Chapter 7
The Meninges
Compartments and Herniation Syndromes
Arachnoid Trabeculae and the Subarachnoid Space
The human nervous system is extremely delicate and lacks the internal connective tissue framework seen in most organs. For protection, the brain and spinal cord are each encased in a bony shell, enveloped by a fibrous coat, and delicately suspended within a fluid compartment. In the living state, the nervous system has a gelatinous consistency, but when treated with fixatives, it becomes firm and easy to handle.
OVERVIEW
The brain and spinal cord are surrounded by the skull and vertebral column, respectively. With the exception of the intervertebral foramina, through which the spinal nerves and their associated vessels pass, and the foramina in the skull, which serve as conduits for arteries, veins, and cranial nerve roots, this bony encasement is complete. The membranous coverings of the central nervous system (CNS), the meninges, are located internal to the skull and vertebral column. The meninges (1) protect the underlying brain and spinal cord; (2) serve as a support framework for important arteries, veins, and sinuses; and (3) enclose a fluid-filled cavity, the subarachnoid space, that is vital to the survival and normal function of the brain and spinal cord.
The presence of this bony and meningeal encasement of the CNS is a double-edged sword. Although these structures offer maximum protection, they can be very unforgiving in the case of trauma or in a disease process. For example, growth of a tumor creates a mass that will increase intracranial pressure and compress or displace various portions of the brain. Something has to give inside the skull when a space-occupying lesion develops, and it is the delicate tissue of the brain that gives. The neurologic deficits that result depend on the location of the mass, the rapidity with which it enlarges, and which parts of the brain are damaged.
DEVELOPMENT OF THE MENINGES
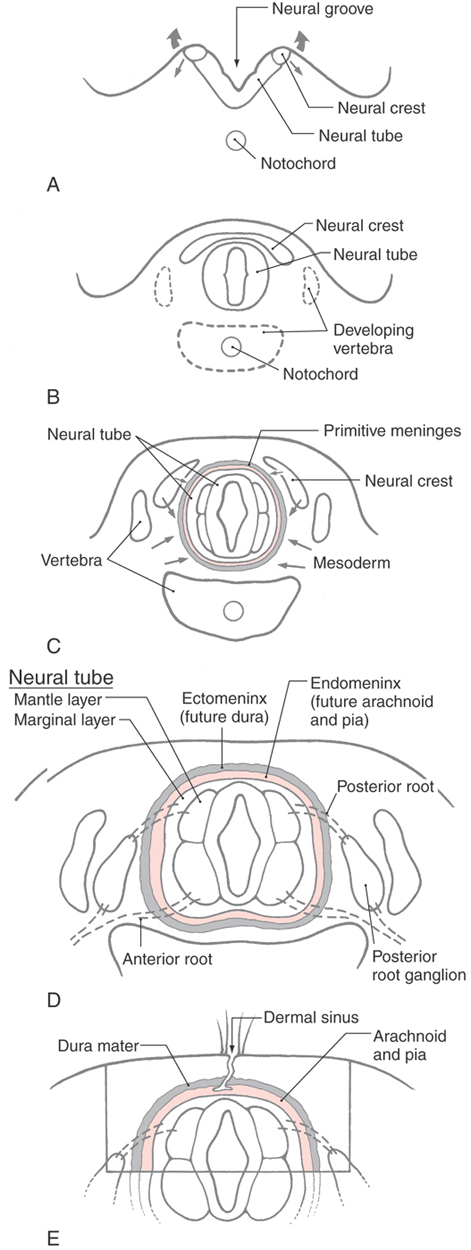
The meninges develop from cells of the neural crest and mesenchyme (mesoderm), which migrate to surround the developing CNS between 20 and 35 days of gestation (Fig. 7-1A-C). Collectively these neural crest and mesodermal cells form the primitive meninges (meninx primitiva). At this stage, no obvious spaces (venous sinuses, subarachnoid space) are present in the meninges. Between 34 and 48 days of gestation, the primitive meninges differentiate into an outer, more compact layer called the ectomeninx and an inner, more reticulated layer called the endomeninx (Fig. 7-1D). As development progresses (45 to 60 days of gestation), the ectomeninx becomes more compact and spaces appear in this layer that correlate with the positions of the future venous sinuses. Concurrently, the endomeninx becomes more reticulated and the spaces that appear in its inner part correspond to the subarachnoid spaces and cisterns of the adult. In general, the ectomeninx will become the dura mater and the endomeninx will form the arachnoid mater and pia mater (the leptomeninges) of the adult nervous system (Fig. 7-1D). By the end of the first trimester, the general plan of the meninges is established.
One developmental defect associated with closure of the neural tube and formation of the meninges in the lumbosacral area is the congenital dermal sinus (also called just dermal sinus) (Fig. 7-1E). This defect is caused by a failure of the ectoderm (future skin) to completely pinch off from the neuroectoderm and the primitive meninges that envelop it. As a result, the meninges are continuous with a narrow, epithelium-lined channel that extends to the skin surface (Fig. 7-1E). Dermal sinuses are sometimes discovered in young patients who have recurrent but unexplained bouts of meningitis. These lesions are surgically removed, and recovery is usually complete.
The ectomeninx around the brain is continuous with the skeletogenous layer that forms the skull. This relationship is maintained in the adult, in whom the dura is intimately adherent to the inner surface of the skull. In the spinal column, the ectomeninx is also initially continuous with the developing vertebrae. However, as development proceeds, the spinal ectomeninx dissociates from the vertebral bodies. A layer of cells remains on the vertebrae to form the periosteum, and the larger part of the ectomeninx condenses to form the spinal dura. The intervening space becomes the spinal epidural space (Fig. 7-2). This space is essential for the administration of epidural anesthetics.
OVERVIEW OF THE MENINGES
In general, the meninges consist of fibroblasts and varying amounts of extracellular connective tissue fibrils. The fibroblasts of each meningeal layer are modified to serve a particular function.
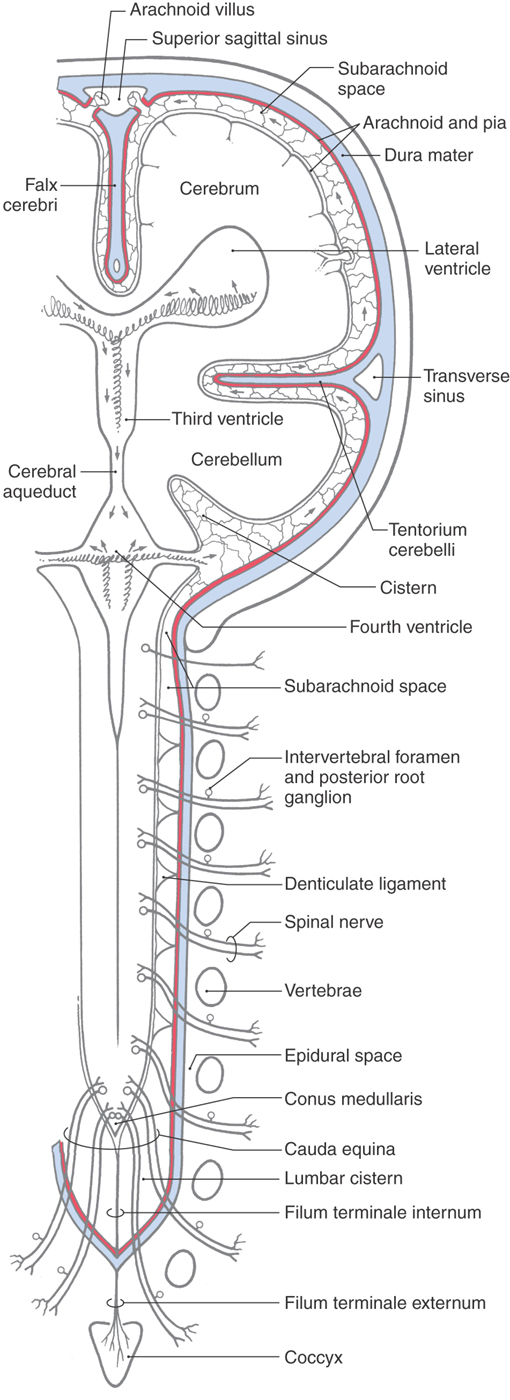
The human meninges are composed of the dura mater, arachnoid mater, and pia mater (Figs. 7-2 and 7-3). The outermost portion, the dura mater, also called the pachymeninx, is adherent to the inner surface of the skull but is separated from the vertebrae by the epidural space (Fig. 7-2). Around the brain the inner portions of the dura give rise to infoldings or septa, such as the falx cerebri and tentorium cerebelli (Fig. 7-2), which separate brain regions from each other. Major venous sinuses are found at the points where these septa originate. Spinal and cranial nerves, as they enter or exit the CNS, must pass through a cuff of the dura that is continuous with the connective tissue of the peripheral nerve. Blood vessels traverse the dura in similar fashion. Rostrally the dura sac is attached to the rim of the foramen magnum. Caudally the sac ends at about the level of the second sacral vertebrae and is attached to the coccyx by the filum terminale externum (or dural part of the filum terminale) (Fig. 7-2).
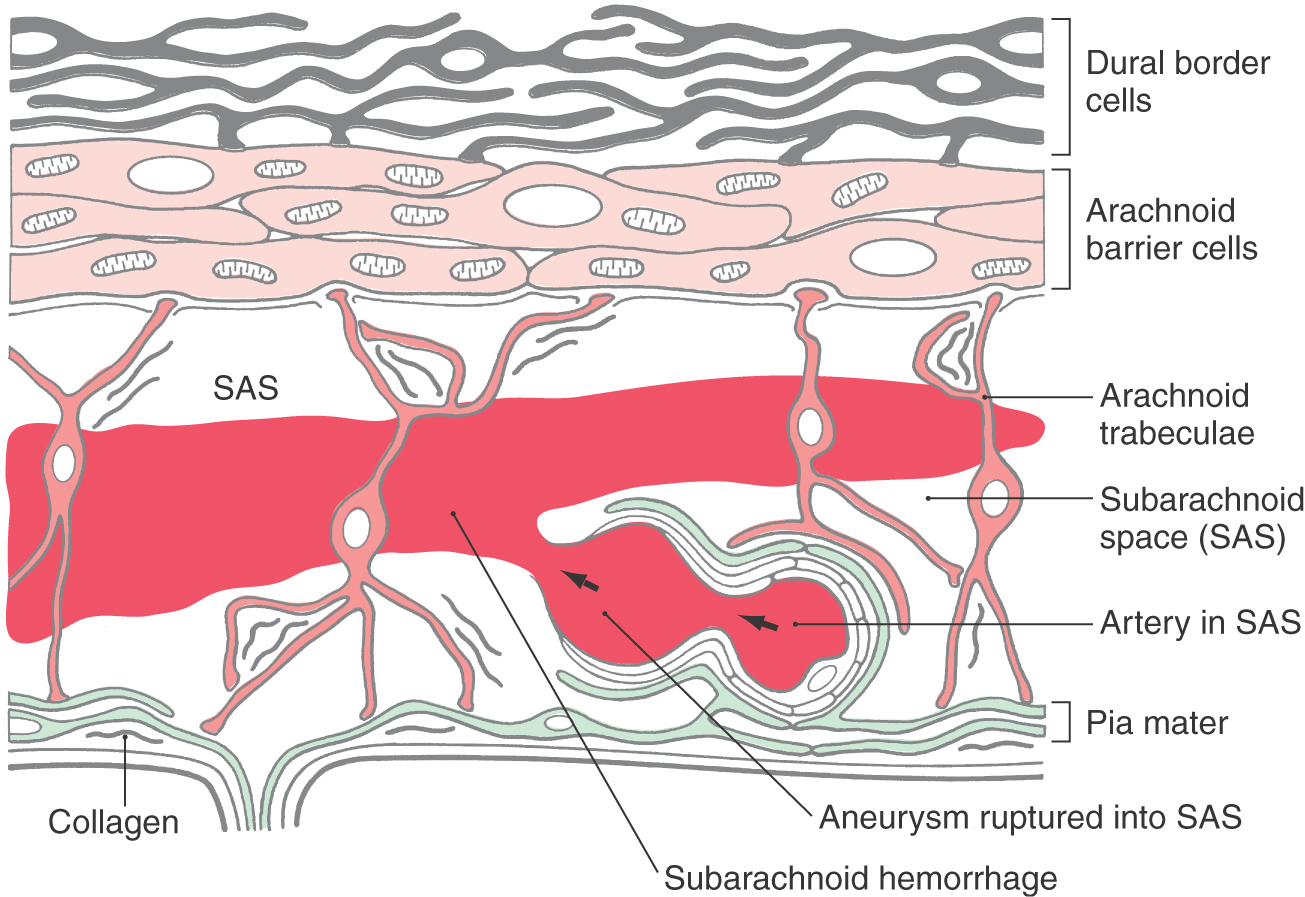
The inner two layers of the meninges, the arachnoid mater and the pia mater (Figs. 7-2 and 7-3), are collectively known as the leptomeninges. This term is also commonly used in clinical medicine (as in leptomeningeal cysts and leptomeningitis). The arachnoid is a thin cellular layer that is attached to the overlying dura but, with the exception of the arachnoid trabeculae, is separated from the pia mater by the subarachnoid space. The arachnoid around the brain is directly continuous with the arachnoid lining the inner surface of the spinal dura (Fig. 7-2). Consequently, the spinal and cerebral subarachnoid spaces are also directly continuous with each other at the foramen magnum. The subarachnoid space contains cerebrospinal fluid (CSF) and vessels and is bridged by fibroblasts of various sizes and shapes that collectively form the arachnoid trabeculae. The arachnoid is avascular and does not contain nerve fibers.
