CHAPTER 82 The Management of Head and Neck Melanoma and Advanced Cutaneous Malignancies
Although the overall trend in the United States demonstrates both a stabilization of cancer incidence and a decrease in cancer death rates, the incidence of cutaneous melanoma continues to rise dramatically on an annual basis.1,2 The diagnosis is growing in men faster than any other malignancy; for women, the increase in incidence is second only to that for lung cancer.3 Overall, cutaneous melanoma remains the sixth leading cause of cancer among men and the seventh cause among women. The current annual budget for melanoma treatment in the United States exceeds $740 million.4
Fortunately, the management of cutaneous melanoma is also one of fastest-evolving fields in cancer, with promising research taking place at both the molecular and clinical levels. Greater understanding of melanoma biology prompted major changes in the staging classification system set forth by the American Joint Committee on Cancer (AJCC).5 Given its association with sun exposure, melanoma is considered a preventable disease. Decreases in incidence and mortality ultimately hinge upon increases in education, prevention, and early diagnosis, as well as improved treatment for advanced disease.
Epidemiology
Despite a decline in overall cancer trends, the incidence of invasive cutaneous melanoma continues to rise dramatically, with an estimated 62,480 new cases anticipated in 2008.1 Over the past 50 years, the annual percentage change in invasive melanoma incidence has steadily increased by 4.3% each year. The lifetime risk for development of invasive melanoma has climbed at an epidemic rate, rising from 1 in 1500 individuals born in 1935 to 1 in 250 born in 1980.6 This trend is expected to continue at a startling rate, with a current estimation that 1 in 41 men and 1 in 61 women will have invasive carcinoma during their lifetimes.
Melanoma is the most lethal form of skin cancer, accounting for 8110 American deaths in 2007, 8420 deaths in 2008, and an expected 8650 deaths in 2009.1,2 These estimates average to approximately one American dying from melanoma every hour. Over the past 50 years, the annual percentage change in mortality rate has increased at a steady rate of 1.8% per year. Melanoma accounts for the second highest increase in mortality rate, especially for men older than 65 years. However, it should not be viewed as a cancer limited to the elderly because 1 in 4 patients are diagnosed before age 40, and it is the third most common cancer among women ages 20 to 39.4,6,7 Consequently, melanoma represents one of the leading cancer causes of lost potential life years, ranking second only to leukemia.
Approximately 25% of all cutaneous melanomas arise in the head and neck region,8 with more than 9000 cases diagnosed annually. The majority involve the cheek, scalp, and neck. A slight male preponderance is consistently reported,9–11 and the median age at diagnosis is 55 years.7 However, juvenile cases account for 1.66% of head and neck melanoma cases, and patients as young as 4 years are diagnosed with these lesions.12,13
Etiology and Risk Factors
Numerous environmental and genetic risk factors have been implicated in the development of cutaneous melanoma.14 They are summarized in Box 82-1.
Box 82-1 Risk Factors Associated with Cutaneous Melanoma
Adapted from Schmalbach CE, Johnson TM, Bradford CR. The management of head and neck melanoma. Curr Probl Surg. 2006;43:784.
Risk Factors
Sun exposure is considered the leading cause of melanoma.15,16 Patients who experienced peeling or blistering sunburns, even during childhood, are at particular risk.17 Associated factors include blond or red hair, green or blue eyes, and fair skin consistent with Fitzpatrick skin types I through III.18 Adults with more than 100 clinically normal-appearing nevi, children with more than 50 clinically normal-appearing nevi, and any patients with atypical or dysplastic nevi are also at risk.
A prior history of melanoma poses a greater risk; 5% to 10% of individuals diagnosed with melanoma experience a second primary melanoma.16 This increased risk remains lifelong, and the melanoma can occur anywhere on the skin. For this reason, long-term follow-up with a thorough total body examination is critical.
Genetics
A genetic component has been implicated in the pathogenesis of melanoma19; 10% to 15% of patients with melanoma report a positive family history.20 The most common chromosomal mutation associated with melanoma involves CDKN2A, which is also known as p16.21 However, the mutation accounts for only a small percentage (0.2%) of melanoma cases observed.22
B-K mole syndrome is a hereditary form of cutaneous melanoma in which members acquire large, irregular, and dysplastic nevi, often in sun-protected regions of the body, such as the scalp and trunk.23 A familial association of melanoma among individuals with atypical nevi has also been coined familial atypical multiple mole-melanoma (FAMMM) syndrome.24 Today, the term “atypical mole syndrome” is applied to familial cases of melanoma. The syndrome is inherited in an autosomally dominant fashion.25,26 The 10-year melanoma risk in this setting is reported to be 10.7%, compared with 0.62% in control patients.27,28 Fitzpatrick and Ortonne18 and Greene and associates29 approximated a 56% cumulative risk from age 20 to 59 years, which predicts that 100% of patients with atypical mole syndrome would have melanoma by age 76.
Xeroderma pigmentosa is a rare hereditary disorder also associated with melanoma; it is inherited in an autosomally recessive fashion.30 The fibroblasts in patients with this disease have a reduced or no ability to repair DNA that has been damaged by ultraviolet light.31 Consequently, multiple primary cutaneous malignancies, including melanoma, basal cell carcinoma, and squamous cell carcinoma, develop. Individuals are usually diagnosed with their first cancer prior to age 10. Unfortunately for patients with xeroderma pigmentosa, the relentless development of skin cancers—as well as other cancers—ultimately leads to death at an early age.
Congenital Nevi
Congenital melanocytic nevi (CMN) are present at birth or appear within the first 6 months of life32; an estimated 1% to 6% of children are born with CMN. The nevi are classified by size: small CMN measure less than1.5 mm in diameter and account for the majority of lesions; medium-sized CMN measure between 1.5 and 19.9 mm in diameter; and large CMN, which are also called giant congenital nevi, measure 20 mm or more. This large size can have significant cosmetic and psychosocial implications.28
Conversely, giant CMN carry an increased risk for melanoma, with an estimated 5% to 20% of patients with these lesions eventually experiencing cancer.33,34 Seventy percent of these individuals are diagnosed before age 10 years.35 Melanoma in the setting of giant CMN originates deep within the epidermis, thus potentially delaying the diagnosis and treatment.
Melanoma Classification
Several histologic subtypes of melanoma are recognized within the head and neck region. It is important to note that if data are corrected for other prognostic variables, such as tumor thickness and ulceration, melanoma subtype does not generally influence prognosis.36
Lentigo maligna (LM) represents intraepidermal or in situ melanoma. Histologically, it is often seen in the background of chronic sun damage. LM is the precursor to invasive lentigo malignant melanoma (LMM). The exact percentage of LMs that progress to invasive LMM remains unknown37; however, it is speculated that, if patients live long enough, all LMs will eventually progress to cancer. LM/LMM is most often found in the head and neck region. Traditionally, the subtype has been associated with older individuals, but the frequency in younger patients is increasing. The LM/LMM pattern warrants special comment, because this subtype is characterized by asymmetric, subclinical, and often extensive involvement of margins with atypical junctional melanocytic hyperplasia. Therefore, management with adequate wide margins can be challenging from both a functional and cosmetic standpoint. Additionally, amelanotic melanoma and invasive desmoplastic melanoma (see later) often arise within LM/LMM.
Desmoplastic-Neurotropic Melanoma
Desmoplastic melanoma (DM) describes a subtype composed of spindle cells, abundant collagen, and features resembling fibromas.38 Some lesions demonstrate a propensity for perineural and endoneural infiltration, which prompted further subclassification of a desmoplastic neurotropic melanoma (DNM) variant.39
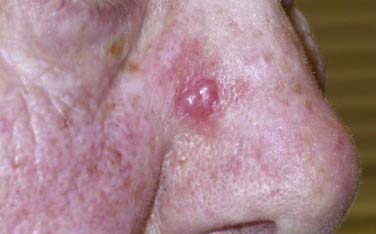
DM/DNM subtypes are rare, accounting for approximately 1% of all cutaneous melanomas.40 However, up to 75% of the lesions manifest in the head and neck region, often in the setting of LM/LMM. The clinical presentation and biologic behavior of these tumors are distinct from those of other cutaneous melanomas. Although amelanotic cases account for only 4% to 5% of cutaneous melanomas, up to 73% of DM/DNMs are amelanotic.40,41 As demonstrated in Figure 82-1, the neoplasms often lack the typical ABCD criteria for melanoma (described later), and they can have a difficult histologic pattern that requires interpretation by an experienced pathologist. Overall, the atypical and challenging appearance of DM/DNM is thought to result in a delay in diagnosis. This fact provides one explanation for the reason that patients with DM/DNM present with thicker, more aggressive tumors.
DM/DNM is known to be locally aggressive and highly infiltrative, often leading to cranial nerve and skull base involvement. Local recurrence has been reported in up to 50% of cases.40 Explanations for this high rate include the association with neurotropism and the failure to recognize and adequately clear peripheral atypical junctional melanocytic hyperplasia margins. Although DM/DNM demonstrates greater tumor thickness at the time of diagnosis, the 12.5% rate of regional lymph node metastasis is lower than that for other melanoma subtypes.39
Mucosal Melanoma
Mucosal melanoma is recognized as a distinct subtype from its cutaneous counterpart. The most common site for mucosal melanoma is the head and neck, with 40% to 50% of cases arising in this location.42 However, less than 2% of all head and neck melanomas are mucosal in origin. Of the 84,836 melanoma cases registered in the National Cancer Database from 1985 to 1994, only 1074 (1.3%) were of the mucosal melanoma variant.43
Although small case numbers limit research to anecdotal reports, several common trends have been noted throughout the literature. The peak age for mucosal melanoma is during the sixth to seventh decade, which is approximately 10 to 15 years later than that for cutaneous melanoma.42,44–47 A slight male preponderance has also been reported.45,47,49 Ethnic differences have been observed; approximately 8.8% of mucosal melanoma cases afflict blacks and Hispanics, a considerable percentage given that less than 3% of all other melanoma variants are diagnosed among this group.48 The relative frequency is also exceedingly high in Japan, where mucosal melanoma accounts for 7.5% of all melanoma cases.50
Within the head and neck region, the most common site of origin is the nasal cavity, where the anterior nasal septum is most often involved, followed by the inferior and middle turbinates.46,49,51,52 The second most common site is the oral cavity, where a predilection for the hard palate and maxillary alveolar gingivae has been found. A review of five major mucosal melanoma series by Batsakis and colleagues51 found laryngeal primary tumors to account for less than 4% of all cases. Within the larynx, the supraglottis was the most common site of origin.
