The Neurologic System
SYSTEMWIDE ELEMENTS
(b) Subcutaneous fascia: Fibrous fatty layer between the skin and galea that contains blood vessels
(c) Galea aponeurotica: Freely movable, tendinous tissue; covers the vertex of the skull; absorbs the force of external trauma
(d) Subaponeurotic or subgaleal space: Contains the diploic and emissary veins
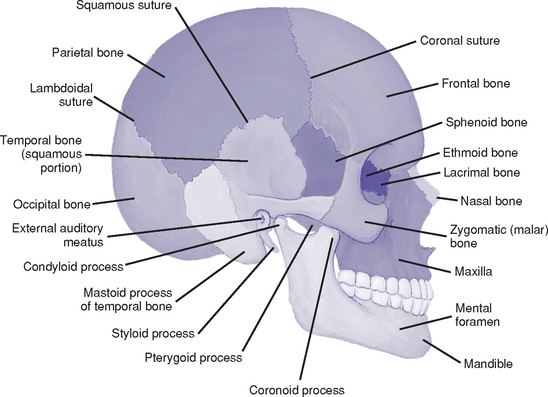
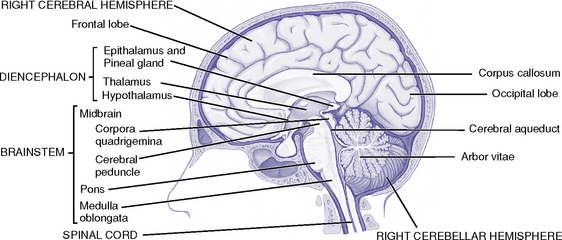
(a) Part of the skull that houses and protects the brain (Figure 4-1)
(b) Bones: Frontal, sphenoid, ethmoid, occipital, two temporal and two parietal bones
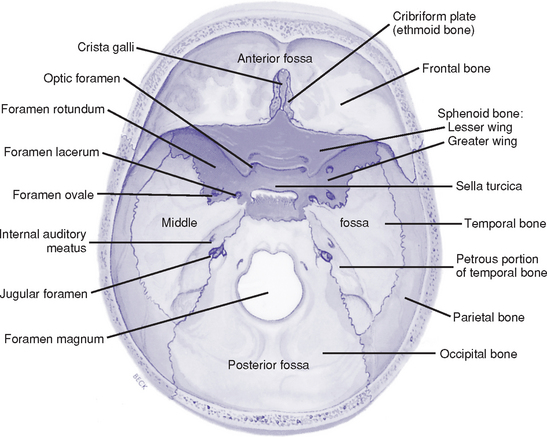
(c) Basilar skull: Base of the skull has three depressions—the anterior, middle, and posterior fossae (Figure 4-2)
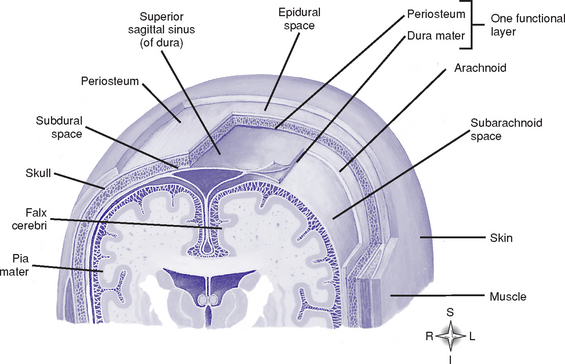
(1) Outermost covering of the brain; consists of two layers of tough fibrous tissue
(2) Outer layer forms the periosteum of the bone
(3) Inner layer folds to form the falx cerebri, tentorium cerebelli, falx cerebelli, and diaphragma sella
(4) Meningeal arteries and venous sinuses lie in clefts formed by the inner and outer layers of the dura mater
(5) Subdural space: Lies between the inner dura mater and the arachnoid mater
(1) Fine, fibrous, elastic layer between the dura mater and pia mater
a) Lies between the arachnoid mater and pia mater; expanded areas of this space form cisterns at the base of the brain
b) Contains blood vessels, including the circle of Willis
c) Contains cerebrospinal fluid (CSF), which completely surrounds the brain and spinal cord; acts as a shock absorber
d) Contains the arachnoid villi: Projections of the arachnoid mater that absorb CSF into the venous system
(a) Telencephalon: Two cerebral hemispheres separated by a longitudinal fissure; joined by the corpus callosum
(1) Functional localization in the cerebral cortex, including cerebral dominance (Table 4-1)
TABLE 4-1
Functional Localization in the Cerebral Cortex
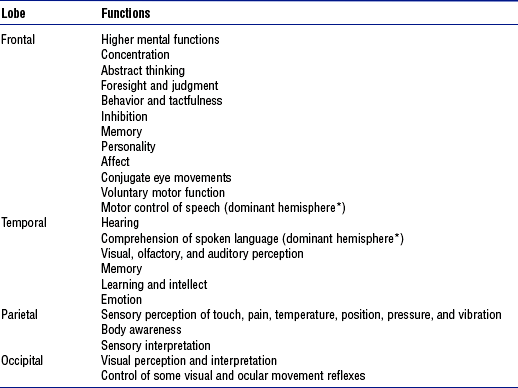
*Cerebral dominance: In right-handed and most left-handed people, the left cerebral hemisphere is dominant for language, mathematical, and analytic functions. The opposite nondominant hemisphere is thought to be concerned with nonverbal, geometric, spatial, visual, and musical functions.
(2) Corpus callosum: Commissural fibers that transfer learned discriminations, sensory experiences, and memory from one cerebral hemisphere to corresponding parts of the other
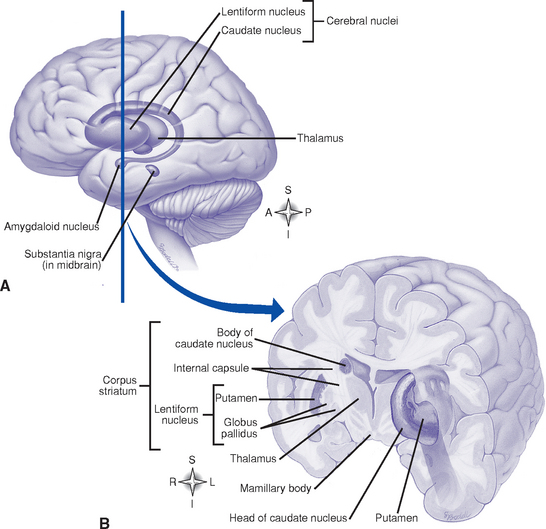
(3) Basal ganglia (basal nuclei) (Figure 4-4)
a) Masses of gray matter; includes the caudate, putamen, globus pallidus, claustrum, amygdaloid, and, functionally, the subthalamic and substantia nigra nuclei
b) Functions: Exert regulating and controlling influences on the coordination of voluntary motion, motor integration, movement initiation, muscle tone, and postural reflexes. A major center of the extrapyramidal motor system.
(1) Thalamus: Two egg-shaped masses of gray matter that abut the lateral walls of the third ventricle; subdivided into several nuclei
a) Certain nuclei receive, integrate, and process sensory input for relay to the cerebral cortex
b) Other nuclei participate in affective aspects of brain function; are functionally related to the association areas of the cortex; or have a role in conscious pain, temperature, and touch awareness, motor function, and the ascending reticular activating system
(2) Hypothalamus: Below the thalamus; regulates
c) Behavior: Part of the limbic system; concerned with aggressive and sexual behavior; elicits physical expressions associated with emotions; may be involved with sleep-wake cycles and circadian rhythm control
d) Autonomic responses: Control center for the autonomic nervous system (ANS); controls numerous visceral and somatic activities (e.g., heart rate, pupil constriction and dilation)
e) Hormonal secretion of the pituitary gland (see Chapter 6)
1) Posterior pituitary gland (neurohypophysis): Stores and releases antidiuretic hormone (ADH) and oxytocin, produced by the hypothalamus. ADH causes vasoconstriction and increases renal water reabsorption. Oxytocin stimulates uterine contraction and milk ejection.
2) Anterior pituitary gland (adenohypophysis): Secretes prolactin and growth-stimulating, thyroid-stimulating, adrenal-stimulating, follicle-stimulating, and luteinizing hormones; hormonal secretion is under the control of pituitary releasing and inhibiting factors produced in the hypothalamus and transported to the anterior pituitary via a pituitary portal system (see Chapter 6)
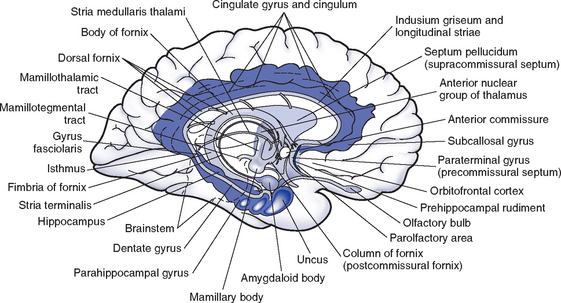
(c) Limbic system (Figure 4-6)
(1) Composed of the limbic lobe (cingulate and parahippocampal gyri) plus structures to which it is anatomically and functionally connected such as the amygdala, hippocampus, fornix, hypothalamus, olfactory tract, and thalamus
(2) Responsible for emotional behavioral responses and accompanying visceral, endocrine, and somatic responses; has a role in basic instinctual drives (e.g., mating, hunger, motivation) and in some aspects of memory and learning
ii. Brainstem (Figure 4-7; also see Figure 4-5)
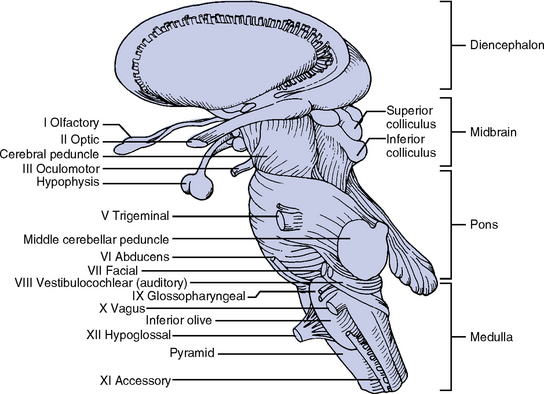
(a) Midbrain (mesencephalon): Located between the diencephalon and pons
(1) Contains nuclei of cranial nerve (CN) III (oculomotor) and CN IV (trochlear) and some CN V (trigeminal) nuclei
(2) Contains motor and sensory pathways
(3) Holds respiratory control centers
(4) Tectal region (inferior and superior colliculi): Concerned with the auditory and visual systems
(5) Connects to the cerebellum via the superior cerebellar peduncles
(b) Pons (metencephalon): Between the midbrain and medulla
(1) Contains nuclei of CN V, VI (abducens), and VII (facial), and some CN VIII (acoustic) nuclei
(2) Middle cerebellar peduncles on its basal surface provide extensive connections between the cerebral cortex and cerebellum, ensuring maximal motor efficiency
(3) Contains motor and sensory pathways
(4) Holds respiratory control centers that help coordinate breathing patterns
(c) Medulla (myelencephalon): Between the pons and spinal cord
(1) Contains nuclei of CN IX (glossopharyngeal), X (vagus), XI (spinal accessory), and XII (hypoglossal) and some nuclei from CN V, VII, and VIII.
