46 The Postanesthesia Care Unit and Beyond
CHILDREN’S RECOVERY FROM ANESTHESIA is substantively different from that of adults. Although both age groups share the key elements of regaining consciousness, controlling pain, and maintaining a patent airway, the nature and timing of these elements are different. In young children, emergence from inhalational agents can be quite rapid because of the increased minute ventilation, increased blood flow to the vessel-rich group (see Chapter 6), and decreased total body muscle and fat stores, whereas emergence from intravenous agents in infants may be delayed because of decreased clearance as a result of reduced liver blood flow and enzyme activity. The quality of the emergence is different in children and adults because agitation (i.e., emergence delirium) after sevoflurane and desflurane is more common in young children than in adults. The criteria used to evaluate emergence from anesthesia or sedation must be consistent with the developmental level of the child. The nature and rapidity of complications that can occur during emergence require careful planning and anticipation of problems. Parents should be considered partners and active participants in effective postoperative management.
Perioperative Environment
Equipment (Table 46-1) and available medications (Tables 46-2 and 46-3) should be standardized throughout the unit and be compatible with transport monitors and other devices used in corresponding high care facilities (e.g., PICU). An important safety precaution is the use of preprinted weight-based emergency drug doses for each child; these rapid reference sheets can be attached to each child’s bed or chart on admission so that a quick dose recommendation is readily available. Alternatively, the electronic record should have precalculated emergency drug doses for each child. This practice may also reduce the risk of drug errors in an emergency situation.
TABLE 46-1 Suggested Essential Bedside Equipment
Oxygen supply with regulated flows
Oxygen facemasks and face tents for spontaneous ventilation (various sizes)
Resuscitation bags, self-inflating (Ambu)
Anesthesia facemasks for positive-pressure ventilation (pediatric sizes: 0, 1, 2, 3; adult sizes: small, medium, large)
Oral airways (sizes 00, 0, 1 to 5)
Nasal airways (sizes 12F to 36F)
Suction and appropriate suction catheters (sizes 6.5F to 14F); tonsil-type (Yankauer) attachment
Needles, syringes, alcohol wipes, Betadine solution, gauze pads
Pulse oximeter and sensors (size appropriate, stick-on type preferred to clip-on type)
Electrocardiograph, monitor and pads
TABLE 46-2 Suggested Emergency Supplies for a Crash Cart or Central Location
Laryngoscopes with blades: Miller 0, 1, 2, 3; Macintosh 2, 3, 4; extra laryngoscope bulbs and batteries
Endotracheal tubes, sizes 2.0-mm internal diameter (ID) through 8-mm ID; cuffed and uncuffed tubes for all sizes when available
Laryngeal mask airways, sizes 1, 1.5, 2, 2.5, 3, 4, 5
ProSeal laryngeal mask airway, sizes 1.5, 2, 2.5, 3, 3.5, 4, 5
Fast-track intubating laryngeal mask airway
Stylet appropriate for each endotracheal tube size
Syringe for endotracheal cuff inflation
Tape and liquid adhesive for endotracheal tube fixation
Intravenous catheter (14 gauge) with 3-mm ID endotracheal tube adapter for emergency cricothyroidotomy (see Fig. 12-25)
Cricothyrotomy kits appropriate for age (see Figs. 12-25 and 12-26; see also E-Figs. 12-5 through 12-10)![]()
Backup resuscitation bags and masks and oral airways for each bedside
Intravenous infusion solutions, tubing, drip chambers
Supplies for intravenous cannulation, catheter sizes 24 to 14 gauge
Cutdown tray, tracheostomy, and suture sets
Central venous catheter insertion sets (3F to 7F single and multiple lumen)
Tube thoracotomy set and system for suction and underwater seal
Defibrillator (adult, child paddles)
Pressure transducer system and oscilloscope monitor
Sterile gowns, gloves, masks, towels, drapes
TABLE 46-3 Suggested Recovery Room Medications
Hydrocortisone, dexamethasone, methylprednisolone
Lidocaine (intravenous and topical)
Propranolol, atenolol, esmolol, labetalol
Succinylcholine and rocuronium
For inhalation: racemic epinephrine (2.2% at 0.05 mL/kg, common in the United States) or epinephrine 1 : 1000 (0.1%), 0.5 mL/kg, maximum of 5 mL
*Alternative or additional medications may be needed.
Nurses, residents, fellows, attending physicians, and other personnel working in the perioperative area should be competent in the provision of neonatal and pediatric advanced life support. The team should participate in mock codes and simulations to train for an emergency. Patient sign outs should be standardized, include checklists, and follow an institution-specific protocol.1 All personnel should be familiar with resuscitative equipment and be able to use it instantaneously. We recommend instituting equipment and policies according to the guidelines for the pediatric perioperative anesthesia environment published by the American Academy of Pediatrics.2
Central Nervous System
Pharmacodynamics of Emergence
Emergence from anesthesia is faster after a relatively insoluble inhalational anesthetic agent such as sevoflurane or desflurane than it is after a more soluble agent such as halothane.3 However, the clinical importance of these differences may be minimal and vary with the duration of anesthesia and the coadministered medications. Differences in the times to discharge from the PACU and the hospital between inhalational agents are even more difficult to detect when specific comparisons are made because so many other factors, such as pain management, agitation, availability of hospital beds, and family circumstances, affect discharge readiness.
The age of the child exerts a minimal influence on the wash-out of inhalational anesthetic agents and has little impact on the rapidity of emergence, although age may be a factor for infants younger than 1 year of age.4 However, the overall clinical implications of age-related differences in emergence are exceedingly difficult to detect.5 The speed of emergence correlates more closely with the duration of anesthesia. The greater the duration of anesthesia, the more the tissue compartments become filled with these anesthetics and the more time it takes to eliminate these anesthetics and for the child to recover. For example, emergence from 30 minutes of sevoflurane anesthesia is significantly faster than emergence from 2 hours of anesthesia, which is more rapid than from 8 hours of anesthesia.6 This relationship between emergence time and the duration of anesthesia has less relevance as inhalational anesthetics have become less soluble (e.g., desflurane).
Emergence from intravenous agents can vary significantly from that of inhalational agents. Several studies have evaluated the quality and rapidity of emergence after intravenous anesthetic agents compared with that after inhalational agents. For outpatient surgery, emergence after propofol anesthesia is as rapid as that after sevoflurane but with far less agitation and pain behaviors.7,8 The recovery characteristics of propofol with remifentanil total intravenous anesthesia have been compared with those after desflurane inhalational anesthesia. Recovery is as rapid as that after desflurane with nitrous oxide, with a similar incidence of nausea and vomiting but with much less agitation.9
Midazolam is rarely used for maintenance of anesthesia but is often used as an oral or intravenous premedication for anxiolysis and amnesia in the preinduction period. There is evidence that the addition of midazolam in the preinduction period to an inhalational or propofol anesthetic may delay early emergence after brief anesthesia. However, this effect of midazolam is attenuated after anesthesia of greater duration, or when considering late emergence, this effect is minimal.10 Midazolam does not affect the incidence of postoperative agitation.11,12
Emergence Agitation or Delirium
Emergence agitation (i.e., emergence delirium [Videos 46-1 and 46-2])![]() was first described in a large cohort of postsurgical patients almost 40 years ago.13 From a clinical perspective, it is often impossible to differentiate pure agitation from delirium. Delirium implies a specific set of thought disorders and hallucinations based on the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV). Despite numerous investigations, differentiating emergence delirium from postoperative pain has also proved difficult. Emergence delirium usually manifests as thrashing, disorientation, crying, and screaming. The child is unable to recognize parents, familiar objects, or surroundings; is inconsolable; and talks irrationally during early emergence from anesthesia. Emergence delirium occurs more often in children (rate of 10% to 20%) than in adults, particularly in those younger than 6 years of age.14,15 It may in part reflect differences in clearance of insoluble inhalational agents from the central nervous system.
was first described in a large cohort of postsurgical patients almost 40 years ago.13 From a clinical perspective, it is often impossible to differentiate pure agitation from delirium. Delirium implies a specific set of thought disorders and hallucinations based on the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV). Despite numerous investigations, differentiating emergence delirium from postoperative pain has also proved difficult. Emergence delirium usually manifests as thrashing, disorientation, crying, and screaming. The child is unable to recognize parents, familiar objects, or surroundings; is inconsolable; and talks irrationally during early emergence from anesthesia. Emergence delirium occurs more often in children (rate of 10% to 20%) than in adults, particularly in those younger than 6 years of age.14,15 It may in part reflect differences in clearance of insoluble inhalational agents from the central nervous system.