The presenting signs and symptoms of mucosal melanoma directly correlate with the anatomic site of origin. The majority of patients with nasal cavity primary tumors present with nasal obstruction and epistaxis.49,51,52 Proptosis, diplopia, pain, and facial deformity are less common and indicate advanced disease. When present, the most common sign of an oral cavity tumor is a mass lesion.52 However, oral cavity mucosal melanoma is often asymptomatic, going undiagnosed until a neck mass develops from metastasis.44,49
Many of the prognostic markers for cutaneous melanoma do not have correlates in mucosal sites.42 Consequently, the cutaneous melanoma staging system set forth by the AJCC (see later) may be less applicable to mucosal melanoma. Most patients with mucosal melanoma present with localized disease, and in only 18.7% is regional spread detected at the time of diagnosis. The high percentage of patients with stage I disease is deceiving, because local recurrence is the major reason for treatment failure.51 Fifty percent of patients have local recurrence, usually within 9 to 12 months of diagnosis, and the overall 5-year survival rate is a dismal 10% to 20%.45–4751 Rich submucosal vasculature and lymphatics are thought to account for the aggressive behavior of mucosal melanoma.51
Unknown Primary
Approximately 2% to 8% of cases of melanoma involve unknown primary sites.43,48,53 In two thirds of the cases, the patients present with regional metastasis in the absence of an identifiable primary lesion or history of melanoma; the remaining third of unknown primary cases involve distant metastasis to sites such as the subcutaneous tissues, lung, and brain.48,53–55 Several theories have been proposed to explain melanoma of unknown primary origin. The identification of melanocytes and nevus cells within the lymph node capsule and visceral organ epithelium lends credence to the belief that unknown primary cases result from melanoma arising de novo at regional and distant sites.43,56 Another clinical scenario is that the primary melanoma lesion undergoes complete spontaneous regression.56,57
The diagnosis of melanoma of unknown origin necessitates a search for the primary site with a total body skin and mucosal evaluation. A history of a previous skin biopsy or a skin lesion that spontaneously disappeared may be helpful. All pathology slides from previously excised lesions should be re-reviewed. The metastatic workup is identical to that for known primary cases (see later). After adjustment of data for tumor stage, melanomas of unknown primary origin share an overall prognosis equivalent to their counterparts with known primary sites.43,53–55
Diagnostic Workup
History
The majority of melanoma lesions are first detected by the patient or his/her significant other.58,59 Less than one fourth of lesions are diagnosed during routine office physical examination, and these latter lesions tend to be thinner.59 Overall, 80% of newly diagnosed melanomas are limited to localized, stage I/II disease.2
The earliest signs of melanoma are a change in color, size, or shape of a lesion, and pruritus is the earliest symptom. Later signs and symptoms, which are usually associated with a more advanced lesion, include bleeding, ulceration, and tenderness. Patients should be questioned about a personal and family history of melanoma. Information about previous skin biopsies, sun exposure, history of blistering sunburns, use of tanning booths, chronic sun exposure, and occupation should be obtained. Schwartz and colleagues59 investigated characteristics of 1515 patients with melanoma and found that 81% elicited a history of at least one sunburn.
Physical Examination
All patients presenting with a suspicious lesion warrant full evaluation of the skin and nodal basins by a physician who is well versed in cutaneous cancers. Thorough evaluation is imperative because up to 8% of newly diagnosed patients have multiple primary cutaneous melanomas.16,60–62
The differential diagnosis for cutaneous melanoma is broad and includes seborrheic keratosis, hemangioma, blue nevus, Spitz nevus, pyogenic granuloma, pigmented basal cell carcinoma, and cutaneous squamous cell carcinoma. The American Cancer Society has published the ABCD checklist to educate both patients and physicians about the early detection of melanoma.63 Under these guidelines, concerning signs for melanoma are as follows:
Although the ABCD checklist is helpful for the identification of melanoma, it does not detect every case.64,65 It is important to realize that a subset of cancers (e.g., amelanotic, desmoplastic, and nodular melanoma) lack the common features of the ABCD list. In one series, 88% patients with melanoma (615 of 696) recalled change in their pigmented lesions prior to melanoma diagnosis.66 Owing to the significance of change, a proposal has been set forth to add “Evolving changes” to the traditional ABCD warning signs.63 Clinicians are hopeful that the new, more comprehensive ABCDE criteria will lead to even further detection of melanoma at an earlier stage.66 Another useful screening tool is the “ugly duckling sign,”67 whereby any pigmented lesion that appears significantly and singularly different from other surrounding lesions should be viewed with a high index of suspicion, even if the lesion lacks the traditional ABCD criteria.
Biopsy
The pathology results from this biopsy then serve as the guide for the second step, which entails wide local excision (WLE) utilizing a 0.5- to 2-cm margin of normal surrounding skin, with or without sentinel lymph node [mapping and] biopsy (SLNB). The role of SLNB ultimately depends on the final microstaging of the primary lesion and is detailed later. Although obtaining wider margins at the time of the initial biopsy seems efficient and cost effective, it is highly discouraged because clinical accuracy is uncertain and removal of significant amounts of skin surrounding the lesion may compromise the ability to accurately stage regional lymph node basins by means of lymphoscintigraphy and SLNB techniques.68 Instead, biopsy with narrow margins in a two-step fashion is advocated.
Metastatic Workup
In an attempt to standardize staging workup for melanoma, a panel of the National Comprehensive Cancer Network (NCCN) has published guidelines.69 The metastatic workup performed at our institution, which accords with these guidelines, is summarized in Table 82-1.
Table 82-1 University of Michigan Guidelines for Workup for Cutaneous Melanoma
| Stage 0 (in situ) | History; physical examination |
| Stage I (<1 mm thickness) | History; physical examination |
| Stage I to II (1-4 mm thick) | History; physical examination |
| Chest radiograph optional | |
| Serum acetate dehydrogenase measurement optional | |
| Stage III (N0; >4 mm thick) | History; physical examination |
| Chest radiograph optional | |
| Serum lactate dehydrogenase measurement optional | |
| Stage III (N+; in transit) | History; physical examination |
| Chest radiograph | |
| Serum lactate dehydrogenase measurement | |
| Other imaging studies, if clinically indicated | |
| Stage IV (distant metastasis) | History; physical examination |
| Chest radiograph | |
| Serum lactate dehydrogenase measurement | |
| Other imaging studies, per clinical trial |
Adapted from Johnson TM, Bradford CR, Gruber SB, et al. Staging work-up, sentinel node biopsy and follow-up tests for melanoma: update of current concepts. Arch Dermatol. 2004;140:107.
Patients presenting with localized stage I disease simply require a thorough history and physical examination. Many physicians advocate a screening chest radiograph, because the lung is the most common site for distant metastasis.68 Although a chest radiograph is an inexpensive, noninvasive means of metastatic evaluation, the rate of detection of occult pulmonary metastasis on chest radiograph in an asymptomatic patient with stage I or II disease is exceedingly low (0.1%).70,71 The high false-positive rate, 15%, necessitates additional and costly evaluations. Evidence supporting the use of other screening modalities, such as computed tomography (CT),72,73 liver-spleen scans, magnetic resonance imaging, and bone scans for patients with limited stage I and II disease is also lacking.74,75 For this reason, the National Comprehensive Cancer Network panel unanimously agreed that a search for visceral metastases, with either a chest radiograph or blood analysis, is not warranted for a patient with in situ or thin, stage IA lesions. A baseline chest radiograph is considered optional for thicker stage IB and II lesions. Imaging studies (CT, magnetic resonance imaging, positron emission tomography) may be obtained in this setting if there are specific concerning signs or symptoms.69 Screening serum lactate dehydrogenase (LDH) measurement carries a 15% false-positive rate, does not correlate with sentinel lymph node (SLN) status, and has not been helpful in detecting occult disease in asymptomatic patients.76 For this reason, our institution recommends LDH measurement only when the history or physical examination reveals jaundice, abdominal pain, or other specific findings that raise concern about distant metastasis.77
Patients with clinically or radiographically suspicious lymph nodes, satellite lesions, or in-transit metastasis (defined as intralymphatic tumor dissemination between the primacy lesion and draining nodal basin) are considered to have stage III disease and are at higher risk for distant metastasis. Fine-needle aspiration is an accurate and cost-effective means of confirming metastatic melanoma within lymph nodes.78 Additional imaging studies should be ordered according to the patient’s history and physical findings. A list of symptoms that warrant a focused investigation of systemic metastasis is summarized in Box 82-2.79 Overall, the yield of screening CT in patients with stage III melanoma has been low77,80–82; however, many physicians obtain the studies to serve as a baseline for future comparison.
Box 82-2 Review of Systems for Melanoma and Metastasis
Adapted from Johnson TM, Chang A, Redman B, et al. Management of melanoma with a multidisciplinary melanoma clinic model. J Am Acad Dermatol. 2000;42:820.
Patients with known stage IV disseminated melanoma require a complete evaluation for systemic metastasis. LDH measurements and chest radiography or chest CT are recommended. CT of the abdomen and pelvis, magnetic resonance imaging of the head, and a positron emission tomography scan (whole body) should also be considered. In this setting, workup is often dictated by clinical trial protocols. Unfortunately, greater survival has not been demonstrated for patients who are asymptomatic when diagnosed with their distant, stage IV disease than for their symptomatic counterparts76; however, this thorough evaluation may lead to a better quality of life.
Prognostic Factors and Tumor Staging
As a result of a greater understanding of the biology of cutaneous melanoma, the AJCC introduced a revised staging system in 2001.5,83 The goal of modifying the classification system was twofold. The main objective was to develop categories based on the most important independent prognostic markers for melanoma. Doing so may make it easier to identify cohorts of patients who have similar survival rates, thereby leading to increased homogeneity in future clinical trials. To achieve this goal, reliable prognostic markers recognized throughout the literature were studied among a cohort of 17,600 patients with melanoma from 13 major cancer institutions.83 This investigation marks the largest analysis of its kind to date. The second goal was to provide physicians with a practical classification system that mirrors clinical practice.
Summary of Revisions
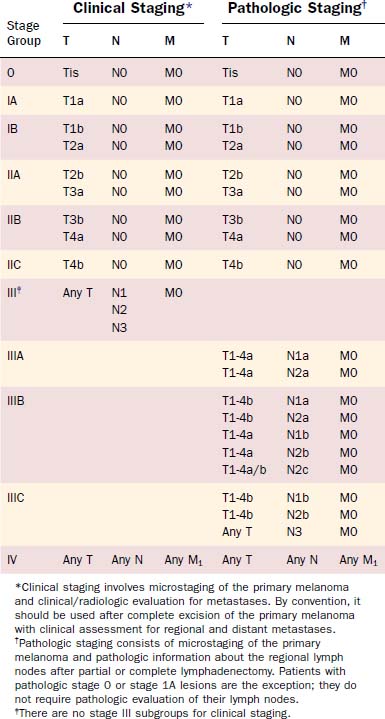
The current AJCC staging system for cutaneous melanoma remains founded upon the traditional tumor, node, metastasis (TNM) classification system (Tables 82-2 and 82-3). Stage I and stage II represent localized disease, stage III is regional disease, and stage IV is reserved for distant metastatic disease. The most important predictors for survival now serve as criteria for the definition of melanoma stage and are summarized in Box 82-3. Major revisions of the staging system are highlighted in Box 82-4.
| T Classification | Thickness (mm) | Ulceration Status |
|---|---|---|
| T1 | ≤ 1.0 mm |
| N Classification | No. of Metastatic Nodes | Nodal Metastatic Mass |
|---|---|---|
| N1 | 1 node | |
| N2 | 2-3 nodes | |
| N3 | 4 or more metastatic nodes, or matted nodes, or in-transit metastasis(es)/satellite(s) with metastatic node(s) |
| M Classification | Site | Serum Lactate Dehydrogenase Level |
|---|---|---|
| M1a | Distant skin, subcutaneous, or nodal metastases | Normal |
| M1b | Lung metastases | Normal |
| M1c |
* Micrometastases are diagnosed after sentinel or elective lymphadenectomy.
