The New Perspectives on the Face+
What Constitutes a Face?
Structural Definitions
There are several ways to view faces. Figure 1-1 shows two fish.14 If two eyes and a mouth define a face, then fish have faces. They may have one or two nostrils, which are not connected to the mouth. However, water entering the nostrils does bathe the olfactory mucosa. Fish lack an ear canal, but the inner ear is present. Weber’s bones connect the swim bladder to the inner ear, and sound is transmitted.10
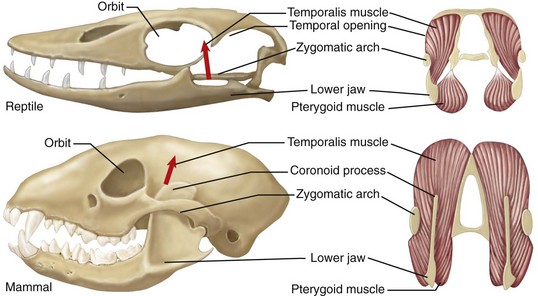
Another definition requires evolutionary transformation of the skull, in which a face is recognizable in mammals, but not in fish, amphibians, or reptiles (Fig. 1-2). The jaw is suspended from the braincase in reptiles. Mammals, however, have three ear ossicles, a secondary palate separating the airway passage from the mouth, and vertical positioning of the dentary.35
Behavioral Definitions
All animals communicate with each other in various ways by tactile, chemical, visual, and acoustical signaling. Insects have heads, but do they have faces? Paper wasps (Polistes dominulus) signal their status to each other by the number of black splotches on their yellow faces (Fig. 1-3).41

Figure 1-3 Wasp faces signal their status to each other by the number of black splotches on their yellow faces. The more blotches, the higher the status. Copyright 2004 Elizabeth Tibbetts. Modified by M. Michael Cohen Jr.
Some define faces by the presence and use of facial muscles, which do not exist in fish, amphibians, reptiles, or birds. In contrast, mammals can suckle and later chew, supported by a muscular tongue and movable lips and cheeks (Fig. 1-4). Often, the muscles in the ears can change positions to aid in hearing; a movable nose for smelling and touching, and facial hair, the vibrissae, are associated with musculature and serve as tactile organs.10

Figure 1-4 Mammals can suckle and later chew, supported by a muscular tongue and movable lips and cheeks.
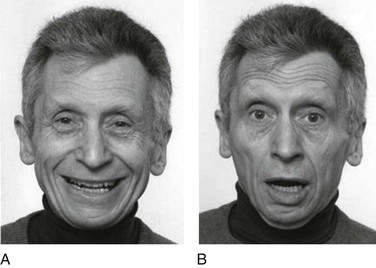
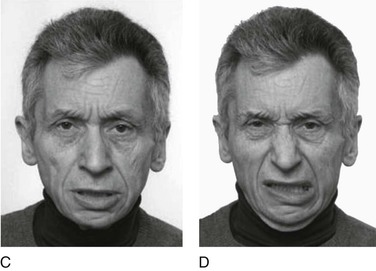
For some, a behavioral definition must include facial expressions, which are found in various primates (Fig. 1-5), including humans (Fig. 1-6).10
Facial Perspectives and Stages in the Life Cycle
Box 1-1 summarizes all possible perspectives from which the face can be described; Box 1-2 lists all the stages in the life cycle; and Box 1-3 lists the origin of some craniofacial components. Figure 1-9 shows embryonic facial development at approximately 42 to 44 days.25 Figure 1-10 shows skull molding in a newborn. Figure 1-11 shows the face of a small child, and Figure 1-12 shows a painting, The Stages of Human Life, by Hans Baldung Grien. In this allegory, a young woman, an old woman, and a dead woman are linked by their hands and arms.4 The dead woman holds an hourglass timer, indicating that life is over.
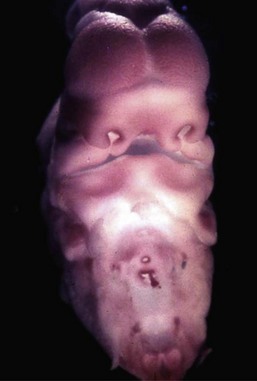
Figure 1-9 Embryo of about 42 to 44 days. The developing face has a frontal area with bulging cerebral hemispheres; a nasodorsal center with nasoseptal and nasozygomatic portions; nasal pits delineated by nasal ridges with premaxillary, medionasal, and lateronasal portions; maxillary primordia; interpremaxillary depression; a premaxillary–maxillary junction; and a lower jaw. Courtesy of Jan E. Jirásek, Prague.
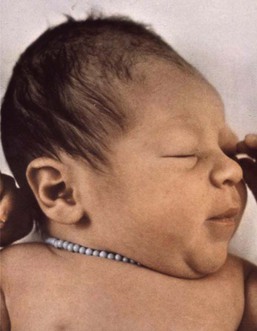
Figure 1-10 Molding of the head caused by compression during passage through the birth canal. From Cohen, 2006.