An emergence delirium scale has been developed and validated that may provide clinicians and investigators with a tool to differentiate emergence delirium from pain.16 Tables 46-4 and 46-5 show two scoring systems used to evaluate emergence behaviors in children. In evaluating emergence delirium with the Pediatric Anesthesia Emergence Delirium (PAED) scale after anesthesia, preliminary evidence suggested that values greater than 10 were consistent with emergence delirium in 37% of patients,17 although that cutoff value has not been useful for others.18 Later evidence suggested that values greater than 12 provided greater sensitivity and specificity.17 In the PICU, evidence suggests that a PAED score greater than 8 predicts emergence delirium.19
TABLE 46-5 Postanesthesia Behavior Assessment Scale
*A higher postanesthesia behavior assessment (PABA) score is associated with a greater degree of postanesthetic distress.
From Przybylo HJ, Martini DR, Mazurek AJ, et al. Assessing behavior in children emerging form anaesthesia: can we apply psychiatric diagnostic techniques? Pediatr Anesth 2003;13:609-16.
Our understanding of emergence delirium or agitation continues to evolve, but it is clear that it occurs after surgical procedures and after procedures that are free from pain, such as magnetic resonance imaging.14,20,21 Emergence delirium appears to occur more frequently after use of less-soluble inhalational anesthetics such as sevoflurane and desflurane than after more-soluble inhalational anesthetics such as halothane and isoflurane,22,23 even though some data suggest otherwise.24 There may be a greater incidence of emergence delirium after painful procedures; emphasizing the difficulty separating agitation due to pain from agitation due to the direct effects of the inhalational agents on the sensorium.25 Emergence delirium occurs more commonly in children younger than 6 years of age than in older children, usually lasts 5 to 15 minutes, and resolves spontaneously if the children are left undisturbed or they are held by their parents.14
Several strategies have been used to decrease the duration and intensity of emergence delirium. Effective regional analgesia, opioids, ketamine, α2-agonists, and propofol can prevent or treat emergence delirium. Low-dose fentanyl (2 µg/kg intranasally or 1 to 2 µg/kg intravenously) decreases the duration and intensity of emergence delirium,26 even in the absence of significant painful stimuli.20 Other adjunctive agents used to treat this phenomenon include ketorolac and acetaminophen (for myringotomy with ventilation tube placement) and midazolam; the effectiveness of midazolam, however, has been mixed.27,28 Dexmedetomidine can decrease the incidence of emergence delirium,29,30 but the cost-effectiveness of this treatment compared with others requires evaluation. Administration of propofol by continuous infusion or by bolus at the end of surgery appears to be preventative,31,32 although these findings have not been consistent.18 The induction dose of propofol administered at the start of the case does not appear to prevent postoperative emergence delirium.33 Regional analgesia in the form of caudal blocks can reduce the incidence of emergence delirium, although this effect is probably related to improved pain control, which eliminates pain as a source of agitation.34,35
Although there is no evidence of long-term consequences, in the current era of fast-tracking anesthesia, emergence delirium can represent a significant time expenditure for nurses in the PACU. Discharge from the PACU may be delayed while waiting for the delirium to wane or for the effects of the interventional drugs to dissipate. Injury to the child who is extremely agitated is a concern. Parental satisfaction decreases when severe emergence delirium occurs. Although the impact of extreme delirium is not fully known, evidence suggests that the incidence of postoperative maladaptive behaviors increases among children who experience marked emergence delirium.36
Respiratory System
Extubation in the Operating Room or Postanesthesia Care Unit
For children who are extubated in the PACU, respiratory insufficiency is the most worrisome and most frequent complication. It comprises approximately two thirds of critical perioperative events when it is associated with emergence from anesthesia.37 Respiratory insufficiency may manifest in the form of difficulty breathing, or it may be more subtle as anxiety, unresponsiveness, tachycardia, bradycardia, hypertension, arrhythmia, or seizures. Cardiac arrest is a late manifestation. When any of these conditions are present, respiratory insufficiency must be considered as the root cause. Hypoxemia, hypoventilation, and upper airway obstruction are the three most common adverse respiratory events that occur in children in the PACU, and this is particularly true for children after tonsillectomy complicated by obesity and possible obstructive sleep apnea and for those who have undergone diagnostic bronchoscopy.
Hypoxemia
Hypoxemia may result from hypoventilation, diffusion hypoxia, upper airway obstruction, bronchospasm, aspiration, pulmonary edema, pneumothorax, atelectasis, or rarely from postobstructive pulmonary edema or pulmonary embolism. Hypoxia occurs more rapidly and may be more profound during emergence from general anesthesia because general anesthesia inhibits the hypoxic and hypercapnic ventilatory drive, reduces functional residual capacity, and alters hypoxic pulmonary vasoconstriction. Shivering may further increase oxygen consumption by a factor of two to five38,39 and exacerbate hemoglobin desaturation.
Postoperative hemoglobin desaturation is more common in children with or recovering from an active upper respiratory tract infection due to increased airway reactivity, atelectasis, and increased secretions than in children without a history of upper respiratory tract infection.40,41 In neonates, hypoxia increases ventilation for approximately 1 minute but then depresses the respiratory drive (i.e., respiratory rate and tidal volume).42 The ventilatory response to hypoxia in formerly preterm infants with severe bronchopulmonary dysplasia who sustained an hypoxic injury is delayed for several months, placing them at particular risk for desaturation in the perioperative period.43
Hypoventilation
Minute ventilation is the product of tidal volume and respiratory rate. It decreases when tidal volume, respiratory rate, or both values decrease. Hypoventilation leads to hypercarbia and promotes alveolar collapse, known as atelectasis. Severe hypoventilation causes respiratory acidosis, hypoxemia, carbon dioxide narcosis, and apnea. The ventilatory response to carbon dioxide depends on the child’s age. For example, during halothane anesthesia and spontaneous ventilation, 3.7% inspired carbon dioxide triggers no ventilatory response in infants younger than 6 months of age, but it triggers a 34% increase in minute ventilation in infants and children older than 6 months of age.44,45
Airway Obstruction
Among the most common and serious problems in the PACU is an extrathoracic airway obstruction. Clinical hallmarks of airway obstruction include hemoglobin desaturation with inspiratory stridor, inspiratory retraction, and paradoxical chest wall motion. Common interventions include stimulating the child, repositioning, suctioning, performing a jaw thrust, insertion of an oral or nasal airway, and application of positive end-expiratory pressure (PEEP) (see Fig. 12-10). If these measures fail, patency of the upper and lower airways should be considered because gas exchange may be compromised by laryngospasm, subglottic narrowing as the result of edema, bronchospasm, atelectasis, or tracheal secretions. Incomplete recovery from general anesthesia or neuromuscular blockade, wound hematoma, and vocal cord paralysis may also lead to upper airway obstruction.