† Macrometastases are defined as clinically detectable nodal metastases confirmed by therapeutic lymphadenectomy or when nodal metastasis exhibits gross extracapsular extension.
Box 82-4 Revisions to the American Joint Committee on Cancer Staging System
From Schmalbach CE, Johnson TM, Bradford CR. The management of head and neck melanoma. Curr Probl Surg. 2006;43:797.
T Classification: Localized Disease
Multivariate analysis of 13,581 patients with localized disease identified tumor thickness and ulceration as the most important predictors of outcome. Overall, tumor thickness was the most powerful prognostic indicator for this subgroup. The 1997 staging system had employed the empirically based Breslow depth of 0.75 mm to differentiate T1 from T2 lesions.84 The revised staging system now uses even-integer cut-points of 1.0, 2.0, and 4.0 mm to delineate T stage. These new cut-points represent the best statistical fit in the correlation of tumor thickness and survival.
In the previous staging system, histologic level of invasion, as represented by the Clark scale (Fig. 82-2), was also used in the definition of T classification. However, the analysis by Balch and associates83 found the histologic level of invasion to be prognostic only for thin T1 lesions. For tumors 1 mm or thinner without ulceration, invasion to Clark level II or III is considered T1a, whereas invasion into the reticular dermis (level IV or V) is considered T1b. With the exception of T1 lesions, Clark level of invasion is no longer used in staging melanoma.
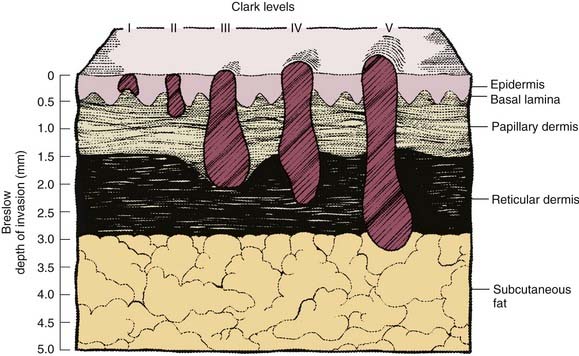
Figure 82-2. Schematic representation of cutaneous melanoma invasion according to Clark level and Breslow microstaging.
Tumor ulceration was the second most important prognostic indicator for patients with localized disease. Ulceration is not a visible crater on gross examination but, rather, a histologic diagnosis in which intact epidermis overlying the melanoma is absent. Survival rates were found to be significantly lower for all patients with ulcerated lesions than for their counterparts without ulceration. In fact, the survival of patients with ulcerated tumors mirrored that of patients with nonulcerated tumors in the next highest T categories; for this reason, ulceration warrants classification of a tumor to a higher stage. The prognostic significance of ulceration85 and the correlation between ulceration and mitotic rate have been reported.86,87 Although mitotic rate was not evaluated during the revision of the AJCC staging system, it may become an important prognostic marker in the future.
Nodal Classification: Regional Disease
Similar multivariate analysis was performed in more than 1000 patients with melanoma diagnosed with lymph node metastasis. The following three prognostic factors met statistical significance, at P < .0001: (1) number of metastatic lymph nodes, (2) tumor burden as represented by microscopic versus macroscopic disease, and (3) primary tumor ulceration. The gross diameter of metastatic nodes was previously used in N classification but is no longer incorporated into the staging system owing to a lack of compelling evidence supporting its predictive value.72,88
Overall, the most important predictive marker for patients with nodal metastasis was the number of positive lymph nodes.83 The greatest difference in 5-year survival rates was used to subclassify the N category. Patients with one metastatic node are now categorized as having N1 disease, patients with two or three metastatic nodes as having N2, and patients with four or more nodes as having N3.
The second most important prognostic indicator for patients with regional metastasis was tumor burden. Patients identified as having microscopic nodal disease, through either SLNB or elective lymphadenectomy, were found to have a significantly better survival than individuals diagnosed with macroscopic disease on the basis of clinical or radiographic examination.83 This difference was so compelling that microscopic versus macroscopic nodal disease is now subclassified within the N category. In addition, the AJCC Melanoma Committee strongly recommends staging with SLNB for patients with T2N0M0, T3N0M0, and T4N0M0 disease before entry into clinical trials.5 The identification of occult nodal disease enables accurate pathologic staging and greater homogeneity among cohorts under clinical investigation.
A fourth criterion now used in the definition of stage III regional disease is intralymphatic metastasis. The presence of satellite metastasis surrounding a primary lesion and in-transit metastasis identified between the primary melanoma and lymph node have been identified as poor prognostic indicators.72,89 Both findings portend a prognosis similar to that for nodal metastasis. Under the new staging system, satellite metastasis and in-transit metastasis are classified as N2c disease, in the absence of nodal disease (N0). If synchronous nodal metastasis is found in a patient with satellite or in-transit metastasis, prognosis is extremely poor. Patients with these findings are automatically classified a having N3 disease, irrespective of the number of synchronous positive lymph nodes.
Overall 5- and 10-year survival rates for patients with metastatic nodal disease were 49% and 37%, respectively.83 However, the range in survival for patients with stage III disease was significant, ranging from 13% for those with ulcerated primary lesion and macroscopic disease identified in four lymph nodes to 69% for patients with nonulcerated primary tumor and microscopic disease confined to one lymph node. This finding confirms previous studies demonstrating that patients with stage III disease are a heterogeneous group.88,90 In addition, it highlights the critical impact on survival afforded by the early diagnosis of regional metastasis.
M Stage: Distant Metastasis
Prognostic factors for disseminated stage IV disease were studied among 1158 patients; the most significant difference was found to be anatomic site of metastasis. Patients with distant metastasis to the skin, subcutaneous tissue, or distant lymph nodes (M1a) had a slightly higher survival rate than patients with lung metastasis (M1b). Patients with metastasis involving other visceral organs (M1c) had the worst prognosis. Previous studies have also identified elevated serum LDH as a marker of poor prognosis.91–94 For this reason, any patient with elevated serum LDH and distant metastasis, regardless of site, is classified as having M1c disease. Additional studies correlated male gender, more than one metastatic site, Karnofsky performance status less than 80, and limited disease-free survival with a poor prognosis.91,95 Overall, survival for patients with distant metastasis is extremely grave, measured in months as opposed to years. The median survival time following the diagnosis of disseminated disease is only 6 to 8 months, with a dismal 5-year survival rate of 6%.91,95 For this reason, stage IV melanoma is not subclassified under the AJCC staging system.
Anatomic Site
Although anatomic site is not used within the formal staging system, prognostic differences have been reported for melanoma.7,96 A review of 6300 cutaneous melanomas treated at the Duke Medical Center identified the worst 10-year survival rate among primary tumors of the head and neck (54%), compared with those of the trunk (61%), lower extremity (71%), and upper extremity (76%).96 A 50% tumor recurrence rate has been reported for head and neck cutaneous melanomas.97,98 This aggressive behavior is likely due to (1) thicker melanoma lesions in the head and neck region than in other sites and (2) inadequate removal of atypical junctional melanocytic hyperplasia at tumor margins, which leads to a higher rate of local recurrence.
Surgical Management of the Primary Tumor
Wide Local Excision and Surgical Margins
The standard of care for primary melanoma is complete surgical excision. However, the extent of surgical margins remains an unanswered question despite numerous retrospective studies, clinical trials, and meta-analyses. Historically, WLE included an extensive, 5-cm surrounding margin of normal tissue, a recommendation based on a 1907 autopsy report of a patient with advanced melanoma.99 The use of 5-cm surgical margins was routine practice until the 1970s, when Breslow and Macht100 challenged the concept by successfully treating 35 patients with thin melanomas through the use of narrower margins.
Two prospective, randomized trials investigating surgical margins for cutaneous melanoma have since followed. The World Health Organization (WHO) sponsored an international trial in which 612 patients with thin melanomas (2 mm) were randomly assigned to surgical excision with either 1-cm margins or margins larger than 3 cm.101 At a mean follow-up of 8 years, disease-free survival and overall survival were reported to be equivalent in the two groups. For this reason, the researchers in this study concluded that wide excision did not influence survival for patients with thin melanomas. For patients with melanomas less than1 mm in thickness, they advocated “narrow,” 1-cm margins to the muscular fascia plane.
Within the WHO trial, a subset of 245 patients had tumors measuring 1.1 to 2.0 mm in thickness. Although a difference in disease-free survival and overall survival was not observed with respect to margins, a local recurrence rate of 3.3% was reported among patients undergoing “narrow” excision. This finding prompted the Intergroup Melanoma Surgical Trial, which prospectively randomly assigned 740 patients with intermediate-thickness (1- to 4-mm) melanomas to WLE with either 2-cm or 4-cm margins.102 Local recurrence rates and 10-year survival were reported to be equivalent in the two groups. This finding led to the recommendation of a 2-cm surgical margin for patients with intermediate-thickness melanomas—those measuring 1.1 to 4.0 mm in.
The latest prospective clinical trial, conducted by the United Kingdom Melanoma Study Group, randomly assigned 900 patients with localized cutaneous melanomas 2 mm or more in thickness to excision with 1-cm or 3-cm margins.103 A statistically significant difference was not identified between the two groups when local, regional, and distant recurrences were compared; overall mortality rates were identical in the two groups. However, when all recurrences (local, in-transit, and nodal) were pooled together, the 1-cm margin group had a statistically higher recurrence rate. This is the first clinical trial comparing tumor margins to report a statistically significant difference in tumor recurrence. From a practical standpoint; however, it is the 1-cm versus 2-cm margin that is debated more often in this clinical setting.104
To date, there is no prospective randomized trial to address the optimal surgical margin for thick (> 4 mm) melanomas. A retrospective study of 278 thick melanomas found that surgical margins greater than 2 cm did not lead to a difference in rates of local recurrence rate, disease-free survival, or overall survival from rates for surgical margins of 2 cm.104 Within this study, 16% of the tumors involved head and neck subsites.
The primary goal of melanoma excision is to eliminate local recurrence secondary to persistent disease. The rate of local recurrence from narrow-margin excisions is admittedly low; however, the consequences are potentially fatal. It is estimated that 100% achievement of ideal margins would lead to a reduction in melanoma-related mortality and a 0.4-year increase in life expectancy for patients with melanoma.105 Although this difference appears small at first glance, it equates to an estimated 11 additional years of life expectancy for patients who would have experienced local recurrence after excision with a 1-cm surgical margin but who instead experienced a disease-free state after excision with a wider surgical margin.
Current guidelines for surgical margins are based on primary tumor thickness (Table 82-4). It is important to realize that these recommendations serve only as guidelines; choices for each case of melanoma must be individualized. The depth of excision includes full-thickness skin and underlying subcutaneous tissue. Resection of fascia, perichondrium, and periosteum is required only in the setting of direct tumor invasion or if the surgical plane was violated during a previous biopsy.106
Table 82-4 Recommended Surgical Margins for Excision of Primary Cutaneous Melanoma
| Tumor Thickness (mm) | Surgical Margin (cm) |
|---|---|
| In situ | 0.5 |
| <1.0 | 1.0 |
| 1.01-2.0 | 1.0-2.0 |
| >2.0 | 2.0 |
LMM warrants special comment, because it has a propensity for wide subclinical spread, which often results in pathologically positive margins.107 Johnson and coworkers108 and Anderson and colleagues36 have reported on the use of the “square” procedure in the treatment of LM and LMM. This staged procedure entails complete excision of the peripheral margin with permanent-section histologic evaluation of 100% of the peripheral margins surrounding the entire tumor.