Evolutionary Considerations
Evolution of the Mammalian Brain
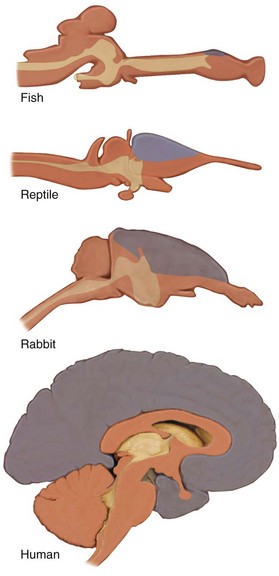
The evolution of the mammalian brain is based on the development of the neocortex (Fig. 1-13), which also resulted in a cranial shape that was different than that of the dinosaurs. The coexistence of dinosaurs and mammals and their competition during the Mesozoic era made mammalian neocortical development possible.5
Molecular Components in Primate Brain and Craniofacial Evolution
The gene Microcephalin (MCPH1) was first identified in its mutant form in which it causes primary microcephaly, but the normal gene was then adaptively found to be important in regulating brain size, and it continues to evolve in humans.18
Homozygous ASPM mutations also cause microcephaly. The normal gene may regulate neural stem cell proliferation and/or differentiation, possibly by mediating spindle-cell assembly during cell division.31
SIGLEC11, a gene involved in sialic acid biology, is expressed in high concentration in microglial cells in the human brain, but only occasionally in the cells of chimpanzees.24
The Ret finger protein-like 1,2,3 (RFPL 1,2,3) genes on chromosome 22 are evolutionary forces that play a role in neocortical development.3
GTF2IRD1—a gene that, when mutated, causes craniofacial anomalies—has been shown in its normal form to be a regulatory determinant of craniofacial development.39
Comparison of Different Skulls
Figure 1-14 shows the orientation of the foramen magnum and the anterior cranial base in a rodent and a human. The foramen magnum is posteriorly placed in the rodent and vertically placed in the human. The anterior cranial floor and the cribriform plate face forward in the rodent and are vertically placed in the human.16 Figure 1-15 shows a dog’s skull compared to a Chevrolet Corvette in contrast to a human skull compared to a camper, indicating an expanded, upright forehead above the face.17
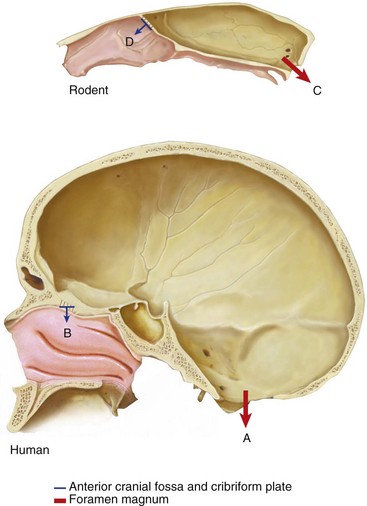
Figure 1-14 Orientation of the foramen magnum and the anterior cranial base in a human and a rodent. The foramen magnum is vertically placed in the human (A) and posteriorly placed in the rodent (C). The anterior cranial floor and the cribriform plate face downward in the human (B) and forward in the rodent (D). Based on Enlow, 1968.


Figure 1-15 Top, Lateral view of a dog skull compared to a Chevrolet Corvette; the face is anterior to the cranium. Bottom, Lateral view of a human skull compared to a camper; the expanded forebrain results in an upright forehead above the face. From Enlow, 1990.
