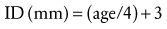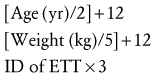12 The Pediatric Airway
Developmental Anatomy of the Airway
The classic works by Negus, Eckenhoff, and Fink and Demarest form the foundation of our knowledge about the structure and function of the pediatric and adult airway.1–3 They suggested that there are five major anatomic differences between the neonatal and the adult airway, which are outlined in this section, although more recent studies suggest that not all of these long-held beliefs are supported by science.2–4 In addition, the relatively large head of an infant negates the need to place anything under the head to achieve a proper “sniffing position.” Older children have airway features that represent a transition between the infant and the adult anatomy.
Tongue
In the past, it was thought that an infant’s tongue is relatively large in proportion to the rest of the oral cavity and therefore can more easily obstruct the airway, especially in a neonate. However, magnetic resonance imaging (MRI) studies have now demonstrated that there is proportional growth of the tongue and other soft tissues in relation to the bony structures of the oral cavity in children 1 to 11 years of age.5 Furthermore, the contribution of the tongue to upper airway obstruction with sedation or induction of anesthesia is relatively minor; much of the obstruction is more likely due to nasopharyngeal and epiglottic collapse, although in a patient of any age the tongue may also contribute to obstruction.6,7
Position of the Larynx
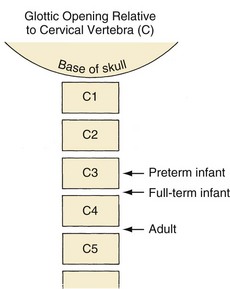
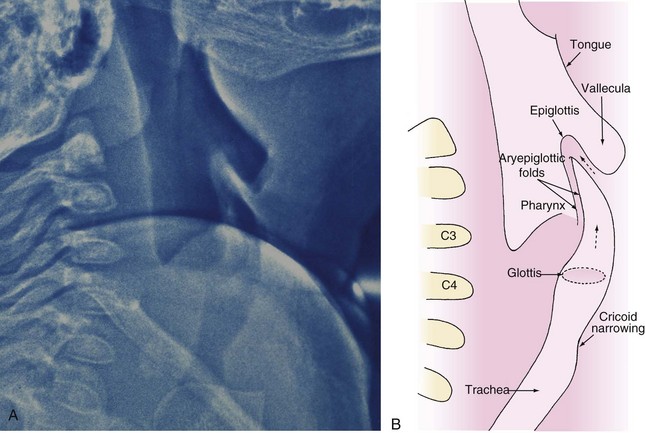
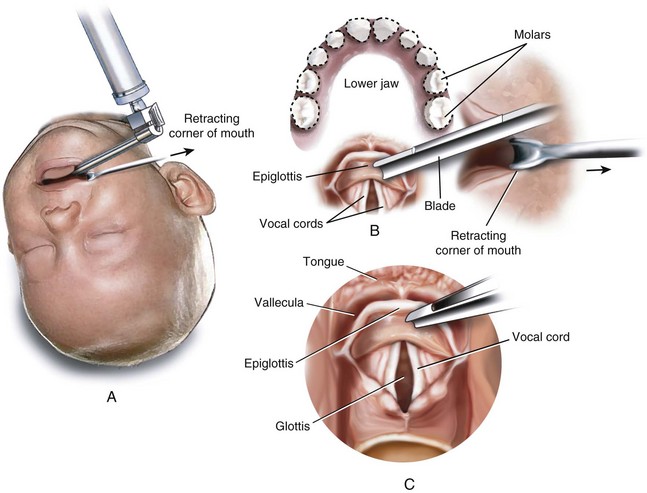
An infant’s larynx is higher (more cephalad) in the neck, classically described at the level of C3-4, than an adult’s larynx, which is at the level of C4-5 (Fig. 12-1). MRI and computed tomography (CT) have confirmed the higher (more cephalad) position of the larynx in children and demonstrated that the hyoid bone is at the C2-3 level in infants and children up to 2 years of age.8 Consequently, the distances between the tongue, hyoid bone, epiglottis, and roof of the mouth are smaller in infants than they are in an older child or adult.
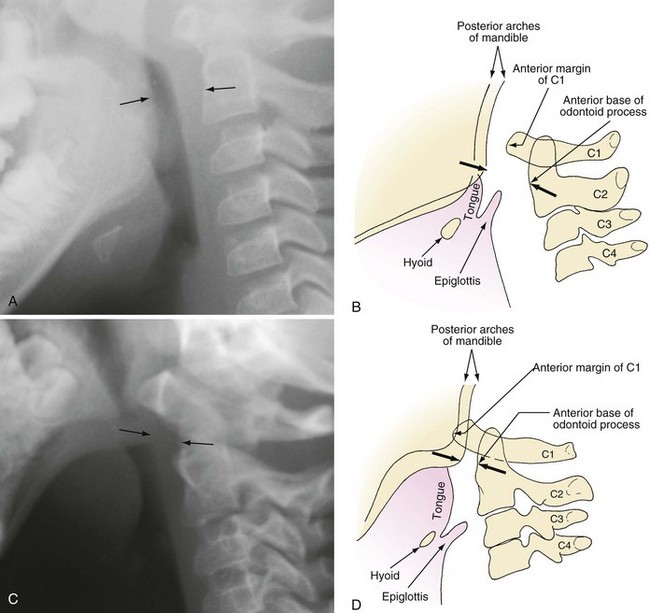
The proximity of the tongue to the more superior larynx also makes visualization of laryngeal structures more difficult, because it produces a more acute angle between the plane of the tongue and the plane of the glottic opening. It is for this reason that a straight laryngoscope blade, which lifts the tongue from the field of view during laryngoscopy, facilitates visualization of an infant’s larynx. This anatomic relationship is further complicated in certain conditions such as the Treacher Collins anomaly and other syndromes associated with mandibular and midfacial hypoplasia that make direct visualization of the glottis difficult and sometimes impossible with standard laryngoscopy (Fig. 12-2). The reason for this difficulty is that with mandibular and midfacial hypoplasia, the base of the tongue is positioned more caudally (known as glossoptosis) and in closer proximity to the laryngeal inlet than normal; the result is an even greater acute angle between the plane of the tongue and the plane of the laryngeal inlet (often 90 degrees) (Fig. 12-3). In this situation, conventional rigid laryngoscopy provides excellent visualization of the esophageal inlet rather than the laryngeal inlet, necessitating the use of special equipment or special techniques to intubate the trachea.
Epiglottis
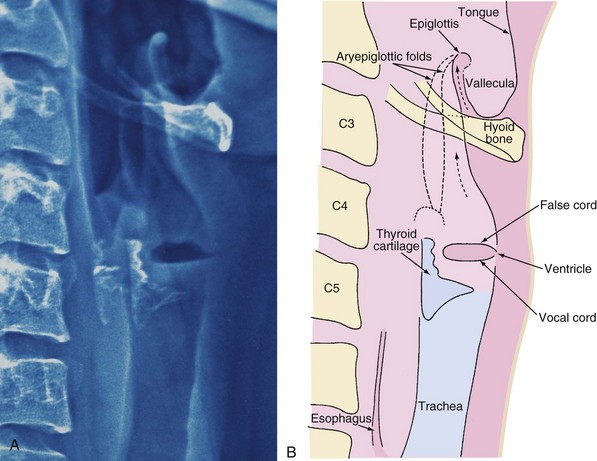
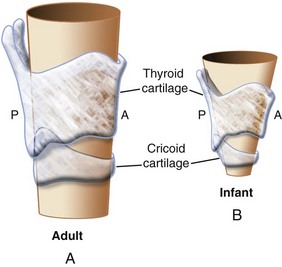
An adult’s epiglottis is flat and broad, and its axis is parallel to that of the trachea (Fig. 12-4), whereas the infant’s epiglottis is narrower, omega shaped, and angled away from the axis of the trachea (Fig. 12-5). It is therefore more difficult to lift the infant’s epiglottis with the tip of a laryngoscope blade.
Vocal Folds
In contrast to the adult, in whom the axis of the vocal folds is perpendicular to that of the trachea, the vocal folds (cords) of an infant are angled such that the anterior insertion is lower (caudad) compared with the posterior insertion (compare Fig. 12-4, A, with Fig. 12-5, A). This anatomic feature alters the angle at which the tracheal tube approaches the laryngeal inlet and occasionally leads to difficulty with tracheal intubation, especially with the nasal approach. In the latter case, the tip of the endotracheal tube (ETT) may be held up at the anterior commissure of the vocal folds.
Subglottis
Classic teaching holds that the narrowest part of an infant’s larynx is the cricoid cartilage; in an adult, it is the rima glottidis. This teaching was supported by an MRI and CT study in young children (<2 years of age) who were sedated with oral medications and breathing spontaneously.8 In contrast, a more recent study in children 2 months to 13 years of age undergoing MRI with propofol sedation and spontaneous respirations reported that the narrowest portions of the pediatric larynx were the glottic opening and the immediate sub–vocal cord level and that this finding did not change relative to the dimensions of the cricoid ring throughout childhood.9 Nonetheless, when a relatively large-diameter tube is inserted into the glottic aperture, the tube passes through the cords but may meet resistance immediately below the cords (e.g., in the subglottic or cricoid ring region). Although these studies demonstrate in vivo physiologic relationships, the cricoid cartilage is functionally the narrowest portion of the upper airway.
Growth of the subglottic airway occurs rapidly during the first 2 years of life; thereafter, growth of the airway is linear.10 At 10 to 12 years of age, the cricoid and thyroid cartilages reach adult proportions, thus eliminating both the angulation of the vocal cords and the narrow subglottic area.
In the adult, the rima glottidis is considered the narrowest part of the airway, and an ETT that traverses the glottis passes into the trachea without resistance. However, in about 70% of adult cadavers, the narrowest portion of the airway was also in the subglottic region.11 The range in diameter for adult females was 10 to 16 mm, and for adult males it was 13 to 19 mm. The likely reason that ETTs pass easily through the glottic opening into the trachea of an adult is that, overall, the narrowest portion of the airway is still larger than the most commonly used ETT sizes. The apparent subglottic narrowing in adults is generally not evident unless there is the need to pass a larger-diameter ETT such as a double-lumen tube. In contrast, in a child, it is common for an ETT to pass easily through the vocal folds (glottic opening) but not through the subglottic region (Fig. 12-6; see Video 12-1). The larynx in both adults and children should be considered funnel shaped, although this configuration is exaggerated and is of greater import in infants and young children.
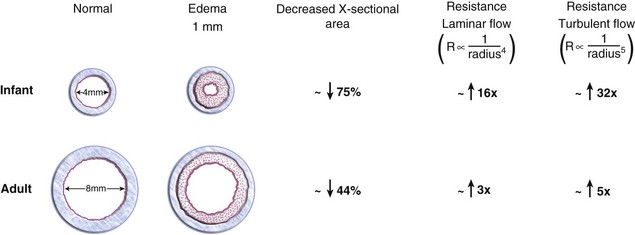
The cricoid is the only complete ring of cartilage in the laryngotracheobronchial tree and is therefore nondistensible. Because the mucosa that lines the upper airway is loose-fitting pseudostratified columnar epithelium, pressure on the mucosa may cause reactive edema that could encroach on the diameter of the lumen. A tight-fitting ETT that compresses the tracheal mucosa at this level may cause inflammation and edema when it is removed, reducing the luminal diameter and increasing the airway resistance at the time of extubation (e.g., postextubation croup). Because the subglottic region in the infant is smaller than in the adult, the same degree of airway edema results in greater resistance in the infant. For example, assuming that the diameter of the cricoid ring in the infant is 4 mm and the diameter of the adult cricoid ring or trachea is 8 mm, 1 mm of edema circumferentially within the airway (i.e., reduction of the diameter of the airway by 2 mm) would decrease the cross-sectional area of the airway in the infant by approximately 75% (to 2 mm), whereas the adult cross-sectional area would decrease by only about 44% (to 6 mm). Physiologically, because the resistance to airflow in the upper airway is turbulent during crying or deep breathing, this reduction in diameter of the upper airway would increase the resistance to flow by the radius to the fifth power, or 32-fold, in the infant, compared with 5-fold in the adult. (Fig. 12-7).2
The Larynx
Anatomy
Structure
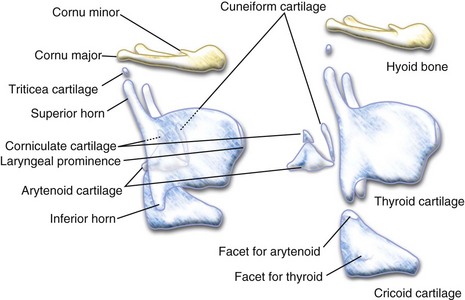
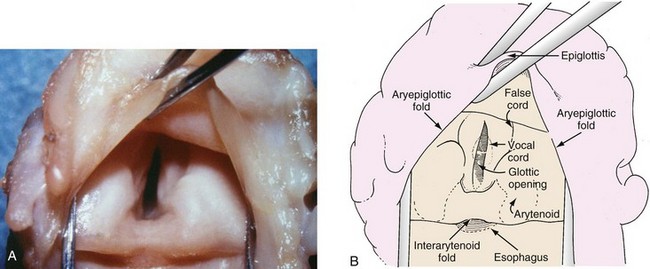
The larynx is composed of one bone (hyoid) and eleven cartilages (the single thyroid, cricoid, and epiglottic cartilages and the paired arytenoid, corniculate, cuneiform, and triticea cartilages). These cartilages are suspended by ligaments from the base of the skull. The body of the cricoid cartilage articulates posteriorly with the inferior cornu of the thyroid cartilage. The paired triangular arytenoid cartilages rest on top of, and articulate with, the superoposterior aspect of the cricoid cartilage. The arytenoid cartilages are protected by the thyroid cartilage (Fig. 12-8). The triticeal cartilages are rounded nodules of cartilage, approximately the size of a pea in adults, located in the margins of the lateral thyrohyoid ligament.
Tissue folds and muscles cover these cartilages. In contrast to adults, but comparable to most mammals, the cartilaginous glottis accounts for 60% to 75% of the length of the vocal folds in children younger than 2 years of age.10 Contraction of the intrinsic laryngeal muscles alters the position and configuration of these tissue folds, thus influencing laryngeal function during respiration, forced voluntary glottic closure (Valsalva maneuver), reflex laryngospasm, swallowing, and phonation (Fig. 12-9).
The laryngeal tissue folds consist of the following:
 Paired aryepiglottic folds extending from the epiglottis posteriorly to the superior surface of the arytenoids (the paired cuneiform and corniculate cartilages lie within for support and reinforcement)
Paired aryepiglottic folds extending from the epiglottis posteriorly to the superior surface of the arytenoids (the paired cuneiform and corniculate cartilages lie within for support and reinforcement)
 Paired vestibular folds (false vocal cords) extending from the thyroid cartilage posteriorly to the superior surface of the arytenoids
Paired vestibular folds (false vocal cords) extending from the thyroid cartilage posteriorly to the superior surface of the arytenoids
 Paired vocal folds (true vocal cords) extending from the posterior surface of the thyroid plate to the anterior projection or vocal process of the arytenoids
Paired vocal folds (true vocal cords) extending from the posterior surface of the thyroid plate to the anterior projection or vocal process of the arytenoids
 A single interarytenoid fold (composed of the interarytenoid muscle covered by tissue) bridging the arytenoid cartilages
A single interarytenoid fold (composed of the interarytenoid muscle covered by tissue) bridging the arytenoid cartilages
 A single thyrohyoid fold extending from the hyoid bone to the thyroid cartilage
A single thyrohyoid fold extending from the hyoid bone to the thyroid cartilage
Histology
The highly vascular mucosa of the mouth is continuous with that of the larynx and trachea. This mucosa consists of squamous, stratified, and pseudostratified ciliated epithelium. The vocal cords are covered with stratified epithelium. The mucosa and submucosa are rich in lymphatic vessels and seromucus-secreting glands, which lubricate the laryngeal folds. The submucosa consists of loose fibrous stroma; therefore, the mucosa is loosely adherent to the underlying structures in most areas. However, the submucosa is scant on the laryngeal surface of the epiglottis and the vocal cords, so the mucosa is tightly adherent in these areas.12,13 Most inflammatory processes of the airway above the level of the vocal cords are limited by the barrier formed by the firm adherence of the mucosa to the vocal cords.13 For example, the inflammation of epiglottitis is usually limited to the supraglottic structures, and the loosely adherent mucosa explains the ease with which localized swelling occurs (see Figs. 31-15 and 31-16). In a similar manner, an inflammatory process of the subglottic region (laryngotracheobronchitis) results in significant subglottic edema in the loosely adherent mucosa of the airway below the vocal cords, but it does not usually spread above the level of the vocal cords (see Fig. 31-14, C).12
Sensory and Motor Innervation
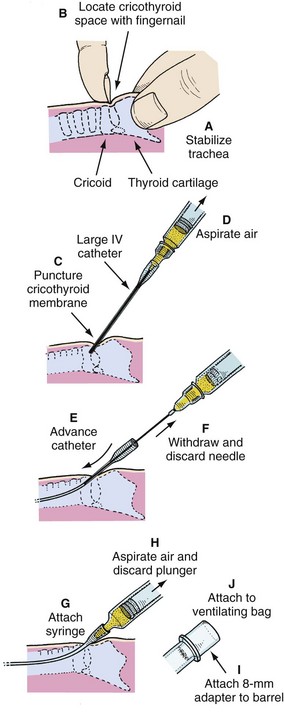
Two branches of the vagus nerve, the recurrent laryngeal and the superior laryngeal nerves, supply both sensory and motor innervation to the larynx. The superior laryngeal nerve has two branches: the internal branch, which provides sensory innervation to the supraglottic region, and the external branch, which supplies motor innervation to the cricothyroid muscle. The recurrent laryngeal nerve provides sensory innervation to the subglottic larynx and motor innervation to all other laryngeal muscles.13,14 Local anesthetic agents injected to block the superior laryngeal nerve result in anesthesia of the supraglottic region down to the inferior margin of the epiglottis and motor blockade of the cricothyroid muscle, which causes relaxation of the vocal cords. Translaryngeal injection of local anesthetic through the cricothyroid membrane or a specific recurrent laryngeal nerve block is required for infraglottic and tracheal anesthesia.15–17
Blood Supply
Laryngeal branches of the superior and inferior thyroid arteries provide the blood supply to the larynx. The recurrent laryngeal nerve and artery lie in close proximity to each other, which accounts for the occasional vocal cord paresis after attempts to control bleeding during thyroidectomy.18
Function
Forced Glottic Closure and Laryngospasm
Glottic closure during forced expiration (forced glottic closure or Valsalva maneuver) is voluntary laryngeal closure and is physiologically similar to involuntary laryngeal closure (laryngospasm). Forced glottic closure occurs at several levels. Contraction of the intrinsic laryngeal muscles results in (1) marked reduction in the interarytenoid distance; (2) anterior rocking and medial movement of the arytenoids, causing apposition of the paired vocal, vestibular, and aryepiglottic folds; (3) longitudinal shortening of the larynx that obliterates the space between the aryepiglottic, vestibular, and vocal folds (like complete closing of a telescope). Contraction of an extrinsic laryngeal muscle, the thyrohyoid, pulls the hyoid bone downward (caudad) and the thyroid cartilage upward (cephalad), leading to further closure.1,3,4,19–22
Closure of the larynx during laryngospasm is similar to, but not identical to that described for voluntary forced glottic closure. There are two important differences. First, laryngospasm is accompanied by an inspiratory effort, which longitudinally separates the vocal from the vestibular folds. Second, in contrast to forced glottic closure, neither the thyroarytenoid muscle (an intrinsic muscle of the larynx) nor the thyrohyoid muscle contracts; thus, apposition of the aryepiglottic folds and median thyrohyoid folds is minimal. These two differences allow the upper portion of the larynx to be left partially open during mild laryngospasm, resulting in the hallmark high-pitched inspiratory stridor (see Video 12-1).1,19 Anterior and upward displacement of the mandible (jaw thrust) longitudinally separates the base of the tongue, the epiglottis, and the aryepiglottic folds from the vocal folds, helping to relieve laryngospasm.20
Swallowing
Glottic closure during swallowing is also similar to that which occurs during forced closure of the glottis. Protection of the glottic opening is achieved primarily by apposition of the laryngeal folds and secondarily by upward (cephalad) movement of the larynx. The upward movement of the larynx brings the thyroid cartilage closer to the hyoid bone, resulting in folding of the epiglottis over the glottic opening.1,19,21,22 With loss of consciousness or deep sedation, the normal protective mechanism of the larynx may be lost or obtunded, thus predisposing to pulmonary aspiration of pharyngeal contents.
