Chapter 13
The Midbrain
Internal Anatomy of the Midbrain
General Regions: Tectum, Tegmentum, and Basis Pedunculi
Summary of Descending Pathways
Diencephalon Junction-Midbrain
Internal Vasculature of the Midbrain
The mesencephalon, or midbrain, is the most rostral portion of the brainstem. It gives rise to cranial nerves III and IV, conducts ascending and descending tracts, and contains nuclei that are essential to motor function. Caudally the midbrain is continuous with the pons, and rostrally it joins the diencephalon. The cerebral aqueduct, the cavity of the midbrain, is continuous rostrally with the third ventricle and caudally with the fourth ventricle. The blood supply to the mesencephalon is primarily from proximal branches of the posterior cerebral arteries (P1 or P2) and from penetrating branches of the posterior communicating artery.
DEVELOPMENT
The mesencephalon (Fig. 13-1) arises early in development as one of the three primary brain vesicles. Immature neurons that arise from the ventricular zone form the intermediate zone and give rise to alar and basal plates, which are continuous with the alar and basal plates of the rhombencephalon (Fig. 13-2). These cell groups are the rostral continuations of the same primitive cell columns described for the metencephalon. The surrounding marginal layer contains the developing axons of cells located in other levels of the neuraxis. The cerebral aqueduct is narrow relative to the fourth ventricle; therefore the basal and alar plates lie anterior and posterior to it, respectively, as in the spinal cord and caudal medulla.
Figure 13-1. Lateral view of the human brain at about 7 weeks of gestation. The midbrain is highlighted.
Basal and Alar Plates
The immature neurons of the alar plate give rise to the quadrigeminal plate, from which the superior and inferior colliculi arise (Fig. 13-2A, upper arrow). In humans, the superior colliculus consists of alternating layers of cells and fibers, whereas the inferior colliculus appears more homogeneous. Immature alar plate neurons also migrate into anterior areas of the developing midbrain to form the red nucleus and the substantia nigra (Fig. 13-2A, lower arrow).
Immature neurons of the basal plate give rise to the somatic efferent (SE) neurons of the oculomotor and trochlear nuclei. In addition, the visceral efferent (VE) preganglionic parasympathetic cells associated with the oculomotor complex also arise from the basal plate (Fig. 13-2).
As the basal and alar plates differentiate, the marginal layer is invaded by axons originating from cells located outside the midbrain. These fibers collect in the anterolateral area of the developing mesencephalon to form an especially prominent bundle, the crus cerebri (Fig. 13-2B).
EXTERNAL FEATURES
Anterior (Ventral) Midbrain
The presence of a pair of large axon bundles, the crura cerebri, is a characteristic feature of the anterior aspect of the midbrain. These bundles emerge from the cerebral hemispheres caudal to the optic tracts, converge slightly toward the midline as they course through the midbrain, and disappear into the basilar pons (Fig. 13-3). The oculomotor nerves exit the medial edge of each crus and pass through the space between the crura: the interpeduncular fossa (Fig. 13-3). Anteriorly, the rostral limit of the midbrain is marked by the exit of the crura cerebri from the cerebral hemispheres and by the caudal edge of the mammillary bodies. The caudal border of the midbrain is formed where each crus enters the basilar pons.
Figure 13-3. Anterior (ventral) views (A, undissected; B, dissected) of the brainstem with emphasis on the midbrain.
The subarachnoid space of the interpeduncular fossa is called the interpeduncular cistern. This cistern contains the oculomotor nerves and the upper part of the basilar artery, including its bifurcation and proximal branches. Numerous vessels penetrate the roof of this fossa and create many small perforations (Fig. 13-3B). This area is frequently called the posterior perforated substance.
Posterior (Dorsal) Midbrain
The posterior surface of the adult midbrain is characterized by four elevations collectively called the corpora quadrigemina (Fig. 13-4). The rostral two elevations are the superior colliculi, and the caudal two are the inferior colliculi. Just caudal to the inferior colliculus, the exit of the trochlear nerve marks the pons-midbrain junction on the posterior surface of the brainstem, whereas the midbrain-diencephalic boundary is formed by the posterior commissure (Fig. 13-5).
Figure 13-5. Sagittal view of the brainstem with emphasis on midbrain structures.
Rostrolaterally, the inferior colliculus is joined to the medial geniculate body of the diencephalon by a fiber bundle called the brachium of the inferior colliculus (Fig. 13-4). The inferior colliculus and the medial geniculate body are part of the auditory system. The brachium of the superior colliculus extends from the optic tract to the superior colliculus in a groove located between the medial geniculate body and pulvinar of the diencephalon (see Figs. 13-4 and 13-14). The superior colliculus, pulvinar, and lateral geniculate body are parts of the visual and visual-motor systems.
On the midline, the pineal gland, a diencephalic structure, extends posteriorly above and between the superior colliculi (Fig. 13-5). Tumors of the pineal may produce noncommunicating (obstructive) hydrocephalus because of compression of the colliculi of the midbrain and resulting occlusion of the cerebral aqueduct.
The subarachnoid space immediately posterior (dorsal) to the colliculi is the quadrigeminal cistern. This cistern contains the exiting trochlear nerves, the great vein of Galen, and distal branches of the posterior cerebral arteries. The ambient cistern is located at the lateral aspect of the midbrain and contains segments P2 to P3 and the superior cerebellar and quadrigeminal arteries (Fig. 13-6). The crural cistern is located between the crus cerebri and the immediately adjacent parahippocampal gyrus; it contains medial posterior choroidal and anterior choroidal arteries and the basal vein (of Rosenthal). The interpeduncular cistern is that part of the subarachnoid space that occupies the interpeduncular fossa (Fig. 13-6).
 Figure 13-6. Major subdivisions of the midbrain and the general positions of cisterns related to the midbrain.
Figure 13-6. Major subdivisions of the midbrain and the general positions of cisterns related to the midbrain.
Vasculature of the Midbrain
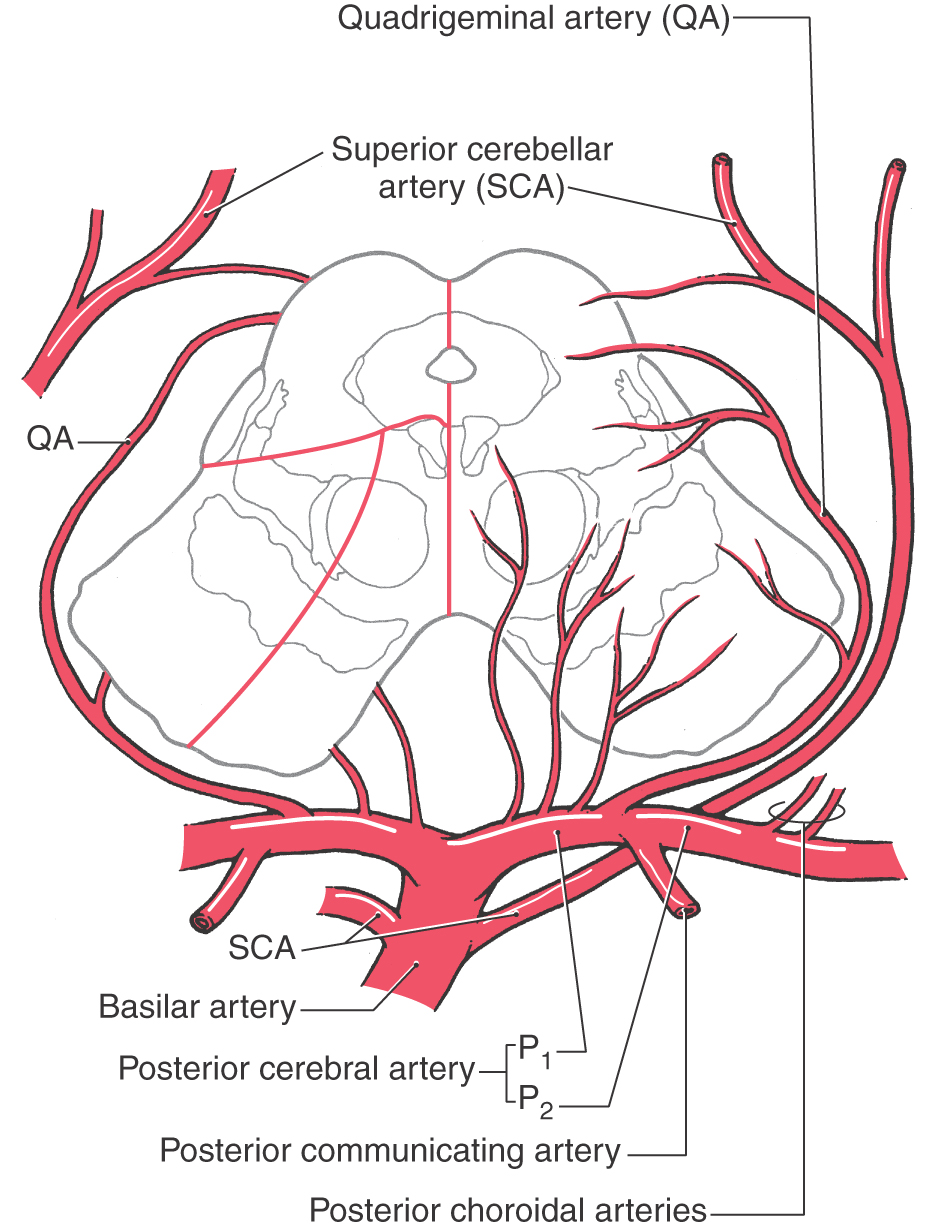
The primary blood supply to the mesencephalon arises via branches of the basilar artery, with smaller branches from the superior cerebellar, anterior choroidal, medial posterior choroidal, and posterior communicating arteries. An important source of blood to the posterior portion of the midbrain is the quadrigeminal artery, a branch of the posterior cerebral artery (P1 segment). Also, the superior cerebellar artery gives rise to branches that serve caudal parts of the posterior midbrain and adjacent regions of the pons.
INTERNAL ANATOMY OF THE MIDBRAIN
General Regions: Tectum, Tegmentum, and Basis Pedunculi
The midbrain is divisible into three regions, which can be appreciated best in cross section. Posterior to the cerebral aqueduct is the tectum (roof) of the midbrain (Figs. 13-5 and 13-6). The characteristic structures of this area are the superior and inferior colliculi. The periaqueductal gray (central gray) is a sleeve of neuron cell bodies that completely surrounds the cerebral aqueduct. The tegmentum of the midbrain extends from the base of the tectum to, but does not include, the substantia nigra. The anterolateral portion of the midbrain on either side is formed by the basis pedunculi, which consists of the substantia nigra and the crus cerebri (Fig. 13-6). In turn, the crus cerebri is composed primarily of descending fibers. The term cerebral peduncle is sometimes used for the crus cerebri, but it actually represents the entire midbrain below the tectum (tegmentum and basis pedunculi).
Summary of Ascending Pathways
The long ascending pathways that traverse the pons and medulla continue through the midbrain (Fig. 13-7). These include the medial lemniscus, the anterolateral system, and the posterior and anterior trigeminothalamic tracts. The lateral lemniscus is also prominent in the caudal midbrain. Other, smaller bundles, such as the medial longitudinal fasciculus, are also present and occupy positions comparable to those seen in lower levels of brainstem.
Figure 13-7. Diagrammatic representation of the brain showing the location and trajectory of three important pathways and the trigeminal nuclei. The color coding for each is continued in Figures 13-8, 13-12, and 13-14.
Summary of Descending Pathways
Descending fibers from the cerebral cortex pass through the midbrain and, as we have seen, are also prominent features of the pons, medulla, and spinal cord (Fig. 13-7). At midbrain levels, these corticospinal, corticonuclear (corticobulbar), and corticopontine fibers are parts of the crus cerebri. Some of the descending fiber bundles that were discussed in the pons and medulla, such as the tectobulbospinal system and the rubrospinal and central tegmental tracts, originate from nuclei of the midbrain.
The following sections describe the anatomy of the midbrain at caudal and rostral brain levels and at the level of the midbrain-diencephalon junction. The anatomy is presented from posterior to anterior at each level, starting with the tectum and proceeding through the tegmentum to the basis pedunculi.
Caudal Midbrain Levels
Transverse sections through the caudal midbrain are characterized by the presence of the inferior colliculus, trochlear nucleus, and decussation of the superior cerebellar peduncle (Figs. 13-8 to 13-10). The inferior colliculus is composed of a large central nucleus bordered posteriorly by a smaller pericentral (dorsal) nucleus and laterally by an external (lateral) nucleus. Lateral lemniscus fibers enter the inferior colliculus from an anterior direction and give this area a goblet-like appearance (Figs. 13-8 and 13-9). The lateral lemniscus provides auditory input to the nuclei of the inferior colliculus, which then transmit this information to the medial geniculate body via fibers of the brachium of the inferior colliculus. A complete tonotopic representation (frequency map) of the cochlea is found in several of the central auditory nuclei (see Chapter 21).