The pia mater is located on the surface of the brain and spinal cord and closely follows all their various grooves and elevations (Figs. 7-2 and 7-3). Around the spinal cord, the pia mater contributes to the formation of the denticulate ligaments and the filum terminale internum (or pial part of the filum terminale) (Fig. 7-2).
DURA MATER
Periosteal and Meningeal Dura
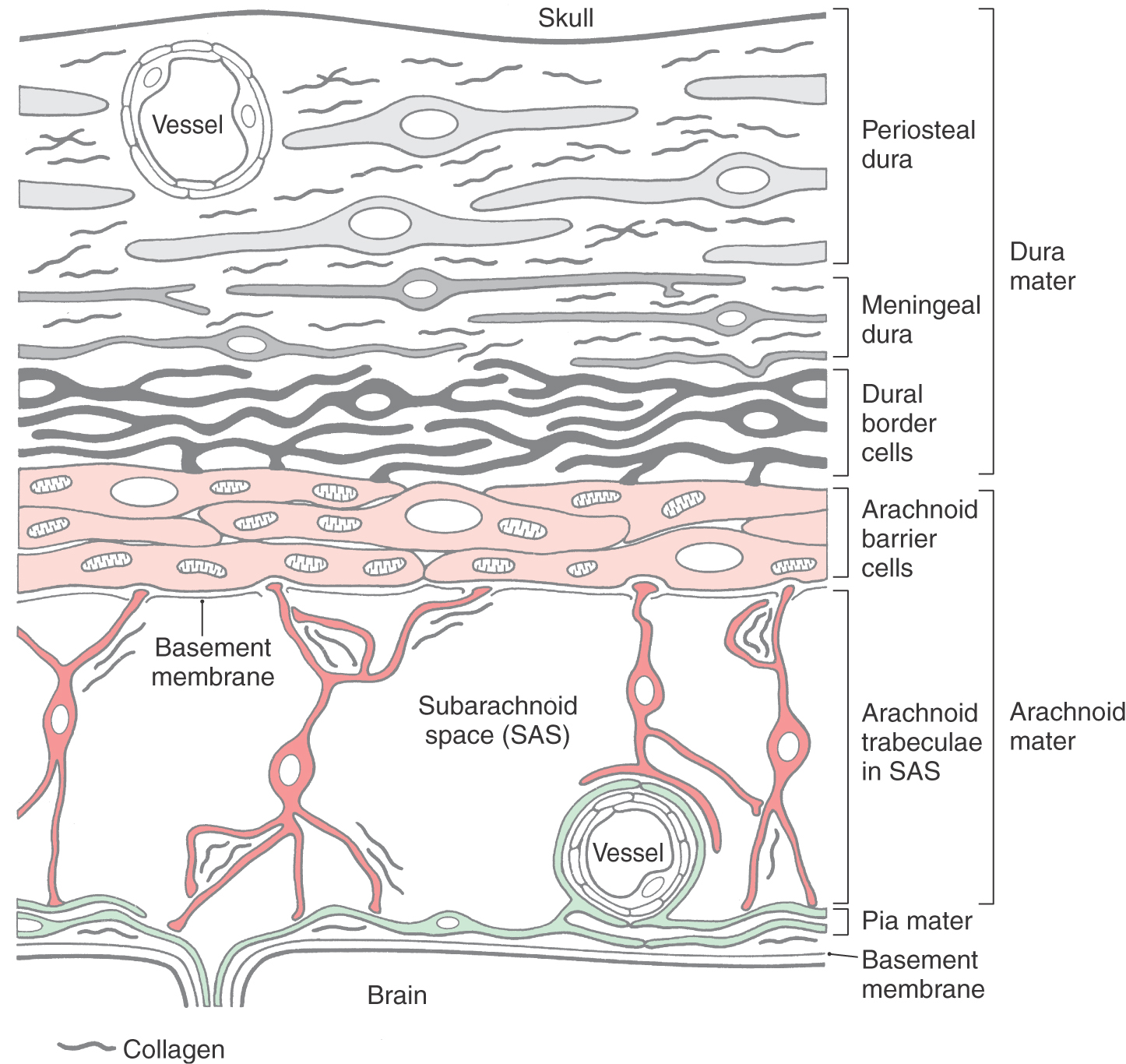
The dura mater (pachymeninx) is composed of elongated fibroblasts and copious amounts of collagen fibrils (Fig. 7-3). This membrane contains blood vessels and nerves and is generally divided into outer (periosteal), inner (meningeal), and border cell portions. There is no distinct border between periosteal and meningeal portions of the dura (Fig. 7-3). Fibroblasts of the periosteal dura are larger and slightly less elongated than other dural cells. This portion of the dura is adherent to the inner surface of the skull, and its attachment is particularly tenacious along suture lines and in the cranial base. In contrast, the fibroblasts of the meningeal dura are more flattened and elongated, their nuclei are smaller, and their cytoplasm may be darker than that of periosteal cells. Although cell junctions are rarely seen between dural fibroblasts, the large amounts of interlacing collagen in periosteal and meningeal portions of the dura give these layers of the meninges great strength.
Dural Border Cell Layer
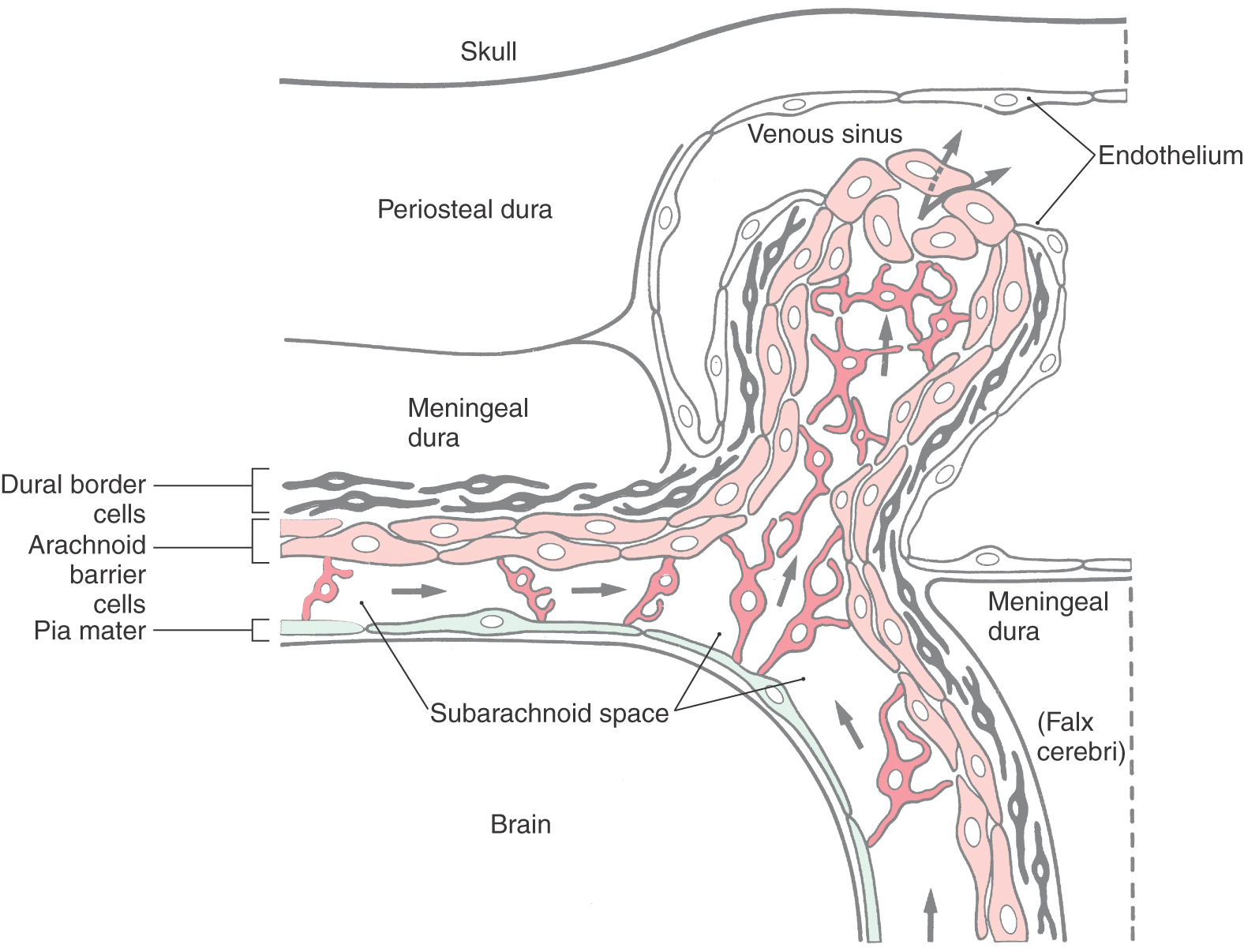
The innermost part of the dura is composed of flattened fibroblasts that have sinuous processes. Collectively these cells form the dural border cell layer (Fig. 7-3). The extracellular spaces between the flattened cell processes of dural border cells contain an amorphous substance but no collagen or elastic fibers. Cell junctions (desmosomes, gap junctions) are occasionally seen between dural border cells and cells of the underlying arachnoid.
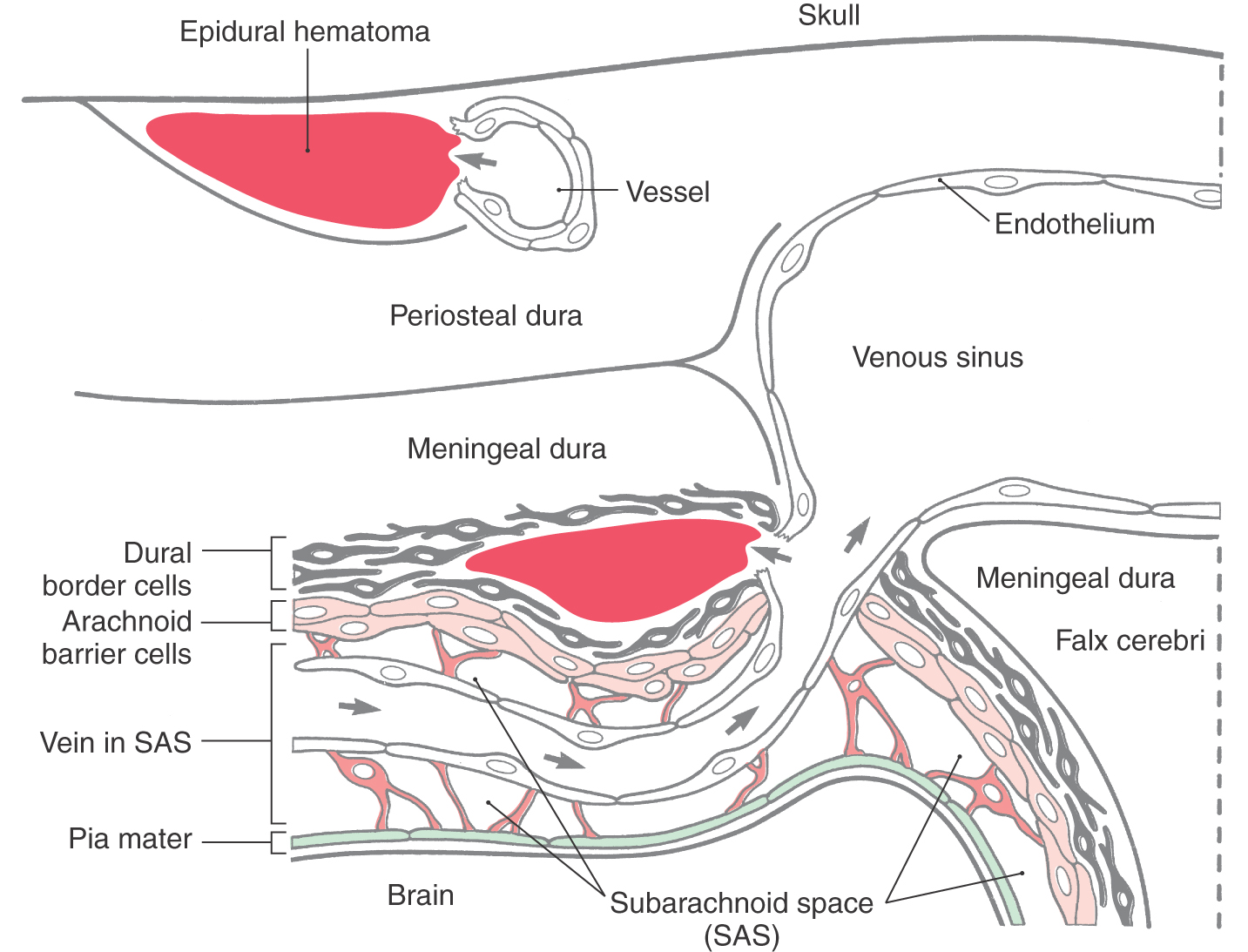
Because of its loose arrangement, enlarged extracellular spaces, and lack of extracellular connective tissue fibrils, the dural border cell layer constitutes a plane of structural weakness at the dura-arachnoid junction. This layer is externally continuous with the meningeal dura and internally continuous with the arachnoid. Consequently, bleeding into this area of the meninges will disrupt and dissect open the dural border cell layer rather than invade the overlying dura or the underlying arachnoid. In the normal (and healthy) human, there is not a naturally occurring, or preexisting, space at the dura-arachnoid interface (Fig. 7-3).