(2) Motor and sensory tracts of spinal cord continue into the medulla
(3) Attaches to the cerebellum via inferior the cerebellar peduncles
(4) Holds respiratory, cardiac, and vasomotor control centers
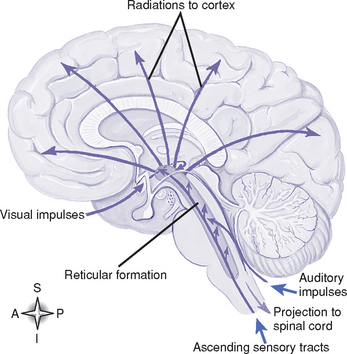
(d) Reticular formation (RF) (Figure 4-8): Diffuse cellular network in the brainstem, with axons projecting to the thalamus and into the cortex; receives input from the cerebrum, spinal cord, other brainstem nuclei, and the cerebellum; has a role in the control of autonomic and endocrine functions, skeletal muscle activity, and visceral and somatic sensation. The reticular activating system is part of the RF.
iii. Cerebellum: Lies in the posterior fossa behind the brainstem; separated from the cerebrum by the tentorium cerebelli
(a) Influences muscle tone in relation to equilibrium, locomotion, posture, and nonstereotyped movements
(b) Important in the synchronization of muscle action to enable coordinated movement
(c) Input is from the spinal cord, brainstem, vestibular system, and cerebral centers; output to the brainstem and thalamus influences spinal and cerebral activities
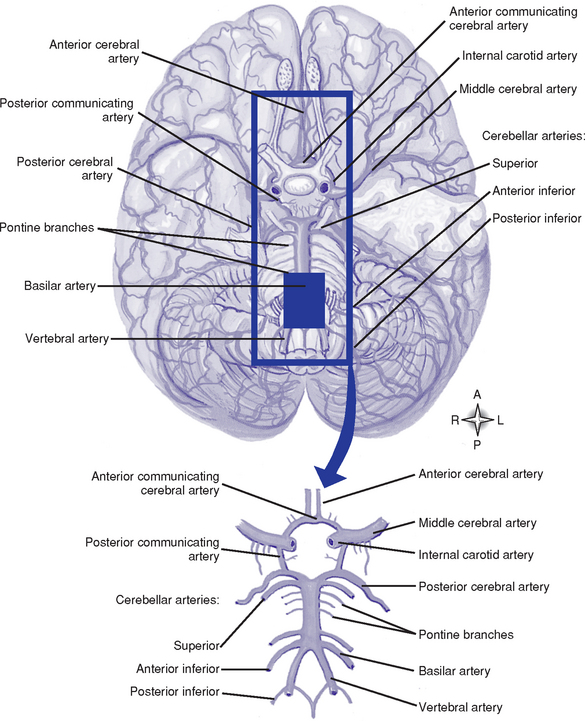
c. Cerebral circulation (Figure 4-9)
i. Arterial system: Supplied by the internal carotid and vertebral arteries
(a) Circle of Willis: Anastomosis of arteries at the base of the brain formed by a short segment of the internal carotid and anterior and posterior cerebral arteries, which are connected by an anterior communicating artery and two posterior communicating arteries. This anastomosis may permit collateral circulation if a carotid or vertebral artery becomes occluded.
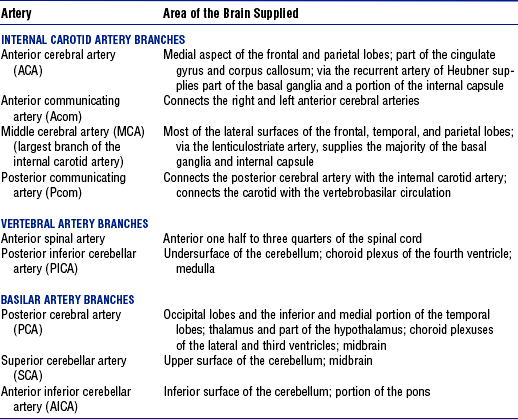
(b) Internal carotid system: Internal carotid arteries arise from the common carotid arteries. Table 4-2 shows the branches of this system and the areas they supply.
(c) Vertebral system: Vertebral arteries arise from the subclavian arteries and join at the lower pontine border to form the basilar artery. Branches of this system and the areas they supply are summarized in Table 4-2.
(d) Branches of the internal carotid, external carotid, and vertebral arteries (e.g., anterior, middle, posterior meningeal arteries) provide blood supply to the meninges
(a) Normal CBF averages 50 ml/100 g of brain tissue per minute
(b) Cerebral perfusion pressure (CPP) and intrinsic regulatory mechanisms affect CBF
(1) CPP: Pressure gradient that drives blood into the brain; calculated as the difference between the mean arterial pressure (MAP) and the intracranial pressure (ICP): CPP = MAP − ICP
(2) Regulatory mechanisms influence the diameter of the cerebrovasculature
a) Pressure or myogenic autoregulation: Alteration in the diameter of the brain’s resistance vessels (arterioles) that maintains a constant CBF over a range of pressures between 50 and 150 mm Hg. Chronic hypertension can increase the upper and lower pressure ranges for the range of autoregulation.
b) Elevated arterial partial pressure of carbon dioxide (Paco2) and hypoxemia (arterial partial pressure of oxygen [Pao2] of <50 mm Hg) cause vasodilatation and increased CBF; decreased Paco2 causes vasoconstriction and reduced CBF
c) Metabolic autoregulation: CBF varies with metabolic activity. Factors that increase the metabolic rate (e.g., seizures, fever) increase CBF; reduced metabolic requirements (e.g., hypothermia) decrease CBF.
(3) Inadequate CBF results in brain tissue ischemia (CBF <18 to 20 ml/100 g/min) and death (CBF <8 to 10 ml/100 g/min)
iii. Venous system: Brain surface drains into the superficial veins; the central interior cerebrum drains into the internal veins beneath the corpus callosum (Figure 4-10). Veins have no valves.
(a) Veins empty into venous sinuses between dural layers (Table 4-3)
TABLE 4-3
Major Venous Drainage Structures, Their Locations, and Areas Drained
| Venous Structure | Location and Area Drained |
| Superior sagittal sinus | Courses along the midline at the superior border of the falx cerebri; superior cerebral veins empty into it |
| Straight sinus | Lies in the midline attachment of the falx cerebri and the tentorium; drains the system of internal cerebral veins (including the inferior sagittal sinus and great cerebral vein of Galen) |
| Transverse sinuses | Lie in the bony groove along the fixed edge of the tentorium cerebelli; drain the straight sinus and the superior sagittal sinus |
| Sigmoid sinuses | Lie on the mastoid process of the temporal bone and jugular process of the occipital bone; receive blood from the transverse sinuses and empty into the internal jugular veins |
| Inferior sagittal sinus | Lies along the free inferior border of the falx cerebri just above the corpus callosum; receives blood from the medial aspects of the hemispheres |
| Emissary veins | Connect the dural sinuses with veins outside the cranial cavity |
(b) Internal jugular veins collect blood from the large dural venous sinuses and return blood to the heart
iv. Blood-brain barrier: Specialized permeability of the brain capillaries that limits transfer of certain substances from blood into brain tissue. Barrier formed by tight junctions between brain capillary endothelial cells, reduced transport mechanisms of these cells, and footlike projections from the astrocytes that encase the capillaries.
(a) Water, carbon dioxide, oxygen, glucose, and lipid-soluble substances cross the cerebral capillaries with ease. Uptake of other substances, such as dyes and ions (e.g., Na+, K+), is much slower.
(b) Regulates the entry or removal of various substances to maintain a homeostatic environment for the central nervous system (CNS)
(c) Clinically significant in treating and diagnosing CNS disease. Blood-brain barrier disruption and increased permeability occurs with brain injury, tumors, infections, and stroke.
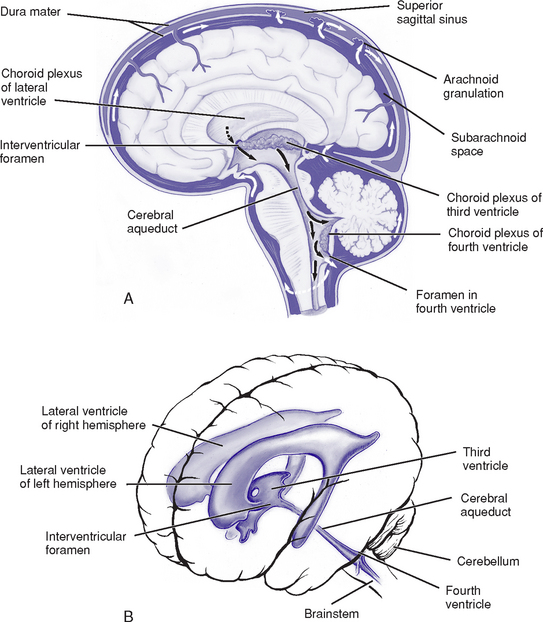
d. Ventricular system and CSF (Figure 4-11)
i. Ventricles: Four cavities containing CSF
(a) Lateral ventricles: Largest ventricles, one in each cerebral hemisphere. The anterior (frontal) horns lie in the frontal lobes; the bodies extend back through the parietal lobes to the posterior (occipital) horns, which project into the occipital lobes; the inferior (temporal) horns lie in the temporal lobes.
(b) Third ventricle: Midline between the two lateral ventricles, surrounded by the diencephalon
(c) Fourth ventricle: In the posterior fossa bordered by the pons, medulla, and superior cerebellar peduncles; continuous with the cerebral aqueduct (aqueduct of Sylvius) superiorly and the central spinal canal inferiorly
(a) Cushions the brain and spinal cord from injury
(b) Provides support and buoyancy for the brain, decreasing its effective weight on the skull
(c) Its displacement out of the cranial cavity (and, to an extent, its increased reabsorption) compensates for increases in intracranial volume and pressure
(d) Regulates the nervous system chemical environment to preserve homeostasis
(e) Enables water-soluble metabolites to diffuse from the brain
(f) Serves as a channel for neurochemical communication within brain
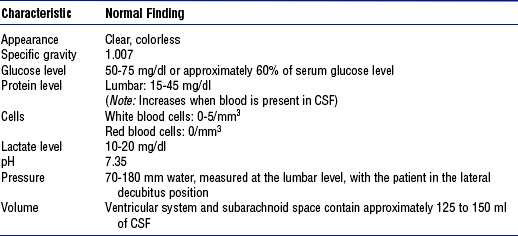
iii. CSF properties: See Table 4-4
(a) Rate of synthesis estimated as 500 ml/day or 22 to 25 ml/hr
(b) Choroid plexus: Tuft of capillaries covered by epithelial cells found in all ventricles; principal source of CSF; lateral ventricles produce most
(c) Small amounts of CSF are produced by the blood vessels of the brain and meningeal linings
v. Circulation and absorption of CSF (see Figure 4-11)
(a) CSF circulates from the lateral ventricles through the interventricular foramina (foramina of Monro) to the third ventricle and to the fourth ventricle via the aqueduct of Sylvius; CSF then circulates to the subarachnoid space via the foramina of Luschka and Magendie
(b) Most CSF is absorbed via the arachnoid villi into the dural sinuses
(c) When CSF pressure exceeds venous pressure, CSF is absorbed through the unidirectional valves of the arachnoid villi
vi. Blood-CSF barrier: Choroid plexus epithelium imposes a barrier analogous to the blood-brain barrier; permits selective transport of substances from the blood into the CSF
i. Brain has high metabolic energy requirements; energy primarily used for neuronal conductive and metabolic activities
ii. At rest, the brain consumes 25% of body glucose and 20% of body oxygen; cerebral oxygen consumption averages 49 ml/min
iii. Brain utilizes glucose as its principal energy source
iv. Minimal storage of oxygen and glucose in the brain necessitates a constant supply for normal neuronal function
v. Anaerobic glucose metabolism (glycolysis) yields insufficient adenosine triphosphate (ATP) to meet cerebral energy demands. Rate of glycolysis increases markedly during hypoxia in an attempt to maintain functional neuronal activity.
