destruction, or interstitial edema of kidney
ii. Tubular functions of reabsorption, secretion, and excretion comprise the following steps in urine formation (Figure 5-3):
(a) Conversion of 180 L of plasma filtered per day to 1 to 2 L of excreted urine
(b) Absorption and secretion by two processes:
(1) Passive mechanisms: Solute moves without the expenditure of metabolic energy
a) Diffusion: Solute following either a concentration or an electrical gradient
1) A solute moves from a solution of higher concentration through a semipermeable membrane to a solution of lower concentration
2) Selectivity of the membrane’s permeability and electrical gradient determine diffusion of the solute
3) The electrical gradient causes a solute to passively migrate to the oppositely charged compartment (e.g., Na+, a positive ion, migrates to a negatively charged compartment, whereas Cl−, a negative ion, moves toward a positively charged compartment)
a) Ion transport requires energy; adenosine triphosphate [ATP]) permits ions to move against the concentration gradient
b) Maximal tubular transport capacity: Active reabsorption mechanisms in the tubule have limited capacity for reabsorption of certain substances such as glucose. Plasma glucose level of 375 mg/min (transport maximum [Tm]), results in no excretion in urine; plasma glucose level above 375 mg/min results in glucose excretion in urine. Tm for glucose can vary from one nephron to another; as a result, glucose can sometimes spill into the urine at lower serum levels.
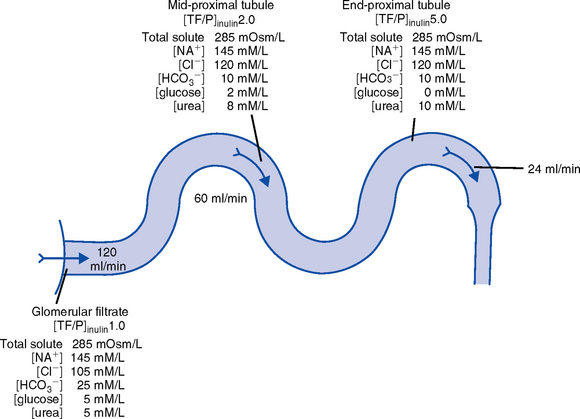
(c) Proximal convoluted tubule
(1) Reabsorbs 60% to 80% of filtrate, which remains isotonic to plasma
(2) Major function is active reabsorption of sodium chloride (NaCl) with passive reabsorption of water
(3) Also reabsorbs glucose, amino acids, phosphates (PO43−), uric acid, potassium ion (K+)
(4) Regulates acid-base balance through reabsorption of carbonic acid (H2CO3) and bicarbonate (HCO3−) and secretion of hydrogen ions (H+)
(5) Secretes K+, ammonium ion (NH4+), organic acids, bases, foreign substances (e.g., drugs)
(1) Variations in length depend on the type of nephron (i.e., juxtamedullary with long loops or cortical with short loops)
a) Descending segment, the thin limb, is permeable to water and impermeable to Na+
b) Ascending segment, the thick limb, has active NaCl pump and is impermeable to water; target site for loop diuretics
(3) Major function is concentration or dilution of urine, accomplished by a countercurrent mechanism that maintains hyperosmolar concentration in the interstitium of the renal medulla
(1) Receives hyposmotic (or hypotonic) urine from the ascending loop of Henle
(3) Water permeability here is controlled by antidiuretic hormone (ADH); Na+ reabsorption is determined by aldosterone
(1) Receives urine, which is isotonic to plasma, from the distal convoluted and collecting tubules
(2) Functions with the distal convoluted tubule; affected by ADH and aldosterone
(3) Final urinary adjustments for composition, tonicity, and volume made here before urine enters the renal pelvis and progresses to the ureter and bladder
2. Renal hemodynamics: Normal blood flow patterns
i. Specialized arrangement of renal blood vessels reflects interdependence of blood supply with kidney function
(a) Kidney: Aorta→segmented renal arteries→interlobar artery→arcuate artery→interlobular artery→(nephron)→interlobular vein→arcuate vein→interlobar vein→renal vein→inferior vena cava
(b) Nephron: Afferent arteriole→glomerular capillary→efferent arteriole→ peritubular capillary→vasa recta adjacent to tubules→interlobular vein→renal vein→inferior vena cava
iii. Juxtaglomerular apparatus: Site of renin synthesis
(a) Specialized cells composed of juxtaglomerular cells and macula densa
(1) Juxtaglomerular granular cells: Smooth muscle cells containing granules of inactive renin
(2) Macula densa: Portion of the distal tubule making contact with afferent arterioles of its respective glomerulus
(b) Responds to arterial BP in afferent and efferent arterioles and to sodium in distal tubule
b. Renal blood flow (RBF) parameters
i. Kidney receives 20% to 25% of cardiac output or 1200 ml/min, which translates to flow rate of 4 ml/g/min to the kidney
ii. Oxygen extraction from renal cells is high, but the amount is not significant enough to account for flow rate; rather, the flow is required to support normal renal function
(a) Cortex: Metabolically active region, receives most (80%) of the blood supply
(b) Medulla: Site of anaerobic metabolism, receives 20% of blood supply
ii. Nephrons: Receive 600 to 650 ml/min of renal plasma flow
d. Intrarenal autoregulation: General principles
i. Mean arterial pressure (MAP) is maintained in a range of 80 to 180 mm Hg to prevent large changes in GFR
ii. Major site of autoregulation is the afferent arteriole
iii. Increase in the renal arterial pressure causes afferent vasoconstriction; decrease causes efferent vasoconstriction, producing an increased GFR/RBF ratio
iv. Changes in vascular tone of the efferent arteriole (primarily vasoconstriction) complement efforts to maintain GFR by compensating for reduced blood flow
v. Autoregulation is essentially absent at an MAP of 70 mm Hg or below
i. Route of nerve supply is along renal blood vessels; renal neurologic intervention is vasoconstrictive
ii. Hypotension decreases systemic arterial pressure, stimulating the carotid sinus and aortic arch baroreceptors to trigger the sympathetic response (release of epinephrine), which decreases RBF and GFR by vasoconstricting both afferent and efferent arterioles
iii. Other factors that stimulate increased sympathetic tone are stress, fear, and exercise
iv. This neuronal effect is not the primary factor in autoregulation; a denervated kidney can be transplanted and still be able to compensate for changes in BP
f. Hormonal modulation of RBF (see Renal Regulation of Blood Pressure)
i. Renin-angiotensin system: A mechanism to sustain systemic BP and plasma volume
(a) Responds to a decreased afferent arteriolar pressure by increasing angiotensin II levels
(b) Angiotensin II vasoconstricts renal blood vessels, particularly the efferent artery, which reduces RBF but increases GFR
ii. Renal prostaglandins: Modulate the effects of vasoactive substances, such as angiotensin II, on the kidney by causing vasodilatation
i. Epinephrine and norepinephrine: Cause efferent arterioles to vasoconstrict, which leads to a rise in the filtration fraction and a dose-related decrease in RBF
ii. Dopamine: Pharmacologic action on RBF is dose-related for renal vasodilatation and increased sodium excretion. Generally has a vasodilatory effect on renal vasculature at dosages between 1 and 4 mcg/kg/min intravenously (IV) (optimal dosage, 3 mcg/kg/min); dosages above 10 mcg/kg/min cause renal vasoconstriction, decreasing RBF and GFR. Dopamine therapy has no impact on the prevention of acute tubular necrosis.
iii. Furosemide and mannitol: Increase GFR initially by increasing blood flow to the kidney and later by decreasing intratubular pressure
iv. Calcium channel blockers: Relax renal arteriole and ameliorate renal failure related to renal transplantation and nephrotoxicity due to radiocontrast dyes or cyclosporine
v. Atrial natriuretic factor (atrial natriuretic peptide, or ANP): Improves function in oliguric acute renal failure (ARF), but not preventive
a. Thirst: Regulator of water intake
i. Thirst center is located in the anterior hypothalamus
ii. Neuronal cells are stimulated by intracellular dehydration, which causes sensation of thirst
iii. Role is maintenance of satiety state (i.e., drinking exact amount of fluid to return body to normal hydration state)
b. ADH: Sodium osmoreceptor mechanism for control of extracellular fluid (ECF) osmolality and sodium concentration
i. ADH is synthesized in the paraventricular and supraoptic nuclei of the hypothalamus and travels along the axons of the supraopticohypophysial tract for storage or release from the posterior pituitary. The supraoptic area of the hypothalamus may overlap with the thirst center, providing integration of the thirst mechanism, osmolality detection, and ADH release.
ii. Release of ADH occurs with the following:
(a) Increased serum osmolality stimulates osmoreceptor cells in the hypothalamus that transmit along the neurohypophysial tracts, leading to ADH release from the posterior pituitary; normal serum osmolality is 285 to 295 mOsm/L
(b) Volume contraction states reverse the inhibitory effect on ADH release; controlled by stretch receptors in the left atrium that activate the ADH mechanism
iii. In the presence of ADH, water reabsorption occurs in the distal tubule and collecting ducts, which results in a hypertonic urine, hypotonic medullary interstitium, and eventual correction of contracted ECF
iv. ADH secretion is inhibited when serum osmolality decreases (water intoxication). When this occurs, the distal tubule and collecting duct become relatively impermeable to water, so that large volumes of hypotonic filtrate are delivered to the collecting duct; this results in dilute urine and excess water loss (compared to extracellular solute concentration), which returns serum osmolality to normal limits.
c. Countercurrent mechanism of the kidney: Mechanism for the concentration and dilution of urine; adjusts urine osmolality from 50 to 1200 mOsm/L
i. Isotonic glomerular filtrate leaves the proximal tubule and enters the loop of Henle at 300 mOsm/L
ii. Descending limb of the loop of Henle is permeable to water only. Water is gradually drawn into the hypertonic medullary interstitium, which gradually increases the osmolality of the filtrate as it becomes dehydrated. At the hairpin turn of the loop, osmolality is dramatically increased by the removal of water and NaCl pump action; osmolality can reach 1000 to 1200 mOsm/L. Concurrently, the medullary interstitium becomes hypotonic.
iii. Thick ascending limb of the loop of Henle is permeable to NaCl but impermeable to water. The medullary interstitium becomes more hypertonic as its sodium concentration is increased by pumping action at the ascending limb.
iv. A dilute filtrate reaches the distal tubule. If ADH is absent, dilute filtrate is excreted unchanged, which results in dilute urine with water excretion in excess of solute. If ADH is present, the collecting duct reabsorbs water and concentrated urine is excreted.