Blood Supply
The arterial supply to the dura of the anterior cranial fossa originates from the cavernous portion of the internal carotid, the ethmoidal arteries (via the ethmoidal foramina), and branches of the ascending pharyngeal artery (via the foramen lacerum). The middle meningeal artery serves the dura of the middle cranial fossa and may be compromised when there is trauma to the skull. It is a branch of the maxillary artery and enters the skull through the foramen spinosum. The accessory meningeal artery (via the foramen ovale) and small branches from the lacrimal artery (via the superior orbital fissure) also serve the dura of the middle fossa. The dura of the posterior fossa is served by small meningeal branches of ascending pharyngeal and occipital arteries and by minute branches of the vertebral arteries.
The spinal dura is served by branches of major arteries (such as vertebral, intercostal, and lumbosacral) that are located close to the vertebral column. These small meningeal arteries enter the vertebral canal via the intervertebral foramina to serve the dura and adjacent structures.
Nerve Supply
The nerve supply to the dura of the anterior and middle fossae is from branches of the trigeminal nerve, Ethmoidal nerves and branches of the maxillary and mandibular nerves innervate the dura of the anterior fossa; the dura of the middle fossa is served mainly by branches from the maxillary and mandibular nerves. The dura of the posterior fossa receives sensory branches from dorsal roots C2 and C3 (and from C1 when this root is present) and may have some innervation from the vagus nerve. The tentorial nerve, a branch of the ophthalmic nerve, courses caudally to serve the tentorium cerebelli. Autonomic fibers to the vessels of the dura originate from the superior cervical ganglia and simply follow the progressive branching patterns of the vessels on which they lie.
Nerves to the spinal dura originate as recurrent branches of the spinal nerve located at that level. These delicate strands pass through the intervertebral foramina and are distributed to the spinal dura and to some adjacent structures.
Dural Infoldings and Sinuses
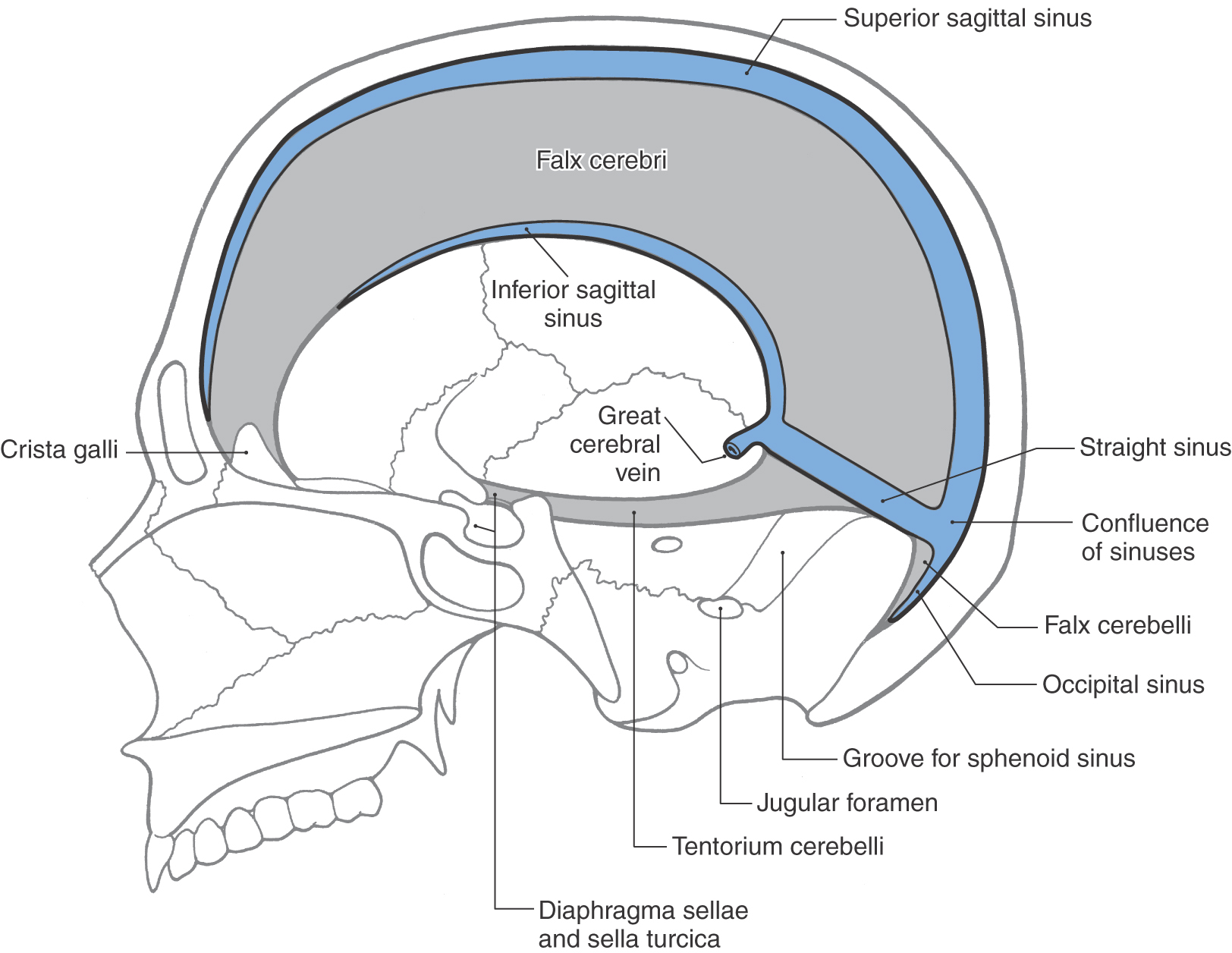
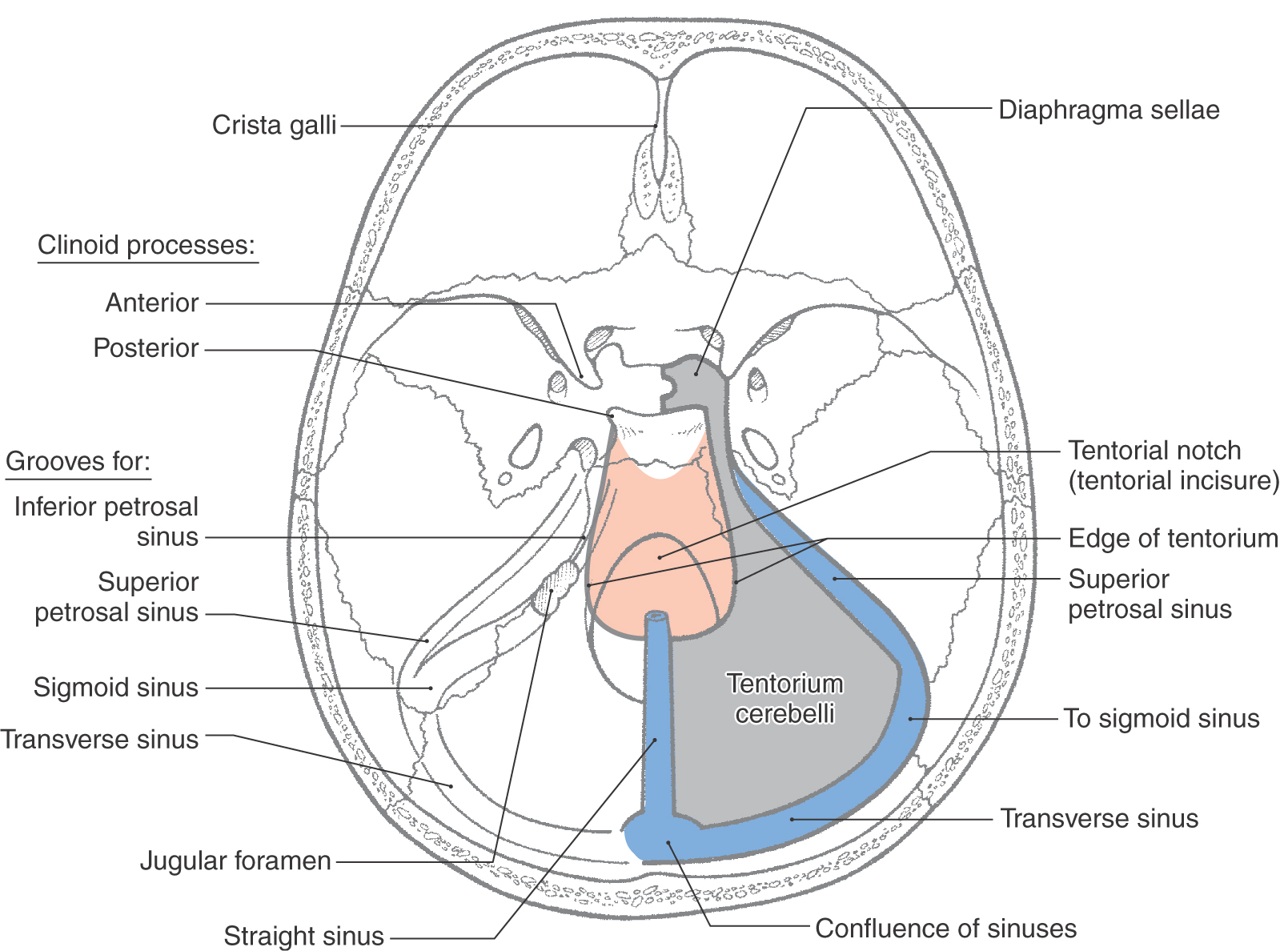
The periosteal dura lines the inner surface of the skull and functions as its periosteum. The meningeal dura is continuous with the periosteal dura but draws away from it at specific locations to form the dural infoldings (or reflections). The largest of these is the falx cerebri (Figs. 7-4 and 7-5A). It is attached to the crista galli rostrally, to the midline of the inner surface of the skull, and to the surface of the tentorium cerebelli caudally. The falx cerebri separates the right hemisphere from the left. The superior sagittal sinus is found where the falx cerebri attaches to the skull, the straight sinus where it fuses with the tentorium cerebelli, and the inferior sagittal sinus at its free edge (Fig. 7-4). Many large superficial veins located on the surface of the cerebral hemispheres empty into the superior sagittal sinus.
