CHAPTER 9 THE PERSISTENT VEGETATIVE STATE (PROLONGED POSTCOMA UNRESPONSIVENESS) AND POSTHYPOXIC BRAIN INJURY
Prolonged survival in a vegetative state is one of the most harrowing events encountered in modern medicine. Although the condition provides intellectual challenges to the expertise of medical ethicists, legal practitioners, and legislators, the patient’s family members and others intimately involved in the daily care of the patient are usually caught in a web of despair, unresolved grief, anger, a pervading sense of futility, and fading hope. Adversarial relationships between medical staff and family may arise, especially if iatrogenic issues such as anesthetic catastrophes are present. Family members may clutch at slim hopes offered by media reports of recovery after prolonged coma or vegetative states. Physicians may be torn by the conflict between limited resources and the pressure to persevere with intensive support, potentially restricting access to patients with substantially better chances of recovery. Decisions to continue or withdraw support are often charged with ethical and medicolegal implications, inasmuch as either course of action can be construed to be contrary to the “best interests” of the patient.
The various terms vegetative state (persistent and permanent) and postcoma unresponsiveness are used to describe the clinical emergence of the patient from deep coma to a state in which the sleep-wake cycle is reestablished but the patient shows no clinical evidence of cognitive interaction with the world around him or her. When the state is deemed to be unremitting (more than 3 months after anoxic brain damage* or more than 12 months after traumatic brain injury) the term permanent vegetative state is recommended. Expert groups in the United States1 and Europe have suggested using only the terms vegetative state and permanent vegetative state because the term persistent has become shrouded with ambiguity. The terms vegetative state and persistent vegetative state do not imply irreversibility; however, the term permanent vegetative state carries prognostic implications.
For the situation in which some minimal level of purposeful response to environmental stimuli is observed, the term minimally conscious state has been advocated.4 The terminological uses vary, and some authors5 have advocated avoiding the term vegetative in view of its potentially pejorative connotations, but the original description6 remains widely accepted.
CRITERIA
Vegetative State
The salient features of the vegetative state† are as follows:
The Minimally Conscious/Responsive State
Patients may improve from the vegetative state to demonstrate clearly discernible evidence of self or environmental awareness. The term minimally conscious state4 or minimally responsive state7 has been proposed to describe the condition of such patients. Minimal criteria such as the ability to follow a simple command, the presence of intelligible verbalization, or sustained visual following have been proposed to identify this state. Such responses may be intermittent and more easily evoked by family than by medical staff. Repeated observations may be necessary to distinguish this state from the vegetative state. Such purposeful responses clearly exclude a diagnosis of vegetative state, but difficulties defining the level of cognitive function sufficient to exceed a diagnosis of the minimally conscious state have limited widespread application of the term. The reestablishment of interactive communication, including orientation to place, the ability to give accurate autobiographical information, and the appropriate use of objects, are generally considered to indicate that the patient is functioning at a level higher than the minimally conscious state.
It is important to note that the lack of behavioral manifestations of self or external awareness cannot by themselves prove the absence of a residual or rudimentary subjective awareness. It may not be possible clinically to distinguish a minimally responsive patient with residual cognitive function from one who lacks adequate afferent processing or efferent control to reliably demonstrate understanding or performance of a requested task. Indeed, Owen and colleagues8 reported three patients with clinical diagnoses of persistent vegetative state who demonstrated “covert cognitive processing” in positron emission tomographic scanning with a facial recognition task. It is also conceivable that patients may satisfy the criteria for the minimally conscious state on a “good” day and, perhaps as a consequence of infection, electrolyte disturbance, or fatigue, show no sign of awareness on a “bad” day.9 Because of such patients, the boundary between the vegetative state and the minimally conscious state may not be as discontinuous as the definitions suggest.
Locked-In Syndrome
The locked-in syndrome refers to a state in which cognitive processing is clearly evident but severe paralysis prevents articulation or gestural expression of awareness. Communication may be achieved by encoding eyelid or ocular movement. In severe cases, the diagnosis may depend on the demonstration of a normal reactive alpha rhythm on the electroencephalogram (EEG).10 Such patients usually have isolated lesions of the ventral pons with preserved cerebral hemispheres. Locked-in syndrome may also occur as a consequence of severe peripheral nerve or muscle disease.
Akinetic Mutism
The term akinetic mutism has been used to describe a range of cases in which varying degrees of consciousness, paralysis, and mutism may be present. Various pathological findings have been described,11 including infarction of the anterior cingulate gyrus.12 Cairns and associates13 described an adolescent with a craniopharyngioma who developed repeated episodes of “silent immobility” with open and apparently attentive eyes and occasional whispered monosyllables whose state was reversed on several occasions by aspiration of the tumor. A similar case with improvement after shunting has been described more recently.14 Akinetic mutism has been divided into frontal and mesencephalic types, the latter distinguished by the presence of vertical gaze palsy or ophthalmoplegia. Cyclosporine neurotoxicity15 and baclofen toxicity16 may each cause a reversible state of akinetic mutism. Severe frontal abulia (for example, caused by bilateral anterior cerebral artery infarction or a ruptured anterior communicating aneurysm) may be impossible to distinguish on clinical criteria from either akinetic mutism or the minimally conscious state.
