CHAPTER 11 The Patient History and Physical Examination
Cervical, Thoracic, and Lumbar
Differential Diagnosis
The differential diagnosis of spinal pain or related symptoms is enormous when considered in a general sense. Numerous anatomic structures may be associated with pain, multiple local or systemic disease processes can affect the spine, and numerous non–spine-related structures or conditions can result in back or neck pain or mimic syndromes related to spine disorders.1–5 In addition, numerous psychosocial factors can produce ongoing pain and disability. The ability to process all of the available possibilities and to develop a relatively short list of diagnostic options depends heavily on the ability to obtain a thorough history and physical examination. It is helpful to begin with an understanding of structures in the spine that can be associated with pain and their patterns of pain referral.
From an anatomic perspective, a structure must be innervated to cause pain. In the spine, the list of discrete anatomic structures with sensory innervation (i.e., potential pain generators) includes muscles, tendons, ligaments, fascia, anulus of the intervertebral discs, bone, zygapophyseal joints, dura mater, nerve roots and dorsal root ganglia, and vascular elements.1,2 All structures of common embryologic segmental origin tend to refer pain in very similar patterns, and the pattern of pain is determined by the nerve supply to the structure.1 The end result is that there is substantial overlap between the referral patterns for anatomic structures of the same spinal level, such as intervertebral discs and zygapophyseal joints, and dermatomal, myotomal, and sclerotomal referral patterns at many spinal levels (Fig. 11–1). The location of pain or radiating symptoms can often be a useful feature in the identification of an affected spinal level, although the location of pain alone does not indicate which particular anatomic structure is the source of the specific symptom.
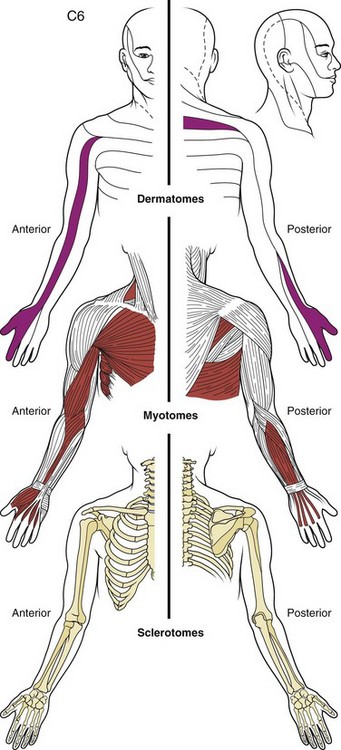
FIGURE 11–1 Drawing of dermatome, myotome, and sclerotome of C6 level showing substantial overlap in distribution.
(From Bland JH: Disorders of the Cervical Spine: Diagnosis and Medical Management, 2nd ed. Philadelphia, WB Saunders, 1994.)
The zygapophyseal joints are one of the best-studied structures in terms of pain referral patterns and relative prevalence in patients with spinal pain. In the cervical spine, the pattern of pain distribution from the stimulation of specific zygapophyseal joints has been described (Fig. 11–2).6 Those results were subsequently validated in a study of patients with cervical complaints based on pain distribution and response to diagnostic blocks.7 Another study using a double-block protocol on patients with persisting symptoms after whiplash injury found that the prevalence of C2-3 zygapophyseal joint pain in patients with headache was 50%; in patients without C2-3 zygapophyseal joint pain, the prevalence of symptoms related to the lower cervical zygapophyseal joints was 49%.8 Although there is far less clinical information on pain associated with thoracic zygapophyseal joints, a similar map of referral patterns has been identified (Fig. 11–3).9
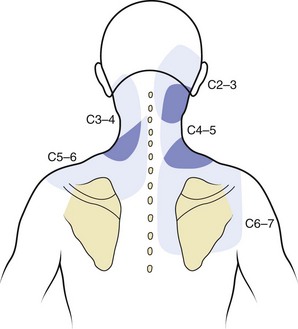
FIGURE 11–2 Map of characteristic areas of pain referred from cervical zygapophyseal joints (C2-3 to C6-7).
(From Dwyer A, Aprill C, Bogduk N: Cervical zygapophyseal joint pain patterns I: A study in normal volunteers. Spine 15:453-457, 1990.)
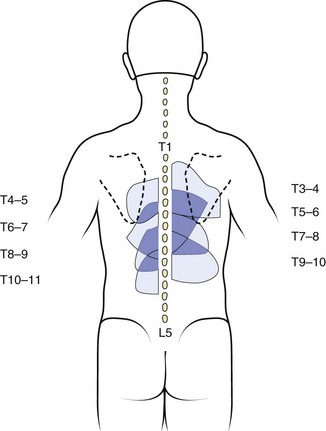
FIGURE 11–3 Map of characteristic areas of pain referred from thoracic zygapophyseal joints (T3-4 to T10-11).
(From Dreyfuss P, Tibiletti C, Dreyer S: Thoracic zygapophyseal joint pain patterns: A study in normal volunteers. Spine 19:807-811, 1994.)
In the lumbar spine, there has also been a great deal of attention directed to the zygapophyseal joints as potential sources of pain, although the relative frequency with which they seem to be primary pain generators is less than for cervical zygapophyseal joints causing pain in patients with chronic whiplash. A more recent study noted a 15% overall prevalence of zygapophyseal pain in a group of 176 patients with chronic low back pain using a diagnostic double-block protocol.10 Although pain associated with lumbar zygapophyseal joints is generally described as occurring with lumbar extension and rotation, the authors of that study did not find any consistent clinical features that were associated with the presence of a positive diagnostic response to injections.10 Stimulation of lumbar zygapophyseal joints can result in either local axial or, far less frequently, radiating pain, and pain referral patterns have been documented.11
Multiple authors have addressed the distribution of pain associated with intervertebral discs. Cloward12 first described cervical discography and noted that pain that seemed to be emanating from irritation of the anulus resulted in radiating pain into the thoracic or scapular regions in distinct patterns (Fig. 11–4). Similar findings were more recently described by others.13 As mentioned previously, pain referral patterns are similar to patterns noted for cervical zygapophyseal joints, with the level of spine pathology, rather than the actual structure involved, affecting the pain referral pattern.
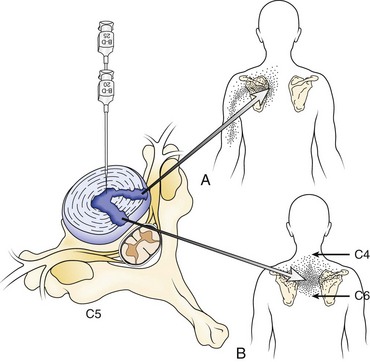
FIGURE 11–4 A and B, Pain referral pattern from posterolateral (A) and central (B) discs.
(From Cloward RB: Cervical discography: A contribution to the etiology and mechanism of neck, shoulder and arm pain. Ann Surg 150:1052-1064, 1959.)
Other pain referral patterns that should be recognized by all physicians treating patients with spine disorders include patterns related to neurologic injury. These patterns are discussed further later in the section on the neurologic examination, but identifying the dermatomal pattern of pain is central to the assessment of individuals with potential nerve root pathology (Fig. 11–5). Nerve root symptoms include paresthesias, burning, hyperalgesia, aching, analgesia, or pain. The ability to identify a dermatomal pattern to the symptoms can help localize the area of spine involvement.