Closure and Reconstruction
With the use of judicious undermining, the majority of surgical sites can be closed primarily. Larger defects may require reconstruction with a split-thickness skin graft, a full-thickness skin graft, a local advancement flap, a regional flap, or free tissue transfer. The method of reconstruction depends on anatomic location, skin color and texture, depth of defect, and patient and surgeon preference. Initially surgeons were reluctant to use grafts at excision sites for fear that surveillance within the surgical bed would be hindered and future diagnosis of recurrence delayed. However, the method of closure has not been shown to affect survival.109 After “clear” margins have been confirmed, surgeons are encouraged to close surgical defects using the technique that they believe will yield the best cosmetic result.
Melanoma of the Ear
Originally, melanoma involving the ear was thought to carry a worse prognosis than that in other head and neck sites.98,110,111 This increased risk was attributed to rich lymphatics, complex anatomic subdivisions of the auricle, and a paucity of subcutaneous tissue between the thin auricular skin and underlying perichondrium.112 Consequently, full-thickness excision or total auriculectomy was often advocated.
After accounting for tumor thickness, later studies demonstrated that melanoma of the ear carries the same prognosis as that in other sites.68,113 In addition, outcome differences were not observed between auricular subsites.112 Retrospective reviews did not demonstrate a difference in local recurrence based on the extent of surgical excision, even when perichondrium was preserved.113 Today, the same prognostic indicators and surgical principles of obtaining wide, clear margins for treatment of cutaneous melanoma can be safely applied to the auricular region.
Surgical Management of Regional Lymph Nodes
Therapeutic Lymph Node Dissection
The most common sites for metastasis of head and neck cutaneous melanoma are the cervical and parotid lymph node basins.114–116 The treatment of choice for regional disease remains a therapeutic lymph node dissection (TLND), which includes draining nodal basins as well as all intervening lymphatics between the primary tumor and the site of regional disease.
The location of the primary tumor dictates the type of TLND and the need for a superficial parotidectomy. Melanomas of the anterolateral scalp, temple, lateral forehead, lateral cheek, and ear, arising anterior to an imaginary coronal plane through the external auditory canals, drain via the parotid nodal basin to the jugular lymph node chain.117 For this reason, a superficial parotidectomy and modified radical neck dissection are both recommended. In the absence of gross tumor involvement or disruption from open biopsy or previous surgical dissection, concerted efforts should be made to preserve the spinal accessory nerve, internal jugular vein, and sternocleidomastoid muscle.117 If the melanoma arises in a more inferior location (e.g., the chin or neck), a superficial parotidectomy is not warranted. Melanomas located on the scalp and occiput—posterior to the imaginary coronal plane through the external auditory canals—can drain to postauricular, suboccipital, and posterior triangle lymph nodes. These nodal basins are not addressed during routine modified radical neck dissection and necessitate a posterolateral neck dissection that includes levels II through V as well as the preceding nodes.118
Elective Lymph Node Dissection
One of the most controversial historic debates about melanoma surrounded the treatment of regional nodal basins in the absence of clinical metastasis (prophylactic treatment of the N0 neck).114,116 Opponents of elective lymph node dissection (ELND) contend that melanoma metastasis is unpredictable. In Fisher’s9 retrospective review of 1444 head and neck patients with melanoma, 16% had distant metastasis in the absence of regional disease. This potential for hematogenous melanoma spread to bypass regional nodal basins theoretically limits the utility of an ELND. Opponents further argue that all four prospective, randomized trials detailed here failed to demonstrate an overall survival benefit for patients undergoing ELND in the absence of regional metastasis.119–124
The WHO Melanoma Group conducted the first prospective, randomized trial (Trial No.1) between 1967 and 1974, in which 535 patients with stage I and II melanoma of the extremity were enrolled.123 A survival difference was not found between patients who underwent WLE and observation, with TLND reserved for the development of gross nodal metastasis, and patients who underwent WLE and ELND. Similarly, surgeons at the Mayo Clinic randomly assigned 171 patients with stage I disease to (1) WLE and observation, (2) WLE and delayed (30 to 60 days) ELND, or (3) WLE with concomitant ELND.122 ELND was not found to provide a survival benefit over observation.
Although both prospective trials represent pioneering research in a challenging area, Balch114 criticized both study designs. At the time of the studies, the prognostic significance of tumor thickness and ulceration was unknown. Later analysis of WHO Melanoma Group Trial No. 1 found significant discrepancy in the distribution of tumor thickness between the two treatment arms. Furthermore, 52% of lesions in the ELND group were ulceration compared with only 19% in the observation group.114 Subsequent reanalysis of these data with respect to tumor thickness and ulceration identified a subset of patients with a 22% better 10-year survival in the setting of ELND. The accuracy of clinical staging within the trial was also questioned because several institutions reported a 30% rate of occult nodal metastasis. This rate is quite high in comparison with rates reported in the literature, which range from 10% to 20%. Lastly, Balch114 argued that the failure to detect a survival benefit with ELND was not surprising, given that both trials included patients with an overall low risk for regional metastasis at the time of diagnosis. Approximately 85% of patients enrolled in WHO Melanoma Group Trial No. 1 were women with extremity melanomas, which are recognized to have a lower rate of metastasis than melanomas at other sites. In addition, the Mayo Clinic study excluded patients with head and neck and midline trunk melanomas.
In order to address these concerns, the Intergroup Melanoma Surgical Trial was initiated.119 This prospective, multi-institutional study involved 740 patients with intermediate tumor thickness (1 to 4 mm) melanomas of the trunk, extremity, or head and neck region. Patients were once again randomly assigned to undergo either WLE and observation or WLE and ELND. Cox regression analysis of the results identified ulceration, site, tumor thickness, and age as independent markers for survival. Overall 5-year survival rates were not found to differ in the two treatment groups. However, a significant survival benefit was found in patients 60 years of age and younger who underwent ELND, especially if their tumors were nonulcerated or measured 1 to 2 mm in thickness. Although this subgroup analysis is criticized for the shortcomings of a retrospective review and the failure to randomize by age, patients were prospectively randomized according to tumor thickness and ulceration.
The fourth prospective trial, initiated in 1982 by the WHO Melanoma Group (Trial No. 14), attempted to study patients truly at high risk for occult nodal metastasis.121 It enrolled 240 patients with trunk melanomas measuring 1.5 mm or more in thickness. A survival difference was not observed between patients randomly assigned to observation and those who underwent ELND. In a multivariate analysis that included sex, age, tumor thickness, and treatment, only sex and tumor thickness were found to have a significant impact on survival. The study did identify a statistically significant 5-year survival difference between patients in whom micrometastasis identified during ELND (47%) and patients in the observation arm who underwent TLND only after the development of gross nodal disease (27%). For this reason, the WHO Melanoma Group advocated early detection of nodal metastasis using procedures such as SLNB (see later).
Statistical power remains one of the greatest challenges in investigating the survival benefits of ELND and early detection of nodal disease in melanoma patients presenting with localized disease in clinical trials conducted to date.125 Only 20% of patients with melanoma presenting with localized disease actually have occult nodal metastasis. It is this 20% who would potentially benefit from early removal of nodal basins. Adjuvant melanoma therapy imparts a survival benefit in 25% to 50% of cases. If a similar survival benefit is applied to the ELND group, 25% to 50% of the 20% of patients with occult disease should benefit. In other words, only 5% to 10% of patients undergoing ELND are expected to experience a survival advantage. Detecting this small difference requires an extremely large clinical trial with enrollment numbering in the thousands. McMasters and colleagues125 point out that the Intergroup Melanoma Surgical Trial, WHO trial, and Mayo Clinic study lacked adequate statistical power to detect this small survival benefit. For this reason, these researchers concluded that the 4% survival benefit observed in the ELND group of the Intergroup Melanoma Surgical Trial study is clinically significant, despite the fact that statistical significance was not reached.
In summary, numerous prospective, randomized trials failed to demonstrate an overall survival benefit for patients undergoing ELND.110,119,121,123,125,126 Therefore, routine ELND is no longer advocated for melanoma. Instead, the procedure has been replaced by SLNB.
Sentinel Lymph Node Biopsy
Sentinel lymph node mapping and biopsy represents a minimally invasive, cost-effective, and efficient means of screening patients for regional metastasis.127 Nodal status is currently recognized as the most important prognostic factor for patients with melanoma.5 Ten percent to 20% of such patients have occult microscopic nodal disease. In an attempt to identify this small group of patients who need TLND while sparing the remaining 80% of patients without regional disease the morbidity associated with a neck dissection, Morton and colleagues126 introduced SLNB for the evaluation of patients with trunk and extremity cutaneous melanoma. The investigators demonstrated that the status of the SLN accurately represented the status of the entire nodal basin from which it was obtained. SLNB is considered the best staging modality for regional disease, with the highest sensitivity and specificity of any modality currently available. In a multivariate model using data from 910 patients with melanoma, Peak and associates128 identified an association between SLN positivity and increasing Breslow depth, younger age, higher mitotic rate, angiolymphatic invasion, and location on the trunk/lower extremities. The indications for SLNB are summarized in Box 82-5. Patients who have metastatic disease or who have undergone previous surgical disruption of the lymphatics or resection with wide margins are not deemed candidates for this screening.
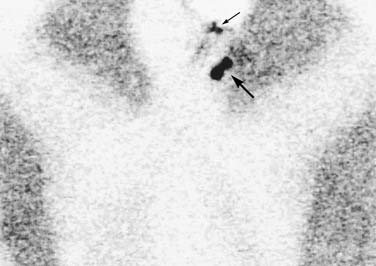
The SLN technique introduced by Morton and others has since evolved to include preoperative lymphoscintigraphy.129 Approximately 2 to 4 hours before surgery, patients undergo intradermal injection of a radioactive colloid into the four quadrants surrounding the primary melanoma tumor. Lymphoscintigraphy is then performed. This nuclear medicine scan enables the surgeon to determine the number, location, and laterality of nodal basins at risk for metastatic disease (Fig. 82-3). It is particularly helpful in the setting of midline head and neck melanomas, for which there is a risk of bilateral lymphatic drainage.
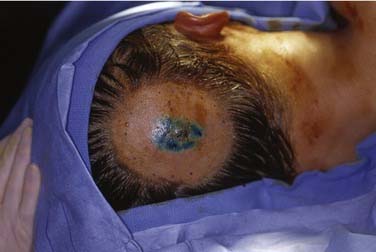
Once the patient is under anesthesia, intraoperative lymphatic mapping with isosulfan blue dye (Lymphazurin 1%; Covidien, Mansfield, MA) or methylene blue dye is performed.126 Approximately 1 mL of dye is injected into the intradermal layer surrounding the primary melanoma lesion (Fig. 82-4). The primary tumor and draining lymphatics are in close proximity within the head and neck region. Therefore, WLE of the primary tumor is performed first to reduce radioactive “shine-through,” which would render the intraoperative gamma probe useless for the identification of SLNs (Fig. 82-5) Shine-through refers to radioactive counts attributable to the primary site injection rather than sentinel lymph nodes.
