The Peripheral Veins
The Peripheral Veins
The peripheral veins may be affected by a variety of disorders which can be assessed by ultrasound. Deep vein thrombosis (DVT) and thromboembolic disease are the most common indications for investigation of the peripheral veins but venous insufficiency and vein mapping are also reasons for examining the veins. Anderson et al.1 found an average annual incidence of 48 initial cases, 36 recurrent cases of DVT and 23 cases of pulmonary embolus per 100 000 population in the Worcester DVT study. The prevalence of varicose veins and chronic venous insufficiency is more difficult to quantify, but it has been estimated that 10–15% of males and 20–25% of females in an unselected Western population over 15 years of age have visible tortuous varicose veins; 2–5% of adult males and 3–7% of females have evidence of moderate or severe chronic venous insufficiency, with a point prevalence for active ulceration of 0.1–0.2%.2
Indications
The indications for ultrasound of the venous system are shown in Box 5-1. The most frequent indication for ultrasound of the veins is for the investigation of possible DVT in the lower limb and, occasionally, in the upper limb – especially if there have been central venous catheters inserted for intensive care monitoring, chemotherapy, dialysis or parenteral feeding. Similarly, indwelling femoral catheters are prone to induce thrombosis and patients should be examined early if this is suspected. Ultrasound provides a non-invasive, reliable method for examining the venous system, particularly with respect to the diagnosis, or exclusion, of dangerous proximal thrombus in symptomatic patients.3 The results for asymptomatic thrombus in the lower limbs are less encouraging and this should be recognised when using ultrasound to screen for DVT in asymptomatic patients.4
Recurrence of varicose veins following surgery can pose many problems for the clinician trying to clarify the venous anatomy. Colour Doppler can be used instead of venography and varicography in most cases and may be the only examination required to define the anatomy and function in patients with recurrent varicose veins.5
The impact of postphlebitis syndromes and chronic venous insufficiency is a rather larger problem than is apparent from its relatively low clinical profile. In one large epidemiological study of 4376 subjects, 62% had some evidence of varicose veins; signs of chronic insufficiency were present in 22%.6 Varicography shows perforator veins which are obviously incompetent and some incompetent superficial and deep venous segments, but ultrasound has the advantage that the segments of the deep and superficial systems can be examined and the direction of blood flow within each segment can be demonstrated. In addition, it is less unpleasant for the patient and allows multiple assessments to be performed without discomfort. The main disadvantage is that it is fairly time-consuming, particularly in complex cases, and requires a significant degree of expertise in order to perform examinations efficiently.
Anatomy and Scanning Technique
The anatomy of the venous system in the limbs is more complex and variable than that of the arteries. The components and nomenclature of the lower limb veins were reviewed by a consensus group in 2002 and their recommendations are used here.7 The meanings of the terms ‘proximal’ and ‘distal’ may cause confusion as the veins start at the periphery and blood flows centrally towards the heart so that ‘upstream’ is peripheral and ‘downstream’ is central, which is the opposite from the situation in the arteries. The convention is that proximal describes locations nearer the heart and distal refers to points further from the heart; these terms are used in this way in this chapter.
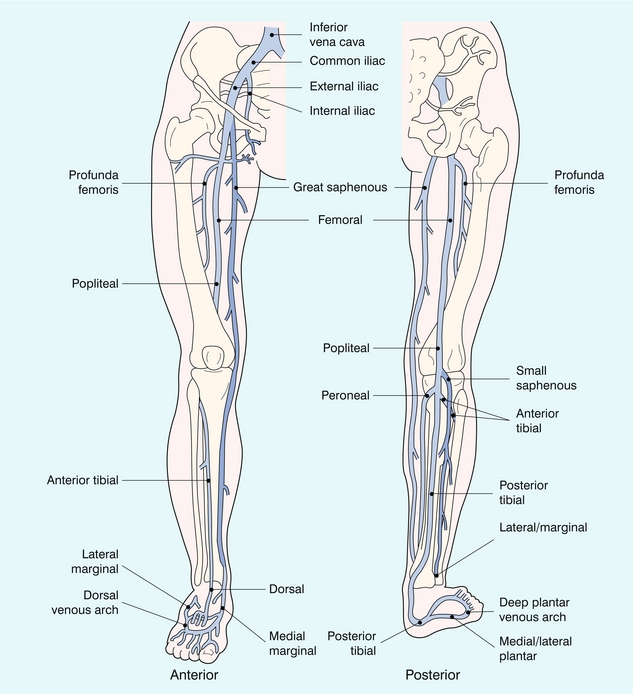
ANATOMY – LOWER LIMB
The veins of the lower limb are divided into deep and superficial systems. These are linked by a variable number of perforator veins which carry blood from the superficial to the deep systems (Fig. 5-1).
The Deep Veins
The anatomy of the lower limb veins is rather variable. Generally the veins accompany the arteries but their number may vary and the communications with other veins along the way can show a variety of patterns; however, a general arrangement is usually apparent. In the calf there are veins running with the main arteries: the posterior tibial, peroneal and anterior tibial veins; there are usually two, occasionally three veins with each artery (Fig. 5-2). In addition there are veins draining the major muscle groups in the posterior calf. These are seen in the upper calf as they pass upwards to join the other deep veins in the lower popliteal region; the gastrocnemius and soleal veins are the largest of these. The gastrocnemius vein is the more superficial and may be mistaken for the small saphenous vein; clues to its true identity are that it is usually accompanied by the artery to the muscle and it can be followed distally down into the muscle rather than outwards to lie subcutaneously on the fascia around the calf, which is the position of the small saphenous vein.
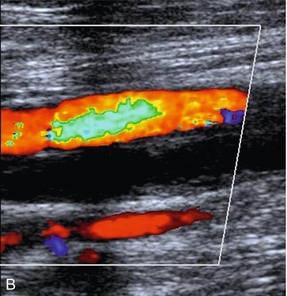
The popliteal vein becomes the femoral vein at the upper border of the popliteal fossa; rarely, the popliteal vein runs more deeply to join with the profunda femoris vein. The femoral vein passes through the femoral canal and runs up the medial aspect of the thigh, posterior to the femoral artery to join with the profunda femoris vein (which can alternatively be called the deep femoral vein) in the femoral triangle below the groin; the profunda femoris vein drains the thigh muscles. The confluence of the femoral and profunda femoris veins to form the common femoral vein is normally a little more caudal than the bifurcation of the common femoral artery into the femoral and profunda femoris arteries. The femoral vein may have significant segments of duplication (Fig. 5-3) along its length in up to 25–30% of subjects,8,9 these dual segments may have a variable relation to the artery, so that they may be overlooked unless care is taken in the examination of the thigh veins with both transverse and longitudinal views being obtained.
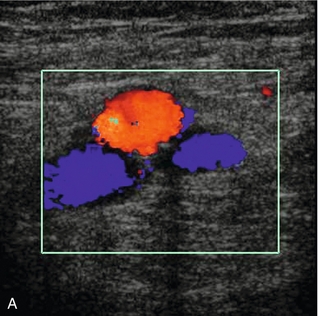
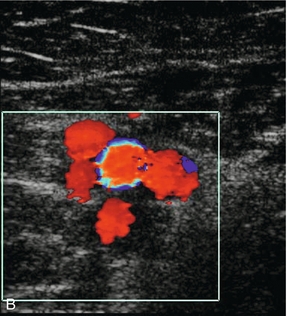
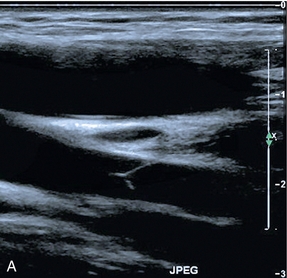
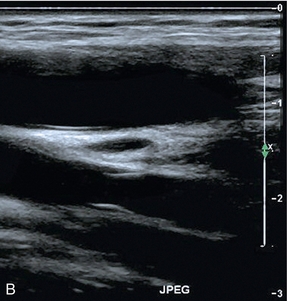
FIGURE 5-3 (A) Transverse view showing dual superficial femoral vein segments; (B) another example of multiple superficial femoral vein segments showing a central artery (aliased colour signal seen as red) with four venous channels adjacent to it.