EPIDEMIOLOGY
The incidence and prevalence of vegetative state in the community vary widely in accordance with the sources of the data. A comprehensive review3 of the epidemiology of vegetative state revealed prevalence rates in the United States ranging from 24 to 168 per million of the population in studies dating from 1990 to 1994. The Multi-Society Task Force on Persistent Vegetative State (1994) accepted an estimate from Ashwal and colleagues17 of 56 to 140 per million.
The incidence of vegetative survivors after all acute causes at 1, 3, and 6 months has been estimated for the United Kingdom, the United States, and France.3 The incidence falls with time as patients either die or attain higher levels of function and is also heavily influenced by the level of head injury in the community. At 1 month after injury, the incidence ranges from 14 per million in the United Kingdom to 67 per million in France. By 6 months after the cerebral insult, the incidence of survival in a vegetative state is 5 per million in the United Kingdom, 17 per million in the United States, and 25 per million in France. The relative contribution of traumatic to nontraumatic causes of vegetative state also varies considerably, depending on the source of the data. Studies have shown the contribution of head injury ranging from 24%18 to 72%.1 Some studies include patients who decline into a vegetative state as a consequence of progressive neurodegenerative disorders, as opposed to progressing to a vegetative state from coma caused by an acute cerebral insult. This and other case ascertainment issues continue to complicate the epidemiological data.
PATHOLOGY
In the setting of cerebral anoxia or diffuse ischemia, there is usually extensive neocortical laminar necrosis and bilateral hippocampal, amygdalar, and thalamic damage.19,20 The Purkinje cell layer of the cerebellum is similarly vulnerable to diffuse ischemic injury. A second pattern of brain damage occurs after a short episode of profound hypotension, in which the ischemic damage is confined to the arterial boundary zones. This produces wedge-shaped zones of ischemia with additional bilateral thalamic damage and varying involvement of the hippocampus.
After severe traumatic brain injury, the brunt of the pathology is subcortical and conforms to the diffuse shearing injuries now known as diffuse axonal injury (DAI), as described by Strich in 195621 and 1961.22 DAI is graded into types I to III; grade I is defined as diffuse subcortical shearing injury without callosal or brainstem involvement, grade II indicates additional focal lesions in the corpus callosum, and grade III indicates additional lesions in both the corpus callosum and the dorsolateral region of the rostral brainstem. Patients with DAI grade I are unlikely to remain in a prolonged vegetative state unless there is coexisting evidence of a hypoxic/ischemic insult. Coexisting evidence of hypoxic/ischemic damage in posttraumatic cases is not an uncommon finding.20 It is thought to reflect respiratory or circulatory failure at the time of head injury, although disturbances of cerebral autoregulation may also play a role. On the other hand, significant brainstem damage is a rare finding in prolonged survival in a vegetative state, which explains the recovery of sleep-wake function and autonomic cardiorespiratory control in this state.
It appears that thalamic damage may play a critical role in the genesis of prolonged postcoma unresponsiveness, as cases with relatively little cortical damage are well described. Adams and associates20 published detailed studies of the pathological findings in both nontraumatic and traumatic cases and compared the latter group with 35 traumatic cases in which patients recovered to a state better than vegetative state before death. In summary, patients who remained in a vegetative state generally had more extensive DAI, thalamic damage, or both. Patients with lesser degrees of subcortical damage nearly always had extensive thalamic damage. Higher functioning patients rarely had both bilateral thalamic damage and extensive DAI, and if thalamic damage was present, it was considered to be of a lesser degree. Thus, extensive bilateral damage to the thalamus appears to be critical for the development of the vegetative state, especially if damage elsewhere is minimal.
DIAGNOSIS
Misdiagnoses of the vegetative state and the minimally conscious state appear to be common.23 Repeated clinical assessments are necessary to clearly establish whether reliable evidence of cognitive function is present in a severely brain-damaged patient. Patients are often in an intensive care environment when the diagnosis is first considered, and varying levels of potentially sedating medications may complicate the assessment. Similarly, metabolic manifestations of other major organ involvement may confound the clinical picture. Neurologists and intensive care physicians may disagree as to whether visual fixation or tracking is present or whether a particular movement is purposeful or reflexive. Nursing staff are often particularly helpful in determining whether an orienting or purposeful response can be consistently linked to a particular stimulus, especially if there is some fluctuation caused by extraneous factors such as seizures or bolus doses of anticonvulsants.