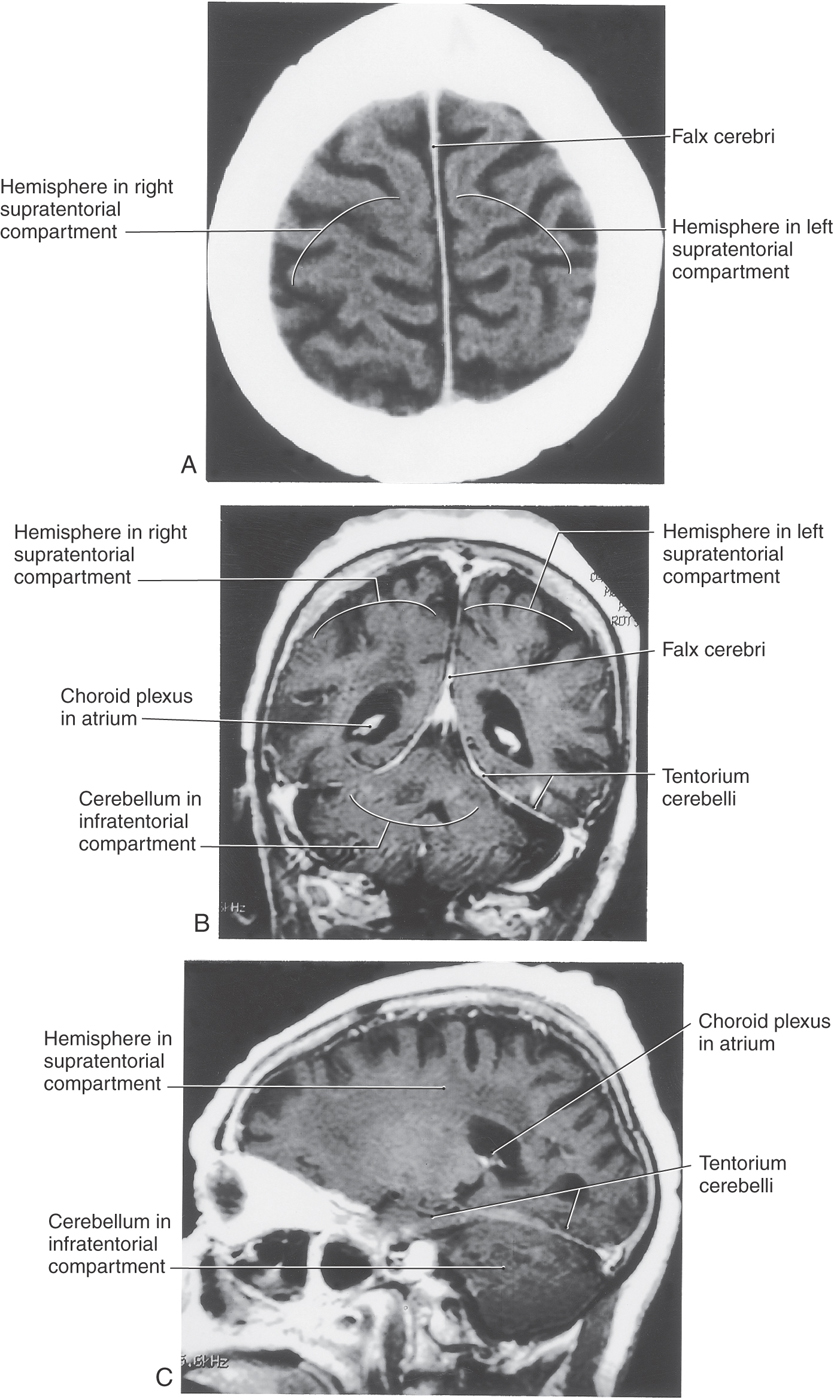
The tentorium cerebelli is the second largest of the dural infoldings (Figs. 7-4 and 7-5B, C). It attaches rostrally to the clinoid processes, rostrolaterally to the petrous portion of the temporal bone (location of the superior petrosal sinus), and caudolaterally to the inner surface of the occipital bone and a small part of the parietal bone (location of the transverse sinus) (Figs. 7-4 and 7-5B, C). The tent shape of the tentorium divides the cranial cavity into supratentorial (above the tentorium) and infratentorial (below the tentorium) compartments (Fig. 7-5B; see also Fig. 7-10). The supratentorial compartment is divided into right and left halves by the falx cerebri (Fig. 7-5A, B). The sweeping edges of the right and left tentoria, as they arch from the clinoid processes to join at the straight sinus, form the tentorial notch (Fig. 7-6). The occipital lobe is above the tentorium, the cerebellum is below it, and the midbrain passes through the tentorial notch.
Located below the tentorium cerebelli on the midline of the occipital bone is the falx cerebelli (Fig. 7-4). This small dural infolding extends into the space found between the cerebellar hemispheres and usually contains a small occipital sinus.
The smallest of the dural infoldings, the diaphragma sellae (Figs. 7-4 and 7-6), forms the roof of the hypophyseal fossa and encircles the stalk of the pituitary. The cavernous sinuses are found on either side of the sella turcica, and the anterior and posterior intercavernous sinuses are found in their respective edges of the diaphragma sellae.
It is emphasized that venous sinuses are endothelium-lined spaces that communicate with each other. In addition, large veins from the surface of the brain empty into the venous sinuses. As they enter the sinus, these veins are attached to a cuff of dura. Consequently, a blow to the head (or a minor bump to the head in an aged person) may cause the brain to shift just enough in the subarachnoid space to tear a vein at the point where it enters the sinus. This tear may allow venous blood to enter the subarachnoid space or may create a hematoma within the dural border cell layer at the dura-arachnoid interface, a subdural hematoma.
Compartments and Herniation Syndromes
The interior of the cranial cavity is divided into a supratentorial compartment located superior to the tentorium cerebelli and consisting of right and left halves (separated by the falx cerebri) and a single infratentorial compartment located inferior to the tentorium cerebelli (Fig. 7-5). The concept of supratentorial and infratentorial compartments, with an understanding of their contents and relationships, is an essential element in the diagnosis of what are commonly called herniation syndromes. In general, a herniation syndrome occurs when there is an intracranial event (hemorrhage, rapid tumor growth, traumatic brain injury) that causes an increase in intracranial pressure, forcing the comparatively gelatinous brain over the edge of a dural reflection. These syndromes are considered in more detail in later chapters.
The following are examples of herniation syndromes related to the supratentorial compartments. A lesion in one cerebral hemisphere may expand toward the midline, deform the falx cerebri, and force the cingulate gyrus under the edge of the falx into the opposite hemisphere; this is a subfalcine or cingulate herniation. In this example, the deficits may reflect occlusion of the adjacent anterior cerebral artery. Central (or transtentorial) herniation is the situation in which the diencephalon is forced downward through the tentorial incisure or notch. This is a neurologic emergency, and in about 90% of patients there is serious disability or death. Uncal herniation is the case when a rapidly expanding lesion, usually a hematoma, forces the uncus, a medial structure of the temporal lobe, over the edge of the tentorium cerebelli with resultant damage to the midbrain. The most common deficits are (1) a decreased level of consciousness, (2) dilation of the pupil and a loss of most eye movement reflecting damage to the ipsilateral oculomotor nerve, and (3) a contralateral hemiplegia reflecting damage to the descending corticospinal fibers. However, this early stage is likely to be followed by serious complications or death.
Cranial Versus Spinal Dura
At the margin of the foramen magnum, the periosteal dura essentially stops, but the meningeal dura continues caudally in the vertebral canal to eventually attach to the inner aspect of the coccyx as the filum terminale externum (dural part of the filum terminale or coccygeal ligament) (Fig. 7-2). The spinal dural sac is anchored rostrally and caudally and is separated from the adjacent vertebrae by an epidural space that contains venous channels, some lymphatics, and fat deposits. There are no dural infoldings around the cord; consequently, there are no venous sinuses in the spinal dura.
ARACHNOID MATER
The arachnoid mater is located internal to the dural border cell layer and is regarded as having two parts (Fig. 7-3). The portion of the arachnoid directly apposed to the dural border cells is the arachnoid barrier cell layer, and the spindly cells that traverse the subarachnoid space constitute the arachnoid trabeculae.
The subarachnoid space is located between the arachnoid barrier cell layer and the pial cells on the surface of the brain or spinal cord. This space contains CSF, many superficial vessels, and the roots of cranial and spinal nerves as they enter or exit the nervous system. Enlarged regions of the subarachnoid space are called subarachnoid cisterns.
Arachnoid Barrier Cell Layer
Fibroblasts of this layer are more plump than the flattened cells of the dura (Fig. 7-3). The arachnoid barrier cell layer is tenuously attached to the dural border cell layer by occasional cell junctions. In contrast, arachnoid barrier cells have closely apposed cell membranes and are joined to each other by numerous tight (occluding) junctions—hence the “barrier” characteristic of this layer. This close apposition of cell membranes excludes any significant extracellular space; consequently, no collagen is found in this layer of the meninges. The tight junctions between these arachnoid cells not only serve as a barrier against the movement of fluids but also impart strength to the membrane.
A basement membrane (basal lamina) is found on the surface of the barrier cell layer that faces the subarachnoid space.
Arachnoid Trabeculae and the Subarachnoid Space
The arachnoid trabeculae are composed of flattened, irregularly shaped fibroblasts that bridge the subarachnoid space in a random fashion (Fig. 7-3). Trabecular cells attach to the barrier layer and may attach to each other, to pial cells, or to blood vessels in the subarachnoid space. Although much of the extracellular collagen associated with trabecular cells is confined in the folded processes of these cells, some may be found free in the subarachnoid space. The attachments of the trabecular cells and their framework of collagen fibrils give added strength to the arachnoid mater.
The subarachnoid space is located internal to the barrier cell layer and external to the pia mater (Figs. 7-2 and 7-3). It contains CSF, trabecular cells and collagen fibrils, arteries and veins, and the roots of cranial nerves. Although some vessels may lie free in the subarachnoid space, most are covered by a thin layer of the leptomeninges (Fig. 7-3). These vessels may be damaged from trauma or may rupture spontaneously, resulting in the spread of blood around the brain; this event is a subarachnoid hemorrhage. CSF is produced by the choroid plexuses of the lateral, third, and fourth ventricles. It exits the ventricular system via the foramina of Magendie and Luschka to enter the subarachnoid space (arrows in Fig. 7-2). After circulating around the brain and spinal cord, CSF reenters the vascular system primarily through the arachnoid villi. The subarachnoid space around the spinal cord is the route used to administer spinal anesthesia.
Although it is common to refer to the brain as “floating” in the CSF of the subarachnoid space, it is actually suspended within this space. The structural basis for this fact is as follows. The dura is adherent to the skull, the arachnoid to the dura, the arachnoid trabeculae to the pia, and the pia to the surface of the brain. Consequently, the brain is suspended, through this chain, within the fluid milieu of the subarachnoid space by the numerous delicate strands of the arachnoid trabeculae. This is possible because the brain loses about 97% of its weight when it is suspended in CSF. For example, a brain that weighs about 1400 g in air will weigh only about 45 to 50 g in fluid.
Because the arachnoid trabeculae are not rigid, the brain may move within the fluid-filled subarachnoid space. In a closed head injury, the brain may move on its trabecular tethers in response to a sudden blow and be subjected to minor damage (concussion or contusion). This injury may result in no or only momentary loss of consciousness. Such a minor injury may be found at the point of the blow or at a site opposite the contact (contrecoup injury).
Arachnoid Villi
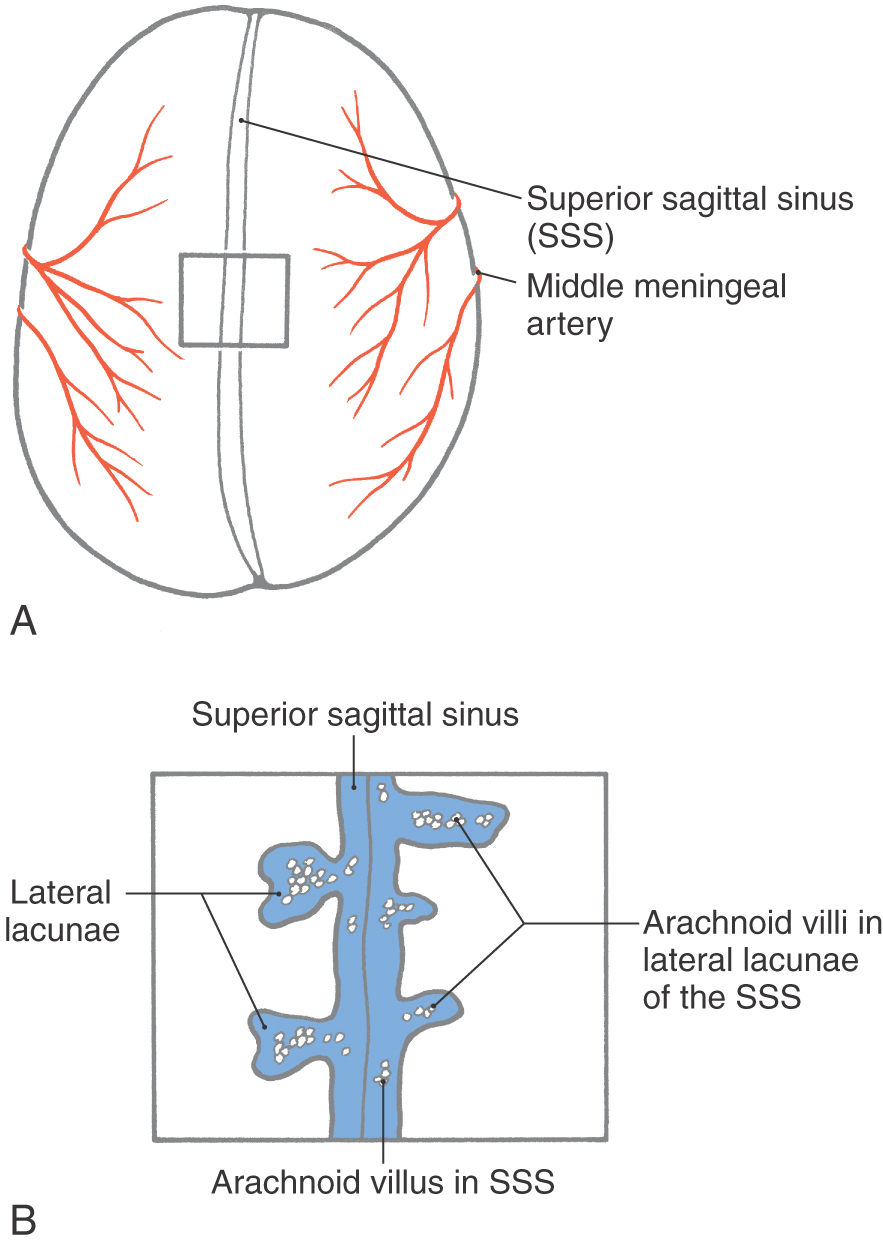
The small specialized portions of the arachnoid that protrude into the superior sagittal sinus through openings in the dura form the arachnoid villi or arachnoid granulations (Figs. 7-7 and 7-8). If they are especially large or calcified (as in older persons), they may be called pacchionian bodies.