Figure 13-8. Cross section of the midbrain at the level of the inferior colliculus. Correlate with Figure 13-7. The anatomic orientation is flipped to illustrate internal structures in a clinical orientation; the clinically important tracts and nuclei are shown on a T2-weighted magnetic resonance image at a comparable level of the inferior colliculus.
 Figure 13-9. A fiber (myelin)–stained cross section of the midbrain at the level of the inferior colliculus. Compare with Figure 13-8.
Figure 13-9. A fiber (myelin)–stained cross section of the midbrain at the level of the inferior colliculus. Compare with Figure 13-8.
 Figure 13-10. A fiber (myelin)–stained cross section of the midbrain at an intercollicular level showing the trochlear nucleus. Compare with Figure 13-8.
Figure 13-10. A fiber (myelin)–stained cross section of the midbrain at an intercollicular level showing the trochlear nucleus. Compare with Figure 13-8.
The periaqueductal gray, or central gray, is a prominent collection of small neurons surrounding the cerebral aqueduct at all midbrain levels (Figs. 13-8 to 13-10, 13-12, and 13-13). It receives somatosensory input, is interconnected with the hypothalamus and thalamus, and projects caudally to brainstem nuclei. Its connections and the presence of a high level of opiate receptor binding activity indicate that the periaqueductal gray plays an important role in the brain mechanisms responsible for the suppression and modulation of pain (analgesia). The cell bodies of the mesencephalic nucleus and their laterally adjacent fibers, the mesencephalic tract, are located in the lateral edge of the periaqueductal gray, and the posterior (dorsal) raphe nucleus is located anteriorly on the midline, adjacent to the trochlear nucleus and medial longitudinal fasciculus (Fig. 13-8).
The central area of the tegmentum at the level of the inferior colliculus is occupied by the decussating fibers of the superior cerebellar peduncle (Figs. 13-8 and 13-9). From this point, the majority of these cerebellar efferent axons pass rostrally as cerebellothalamic fibers to targets in the midbrain and thalamus, although some turn caudally and enter the pons and medulla. Many of these cerebellar efferent fibers that turn caudally descend as a component of the central tegmental tract; this tract also contains other connections, such as those from the red nucleus to the ipsilateral inferior olivary complex (rubroolivary fibers). At the level of this decussation, the somatic efferent (SE) cell bodies of the trochlear nucleus form an oval cell group nestled in the fibers of the medial longitudinal fasciculus (Figs. 13-8 and 13-10). The axons of trochlear motor neurons pass laterally and posteriorly around the periaqueductal gray to cross the midline before exiting the brainstem just caudal to the inferior colliculus. They innervate the superior oblique muscle. Tectobulbospinal fibers are ventral to the medial longitudinal fasciculus, and as their name states, the fibers of the central tegmental tract occupy the center of the tegmentum (Figs. 13-8 to 13-10).
Immediately anterior to the decussation of the superior cerebellar peduncle are the interpeduncular nucleus and rubrospinal fibers (Fig. 13-8). The interpeduncular nucleus is related to the limbic system, a part of the brain that functions in the control of emotional behavior.
Located in the anterolateral and lateral portions of the midbrain tegmentum are fibers of the anterolateral system and medial lemniscus (Fig. 13-8). The anterolateral system conveys pain and temperature signals from the contralateral side of the body. Most of its fibers terminate in the dorsal thalamus and therefore pass through the midbrain, but it also contains spinomesencephalic fibers that end in the midbrain. Some of these fibers synapse in the reticular nuclei of the midbrain and pons (spinoreticular fibers), some synapse in the tectum (spinotectal fibers), and some synapse in the periaqueductal gray. The last fibers represent an important link in the pathways involved in the inhibition of pain at medullary and spinal levels. Medial lemniscal fibers have shifted from a horizontal position, characteristic of the rostral pons, to the anteromedial to posterolateral orientation of the midbrain. The somatotopy of fibers in the medial lemniscus follows accordingly (Fig. 13-11).
 Figure 13-11. The orientation of the medial lemniscus in the midbrain compared with that in the pons and medulla.
Figure 13-11. The orientation of the medial lemniscus in the midbrain compared with that in the pons and medulla.
Posterior and anterior trigeminothalamic tracts are located adjacent to the central tegmental tract and the medial lemniscus, respectively (Figs. 13-8 and 13-9). These bundles are somewhat diffusely arranged and convey crossed (anterior) and uncrossed (posterior) somatosensory information from the face.
The base of the midbrain on either side is formed by the basis pedunculi, which consists of the substantia nigra and the crus cerebri (Fig. 13-8). The substantia nigra is related to motor function and is considered in the next section of this chapter. The crus cerebri contains corticospinal, corticonuclear (corticobulbar), and corticopontine fibers. The first two fiber populations are found in the middle third of the crus cerebri. Corticospinal fibers projecting to lumbosacral cord levels (lower extremity representation) are found laterally, and corticonuclear fibers that project to cranial nerve nuclei (face representation) are located more medially. Corticospinal fibers projecting to cervical cord levels (upper extremity representation) occupy an intermediate position. Corticopontine fibers in the medial third of the crus arise from the frontal lobe (frontopontine), whereas those in the lateral third originate from parietal, occipital, and temporal lobes (parietopontine, occipitopontine, and temporopontine).
Rostral Midbrain Levels
Transverse sections through the rostral midbrain are characterized by the presence of the superior colliculus, red nucleus, and oculomotor nuclei (Figs. 13-12 and 13-13). The paired superior colliculi are composed of alternating layers of gray matter (cells) and white matter (fibers). The “superficial” three layers (I to III) receive input from the retina and visual cortices and project to the thalamus. The “deep” four layers (IV to VII) subserve gaze changes including eye movements and project to the thalamus, brainstem, and spinal cord. Descending crossed projections from the tectum pass to a variety of brainstem areas (as tectoreticular and tectoolivary fibers) and—although this element is minor in humans—to the cervical spinal cord as tectospinal fibers. Together, these fibers constitute the tectobulbospinal system of the brainstem; the name “tectobulbospinal” reflects the diversity of the fibers in this bundle. Rubrospinal fibers originate from the contralateral red nucleus; these fibers descend to medullary and spinal levels, where they influence the activity of motor neurons innervating skeletal muscles.
 Figure 13-12. Cross section of the midbrain at the level of the superior colliculus. Correlate with Figure 13-7.
Figure 13-12. Cross section of the midbrain at the level of the superior colliculus. Correlate with Figure 13-7.
 Figure 13-13. A fiber (myelin)–stained cross section of the midbrain at the level of the superior colliculus. This plane of section is between those illustrated in Figures 13-12 and 13-14.
Figure 13-13. A fiber (myelin)–stained cross section of the midbrain at the level of the superior colliculus. This plane of section is between those illustrated in Figures 13-12 and 13-14.
Anterior to the periaqueductal gray, the oculomotor complex forms a V-shaped region between the medial longitudinal fasciculi (Figs. 13-12 and 13-14). This complex consists of the somatic efferent (SE) cells of the oculomotor nucleus and visceral efferent (VE) cells classically referred to as the Edinger–Westphal nucleus. It is now known that this nucleus is composed of two portions. The first is the Edinger-Westphal preganglionic cells (EWpg) that provide preganglionic parasympathetic fibers to the ciliary ganglion and influence visceromotor functions mediated by the oculomotor nerve, most notably the efferent limb of the pupillary light reflex. The second is the Edinger–Westphal centrally projecting cells (EWcp) that project to a variety of brainstem centers, such as the inferior olivary, posterior column, parabrachial, and spinal trigeminal nuclei and the spinal cord. These fibers function broadly in areas of behavior such as stress, eating, and drinking.
Figure 13-14. Cross section of the midbrain at the mesencephalon-diencephalon junction. Correlate with Figure 13-7. The anatomic orientation is flipped to illustrate internal structures in a clinical orientation; the clinically important tracts and nuclei are shown on a T2-weighted magnetic resonance image at a comparable level of the superior colliculus and the mesencephalon-diencephalon junction. This magnetic resonance image, as also shown in the corresponding drawing, contains midbrain structures as well as caudal diencephalic structures.
Oculomotor fibers arch through and medial to the red nucleus, emerge from the brainstem at the medial edge of the basis pedunculi, and innervate four of the six extraocular muscles. The Edinger-Westphal nucleus (Figs. 13-13 and 13-14) lies posterior to the oculomotor nucleus; its preganglionic cell group provides the preganglionic parasympathetic fibers that travel to the ciliary ganglion via the oculomotor nerve. These parasympathetic fibers are located on the perimeter of the oculomotor nerve and consequently are the first to be affected in a compression injury to this nerve. The postganglionic fibers from the ciliary ganglion innervate the sphincter pupillae and ciliary muscles.
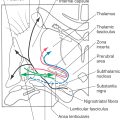
Several other cell groups are located close to the main oculomotor nucleus (Figs. 13-13 and 13-15). These include (1) the nucleus of Darkschewitsch situated within the ventrolateral border of the periaqueductal gray, (2) the interstitial nucleus of Cajal located adjacent to the fibers of the medial longitudinal fasciculus, and (3) the nucleus of the posterior commissure, which is one of the pretectal nuclei. The last two nuclei are involved in the control of eye movements.
The red nucleus, a prominent structure in the midbrain tegmentum at this level (Figs. 13-12 to 13-15), is so named because in the unfixed brain the dense vascularity of the region gives it a pink color. It is generally composed of caudal magnocellular (large-celled) and rostral parvocellular (small-celled) regions. The red nucleus is involved in motor function and has extensive connections throughout the neuraxis. Its efferents include the rubrospinal tract, which travels to the contralateral spinal cord, and rubroolivary fibers, which descend in the central tegmental tract to the ipsilateral inferior olivary complex. Rubrospinal fibers arise from the magnocellular portion of the red nucleus and rubroolivary fibers from its parvocellular portion. Afferents to the red nucleus arise primarily from the contralateral cerebellar nuclei and the ipsilateral cerebral cortex.
The major tracts of the caudal midbrain tegmentum are present in similar locations at rostral midbrain levels (Fig. 13-12). Posterior and anterior tegmental decussations cross the midline at the levels of the posterior and anterior limits of the red nuclei, respectively. The posterior decussation carries tectobulbospinal fibers, and the anterior decussation carries rubrospinal fibers. Immediately lateral to the red nucleus are cerebellorubral and cerebellothalamic fibers. These are crossed ascending fibers from the decussation of the superior cerebellar peduncle. Posterior (dorsal) and anterior (ventral) trigeminothalamic fibers and the medial lemniscus occupy central and more lateral areas of the tegmentum (Fig. 13-12). Fibers of the anterolateral system are located at the lateral extreme of the medial lemniscus, and at this level, spinotectal fibers enter the deep layers of the superior colliculus (Fig. 13-13).
The structure of the basis pedunculi and the organization of fibers in the crus cerebri are the same in the rostral midbrain as more caudally (Fig. 13-12). The substantia nigra is functionally associated with the basal nuclei and is commonly divided into a compact part (pars compacta) and a reticular part (pars reticulata)(Figs. 13-12 and 13-14). Cells of the reticular part project to the superior colliculus, thalamus, and pontine reticular formation, whereas those of the compact part project diffusely to the caudate nucleus and putamen, where their synaptic terminals release dopamine. Parkinson disease, a deficit characterized by tremor and difficulty in initiating or terminating movement, is associated with the loss of dopamine-containing cells in the compact part.
Midbrain-Diencephalon Junction
The major tracts and nuclei seen at the level of the superior colliculus are still present in their same locations at the mesencephalic-diencephalic interface. A comparison of Figures 13-12 and 13-14 reveals these similarities.
Groups of cells related primarily to the visual system and collectively referred to as the pretectal nuclei are located at the rostral extent of the superior colliculus laterally adjacent to the posterior commissure (Fig. 13-15). Indeed, the nucleus of the posterior commissure is one of the pretectal nuclei. The pretectal nuclei include a controlling center for the pupillary light reflex. Neurons involved in this reflex receive retinal inputs and project bilaterally to visceral motor (parasympathetic) cells in the EWpg.