NEUROPHYSIOLOGICAL ASSESSMENT
Electroencephalography
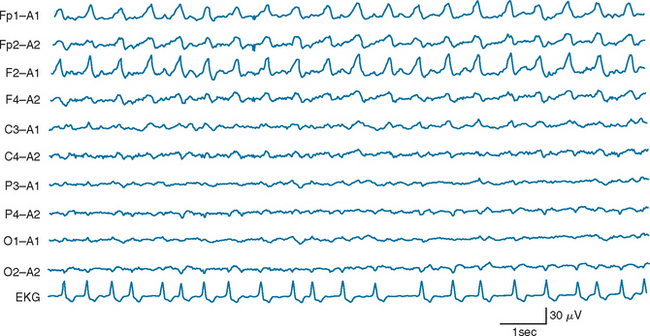
Certain electroencephalographic patterns are known to be associated with extremely grave outcomes. Care must be taken to ensure that sedative medications, hypothermia, and severe metabolic disturbances are not confounding the interpretation of the record. In the setting of anoxic/ischemic cerebral injury, several electroencephalographic patterns have been associated with either a fatal outcome or, at best, survival with severe neurological sequelae.24 Of these patterns, the isoelectric or “flat” EEG after the first 24 hours is invariably associated with a poor outcome. The burst-suppression pattern (Fig. 9-1), especially if accompanied by generalized (typically facial and axial) myoclonic status25; the continuous bilateral periodic EEG or generalized epileptiform EEG (Fig. 9-2); and alpha (Fig. 9-3) and theta coma (Fig. 9-4) patterns usually but not invariably indicate a poor prognosis. Serial EEGs are sometimes necessary to identify a deteriorating trend.
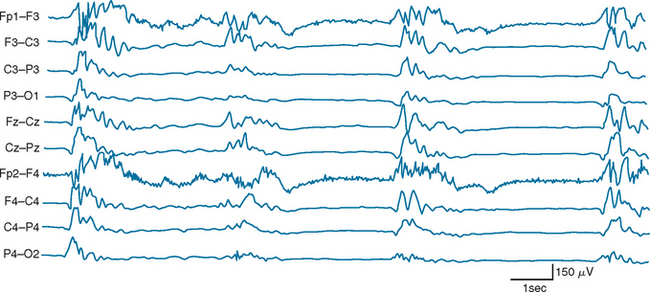
Figure 9-1 Burst suppression electroencephalographic pattern two days after prolonged cardiac arrest.
(Reproduced with permission from the American Journal of EEG Technology. Drury I.: The EEG in hypoxic-ischemic encephalopathy. Am J EEG Technol 1988; 28:129-137.)
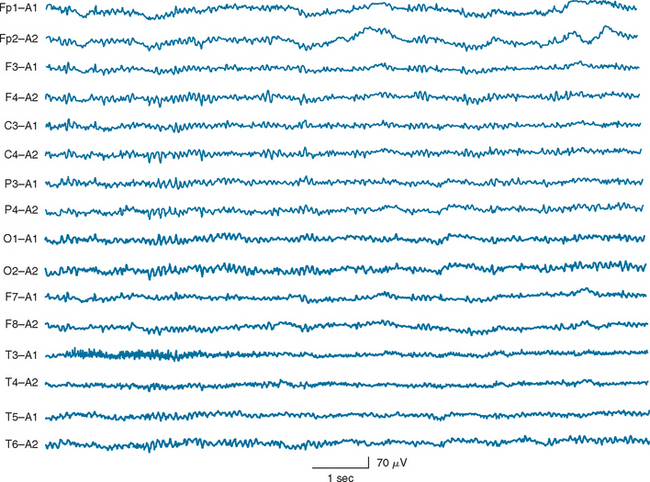
Figure 9-3 Diffuse unreactive alpha frequency activity on electroencephalography after cardiac arrest.
(Reproduced with permission from the American Journal of EEG Technology.)
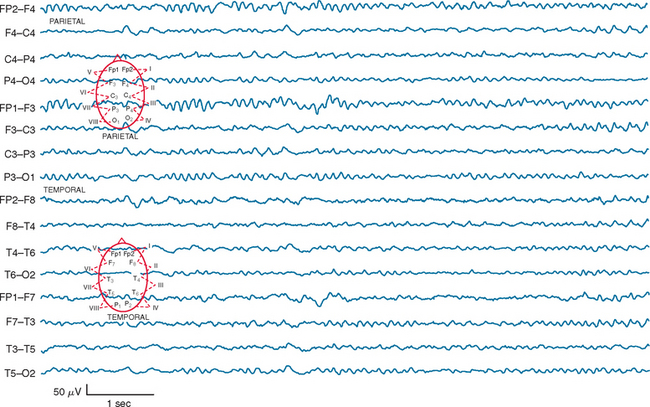
Figure 9-4 Diffuse unreactive theta slowing on EEG in an elderly patient after prolonged cardiac arrest.
The isoelectric EEG is used in many countries as a confirmatory test for brain death, and the technique has been standardized.26 The recording must be undertaken for a minimum of 30 minutes at a sensitivity of 2 μV/mm using a minimum of eight channels with double-distance (>10 cm) electrodes in the 10-to-20 montage with filters set at less than 1 Hz and at 70 Hz. At these sensitivities, artifact is common, and the technician must take great care to identify all sources of artifact. ECG artifact is usually present in all channels, and this can usually be readily distinguished from activity of cerebral origin.