Arachnoid villi extend into the sinus through tight cuffs in the meningeal dura and are found just off the midline or in cul-de-sacs (the lateral or venous lacunae) of the sinus (Figs. 7-7 and 7-8). The vast majority of arachnoid villi are located in the lateral lacunae of the superior sagittal sinus (see Fig. 7-11). The space in the center of each villus is continuous with the subarachnoid space around the brain. This space is enclosed in a layer of cells that are markedly similar to arachnoid barrier cells, and these arachnoid cells, in turn, are surrounded by a capsule of cells that are essentially the same as dural border cells. These two layers are continuous with their respective meningeal layers through the stalk of the villus (Fig. 7-7). The endothelial lining of the sinus is reflected onto the villus and may cover this structure entirely or may leave a few arachnoid cells exposed; the exposed cells are called arachnoid cap cells. The endothelium covering the villus sits on a basement membrane, beneath which some extracellular collagen may be found.
Arachnoid villi are structurally adapted for the transport of CSF from the subarachnoid space into the venous circulation (Fig. 7-7). CSF moves only from the villus into the sinus. The two routes of fluid movement are through small intercellular channels located between cells and by way of a vacuole-mediated transport of fluid and other elements (bacteria, blood cells) through villus cells. As CSF traverses the villus, it moves down a pressure gradient from a point of higher pressure (the subarachnoid space) to a point of lower pressure (the venous sinus). If the pressure on the venous side exceeds that on the subarachnoid space side, the flow of CSF will slow or stop. Venous blood, however, never flows from the sinus into the subarachnoid space.
MENINGIOMA
Meningiomas (tumors of the meninges) are characterized as slow-growing, benign (usually), extraaxial (located outside the brain substance) tumors that may be calcified and may result in abnormal growth of adjacent bone (hyperostosis). The treatment of choice is surgical removal, which usually results in a complete cure. These growths are primary intracranial tumors but not primary tumors of the brain. This means that these tumors originate from structures that are intimately associated with the CNS (indeed, portions of the meninges arise from the neural crests that are derived from the neural plate) but do not originate from the brain substance itself.
Origins and Locations
Meningiomas arise from arachnoid cells found in the villi, at points where blood vessels and cranial nerves traverse the dura, and along the base of the skull. In clinical parlance, these specific cells are called arachnoid cap cells. As one would expect, most meningiomas (about 90%) are found in the cranial cavity or in association with the spinal cord (about 9%). Ectopic meningiomas are tumors with the histologic features of meningiomas that are found outside the brain and spinal cord (about 1%).
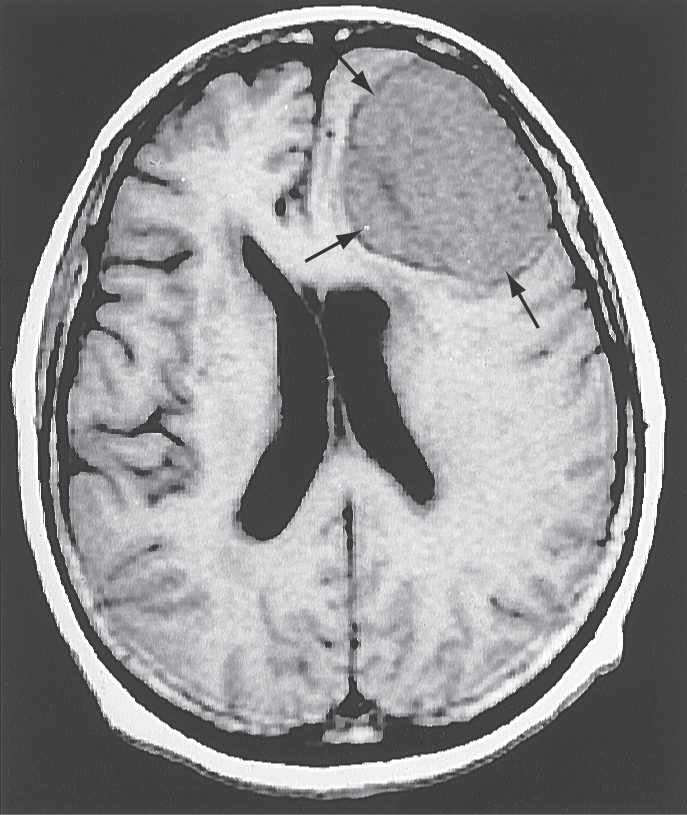
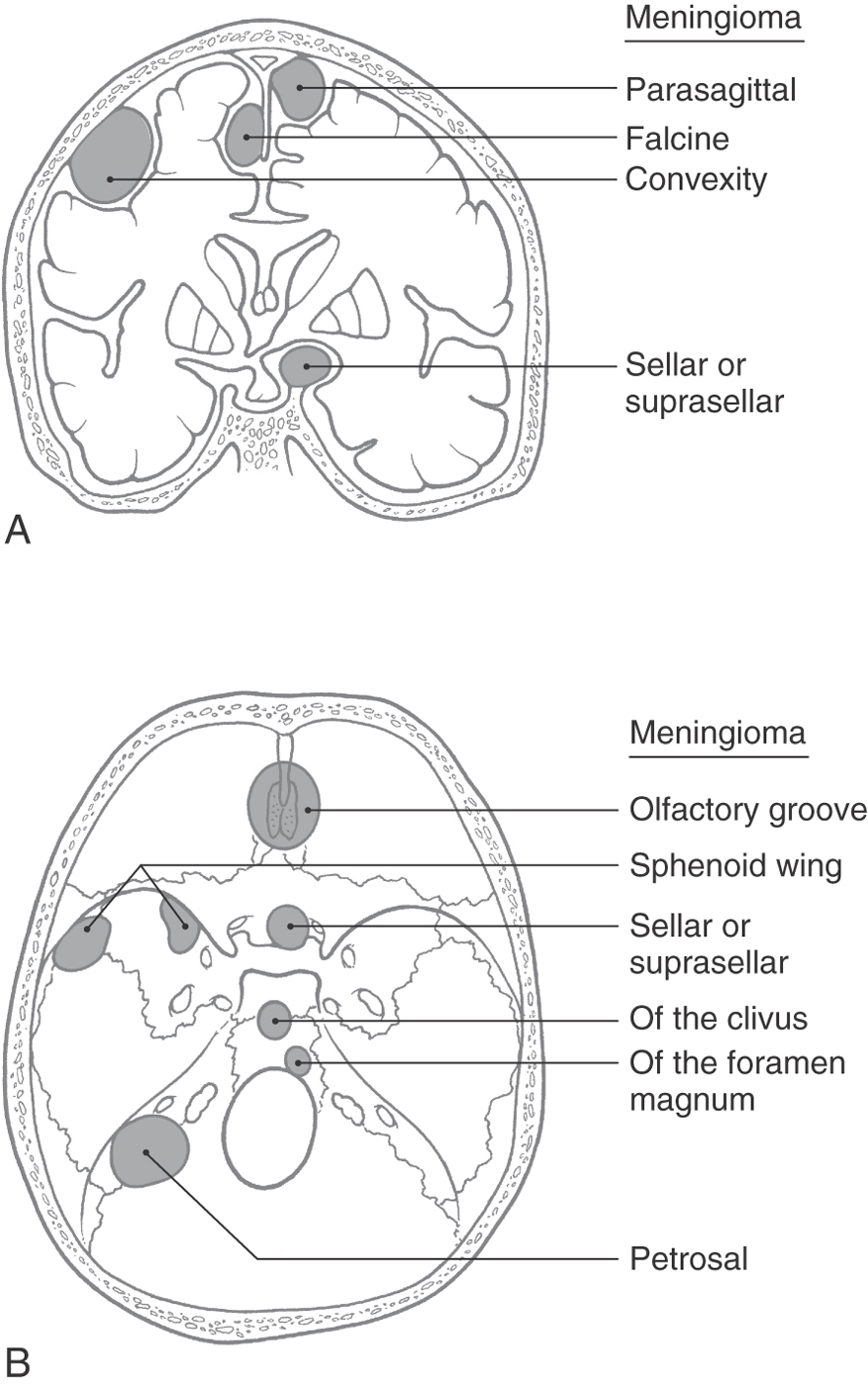
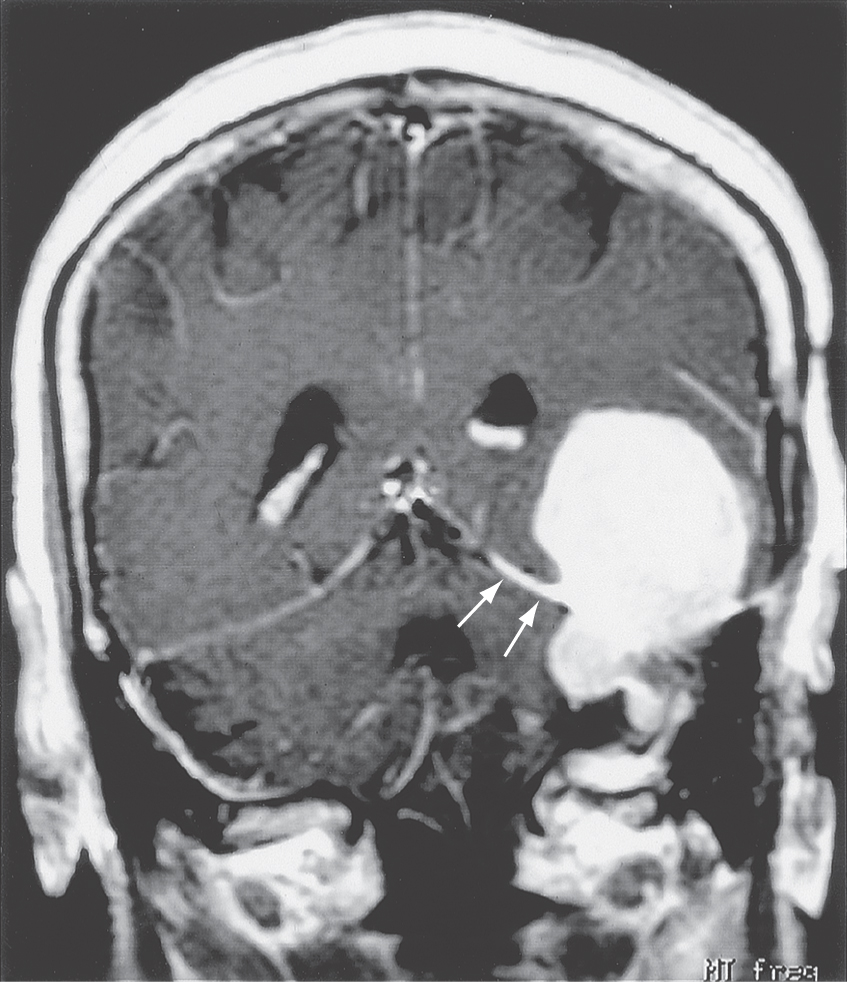
In descending order of occurrence, meningiomas are found in the following locations: parasagittal (about 21%), convexity (15%), tuberculum sellae (13%), ridge of the sphenoid (12%), olfactory groove (10%), and falx cerebri (8%) (Fig. 7-9A). It is common to refer to these tumors by their location, for example, a parasagittal meningioma, a convexity meningioma, and a tentorial meningioma (Figs. 7-9 and 7-10). Convexity meningiomas may also be designated by the lobe in which they are located, such as a frontal lobe meningioma (Fig. 7-11). In this type of meningioma, the deficits experienced by the patient may reflect characteristics of the lobe involved. Because of the position of the tentorium cerebelli, a meningioma of this structure (tentorial meningioma) may extend into supratentorial and infratentorial compartments (Fig. 7-10).
Patients presenting with meningiomas are typically in the range of 40 to 60 years (peak incidence at about 45 years) and, by a small margin, are more likely to be female (ratio of 3:2). These tumors are usually single, but some patients may have more than one. Multiple meningiomas may be seen in patients with neurofibromatosis (of the central type); these patients may also have bilateral vestibular schwannomas.