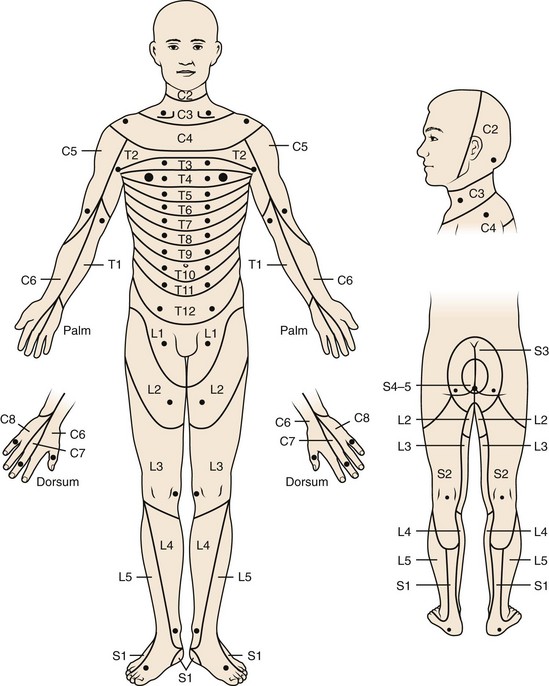
FIGURE 11–5 Dermatomal distribution and key sensory points.
(From American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury [reprint]. Chicago, American Spinal Injury Association, 2008.)
The clinical utility of pain provocation is uncertain because there are inherent problems with this approach owing to the complex nature of pain perception.1,14–18 The identification of a “pain generator” in individuals with chronic spinal pain can be difficult. In contrast to cutaneous sensation, nociceptive signals from deep somatic structures such as joint capsules, fascia, and periosteum are carried by relatively few primary afferent fibers, resulting in only vague localization of pain.16 Additionally, there is the issue of convergence, in which a single dorsal horn cell may receive synaptic input from afferent fibers that innervate many structures and can result in multiple structures producing similar patterns of pain perception. This convergence makes it extremely difficult to validate a single entity as “the cause” of an individual’s pain because the stimulation of any one of numerous structures may result in identical perceptions of pain.16 These issues become even more complex when additional potential neurologic and psychological changes that can occur with chronic pain are involved.
Biomedical Factors and the Medical History
“Red Flags”—What Not to Miss
It is essential to identify all conditions that pose a substantial, imminent risk for further harm to the patient. Many authors have identified specific “red flags” in the history of patients with low back complaints that indicate the presence of such a condition; these include infection, tumor, fracture, cauda equina injury, and progressive neurologic injury such as motor loss or myelopathy (Table 11–1).19–22 “Red flags” for the possibility of cancer include age older than 50 years, previous cancer history, unexplained weight loss, pain not relieved by bed rest, duration of pain for more than 1 month, and failure of conservative therapy after 1 month.22 The combined sensitivity of age older than 50 years, history of cancer, unexplained weight loss, and failure of conservative therapy is nearly 100%.22 A history of smoking also increases a patient’s risk for cancer.21 Although similar data are unavailable for cervical and thoracic pain, the same factors seem relevant.
TABLE 11–1 “Red Flags”: Emergent or Urgent Medical Conditions That Need to Be Identified Promptly in All Patients Presenting with Possible Issues Related to the Spine
| Symptom or Finding | Possible Significance |
|---|---|
| History of cancer | Cancer |
| Unexplained weight loss | |
| Age >50 yr | |
| Failure to respond to >1 mo of conservative care | |
| Duration of pain >1 mo | |
| No pain relief with bed rest | |
| Night pain | |
| History of smoking | |
| Known osteopenia or osteoporosis | Fracture |
| History of corticosteroid use | |
| Age >50 yr | |
| DISH or ankylosing spondylitis | |
| Trauma (major in younger individual, minor in older individual) | |
| Fever | Infection |
| Illicit use of intravenous or percutaneously injected drugs | |
| Recent or known infection | |
| Immunosuppressive illness | |
| Use of immunosuppressive medications | |
| Tuberculosis exposure | |
| Progressive weakness in limbs | Cauda equina or spinal cord injury |
| Progressive balance deficit or loss of coordination | |
| Bowel or bladder dysfunction or urinary retention | |
| Sexual dysfunction | |
| Numbness or paresthesias in perineum or saddle anesthesia | |
| Significant weakness of major muscle group or progressive motor loss in limb | Severe or progressive radiculopathy |
DISH, diffuse idiopathic skeletal hyperostosis.
Spine infections, including discitis, osteomyelitis, and epidural abscess, are usually blood-borne from other regions.22 Important risk factors for infection include the use of illicit intravenous drugs, active or recent infection elsewhere (e.g., urinary tract, pulmonary, skin, dental), and immunosuppression (owing to either medications or illness affecting the immune system).19,22 Additional risk factors for infection include diabetes and history of tuberculosis or exposure to a region endemic for tuberculosis.
The risk of fracture is elevated in patients older than 50 years, particularly patients older than 70 years.22 Patients with a history of corticosteroid use or known osteopenia or osteoporosis are also at increased risk for fracture. A history of fracture in a younger individual after major trauma, in an older individual after minor trauma, or in anyone with the potential for reduced bone density should also be considered a risk factor. Trauma and fracture risk are discussed further elsewhere in this book.
Significant neurologic injuries include cauda equina syndrome, progressive radiculopathy, or myelopathy. Cauda equina syndrome should be considered in a patient with saddle anesthesia; bowel, bladder, or sexual dysfunction; or significant lower extremity pain and weakness, particularly if bilateral.19,22 Progressive neurologic loss from nerve root compression is an indication for urgent surgical intervention and needs to be identified promptly. Myelopathy can present in various ways, including hand paresthesias or decreased fine motor control; lower extremity weakness or gait instability; sensory alterations in the trunk or extremities; or changes in bowel, bladder, or sexual function.23
Axial Versus Radicular Pain
The distinction between axial and radicular pain is fundamental in assessing a patient with a potentially neurogenic problem. Axial pain in the cervical, thoracic, or lumbar region suggests a different etiology, evaluation, diagnosis, and potentially treatment than radicular pain. For all levels of the spine, pathology involving the musculotendinous and ligamentous structures, zygapophyseal joints, vertebrae, and anulus of the intervertebral discs tends to cause axial pain. Other structures in the cervical and thoracic regions that can result in axial pain include soft tissue structures in the neck; vascular structures (e.g., aorta or carotid arteries); portions of the brachial plexus such as the long thoracic or suprascapular nerves; the proximal portion of ribs; costovertebral or costotransverse articulations; various structures within the shoulder; and various visceral structures, including the pancreas, gallbladder, lung and pleura, and stomach or duodenum (Fig. 11–6).
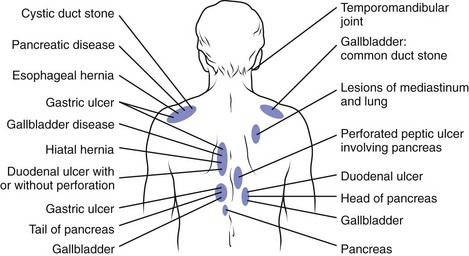
FIGURE 11–6 Posterior referral sites from distant visceral or somatic structures.
(From Nakano KK: Neck pain. In Kelley WN, Harris ED Jr, Ruddy S, et al [eds]: Textbook of Rheumatology, 4th ed. Philadelphia, WB Saunders, 1993.)
