CHAPTER 26 The Painful Medial Unicompartmental Knee Arthroplasty
 Careful implant sizing in the medial-lateral position is critical to avoid overhang and possibly pain following medial mobile-bearing UKA.
Careful implant sizing in the medial-lateral position is critical to avoid overhang and possibly pain following medial mobile-bearing UKA. Many patients undergoing UKA have anterior knee pain; medial PFJ changes are associated with outcomes similar to those without changes, and pain resolves in most patients.
Many patients undergoing UKA have anterior knee pain; medial PFJ changes are associated with outcomes similar to those without changes, and pain resolves in most patients. Aspirations should be performed to examine for current hemarthroses, infection, and crystalline arthropathy.
Aspirations should be performed to examine for current hemarthroses, infection, and crystalline arthropathy.Introduction
Long-term survivorship of unicompartmental knee arthroplasty (UKA) has increased with contemporary prosthetic designs and improved patient selection and has been reported to be between 96% and 98% at 10–13 years of follow-up.1–4 Failure leading to revision in UKA has been ascribed to progression of arthritis in retained compartments, polyethylene wear, patient selection, implant malpositioning, loosening, fracture, and persistent pain.1–5 This chapter describes the evaluation and management of a painful UKA. A differential diagnosis is outlined in Box 26–1. Clinical and radiographic evaluations should seek to understand the diagnoses that may account for pain with well-positioned and stable implants. In addition, the assumption that the painful UKA will be solved with a conversion to a total knee arthroplasty (TKA) is discussed.
Often the evaluation of a painful UKA may lead the surgeon biased against medial UKA to believe that a TKA should have been indicated as the original arthroplasty. The reality, however, is that not all TKAs are pain free,6 with a series by Price et al. reporting pain at midterm follow-up at 41%. In addition, patient satisfaction following TKA may not be as high as assumed by many surgeons (many of whom do not perform UKAs), as Bourne et al. reported a 19% patient dissatisfaction rate in a large cohort of TKAs.7 Finally, the decision to revise a painful UKA to a TKA may have a lower threshold than a painful TKA and must be carefully considered. This “threshold for revision” may underscore higher revision rates in national registry data. A UKA may also have been performed prematurely without full-thickness cartilage loss and result in incomplete pain relief following the arthroplasty.8
Clinical Evaluation
The “appropriate” indications for medial UKA have long been debated.9–12 Some have suggested a combination of patient factors and examination findings that may limit the number of appropriate candidates to 4–6% of varus knees.9–12 Others have followed more physiologic criteria as documented by “anteromedial osteoarthritis” with intact collateral and cruciate ligaments, which may increase the percentage of varus knees that are appropriate candidates for medial UKA to as high as 30%. The published long-term data support the latter approach.4 The status of the patellofemoral joint (PFJ) as a contraindication to UKA is misunderstood, and long-term data by Beard et al.13,14 shed important light on this subject, demonstrating that anterior knee pain resolves after medial UKA with central and medial PFJ degeneration. Further, anterior knee pain does not correlate with intraoperative or radiographic findings in patients with anteromedial osteoarthritis (OA). There are no published data to refute this approach of largely ignoring the PFJ in patients with anteromedial OA of the knee and proceeding with medial UKA.
Radiographic Evaluation
Weight-bearing anteroposterior, lateral, and sunrise patellar radiographic views should be obtained and assessed. Review of the original preoperative radiographs is also quite helpful as partial-thickness cartilage loss has been associated with inconsistent pain relief following UKA.8 This may be an indication that the early medial degenerative changes did not fully account for the preoperative knee pain leading to the UKA. Tibial implant overhang greater than 3 mm has been associated with increased pain following medial mobile-bearing UKA.15 Correlation of pain and implant position—most notably overhang with other implant designs—is needed. Careful implant sizing in the medial-lateral position is important to avoid this scenario.