If the airway is not cleared by any of the previously described maneuvers, placement of a tracheal tube preceded by administration of oxygen by mask with continuous positive airway pressure and indicated medications may be necessary. Postobstructive pulmonary edema is a complication of acute upper airway obstruction and the relief of chronic airway obstruction after tonsillectomy. The mechanism appears to be generation of extreme negative intrathoracic pressure against a closed glottis or obstructed airway and its sudden release, resulting in a dramatic increase in pulmonary blood flow and causing noncardiogenic or neurogenic pulmonary edema. This complication should be suspected when significant hypoxia, persistent tachypnea, or tachycardia follows a prolonged episode of laryngospasm, airway obstruction, or tonsillectomy and the child has pink, frothy secretions. Treatment of noncardiogenic pulmonary edema includes tracheal intubation, positive-pressure ventilation with PEEP, 100% oxygen to maintain an adequate oxygen tension, furosemide, and morphine. Furosemide (1 to 2 mg/kg) should be given immediately intravenously because it is thought to act instantaneously by decreasing venous return to the heart by direct venodilatation.46–48
Postintubation croup or subglottic edema has been associated with factors such as traumatic intubation, tight-fitting tracheal tubes, multiple intubation attempts, coughing with an in situ tracheal tube, a change in the child’s position during surgery, prolonged duration of intubation, surgery of the head and neck, and a history of croup.49 Treatment should be initiated with the inhalation of cool mist. If the symptoms do not abate, nebulized epinephrine should be administered, although its effects are temporary and its repeated use may be followed by rebound edema. The use of nebulized epinephrine indicates the need for a prolonged period of observation. Outpatients may have to be admitted to the hospital overnight or observed for an extended period.
Discharge of Preterm Infants from the Postanesthesia Care Unit
Preterm infants (<37 weeks gestation at birth) are at risk for apnea after sedation and general anesthesia.50,51 As the PCA (i.e., age since conception) increases, the risk for apnea decreases.52 Guidelines are lacking because there are inadequate randomized, controlled trials and underpowered institutional studies, and apnea has been reported even after more modern anesthetic agents (i.e., desflurane and sevoflurane). However, it is recommended that formerly preterm infants who are 55 to 60 weeks PCA who are not anemic and not experiencing apnea be observed for an extended period and, if stable, later discharged. Infants younger than 55 weeks PCA, those who are anemic (hematocrit <30%), and those with ongoing apnea should be admitted for monitoring.53–59 Prophylactic administration of caffeine (10 mg/kg intravenously) may reduce the risk of apnea after general anesthesia for infants at high risk,60,61 although it should not supplant postoperative admission and monitoring. Administration should be discussed with neonatologists, because it does not change management (i.e., infants are monitored in the hospital), and the administration of caffeine resets the number of apnea or bradycardia-free days (i.e., used as a discharge criterion for preterm infants) to zero. Preterm infants younger than 55 weeks PCA, particularly those with anemia or those with major cardiorespiratory or neurologic disorders, should be admitted and monitored for at least 12 apnea-free hours after general or regional anesthesia or sedation (see Chapter 4).54,62
Although preterm infants who undergo surgery under spinal anesthesia have fewer respiratory and cardiovascular complications compared with those undergoing general anesthesia,54,55,58,61 the infants remain at risk for apnea. It is unknown whether the risk is greater after a spinal anesthetic without supplemental sedation (with no other medication given) than the preoperative baseline risk. Similarly, caudal anesthesia has been reported as an effective alternative to spinal anesthesia in preterm infants undergoing herniotomy.62,63 Infants who have received a spinal anesthetic supplemented with ketamine or midazolam are at greater risk for apnea than those who received no supplemental sedation. Despite evidence of a reduced risk of apnea after regional anesthesia61,64 and no postdischarge complications on the day of surgery in some institutions,61,65 there is insufficient evidence to make general recommendations regarding this practice. Our recommendation is to admit and monitor these infants.
Cardiovascular System
Bradycardia
Bradycardia is the most common dysrhythmia in children and requires immediate attention because of its association with decreased cardiac output. Until proved otherwise, the most common cause of bradycardia in infants and children is hypoxemia. Other possible causes for the bradycardia include vagal responses (e.g., passage of a nasogastric tube), medications (e.g., neostigmine, β-adrenergic blockade, high spinal blockade, opioids such as fentanyl), increased intracranial pressure, and high neuraxial anesthetic block. The definition of bradycardia depends on the age of the child; the incidence decreases with increasing age (see Chapter 2).
Treatment is directed at correcting the underlying cause, including the administration of oxygen and ensuring a patent airway. Bradycardia should be immediately treated with oxygen and, if necessary, with ventilation. If intervention with oxygen does not immediately restore the heart rate, atropine (0.02 mg/kg) should be administered; and if no response is observed within 30 seconds, administration of epinephrine (2 to 10 µg/kg) is indicated. For symptomatic bradycardia (e.g., hypotension, decreased level of consciousness), immediate administration of epinephrine is indicated. If there is no response to epinephrine, chest compressions should be instituted and standard cardiopulmonary resuscitation algorithms followed (see Chapter 39).
Tachycardia
Tachycardia is an important postoperative sign that is a marker for one of several disorders, such as inadequate cardiac output or oxygen delivery, or it may be a response to pain or a direct drug effect (e.g., epinephrine, atropine). Tachycardia may occur in response to hypoxemia, hypercarbia, hypovolemia, hypervolemia, emergence delirium, anxiety, sepsis, fever, a full bladder, a previously unrecognized cardiac conduction abnormality (see Chapters 14 and 16), or heart failure. The threshold for diagnosing tachycardia varies with the age of the child, and the threshold decreases with age.
Other Arrhythmias
With the exception of bradycardia and tachycardia, postoperative arrhythmias are rare in children. Isolated premature ventricular or atrial beats may be observed in the PACU and, unless they progress, are not important. Multifocal premature ventricular beats are uncommon in children. They may occur as a result of inadequately treated pain, cardiac conduction defects, or in rare instances, may be a harbinger of malignant hyperthermia (see Chapters 14, 16, and 40), acute rhabdomyolysis with hyperkalemia, inadequately treated pain, a congenital conduction defect, or a structural cardiac defect. Electrolyte and arterial blood gas status should be checked. Children with known congenital heart disease should have continuous ECG monitoring in the PACU (see Chapters 14 and 16); all arrhythmias should be recorded and a cardiologist consulted because this may be the first manifestation of a developing ectopic focus.
Blood Pressure Control
Hypotension
The anesthesiologist should be familiar with the normal blood pressure ranges of infants and children (see Chapter 2). The measurement should be obtained with an appropriately sized blood pressure cuff; the width of the cuff should be two thirds of the length of the upper arm. An improperly sized cuff produces spurious readings. Small cuffs may yield a false high reading, whereas large cuffs may yield a false low reading. Proper placement of the cuff is essential to avoid errors in interpretation.
The most common cause of hypotension in children is hypovolemia from inadequate replacement of blood and fluids lost during the surgical procedure or ongoing blood loss. Clinical hallmarks of hypovolemia are tachycardia, urine output of less than 0.5 to 1 mL/kg/hr, slow capillary refill (>3 seconds), and narrowing of the pulse pressure. If the hematocrit is adequate, hypovolemia may be treated with an initial bolus of 10 to 20 mL/kg of isotonic crystalloid solution or albumin. This may be repeated until the blood pressure is normalized. If the hematocrit is inadequate, packed red blood cells (PRBCs) or whole blood should be administered. In this case, a rough guide for the volume of blood required is 4 mL/kg of packed cells or 6 mL/kg of whole blood to raise the hemoglobin 1 g/dL in children and adults (see Chapter 10). To achieve a desired hematocrit more precisely, the volume of PRBCs may be estimated as follows:
Renal System
Complications related to the renal system are rare in the postoperative period. The most likely cause of low urine output (<0.5 to 1 mL/kg/hr) is hypovolemia as discussed previously (e.g., postoperative hypotension). Mechanical obstruction downstream from the kidneys may result from direct surgical interference or a misplaced or dysfunctional urinary catheter (i.e., blood clot or kink). If the child has regional (spinal or epidural) anesthesia that includes an opioid and there is no urinary catheter in place, placement of a Foley or straight catheter may be indicated. Renal failure is a rare possibility in children who have had major operations or have systemic inflammatory disease. If screening tests such as blood urea nitrogen, serum creatinine, and urine analysis suggest renal insufficiency, a pediatric nephrologist should be consulted (see also Chapter 26).