Phonation
Phonation is accomplished by alteration of the angle between the thyroid and cricoid cartilages (the cricothyroid angle) and by medial movement of the arytenoids during expiration.1,14,23 These movements result in fine alterations in vocal fold tension during movement of air, causing vibration of the vocal folds. Lesions or malfunctions of the vocal folds (e.g., inflammation, papilloma, paresis) therefore affect phonation. Phonation is the only laryngeal function that alters the cricothyroid angle.1Therefore, despite significant airway obstruction during inspiration, it may still be possible to phonate.
Physiology of the Respiratory System
Obligate Nasal Breathing
Infants are considered to be obligate nasal breathers.24,25 Obstruction of their anterior or posterior nares (nasal congestion, stenosis, choanal atresia) can cause asphyxia.26–28 Immaturity of coordination between respiratory efforts and oropharyngeal motor and sensory input accounts in part for obligate nasal breathing.29 Furthermore, because the larynx is higher (more cephalad) in the neck of an infant and oropharyngeal structures are closer together, the tongue rests against the roof of the mouth during quiet respiration, resulting in oral airway obstruction.25 Multiple sites of pharyngeal airway obstruction may also contribute to airway obstruction when the infant attempts to breathe against a partially obstructed upper airway or with relaxation of upper airway muscle tone after sedation or induction of anesthesia.30–34
As the infant matures, the ability to coordinate respiratory and oral function increases. The larynx enlarges and moves down lower (more caudad) in the neck as the cervical spine lengthens and the infant begins to breathe adequately through the mouth. This maturation occurs by age 3 to 5 months. Studies have shown that the ability to breathe through the mouth when the nares are obstructed is age dependent: 8% of preterm infants of 31 to 32 weeks postconceptional age were able to breathe through the mouth in response to nasal occlusion, compared with 28% of more mature preterm infants of 35 to 36 weeks postconceptional age35; approximately 40% of full-term infants can switch from nasal to oral breathing.36 However, more recent data contradict these earlier data. Slow and fast nasal occlusion applied to 17 healthy preterm infants (gestational age, 32 ± 1 weeks; postnatal age, 12 ± 2 days) led to a switch from nasal to oral breathing. The authors attributed the difference in findings to the more extended observation period in their study (>15 seconds).37 The presence of a nasogastric tube may also significantly affect the infant’s breathing if the “unobstructed” nasal passage has an existing underlying obstruction.
Tracheal and Bronchial Function
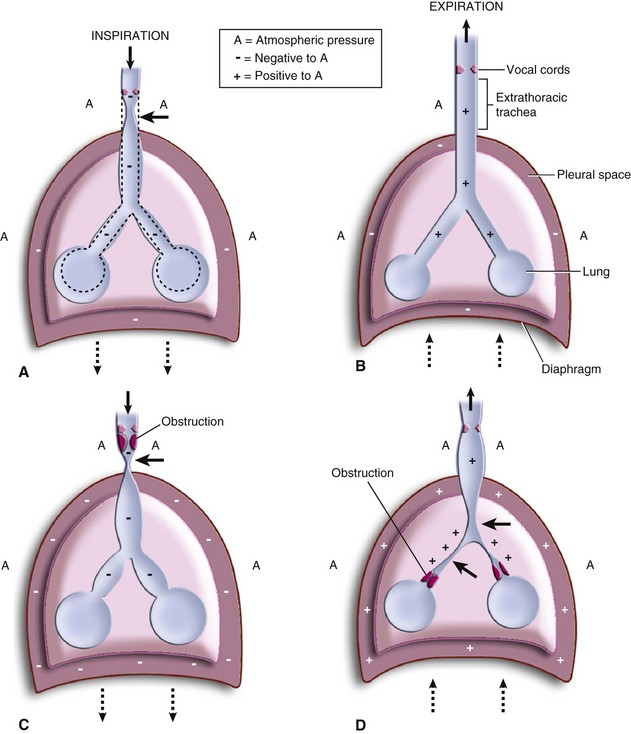
Tracheal and bronchial diameters are a function of elasticity and of distending or compressive forces (Fig. 12-10). The larynx, trachea, and bronchi in the infant are quite compliant compared with those in the adult and therefore are more subject to distention and compression forces.24,38,39 The intrathoracic trachea is subject to stresses that are different from those in the extrathoracic portion.38 During expiration, intrathoracic pressure remains slightly negative, thus maintaining patency of the intrathoracic trachea and bronchi (see Fig. 12-10, B). During inspiration, a greater negative intrathoracic pressure dilates and stretches the intrathoracic trachea and bronchi.40 The extrathoracic trachea at the thoracic inlet is slightly narrowed by dynamic compression that results from the differential between intratracheal pressure and atmospheric pressure. However, the cartilages of the trachea, along with the muscles and soft tissues of the neck, maintain patency of the airway (see Fig. 12-10, A).
Obstruction of the extrathoracic upper airway that can occur with epiglottitis, laryngotracheobronchitis, or an extrathoracic foreign body alters normal airway dynamics. Inspiration against an obstruction results in more negative intrathoracic pressure, further dilating the intrathoracic airways. Clinically, the net effect is a dynamic collapse of the extrathoracic trachea below the level of the obstruction. This collapse is maximal at the thoracic inlet, where the greatest pressure gradient exists between negative intratracheal and atmospheric pressures. As a result, inspiratory stridor is prominent (see Fig. 12-10, C, and Video 12-1)![]() .38–45 With intrathoracic tracheal obstruction (e.g., foreign body, vascular ring) (see Video 12-1), stridor may occur during both inspiration and expiration.46–49 In lower airway obstruction (e.g., asthma, bronchiolitis), significant intrathoracic tracheal and bronchial collapse may occur as a result of the prolonged expiratory phase and greatly increased positive extraluminal pressure (see Fig. 12-10, D).50 In addition, because the airways in children are very compliant, they may be more susceptible to closure during bronchial smooth muscle contraction (e.g., with reactive airway disease). Preterm and term infants may experience airway closure even during quiet respirations.
.38–45 With intrathoracic tracheal obstruction (e.g., foreign body, vascular ring) (see Video 12-1), stridor may occur during both inspiration and expiration.46–49 In lower airway obstruction (e.g., asthma, bronchiolitis), significant intrathoracic tracheal and bronchial collapse may occur as a result of the prolonged expiratory phase and greatly increased positive extraluminal pressure (see Fig. 12-10, D).50 In addition, because the airways in children are very compliant, they may be more susceptible to closure during bronchial smooth muscle contraction (e.g., with reactive airway disease). Preterm and term infants may experience airway closure even during quiet respirations.
Avoiding dynamic airway collapse is particularly important. The very compliant trachea and bronchi of an infant or child are prone to collapse, particularly at the extremes of transluminal pressures that may occur when a child is crying vigorously. The susceptibility of a child to these dynamic forces on the airway is inversely related to age, with preterm infants being most susceptible and adults being least susceptible.51 For this reason, it is essential that children with airway obstruction remain calm. Skill and understanding are required on the parts of the parents, nursing staff, and physicians. Sedatives and opioids should be used with caution before insertion of an ETT, because they may depress or ablate the life-sustaining voluntary efforts to breathe, resulting in significant morbidity or mortality.
Work of Breathing
Work of breathing (WOB) may be defined as the product of pressure and volume. It may be analyzed by plotting transpulmonary pressure against tidal volume. The WOB per kilogram body weight is similar in infants and adults. However, the oxygen consumption of a full-term neonate (4 to 6 mL/kg/min) is twice that of an adult (2 to 3 mL/kg/min).52 This greater oxygen consumption (and greater carbon dioxide production) in infants accounts in part for their increased respiratory frequency compared with older children. In preterm infants, the oxygen consumption related to breathing is three times that in adults.53
The location of airway resistance within the tracheobronchial tree differs between infants and adults. The nasal passages account for 25% of the total resistance to airflow in a neonate, compared with 60% in an adult.25,54 In infants, most resistance to airflow occurs in the bronchi and small airways. This results from the relatively smaller diameter of the airways and the greater compliance of the supporting structures of the trachea and bronchi.24,55,56 In particular, the chest wall of a neonate is very compliant; the ribs provide less support to maintain negative intrathoracic pressure. This lack of negative intrathoracic pressure combined with the increased compliance of the bronchi can lead to functional airway closure with every breath.57–59 In infants and children, therefore, small-airway resistance accounts for most of the WOB, whereas in adults, the nasal passages provide the major proportion of flow resistance.25,57,58,60–65
In the presence of increased airway resistance or decreased lung compliance, an increased transpulmonary pressure is required to produce a given tidal volume, and therefore the WOB is increased. Any change in the airway that increases the WOB may lead to respiratory failure. Recall that the WOB (resistance to air flow) is inversely proportional to the fourth power of the radius of the lumen during laminar flow (beyond the fifth bronchial division) and proportional to the fifth power of the radius during turbulent flow (upper airway to the fifth bronchial division). Because the diameter of the airways in infants is smaller than in adults, pathologic narrowing of the airways in infants exerts a greater adverse effect on the WOB. Increase in the WOB may also occur with a long ETT of small diameter, an obstructed ETT, or a narrowed airway. All of these situations increase oxygen consumption, which in turn increases oxygen demand.66 The increased oxygen demand is initially addressed by an increase in respiratory rate, but the increased WOB may not be sustainable. The end result may be exhaustion, which leads to respiratory failure (CO2 retention and hypoxemia).
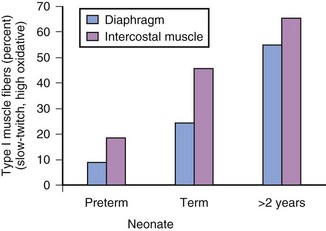
The difference in histology of the diaphragm and intercostal muscles of preterm and full-term infants compared with older children also contributes to increased susceptibility of infants to respiratory fatigue or failure. Type I muscle fibers permit prolonged repetitive movement; for example, long-distance runners through repeated exercise increase the proportion of type I muscle fibers in their legs. The percentage of type I muscle fibers in the diaphragm and intercostal muscles changes with age (preterm infants < full-term infants < 2-year-old children) (Fig. 12-11). Any condition that increases the WOB in neonates and infants may fatigue the respiratory muscles and precipitate respiratory failure more readily than in an adult.67–69
Airway Obstruction during Anesthesia
Airway obstruction during anesthesia or loss of consciousness appears to be most frequently related to loss of muscle tone in the pharyngeal and laryngeal structures rather than apposition of the tongue to the posterior pharyngeal wall.30,31,70,71 The progressive loss of tone with deepening anesthesia results in progressive airway obstruction primarily at the level of the soft palate and the epiglottis.30,31,34,70,72,73 In children, the pharyngeal airway space decreases in a dose-dependent manner with increasing concentrations of both sevoflurane and propofol anesthesia.74–76 This reduction in pharyngeal space has been observed mainly in the anteroposterior dimension. As the depth of propofol anesthesia in children increases, upper airway narrowing occurs throughout the entire upper airway but is most pronounced in the hypopharynx at the level of the epiglottis. Extension of the head at the atlantooccipital joint with anterior displacement of the cervical spine (sniffing position) improves hypopharyngeal airway patency but does not necessarily change the position of the tongue. This observation supports the concept that upper airway obstruction is not primarily caused by changes in tongue position but rather by collapse of the pharyngeal structures.32–34
Pharyngeal airway obstruction also occurs during obstructive sleep apnea in infants and adults.29,77 The sniffing position increases the cross-sectional area and decreases the closing pressure of both the retropalatal and the retroglossal space in anesthetized adults with obstructive sleep apnea.78 The application of continuous positive airway pressure (CPAP) is a common method to overcome such airway obstruction (see Figs. 31-6 and 31-7). During propofol anesthesia in children, CPAP works primarily by increasing the transverse dimension of the airway.75 This occurs despite the fact that anesthesia obstructs the airway mostly by narrowing the anteroposterior dimension. Chin lift and jaw thrust also improve airway patency in anesthetized children with adenotonsillar hypertrophy.79–81 Lateral positioning (also known as the “recovery position”) dramatically enhances the effects of these airway maneuvers80,81; lateral positioning alone improves airway dimensions.6 Compared with chin lift and CPAP, the jaw thrust maneuver is known to be the most effective means to improve airway patency and ventilation in children undergoing adenoidectomy.79 (See Video 4-3, A and B.)![]()
Evaluation of the Airway
A history and physical examination with specific reference to the airway should be performed in all children who require sedation or anesthesia. In particular, a history of a congenital syndrome or physical findings of a congenital anomaly (e.g., microtia, which has been associated with difficult laryngoscopy82) should alert the practitioner to the possibility of difficulties with airway management. In special situations, radiologic and laboratory studies are required to further evaluate and clarify a disorder revealed by the history and physical examination. Although many methods exist for evaluating and predicting the difficult airway (DA) in adults,83–87 no published studies have assessed the use of any of these techniques in children.88,89 Routine evaluation of the airway in all children, followed by correlation with any airway problems occurring during anesthetic management, helps the practitioner to develop experience. This experience then may be used to identify in the future children who might have airway difficulties during or after anesthesia.