For the lumbar spine, the hip and pelvic structures must be considered as potential sources of low back, buttocks, or posterolateral hip pain. Particular sources of low back or buttock pain related to the bony pelvis include the sacroiliac joints, the sacrum (e.g., stress fractures), the ilia, and the hip joints. Other structures and processes that can result in low back pain include the kidneys and ureters; the pancreas; gastric ulcers; vascular abnormalities (e.g., aortic aneurysm); and retroperitoneal processes such as hematoma, endometriosis, or lymphadenopathy associated with malignancy.24
As with upper extremity pain, lower extremity radicular pain often has different etiologies and generally implies involvement of the lumbosacral nerve roots, the conus medullaris, or the spine. The lumbar zygapophyseal joints and the sacroiliac joints also may occasionally be associated with radicular leg pain.11,25 Distal lower extremity symptoms also may arise from intra-articular hip pathology; greater trochanteric bursitis; vascular pathology (e.g., vascular claudication); peripheral nerve injuries; compartment syndrome; local musculotendinous, ligamentous, or bony structures; and pelvic causes such as endometriosis.
Patient Demographics
Growth and development have a profound impact on the development and approach to various processes, such as spondylolisthesis, scoliosis, and Scheuermann kyphosis. In contrast to the adult spine, the developing bony spine is relatively more prone to injury than some soft tissue structures. In a study by Micheli and Wood,26 47% of adolescents presenting to a pediatric sports medicine clinic were diagnosed with spondylolysis and only 11% had disc abnormalities compared with 48% of adults presenting to a low back pain clinic who were thought to have disc pathology. Generally, symptomatic isthmic spondylolysis is almost entirely seen in older children, adolescents, or young adults, although the rate of pars defects identified in the general population does not change substantially between the ages of 20 and 80.27,28 Although 50% or more of children may be affected by low back pain by age 15,29,30 significant spinal pain in children is uncommon and should raise concern for the presence of serious medical pathology.31,32 Infection, neoplasm, rheumatologic conditions such as ankylosing spondylitis and juvenile rheumatoid arthritis, and other nonspine sources of pain may be more common in children and adolescents than in adults.31,32
In adults, the frequency of certain spine conditions varies by age group. Disc herniations are most frequent during the 4th and 5th decades, although they can affect individuals in their 50s and 60s or children and young adults.33 Degenerative spinal stenosis and degenerative spondylolisthesis tend to present later in life. As mentioned previously, some medical conditions, including ankylosing spondylitis, spondylitis associated with inflammatory bowel disease, and tumors such as osteoid osteoma and osteoblastoma, tend to manifest in younger adults (20s and 30s). Other conditions, such as osteoporosis, polymyalgia rheumatica, metastatic cancer, or multiple myeloma, tend to occur in older adults (40s and 50s or older) (Fig. 11–7).3,34
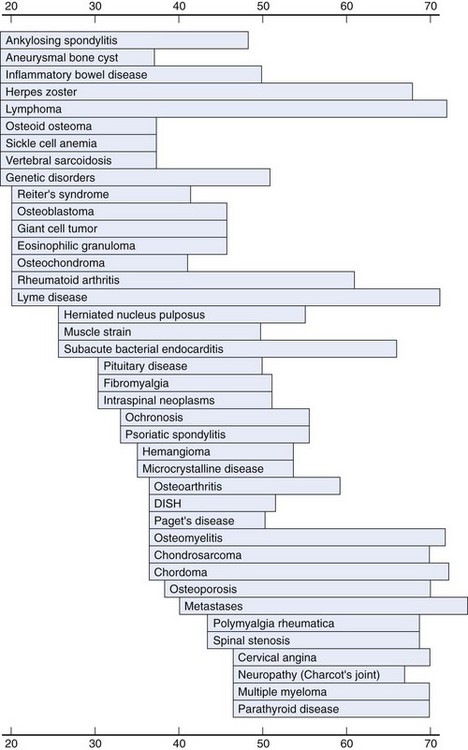
FIGURE 11–7 Age at peak incidence of neck pain associated with various disorders.
(From Borenstein DG, Wiesel SW, Boden SD: Neck Pain: Medical Diagnosis and Comprehensive Management. Philadelphia, WB Saunders, 1996.)
Gender is a factor in many spine pathologies. Osteoporosis is more common in women than in men, and osteoporotic fractures are more common in women. Neck pain also has been noted to be more prevalent in women than in men.35,36 Rheumatoid arthritis, polymyalgia rheumatica, and endocrine disorders also tend to occur more frequently in women.34 Spondyloarthropathies; infections; and various spine tumors, such as multiple myeloma, lymphoma, osteoblastoma, and eosinophilic granuloma, occur more frequently in men.
Demographic factors such as race, ethnicity, and cultural milieu may also play a role in the prevalence of some spine disorders but are less well studied. Whites tend to have higher rates of osteoporosis than some other races, and metabolic conditions such as Gaucher disease can be associated with certain ethnic groups.3 Whites have a higher rate of spondylolysis than African Americans.27 The prevalence of low back pain also varies in different parts of the world, with industrialized regions reporting a higher prevalence of low back complaints than rural, low-income areas.37 Pain perception, disability, and other effects of pain on individuals vary widely and depend on many cultural and social factors.
Past Medical History
In addition to identifying prior surgical procedures, it is important to identify all past and current medical conditions because many medical problems can be associated with spine issues and can affect care of a patient with a spine disorder. As noted previously, a history of cancer, recent infection, or disease processes that affect the immune system or may require immunosuppressive medications can be associated with significant spine problems. Other medical conditions, such as osteoporosis, ankylosing spondylitis, and diffuse idiopathic skeletal hyperostosis, may place patients at increased risk for spine fracture.38 Some congenital or genetic syndromes, such as Marfan syndrome and Down syndrome, can be associated with spine anomalies that must be identified. Vascular disease, such as vascular claudication or aortic aneurysm, can produce symptoms that mimic spine pathology. Other disorders, including cardiac or pulmonary disease, renal disorders, skin conditions, gastric ulcers, diabetes, and hepatic disorders, may have an impact on potential treatment options and may preclude certain therapies. Clinicians need to be aware of all facets of a patient’s medical history and the potential influence that medical issues may have on the care of the patient.
Family History
The family history is a necessary component of a complete medical history. Although back pain and many other spinal conditions are common in the general population, data suggest possible genetic risk factors for lumbar degenerative disc disease.39 A family history of rheumatologic diseases, particularly conditions associated with HLA B-27 such as ankylosing spondylitis, Reiter syndrome, and inflammatory bowel disease, can suggest a tendency for, or risk of, developing a similar process.3,40 Other inheritable diseases, including certain neuromuscular diseases, may be associated with progressive spinal deformity, and patients with a genetic predisposition for certain medical conditions (e.g., vascular disease, specific cancers) may also present additional diagnostic considerations.
“Yellow Flags”—Predictors of Poor Outcome in the Patient’s History
Numerous factors in a patient’s history have been identified as potential predictors of poor outcome in the treatment of spinal pain. These factors are known as “yellow flags” (Table 11–2).41 The presence of more than one of these factors in a patient is a strong predictor of poor outcome and chronic pain and disability.41 These “yellow flags” include issues related to the nature of the patient’s injury and general medical health, occupational and social issues, and psychological factors. It is imperative to identify these factors, if present, early in the course of evaluation and treatment of patients with spinal pain because they have been shown to be more powerful predictors of outcome than other biomedical issues.42–44
TABLE 11–2 “Yellow Flags”: Potential Predictors of Poor Outcomes or Persisting Pain and Disability, Particularly When More than One Is Present
| Biomedical Factors |
From Gaunt AM: Caring for patients who have acute and subacute low back pain. CME Bull 7:1-7, 2008.