General Histologic Features
The vast majority of meningiomas are histologically characterized as benign (about 95%) and are rarely diagnosed as malignant (1.5% to 2.0%). However, when these tumors are malignant, they may invade brain tissue or the dura, thereby significantly complicating their treatment. Malignant tumors contain many mitotic figures and may metastasize to distant sites.
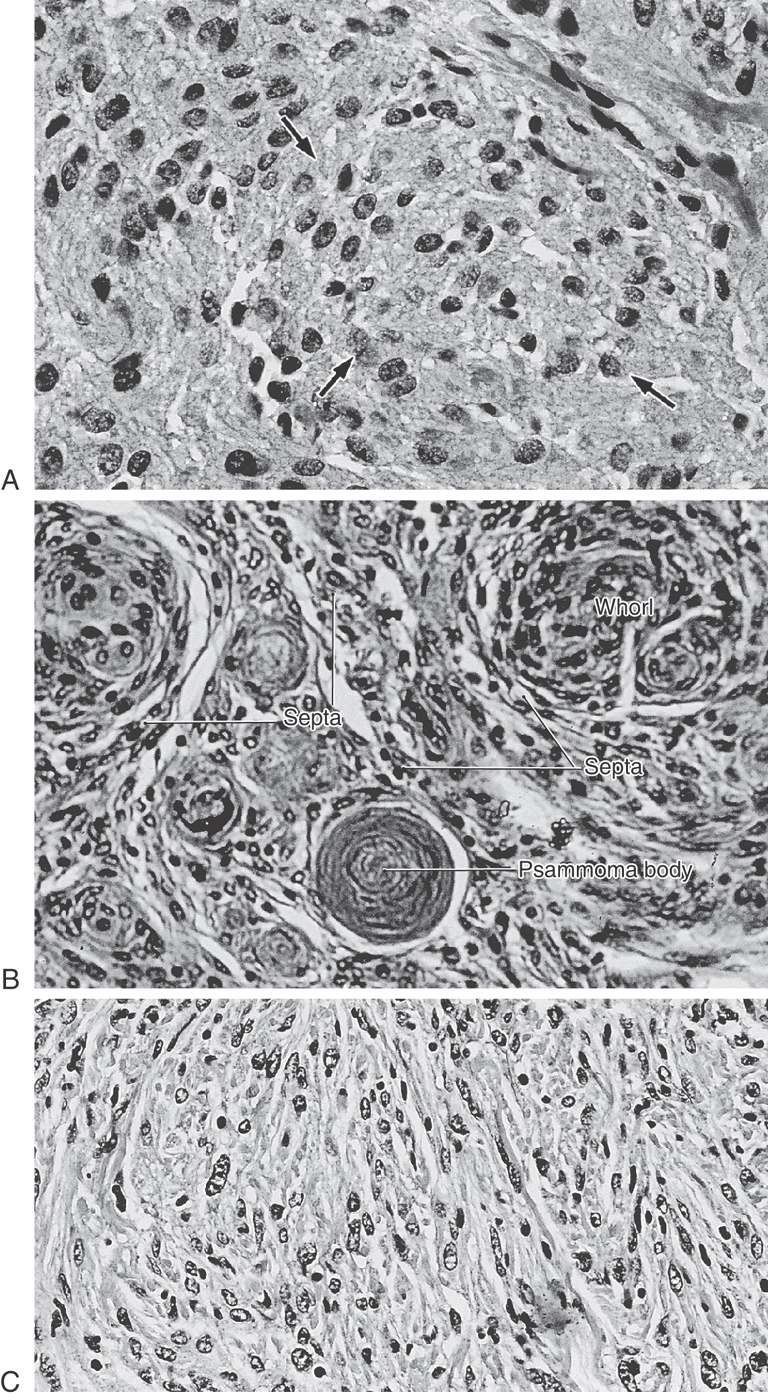
On the basis of their histologic characteristics, meningiomas can be divided into three general types. There are variations on these types, but these variations are beyond the scope of this book. Meningotheliomatous (or syncytial) meningiomas are composed of polygon-shaped cells with large, centrally located nuclei that contain nucleoli and sometimes vacuoles (Fig. 7-12A). These cells are arranged in sheets, and some cells form small concentric aggregations suggesting whorls. In many areas of these tumors, the borders between cells are obscured, giving the tumors the appearance of a syncytium. Transitional meningiomas have an appearance intermediate between syncytial and fibrous meningiomas and are characterized by cells arranged in tight concentric whorl formations separated by thin septa of spindle-shaped cells (Fig. 7-12B). These whorls may form around a centrally located cell or a small blood vessel. Psammoma bodies (Greek for “grains of sand”), made up of concentric layers of calcium, are also seen in this type of meningioma. Fibroblastic (or fibrous) meningiomas contain layers of long spindle-shaped cells with elongated nuclei; in some areas the sheets are many cells thick and contain large amounts of collagen (Fig. 7-12C). Whorl formations and psammoma bodies may also be present in this tumor.
Symptoms and Treatment
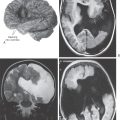
Because meningiomas are slow growing, symptoms may appear very slowly or not at all. It is not uncommon to see meningioma as an incidental finding in patients who have died of other causes or who have been subjected to imaging studies for some other problem, such as trauma or stroke. Contrast medium–enhanced computed tomography (CT) and magnetic resonance imaging (MRI) are especially valuable in the evaluation of this tumor; edema surrounding the lesion is especially evident in MRI. A diagnosis may require histologic confirmation.
Neurologic symptoms or signs in patients with meningioma are generally due to compression of brain structures, involvement of cranial nerves, or secondary causes such as edema. In addition, these patients may present with seizures or with slowly developing personality or behavioral changes that may (or may not) accompany specific deficits related to cranial nerve or long tract involvement.
The treatment of choice for meningioma is surgical removal. The location of the mass may dictate the ease or difficulty of its removal. A convexity meningioma is rather straightforward, whereas a parasagittal tumor is more complex because of its potential involvement of the superior sagittal sinus. In like manner, meningiomas in the region of the cavernous sinus may involve branches of cranial nerves III, IV, V, and VI or the internal carotid artery; a tumor of the sella may envelop optic structures. Radiation therapy may be used to treat specific types of meningiomas, but chemotherapy has not proved to be effective at present.
MENINGEAL HEMORRHAGES
At this point, it is appropriate to consider meningeal hemorrhages that are specifically related to the dura-skull interface and to the arachnoid-dura interface. These lesions share the common feature of being most likely caused by trauma.
Extradural and “Subdural” Hemorrhages
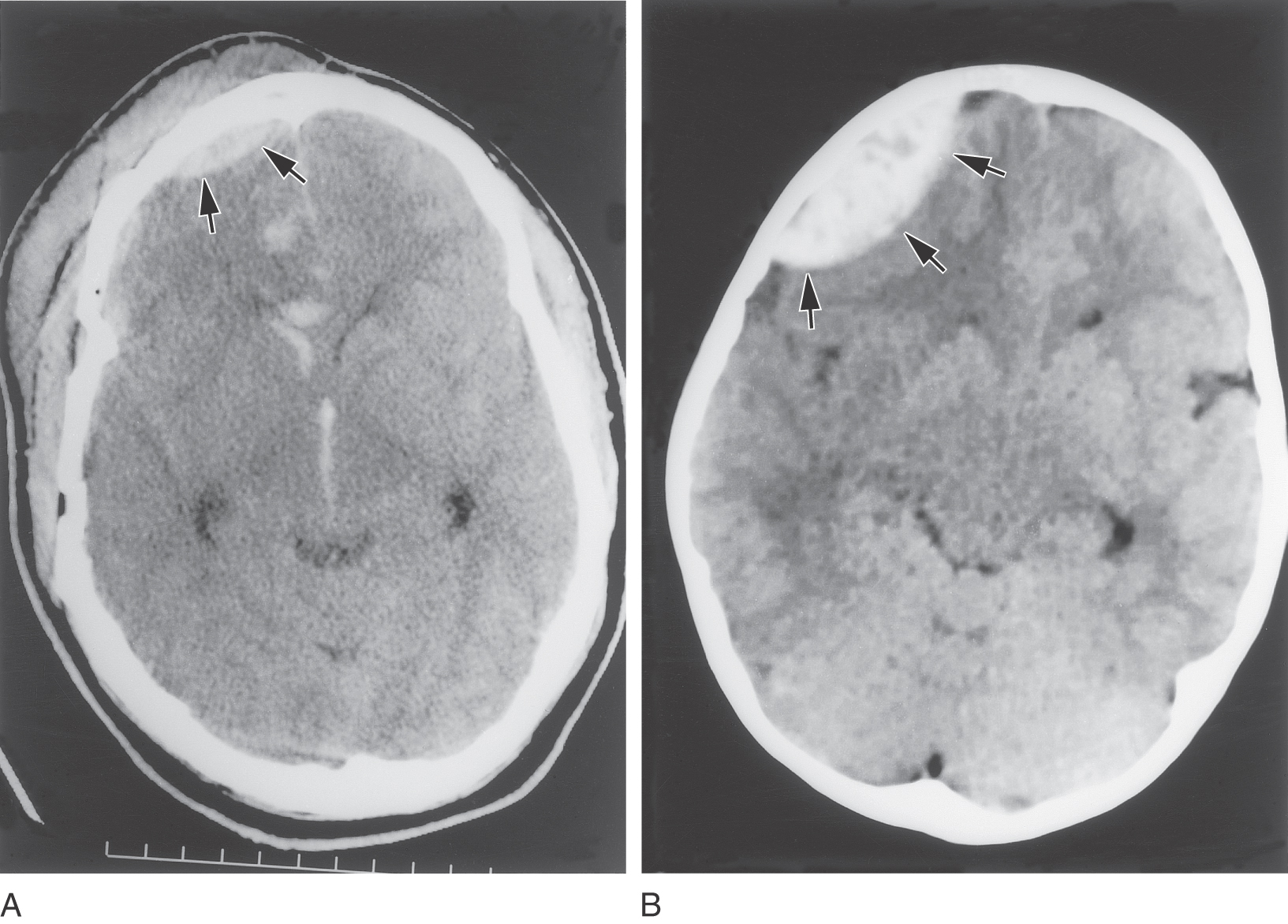
If we exclude, for the moment, subarachnoid hemorrhages, which are considered later in this chapter, meningeal hemorrhages can be generally described as extravasated blood that strips the dura from the skull or dissects open the dural border cell layer (Fig. 7-13). The most common cause in both situations is an injury to the head, with or without skull fracture. In a head injury, the periosteal dura may be loosened from the skull with consequent damage to a major artery; the middle and accessory meningeal arteries are common victims. Extravascular blood dissects the periosteal dura from the skull and collects to form an extradural (epidural) hematoma (Figs. 7-13 and 7-14). These lesions tend to be lenticular and appear “short and wide” owing to the fact that they do not cross the dural attachment at suture lines (Fig. 7-14). The neurologic deficits seen in patients with epidural hemorrhage are usually those characteristic of increased intracranial pressure. These deficits are, in order of occurrence, headache, confusion and disorientation, lethargy, and finally a state of unresponsiveness. In some cases of head trauma, the patient may initially be rendered unconscious, followed by a lucid interval (the patient is wide awake and conversant), then subsequently deteriorate rapidly and die; this is called talk and die. Keeping this in mind, it is essential to observe these patients closely.
Figure 7-14. A and B, Examples of epidural hematomas (arrows) in CT scans on the patient’s right side. The smaller lesion in A is obviously of traumatic origin; this patient has soft tissue damage, a fractured skull, blood in the substance of the brain, and blood in the anterior horn of the lateral ventricle and in the third ventricle. The cause of the larger lesion (B) is not obvious. Compare the shape of these lesions with that of a “subdural” hematoma in Figure 7-15.