The continuous bilateral periodic EEG and generalized epileptiform records can be confused with nonconvulsive electrographic status epilepticus. The distinction may well be semantic. In general, patients who develop clinically subtle or nonconvulsive status epilepticus after anoxic cerebral injury do not do well, and aggressive trials of anticonvulsants are typically futile. If doubts remain on clinical grounds (e.g., a known epileptic patient who suffers a near-drowning event), a suitable trial of an anticonvulsant therapy is prudent, and SSEPs can be used as a more reliable guide to prognosis. Periodic complexes are also characteristic of advanced renal and hepatic failure, but these are readily ruled out by appropriate biochemical investigations.
Apart from the technical difficulties often experienced in obtaining good-quality EEGs in patients with severe traumatic brain injury, prognostication for this group based on electroencephalographic findings is recognized to be less reliable. The presence of reactivity to auditory, visual, or noxious stimulation, which may be either “attenuation” or “paradoxical” (decrease or increase in background amplitude after stimulation), implies a better outcome.27 Such a finding suggests that the patient is very unlikely to remain in merely a permanent vegetative state after emerging from coma. Electroencephalographic reactivity has been demonstrated in the cat to depend on an intact reticular activating system and preserved thalamocortical pathways.28 Head-injured patients with less favorable electroencephalographic findings may still do well, however, and in this group the additional use of other neurophysiological parameters—in particular, SSEPs—may help refine prognostication.
SOMATOSENSORY EVOKED POTENTIALS
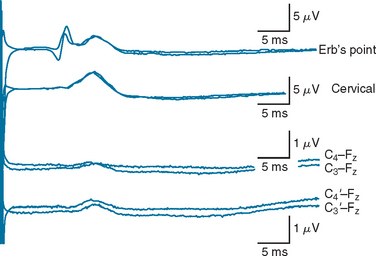
The bilateral absence of thalamocortical waveforms (N19/P22 or N1) in comatose patients after either anoxic cerebral injury or severe head injury29 indicates an extremely bleak prognostic outcome. If the study is performed with appropriate care, this is the most robust electrophysiological finding in the intensive care environment. In the setting of coma after anoxia, patients with this finding do not improve beyond the vegetative state. Repeated studies30 have shown this finding to have 100% specificity when the SSEPs are performed after 24 hours of coma. In patients with traumatic brain injury, care should be taken to rule out significant cervical trauma or brainstem pathology that may interrupt large fiber sensory pathways at that level and hence may complicate SSEP interpretation. Access and technical factors such as prominent scalp edema or hematoma can also make results of SSEP studies more difficult to interpret in head injury. In addition, the use of barbiturate-induced coma in severely head-injured patients may attenuate low-amplitude waveforms (B. Day, personal observation, 2003); therefore, SSEP studies for prognostication in these patients should be delayed until the barbiturate-induced coma therapy is withdrawn. Well-formed, normal-latency Erb’s point potentials and cervical potentials are important quality assurance requirements before prognostication can be made without thalamocortical waveforms (Fig. 9-5). Although higher stimulus intensities can be used in comatose patients, the stimulus frequency should be less than 5 Hz. If these technical issues do not complicate interpretation, bilateral absence of cortical SSEP responses is also 100% specific for an outcome no better than vegetative state in patients with traumatic brain injury.
The high specificity of the bilateral absence of cortical waveforms is, however, accompanied by a low sensitivity (28% to 73%).30 Many patients with preserved but abnormal cortical waveforms nonetheless have very poor neurological outcomes, and the finding of normal cortical responses is not necessarily predictive of a favorable outcome. Unilateral delayed or low-amplitude cortical responses tend to be associated with a more severe residual neurological deficit in the stimulated limb, whereas a well-formed normal-latency cortical waveform is suggestive of a higher level of function in the corresponding limb, but the positive predictive value of such findings varies sufficiently between studies that their clinical utility is limited.
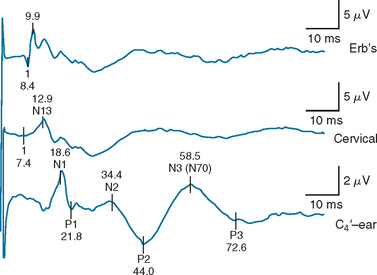
For patients in whom the cortical waveforms (N19/P22 or N1) are present, some attempt to improve the sensitivity of SSEP studies has been undertaken by examining later waveforms, particularly the N70 (also called N3)31,32 (Fig. 9-6). No patient with bilaterally absent N19/P22 or N70 potentials “awakened” (“awakening” being defined as following commands or having comprehensible speech). No patient with absent N19/P22 potentials had preserved N70 waveforms. Patients in whom the N70 waveforms were present but prolonged beyond 176 milliseconds did not “awaken.” When the absence of the N70 or an N70 latency of more than 176 milliseconds was used instead of bilaterally absent N19/P22 potentials for predicting nonawakening, the sensitivity of SSEP testing increased from 55% to 67%, and the specificity remained at 100%. Identification of the N70 waveform and determination of its peak latency can be technically more difficult, however, and it is more sensitive to the effects of anesthetic medications than is the N19/P22 waveform.