Gastrointestinal System
Incidence of Postoperative Nausea and Vomiting
Postoperative nausea and vomiting (PONV) is one of the most bothersome adverse effects of anesthesia and surgery. Unlike adults, most children are unfamiliar with and have never experienced nausea. It is unlikely that they will warn the PACU personnel that they are nauseated. In children, vomiting and complaining about a “sore tummy” are likely the first and only manifestations of gastrointestinal upset. Among children, PONV is inversely related to age.66 The incidence of PONV is small in very young children, increases throughout childhood, and reaches a zenith in adolescents, for whom the incidence exceeds that for adults.66
The type of surgery influences the incidence of PONV. The incidence of PONV in children is greatest after tonsillectomy, strabismus repair, hernia repair, orchiopexy, microtia, and middle ear procedures.67 Before puberty, there are no gender-related differences in PONV; after puberty, girls experience much more PONV than boys. The medical complications of PONV include pulmonary aspiration, dehydration, electrolyte imbalance, fatigue, wound disruption, and esophageal tears. PONV can produce psychological effects that may produce anxiety in the children and parents and lead them to avoid further surgery. The cost implications of PONV can be major because of delayed recovery and discharge, increased medical care, and reoperation. Although these problems are seldom life-threatening, the cumulative costs in terms of prolonged PACU stays, unplanned admissions, and patient dissatisfaction are serious.68
Evidence-Based Consensus Management
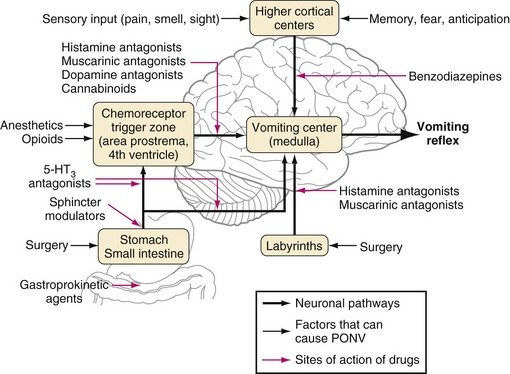
Management of PONV is complex, and many treatment strategies have been formulated (Fig. 46-1). Most have been shown to be effective in one study or another. However, the superiority of some treatments over others has not been established, in part because of study design flaws such as inadequate dosing, small sample sizes, or various periods of observations and data collection; some studies monitored PONV only during the first few hours after surgery, whereas others monitored the children for 24 to 48 hours after surgery. To make sense of the conflicting data that exist, a consensus-based management strategy for the prevention and management of PONV has been devised.69 These guidelines advise first identifying the children at significant risk for PONV as outlined earlier; prophylaxis for PONV is recommended for children in high-risk categories. Studies frequently focus on postoperative vomiting as the primary outcome because nausea may be difficult to identify in children.
The consensus guidelines recognize that the choice of anesthetic can influence the incidence of PONV in children. Propofol-based anesthesia during operations associated with a large incidence of PONV dramatically reduces the incidence of PONV compared with isoflurane-based anesthesia, even when both groups are given prophylactic 5-hydroxytryptamine type 3 (5-HT3) receptor inhibitors.70 Similarly, multimodal therapy that is a combination of PONV treatment strategies is more effective than a single-treatment strategy.71 For instance, the combination of propofol anesthesia plus ondansetron has been shown to significantly reduce the incidence of PONV compared with the use of propofol alone (7% versus 22%).72 The combination of dexamethasone and ondansetron is more effective than either intervention in isolation and permits the dose of ondansetron to be reduced by 50%.73 A slightly more contentious effect has been the elimination of nitrous oxide, which decreases the incidence of PONV among those undergoing highly emetogenic surgery, with a number needed to treat of only five patients.74 However, that meta-analysis also revealed a 2% incidence of awareness under anesthesia if nitrous oxide was omitted.74
Other strategies recommended to decrease the rate of PONV include the use of the smallest dose of opioids that still provides adequate pain control and the use of regional anesthesia if possible. The use of nonopioids such as acetaminophen, ketamine, and ketorolac should be considered. Adequate parenteral hydration and avoidance of early postoperative fluid ingestion can reduce the incidence of PONV (see Chapter 4).
Prophylactic Therapy
Ondansetron has been studied extensively and shown to decrease early and late PONV at doses of 50 to 100 µg/kg.75 Because the 5-HT3 receptor antagonists as a group have greater efficacy in the prevention of vomiting than nausea, they are the drugs of first choice for prophylaxis in children. Dexamethasone also is effective in decreasing PONV.76 Administration of dexamethasone alone or in combination with other antiemetics can extend the period of effective treatment up to 24 hours. In a systematic review, Steward and associates demonstrated that children who received a single dose of dexamethasone (0.15 to 1 mg/kg) were two times less likely to vomit after tonsillectomy and adenoidectomy than those who did not receive dexamethasone.77,78 In a randomized, prospective dose-finding study of dexamethasone administered to children undergoing tonsillectomy, there was no difference in the incidence of vomiting after prophylactic doses of dexamethasone between 0.0625 and 1.0 mg/kg (see Fig. 31-5)79; a similar trial has not been conducted in the PACU for children who are already vomiting. Before the black box warning was added for droperidol, it was also recommended for prophylaxis of PONV in the United States,80 but for medicolegal reasons alone, it is no longer a first-tier antiemetic. Droperidol is commonly used in low doses, which limit extrapyramidal and sedation side effects.
Adequate fluid resuscitation plays in important role in PONV prevention. Children given 10 mL/kg of lactated Ringer’s solution during strabismus correction had more PONV than those given 30 mL/kg (54% versus 22%).81
The most effective prophylaxis strategy in children at moderate or high risk for PONV is to use combination therapy that includes hydration, a 5-HT3 receptor antagonist, and a second drug such dexamethasone. Antiemetic rescue therapy should be administered to children who vomit after surgery. An emetic episode more than 6 hours postoperatively can be treated with any of the drugs used for prophylaxis except dexamethasone and transdermal scopolamine.82
Rescue Therapy
The consensus panel recommends that children who did not receive intraoperative prophylaxis or those who fail prophylaxis should receive a 5-HT3 receptor antagonist at the first signs of PONV.69 The recommended dose should be one fourth of that used for prophylaxis. For all other therapies, the data on efficacy for rescue are sparse, and doses are unknown. In adults, promethazine and droperidol have been as effective as ondansetron in the general surgical population, but comparable studies have not been conducted in children. The sedative properties of promethazine may last for many hours and may be a problem for patients with OSA.
Alternative Treatments
Alternative methods for nausea and vomiting prophylaxis deserve consideration. Isopropyl alcohol reduces PONV, although the effect is transient.83 In a meta-analysis of alternative antinausea and vomiting techniques, acupuncture, electroacupuncture, transcutaneous electrical nerve stimulation, acupoint stimulation, and acupressure each exert antiemetic effects compared with placebo in adults, but not in children.84
Postoperative Care and Discharge
Pain Management in the Postanesthesia Care Unit
Acute postoperative pain management strategies are discussed in detail in Chapter 43. A child’s level of pain (or the perception of pain) changes more rapidly in the PACU than in any other unit of the hospital. Frequent and consistent use of pain scores for children of all ages, including those with developmental disabilities, is essential. Many pain scales have been validated for use in children. More important than the specific scale employed, the scale should be used consistently and follow simple principles. For instance, children who are verbal and developmentally appropriate should be encouraged to describe their pain using a self-report scale (e.g., Oucher scale). Young children or those without verbal skills should be assessed using an objective pain behavior scale (e.g., Face, Legs, Activity, Cry, Consolability [FLACC] scale).85,86 Just as important is the consistent application of protocols to treat pain; treatment of a given pain level should not vary from shift to shift or from one nurse to another.87
As with other areas of pediatric pain control, a multimodal approach to postoperative pain is recommended. A plan for pain management should be discussed among the family, surgical team, and anesthesia team before surgery.88 Depending on the surgery, the plan may include any or all of the following: acetaminophen, nonsteroidal agents, local anesthesia, nerve blocks, regional anesthesia, clonidine, opioids, patient-controlled analgesia, and patient-controlled epidural analgesia.