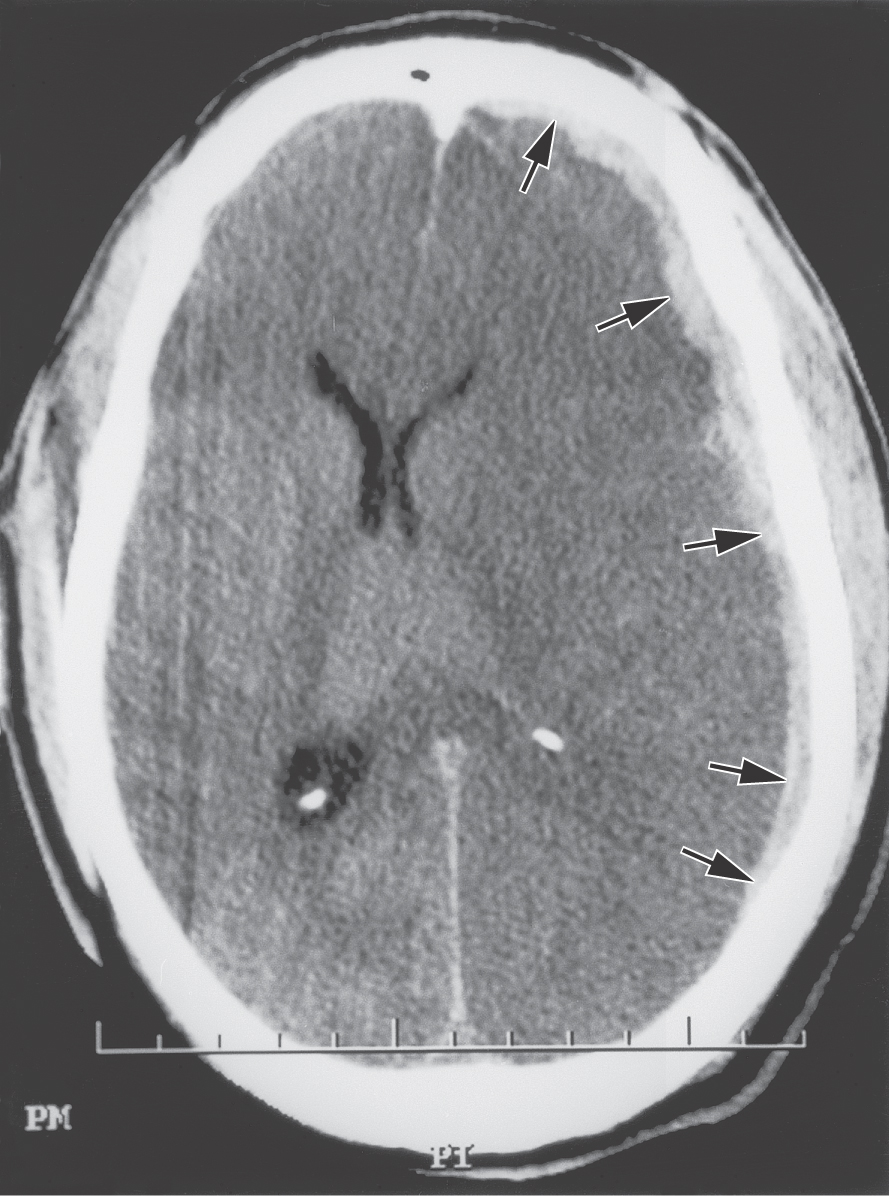
In contrast to extradural hemorrhages, bleeding into the meninges at the junction of the arachnoid with the dura originates mainly from venous structures. A common cause is the tearing of “bridging veins” as they pass through the subarachnoid space and enter a dural venous sinus (Fig. 7-13). Although these lesions are commonly called subdural, as noted previously, there is no naturally occurring space at the arachnoid-dura junction. Hematomas at this junction are usually caused by extravasated blood that splits open the dural border cell layer (Figs. 7-13 and 7-15). In contrast to epidural lesions, so-called subdural hematomas appear “long and thin” because they are not constrained by any dural attachments (Fig. 7-15). This extravascular blood does not collect within a preexisting space but rather creates a space at the dura-arachnoid junction. Because these so-called subdural hematomas are usually found within a specific layer of cells, they actually constitute “dural border” hematomas. These lesions generally contain blood in their central area and myofibroblasts, fibroblasts, mast cells, proliferating blood vessels, and dural border cells in the surrounding capsule.
Figure 7-15. An example of a so-called subdural hematoma (arrows) in CT scan on the patient’s left side. This lesion is long and thin and extends for considerable distance over the surface of the hemisphere by dissecting through the dural border cell layer; note the shift in the midline. Compare the shape of this lesion with that of the epidural hematomas in Figure 7-14.
Hygroma
Trauma to the skull may also result in tearing of the arachnoid membrane. In such instances, CSF, which is under pressure (100 to 150 mm H2O in a recumbent position), also may dissect open and collect within the dural border cell layer. These lesions are called hygromas.
Pia Mater
The pia mater consists of flattened cells with long, equally flattened processes that closely follow all the surface features of the brain and spinal cord (Fig. 7-3). The pia and arachnoid together constitute the leptomeninges. Vessels in the subarachnoid space (Fig. 7-3) may be covered by a single layer of pial cells, may be enveloped by several layers of leptomeningeal cells, or may lie free in this space. The pia is separated from the brain surface by a glial basement membrane and by occasional places where pial cells pull away from the brain to form a small subpial space. Pial cells at the brain surface may be arranged in a single layer or in several layers. Single pial cell processes and their subjacent collagen correspond to the pia intima; these closely follow surface features of the brain and spinal cord. When there are several tiers of pial cell processes, the outer layers correspond to the epipial layer. In general, the pia is thicker on the spinal cord than on the brain.
Where small vessels penetrate the surface of the brain and spinal cord, they pull along a small envelope of pial cell processes and extracellular space. These perivascular spaces (Virchow-Robin spaces) extend for varying distances into the parenchyma of the nervous system and may serve as conduits for the movement of extracellular fluid between the subarachnoid space and the minute spaces around neurons and glial cells.
The spinal cord is anchored in the subarachnoid space by three structures: two pial modifications plus a reticulated septum of arachnoid cell processes that attaches to the posterior midline of the cord. The first of the pial structures, the denticulate ligaments, run longitudinally along each side of the spinal cord about midway between the posterior and anterior roots and attach to the inner surface of the arachnoid-lined dural sac (Fig. 7-2). From each ligament a series of 20 to 22 structures, shaped much like shark’s teeth, extend laterally to attach to the inner surface of the arachnoid-lined dural sac. Second, extending caudally from the conus medullaris is a tough strand composed primarily of pia; this is the filum terminale internum (pial part of the filum terminale). The filum terminale internum attaches to the caudal end of the dural sac, which in turn attaches to the coccyx as the filum terminale externum (dural part of the filum terminale or coccygeal ligament) (Fig. 7-2). Together, these anchoring structures serve a function analogous to that of the arachnoid trabeculae around the brain.
The large space caudal to the conus medullaris, which contains CSF, posterior and anterior roots (constituting the cauda equina), and the filum terminale internum, is the lumbar cistern (Fig. 7-2). The retrieval of CSF is an important diagnostic tool for evaluation of a variety of CNS disorders. A needle introduced into the lumbar cistern (spinal tap or lumbar puncture) through the third to fourth or fourth to fifth lumbar interspace is the primary method used to collect a sample of CSF from this cistern (see Fig. 9-2).
Cisterns, Subarachnoid Hemorrhages, and Meningitis
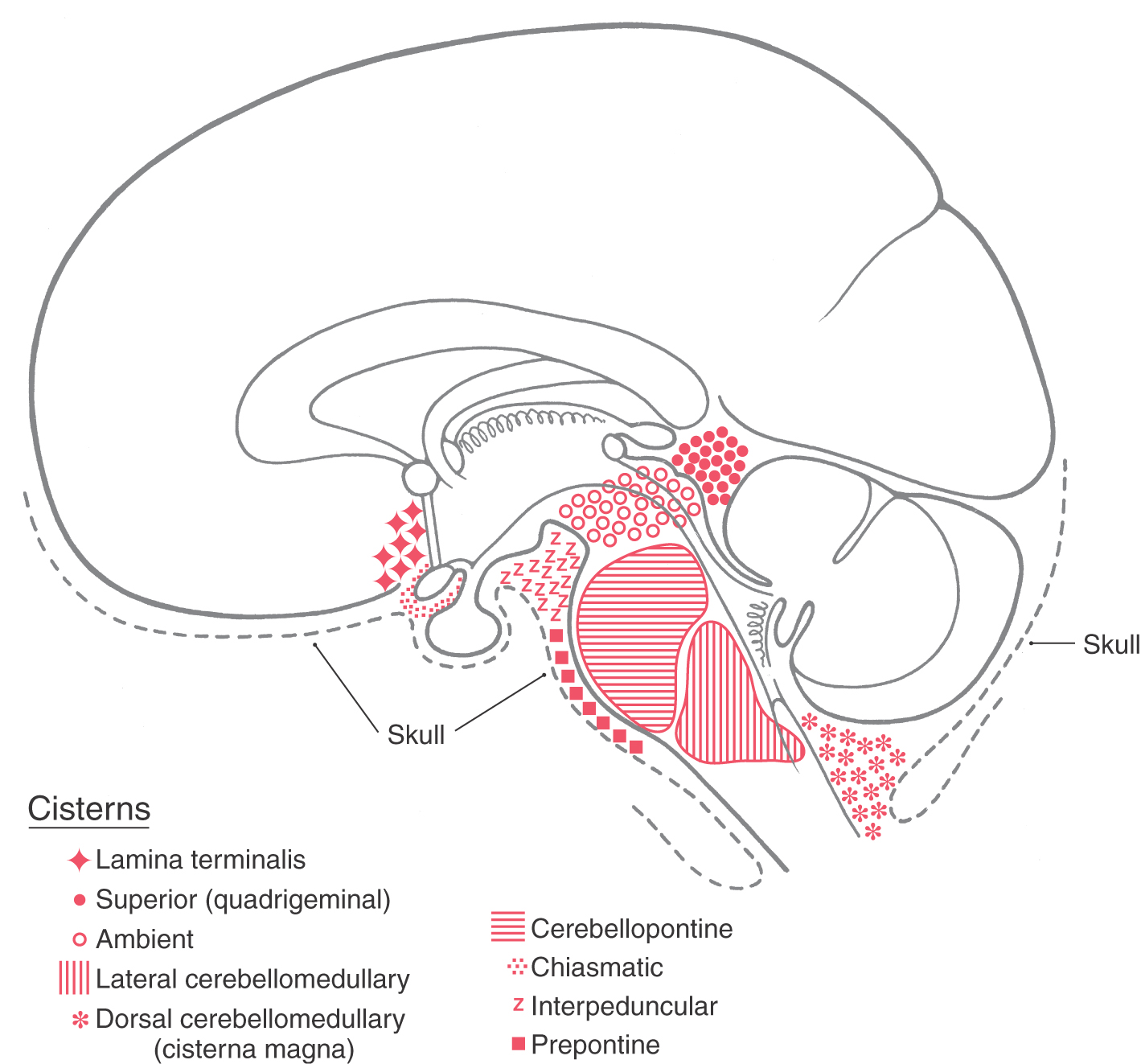
The subarachnoid space is the thin envelope of space located between the arachnoid and pia (Figs. 7-2 and 7-7). This space has a number of naturally enlarged regions called subarachnoid cisterns, which contain CSF, arteries and veins, and in some cases cranial nerve roots (Fig. 7-16; Table 7-1). Cisterns occur where the brain draws away from the skull as part of its natural variation in shape, thus enlarging the subarachnoid space. In addition to discussing cisterns, we shall consider subarachnoid hemorrhage and meningitis at this point because these clinical problems are most specifically related to the leptomeninges.