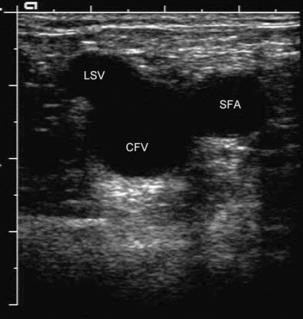
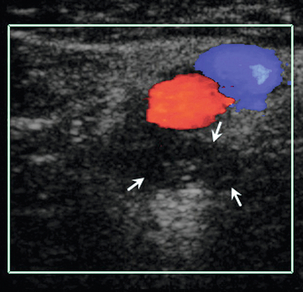
In the pelvis and groin, the anatomy is generally consistent. The femoral vein and profunda femoris vein join to form the common femoral vein, which lies medial to the common femoral artery. The common femoral vein is joined by the great saphenous vein at the saphenofemoral junction; the appearance of the common femoral vein, great saphenous vein and the common femoral artery in transverse section is sometimes referred to as the ‘Mickey Mouse’ view (Fig. 5-4). The common femoral vein is also joined by veins from the muscles around the hip. These veins are variable in size and number, occasionally one of these is large enough to be confused with the great saphenous vein or profunda femoris vein but careful attention to the anatomy should clarify the situation. The common femoral vein becomes the external iliac vein after it has passed under the inguinal ligament, and then it passes posteriorly along the posterior pelvis, running alongside the external iliac artery. The internal iliac vein, which drains the pelvic structures, joins with the external iliac vein deep in the pelvis to form the common iliac vein (Fig. 5-5). The two common iliac veins then join at the level of the aortic bifurcation to form the inferior vena cava, which normally passes cranially on the right side of the aorta. The left common iliac vein passes behind the right common iliac artery just distal to this confluence. In a small number of individuals this confluence does not occur and the two common iliac veins continue cranially as dual inferior venae cavae; this reflects the arrangement of paired cardinal veins in the embryo.
The deep veins have a series of valves along their course (Fig. 5-6). These are somewhat variable in their number and location. They are most numerous in the veins below the knee; in the thigh, the femoral vein usually has one just below the confluence with the profunda femoris vein and at several levels below this. The iliac veins, in contrast, have relatively few valves;10 rarely a valve may be seen in the inferior vena cava.
The Superficial Veins
The two main superficial venous channels in the lower limb are the great and small saphenous veins. The great saphenous vein arises from the medial aspect of the dorsal venous arch of the foot and passes in front of the medial malleolus to run up the medial aspect of the calf and knee into the thigh. In the upper thigh, the great saphenous vein curves laterally and deeply to join the common femoral vein just below the inguinal ligament. The great saphenous vein has two components in the calf: the posterior division passes up from the medial malleolus and communicates with the perforator veins; the anterior division usually joins the posterior division just below the level of the knee joint. Duplication of the great saphenous vein can be seen in the thigh in up to 50% of people,11 this usually takes the form of parallel channels. The great saphenous vein receives many superficial tributaries and is connected to the deep veins by perforating veins; some of these tributaries in the thigh can be quite prominent and may be mistaken for the main vein if their true nature is not recognised. In the region of the saphenofemoral junction the great saphenous vein receives several tributaries draining the groin, lower abdominal wall and perineum. These veins are of significance in the recurrence of varicose veins following high ligation, as they provide a network of collateral channels which may bypass the resected segment.
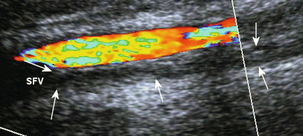
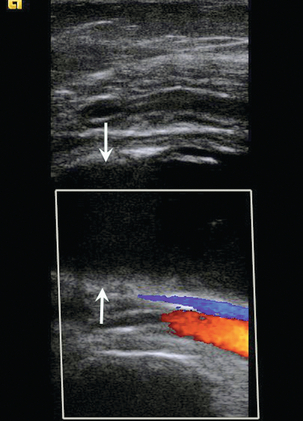
The small saphenous vein arises from the lateral aspect of the dorsal venous arch of the foot, passing below and behind the lateral malleolus to run up the posterolateral aspect of the calf to the popliteal fossa, where it passes through the deep fascia to join the popliteal vein. Classically, it enters the lateral aspect of the popliteal vein at the level of the popliteal skin crease, or within a few centimetres above this but the level of the confluence can be quite variable. It can be distinguished from the posterior muscle venous sinuses as it does not have an accompanying artery and it is seen to lie within the fascial triangle in the posterior thigh defined by the deep muscular fascia and the superficial fascia (Fig. 5-7). Occasionally there is a thigh extension of the small saphenous vein, passing upwards to join the profunda femoris vein in the lower thigh – a Giacomini vein.12 Burihan and Baptista-Silva13 dissected 200 adult cadaver legs and reported 20 different patterns of termination of the small saphenous vein. In 27.5% of legs the small saphenous vein terminated in the principal deep vein of the leg (popliteal or lower femoral vein), in 25% of legs the small saphenous vein, or a branch arising from it, communicated with the great saphenous vein. In the remaining legs, there was a wide variety and combination of communications with other veins, including the deep femoral vein, the mid-thigh perforator vein, muscular veins and even the inferior gluteal vein in three legs. Other studies have shown that Giacomini veins can be affected by varicose disease with reflux either upwards or downwards in the thigh to the greater and lesser saphenous veins respectively.14
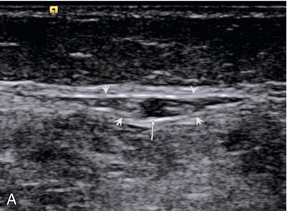
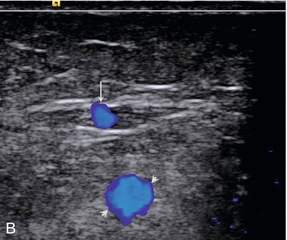
FIGURE 5-7 (A) The small saphenous vein (arrow) in the posterior calf lying in the fascial triangle (arrowheads) formed between the superficial fascia and the deeper muscular fascia; (B) colour Doppler showing the small saphenous vein (arrow) and a muscule vein (arrowheads) deep to the fascia which might be mistaken for the SSV if its location is not recognised.
The Perforating Veins
The perforating veins connect the superficial veins to the deep veins. They are numerous and very variable in both size and location. In the past, they were often known by eponymous designations15 but with the revised nomenclature they are now identified by their anatomical location – for example: medial, lateral, or anterior ankle perforator – full details are given in the consensus statement on venous nomenclature.7 They are normally less than 5 mm in diameter and blood flows inwards from the superficial to the deep systems.
SCANNING TECHNIQUE – LOWER LIMB
The technique varies depending on the clinical indication. The most common indication is the diagnosis or exclusion of DVT in the lower limb. This section therefore concentrates on this aspect and variations in technique for other indications will be dealt with in subsequent sections (Box 5-2). A 7–10 MHz linear transducer will normally provide sufficient penetration, although in large or oedematous thighs a lower frequency may be required. It is important to ensure that the system is set up for the slower velocities found in veins, rather than the significantly higher arterial velocities.
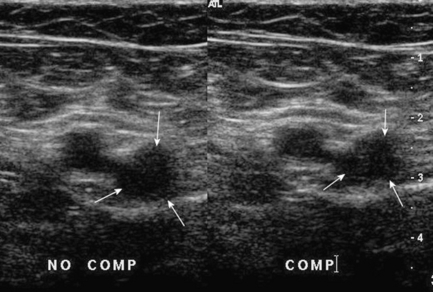
There are three components to the ultrasound examination of the veins for DVT: imaging, Doppler and compression. Thrombus may be seen in the vein, Doppler may show abnormal, or absent, flow signals and compression refers to the fact that a normal vein is easily compressible – light pressure with the transducer will obliterate the lumen of the vein, whereas thrombus in the lumen will prevent apposition of the walls. Two points should be noted in relation to compression: first, compression should be performed in the transverse plane (Fig. 5-8) for the reason that if it is done in the longitudinal plane a thrombosed vein may disappear as it is no longer in the scan plane, rather than because it has been compressed. Second, fresh thrombus is soft and gelatinous, so that firm pressure can produce a degree of compression, which may give a false impression of patency. The use of colour Doppler will clarify this situation. A further reason for scanning in the transverse plane is that dual segments of the superficial femoral vein will be identified more reliably.
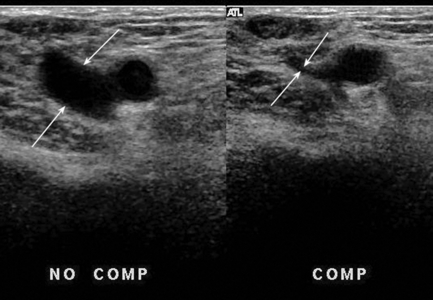
FIGURE 5-8 Normal compression: the lumen of the vein (arrows) is completely obliterated by pressure from the transducer.