Following WLE of the primary melanoma, the surgeon uses handheld gamma probe to identify the site(s) of sentinel lymph node(s). The procedure of identifying and removing sentinel lymph nodes continues until the gamma counts per minute (cpm) in the lymph node basin are equal or less than 10% of the “hottest” sentinel lymph node removed. We advocate this 10% rule because our data suggest that for surgeons using radioactive colloid who routinely terminate the procedure after identifying only the hottest node, or when the cpm values for nodes drop below one-half to one-third the cpm of the hottest node, the false-negative rates are higher.130 The SLNs are removed with the use of 1- to 3-cm incisions overlying the areas of increased radioactivity. A preauricular incision is recommended for SLNB in the parotid region. SLNs are then identified through the use of a combination of the gamma probe and visual cues from the blue dye (see Fig. 82-5). Each SLN is individually dissected from surrounding tissue.
At our institution, all SLNs are sent for histologic evaluation using permanent sections. Frozen sections are less reliable, carrying a false-negative rate between 5% and 10%.131 The evaluation involves serial sectioning (5-µm thick sections) and staining with hematoxylin and eosin. Special melanoma immunohistochemical staining for S-100 protein and melan-A (MART-1 [melanoma antigen recognized by T cells]) are performed for all SLNs with negative response to hematoxylin and eosin staining. This panel was chosen after pathologic evaluation of 99 positive SLNs from 72 patients treated at our institution.132 The sensitivities for S-100, melan-A, and HMB-45 monoclonal antibody were found to be 97%, 96%, and 75%, respectively. In addition, HMB-45 stained a smaller percentage of cells (25% to 75%), with weaker intensity compared with S-100 and melan-A; for this reason, we no longer routinely stain for HMB-45.
Patients with a positive SLN result are returned to the operating room within 2 weeks of diagnosis for definitive TLND; patients with a negative result are followed clinically. An alternative to this two-stage technique is immediate TLND on the basis of frozen-section evaluation of the SLNs. However, it is important to realize that the reliability of frozen sections for melanoma analysis has been questioned,133 and permanent sections remain the “gold standard.”
The pathologist plays an extremely critical role in the success of SLNB. Occult lymphatic metastasis from cutaneous melanoma can be difficult to detect and warrants rigorous pathologic analysis, including serial sectioning, special immunohistochemical study when indicated, and interpretation by an experienced pathologist. Wagner and associates134 reported the mean tumor volume in SLNs positive for metastatic melanoma to be only 4.7 mm3. Joseph and colleagues66 reported the identification of only 73% of metastatic SLNs with the use of standard hematoxylin and eosin staining alone. In our study, 20 of the 97 positive SLNs (21%) tested negative on initial hematoxylin and eosin staining.132 This high false-negative rate highlights the importance of immunohistochemical staining for accurate diagnosis of occult nodal disease.
The histologic analysis of SLNs is more thorough, complete, and practical than the traditional evaluation of the entire lymphadenectomy specimen, because the technique provides the pathologist with a limited number of nodes to evaluate thoroughly.135 SLNB is particularly helpful in the diagnosis of occult DM/DNM. For this subtype, a focused histopathologic evaluation is of particular importance because the microscopic features are quite variable, often lack resemblance to the primary tumor, are limited to a paucity of tumor cells, and demonstrate inconsistencies on HMB-45 and melan-A staining.136
SLNB is a team effort involving experienced surgeons, nuclear medicine staff, and pathologists. The experience and technical skill of the surgeon is vital. Morton and associates127 previously suggested a 30-case learning curve. However, long-term follow-up of their international Multicenter Selective Lymphadenectomy Trial (MSLT-1) found the 30-case learning curve to be too shallow. Analysis of the first 25 cases performed at the 10 highest-volume centers in the trial revealed a nodal basin recurrence rate of 10.3%.131 This false-negative rate dropped to 5.2% after 25 additional cases. The researchers now conclude that a 55-case learning curve is required in order to achieve at least 95% accuracy with SLNB.
A multivariate analysis involving patients with stage I and II melanoma by Gershenwald and coworkers137 found the pathologic status (positive or negative for metastasis) of the SLN to be the most important prognostic factor for both recurrence and overall survival. For patients with stage III melanoma, survival was better in those with occult microscopic disease than in those with palpable macroscopic disease.5 This survival difference was so compelling that the AJCC has now incorporated SLNB into the revised staging system for cutaneous melanoma.
Although SLNB has a defined role in the evaluation of cutaneous melanoma of the trunk and extremities, several questions have been posed with respect to its application in the head and neck region.127 The complexity of the head and neck lymphatic system has caused concern about the reliability of the SLNB to accurately reflect the status of the entire nodal basin. The interlacing network of cervical lymphatic vessels is often deemed to be watershed in nature. The complexity of this lymphatic system was demonstrated by O’Brien and colleagues,138 who reported a 34% discordance between the clinical prediction of lymphatic drainage and lymphoscintigraphy findings in 97 cases of head and neck cutaneous melanoma. The popularity of SLNB in the head and neck region has also been limited by concern surrounding damage to vital structures such as the facial nerve,139 technical difficulties,139 and the necessity for nuclear medicine staff as well as pathologists who specialize in SLNB technique.
Our experience in staging 80 cases of head and neck cutaneous melanoma demonstrated that the complexity of the head and neck anatomy does not preclude the use of SLNB for the staging of cutaneous melanoma.140 SLNB in the head and neck region accurately predicted the status of the nodal basin. Fourteen of 80 patients (17.5%) had a positive sentinel lymph node. Of 66 (4.5%) patients with negative SLNB results, only 3 experienced regional recurrence. The 17.5% positivity rate of SLNs and the 4.5% false-negative rate both mirror the results that SLNB achieved in other anatomic sites, such as the trunk and extremities.135,141 Similar success in the application of SLNB for head and neck cutaneous melanoma has been reported by others,142,143 and the technique has been applied successfully in pediatric head and neck cases.13
Approximately 25% to 30% of head and neck cutaneous melanomas drain to lymph nodes within the parotid bed.138,140 Potential injury to the facial nerve from SLN biopsy has led some surgeons to advocate superficial parotidectomy over the mapping procedure.139 In our retrospective analysis, staging with SNLB was successful in 28 of 30 (93.3%) patients whose tumors were draining to the parotid nodal basin.140 One patient required a superficial parotidectomy owing to the location of the SLN deep to the facial nerve, and a second patient experienced significant bleeding from surrounding parotid tissue, which could have put the facial nerve at increased risk. A total of 39 nodes from the parotid basins of these 28 patients were removed without facial nerve injury. Continuous facial nerve monitoring for SLNB within the parotid nodal basin can be helpful when the biopsy is performed within the parotid bed.
Concern has also been expressed that SLNB causes inflammation and fibrosis that could place the facial nerve at increased risk when reoperation is required to definitively treat the parotid basin in the setting of a pathologically positive SLN.139 In our experience, all patients with a positive parotid SLN underwent superficial parotidectomy as a subsequent procedure without facial nerve injury. Our findings are consistent with those of other reports demonstrating that SLNB can reliably and safely be performed within the parotid nodal basin.144,145
Other researchers have suggested that SLNB increases the risk of in-transit metastasis (ITM), which is defined as intralymphatic tumor dissemination within cutaneous or subcutaneous tissue located between the primary lesion and draining nodal basin.146–150 It is theorized that ITM develops when melanoma cells detach from the primary lesion and become lodged in the dermal plexus of lymphatics prior to reaching the lymph nodes. ITM presents a therapeutic challenge and carries a poor prognosis, as indicated by the changes to the AJCC staging system previously described.5 Original reports citing increased ITM following SLNB must by viewed with caution, because the studies often entailed pooled data from small cohorts and failed to control for important prognostic factors such as tumor thickness and ulceration.61,148 A later prospective review comparing 4412 patients undergoing WLE alone, WLE with SLNB, or ELND identified a correlation between ITM and increasing Breslow depth, Clark level, and T stage.61 A statistically significant difference between ITM and tumor recurrence was not found among the treatment groups, once adjustment of data was made for T stage, age, sex, tumor thickness, and site. Additional studies have concluded that it is the tumor biology, rather than the surgical procedure (SLNB; ELND), which dictates melanoma metastatic behavior.148,151 Finally, a correlation between ITM and SLNB was not reported with MSLT-1, thus negating this concern.131
The impact of SLNB on overall survival remains to be determined. The answer will hopefully be provided through the multi-institutional Sunbelt Melanoma Trial, which is a prospective, randomized clinical trial that utilizes SLN staging to determine the need for adjuvant therapy.152 While we await these results, McMasters and colleagues153 outline the following four compelling reasons to use of SLNB for accurate regional staging of cutaneous melanoma.
The fifth and final analysis of MSLT-I will provide additional insight into the potential therapeutic benefit of SLNB.131 Publication of the MSLT-I interim analysis is exciting in that it is the first randomized, prospective trial to demonstrate that SLNB accurately identifies occult nodal metastasis that would lead to advanced, palpable nodal disease if left in situ. The researchers argue that there is no reason not to perform SLNB because a zero mortality rate was reported, and the complication rate for SLNB (10%) was significantly lower than that for TLND (37%). Morton and colleagues131 concluded that SLNB should be considered the standard of care for regional staging of primary cutaneous melanoma—the key term being “staging.” At present SLNB is a diagnostic tool for regional staging, not a therapeutic modality. Although it is not 100% accurate, SLNB is the most reliable means for regional staging. It is more sensitive and specific than CT, magnetic resonance imaging, positron emission tomography, ELND, and clinical examination.154 McMasters astutely points out that we do not expect a survival benefit from any other cancer staging test, and therefore we should not ask the same of SLNB.
Future Investigations
SLNB research endeavors hold exciting and great promise for the future. In an effort to further investigate the therapeutic potential of SLNB, research efforts have focused on identifying markers of both the primary lesion and SLN, which are predictive of tumor-containing non-SLNs.155–157 Absence of these markers would then allow identification of the subset of patients with positive SLNB results, in whom further treatment with a formal TLND may not be warranted. Unfortunately, current studies have not identified a consistent and 100% accurate marker. For this reason, the MSLT-II trial, currently under way, is designed to investigate the indications for TLND following a positive SLNB result. Specifically, it will determine whether immediate TLND provides a survival benefit over diligent postoperative, ultrasonographic monitoring of the draining nodal basins.131
Molecular staging of melanoma is also gaining increased interest. Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of SLNs for melanoma-associated genes such as MART-1, tyrosinase, microphthalmia-associated transcription factor (MIFT), and tyrosinase-related protein 2 (TRP-2) has proved helpful in identifying a subset of patients who have occult nodal disease at a submicroscopic level, which cannot be detected with traditional immunohistochemical staining.84,158,159 In one study, in 30% (49 of 162) of patients in whom results of immunohistochemical staining of SLNs were negative, at least one melanoma marker was identified with RT-PCR.160 This subset of patients experienced a higher rate of tumor recurrence. Currently, RT-PCR lacks specificity. The reason for the high false-positive rate may be the inability of RT-PCR to differentiate melanoma cells from occult benign nevus cells.16 Through future research efforts, molecular staging may prove helpful in the identification of a subset of high-risk patients with melanoma in whom nodal or distant metastases develop despite presentation with a thin primary tumor. This information has the potential to change the manner in which these individuals are counseled both with respect to adjuvant therapy and follow-up surveillance.65
Surgical Management of Distant Metastasis
Patients with stage IV melanoma involving distant sites have an exceedingly grave prognosis. Surgical treatment has a limited role in the setting of disseminated disease. It has successfully been used as a means of palliative treatment in patients suffering from brain, lung, gastrointestinal, subcutaneous soft tissue, and distant lymph node metastasis.161 Success of surgery in the palliative setting depends heavily on appropriate patient selection; it should be considered only if clearly identifiable and specific symptoms are associated with a metastatic lesion. Other considerations are surgical morbidity, expected quality of life, expected survival, and, most important, the patient’s wishes.162 It is imperative that the patient and his or her family understand that the goal of surgery is palliative in nature.