a. Sodium regulation: Normal serum concentration is 136 to 145 mEq/L solute
i. Na+ is the major extracellular cation and osmotically active solute. Because variation in body sodium can be associated with an exchange of water between intracellular and extracellular compartments, sodium affects ECF volume.
ii. Renal reabsorption sites: Normal percentages of reabsorbed filtered sodium
iii. Major factors that influence Na+ excretion include GFR, the sympathetic nervous system, aldosterone, the renin-angiotensin-aldosterone system, vasopressin (ADH), and ANP (a peptide hormone that plays a role in regulating and monitoring fluid, electrolyte, and cardiovascular balance)
iv. Sodium reabsorption increases at the renal tubules under the following conditions:
(a) Decreased GFR secondary to renal hypoperfusion (e.g., shock): Less sodium is delivered to the renal tubules, and less is excreted
(b) Secretion of aldosterone (a mineralocorticoid secreted by the adrenal cortex)
(1) Major effects are to increase renal tubular reabsorption of Na+ and to control selective renal excretion of K+
(2) Increases Na+ in ECF, which promotes water reabsorption; at the same time, K+ is secreted into the distal tubule and collecting duct to be excreted
(3) Regulated by K+ concentration in the ECF, the renin-angiotensin-aldosterone mechanism, total body sodium, and adrenocorticotropic hormone (ACTH)
(c) ANP action: Causes natriuretic, diuretic, and hypotensive effects secondary to its potent vasodilatory properties; the increased urinary excretion of Na+ is matched by an accompanying loss of K+ and PO43−
v. Sodium reabsorption decreases at the renal tubules under the following conditions:
(a) Increased GFR (excess ECF volume): Increases renal perfusion and GFR; more sodium is delivered to the renal tubules and more is excreted in urine
(b) Inhibition of aldosterone secretion, which results in renal Na+ excretion
(c) Secretion of ANP and ADH, administration of diuretics, especially loop-affecting diuretics
b. Potassium regulation: Normal serum concentration is 3.5 to 5.5 mEq/L
i. Potassium is a major intracellular cation (K+) necessary for the maintenance of osmolality and electroneutrality of cells
ii. Renal transport sites: K+ is actively reabsorbed in the proximal tubule (60% to 70%) and thick ascending loop (10%); active and passive secretion in the distal tubule and collecting duct maintain the electroneutrality of urine. This electrical gradient is determined primarily by reabsorption of Na+ from urine.
iii. Factors enhancing K+ excretion
(a) Increase in cellular potassium via increased exchange with Na+ (K+ excreted in urine whereas Na+ is reabsorbed) or via acute metabolic or respiratory alkalosis (causes movement of K+ ions into cells)
(b) High-volume tubular flow rates in the distal portion of the nephron: Increase the number of available K+ ions and thus increase the excretion of potassium
(c) Aldosterone (provides feedback mechanism for maintenance of K+ in ECF)
(1) Elevation of serum potassium stimulates the secretion of aldosterone
(2) Aldosterone acts on the distal nephrons and collecting ducts, enhancing the retention of Na+ and excretion of K+
(3) Excretion of excess K+ eventually returns levels to normal
(d) Hydrogen ions: Alkalemia (associated decrease in H+) stimulates K+ secretion
(e) Diuretics: Loop and thiazide diuretics block NaCl and waste reabsorption, increasing tubular flow and secretion of K+
c. Calcium regulation: Normal serum concentration is 8.5 to 10.5 mg/dl or 2.20 to 2.60 mmol/L
i. Major functions of calcium ions (Ca2+): Generation of cardiac action potential and pacemaker function, contraction of cardiac and vascular smooth muscle, transmission of nerve impulses, blood coagulation, formation of bones and teeth, and maintenance of cellular permeability
ii. Total serum Ca2+: 40% bound to protein, 50% ionized, and 10% combined with carbonate, phosphate, citrate, and various ions
iii. Renal transport sites: 98% of filtered Ca2+ is reabsorbed. Reabsorptive pathways are similar to those for sodium transport. Most active reabsorption occurs in the proximal tubule. Other sites include the loop (20% to 25%) and the distal tubule (10%).
iv. Factors influencing Ca2+ reabsorption:
(1) Decrease in serum calcium stimulates secretion of PTH
(2) PTH stimulates tubular reabsorption of Ca2+ at the distal portion of the nephron, stimulates increased phosphate excretion, and mobilizes calcium and phosphate from bone
(b) Vitamin D: Calcium absorption from the small intestine depends on the presence of activated vitamin D (1,25-dihydroxycholecalciferol)
(1) Activation process: Absorption of ultraviolet light converts 7-dehydrocholesterol in skin to cholecalciferol. The liver hydroxylates vitamin D to form 25-hydroxycholecalciferol. The kidney further hydroxylates to the final activated form of vitamin D (1,25-dihydroxycholecalciferol) in the proximal tubule. PTH stimulates this activation process.
(2) Decreased serum calcium level reduces urinary Ca2+ excretion, so activated vitamin D must be available to absorb Ca2+ from the small intestine to maintain adequate serum calcium levels
(c) Corticosteroid effect: Large doses decrease Ca2+ absorption in the intestines; may influence the activation of vitamin D in the liver
(d) Diuretic effect: Diuretics can cause Na+ and Ca2+ excretion. Ultimate effect of reduced serum calcium is decreased excretion. A decrease in total body fluid volume leads to diminished GFR and reduced calcium excretion.
d. Phosphate regulation: Normal serum concentration is 3.0 to 4.5 mg/dl
i. About 90% of phosphate is found in bone, 10% in intracellular and extracellular fluid spaces. Phosphates (PO43−) play significant role in intracellular energy production and may also influence DNA, RNA, and genetic code information. Phosphates are used by the kidneys to buffer H+.
ii. Renal transport sites: Reabsorption of phosphate is an active process that occurs in the proximal tubule and requires Na+. Factors influencing phosphate excretion include the following:
e. Magnesium regulation: Normal serum concentration is 1.5 to 2.2 mEq/L
i. The magnesium ion (Mg2+) is the second major intracellular cation and is a significant factor in cellular enzyme systems and biochemical reactions
ii. Mg2+ may have a role in the management of acute myocardial infarction (MI), because magnesium administration decreases the mortality rate in MI by 24% and improves ventricular function by 25%. Benefits may be attributed to magnesium’s ability to enhance coronary blood flow, conserve potassium, improve cellular function, and diminish dysrhythmias.
iii. Renal transport site: The reabsorptive process is similar to that of ca2+ and is linked to Na+ reabsorption along the renal tubules
iv. Factors influencing reabsorption include the availability of sodium (Na+ is necessary for reabsorption) and the availability of PTH (has minimal effect on Mg2+ reabsorption)
f. Chloride regulation: Normal serum concentration is 96 to 106 mEq/L
5. Excretion of metabolic waste products: Excretion is a primary renal function. The kidney excretes more than 200 metabolic waste products. The products measured for interpretation of renal function are blood urea nitrogen (BUN) and serum creatinine.
a. Urea: Nitrogen waste product of protein metabolism filtered and reabsorbed along the entire nephron
i. Is an unreliable indicator of GFR, because urea excretion is influenced by
(a) Urine flow (decrease in urine flow rate may allow for reabsorption of urea)
(b) Extrarenal factors (e.g., hypoperfusion states or drugs such as corticosteroids)
(c) Gastrointestinal (GI) bleeding or catabolic states such as fever or infection
ii. Elevation in BUN level without an associated rise in creatinine level (>25:1 ratio) suggests
(a) Volume depletion, low renal perfusion pressure
(b) Severe catabolic process or trauma with massive muscle injury (e.g., burns)
iii. Elevated levels of both BUN and creatinine (at a 10:1 ratio) indicate renal disease
b. Creatinine: A waste product of muscle metabolism
i. Amount produced daily is proportional to muscle mass, and production occurs at a constant rate
ii. Normal kidney excretes creatinine at a rate equal to RBF or GFR
iii. Creatinine is freely filtered, so its production normally equals its excretion, which makes it a reliable indicator of kidney function
iv. Elevated serum creatinine level is directly correlated with deterioration in renal function
6. Renal regulation of acid-base balance: The kidneys regulate acid-base balance by minimizing wide variations in body fluid balance in conjunction with retaining or excreting hydrogen ions. Acid-base balance is also regulated by the lungs and the body buffers (serum bicarbonate, blood, and plasma proteins)
a. Bicarbonate (HCO3−) reabsorption
i. Primarily occurs in the proximal tubule with less in the distal tubule; occurs with reabsorption of Na+
ii. Occurs if the filtrate contains more than 28 mEq/L (Tm) as in acidemia, volume contraction
i. Passive secretion occurs in the proximal tubule; active secretion occurs distally in exchange for Na+
ii. Acid is buffered by ammonia (NH3+) or phosphate (HPO42−) before excretion, which provides for hydrogen (H+) excretion without lowering pH
iii. H+ secretion is increased during acidemia and decreased during alkalemia
c. Renal buffers of hydrogen ions
i. Buffers that are filtered by the glomerulus
(a) HCO3− is completely reabsorbed (up to 28 mEq/L)
(b) Phosphate (PO43−) is secreted and then reacts with hydrogen
ii. Buffers produced by the kidney tubule
(a) HCO3− can be synthesized in the distal tubule when H+, excreted into urine as HCO3−, is delivered by ECF with Na+. H+ and HCO3− both come from the distal tubule cell as a result of ionization of carbonic acid (H2CO3); thus
where CA is carbonic anhydrase
(b) Carbonic acid comes from hydration of carbon dioxide (CO2) via CA:
(c) CO2 is derived from either cellular metabolism or dissolved CO2 in venous blood; thus new HCO3− can be made in the distal tubule from extraurinary sources
d. Summary of renal responses to acidemia
i. H+ secretion is increased at the distal tubule with increased excretion of titratable acids (HPO42−)
ii. All HCO3− is reabsorbed in the proximal tubule
iii. Ammonium is produced to accommodate H+ excretion: NH3+ + H+ E NH4+
iv. Urinary pH can be as low as 4.5 for excretion of a more acid urine in the presence of acidemia
7. Renal regulation of blood pressure: Renal regulation of BP involves five mechanisms:
a. Maintenance of volume and composition of ECF
i. Normal plasma volume is essential for control of BP
ii. Alterations in plasma volume eventually affect BP. Reduction of plasma volume lowers arterial BP, leading to compensation by vasoconstriction. Expansion of plasma volume increases cardiac preload and, in accordance with Starling’s curve, raises BP.
