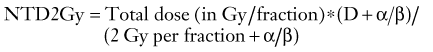CHAPTER 249 The Radiobiology and Physics of Radiosurgery
Radiosurgery is a concept devised by Lars Leksell1 that involves destruction of intracranial targets and induction of desired biologic effects in target tissue with the use of a single high dose of cross-firing ionizing beams through the intact skull. The concept of radiosurgery has since been extended to include treatment with one to five fractions and now involves targeting of extracranial sites as well.
Types of Ionizing Radiation
Electromagnetic Radiation
X-rays and gamma rays differ only in their manner of production. X-rays are produced either as a result of the interaction between a high-speed electron and a nucleus (bremsstrahlung x-rays) or as a result of electrons in the outer shell of an ionized atom falling from a high- to a low-energy level to fill a vacancy created by an electron that has been ejected (characteristic x-rays). X-rays may be a by-product of radioactive decay or may be created by human intervention. For example, linear accelerators (LINACs) generate x-rays by accelerating electrons and directing them to strike a target composed of a substance with high atomic number. The electrons interact with the target nuclei and generate (primarily) bremsstrahlung and (secondarily) characteristic x-rays.2
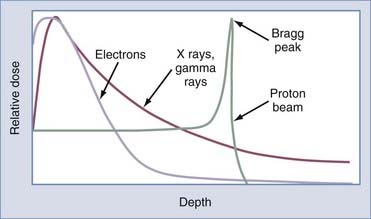
High-energy photons (>1 MV) are useful in radiation therapy because they deposit a significant amount of energy at depth in tissue, so they can be used to treat tumors deep within the body. In addition, high-energy photons exhibit a property called the build-up region when they enter tissue because the electrons liberated by the interacting photons near the skin surface are propelled in a mostly forward direction and deposit their energy deeper in tissue. This gives photons an advantage known as the “skin-sparing” effect (Fig. 249-1).
Particle Radiation
High-energy electrons are usually produced in LINACs by replacing the high–atomic number target (usually tungsten), which results in x-ray production, with a foil that serves to scatter the electrons in a desired pattern. Electrons begin depositing appreciable dose near the surface of tissue, have a predictable range at which they deposit the majority of their energy, and exhibit rapid dose falloff. This gives electron therapy a particular advantage in the treatment of cutaneous or subcutaneous lesions (Fig. 249-1).
High-energy protons are produced in particle accelerators such as cyclotrons. Protons are much more massive particles than electrons. Therefore, at a particular velocity, protons have much greater kinetic energy and do not scatter as easily. Hence, protons can potentially cause less damage to surrounding tissue. In addition, most of the energy absorption from protons occurs at the distal end (over the last few millimeters) of the particle track. The precisely defined area of intense ionization at the end of the track after the passage of protons is called a Bragg peak (Fig. 249-1). After the Bragg peak the deposited energy falls off quickly, so protons have a defined range in tissue with essentially no exit dose. To treat the entire thickness of a tumor, the proton beam may be altered to spread the Bragg peak out to the desired range of depth. By taking advantage of the Bragg peak effect, as well as cross-firing of a number of proton beams, a well-localized volume of high radiation delivery can be produced and has been applied in a radiosurgical setting.3,4
Radiobiology
Radiobiology of Conventional Radiotherapy
The probability of cell survival after single doses of radiation is a function of the absorbed dose, measured in the unit gray (Gy). Typical mammalian cell survival curves obtained after single-dose irradiation in culture have a characteristic shape that includes a low-dose shoulder region followed by a steeply sloped region at higher doses.5,6 The shoulder region is interpreted as an accumulation of sublethal damage at low doses, with lethality resulting from the interaction of two or more such sublethal events. As noted previously, single-strand breaks in DNA may be repaired and therefore represent sublethal damage to the cell. However, double-strand breaks may result in cellular changes, including cell death. Such a model can be described by the following probabilistic equation in which probability (cure or complication) = exp(−K*exp[−αD − βD2])] (“exp” represents exponential, K equals the number of clonogens, “α“ and “β“ are constants related to single-event cell killing and cell killing through the interaction of sublethal events, respectively, and “D” represents dose). The α/β ratio is the single dose at which overall cell killing is equally attributable to both components of cell killing (αD = βD2 or D = α/β).7 The validity of the linear quadratic formula for single-dose radiosurgery has been questioned, however.8 Nevertheless, it still provides a meaningful method to relate radiosurgery to fractionated radiation schemes.