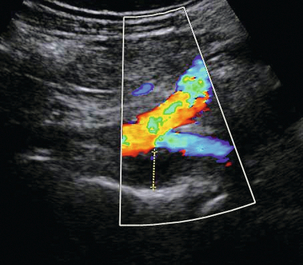
The examination begins at the groin, where the common femoral vein is located on a transverse scan and compressed. Compression is then repeated at intervals of 3–5 cm down the length of the thigh to the adductor canal. At this point the superficial femoral vein is difficult to compress from an anterior approach as it is well supported by the bulk of the anterior thigh muscles. Compression is better achieved in this region by placing a hand behind the medial thigh and pushing up with the fingers against the transducer. The scan plane is then changed to longitudinal and the vein examined with colour Doppler, or power Doppler, as the transducer is moved up the thigh. If the iliac veins are not being formally examined it is useful to obtain a spectral waveform in quiet respiration from the common femoral vein to confirm cardiac and respiratory flow variation being transmitted down patent iliac veins from the chest. Squeezing the calf gently will augment flow and allow easier detection of areas of flow or thrombosis; alternatively, the patient can be asked to plantar-flex their toes, which results in calf muscle contraction and emptying of the calf veins. Colour Doppler is often sufficient, in conjunction with the findings on compression, to confirm or exclude a diagnosis of DVT (Fig. 5-9). If there is any doubt then a spectral assessment will allow a better appreciation of damped flow, absent respiratory variation and impaired augmentation.
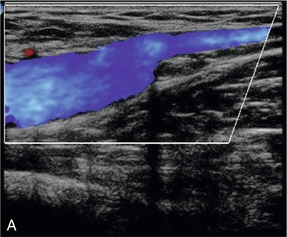
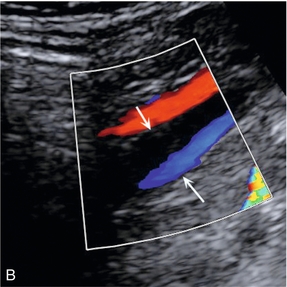
FIGURE 5-9 The common femoral vein showing (A) complete colour fill-in across the vein lumen in a normal vein: (B) only a small residual lumen in a partially thrombosed vein.
Once the thigh veins have been examined the patient is turned into a lateral position, with the medial aspect of the leg being examined uppermost, so that the popliteal veins can be examined. Again, compression and colour Doppler are used to assess the veins. Some patients, particularly postoperative hip patients, may not be able to move into a decubitus position. In these cases the popliteal veins can be examined with the knee partially flexed up off the couch, with external rotation, if possible, so that the transducer can be positioned in the popliteal fossa; a curved array can be of benefit in gaining access in this situation. Alternatively, the leg can be elevated and supported off the couch by an assistant. In addition to the popliteal vein, the main muscular veins draining soleus and gastrocnemius should be assessed, especially if there is pain and tenderness associated with the posterior calf muscles (Fig. 5-10).
The calf veins can be examined after the popliteal vein with the patient in the decubitus position on a tilted couch, or in the supine position with the knee flexed up off the mattress, if the patient is relatively immobile. Alternatively, the patient can sit on the couch with their legs over the side so that the dependent calf veins are well distended. The posterior tibial and deeper peroneal vessels are most easily located by scanning in the transverse plane from the medial side of the calf and identifying the arterial signals on colour Doppler (Fig. 4-2). These veins may also be located on a longitudinal scan; again the arterial signal provides a useful guide to the position of the veins. If there are difficulties identifying the posterior tibial veins at the mid-calf level then scanning the lower calf just above the medial malleolus, where the vessels are superficial and constant in location, may be of value; the posterior tibial vessels can then be followed back up the calf with augmentation of flow as necessary in order to assess patency. In the mid- and lower calf, squeezing the calf can produce motion artefacts from movement of the calf muscles which obscure the flow signals from the veins; in these cases, squeezing the foot will produce adequate augmentation of flow. The anterior tibial veins are examined from an anterolateral approach: scanning transversely, the tibia, fibula and interosseous membrane are identified. The anterior tibial vessels are found on the superficial aspect of the interosseous membrane, although it should be noted that these veins are rarely involved in DVT in isolation from the other calf veins. The peroneal veins may also be visualised deep to the interosseous membrane in many patients from this anterolateral aspect, allowing their examination if they have not been identified from a posteromedial approach; a posterolateral approach is also of value in identifying the deeply situated peroneal veins in some patients.
The iliac veins are examined by following the external iliac vein upwards from the common femoral vein into the pelvis. A 3–5 MHz transducer is usually necessary for adequate penetration. Firm pressure may be required to displace bowel gas. This may produce narrowing or effacement of the more superficial segments of vein, resulting in an absence of signal and a possible false diagnosis of occlusion. If the pelvic veins are difficult to trace superiorly then the common iliac vein can usually be identified just distal to the inferior vena cava and aortic bifurcation; this can then be followed peripherally. In some patients it is impossible to identify the deeper pelvic portion of the iliac veins; however, if there is a patent external iliac vein which shows respiratory variation with good augmentation and a patent upper common iliac vein, then it is highly unlikely that there is significant thrombus in the invisible segment. Transvaginal scanning will show the deeper pelvic veins and may be considered if there is a need to visualise these vessels directly. In thinner patients, or patients with good pelvic access, the proximal internal iliac vein may be seen joining the external iliac vein in the pelvis (Fig. 5-5). The inferior vena cava is examined if thrombus is seen extending into this vessel. It is important, whenever thrombus is diagnosed in a leg vein, that the proximal extent of the clot is identified, as this may have a significant impact on management decisions in relation to anticoagulation therapy, or the placing of a filter.
ANATOMY – UPPER LIMB
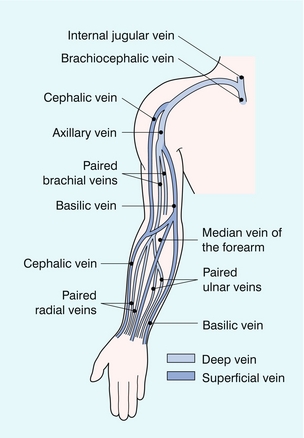
The veins of the upper limb are also divided into deep and superficial groups (Fig. 5-11). The deep veins are usually paired and accompany the arteries: the radial, ulnar and brachial veins; with the axillary, subclavian and brachiocephalic veins more centrally. There is a variable pattern of communicating veins between the deep veins and between the deep and the superficial veins. The superficial system is more variable than in the leg but there are usually two main vessels: the cephalic vein on the radial aspect of the arm and the basilic vein on the ulnar side. These communicate at the cubital fossa by way of the median cubital vein and they also communicate with the deep brachial veins at this level. The basilic vein pierces the deep fascia on the medial aspect of the mid-upper arm to join the brachial veins and this combined venous channel becomes the axillary vein when it enters the axilla. The cephalic vein passes more cranially along the lateral aspect of the biceps. At the level of pectoralis major it turns medially and deeply to pierce the clavipectoral fascia below the clavicle and joins the upper axillary vein. The axillary vein also receives other tributaries from the region of the shoulder joint and the lateral chest wall.
Diagnosis of Deep Vein Thrombosis
Clinical diagnosis of DVT is inaccurate and clinical scoring systems, such as the Wells Score (Box 5-3), have been introduced and refined to stratify risk more accurately.16 In addition measurements of serum D-dimer can be used to further refine the selection of patients more likely to have a DVT who will benefit from an ultrasound scan.17 Patients with a low probability for DVT should have a D-dimer estimation. If this is negative, they are highly unlikely to have a DVT and do not require scanning; if the D-dimer is positive, or the patient has an intermediate or high probability score for DVT, then a scan should be performed. D-dimer levels are less useful in patients with many pre-existing conditions, or who have recently undergone surgery as false positives are more common (Box 5-4).
The diagnosis of normal or thrombosed veins is based on the compressibility of the veins, the appearance of the veins and the changes which occur to the spectral and colour Doppler findings. The main changes associated with DVT are shown in Box 5-5. The lower limb is examined for possible thrombosis much more frequently than the upper limb, although the principles and features described are also applicable to the arm veins.
COMPRESSIBILITY
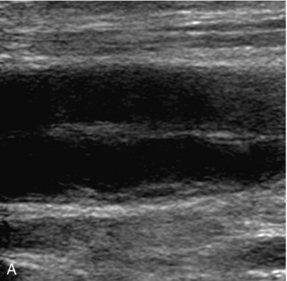
As noted above, a normal vein is easily compressible with only mild to moderate pressure from the transducer, so that the lumen is completely obliterated. A vein filled with thrombus will be held open (Fig. 5-12), although it must be remembered that fresh thrombus has the consistency of jelly, so that it can be compressed to some extent by strong pressure.
APPEARANCES OF THE VEIN AND THE VEIN LUMEN
The lumen of a normal vein is usually anechoic and, on colour Doppler, the whole lumen of the vein should be filled with colour, particularly on augmentation of flow. Although fresh thrombus is anechoic, or hypoechoic, it becomes increasingly echogenic as it matures. In addition fresh thrombus has a tendency to expand the vein and make it look rounder and fuller than a normal vessel.18 This is accentuated at the upper end of the thrombus where the patent lumen above the clot may be relatively poorly filled with blood due to the distal obstruction by the thrombus (Fig. 5-13).