Patients who report more widespread symptoms of neck or back pain, who have more severe pain or disability at the onset of their injury, or who have higher rates of concurrent comorbidities tend to have a higher risk of developing protracted pain complaints or disability.43–46 For low back pain specifically, dominant medical factors associated with the development of protracted pain or disability seem to be the presence of severe leg pain and a history of prior episodes of low back pain.43,45,47 Some distinct occupational factors that have been shown to be related to the development of chronic pain include heavy physical workload, unavailability of light duties on return to work, perceived poor working environment or job dissatisfaction, a low level of education, and a short time of employment on the job.43–45,47,48 The amount of time off work from an injury also has a negative correlation with return to work rates.49,50
As noted previously, psychological factors seem to play a substantial role in the development of chronic spinal pain. In a review on this topic, Linton42 noted that psychological variables are clearly linked to the transition from acute to chronic pain and generally have a stronger impact on chronicity than medical or biomechanical factors. Pertinent emotional factors cited include depression, anxiety, distress, and self-perceived poor health. Cognitive and behavioral factors also apparently play a key role in the development of a chronic pain state; these include a passive coping style, “catastrophizing,” and fear-avoidance beliefs (beliefs that certain activities should be avoided owing to fear of injury). A history of sexual or physical abuse also may be related to chronic pain and disability.42 A systematic review of psychosocial factors found that psychological distress, depressed mood, and somatization were associated with the transition to chronic low back pain.51
Despite the high prevalence of psychopathology in patients with chronic pain, there does not seem to be a premorbid “pain-prone” personality; the depressive features of chronic spinal pain generally seem to arise more as a consequence, rather than a cause, of the pain state.42,52,53 One study did identify premorbid depression, however, as an independent, robust risk factor for the onset of an episode of troublesome neck or low back pain.54
From a strictly surgical perspective, the outcomes of lumbar surgical procedures are influenced by numerous factors completely unrelated to the anatomy or pathophysiology of the spine. The results of lumbar discography are influenced by psychosocial variables to such a large degree that there are concerns about the validity of the procedure.15 Factors identified as predictors of poor outcome from surgical intervention in the lumbar spine include low level of education, low income at the time of injury, the presence of pending litigation, the presence of an industrial injury, and depression.55–58 Surgical outcomes have also been found to be worse in geographic regions with higher rates of surgical intervention.59 From a clinical standpoint, it is important to identify predictors of poor outcome or chronicity to provide appropriate care to address these issues and to avoid invasive care that is highly unlikely to be helpful and could contribute to the perpetuation of chronic pain and disability.
Obtaining a Psychosocial History
It is dangerous to assume that a patient’s presenting symptoms are solely the result of the injury that led to the consultation. Patients in whom disability greatly exceeds that expected on the basis of objective findings have been shown to be much more likely to have encountered childhood abuse and conflict, parental job stress, or a difficult divorce. Pain is an experience that is influenced by everything that is currently occurring in the life of the patient. Equally or sometimes more important is everything that has gone on in the patient’s life in the past. In a study of more than 25,000 subjects in 14 countries, the World Health Organization found that physical disability is more closely associated with psychological factors than with medical diagnosis.60 An appreciation of the power of this observation is extremely valuable.
It has been estimated that approximately 50% of patients with chronic pain in rehabilitation and family practice settings have a personality disorder, as documented through structured interviews and psychological testing.61 Thorough evaluation of patients with back pain needs to include some form of psychological testing because psychological factors play a critical role in patient recovery from illness or injury. Ignoring either the physical or the psychological components of pain in diagnosis and treatment is a prescription for failure, disappointment, and dissatisfaction. Several psychological test instruments are available for this purpose.
Additional Assessment Tools
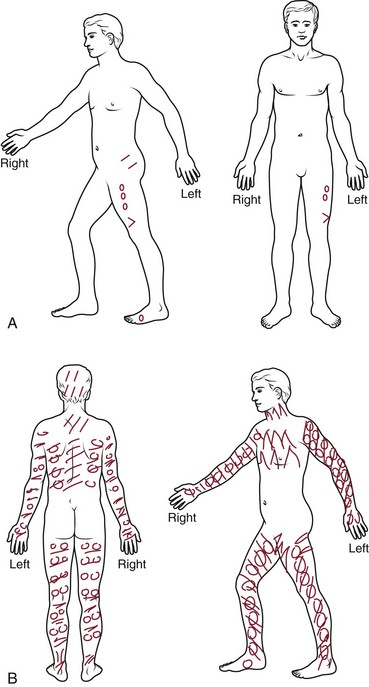
Pain drawings have been used since the 1940s, and research into their significance has provided mixed results.62 Although there are data supporting an association between psychological distress and widespread, nonanatomic markings on the pain drawing, there is contradictory evidence in other studies. Data are also contradictory on the usefulness of pain drawings in predicting surgical outcomes.62 Pain drawings have been assessed using various means and have been shown to have relatively high repeatability.62,63 Intraobserver reliability is relatively good, although interobserver reliability is more questionable, particularly for qualitative assessments.62,63 Although the presence of widespread or nonanatomic patterns of pain on these drawings may be of some use in identifying pain intensity and the presence of depression or psychological distress, one systematic review did not find evidence to support their use as a formal psychological assessment tool (Fig 11–8).62,64,65
A variety of pain scales may be used in patient assessment. Various visual analog scales have been reported. The Million Visual Analog Scale has been shown to have good reliability, validity, and responsiveness.66 The McGill Pain Questionnaire has also been widely used and is well validated. This questionnaire provides a quantitative assessment using numerous descriptors of pain over three separate domains that are identified by the test taker and scored.34,66 Other scales identifying the “bothersomeness” of pain and the bodily pain item in the Medical Outcomes Study 36-item Short Form Health Survey (SF-36) have also been applied in the assessment of patients with spine disorders.67
Numerous functional scales exist, including the Oswestry Low Back Pain Disability Questionnaire, the modified Roland scale, the Neck Disability Index, the Sickness Impact Profile and the related Disability Questionnaire, and the SF-36.66–70 The Oswestry questionnaire, which uses self-rated functional impairment in numerous activities of daily living, has been shown to be valid and responsive and is generally easy to administer and score.66,68 The modified Roland scale, which consists of 24 “yes” or “no” questions regarding the functional impact of back pain, was originally derived from the Sickness Impact Profile, has been well validated, has a high responsiveness, and is very easy to score.66,67
Several brief psychological scales are also useful. The presence of fear-avoidance beliefs and catastrophizing are particularly important in the development and maintenance of chronic pain and disability. The Fear-Avoidance Beliefs Questionnaire71 and the Pain Catastrophizing Scale72 are validated assessment tools that can be used to quantify these factors.
The Battery for Health Improvement (BHI-2) is a self-report multiple-choice instrument designed for assessment of medical patients. It is intended to provide one source of clinical hypotheses that professionals can use to explore the interrelationships between a patient’s psychological and medical conditions. The information can be particularly useful in helping to determine factors that may be influencing an inexplicable delay in recovery of an injured patient. The Opioid Risk Tool is clinically relevant and easily employed during the interview.73
Physical Examination
Observation
Spine range of motion should be assessed for all relevant spine segments. There is debate as to what constitutes “normal” range of spine motion and the significance of any perceived restriction of motion. In the lumbar spine, range of motion has been variably reported by using inclinometry, measuring the distance from the fingertips to the floor, assessing segmental motion, measuring dynamic motion, measuring motion with the pelvis restrained, radiographic measurement, and using variations of the Schober test (measuring the change in distance between a mark over the S1 spinous process and one made 10 cm above this in standing that occurs between standing and flexion).74–77
The value of range of motion measurements is questionable, however, because some data do indicate that there is no consistent relationship between range of motion and physical or functional impairment in subjects with chronic low back pain.75 Range of motion generally seems to decline with age, further complicating attempts at establishing normative data.74 Gross lumbar motions generally include motion from the hips and lower extremities, and any lateral flexion or rotation involves coupled motion at multiple levels, making it difficult to assess these reliably. It is important to examine hip motion, however, because painful and restricted hip motion, particularly in flexion with internal rotation, that mimics the patient’s usual pain would generally implicate the hip as the source of pain.