Clinical Evaluation
 Presence of an upper respiratory tract infection (predisposition to coughing, laryngospasm, bronchospasm, and desaturation during anesthesia or to postintubation subglottic edema or postoperative desaturation)90–94
Presence of an upper respiratory tract infection (predisposition to coughing, laryngospasm, bronchospasm, and desaturation during anesthesia or to postintubation subglottic edema or postoperative desaturation)90–94
 Snoring or noisy breathing (adenoidal hypertrophy, upper airway obstruction, obstructive sleep apnea, pulmonary hypertension)
Snoring or noisy breathing (adenoidal hypertrophy, upper airway obstruction, obstructive sleep apnea, pulmonary hypertension)
 Presence and nature of cough (“croupy” cough may indicate subglottic stenosis or previous tracheoesophageal fistula repair; productive cough may indicate bronchitis or pneumonia; chronicity affects the differential diagnosis [e.g., the sudden onset of a persistent cough may indicate foreign-body aspiration])
Presence and nature of cough (“croupy” cough may indicate subglottic stenosis or previous tracheoesophageal fistula repair; productive cough may indicate bronchitis or pneumonia; chronicity affects the differential diagnosis [e.g., the sudden onset of a persistent cough may indicate foreign-body aspiration])
 Past episodes of croup (postintubation croup, subglottic stenosis)
Past episodes of croup (postintubation croup, subglottic stenosis)
 Inspiratory stridor, usually high pitched (subglottic narrowing [see Video 12-1], laryngomalacia [see Video 12-1], macroglossia, laryngeal web [Video 12-2]
Inspiratory stridor, usually high pitched (subglottic narrowing [see Video 12-1], laryngomalacia [see Video 12-1], macroglossia, laryngeal web [Video 12-2]![]() , extrathoracic foreign body or extrathoracic tracheal compression)
, extrathoracic foreign body or extrathoracic tracheal compression)
 Hoarse voice (laryngitis, vocal cord palsy, papillomatosis [see Video 12-1], granuloma [see Video 12-1])
Hoarse voice (laryngitis, vocal cord palsy, papillomatosis [see Video 12-1], granuloma [see Video 12-1])
 Asthma and bronchodilator therapy (bronchospasm)
Asthma and bronchodilator therapy (bronchospasm)
 Repeated pneumonias (incompetent larynx with aspiration, gastroesophageal reflux, cystic fibrosis, bronchiectasis, residual tracheoesophageal fistula, pulmonary sequestration, immune suppression, congenital heart disease)
Repeated pneumonias (incompetent larynx with aspiration, gastroesophageal reflux, cystic fibrosis, bronchiectasis, residual tracheoesophageal fistula, pulmonary sequestration, immune suppression, congenital heart disease)
 History of foreign-body aspiration (increased airway reactivity, airway obstruction, impaired neurologic function)
History of foreign-body aspiration (increased airway reactivity, airway obstruction, impaired neurologic function)
 History of aspiration (laryngeal edema [Video 12-3]
History of aspiration (laryngeal edema [Video 12-3]![]() , laryngeal cleft
, laryngeal cleft
 Previous anesthetic problems, particularly related to the airway (difficult intubation, difficulty with mask ventilation, failed or problematic extubation)
Previous anesthetic problems, particularly related to the airway (difficult intubation, difficulty with mask ventilation, failed or problematic extubation)
 Atopy, allergy (increased airway reactivity)
Atopy, allergy (increased airway reactivity)
 History of smoking by primary caregivers (increased airway resistance, increased propensity to desaturation)95
History of smoking by primary caregivers (increased airway resistance, increased propensity to desaturation)95
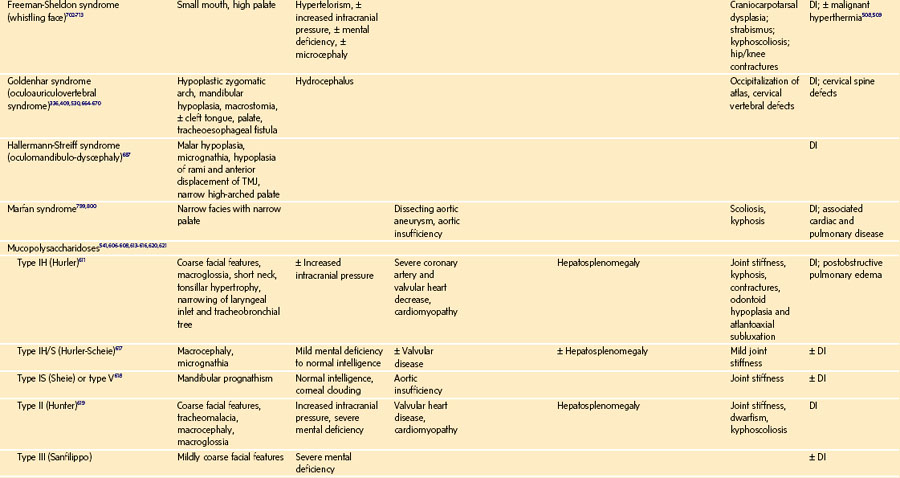
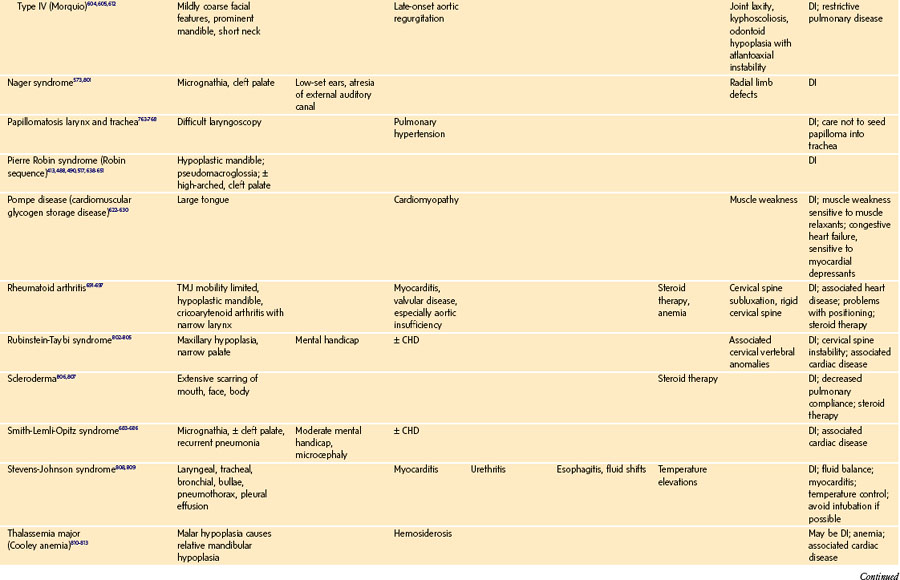
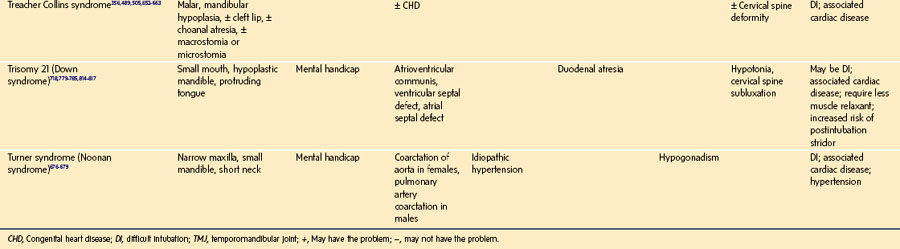
 History of a congenital syndrome (many are associated with DA management)
History of a congenital syndrome (many are associated with DA management)
The physical examination should include the following observations:
 Presence or absence of nasal flaring
Presence or absence of nasal flaring
 Presence or absence of mouth breathing
Presence or absence of mouth breathing
 Presence or absence of retractions (suprasternal, intercostal, subcostal [see Video 12-1])
Presence or absence of retractions (suprasternal, intercostal, subcostal [see Video 12-1])
 Presence or absence of voice change
Presence or absence of voice change
 Size of tongue and its relationship to other pharyngeal structures (Mallampati Score)
Size of tongue and its relationship to other pharyngeal structures (Mallampati Score)
 Loose or missing teeth (see Fig. 12-12, B)
Loose or missing teeth (see Fig. 12-12, B)
 Size and configuration of palate
Size and configuration of palate
 Size and configuration of mandible
Size and configuration of mandible
 Location of larynx in relation to the mandible (see Fig. 12-12, C)
Location of larynx in relation to the mandible (see Fig. 12-12, C)
 Presence of stridor and, if present:
Presence of stridor and, if present:
 Is stridor predominantly inspiratory, suggesting an upper airway (extrathoracic) lesion (epiglottitis, croup, extrathoracic foreign body)?
Is stridor predominantly inspiratory, suggesting an upper airway (extrathoracic) lesion (epiglottitis, croup, extrathoracic foreign body)? Is stridor both inspiratory and expiratory, suggesting an intrathoracic lesion (aspirated foreign body, vascular ring, or large esophageal foreign body)? (see Video 12-1)
Is stridor both inspiratory and expiratory, suggesting an intrathoracic lesion (aspirated foreign body, vascular ring, or large esophageal foreign body)? (see Video 12-1) Is the expiratory phase prolonged or stridor predominantly expiratory, suggesting lower airway disease?
Is the expiratory phase prolonged or stridor predominantly expiratory, suggesting lower airway disease? Baseline oxygen saturation in room air
Baseline oxygen saturation in room air
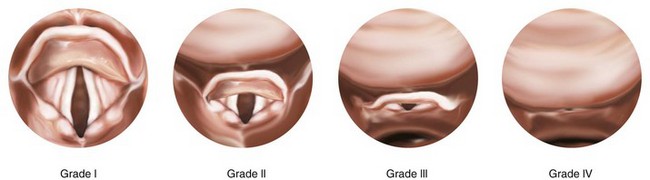
 Microtia: Bilateral but not unilateral microtia is associated with difficulty in visualizing the laryngeal inlet (grade 3 or 4 in the Cormack-Lehane classification, see Fig. 12-22).82 Five (42%) of 12 children with bilateral microtia were found to have a difficult laryngeal view, compared with 2 (2.5%) of 81 children with unilateral microtia and 0 of 93 children without microtia.82 Microtia may represent a mild form of hemifacial microsomia and its associated mandibular hypoplasia. The advantage of understanding this association is that ear deformity is often a more easily recognized clinical finding than mandibular hypoplasia.
Microtia: Bilateral but not unilateral microtia is associated with difficulty in visualizing the laryngeal inlet (grade 3 or 4 in the Cormack-Lehane classification, see Fig. 12-22).82 Five (42%) of 12 children with bilateral microtia were found to have a difficult laryngeal view, compared with 2 (2.5%) of 81 children with unilateral microtia and 0 of 93 children without microtia.82 Microtia may represent a mild form of hemifacial microsomia and its associated mandibular hypoplasia. The advantage of understanding this association is that ear deformity is often a more easily recognized clinical finding than mandibular hypoplasia.
 Global appearance: Are there congenital anomalies that may fit a recognizable syndrome? The finding of one anomaly mandates a search for others. If a congenital syndrome is diagnosed, specific anesthetic implications must be considered (see E-Appendix 12-1, which is available online).
Global appearance: Are there congenital anomalies that may fit a recognizable syndrome? The finding of one anomaly mandates a search for others. If a congenital syndrome is diagnosed, specific anesthetic implications must be considered (see E-Appendix 12-1, which is available online).
Diagnostic Testing
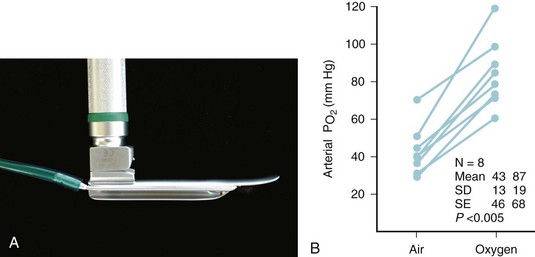
Routine evaluation of the airway usually requires only a careful history and physical examination. In the presence of airway pathology, however, laboratory and radiologic evaluation can be extremely valuable. Radiographs of the upper airway (anteroposterior and lateral films and fluoroscopy) may provide evidence about the site and cause of airway obstruction. When necessary, MRI and CT provide more detailed information.96–112 Radiologic airway examination in a child with a compromised airway may be undertaken only if there is no immediate threat to the child’s safety and only in the presence of skilled and appropriately equipped personnel able to manage the airway. Securing the airway through tracheal intubation must not be postponed in order to obtain a radiologic diagnosis when the child has severely compromised air exchange. Blood gas analysis is occasionally of value in assessing the degree of physiologic compromise, especially with chronic airway obstruction and compensated respiratory acidosis. Performing an arterial (or venous) puncture for blood gas analysis, although providing helpful information, is often upsetting to the child and may risk aggravation of the underlying airway obstruction through dynamic airway collapse. Candidates for blood gas analysis must be carefully selected and the procedure skillfully performed.
Airway Management: The Normal Airway
Mask Ventilation
Admonitions against extreme positions of the infant’s head during bag-and-mask ventilation are intended to minimize the risk of stretching and thus narrowing and obstructing the very compliant infant trachea. However, a study of 18 healthy, full-term infants younger than 4 months of age showed that the tracheal dimensions did not change when the head position changed.113 Therefore, stretching of the trachea may not result in narrowing of the tracheal lumen in otherwise healthy infants (Video 12-4)![]() . However, this study did not examine the effects of these head positions on the supraglottic airway or in the preterm infant. It is possible that these maneuvers (head extension) could result in supraglottic airway obstruction in some children.
. However, this study did not examine the effects of these head positions on the supraglottic airway or in the preterm infant. It is possible that these maneuvers (head extension) could result in supraglottic airway obstruction in some children.
Oropharyngeal Airways
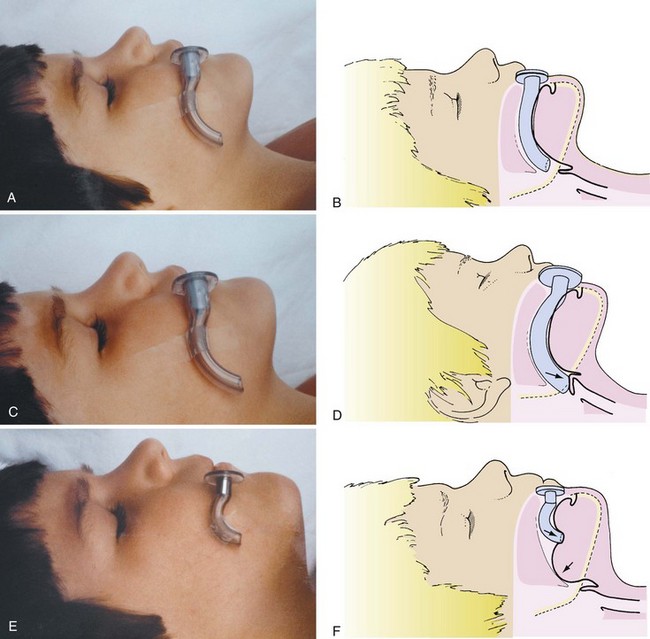
An infant’s tongue may obstruct the airway during induction of anesthesia or loss of consciousness. An oropharyngeal airway of appropriate size (or a supraglottic airway [SGA] such as the laryngeal mask airway [LMA; LMA North America, San Diego]) may be inserted to relieve the obstruction. By holding the oral airway as shown in Figure 12-13, one can estimate the appropriate size for the child; airways one size larger and one size smaller should be readily available as well. A tongue depressor may be inserted over the tongue to facilitate insertion of the oral airway by preventing downfolding of the tongue, which could impair venous and lymphatic drainage, causing tongue swelling and airway obstruction. If the airway device is too long, it may push the epiglottis into the glottic aperture, creating an additional site of airway obstruction or causing traumatic epiglottitis, or the tip may impinge on the uvula, causing uvular swelling and airway obstruction (see Fig. 12-13, C, D).114,115 If the airway device is too short, it may rest against the base of the tongue, forcing it posteriorly against the roof of the mouth and further aggravating airway obstruction (Fig. 12-13, E, F). Oral airways should not be considered panaceas for upper airway obstruction. Care must be taken to avoid trauma to the lips and tongue, which may be caught between the teeth and the flange of the airway. An oral airway is also used to protect an ETT from compression by the child’s teeth, and it serves to separate the mandible and maxilla to facilitate oropharyngeal suctioning.
Tracheal Intubation
Technique
As previously discussed, because of differences in anatomy, there are differences in techniques for intubating the trachea of infants and children compared with adults.1–4,20–22,101,116,117 Because of the smaller dimensions of the pediatric airway, there is increased risk of obstruction with trauma to the airway structures. A technique to be avoided is that in which the blade is advanced into the esophagus with laryngeal visualization achieved during withdrawal of the blade. This maneuver may result in laryngeal trauma when the tip of the blade scrapes the arytenoids and aryepiglottic folds.
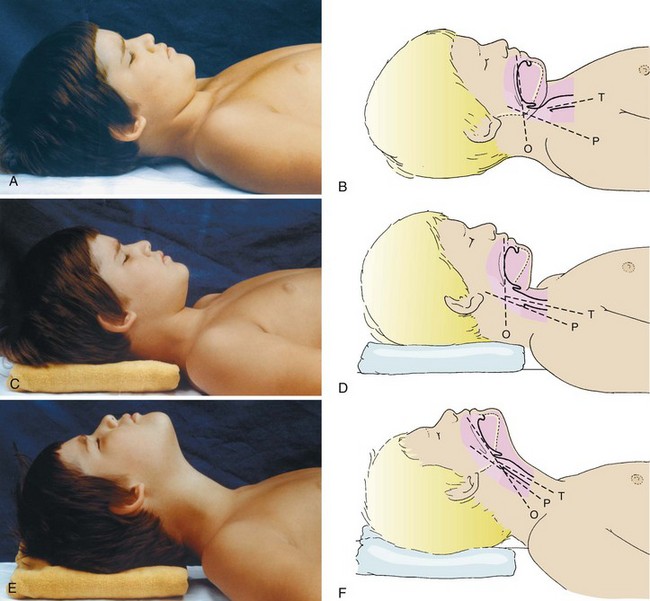
Optimal positioning for laryngoscopy changes with age. The trachea of older children (≥6 years) and adults is most easily exposed when a folded blanket or pillow is placed beneath the occiput of the head (5 to 10 cm elevation), displacing the cervical spine anteriorly.118 Extension of the head at the atlantooccipital joint produces the classic “sniffing” position.101,119,120 These movements align three axes: those of the mouth or oral (O), pharynx (P), and trachea (T). Once aligned, these three axes permit direct visualization of laryngeal structures. They also result in improved hypopharyngeal patency.32,34,70,78,119,120 Figure 12-14 demonstrates maneuvers for positioning the head during airway management. In infants and younger children, it is usually unnecessary to elevate the head because the occiput is large in proportion to the trunk, resulting in adequate anterior displacement of the cervical spine; head extension at the atlantooccipital joint alone aligns the airway axes. If the occiput is displaced excessively, exposure of the glottis may actually be hindered. In neonates, it is helpful for an assistant to hold the patient’s shoulders flat on the operating room table with the head slightly extended. Some practitioners have adopted the practice of placing a rolled towel under the shoulders of neonates to facilitate tracheal intubation. This technique may be disadvantageous if the laryngoscopist stands but may be advantageous if he or she is seated.