Several prognostic markers have been identified in patients with disseminated stage IV melanoma.162,163 These markers are reflected in the AJCC staging system previously described above and should serve as a guide for the consideration of surgical resection of distant tumors. Patients with metastatic disease limited to one or two isolated sites experience a better prognosis as than patients who have multiple metastatic lesions. A short disease-free interval is associated with an overall poor prognosis. Patients in whom distant metastasis develops within a year of initial diagnosis have a poor prognosis, even when complete resection of the metastatic lesion is achieved.162 Lastly, the anatomic site of the metastatic lesion is of importance. Patients with metastatic spread to nonvisceral sites (e.g., distant subcutaneous tissues, lymph nodes) have a better prognosis than those with visceral metastasis. Within the group of patients suffering from visceral metastasis, individuals with pulmonary lesions experience better survival than those with lesions at other visceral sites.
Radiation Therapy
Melanoma has traditionally been classified as a radioresistant tumor.8,164 Although adjuvant irradiation has not been shown to have an impact on survival,165 researchers at the MD Anderson Cancer Center completed a phase II clinical trial supporting the efficacy of hypofractionated radiation as an adjuvant to surgery for patients with head and neck cutaneous melanoma at high risk for local/regional recurrence.166–168 Local/regional control was achieved in 88% of patients, which is an improvement over the historic control rates of 50% to 70%.10,169 Late complications of irradiation were rare (3 of 174 patients) and included moderate neck fibrosis, mild ipsilateral hearing loss, and transient exposure of external auditory canal cartilage. Local/regional control is important, because recurrence can significantly affect quality of life by causing pain, wound breakdown, and socially debilitating cosmetic disfigurement.170,171 However, no overall survival benefit has been demonstrated.
Ultimately the survival benefit achieved with adjuvant radiation therapy will be determined through the prospective, randomized phase III clinical trial (E3697) currently being conducted by the Radiation Therapy Oncology Group.170 Until this trial is completed, most authorities advocate the use of adjuvant irradiation for patients demonstrating adverse prognostic markers such as neurotropism, extracapsular spread, multiple node involvement (>4), or recurrence.172–174 These patients are often eligible to receive adjuvant interferon-α 2b (see later), which is thought to act as a radiosensitizer. Therefore, it is common practice to delay radiation therapy until the 4-week induction phase of interferon therapy is complete.175
On very rare occasions, primary irradiation can be used to treat extensive LM or LMM in an elderly patient who is not deemed a surgical candidate.176 Radiation therapy can also be administered as palliative treatment in patients suffering from systemic stage IV disease, especially in the setting of brain metastasis, bone metastasis, spinal cord compression, or isolated, symptomatic visceral metastasis.2
Chemotherapy
Melanoma is a relatively chemoresistant tumor.177–179 A small subset of patients is thought to benefit from chemotherapy; however, a regimen shown definitively to have an impact on survival has not emerged. The main role of chemotherapy remains as palliative treatment in the setting of disseminated stage IV disease.
Dacarbazine (DTIC) is currently the only chemotherapeutic agent approved for the treatment of advanced stage IV melanoma. Response rates following DTIC administration are modest at best, ranging from 10% to 20%.8,87,180–182 This prognosis has not changed over the past 20 years, despite dedicated research efforts using a host of chemotherapeutic agent regimens.178 Overall, less than 5% of individuals with stage IV melanoma experience a complete response with DTIC therapy.
Immunotherapy
Classically, immunotherapy is divided into two categories. Specific immunotherapeutic agents up-regulate the antibody and cytotoxic T-cell immune responses specifically to the patient’s tumor or to known melanoma antigens; the majority of melanoma vaccines fall into this category. By contrast, nonspecific immunotherapeutic agents such as interferon, interleukin, and microbacterial products stimulate the host’s immune system without targeting melanoma tumor antigens. These agents are often administered in concert with specific immunotherapeutic agents in an attempt to augment the immune system.175,182
Interferon
Despite myriad clinical trials involving adjuvant regimens, high-dose interferon-α 2b remains the only adjuvant treatment approved by the U.S. Food and Drug Administration (FDA) for stage III melanoma. It functions as a biologic response modifier; the mechanism of action includes direct antiproliferative effects, immune stimulation through the enhancement of natural killer cells, increased histocompatibility antigen expression on melanoma cells, macrophage phagocytosis, and enhanced T-cell–mediated cytotoxicity.107,183 Although all three types of interferon (α, β, and γ) demonstrate antitumor activity,183 Interferon-α 2b is the only treatment that is currently approved for the adjuvant treatment of patients with melanoma at high risk for recurrence after surgery.
Three large clinical trials involving adjuvant interferon-α 2b have been conducted by the Eastern Cooperative Oncology Group (ECOG).184–186 In brief, ECOG trial E1684 was the first study to demonstrate the efficacy of interferon-α 2b.184 The regimen consisted of high-dose interferon (20 million units/m2/day) administered intravenously, 5 days a week, for 4 weeks. Maintenance treatment followed, consisting of 48 weeks of subcutaneous interferon-α 2b (10 MU/m2/day) administered 3 days a week. The prolonged disease-free survival rate and overall survival rate in the interferon-α 2b arm of E1684 ultimately led to FDA approval of the adjuvant therapy.
Although the follow-up trial E1690 did not confirm the efficacy of high-dose interferon-α 2b,185 the results require careful interpretation.153 Unlike in E1684, patients enrolled in E1690 did not require pathologic staging with ELND or SLNB, nor were they stratified according to ulceration. In addition, a disproportionate number of individuals from the observation arm crossed over into the interferon-α 2b arm to receive salvage therapy for recurrent disease. Any therapeutic benefit provided to this subgroup by interferon-α 2b went unrecognized, because the crossover patients remained in the observation arm for the purposes of statistical analysis.
The latest and largest of the three studies, ECOG 1694,186 once again confirmed the efficacy of high-dose interferon-α 2b. In fact, the difference between rates of relapse-free and overall survival observed in the high-dose interferon-α 2b control arm in the GMK ganglioside vaccine treatment arm was so compelling that the Data Safety Monitoring Committee terminated the trial early.
With close follow-up, dose modification, and pharmacologic intervention, the majority of patients with melanoma are able to tolerate the 1-year course of interferon-α 2b.153,175,185,186 Almost all individuals experience flulike symptoms (fevers, chills, malaise) during the initial treatment course. Severe and intolerable chronic fatigue occurs in 20% to 30% of patients, and an additional 2% to 10% experience neurologic and psychiatric side effects, including depression, anxiety, suicidal ideation, and difficulty with cognition. Myelosuppression, thyroid dysfunction, and elevated liver enzyme values require close monitoring. Contraindications include a history of myocardial infarction or dysrhythmia, liver disease, central nervous system disorders, and severe psychiatric illness.175 Approximately 50% of patients require a dose reduction or delay as a result of these side effects. The majority of patients with melanoma are willing to accept these side effects, given the potential benefit of interferon-α 2b.187
Although clinical trials continue to investigate alternative dosages and schedules,188,189 high-dose interferon-α 2b remains the only proven adjuvant treatment for melanoma, with relapse-free survival prolonged by approximately 10%.190 It remains the standard of care for adjuvant therapy against which all interventions must be compared, ideally in the setting of prospective, randomized trials.191 Every patient with regional lymph node metastasis or a primary tumor thickness greater than 4 mm should be informed of the option to receive postoperative interferon-α 2b. To enable the patient to make an educated decision, the surgeon must have an objective discussion about the side effects of interferon-α 2b and the opportunities to enroll in other clinical trials.
Interleukin-2 and Other Cytokines
Interleukin-2 (IL-2) is another form of immunotherapy that is used in the primary treatment of disseminated stage IV disease. Unlike interferon, IL-2 lacks direct antitumor activity and in vitro activity.107 However, in vivo, IL-2 stimulates the host’s immune system by activating effector cells such as natural killer cells, monocytes, cytotoxic T cells, and helper T cells. It also induces cytokines such as interferon-γ and tumor necrosis factor-α.107,192
Rosenberg and colleagues,193 of the Surgery Branch of the National Cancer Institute, successfully used high-dose IL-2 to treat 134 patients with melanoma. An overall response was observed in 17% of patients; 10% experienced a partial response, and an additional 7% had complete regression. The therapeutic benefit was substantial, lasting between 2 and 8 years.
The side effects of IL-2 are significant and potentially lethal. Acute toxic effects include myocardial infarction, arrhythmia, respiratory distress, hypotension, capillary leak syndrome, nephrotoxicity, hepatic toxicity, and sepsis.192,194 Others are anemia, thrombocytopenia, nausea, emesis, diarrhea, myalgia/arthralgia, skin erythema, and pruritus. Only patients who demonstrate excellent cardiopulmonary health and performance status should be considered for clinical trials involving IL-2.
Subsequent efforts to enhance the response to IL-2 by altering the schedule and dose and by combining it with other therapeutic agents (e.g., lymphokine-activated killer cells) have not proved beneficial.107,182,192,194 In addition to IL-2, other cytokines have been studied alone and in combination with various chemotherapeutic agents, including IL-1, IL-4, IL-6, and tumor necrosis factor-α.192 Significant therapeutic benefit has not been demonstrated for any.
Future Immunotherapy Investigations
Significant advances in the field of melanoma research have been achieved in recent years.175,182,183,195,196 Intense efforts in the areas of melanoma vaccination, gene therapy, and HLA immunoprinting will likely play important roles in future research endeavors. Until therapeutic efficacy achieves that of interferon-α 2b, all other adjuvant agents should be administered to high-risk patients only in the setting of well-designed clinical trials. Given the heterogeneous melanoma patient population, myriad immunotherapy regimens, and the limited survival of high-risk patients with melanoma, multi-institutional studies with accurate clinical staging will be imperative.
Follow-Up and Surveillance
The primary goals of melanoma follow-up are the early detection of local/regional tumor recurrence, the early identification of second primary tumors (including melanoma and other skin cancers), continuing patient education, and psychological support.197 Each follow-up visit should include an inquiry into new or changing skin lesions. A review of systems (see Box 82-2) as they relate to distant metastasis should be performed.79 A thorough examination of the skin and mucosa is required, with particular attention paid to the original melanoma site and associated draining nodal basins. Each follow-up visit should be viewed as an opportunity to reducate patients about the ABCD melanoma warning signs and the importance of monthly skin self-examination. Education about sun exposure—including using sunscreen, avoiding peak sunlight hours, seeking shade, wearing protective clothing, and the potential risks of tanning booths—should be emphasized.
Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622.
Balch CM, Buzaid AC, Soong SJ. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635.
Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902.
Fisher SR. Elective, therapeutic, and delayed lymph node dissection for malignant melanoma of the head and neck: analysis of 1444 patients from 1970 to 1998. Laryngoscope. 2002;112:99.
Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976.
Johnson TM, Bradford CR, Bruber SB, et al. Staging work-up, sentinel node biopsy and follow-up tests for melanoma: update of current concepts. Arch Dermatol. 2004;140:107.
Kang S, Barnhill RL, Mihm MCJr, et al. Multiple primary cutaneous melanoma. Cancer. 1992;70:1911.
Kennedy C, Bajdik CD, Willemze R, et al. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003;120:1087.
MacKie RM. Clinical recognition of early invasive melanoma. BMJ. 1990;301:1005.
McMasters KM, Noyes RD, Reintgen DS, et al. Sunbelt Melanoma Trial. Lessons learned from the Sunbelt Melanoma Trial. J Surg Oncol. 2004;86:212.
McMasters KM, Reintgen DS, Ross MI, et al. Sentinel lymph node biopsy for melanoma: controversy despite widespread agreement. J Clin Oncol. 2001;19:2851.
Morton DL, Davtyan DG, Wanek LA, et al. Multivariate analysis of the relationship between survival and the microstage of primary melanoma by Clark level and Breslow thickness. Cancer. 1993;71:3737.
Morton DL, Cochran AJ, Thompson JF, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307.
Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma accuracy and morbidity in MSLT-I: an international multicenter trial. Ann Surg. 2005;242:302.
Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma. Ann Surg. 1999;230:453.
Moschos SJ, Kirkwood JM. Present status and future prospects for adjuvant therapy of melanoma: time to build upon the foundation of high dose interferon alfa-2b. J Clin Oncol. 2004;22:11.
2002 National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology—v. 1. 2008: Melanoma. Rockledge PA: NCCN, Inc., 2002. Updates available at www.nccn.org
Ostmeier Ostmeier H, Fuchs B, Otto F, et al. Can immunohistochemical markers and mitotic rate improve prognostic precision in patients with primary melanoma? Cancer. 1999;85:2391.
Paek SC, Griffith KA, Johnson TM, et al. The impact of factors beyond Breslow depth on predicting sentinel lymph node positivity in melanoma. Cancer. 2007;109:100.
Reintgen D, Pendas S, Jakub J. National trials involving lymphatic mapping for melanoma: the Multicenter Selective Lymphadenectomy Trial, The Sunbelt Melanoma Trial, And The Florida Melanoma Trial. Semin Oncol. 2004;31:363.
Rigel Rigel DS. The effect of sunscreen on melanoma risk. Dermatol Clin. 2002;20:601.
Rigel DS, Carucci JA. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA Cancer J Clin. 2000;50:215.
Sabel MS, Strecher VJ, Schwartz JL, et al. Predictors of nonsentinel lymph node positivity in patients with a positive sentinel node for melanoma. J Am Coll Surg. 2005;201:37.
Schmalbach CE, Nussenbaum B, Rees RS, et al. Reliability of sentinel lymph node mapping with biopsy for head and neck cutaneous melanoma. Arch Otolaryngol Head Neck Surg. 2003;129:61.
Schmalbach CE, Johnson TM, Bradford CR. The management of head and neck melanoma. Curr Probl Surg. 2006;43:771.
Terhune Terhune MH, Swanson N, Johnson TM. Use of chest radiography in the initial evaluation of patients with localized melanoma. Arch Dermatol. 1998;134:569.
Thomas Thomas JM, Clark MA. Sentinel lymph node biopsy: not yet standard of care for melanoma. BJM. 2004;329:170.
1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71.
2. National Cancer Institutes. Surveillance, Epidemiology and End Results. Available at http://seer.cancer.gov/statfacts/html/melan.html, 2008.
3. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43.
4. Tsao H, Rogers GS, Sober AJ. An estimate of the annual direct cost of treating cutaneous melanoma. J Am Acad Dermatol. 1998;38:669.
5. Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635.
6. Rigel DS, Carucci JA. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA Cancer J Clin. 2000;50:215.
7. Fisher SR, O’Brien CJ. Head and neck melanoma. In: Balch CM, Houghton AN, Sober AJ, Soong SJ, editors. Cutaneous Melanoma. St. Louis: Quality Medical Publishing, 1998.
8. Lentsch EJ, Myers JN. Melanoma of the head and neck: current concepts in diagnosis and management. Laryngoscope. 2001;111:1209.
9. Fisher SR. Elective, therapeutic, and delayed lymph node dissection for malignant melanoma of the head and neck: analysis of 1444 patients from 1970 to 1998. Laryngoscope. 2002;112:99.
10. O’Brien CJ, Coates AS, Petersen-Schaefer K, et al. Experience with 998 cutaneous melanomas of the head and neck over 30 years. Am J Surg. 1991;162:310.
11. Peralta EA, Yarington T, Glenn MG. Malignant melanoma of the head and neck: effect of treatment on survival. Laryngoscope. 1998;108:220.
12. Fisher SR, Reintgen DS, Seigler HF. Juvenile malignant melanoma of the head and neck. Laryngoscope. 1988;98:184.
13. Pacella SJ, Lowe L, Bradford C. The utility of sentinel lymph node biopsy in head and neck melanoma in the pediatric population. Plast Reconstr Surg. 2003;112:1257.
14. Tucker MA, Goldstein AM. Melanoma etiology: where are we? Oncogene. 2003;22:3042.
15. Berwick M. Current trends, risk factors, and environmental cancers. In: Balch CM, Houghton AN, Sober AJ, Soong SJ, editors. Cutaneous Melanoma. St. Louis: Quality Medical Publishing, 1998.
16. Johnson TM, Hamilton T, Lowe L. Multiple primary melanomas. J Am Acad Dermatol. 1998;39:422.
17. Rigel DS. Identification of those at highest risk for development of malignant melanoma. Adv Dermatol. 1995;10:151.
18. Fitzpatrick TB, Ortonne JP. Normal skin color and general considerations of pigmentary disorders. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz AI, editors. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw-Hill, 2003.
19. Kraehn GM, Schartl M, Peter RU. Human malignant melanoma: a genetic disease? Cancer. 1995;75:1228.
20. Piepkorn K. Melanoma genetics: an update with focus on the CDKN2A(p16)/ARF tumor suppressors. J Am Acad Dermatol. 2000;42:705.
21. Kamb A, Gruis NA, Weaver-Feldhaus J, et al. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264:436.
22. Aitken J, Welch J, Duffy D, et al. CDKN2A variants in a population-based sample of Queensland families with melanoma. J Natl Cancer Inst. 1999;91:446.
23. Clark WHJr, Reimer RR, Greene MH, et al. Origin of familial malignant melanoma from heritable melanocytic lesions: the B-K mole syndrome. Arch Dermatol. 1978;114:732.
24. Lynch HT, Frichot BC, Lynch JF. Familial atypical multiple mole-melanoma syndrome. J Med Genetics. 1978;15:352.
25. Greene MH, Goldin LR, Clark WHJr, et al. Familial cutaneous malignant melanoma: autosomal dominant trait possibly lined to the Rh locus. Proc Natl Acad Sci U S A. 1983;80:6071.
26. Tsao H, Sober AJ. Atypical melanocytic nevi. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz AI, editors. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw-Hill, 2003.
27. Marghoob AA, Kopf AW, Rigel DS, et al. Risk of cutaneous malignant melanoma in patients with “classic” atypical mole syndrome: a case-control study. Arch Dermatol. 1994;130:993.
28. Marghoob AA. Congenital melanocytic nevi: evaluation and management. Dermatol Clin. 2002;20:4.
29. Greene MH, Clark WHJr, Tucker MA, et al. High risk of malignant melanoma in melanoma-prone families with dysplastic nevi. Ann Intern Med. 1985;102:458.
30. Kraemer KH, Levy DD, Parris CN, et al. Xeroderma pigmentosum and related disorders: examining the linkage between defective DNA repair and cancer. J Invest Dermatol. 1994;103:96.
31. Cleaver JE. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968;218:652.
32. Rhodes AR, Albert LS, Weinstock MA. Congenital nevomelanocytic nevi: proportionate area expansion during infancy and early childhood. J Am Acad Dermatol. 1996;34:51.
33. Bittencourt FV, Marghoob AA, Kopf AW, et al. Large congenital melanocytic nevi and the risk of developing malignant melanoma and neurocutaneous melanocytosis. Pediatrics. 2000;106:736.
34. Consensus Conference: Precursors to malignant melanoma. JAMA. 1984;251:1864.
35. Marghoob AA, Schoenbach SP, Kopf AW, et al. Large congenital melanocytic nevi and the risk of developing malignant melanoma: a prospective study and review of the world literature. J Invest Dermatol. 1995;104:563.
36. Anderson KW, Baker SR, Lowe L, et al. Treatment of head and neck melanoma, lentigo maligna subtype, a practical surgical technique. Arch Facial Plast Surg. 2001;3:202.
37. Cohen LM. Lentigo maligna and lentigo maligna melanoma. J Dermatol. 1995;33:923.
38. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of spindle cell melanoma). Cancer. 1971;28:914.
39. Reed JG, Leonard DD. Neurotropic melanoma: a variant of desmoplastic melanoma. Am J Surg Pathol. 1979;3:310.
40. Carlson JA, Dickersin GR, Sober AJ, et al. Desmoplastic neurotropic melanoma, a clinicopathologic analysis of 28 cases. Cancer. 1995;75:478.
41. Quinn MJ, Crotty KA, Thompson JG, et al. Desmoplastic and desmoplastic neurotropic melanoma: experience with 280 patients. Cancer. 1998;83:1128.
42. Ross MI, Stern SJ. Mucosal melanomas. In: Balch CM, Houghton AN, Sober AJ, Soong SJ, editors. Cutaneous Melanoma. St. Louis: Quality Medical Publishing, 1998.
43. Chang AE, Karnell LH, Menck HR. The National Cancer Database report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664.
44. Berthelsen A, Andersen AP, Jensen TS, et al. Melanomas of the mucosa in the oral cavity and the upper respiratory passages. Cancer. 1984;54:907.
45. Hoyt DJ, Jordan T, Fisher SR. Mucosal melanoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1989;115:1096.
46. Manolidis S, Donald PJ. Malignant mucosal melanoma of the head and neck: review of the literature and report of 14 patients. Cancer. 1997;80:1373.
47. Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24:247.
48. Chang P, Knapper WH. Metastatic melanoma of unknown primary. Cancer. 1982;49:1106.
49. Stern SJ, Guillamondegui OM. Mucosal melanoma of the head and neck. Head Neck. 1991;13:22.
50. Kato T, Takematsu H, Tomita Y, et al. Malignant melanoma of mucous membranes. Arch Dermatol. 1987;123:216.
51. Batsakis JG, Regezi JA, Solomon AR, et al. The pathology of head and neck tumors: mucosal melanoma, part 13. Head Neck Surg. 1982;4:404.
52. Lee SP, Shimizu KT, Tran LM, et al. Mucosal melanoma of the head and neck: the impact of local control on survival. Laryngoscope. 1994;104:121.
53. Norman J, Cruse CW, Wells KE, et al. Metastatic melanoma with an unknown primary. Ann Plast Surg. 1992;28:81.
54. Nasri S, Namazie A, Dulguerov P, et al. Malignant melanoma of cervical and parotid lymph nodes with an unknown primary site. Laryngoscope. 1994;104:1194.