vi. Within seconds to minutes of anoxia, the energy-dependent sodium-potassium pump fails; cytotoxic cerebral edema results
vii. Hypoglycemia causes neuronal dysfunction and may lead to convulsions, coma, and death
f. Cells of the nervous system
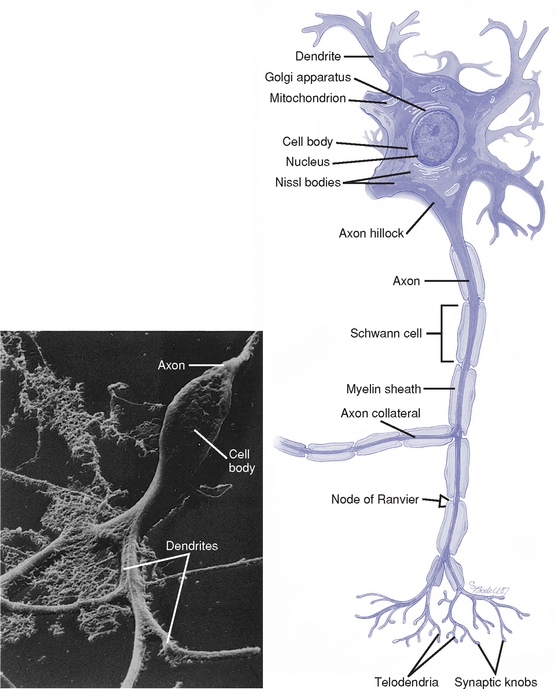
i. Neuron: Basic functional unit of the nervous system; transmits nerve impulses
(a) Components of each cell (Figure 4-12)
(1) Cell body: Carries out the metabolic functions of the cell; contains a nucleus, cytoplasm, and organelles surrounded by a lipoprotein cell membrane
(2) Dendrites: Short branching extensions of the cell body; conduct impulses toward the cell body
(3) Axon hillock: Thickened area of the cell body from which the axon originates
(4) Axon: Long extension of the cell body; conducts impulses away from the cell body; usually myelinated. Outside the brain, axons are also covered with neurilemma. Branch into several processes at the terminal end.
(5) Myelin sheath: White protein-lipid complex that surrounds some axons; laid down by oligodendrocytes in the CNS and by Schwann cells in the PNS
(6) Nodes of Ranvier: Periodic interruptions in the myelin covering along the axon. Impulses are conducted from node to node (saltatory conduction), which makes conduction more rapid and efficient.
(7) Synaptic knobs: At the terminal ends of the axon; contain vesicles that store neurotransmitter substances
ii. Neuroglial cells: Support, nourish, and protect the neurons; about 5 to 10 times as numerous as neurons. Four types:
(a) Microglia: Phagocytize tissue debris when nervous tissue is damaged
(b) Oligodendroglia: Responsible for myelin formation on axons in the CNS
(c) Astrocytes: Contribute to the structure of the blood-brain barrier. Provide nutrients for neurons. Constitute the structural and supporting framework for nerve cells and capillaries. Remove excess potassium and neurotransmitters. Contribute to scar formation in response to neuronal cell injury or death.
(d) Ependyma: Line the ventricles of the brain and the central canal of the spinal cord. Regulate the flow of substances from these cavities into the brain. Aid in CSF production.
g. Synaptic transmission of impulses: Unidirectional conduction of an impulse from a presynaptic neuron across a junction or synapse to a postsynaptic neuron
i. Resting membrane potential (RMP): Voltage difference across the cell membrane when the neuron is resting. Determined by the difference in ion concentrations on either side of the membrane. At rest, cells are positively charged outside and negatively charged inside.
ii. Depolarization: Stimulus causes sodium channels to open, which results in an intracellular influx of sodium ions (Na+)
(a) These ionic fluxes decrease RMP
(b) This depolarization is called the excitatory postsynaptic potential (EPSP)
iii. Action potential: If a transient voltage change that occurs with depolarization is of sufficient magnitude (threshold level), an action potential is produced and transmitted (conducted as an impulse) along the nerve fibers in an active, self-propagating process
iv. Summation: Simultaneous excitation of numerous excitatory presynaptic terminals (or rapidly successive discharges from the same presynaptic terminal) can add together to cause a progressive increase in the postsynaptic potential that may eventually reach threshold to generate an action potential
v. Neurotransmitters (Table 4-5): Chemicals secreted by presynaptic knobs or vesicles (usually located at the axon terminal) that excite, inhibit, or modify the response of a postsynaptic neuron. When an action potential reaches the synaptic knob, calcium channels are opened, allowing Ca++ influx into the knob, which triggers neurotransmitter release.
TABLE 4-5
Major Neurotransmitters: Type, Location, and Action

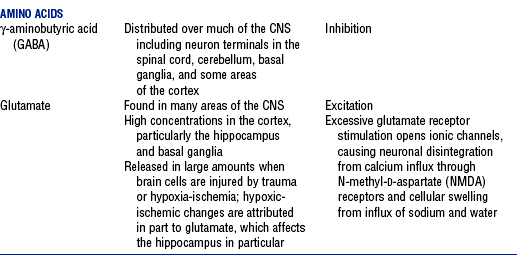
*Action is determined by the postsynaptic receptor rather than the neurotransmitter.
(a) Transmitter diffuses across the synapse and binds with postsynaptic membrane receptors, which causes certain ion channels to open
(b) Excitatory neurotransmitters: Open sodium and potassium channels, which results in postsynaptic membrane depolarization
(a) Absolute refractory period: Membrane is unresponsive to any stimulus, so that the neuron is incapable of producing an action potential. Occurs for a fraction of a second after the membrane surpasses the threshold potential. Limits the frequency of the impulses that a cell can generate.
(b) Relative refractory period: Neuron can be excited again but only by a very strong stimulus (i.e., summation above threshold); occurs during membrane repolarization
(a) At the peak of an action potential, the cell membrane again becomes impermeable to Na+; potassium channels open and allow rapid efflux of K+ from the cell, which thereby reestablishes the RMP
(b) RMP returns with the aid of the sodium–potassium–adenosine triphosphatase (ATPase) pump, which pumps Na+ out of the cell and K+ into the cell
(a) Inhibitory postsynaptic potential (IPSP)
(1) Inhibitory neurotransmitters open potassium and/or chloride channels; this causes increased negativity of the membrane potential, which results in hyperpolarization of the cell membrane
(2) Decreases excitability and inhibits impulse transmission
(b) Presynaptic inhibition: Reduced amount of neurotransmitter is released; this reduces the magnitude of the EPSP to subthreshold levels
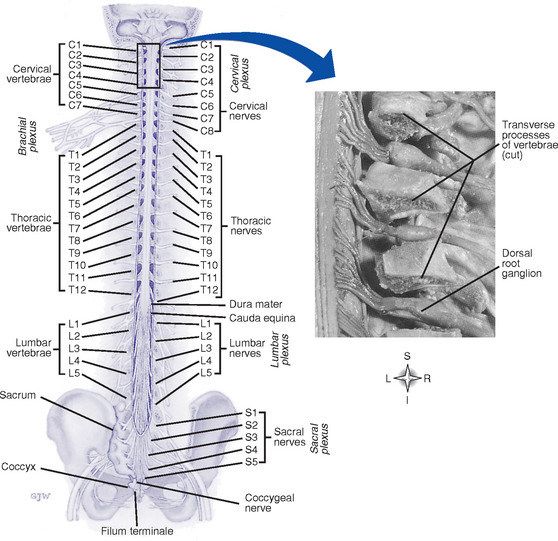
a. Vertebral column (Figure 4-13)
(1) Support the head and neck; smallest vertebrae
(2) Atlas (first cervical vertebra): Supports the head; articulates with the occipital bone superiorly and the axis inferiorly
(b) Thoracic: Twelve vertebrae; articulate with the ribs; support the chest muscles
(c) Lumbar: Five vertebrae; support the lower back muscles; the largest and strongest vertebrae
(d) Sacral: Five fused vertebrae; form a large triangular bone, the sacrum
ii. Anatomic features of a typical vertebra
(a) Body: Flat round, solid portion; lies anteriorly
(b) Arch: Posterior part of the vertebra. Consists of:
(1) Pedicles: Two short bony projections that extend posterior from the body
(2) Lamina: Join each pedicle and fuse posteriorly at the midline to complete the arch; processes project from the laminae
(3) Spinous process: Midline projection protruding posteriorly from the laminae
(4) Transverse processes: Projections from the laminae on each side of the vertebrae
(5) Articular processes (facets): Projections from the laminae that protrude upward or downward (superior or inferior articulating processes); inferior processes articulate with the superior processes of the vertebra directly below
(c) Intervertebral foramina: Openings between the vertebrae through which spinal nerves pass
(d) Spinal foramina: Opening between the arch and the body through which the spinal cord passes
(a) Fibrocartilage layer between the bodies of adjoining vertebrae
(c) Composed of the annulus fibrosus (tough outer layer) and nucleus pulposus (gelatinous inner layer)
iv. Spinal ligaments: Hold the vertebrae and discs in alignment; prevent excessive spinal flexion or extension
i. Location: Extends from the superior border of the atlas to the first or second lumbar vertebra
(a) Continuous with the medulla oblongata
(b) Conus medullaris: Caudal end of the spinal cord
(c) Central canal: In the center of the spinal cord; contains CSF and is continuous with the fourth ventricle
(d) Filum terminale: Nonneural filament that extends downward from the conus medullaris and attaches to the coccyx; helps maintain the position of spinal cord during trunk movement
ii. Meninges: Continuous with the layers covering the brain
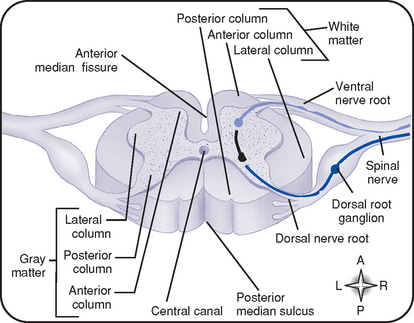
iii. Gray matter (Figure 4-14)
(a) An H-shaped, internal mass of gray substance surrounded by white matter; consists of cell bodies and their dendrites and axons
(b) Anterior gray column (anterior horn): Contains cell bodies of efferent motor fibers
(c) Lateral column: Contains preganglionic fibers of the ANS
(d) Posterior gray column (posterior horn): Contains cell bodies of afferent sensory fibers
iv. White matter (see Figure 4-14)
(a) Composed of three longitudinal columns (funiculi): Anterior, lateral, and posterior
(b) Contains mostly myelinated axons
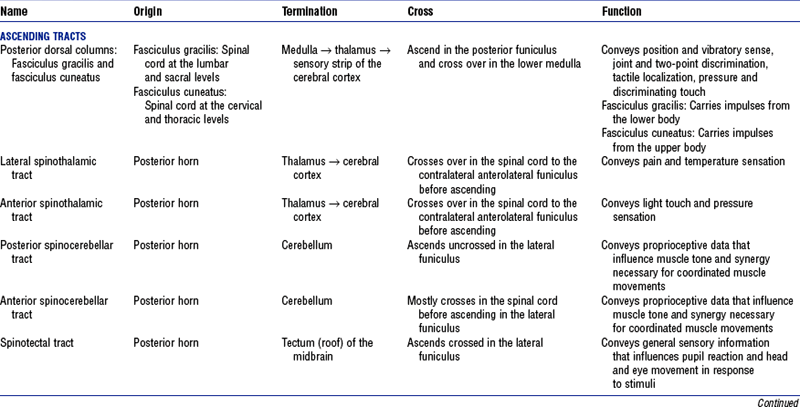
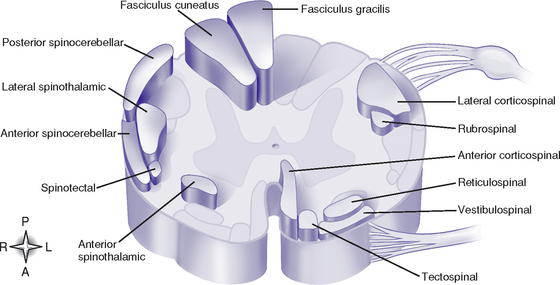
(c) Funiculi contain tracts (fasciculi): Composed of axons with similar origin, course, and termination that perform specific functions; clinically significant tracts are summarized in Table 4-6; classified as follows (Figure 4-15):
(1) Ascending or sensory tracts: Pathways to the brain for impulses that enter the cord via the dorsal roots of the spinal nerves
(2) Descending or motor tracts: Transmit impulses from the brain to the motor neurons of the spinal cord that exit via the ventral root of the spinal nerves
(d) Most tracts are named to indicate the column in which the tract travels, the location of its cells of origin, and the location of axon termination
v. Upper and lower motor neurons
(a) Lower motor neurons (LMNs): Spinal and cranial motor neurons that directly innervate muscles. LMN lesions cause flaccid paralysis, muscular atrophy, absent reflexes.