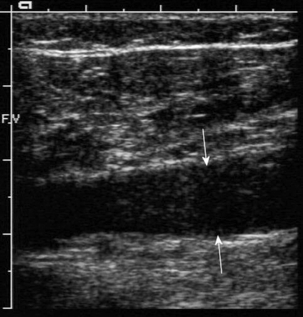
FIGURE 5-13 A vein containing thrombus: low-level echoes are seen in the clot (arrows), the patent lumen above the thrombus is narrower than the thrombosed segment.
Fresh thrombus is not particularly adherent to the vein wall, so that some blood may be seen around the periphery of the clot in the vein on colour Doppler. Another appearance which may be seen in early thrombosis is that of a thin tail of thrombus extending up the vein from its origin and lying free in the lumen of the vein (Fig. 5-14). Older thrombus becomes increasingly echogenic, adherent to the vein wall and contracts as it becomes more organised and fibrotic. This may result in the vein being reduced to a relatively small echoic structure that may be difficult to locate. Alternatively, the thrombus may retract to one side of the vein, resulting in an asymmetric lumen on colour Doppler.
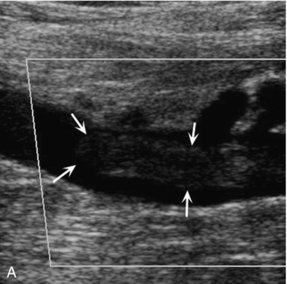
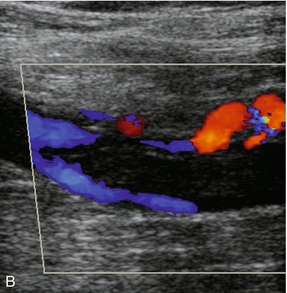
FIGURE 5-14 (A) A tail of thrombus extending up the vein (arrows) which is not sufficiently large to produce any obstruction to flow and could be overlooked if visualisation of this area was poor; (B) power Doppler image of the same thrombus showing flow around it.
Normal valves may be seen moving gently in the currents from blood passing them, particularly in the larger thigh veins (Fig. 5-6). One of the earliest sites of deep vein thrombus formation is in the sinus above a valve cusp, so that apparent rigidity or fixation of a cusp should raise the suspicion of possible early DVT and a careful examination of the area should be undertaken.
SPECTRAL DOPPLER FINDINGS
Spontaneous Flow and Respiratory Variation
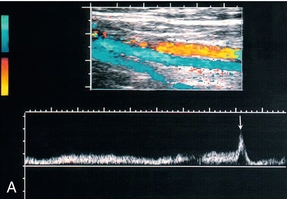
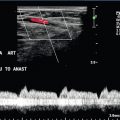
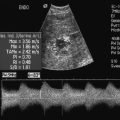
Even at rest and with some head-up tilt there should still be spontaneous flow along the vein which shows some respiratory variation or phasicity, particularly in the proximal leg veins. This variation is produced by the intra-abdominal pressure changes on respiration and is the opposite of the changes found in the jugular vein and arm veins. On inspiration the diaphragm descends and the intra-abdominal pressure rises; this results in decreased flow from the leg veins into the abdomen. On expiration the intra-abdominal pressure decreases and flow from the legs increases. Similarly, if the patient holds their breath, flow in the leg veins slows and may cease until the patient relaxes, when there is relatively high flow from the legs. As well as changes in flow secondary to respiration, there is also variation of flow secondary to cardiac contractions (Fig. 5-15A). Respiratory variations in flow are sometimes referred to as phasicity and cardiac variations as periodicity.
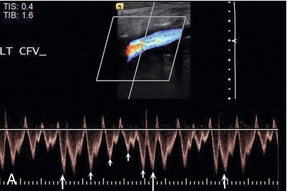
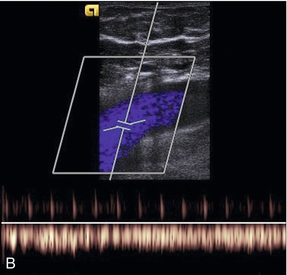
FIGURE 5-15 Spectral Doppler traces from different common femoral veins: (A) Prominent cardiac pulsation or periodicity (small arrows) and respiratory variation or phasicity (large arrows); (B) Flattened trace in a patient with segmental iliac vein thrombosis.
If there is thrombus occluding the vein there will not be any flow detected in the vein lumen at the level of the thrombus. Sometimes thrombosis is segmental, with a segment of iliac vein or superficial femoral vein occluded but with patent veins below this level; there is a higher incidence of this in pregnant patients and patients with pelvic tumours. Patent segments below the thrombus may show some slow antegrade flow, particularly if collateral channels are adequate, but this does not show any respiratory or cardiac variation and the augmentation response is damped (Fig. 15-15B).
Augmentation
Normal venous flow is slow and can be improved by compression distal to the point of assessment. There are various techniques for achieving this which are discussed further in the section on chronic venous insufficiency, but for the assessment of possible DVT manual compression of the calf is usually sufficient. The muscles of the calf are squeezed rapidly and firmly in order to propel blood up the veins. In a normal venous system there will be a rapid rise and fall in the frequency shift; whereas if there is a thrombosed segment in the veins, this will increase resistance to flow with damping, or absence, of the augmentation response (Fig. 5-16). It should be remembered that increased resistance to flow anywhere in the vein above the point of compression will result in impaired augmentation and the thrombus may be above or below the point of examination. Therefore, the demonstration of impaired augmentation should lead to a careful search for thrombus in that limb; particularly in the calf or iliac segments. The squeeze of the calf muscles should not be violent, or excessive, as patients will often have tender or painful calves; in addition there is a small potential risk of dislodging a fresh friable thrombus, producing a pulmonary embolus. The risk of this is small and reports of this type of event are few.19
DISTINCTION OF ACUTE FROM CHRONIC THROMBUS
The features which suggest older, rather than fresh, thrombus are given in Table 5-1. However, it is not always possible to define the age of a thrombus and, in these cases, the management of the patient must be based on the clinical picture.
TABLE 5-1
Distinction between Acute and Chronic Thrombus
| Acute | Chronic |
| Anechoic or hypoechoic | Increasingly echogenic |
| Expansion of the vein | Contraction of the vein |
| Some compression possible | Incompressible |
| Thrombus ‘tail’ in lumen | Clot adherent around the wall of the vein |
| Absent or minimal collaterals | Collateral channels in the tissues |
Fresh thrombus is hypoechoic or anechoic. It may not be attached to the wall around the whole circumference of the vein but if it fills the vein, the vein is a little expanded (Fig. 5-13).18,20 Increased flow may be detected in the profunda femoris vein, or saphenous veins. As thrombus matures it becomes increasingly echogenic and starts to contract as it becomes organised. Longitudinal studies of thrombosed veins show that some 64–75% of veins will recanalise completely, or in part, by 1 year after thrombosis,21 although valvular incompetence will be found at some level in the majority of these.22 The remaining veins will show varying degrees of recanalisation, with a thickened irregular wall around an uneven lumen (Fig. 5-17); or remain as fibrotic, permanently occluded structures (Fig. 5-18). Abnormal collateral venous channels will develop in the soft tissues around any segments which are significantly obstructed for any length of time (Fig. 5-19).
UPPER LIMB AND JUGULAR VEIN THROMBOSIS
The same principles apply to examination of the upper limb and neck veins. Lack of compressibility of the deep veins of the arm and neck and/or absence of flow on colour or power Doppler are diagnostic of thrombosis (Fig. 5-20). The larger, more proximal veins, such as the axillary and subclavian, cannot be compressed due to their location; diagnosis of thrombosis in these vessels will therefore depend on careful assessment using colour or power Doppler (Fig. 5-21). Indirect signs of thrombosis include loss of respiratory phasicity or cardiac variation, which indicates proximal occlusion and are useful if central vein (innominate or superior vena cava) thrombosis is suspected. Respiratory phasicity can be modified by asking the patient to breathe deeply, hold their breath or perform a Valsalva manoeuvre. Comparison with the other side may be helpful, assuming that this is normal.
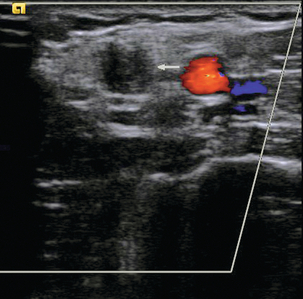
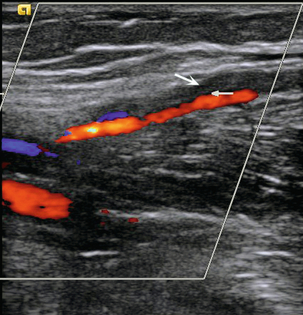
FIGURE 5-20 Transverse view of the brachial artery and its accompanying veins; one of the veins is thrombosed (arrow).