Several structures appear at this junction that signal the transition from midbrain to thalamus (Fig. 13-14). Laterally, a small part of the pulvinar (a thalamic nucleus) is present, and portions of the medial and lateral geniculate nuclei also appear in the same plane. Fibers of the brachium of the inferior colliculus convey auditory signals to the medial geniculate nucleus. The optic tract contains visual fibers, some of which terminate in the lateral geniculate nucleus, whereas others continue into the superior colliculus and pretectal area via the brachium of the superior colliculus (Figs. 13-4 and 13-14). The anterior (ventral) tegmental nucleus (of Tsai) is a diffuse cell group located anteromedially to the red nucleus and rostrally continuous with the lateral hypothalamic area. Many of the cells of this nucleus use dopamine as a neurotransmitter and receive input from and project to hypothalamic and limbic structures that function in emotional behavior.
Reticular and Raphe Nuclei
The reticular formation of the midbrain tegmentum is composed of the cuneiform and subcuneiform nuclei (Fig. 13-16). The midbrain reticular formation participates in the ascending systems that regulate states of consciousness. Many of these neurons project to the thalamus, especially the thalamic reticular nucleus, and the hypothalamus. This ascending fiber system is largely responsible for maintaining an alert, wakeful state and thus forms part of the ascending reticular activating system. Lesions involving the midbrain reticular formation can result in hypersomnia, which is characterized by slow respiration and an electroencephalographic pattern (large-amplitude slow waves) indicative of a sleep state.
Located in anterior parts of the periaqueductal gray, the posterior (dorsal) raphe nucleus extends from the rostral pons into the caudal midbrain (Fig. 13-16). The serotoninergic cells of this nucleus project to wide areas of the cerebral cortex, where they modulate neuronal activity involved in sleep-dream cycles. Serotonin-, enkephalin-, and cholecystokinin-containing cells are also found in the central (periaqueductal) gray, and indeed, some neuroscientists consider the periaqueductal gray to function in concert with the raphe nuclei.
Internal Vasculature of the Midbrain
The blood supply to the midbrain originates from the basilar artery and its major branches (the quadrigeminal and superior cerebellar arteries) and from the anterior choroidal artery, which is a branch of the internal carotid, and the medial posterior choroidal artery, which is usually a branch of P2 (Fig. 13-17). Medial regions of the midbrain receive numerous small branches from the P1 segment of the posterior cerebral artery and from the posterior communicating artery. These paramedian branches constitute the posteromedial group of branches from the circle of Willis. Included in their territory are the oculomotor, trochlear, and Edinger-Westphal nuclei; the exiting oculomotor fibers; the red nucleus; and medial aspects of the substantia nigra and crus cerebri (Fig. 13-17).
 Figure 13-17. Blood supply of the midbrain. Arteries are shown mainly on the right and the territories served by each on the left. The anterior choroidal artery, which is a branch of the internal carotid and follows the general route of the optic tract (Fig. 13-3B), also sends branches to the lateral portions of the midbrain.
Figure 13-17. Blood supply of the midbrain. Arteries are shown mainly on the right and the territories served by each on the left. The anterior choroidal artery, which is a branch of the internal carotid and follows the general route of the optic tract (Fig. 13-3B), also sends branches to the lateral portions of the midbrain.
Ventrolateral regions of the midbrain are served by penetrating branches of the quadrigeminal artery (Fig. 13-17), the anterior choroidal artery, and the medial posterior choroidal artery. The region served by these branches includes the lateral parts of the crus and substantia nigra and the medial lemniscus.
The posterior midbrain is served primarily by the quadrigeminal artery (collicular artery), which typically arises from P1 (Fig. 13-17). Much of the periaqueductal gray, the nuclei of the superior and inferior colliculi, the anterolateral system, and the brachium of the inferior colliculus are served by quadrigeminal branches. Additional blood supply to the area surrounding the exit of the trochlear nerve and the inferior colliculus arises from medial branches of the superior cerebellar artery.
VASCULAR SYNDROMES OF THE MIDBRAIN
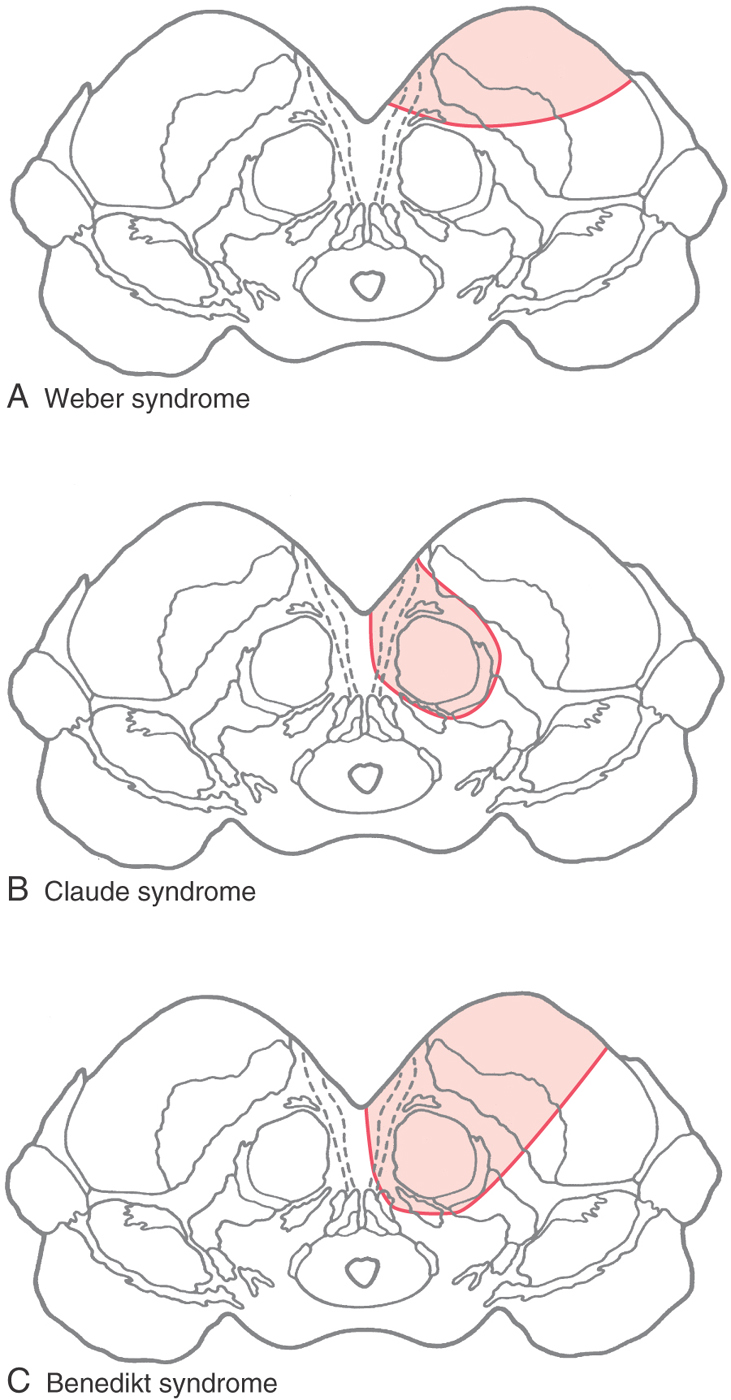
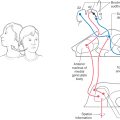
Occlusion of vessels serving the medial portions of the midbrain may result in the Weber syndrome (Fig. 13-18A). This produces an ipsilateral paralysis of all extraocular muscles except the lateral rectus and superior oblique, reflecting damage to the exiting root of the oculomotor nerve and a paralysis of the contralateral extremities, indicating damage to corticospinal fibers in the crus cerebri. The ipsilateral pupil is also dilated. This lesion also includes damage to corticonuclear fibers in the crus, resulting in a weakness of the facial muscles of the lower half of the face and a deviation of the tongue when it is protruded, both on the side contralateral to the lesion (see also Chapter 25).
If the vascular lesion is located in the more central area of the midbrain, the structures damaged include the fibers of the oculomotor nerve, the red nucleus, and the cerebellothalamic fibers. Collectively, the deficits seen after a lesion in this area constitute the Claude syndrome (Fig. 13-18B). Deficits include an ipsilateral paralysis of most eye movements; the eye is directed down and out with a dilated pupil (oculomotor nerve) and a contralateral ataxia, tremor, and incoordination that results from the damage to the red nucleus and cerebellothalamic fibers (see Chapter 24 and 25).
A large lesion that includes the territories of both the Weber and Claude syndromes results in a constellation of deficits called the Benedikt syndrome (Fig. 13-18C). These include an ipsilateral paralysis of most eye movements, contralateral weakness of the extremities, and contralateral tremor and ataxia. In each of these lesions, it is possible that the VE preganglionic parasympathetic fibers arising in the Edinger-Westphal preganglionic nucleus may be damaged. Thus the pupil on the side of the lesion will be dilated because of the action of the intact sympathetic input to the dilator muscle of the iris.
HERNIATION SYNDROMES RELATED TO THE MIDBRAIN
The midbrain lies at the intersection of the right and left supratentorial compartments with the infratentorial compartment. In this position it traverses the tentorial notch, extending from the caudal diencephalon to the rostral pons. The herniation syndromes related to the midbrain are central or transtentorial herniation, upward cerebellar herniation, and uncal herniation.
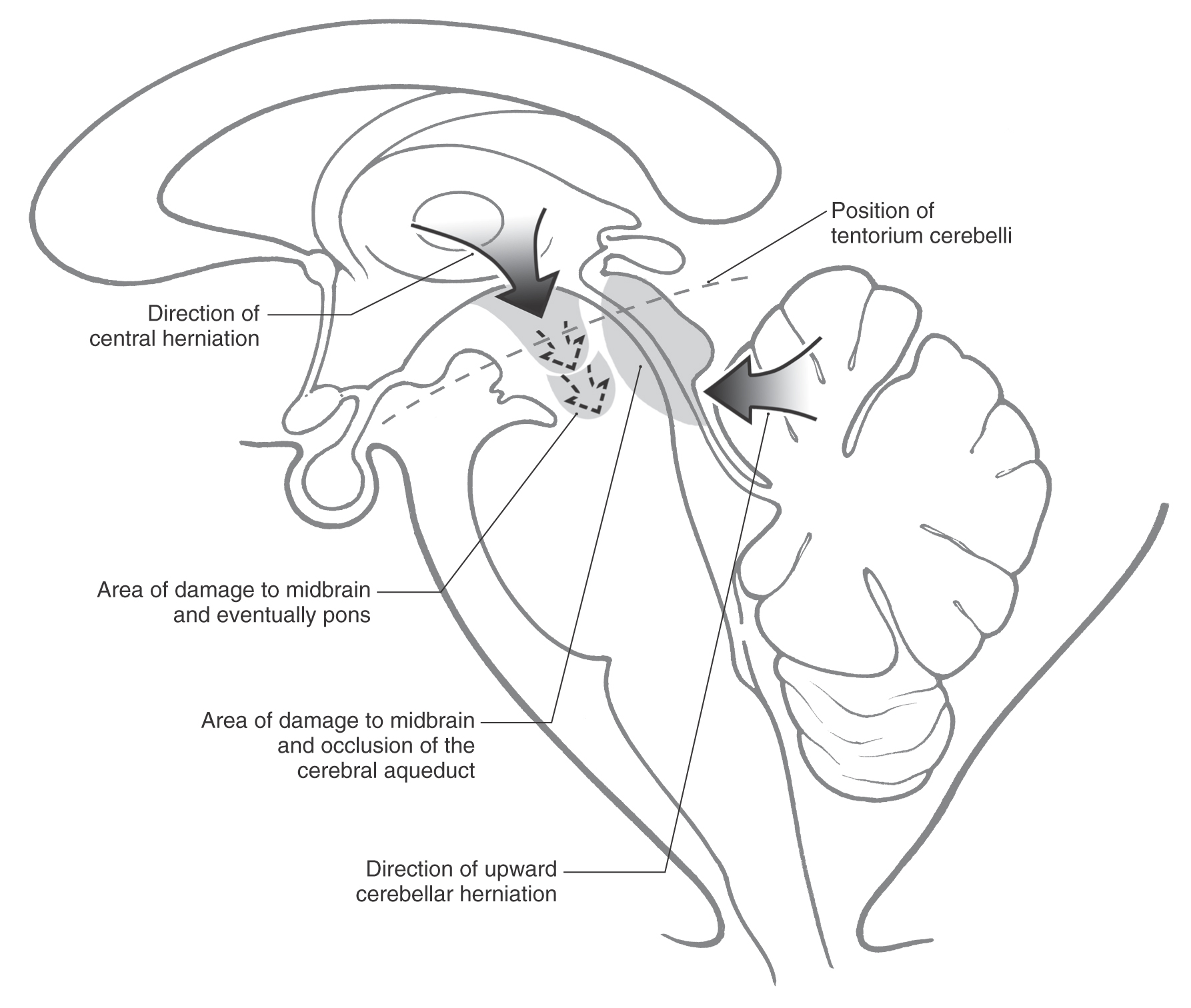
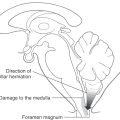
Central (or transtentorial) herniation (Fig. 13-19) may follow a large hemorrhage into the hemisphere or a large, rapidly growing tumor. Essentially, the enlarging mass (be it hemorrhage or tumor) forces the diencephalon downward through the tentorial incisure and into the midbrain. Before the herniation, the patient is less alert (decreased level of consciousness) and may have altered respiratory patterns and eye movements. The patient responds to noxious stimuli and may have hyperactive muscle stretch reflexes. As intracranial pressure increases, the patient may become decorticate (lower extremities, trunk, and neck extended; upper extremities flexed). The herniation traverses the tentorial notch and impinges on the midbrain, beginning a series of catastrophic events. The patient is comatose, pupils are dilated and fixed, and respiration is irregular with changes in depth and rate (Cheyne-Stokes) or periods of tachypnea and eventual apnea; the patient becomes decerebrate with all four extremities extended (see Chapter 24). As the cone of ischemia extends into and through the midbrain, the deficits are exacerbated and the likelihood of survival is greatly diminished.