Despite these substantial limitations, it is still important to assess active spine motion in flexion, extension, rotation, and lateral flexion. Along with absolute degrees of movement, the examiner can assess symmetry of motion, preferred movement patterns, pain or symptom reproduction associated with motion, the relative contributions of associated body segments to motion (e.g., hips), motor control, and inconsistencies between movement noted on formal examination and that seen during casual observation or while the patient is otherwise distracted. Generally, patient motion should be assessed actively within the patient’s range of comfort. There is little or no role for passive range of motion because this adds little to the clinical assessment and may place the patient at risk for further injury.34
For cervical and thoracic complaints, it is also important to assess shoulder and scapular motion. Shoulder range of motion can be assessed actively by flexion and abduction along with passive motion of the glenohumeral joint. Scapular position at rest and with various arm positions can reveal abnormal movement patterns and may indicate problems with scapulothoracic function, other shoulder joint complex disorders, or neurologic injury affecting the parascapular musculature (e.g., a long thoracic or spinal accessory nerve injury). Scapulothoracic dysfunction of various kinds may also be a source of pain in patients with thoracic complaints.78 Reproduction of a patient’s shoulder region pain by passive shoulder motion, particularly if it is restricted, would generally implicate the shoulder rather than the neck as the source of pain. Patients with a cervical radiculopathy obtain relief with ipsilateral shoulder abduction (the shoulder abduction relief maneuver); patients with intrinsic shoulder pathology often have reproduction of pain with shoulder abduction.
Palpation
The relevant areas of the patient’s spine and related structures should be palpated with the patient standing or, when appropriate, in side-lying or prone position. Palpation may aid in the localization of the patient’s symptoms, the identification of an injured structure, or the identification of associated soft tissue or bony abnormalities. It should be noted whether tenderness is elicited in the midline or to either side of midline, potentially differentiating between spinal pain and pain from an adjacent soft tissue source.34 Localized tenderness should be distinguished from diffuse tenderness, the latter being less consistent with a focal injury.
In the cervical spine, palpation should include the occipital region; the anterior neck; the clavicular, supraclavicular, and scapular regions; and the areas of the associated cervicothoracic musculature.34 In the thoracic region, palpation should also extend across the posterior ribs to identify focal bony tenderness that may suggest rib pathology rather than spine pathology. Pain with palpation or percussion of the costovertebral angle may suggest renal pathology.79 Spondylolisthesis can frequently be appreciated by a palpable step-off of the spinous processes in the lumbar spine. In the lumbar region, palpation should include not only the lumbar spine but also the iliac crests, sacrum, sacroiliac joints, ischial tuberosities, proximal hamstring, and greater trochanteric areas, as indicated, to assess for the possibility of contributing problems from these regions. Trochanteric pain may mimic pain from a spine etiology.
Neurologic Examination
As with the general medical examination, the neurologic examination may cover a wide range of factors, depending on the particular presenting problem. The most common neurologic manifestations of spine pathology generally involve the spinal nerve roots or the spinal cord, resulting in radicular or myelopathic findings on examination. The symptoms resulting from spine pathology may frequently overlap, however, with symptoms of various peripheral nerve processes, central nervous system disease, or anterior horn cell disease. An examiner needs to be aware of the clinical presentations and neurologic findings associated with these disorders. A full discussion of all relevant examination techniques and neurologic pathology is beyond the scope of this chapter but can be found in general neurology texts.80,81 This section focuses on findings more directly related to spine pathology.
A thorough understanding of dermatomal patterns is essential for all clinicians examining spine patients. As a reference, the key sensory points identified by the American Spinal Injury Association (ASIA)82 can be helpful in assessing or screening patients with spine pathology (see Fig. 11–5). Soft touch and pin-prick sensation can be assessed well in most patients, and the examiner should distinguish between a dermatomal distribution suggesting nerve root pathology, a stocking or stocking-and-glove distribution suggesting peripheral polyneuropathy, multiple nerve distribution suggesting alternative peripheral nerve pathology, or a nonorganic distribution. Proprioception, vibration, position sense, and temperature sensation may also be tested, particularly when there is concern for a spinal cord or central nervous system process or a peripheral neuropathy.
Motor examination consists of several parts, including strength, tone, coordination, muscle bulk, and involuntary movements.79 Strength is the modality most generally assessed by clinicians, but all portions of the motor examination may be important in some patients with spine disorders. Involuntary movements may be noted in patients with cervical dystonia or in various neurologic diseases that may affect function, such as Parkinson disease. The presence or absence of focal muscle atrophy should be noted in all patients. The mere presence of focal atrophy implies neurologic injury or disease, and the distribution of atrophic muscles can be helpful in defining the type of pathology present. Fasciculations associated with atrophic muscles imply the presence of lower motor neuron injury. Muscle tone can be affected by many neurologic processes. Reduced tone suggests lower motor neuron involvement, whereas increased tone or spasticity is seen with upper motor neuron disease. Coordination may be disrupted by numerous pathways, generally involving the cerebellum or its pathways, but weakness, proprioceptive loss, and cognitive disturbance may also affect motor performance on tests of coordination. Clinical methods to assess coordination include rapid alternating hand and foot movements and finger-to-nose testing.79
Strength is generally graded on a scale of 1 to 5 as follows:79
Active movement is generally meant to imply joint motion through the full available range of motion. For some muscle groups, patients can often have significant loss of strength that is not detectable by providing manual resistance with the examiner’s arms, and other test maneuvers may be necessary to identify more subtle weakness. Examples of such maneuvers would be having the patient do a partial squat or arise from sitting without using the upper extremities to assess for weakness in the knee extensors. Beevor sign (in which the umbilicus moves craniad during contraction of the abdominal muscles with supine neck flexion) indicates weakness of the lower abdominal muscles.23
Reflex testing can further aid in the localization of neurologic injury and help distinguish upper motor neuron from lower motor neuron disease. In lower motor neuron injuries, deep tendon reflexes of affected regions are generally reduced, whereas they are brisk in upper motor neuron injuries. Babinski response to appropriate plantar stimulation, Hoffman sign in the hand, and clonus all can indicate the presence of upper motor neuron injury. As with other physical examination findings, the sensitivity and specificity of these findings are limited for any particular condition. In a study assessing the prevalence of physical examination findings in cervical myelopathy treated surgically, it was noted that 21% of the patients had no myelopathic findings on examination. Of the findings just mentioned, Hoffman sign was the most sensitive (59%), whereas Babinski response had very low sensitivity (13%) but was highly specific.83 Various other reflexes, including abdominal, cremasteric, and palmomental, can also be used as part of the neurologic examination where appropriate.
Although a neurologic injury often manifests as either an upper or a lower motor neuron lesion, it can also manifest with a mixed pattern of upper and lower motor neuron features, as can be seen with amyotrophic lateral sclerosis. The segmental distribution of commonly tested deep tendon reflexes is as follows:79
For the most part, the sensitivity and specificity of isolated tests for sensation, strength, and reflexes are relatively limited in the assessment of spine conditions, particularly when any one single test is considered.22,77,84 There may be more utility in combining a variety of findings across multiple modalities, especially when the findings are consistently reproducible. The degree of consistency between examination findings, history, imaging results, and self-reported levels of pain and disability for affected patients should always be considered when clinical decisions on care are made.