BIOCHEMICAL MARKERS
Cerebrospinal Fluid Creatine Kinase BB activity
Elevated creatine kinase BB (205 U/L) isoenzyme activity measured in the cerebrospinal fluid 48 to 72 hours after cardiopulmonary arrest has been reported to have 100% specificity and 48% sensitivity for never awakening33 (as defined previously). Using a lower cutoff increases the sensitivity but lowers the specificity. Using this parameter in conjunction with SSEP studies further increases sensitivity to 78% without sacrificing the 100% specificity required for making decisions about withdrawal of life support.31
Serum Astroglial S-100 Protein
The calcium-binding modulator protein S-100 is an established biochemical marker of central nervous system injury.34 Increased serum levels (0.2 μg/L) in comatose patients 24 to 48 hours after out-of-hospital cardiopulmonary arrest were invariably associated with a fatal outcome within 14 days.35
Serum Neuron-Specific Enolase
Serum concentrations of neuron-specific enolase exceeding 33 ng/mL were also predictive of a poor prognostic outcome in comatose patients, with a high degree of specificity (100%), when measured within 72 hours after out-of-hospital cardiopulmonary arrest.36
The role of biochemical markers, especially when used in conjunction with neurophysiological tests, is likely to become increasingly important in establishing the early prediction of outcome from coma caused by anoxic ischemic injury or severe head injury.30
IMAGING STUDIES
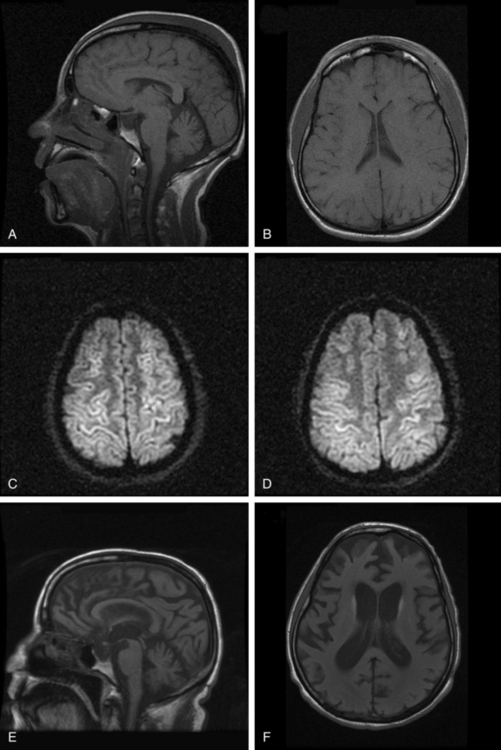
Although computed tomographic and magnetic resonance imaging (MRI) studies are often routinely performed in patients with prolonged postcoma unresponsiveness after hypoxic brain injury, these studies only rarely provide useful prognostic information. Diffusion-weighted MRI in the acute stage when the patient is still comatose may show diffuse cortical enhancement that is consistent with laminar infarction (Fig. 9-7A-D). Late imaging usually shows diffuse cortical atrophy (see Fig. 9-7E and F), but correlation between the degree of atrophy and outcome is imprecise. In one notable case, a 60-year-old academician was able to resume his career after emerging from a vegetative state of 8 weeks’ duration, despite generalized cerebral atrophy evident on the computed tomographic head scan.37
For patients in a posttraumatic vegetative state, more extensive MRI changes of DAI involving the corpus callosum and dorsolateral brainstem are correlated with continuing postcoma unresponsiveness.38
Magnetic resonance spectroscopy and diffusion tensor imaging have yet to be subjected to prospective longitudinal randomized studies in patients with prolonged postcoma unresponsiveness after anoxic cerebral injury or head injury, although early studies39 suggest that such techniques hold promise.
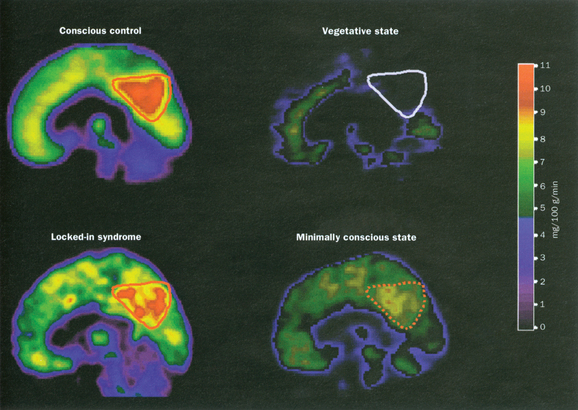
Measurements of cerebral metabolism and brain activation with positron emission tomographic scanning and functional MRI studies after sensory stimulation are providing important insights into the pathophysiology of the vegetative state (see Laureys et al, 2004).40 These techniques are methodologically complex and careful interpretation is required, but important differences between the vegetative state and the minimally conscious state are beginning to emerge. Overall, patients who remain in a vegetative state have cerebral metabolic rates of the order of 30% to 40% of normal41 (Fig. 9-8), marginally lower than those in minimally conscious patients. A notable finding in the vegetative state is the extent of the metabolic impairment in the polymodal association cortices. These regions are important for higher order processing of incoming afferent information necessary for orientation, attention, recognition, memory, and language42 (Fig. 9-9). Such studies will potentially provide important new insights into prognostic and ethical issues, such as the presence of “covert cognitive processing”; however, current methodological complexities and problems with analysis and interpretation limit their clinical application.