Upward cerebellar herniation (Fig. 13-19) may result from an expanding mass in the posterior fossa. In this situation, medial portions of the cerebellum pass upward through the tentorial notch and compress the midbrain. There is the eventual possibility of cerebellar infarction from compression of the superior cerebellar arteries and hydrocephalus from obstruction of the cerebral aqueduct. If the primary cause of the herniation is treated in a timely manner, there is usually a good outcome.
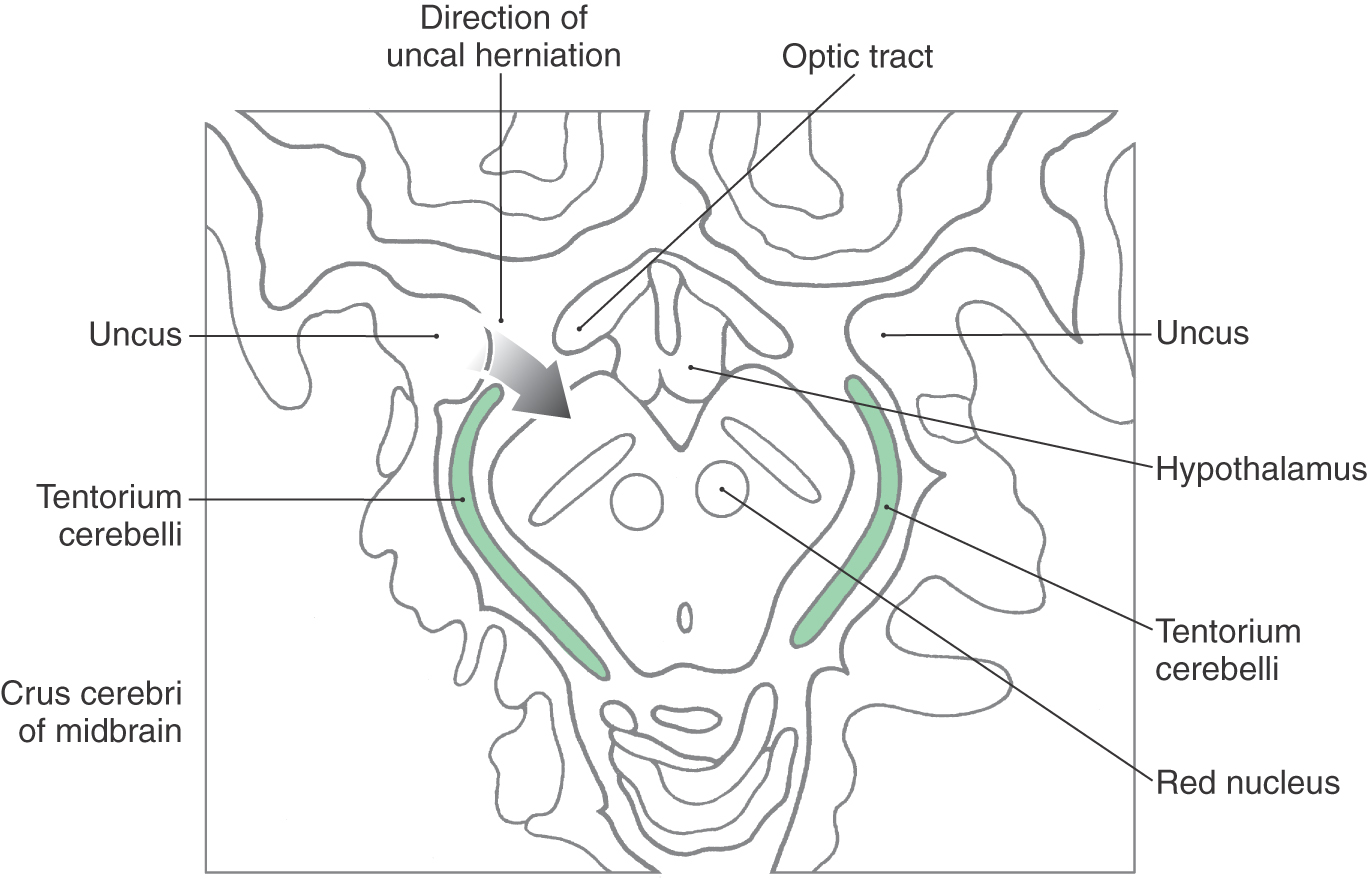