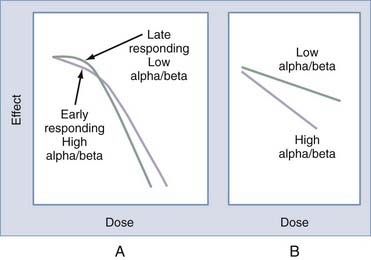
The α/β ratio varies depending on the tumor and normal tissue type (Fig. 249-2). Late-responding tissues such as the brain or spinal cord have an α/β ratio of approximately 2, whereas many tumors have an α/β ratio of nearly 10. The α/β ratio for skin or mucosa is approximately 5 to 8. Tumors with a low α/β ratio (i.e., a small α or single-hit component for radiation kinetics) will have less of a desired effect when a scheme involving a low radiation dose per fraction is used than when comparable tissues with a high α/β ratio are treated. The dose may be normalized to a scheme of 2 Gy per fraction (NTD2Gy) by using the following equation9:
Conventional fractionated radiation therapy relies on the four R’s of radiobiology: repair of nonlethal injury, reoxygenation of hypoxic tumor cells, repopulation of tumor cells, and reassortment of tumor cells into more susceptible phases of the cell cycle. There are advantages and disadvantages to fractionated radiation therapy and radiosurgery. Depending on the clinical scenario, one may prove superior to the other. Certainly, there seems to be little advantage to fractionation for functional cases (e.g., trigeminal neuralgia) or for the treatment of patients with arteriovenous malformations (AVMs).8
Modalities of Radiation Therapy
Stereotactic Radiosurgery
Leksell published his paper on radiosurgery in 1951.1 He described coupling of the stereotactic technique with narrow beams of radiant energy to target an area in the brain. The points of entry of the beams were distributed over the convexity of the patient’s skull. At the time, Leksell was using x-ray radiation as his energy source.
In the perspective of the past half century, Leksell’s paper turned out “to be a milestone in contemporary neurosurgery”10 that totally changed our way of viewing the management of neurosurgical pathology and forced “reluctant neurosurgeons to consider major changes in classic thinking about the proper care of many illnesses including vascular malformations, cavernous sinus meningiomas and vestibular schwannomas.”11
Radiosurgery proved successful after development of the Gamma Knife and modification of LINACs and cyclotrons for conformal delivery of beams to intracranial targets, as well as targets in other parts of the body. Radiosurgery is a minimally invasive technique designed by Lars Leksell to deliver a destructive amount of radiation to intracranial lesions that may be inaccessible or unsuitable for open surgery. Undoubtedly, the experience of delivering ether anesthesia to neurosurgical patients for Dr. Olivecrona motivated Leksell to devise a technique associated with fewer complications than occur with open surgery. A passage from Leksell’s autobiography proved that the idea of a minimally invasive neurosurgical approach was on his mind for some time. At the first Scandinavian neurosurgical meeting held in Oslo, Leksell left the conference room during a less than exciting presentation and decided to walk in a garden. While on this walk, Leksell met Sir Hugh Cairns. Leksell confessed to Cairns his doubts concerning the state of neurosurgical techniques available at the time and was convinced that something new had to be developed. He explained his plans to mechanically direct a probe into the brain by using perhaps the brain’s own electrical activity and ablate pain pathways. He also mused about the idea of using narrow-beam x-rays or ultrasound as the physical agent and doing away with the probe entirely. His enthusiasm and ideas were given a warm reception by Cairns, and the encouraged young Leksell started work that led to the development of an “arc-radius” type of stereotactic system. Leksell wrote, “I was born under the sign of the ‘archer’ and looked forward to sharpshoot into the brain.”12
In the following 10 years, Leksell made considerable progress in the treatment of deep brain structures with a single heavy dose of radiation. He collaborated with physicists Kurt Liden and Borje Larsson to use a proton beam for radiosurgery.13–15 The first stereotactic proton beam operation was performed at the Gustaf Werner Institute in Uppsala in 1960. Leksell found the synchrotron too awkward and expensive for widespread use and consequently developed a similar technique based on the LINAC. At the time, however, LINACs lacked the precision needed for use by neurosurgeons. Leksell also tried focused beam ultrasound, but this too lacked precision and required that a cranial defect be made before its use.