b. Aldosterone–body sodium balance, which determines ECF volume: Aldosterone stimulates renal tubular reabsorption of Na+ in exchange for excretion of primarily K+ ions
c. Renin-angiotensin-aldosterone system: Preserves BP and avoids serious volume reduction
i. Juxtaglomerular apparatus: Granular cells contain inactivated renin. Factors that trigger juxtaglomerular cells to release renin reflect diminished GFR (e.g., reduced arterial BP in afferent and efferent arterioles, reduced Na+ content or concentration at distal tubule, sympathetic stimulation of kidneys).
ii. Renin, an enzyme, is released from juxtaglomerular cells into the afferent arteriole
iii. On entering the circulation, renin acts on angiotensinogen to split away the vasoactive peptide angiotensin I and convert it to angiotensin II. Requires the presence of angiotensin-converting enzyme (ACE), found primarily in the lung and liver but also in the kidney and all blood vessels. Angiotensin II is a potent systemic vasoconstrictor.
iv. Circulatory effect of angiotensin II on arterial BP
(a) Significant peripheral arteriole constriction with moderate venous constriction occurs, which results in the reduction of vascular volume
(b) Renal arteriolar constriction results in the renal retention of sodium and water; this expands ECF volume, thus increasing arterial BP
v. Fluid volume response to angiotensin II restores effective circulating volume in the following ways:
d. Renal prostaglandins: Modulating effect
i. Major renal prostaglandins are prostaglandins E2, D2, I2 (vasodilators) and A2 (vasoconstrictor)
ii. Physiologic role is modulation, amplification, and inhibition. Vasoactive substances (angiotensin, norepinephrine, bradykinins) stimulate the synthesis and release of prostaglandins. Prostaglandins modulate the action of the vasoactive substances.
iii. Prostaglandins diminish arterial BP and increase RBF by arterial vasodilation and inhibition of the distal tubules’ response to ADH. Suppressed ADH response leads to sodium and water excretion, which ultimately decreases the effective circulatory volume.
iv. Pharmacologic prostaglandin inhibitors are the nonsteroidal antiinflammatory drugs (NSAIDs). In cases of compromised renal function avoid the use of NSAIDs (i.e., salicylic acid, ibuprofen [Motrin], indomethacin [Indocin], and naproxen [Naprosyn]).
v. Loop diuretics stimulate prostaglandin secretion, which leads to vasodilation and decreased preload
e. Kallikrein-kinin system: Renal kallikreins are proteases that release kinins and are excreted in the urine. Kinins stimulate both the renin-angiotensin and prostaglandin systems, appearing to link renal hemodynamics and fluid-electrolyte excretion.
8. Red blood cell synthesis and maturation
a. Erythropoietin secretion: Stimulates the production of erythrocytes in the bone marrow and prolongs the life of erythrocytes
b. Mechanism of erythropoietin synthesis and secretion
i. Renal cortical interstitial cells produce erythropoietin, a glycosylated, 165-amino-acid protein
ii. Renal erythropoietin production accounts for 90% of RBC production; the remaining 10% is produced by the liver
iii. Hypoxia stimulates renal erythropoietin production; the liver is not as responsive to hypoxia and therefore cannot support erythropoiesis in renal failure
c. Erythropoietin deficiency: Primary cause of anemia in chronic renal failure (CRF); bleeding is the second most common cause
a. Age-related changes can occur as early as 20 to 40 years of age. Changes include a decrease in tubular length and, at and over age 40 years, a progressive decrease in the percentage of glomeruli. Generally, renal function is diminished by 10% at age 65; may diminish further with aging.
b. Renal response in the elderly
i. Decreased renal mass associated with a diminished number of nephrons
ii. Decreased GFR; diminished RBF secondary to age-related changes in vasculature
iii. Diminished creatinine production (10 mL/min/1.73 m2 per decade) and diminished ability to excrete creatinine; therefore, change in serum creatinine level may not be evident. Uric acid levels are slightly increased.
iv. Decreased serum renin and aldosterone levels reduce the ability to conserve sodium, impair urinary water excretion, and limit urinary concentration
Patient Assessment
i. Previous health problems: Indicate the presence of or predisposition to renal disease
(a) Kidney and/or urinary tract disease
(1) Hypertension: BP control and treatment may prevent or halt renal damage; hypertension develops in 70% to 80% of patients with advanced renal failure
(c) Diabetes mellitus: Renal disease caused by vascular disease alterations, infection, or neuropathy
(d) Immunologic disorders, recent infections (streptococcal)
(e) Pulmonary disease (Goodpasture’s syndrome)
(f) Allergies, recent blood transfusions (history of incompatibility reaction)
(g) Other: Toxemia of pregnancy, renal transplantation, anemia, recent surgery, dialysis, exposure to drugs and toxins, renal calculi, azotemia, hematuria, exposure to chemicals or poisons
ii. History of specific signs and symptoms
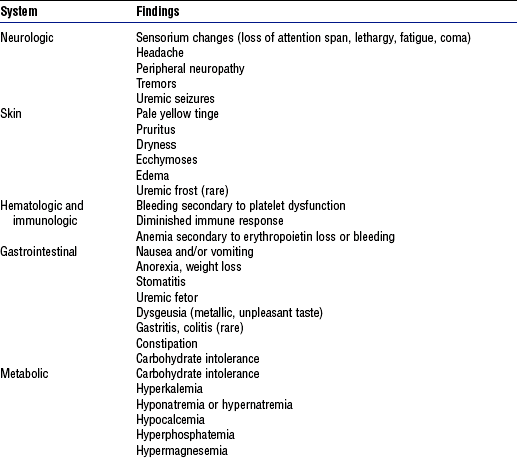
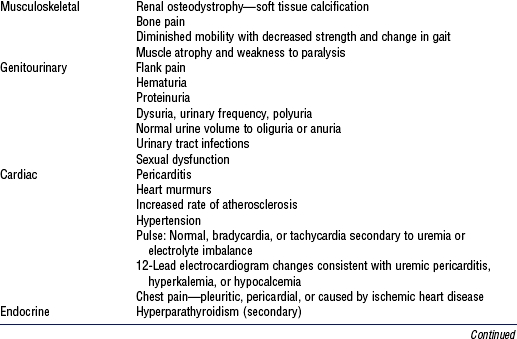
(a) Signs and symptoms of urinary tract disorders
(2) Abnormal appearance of urine
c) Biliuria or bilirubinuria (orange)
d) Myoglobinuria (usually clear; red-brown urine; Hematest positive)
(3) Urine frequency, urgency, incontinence, hesitancy; nocturia
a) Normal volume: Approximately 1500 ml/24 hr
b) Oliguria: Less than 400 ml/24 hr
c) Anuria: Less than 50 ml to no output over 24 hours
d) Polyuria: Excessive output exceeding 24-hour intake
e) Nonoliguria: Normal or excess urine volume in the presence of ARF
(7) Pain in costovertebral angle, flank, or groin
(8) Pattern of weight gain or loss; dry weight is the ideal weight that minimizes symptomatology for a patient with renal failure as achieved by a dialysis treatment
b. Family health history: Genetic renal disease accounts for about 30% of azotemia. Genetically transmitted diseases that can cause or precipitate renal disease include the following:
i. Cardiovascular disease, hypertension
v. Polycystic kidney disease and medullary cystic disease
i. Social history: Sexual activity prior to renal disease and sexual dysfunction related to renal disease
i. Nephrotoxic agents: Radiocontrast dye and antibiotic therapy (tetracyclines, aminoglycosides, gentamicin, amphotericin B)
ii. Diuretics, antihypertensives
iii. Cardiac glycosides (digoxin), antiarrhythmic agents
2. Nursing examination of patient
(a) Diminished level of consciousness (lethargy, coma)
(1) Abnormal color: Grayish tinge from anemia, yellowish tinge if retained carotenoids or urochrome pigments in uremia
(c) Eye: Cataracts, periorbital edema
(d) Ear: Nerve deafness (Alport’s syndrome)
(f) Respiration: May see rate and pattern similar to Kussmaul’s respirations
(g) Muscle tremors, weakness, weight loss with uremic syndrome
(h) Tetany: Positive Chvostek’s and Trousseau’s signs; rarely observed; result from severe hypocalcemia or very rapid correction of acidosis
(i) Asterixis: Indicates progressive uremic state
(1) Ask the patient to face the examiner and raise the upper extremities in a fixed hyperextension position
(2) Palms (fingers separated) must be visible to the examiner
(3) Positive sign—irregular movements of the wrists, flapping movements of the fingers—occurs within 30 seconds
(j) Fatigue: Occurs with activities of daily living and exercise, and at rest
ii. Palpation: To determine size and shape of the kidney and to check for tenderness, cysts, and masses
(a) Right kidney is easier to palpate because it is lower in the abdomen
(b) Palpate the bladder for urinary distention due to obstruction
(c) Palpate the flank area to elicit tenderness or pain
(d) Palpate pulses for a baseline reading and to determine abnormalities
iv. Auscultation: Listen for aortic and renal artery bruits (heard in flanks or intercostal regions of anterior abdomen)
b. Monitoring data: Intake and output (I&O), hemodynamics, body weight, central venous pressure (CVP) and/or pulmonary artery occlusion pressure to determine relationship between cardiac filling pressures and hydration status; correlate findings with daily weight
3. Appraisal of patient characteristics: Patients with acute, life-threatening renal problems come to critical care units with a wide range of biochemical, metabolic, and psychosocial clinical characteristics. During their stay, their clinical status may significantly improve or deteriorate, slowly or abruptly change, involve one or all life-sustaining functions, and be readily or nearly impossible to monitor with precision. Some attributes of patients with acute renal disorders that the nurse needs to assess are the following:
i. Level 1—Minimally resilient: Any patient with end-stage CRF on hemodialysis, who is septic with two other forms of organ failure, such as acute respiratory distress syndrome and cirrhosis
ii. Level 3—Moderately resilient: A 58-year-old patient on postoperative day 3 after uncomplicated coronary artery bypass graft surgery who received prophylactic intravenous therapy intraoperatively to prevent renal involvement but still developed and is recovering from nonoliguric ARF
iii. Level 5—Highly resilient: Any patient with an isolated episode of a prerenal ARF secondary to dehydration with no significant alteration in hemodynamics, receiving hydration
i. Level 1—Highly vulnerable: A 65-year-old male diabetic patient with cardiovascular complications who has rejected his renal transplant and is unable to tolerate hemodialysis
ii. Level 3—Moderately vulnerable: A 24-year-old female with acute postrenal failure from infected renal calculi who is scheduled for surgery today