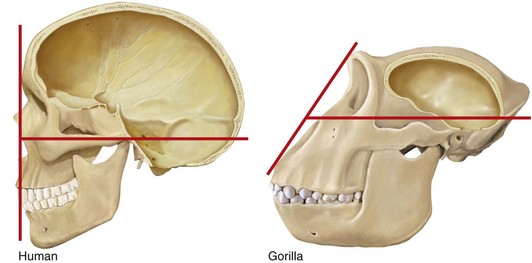
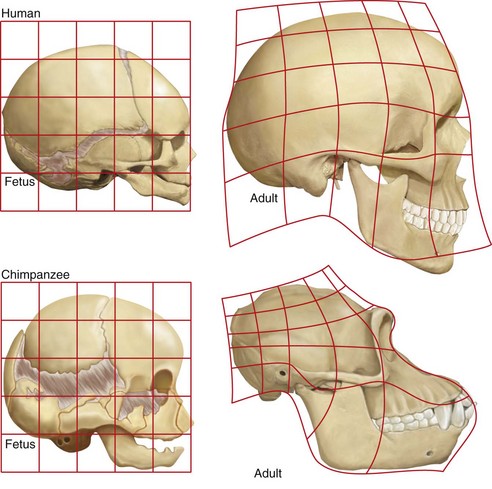
In Figure 1-16, expansion of the brain and a reduction in human facial prominence results in a flat facial profile compared with a smaller brain and a more protruding gorilla face, resulting in a sloping facial angle.10 Figure 1-17 compares the growth pattern of a fetal chimp with that of a human fetus. Both fetuses look similar. However, compared with the adult chimp, the adult human more closely resembles its own fetal pattern. Neoteny—a slowdown in the growth rate with a delay in maturation—has occurred in human evolution.23
Comparison of Homo sapiens, Homo neanderthalensis, and Homo floresiensis
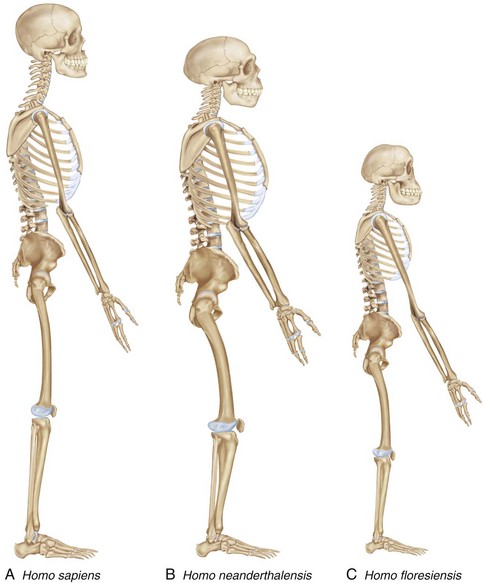
Evidence now shows that there are three separate species of hominins. Figure 1-18 compares the skulls of Homo neanderthalensis with Homo sapiens. The braincase of modern humans is relatively shorter, and the forehead is rounder, higher, and has a nearly vertical slope. Neanderthals have large brow ridges, projecting midface, elongated skull, occipital protuberance, a skull capacity 10% greater than that of modern humans, and a distinctive bony labyrinth not found in humans.27 DNA sequence comparisons show that Neanderthals fall outside the variation of modern humans. Molecular divergence provides a date of over 500,000 years.28
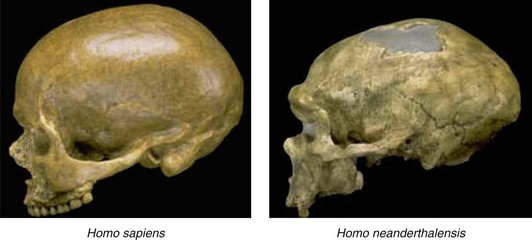
Figure 1-18 Lateral view of the skulls of Homo sapiens and Homo neanderthalensis. See text for description.
Figure 1-19 contrasts the phenotypes of the different species of hominins. Homo neanderthalensis has an elongated skull with a larger endocranial capacity than in humans; a distinctive bony labyrinth not found in humans (not shown); a robust skeleton with a barrel-shaped ribcage; a long superior pubic ramus; long clavicles; thick, bowed femoral shafts; large patellas; and large, round, terminal phalanges (fingers). Homo floresiensis has a tiny skull with a small endocranial capacity (380 cm3 to 430 cm3), a chinless mandible, a diminutive body (~1 m in height), long arms in relation to the legs (arms hang almost to the knees), unusual shoulders, a wider pelvis than in humans, and large feet (more than 7½ inches long and out of proportion with the short lower limbs, flat feet, and a stubby great toe).10
Craniofacial Growth and Development
Comparative Skull Size with Age
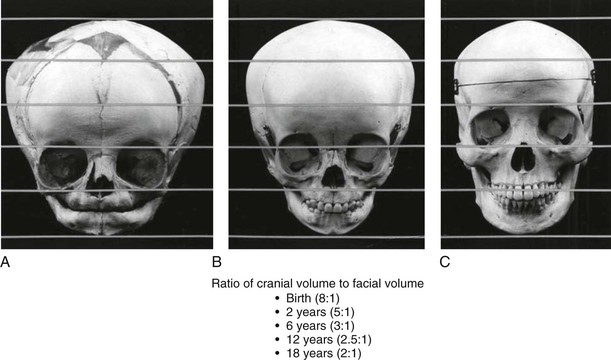
In the newborn skull (Fig. 1-20), the cranium and the orbits are relatively large, and the face is diminutive. In early childhood, the cranium and the orbits remain relatively large, but the eruption of the primary dentition enlarges the facial skeleton. In the adult skull, the facial skeleton is well-developed with relatively less prominence of the orbits and cranium. The ratio of the cranial volume to the facial volume changes during growth, and these ratios appear in Figure 1-20 under the skulls.10
Bone Modification with Age
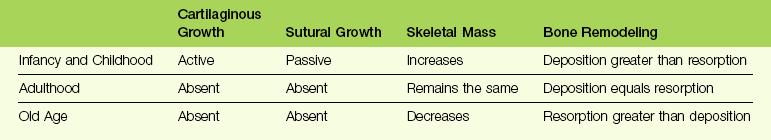
Bone modification of the craniofacial skeleton occurs normally with age (summarized in Table 1-1). Table 1-2 lists some examples of abnormal alterations in the craniofacial skeleton with age.10