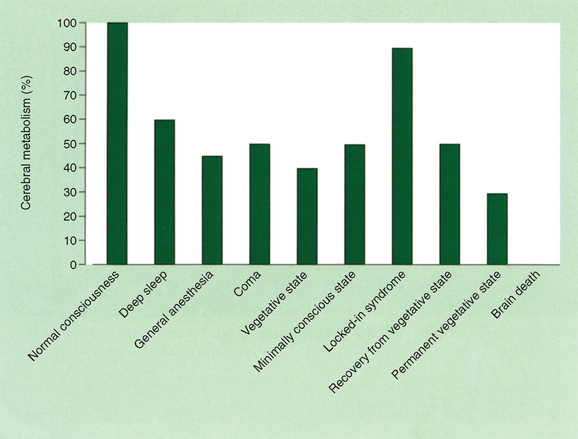
Figure 9-8 Cerebral metabolism in various states.
(From Laureys S, Owen AM, Schiff ND: Brain function in coma, vegetative state and related disorders. Lancet Neurol 2004; 3:537-546. Copyright 2004. Reprinted with permission from the American Academy of Opthalmology.)
PROGNOSTIC, MANAGEMENT, AND ETHICAL CONSIDERATIONS
The phenomenon of prolonged survival in a vegetative state after coma is a direct consequence of advances in modern medical practice, particularly of the popular promotion of techniques such as cardiopulmonary resuscitation and highly expeditious emergency rescue and retrieval systems, coupled with access to sophisticated intensive care facilities. As a consequence, many busy intensive care units are faced on a daily basis with acutely comatose patients with potentially very poor but ultimately uncertain prognoses for independent recovery. Although ethical concerns dictate that economic considerations be set aside in the management of such patients, many intensive care services in large tertiary hospitals have problems with both significant access and cost containment. Ongoing futile management represents a serious misallocation of limited health care resources, as well as an unnecessary addition to the family’s distress. There is little doubt that prolonged survival in a vegetative state represents a disastrous outcome, particularly if it results from overly zealous application of intensive care support of comatose patients in the presence of extremely grave prognostic indicators. There is therefore an urgent need to refine neurophysiological, biochemical, and cerebral metabolic activity imaging studies further as early prognostic indicators to assist clinicians and families make the painful decisions regarding ongoing support.
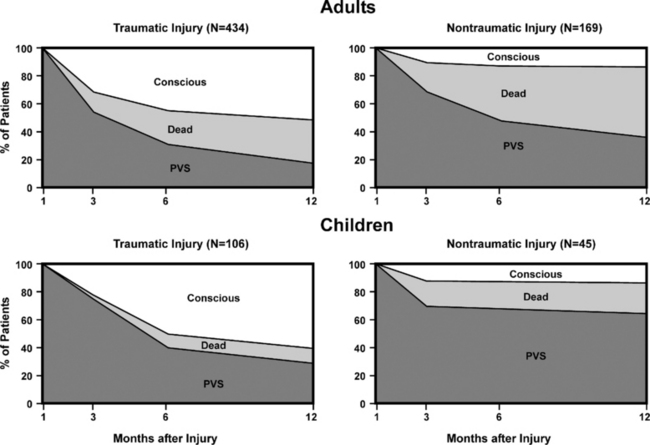
Once the patient has emerged from coma into a vegetative state with autonomous respiratory function, the ethical issues regarding support become much more vexed. As indicated previously, the vegetative state, especially in its early phase, cannot be considered irreversible. The significant difference in the chances for recovery from the vegetative state that arise as a consequence of anoxic cerebral injury as distinct from that caused by head injury is reflected in the difference in elapsed time before the vegetative state can be considered permanent (Fig. 9-10). Even after these times have elapsed, there are occasional single case reports of late recovery. Review of many of these cases reveals a small error rate for declaring permanence.3 Since the Multi-Society Task Force review of late recovery cases in 1994, only two cases of late recovery have been reported,43,44 which perhaps reflects more widespread acceptance of the definitional criteria and time constraints on the determination of permanence.