Baarslag et al.23 compared colour Doppler with venography and found 82% sensitivity and 82% specificity for the diagnosis of upper limb DVT; 63% of the patients who had thrombosis had an associated malignant disease and in 14% of those with thrombosis this was associated with an in-dwelling central venous catheter in patients without malignant disease. There is a low risk of clinically significant pulmonary embolus from upper limb DVT; in one series of 65 patients with arm vein thrombosis, none of the patients were found to have symptomatic pulmonary emboli.24
Problems and Pitfalls in the Diagnosis of Deep Vein Thrombosis
Some of these have been discussed already; however, the value of ultrasound as a technique for the diagnosis of DVT depends on the operator performing a careful, complete examination, being aware of potential pitfalls and recognising when a less than adequate examination has been performed. The main problem areas which should be remembered are shown in Box 5-6.
Duplicated Venous Segments
Dual segments of femoral vein may be overlooked unless they are actively sought with transverse scanning. If they are not recognised, then one component may be patent and seen on colour Doppler, whereas the other component may contain thrombus and be overlooked (Fig. 5-22).
Non-occlusive Thrombus
Similarly, non-occlusive thrombus may be missed if the vein is not seen adequately. If there is only a small amount of thrombus in the vein then good flow signals will be obtained on spectral and colour Doppler and the presence of the thrombus may not be recognised (Fig. 5-14). This is particularly important in obese or oedematous legs.
Isolated Calf Vein Thrombus
The calf veins are multitudinous in number and variable in their anatomy. Even with a careful, patient, time-consuming examination it is difficult to exclude completely the presence of a small segmental thrombus in a calf vein or muscular sinus (Fig. 5-10, Fig. 5-23). In a mobile patient with a little calf tenderness or swelling this is not a problem, as the body’s normal thrombolytic mechanisms will probably clear this. However, in a patient who is immobile following surgery or a stroke, a small segmental calf thrombus indicates that the clotting cascade has been activated and there is a possibility that this small thrombus may increase in size, resulting in a significant, occlusive thrombus. Therefore a follow-up scan should be considered in these patients in order to identify any progression of thrombus from the calf. A study by Labropoulos et al.25 reviewed 5250 patients; isolated calf vein thrombus was found in 4.8% (282 limbs in 251 patients). In these patients, variable patterns of involvement of the calf veins were demonstrated with the soleal veins involved in 20% of cases, gastrocnemius veins in 17%, peroneal veins in 15% and the posterior tibial veins in 12%; in 64% of these positive cases, only a single vein group was involved.
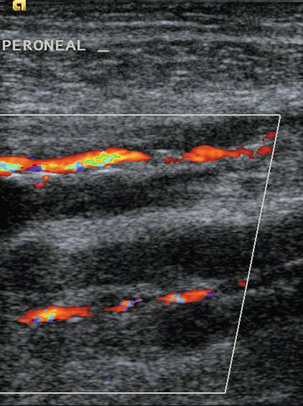
FIGURE 5-23 Calf vein thrombosis: Flow is seen in the posterior tibial and peroneal arteries but not in the accompanying veins.
A review by Scarvelis et al.,26 discussing the management of patients with deep vein thrombosis, comments that only 1–2% of patients who have a negative initial ultrasound will be confirmed to have a proximal DVT upon serial testing, so serial examinations are not cost-effective. However, whilst re-scanning should not be a routine expectation, it should be considered in cases with a high clinical probability or clinical concern and an initial negative scan.
Asymptomatic Thrombus
The accuracy of Doppler in the detection of asymptomatic thrombus is less impressive than that for symptomatic thrombus,4,27,28 and the technique is therefore generally inadequate as a screening tool for the detection of asymptomatic thrombus. This is probably because asymptomatic thrombi are more likely to be small and non-occlusive; in addition, there is a higher incidence of distal thrombi in the calf veins, which may be more difficult to demonstrate with ultrasound.3
Segmental Iliac Vein Thrombus
The external and common iliac veins may not be demonstrated in their entirety due to obesity or overlying bowel gas. Care must be taken to exclude segmental iliac vein thrombosis (Fig. 5-24), especially if this is a possibility following pelvic surgery; although it is rare for iliac thrombosis not to include the common femoral vein, segmental iliac thrombosis can occur and should be sought in patients with a good clinical picture for DVT but a negative scan of the femoral and popliteal veins. An indicator of segmental iliac thrombosis is loss of cardiac and respiratory variation (Fig. 5-15) and an impaired augmentation response in the common femoral vein. In some patients segmental iliac vein thrombosis may be associated with a structural web or spur in the left common iliac vein wall at the point where it is crossed by the right common iliac artery – May–Thurner syndrome.29 The internal iliac veins are difficult to assess but any thrombus arising in these, which extends into the common iliac vein and significantly impedes blood flow, may be suggested by an impaired augmentation response in the femoral veins, or loss of respiratory variation on deep breathing or panting. However, non-occlusive thrombus which is insufficient to produce this effect may be overlooked; transvaginal scanning may be of value in difficult cases. It is important that the proximal extent of any thrombus is defined so that any subsequent extension can be appreciated. In addition, insertion of a caval filter might be considered and it is important to know if access is possible from the groin through the iliac veins and lower IVC. Once a filter has been inserted, the subsequent patency of the cava and iliac veins can be assessed using ultrasound (Fig. 5-25).
Pregnancy
During pregnancy several factors are present which increase the risk of thrombosis. These include changes to the coagulation system and physiological changes to venous flow in the leg veins due to a combination of hormonal effects and pressure from the enlarging uterus.30 Some of the technical aspects relating to the ultrasound diagnosis of thrombosis associated with pregnancy have already been discussed. There is also an increased tendency to develop segmental proximal thromboses in the iliac and upper femoral veins. This is more common on the left side,31,32 perhaps reflecting the additional potential compression from the right common iliac artery, which crosses the left common iliac vein just beyond the aortic bifurcation; in one study32 only 18% of deep vein thromboses were confined to the right leg. If isolated iliac thrombosis is suspected and the ultrasound examination is less than adequate, then consideration should be given to further imaging with magnetic resonance (MR), or contrast venography.30 Patients who have undergone caesarean section will have a higher risk of developing a DVT.
OTHER CAUSES OF LEG SWELLING, PAIN OR TENDERNESS
Unlike venography, ultrasound allows examination of other structures in the pelvis and leg. Other pathologies may be seen which account for the patient’s symptoms of a swollen, or painful, tender leg; these are given in Box 5-7. It is important to remember that, even if a ruptured popliteal cyst is seen (Fig. 5-26), or a superficial thrombophlebitis is demonstrated (Fig. 5-27), the deep veins must still be examined carefully, as a coexistent DVT may otherwise be overlooked. Labropoulos et al.33 demonstrated popliteal cysts in 3% of asymptomatic individuals, rising to 10% of patients with symptoms of possible DVT and 20% of patients with painful knees. Langsfeld et al.34 found popliteal cysts in 3% of patients being examined for possible DVT, 7% of those with cysts had a coexisting DVT.
Accuracy in Relation to Other Techniques
Despite these potential problems, ultrasound is a good non-invasive method for the diagnosis of symptomatic DVT, especially between the lower popliteal region and the groin.3 The key to its value in any given department is that the sonographers must not only be well trained in the technique, but must also be able to recognise an inadequate examination so that appropriate further measures, such as venography or a repeat scan, can be arranged. Should venography be required to clarify areas of doubt, this can be focused on the area of concern identified at the ultrasound examination and only a limited examination may be required.
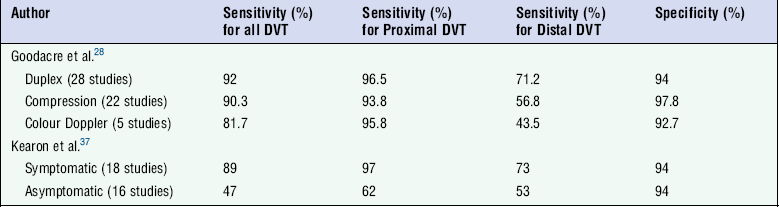
Many studies have shown that, in comparison to venography, ultrasound is an accurate technique for the diagnosis of symptomatic DVT in the femoropopliteal segments.3 Used alone, compression is an accurate method for detecting DVT, with sensitivities of 89% and specificity of 100% being reported for proximal thrombosis,35 and sensitivities of 86–92% and specificities of 96–100% for careful examination of the calf veins.36 The additional use of colour Doppler allows very accurate diagnosis of DVT, particularly in the femoropopliteal segments. With the development of colour Doppler techniques, further studies have shown the value of ultrasound and that the calf veins can be examined satisfactorily in most cases (Table 5-2).28,37 The need for an adequate examination must be emphasised. In one study, the initial results in the calf were significantly less accurate than the results for the femoropopliteal segment, but when the examinations were reviewed and only those which were technically adequate were considered, the overall accuracy improved markedly and reached a similar level to that obtained in the upper part of the limb.38 In another study,39 32% of studies of calf veins were inadequate; if these were excluded then ultrasound showed 93% sensitivity, 98% specificity and 97% accuracy for the diagnosis of lower limb DVT.