iii. Level 5—Minimally vulnerable: A 32-year-old single female with a urinary tract infection (UTI)
i. Level 1—Minimally stable: Any cardiogenic shock patient with oliguric ARF on intraaortic balloon pump and continuous renal replacement therapy
ii. Level 3—Moderately stable: An end-stage CRF patient with malignant hypertension whose BP is beginning to respond to a new therapeutic regimen of antihypertensive agents including ACE inhibitors
iii. Level 5—Highly stable: A 36-year-old patient with lupus nephritis in early stages of CRF who requires a protein-restricted diet and is compliant with the medical regimen
i. Level 1—Highly complex: A 36-year-old elementary school teacher with chronic diabetic renal disease who decided to terminate dialysis treatment and is now in a uremic coma, and whose family does not agree with her decision
ii. Level 3—Moderately complex: A 21-year-old college student who is approaching end-stage renal failure and feels conflicted because two of his younger siblings are a tissue match and are both eager to donate a kidney
iii. Level 5—Minimally complex: A 46-year-old male with CRF who complies with his medical regimen, is dialyzed at home by his supportive wife, and is now admitted for an acute repair of his arteriovenous fistula
i. Level 1—Few resources: A 50-year-old Russian immigrant who has been in the United States 2 weeks when it is determined that he requires hemodialysis; he is not insured and is not eligible for state assistance, and his family cannot offer financial support
ii. Level 3—Moderate resources: A middle-aged patient who needs renal transplantation; he has four siblings who offered to donate but none is a tissue match, so he places himself on the cadaveric organ donor list
iii. Level 5—Many resources: Mrs. Jones, a well-respected elementary school nurse for the past 15 years, who has been effectively managing her own continuous ambulatory peritoneal dialysis for 5 years
i. Level 1—No participation: A 26-year-old quadriplegic with nephrotoxic acute tubular necrosis (ATN) secondary to carbenicillin administered for an antibiotic-resistant UTI requires 2 weeks of dialysis after which kidney function should return
ii. Level 3—Moderate participation: A blind man with diabetes is taught how to perform his own peritoneal dialysis at night with the assistance of his wife
iii. Level 5—Full participation: A patient who has had a successful renal transplant for the past 3 years makes a practice of visiting local hemodialysis units and teaching the benefits of transplantation as a treatment option
g. Participation in decision making
i. Level 1—No participation: A 70-year-old mentally retarded male patient with advanced chronic kidney disease is approaching the need for dialysis and lives with his 95-year-old mother, who has periods of senility
ii. Level 3—Moderate participation: A 32-year-old female recently diagnosed with renal cancer has a history of admissions for psychosis and is estranged from most of her family except her 29-year-old sister
iii. Level 5—Full participation: After running a marathon, the patient spends a week in the intensive care unit (ICU) recovering from an episode of ARF secondary to rhabdomyolysis; he is surrounded by his family, friends, and representatives from his community, who offer their support during his recovery at home
i. Level 1—Not predictable: Any critically ill patient who develops ATN after an extensive period of hypotension and experiences repeated episodes of severe dehydration, systemic infection, and exposure to nephrotoxic agents during recovery
ii. Level 3—Moderately predictable: Any critically ill patient who develops contrast media–induced ATN, when renal involvement is identified early and hydration and loop diuretics are administered
iii. Level 5—Highly predictable: A critically ill patient with a relatively uncomplicated condition who develops nonoliguric ATN after a short episode of hypotension, easily reversed, and has an uneventful recovery period
(a) Complete blood count: Reduced hematocrit and hemoglobin levels may reflect bleeding or a lack of erythropoietin
(b) Serum creatinine: To estimate GFR (normal level, 0.6 to 1.2 mg/dl)
(1) Creatinine excretion is proportional to its production
(2) A significant elevation in creatinine level is associated with renal disease and correlates with percentage of nephrons damaged
(c) BUN: Normal level, 10 to 20 mg/dl (Table 5-1)
(1) Prerenal problem: Ratio of BUN to serum creatinine equal to or greater than 25:1 suggests extrarenal problem (dehydration, catabolic state). Elevation in both BUN and creatinine results from decreased GFR.
(d) Cystatin C: New test to determine GFR
(1) Cystatin C is a nonglycosylated basic protein continually produced by nucleated cells
(2) Freely filtered by glomeruli, metabolized by tubules
(3) Serum levels not affected by age, sex, or muscle mass
(4) More reliable indicator of renal function than creatinine
(5) Used to detect mild reductions in GFR, especially in renal transplant patients
(e) Serum chemistry tests (calcium, phosphate, alkaline phosphatase, bilirubin, uric acid, sodium, potassium, chloride, carbon dioxide, magnesium, glucose, cholesterol)
(f) Baseline arterial blood gas (ABG) levels, clotting profile
(a) Visual examination for color and clarity
(b) Osmolality (50 to 1200 mOsm/kg)
(c) SG: Wide range of normal values (1.003 to 1.030); provides reasonable estimate of urinary osmolality; actually measures density
(1) Low normal (<1.010): Suspect diabetes insipidus, overhydration, or heart failure
(2) Above normal (>1.030): Occurs in proteinuria, glycosuria, severe dehydration, presence of x-ray contrast medium
(d) Creatinine clearance (Ccr): 24-hour urine collection
(1) Purpose: To determine the presence and progression of renal disease, estimate percentage of functioning nephrons, or determine specific medication dosages
(2) In 24 hours, the following occurs:
Ucr = amount of urinary creatinine excreted
(3) In average-size patients, a satisfactory 24-hour urine collection always has approximately 1 g of creatinine, regardless of the degree of renal function
(4) Cockcroft-Gault formula for estimation of Ccr: See Table 5-2
TABLE 5-2
Cockcroft-Gault Formula for Estimation of Creatinine Clearance Without Urine Specimen
| Gender | Formula |
| Male | Ccr (in ml/min) = ([140 – age in years] × weight in kg) ÷ (Pcr in mg/dl × 72) |
| Female | Ccr (in ml/min) = ([140 – age in years] × weight in kg) ÷ (Pcr in mg/dl × 72) × 0.85 |
(e) Culture and sensitivity: Check for infection
(f) pH (normal range, 4.5 to 8; average value, 6); alkaline urine is frequently seen with infection; in absence of infection, possibly indicates renal tubular acidosis if both alkaline urine and systemic acidosis are present
(g) Glucose: In urine when renal threshold for glucose exceeded
(h) Acetone: In urine with starvation or diabetic ketoacidosis; a false-positive result can occur in patients taking salicylates
(i) Protein: Expressed quantitatively as 1+ to 4+; diagnostic for the presence of glomerular membrane disease (nephritic syndrome) and allows the detection of myeloma proteins causing renal failure
(1) Measure urinary concentrations of Na+, K+, Cl−
(2) Screening test for tubular function; assess the kidney’s ability to conserve sodium and concentrate urine
(1) Casts: Precipitations of protein in the kidney that take the shape of the tubules in which they are formed
a) Hyaline casts: Entirely protein; small amounts are normal in urine; if large amounts, suspect significant proteinuria such as albumin or myeloma protein in urine
b) Erythrocyte casts: Diagnostic for active glomerulonephritis or vasculitis
c) Leukocyte casts: Indicative of an infectious process and intrarenal inflammation
d) Granular casts: Small number, possibly the result of degenerating erythrocyte or leukocyte casts indicative of an infectious process or an allergic interstitial nephritis
(2) Bacteria: Presence determined by Gram stain
(3) Erythrocytes: Small numbers normal; in abundance during active glomerulonephritis, interstitial nephritis, malignancies, and infection
(4) Leukocytes: Small numbers normal; present in infection and interstitial nephritis
(5) Renal epithelial cells: Rarely seen; present in abundance during ATN, nephrotoxic injury, and allergic reaction in the kidney
(6) Crystals: Seen in diseases of stone formation or following certain intoxications
(l) Nucleomatrix test: Noninvasive, quantitative, painless examination for transitional cell cancer of the bladder
i. Plain abdominal x-ray study: Determines position, shape, and size of the kidney and identifies calcification in the urinary system
ii. Intravenous pyelography (IVP)
(a) Visualizes the urinary tract for diagnosing partial obstruction, renovascular hypertension, tumor, cyst, congenital abnormality
(b) Complications include allergic reaction to dye, dehydration
(c) Contraindicated in the presence of the following:
(1) Poor renal function: Dye’s dehydrating effect and nephrotoxicity may further compromise function
(2) Multiple myeloma: IVP dye may precipitate myeloma protein in the kidney
(3) Pregnancy: Abdominal irradiation should be avoided
(4) Heart failure: Osmotic effect of dye can compromise cardiac function by expanding vascular volume
(6) Sickle cell anemia: Dye’s elevation of renal oncotic pressure can promote renal tissue sickling, infarction
iii. High-excretion tomography: Indicated when kidneys cannot be readily visualized on IVP
iv. Renal scan: Determines renal perfusion and function; can provide information about obstructions and renal masses. Radioactive dye is taken up by normal kidney tubule cells. A decrease in uptake indicates hypoperfusion. Often used to assess renal transplants.