The validity of the three-axis theory (alignment of the O, P, and T) to describe the optimal intubating position in adults has been challenged.121–124 Some authors question the notion that elevation of the occiput improves conditions for visualization of the laryngeal inlet based on evidence from both MRI and clinical investigations.121,123 However, one MRI study in children with an LMA in place found that slight head extension improved the alignment of the glottic and pharyngeal axes but worsened the alignment of the pharyngeal and laryngeal axes.125 In a study of adults, neck extension alone was adequate for visualization of the larynx in most patients, but for obese patients and those with limited neck extension, an optimal intubating position was not determined.121 Others favor the sniffing position but with varying support for the three-axis theory.126–132 Even if the tracheas of only a few patients are intubated more easily when placed in the sniffing position compared with simple head extension, the current routine application of the sniffing position appears to be the best clinical practice.
Laryngoscopy can be performed while the child is awake, anesthetized and breathing spontaneously, or anesthetized and paralyzed. Most tracheal intubations in children who are awake are performed in neonates, an approach that is not usually feasible or humane in older awake and uncooperative children. Awake intubation in the neonate is generally well tolerated if it is performed smoothly and rapidly; however, an international consensus group and others have cautioned against this practice unless intravenous (IV) access is not available or there is a life-threatening situation.133–136 Data suggest that preterm and term infants are better managed with sedation and paralysis to minimize adverse hemodynamic responses.137–141
Selection of Laryngoscope Blade
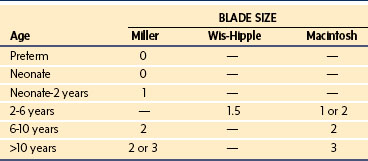
A straight blade is generally more suitable for use in infants and young children than a curved blade because it better elevates the base of the tongue to expose the glottic opening. Curved blades are satisfactory in older children. The blade size chosen depends on the age and body mass of the child and the preference of the anesthesiologist. Table 12-1 presents the ranges commonly used.
Endotracheal Tubes
Since 1967, all materials used in the manufacture of ETTs have been subjected to rabbit muscle implantation testing in accordance with the standards promulgated by the Z79 Committee.141a If the material causes an inflammatory response in the rabbits, it cannot be used in the manufacture of ETTs. This has resulted in the elimination of organometallic constituents, which were used in the manufacture of red rubber ETTs.
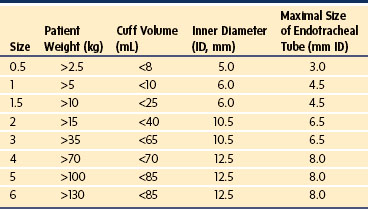
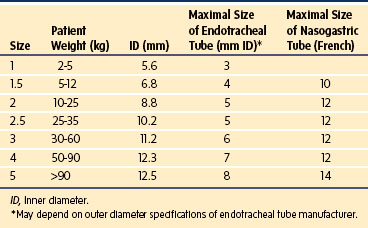
Selection of the proper size of an ETT depends on the individual child.142 The only size requirement for a manufacturer is a standardized inner diameter (ID). The external (outer) diameter (OD) varies among manufacturers, depending on the material from which the ETT is constructed. This diversity in OD mandates checking for proper ETT size and leakage around the tube. An appropriately sized uncuffed ETT may be approximated according to the child’s age and weight (Table 12-2).143
TABLE 12-2 Endotracheal Tubes (ETTs) Used in Infants and Children
| Age | Size (mm ID) Uncuffed | Size (mm ID) Cuffed |
|---|---|---|
| Preterm | ||
| 1000 g | 2.5 | |
| 1000-2500 g | 3.0 | |
| Neonate-6 months | 3.0-3.5 | 3.0-3.5* |
| 6 month-1 year | 3.5-4.0 | 3.0-4.0 |
| 1-2 years | 4.0-5.0 | 3.5-4.5 |
| >2 years | (age in years + 16)/4 | (age in years/4) +3 |
ID, Inner diameter.
*In some neonates, a cuffed ETT may not have a leak below 30 cm H2O, and therefore an uncuffed ETT may be more appropriate.
ETTs one-half mm ID greater or less than the anticipated size should be available because of variability in the size of the airway. Use of the diameter of the terminal phalanx of either the second or fifth digit is unreliable.144 Children with Down syndrome often require an ETT with a diameter smaller than anticipated.145 After tracheal intubation and stable cardiorespiratory indices are obtained, a sustained inflation to 20 to 25 cm H2O (short-term intubation perhaps as high as 35 cm H2O) should be applied to detect an audible or auscultatory air leak over the glottis. If no leak is detected, the ETT should be exchanged for one with an ID 0.5 mm smaller. An air leak at this pressure is recommended because it is believed to approximate the capillary pressure of the adult tracheal mucosa. If lateral wall pressure exceeds this amount, ischemic damage to the subglottic mucosa may occur.146 Be aware, however, that if the trachea has been intubated without muscle relaxants, laryngospasm around the ETT may prevent any gas leak and mimic a tight-fitting ETT.147 If such a situation is suspected, the anesthetic depth should be increased, and an air leak may become evident. Changes in head position may also increase or decrease the leak.147 These maneuvers are important for making the occasional diagnosis of unrecognized subglottic stenosis (see Fig. 36-3, and Videos 12-1 and 12-5![]() ).
).
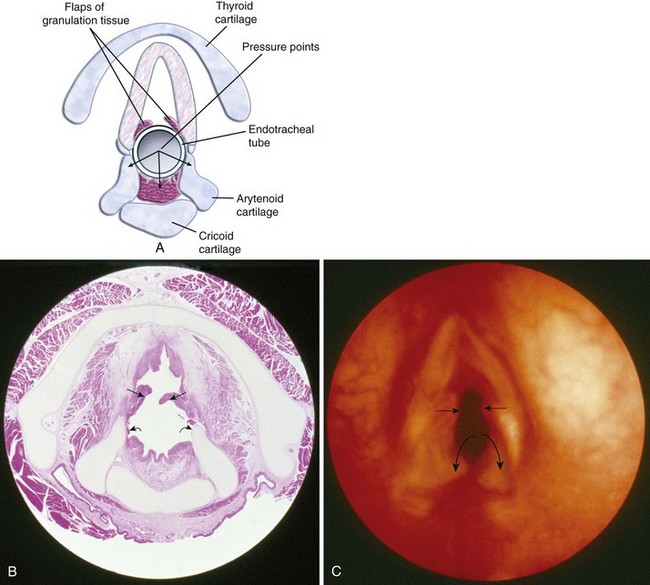
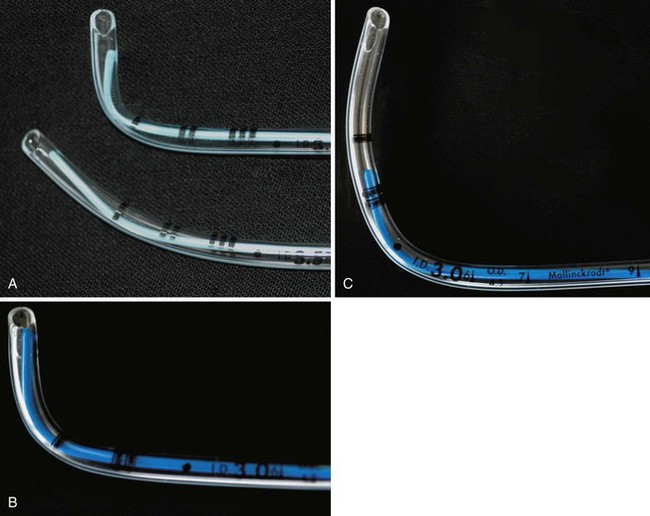
Traditional teaching has advocated the use of uncuffed ETTs for children younger than 8 years, because an uncuffed ETT with an air leak exerts minimal pressure on the internal surface of the cricoid cartilage and thus poses potentially less risk for postextubation edema (croup).143,146,148 An uncuffed ETT also allows insertion of a tube with a larger ID, resulting in less airway resistance, although this holds relevance only for a spontaneously breathing child.149 However, more recent clinical data and clinical practice have challenged these assumptions150–158; a number of studies demonstrated no differences in the incidence of postextubation complications after cuffed and uncuffed tubes.150,155 Cited advantages of cuffed ETTs include decreased numbers of laryngoscopies and intubations to determine the appropriate size for the ETT, reduced subglottic pressure, reduced operating room pollution and costs of anesthetic agents, decreased risk of aspiration, accurate control of carbon dioxide tension (Pco2), better ability to accurately measure the sophisticated physiologic respiratory functions of modern ventilators, absolute ability to deliver increased airway pressures in children with restrictive lung disease, the ability to control cuff pressure, and no increased risk of postextubation stridor.150–156,159–161
A drawback of cuffed tubes is the greater variability in functional OD compared with uncuffed tubes because of differences in cuff shape, size, and inflation characteristics.162 In general, if a cuffed ETT is inserted, an ETT with a smaller ID should be selected to compensate for the ETT cuff. One study found a 99% rate of appropriate cuffed tube size selection for full-term infants through children 8 years of age using the following formula155:
To overcome the shortcomings of the many pediatric cuffed tubes, the MICROCUFF ETT (MICROCUFF, PET, I-MPEDC; MICROCUFF GmbH, Weinheim, Germany, distributed by Kimberly-Clark USA) was designed with a high volume/low pressure cuff that is more distally placed along the shaft of the ETT to better accommodate the anatomy of the airway in infants and children (Fig. 12-15).163 The ultrathin polyurethane cuff (10 µm) allows tracheal sealing at low pressures and provides a uniform and complete surface contact with minimal formation of cuff folds.157,159,163–166 At 20 cm H2O inflation pressure, the cuffs have a cross-sectional cuff area of approximately150% of the maximal internal tracheal cross-sectional area. Uninflated, the cuff adds only a minimal amount to the OD of the ETT. Shortened cuffs and the elimination of a Murphy eye allow a more distal position of the cuff, thereby theoretically reducing the risk of pressure being applied to the cricoid ring and adjacent mucosa.167 The location of the cuff on the shaft of the tube helps to ensure cuff placement below the subglottis, perhaps with the advantage of less risk for endobronchial intubation or intralaryngeal cuff position. An anatomically based depth mark on the surface of the tube helps to guide correct placement.
An investigation of this specially designed ETT for children used the following guidelines to select cuffed ETT sizes159:
Use of these formulas resulted in the need to reintubate to change tube size in 1.6% of children (6/500).159 The incidence of postintubation croup was 0.4% (2/500 children). In a randomized, multicenter study in 2246 young children (mean age, 1.9 years), investigators reported a similar findings for cuffed (MICROCUFF) and uncuffed tubes: postextubation stridor, 4.4% and 4.7%, respectively, and ETT exchange, 2.1% and 30.8%.161 However, in a cost/benefit analysis of the MICROCUFF tube (three to six times that of standard ETTs), the reduction in anesthetic cost offset the cost of the MICROCUFF ETT.160
As a rule, if a cuffed ETT is chosen, the cuff should be inflated to the minimal pressure that seals the air leak; with the MICROCUFF ETT, this appears to be approximately 10.6 cm H2O.159,161,168,169 This air leak must be reevaluated during the anesthetic procedure if nitrous oxide is used, because the gas may diffuse into the cuff, producing excessive tracheal mucosal pressure.168–170 In fact, the MICROCUFF ultrathin polyurethane ETT cuff has greater permeability for nitrous oxide than conventional polyvinyl chloride cuffs and therefore may increase the cuff pressure more rapidly than other cuffed tubes. However, with the cuff of the MICROCUFF ETT sealing the air leak at approximately 10 cm H2O in children, the time interval to reach 25 cm H2O cuff pressure was greater than with a conventional cuffed ETT.171 Routinely checking cuff pressure or filling the cuff with nitrous oxide is recommended.172 A pressure relief valve that can be connected to the pilot balloon of a cuffed ETT to limit cuff pressures to 20 cm H2O when nitrous oxide is used has been described.173
Endotracheal Tube Insertion Distance
The length of the trachea (vocal cords to carina) in neonates and children up to 1 year of age varies from 5 to 9 cm.40 In most infants 3 months to 1 year of age, if the 10-cm mark of the ETT is placed at the alveolar ridge, the tip of the tube rests above the carina. In preterm and full-term infants, the distance is less. In children 2 years old, 12 cm is usually appropriate. An easy way to remember these lengths is 10 for a newborn, 11 for a 1-year old, and 12 for a 2-year old. After 2 years of age, the correct length of insertion (in centimeters) for oral intubation may be approximated by formulas based on age or weight (Table 12-3)174–177:
TABLE 12-3 Distance for Insertion of an Oral Endotracheal Tube by Patient Age
| Age | Approximate Distance of Insertion (cm) Even with Alveolar Ridge |
|---|---|
| Preterm <1000 g | 6-7 |
| Preterm 1000-2000 g | 7-9 |
| Term newborn | 9-10 |
| 1 year | 11-12 |
| 2 years | 12-13 |
| 6 years | 15-16 |
| 10 years | 17-18 |
| 16 years | 18-20 |
| 20 years | 20-22 |
Some practitioners suggest using anatomic markers to choose the appropriate depth for the tube in the trachea in neonates.178,179 An advantage of anatomic measurements is that the infant’s weight may not be available immediately after birth or in sick neonates who present to the emergency department with urgent respiratory or cardiac compromise. One study that used chest radiographs to evaluate final ETT position determined that the length of the foot was as accurate as weight-based formulas to determine the depth of insertion for a nasotracheal tube (44% versus 56% rate of optimal placement, and 83% versus 72% satisfactory placement).179 Alternatively, the nasal-tragus length (the distance from the base of the nasal septum to the tip of the tragus) or the sternal length (the distance from the suprasternal notch to the tip of the xiphoid process) predicted the depth of insertion of the ETT. Either distance plus 1 cm accurately estimated oral ETT tube insertion distance; either distance plus 2 cm accurately estimated nasotracheal tube insertion distance.178 Both measurements compared favorably with weight-based formulas when tube position was verified by chest radiography.
After the ETT has been inserted and the first strip of adhesive tape has been applied to secure it, one must observe for symmetry of chest expansion and auscultate for equality of breath sounds in the axillae and apices (not on the anterior chest wall). The anterior chest wall in the child is not used to verify tracheal intubation because breath sounds may reverberate across the precordium in small children, precluding the diagnosis of an endobronchial intubation. A CO2 monitor confirms intratracheal positioning but does not confirm that the tip of the ETT is not in an endobronchial position. A capnogram that diminishes during the first few breaths suggests an esophageal intubation. Unexpectedly increased airway pressures, persistent desaturation, and asymmetrical chest wall movement all suggest an endobronchial intubation. Visible humidity on the walls of the ETT during expiration also confirms tracheal placement, but the humidity may not be visible in younger infants. It is also important to auscultate over the stomach and to observe for desaturation or cyanosis. Once satisfactory position is achieved, a second strip of tape ensures secure fixation (Fig. 12-16).