Uncal herniation results from an expanding lesion (usually a hematoma) in the hemisphere, frequently the temporal lobe, that forces the uncus over the edge of the tentorium and into the midbrain (Fig. 13-20). The initial finding is a dilated pupil (pressure on the third nerve) on the side of the herniation followed by a paralysis of most eye movement in the eye with the dilated pupil. As the herniation progresses, corticospinal fibers in the crus cerebri are compressed, resulting in a weakness of the contralateral upper and lower extremities. In addition, cranial nerve signs such as a central seven (lower facial muscles contralaterally), difficulty swallowing, and deviation of the tongue contralaterally on protrusion may also appear as a result of damage to corticonuclear fibers in the crus cerebri. If the cause of the uncal herniation is not treated, the deficits may escalate into those described earlier for central herniation with similar consequences.
Sources and Additional Reading
Bobillier P, Seguin S, Petitjean F, Salvert D, Tovret M, Jouvet M. The raphe nuclei of the cat brain stem: A topographical atlas of their efferent projections as revealed by autoradiography. Brain Res. 1976;113:449–486.
Brodal A. Neurological Anatomy. 3rd ed New York: Oxford University Press; 1981.
Duvernoy HM. Human Brainstem Vessels. Berlin: Springer-Verlag; 1978.
Leblanc A. The Cranial Nerves: Anatomy, Imaging, Vascularisation. New York: Springer; 1995.
Nieuwenhuys R. Chemoarchitecture of the Brain. Berlin: Springer-Verlag; 1985.
Olszewski J, Baxter D. Cytoarchitecture of the Human Brain Stem. 2nd ed Basel: Karger; 1982.
Parent A. Carpenter’s Human Neuroanatomy. 9th ed Baltimore: Williams & Wilkins; 1996.
Paxinos G, ed. The Human Nervous System. San Diego: Academic Press; 1990. chapters 7–14

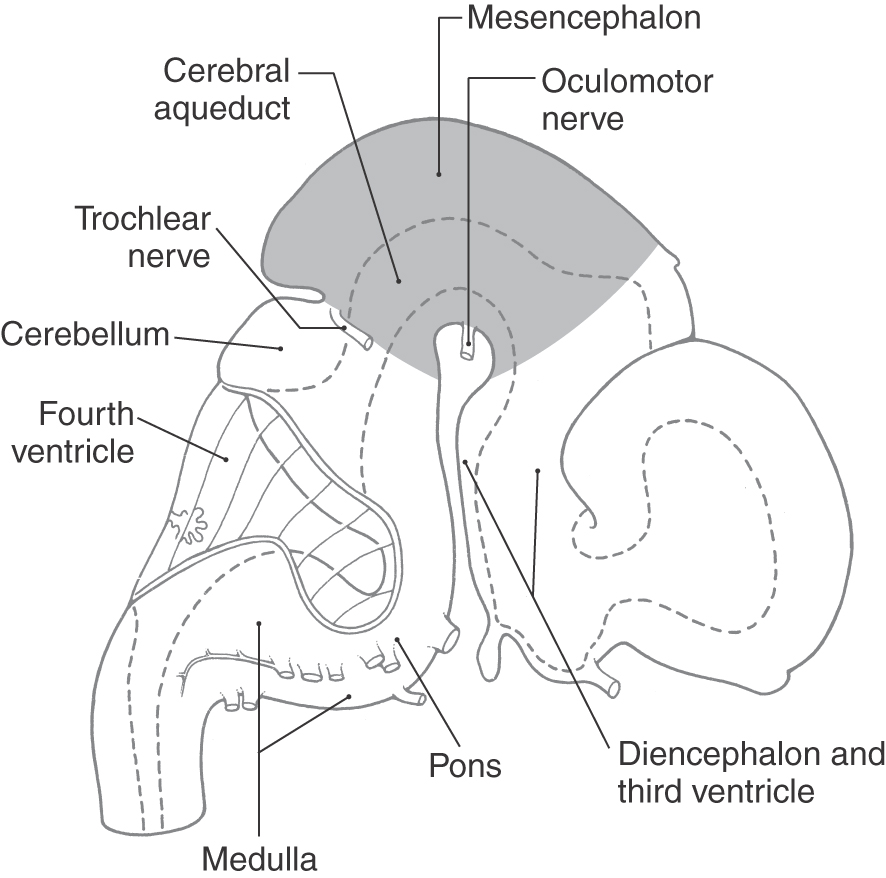
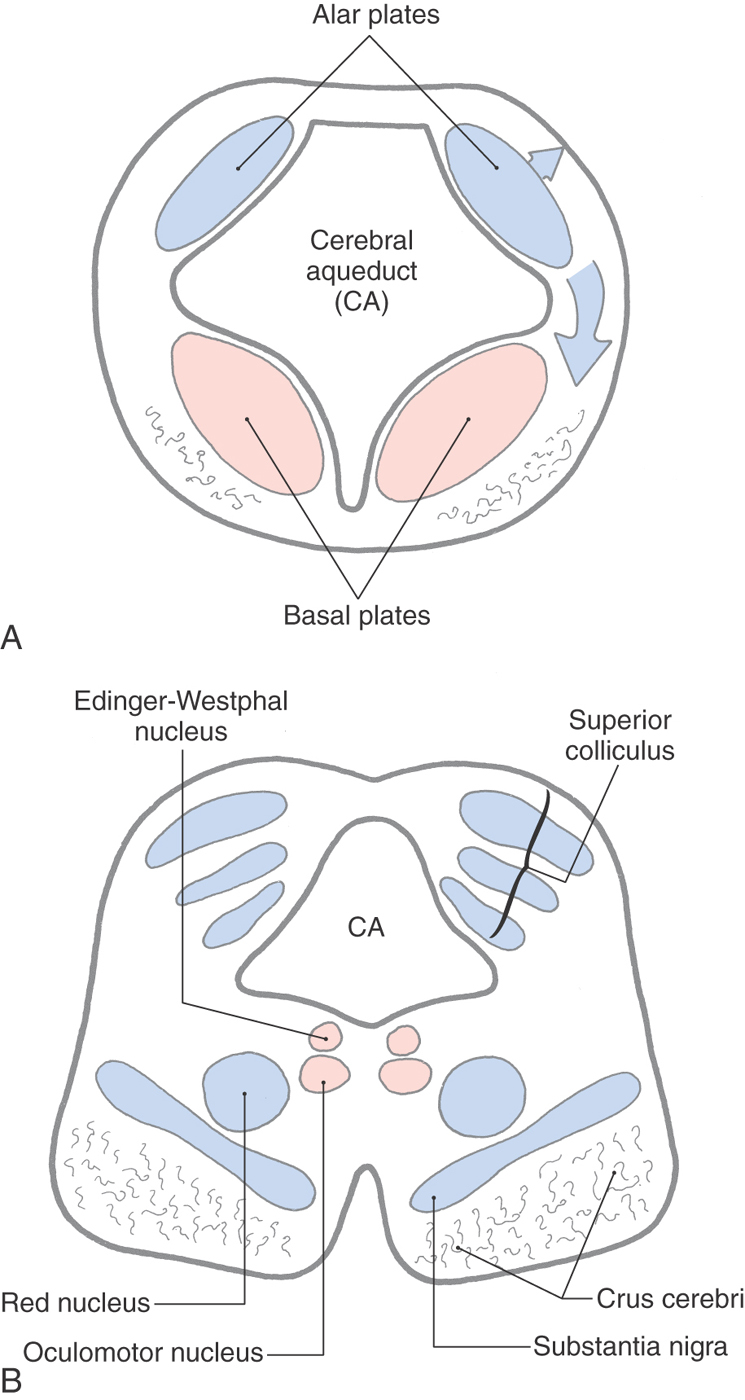