v. Retrograde pyelography: Used to examine upper region of collecting system
vi. Retrograde urethrography: Used to examine the urethra
vii. Cystoscopy: Detects bladder or urethral pathology
viii. Renal arteriography (angiography): Identifies tumors and distinguishes type of renal or renovascular disease. Potential complications can be serious:
(a) Allergic reaction to dye can cause same complications seen with IVP dye
(b) Puncture of a peripheral artery, with consequent hematoma, embolism, or thrombus formation, is the greatest technical risk
ix. Voiding cystourethrography: Identifies abnormalities of lower urinary tract, urethra, bladder to detect reflux and residual urine
x. Diagnostic ultrasonography: Identifies hydronephrosis, differentiates solid and cystic tumors, localizes cysts or fluid collections
xi. Computed tomography (CT): Identifies tumors and other pathologic conditions that create variations in body density (e.g., abscess or lymphocele); used in renal trauma to determine reason for acute flank pain
xii. Magnetic resonance imaging (MRI)
(a) Provides better tissue characterization than CT; provides direct imaging in several planes for detection of renal cystic disease, inflammatory processes, and renal cell carcinoma
(b) Detects alterations in blood flow (i.e., slow or absent flow)
xiii. Magnetic resonance urography: A form of magnetic imaging that offers results similar to those of an IVP, without the use of dye
xiv. Chest radiography: Identifies pulmonary edema, cardiomegaly, left ventricular hypertrophy, uremic lung, Goodpasture’s disease, and infection
Patient Care
1. Overhydration: A state in which an individual experiences fluid retention and edema because kidneys are unable to excrete excess body water
ii. Weight gain with oliguria or anuria, low SG (≤1.015), dilute urine
iii. Elevated BP, bounding pulses, neck vein distention; elevated CVP, pulmonary artery pressure (PAP), and pulmonary artery occlusive pressure (PAOP), and muffled heart sounds
iv. Edema: Peripheral, anasarcal, ascitic, periorbital, pulmonary
v. Dyspnea, orthopnea, crackles on auscultation, pulmonary congestion
vi. Decreased (diluted) hemoglobin, hematocrit, and electrolyte values
vii. Anxiety, restlessness, stupor (seen with water intoxication)
i. Patient maintains dry weight
ii. BP, CVP, PAP, and PAOP are normal
c. Collaborating professionals on health care team
ii. Critical care intensivist or physician
iii. Nurse practitioner, clinical nurse specialist, or physician assistant
i. Identify presence of common causes of fluid volume excess
(a) Expanded total body water volume secondary to renal failure with oliguria or anuria
(b) Expanded blood volume due to renal sodium retention
(c) Lower plasma oncotic pressure due to loss of plasma proteins
ii. Document I&O; compare with daily weight; consider insensible losses—fluid losses via lungs, skin, and bowel (600 to 800 ml/day)
(a) Urine volume, urinalysis, creatinine clearance, and BUN/creatinine ratio
iv. Restrict fluids in overhydration associated with impaired renal function, impaired cardiac function, or syndrome of inappropriate secretion of antidiuretic hormone (SIADH)
v. Administer diuretics (preferably loop) if renal response is a GFR of 25 ml/min or higher
vi. Consider acute dialysis with ultrafiltration for rapid volume removal
2. Dehydration: A state in which an individual experiences vascular, cellular, or intracellular volume depletion due to active fluid loss. Dehydration may occur in the diuretic phase of ARF or as a result of aggressive diuretic therapy.
ii. Weight loss with elevated SG (≥1.020), concentrated urine, variable urinary output
(a) Polyuric phase: Large volume of dilute urine with low SG
(b) Dehydration with normal renal function: Oliguria, concentrated urine with an elevated SG
iii. Hypotension, increased pulse, decreased CVP
i. Patient’s weight is normal and stable
ii. Vital signs and hemodynamic parameters are normal
iii. Fluid balance and urine output are within normal limits (WNL)
c. Collaborating professionals on health care team: See Overhydration
i. Identify common causes of fluid deficit
ii. Document I&O; compare with daily weight
(a) Fluid challenge to increase RBF and urinary excretion
(b) Caution for fluid challenge: Monitor for pulmonary edema and renal failure unresponsive to volume expansion (i.e., no increase in urinary output)
(c) Follow with replacement fluid therapy until volume goal achieved, then proceed to maintenance fluid regimen
a. Description of problem: Malnutrition is associated with increased morbidity in CRF, especially in the presence of hypoalbuminemia. Dietary protein intake is restricted to preserve kidney function in early stages of chronic kidney disease. Protein restriction can contribute to malnutrition.
i. Patient’s intake meets nutritional requirements
ii. Patient maintains stable baseline weight and adequate muscle mass
iii. Serum protein and albumin, BUN, and creatinine levels are normal
c. Collaborating professionals on health care team: See Overhydration
i. Identify cause of inadequate nutritional intake; direct care there
ii. Teach appetite-enhancing measures
(a) Provide oral hygiene prior to meals
(b) Give small, frequent meals
(c) Identify preferred foods, especially those high in complex carbohydrates and essential amino acids
iii. Teach the necessary elements of the renal patient’s diet
(a) Essential amino acids, adequate calories, vitamin and iron supplements (folic acid, multivitamins) as warranted
(b) Adjusted protein and electrolyte intake (Na+ and K+) to avoid uremic symptoms and electrolyte imbalances. Excessively diminished protein intake causes use of protein stored in muscles, which leads to body muscle wasting. Providing increased calories can help avoid this situation.
iv. Monitor pattern of changes in weight and nutritional intake
i. In renal failure, the hypertensive state (diastolic BP >90 mm Hg, systolic BP >140 mm Hg) is usually created by fluid retention and/or stimulation of the renin-angiotensin mechanism; preexisting hypertension is common
b. Goals of care (see Chapter 3): Goal in CRF is systolic BP 130 mm Hg or lower and diastolic BP 80 to 85 mm Hg or lower
c. Collaborating professionals on health care team: See Chapter 3
d. Interventions (see Chapter 3): Treatment of hypertension in an aggressive manner with a diuretic, ACE inhibitor, β-blocker, and/or possibly calcium channel blocker has the benefit of slowing the progression of CRF
i. Administer diuretics, as ordered, to treat edema and hypertension
(a) General characteristics of diuretics
(1) Inhibit the active transport of sodium or chloride, resulting in an increase in urine output
(2) The diuretic effect reduces effective plasma circulating volume, thereby lowering BP
(c) Types of diuretics: Used as single therapy to treat hypertension or with other antihypertensive agents to enhance their therapeutic effect
(1) Osmotic diuretic: A nonabsorbable solute (mannitol)
a) Exerts an osmotic effect, causing water diuresis in excess of NaCl
b) Side effects: Blurred vision, rhinitis, rebound plasma volume expansion, thirst, urinary retention, and fluid and electrolyte imbalance
(2) Loop diuretics: The most potent diuretics available (furosemide, indapamide, bumetanide, torsemide, and ethacrynic acid). The primary site of action is the thick segment of the medullary ascending loop of Henle.
a) Block the reabsorption of NaCl, thus contributing to a large diuresis of isotonic urine; potassium excretion also enhanced
b) Increase RBF by stimulating increased secretion of prostaglandin, which exerts a vasodilatory effect on renal vasculature leading to reduction in preload
c) Vasodilatory effect of loop diuretics can be minimized, if the cardiovascular effect is negative, by the administration of ACE inhibitors
d) Increase GFR even with a decrease in ECF volume, because the tubuloglomerular feedback mechanism is blocked
e) Side effects: Volume depletion, agranulocytosis, thrombocytopenia, transient deafness, abdominal discomfort, hypokalemia, hypomagnesemia, metabolic alkalosis, and hyperglycemia
f) Prolonged use without electrolyte replacement results in all other electrolyte imbalances
(3) Thiazides (hydrochlorothiazide, chlorthalidone, and metolazone)
a) Sodium reabsorption inhibited in the ascending loop of Henle and the beginning portion of the distal tubule
b) Increased potassium excretion occurs with a weak carbonic anhydrase inhibitory effect
c) Side effects: Rashes, leukopenia, thrombocytopenia, hypercalcemia, and acute pancreatitis
(4) Potassium-sparing diuretics (spironolactone, amiloride, triamterene): Aldosterone inhibitors
a) Promote Na+ secretion into the distal tubule and K+ reabsorption; cause mild diuresis and protect K+ level
b) Usually selected for patients receiving digoxin and diuretic therapy who cannot tolerate low serum K+ levels or when a mild diuretic effect is desirable
c) Side effects: Hyperkalemia, hyponatremia, headache, rash, nausea, diarrhea, urticaria, and gynecomastia or menstrual disturbances
(5) Carbonic anhydrase inhibitors (acetazolamide sodium)
a) Inhibit the enzyme carbonic anhydrase
b) Increase the excretion of Na+ by interfering with HCO3− reabsorption. Sodium bicarbonate is lost in the urine, which creates a hyperchloremic metabolic acidosis
c) Are beneficial when an alkaline urine is desirable, such as with metabolic alkalosis
d) Side effects: Hyperchloremic acidosis, renal calculi, rash, nausea, vomiting, anorexia, diminished renal function
(6) Other agents: Pharmacologic agents that increase both cardiac output and GFR contribute to diuresis (e.g., xanthines [theophylline, aminophylline] and digoxin)
(d) General nursing considerations in the administration of diuretics
(1) Collaborate with the physician to determine the weight and fluid balance desired at the conclusion of diuretic therapy
(2) Observe for fluid, electrolyte, and acid-base disorders
(3) Maintain I&O records; correlate with daily weights
(4) Monitor serum K+ levels, especially if the patient is taking digoxin (hypokalemia increases risk of digitalis toxicity)
(5) Administer potent or high doses of diuretics in the early morning or afternoon unless a Foley catheter is in place
(6) Monitor BP during aggressive diuresis because hypotension can indicate dehydration and impending circulatory collapse
(7) Advise the patient to report the onset of side effects such as difficulty hearing
(8) Be aware that a diminished response to diuretics may be related to electrolyte imbalances, particularly hyponatremia, hypochloremia, and hypokalemia
ii. Administer antihypertensive agents as ordered (see Chapter 3)
5. Metabolic acidosis: A condition commonly associated with renal failure caused by the inability of the kidney to excrete hydrogen ions (see Chapter 2)
6. Anemia: In renal disease, anemia is related primarily to a lack of erythropoietin synthesis and secretion by the kidney but can also be caused by actual blood loss (e.g., stress ulcer)
a. Description of problem: See Chapter 7
b. Goals of care: See Chapter 7
c. Collaborating professionals on health care team: See Overhydration; include hematology consult if the patient is unresponsive to therapies
i. Identify common causes of anemia associated with renal failure
ii. Treat chronic anemia associated with renal failure
(a) Oral or IV iron unless the patient has excess body iron stores
(b) Folic acid and pyridoxine (vitamin B6): Important, especially in dialysis patients, because these are dialyzable vitamins
(c) Epogen (recombinant human erythropoietin): Stimulates erythrocyte production and prevents the anemia of CRF; effect does not begin until 2 to 6 weeks, with peak results in 3 months after administration; as a result, it is not used in ARF
a. Description of problem: Uremic state results from the kidney’s inability to excrete toxic waste products; uremic symptoms usually occur at BUN levels above 100 mg/dl or at a GFR below 10 to 15 ml/min (Table 5-3)
b. Goals of care: BUN level is maintained below 100 mg/dl or at a level that minimizes uremic symptoms
c. Collaborating professionals on health care team: See Overhydration
d. Interventions: Based on minimizing azotemia and preventing dehydration
i. Restrict oral protein intake
ii. Remove blood if it is present in the GI tract because this is another protein source that can be metabolized to ammonia and urea. These metabolites cannot be handled by diseased kidneys.