We have observed a number of children whose ETT moved into a main stem bronchus after initial correct position during repositioning for the surgical procedure; this manifested as a slight but persistent decrease in oxygen saturation (e.g., from 100% to a range of 93% to 95%). Several studies have demonstrated that simple flexion or extension of the neck can move the ETT sufficiently to cause an endobronchial intubation or dislodgement of the tube from the trachea, respectively.180–182 When a small but persistent change in oxygen saturation is noted, rather than increase the inspired oxygen concentration (Fio2), one must first investigate the cause and reassess the position of the ETT.183
Complications of Tracheal Intubation
Postintubation Croup
Perioperative postintubation croup (also referred to as postextubation croup) occurs in 0.1% to 1% of children.156,159,184,185 Factors associated with increased risk of croup include an ETT with an OD that is too large for the child’s airway (no leak at >25 cm H2O pressure or resistance at the time of insertion), changes in position during the procedure, a position other than supine, repeated attempts at intubation, traumatic intubation, patient age between 1 and 4 years, duration of surgery greater than 1 hour, coughing on the ETT, and previous history of croup.171,172 Concurrent upper respiratory infection has been variously reported as a risk factor and as unrelated.90,185
Treatment of postintubation croup consists of nebulized epinephrine and dexamethasone. The rationale for this treatment is based primarily on experience with the treatment of infectious croup.186–195 Caution should be exercised when translating treatments from one type of croup to another, because the two types of croup are not identical processes, and efficacy of the interventions for the treatment of postintubation croup has not been proved in controlled trials. Studies that examined the effect of dexamethasone given before extubation in children with prolonged intubation are conflicting.196–199 Methylprednisolone given intramuscularly for the same indication has been reported to reduce postintubation stridor.200
Laryngotracheal (Subglottic) Stenosis
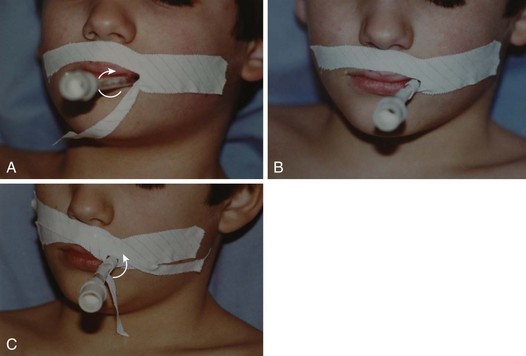
Ninety percent of acquired subglottic stenoses are the result of tracheal intubation, particularly prolonged intubation (see Videos 12-1 and 12-2).201–205 The incidence of subglottic stenosis after prolonged intubation in preterm neonates is reduced because the cricoid cartilage is relatively immature. At this age, the cartilage structure is hypercellular and the matrix has a large fluid content, making the structures more resilient and less susceptible to ischemic injury.206
The pathogenesis of acquired subglottic stenosis is ischemic injury secondary to lateral wall pressure from the ETT. Ischemia results in edema, necrosis, and ulcerations of the mucosa. Secondary infection results in exposure of the cartilage. Within 48 hours, granulation tissue begins to form within these ulcerations. Ultimately, scar tissue forms, resulting in narrowing of the airway (Fig. 12-17).207–209 Specimens obtained from partial cricotracheal resection in children were found to have severe and sclerotic scarring with squamous metaplasia of the epithelium, loss of glands and elastic mantle fibers (tunica elastica), and dilation of the remaining glands with formation of cysts.210 Also, the cricoid cartilage was affected on the internal and external side, with irreversible loss of perichondrium on the inside and resorption by macrophages of cartilage on both sides.194
Factors that predispose to subglottic stenosis include use of an ETT that is too large, laryngeal trauma (e.g., traumatic intubation, chemical or thermal inhalation, external trauma, surgical trauma, gastric reflux),211–213 prolonged intubation (particularly greater than 25 days), repeated intubation, sepsis and infection, chronic illness, and chronic inflammatory disease.204,214,215
Laryngeal Mask Airway
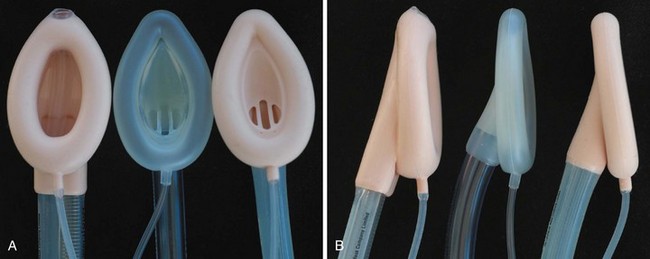
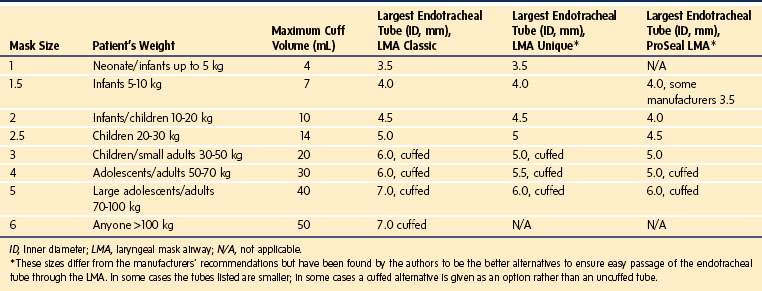
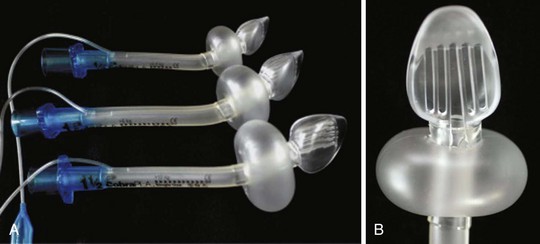
The LMA has become a standard alternative for airway management during general anesthesia.216–221 A variety of types have been introduced into practice since the development of the original LMA, which is now called the LMA Classic (Fig. 12-18). These include the disposable LMA Unique, the ProSeal LMA (PLMA, described later), the Flexible LMA, the LMA Supreme (discussed later), and the intubating LMA Fastrach. The Fastrach is available in sizes 3, 4, and 5 and has been described for use in children who weigh more than 40 kg.222 The LMA Classic is made of medical-grade silicone and consists of a large-bore tubular structure (barrel) that has a 15-mm adapter at its proximal end and an elliptical, mask-like device that fits over the laryngeal inlet at its distal end. All masks are inflated by means of a valved pilot tube and balloon. The LMA Classic and the PLMA can be sterilized for reuse up to 40 times. The LMA Classic is available in eight sizes. Guidelines for selecting the appropriate mask for children are based on weight (Table 12-4). A number of other manufacturers have developed similar devices; however, there is a dearth of comparative data available for children.
The LMA has been used for many different surgeries.223 Some suggest that an LMA can be used for any case in which spontaneous ventilation is appropriate or any case that might reasonably be managed by face mask. Advantages of the LMA over the facemask are that it frees the anesthesiologist’s hands for other tasks and that it may be associated with less operating room pollution compared with mask ventilation.224,225 The use of controlled ventilation with the LMA Classic has also been described.226,227 However, this practice is more controversial than its use in spontaneously breathing children because of the risk of insufflation of ventilated gas into the stomach and resultant regurgitation.228–230 Insufflation of gas into the stomach is more likely if high ventilating pressures are used or required (i.e., pressures greater than the pressure that produces an audible air leak).226,231 Clinically undetected LMA Classic malpositioning has been reported to be a significant risk factor for gastric air insufflation in children between 3 and 11 years of age undergoing positive-pressure ventilation, especially at peak inspiratory pressures greater than 17 cm H2O.232 Controlled ventilation with an LMA (with or without neuromuscular blockade) is easily accomplished when there is a relatively good seal and lung inflating pressures are less than about 17 cm H2O.
The PLMA was designed to improve sealing pressures and to provide a conduit for evacuation of stomach contents; these features make it more appealing for use with positive-pressure ventilation. In sizes 3 and larger, there is a second dorsal cuff to increase the seal pressure of the glottic mask. The dorsal and ventral cuffs communicate, allowing simultaneous inflation by a single pilot balloon. In the smaller sizes, there is no second dorsal cuff but the profile of the mask has been altered to improve sealing. The PLMA is reported to be easy to insert, to allow greater airway pressures with positive-pressure ventilation, and to provide better protection against gastric insufflation.233,234 A number of studies support the efficacy of the PLMA for use in children for both spontaneous and controlled ventilation.234–238 In children, the PLMA is reported to be similar to the LMA Classic in ease of insertion, confirmation of proper position by fiberoptic visualization, and frequency of mucosal trauma. The advantage is that oropharyngeal leak pressure is greater and gastric insufflation is less common with the PLMA.234,236,238 In children, the ability to provide pressure support ventilation with the PLMA during anesthesia also improves gas exchange and reduces WOB compared with the application of CPAP.239,240 The greater sealing pressure may also protect against aspiration, as was reported in a 5-year-old child after inguinal hernia repair.241 Pediatric gastroscopy is reported to be quicker and to involve fewer airway complications when performed around the PLMA compared with nasal cannulas and with a conventional approach utilizing an anesthetic technique in which children breathed a sevoflurane-air-oxygen mixture spontaneously with 1-mg/kg IV boluses of propofol.242
Flexible diagnostic and therapeutic bronchoscopy, radiation therapy, radiologic procedures, ear/nose/throat surgeries, and ophthalmologic procedures are the most commonly described pediatric indications for the LMA.221,223,243–246 An advantage of the LMA for securing the airway in ophthalmologic surgery is that it is associated with no increase in intraocular pressure, in contrast to endotracheal intubation.247 The advantage of the LMA for diagnostic and therapeutic flexible bronchoscopy is that it provides a conduit for oxygenation and ventilation while allowing a larger bronchoscope to be used than can be passed through an age-appropriate ETT.244,245,248,249 It also allows visualization and evaluation of the laryngeal structures. Some LMAs may provide better bronchoscopic conditions than others due to their material or preconfigured shape.250 For children requiring frequent anesthesias over a short period, as in radiation therapy, the LMA provides a secure airway without the trauma of repeated intubation.221 The LMA has also been advocated for use in place of intubation in children who are at increased risk for bronchial airway reactivity (e.g., upper respiratory tract infection, history of reactive airway disease).251–254 However, caution is required in children with an upper respiratory tract infection because the risk of laryngospasm remains substantive.
The LMA has also become an important tool in the management of the DA, particularly in neonates (see later discussion)(Video 12-6)![]() . However, it should be noted that the LMA is an SGA device and, as such, does not reliably protect against pulmonary aspiration of gastric contents.228–230 Because of their gastric access lumens, the PLMA and the LMA Supreme may be better alternatives in this setting, but formal study in children has not been done.255
. However, it should be noted that the LMA is an SGA device and, as such, does not reliably protect against pulmonary aspiration of gastric contents.228–230 Because of their gastric access lumens, the PLMA and the LMA Supreme may be better alternatives in this setting, but formal study in children has not been done.255
The recommended insertion technique for the LMA is the same for children as for adults (see Video 12-6). The correct technique mimics deglutition or swallowing of food.256 The cuff is completely deflated, and the posterior surface of the mask is well lubricated. These actions mimic lubrication of a food bolus with saliva and formation of a soft, flattened, wedge-shaped bolus. The child is placed in the age-appropriate intubating position. Induction may proceed by inhalation of halothane or sevoflurane or by IV propofol (3 to 5 mg/kg).223,257 The nondominant hand is used to extend the head and flex the neck (sniffing position). This head position mimics the elevation of the larynx, neck flexion, and head extension that occurs with swallowing. The LMA is inserted with the mask aperture facing anteriorly (toward the tongue). The index finger of the insertion hand should be placed in the cleft between the mask and the barrel. With the index finger, the LMA is pushed upward and backward, toward the top of the child’s head. This flattens the mask against the palate. Continued backward pressure (toward the top of the child’s head) guides the LMA along the palate and down into the upper esophageal sphincter. It is essential that pressure be applied to force the LMA against the roof of the mouth. The mask is advanced along the palate until some resistance is felt. These actions mimic the propulsion of a food bolus into the hypopharynx caused by tongue pressure, first upward and backward, then downward in an arc. When resistance is felt, air is injected into the mask cuff (see Table 12-4 for size recommendations and maximum recommended inflation volumes).
Several reports claim that when the traditional insertion technique is used in children, the LMA frequently hangs up in the posterior pharynx, making proper positioning difficult.258,259 Therefore, other insertion techniques have been described. The rotational or reverse technique for children has been advocated to be simpler and more successful than the traditional placement technique.260 The LMA is placed in the mouth with the cuff facing the hard palate (the opposite of the traditional technique). It is then advanced and rotated into position simultaneously (Video 12-7)![]() .258,259,261 A partial mask inflation technique has also been advocated as more successful than the traditional (mask deflated) technique.261–264 The LMA is left partially inflated to smooth the edges of the mask and then is inserted in the usual manner,262,263 or in a lateral manner and then rotated and advanced,264 or with a complete 180-degree rotation.261 For placement of the PLMA, the rotational technique was found to have no advantage over the standard technique in children.265 A jaw thrust maneuver and the use of a rigid laryngoscope have also been advocated to assist in placement of the LMA Classic.266
.258,259,261 A partial mask inflation technique has also been advocated as more successful than the traditional (mask deflated) technique.261–264 The LMA is left partially inflated to smooth the edges of the mask and then is inserted in the usual manner,262,263 or in a lateral manner and then rotated and advanced,264 or with a complete 180-degree rotation.261 For placement of the PLMA, the rotational technique was found to have no advantage over the standard technique in children.265 A jaw thrust maneuver and the use of a rigid laryngoscope have also been advocated to assist in placement of the LMA Classic.266
LMA use can result in injuries to upper airway structures267–269 and in damage to the recurrent laryngeal270 or the hypoglossal nerves.271 The incidence of sore throat may be equal to or greater than that seen with tracheal intubation.272–274 LMA use in infants requires special caution. A review of the use of the size 1 LMA in 50 infants found that the LMA sometimes migrated over time, even after apparent correct initial placement; delayed airway obstruction occurred in 12 infants after apparent successful placement.275 Vigilance is required to prevent loss of the airway.
LMA placement has been used successfully for neonatal resuscitation276–278; it may be an easier skill to acquire than bag-and-mask ventilation.279,280 Given the recent recognition280a that chest compressions are the most important factor in successful outcomes after cardiac arrest and the need to avoid interrupting compressions during cardiopulmonary resuscitation, the LMA may assume a greater role in airway management in cardiac arrest in the neonate. The LMA has also been used to deliver surfactant to neonates with respiratory distress syndrome,281 for longer-term intensive care management of neonates with DAs,282–284 and for intrahospital transport of neonates with DAs.285
The LMA Fastrach was specifically designed to allow the blind passage of an ETT in an emergent situation in which direct laryngoscopy is not possible or in patients with cervical spine immobilization.178,286–288 This is a rigid device with a fixed angulation designed primarily for adults (available in sizes 3, 4, and 5). It requires special, flexible ETTs with an ID of 6.0 to 8.0 mm.
The timing for removal of the LMA in children is controversial. Experts have advocated both “awake” and “deep” removal.289–294 Awake removal ensures return of protective reflexes but with the attendant problems of airway reactivity. Deep removal avoids excessive airway reactivity and potential laryngospasm but may increase the risk of aspiration or airway obstruction (or both) as the child emerges from anesthesia later in the recovery room. One author suggested leaving the cuff inflated until the child begins swallowing or is able to open the mouth on command as a means for reducing the potential for laryngospasm. The proposed mechanism is that secretions are swept away from the larynx, reducing the stimulus for larygospasm.294 Lubrication of the cuff with 2% lidocaine jelly or the addition of an intravenously administered opioid to the anesthetic may reduce coughing and laryngeal stimulation on emergence.262
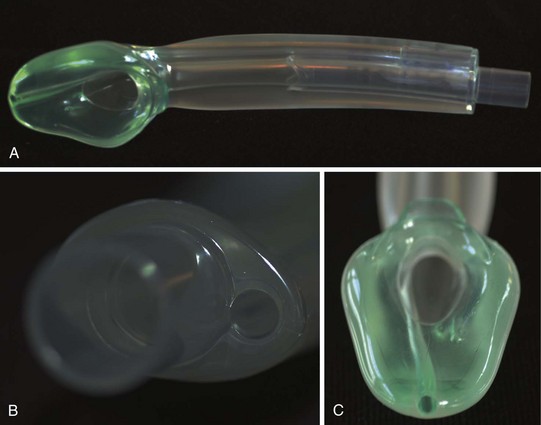
The LMA Supreme is a single-use, curved laryngeal mask with an elliptical airway tube and an integrated drain tube that extends to the tip of the mask bowl. The proximal end of the airway tube consists of a bite block, which should lie between the teeth when the mask is properly positioned. A fixation tab allows the mask to be secured to the face. The deflated mask is held at the fixation tab and is inserted along the palate into the pharynx in a fashion similar to that used for the LMA Classic. Once in place, the cuff is inflated and the mask position is confirmed to be appropriate with the use of simple confirmatory tests (Fig. 12-19). An appropriately positioned mask forms a leak-free seal with the glottis, and the mask tip is embedded in the upper esophageal sphincter. A simple test to confirm the position of the mask is the suprasternal notch test, wherein a small amount of water-soluble lubricant is applied to the drain tube of the airway. Application of slight pressure in the suprasternal notch should result in a slight up-and-down movement of the applied lubricant on the drain tube. This confirms that the drain tube is contiguous with and adequately sealed in the upper esophageal sphincter. The ability to easily place a gastric tube through the drain tube further confirms correct positioning of the airway. Suction should not be applied to the gastric tube until it has been advanced into the stomach; this prevents collapse of the drain tube and potential injury to the upper esophageal sphincter. The LMA Supreme is now available in all pediatric sizes. A study comparing the LMA Supreme with the PLMA and the Classic LMAs in a neonatal manikin model demonstrated higher inflation pressures and shorter insertion times with the LMA Supreme.295 Although the LMA Supreme has been found to be effective in adult populations,296–299 there are currently no human evaluations of the pediatric sizes.
Other Supraglottic Airway Devices
Many other manufacturers have created their own versions of a SGA device similar to the LMA Classic. Some of these devices have design features that make them better conduits for tracheal intubation than the LMA Classic. Some have larger-diameter airway tubes that allow passage of larger (and cuffed) ETTs, lack glottic aperture bars (which can impede ETT advancement during an intubation attempt), and shorter airway tubes. Some devices also offer advantages in terms of cost.300–302 Those SGAs that have a different design and different mechanism for maintaining the airway are discussed next.
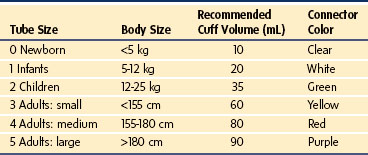
The Laryngeal Tube
The Laryngeal Tube (LT; VBM Medizintechnik GmbH, Sulz, Germany) is designed to secure a patent airway during either spontaneous breathing or controlled ventilation. This device is available with a single lumen for ventilation only or with a double-lumen tube that also allows suction of gastric contents. This system seals the esophagus at the distal end with a small cuff attached at the tip (distal cuff), and a larger balloon cuff at the middle part of the tube (proximal cuff) stabilizes the device and blocks the oropharynx and nasopharynx. The two openings that lie between the cuffs are positioned so that the more distal opening faces the glottis. The cuffs are inflated through a single pilot tube and balloon, through which cuff pressure can be monitored. There are three black lines on the tube, near a standard 15-mm connector, that indicate adequate depth of insertion when aligned with the teeth. The nondisposable device is made of silicone (latex free) and is reusable up to 50 times after sterilization in an autoclave. There are four variations: (1) standard single-lumen, reusable (LT); (2) single-lumen, disposable (LT-D); (3) double-lumen with drain tube, reusable (LT-suction II, or LTS II); and (4) double-lumen with drain tube, disposable (LTS-D) (E-Fig. 12-1).303 It is available in six sizes, suitable for neonates up to large adults (Table 12-5).
The LT should be inserted while the child’s head and neck are placed in the sniffing or neutral position. The tip of a well-lubricated LT is placed against the hard palate behind the upper incisors. The device is then slid down the center of the mouth until resistance is felt or the device is almost fully inserted. After connection to the anesthesia circuit, proper placement is confirmed by assessing ease of ventilation. Some adjustment (usually slight withdrawal) may be required to provide optimal ease of ventilation. Care should be taken not to push the tongue backward into the posterior pharynx. Ease of insertion of the standard LT is reported to be comparable to that of the LMA Classic, although the LT may require more readjustments of its position to obtain a clear airway.304,305 The incidence of complications with the two devices appears to be similar.304 The LT may provide a better seal than the Classic LMA.306 Compared with the PLMA, the LT may be less effective and more difficult to insert.307–309 Although the LT-suction device may have similar success to the PLMA,310 there are scant data in children.311–316 An initial report of its use in children ages 2 to 12 years found a successful placement rate of 96% (77/80 children). Complications occurred in two children; one had laryngospasm that resolved with deepening of the anesthetic, and the other complained of mild difficulty with swallowing postoperatively.312 A study comparing the LT with the LMA found it to be less effective for either spontaneous or assisted ventilation and for fiberoptic evaluation of the airway in children younger than 10 years of age.311 A study of 70 children using sizes 0 to 3 reported failure to place the LT in 12% of children. Failures were caused by inability to ventilate, hypoxemia, gastric insufflation, cough, and laryngospasm or stridor, particularly for children weighing less than 10 kg; therefore, the LT was not recommended for children of this size.313 Although the manufacturer states that a flexible fiberoptic bronchoscope (FOB) may be passed through the device, the openings are of insufficient size to permit passage of an ETT.