The next logical step was to look for another radiation source. Leksell turned to 60Co. The first stereotactic Gamma Knife unit was installed in Sophiahemmet Hospital in 1968.16 The unit was originally intended for functional neurosurgery (Larsson and colleagues, 1974). However, the applications were quickly expanded to include AVMs and certain brain tumors.17,18 The first Gamma Knife yielded fairly promising results, and an improved second Gamma Knife unit was built and installed at the Karolinska Institute in 1974. This unit proved to be both reliable and easy to use. Leksell wrote that, “Maybe the most important lesson learnt at the Karolinska is that the simplicity of using the Gamma Unit makes this integration possible and that the same individual can be a competent microsurgeon and also a stereotactic radiosurgeon. Someone competent in both techniques is best fitted to decide where the boundaries between the two methods should lie.”16
In 1983 at a hospital in Buenos Aires, Betti and Derechinsky introduced the concept of a modified LINAC for radiosurgery.19,20 This system relied on a 10-MV LINAC and used a chair-based Talairach stereotactic frame.21 Other innovative developments in LINAC-based radiosurgical devices quickly followed from Hartman and colleagues in Heidelberg, Barcia-Salorio and associates in Valencia, Colombo and coworkers in Vincenza, Podgorsak and colleagues in Canada, and Friedman and Bova in Florida.19,20,22–26 At each of these centers, neurosurgeons played a critical role in the refinement of LINACs for radiosurgery. Similarly, the multileaf collimator for LINACs was designed in part to achieve conformality. Innovative work in the field of radiosurgery involving the use of heavy particles from cyclotrons was also conducted by Raymond Kjellberg, Jay Loeffler, and Jacob Fabrikant.
Radiosurgery is possible because of the synthesis of two concepts: stereotaxis as applied to delivery of radiation and three-dimensional visualization. Stereotaxis allows precise localization of a target point to be determined within a coordinate system known as stereotactic space. This is typically accomplished by attaching a rigid stereotactic head ring to the patient. The head ring is of known dimensions, and by affixing it to the patient’s head a physical correspondence between the stereotactic coordinate system and the volume of the head is established. A number of stereotactic systems have been developed, including the Spiegel-Wycis frame,27 the Leksell stereotactic frame,28 the Talairach frame,29 and the Todd-Wells frame, which eventually became the CRW and BRW frames.30
Linear Accelerator–Based Radiosurgery
LINACs were first proposed as radiation sources for radiosurgery by Larsson and coauthors in 1974. The earliest reports on clinical LINAC-based radiosurgery were published in 1983 by Betti and Derenchinsky20 and in 1985 by Colombo and coworkers31 and Hartmann and associates.24 The LINACs used for radiosurgery are usually modified from machines used for routine cancer therapy to achieve smaller beam sizes and more precise positioning specifications.
Gamma Knife Radiosurgery
Operational Principles
The Gamma Knife operates by precisely aligning the gamma-ray emissions from an array of 60Co sources so they intersect at a point called the focus point. Each individual beam has a fairly low dose rate; however, summation of the beams at the focus point creates a very high dose rate. By spreading the energy of a treatment among the beams (either 201 or 192 beams, depending on the model of the unit), it is possible to achieve a high radiation dose within the target volume while largely sparing normal brain because the dose quickly falls to a low level as the distance from the focus (or isocenter) increases. Dosimetry in Gamma Knife radiosurgery is quite different from that in traditional fractionated radiation therapy, which focuses on dose homogeneity within the target volume; the steep dose gradient achieved by the Gamma Knife means that the dose within the target is quite inhomogeneous.32
Gamma Knife Description
The Gamma Knife unit (Fig. 249-3) itself consists of several main components: a large spherical shield (the bulk of the unit) that contains the array of cobalt sources and protects the patient and operational staff from gamma emissions, a central body that actually holds the source array and contains the primary collimation system that directs the gamma rays to the focus point, a treatment table that moves the patient’s head in and out of the unit (and in more recent units precisely positions the head so that the target is at the focus point), a control suite to allow operational control of the unit, and a treatment planning system that allows the neurosurgeon to create appropriate dose distributions.