Acetaminophen and nonsteroidal drugs act through inhibition of prostaglandins and their metabolites. Most of these drugs are given orally and should be given preoperatively or intraoperatively to be effective in the PACU. Occasionally, they may be indicated in the PACU if they were not administered before arrival. Oral acetaminophen (15 mg/kg) or ibuprofen (10 mg/kg) has been shown to decrease opioid requirements by 20% to 30% after a variety of surgical procedures. Intravenous acetaminophen has become available in the United States for children 2 years of age or older, and it is likely to become a popular analgesic for mild to moderate pain in the PACU and as an opioid-sparing drug.89,90 The U.S. Food and Drug Administration (FDA) has approved the use of intravenous acetaminophen for children 2 years or older for the treatment of mild to moderate pain or fever. The intravenous dose of 15 mg/kg every 6 hours is recommended for patients weighing less than 50 kg and should be administered over 15 minutes.91 Studies on intravenous formulations of propacetamol and paracetamol have mostly been conducted in the European Union since drug approval in 2002. Several randomized, controlled studies and meta-analyses have demonstrated efficacy of intravenous administration in adults and children for the treatment of mild to moderate pain or fever.92,93 It is effective as an adjuvant to other analgesic modalities for moderate to severe pain.94
Acetaminophen can also be given rectally in doses of 35 to 45 mg/kg; however, because absorption varies and is delayed (i.e., peak concentration at 60 to 180 minutes after rectal administration), this route is not recommended for use in the PACU.95 Because of the pharmacokinetics of the rectal route, a greater interval (6 hours) between doses is recommended, and subsequent doses are reduced (20 mg/kg) so that the total dose per 24 hours does not exceed 100 mg/kg.96 There are no data to provide guidance for rectal acetaminophen beyond 24 hours. If a child has received rectal acetaminophen, the first oral dose should be delayed until 6 hours after the rectal dose.
The nonsteroidal antiinflammatory drug ketorolac can decrease opioid requirements by approximately 30%. The recommended dosage is 0.2 to 0.5 mg/kg, which is given intravenously every 6 hours.97 Caution is warranted in postoperative children with significant bleeding or a history of renal insufficiency. The manufacturer recommends limiting the total doses to 15 mg for children weighing less than 50 kg and to 30 mg for children weighing more than 50 kg.98
Opioids are indicated during the immediate postoperative period for any procedure in which moderate or severe pain is not being managed by other means. Morphine, fentanyl, and hydromorphone have a long history of safe use for infants and children in the PACU. Repeated doses of meperidine are not recommended for children because of the potential for seizures from epileptogenic metabolites (e.g., normeperidine).99 Opioid dosing should be initiated according to body weight, physiologic development, underlying medical or surgical conditions, coadministered medications, and severity of pain. The goal should be effective and rapid pain relief. Subsequent dosing of the medications should be titrated based on response to the initial dose. Administration of multiple, small, ineffective doses results in prolongation of pain, stress, and anxiety without improving the safety of care provided. With this caveat in mind, patient-controlled analgesia and patient-controlled epidural analgesia (see Chapters 41 and 43) may be used in the PACU environment, but either intervention should be started only after acute pain has been adequately treated. The small doses administered by patient-controlled analgesia typically are not adequate to completely treat acute postoperative pain and may add to a sense that the method is not working for a given child.100
Regional analgesia is a common mode of intraoperative pain control in the child that extends into the PACU (see Chapters 41 to 43). The PACU personnel need to know how well it is working, and this assessment can be approached in a systematic manner.
The regional block should be placed in a manner that provides analgesia for the surgical incision site and visceral pain. Evidence that the regional block is effective should first be detected during surgery. If the regional block is effective, the anesthetic requirements are usually reduced. For instance, a caudal block is not effective for a midabdominal incision unless the catheter has been threaded to the region of the incision or relatively large volumes of local anesthetic have been administered. The addition of hydrophilic opioids or clonidine may extend the level of analgesia to some extent over the course of several hours; however, a block that is many dermatome segments away from the site of the surgical incision is unlikely to remain adequate for long. The addition of opioids to an epidural or spinal block increases the risk of pruritus, urinary retention, and emesis. Similarly, visceral pain such as bladder spasms (which have thoracic innervation) or the sore throat after intubation are not attenuated by a lumbar epidural catheter and must be managed by other measures.101
The anesthesiologist must verify that the catheter is in the epidural space. Older children can be questioned about their sensation level using ice or other cold sensation to determine the level of sympathectomy. Preverbal or developmentally disabled patients require some other objective form of confirmation. Previous reports have focused on electrical stimulation through the epidural catheter at the time of catheter placement to determine the level of insertion.102 Ultrasound methods for detecting epidural catheter placement have been described.103 Perhaps most practical in the PACU may be radiographic confirmation of the dermatome level of the tip of the catheter, often with the use of an appropriate contrast material (i.e., epidurogram) to ensure appropriate placement in the epidural space.104,105 A small amount (<1 mL) of contrast (e.g., Omnipaque 180 or 240) can be infused into the catheter while one radiograph is taken to confirm placement (see Fig. 41-7).105
Temperature Management
Intraoperative normothermia is key to maintaining a normal temperature postoperatively. Hypothermia is associated with discomfort, bleeding, infections, altered metabolism of drugs, delayed return of cognitive functions, and prolonged recovery.106–110 Because about 90% of heat loss occurs through the skin, only heat exchange through the skin provides an adequate way of warming children. This method of warming is enhanced by the vasodilation properties of most anesthetic agents. Forced-air warming blankets are the most effective way of maintaining body temperature in children.111 Given the vasoconstriction that occurs after anesthesia, attempts at warming are less effective postoperatively than intraoperatively, and most of the detrimental physiologic changes have already taken place. A growing body of literature documents the detrimental effects of hypothermia and shows that it is best when children arrive in the PACU with a normal body temperature.
Discharge Criteria
The recovery process and discharge criteria vary from institution to institution. Various criteria are used to determine readiness for discharge from the PACU. Some institutions require an assessment by a physician before discharge for all patients, but others require an evaluation only if routine discharge criteria are not met. The modified Aldrete scale is the most common system used to assess discharge readiness, but specific criteria depend on the particular situation or environment to which the child will be discharged. For example, a child with a slight degree of postextubation croup or stridor may be discharged for monitoring on a pediatric floor or ICU, but the same child is not discharged to parental care and a 2-hour drive home. The criteria for discharge of children to a general inpatient setting are summarized in Table 46-6. For outpatients, these criteria hold, and the additional criteria outlined in Table 46-7 usually must be met before discharge.
TABLE 46-6 Discharge Criteria for Inpatients
1. Recovery of airway and respiratory reflexes adequate to support gas exchange and to protect against aspiration of secretions, vomitus, or blood
2. Stability of circulation and control of any surgical bleeding
3. Absence of anticipated instability in criteria 1 and 2
4. Reasonable control of pain and vomiting
5. Appropriate duration of observation after opioid or naloxone flumazenil administration (minimum of 60 minutes after intravenous naloxone and up to 2 hours after flumazenil)
6. Return to baseline level of consciousness unless transfer is to an intensive care unit environment
TABLE 46-7 Discharge Criteria for Outpatients
1. Cardiovascular function and airway patency are satisfactory and stable.
2. The child is easily arousable, and protective reflexes are intact.
3. The child can talk (if age appropriate).
4. The child can sit up unaided (if age appropriate).
5. For a very young or handicapped child, incapable of the usually expected responses, the preanesthetic level of responsiveness or a level as close as possible to the normal level for that child should be achieved unless the child is to be transferred to another monitored location.
6. The state of hydration is adequate.
7. It may be permissible for parents to carry their children without full recovery of gait (parents must be advised that the child is at risk of injury if improperly supervised).