TABLE 1-2
Some Examples of Abnormal Alterations in the Craniofacial Skeleton with Age
| Abnormal Skeletal Condition | Pathologic Process | |
| Infancy and Childhood | Achondroplasia | Bony midface deficiency secondary to hypoplasia of cartilaginous nasal capsule and cranial base |
| Craniosynostosis | Premature sutural fusion by bone deposition | |
| Adulthood | Acromegaly | Bone deposition dramatically exceeds bone resorption |
| Hemifacial atrophy | Localized bone resorption exceeds bone deposition | |
| Old Age | Paget disease of bone | Bone deposition exceeds bone resorption |
| Senile osteoporosis | Bone resorption exceeds bone deposition |
Alterations in the Face with Age
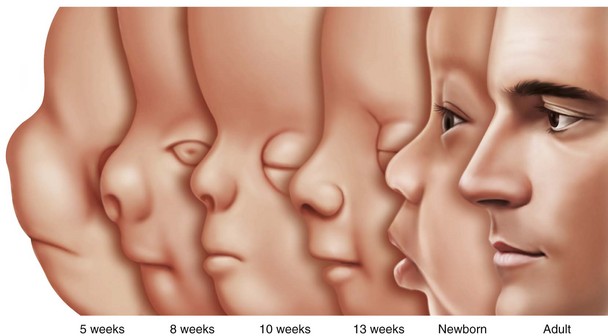
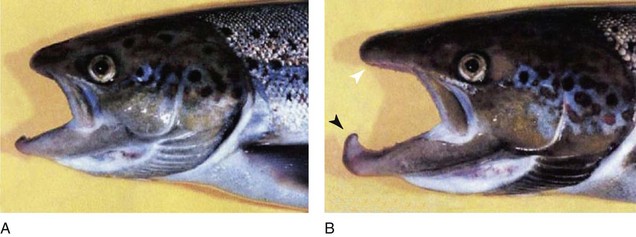
The changing proportions of head size to body size are illustrated in Figure 1-21. From the 2-month-old embryo to the 22-year-old adult, the relative head size decreases significantly, but it is greatest in the fetus and the infant. Figure 1-22 illustrates line drawings of the facial profile showing alterations from 5 months of age to adulthood, with striking changes in the nose and chin with age.10 The lower jaw in the male Atlantic salmon is extraordinary. There is rapid and pronounced growth during adult life (Fig. 1-23), when the starving salmon migrate upriver for spawning.42
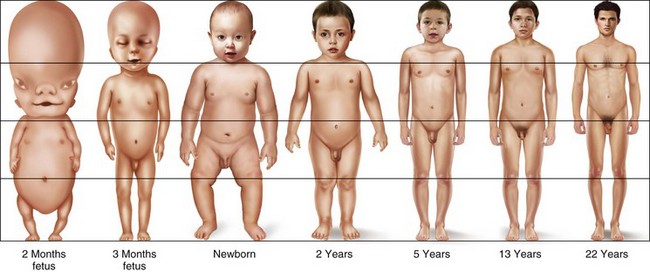
Figure 1-21 Diagram illustrating the changing proportions of head size and body size. From the 2-month-old embryo to the 22-year-old adult, the relative head size decreases significantly, but it is greatest in the fetus and the infant. Based on Scammon, 1953.
Facial Asymmetry
Those psychologists who study faces and state that “beautiful faces are symmetric” don’t know what they’re talking about, because all normal faces are asymmetric. With respect to the normal face, subtle degrees of asymmetry become particularly evident when properly oriented frontal photographs are divided along the median plane and reprocessed, each side being paired with its mirror image (Fig. 1-24).10
The Skull as a Community of Bones versus the Skull as a Community of Joints
According to Pruzansky,34 the skull is a community of bones separated by joints, but according to Moffett32 the skull is a community of joints separated by bones. Which view is correct? They are simply different contexts in which to view the development of the skull.10
Pruzansky34 stated the following:
Achondroplasia, caused by several FGFR3 gain-of-function mutations (particularly Gly380 Arg9) illustrates what Pruzansky34 meant (Fig. 1-25): if one member of the community is affected, inevitably other parts will suffer. In this case, the nasal capsule and cranial base, both cartilaginous in origin, have a secondary effect on membrane bones, because all craniofacial bones articulate with one another. Thus, in achondroplasia, the hypoplastic nasal capsule and short anterior cranial fossa result in midface deficiency.7,9,10
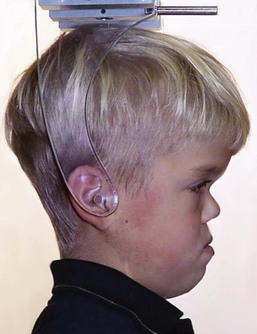
Figure 1-25 Midface deficiency secondary to hypoplasia of the nasal capsule in achondroplasia. From Cohen, 2000.