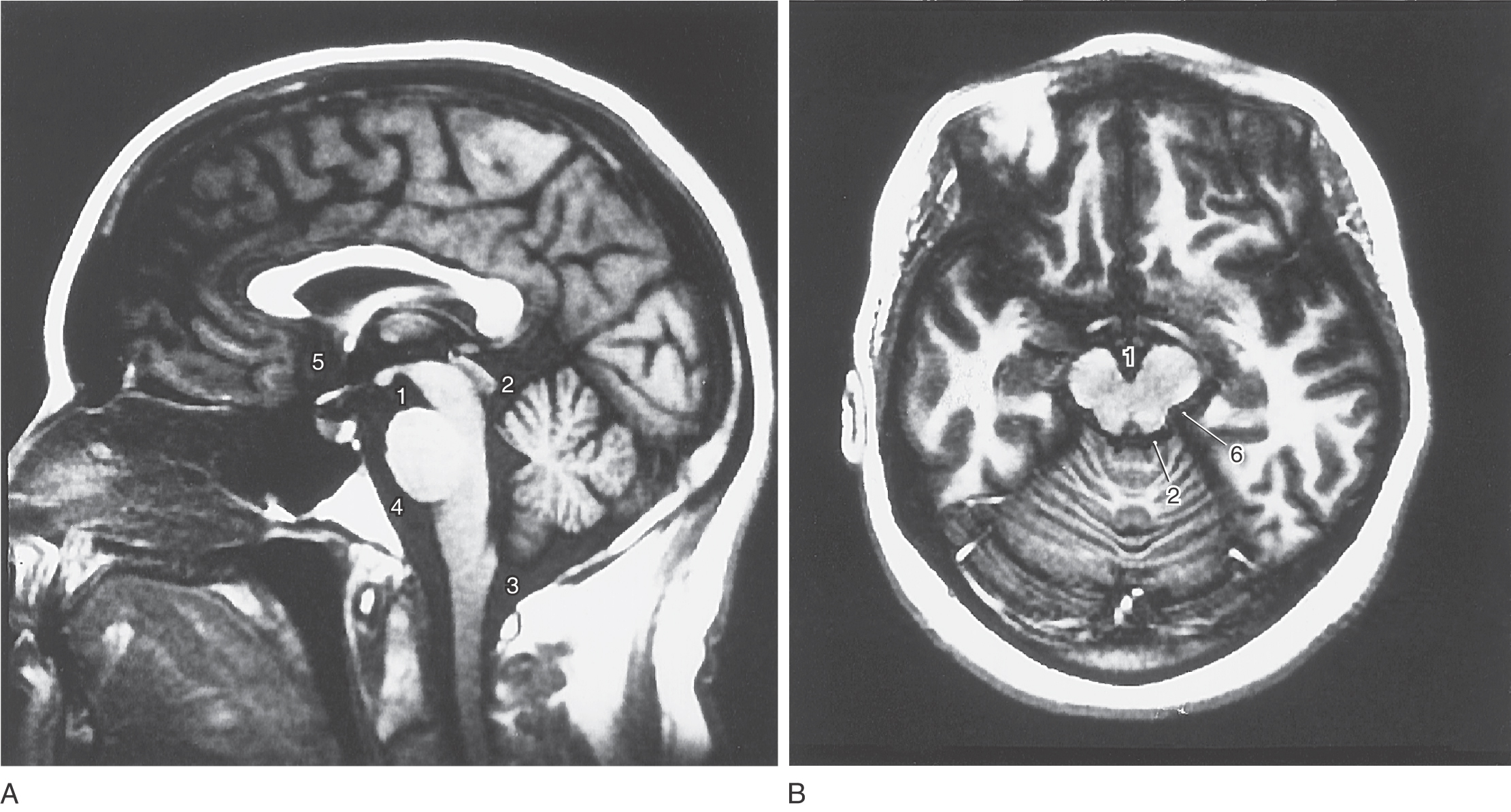
Figure 7-16. The locations of the major subarachnoid cisterns in relation to brain structures. Although the cerebellopontine, lateral cerebellomedullary, and ambient cisterns are located on the lateral aspect of the brainstem, their approximate positions are indicated on this midsagittal view. Compare with Table 7-1. (From Haines DE, Fredrickson RG: The meninges. In Al-Mefty O [ed]: Meningiomas. New York, Raven Press, 1991.)
Table 7-1 Some Principal Cisterns and the Main Arteries, Veins, Cranial Nerves, and Other Structures Associated with Them
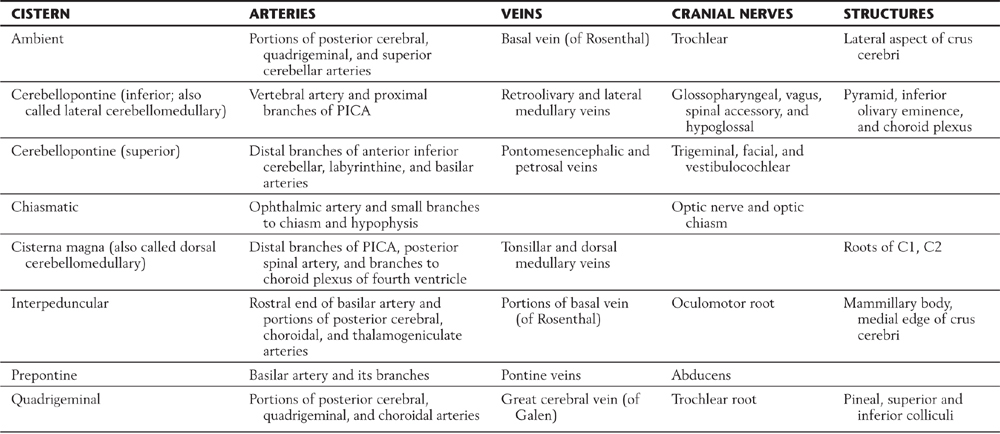
Data from Yasargil MG: Microneurosurgery, vol I, Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain, Diagnostic Studies, General Operative Techniques and Pathological Considerations of the Intracranial Aneurysms, Stuttgart, 1984, Georg Thieme.
PICA, posterior inferior cerebellar artery.
Cisterns
Cisterns are usually named according to the structures on which they border. For example, the interpeduncular cistern is found in the interpeduncular fossa, and the dorsal cerebellomedullary cistern (cisterna magna) is found between the cerebellum and the medulla (Fig. 7-16). Typically, the shapes of cisterns, as seen on MRI and CT, are determined by the corresponding shapes of surrounding brain structures (Fig. 7-17); this characteristic relationship is useful in diagnosis. The cisterna magna is a potential source of CSF if the lumbar cistern is not accessible. In a cisternal puncture, a needle is carefully introduced into the cisterna magna through the atlantooccipital membrane and a sample of fluid is withdrawn.
Cisterns are bordered by particular brain structures, contain segments of major vessels, and may also contain cranial nerve roots or other structures (Table 7-1). Consequently, a progressively enlarging aneurysm or a slow hemorrhage into a particular cistern may result in signs or symptoms related to the structures found in or next to the cistern. For example, an aneurysm protruding into the interpeduncular cistern may affect the oculomotor nerve (Table 7-1) and consequently eye movements or pupil size.
Subarachnoid Hemorrhage
A subarachnoid hemorrhage is an extravasation of blood (usually arterial) into the subarachnoid space (Figs. 7-18 and 7-19). The most common cause of blood in the subarachnoid space (subarachnoid hemorrhage) is trauma; the second most common cause of subarachnoid blood is rupture of an intracranial aneurysm (also called nontraumatic or spontaneous subarachnoid hemorrhage).
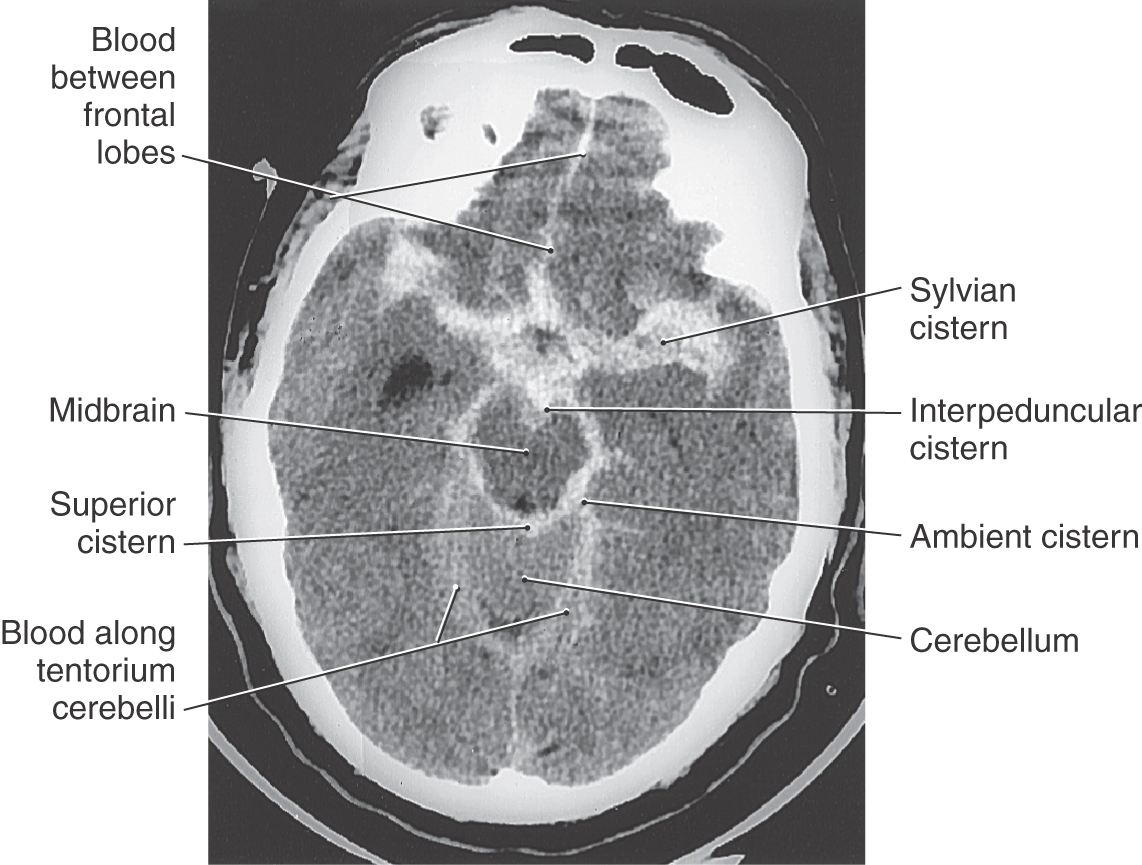
In traumatic subarachnoid hemorrhage, the source of the blood may be due to damage to large veins. In either case, blood is extruded into the subarachnoid space and may be sequestered in cisterns or migrate through the subarachnoid space and subsequently may be seen, as hyperdense areas, on imaging studies to outline structures such as brain divisions or dural reflections (Fig. 7-19). Aneurysms are clearly defined dilations in the walls of arteries (Fig. 7-18). Although some aneurysms are thought to be congenital, they may also be caused by an ongoing pathologic process or by trauma or may be secondary to a general systemic problem such as hypertension. Subarachnoid hemorrhage from a ruptured aneurysm is most common in persons between 40 and 65 years of age and is a potentially catastrophic event. About one third of affected patients die before or soon after admission to a medical facility, about one third have permanent and significant disabilities (cognitive, motor), and about one third may recover with minimal neurologic sequelae.
The occurrence of a subarachnoid hemorrhage, consequent to aneurysm rupture, may be signaled by a sudden excruciating headache, neck stiffness, vomiting or nausea, and a depression or loss of consciousness. Patients who do not become unconscious at the time of the hemorrhage may describe the headache as “explosive and awful,” like a “thunderclap” in my head, or “the absolutely worst headache I have ever had.” Bloody CSF obtained by lumbar or cisternal puncture is diagnostic of subarachnoid hemorrhage, and blood can be clearly identified in the subarachnoid space on CT examination (Fig. 7-19). In some cases a patient may have warning signs and symptoms of an impending subarachnoid hemorrhage (leaking aneurysm). These include intermittent headache, nausea or vomiting, and fainting spells (syncope). In persons with neurologic signs that can be traced to an aneurysm, the treatment of choice is to clip the aneurysm or its stalk, thereby separating it from the cerebral circulation.
Meningitis
Meningeal infection may be of either bacterial or viral origin or occur as a sequel to some other disease process, such as tuberculosis or fungal infections. With bacterial meningitis, the meningeal infection is most often located in the subarachnoid space and involves the arachnoid and pia; hence the designation leptomeningitis (Fig. 7-20). If the infection involves the dura mater, it is called pachymeningitis.
Such infections may result from a variety of causes, including trauma (which may introduce bacteria into the head or spine such as Staphylococcus aureus), septicemia, and metastasis from another site of infection in the body. Bacterial meningitis is generally classified as acute or subacute, depending on how rapidly the disease progresses. The more common causative agents are Streptococcus agalactiae and Escherichia coli (birth to 2 years) and Streptococcus pneumoniae and Neisseria meningitidis (2 years to >50 years).
Signs of acute bacterial meningitis include elevated temperature, alternating chills and fever, and headache; the patient is acutely ill and may have a depressed level of consciousness. These signs and symptoms seen in concert with increased CSF pressure and cloudy CSF containing many white blood cells, increased protein, and bacteria are diagnostic of the disease. The inflammatory process may result in thickening of the leptomeninges with consequent partial obstruction of CSF flow and signs of hydrocephalus (Fig. 7-20). Although the death rate is low in acute cases with proper treatment, the patient may become ill suddenly and may die within 2 days in rapidly advancing cases.
Viral meningitis is caused by a range of viral agents, is most commonly seen in younger patients (younger than 25 years), and is a disease for which no antiviral medications are available. The patient becomes ill during a period of days and experiences fever, headaches of increasing intensity, and confusion and possibly an altered level of consciousness. In a minority of cases, more serious signs and symptoms may be seen, such as seizures, rigidity, or cranial nerve palsies. Treatment in mild cases is supportive and generally focuses on medications for fever, pain, and general discomfort. After an acute period of 1 to 2 weeks, the signs and symptoms moderate, and the patient generally recovers without permanent deficits.
Sources and Additional Reading
Alcolado R, Weller RO, Parrish EP, Garrod D. The cranial arachnoid and pia mater in man: Anatomical and ultrastructural observations. Neuropathol Appl Neurobiol. 1988;14:1–17.
Al-Mefty O. Meningiomas. New York: Raven Press; 1991.
Haines DE. On the question of a subdural space. Anat Rec. 1991;230:3–21.
Nicholas DS, Weller RO. The fine anatomy of the human spinal meninges. J Neurosurg. 1988;69:276–282.
Williams PL, ed. Gray’s Anatomy. ed 38 New York: Churchill Livingstone; 1995.