8. Control of pain should be achieved to permit adequate analgesia by the oral route thereafter.
9. Control of nausea and vomiting should be achieved to allow for oral hydration (see “Discharge Criteria” in text).
The requirements for children to eat, drink, and void before leaving the secondary recovery area significantly delay discharge. Efforts should be made to reinstate volume homeostasis during surgery, negating any physiologic imperative for oral intake in the immediate postoperative period. Postoperative maintenance fluids should consist of isotonic rather than hypotonic solutions for those expected to remain as inpatients to reduce the risk of hyponatremia (see also Chapter 8).112 Other than children who are at high risk for urinary retention (e.g., history of urinary retention, urethral surgery), there is little evidence that discharge before voiding results in readmission for voiding problems, and this requirement is therefore no longer part of standard discharge criteria.113 Children who have received a caudal block for surgery are likewise at low risk for urinary retention as long as opioids have not been added to the caudal medication.114
Although there are few data on the current status of recovery processes across the country, there appears to be a trend toward one-stage (fast-track) recovery for pediatric outpatients.115,116 This process allows selected children to bypass the first-stage recovery and go directly to the second-stage unit based on appropriate level of consciousness, physical activity, vital signs, respiratory status, and pain control (Table 46-8). This approach has proved successful and quite safe, although appropriate attention to issues such as pain control must be addressed when initiating such a program.
| Criteria | Score |
|---|---|
| Level of Consciousness | |
| Aware and oriented | 2 |
| Arousable with minimal stimulation | 1 |
| Responsive only to tactile stimulation | 0 |
| Physical Activity | |
| Able to move all extremities on command | 2 |
| Some weakness in movement of extremities | 1 |
| Unable to voluntarily move extremities | 0 |
| Hemodynamic Stability | |
| Blood pressure <15% of baseline MAP value | 2 |
| Blood pressure 15% to 30% of baseline MAP value | 1 |
| Blood pressure >30% of baseline MAP value | 0 |
| Respiratory Stability | |
| Able to breathe deeply | 2 |
| Tachypneic with good cough | 1 |
| Dyspneic with weak cough | 0 |
| Oxygen Saturation Status | |
| Maintains value >95% on room air | 2 |
| Requires supplemental oxygen (nasal prongs) | 1 |
| Saturation <90% with supplemental oxygen | 0 |
| Postoperative Pain Assessment | |
| None or mild discomfort | 2 |
| Moderate to severe pain controlled with intravenous analgesics | 1 |
| Persistent, severe pain | 0 |
| Postoperative Emetic Symptoms | |
| None or mild nausea with no active vomiting | 2 |
| Transient vomiting or retching | 1 |
| Persistent, moderate to severe nausea and vomiting | 0 |
| Total* | 14 |
MAP, Mean arterial pressure; PACU, postanesthesia care unit.
*Pediatric patients must score 14 to bypass the phase 1 (PACU) recovery unit to be admitted directly to the step-down care unit.
From White PF, Song D. New criteria for fast-tracking after outpatient anesthesia: a comparison with the modified Aldrete’s scoring system. Anesth Analg 1998;88:1069-72.
Anderson BJ, Woolard GA, Holford NH. Pharmacokinetics of rectal paracetamol after major surgery in children. Paediatr Anaesth. 1995;5:237–242.
Choong K, Arora S, Cheng J, et al. Hypotonic versus isotonic maintenance fluids after surgery for children: a randomized controlled trial. Pediatrics. 2011;128:857–866.
Cravero JP, Beach M, Thyr B, Whalen K. The effect of small dose fentanyl on the emergence characteristics of pediatric patients after sevoflurane anesthesia without surgery. Anesth Analg. 2003;97:364–367.
Gan TJ, Meyer T, Apfel CC, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97:62–71.
Hackel A, Badgwell JM, Binding RR, et al. Guidelines for the pediatric perioperative anesthesia environment. American Academy of Pediatrics. Section on Anesthesiology. Pediatrics. 1999;103:512–515.
Kain ZN, Caldwell-Andrews AA, Maranets I, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99:1648–1654.
McNicol ED, Tzortzopoulou A, Cepeda MS, et al. Single-dose intravenous paracetamol or propacetamol for prevention or treatment of postoperative pain: a systemic review and meta-analysis. Br J Anaesth. 2011;106:764–775.
1 Catchpole KR, de Leval MR, McEwan A, et al. Patient handover from surgery to instensive care: using Formula 1 pit-stop and aviation models to improve safety and quality. Pediatr Anaesth. 2007;17:470–478.
2 Hackel A, Badgwell JM, Binding RR, et al. Guidelines for the pediatric perioperative anesthesia environment. American Academy of Pediatrics. Section on Anesthesiology. Pediatrics. 1999;103:512–515.
3 Nordmann GR, Read JA, Sale SM, Stoddart PA, Wolf AR. Emergence and recovery in children after desflurane and isoflurane anaesthesia: effect of anaesthetic duration. Br J Anaesth. 2006;96:779–785.
4 Lerman J, Sikich N, Kleinman S, Yentis S. The pharmacology of sevoflurane in infants and children. Anesthesiology. 1994;80:814–824.
5 Behne M, Wilke HJ, Harder S. Clinical pharmacokinetics of sevoflurane. Clin Pharmacokinet. 1999;36:13–26.
6 Eger EI, II., Gong D, Koblin DD, et al. The effect of anaesthetic duration on kinetic and recovery characteristics of desflurane versus sevoflurane and on the kinetic characteristics of compound A in volunteers. Anesth Analg. 1998;98:414–421.
7 Uezono S, Goto T, Terui K, et al. Emergence agitation after sevoflurane versus propofol in pediatric patients. Anesth Analg. 2000;91:563–566.
8 Picard V, Dumont L, Pellegrini M. Quality of recovery in children: sevoflurane versus propofol. Acta Anaesthesiol Scand. 2000;44:307–310.
9 Grundmann U, Uth M, Eichner A, et al. Total intravenous anaesthesia with propofol and remifentanil in paediatric patients: a comparison with a desflurane-nitrous oxide inhalation anaesthesia. Acta Anaesthesiol Scand. 1998;42:845–850.
10 Coté CJ, Cohen IT, Suresh S, et al. A comparison of three doses of a commercially prepared oral midazolam syrup in children. Anesth Analg. 2002;94:37–43.
11 Bevan JC, Veall GR, Macnab AJ, et al. Midazolam premedication delays recovery after propofol without modifying involuntary movements. Anesth Analg. 1997;85:50–54.
12 Viitanen H, Annila P, Viitanen M, Yli-Hankala A. Midazolam premedication delays recovery from propofol-induced sevoflurane anesthesia in children 1-3 yr. Can J Anaesth. 1999;46:766–771.
13 Eckenoff JE, Kneale DH, Dripps RD. The incidence and etiology of postanesthetic excitement. Anesthesiology. 1961;22:667–673.
14 Cravero J, Surgenor S, Whalen K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: a comparison with halothane. Paediatr Anaesth. 2000;10:419–424.
15 Przybylo HJ, Martini DR, Mazurek AJ, et al. Assessing behaviour in children emerging from anaesthesia: can we apply psychiatric diagnostic techniques? Paediatr Anaesth. 2003;13:609–616.
16 Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100:1138–1145.
17 Bajwa SA, Costi D, Cyna AM. A comparison of emergence delirium scales following general anesthesia in children. Paediatr Anaesth. 2010;20:704–711.
18 Pieters BJ, Penn E, Nicklaus P, et al. Emergence delirium and postoperative pain in children undergoing adenotonsillectomy: a comparison of propofol vs sevoflurane anesthesia. Paediatr Anaesth. 2010;20:944–950.
19 Janssen NJ, Tan EY, Staal M, et al. On the utility of diagnostic instruments for pediatric delirium in critical illness: an evaluation of the Pediatric Anesthesia Emergence Delirium Scale, the Delirium Rating Scale 88, and the Delirium Rating Scale-Revised R-98. Intensive Care Med. 2011;37:1331–1338.
20 Cravero JP, Beach M, Thyr B, Whalen K. The effect of small dose fentanyl on the emergence characteristics of pediatric patients after sevoflurane anesthesia without surgery. Anesth Analg. 2003;97:364–367.
21 Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104:84–91.
22 Cravero JP, Beach M, Dodge CP, Whalen K. Emergence characteristics of sevoflurane compared to halothane in pediatric patients undergoing bilateral pressure equalization tube insertion. J Clin Anesth. 2000;12:397–401.
23 Murray DJ, Cole JW, Shrock CD, Snider RJ, Martini JA. Sevoflurane versus halothane: effect of oxycodone premedication on emergence behaviour in children. Paediatr Anaesth. 2002;12:308–312.