(b) Upper motor neurons (UMNs): Located completely in the CNS; regulate LMN activity. UMN lesions are associated with spastic paralysis, clonus, increased tone, hyperactive reflexes, Babinski’s sign.
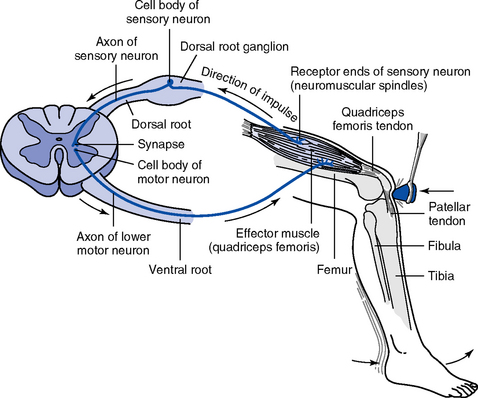
i. Reflex arc: Requires a receptor, sensory neuron, motor neuron, and effector (e.g., muscle or gland) (Figure 4-16)
ii. Monosynaptic reflex arc: Direct synapse between the afferent and efferent neurons
(a) Stimulation of afferent nerve fibers sends impulses to the spinal cord through the dorsal roots of spinal nerves
(b) Impulse synapses with anterior motor neurons, sending out an efferent discharge confined to the axons supplying the muscle from which the afferent impulse originated
(a) More than one synapse required to complete the reflex arc
(b) Most reflexes are polysynaptic; may involve interneurons (neurons in the CNS that transmit impulses from a sensory neuron to or toward a motor neuron) and multiple spinal segments and/or areas of brain
iv. Reciprocal innervation: Impulses that excite motor neurons supplying a particular muscle also inhibit motor neurons of antagonistic muscles
3. Peripheral nervous system (PNS)
i. Thirty-one symmetrically arranged pairs of nerves, each possessing a sensory (dorsal) root and a motor (ventral) root: 8 cervical pairs, 12 thoracic pairs, 5 lumbar pairs, 5 sacral pairs, 1 coccygeal pair (see Figure 4-13)
ii. Fibers of the spinal nerves
(a) Motor fibers: Originate in the anterior gray column of the spinal cord, form the ventral root of the spinal nerve, and pass to skeletal muscles
(b) Sensory fibers: Originate in the spinal ganglia of the dorsal roots; peripheral branches distribute to visceral and somatic structures as mediators of sensory impulses to the CNS
(d) Cauda equina: Spinal nerves that arise from the lumbosacral portion of the spinal cord contained within the lumbar cistern
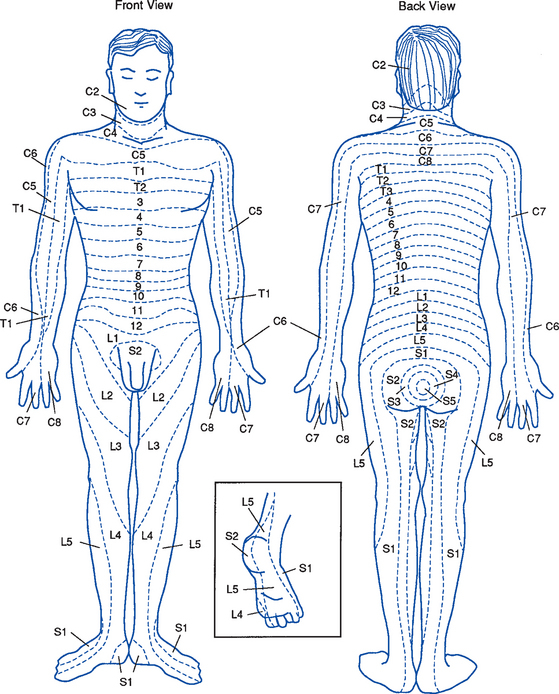
iii. Dermatomes (Figure 4-17): Skin areas supplied by the dorsal root (sensory fibers) of a given spinal nerve; adjacent dermatomes overlap
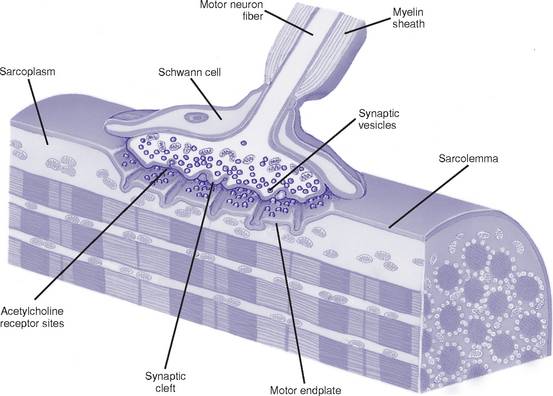
b. Neuromuscular transmission (Figure 4-18)
i. Physiologic anatomy at the neuromuscular junction
(a) Motor end plate: Distal end of motor axon loses its myelin sheath and flattens out at the end lying close to the muscle fiber membrane (sarcolemma)
(b) Synaptic cleft: Space between the motor endplate and the muscle fiber membrane
(c) Synaptic gutter: Invagination of the muscle fiber membrane where numerous folds increase the surface area available for neurotransmitter to act
(d) Vesicles: Nerve terminal structures that store and release the neurotransmitter acetylcholine (ACh)
ii. When an action potential reaches the neuromuscular junction, vesicles release ACh into the synaptic cleft. Amount released depends on the magnitude of the action potential and the presence of calcium. ACh attaches to receptor sites on the postjunctional muscle membrane and increases its permeability to Na+, K+, and other ions.
iii. End-plate potential: Motor nerve action potential that is local (e.g., nonpropagated) and graded, rather than all or nothing
iv. Muscle contraction: Action potentials subsequently form on either side of the endplate and conduct in both directions along the muscle fiber, initiating a series of events that result in muscle contraction
v. Acetylcholinesterase: Catalyzes the hydrolysis of ACh to choline and acetic acid and thus limits the duration of ACh action on the endplate, which ensures production of only one action potential
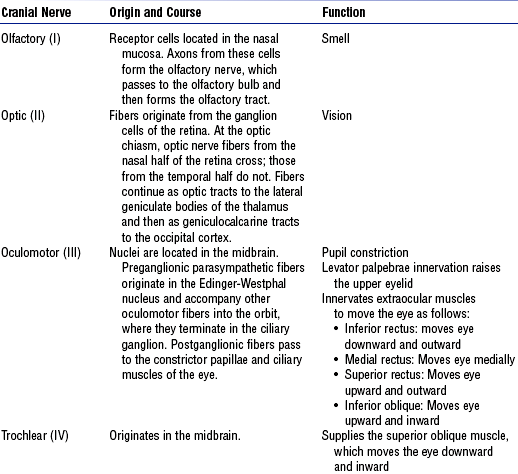
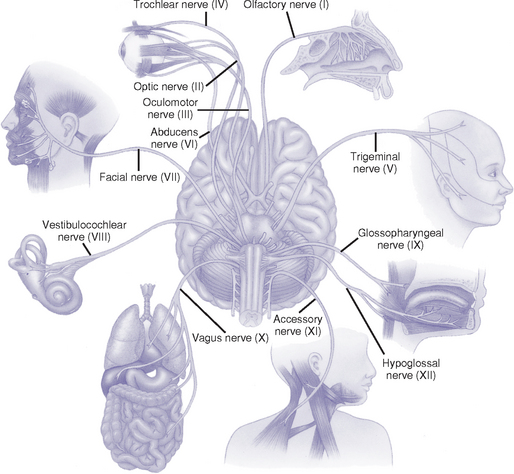
c. Cranial nerves: 12 pairs of nerves considered part of the PNS (Figure 4-19 and Table 4-8)
(a) Composed of two neuron chains
(b) Preganglionic cell bodies are located within the lateral gray column of the spinal cord or brainstem nuclei
(c) Most preganglionic axons are myelinated and synapse on the cell bodies of postganglionic neurons outside the CNS
(d) Axons of postganglionic neurons terminate on visceral effectors (i.e., smooth and cardiac muscle, glandular epithelium)
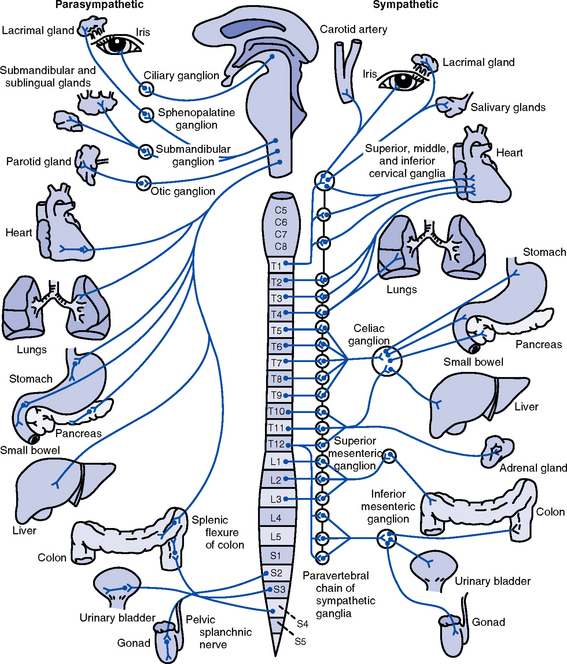
(a) Sympathetic (thoracolumbar)
(1) Preganglionic axons emerge from cell bodies within the lateral horn of the spinal cord gray matter at the thoracic and upper two lumbar levels. Axons leave the spinal cord via the ventral roots and pass to
a) Paravertebral sympathetic ganglion chain via white rami communicantes, ending on cell bodies of postganglionic neurons
b) Collateral ganglia, ending on postganglionic neurons closer to the viscera
c) Adrenal medulla, ending on modified postganglionic neurons that are secretory endocrine cells
(2) Postganglionic axons pass to
a) Viscera via the sympathetic nerves
b) Gray rami communicantes, which return to the spinal nerve and are distributed to autonomic effectors in areas supplied by these nerves
TABLE 4-9
Autonomic Nervous System Effects on Various Effector Sites
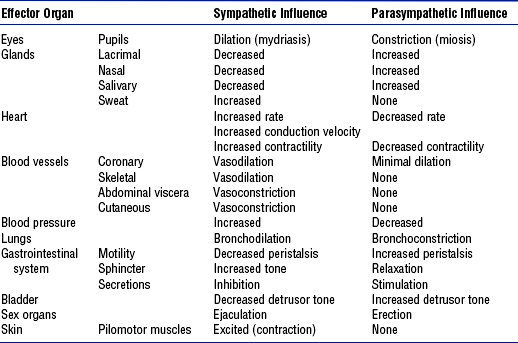
From Russo-McCourt T: Spinal cord injuries. In McQuillan KA, Von Rueden KT, Hartsock RL, et al, editors: Trauma nursing: from resuscitation through rehabilitation, ed 3, Philadelphia, 2002, Saunders, p 512.