The Cobra Perilaryngeal Airway
The Cobra Perilaryngeal Airway (CobraPLA; Engineered Medical Systems, Indianapolis, Ind.) is a disposable SGA that is marketed for the same indications as the LMA Classic but creates a seal more cephalad in the hypopharynx using a cylindrical inflatable cuff. The distal end of the device sits over the larynx, but the distal end is not inflatable (E-Fig. 12-2).301,317,318 An initial report comparing the CobraPLA with the LMA Classic found that insertion time, airway adequacy, and number of repositioning attempts were similar. Peak airway sealing pressure was significantly greater with the CobraPLA; the authors concluded that the CobraPLA has better airway sealing capabilities than the LMA Classic.319 A more recent group of investigators studying the CobraPLA in adults raised concerns about both the design and the safety of this device, particularly during controlled ventilation. After studying 29 patients, investigations were suspended and later stopped after two cases of significant pulmonary aspiration occurred in patients while using the CobraPLA.320 The device is available in eight sizes and can be used in infants as small as 2.5 kg (Table 12-6).317 The distal grill has a long center slit that is specifically designed to allow passage of a fiberoptic bronchoscope (FOB) and ETT (the size 0.5 neonate CobraPLA allows easy passage of a 3.5 uncuffed ETT). In a pediatric study, the orientation of the larynx as viewed through the CobraPLA using video was obtained in 45 infants and children. An acceptable view of the airway was obtained in all subjects, but the laryngeal view was almost obstructed or completely obstructed by the folding of the epiglottis over the glottic opening in 77% of children weighing less than 10 kg. (A similar problem was encountered in approximately 80% of children managed with the LMA Classic as well.321) The investigators suggested extra vigilance to prevent airway obstruction in small children. Also, because the grill bars of the CobraPLA were closely apposed to the epiglottis and supraglottic structures in almost all subjects, it was suggested that removal of the device in a deeper plane of anesthesia may minimize laryngeal stimulation. Further study of this device in children, particularly infants, is needed to define its role compared with the LMA.322–324
The i-gel
The i-gel SGA (Intersurgical, Liverpool, N.Y.) consists of a dual-channeled, noninflatable laryngeal mask made from a gel-like thermoplastic elastomer (Fig. 12-20). It has a built-in bite block and is available in sizes 1, 1.5, 2, 3, 4, and 5 (Table 12-7). One channel functions as the airway tube while the second channel exits at the tip of the device and provides gastric access when seated properly. The lubricated posterior surface of the i-gel is inserted along the palate into the posterior pharynx. An observational study in 50 children reported easy insertion in all patients with a mean leak pressure of 25 cm H2O; gastric access was successfully obtained in all children.325 A study that compared the i-gel to the Ambu AuraOnce laryngeal mask (Ambu, Glen Burnie, Md.) showed higher leak pressures and longer insertion times with the i-gel. The authors noted a tendency for the i-gel to slide out and recommended taping to prevent dislodgment intraoperatively.326
Airway Management: The Abnormal Airway
Classifying the Abnormal Pediatric Airway
It is important to recognize circumstances that may cause airway obstruction or difficult laryngoscopy. Conditions that predispose to airway problems may be grouped according to anatomic location and may result from congenital, inflammatory, traumatic, metabolic, or neoplastic disorders. Tables 12-8 and 12-9 list the more common pediatric airway problems according to anatomic location. E-Appendix 12-1 lists the more common pediatric syndromes and associated anesthetic considerations; more complete information may be obtained elsewhere.327–330
TABLE 12-8 Pediatric Airway Pathology Related to Anatomic Site
| Anatomic Site | Etiology | Clinical Condition |
|---|---|---|
| Nasopharynx | Congenital | Choanal atresia, stenosis,26,27,576–578 encephalocele579–584 |
| Traumatic | Foreign body, trauma585–587 | |
| Inflammatory | Adenoidal hypertrophy,585,588 nasal congestion28 | |
| Neoplastic | Teratoma589–591 | |
| Tongue | Congenital | Hemangioma, Down syndrome |
| Traumatic | Burn, laceration, lymphatic/venous obstruction114,115,585,592–597 | |
| Metabolic | Beckwith-Wiedemann syndrome,504,598–603 hypothyroidism, mucopolysaccharidosis,541,604–621 glycogen storage disease,622–630 gangliosidosis,631–634 congenital hypothyroidism | |
| Neoplastic | Cystic hygroma,635–637 cystic teratoma | |
| Mandible/maxilla | Congenital hypoplasia | Pierre Robin syndrome,413,488,490,517,638–651 Treacher Collins syndrome,356,489,505,652–663 Goldenhar syndrome,336,409,530,664–670 Apert syndrome, achondroplasia,492,671–675 Turner syndrome,676–679 Cornelia de Lange syndrome,680–682 Smith-Lemli-Opitz syndrome,683–686 Hallermann-Streiff syndrome,687 Crouzon syndrome688 |
| Traumatic | Fracture,689 neck burn with contractures597,690 | |
| Inflammatory | Juvenile rheumatoid arthritis691–697 | |
| Neoplastic | Tumors, cherubism698,699 | |
| Pharynx/larynx | Congenital | Laryngomalacia (infantile larynx) (see Video 12-1),585,700,701 Freeman-Sheldon syndrome (whistling face),702–713 laryngeal stenosis,585,714 laryngocele,700 laryngeal web,700,715–717 hemangioma718 |
| Traumatic | Dislocated/fractured larynx,585,719–726 foreign body,46,47,585,727–735 inhalation injury (burn),592–595,597,690,736 postintubation edema/granuloma/stenosis,737–747 swelling of uvula,114 soft palate trauma, epidermolysis bullosa748–756 | |
| Inflammatory | Epiglottitis,42–44,757–760 acute tonsillitis,588 peritonsillar abscess,761,762 retropharyngeal abscess, diphtheritic membrane, laryngeal polyposis763–768 | |
| Metabolic | Hypocalcemic laryngospasm39 | |
| Neoplastic | Tumors | |
| Neurologic | Vocal cord paralysis, Arnold-Chiari malformation769–772 | |
| Trachea | Congenital | Vascular ring,48,49 tracheal stenosis or complete tracheal rings (Video 12-8) |
| Inflammatory | Laryngotracheobronchitis (viral),41,44,45,185,775–777 bacterial tracheitis | |
| Neoplastic | Mediastinal tumors: neurofibroma,778 paratracheal nodes (lymphoma) |
TABLE 12-9 Cervical Spine Anomalies*
| Etiology | Clinical Condition |
|---|---|
| Congenital | Down syndrome,779–785 Klippel-Feil malformation,786 Goldenhar syndrome,336,409,530,664–670 torticollis |
| Traumatic | Fracture, subluxation,719–723787 neck burn contracture |
| Inflammatory | Rheumatoid arthritis691–697 |
| Metabolic | Mucopolysaccharidosis (Morquio syndrome)604–619788 |
*Abnormalities of the cervical spine may limit extension and flexion, thereby contributing to the difficulties of airway management; a significant percentage of infants with Down syndrome have atlantoaxial instability.787
Management Principles
For any laryngoscopy, but especially for a DA, the proper array of equipment must always be available. We advocate the creation of a DA cart stocked with equipment useful in the management of the DA for children of all sizes and ages. Suggestions for contents are listed in Table 12-10. The approach to a DA, as described earlier, must include a careful history and physical examination and, when indicated, radiologic evaluation. In the past, lateral neck xerograms have been useful in delineating anatomic aberrations; however, ultrasound, MRI, and CT imaging have replaced this modality.97–99,101–112,120,331–336 In addition to the airway pathology, the pathophysiology of the congenital syndrome or associated disease process must be fully evaluated.
TABLE 12-10 Items to Consider for an Emergency Intubation Cart
Transtracheal jet-ventilation catheters* (VBM)—infant (16 gauge), child (14 gauge), adult (13 gauge)
Emergency Transtracheal Airway Catheter (Cook)
Magill forceps—adult and pediatric sizes
Miller blades—sizes 0, 1, 2, 3, 4
Macintosh blades—sizes 1, 2, 3, 4
Wis-Hipple blades—sizes 1, 1.5
Oxyscope blades—sizes 0, 1 (with oxygen tubing)
Albuterol adapters for metered-dose administration (3)
Intravenous catheters, 10 of each commonly used sizes (24g, 22g, 20g, 18g, 16g)*
Enk Oxygen Flow Modulation set (Cook)
ETT exchange catheters with both Luer-Lok and 15-mm OD adapters—sizes (mm OD) 3, 4, 5, 7
Retrograde catheter with extra guidewire
Extension cord with converter from Hubble to three-prong plug
Other equipment to consider: lighted stylets and optical stylets (see text).
Cook, Cook Critical Care, Bloomington, Ind.; ETT, endotracheal tube; F, French; ID, inner diameter; LMA, laryngeal mask airway, LMA North America, San Diego; OD, outer diameter; VBM, VBM Medizintechnik GmbH, Sulz, Germany.
Modified from Department of Pediatric Anesthesiology. The difficult airway cart. Children’s Memorial Hospital, Chicago.
The safest approach to managing a DA is to formulate a plan that includes several contingencies for failure or loss of the airway and to have skilled help available, especially a surgeon who is experienced in performing pediatric bronchoscopy and tracheostomy. To maximize success and safety, a skilled assistant should help to position the child, facilitate airway management, and observe the monitors and the child’s vital signs. To direct an assistant, there should be clear communication about the airway management plan and specific details about maneuvers needed to facilitate the process. Familiarity with DA algorithms and DA management reviews can help the practitioner formulate a reasonable plan and ensure that no viable management options are missed.337–343
Certain principles apply to the care of any child in whom difficulty with airway management is anticipated. In most circumstances, an awake or mildly sedated approach would be the primary management strategy for the anticipated DA if airway concerns were considered in isolation. However, often the practitioner who is caring for a child is restricted from choosing an awake approach because of difficulty obtaining the child’s cooperation. Assisted spontaneous ventilation during general anesthesia is the preferred technique when abnormal airway anatomy is present and difficulty with patient cooperation is anticipated; it provides adequate oxygenation while the airway is evaluated for the appropriate approach to tracheal intubation. Therefore, the first choice for management of a potential DA, whether the child is sedated or under general anesthesia, is to maintain spontaneous ventilation.338,343 There are two reasons for maintaining spontaneous gas exchange. First, neuromuscular blockade may result in total airway obstruction due to loss of tone of the tongue, pharyngeal and laryngeal muscles, and suspensory ligaments. This obstruction may not be easily alleviated with manual ventilation of the lungs. Neuromuscular blockade should not be used if airway obstruction or the potential for airway obstruction exists.34 Second, if a child is paralyzed, the loss of spontaneous breath sounds eliminates a valuable guide to locating the glottis. For example, in children with craniofacial anomalies or cervical burn contractures, one may be able to visualize only the tip of the epiglottis with standard rigid laryngoscopy. In such cases, if specialized airway management equipment is unavailable, shaping the ETT tip into a 90-degree angle with a stylet (Fig. 12-21), placing the tip behind the epiglottis (or the center of the base of the tongue if the epiglottis is not visible), and then listening for breath sounds with the ear near the proximal end of the ETT often allows the practitioner to “blindly” locate the glottic opening and trachea.
If the child is able to cooperate while mildly sedated, there are several options for airway management. An opioid-benzodiazepine combination will blunt airway reactivity, decrease discomfort, and provide anxiolysis and amnesia. These combinations, particularly fentanyl and midazolam, are effective for sedation of adolescents and mature preteens.344 Dosing is based on weight and is guided by clinical parameters, including preexisting medical conditions. However, benzodiazepine-opioid sedation may not suffice for a frightened young child, because the dose requirement for sedation may exceed the dose that causes apnea. Alternatively, ketamine, which provides both hypnosis and analgesia, may be used alone or in conjunction with midazolam.344 Ketamine usually preserves adequate spontaneous ventilation and upper airway patency345 while preventing laryngeal reactions to airway manipulation. Ketamine and midazolam should be slowly titrated to effect so as to avoid oversedation and apnea.346 Midazolam takes almost 5 minutes to achieve peak electroencephalographic effects, necessitating adequate time between incremental doses (see Fig. 47-12).347,348 Ketamine is usually titrated in doses of 0.25 to 0.5 mg/kg IV every 2 minutes. Although there is a large incidence of psychomimetic emergence reactions in adults, these reactions are less common in children, particularly if ketamine is combined with midazolam. Ketamine may increase secretions that enhance airway reactivity and interfere with video-based airway management. Antisialagogue administration may mitigate these effects. In addition, the anticholinergic effect of atropine or glycopyrrolate will blunt reflex bradycardia that can occur with airway manipulation. Dexmedetomidine may be administered as the sole sedating agent or combined with reduced doses of other sedatives or opioids to be effective for sedation while maintaining spontaneous respiration during fiberoptic intubation in adults and children.349–356
Topical anesthesia may be used in conjunction with sedation or general anesthesia to blunt airway reactivity in those children in whom spontaneous ventilation is preserved. Useful methods for providing topical anesthesia to the airway include (1) nebulized lidocaine; (2) topical application of local anesthetic sprays, jellies, or ointments; (3) translaryngeal delivery of lidocaine (Video 12-9)![]() ; (4) “spray as you go” with lidocaine injected onto the surface of the larynx and vocal cords through the channel of an FOB usually used for suctioning or administering oxygen; and (5) superior laryngeal nerve block.344 Caution is required to avoid delivering a toxic dose of local anesthetic. Maximum doses of the local anesthetic are based on the patient’s weight and should be calculated in advance (see Table 41-2). Lidocaine seems to have the best safety profile; we limit our maximum dose to 5 mg/kg. We do not recommend the use of benzocaine (Cetacaine) local anesthetic spray in children weighing less than 40 kg, because it is associated with methemoglobinemia, and it is difficult to titrate or limit the administered dose.88,357
; (4) “spray as you go” with lidocaine injected onto the surface of the larynx and vocal cords through the channel of an FOB usually used for suctioning or administering oxygen; and (5) superior laryngeal nerve block.344 Caution is required to avoid delivering a toxic dose of local anesthetic. Maximum doses of the local anesthetic are based on the patient’s weight and should be calculated in advance (see Table 41-2). Lidocaine seems to have the best safety profile; we limit our maximum dose to 5 mg/kg. We do not recommend the use of benzocaine (Cetacaine) local anesthetic spray in children weighing less than 40 kg, because it is associated with methemoglobinemia, and it is difficult to titrate or limit the administered dose.88,357
If one is unable to secure tracheal intubation, it is important to recognize the limits of one’s ability. Do not hesitate to seek assistance from a colleague or request the surgeon to perform a tracheostomy or bronchoscopy. As an alternative, the child can be awakened and referred to a major pediatric center. In an urgent, life-threatening situation, placement of an SGA device or percutaneous cricothyroidotomy can be life-saving (see “The Unexpected Difficult Intubation”).218,248,249,358,359
Documentation
1. Whether mask ventilation was attempted, and if so, whether there was any difficulty
2. Special maneuvers that were required for successful mask ventilation
3. Special maneuvers that were not helpful with mask ventilation
4. Any difficulty with tracheal intubation
5. Special techniques that were required for successful intubation
6. Special techniques that were not helpful for intubation
7. Grade of laryngoscopic view of laryngeal structures during direct laryngoscopy (Fig. 12-22)
In addition to discussion with the family and the child (when age appropriate), a letter should be given to the family and child outlining the difficulties with the airway, describing how the airway was managed, and referring them to the MedicAlert Foundation registry.320 This should be copied and circulated to the medical record and to the MedicAlert registry. In the United States, the MedicAlert registry for difficult airway/difficult intubation can be reached by telephone at 1-888-633-4298. Similar registries are also being formed internationally.360 The MedicAlert registration form asks for clinical details about the type of airway difficulty as well as which maneuvers were successful in management and which were not. Any practitioner who provides airway management to the registered patient can update this information at any time. Although scoring systems used in adults83–87 have not been thoroughly investigated in all age groups,88,361 it is useful to describe in detail the view of the larynx that was achieved and how it was achieved (e.g., blade type, size, external laryngeal manipulation).