24 Meyer RR, Munster P, Werner C, Brambrink AM. Isoflurane is associated with a similar incidence of emergence agitation/delirium as sevoflurane in young children—a randomized controlled study. Paediatr Anaesth. 2007;17:56–60.
25 Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. 2003;96:1625–1630.
26 Cohen IT, Finkel JC, Hannallah RS, Hummer KA, Patel KM. The effect of fentanyl on the emergence characteristics after desflurane or sevoflurane anesthesia in children. Anesth Analg. 2002;94:1178–1181.
27 Hollman GA. Oral midazolam and emergence delirium. Ann Emerg Med. 1995;25:853–854.
28 Ko YP, Huang CJ, Hung YC, et al. Premedication with low-dose oral midazolam reduces the incidence and severity of emergence agitation in pediatric patients following sevoflurane anesthesia. Acta Anaesthesiol Sin. 2001;39:169–177.
29 Ibacache ME, Munoz HR, Brandes V, Morales AL. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg. 2004;98:60–63.
30 Isik B, Arslan M, Tunga AD, Kurtipek O. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr Anaesth. 2006;16:748–753. erratum in Paediatr Anaesth 2006;16:811
31 Aouad MT, Yazbeck-Karam VG, Nasr VG, et al. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology. 2007;107:733–738.
32 Lee CJ, Lee SE, Oh MK, et al. The effect of propofol on emergence agitation in children receiving sevoflurane for adenotonsillectomy. Korean J Anesthesiol. 2010;59:75–81.
33 Lee CJ, San E, Min KO. The effect of propofol on emergence agitation in children receiving sevoflurane for adenotonsillectomy. Korean J Anesthesiol. 2010;59:75–81.
34 Aouad MT, Kanazi GE, Siddik-Sayyid SM, et al. Preoperative caudal block prevents emergence agitation in children following sevoflurane anesthesia. Acta Anaesthesiol Scand. 2005;49:300–304.
35 Vlajkovic GP, Sindjelic RP. Emergence delirium in children: Many questions, few answers. Anesth Analg. 2007;104:84–91.
36 Kain ZN, Caldwell-Andrews AA, Maranets I, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99:1648–1654.
37 Tay CL, Tan GM, Ng SB. Critical incidents in paediatric anaesthesia: an audit of 10,000 anaesthetics in Singapore. Paediatr Anaesth. 2001;11:711–718.
38 Akin A, Esmaoglu A, Boyaci A. Postoperative shivering in children and causative factors. Pediatr Anesth. 2005;15:1089–1093.
39 Kranke P, Eberhart LHJ, Roewer N, Tramer MR. Postoperative shivering in children: a review on pharmacologic prevention and treatment. Paediatric Drugs. 2003;5:373–383.
40 Bryson GL, Chung F, Cox RG, et al. Patient selection in ambulatory anesthesia—an evidence-based review: II. Can J Anaesth. 2004;51:782–794.
41 Tait AR. Anesthetic management of the child with an upper respiratory tract infection. Curr Opin Anaesthesiol. 2005;18:603–607.
42 Epstein RA, Hyman AI. Ventilatory requirements of critically ill neonates. Anesthesiology. 1980;53:379–384.
43 Keens TG, Bryan AC, Levison H, Ianuzzo CD. Developmental pattern of muscle fiber types in human ventilatory muscles. J Appl Physiol. 1978;44:909–913.
44 Lindahl SG, Charlton AJ, Hatch DJ. Ventilatory responses to rebreathing and carbon dioxide inhalation during anaesthesia in children. Br J Anaesth. 1985;57:1188–1196.
45 Lindahl SG, Johannesson GP. Ventilatory CO2 response, respiratory drive and timing in children anaesthetized with halothane, enflurane or isoflurane. Eur J Anaesthesiol. 1987;4:313–326.
46 McGonagle M, Kennedy TL. Laryngospasm induced pulmonary edema. Laryngoscope. 1984;94:1583–1585.
47 Mehta VM, Har-El G, Goldstein NA. Postobstructive pulmonary edema after laryngospasm in the otolaryngology patient. Laryngoscope. 2006;116:1693–1696.
48 Silva PS, Monteiro Neto H, Andrade MM, Neves CV. Negative-pressure pulmonary edema: a rare complication of upper airway obstruction in children. Pediatr Emerg Care. 2005;21:751–754.
49 Koka BV, Jeon IS, Andre JM, et al. Postintubation croup in children. Anesth Analg. 1977;56:501–505.
50 Gregory GA, Steward DJ. Life-threatening perioperative apnea in the ex-“premie.”. Anesthesiology. 1983;59:495–498.
51 Kurth CD, Spitzer AR, Broennle AM, Downes JJ. Postoperative apnea in preterm infants. Anesthesiology. 1987;66:483–488.
52 Coté CJ, Zaslavsky A, Downes JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy: a combined analysis. Anesthesiology. 1995;82:809–822.
53 Krane EJ, Haberkern CM, Jacobson LE. Postoperative apnea, bradycardia, and oxygen desaturation in formerly premature infants: prospective comparison of spinal and general anesthesia. Anesth Analg. 1995;80:7–13.
54 Kurth CD, LeBard SE. Association of postoperative apnea, airway obstruction, and hypoxeemia in former premature infants. Anesthesiology. 1991;75:22–26.
55 Malviya S, Swartz J, Lerman J. Are all preterm infants younger than 60 weeks postconceptual age at risk for postanesthetic apnea? Anesthesiology. 1993;78:1076–1081.
56 Welborn LG, Greenspun JC. Anesthesia and apnea: perioperative considerations in the former preterm infant. Pediatr Clin North Am. 1994;41:181–198.
57 Welborn LG, Hannallah RS, Luban NL, et al. Anemia and postoperative apnea in former preterm infants. Anesthesiology. 1991;74:1003–1006.
58 Welborn LG, Rice LJ, Hannallah RS, et al. Postoperative apnea in former preterm infants: Prospective comparison of spinal and general anesthesia. Anesthesiology. 1990;72:838–842.
59 Henderson-Smart DJ, Steer P. Postoperative caffeine for preventing apnea in preterm infants. Cochrane Database Syst Rev. 4, 2000. CD000048
60 Henderson-Smart DJ, Steer P. Prophylactic caffeine to prevent postoperative apnea following general anesthesia in preterm infants. Cochrane Database Syst Rev. 4, 2001. CD000048
61 William JM, Stoddart PA, Williams SA, Wolf AR. Postoperative recovery after inguinal herniotomy in ex-premature infants: comparison between sevoflurane and spinal anaesthesia. Br J Anaesth. 2001;86:366–371.
62 Walther-Larsen S, Rasmussen LS. The former preterm infant and risk of postoperative apnoea: recommendations for management. Acta Anaesthesiol Scand. 2006;50:888–893.
63 Hoelzle M, Weiss M, Dillier C, Gerber A. Comparison of awake spinal with awake caudal anesthesia in preterm and ex-preterm infants for herniotomy. Pediatr Anesth. 2010;20:620–624.
64 Williams RK, Adams DC, Aladjem EV, et al. The safety and efficacy of spinal anesthesia for surgery in infants: the Vermont Infant Spinal Registry. Anesth Analg. 2006;102:67–71.
65 Frumiento C, Abajian JC, Vane DW. Spinal anesthesia for preterm infants undergoing inguinal hernia repair. Arch Surg. 135, 2000. 445–441
66 Lerman J. Surgical and patient factors involved in postoperative nausea and vomiting. Br J Anaesth. 1992;69:24S–32S.
67 Shende D, Bharti N, Kathirvel S, Madan R. Combination of droperidol and ondansetron reduces PONV after pediatric strabismus surgery more than single drug therapy. Acta Anaesthesiol Scand. 2001;45:756–760.
68 D’Errico C, Voepel-Lewis TD, Siewert M, Malviya S. Prolonged recovery stay and unplanned admission of the pediatric surgical outpatient: an observational study. J Clin Anesth. 1998;10:482–487.
69 Gan TJ, Meyer T, Apfel CC, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97:62–71.
70 Erb TO, Hall JM, Ing RJ, et al. Postoperative nausea and vomiting in children and adolescents undergoing radiofrequency catheter ablation: a randomized comparison of propofol and isoflurane based anesthetics. Anesth Analg. 2002;95:1577–1581.