Special Tests and Provocative Maneuvers
Lhermitte sign, although more technically a symptom, is the presence of an electric shock–type sensation radiating into the limbs with cervical flexion. Although first described in a patient with multiple sclerosis, this sign is associated with various spinal cord lesions.23,34 If elicited with neck flexion, this sign should raise concern for the presence of a cervical cord lesion. If elicited with trunk flexion, this may indicate a thoracic cord lesion.23
Spurling maneuver is a test for cervical nerve root compression or irritation. A positive test is elicited by extending, rotating, and laterally bending the head to one side with reproduction of radicular pain into the affected ipsilateral extremity.23,34 One study comparing Spurling maneuver with the results of electrodiagnostic testing found that the maneuver had poor sensitivity (30%) but good specificity (93%) in the diagnosis of electrodiagnostically confirmed cervical radiculopathy.85
Valsalva maneuver is performed by having a patient hold his or her breath and bear down. A reproduction of the patient’s radicular symptoms or spinal pain with this maneuver is believed to indicate a space-occupying lesion, such as a disc herniation, in the spinal canal.23,34
Dural tension signs are frequently used to assess lumbar spine pathology. Many different maneuvers have been described. A supine straight-leg raise is performed by elevating the leg with knee extended and assessing for the reproduction of pain into the leg. The test is considered positive if pain occurs between 30 degrees and 70 degrees of elevation because no true change in tension on the nerve roots is believed to occur outside of this range.3,77 Variations on this test include Lasègue sign or Bragard sign, which involves raising the leg to the point of symptom reproduction and then lowering the leg slightly and dorsiflexing the foot passively; a positive test results in reproduction of the patient’s radiating leg pain.3,86 Other variants include internally rotating the leg to increase “dural tension,” raising the leg with knee flexed and then slowly extending the knee to the point of reproduction of leg pain (also sometimes referred to as Lasègue sign), and either relieving pain by flexing the already extended knee at the point of symptom reproduction or eliciting pain by pressing on the popliteal fossa of the elevated leg with the knee partially flexed (both varyingly called the bowstring sign).3,77,84,86
Additional tests include the crossed straight-leg raise, in which symptoms are reproduced in the symptomatic leg by performing a supine straight-leg raise on the contralateral leg, and the femoral nerve stretch test or reverse straight-leg raise, in which the patient is prone and the knee is passively flexed, with a positive test reproducing pain into the anterior thigh. A positive straight-leg raise test and its variations indicates tension on the lower lumbar roots and upper sacral root (L4, L5, and S1 nerve roots). A positive femoral nerve stretch test is the equivalent tension sign for the upper lumbar (L2-4) nerve roots.3,77,84
Numerous studies have looked at the sensitivity and specificity of some of the above-mentioned maneuvers. As might be surmised by the varying descriptions and terminology, there are some difficulties with consistency in the literature. Overall, the ipsilateral straight-leg raise test has a good sensitivity of 72% to 97% but a poorer specificity of 11% to 66%.84 The crossed straight-leg raise test is less sensitive (23% to 42%) but more specific (85% to 100%) than the ipsilateral straight-leg raise.77,84
Tests proposed for assessing the sacroiliac joint include Gillet, Patrick, and Gaenslen tests. Although the sacroiliac joint can be a source of pain, the diagnosis of “sacroiliac joint dysfunction” is debated as a true pathologic entity. Dreyfuss and colleagues25 studied numerous supposedly diagnostic tests for this condition, including the Gillet, Patrick, and Gaenslen tests, and compared the responses on these test maneuvers with the results of fluoroscopically guided sacroiliac joint blocks. They found that no historical feature, none of the diagnostic tests performed, and no combination of these tests showed any significant and reliable diagnostic value.
Nonorganic Signs
Chronic pain behavior is often believed to display common physical examination findings suggesting symptom magnification and psychological distress, possibly an expression of suffering.87,88 Waddell and colleagues87 defined and studied a group of five findings on physical examination, commonly known as Waddell signs. These findings consist of a superficial or nonanatomic distribution of tenderness; a nonanatomic motor or sensory impairment (regional disturbance); excessive verbalization of pain or gesturing (overreaction); production of pain complaints by tests that simulate only a specific movement, such as low back pain that occurs with axial loading on the crown of the head (simulation); and inconsistent reports of pain when the same movement is performed in different positions, such as a straight-leg raise in a seated versus supine position (distraction).87
The presence of three or more of these signs indicates a nonorganic component to an individual’s pain complaints. The presence of Waddell signs does not mean, however, that there is no significant organic pathology present or that the patient is malingering, and objective clinical signs may be present as well. Although some studies have found these maneuvers to be reproducible, an evidence-based review by Fishbain and colleagues89 noted that these findings do not correlate with psychological distress or secondary gain, and they do not discriminate nonorganic from organic problems. They are associated with poorer treatment outcomes and higher pain levels. Although these maneuvers may be useful, the clinician should be wary of placing too much emphasis on any one part of the physical examination.
Additional Orthopaedic Assessment
Depending on the area of the spine involved, it is frequently important to cover additional areas of the orthopaedic examination. As was previously mentioned, examination of the shoulder complex is often necessary in evaluating the cervical and thoracic spine. Following the concept of the kinetic chain, it is also often helpful to assess multiple other joint structures and movement patterns from the feet up through the trunk to the neck, depending on the individual patient’s situation.90 For the lumbar spine, examination of the hip is also generally important, although examination of more distal lower extremity structures and more cranial regions of the spine and upper extremities may be necessary as well. Because other conditions such as carpal tunnel syndrome, ulnar neuropathy, brachial plexopathy, peroneal neuropathy, and femoral nerve injury (among others) can masquerade as radiculopathies, examination for these entities is also often indicated. As noted previously, an appropriate history can help greatly in defining the scope of examination necessary to evaluate a particular patient.
There is a large body of literature on manual orthopaedic examination.91,92 These techniques generally are poorly validated and of uncertain correlation to some of the more “objective” findings noted earlier. A systematic review of the literature on the reliability of palpatory examination maneuvers found that most procedures have moderate or strong evidence for low reliability.93 The authors noted that “a consistent finding from work in this field is the generally low reliability of palpation-based assessment.”93 These techniques may be helpful in certain treatment paradigms, however, and they may be more useful when symptom response with repeated movements is considered.93 Another systematic review assessed the literature on chiropractic tests of the lumbar spine and found insufficient evidence on the reliability and validity of these tests to support their clinical role.94
Conclusions
Key Points
1 Bogduk N. The anatomy and pathophysiology of neck pain. Phys Med Rehabil Clin N Am. 2003;14:455-472.
This is a concise overview of some important issues in assessing patients with neck pain.
2 Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268:760-765.
3 Linton SJ. A review of psychosocial risk factors in back and neck pain. Spine. 2000;25:1148-1156.
4 Gatchel RJ, editor. Compendium of Outcome Instruments for Assessment and Research of Spinal Disorders. La Grange, IL: North American Spine Society, 2001.
5 Solomon J, Nadler SF, Press J. Physical examination: Of the lumbar spine. In: Malanga G, Nadler SF, editors. Musculoskeletal Physical Examination: An Evidence-based Approach. Philadelphia: Hanley & Belfus; 2006:189-226.
6 American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury (reprint). Chicago: American Spinal Injury Association; 2008.