Anterior Knee Pain and Radiographic Appearance
Anterior knee pain and radiographic signs of PFJ degeneration have been considered contraindications to UKA and a possible source of pain after UKA. It is interesting to reflect on how these unnecessary contraindications developed. Kozinn and Scott10 suggested PFJ degeneration as a contraindication to a medial UKA and therefore have no data to support that ignoring anterior knee pain and PFJ degeneration leads to persistent pain and progressive deterioration of the knee. Beard et al.,13,14 however, reported on the outcomes of medial UKA in such patients. These data, rather than prior hypotheses and historical traditions, should dictate our clinical practice. These authors found that over half (54%) of patients undergoing UKA had anterior knee pain. Furthermore, 54% of patients had degenerative changes observed on preoperative skyline radiographs. Medial PFJ changes were associated with outcomes similar to those knees without changes. Furthermore, anterior knee pain resolved in all patients. Our historical suspicion of all anterior knee pain originating from the PFJ appears not to be supported by published evidence as it resolved following medial UKA for anteromedial OA.13
Pain and Radiolucencies
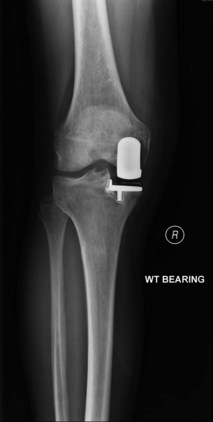
An important consideration is the radiographic appearance of a medial UKA. In the United States, the majority of surgeons follow their UKA patients with standard radiographs. However, radiolucent lines (RLL) often are detected only on “screened” radiographs16 (Fig. 26–1). The Oxford group (Nuffield Orthopaedic Centre) has long utilized a screened or fluoroscopic imaging system to track the radiographic appearance of medial UKAs in the long term. This is a critical point as radiolucencies beneath the tibial tray are often misinterpreted to be a source of pain and result in unnecessary revision.17 Progressive RLL with implant migration indicates implant loosening; however, a stable radiolucency often does not. Retrieval of well-functioning implants has demonstrated a partial fibrocartilage layer that may be a biologic response to loading conditions.16 In other words, RLL are quite common and not always indicative of a pathologic process. The incidence of RLL is highly variable and is directly related to screened (fluoroscopic) versus nonscreened films. Small changes in the tilt of the x-ray beam may obscure RLL. RLL alone must be treated with great care as pain and the presence of RLL after UKA have not been clearly linked.
Additional Radiographic and Related Evaluations
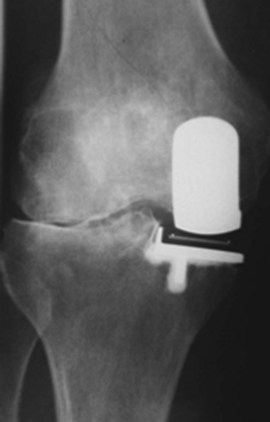
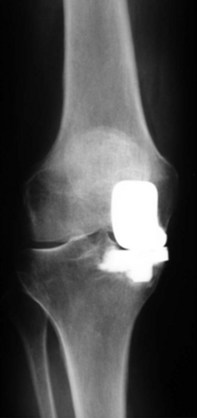
The lateral compartment of the knee may show joint space collapse in the second decade and lead to revision.18,19 It may also be an indication of unrecognized inflammatory arthritis (Fig. 26–2). Polymer wear is also noted (Fig. 26–3) on standard radiographs and is more common in fixed-bearing devices, especially in those with oxidized polyethylene and long shelf lives prior to implantation. Bone scans may be “hot” or show increased activity in the medial compartment for many years after UKA and must be interpreted with caution in the face of a painful UKA. Aspirations should be performed to examine for recurrent hemarthroses, infection, and crystalline arthropathy. Routine synovial fluid analysis is performed.
Biomechanics of UKA Loading … A Possible Explanation of Pain?
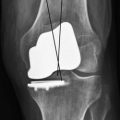
The in vivo loading conditions of a medial UKA and the associated osseous and soft tissue adaptations are not well understood. We have hypothesized that changes in loading, as quantified by osseous stresses and strains, may at least in part explain the persistence and resolution of pain following a medial UKA. We have reported on the effects of significant overload of the tibial bone leading to implant subsidence and bony collapse in some fixed-bearing UKA designs.5 In our study of 32 consecutive UKA revisions of both metal-backed and all-polyethylene tibial component designs, we observed medial tibia collapse in 47% of the cases studied (Fig. 26–4); half of these occurred early in the postoperative course, within 16 months of the surgery. We found increased tibial slope was associated with tibial collapse, with increased posterior slope in those cases that collapsed posteriorly as opposed to in anterior collapse. Hernigou et al.20 reported similar influences of tibial slope on failure mechanisms following a UKA. While some studies have reported relatively clear-cut conversion to TKA for common UKA failure modes, this study noted the increased complexity of revision in cases of medial collapse as requiring screws, augments, and ancillary bone cement during revision to TKA. We have also quantified the influence of knee kinematics21 and metal backing on the loading patterns of metal-backed mobile-bearing and all-polyethylene fixed-bearing medial UKAs.22 We found metal-backed tibial implants demonstrated more diffuse loading distributions while all-polyethylene tibial components had more focal loading concentrations (Fig. 26–5). We simulated the flexion and extension of the knee experienced during gait and dynamic loading conditions and demonstrated that both component designs demonstrated significantly different loading patterns based on loading contact position. More work is needed in this area to further characterize the effects of these laboratory findings and postoperative pain in a medial UKA.
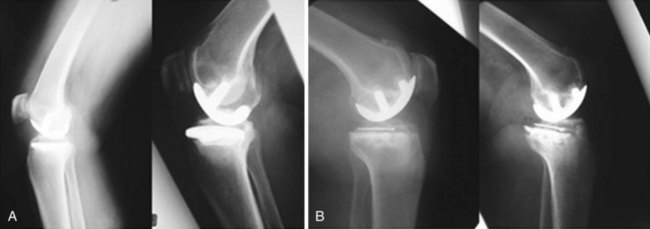
Figure 26–4 (A) UKA tibial component failure due to posterior tibial collapse. (B) Anterior tibial bony collapse.