iii. Consider dialysis to maintain BUN level below 100 mg/dl. In each patient, uremic symptoms develop at individual levels of BUN and creatinine. Identify these values, then strive to maintain BUN and creatinine below those levels.
a. Description of problem: Major cause of death in patients with ARF and can seriously compromise patients with CRF (see Chapter 7)
b. Interventions: See Chapter 7
i. Keep in mind: Patients with renal failure have an impaired immune response from uremic toxins and reduced phagocytosis by the reticuloendothelial system
ii. Implement the following precautions
(a) Obtain a urine specimen for culture on admission: UTI may be asymptomatic
(b) Prevent introduction of microorganisms; avoid indwelling urinary catheters and unnecessary invasive monitoring procedures
(c) Use an aseptic technique for urinary and intravenous catheter care
(d) Maintain the BUN level at 80 to 100 mg/dl or lower to minimize susceptibility to infection
(e) Implement isolation techniques for hepatitis antigen–positive patients receiving hemodialysis
9. Bone disease—osteomalacia, osteitis fibrosa: Chronic hypocalcemia can precipitate hyperparathyroidism, which leads to the mobilization of calcium from the bone and results in softening of the bone (osteomalacia)
i. History of chronic hypocalcemia, hyperparathyroidism, or both
ii. Bone pain, fractures, and radiologic examination of the skull, hands, and feet revealing signs of demineralization
c. Collaborating professionals on health care team: See Overhydration
d. Interventions: See Electrolyte Imbalances—Calcium Imbalance: Hypocalcemia and Electrolyte Imbalances—Phosphate Imbalance: Hyperphosphatemia later in this chapter
10. Altered metabolism and excretion of pharmacologic agents related to renal failure
i. Kidneys unable to metabolize or excrete pharmacologic agents
ii. Unusual untoward effects may include enhanced sensitivity to drugs
iii. Active or toxic metabolites of a medication retained
iv. Increased azotemia due to elevation in metabolic wastes from drug usage
c. Collaborating professionals on health care team: See Overhydration; also Pharmacologist
i. Recognize alterations in the body’s use of drugs during renal failure
(a) Distribution of drugs in a uremic state
(1) Decreased stores of body fat affect distribution of lipid-soluble drugs
(2) Low cardiac output states reduce renal metabolism or excretion of drugs
(3) Acidemia alters tissue uptake of drugs
(4) Increased body water has a dilutional effect
(5) Decreased protein binding causes competition by various drugs for tissue binding sites, leading to a higher concentration of unbound drugs
ii. Follow general principles for drug administration during renal insufficiency
(b) Increase intervals between doses
(c) Question orders for nephrotoxic agents (i.e., NSAIDs, meperidine)
(d) Closely observe patients to recognize toxicity due to drug accumulation
(e) Report any untoward signs, especially elevated serum creatinine level, so the drug can be reconsidered, reduced in dosage, or discontinued
(f) Monitor serum drug levels, especially in situations requiring a specific drug concentration (e.g., antibiotics, digoxin, procaine)
(g) To ensure a more stable serum concentration, administer initial loading doses of drugs that have a long half-life (e.g., digoxin)
e. Evaluation of patient care: Patient tolerates pharmacologic therapy with no untoward drug effects
11. Ineffective patient and family coping (see also Chapter 10)
i. Insufficient, ineffective, or compromised support, comfort, assistance, or encouragement, usually by a supportive primary person (family member or close friend). The patient may need to manage adaptive tasks related to the stress of renal failure on the patient and the family.
ii. Signs of maladaptive patient coping
(a) Verbalization of the inability to cope or to ask for help
(b) Inability to meet role expectations and solve problems
(c) Diminished communication and socialization
(d) Destructive behavior toward self or others (i.e., suicide attempt)
iii. Signs of maladaptive family coping
(a) Patient communicates concern about the family’s response to his or her disease
(b) Family members demonstrate preoccupation with their own personal reactions—fear, anticipatory grief, guilt, anxiety
(c) Family has inadequate understanding of the patient’s condition, or therapy interferes with effective supportive behaviors
(d) Family withdraws from communication with the patient or demonstrates overprotective or underprotective behaviors
i. Patient demonstrates increased functional independence, compliance with treatment regimen, and participation in programs that enhance quality of life (e.g., exercise or rehabilitation program)
ii. Patient appropriately expresses ideas, feelings, and needs, participates in family activities, and accepts family support, as appropriate
iii. Patient and family adjust to any necessary role changes
c. Collaborating professionals on health care team (see also Overhydration)
i. Identify common causes of stress in the patient and family
(a) Life-threatening nature of renal disease
(b) Inability to perform activities of daily living
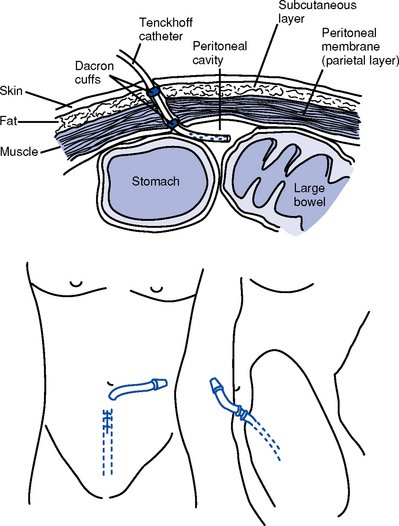
(c) Restrictions caused by a shunt, a fistula, or a Tenckhoff catheter; demands of dialysis schedule and other treatments
(d) Reversal in family roles, effects on sexual behavior and sexuality, and questions regarding ability to maintain or return to work
ii. Recognize that psychologic consequences of renal disease and its treatment include denial, depression, and dependency, and that the suicide rate among patients maintained with hemodialysis is believed to be 100 times that of the general population
iii. Assess the patient’s ability to cope with renal disease (see Chapter 10)
iv. Specific nursing interventions to support adaptation of the patient with renal failure
SPECIFIC PATIENT HEALTH PROBLEMS
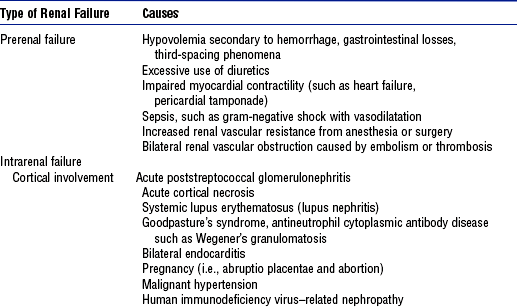
i. Cortical involvement of vascular, infectious, or immunologic processes
(a) Causes renal capillary swelling and cellular proliferation, which eventually decrease the GFR
(b) Edema and cellular debris obstruct the glomeruli, which results in oliguria
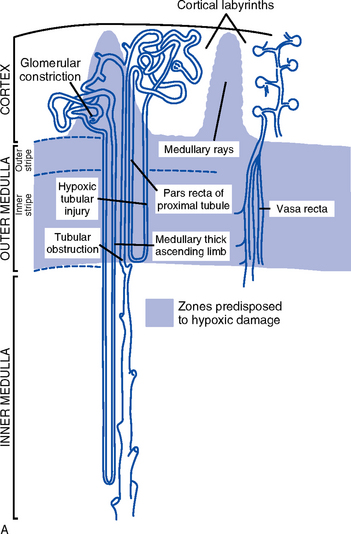
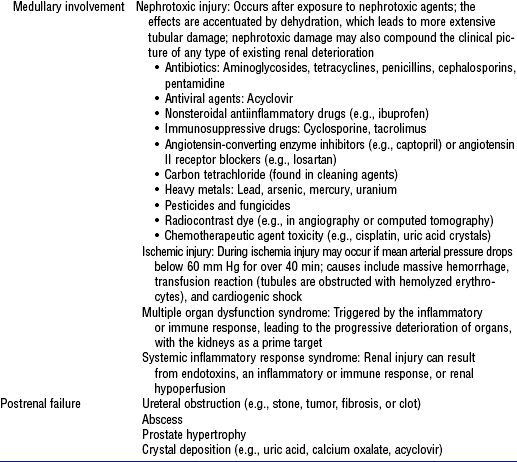
ii. Medullary involvement after prolonged ischemia or hypoperfusion or nephrotoxic injury to the tubular portion of the nephrons (Figure 5-4, A)
(a) Medullary hemodynamics: Hypoperfusion states and oxygen insufficiency disrupt the fine balance between limited oxygen supply and high oxygen consumption in the outer medullary region; may contribute to ARF from hypoxic medullary damage
(1) Conditions predisposing to hypoperfusion
(2) Pharmacologic agents can also alter medullary hemodynamics, especially if administered in absence of volume depletion (i.e., furosemide, mannitol, dopamine). Other substances suspected of improving medullary hemodynamics are nitric oxide, which is normally produced by the macula densa to control glomerular blood flow and renin release, and ANP, an endogenous vasodilator.