In the most recent PERFEXION Gamma Knife, the external collimation system has been replaced by a single, internal collimating structure with precisely machined individual collimators (4, 8, and 16 mm). The 60Co source array has been split into eight sectors with source holders that can slide on linear bearings driven by motors at the rear of the unit to align the sector with any of the available collimator sizes or a “blocked” position. Thus, each of the eight sectors may be configured independently of the others so that a “composite” isocenter can be created that is composed of multiple field sizes. Shielding with plugs (a highly manual process) has similarly been replaced by the fully automated process of setting a sector to the blocked position.33
Adverse Effects of Radiation
Central Nervous System Toxicity of Fractionated Radiation Therapy
Another late complication is diffuse cerebral atrophy. Diffuse cerebral atrophy is clinically associated with cognitive decline, personality changes, and gait disturbances. In children, radiation therapy, surgery, and the intracranial tumor contribute to intellectual decline. The radiation-associated decline in intelligence is generally in the area of performance, especially visuospatial integration.34–36 Because myelination is not complete until the age of 2 to 3 years, young children are at greater risk.37 A decline in verbal IQ has also been noted. As in adults, previous acquired knowledge is usually preserved, but there is a deficit in acquiring new skills.38
Complications after Radiosurgery
Radiation-induced changes are characterized by a bright signal on T2-weighted MRI. In cases in which it is associated with contrast enhancement on T1-weighted MRI, it presumably represents radiation-induced injury with an associated breakdown of the blood-brain barrier. Guo reported that radiation-induced changes can be observed in 47% of patients after Gamma Knife radiosurgery for AVMs. The onset of these changes occurred 3 to 15 months after treatment in the majority of patients (92%) and more than 26 months after treatment in 8%.39 Progressive resolution of the radiation-induced effects is the usual course. The clinical manifestations included headache, symptoms of raised intracranial pressure, and focal neurological deficits. In a small percentage of patients, it is associated with focal damage to neural tissue. Neurological deficits were still present at the time of the last follow-up in 3% of patients.
Flickinger and associates evaluated follow-up imaging and clinical data in 307 patients with AVMs treated by Gamma Knife radiosurgery.40 Radiation-induced changes developed in 30.5% of patients and were symptomatic in 10.7%. The changes resolved within 3 years and did so significantly less often in patients with symptoms (52.8%) than in asymptomatic patients (94.8%). The 7-year actuarial rate for the development of persistent symptomatic radiation-induced changes was 5.05%. Multivariate logistic regression modeling found that the 12-Gy volume was the only independent variable that significantly correlated with the changes whereas symptomatic radiation-induced changes were correlated with both 12-Gy volume and AVM location. They suggested that complications from radiosurgery for AVMs can be predicted with a statistical model relating the risk for development of symptomatic imaging changes after radiosurgery to 12-Gy treatment volume and location.
Cranial Nerves
The largest experience on the radiation tolerance of cranial nerves comes from radiosurgical studies on the trigeminal and facial nerves. In our series of 151 patients who underwent radiosurgery for trigeminal neuralgia, 12 patients (8%) had new-onset facial numbness after treatment.41 Norén and associates analyzed risk factors for facial and trigeminal neuropathy in patients with tumors receiving 12 to 20 Gy and concluded that the most significant factor is the length of the nerve irradiated, not the volume of tumor or dose.42
In treating patients with AVMs, meningiomas, and secretory pituitary adenomas, doses between 20 and 25 Gy were delivered to the cranial nerves in the parasellar region without complications. Tishler and colleagues noted that the maximum dose delivered to the cranial nerves was associated with neurologic deficits in 29 patients after LINAC radiosurgery and 33 patients after Gamma Knife radiosurgery.43 Twelve new neuropathies were observed that were related to nerves in the parasellar region, but they were all unrelated to a maximum dose in the range of 10 to 40 Gy. The conclusion of this study was that doses up to 40 Gy are relatively safe for nerves in the parasellar region. In our recently published studies on radiosurgery for secretory pituitary tumors, factors statistically related to visual dysfunction after Gamma Knife radiosurgery included an increasing maximal dose, decreasing number of isocenters, and more than one Gamma Knife radiosurgery or previous radiation therapy.44,45 The oculomotor nerves are the most radiosensitive nerves, followed by the optic, trochlear, and abducens nerves.