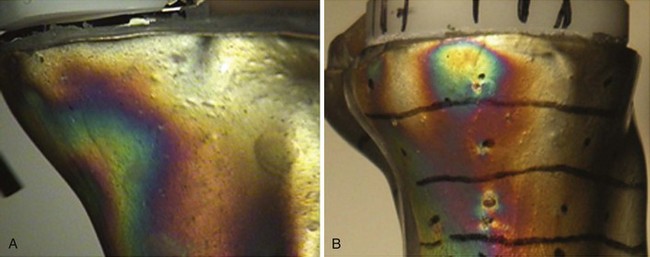
Figure 26–5 (A) Diffuse strain distribution during loading of a metal-backed UKA tibial component with the bearing in the anterior position simulating heel strike and knee extension.21 (B) Localized strain distribution during loading of an all-polyethylene UKA tibial component in a posterior position simulating deep flexion and femoral roll-back.22
1 Squire MW, Callaghan JJ, Goetz DD, et al, Unicompartmental knee replacement: a minimum 15 year follow-up study. Clin Orthop Relat Res, 367, 1999, 61-72.
2 Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg [Am]. 2005;87:999-1006.
3 Price AJ, Waite JC, Svärd U, Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res, 435, 2005, 171-180.
4 Murray DW, Goodfellow JW, O’Connor JJ. The Oxford medial unicompartmental arthroplasty: a ten-year survival study. J Bone Joint Surg [Br]. 1998;80:983-989.
5 Aleto TJ, Berend ME, Ritter MA, et al. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23:159-163.
6 Price AJ, Longino D, Rees J, et al. Are pain and function better measures of outcome than revision rates after TKR in the younger patient? Knee. 2010;17:196-199.
7 Bourne RB, Chesworth BM, Davis AM, et al, Patient satisfaction after total knee arthroplasty: who is satisfied and who is not?. Clin Orthop Relat Res, 468, 2010, 57-63.
8 Pandit H, Gulati A, Jenkins C, et al. Unicompartmental knee replacement for patients with partial thickness cartilage loss in the affected compartment. Knee. 2010. June 1. [Epub ahead of print]
9 Stern SH, Becker MW, Insall JN, Unicondylar knee arthroplasty: an evaluation of selection criteria. Clin Orthop Relat Res, 286, 1993, 143-148.
10 Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg [Am]. 1989;71:145-150.
11 Kozinn SC, Marx C, Scott RD. Unicompartmental knee arthroplasty: a 4.5–6-year follow-up study with a metal-backed tibial component. J Arthroplasty. 1989;4(Suppl):S1-S10.
12 Kozinn SC, Scott RD. Surgical treatment of unicompartmental degenerative arthritis of the knee. Rheum Dis Clin North Am. 1988;14:545-564.
13 Beard DJ, Pandit H, Ostlere S, et al. Pre-operative clinical and radiological assessment of the patellofemoral joint in unicompartmental knee replacement and its influence on outcome. J Bone Joint Surg [Br]. 2007;89:1602-1607.
14 Beard DJ, Pandit H, Gill HS, et al. The influence of the presence and severity of pre-existing patellofemoral degenerative changes on the outcome of the Oxford medial unicompartmental knee replacement. J Bone Joint Surg [Br]. 2007;89:1597-1601.
15 Chau R, Gulati A, Pandit H, et al. Tibial component overhang following unicompartmental knee replacement—does it matter? Knee. 2009;16:310-313.
16 Tibrewal SB, Grant KA, Goodfellow JW. The radiolucent line beneath the tibial components of the Oxford meniscal knee. J Bone Joint Surg [Br]. 1984;66:523-528.
17 Gulati A, Chau R, Pandit HG, et al. The incidence of physiological radiolucency following Oxford unicompartmental knee replacement and its relationship to outcome. J Bone Joint Surg [Br]. 2009;91:896-902.
18 Emerson RHJr, Higgins LL. Unicompartmental knee arthroplasty with the Oxford prosthesis in patients with medial compartment arthritis. J Bone Joint Surg [Am]. 2008;90:118-122.
19 Collier MB, Eickmann TH, Anbari KK, Engh GA, Lateral tibiofemoral compartment narrowing after medial unicondylar arthroplasty. Clin Orthop Relat Res, 464, 2007, 43-52.
20 Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. Bone Joint Surg [Am]. 2004;86:506-511.
21 Small SR, Berend ME, Ritter MA, Buckley CA. Bearing mobility affects tibial strain in mobile-bearing unicompartmental knee arthroplasty. Surg Technol Int. 2010;19:185-190.
22 Small SR, Berend ME, Ritter MA, et al. Metal backing significantly decreases tibial strain in medial unicompartmental knee arthroplasty model. J Arthoplasty. 2010. September 14. [Epub ahead of print]