1 Bogduk N. The anatomy and pathophysiology of neck pain. Phys Med Rehabil Clin N Am. 2003;14:455-472.
2 Bogduk N. Clinical Anatomy of the Lumbar Spine and Sacrum, 3rd ed. New York: Churchill-Livingstone; 1997.
3 Borenstein DG, Wiesel SW. Low Back Pain: Medical Diagnosis and Comprehensive Management. Philadelphia: WB Saunders; 1989.
4 Liss H, Liss D, Pavell J. History and past medical history. In: Cole AJ, Herring SA, editors. The Low Back Pain Handbook. 2nd ed. Philadelphia: Hanley & Belfus; 2003:49-67.
5 Weinstein SM, Herring SA, Standaert CJ. Low back pain. In: Delisa JA, Gans BM, Walsh NE, editors. Physical Medicine and Rehabilitation: Principles and Practice. 4th ed. Philadelphia: Lippincott-Williams & Wilkins; 2005:653-678.
6 Dwyer A, Aprill C, Bogduk N. Cervical zygapophyseal joint pain patterns I: A study in normal volunteers. Spine. 1990;15:453-457.
7 Aprill C, Dwyer A, Bogdul N. Cervical zygapophyseal joint pain patterns II: A clinical evaluation. Spine. 1990;15:458-461.
8 Lord SM, Barnsley L, Wallis BJ, et al. Chronic cervical zygapophyseal joint pain after whiplash: A placebo-controlled prevalence study. Spine. 1996;21:1737-1745.
9 Dreyfuss P, Tibiletti C, Dreyer S. Thoracic zygapophyseal joint pain patterns: A study in normal volunteers. Spine. 1994;19:807-811.
10 Schwarzer AC, Aprill CN, Derby R, et al. Clinical features of patients with pain stemming from the lumbar zygapophysial joints: Is the lumbar facet syndrome a clinical entity? Spine. 1994;19:1132-1137.
11 Marks R. Distribution of pain provoked from lumbar facet joints and related structures during diagnostic spinal infiltration. Pain. 1989;39:37-40.
12 Cloward RB. Cervical discography: A contribution to the etiology and mechanism of neck, shoulder and arm pain. Ann Surg. 1959;150:1052-1064.
13 Grubb SA, Kelly CK. Cervical discography: Clinical implications from 12 years of experience. Spine. 2000;25:1382-1389.
14 Campbell JN. Nerve lesions and the generation of pain. Muscle Nerve. 2001;24:1261-1273.
15 Carragee EJ, Alamin TF. Discography: A review. Spine J. 2001;1:364-372.
16 Hogan Q. Back pain: Beguiling physiology (and politics). Reg Anesth. 1997;22:395-399.
17 Sinclair JD. Chronic noncancer pain basics for the primary care physician. Primary Care Rep. 2002;8:63-73.
18 Sheather-Reid RB, Cohen ML. Psychophysiological evidence for a neuropathic component of chronic neck pain. Pain. 1998;75:341-347.
19 Agency for Health Care Policy and Research. Acute Low Back Problems in Adults: Assessment and Treatment. Washington, DC: US Department of Health and Human Services; 1994.
20 Akuthota V, Willick SE, Harden RN. The adult spine: A practical approach to low back pain. In: Rucker KS, Cole AJ, Weinstein SM, editors. Low Back Pain: A Symptom-based Approach to Diagnosis and Treatment. Boston: Butterworth-Heinemann; 2001:15-41.
21 Carragee EJ, Hannibal M. Diagnostic evaluation of low back pain. Orthop Clin North Am. 2004;35:7-16.
22 Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268:760-765.
23 Bland JH. Disorders of the Cervical Spine: Diagnosis and Medical Management, 2nd ed. Philadelphia: WB Saunders; 1994.
24 Mazanec D. Pseudospine pain: Conditions that mimic spine pain. In: Cole AJ, Herring SA, editors. The Low Back Pain Handbook. 2nd ed. Philadelphia: Hanley & Belfus; 2003:117-131.
25 Dreyfuss P, Michaelsen M, Pauza K, et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21:2594-2602.
26 Micheli LJ. Back pain in young athletes: Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149:15-18.
27 Roche MA, Rowe GG. The incidence of separate neural arch and coincident bone variations: A survey of 4,200 skeletons. Anat Rec. 1951;109:233-252.
28 Standaert CJ, Herring SA. Spondylolysis: A critical review. Br J Sports Med. 2000;34:415-422.
29 Burton AK, Clarke RD, McClune TD, et al. The natural history of low back pain in adolescents. Spine. 1996;21:2323-2328.
30 Kovacs FM, Gestoso M, Gil del Real MT, et al. Risk factors for non-specific low back pain in schoolchildren and their parents: A population based study. Pain. 2003;103:259-268.
31 Andersen SJ. Adolescent lumbar spine disorders. In: Rucker KS, Cole AJ, Weinstein SM, editors. Low Back Pain: A Symptom-based Approach to Diagnosis and Treatment. Boston: Butterworth-Heinemann; 2001:3-14.
32 Hosalkar H, Dormans J. Back pain in children requires extensive workup. Biomechanics. 2003;10:51-58.
33 Malanga GA, Nadler SF, Ageson T. Epidemiology. In: Cole AJ, Herring SA, editors. The Low Back Pain Handbook. 2nd ed. Philadelphia: Hanley & Belfus; 2003:1-7.
34 Borenstein DG, Wiesel SW, Boden SD. Neck Pain: Medical Diagnosis and Comprehensive Management. Philadelphia: WB Saunders; 1996.
35 Bovim G, Schrader H, Sand T. Neck pain in the general population. Spine. 1994;19:1307-1309.
36 Makela M, Heliovaara M, Sievers K, et al. Prevalence, determinants, and consequences of chronic neck pain in Finland. Am J Epidemiol. 1991;134:1356-1367.
37 Volinn E. The epidemiology of low back pain in the rest of the world: A review of surveys in low- and middle-income countries. Spine. 1997;22:1747-1754.
38 Belanger TA, Rowe DE. Diffuse idiopathic skeletal hyperostosis: Musculoskeletal manifestations. J Am Acad Orthop Surg. 2001;9:258-267.
39 Paassilta P, Lohiniva J, Goring HH, et al. Identification of a novel common genetic risk factor for lumbar disk disease. JAMA. 2001;285:1843-1849.
40 Canoso JJ. Rheumatology in Primary Care. Philadelphia: WB Saunders; 1997.
41 Gaunt AM. Caring for patients who have acute and subacute low back pain. CME Bull. 2008;7:1-7.
42 Linton SJ. A review of psychosocial risk factors in back and neck pain. Spine. 2000;25:1148-1156.
43 Valat JP, Goupille P, Vedere V. Low back pain: risk factors for chronicity. Rev Rheum [Engl Ed]. 1997;64:189-194.
44 van der Giezen AM, Bouter LM, Nijhuis FJN. Prediction of return-to-work of low back pain patients sicklisted 3-4 months. Pain. 2000;87:285-294.
45 Fransen M, Woodward M, Norton R, et al. Risk factors associated with the transition from acute to chronic occupational back pain. Spine. 2002;27:92-98.
46 Radanov BP, Sturzenegger M. The effect of accident mechanisms and initial findings on the long-term outcome of whiplash injury. J Musculoskelet Pain. 1996;4:47-59.
47 McIntosh G, Frank J, Hogg-Johnson S, et al. Prognostic factors for time receiving workers’ compensation benefits in a cohort of patients with low back pain. Spine. 2000;25:147-157.
48 Krause N, Ragland DR, Greiner BA, et al. Physical workload and ergonomic factors associated with prevalence of back and neck pain in urban transit operators. Spine. 1997;22:2117-2126.