(b) Parasympathetic (craniosacral) (see Table 4-9)
(1) Preganglionic cell bodies are located in brainstem nuclei and the lateral gray columns of the middle three sacral spinal cord segments (S2 to S4)
(2) Preganglionic fibers end on short postganglionic neurons located on or near visceral structures
(3) Supplies visceral structures in the head via the oculomotor (III), facial (VII), and glossopharyngeal (IX) cranial nerves and those in the thorax and upper abdomen via the vagus (X) nerve
(4) Sacral outflow supplies the pelvic viscera via the pelvic branches of S2 to S4
(5) Produces localized reactions rather than the mass action of sympathetic stimulation
iii. Chemical mediation: ANS is divided into cholinergic and adrenergic divisions based on the neurotransmitter released
a. Core Curriculum focuses primarily on acute (nociceptive or physiologic) pain. Chronic pain is discussed briefly because unrelieved acute pain can result in the development of chronic (pathologic) pain.
b. Acute pain results when mechanisms such as surgery, trauma, or disease cause inflammation and cellular damage
i. Warns of potential for, or extent of, tissue damage; initiates protective behaviors to minimize damage and promote tissue repair
ii. Usual characteristics include the following:
(a) Activates sympathetic nervous system (autonomic, involuntary) responses, such as increased heart rate, blood pressure (BP), respiratory rate
(b) Proportional to intensity and extent of stimuli; however, wide variations exist among patients
(c) Has a beginning and end, with resolution or healing of the underlying pathologic condition. Research indicates that
iii. Classifications of acute pain
(a) Somatic: Arising from tissues (e.g., skin, muscle, bone). Descriptors include sharp, dull, aching, cramping. Is localized or diffuse; may radiate.
(b) Visceral: Arising from organs (e.g., liver, pancreas, bowel). Descriptors include sharp, stabbing, deep ache. Is well or poorly localized; may be referred.
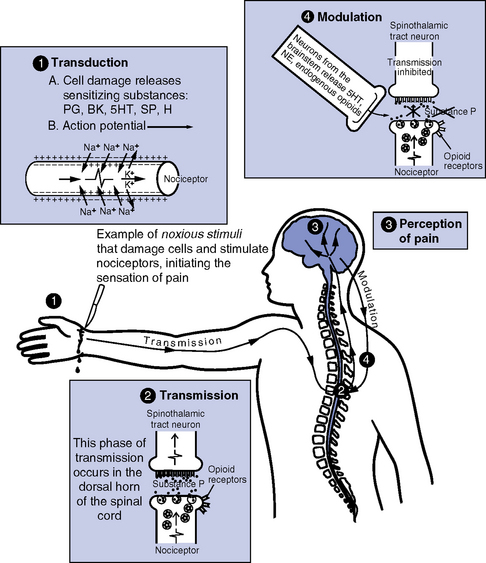
(a) Nociception: Neurochemical process of transmitting the pain response to a peripheral noxious (thermal, mechanical, or chemical) stimulus to an intact spinal cord and brain
(1) Pain perception requires cortical integration of many factors (e.g., physiologic factors, psychosocial factors, past experiences); often results in emotional responses (e.g., anger, anxiety)
(2) Nociception does not always result in pain (e.g., pain perception can be blunted by endogenous endorphin release); pain can occur without nociception (e.g., phantom limb pain)
(b) Nociceptors: Peripheral neurons (primary afferent peripheral fibers) that sense unpleasant or potentially damaging noxious stimuli. Examples are
(1) A delta: Large, thinly myelinated fibers that rapidly conduct impulses generated in response to mechanical or thermal stimuli; usually results in sharp, well-localized pain
(2) C: Smaller, unmyelinated fibers that slowly conduct impulses generated in response to all noxious stimuli; usually poorly localized, dull, aching pain; 75% of nociceptors are C fibers (Munden, Eggenberger, Goldberg, et al, 2003)
(c) Peripheral sensitization: Lowering of nociceptor activation threshold following exposure to chemical mediators or repeated noxious stimuli
(d) Four basic stages of nociception are described in Table 4-10 and illustrated in Figure 4-21
TABLE 4-10
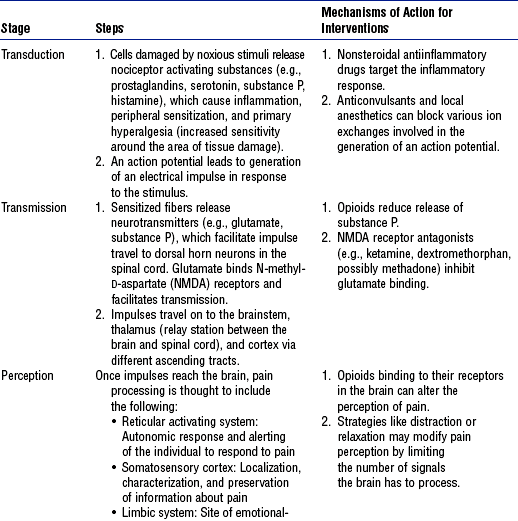

Compiled from Ballantyne J, Fishman SM, Abdi S, editors: The Massachusetts General Hospital handbook of pain management, Philadelphia, 2002, Lippincott Williams & Wilkins; Blakely WP, Page GG: Pathophysiology of pain in critically ill patients, Crit Care Nurs Clin North Am 12(2):167–179, 2001; McCaffery M, Pasero C: Pain: clinical manual, ed 2, St Louis, 1999, Mosby; Melzack R, Wall PD, editors: Handbook of pain management, Edinburgh, 2003, Churchill Livingstone; and National Pharmaceutical Council: Pain: current understanding of assessment, management, and treatment, Reston, Va, 2001, Author. Churchill Livingstone
c. Chronic pain: Pain state persisting beyond the period of healing
i. Serves no useful purpose (St. Marie, 2002)
ii. Usual characteristics (McCaffery and Pasero, 1999)
(a) Neither tissue pathology nor sympathetic nervous system activation is identifiable
(b) Represents a disproportionate response to the stimulus and/or physical findings
(c) Possibly indicates interpersonal or psychologic problems
iii. Often involves neuropathic pain: Abnormal processing of sensory input in PNS or CNS due to injury or impairment
(a) Described as either continuous or intermittent sensations (e.g., burning, shooting, shocklike, tingling, jabbing)
(b) Examples include pain caused by nerve root compression, diabetic neuropathy, Guillain-Barré syndrome; phantom limb pain, complex regional pain syndrome
Patient Assessment
i. Current symptoms, including chronologic sequence of onset, duration, location, and frequency
ii. Factors that relieve or exacerbate symptoms
iii. Difficulties performing activities of daily living (ADLs)
b. Patient health history: Significant medical and surgical history, including traumatic injury and childhood diseases
c. Medication history: Use of over-the-counter and prescription drugs, nutritional and herbal supplements, including amount, frequency, duration, last dose, effectiveness, adverse response. Especially note use of analgesics, anticonvulsants, tranquilizers, sedatives, anticoagulants, platelet aggregation inhibitors, stimulants, antihypertensives, cardiac medications.
e. Family history: Note history of disease that may impact current illness (e.g., cardiac disease, stroke [especially early onset], aneurysms, arteriovenous malformations, seizures, migraines, dementia, autoimmune disorders)
i. Significant others affected by the patient’s illness
ii. Support systems available to assist the patient and family
iii. Alcohol and tobacco use: Past and present, amount, duration
iv. Illicit drug use or abuse: Particularly cocaine, amphetamines
v. Type of work; impact of symptoms on work
vi. Hobbies, recreational activities
vii. Current dwelling, including layout and number of stairs
2. Nursing examination of patient
i. First ABC: Evaluate airway patency, sufficiency of breathing and circulation
ii. Inspection then palpation of the head, face, and spine: Shape, symmetry, bony contour, coloration and skin integrity; irregularities may indicate injury, ventricular shunt, previous surgery, or congenital abnormality. Note nares or ear drainage.
iii. Auscultation: Heart for murmurs and clicks; carotid arteries and over eyes for bruits
iv. Assessment of neurologic function
(a) Level of consciousness (LOC) (Box 4-1)
(b) Glasgow Coma Scale (GCS) (Table 4-11): Used to assess LOC; total score also used to classify severity of brain injury. Limitations include inability to assess eye opening in patients with periorbital swelling or verbal response in intubated patients. Hypoxia, hypotension, hypothermia, drug intoxication, postictal state, and administration of sedatives, analgesics, or paralytic agents can interfere with GCS responses. Presence of any confounding variable should be noted when reporting score. Neurologic deterioration that affects only one side of the body is not reflected in GCS score.
TABLE 4-11

*Rigid flexion, internal rotation, and adduction of the upper extremity; extension, internal rotation, and plantar flexion of the lower extremity.
†Rigid extension, adduction, and internal rotation of the upper extremity; extension, internal rotation, and plantar flexion of the lower extremity.