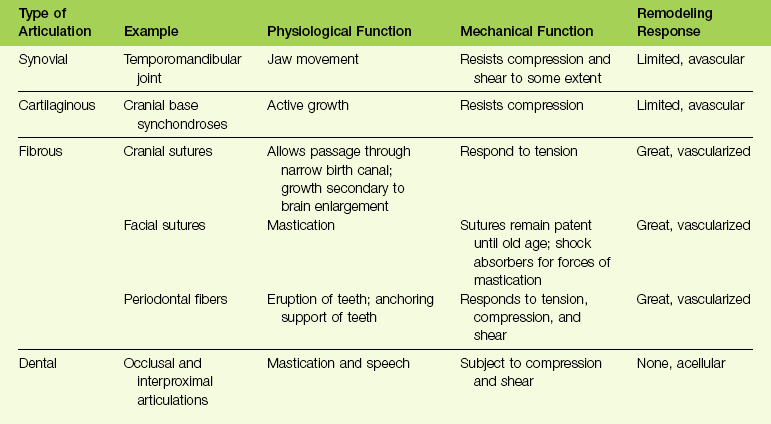
The skull may also be considered a community of joints separated by bones. Types of joints, known as craniofacial articulations, include synovial, cartilaginous, fibrous, and dental.32 Table 1-3 summarizes craniofacial articulations together with their physiologic and mechanical functions and their remodeling responses.10
Dysmorphic Faces
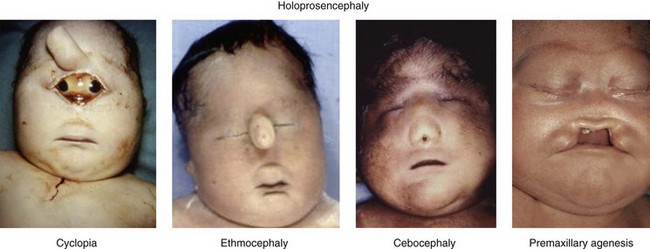
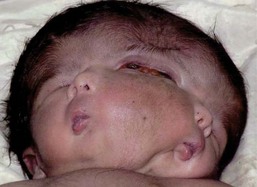
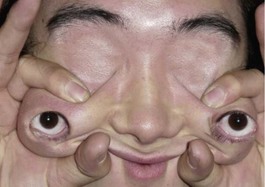
Four different types of faces are contrasted in Figure 1-26: (1) the normal face; (2) Down syndrome with minor anomalies; (3) Crouzon syndrome; and (4) a bizarre prenatally determined face. Three views of Williams syndrome are illustrated in Figure 1-27. The combination of minor facial anomalies—strabismus, anteverted nares, and thick lips—are evident in all three patients. In contrast, the severe facial anomalies associated with holoprosencephaly—cyclopia, ethmocephaly, cebocephaly, and premaxillary agenesis—are shown in Figure 1-28.6,8,10–12 A newborn with diprosopus—a double face with two nostrils, four eyes, two noses, and two mouths—is illustrated in Figure 1-29. The most bizarre case of all is shown in Figure 1-30. This patient is missing his lateral orbital walls, and he is able to pull his eyes apart laterally without ripping his optic heads from the back of his eyeballs. How he does this is mysterious, but I suspect he started doing this very early in childhood and gradually increased the distance over time.
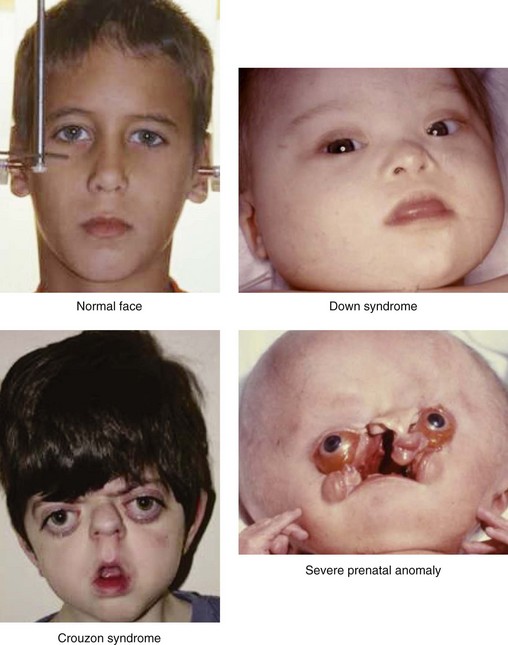
Figure 1-26 Four kinds of faces with different implications. A normal child’s face is shown. Down syndrome involves many minor facial anomalies that are diagnostic. Crouzon syndrome is severe but can be treated successfully with surgery. There is no effective treatment for the infant with severe facial anomalies and hydrocephalus. Based on the work of M. Michael Cohen Jr. Crouzon syndrome is by courtesy of Bonnie Padwa, Boston.