There are few useful data to guide clinicians in predicting recovery from a vegetative state once the diagnosis is established. It is generally considered that the prognosis for emergence is better after trauma than after hypoxia and that younger patients have a better chance of recovery. A short duration of coma before eye opening has been postulated to indicate an improved likelihood of further gains in function beyond a vegetative state.5 Recovery of visual pursuit or of a blink response to visual threat are often the first signs of recovery beyond vegetative state, and the early finding of such signs may suggest a better chance of further gains. Evidence on the quality of recovery from vegetative state is also very limited. A large study from France of 522 patients who were vegetative 1 month after head injury showed that although 61% had regained consciousness after 1 year of follow-up, only 14% had become independent. The statistics were markedly affected by age: only 5% of adults became independent, whereas 24% of those younger than 20 years reached this level.45 In the nontraumatic vegetative state, only 1% of patients who remained vegetative at 3 months and none who remained vegetative at 6 months achieved an independent outcome by 1 year.1
Conversely, very prolonged survival in the vegetative state is also unusual, although survival (up to 41 years)46 has been described. In the Multi-Society Task Force review, mean survival in a vegetative state after acute cerebral injury ranged from 2 to 5 years1 with a 70% rate of mortality by 3 years and an 84% rate of mortality by 5 years. However, long-term survival figures are likely to be heavily influenced by decisions to limit treatment.
The decision to withdraw or withhold support (usually only artificial hydration and nutritional support, but occasionally antibiotics for intercurrent infections) from a patient in the permanent vegetative state is an emotional, ethical, and medicolegal challenge. The relevant legal protocols vary between jurisdictions. The ethical considerations hinge on the concepts of patient autonomy, the presence of advance directives, the wishes of family or other proxies, and the ability of a surrogate to act on behalf of an incompetent patient. A full discussion of the ethical and legal issues involved is beyond the scope of this chapter but is well covered in several monographs.3,47
Jennett B. The Vegetative State: Medical Facts, Ethical and Legal Dilemmas. Cambridge, UK: Cambridge University Press, 2002.
Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state and related disorders. Lancet Neurol. 2004;3:537-546.
The Multi-Society Task Force on Persistent Vegetative State. Medical aspects of the persistent vegetative state. Parts 1 and 2. N Engl J Med. 1994;330:1499-1508. and 1572–1579.
1 Multi-Society Task Force on Persistent Vegetative State. Medical aspects of the persistent vegetative state. Parts 1 and 2. N Engl J Med. 1994;330:1499-1508. and 1572–1579.
2 Royal College of Physicians of London. The Vegetative State, Guidance on Diagnosis and Management. Clin Med. 2003;3:249-254.
3 Jennett B. The Vegetative State: Medical Facts, Ethical and Legal Dilemmas. Cambridge, UK: Cambridge University Press, 2002.
4 Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349-353.
5 National Health and Medical Research Council. Postcoma unresponsiveness (vegetative state): a clinical frame work for diagnosis. Canberra, Australia: National Health and Medical Research Council, 2004.
6 Jennett B, Plum F. Persistent vegetative state after brain damage: a syndrome in search of a name. Lancet. 1972;299:734-737.
7 American Congress of Rehabilitation Medicine. Recommendations for use of uniform nomenclature pertinent to patients with severe alterations in consciousness. Arch Phys Med Rehabil. 1995:76205-76209.
8 Owen AM, Menon DK, Johnsrude IS, et al. Detecting residual cognitive function in persistent vegetative state. Neurocase. 2002;8:394-403.
9 Thomasma DC, Brumlik J. Ethical issues in the treatment of patients with a remitting vegetative state. Am J Med. 1984;77:373-377.
10 Bauer G, Gerstenbrand F, Rumpl E. Varieties in locked-in syndrome. J Neurol. 1971;221:77-91.
11 Plum F, Posner JB. The Diagnosis of Stupor and Coma, 2nd ed., Philadelphia: FA Davis; 1972:24-25.
12 Freeman FR. Akinetic mutism and bilateral anterior cerebral artery occlusion. J Neurol Neurosurg Psychiatry. 1971;34:693-698.
13 Cairns H, Oldfield RC, Pennybaker JP, et al. Akinetic mutism with an epidermoid cyst of the third ventricle. Brain. 1941;64:237-290.
14 Abekura M. Akinetic mutism and magnetic resonance imaging in obstructive hydrocephalus: case illustration. J Neurosurg. 1998;88:161.
15 Bird GL, Meadows J, Goka J, et al. Cyclosporin associated akinetic mutism and extrapyramidal syndrome after liver transplantation. J Neurol Neurosurg Psychiatry. 1990;53:1068-1071.
16 Rubin DI, So EL. Reversible akinetic mutism possibly induced by baclofen. Pharmacotherapy. 1999;19:468-470.
17 Ashwal S, Bale JF, Coulter DL, et al. The persistent vegetative state in children: report of the Child Neurology Society Ethics Committee. Ann Neurol. 1992;32:570-576.
18 Sato S, Imamura H, Ueki K, et al. Epidemiological survey of vegetative state patients in the Tokohu District, Japan. Neurol Med Chir (Tokyo). 1979;8:327-333.
19 Kinney HC, Samuels MA. Neuropathology of the persistent vegetative state—a review. J Neuropathol Exp Neurol. 1994;53:548-558.