(1) Assess size and contour of muscles: Note atrophy, hypertrophy, asymmetry, and joint malalignments
(2) Observe for involuntary movements, such as fasciculations, tics, tremors, abnormal positioning
(3) Determine motor response to stimuli
a) Ability to follow simple commands such as “Hold up two fingers.” Do not ask the patient to squeeze your hand because this may be a reflex response to palmar stimulation.
b) Localization: Able to locate a noxious stimulus (e.g., deep pain stimulus) and attempt to remove it; indicates cortical dysfunction
c) Withdraws: Pulls limb(s) away from painful stimuli with normal flexor movement; indicates extensive cortical damage
d) Abnormal flexion (decorticate posturing; see Table 4-11): Associated with lesions to the corticospinal tract just above the brainstem near or in the cerebral hemispheres, in the area of the diencephalon
e) Extensor (decerebrate) posturing (see Table 4-11): Indicates damage to the midbrain or upper pons
f) No response: Associated with lower brainstem or high spinal cord dysfunction
(4) Strength testing (if the patient is able to follow commands)
a) Evaluate the integrity and function of UMNs and LMNs that innervate a specific muscle or muscle group (Table 4-12)
TABLE 4-12
Muscle Groups, Associated Level of Spinal Cord Innervation, and Method of Testing
| Muscle(s) Tested | Primary Level(s) of Spinal Nerve Innervation | Method of Testing |
| Deltoids | C5 | Raising of arms |
| Biceps | C5 | Flexion of elbow |
| Wrist extensors | C6 | Extension of wrist |
| Triceps | C7 | Extension of elbow |
| Hand intrinsics | C8-T1 | Hand squeezing, finger flexion, finger abduction |
| Iliopsoas | L1, L2 | Hip flexion |
| Hip adductors | L2-L4 | Adduction of hips (squeezing legs together) |
| Hip abductors | L4, L5, S1 | Abduction of hips (separating hips) |
| Quadriceps | L3, L4 | Knee extension |
| Hamstrings | L5, S1, S2 | Knee flexion |
| Tibialis anterior | L4, L5 | Dorsiflexion of foot |
| Extensor hallucis longus | L5 | Extension of great toe |
| Gastrocnemius | S1 | Plantar flexion of foot |
Adapted from McIlvoy L, Meyer K, McQuillan KA: Traumatic spine injuries. In Bader MK, Littlejohns LR, editors: AANN core curriculum for neuroscience nursing, ed 4, St Louis, 2004, Saunders, p 345.
b) Grade strength on a 0 to 5 scale (Table 4-13)
TABLE 4-13
| Score | Muscle Function |
| 0 | Absent, no muscle contraction |
| 1 | Contraction of muscle felt or seen |
| 2 | Movement through full range of motion with gravity removed |
| 3 | Movement through full range of motion against gravity |
| 4 | Movement against resistance but can be overcome |
| 5 | Full strength against resistance |
c) Note whether weakness follows a distributional pattern (proximal-distal, right-left, or upper-lower extremity)
(5) Strength testing for a patient unable to follow commands:
a) Observe which extremities move spontaneously or to noxious stimuli
b) Hemiparesis or hemiplegia may be detected by lifting both arms off the bed and releasing them simultaneously. The limb on the hemiparetic side will fall more quickly and more limply than that on the normal side.
(6) Muscle tone: State of muscle tension assessed by palpating muscles at rest and during passive range-of-motion (ROM) movement; possible abnormalities include:
a) Rigidity: Increased muscular resistance throughout passive ROM movement; seen with a basal ganglia lesion
b) Spasticity: Increased muscular resistance to joint movement, often followed by release of resistance; increased tone indicates corticospinal tract lesion
c) Hypotonia (flaccidity): Decreased muscle tone associated with LMN lesions, cerebellar dysfunction, or spinal shock related to acute spinal cord injury
(7) Deep tendon or muscle stretch reflexes: Elicited by percussing the tendon with a reflex hammer, which causes stretching of the muscle spindles and subsequent contraction of muscle fibers when the monosynaptic reflex arc is intact. Compare responses side to side.
a) Hyperreflexia usually indicates UMN lesion
b) May be diminished initially after an acute intracranial injury due to cerebral shock or at and below the level of spinal cord injury due to spinal shock
c) Areflexia most often due to LMN lesions
d) Deep tendon reflexes commonly tested: See Table 4-14
TABLE 4-14
Deep Tendon or Muscle Stretch Reflexes and Level of Spinal Cord Innervation
| Reflex | Level of Spinal Cord Innervation |
| Biceps | C5, C6 |
| Brachioradialis | C5, C6 |
| Triceps | C7, C8 |
| Quadriceps (patellar) | L2-L4 |
| Achilles (ankle jerk) | S1, S2 |
e) Grade deep tendon reflexes on a 0 to 4 scale: See Table 4-15
TABLE 4-15
Grading Scale for Strength of Deep Tendon Reflexes
| Score | Reflex Response |
| 4+ | Hyperreactive, clonus |
| 3+ | Very brisk |
| 2+ | Normal, average |
| 1+ | Diminished |
| 0 | No response, flaccid |
From McIlvoy L, Meyer K, McQuillan KA: Traumatic spine injuries. In Bader MK, Littlejohns LR, editors: AANN core curriculum for neuroscience nursing, ed 4, St Louis, 2004, Saunders, p 346.
(8) Superficial reflexes: Tested by stroking the skin with a moderately sharp object (Table 4-16). These reflexes are lost or abnormal with UMN or LMN lesions.
TABLE 4-16
Superficial Reflexes, Level of Spinal Nerve Innervation, and Method for Assessment
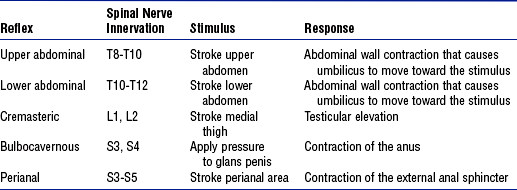
Adapted from McIlvoy L, Meyer K, McQuillan KA: Traumatic spine injuries. In Bader MK, Littlejohns LR, editors: AANN core curriculum for neuroscience nursing, ed 4, St Louis, 2004, Saunders, p 347.
(9) Pathologic reflexes: See Box 4-2
(1) In an unresponsive or uncooperative patient, a cursory sensory examination is performed by noting the patient’s response to painful stimuli applied while performing various interventions (e.g., venipuncture)
(2) In an awake, cooperative patient able to understand and follow commands, a complete sensory assessment can be performed. Test with the patient’s eyes closed and compare one side of the body with the other
(3) Sensory function is scored using a 0 to 2 scale: See Table 4-17
TABLE 4-17
| Score | Sensory Function |
| 0 | Absent |
| 1 | Impaired or hyperesthetic |
| 2 | Normal or intact |
Adapted from McIlvoy L, Meyer K, McQuillan KA: Traumatic spine injuries. In Bader MK, Littlejohns LR, editors: AANN core curriculum for neuroscience nursing, ed 4, St Louis, 2004, Saunders, p 344.
(4) When possible, delineate sensory impairments based on dermatome distribution (see Figure 4-17)
(5) Spinothalamic tracts: See Box 4-3
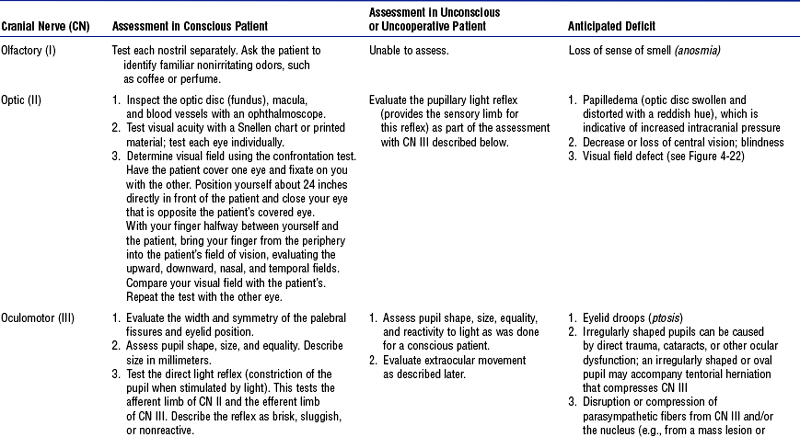
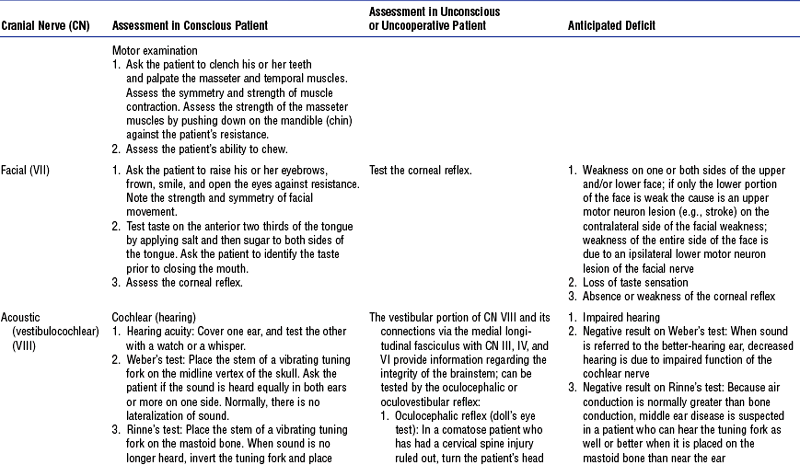
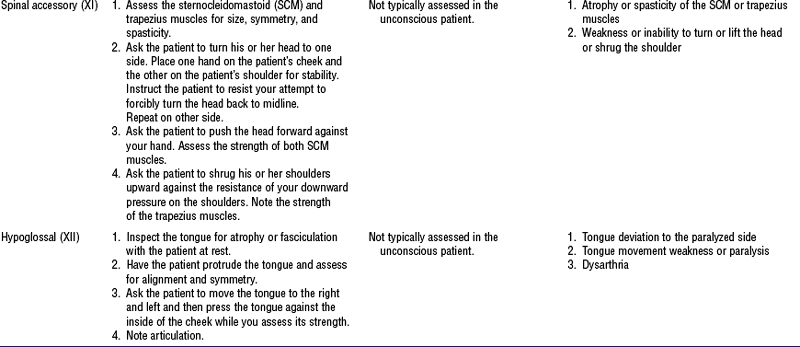
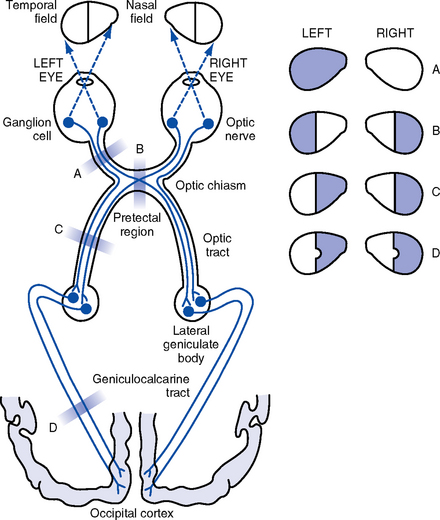
(e) Cranial nerves: See Table 4-18 and Figure 4-22
(f) Eye and pupil signs: In addition to cranial nerve assessment, other findings may include the following:
(1) Pupil abnormalities (Table 4-19)
TABLE 4-19
Pupil Abnormalities Associated with Specific Areas of Brain Dysfunction
| Pupil Finding | Related Brain Dysfunction |
| Small, equal, reactive | Bilateral diencephalic damage that affects the sympathetic innervation originating from the hypothalamus; metabolic dysfunction |
| Nonreactive, midpositioned | Midbrain damage |
| Fixed and dilated | Ipsilateral oculomotor (cranial nerve III) compression or injury |
| Bilateral fixed and dilated | Brain anoxia and ischemia; bilateral cranial nerve III compression |
| Pinpoint, nonreactive | Pons damage, often from hemorrhage or ischemia that interrupts the sympathetic nervous system pathways |
| One pupil is smaller that the other, but both reactive to light; associated with ptosis and an inability to sweat on the same side as the smaller pupil (Horner’s syndrome) | Interruption of ipsilateral sympathetic innervation that can be caused by a lesion of the anterolateral cervical spinal cord or lateral medulla, damage to the hypothalamus, or occlusion or dissection of the internal carotid artery |
(2) Gaze deviation or gaze preference: Horizontal or vertical gaze deviations indicate a cortical or brainstem lesion
a) Eyes deviate toward the side of a destructive hemispheric lesion affecting the frontal gaze centers
b) Gaze deviates away from irritative foci (seizures) affecting the frontal gaze centers
c) Inability to gaze upward is associated with dorsal midbrain lesions
d) Eyes deviate away from the side of a unilateral pons lesion
a) Hyperthermia increases cerebral oxygen consumption by 10% for every 1.8° F (1° C) elevation. Higher metabolic demand increases the risk for CNS ischemia. Neurogenic fever can be caused by damage to the hypothalamus, where the thermoregulation center is located.
b) Hypothermia, if extreme, can lead to cardiac dysrhythmias, coagulopathies, other complications. Seen with hypothalamic lesions, spinal cord injury with autonomic dysfunction, and metabolic or toxic encephalopathy.