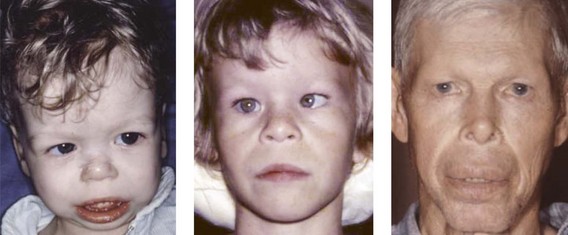
Figure 1-27 Three patients with Williams syndrome of different ages. All have strabismus, anteverted nares, and thick lips. From Cohen, 1997, 2002, 2006, and 2007, a and b.
The Outer Limits in Facial Surgery
The problems surrounding complete facial transplantation are technical, psychological, and ethical. Earlier, facial transplantation was rejected by many institutions, with the exception of the Cleveland Clinic’s Institutional Review Board, and I have discussed the concept of facial transplantation and its associated problems elsewhere.10 Although it was rejected earlier by the French National Ethics Advisory Committee, the door was left open for a severely disfigured woman bitten by a dog for which she received a lower facial transplant. Since then, at least seven facial transplants have been carried out. The result of a facial transplant after a gunshot wound is shown in Figure 1-31.
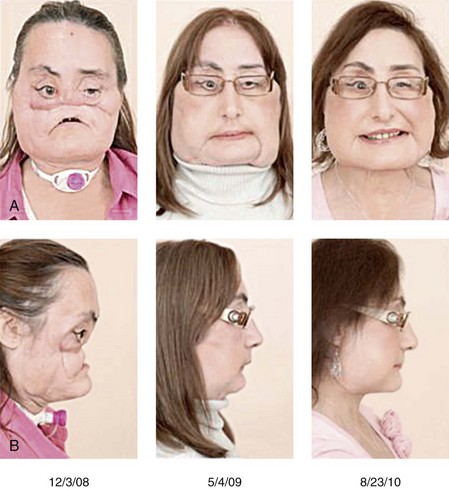
Figure 1-31 A, Three frontal views by dates. 12/3/08: A 45-year-old woman with a shotgun blast to the face. She underwent a nearly total facial transplantation, including a composite LeFort III midfacial skeleton, overlying skin, soft tissue, nose, lower eyelids, upper lip, total infraorbital floor, both zygomas, both parotid glands, the anterior maxilla with central maxillary incisors, the whole alveolus, the anterior hard palate, and intraoral mucosa. Note tracheostomy. 5/4/09: Second facial transplantation with construction of the nose and lips. 8/23/10: Reduction of facial fat and better facial appearance. B, Three profile views with the same dates as in A. 5/4/09: Note better projection of the nose. 8/23/10: Mandibular advancement with normal lower facial appearance. Both the upper and lower portions of the face are balanced. Courtesy of the Cleveland Clinic.
Making Blind People See Again
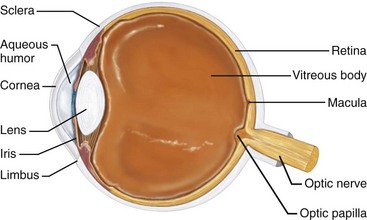
The cornea covers the eye globe and a narrow zone between the cornea and the conjunctiva known as the limbus (Fig. 1-32), which is a source of stem cells for corneal epithelium. In recent years, new techniques that can restore vision in certain types of blindness have come to the fore. In a surgical procedure called limbal cell transplantation, extraction of stem cells from the healthy contralateral eye of a patient or from a relative in the family is transplanted into a patient’s eye with corneal degeneration, blindness, or some other ocular disease. The stem cells then differentiate into corneal epithelial cells and improve vision.19 The limbus itself can be destroyed by chemical burns, by thermal burns, or by infection, which results in corneal stem-cell deficiency. However, it has been recently reported that a viable alternative source of cells for transplantation consists of limbal cells maintained in culture.36 A biosynthetic cornea created from human collagen has also been developed. It mimics the protein’s scaffolding and can be used to trigger regeneration of a patient’s own corneal cells.20
Age-related macular degeneration is a major cause of blindness. Although there is no treatment for the avascular type, the neovascular type results from an imbalance in antiangiogenic and proangiogenic factors and can be treated. Intravitreal injection of VEGF inhibitors results in significant recovery of vision in 30% to 40% of patients.40
Leber congenital amaurosis is an inherited group of rod–cone dystrophies resulting in congenital blindness. One form caused by the RPE65 gene accounts for 16% of the cases. Using rAAV as a vector for retinal gene therapy, a cannula is passed through the front of the eye and across the vitreous gel. Working copies of the gene are injected into the back of the eye. Maguire and colleagues30 reported improvement in visual acuity for all patients.