The Unexpected Difficult Intubation
With careful preoperative evaluation and planning, the unexpected pediatric DA should be a rare occurrence. However, the practitioner should always be prepared for this potentially life-threatening event. Because the unexpected DA occurs after the anesthesia (plan A) has been initiated, many of the management decisions required for the anticipated DA have already been made. Of primary importance is maintaining adequate oxygenation while a definitive course of action is pursued (i.e., plan B, plan C, and so on). A reasonable decision tree, based on the DA algorithm of the American Society of Anesthesiologists (ASA), is presented in Figure 12-23. An important difference between infants and adults should be noted in this scenario. Because infants have an increased metabolic rate and decreased functional residual capacity, the time between the loss of the airway and resultant hypoxemia with potential secondary neurologic injury is significantly diminished compared with adults.362 In a mathematical model, the approximate time to zero oxygen saturation from an Fio2 of 90% is 4 minutes in a 10-kg child, whereas the same process in a healthy 70-kg adult takes almost 10 minutes.360,363
Extubation of the Child with the Difficult Airway
A Cook airway exchange catheter with Rapi-Fit adapter (Cook Critical Care, Bloomington, Ind.) is a hollow plastic guide with holes on its distal end that may be useful as a bridge to extubation because the adapter on its proximal end allows the placement of either a Luer-Lok connector for connection to a jet ventilator or a 15-mm adapter for connection to a standard anesthesia ventilating system (E-Fig. 12-3).364–367 It is available in a variety of sizes to allow the exchange of ETTs with 3.0 mm ID or larger. This can be used for oxygenation and ventilation and as a guide to reinsertion of the ETT if the child’s ventilatory efforts are inadequate or if airway obstruction occurs.368 However, caution is required when using this device for jet ventilation, because significant barotrauma has been reported.369,370
An alternative to jet ventilation is the Enk Oxygen Flow Modulation set (Cook Critical Care), which allows flow from a standard low-pressure flow meter to be adjusted by occluding holes in the delivery system with the thumb and forefinger (E-Fig. 12-4, A). As a potential substitute for the Enk device, one could cut a side hole in the plastic oxygen delivery tubing to create a similar low-pressure oxygen delivery system (see E-Fig. 12-4, B). Pneumothorax, pneumomediastinum, and deaths have occurred when jet ventilation was used with an airway exchange catheter.371 One report suggested that only insufflation or gentle manual ventilation should be used initially and that jet ventilation should be reserved for situations in which these techniques are ineffective. These authors also recommended that the optimal management was reintubation.372
Special Techniques for Ventilation
Multi-handed Mask Ventilation Techniques
Multi-handed mask ventilation techniques can provide an effective temporizing measure until the airway is secured or the child is awakened. One person uses both hands to maintain an adequate mask fit, and a second person compresses the reservoir bag (Fig. 12-24). This can also be accomplished by having a single provider use two hands on the mask while the anesthesia ventilator is activated.373 Occasionally, a second person is required to perform a jaw thrust with one hand while compressing the anesthesia bag with the other. Rarely, a third person may be required to compress the anesthesia bag with two hands (in order to generate a greater peak inflation pressure) while the first person holds the mask with two hands and the second person performs a two-handed jaw thrust.373
Laryngeal Mask Airway
The LMA has revolutionized DA management in children. Numerous case reports and extensive clinical experience attest to the value of the LMA for establishing an airway when both ventilation and intubation are extremely difficult or impossible.340,374–376 The LMA has been described as a tool for use in both the nonemergency pathway (cannot intubate, can ventilate) and the emergency pathway (cannot intubate, cannot ventilate [CICV]) of the ASA DA algorithm.338,340 Use has been described in the awake child (LMA insertion in awake infants with Pierre Robin syndrome [Robin sequence])377 (Video 12-10)![]() and in the anesthetized child with a known or suspected DA. It can be used as the definitive airway in some circumstances, as a conduit for intubation, or as a temporizing airway while other options are pursued (e.g., a surgical airway). There are now many other SGA devices reported to be useful in the management of the child with a DA, but comparative studies in pediatrics are lacking.255,378
and in the anesthetized child with a known or suspected DA. It can be used as the definitive airway in some circumstances, as a conduit for intubation, or as a temporizing airway while other options are pursued (e.g., a surgical airway). There are now many other SGA devices reported to be useful in the management of the child with a DA, but comparative studies in pediatrics are lacking.255,378
Percutaneous Needle Cricothyroidotomy
In 1992, the American Heart Association changed its recommendations for emergency airway management to a percutaneous needle cricothyroidotomy over a surgical cricothyroidotomy because it was believed that the former entails less risk of injury to vital structures such as the carotid arteries or jugular veins, particularly in the hands of nonsurgical trained practitioners. In addition, most practitioners can more rapidly perform the percutaneous procedure. However, the cricothyroid membrane has a relatively small width in infants and children. Attempts at cricothyroidotomy may readily damage cricoid and thyroid cartilages, resulting in laryngeal stenosis and permanent damage to the speech mechanism. Therefore, this procedure should be reserved for use only under emergency circumstances.379,380
Because percutaneous needle cricothyroidotomy is rarely used, it is recommended that experience be gained with patient simulators or in animal models, because success in the hands of the inexperienced is not assured.381,382 A schema of this procedure is presented in Figure 12-25. A commercial product called the Jet-Ventilation-Catheter (VBM) is available in three sizes: 16, 14, and 13 gauge. It consists of a slightly curved puncture needle within a Teflon, kink-resistant cannula nearly identical to an IV catheter (Fig. 12-26, A). This cannula has two lateral eyes at its distal end and a combined Luer-Lok and 15-mm adapter at its proximal end (see Fig. 12-26, B). It also has a fixation flange and foam neck tape to secure the airway.
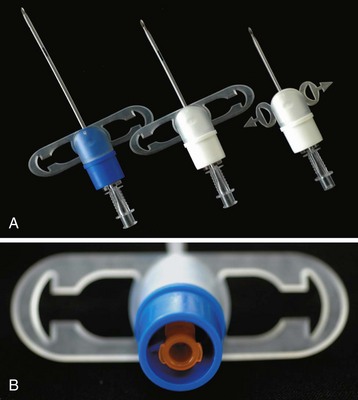
FIGURE 12-26 A, The Jet-Ventilation-Catheter (VBM Medizintechnik GmbH, Sulz, Germany) is available in three sizes: From left to right: 13 gauge (adult), 14 gauge (child), and 16 gauge (infant). It consists of a slightly curved puncture needle within a Teflon, kink-resistant cannula. The procedure for insertion is similar to that described in Figure 12-25. B, This cannula has two lateral eyes at its distal end and a combined Luer-Lok and 15-mm adapter (surrounding the Luer-Lok) at its proximal end, allowing either jet or standard ventilation. It also has a fixation flange and foam neck tape to secure the airway.
Percutaneous needle cricothyroidotomy provides only a means for oxygen insufflation and does not reliably provide adequate ventilation. In the spontaneously breathing patient, simple delivery of intratracheal oxygen (1 to 2 L/min) may be sufficient in the short term, because hypercarbia is generally well tolerated by healthy children.379,383,384 A number of children with arterial CO2 values well above 150 mm Hg have survived neurologically intact when adequate oxygenation was maintained.384 Therefore, simple oxygenation without attempts at ventilation may be all that is required to sustain life (Fig. 12-27). For the child without respiratory effort, there is a need to provide ventilation in addition to oxygenation. An Ambu bag with the pop-off disabled can provide limited ventilation through a percutaneous catheter, but these devices will be ineffective at standard pop-off pressures.379,385 Extremely high ventilating pressures are required, but midtracheal pressures are significantly lower (10 to 16 cm H2O).379 A percutaneous cricothyroidotomy catheter can also be used with a jet ventilation system. Jet ventilation via a catheter passed through a narrow glottic opening has been described.386–389
If upper airway obstruction is present (e.g., after multiple unsuccessful attempts at rigid laryngoscopy), there will be a limited pathway for the egress of air and oxygen, and barotrauma may result from insufflation of oxygen or attempts at ventilation. Very serious morbidity and mortality may result from massive subcutaneous emphysema or tension pneumothorax.390,391 Therefore, jet ventilation must be used with extreme caution in infants and children.392
Another IV catheter–type emergency airway device is the Emergency Transtracheal Airway Catheter (Cook Critical Care), which consists of a 6-F reinforced catheter that is advanced over a 15-gauge needle similar to the devices described earlier (E-Fig. 12-5). One study simulated use of the Enk Oxygen Flow Modulation set with a variety of IV cannulae and the Emergency Transtracheal Airway Catheter. The investigators concluded that the device worked best when all holes on the Enk device were occluded simultaneously and that minimum flow should never be less than 1 L/min. They also suggested that initial fresh gas flow be set to 1 L/min and then adjusted up or down to effect.393 Successful ventilation with uncuffed devices may depend on the patency of the upper airway (i.e., the greater the patency, the less the effectiveness of ventilation in the nonbreathing patient).394 None of these devices has been examined in controlled trials to confirm efficacy, in part because these events are so rare. Therefore, we recommend training on simulators so that each practitioner can determine what device is best in his or her hands.

E-Figure 12-5 Top, A transtracheal kink-resistant catheter is available in a 6-F external diameter (Emergency Transtracheal Airway Catheter; Cook Critical Care, Bloomington, Ind.). The system is similar to a simple intravenous catheter device inserted percutaneously with a syringe (bottom). Aspiration of air confirms tracheal position, and the catheter is advanced as described in Figure 12-25. The catheter can be attached to either a jet ventilation system (top) or the system described in Figure 12-25.
A number of percutaneous emergency airway devices are available that use a short but large-diameter needle or a needle, guidewire, and dilator to aid insertion of a percutaneous airway.381,395–397 The Quicktrach (Rüsch) is a device that consists of a tapered 2- or 4-mm catheter with a fixation flange for securing with cloth tape. A removable plastic stopper is designed to limit the depth of needle insertion. This device requires several steps: puncture of the skin, aspiration for air, removal of the stopper, removal of the needle/syringe, and attachment to standard 22-mm connector. A flexible connector is also provided (E-Fig. 12-6). A rabbit model to simulate infant cricothyroidotomy found success in all attempts but 2 of 10 attempts resulted in fracture of the cricoid cartilage and 1 resulted in damage to the mucosa of the posterior tracheal wall.398 Conversely, this device has been shown to allow more rapid establishment of an airway than other devices that use a Seldinger technique.399,400 A larger, cuffed adult model is now available (E-Fig. 12-7).
E-Figure 12-6 QuickTrach Emergency Cricothyroidotomy Device (Rüsch Inc., Duluth, Ga.) is available in three sizes (A and B, bottom to top): 1-mm neonatal, 2-mm toddler, and 4-mm older child. This device is similar to the devices presented in Figure 12-25, but the diameter of the introducing needle is larger and the final size catheter also has a larger diameter. A white plastic stopper (C) is designed to prevent excessive depth of insertion and is removed after successful entry into the trachea. The fixed curve of the large needle requires a change in angle from 90 degrees with the skin at insertion to 60 degrees with the skin after entry into the trachea. The built-in flange and strap allow rapid fixation. The size of these devices would seem to limit their utility to larger children and adults, and it would appear that some training on a simulator would be advised.
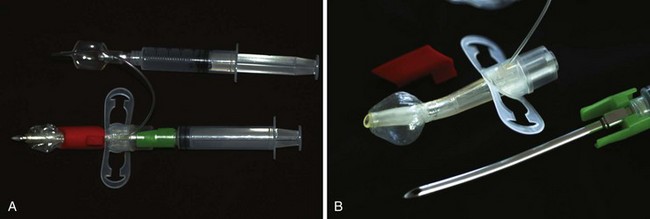
E-Figure 12-7 A and B, QuickTrach Emergency Cricothyrotomy Device for older patients. The device is shown assembled (A) and disassembled (B). The principles of insertion are similar to those described in Figure 12-25, but this device provides a much larger airway with a cuff, allowing better seal. It would appear that this device would only be appropriate for teenagers due to the size of the insertion needle.
Other devices that use the Seldinger technique (i.e., needle, guidewire, scalpel incision of the skin, and passage of a dilator and tracheostomy tube) are the Arndt and Melker devices (Cook Critical Care).401 These devices provide a 3.0-mm ID airway that is sufficient for ventilation as well as oxygenation (E-Fig. 12-8). However, the time required to insert such devices may be longer than for simpler devices and may be inappropriate for immediate rapid establishment of an airway.381,399,400 In contrast, a porcine cadaver study found greater comfort with this technique compared with a scalpel technique.402 These devices are useful for elective percutaneous tracheostomy.403
Another device, Pertrach (Engineered Medical Systems, Indianapolis, Ind.), uses a split needle on a syringe to puncture the cricothyroid membrane (E-Fig. 12-9). A skin incision is made, and an introducer with tracheostomy tube (3.0-mm ID) is directed into the trachea, splitting the needle, which is removed. The introducer is then removed, and the airway is secured with tracheostomy tape. There are no case reports in the literature to determine the ease or difficulty of insertion in children, but the multiple steps required suggest that it may be a device for elective tracheostomy rather than emergent establishment of a surgical airway.
Another percutaneous tracheostomy device is the Bivona Pedia-Trake kit (Smiths Medical, St. Paul) (E-Fig. 12-10). A skin incision is made with a scalpel, and a large needle is introduced with a skin dilator, which in theory opens the incision sufficiently to allow passage of an obturator and a 3.0-, 4.0-, or 5.0-mm ID tracheostomy tube (with or without cuff). This device appears sufficiently complicated so as to not be useful in a CICV emergency; it might be better suited when there is an urgent need to establish surgical access to the airway.
Other devices with limited pediatric use395 are kits designed to place a full-sized tracheostomy tube, such as the Nu-Trake (International Medical Devices, Northridge, Calif.) and Abelson (Gilbert Surgical Instruments, Bellmawr, N.J.) devices. They may potentially cause tracheal or laryngeal injury in small patients because of the relatively large size of the needle; again, little experience in children has been published.396,397,404
Laryngeal Mask Airway versus Percutaneous Needle Cricothyroidotomy and Transtracheal Jet Ventilation
The LMA Classic has proved to be an extremely useful device in airway emergencies. In contrast to percutaneous needle cricothyroidotomy, it is an effective device for ventilation as well as a conduit for intubation. The LMA is easily inserted and requires a relatively low level of skill, as demonstrated in numerous studies comparing this technique with other airway management skills (e.g., mask ventilation, endotracheal intubation). More importantly, in contrast to transtracheal jet ventilation, the complication rate with the LMA Classic is exceedingly low.405 However, this is an SGA device. If glottic or subglottic obstruction to ventilation is present, it will be ineffective, and a surgical airway with or without transtracheal jet ventilation is still the emergency technique of choice. Since the introduction of the LMA, clinical experience suggests that if glottic or subglottic pathology is not suspected, LMA placement to establish ventilation may be appropriately attempted first.340
Surgical Airway
Establishing a surgical airway emergently requires great technical skill. It is difficult to perform quickly, and other methods to provide oxygenation should be pursued first. In nonemergent airway management for children, a tracheostomy is preferred to a cricothyroidotomy because of fewer long-term complications and better results with later decannulation of the airway.381,406
Anterior Commissure Scope and Rigid Ventilating Bronchoscope
Two pieces of equipment used by otolaryngologists that can assist in visualizing the larynx and providing a method of ventilation are the anterior commissure scope and the rigid ventilating bronchoscope. The anterior commissure scope is a rigid, tubular, straight-blade laryngoscope with a light at the tip. The technique to place the anterior commissure scope and the advantages for visualization are similar to those described later for the straight blade used with the retromolar approach.407
Special Techniques for Intubation
Rigid Laryngoscopy
The rigid laryngoscope is the most familiar and most universally available piece of airway equipment; therefore, it is critical for the practitioner to become facile in its use and to know a variety of techniques. Some suggestions are reviewed here. It is reasonable to take a second look with the rigid laryngoscope after an unexpected failed intubation; however, a good rule is to always change something about the approach that may improve visualization. In the past, awake rigid intubation was the traditional approach to the problematic neonatal airway, but this approach should be used only in an extreme emergency or when IV access is not available.133–136 Some anatomic features are completely unfavorable for success with the rigid laryngoscope, regardless of technique. Repeated unsuccessful attempts should be avoided, because this can lead to airway trauma and edema. Because infants and children already have smaller airway structures, they are uniquely susceptible to a rapid progression from “cannot intubate, can ventilate” to the CICV scenario.
Optimal External Laryngeal Manipulation
Pressure can be applied externally to the larynx during the intubation to maximize visualization of the larynx.408 Optimal external laryngeal manipulation (OELM) is particularly helpful for children with immobile or shortened necks and for infants. Either an assistant or the laryngoscopist can perform OELM. When the laryngoscopist performs the maneuver, the assistant either can pass the ETT into the glottis while the laryngoscopist maintains OELM or OELM can be assumed by the assistant to allow the laryngoscopist to pass the ETT.408 OELM may also be used in conjunction with other, more advanced airway devices such as the GlideScope (discussed later).409–411
Intubation Guides
Intubation guides include plastic-coated, flexible metal stylets and the gum elastic bougie. These can be used for blind placement of the ETT under the epiglottis. A flexible stylet is placed inside the ETT and preformed to shape the ETT tip to one that will optimize intubation success (see Fig. 12-21). A hockey-stick configuration is frequently useful, particularly if only the epiglottis or the most posterior portion of the glottis can be visualized. The gum elastic bougie has a preformed, angled tip. It is placed alone and then the ETT is threaded over it and into the trachea (E-Fig. 12-11). When the bougie is successfully placed in the trachea, one can detect the subtle “bumpy” feel of the bougie making contact with the anterior tracheal rings. This device may also be used to facilitate intubation through an LMA or as an adjunct to other airway devices.412–415
Oxyscope
The Oxyscope (Heine Optotechnik, Herrsching, Germany) is a Miller 1 laryngoscope blade with an insufflation channel along its length so that it may be attached to an oxygen source. An Oxyscope provides increased Fio2 and is particularly useful for intubation in spontaneously breathing neonates with high oxygen consumption in whom desaturation may rapidly develop (E-Fig. 12-12).416–418
Dental Mirror
The authors of one report used a short-handled dental mirror (no. 3, Storz Instrument Company, Manchester, England) to assist in the indirect visualization of the larynx of a 10-week-old infant. Laryngoscopy was impossible with a Miller no. 1 blade. The infant was returned to spontaneous ventilation, a Macintosh no. 1 blade was used to expose the pharynx, and the mirror was used to visualize the larynx. A styleted ETT was then passed into the glottis under indirect vision.419
Retromolar, Paraglossal, or Lateral Approach using a Straight Blade
Use of a straight blade in a retromolar approach may allow glottic visualization when the classic rigid intubation technique fails, particularly if the difficulty is secondary to a large tongue or small mandible (Fig. 12-28)407,410,413,420–424 With the child’s head turned slightly to the left, a no. 1 Miller blade is introduced into the extreme right side of the mouth. It is advanced in the space between the tongue and the lateral pharyngeal wall; the tongue is swept completely to the left and is essentially bypassed. It is very helpful to have an assistant pull back the right corner of the mouth with a small retractor (e.g., Senn retractor) to increase the space for ETT placement. The blade is advanced while staying to the right, overlying the bicuspids and lateral incisors until the epiglottis or the glottis is visualized. When the epiglottis comes in view, it is lifted with the blade tip to expose the glottic opening. It is extremely unlikely that pressure from the laryngoscope blade on the bicuspids and lateral incisors could loosen these teeth, because they have double roots or longer roots than the central incisors.