In a review of outcomes following negative femoropopliteal ultrasound examinations, Gottlieb and Widjaja40 showed that only 0.7% of cases developed a subsequent pulmonary embolus, they also reviewed 1797 similar patients reported in the literature and noted that only four (0.2%) of these had developed a pulmonary embolus following a negative ultrasound examination of the thigh area in patients symptomatic for DVT.
It is important to draw a distinction between the accuracy of ultrasound for the diagnosis of symptomatic thrombus and asymptomatic thrombus. The results for the latter are less good as, almost by definition, asymptomatic thrombus will be non-occlusive in many cases and therefore easier to miss. Weinmann et al. noted an overall sensitivity in six reported series of only 59% for proximal thrombus, although the specificity was 98%.4 In addition, asymptomatic thrombus may be small, or involve one or only a few calf vein segments. A further review by Wells of 17 screening studies in orthopaedic patients showed a sensitivity of 62%, specificity of 97% and a positive predictive value of 66% in those studies which had been carried out with an adequate scientific method.41
The continuing developments with MR imaging (MRI) and multislice computed tomography (CT) mean that it is now feasible to consider using these for the diagnosis of DVT. Several authors have suggested that performing a CT scan of the pelvis and upper legs in patients undergoing CT pulmonary arteriography for pulmonary embolus is a satisfactory way to confirm or exclude the presence of significant proximal thrombus in the leg and pelvic veins.42,43 However, this technique would not be practical for the assessment of all cases of possible DVT and considerations relating to radiation dose and contrast injection would need to be taken into account. Similarly, MR venography is also of some value44,45 as it shows not only the thrombus in the lumen of the vein as a filling defect but can also show thrombus directly due to the methaemoglobin present within the thrombus; in addition it also shows the perivascular inflammatory reaction to acute thrombosis.46 As with CT, MR venography is not practical or suitable for initial assessment of all patients with possible DVT, although incidental findings of DVT in abdominal and pelvic examinations can easily be recognised and research into its role is continuing.
Recurrent Varicose Veins and Chronic Venous Insufficiency
The venous system of the lower limb is relatively fragile and easily damaged by a variety of insults including thrombosis, trauma and inflammation. Previous thrombosis may not clear completely, resulting in chronic obstruction and damage to the valves. In limbs affected by DVT, 50–80% will recanalise months or years after the event; chronic sequelae are most often ascribed to reflux rather than to residual obstruction, although both play a part in the development of chronic problems.47 This damage results in loss of the protective action of the valves so that a continuous column of blood is present between the heart and the tissues of the calf, ankle and foot. In the erect position this may extend over 1.25 m and the hydrostatic pressure exerted on the tissues interferes with the circulation of blood in the capillaries, the transfer of nutrients and waste matter between blood and the tissues and may also promote local inflammatory responses in the tissues. These changes result in the development of varicose veins, varicose eczema and, ultimately, varicose ulceration. Treatment options include standard varicose vein surgical techniques, pressure stockings, dressings and venous reconstruction techniques. The pattern of damaged and incompetent veins can be defined using Doppler ultrasound to examine the deep and superficial veins in order to identify thrombosed or partially recanalised veins. Incompetent venous segments, together with incompetent perforating veins, can be mapped out and appropriate surgical or medical techniques applied. Approximately 1% of the population will have venous leg ulceration at some point in their lives,2 and up to 22% will have evidence of chronic venous insufficiency.5
Diagnosis and assessment of primary varicose veins have traditionally been based on clinical assessment in conjunction with hand-held Doppler devices, but it has been shown that a formal colour Doppler assessment prior to surgery will alter the proposed operative procedure in a number of cases and improve the overall results of surgery for primary varicose veins. Blomgren et al.48 showed that over a 7-year follow-up period, 34% of legs which did not have preoperative duplex required reoperation, compared with 13% of legs on which pre-operative duplex had been carried out. However, applying this principle to all cases of primary varicose veins would result in a heavy workload, so some consideration needs to be given to patient selection and scanning only those in whom there are incomplete, or conflicting clinical findings;49 or in whom endovascular ablation is to be performed so that an accurate assessment of vessel calibre and anatomy can be made.
Treatment options for varicose veins are no longer restricted to surgical ablation or stripping of the affected vein. Laser or radiofrequency ablation, as well as foam sclerotherapy have been shown to be as effective as surgery.50 Ultrasound has a major role in the localisation of catheters and ablation devices, as well as monitoring the progress of these treatments.51
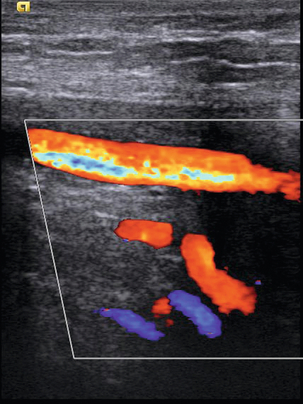
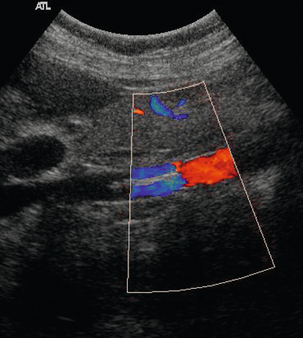
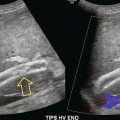
Recurrence of varicose veins after surgery or sclerotherapy may occur. Three main patterns of recurrence have been described.52 A patent long saphenous vein may be present, suggesting that it has been missed at the time of the operation. Small collateral veins along the line of the long saphenous vein may enlarge to reconstitute the path of the vein (Fig. 5-28). Finally, drainage can occur through venous collaterals which pass along a variety of courses remote from the normal line of the vein. Colour Doppler is useful to assess the pattern of recurrence, so that appropriate surgical intervention may be planned.53
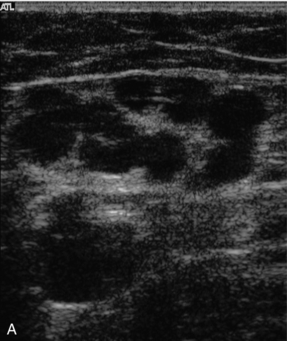
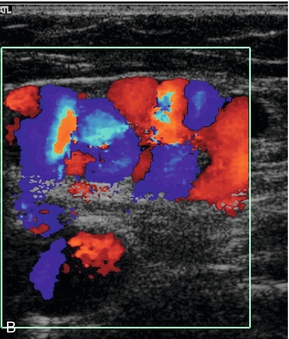
FIGURE 5-28 Collateral channels at the saphenofemoral junction following previous surgery: (A) B-scan image; (B) colour Doppler image.
TECHNIQUE OF EXAMINATION
Various techniques can be used to assess competence or incompetence of a venous segment.54 The most convenient method for general assessment is to squeeze firmly, then release the patient’s calf, or lower thigh, to promote forward flow. Incompetent valves will allow reverse flow back through them after forward flow has ceased (Fig. 5-29), whereas competent valves will stop any reverse flow. Pressure cuffs that can be inflated and deflated rapidly can be used to produce a similar effect and produce a more standardised stimulus than manual compression.55 They can also be used to compress a segment of leg in order to squeeze out the venous blood and then released suddenly so that any incompetent segments will show up by reversed filling from above. Alternatively, proximal compression may be applied to induce reverse flow. Getting the patient to perform a Valsalva manoeuvre will also show incompetent segments but there are two disadvantages to this technique. First, the effect will only demonstrate reverse flow as far as the first competent valve, so that any incompetent segments below this will not be demonstrated. Second, it is quite difficult to explain to many patients the exact nature and method for performing a Valsalva. Asking the patient to blow into a high-resistance spirometer circuit can produce the desired sudden increase in intra-abdominal pressure and is easier for many patients to understand. In some patients reflux will be seen simply with inspiration.