Several animal studies show great promise for future treatment. The development of retinal cell transplants has been studied in rabbits,38 and retinal repair by transplantation of photoreceptor precursors has been studied in mice.29
Prosopagnosia
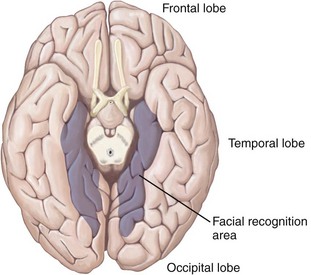
The ability to identify human faces is remarkable. Although the face ages with time, it is possible to instantly identify a face of someone who has not been seen for 25 or 30 years. An area in the brain that governs facial recognition is found at the base of the cerebral cortex (Fig. 1-33). Any lesion that destroys this area impairs the ability to identify faces but has almost no other effects, although the dimming of vision may occur in some affected individuals. The patient can read, name objects, and match a full-face picture with a profile picture of the same person. Only the ability to recognize specific faces is lost; this is a neurological condition known as prosopagnosia. An affected man who cannot recognize his own wife can see her, but he can only recognize who she is when he hears her speak.21 An epidemiologic study has found a prevalence rate of prosopagnosia of 2.47%; of 17 cases, 14 subjects had at least one first-degree relative with prosopagnosia.26
The New Psychiatric Genetics
Because psychiatric thinking began by attributing behavioral problems to earlier psychological events, it was the last bastion to be stormed by molecular genetics. Behavioral disorders are now being delineated at a molecular level, but, like the early delineation of physical genetic disorders, progress in psychiatric genetics only accounts for a small percentage of cases at present. Disorders with molecular findings include intellectual disability, schizophrenia, bipolar disorder, Alzheimer disease, autism, Parkinson disease, Huntington disease, and frontotemporal dementia.13
Artistic Perspective
Figure 1-34 compares two Renaissance paintings: a Florentine Portrait of a Man with a Medal of Cosimo the Elder by Botticelli and a Venetian Portrait of Jacopo Soranzo by Tintoretto. The difference in painting style is striking. In the Florentine painting, the outlines of the face are sharply drawn with a three-dimensional sculptural quality together with a sense of palpability. In contrast, in the Venetian painting, the lines of the face are soft and less distinct but with a sense of atmosphere surrounding the figure.2

Figure 1-34 Left, Close-up of a Florentine Portrait of a Man with a Medal of Cosimo the Elder by Botticelli. Right, Close-up of a Venetian Portrait of Jacopo Soranzo, by Tintoretto. For stylistic interpretation, see text. A, From the Uffizi Gallery, Florence Inv 1890 no. 1,488; B, from Gallerie del’ Accademia, Venice. Image source: Art Resource, NY.
References
1. Arneson, DJ. Stories: Tweety and Sylvester. Middleton, Conn: Xerox Education Publications; 1971.
2. Berenson, B. Italian Painters of the Renaissance. New York: Meridian; 1976.
3. Bonnefont, J, Nikolaev, SI, Perrier, AL, Guo, S, Cartier, L, Sorce, S, Laforge, T, Aubry, L, Khaitovich, P, Peschanski, M, Stylianos, E, Antonarakis, E, Karl-Heinz, K. Evolutionary forces shape the human RFPL 1,2,3 genes toward a role in neocortex development. Am J Hum Genet. 2008; 83:208–218.
4. Buendia, JR. Basic Guide to the Prado. Madrid: Silex; 1989.
5. Campbell, B. Human Evolution. Chicago: Aldine; 1970.
6. Cohen, MM, Jr. The Child with Multiple Birth Defects, ed 2. New York: Oxford University Press; 1997.
7. Cohen, MM, Jr., MacClean, RE. Craniosynostosis: Diagnosis, Evaluation, and Management. New York: Oxford University Press; 2000.
8. Cohen, MM, Jr. Perspectives on Craniofacial Anomalies, Syndromes, and Other Disorders. In: Lin KY, Ogle RC, Jane JA, eds. Craniofacial Surgery: Science and Surgical Technique. Philadelphia: W. B. Saunders Company; 2002:3–38.
9. Cohen, MM, Jr. FGFs/FGFRs and Associated Disorders. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, eds. Inborn Errors of Development. New York: Oxford University Press; 2004:380–400.
10. Cohen, MM, Jr. Perspectives on the Face. New York: Oxford University Press; 2006.
11. Cohen, MM, Jr. Craniofacial Disorders. In: Rimoin DL, Conner JM, Pyeritz RE, Korf BR, eds. Emory and Rimoin’s Principles and Practice of Medical Genetics. ed 5. Philadelphia: Elsevier; 2007:3303–3348.
12. Cohen, MM, Jr. Craniofacial Abnormalities. In Potter’s Pathology of the Fetus, Infant, and Child, ed 2, Philadelphia: Elsevier; 2007:885–918.