Radiation-Induced Neoplasia
Cahan and associates defined the criteria for a tumor to be considered a result of irradiation: (1) the tumor must occur in the irradiation field; (2) it cannot be present before irradiation; (3) any primary tumor must differ histologically from the induced tumor; and (4) there must be no genetic predisposition for the occurrence of a secondary malignancy or tumor progression.46 Studies from Israel have shown that radiation doses as low as 1.5 Gy to the brain after the treatment of tinea capitis increase the risk for malignant tumors or meningiomas even 30 year after exposure.47 Brada and coworkers conducted a long-term study and reported that the risk for a second tumor after fractionated radiotherapy for pituitary adenoma was 1.3% after 10 years and 1.9% after 20 years.48
Based on the literature, the incidence of radiosurgery-induced neoplasia ranges between 0 and 3 per 200,000 patients; however, the true incidence is likely to be higher because few of these 200,000 patients treated with radiosurgery were monitored over a long period.49 Rowe presented the results of a study in which he cross-referenced patients treated by radiosurgery in Sheffield against England’s national mortality and cancer databases. With a group of 5000 patients and more than 30,000 patient-years of data, 1 patient was found to have new malignant brain tumors.50 However, data covering at least 10 years were available on only 1200 of these patients.
We reviewed 1333 patients with AVMs treated with the Gamma Knife and monitored with sequential MRI. A subset of 288 patients in this group underwent neuroimaging and participated in clinical follow-up for at least 10 years.51 In 2 patients, radiosurgically induced neoplasia was identified. Each of the patients was found to have an incidental, uniformly enhancing, dura-based mass lesion. These lesions displayed the imaging characteristics of a meningioma. From our series, if we conservatively estimate the radiosurgery-induced lesions that would be evident within a 10-year interval, our incidence of radiosurgery-induced neoplasia would 2 in 2880 person-years or 69 in 100,000 person-years. Thus, there is a 0.7% chance that a radiation-induced tumor may develop within 10 years after Gamma Knife radiosurgery. This is less than the 1.3% risk over the first 10 years or 1.9% over 20 years detailed by Brada and colleagues in radiotherapy series. However, our results encompass a follow-up period of just 10 years. Therefore, even though the risk for radiosurgery-induced secondary tumors is low, it must be weighed in the treatment of pediatric patients and in those with benign tumors and a long life expectancy.
Betti O, Derechinsky V. [Multiple-beam stereotaxic irradiation.]. Neurochirurgie. 1983;29:295-298.
Brada M, Rajan B, Traish D, et al. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf). 1993;38:571-578.
Colombo F, Benedetti A, Pozza F, et al. External stereotactic irradiation by linear accelerator. Neurosurgery. 1985;16:154-160.
Colombo F, Benedetti A, Pozza F, et al. Stereotactic radiosurgery utilizing a linear accelerator. Appl Neurophysiol. 1985;48:133-145.
Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol. 1985;58:515-528.
Flickinger JC, Kalend A. Use of normalized total dose to represent the biological effect of fractionated radiotherapy. Radiother Oncol. 1990;17:339-347.
Flickinger JC, Kondziolka D, Maitz AH, et al. Analysis of neurological sequelae from radiosurgery of arteriovenous malformations: how location affects outcome. Int J Radiat Oncol Biol Phys. 1998;40:273-278.
Guo WY. Radiological aspects of gamma knife radiosurgery for arteriovenous malformations and other non-tumoural disorders of the brain. Acta Radiol Suppl. 1993;388:1-34.
Hall EJ, Giaccia A. Radiobiology for the Radiologist, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
Hartmann GH, Schlegel W, Sturm V, et al. Cerebral radiation surgery using moving field irradiation at a linear accelerator facility. Int J Radiat Oncol Biol Phys. 1985;11:1185-1192.
Kjellberg RN, Koehler AM, Preston WM, et al. Stereotaxic instrument for use with the Bragg peak of a proton beam. Confin Neurol. 1962;22:183-189.
Kjellberg RN, Sweet WH, Preston WM, et al. The Bragg peak of a proton beam in intracranial therapy of tumors. Trans Am Neurol Assoc. 1962;87:216-218.