(b) Tubular necrosis produced as localized damage in patchy pattern (actual necrosis) or in apoptosis as disruption of cellular function (usually in the distal tubules): Extent of the damage differs in nephrotoxic injury, ischemia or hypoperfusion, sepsis-associated states, and multiple organ failure
(1) Nephrotoxic injury affects the epithelial cellular layer (can regenerate)
(2) Ischemia and hypoperfusion alter renal tubular cells and damage the tubular basement membrane (cannot regenerate)
a) Cellular injury may involve several factors: ATP depletion, oxygen free radical formation, loss of epithelial cell polarity, and increased calcium levels; apoptosis causes DNA fragmentation and cytoplasmic condensation
b) ATP depletion: Begins 30 seconds after the kidney is hypoperfused; normal homeostatic benefits of cellular ATP (preservation of cellular volume, ionic composition, membrane integrity) are lost
c) Oxidative metabolism produces oxygen free radicals
1) Because these substances are highly reactive and volatile, intracellular mechanisms (enzyme systems and antioxidants) exist for their rapid breakdown and destruction
2) Left unopposed, as during ischemic events, these radicals disrupt cellular functioning (e.g., during ischemia, the renal cell is unstable and unable to protect itself from oxygen free radicals, which results in renal cell injury)
d) Loss of epithelial cell polarity: Ischemia alters the passage of water, electrolytes, and other charged elements through the tubule’s epithelial wall, which leads to a concentration defect
e) Increased calcium levels: Ischemic and hypoperfusion states lead to a rise in intracellular calcium levels that causes renal vasoconstriction and a decrease in GFR
(3) Systemic inflammatory response syndrome (SIRS): Released endotoxins significantly reduce renal perfusion, and renal vasoactive substances alter renal cellular metabolism and constrict renal vasculature (see Chapter 9)
(4) Multiorgan dysfunction syndrome results in rapid and progressive deterioration of renal function (see Chapter 9)
(c) Phases of recovery: Classic form of ARF has four phases, whereas nonoliguric form has only three; the nonoliguric phase seems to be synonymous with the diuretic phase, which suggests that nonoliguric ARF reflects less tubular damage so recovery is more rapid
(d) Onset, or initial phase, precedes the actual necrotic injury and correlates with a major alteration in renal hemodynamics
(1) Associated with a decrease in RBF and GFR
(2) Most important factor altering RBF is decrease in cardiac output
(3) Other mechanisms contributing to decreased renal perfusion are increased sympathetic activity and renal vascular resistance
(4) A consistent increase in cardiac output during this phase will maintain an increase in RBF and protect the patient from impending ARF
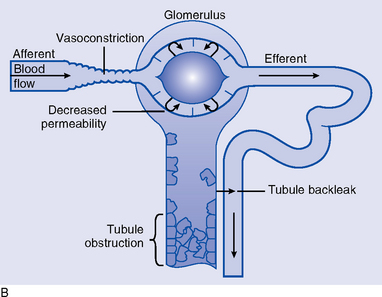
(e) Oliguric phase reflects four processes (Figure 5-4, B)
(1) Obstruction of tubules by cellular debris, tubular casts, or tissue swelling
(2) Reabsorption or back-leak of urine filtration through the damaged tubular epithelium and into circulation
(3) Tubular cell damage with development of necrotic, patchy areas; the cell leaks ATP and K+, edema is present, mitochondria are altered, and calcium leaks into the cell
(4) Renal vasoconstriction continues and may contribute to the decreased GFR
(f) Nonoliguric phase reflects less tubular damage; symptomatology resembles that of the diuretic phase
(1) Urine output may exceed 1 L/hr
(2) Solute present in urine at approximately 350 mOsm/L
(3) Creatinine clearance is as high as 15 ml/min, and Na+ excretion is low
(4) Hyperkalemia remains a significant problem
(5) Phase of short duration; recovery phase reached in 5 to 8 days
(g) Diuretic phase: Signifies that tubular function is returning
c. Postrenal conditions: Associated with obstruction of the urinary collecting system
i. Partial obstruction: Can increase renal interstitial pressure, increasing opposing forces of glomerular filtration; result is diminished urine output
ii. Complete obstruction: Impediment to urine flow accompanies bilateral kidney involvement; the “back-up” pressure of urine compresses the kidneys
2. Etiology and risk factors: See Table 5-4
a. Malaise, fatigue, lethargy, confusion
b. Twitching and/or weakness secondary to metabolic acidosis
d. Change in urine color and/or volume: Oliguria (<400 ml/24 hr); nonoliguria (excess, dilute urine); anuria (no urine output or <100 ml/24 hr); or hematuria
i. Dysrhythmias secondary to electrolyte imbalance or heart failure
ii. Change in pulse rate (either tachycardia or bradycardia)
f. Skin changes: Dry skin, edema, pallor, bruising, uremic frost (rare), pruritus
h. Local or systemic infection presenting with shaking, chills, and fever
i. Abdominal distention secondary to enlarged bladder, obstruction
ii. Intrarenal—cortical disease
(1) Urinary sodium level less than 10 mEq/L
(3) Moderate to heavy proteinuria
(b) Serum BUN and creatinine levels elevated but remain in 10:1 ratio
iii. Intrarenal—medullary disease
(a) Serum BUN and creatinine levels elevated with complete obstruction
(b) Bacteriologic report showing significant positive results for a specific organism
(a) Antistreptolysin O titer: To diagnose recent streptococcal infection (may cause poststreptococcal glomerulonephritis)
(b) Antiglomerular basement membrane titers: To diagnose Goodpasture’s syndrome, a devastating disease of pulmonary hemorrhage and renal failure
(c) Antineutrophil cytoplasmic antibody test for pulmonary and renal failure
(d) Serum studies for complement components: A fall in complement levels is seen in active complement-mediated glomerulonephritis
(e) Serum electrophoresis for immunoglobulin levels: Abnormal proteins (as in multiple myeloma) can damage kidneys
(f) Hepatitis serologic tests: Hepatitis B and C cause kidney disease
b. Radiologic: To rule out obstruction as a cause of oliguria or anuria, because immediate treatment may reverse renal failure. Kidney size provides diagnostic information, because small kidneys imply chronic rather than acute renal failure (see Diagnostic Studies under Patient Assessment)
6. Collaborating professionals on health care team
a. Critical care nurse, clinical specialist or physician assistant, case manager, hemodialysis nurse
b. Physicians: Intensivist, nephrologist (higher mortality when renal consult delayed >48 hours), hematology consult (if blood dyscrasias accompany ARF), vascular surgery consultant
a. Anticipated patient trajectory: Patients with ARF experience rapid decline, with recovery from 8 days for nonoliguric ATN and from 2 weeks to 3 months for oliguric ATN. Transfer or discharge varies with the stage of renal recovery. Expect patients with ARF to have needs in numerous areas:
i. Skin care: Impaired skin integrity due to uremia, malnutrition, immobility
(a) Assess for uremic effects on skin integrity (Table 5-3)
ii. Nutrition: ARF is associated with accelerated protein catabolism that contributes to negative nitrogen balance and uncontrollably high BUN levels usually indicative of a hypercatabolic state. Repeated elevations of BUN over 100 mg/dl despite routine dialysis correlate with evidence of rapid muscle wasting and indicate the need for higher levels of protein consumption, together with a continuous form of dialysis.
(a) Maintain protein intake at a minimum of 0.6 to 0.8 g/kg of body weight; administer higher amounts of protein during hypercatabolism
(b) Provide total calories of 30 to 35 kcal/kg/day of a carbohydrate and lipid combination while controlling glucose and triglyceride intake
(c) Be aware that hyperalimentation and daily dialysis have been associated with increased survival rates in ARF as well as promotion of renal tubular cell regeneration. Hyperalimentation requirements include consumption of large amounts of both essential and nonessential amino acids.
(d) Give IV glucose and lipid solution to augment caloric and nutritional intake, thereby reducing the need for protein in hypercatabolic states
(e) Maintain fluid restriction by limiting non–electrolyte-containing fluids
(f) Administer water-soluble vitamins. Avoid excessive doses of vitamin C (not exceeding 250 mg/day), which may exacerbate ARF. Be cautious with vitamin A, because excessive intake in the absence of renal excretion can lead to vitamin A toxicity.
(g) Monitor serum protein, albumin, hematocrit, and urea levels and weigh daily to assess the effectiveness of nutritional therapy
iii. Infection control: Uremia increases patient susceptibility to infection
(a) Assess for BUN levels over 80 to 100 mg/dl because these are associated with an increased risk of infection
(b) Monitor for early signs of septic shock (SIRS)
(c) Monitor serum protein and albumin levels, because inadequate levels have an immunosuppressive effect
(a) Teach patient and family members or significant others the following:
(1) Etiology and course of the disease
(2) Dietary and fluid restriction requirements
(b) Assess and prepare the home for patient care and, if appropriate, for dialysis
(c) Make the patient and family aware of community resources (e.g., national or local kidney foundation [http://www.kidney.org], local dialysis center)
(d) Assist in patient transition to rehabilitation and/or home care
(a) Use pharmacologic agents with adequate fluid replacement to reestablish or augment RBF. This does not protect the tubules from damage but may limit the extent of damage, creating nonoliguric ATN.
(1) Renal-dose dopamine: No longer the therapy of choice for prevention or treatment. Research (Kellum and Decker, 2001) reveals that dopamine has no benefit in treating ARF. Dopamine may actually compromise the kidney by moving oxygen to the renal medulla; can cause tachycardia and mesenteric ischemia and does not decrease mortality.
(2) Diuretics: Studies (Mehta et al, 2002; Singri, Ahya, and Levin, 2003) question their effectiveness in treating ARF
1) Traditional diuretics (mannitol, loop diuretics): Used to convert oliguria to nonoliguria
2) Mannitol: Protects the kidney by preventing the buildup of cellular debris, reducing tubular obstruction, and augmenting blood flow. Preserves mitochondrial function via osmotic effect; limits recovery ischemia and free radical production. Administer with caution; may precipitate pulmonary edema.