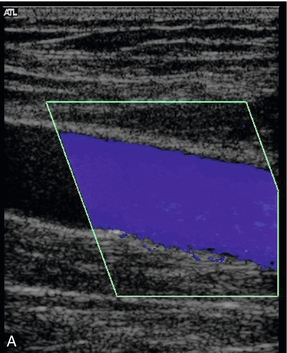
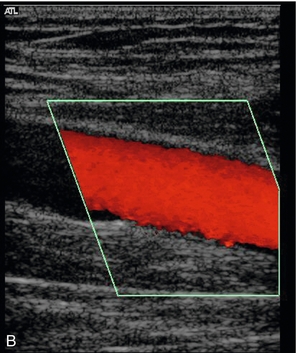
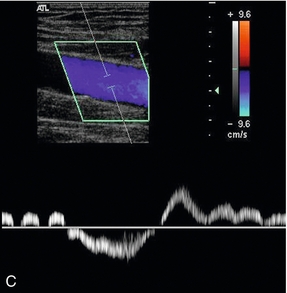
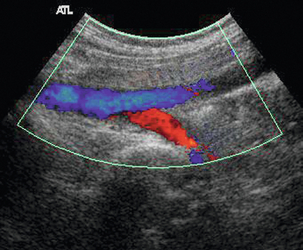
FIGURE 5-29 An incompetent segment of superficial femoral vein showing forward (A, blue) and reverse flow (B, red); (C) the spectral tracing with reverse flow below the baseline lasting for approximately 3 s.
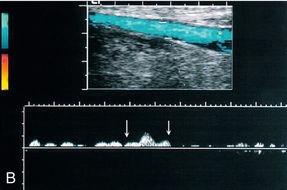
Reflux can be defined as reverse flow occurring after the cessation of forward flow. It is generally held to be significant if it lasts > 0.5 s in the superficial veins, deep femoral and calf veins. For the femoropopliteal veins, a cut off of 1.0 s is used as their larger diameters and smaller number of valves are thought to contribute to slower valve closure rates.56 Shorter periods of reversed flow may be seen in normal veins and represent the short period as the valve cusps come together and blood in the venous segment settles under the influence of gravity. Reflux should not be confused with the reversed flow which occurs with turbulence, particularly in the common femoral vein and popliteal veins. The difference is usually apparent on colour Doppler, and turbulence is seen on spectral Doppler as reverse flow occurring at the same time as forward flow (Fig. 5-30).
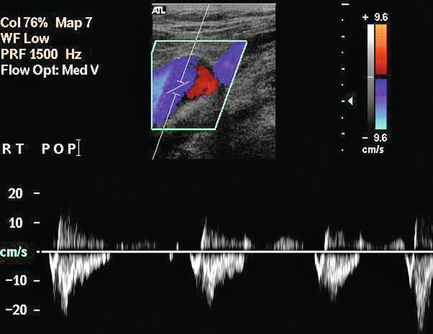
FIGURE 5-30 Turbulence in a vein showing both red and blue signal in the lumen on colour Doppler and simultaneous forward and reversed flow on the spectral display.
The examination begins in the groin,57 where the common femoral vein, profunda femoris vein and saphenofemoral junction are identified and assessed. If there is a history of previous venous surgery the details are sometimes uncertain, or even wrong, and the region of the saphenofemoral junction should be examined carefully to assess the type of surgery, whether it was successful and whether there are any significant collaterals, or recanalised segments which are incompetent. The loss of the normal smooth curve of the great saphenous vein as it passes laterally and deeply towards the common femoral vein is suggestive of previous surgery with subsequent recanalisation or collateral formation.
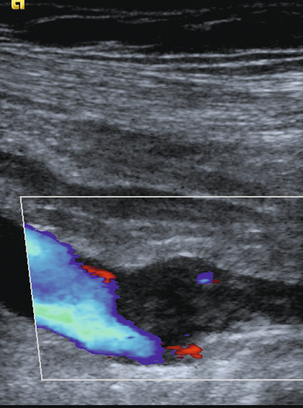
The patency and competence of the deep and superficial veins of the thigh are then assessed down to the level of the knee. Whilst examining the great saphenous vein the presence of incompetent perforators should be sought (Fig. 5-31), especially if the vein becomes incompetent at a level below the saphenofemoral junction. These can be identified most easily by scanning down the vein transversely whilst applying recurrent compression to the calf or lower thigh and looking for outward flow with colour Doppler. The commonest of these perforating veins is in the lower thigh at the level of the junction of the middle and lower thirds of the great saphenous vein and is called the mid-thigh perforator vein. The use of tourniquets may help clarify difficult cases but this is not usually required with colour Doppler.
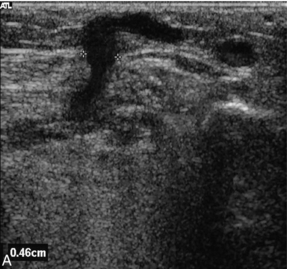
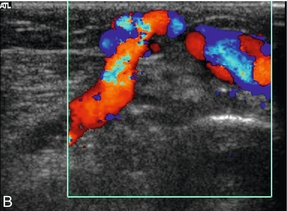
FIGURE 5-31 (A) An incompetent calf perforating vein with a diameter of approximately 5 mm passing through the superficial fascia; (B) colour Doppler shows flow passing from the deep to the superficial veins.
The findings can be recorded on a diagram of the main lower limb veins where presence of any significant reflux, together with a note of the reflux time, can be indicated on the form (Fig. 5-32).
Saphenous Vein Mapping
The long saphenous vein is the preferred conduit for arterial bypass grafting in the coronary arteries and lower limb. If there is any doubt concerning the suitability of the vein for the procedure, ultrasound can be used to assess the calibre and available length of the vein. Ideally the vein should be more than 3–4 mm wide for much of its length and more than 2 mm at the ankle if a long, femorodistal graft is being considered.58 The aim of the examination depends on the surgical procedure being contemplated. If the vein is to be removed for a coronary artery or reversed lower limb arterial graft, then the examination can be limited to confirming the presence of the vein and assessing its calibre over the required length. If an in situ lower limb arterial graft is to be performed then a much more detailed examination is required in order to identify perforating veins and superficial branches communicating with the main vein, as these must be ligated during the operation to stop arteriovenous fistulae developing.
TECHNIQUE
The examination is performed with the patient standing, if possible, as this produces distension of the vein, allowing easier location due to dilatation and a better estimation of the calibre of the vessel. If the patient is unable to stand they can be examined sitting with their legs over the side of the couch; if this is not possible then they can be assessed lying supine with a low-pressure tourniquet applied in order to produce distension of the superficial veins.59 Some operators prefer to mark out the vein with the patient supine as this approximates better to the position during surgery.
REFERENCES
1. Anderson, F. A., Jr., Wheeler, H. B., Goldberg, R. J., et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism: the Worcester DVT study. Arch Intern Med. 1991; 151:933–938.
2. Callam, M. J., Ruckley, C. V. The epidemiology of chronic venous disease. In: A textbook of vascular medicine. London: Arnold; 1996:562–579.
3. Baxter, G. M. The role of ultrasound in deep vein thrombosis Editorial. Clin Radiol. 1997; 52:1–3.
4. Weinmann, E. E., Salzman, E. W. Deep vein thrombosis: a review. New Engl J Med. 1994; 331:1630–1641.
5. Phillips, G. W. L., Paige, J., Molan, M. P. A comparison of colour duplex ultrasound with venography and varicography in the assessment of varicose veins. Clin Radiol. 1995; 50:20–25.
6. Da Silva, A., Widmer, L. K., Martin, H., et al. Varicose veins and chronic insufficiency: prevalence and risk factors in 4376 subjects of the Basle Study II. Vasa. 1974; 3(2):118–125.
7. Caggiatti, A., Bergan, J. J., Gloviczki, P., et al. Nomenclature of the veins of the lower limbs: An international interdisciplinary consensus statement. J Vasc Surg. 2002; 36:416–422.
8. Gordon, A. C., Wright, I., Pugh, N. D. Duplication of the superficial femoral vein: recognition with duplex ultrasonography. Clin Radiol. 1996; 51:622–624.
9. Quinlan, D. J., Alikhan, R., Gishen, P., et al. Variations in lower limb venous anatomy: implications for US diagnosis of deep vein thrombosis. Radiology. 2003; 228:443–448.
10. Basmajian, J. V. Distribution of valves in femoral, external iliac and common iliac veins and their relationship to varicose veins. Surg Gynecol Obstet. 1952; 85:537–542.
11. Corrales, N. E., Irvine, A., McGuiness, C. L., et al. Incidence and pattern of long saphenous vein duplication and its possible implications for recurrence after varicose vein surgery. Br J Surg. 2002; 89:323–326.
12. Giacomini, C. Osservazioni anatomische per service allo studio della circulazioni venosa delle extremitá inferiori. Torino: Tip V Vercillino; 1873.
13. Burihan, E., Baptista-Silva, J. C. C. Anatomical study of the small saphenous vein (saphena parva): types of termination. Phlebology. 1995; 10(Suppl. 1):57–60.
14. Georgiev, M., Myers, K. A., Belcaro, G. The thigh extension of the lesser saphenous vein: From Giacomini’s observations to ultrasound scan imaging. J Vasc Surg. 2003; 37:558–563.