49 McGill CM. Industrial back problems: A control program. J Occup Med. 1968;10:174-178.
50 Waddell G. Epidemiology: A new clinical model for the treatment of low back pain. In: Weinstein JN, Wiesel SW, editors. The Lumbar Spine: The International Society for the Study of the Lumbar Spine. Philadelphia: Saunders; 1990:38-56.
51 Pincus T, Burton AK, Vogel S, et al. A systematic review of psychosocial factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine. 2002;27:E109-E120.
52 Gatchel RJ, Polatin PB, Mayer TG. The dominant role of psychosocial risk factors in the development of chronic low back pain disability. Spine. 1995;20:2702-2709.
53 Wallis BJ, Lord SM, Bogduk N. Resolution of psychological distress of whiplash patients following treatment by radiofrequency neurotomy: A randomized, double-blind, placebo-controlled trial. Pain. 1997;73:15-22.
54 Carroll LJ, Cassidy JD. Cote P: Depression as a risk factor for onset of an episode of troublesome neck and low back pain. Pain. 2004;107:134-139.
55 DeBerard MS, Masters KS, Colledge AL, et al. Outcomes of posterolateral lumbar fusion in Utah patients receiving workers’ compensation: A retrospective cohort study. Spine. 2001;26:738-747.
56 Junge A, Dvorak J, Ahrens S. Predictors of bad and good outcomes of lumbar disc surgery: A prospective clinical study with recommendations for screening to avoid bad outcomes. Spine. 1995;20:460-468.
57 Loupasis GA, Stamos K, Katonis PG, et al. Seven to 20-year outcome of lumbar discectomy. Spine. 1999;24:2313-2317.
58 Pappas CTE, Harrington T, Sonntag VK. Outcome analysis in 654 surgically treated lumbar disc herniations. Neurosurgery. 1992;30:862-866.
59 Keller RB, Atlas SJ, Soule DN, et al. Relationship between rates and outcomes of operative treatment for lumbar disc herniation and spinal stenosis. J Bone Joint Surg Am. 1999;81:752-762.
60 Fishbain DA, Goldberg M, Meagher BR, et al. Male and female chronic pain patients categorized by DSN-III psychiatric diagnostic criteria. Pain. 1986;26:181-197.
61 Ormel J, VonKorff M, Ustun TB, et al. Common mental disorders and disability across cultures: Results from the WHO Collaborative Study on Psychological Problems in General Health Care. JAMA. 1994;272:1741-1748.
62 Hagg O, Fritzell P, Hedlund R, et al. Pain-drawing does not predict the outcome of fusion surgery for chronic low-back pain: A report from the Swedish Lumbar Spine Study. Eur Spine J. 2003;12:2-11.
63 Ohnmeiss DD. Repeatability of pain drawings in a low back pain population. Spine. 2000;25:980-988.
64 Dahl B, Gehrchen PM, Kiaer T, et al. Nonorganic pain drawings are associated with low psychological scores on the preoperative SF-36 questionnaire in patients with chronic low back pain. Eur Spine J. 2001;10:211-214.
65 Carnes D, Ashbey D, Underwood M. A systematic review of pain drawing literature: Should pain drawings be used for psychologic screening? Clin J Pain. 2006;22:449-457.
66 Gatchel RJ, editor. Compendium of Outcome Instruments for Assessment and Research of Spinal Disorders. La Grange, IL: North American Spine Society, 2001.
67 Patrick DL, Deyo RA, Atlas SJ, et al. Assessing health-related quality of life in patients with sciatica. Spine. 1995;20:1899-1908.
68 Fairbank JCT, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271-273.
69 Millard RW. A critical review of questionnaires for assessing pain-related disability. J Occup Rehabil. 1991;1:289-302.
70 Vernon H, Mior S. The neck disability index: A study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409-415.
71 Waddell G, Newton M, Henderson I, et al. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic back pain and disability. Pain. 1993;52:157-168.
72 Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: Development and validation. Psychol Assess. 1995;7:524-532.
73 Webster LR, Webster RM. Predicting aberrant behaviours in opioid treated patients: Preliminary validation of the opioid risk tool. Pain Med. 2005;6:432-442.
74 McGregor AH, McCarthy ID, Hughes SP. Motion characteristics of the lumbar spine in the normal population. Spine. 1995;20:2421-2428.
75 Nattrass CL, Nitschke JE, Disler PB, et al. Lumbar spine range of motion as a measure of physical and functional impairment: An investigation of validity. Clin Rehabil. 1999;13:211-218.
76 Ng JKF, Kippers V, Richardson CA, et al. Range of motion and lordosis of the lumbar spine: Reliability and measurement of normative values. Spine. 2001;26:53-60.
77 Solomon J, Nadler SF, Press J. Physical examination: Of the lumbar spine. In: Malanga G, Nadler SF, editors. Musculoskeletal Physical Examination: An Evidence-based Approach. Philadelphia: Hanley & Belfus; 2006:189-226.
78 Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: Spectrum of pathology part III: The SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19:641-661.
79 Bates B. A Guide to Physical Examination and History Taking, 5th ed. Philadelphia: JB Lippincott; 1991.
80 Bradley WG, Daroff RB, Fenichel GM, et al, editors. Neurology in Clinical Practice: Principles of Diagnosis and Management, 5th ed, Boston: Butterworth Heinemann, 2007.
81 Goetz CG, editor. Textbook of Clinical Neurology, 3rd ed, Philadelphia: WB Saunders, 2007.
82 American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury (reprint). Chicago, American Spinal Injury Association, 2008.
83 Rhee JM, Heflin JA, Hamasaki T, et al. Prevalence of physical signs in cervical myelopathy: A prospective, controlled study. Spine. 2009;34:890-895.
84 Andersson GBJ, Deyo RA. History and physical examination in patients with herniated lumbar discs. Spine. 1996;21:10S-18S.
85 Tong HC, Haig AJ, Yamakawa K. The Spurling test and cervical radiculopathy. Spine. 2002;27:156-159.
86 Supik LF, Broom MJ. Sciatic tension signs and lumbar disc herniation. Spine. 1994;19:1066-1069.
87 Waddell G, McCulloch JA, Kummel E, et al. Nonorganic physical signs in low-back pain. Spine. 1980;5:117-125.
88 Maruta T, Goldman S, Chan CW, et al. Waddell’s nonorganic signs and Minnesota Multiphasic Personality Inventory profiles in patients with chronic low back pain. Spine. 1997;22:72-75.
89 Fishbain DA, Cole B, Cutler RB, et al. A structured evidence-based review on the meaning of nonorganic physical signs: Waddell signs. Pain Med. 2003;4:141-181.
90 Kibler WB. Determining the extent of the functional deficit. In: Kibler WB, Herring SA, Press JM, et al, editors. Functional Rehabilitation of Sports and Musculoskeletal Injuries. Gaithersburg, MD: Aspen; 1998:1-8.
91 Basmajian JV, Nyberg R, editors. Rational Manual Therapies. Baltimore: William & Wilkins, 1993.
92 Brieve GP. Mobilization of the Spine, 4th ed. New York: Churchill-Livingstone; 1984.
93 May S, Littlewood C, Bishop A. Reliability of procedures used in the physical examination of non-specific low back pain: A systematic review. Aust J Physiother. 2006;52:91-102.
94 Hestbaek L, Leboeuf-Yde C. Are chiropractic tests for the lumbo-pelvic spine reliable and valid? A systematic critical literature review. J Manipulative Physiol Ther. 2000;23:258-275.