20 Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123:1327-1338.
21 Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurol Neurosurg Psychiatry. 1956;19:163-185.
22 Strich SJ. Shearing of nerve fibres as a cause of brain damage due to head injury. Lancet. 1961;278:443-448.
23 Andrews K. International Working Party on the Management of the Vegetative State: summary report. Brain Injury. 1996;10:797-806.
24 Young GB. The EEG in coma. J Clin Neurophysiol. 2000;17:473-485.
25 Wijdicks EF, Parisi JE, Sharbrough FW. Prognostic value of myoclonus status in comatose survivors of cardiac arrest. Ann Neurol. 1994;35:239-243.
26 Silverman D, Saunders MG, Schwab RS, et al. Cerebral death and the electroencephalogram: Report of the ad hoc committee of the American Electroencephalographic Society on EEG Criteria for determination of cerebral death. JAMA. 1969;209:1505-1510.
27 Gutling E, Gonser A, Imhof HG, et al. EEG reactivity in the prognosis of severe head injury. Neurology. 1995;45:915-918.
28 Moruzzi G, Magoun HW. Brainstem reticular formation and activation of the EEG. 1949. J Neuropsychiatry Clin Neurosci. 1995;7:251-267.
29 Carter BG, Butt W. Review of the use of somatosensory evoked potentials in the prediction of outcome after severe brain injury. Crit Care Med. 2001;29:178-186.
30 Zandbergen EGJ, de Haan RJ, Stoutenbeek CP, et al. Systematic review of early prediction of poor outcome in anoxicischaemic coma. Lancet. 1998;352:1808-1812.
31 Sherman AL, Tirschwell DL, Micklesen PJ, et al. Somatosensory potentials, CSF creatine kinase BB activity and awakening after cardiac arrest. Neurology. 2000;54:889-894.
32 Madl C, Kramer L, Yeganehfar W, et al. Detection of nontraumatic comatose patients with no benefit of intensive care treatment by recording of sensory evoked potentials. Arch Neurol. 1996;53:512-516.
33 Tirshwell DL, Longstreth WTJr, Rauch-Matthews ME, et al. Cerebrospinal fluid creatine kinase BB isoenzyme activity and neurological prognosis after cardiac arrest. Neurology. 1997;48:352-357.
34 Raabe A, Grolms C, Sorge O, et al. Serum S-100B protein in severe head injury. Neurosurgery. 1999;45:477-483.
35 Rosen H, Rosengren L, Herlitz J, et al. Increased serum levels of the S-100 protein are associated with hypoxic brain damage after cardiac arrest. Stroke. 1998;29:473-477.
36 Fogel W, Krieger D, Veith M, et al. Serum neuron-specific enolase as early predictor of outcome after cardiac arrest. Crit Care Med. 1997;25:1133-1138.
37 Falk RH. Physical and intellectual recovery following prolonged hypoxic coma. Postgrad Med J. 1990;66:384-386.
38 Kampfl A, Schmutzhard E, Franz G, et al. Prediction of recovery from post traumatic vegetative state with cerebral magnetic-resonance imaging. Lancet. 1998;351:1763-1767.
39 Ricci R, Barbarella G, Musi P, et al. Localised proton MR spectroscopy of brain metabolism in vegetative patients. Neuroradiology. 1997;39:313-319.
40 Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state and related disorders. Lancet Neurol. 2004;3:537-546.
41 Tommasino C, Grana C, Lucignani G, et al. Regional cerebral metabolism of glucose in comatose and vegetative state patients. J Neurosurg Anesthesiol. 1995;7:109-116.
42 Baars B, Ramsøy T, Laureys S. Brain, conscious experience and the observing self. Trends Neurosci. 2003;26:671-675.
43 Childs NL, Mercer WN. Brief report: late improvement in consciousness after posttraumatic vegetative state. N Engl J Med. 1996;334:24-25.
44 Dyer C. Hillsborough survivor emerges from permanent vegetative state. BMJ. 1997;314:996.
45 Danze F, Veys B, Lebrun T, et al. Prognostic factors of posttraumatic vegetative states: 522 cases. Neurochirurgie. 1994;40:348-357.
46 Sibbison JB. USA: right to live, or right to die? Lancet. 1991;337:102-103.
47 Bernat JL. Ethical Issues in Neurology, 2nd ed. Boston: Butterworth Heinemann, 2002.
* Some authorities, such as the Royal College of Physicians of London,2 recommend that a 6-month period for nontraumatic etiologies elapse before the declaration of permanence. However, Jennett3 (2002, p 61) states that “the Task Force concluded from analysis of the 754 cases reviewed that the vegetative state could reasonably be declared permanent 3 months after nontraumatic damage and 12 months after head injury in both children and adults.” He further stated (p 64) that “given the interest in late recoveries provoked by the time limits proposed by the Task Force for declaring permanence, it might have been expected that most exceptions to these since then would have been reported, but only two have been.”
† For a full description of the various diagnostic criteria used internationally, see Jennett (2002).3