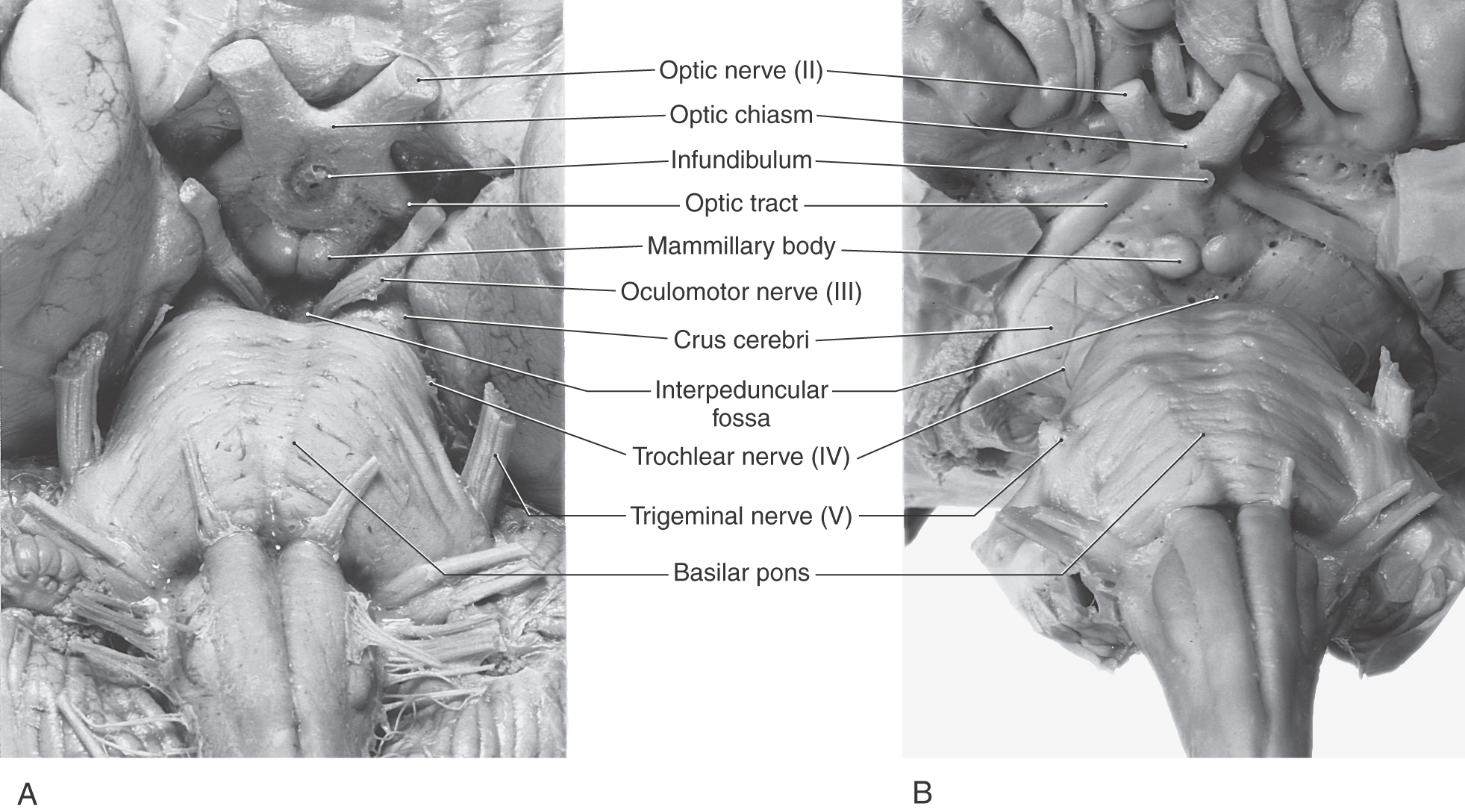
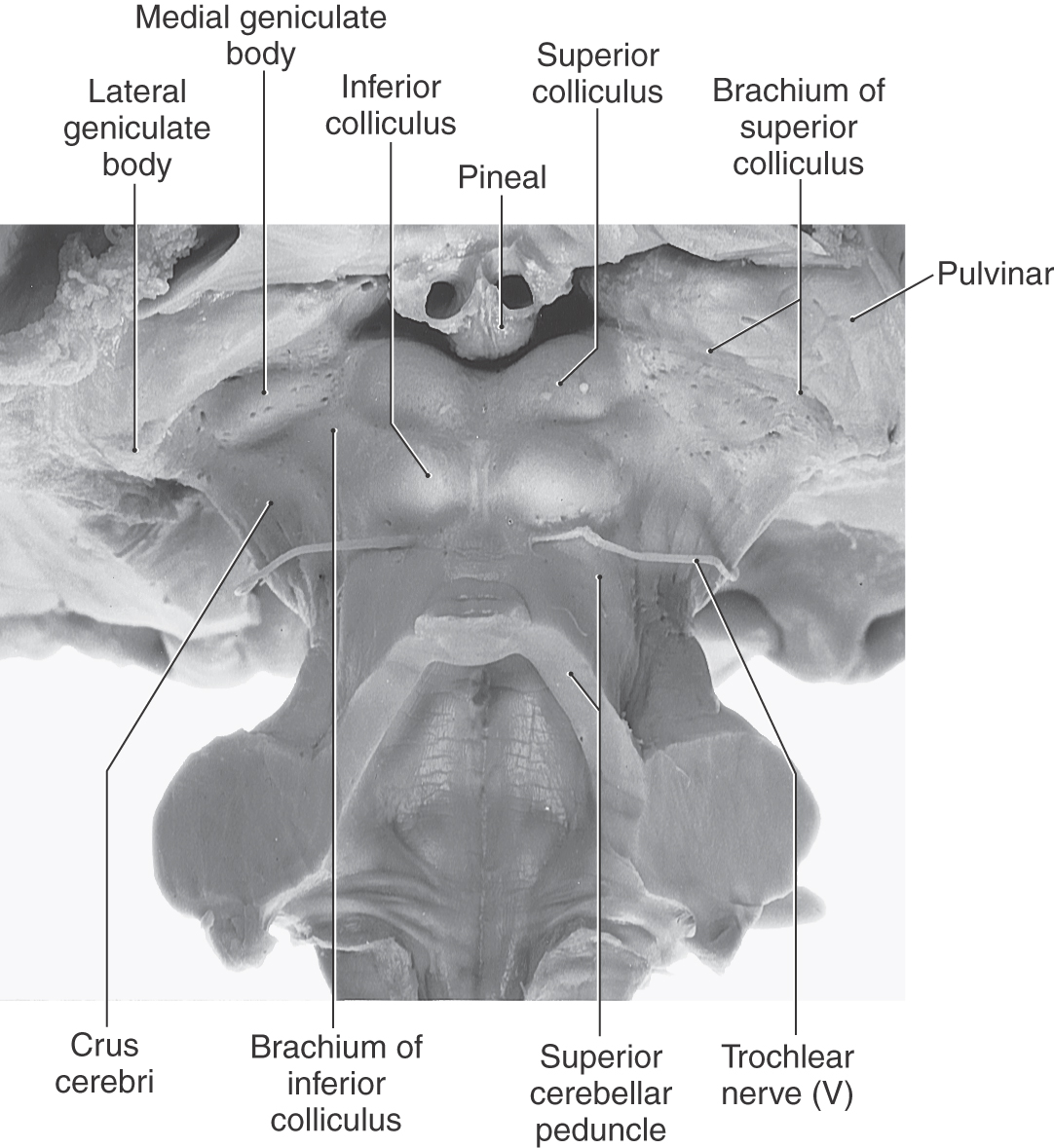
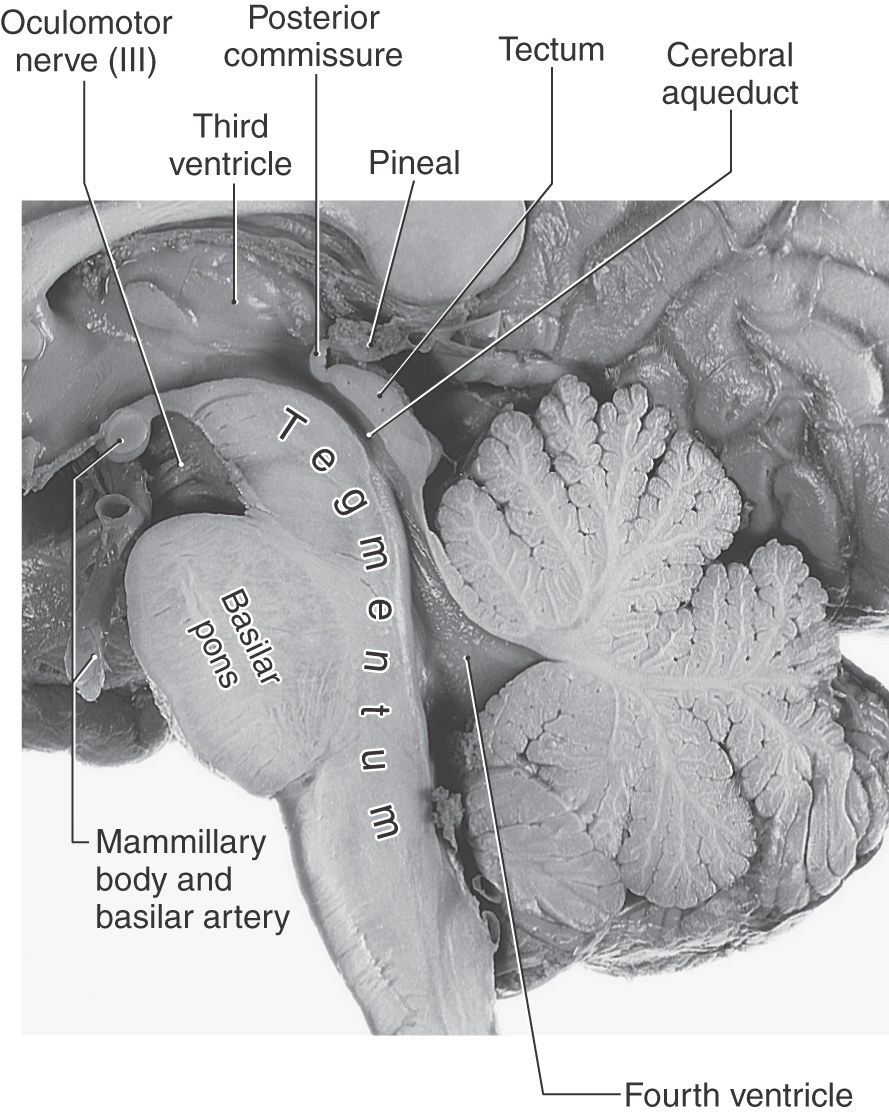
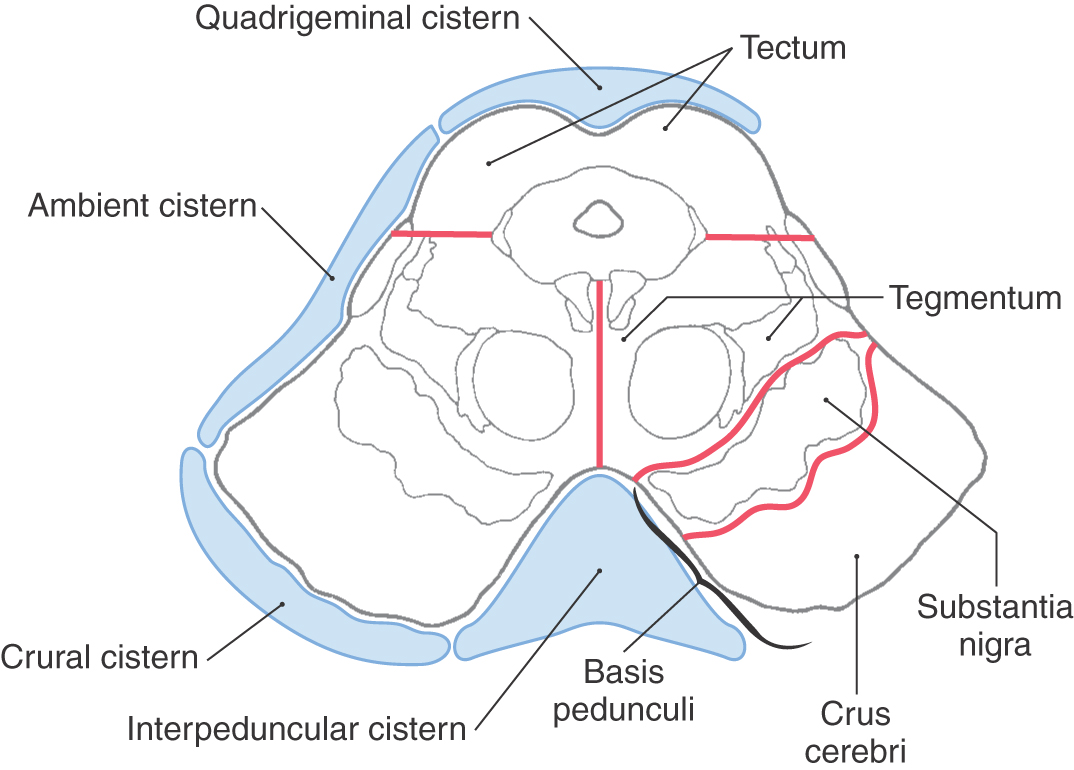
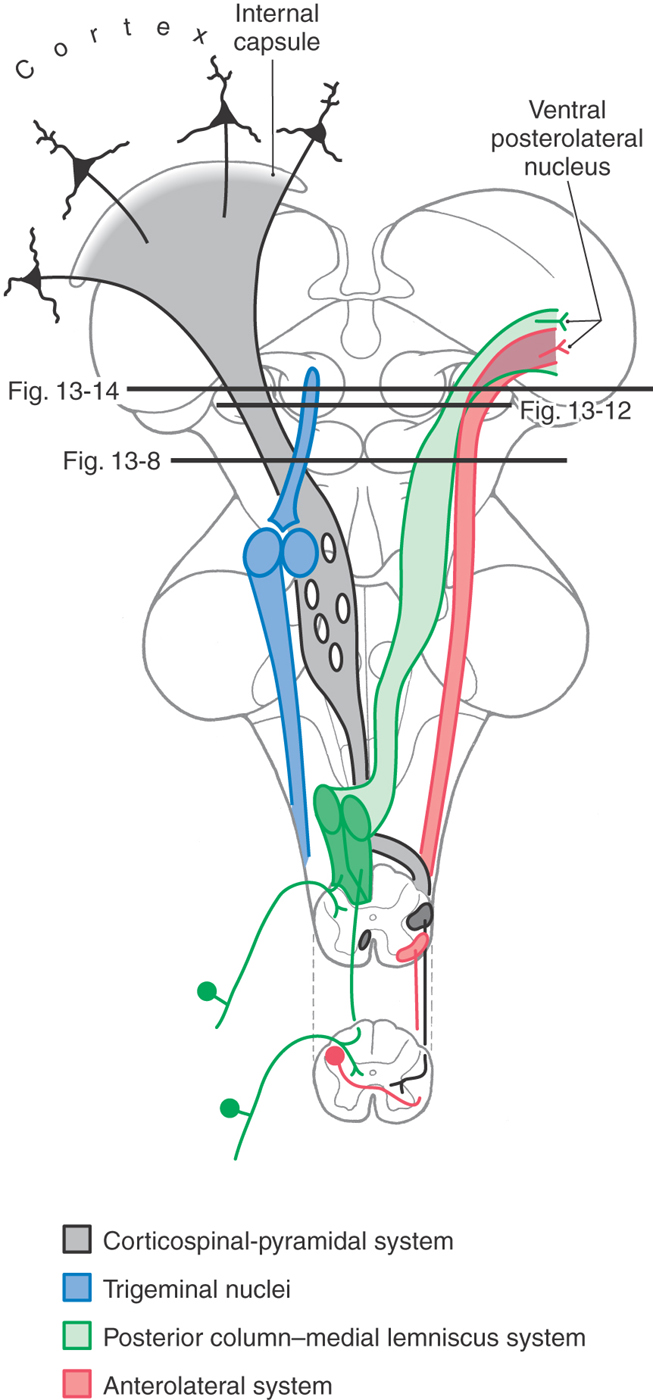
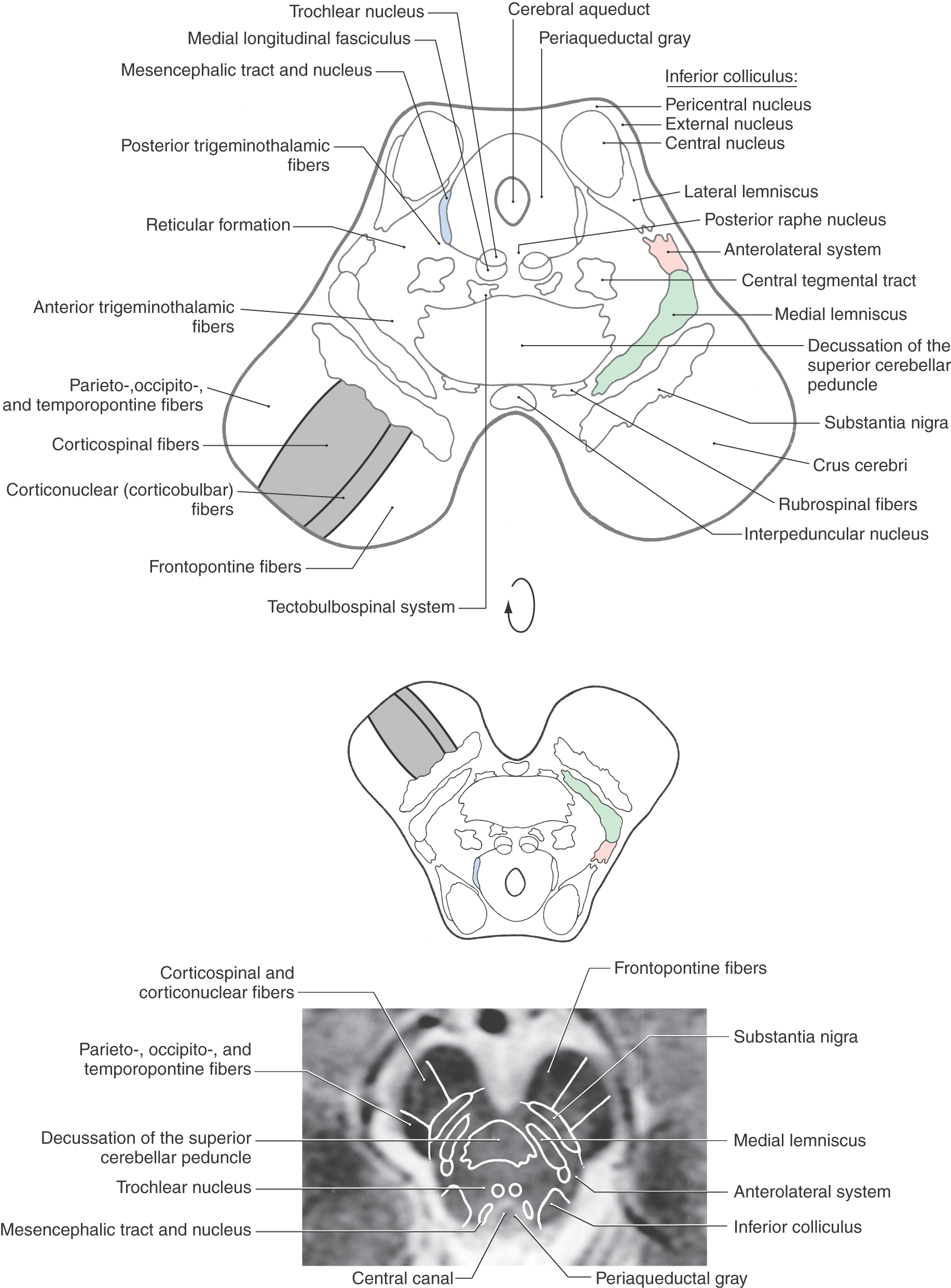
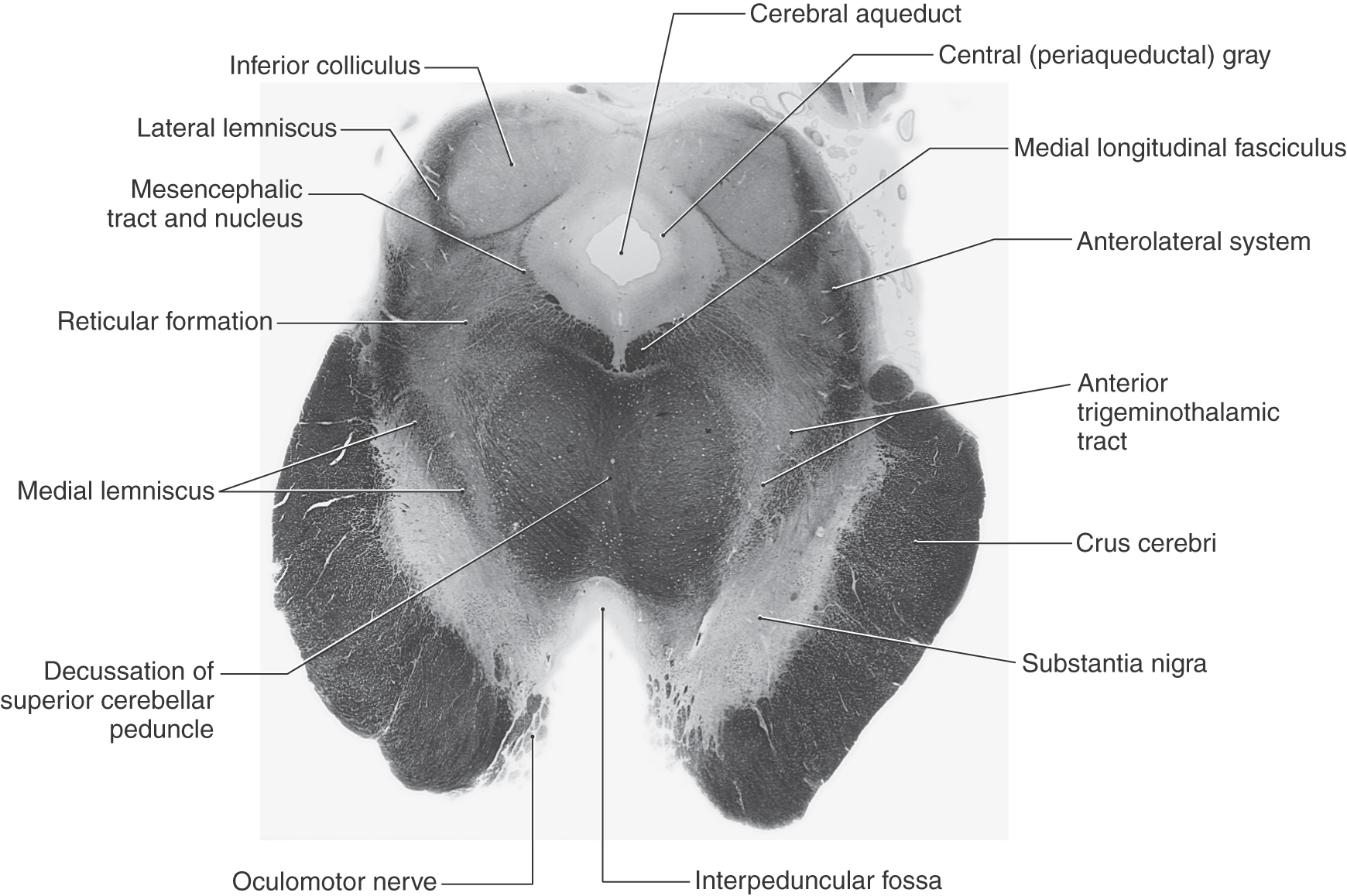
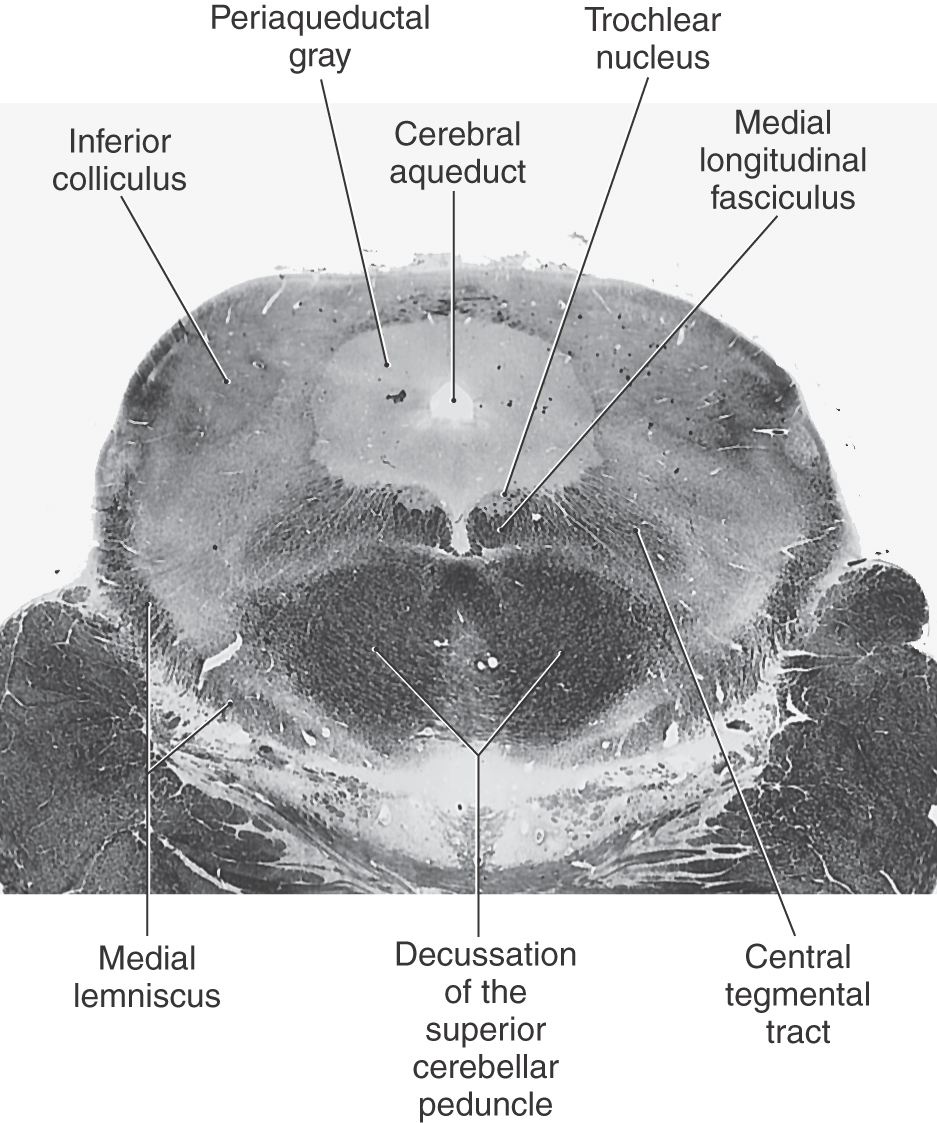
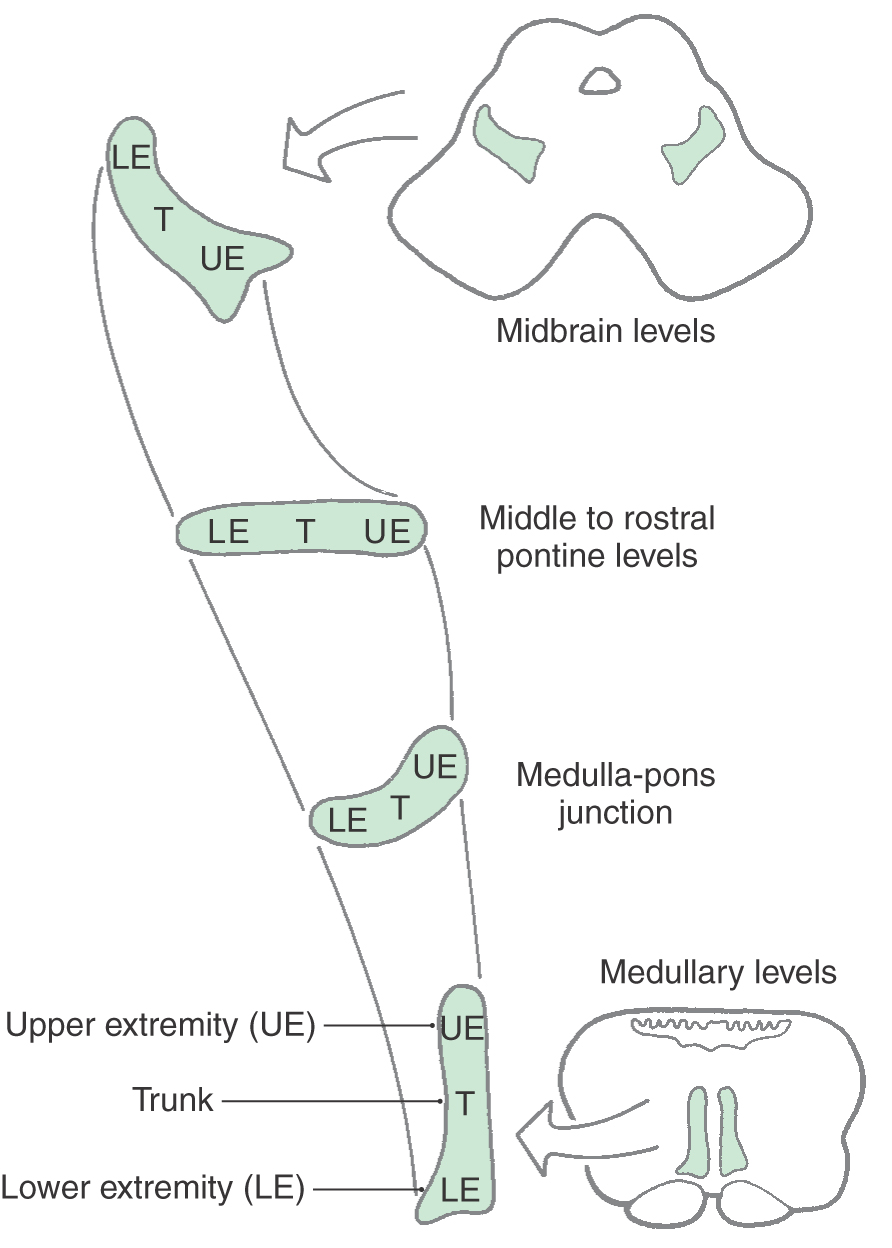
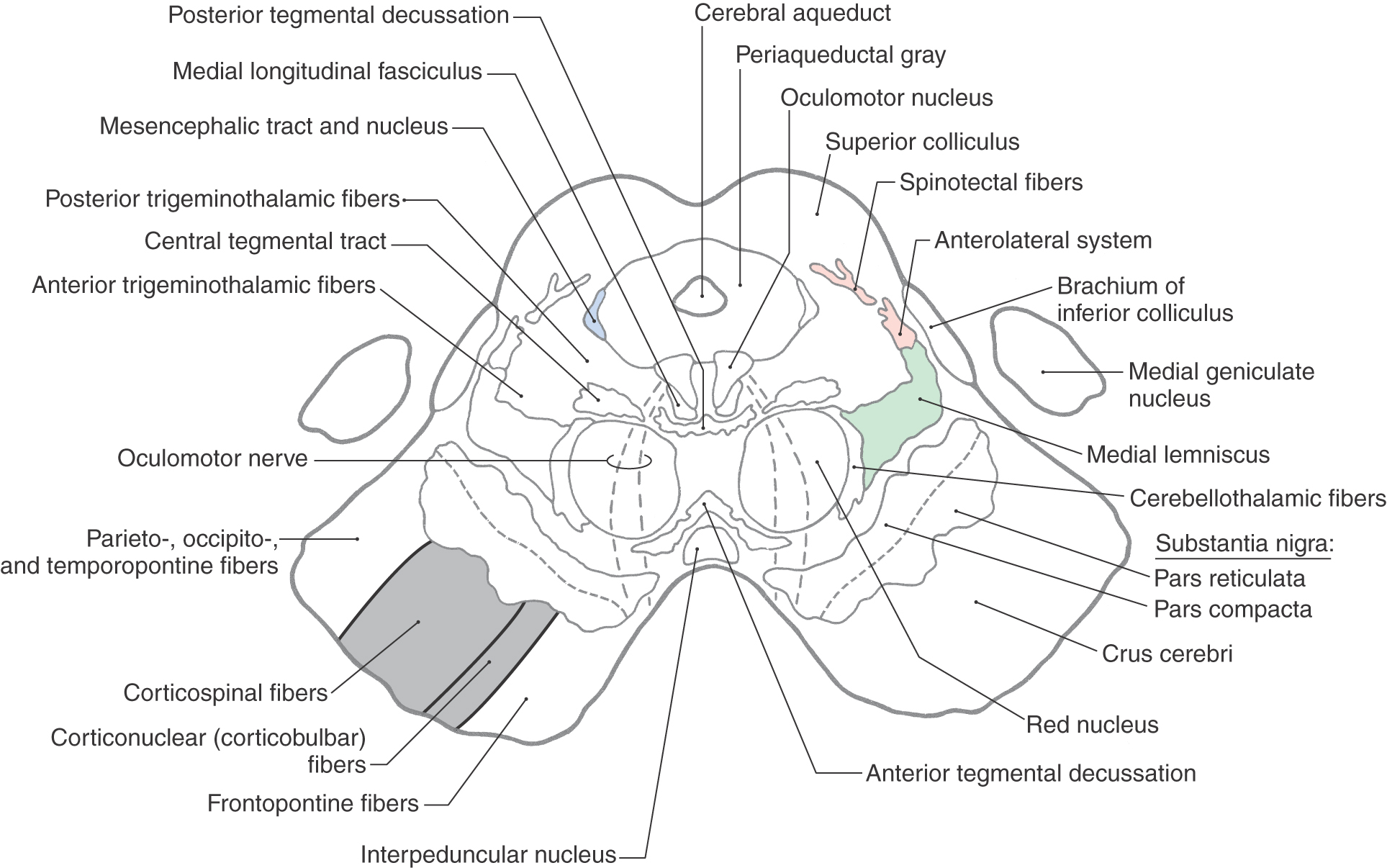
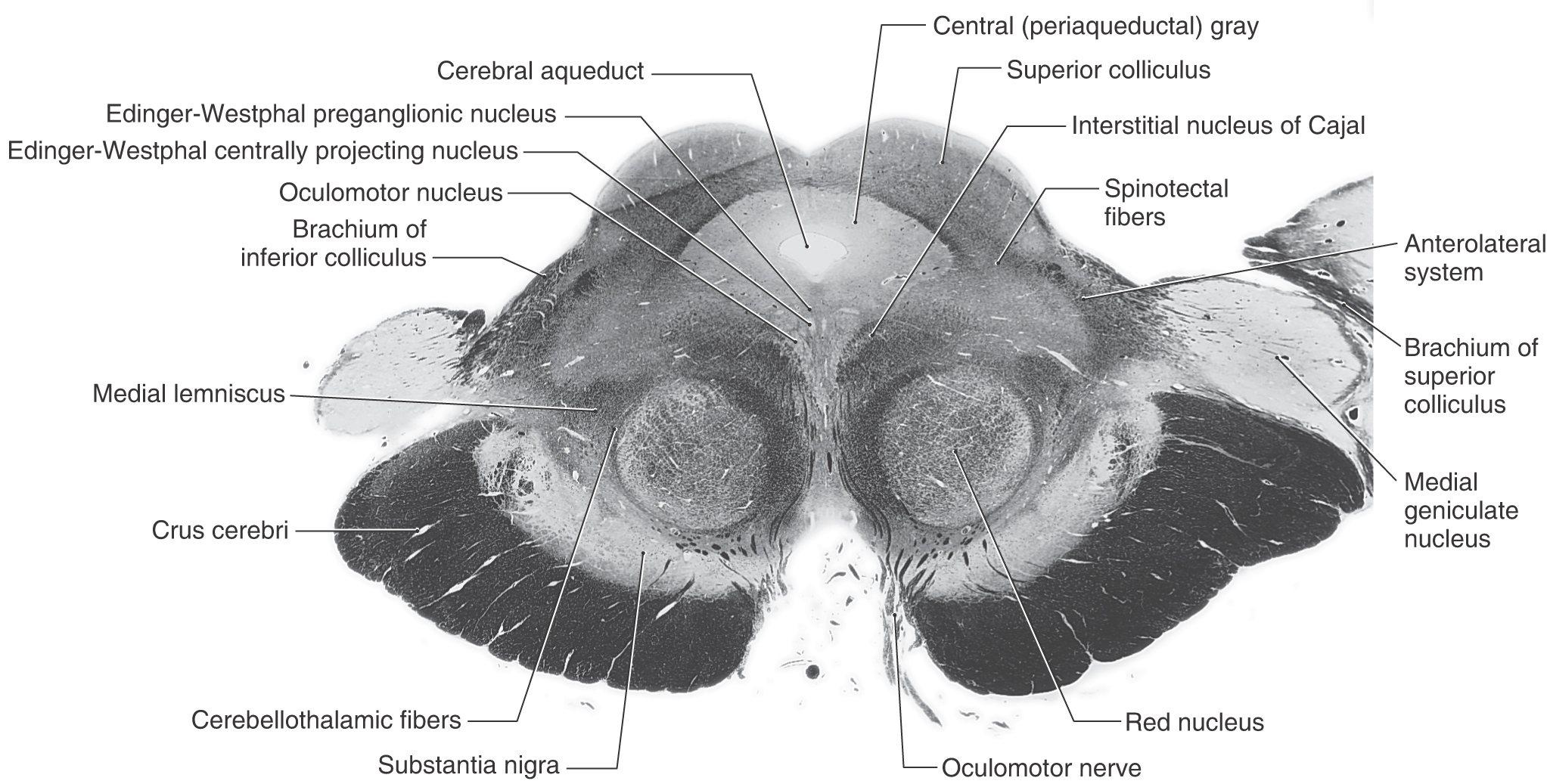
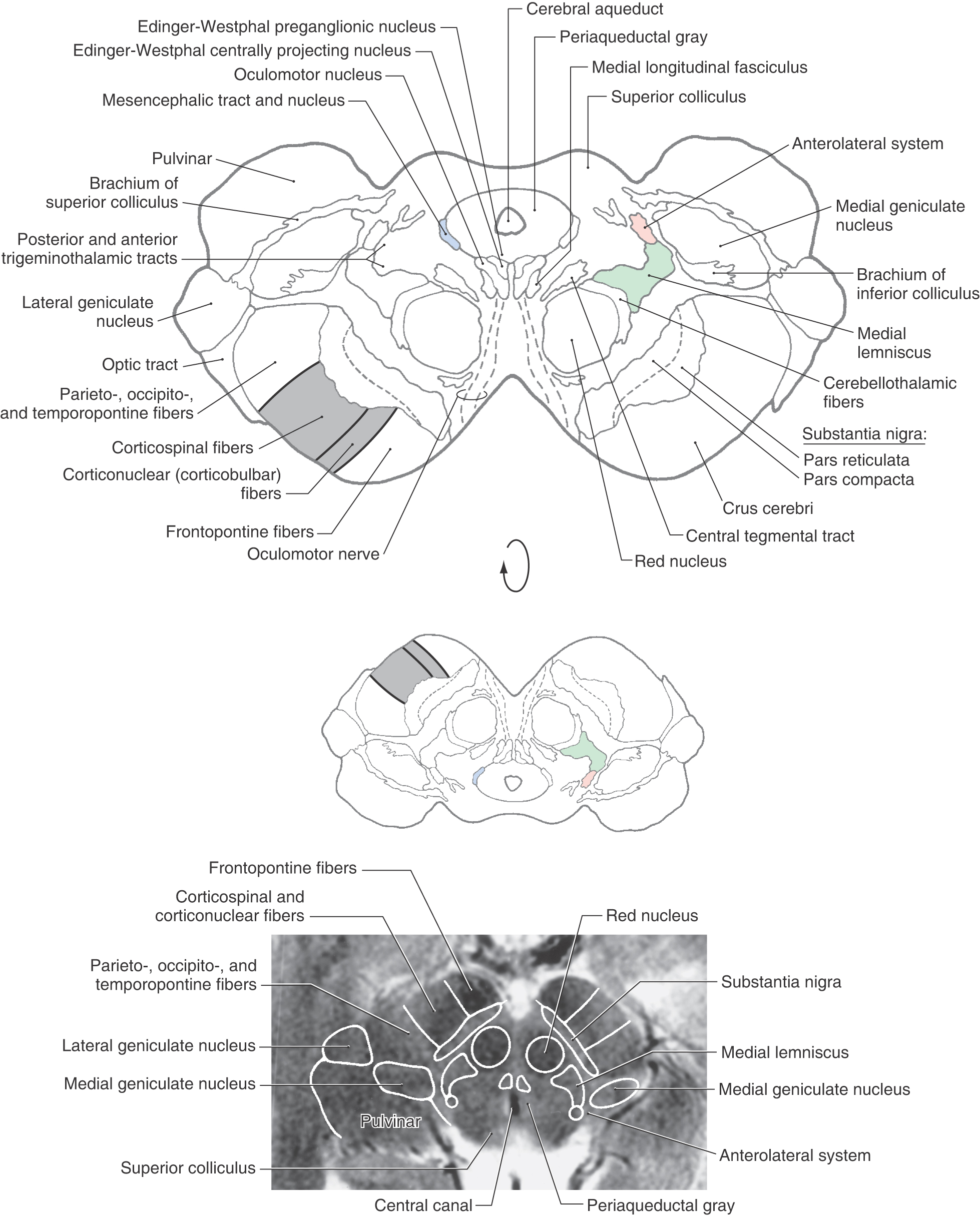
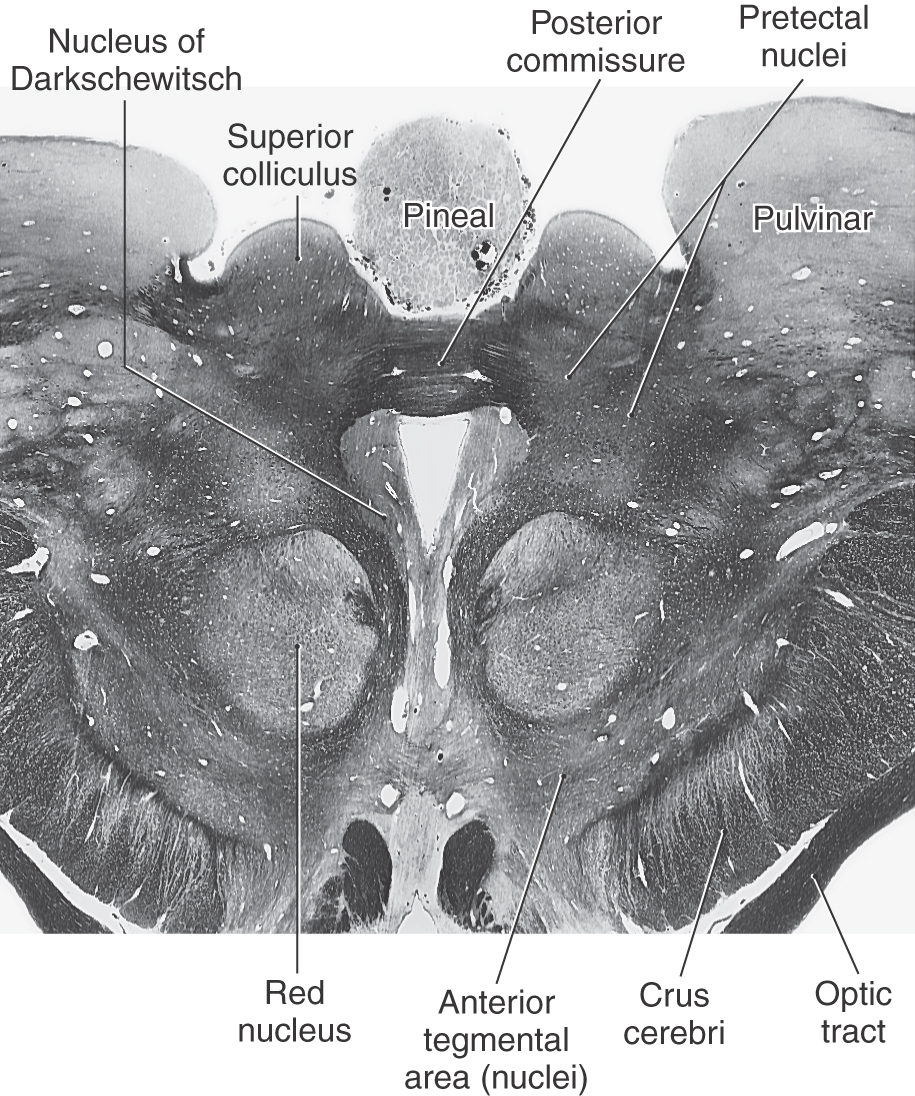
 Figure 13-15.
Figure 13-15. 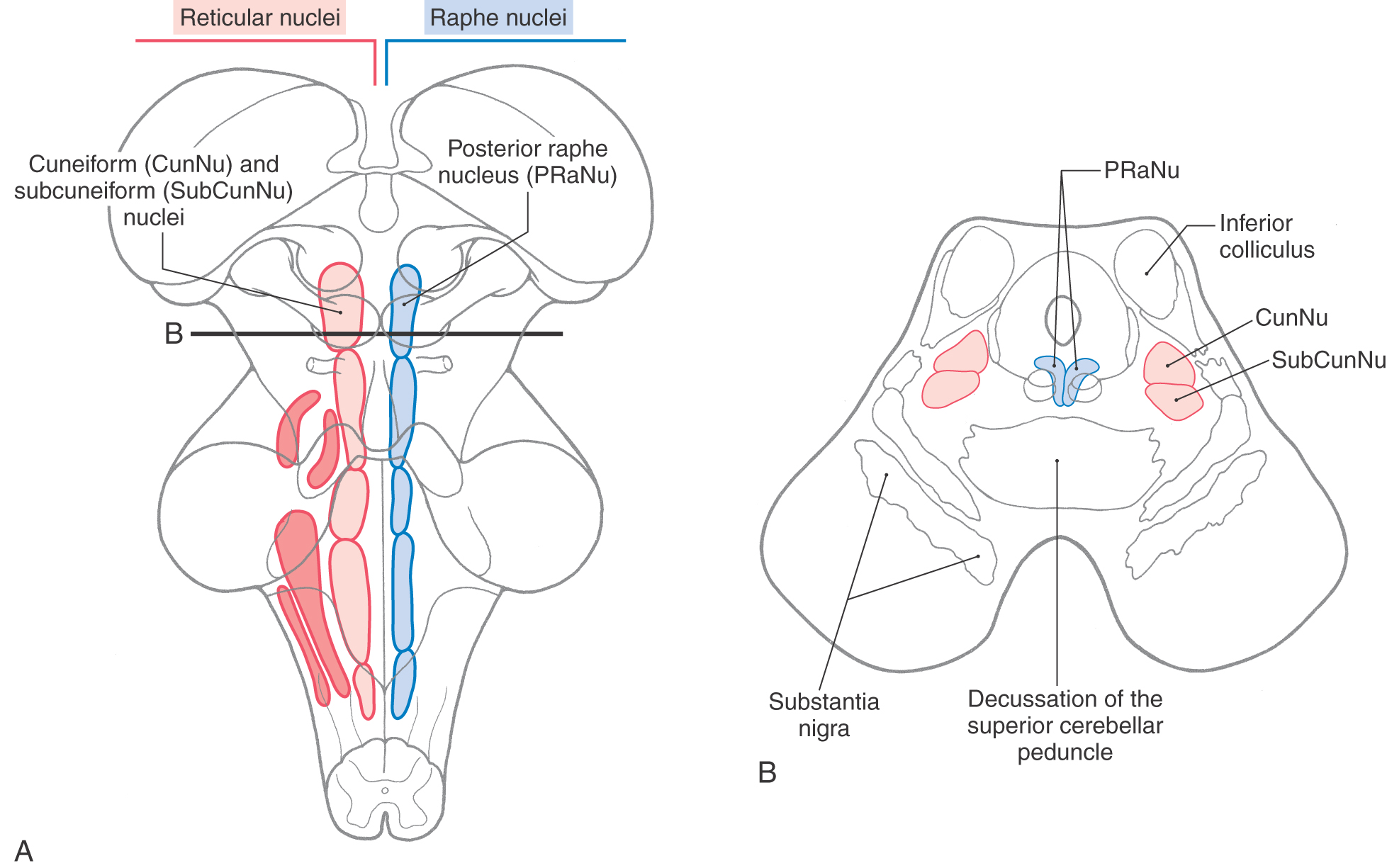
 Figure 13-16.
Figure 13-16.