13. Cohen, MM, Jr., Mental retardation, psychiatric disorders, neurodegenerative disorders, and genetics. 2010.
14. Doubilet, D. Fishface. New York: Phaidon Press; 2003.
15. Elffers, J. Play with Your Food. New York: Stewart, Tabori & Chang; 1997.
16. Enlow, DH. The Human Face. New York: Harper & Row; 1968.
17. Enlow, DH. Facial Growth, ed 3. Philadelphia: Saunders; 1990.
18. Evans, PD, Gilbert, SL, Mekel-Bobrov, N, Vallender, EJ, Anderson, JR, Vaez-Azizi, LM, Tishkoff, SA, Hudson, RR, Lahn, BT. Microcephalin, a gene regulating brain size, continues to evolve adaptively in humans. Science. 2005; 309:1717–1720.
19. Ezhkova, E, Fuchs, E. An eye to treating blindness. Nature. 2010; 466:567–568.
20. Fagerholm, P, Lagali, NS, Merrett, K, Jackson, WB, Munger, R, Liu, Y, Polarek, JW, Söderqvist, M, Griffith, M. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2, 2010.
21. Geschwind, N. Specialization of the human brain. In: The Brain: A Scientific American Book. San Francisco: W. H. Freeman and Co. ; 1979:108–117.
22. Gilbert CA: All Is Vanity. New York: House of Art.
23. Gould, SJ. Ontogeny and Phylogeny. Cambridge, UK: Harvard University Press; 1977.
24. Hayakawa, T, Angata, T, Lewis, AL, Mikkelsen, TS, Varki, NM, Varki, A. A human-specific gene in microglia. Science. 2005; 309:1693.
25. Jirásek, JE. Atlas of Human Prenatal Morphogenesis. Boston: Martinus Nijhoff; 1983.
26. Kenerknecht, I, Grueter, T, Welling, B, Wentzek, S, Horst, J, Edwards, S, Gueter, M. First report of prevalence of non-syndromic hereditary prosopagnosia (HPA). Am J Med Genet. 2006; 140A:1617–1622.
27. Klein, RG. Whither the Neanderthals? Science. 2003; 299:1525–1527.
28. Krings, M, Capelli, C, Tschentdcher, F, Geisert, H, Meyer, S, von Haeseler, A, Grossschmidt, K, Possnert, G, Paunovic, M, Pääbo, S. A view of Neanderthal genetic diversity. Nat Genet. 2004; 26:144–146.
29. MacLaren, RE, Pearson, RA, MacNeil, A, Douglas, RH, Salt, TE, Akimoto, M, Swaroop, A, Sowden, JC, Ali, RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006; 444:203–207.
30. Maguire, AM, Simonelli, F, Pierce, EA, Pugh, EN, Mingozzi, F, Benicelli, J, Banfi, S, Marshall, KA, Tesa, F, Surace, EM. Safety and efficiency of gene transfer for Leber congenital amaurosis. New Engl J Med. 2008; 358:2240–2248.
31. Mekel-Bobrov, N, Gilbert, SL, Evans, PD, Vallender, EJ, Anderson, JR, Hudson, RR, Tishkoff, SA, Lahn, BT. Ongoing adaptive evolution of ASPM, a brain size determinant in Homo sapiens. Science. 2005; 309:1720.
32. Moffett, BC, Jr. Syllabus for course in fundamentals of skeletal biology related to clinical dentistry. University of Washington; 1973.
33. Parr, LA, Heintz, M. Rhesus monkey expressions. Anim Behav. 2009; 77:1507–1513.
34. Pruzansky, S. Clinical investigation of the experiments of nature. ASHA Report. 1973; 8:62–94.
35. Radinsky, LB. The Evolution of Vertebrate Design. Chicago: University of Chicago Press; 1987.
36. Rama, P, Matuska, S, Paganoni, G, Spinelli, A, De Luca, M, Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. New Engl J Med. 2010; 363:147–155.
37. Scammon, R. Section 1 in Human Anatomy by H. Morris, ed 11. Philadelphia: Blakiston; 1953.
38. Sharma, RK. Developmental expression of GABAA receptors in retinal transplants. Ophthalmic Res. 2000; 32:45–51.
39. Tassabehji, M, Hammond, P, Karmiloff-Smith, A, Thompson, P, Thorgeirsson, SS, Durkin, ME, Popescu, NC, Hutton, T, Read, AP, Macconochie, M, Donnai, D. GTF2IRD1 in craniofacial development of humans and mice. Science. 2005; 310:1184–1187.
40. Thumann, G. Nonviral gene therapy for age-related macular degeneration. Expert Rev Ophthalmol. 2011; 6:81–93.
41. Tibbetts, EA, Dale, J. A socially enforced signal of quality in a paper wasp. Nature. 2004; 432:218–222.
42. Witten, PE, Hall, BK. Seasonal changes in the lower jaw skeleton in male Atlantic salmon (Salmo salar L. ): Remodeling and regression of the kype after spawning. J Anat. 2003; 203:435–450.