Larsson B, Leksell L, Rexed B, et al. Effect of high energy protons on the spinal cord. Acta Radiol. 1959;51:52-64.
Larsson B, Leksell L, Rexed B, et al. The high-energy proton beam as a neurosurgical tool. Nature. 1958;182:1222-1223.
Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
Leksell L, Larsson B, Andersson B, et al. Lesions in the depth of the brain produced by a beam of high energy protons. Acta Radiol. 1960;54:251-264.
Mehta MP. The physical, biologic, and clinical basis of radiosurgery. Curr Probl Cancer. 1995;19:265-329.
Sheehan J, Steiner L, Laws ER. Pituitary adenomas: is Gamma Knife radiosurgery safe? Nat Clin Pract Endocrinol Metab. 2005;1:2-3.
Steiner L, Leksell L, Greitz T, et al. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Report of a case. Acta Chir Scand. 1972;138:459-464.
Wu A. Physics and dosimetry of the gamma knife. Neurosurg Clin N Am. 1992;3:35-50.
1 Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
2 Khan FM. The Physics of Radiation Therapy, 3rd ed. Baltimore: Lippincott Williams & Wilkins; 2003.
3 Kjellberg RN, Koehler AM, Preston WM, et al. Stereotaxic instrument for use with the Bragg peak of a proton beam. Confin Neurol. 1962;22:183-189.
4 Kjellberg RN, Sweet WH, Preston WM, et al. The Bragg peak of a proton beam in intracranial therapy of tumors. Trans Am Neurol Assoc. 1962;87:216-218.
5 Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol. 1985;58:515-528.
6 Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679-694.
7 Hall EJ, Giaccia A. Radiobiology for the Radiologist, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
8 Hall EJ, Brenner DJ. The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys. 1993;25:381-385.
9 Flickinger JC, Kalend A. Use of normalized total dose to represent the biological effect of fractionated radiotherapy. Radiother Oncol. 1990;17:339-347.
10 Samii M, Matthies C. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92:892-894.
11 Malis L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92:894-895.
12 Steiner L, Prasad D, Lindquist C, et al. Gamma Knife Surgery in Vascular, Neoplastic, and Functional Disorders of the Nervous System, 3rd ed. Philadelphia: Saunders; 1995.
13 Larsson B, Leksell L, Rexed B, et al. Effect of high energy protons on the spinal cord. Acta Radiol. 1959;51:52-64.
14 Larsson B, Leksell L, Rexed B, et al. The high-energy proton beam as a neurosurgical tool. Nature. 1958;182:1222-1223.
15 Leksell L, Larsson B, Andersson B, et al. Lesions in the depth of the brain produced by a beam of high energy protons. Acta Radiol. 1960;54:251-264.
16 Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry. 1983;46:797-803.
17 Leksell L. A note on the treatment of acoustic tumours. Acta Chir Scand. 1971;137:763-765.
18 Steiner L, Leksell L, Greitz T, et al. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Report of a case. Acta Chir Scand. 1972;138:459-464.
19 Betti O. Historique de la radiochirurgie. Cancer Radiother. 1998;2:101-104.
20 Betti O, Derechinsky V. Multiple-beam stereotaxic irradiation. Neurochirurgie. 1983;29:295-298.
21 Mehta MP. The physical, biologic, and clinical basis of radiosurgery. Curr Probl Cancer. 1995;19:265-329.
22 Barcia-Salorio J, Broseta J, Hernandez G. Radiosurgical treatment in huge acoustic neuromas. In: Szilka G, editor. INSERM Symposium on Stereotactic Irradiations, Vol 12. Amsterdam: Elsevier/North Holland Biomedical Press; 1979:245-249.
23 Colombo F, Benedetti A, Pozza F, et al. External stereotactic irradiation by linear accelerator. Neurosurgery. 1985;16:154-160.
24 Hartmann GH, Schlegel W, Sturm V, et al. Cerebral radiation surgery using moving field irradiation at a linear accelerator facility. Int J Radiat Oncol Biol Phys. 1985;11:1185-1192.
25 Podgorsak EB, Olivier A, Pla M, et al. Physical aspects of dynamic stereotactic radiosurgery. Appl Neurophysiol. 1987;50:263-268.