(2) Respirations: Respiratory dysrhythmias often correlate with lesions at specific locations in the brain, although effects may vary and may be influenced by other factors (Table 4-20)
TABLE 4-20
Respiratory Patterns Associated with Specific Areas of Brain Dysfunction
| Breathing Pattern | Description | Location of Brain Lesion or Type of Dysfunction |
| Cheyne-Stokes | Regular cycles of respirations that gradually increase in depth to hyperpnea and then decrease in depth to periods of apnea | Usually bilateral lesions deep within the cerebral hemispheres, basal ganglia, or diencephalon; metabolic disorders |
| Central neurogenic hyperventilation | Deep, rapid respirations | Midbrain, upper pons |
| Apneustic | Prolonged inspiration followed by a 2- to 3-sec pause; occasionally may alternate with an expiratory pause | Pons |
| Cluster | Cluster of irregular breaths followed by an apneic period lasting a variable amount of time | Lower pons or upper medulla |
| Ataxic or irregular | Irregular, unpredictable pattern of shallow and deep respirations and pauses | Medulla |
Adapted from McQuillan KA, Mitchell PH: Traumatic brain injuries. In McQuillan KA, Von Rueden KT, Hartsock RL, et al, editors: Trauma nursing from resuscitation through rehabilitation, ed 3, St Louis, 2002, Saunders, p 420.
a) Both are notoriously unreliable parameters in detecting CNS disease or neurologic deterioration. May change late in the course of increased ICP and thus are of limited clinical use.
b) Cardiac dysrhythmias may be seen with neurologic disorders, particularly with blood in the CSF, after posterior fossa surgery, with increased ICP, or with stroke in certain locations
c) Tachycardia and hypertension may be seen with injury or compression of the hypothalamus that results in sympathetic nervous system stimulation
d) Cushing’s response occurs when intracranial hypertension causes compression of the medullary vasomotor center. Systolic pressure rises, widens pulse pressure, and may slow pulse.
a) Pain that is assessed at regular intervals and treated with the same zeal as abnormalities in other vital signs has a much better chance of being treated effectively (Campbell, 1995)
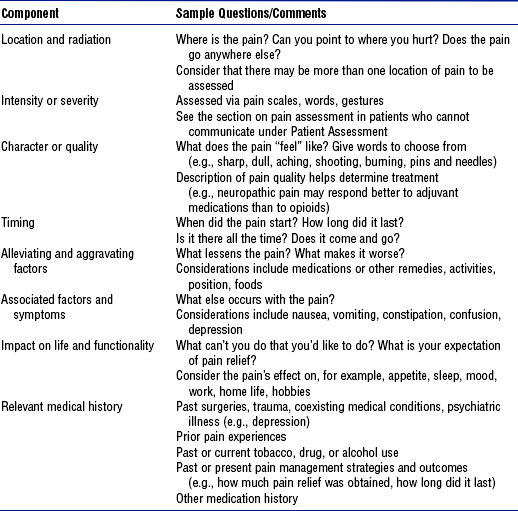
b) Key concepts in pain assessment: See Box 4-4
c) Components of pain assessment: See Table 4-21
d) Adequate pain assessment poses a special challenge in the critically ill patient
e) Acute pain assessment tools
1) Pain tools should estimate the severity of pain and accurately reflect changes in pain intensity following interventions
2) General guidelines for successful use:
a) Tool should be valid, reliable, and appropriate for the patient’s age and cognitive, cultural, developmental, and physical status
b) Patient and caregivers should be educated on tool use and purpose
c) Whenever possible, the same tool should be used consistently with a given patient
d) Whenever possible, a pain tool in the patient’s language should be used
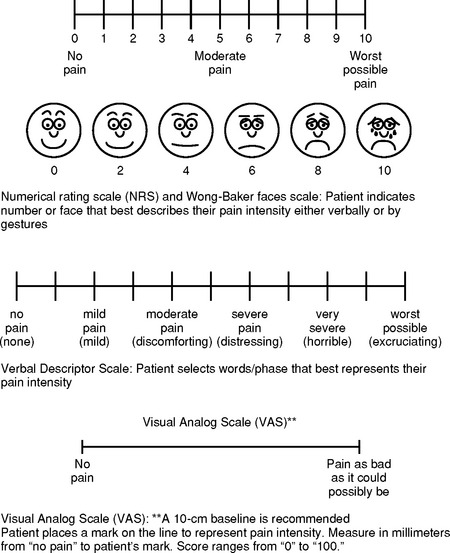
3) Unidimensional tools measure a single element of pain (e.g., intensity). Figure 4-23 shows examples of reliable and valid self-reporting tools for measuring intensity.
d) Visual analogue scale (0 to 100): May be difficult for critically ill patients to use
4) Multidimensional tools measure characteristics and effects of pain on the patient’s life. More appropriate with complex pain situations than for critically ill patients. Examples include the following:
f) Pain assessment when the patient cannot communicate
1) Lack of self-report can lead to undertreatment
2) When self-report is not possible, assume pain is present based on the presence of a painful condition or procedure (American Pain Society, 2003)
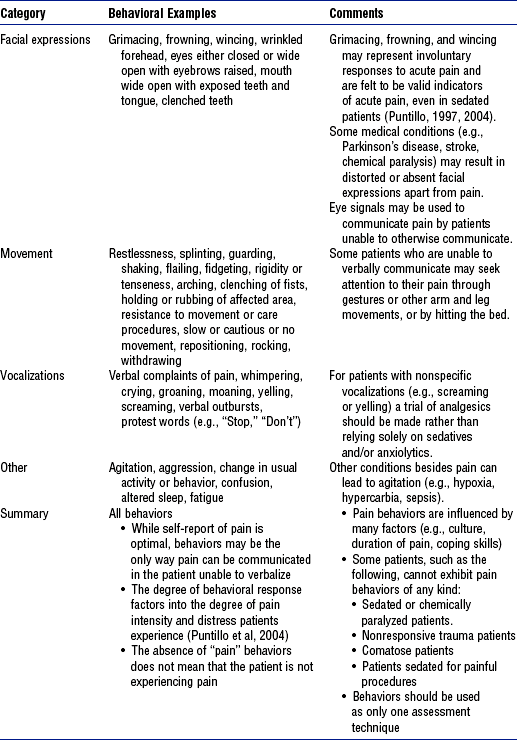
3) Behavioral and physiologic signs are not always reliable indicators of pain but may be useful to confirm suspicion of pain or evaluate the response to intervention when no alternative exists
a) Behaviors are often more reliable indicators than physiologic signs
b) Physiologic signs are temporary, may resolve before pain resolves, and may reflect other phenomena (e.g., anxiety)
4) Hierarchy of importance of basic measures of pain intensity (criteria are listed in order of reliability) (McCaffery and Pasero, 1999):
a) Patient’s self-report of pain
b) Pathology (e.g., fractures, incisions)
c) Behaviors (e.g., grimacing, frowning, wincing) (Table 4-22)
d) Report of a parent, family member, or other person close to the patient (“proxy pain rating”)
e) Physiologic indices (e.g., increased heart rate, BP) (Table 4-23)
TABLE 4-23
Harmful Effects and Clinical Manifestations of Acute Pain
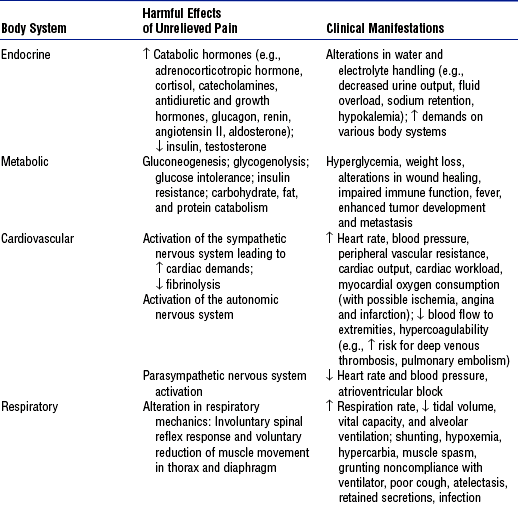
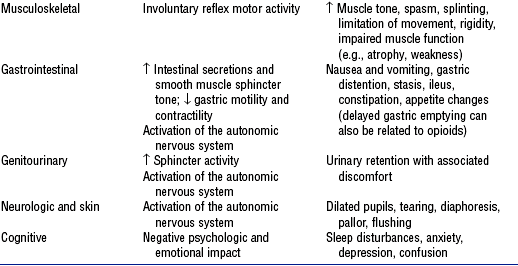
Compiled from Graf C, Puntillo K: Pain in the older adult in the intensive care unit, Crit Care Clin 19(4):749–770, 2003; Hamill-Ruth RJ, Marohn ML: Evaluation of pain in the critically ill patient, Crit Care Clin 15(1):35–54, 1999; McCaffery M, Pasero C: Pain: clinical manual, ed 2, St Louis, 1999, Mosby; Melzack R, Wall PD, editors: Handbook of pain management, Edinburgh, 2003, Churchill Livingstone; Pasero C: Pain in the critically ill patient, J Perianesth Nurs 18(6):422–425, 2003; and Payen, Olivier, Bosson, et al, 2001.
5) Behavioral pain tools designed for use in critically ill patients require further testing before general use can be recommended (Pasero, 2004a)
a) If used, evaluate each time a low score is obtained with potentially painful conditions as patients may not be able to demonstrate specific behaviors required for scoring (Pasero, 2004a)
b) Examples include Behavioral Pain Scale (Payen et al, 2001), Nonverbal Pain Scale (Odhner et al, 2003), Checklist of Nonverbal Pain Indicators (Feldt, 2000)
i. ICP monitoring: See specific patient health problems
ii. Jugular venous oxygen saturation (Sjo2)
(a) Principle: Assesses the coupling or uncoupling of cerebral oxygen delivery (CBF) and cerebral oxygen consumption (metabolism). Placement of a fiberoptic catheter into the jugular bulb allows for continuous measurement of Sjo2 and intermittent venous blood gas sampling. Reflects a global view of oxygenation in the cerebral hemisphere on the side of catheter placement.
(b) Clinical uses: May detect episodes of uncoupling between CBF and metabolism indicating cerebral ischemia (CBF is less than the metabolic demand) or hyperemia (CBF exceeds the metabolic demand). Factors that decrease cerebral oxygen supply (i.e., hypoxemia, anemia, insufficient cerebral perfusion) or increase cerebral metabolic demand (e.g., seizures, hyperthermia) lower Sjo2 and can cause ischemia. Factors that increase oxygen supply (i.e., increased CBF, hyperoxia) or reduce cerebral oxygen demand (e.g., large area of cerebral infarction, hypothermia) raise Sjo2 and can cause hyperemia.
(1) Normal Sjo2 is 55% to 75%; Sjo2 below 55% is indicative of global ischemia and warrants treatment; Sjo2 above 80% indicates hyperemia
(2) Cerebral extraction of oxygen (CEo2): Calculated by subtracting Sjo2 from arterial oxygen saturation (Sao2). Normal = 24% to 40%; lower than 24% indicates hyperemia; higher than 40% indicates cerebral oxygen supply insufficient for demand.