71 Kovac AL. Management of postoperative nausea and vomiting in children. Pediatr Drugs. 2007;9:47–69.
72 Barst SM, Leiderman JU, Markowitz A, et al. Ondansetron with propofol reduces the incidence of emesis in children following tonsillectomy. Can J Anaesth. 1999;46:359–362.
73 Splinter WM, Rhine EJ. Low-dose ondansetron with dexamethasone more effectively decreases vomiting after strabismus surgery in children than does high-dose ondansetron. Anesthesiology. 1998;88:72–75.
74 Tramer MR, Moore RA, Reynolds DJ, McQuay HJ. A quantitative systematic review of ondansetron in treatment of established postoperative nausea and vomiting. BMJ. 1997;314:1088–1092.
75 Tramer MR, Reynolds DJ, Moore RA, McQuay HJ. Efficacy, dose response, and safety of ondansetron in prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized placebo-controlled trials. Anesthesiology. 1997;87:1277–1289.
76 Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg. 2000;90:186–194.
77 Steward DL, Welge JA, Myer CM. Do steroids reduce morbidity of tonsillectomy? Meta-analysis of randomized trials. Laryngoscope. 2001;111:1712–1718.
78 Steward DL, Welge JA, Myer CM. Steroids for improving recovery following tonsillectomy in children. Cochrane Database Syst Rev. 1, 2003. CD003997
79 Kim MS, Coté CJ, Cristoloveanu C, et al. There is no dose-escalation response to dexamethasone (0.0625-1.0 mg/kg) in pediatric tonsillectomy or adenotonsillectomy patients for preventing vomiting, reducing pain, shortening time to first liquid intake, or the incidence of voice change. Anesth Analg. 2007;104:1052–1058.
80 Henzi I, Sonderegger J, Tramer MR. Efficacy, dose-response, and adverse effects of droperidol for prevention of postoperative nausea and vomiting. Can J Anaesth. 2000;47:537–551.
81 Goodarzi M, Matar MM, Shafa M, Townsend JE, Gonzalez I. A prospective randomized blinded study of the effect of intravenous fluid therapy on postoperative nausea and vomiting in children undergoing strabismus surgery. Paediatr Anaesth. 2006;16:49–53.
82 Habib AS, Gan TJ. Evidence-based management of postoperative nausea and vomiting. Can J Anaesth. 2004;51:326–341.
83 Wang SM, Hofstadter MB, Kain ZN. An alternative method to alleviate postoperative nausea and vomiting in children. J Clin Anesth. 1999;11:231–234.
84 Lee A, Done ML. The use of nonpharmacologic techniques to prevent postoperative nausea and vomiting: a meta-analysis. Anesth Analg. 1999;88:1200–1202.
85 Manworren RC, Hynan LS. Clinical validation of FLACC: preverbal patient pain scale. Pediatr Nurs. 2003;29:140–146.
86 Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–297.
87 Knowles R. Standardization of pain management in the postanesthesia care unit. J Perianesth Nurs. 1996;11:390–398.
88 Committee on Psychosocial Aspects of Child and Family Health. The assessment and management of acute pain in infants, children, and adolescents. Pediatrics. 2001;108:793–797.
89 Viitanen H, Tuominen N, Vaaraniemi H, et al. Analgesic efficacy of rectal acetaminophen and ibuprofen alone or in combination for paediatric day-case adenoidectomy. Br J Anaesth. 2003;91:363–367.
90 Hernandez-Palazon J, Tortosa JA, Martinez-Lage JF, Perez-Flores D. Intravenous administration of propacetamol reduces morphine consumption after spinal fusion surgery. Anesth Analg. 2001;92:1473–1476.
91 Duggan ST, Scott LJ. Intravenous paracetamol (acetaminophen). Drugs. 2009;69:101–103.
92 Wininger SJ, Miller H, Minkowitz HS. A randomized, double-blind, placebo-controlled, multicenter, repeat-dose study of two intravenous acetaminophen dosing regimes for the treatment of pain after abdominal laparoscopic surgery. Clin Ther. 2010;32:2348–2369.
93 McNicol ED, Tzortzopoulou A, Cepeda MS, et al. Single-dose intravenous paracetamol or propacetamol for prevention or treatment of postoperative pain: a systemic review and meta-analysis. Br J Anaesth. 2011;106:764–775.
94 Uysai HY, Takmaz SA, Yaman F, Baltaci B, Basar H. The efficacy of intravenous paracetamol versus tramadol for postoperative analgesia after adenotonsillectomy in children. J Clin Anesth. 2011;23:53–57.
95 Birmingham PK, Tobin MJ, Henthorn TK, et al. Twenty-four-hour pharmacokinetics of rectal acetaminophen in children: an old drug with new recommendations. Anesthesiology. 1997;87:244–252.
96 Beck DH, Schenk MR, Hagemann K, et al. The pharmacokinetics and analgesic efficacy of larger dose rectal acetaminophen (40 mg/kg) in adults: a double-blinded, randomized study. Anesth Analg. 2000;90:431–436.
97 Kokki H. Nonsteroidal anti-inflammatory drugs for postoperative pain: a focus on children. Paediatr Drugs. 2003;5:103–123.
98 Litalien C, Jacqz-Aigrain E. Risks and benefits of nonsteroidal antiinflammatory drugs in children: a comparison with paracetamol. Paediatr Drugs. 2001;3:817–858.
99 Armstrong PJ, Bersten A. Normeperidine toxicity. Anesth Analg. 1986;65:536–538.
100 Lehr VT, BeVier P. Patient-controlled analgesia for the pediatric patient. Orthop Nurs. 2003;22:298–304.
101 Park JM, Houck CS, Sethna NF, et al. Ketorolac suppresses postoperative bladder spasms after pediatric ureteral reimplantation. Anesth Analg. 2000;91:11–15.
102 Tsui BC, Gupta S, Finucane B. Confirmation of epidural catheter placement using nerve stimulation. Can J Anaesth. 1998;45:640–644.
103 Chawathe MS, Jones RM, Gildersleve CD, et al. Detection of epidural catheters with ultrasound in children. Paediatr Anaesth. 2003;13:681–684.
104 Taenzer AH. Inadvertent spinal anesthesia during continuous epidural anesthesia in an infant. Anesthesiology. 2003;98:1014–1015.
105 Taenzer AH, Kovarik D, Clark C. Experience with 724 epidurograms for epidural catheter placement in pediatric anesthesia. Reg Anesth Pain Med. 2010;35:432–435.
106 Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318–1323.
107 Schmied H, Kurz A, Sessler DI, et al. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–292.
108 Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–1215.
109 Kurz A, Sessler DI, Narzt E, et al. Postoperative hemodynamic and thermoregulatory consequences of intraoperative core hypothermia. J Clin Anesth. 1995;7:359–366.
110 Winkler M, Akca O, Birkenberg B, et al. Aggressive warming reduces blood loss during hip arthroplasty. Anesth Analg. 2000;91:978–984.
111 Kurz A, Kurz M, Poeschl G, et al. Forced-air warming maintains intraoperative normothermia better than circulating-water mattresses. Anesth Analg. 1993;77:89–95.
112 Choong K, Arora S, Cheng J, et al. Hypotonic versus isotonic maintenance fluids after surgery for children: a randomized controlled trial. Pediatrics. 2011;128:857–866.
113 Marshall SI, Chung F. Discharge criteria and complications after ambulatory surgery. Anesth Analg. 1999;88:508–517.
114 Fritz WT, George L, Krull N, et al. Utilization of a home nursing protocol allows ambulatory surgery patients to be discharged prior to voiding. Anesth Analg. 1997;84:S6.
115 Patel RI, Verghese ST, Hannallah RS, Aregawl A, Patel KM. Fast-tracking children after ambulatory surgery. Anesth Analg. 2001;92:918–922.
116 White PF, Song D. New criteria for fast-tracking after outpatient anesthesia: a comparison with the modified Aldrete’s scoring system. Anesth Analg. 1999;88:1069–1072.