26 Rahman M, Murad GJ, Bova F, et al. Stereotactic radiosurgery and the linear accelerator: accelerating electrons in neurosurgery. Neurosurg Focus. 2009;27:E13.
27 Spiegel EA, Wycis HT, Marks M, et al. Stereotaxic apparatus for operations on the human brain. Science. 1947;106:349-350.
28 Leksell L. A stereotactic apparatus for intracerebral surgery. Acta Chir Scand. 1949;99:229-233.
29 Talairach J, Szikla G. Application of stereotactic concepts to the surgery of epilepsy. Acta Neurochir Suppl (Wien). 1980;30:35-54.
30 Brown RA. A stereotactic head frame for use with CT body scanners. Invest Radiol. 1979;14:300-304.
31 Colombo F, Benedetti A, Pozza F, et al. Stereotactic radiosurgery utilizing a linear accelerator. Appl Neurophysiol. 1985;48:133-145.
32 Wu A. Physics and dosimetry of the gamma knife. Neurosurg Clin N Am. 1992;3:35-50.
33 Leksell Gamma Knife Perfexion: System Description, in article No 1002703. Stockholm: Elekta, AB, 2006.
34 Eiser C. Intellectual abilities among survivors of childhood leukaemia as a function of CNS irradiation. Arch Dis Child. 1978;53:391-395.
35 Hirsch JF, Renier D, Czernichow P, et al. Medulloblastoma in childhood. Survival and functional results. Acta Neurochir (Wien). 1979;48:1-15.
36 Spunberg JJ, Chang CH, Goldman M, et al. Quality of long-term survival following irradiation for intracranial tumors in children under the age of two. Int J Radiat Oncol Biol Phys. 1981;7:727-736.
37 Danoff BF, Cowchock FS, Marquette C, et al. Assessment of the long-term effects of primary radiation therapy for brain tumors in children. Cancer. 1982;49:1580-1586.
38 Mulhern RK, Wasserman AL, Fairclough D, et al. Memory function in disease-free survivors of childhood acute lymphocytic leukemia given CNS prophylaxis with or without 1,800 cGy cranial irradiation. J Clin Oncol. 1988;6:315-320.
39 Guo WY. Radiological aspects of gamma knife radiosurgery for arteriovenous malformations and other non-tumoural disorders of the brain. Acta Radiol Suppl. 1993;388:1-34.
40 Flickinger JC, Kondziolka D, Maitz AH, et al. Analysis of neurological sequelae from radiosurgery of arteriovenous malformations: how location affects outcome. Int J Radiat Oncol Biol Phys. 1998;40:273-278.
41 Sheehan J, Pan HC, Stroila M, et al. Gamma knife surgery for trigeminal neuralgia: outcomes and prognostic factors. J Neurosurg. 2005;102:434-441.
42 Norén G, Greitz D, Hirsch A, et al. Gamma knife surgery in acoustic tumours. Acta Neurochir Suppl (Wien). 1993;58:104-107.
43 Tishler RB, Loeffler JS, Lunsford LD, et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215-221.
44 Jagannathan J, Sheehan JP, Pouratian N, et al. Gamma Knife surgery for Cushing’s disease. J Neurosurg. 2007;106:980-987.
45 Sheehan J, Steiner L, Laws ER. Pituitary adenomas: is Gamma Knife radiosurgery safe? Nat Clin Pract Endocrinol Metab. 2005;1:2-3.
46 Cahan WG, Woodard HW, Higinbotham NL, et al. Sarcoma arising in irradiated bone: report of eleven cases. Cancer. 1998;82:8-34.
47 Modan B, Baidatz D, Mart H, et al. Radiation-induced head and neck tumours. Lancet. 1974;1:277-279.
48 Brada M, Rajan B, Traish D, et al. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf). 1993;38:571-578.
49 Ganz JC. Gamma knife radiosurgery and its possible relationship to malignancy: a review. J Neurosurg. 2002;97:644-652.
50 Rowe J, Grainger A, Walton L, et al. Risk of malignancy after gamma knife stereotactic radiosurgery. Neurosurgery. 2007;60:60-65.
51 Sheehan J, Yen CP, Steiner L. Gamma knife surgery-induced meningioma. Report of two cases and review of the literature. J Neurosurg. 2006;105:325-329.