(3) When venous and arterial blood gas levels are analyzed simultaneously, arteriovenous oxygen difference (AVDo2) can be calculated. Abbreviated formula: AVDo2 = 1.34 (Sao2 − Sjo2) hemoglobin/100. Normal AVDo2 is 4.5 to 8.5ml/dl; less than 4.5 ml/dl indicates hyperemia and more than 8.5 ml/dl indicates cerebral oxygen supply insufficient for demand.
(4) Knowledge of uncoupling between cerebral oxygen delivery and demand can prompt implementation of interventions to improve the balance and prevent ischemia or hyperemia (Box 4-5)
(c) Postprocedure care: After insertion, lateral cervical radiograph confirms location of the catheter tip. Calibrate the continuous Sjo2 monitor. Because readings may be inaccurate due to poor catheter position or improper calibration, a strategy for troubleshooting any abnormal value should be established. Potential complications include infection, bleeding, vascular injury, and thrombosis.
iii. Partial pressure of brain tissue oxygen (Pbto2)
(a) Principle: Oxygen-sensing probe inserted into brain parenchyma continuously monitors Pbto2 around the tip. Provides data regarding the balance between oxygen delivery to the cerebral extracellular space and oxygen consumption by cerebral tissue.
(b) Clinical uses: Normal Pbto2 varies depending on the brand of monitor used. Depth and duration of low Pbto2 correlate with a poorer outcome from brain injury. When cerebral hypoxia is recognized, interventions to improve cerebral oxygen delivery and minimize cerebral oxygen consumption can be instituted (Box 4-6). Use of the device may be beneficial for patients with severe traumatic brain injury (TBI), stroke, tumor, or subarachnoid hemorrhage.
(c) Postprocedure care: Observe for potential complications, including infection, hemorrhage
iv. Near-infrared cerebral spectroscopy
(a) Principle: Sensor placed on the scalp emits near-infrared light that penetrates the scalp and skull to measure oxygen saturation in underlying brain tissue
(b) Clinical uses: Normal values are ill-defined but changes in trends may indicate alterations in regional cerebral oxygen saturation. Measurements may be unreliable due to stray light, sensor disruption, drift, movement artifact, temperature changes, or contamination from extracranial circulation.
v. Continuous electroencephalographic (EEG) monitoring
(a) Principle: Continuously monitors electrical activity of the brain by means of electrodes attached to the scalp
(b) Clinical uses: Identifies the onset of seizures or cerebral ischemia so appropriate interventions can be initiated. Monitors burst activity in patients in drug-induced coma for refractory increased ICP so that the minimal amount of barbiturate or propofol to achieve the desired burst suppression is administered.
(c) Care during procedure: Move the patient carefully to avoid dislodging the electrodes; EEG readings are influenced by electrical, environmental, movement, and biologic artifacts
vi. Transcranial Doppler ultrasonography (TCD)
(a) Principle: Probe positioned over thin areas of the cranium emits a low-frequency pulsed ultrasonic signal to measure the direction and velocity of blood flow through underlying major vessels
(b) Clinical uses: High velocities correlate with cerebral vasospasm or hyperemia. If middle cerebral artery (MCA) velocity is higher than 120 cm/sec, the ratio of MCA to internal carotid artery (ICA) velocity can be calculated. A ratio of 3 or higher indicates cerebral vasospasm and less than 3 suggests hyperemia. Can detect emboli, stenotic or occluded vessels, and other vascular anomalies. May reveal blood flow alterations indicating ICP elevations. May also be used to evaluate cerebral autoregulation and as a confirmatory test of brain death.
(1) Advantages: Noninvasive, portable, relatively inexpensive, can be safely repeated often or used continuously to monitor changes
(2) Disadvantages: Absolute velocities vary depending on many factors—age, hematocrit, Paco2, cardiac output, and the metabolic activity of the brain tissue supplied by the artery being insonated. Difficult to distinguish anatomic variations of arteries from arterial disease; does not detect bilaterally symmetric disease, long regions of vasoconstriction or stenosis, or distal artery disease.
(c) Preprocedure and postprocedure care: No specific care required; no known complications
vii. Continuous CBF monitoring
(a) Principle: Sensor placed on the surface of the brain continuously measures regional CBF
(1) Thermal diffusion flowmetry: Temperature variation between two plates on a brain surface sensor provides an inverse measure proportional to CBF
(2) Laser Doppler flowmetry: Laser Doppler probe positioned on the brain surface or in the parenchyma measures CBF
(b) Clinical uses: Provides a continuous measure of regional CBF that can be used to evaluate autoregulation and detect local brain ischemia. Can guide therapy to avoid brain ischemia.
(c) Postprocedure care: Observe for potential complications, including infection or CSF leakage. Troubleshoot abnormal values; data may be unreliable if the probe loses contact with the brain surface or comes in contact with large blood vessels. Alterations in hematocrit, strong external light, or probe movement artifact can influence laser Doppler measurements.
3. Appraisal of patient characteristics: Patients with acute, life-threatening neurologic problems enter critical care units with a wide range of clinical characteristics. During their stay, their clinical status may slowly or abruptly improve or deteriorate. Changes in the patient’s condition may involve one or all life sustaining functions, and functions can be easy or nearly impossible to monitor with precision. Examples of clinical attributes that the nurse should assess when caring for a patient with an acute neurologic disorder are the following:
i. Level 1—Minimally resilient: A frail, 84-year-old female, hit by a car 10 days earlier, who suffered a complete transection of the spinal cord at the C4-C5 level and has developed respiratory and renal failure
ii. Level 3—Moderately resilient: A 35-year-old woman being treated for Guillain-Barré syndrome who has required ventilator support for the past 7 days. She now has a low-grade fever, elevated white blood cell (WBC) levels, and purulent sputum.
iii. Level 5—Highly resilient: An otherwise healthy, alert, and oriented 17-year-old male complaining of nausea who has been admitted for overnight monitoring following a concussion sustained while playing football
i. Level 1—Highly vulnerable: A 41-year-old college professor diagnosed with a fast-growing, invasive grade IV glioblastoma 3 months earlier is admitted from the oncology clinic, where he suddenly became unresponsive during a chemotherapy session
ii. Level 3—Moderately vulnerable: A 37-year-old electrician admitted directly from the emergency department following a grand mal seizure preceded by complaints of severe headache and confusion who has been diagnosed with an arteriovenous malformation (AVM). He is awaiting further diagnostic studies and intervention for the lesion.
iii. Level 5—Minimally vulnerable: A 23-year-old female rugby player who is alert and oriented 2 days after evacuation of a small epidural hematoma
i. Level 1—Minimally stable: A 32-year-old male who suffered a severe brain injury and bilateral pulmonary contusions 2 days earlier in a motor vehicle crash. He is now having problems with increased ICP above 25 mm Hg that is unresponsive to therapy and respiratory failure requiring increasing ventilator support.
ii. Level 3—Moderately stable: A 50-year-old woman 1 week after a subarachnoid hemorrhage (SAH) and aneurysm clipping who has developed a pronator drift secondary to vasospasm that resolves with hypertensive, hypervolemic therapy
iii. Level 5—Highly stable: A 16-year-old boy who fell off the back of a truck and hit his head, suffering a traumatic SAH. He was unconscious for 15 minutes and confused for 24 hours but is now awake and neurologically intact.
i. Level 1—Highly complex: A 17-year-old boy who jumped off a third-floor balcony while intoxicated and suffered a C4-C5 subluxation with complete spinal cord injury. He is in neurogenic shock, has a temperature of 93° F (33.9° C), has an ileus, and requires increasing ventilator support.
ii. Level 3—Moderately complex: A 60-year-old diabetic woman with a hemorrhagic hypertensive stroke who develops hydrocephalus
iii. Level 5—Minimally complex: A 40-year-old woman with a 1-cm meningioma resected 2 days earlier who is anxious to go home
i. Level 1—Few resources: A 59-year-old homeless man found in a vacant lot with an alcohol level of 320 mg% and an acute on chronic subdural hematoma. Two days after admission, his GCS score is 6 while he is intubated.
ii. Level 3—Moderate resources: A 30-year-old schoolteacher with a small right parietal AVM that hemorrhaged 1 week earlier who is now neurologically intact. She is not married and has no family in the area, but a close friend has been visiting. She is insured and is a candidate for outpatient focused-beam radiation therapy.
iii. Level 5—Many resources: A 35-year-old woman who suffered moderate TBI 5 days earlier and has a right hemiparesis and expressive aphasia that will require in-patient rehabilitation. She is married, has insurance, and has two sisters and parents who live near her.
i. Level 1—No participation: A 28-year-old transient worker with no known family in the United States who is comatose and areflexic subsequent to massive head and neck trauma suffered in an automobile crash today
ii. Level 3—Moderate level of participation: A non–English-speaking 76-year-old, newly diagnosed with diabetes, who will be discharged home tomorrow and has moderate expressive dysphasia and right hemiparesis following an ischemic stroke. The patient’s primary support person at home is a daughter who works as a nursing assistant and speaks some English.
iii. Level 5—Full participation: A 56-year-old high school teacher who developed acute neurologic deterioration due to infectious meningitis contracted from a student and who is now fully alert and cooperative, recovering rapidly, and planning the details of her home care with the help of her daughter, an agency nurse
g. Participation in decision making
i. Level 1—No participation: A 55-year-old man who had a compressor explode in his face at work 1 week earlier and who has a GCS score of 4 while intubated
ii. Level 3—Moderate level of participation: A 46-year-old woman with an anterior communicating artery aneurysm that ruptured 19 days earlier who has moderate vasospasm and is lethargic but is otherwise neurologically intact
iii. Level 5—Full participation: A 45-year-old college professor who just had surgery for a right hemisphere glioblastoma, has CN VII paresis, and had three seizures but is otherwise neurologically intact. He has asked about experimental research protocols available to him.
i. Level 1—Not predictable: A 60-year-old woman who is transferred from an outside hospital with an anterior communicating artery aneurysm and grade III SAH suffered 5 days earlier. She is having vasospasm and has developed hydrocephalus. Her aneurysm has not been clipped.
ii. Level 3—Moderately predictable: An 18-year-old high school senior who suffered TBI 3 days earlier and is beginning to localize in response to noxious stimuli. His ICP varies from 10 to 25 mm Hg and is controlled with CSF drainage and sedation.
iii. Level 5—Highly predictable: A 25-year-old woman who suffered a linear skull fracture and brain injury 1 week earlier and is now fully awake, alert, and cooperative
i. Complete blood count (CBC) and differential
iii. Blood chemistry tests, including osmolality, electrolyte levels
iv. Clotting profile, including prothrombin time (PT), international normalized ratio (INR), partial thromboplastin time (PTT), d-dimer levels, fibrinogen levels
v. Arterial blood gas (ABG) levels
viii. CSF analysis: Compare with normal values and request a culture and sensitivity test
i. Skull series: In the absence of computed tomographic (CT) scans, skull radiographs may be useful in diagnosing skull abnormalities (e.g., fractures, erosion), noting shift of the pineal gland, and detecting intracranial air or abnormal calcifications
ii. Spine series: Assesses vertebral integrity and alignment to diagnose fractures, dislocations, bony defects, or degenerative processes; CT (Box 4-7) or magnetic resonance imaging (MRI) (Box 4-8 and Table 4-24) often used to further delineate abnormalities