15. Linton, R. R. The communicating veins of the lower leg and the operative technique for their ligation. Ann Surg. 1938; 107:582–593.
16. Wells, P. S., Anderson, D. R., Rodger, M., et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost. 2000; 83:416–420.
17. Wells, P. S., Anderson, D. R., Rodgers, M., et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. NEJM. 2003; 349:1227–1235.
18. Hertzberg, B. S., Kliewer, M. A., DeLong, D. M., et al. Sonographic assessment of lower limb vein diameters: implications for the diagnosis and characterization of deep venous thrombosis. Am J Roentgenol. 1997; 168:1253–1257.
19. Perlin, S. J. Pulmonary embolism during compression US of the lower extremity. Radiology. 1992; 184:165–166.
20. Zwiebel, W. J., Priest, D. L. Colour duplex sonography of extremity veins. Semin Ultrasonogr CT MR. 1990; 11:136–137.
21. Rosfors, S., Eriksson, M., Leijd, B., et al. A prospective follow-up study of acute deep venous thrombosis using colour duplex ultrasound, phlebography and venous occlusion plethysmography. Internat Angiol. 1997; 16:39–44.
22. Franzeck, U. K., Schalch, I., Jager, K. A., et al. Prospective 12-year follow-up study of clinical and haemodynamic sequelae after deep vein thrombosis in low-risk patients (Zurich study). Circulation. 1996; 93:74–79.
23. Baarslag, H. J., van Beek, E. J., Koopman, M. M., et al. Prospective study of color duplex ultrasonography compared with contrast venography in patients suspected of having deep venous thrombosis of the upper extremities. Ann Intern Med. 2002; 136:865–872.
24. Mustafa, S., Stein, P. D., Patel, K. C., et al. Upper extremity deep venous thrombosis. Chest. 2003; 123:1953–1956.
25. Labropoulos, N., Webb, K. M., Kang, S. S., et al. Patterns and distribution of isolated calf deep vein thrombosis. J Vasc Surg. 1999; 30:787–791.
26. Scarvelis, D., Wells, P. S. Diagnosis and treatment of deep-vein thrombosis. CMAJ. 2006; 175:1087–1092.
27. Davidson, B. L., Elliot, C. G., Lensing, A. W. A. Low accuracy of colour Doppler ultrasound in the detection of proximal leg vein thrombosis in asymptomatic high-risk patients. Ann Intern Med. 1992; 117:735–738.
28. Goodacre, S., Sampson, F., Stevenson, M., et al. Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis. Health Technol Assess. 2006; 10:37–42.
29. May, R., Thurner, J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957; 8:419–427.
30. James, A. H., Jamison, M. G., Brancaio, L. R., et al. Venous thromboembolism during pregnancy and the postpartum period: Incidence, risk factor, and mortality. Am J Obstet Gynecol. 2006; 194:1311–1315.
31. Polak, J. F., Wilkinson, D. L. Ultrasonographic diagnosis of symptomatic deep venous thrombosis in pregnancy. Am J Obstet Gynecol. 1991; 165:625–629.
32. Ray, J. G., Chan, W. S. Deep vein thrombosis during pregnancy and the pueperium: a meta-analysis of the period of risk and leg of presentation. Obstet Gynecol Surv. 1999; 54:265–271.
33. Labropoulos, N., Shifrin, D. A., Paxinos, O., et al. New insights into the development of popliteal cysts. Br J Surg. 2004; 91:1313–1318.
34. Langsfeld, M., Matteson, B., Johnson, W., et al. Baker’s cysts mimicking the symptoms of deep vein thrombosis: diagnosis with venous duplex scanning. J Vasc Surg. 1997; 25:658–662.
35. Cronan, J. J., Dorfman, G. S., Scola, F. H., et al. Deep venous thrombosis: US assessment using vein compression. Radiology. 1987; 162:191–194.
36. Atri, M., Herba, M. J., Reinhold, C., et al. Accuracy of sonography in the evaluation of calf deep vein thrombosis in both postoperative surveillance and symptomatic patients. Am J Radiol. 1996; 166:1361–1367.
37. Kearon, C., Julian, J. A., Newman, T. E., et al. Non-invasive diagnosis of deep vein thrombosis. Annals Int Med. 1998; 128:663–667.
38. Rose, S. C., Zweibel, W. J., Nelson, B. D., et al. Symptomatic lower limb venous thrombosis: accuracy, limitations and role of colour duplex flow imaging in the diagnosis. Radiology. 1990; 175:639–644.
39. Theodorou, S. J., Theodorou, D. J., Kakitsubata, Y. Sonography and venography of the lower extremities for diagnosing deep vein thrombosis in symptomatic patients. Clin Imaging. 2003; 27:180–183.
40. Gottlieb, R. H., Widjaja, J. Clinical outcomes of untreated symptomatic patients with negative findings on sonography of the thigh for deep vein thrombosis: our experience and a review of the literature. AJR Am J Roentgenol. 1999; 172:1601–1604.
41. Wells, P. S., Lensing, A. W., Davidson, B. L., et al. Accuracy of ultrasound for the diagnosis of deep vein thrombosis in asymptomatic patients after orthopaedic surgery: A meta-analysis. Ann Intern Med. 1995; 122:47–53.
42. Thomas, S. M., Goodacre, S. W., Sampson, F. C., et al. Diagnostic value of CT for deep vein thrombosis: results of a systematic review and meta-analysis. Clin Radiol. 2008; 63:299–304.
43. Lim, K. E., Hsu, W. C., Hsu, Y. Y., et al. Deep venous thrombosis: comparison of indirect multidetector CT venography and sonography of lower extremities in 26 patients. Clin Imaging. 2004; 28:439–444.
44. Fraser, D. G., Moody, A. R., Davidson, I. R., et al. Deep venous thrombosis: diagnosis by using venous enhanced subtracted peak arterial MR venography versus conventional venography. Radiology. 2003; 226:812–820.
45. Sampson, F. C., Goodacre, S. W., Thomas, S. M., et al. Accuracy of MRI in diagnosis of suspected deep vein thrombosis: systematic review and meta-analysis. Eur Radiol. 2007; 17:175–181.
46. Froehlich, J. B., Prince, M. R., Greenfield, L. J., et al. ‘Bull’s-eye’ sign on gadolinium-enhanced magnetic resonance venography determines thrombus presence and age: a preliminary study. J Vasc Surg. 1997; 26:809–816.
47. Nicolaides, A. N. Investigation of chronic insufficiency: a consensus statement. Circulation. 2000; 102:e126–e163.
48. Blomgren, L., Johansson, G., Emanuelson, L., et al. Late follow up of a randomized trial of routine duplex imaging before varicose vein surgery. Br J Surg. 2011; 98:1112–1116.
49. Kent, P. J., Weston, M. J. Duplex scanning may be used selectively in patients with primary varicose veins. Ann R Coll Surg Engl. 1998; 80:388–393.
50. Rasmussen, L. H., Lawaertz, M., Bjoern, B., et al. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011; 98:1079–1087.
51. Mowatt-Larssen, E., Shortell, C. K. Treatment of primary varicose veins has changed with the introduction of new techniques. Semin Vasc Surg. 2012; 25:18–24.
52. Stonebridge, P. A., Chalmers, N., Beggs, I., et al. Recurrent varicose veins: a varicographic analysis leading to a new, practical classification. Br J Surg. 1995; 82:60–62.
53. Bradbury, A. W., Stonebridge, P. A., Callam, M. J., et al. Recurrent varicose veins: assessment of the saphenofemoral junction. Br J Surg. 1994; 81:373–375.
54. Allan, P. L. The role of ultrasound in the assessment of chronic venous insufficiency. Ultrasound Q. 2001; 17:3–10.
55. Markel, A., Meissner, M. H., Manzo, R. A., et al. A comparison of the cuff deflation method with Valsalva’s maneuver and limb compression in detecting venous valvular reflux. Arch Surg. 1994; 129:701–705.
56. Labropoulos, N., Tiongson, J., Pryor, L., et al. Definition of venous reflux in lower-extremity veins. J Vasc Surg. 2003; 38:793–798.
57. Coleridge-Smith, P., Labropoulos, N., Partsch, H., et al. Duplex ultrasound of the veins in chronic venous disease of the lower limbs – UIP consensus document. Part 1: Basic Principles. Eur J Vasc Endovasc Surg. 2006; 31:83–92.
58. Leopold, P. W., Shandall, A., Kupinski, A. M., et al. Role of B-mode venous mapping in infrainguinal in situ vein arterial bypasses. Br J Surg. 1989; 76:305–307.
59. Hoballah, J., Corry, D. C., Rossley, N., et al. Duplex saphenous vein mapping: venous occlusion and dependent position facilitate imaging. Vasc Endovasc Surg. 2002; 36:377–380.