At this point in the laryngoscopy, it may be possible to move the proximal end of the blade toward the midline of the mouth to increase room for ETT placement and manipulation, although care must be taken to avoid applying pressure on the central incisors of the maxilla, lest they become loosened. If the glottis is not visualized, the head can be rotated farther to the left and the blade can be kept lateral to improve visualization. The ETT should be styleted and formed into a 90-degree bend configuration to assist in placement (see Fig. 12-21), particularly if the view of the glottis is only partial. A shorter-length blade (usually a Miller no. 1, even in older children) than that used in the traditional midline approach is chosen because the distance to the glottis with this method is greatly shortened.
Several mechanisms are responsible for the improved view of the glottis with the retromolar approach to laryngoscopy. First, there is a reduced need for soft tissue displacement and compression because the lateral placement of the blade bypasses the tongue. This approach to an improved view of the glottic opening is particularly useful for children with micrognathia.410,413,421,423,424 In such children, the space available to displace the tongue is reduced compared with the traditional midline approach to rigid laryngoscopy. Second, there is an improved line of visualization because the incisors and maxillary structures are bypassed by lateral blade placement and by shifting of the head to the left. Third, the use of a straight blade avoids the possible intrusion of a curved blade into the line of site.407,420–422 Finally, both the angle and the distance from the insertion of the straight blade at the right commissure of the mouth are reduced, facilitating an easier view of the glottic opening, particularly during a difficult intubation, compared with inserting the blade in the midline.
Fiberoptic Laryngoscopy
An advantage of using the FOB for intubation of the child with a DA is that it does not require extensive head or neck manipulation and is therefore useful in children who have cervical inflexibility (Klippel-Feil syndrome) or cervical instability (Down syndrome, achondroplastic dwarfism, trauma) (Video 12-9 and 12-11)![]() . The technique is also versatile because the flexible instrument conforms to a variety of abnormal airways; it is well tolerated by the sedated, spontaneously breathing child.344
. The technique is also versatile because the flexible instrument conforms to a variety of abnormal airways; it is well tolerated by the sedated, spontaneously breathing child.344
Its disadvantage is that the fiberoptic bundle is small, permitting only a limited field of vision. For this reason, the presence of blood or copious secretions may render the FOB ineffective. In addition, use of the FOB requires extensive experience and practice in normal airways first. Our experience suggests that each size of available FOB should be used in at least 20 normal airways before use is attempted in an abnormal airway.425 Practice with all available sizes of FOBs is important because the manual skills required for each size differ somewhat. Also, FOBs are fragile and expensive. Great care must be taken when using and storing FOBs to prevent breakage of the fiberoptic bundles and the adjustable tip mechanism. They must also be sterilized between uses to maintain the patency of the working channel and clear, bright vision through the fiberoptic bundles and to avoid transmission of infection.
Ancillary Equipment
Endoscopy masks can be used to provide oxygen to the spontaneously breathing child and to ventilate the paralyzed child during fiberoptic laryngoscopy. The Frei endoscopy mask (E-Fig. 12-13) and the Patil-Syracuse endoscopy mask are commercially available.426,427 Patil-Syracuse masks are available in a child size but are too large for most children younger than age 4 years.88 The Frei mask configuration allows the FOB to be placed in a central position, overlying the nose and mouth, which is more favorable for tracheal intubation. The clear membrane with the hole for the bronchoscope can be rotated to allow either oral or nasal approaches. Alternatively, a disposable facemask can be combined with a bronchoscopic swivel adapter.428 The FOB can then be passed through the diaphragm of the adapter while ventilation is maintained via the anesthesia circuit. There are two types of commercially available adapters. One type attaches directly to an anesthesia mask. The other type is designed to attach to the ETT and can be modified to fit on the anesthesia mask by the use of a 15- to 22-mm adapter.
Commercial oral airways designed for use in bronchoscopy are available for pediatric patients (IMD Inc., Park City, Utah); however, there are only three sizes (infant, child, and adult), and no studies have evaluated their usefulness in assisting fiberoptic intubation in children. Guedel airways can also be modified for use as oral intubation guides.428 A strip is cut from the convex surface of the airway to create a channel for placement of the FOB. This modified airway may be used to maintain a midline approach to the glottis; however, it is ineffective as a bite block.
Direct Technique
The optimal position of the child for fiberoptic bronchoscopy is different from the position for rigid laryngoscopy. The head should be flat on the table and slightly extended at the atlantooccipital joint to prevent the epiglottis from obstructing a view of the glottic opening.429 If an oral approach is selected, it is vital that the FOB pass in the midline. A nasal approach may make midline placement simpler and avoids the risk of the child’s biting the FOB or ETT. One useful technique is to apply oxymetazoline to the naris, place a lubricated nasal trumpet into the nares, and then insert a 15-mm connector from a tracheal tube into the nasal trumpet. This allows oxygen and inhalation agent to be delivered to the child while performing standard fiberoptic laryngoscopy (see Videos 12-9 and 12-11). However, the oral approach offers several advantages over the nasal approach, including avoidance of shearing adenoidal tissue and nasal bleeding. The oral approach may also be less stimulating and better tolerated than the nasal approach. If nasal intubation is chosen in a young child, a topical vasoconstrictor will reduce the risk of bleeding. With both approaches, an assistant should perform a jaw thrust to open the posterior pharyngeal and supraglottic spaces. Alternatively, a bite block or intubating airway may be used. Occasionally, the best view is obtained by direct traction on the tongue, which optimally opens the posterior pharynx. This can be accomplished by grasping the tongue with a gauze, plastic forceps, a stitch through the tongue, or application of high suction to the underside or tip of the tongue (Fig. 12-29).430
Once the FOB is introduced into the airway, a common problem is resistance to passage of the ETT. To minimize this occurrence, the ETT should be loaded onto the scope with the bevel facing down (Murphy eye up) for oral intubation and bevel facing up for nasal intubation.431,432 This can be remembered by the mnemonic UNDO: bevel Up for Nasal intubation, Down for Oral intubation (see Video 12-10).433 If persistent resistance is encountered while attempting to pass the ETT past the glottic opening, the ETT should be rotated 90 to 180 degrees to place the bevel in a more favorable orientation for passage through the vocal cords. The depth of anesthesia or sedation as well as oxygen saturation (pulse oximetry) must be carefully monitored throughout the procedure. Arrhythmias may be avoided by providing an adequate depth of analgesia/anesthesia and ensuring a patent airway.
Fiberoptic laryngoscopy techniques should be perfected on manikins and on children with normal anatomy before such techniques are attempted on children with pathologic airway anatomy.434–440 In a study that compared the time to intubation and complications in 40 infants with the Miller no. 1 laryngoscope or the Olympus LFP FOB, the time to intubation was slightly greater with the FOB (22.8 versus 13.6 seconds), whereas the complication rate was similar.441 The authors concluded that routine use of the FOB for intubation in normal infants is a safe and reasonable method to gain and maintain skills with this technique.441 For children, video-assisted fiberoptic intubation appears to offer advantages over fiberoptic intubation using a traditional eyepiece. These include faster mastering of this skill and an overall improved success rate when compared with the traditional method.425,442
Staged Techniques
Staged methods of fiberoptic intubation can be used in infants and small children when the available FOBs are too large to pass through the appropriately sized ETT.443 One method requires an FOB with a working channel and a cardiac vascular catheter with guidewire. The guidewire is passed through the working channel of the FOB to within 1 inch of its tip. The FOB is then introduced into the mouth and positioned above the vocal cords. The guidewire is advanced under direct observation through the glottis into the trachea. The FOB is removed, leaving the guidewire in place. The lungs are ventilated by mask while an assistant passes the cardiac catheter over the guidewire (to stiffen the guidewire and facilitate passage of the ETT). The ETT is threaded over the catheter/guidewire combination, which is then removed, leaving the ETT in place.443–445 Some authors have found that threading of the cardiac catheter over the guidewire is unnecessary. A modification of this technique when the FOB has no working channel has also been described.446 The authors used an 8-F red rubber catheter attached by waterproof tape to the insertion cord of the FOB proximal to the flexible tip. The larynx was visualized with the FOB, and a guidewire was threaded through the rubber catheter into the trachea. With the guidewire in position, the FOB (with the red rubber catheter) was withdrawn, and an ETT was passed over the guidewire into the trachea.
Another alternative for intubation when the FOB is too large to pass through the appropriately sized ETT is intubation under fiberoptic observation.447–449 The FOB is introduced through one naris to visually aid the placement of the ETT that is passed through the other naris and manipulated into the glottis. Alternatively, if the observed ETT is not easily passed into the glottis, a small catheter may be more easily manipulated into the glottis and used as a stylet to pass the ETT into the trachea. Spontaneous ventilation is preserved, and oxygen can be administered via the ETT during the intubation. This technique was used successfully in two neonates, one with congenital fusion of the jaws and a second with Dandy-Walker syndrome associated with Klippel-Feil syndrome, micrognathia, hypoplasia of the soft palate, and anteversion of the uvula.447,448 More recently, an adult video-FOB was used to intubate the trachea of a toddler with temporomandibular joint ankylosis.450
If the available FOB is both too large and lacks a working channel, another staged fiberoptic intubation technique can be used. The FOB is loaded with an ETT that is larger than the larynx of the infant. The larynx is visualized with the FOB, and the ETT is advanced and positioned just above the vocal cords. The FOB is then removed, and an ETT changer or catheter is advanced into the trachea through the larger ETT. The larger ETT is then removed, and the appropriately sized ETT for the child is threaded over the tube changer or catheter into the trachea. This technique was used successfully in a 6-month-old infant whose operation had previously been canceled because of failure to intubate.451
Lighted Stylet
A lighted stylet (light wand) is a useful adjunct for managing the pediatric DA (E-Fig. 12-14).452–456 A number of these devices are available: Trachlight (Laerdol Medical Corporation, Wappingers Falls, N.Y.), Surch-Lite Lighted Intubation Stylet (Aaron Medical Industries, St. Petersburg, Fla.), Light Wand (Vital Signs, Totowa, N.J.), and Trachlite (Rüsch, Tuttlingen, Germany). These devices essentially comprise a malleable stylet with a high-intensity light at the tip; the stylet is shaped into a curve similar to that anticipated for successful passage into the laryngeal inlet (45 to 90 degrees). To begin, an ETT is passed over a well-lubricated lighted stylet. The tip of the stylet should remain within the tip of the ETT to minimize the potential for airway trauma. The room lights should be dimmed at this time to ensure that the stylet is visible when the wand is in the mouth. The lighted stylet is then introduced into the mouth while the proximal end of the stylet is flat against the cheek. As the stylet is inserted into the mouth, the proximal end of the handle is rotated counterclockwise until it is upright. The stylet continues to pass through the oropharynx, following the curvature of the tongue. If the tip of the lighted stylet is not in the proper position (e.g., in the esophagus), a diffuse light or no light will be observed on the surface of the neck. Proper position is usually ensured when a sharp, well-defined, bright circle or cone of light is observed transilluminating the neck directly in the midline at the level of the cricothyroid membrane (see E-Fig. 12-14). Once proper position is ensured, the ETT is gently advanced and the stylet is removed.455,457
This technique is useful in those children in whom there is no intrinsic laryngeal or airway pathology but in whom visualization is anticipated to be difficult. The light wand may also be of value in children with a fracture of the cervical spine, because tracheal intubation can be accomplished with minimal movement of the neck.458 The hemodynamic response to intubation with this technique is similar to that observed with rigid laryngoscopy.454 The limitations of this technique are that it is a blind technique, the diameter of the device limits it use to larger-sized ETTs, and it may require multiple attempts; however, the success rate markedly increases with experience.455,457 The most common cause of difficulty in passing the ETT is that the tube hangs up on the epiglottis. When this occurs, the lighted stylet may be withdrawn and its position slightly adjusted more posterior to allow passage behind and beyond the epiglottis. Alternatively, the ETT can be rotated along the long axis of the stylet so that the bevel is facing up. Our advice for using this technique is similar to that for bronchoscopy: it should be used in children with normal anatomy to gain the necessary experience required for managing children with abnormal airway anatomy. This adjunct has also been combined with an LMA to guide the stylet into the trachea.459
Bullard Laryngoscope
The Bullard laryngoscope is used for direct visualization of the laryngeal inlet in children with airway pathology (E-Fig. 12-15). It is available in three sizes: adult, pediatric, and pediatric long. This instrument combines fiberoptic bundles and mirrors. It is positioned within the larynx like a laryngoscope blade, and the direction of force used to displace the tongue is similar to that used with a standard laryngoscope, although this is not intuitively obvious from its configuration. It is designed to provide visualization around a 90-degree bend at the tip (i.e., around the base of the tongue). This configuration may be helpful for direct visualization of the larynx in children with mandibular hypoplasia syndromes (e.g., Robin sequence, Treacher Collins syndrome,460 Goldenhar syndrome, cervical fracture restricting motion), when the acute angulation of the base of the tongue to the glottic opening is exaggerated, and in children with congenital trismus (Hecht syndrome).461 The technique used in children is different from that used in adults. Once the laryngeal inlet is visualized, a styleted ETT, with a bent configuration similar to the curve of the Bullard laryngoscope (see Fig. 12-21, B), is inserted just to the side of the Bullard laryngoscope blade and advanced under direct vision into the trachea. Success with this instrument is directly proportional to the experience of the anesthesiologist, because the perspective seen through this laryngoscope, the method of visualization, and the indirect method of ETT placement are so different from standard laryngoscopy.462,463
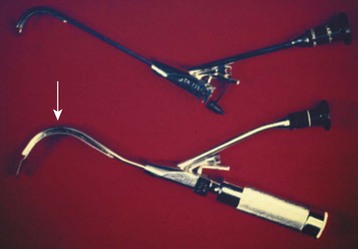
E-Figure 12-15 The Bullard laryngoscope is available in three sizes: adult, pediatric, and pediatric long. The device shown at the top is the adult version, which has an attached endotracheal tube guide; the pediatric versions do not have this guide. This instrument combines fiberoptic techniques and mirrors; the fiberoptic bundles are contained within an instrument that functions and is positioned within the larynx like a laryngoscope blade. It is designed to provide visualization around a 90-degree bend at the tip (arrow) (i.e., around the base of the tongue). This configuration may be helpful for direct visualization of the larynx in children with mandibular hypoplasia syndromes (e.g., Pierre Robin syndrome, Treacher Collins syndrome, Goldenhar syndrome, cervical fracture restricting motion) when the acute angulation of the base of the tongue to the glottic opening is exaggerated. Once the laryngeal inlet is visualized, a styleted endotracheal tube with a bent configuration similar to the curve of the Bullard laryngoscope (see Fig. 12-21, B and C) is inserted just to the side of the Bullard laryngoscope blade and advanced under direct vision into the trachea.
Despite the availability of pediatric Bullard layngoscopes,464 adult scopes have also been successfully used in children.465,466 Although tracheal intubation in children 1 to 5 years of age with a Bullard laryngoscope takes more time than with a Wis-Hipple 1.5 blade, the adult Bullard laryngoscope complements the Wis-Hipple 1.5 blade. Occasionally, the Bullard laryngoscope provides a superior laryngeal view and thus allows successful intubation when a failure with the Wis-Hipple blade occurs. When multiple passes of the tube off the adult Bullard laryngoscope were required, this is usually because of contact with the right aryepiglottic fold or anterior vocal cord. The latter appears to be more problematic when the adult laryngoscope is used in children.465,466
Additional limitations of the Bullard laryngoscope are that the ETT can partially obstruct the view of the larynx during insertion and that it can be used only for oral intubation.464 An advantage is that there appears to be minimal motion of the cervical spine in patients with cervical spine disarticulations.467–469
Retrograde Wire-Guided Intubation
The technique of retrograde wire-guided intubation utilizes transtracheal passage of an IV catheter through the cricothyroid membrane into the larynx and retrograde passage of a guidewire from a Seldinger vascular cannulation set to create a guide for intubation.470–477 A commercial kit is available for use with ETTs that are 5 mm ID or larger (Cook Critical Care). This technique is rarely used in children because of the greater compressibility of the trachea and the increased risk of posterior tracheal wall perforation by the catheter in children compared with adults.
Video and Indirect Intubating Devices
Advances in technology have led to the reduction in size of video cameras and optical lenses. The integration of these devices into various laryngoscopes and stylets has produced several enhanced tools for securing the airway in children.478 Several of these new scopes improve visualization during laryngoscopy412,479–485 and perform better than traditional laryngoscopy in children with difficult direct laryngoscopy.486–492 However, they are often associated with prolonged time to intubation compared with direct laryngoscopy, and their utility is limited in the presence of blood and secretions. These new devices can be categorized as (1) video laryngoscopes, which incorporate a video camera into the tip of the device; (2) optical laryngoscopes, which use a series of mirrors, prisms, or both to transmit the image from the tip of the device; and (3) optical stylets, which incorporate video or optical systems into a rigid or malleable stylet.