3) Furosemide (Lasix): Acts as both a diuretic and an augmentor of RBF; maximum dosage should not exceed 4 mg/kg/min
b) Diuresis encourages removal of sloughed tubular cells, eliminating tubular obstruction
c) Volume replacement needs to be a priority before administering diuretics; a trial of diuretics can be attempted but should be limited when effectiveness is in question
d) Monitor and report changes in urine output (onset of oliguria, nonoliguria, or anuria)
(b) Metabolism and excretion of pharmacologic agents may be altered in ARF (see Patient Care)
(a) Prevention modalities for ARF: Remain the best intervention; preservation of renal function is the desired outcome
(1) Identify patients at higher risk for ARF
a) Hemodynamic instability; blood loss or hypotension in surgical patients
b) Multiple trauma, multiorgan dysfunction syndrome, rhabdomyolysis
(2) Monitor for prerenal or onset stage of ATN (see Diagnostic Study Findings for prerenal failure)
(b) Correct hypotension and/or renal hypoperfusion by fluid administration and/or pharmacologic agents
(1) Fluid administration: The single best modality for reinstating renal perfusion is to increase cardiac output through the administration of fluids, especially in preventing radiocontrast-associated ARF
a) Consider the following fluids: Normal saline, colloids (either albumin or dextran) and/or blood products
b) Monitor patient response to administration of as much as 1 to 2 L normal saline over 2 or more hours; observe for nonresponse to volume expansion or pulmonary edema
(2) Pharmacologic agents: Include calcium channel blockers, ANP
a) Dopamine not proven to be clinically useful
b) Calcium channel blockers vasodilate renal vasculature and augment renal function; found to be useful in ARF secondary to renal transplantation, radiocontrast nephrotoxicity, and cyclosporine use
c) ANP use associated with improvement in oliguric rather than nonoliguric ARF; beneficial in management of heart failure
d) Mucomyst (N-acetylcysteine) beneficial in the prevention of ARF secondary to IV radiocontrast nephrotoxicity; consider 600 mg by mouth twice daily
(c) Determine the need for hemodialysis: Early initiation of any form of dialysis is beneficial for the prevention and management of acute and chronic renal failure
(1) Indications for which hemodialysis remains the initial treatment of choice
(d) Initiate hemodialysis (Figure 5-5)
(1) Principles of hemodialysis: Include osmosis (optional), diffusion, and convection-ultrafiltration
a) Osmosis: Movement of water across a semipermeable membrane from an area of lesser to an area of greater osmolality
b) Diffusion: Movement of molecules from area of higher to an area of lower concentration
c) Ultrafiltration and convection: Movement of particles through a semipermeable membrane by hydrostatic pressure
(2) Hemodynamics: By means of vascular access and a blood pump, about 300 ml of blood travels through an extracorporeal dialyzer, which removes wastes, toxic substances, excess electrolytes, metabolic products, and pharmacologic agents and then returns the blood to the systemic circulation
a) Prior to the procedure, heparinization is performed to keep blood anticoagulated within the hemodialysis machine (regional heparinization)
b) For patients without complications, 5000 units heparin is administered to start and 2000 units/hr is given while the patient is on the machine (general heparinization); dosage may be adjusted to meet the needs of the individual patient
(4) Vascular access for dialysis
a) Central venous access (i.e., dual-lumen internal jugular, femoral, or subclavian catheter): For emergent dialysis or temporarily after failure of a permanent catheter while awaiting repair or replacement
1) Blood flow must range from 200 to 500 mL/min to accommodate hemodialysis
2) Double- or triple-lumen catheter requires the use of a large vein, such as the femoral vein, which limits ambulation and carries the risk of dislodgement, infection, and kinking; other sites include the right or left subclavian and right or left jugular vein
3) Palpate peripheral pulses in the cannulated extremity
4) Observe for bleeding or hematoma formation; if it occurs, apply pressure dressing and notify the physician
5) Properly position the catheter to avoid dislodgment during the dialysis procedure
6) If the femoral vein catheter is to be maintained after dialysis, connect it to a pressurized IV flow system. Add a low dose of heparin (500 U/L) to the solution. Maintain a secure aseptic dressing to minimize the risk of infection. No standing or ambulation is allowed while the catheter is in place.
7) On removal of a femoral catheter, apply direct pressure to the puncture site for 5 to 10 minutes (or the time needed to stop the bleeding after dialysis and after the period of heparinization). Complete this procedure with the application of a pressure dressing and a period of bed rest.
b) Permanent vascular access: An arteriovenous fistula is usually placed in an upper rather than a lower extremity
1) Surgical procedure with anastomosis of an artery to a vein, or an artificial vascular graft is used
2) Do not perform venipuncture, start IV therapy, give injections, or take BP with a cuff on the arm with a fistula; post this information on signage above bed
3) Palpate the thrill or auscultate the bruit to confirm patency
4) Avoid circumferential dressings and restrictive clothing
5) Report bleeding, skin discoloration, drainage, and other signs of infection; culture the drainage
c) External permanent vascular access: An arteriovenous shunt is rarely selected
1) Auscultate for the bruit or palpate for the thrill to assess shunt patency
2) Promptly report any suspicion of clotting (color change of blood, separation of serum from erythrocytes, absence of pulsations in tubing)
3) Hydrate adequately to minimize clotting
4) Change the sterile dressing over the shunt at least daily; reinforce the dressing as necessary
5) Do not perform venipuncture, give IV therapy, give injections, or take BP with a cuff on the shunt arm
(5) Hemodialysis membrane compatibility
(6) Frequency: ARF may require daily dialysis or a one-time dialysis treatment to resolve an acute problem, such as a hyperkalemic episode
(e) Continuous renal replacement treatment (CRRT)
a) Form of dialysis specifically developed for the critically ill, hemodynamically unstable patient
b) May also be selected when both hemodialysis and peritoneal dialysis are contraindicated
c) Associated with a shorter length of stay and less resource utilization, but not with lower mortality
a) Conditions of fluid overload or cardiovascular instability requiring a continuous method of fluid removal or compensation for azotemia (e.g., ATN)
b) Ascites, diuretic-resistant edema, acute pulmonary edema
c) Post cardiac surgery, recent acute MI
d) Inability to tolerate the cardiovascular impact of rapid fluid losses associated with hemodialysis or failure of a trial of hemodialysis
(3) Contraindications: Rare; hematocrit over 45% is a contraindication for manual forms of CRRT (i.e., continuous arteriovenous hemofiltration, continuous arteriovenous hemodialysis)
a) Slow continuous ultrafiltration (SCUF)
b) Continuous arteriovenous hemofiltration (CAVH)
c) Continuous arteriovenous hemodialysis (or hemodiafiltration) (CAVHD)
d) Continuous venovenous hemofiltration (CVVH)
e) Continuous venovenous hemodialysis (CVVHD) or hemodiafiltration (CVVHDF); involves the use of a hollow-fiber hemofilter capable of rapid fluid removal during hypotensive or low blood flow states
(5) Principles: See Principles of Hemodialysis
a) Heparin used in SCUF, CAVH, and CAVHD
b) Heparin or trisodium citrate used in CRRT machine forms of CVVH, CVVHD, and CVVHF
c) Trisodium citrate causes binding of serum calcium; therefore, must monitor calcium levels. Calcium administration may be necessary.
(7) Frequency: A continuous dialysis form providing the ability to dialyze 24 hours a day and 7 days a week; the advantage in the critically ill is homeostasis, with avoidance of erratic swings in the levels of toxic substances
1) Use the principle of ultrafiltration, the exchange of primarily plasma water along with particles (e.g., K+, BUN, creatinine) by convection
2) Exchange rate depends on membrane area, fiber diameter, hematocrit, plasma protein concentration, pressure gradient, and blood flow rate
b) CAVHD: Incorporates peritoneal dialysis fluid with ultrafiltration, thus combining the principles of diffusion and convection. Peritoneal dialysis fluid administration is regulated by a volumetric pump at 15 ml/min. The dialysis fluid enters the ultrafiltration compartment of the hemofilter and flows in the opposite direction to the blood flow.
c) CVVH: Used more often than CAVH; an adaptation of the previous method. The replacement fluid is often electrolyte or bicarbonate based. Uses a blood pump with a venovenous blood access and administration of replacement fluid. Use of a single venovenous puncture is an advantage over CAVH, which requires arterial and venous punctures. Ultrafiltration is the primary principle involved, and solute is removed by convection. No dialysate is used (Figure 5-6).
d) CVVHD: Uses a blood pump in conjunction with the dialysate flowing countercurrently to the blood for the ultrafiltration, diffusion, and osmotic dialysis effect. No replacement fluid is used.
e) CVVHDF: Similar to CVVHD; uses a blood pump and dialysate flowing countercurrently to remove and replace high volumes of fluid hourly. Solute removal is via both convection and diffusion. Replacement fluid is used.
f) Overview of the method for CRRT
1) Prepare the patient: Explain the procedure. Obtain baseline serum analyses, clotting time, blood chemistry analyses, ABG levels, and complete blood count. Administer a loading dose of heparin.
2) Prepare the hemofilter, apply the blood pump if initiating CVVH or CVVHD, and connect to the vascular access properly
3) Attach the peritoneal dialysis fluid infusion if initiating CAVHD
4) Determine the blood flow through the hemofilter and the resulting ultrafiltration rate, and begin fluid replacement therapy
5) Monitor fluid replacement according to the patient’s condition and desired rate of filtrate output to prevent circulatory collapse
6) Regulate BP, oncotic pressure, and ultrafiltration compartment to optimize the amount of filtrate (according to the prescribed dialyzing device)
g) Potential complications include clotting, hypotension, air entry, blood leak
(f) Peritoneal dialysis (PD): Effective in the critically ill for maintaining homeostasis; however, if hemodialysis is contraindicated, CRRT is generally used (CVVH); a combination of PD (for solute removal) and hemodialysis (for ultrafiltration) also effective in ARF (Figure 5-7)
b) Electrolyte or acid-base imbalance
c) Acute or chronic renal failure
d) Intoxication from dialyzable drugs and poisons
(2) Contraindications: Bleeding disorder, abdominal adhesions, recent peritoneal surgery
(3) Principles of PD: Primarily osmosis and diffusion
(4) Description: Dialysate is instilled into the peritoneal cavity through a catheter, allowed to “pool” (usually for a minimum of 30 minutes), then drained. New dialysate is infused, which initiates the next cycle.
(5) Anticoagulation: Minimal amount of heparin required
(6) Frequency: Continuous form of dialysis; dialysis sessions can last 3 to 4 days or longer depending on the needs of the patient
ii. Uremic pericarditis with effusion
(a) Mechanism: Uremic toxins on myocardium result in pericarditis with or without pericardial effusion
(a) Mechanism: Actual bleeding secondary to primary cause or uremia, septic shock, or SIRS, which is commonly associated with the onset of disseminated intravascular coagulation. Lack of erythropoietin observed at onset of ARF.
(a) Mechanism: Inability of kidneys to excrete electrolytes and concentrate urine in ARF
(b) Management: See later sections on hyperkalemia, hypocalcemia, hyperphosphatemia, and hypermagnesemia, which are the most common imbalances
(a) Mechanism: During ARF, sleep is interrupted by the intensity of care, critical care environment, sleep apnea, and uremic condition (e.g., tremors, restless leg syndrome). Nursing research reveals an increase in the number of recalled nightmares the night prior to dialysis at the uremic peak, which results in a disturbed sleep pattern. Disruption of sleep interferes with the healing process and quality of life.
vii. Altered metabolism and excretion of pharmacologic agents: See Patient Care
a. Renal perfusion is improved to prevent prerenal failure and ATN
b. Urine output exceeds 30 ml/hr with normal concentration and volume; balanced 24-hour I&O record coincides with daily weight
c. Weight is stable, with no evidence of muscle wasting; nutrition is adequate
d. Electrolyte balance and metabolic acidosis are WNL or minimized at asymptomatic levels
e. Dialysis is tolerated and corrects or maintains asymptomatic fluid, electrolyte, and acid-base balance
f. Infection-free skin is dry, clean, and intact with no itching; wound healing is progressing
g. Patient is free of major anxiety, coping satisfactorily with illness, participating in care, using effective support systems, and not suffering from sleep deprivation
h. Patient has knowledge of ARF and treatment and is compliant with disease management expectations