55. Santini H, Byers RM, Wolf PF. Melanoma metastatic to cervical and parotid nodes from an unknown primary site. Am J Surg. 1985;150:510.
56. Jonk A, Kroon BBR, Rümke P, et al. Lymph node metastasis from melanoma with an unknown primary site. Br J Surg. 1990;77:665.
57. Bulkey GB, Cohen MH, Banks PM, et al. Long-term spontaneous regression of malignant melanoma with visceral metastasis: report of a case with immunologic profile. Cancer. 1975;36:485.
58. Koh HK, Miller DR, Geller AC, et al. Who discovers melanoma? Patterns from a population-based survey. J Am Acad Dermatol. 1992;26:914.
59. Schwartz JL, Wang TS, Hamilton TA, et al. Thin primary cutaneous melanomas: associated detection patterns, lesion characteristics, and patient characteristics. Cancer. 2002;95:1562.
60. Beardmore GL, Cavis NC. Multiple primary cutaneous melanomas. Arch Dermatol. 1975;111:603.
61. Kang JC, Wanek LA, Essner R, et al. Sentinel lymphadenectomy does not increase the incidence of in-transit metastases in primary melanoma. J Clin Oncol. 2005;23:4764.
62. Moseley HS, Giuliano AE, Storm FK, et al. Multiple primary melanoma. Cancer. 1979;43:939.
63. Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma. JAMA. 2004;292:2771-2776.
64. McGovern TW, Litaker MS. Clinical predictors of malignant pigmented lesions: a comparison of the Glasgow seven-point checklist and the American Cancer Society’s ABCDs of pigmented lesions. J Dermatol Surg Oncol. 1992;18:22.
65. Whited JD, Grichnik JM. Does this patient have a mole or a melanoma? JAMA. 1998;279:696.
66. Joseph E, Brobeil A, Glass F, et al. Results of complete lymph node dissection in 83 melanoma patients with positive sentinel nodes. Ann Surg Oncol. 1998;5:119.
67. Grob JJ. The ‘ugly ducking’ sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998;134:103.
68. Stadelmann WK, McMasters K, Digenis AG. Cutaneous melanoma of the head and neck: advances in evaluation and treatment. Plastic Reconstr Surg. 2000;5:2105.
69. National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology—v. 1.2008: Melanoma. Rockledge, PA: NCCN, Inc., 2002. Updates available at www.nccn.org
70. Ardizzoni A, Grimaldi A, Repetto L, et al. Stage I-II melanoma: the value of metastatic work-up. Oncology. 1987;44:87.
71. Khansur T, Sanders J, Das SK. Evaluation of staging workup in malignant melanoma. Arch Surg. 1989;124:847.
72. Buzaid AC, Ross MI, Balch CM, et al. Critical analysis of the current American Joint Committee on Cancer staging system for cutaneous melanoma and proposal of a new staging system. J Clin Oncol. 1997;15:1039.
73. Curtis AM, Ravin CE, Deering TF, et al. The efficacy of full-lung tomography in the detection of early metastatic disease from melanoma. Radiology. 1982;144:27.
74. Au FC, Maier WP, Malmud LS, et al. Preoperative nuclear scans in patients with melanoma. Cancer. 1984;53:2095.
75. Roth JA, Eilber FR, Bennett LR, et al. Radionuclide photoscanning: usefulness in preoperative evaluation of melanoma patients. Arch Surg. 1975;110:1211.
76. Johnson TM, Bradford CR, Bruber SB, et al. Staging work-up, sentinel node biopsy and follow-up tests for melanoma: update of current concepts. Arch Dermatol. 2004;140:107.
77. Wang TS, Johnson TM, Cascade PN, et al. Evaluation of staging chest radiographs and serum lactate dehydrogenase for localized melanoma. J Am Acad Dermatol. 2004;51:399.
78. Basler GC, Fader DJ, Yahanda A, et al. The utility of fine needle aspiration in the diagnosis of melanoma metastatic to lymph nodes. J Am Acad Dermatol. 1997;36:403.
79. Johnson TM, Chang A, Redman B, et al. Management of melanoma with a multidisciplinary melanoma clinic model. J Am Acad Dermatol. 2000;42:820.
80. Johnson TM, Fader DJ, Chang AE, et al. Computed tomography in staging patients with melanoma metastatic to regional nodes. Ann Surg Oncol. 1997;4:396.
81. Kuvshinoff BW, Kurtz C, Coit DG. Computed tomography in evaluation of patients with stage III melanoma. Ann Surg Oncol. 1997;4:252.
82. Buzaid AC, Sandler AB, Mani S, et al. Role of computed tomography in the staging of primary melanoma. J Clin Oncol. 1993;11:638.
83. Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622.
84. Bostick PJ, Morton DL, Turner RR, et al. Prognostic significance of occult metastases detected by sentinel lymphadenectomy and reverse transcriptase-polymerase chain reaction in early stage melanoma patients. J Clin Oncol. 1999;17:3238.
85. Balch CM, Wilkerson JA, Murad TM, et al. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980;45:3012.
86. Azzola MF, Shaw HM, Thompson JF. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer. 2003;97:1488.
87. McClay EF, McClay MT. Systemic chemotherapy for the treatment of metastatic melanoma. Semin Oncol. 1996;23:744.
88. Buzaid AC, Tinoco LA, Jendiroba D, et al. Prognostic value of size of lymph node metastases in patients with cutaneous melanoma. J Clin Oncol. 1995;13:2361.
89. Harrist T, Rigel D, Day CJr, et al. Microscopic satellites are more highly associated with regional lymph node metastases than with primary melanoma thickness. Cancer. 1984;53:2183.
90. Morton DL, Davtyan DG, Wanek LA, et al. Multivariate analysis of the relationship between survival and the microstage of primary melanoma by Clark level and Breslow thickness. Cancer. 1993;71:3737.
91. Brand CU, Ellwanger U, Stroebel W, et al. Prolonged survival of 2 years or longer for patients with disseminated melanoma, an analysis of related prognostic factors. Cancer. 1997;79:2345.
92. Deichmann M, Benner A, Bock M, et al. S100-Beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. J Clin Oncol. 1999;17:1891.
93. Eton O, Legha SS, Moon TE, et al. Prognostic factors for survival of patients treated systemically for disseminated melanoma. J Clin Oncol. 1998;16:1103.
94. Sirott MN, Bajorin DF, Wong GYC, et al. Prognostic factors in patients with metastatic malignant melanoma: a multivariate analysis. Cancer. 1993;72:3091.
95. Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181:193.
96. Garbe C, Büttner P, Bertz J, et al. Primary cutaneous melanoma: prognostic classification of anatomic location. Cancer. 1995;75:2492.
97. Fisher SR. Cutaneous malignant melanoma of the head and neck. Laryngoscope. 1989;99:822.
98. Fisher SR, Seigler HF, George SL. Therapeutic and prognostic considerations of head and neck melanoma. Ann Plast Surg. 1992;28:79.
99. Handley WS. The pathology of melanotic growths in relation to their operative treatment. Lancet. 1907;1:927.
100. Breslow A, Macht SD. Optimal size of resection for thin cutaneous melanoma. Surg Gynecol Obstet. 1977;145:691.
101. Veronesi U, Cascinelli N, Adamus J, et al. Thin stage I primary cutaneous malignant melanoma: comparison of excision with margins of 1 or 3 cm. N Engl J Med. 1988;318:1159.
102. Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1-4 mm melanomas. Ann Surg Oncol. 2001;8:101.
103. Johnson TM, Newton-Bishop J, A’Hern R, et al. United Kingdom Melanoma Study Group; British Association of Plastic Surgeons; Scottish Cancer Therapy Network. Excision margins in high-risk malignant melanoma. N Engl J Med. 2004;350:757-766.
104. Heaton KM, Sussman JJ, Gershenwald JE, et al. Surgical margins and prognostic factors in patients with thick (>4mm) primary melanomas. Ann Surg Oncol. 1998;5:322.
105. Barzilai DA, Singer ME. The potential impact on melanoma mortality of reducing rates of suboptimal excision margins. J Invest Dermatol. 2003;120:1067.
106. Kenady DE, Brown BE, McBride CM. Excision of underlying fascia with a primary malignant melanoma: effect on recurrence and survival rates. Surgery. 1982;92:615.
107. Johnson TM, Smith JWII, Nelson BR, et al. Current therapy for cutaneous melanoma. J Am Acad Dermatol. 1995;32:689.
108. Johnson TM, Headington JT, Baker SR, et al. Usefulness of the staged excision for lentigo maligna and lentigo maligna melanoma: the “square” procedure. J Am Acad Dermatol. 1997;37:758.
109. Lent WM, Ariyan S. Flap reconstruction following wide local excision for primary malignant melanoma of the head and neck region. Ann Plast Surg. 1994;33:23.
110. Urist MN, Balch CM, Soong S, et al. Head and neck melanoma in 534 clinical stage I patients. Ann Surg. 1984;200:769.
111. Wanebo HJ, Cooper PH, Young DV, et al. Prognostic factors in head and neck melanoma. Cancer. 1988;62:831.
112. Byers RM, Smith JL, Russell N, et al. Malignant melanoma of the external ear, review of 102 cases. Am J Surg. 1980;140:518.
113. Cole DJ, Mackay GJ, Walker BF, et al. Melanoma of the external ear. J Surg Oncol. 1992;50:110.
114. Balch CM. The role of elective lymph node dissection in melanoma: rationale, results, and controversies. J Clin Oncol. 1988;6:163.
115. Fisher SR. Elective, therapeutic, and delayed lymph node dissection for malignant melanoma of the head and neck: analysis of 1444 patients from 1970 to 1998. Laryngoscope. 2002;112:99.
116. Myers JN. Value of neck dissection in the treatment of patients with intermediate-thickness cutaneous malignant melanoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1999;25:110.
117. Byers RM. Treatment of the neck in melanoma. Otolaryngol Clin North Am. 1998;31:833.
118. Goepfert H, Jesse RH, Ballantyne AJ. Posterolateral neck dissection. Arch Otolaryngol. 1980;106:618.
119. Balch CM, Soong SJ, Bartolucci AA, et al. Efficacy of an elective regional lymph node dissection of 1 to 4 mm thick melanomas for patients 60 years of age and younger. Ann Surg. 1996;224:255.
120. Balch CM, Soong SJ, Ross MI, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm): Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7:87.
121. Cascinelli N, Morabito A, Santinami M, et al. Immediate or delayed dissection of regional node in patients with melanoma of the trunk: a randomized trial—WHO Melanoma Programme. Lancet. 1998;351:793.
122. Sim FH, Taylor WF, Ivins JC, et al. A prospective randomized study of the efficacy of routine elective lymphadenopathy in management of malignant melanoma. Cancer. 1978;41:948.
123. Veronesi U, Adamus J, Bandiera DC, et al. Inefficacy of immediate node dissection in stage I melanoma of the limbs. N Engl J Med. 1977;297:627.
124. Veronesi U, Adamus J, Bandiera DC, et al. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer. 1982;49:2420.
125. McMasters KM, Sondak VK, Lotze MT, et al. Recent advances in melanoma staging and therapy. Ann Surg Oncol. 1999;6:467.
126. Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Ann Surg. 1992;127:392.
127. Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma. Ann Surg. 1999;230:453.
128. Peak SC, Griffith KA, Johnson TM, et al. The impact of factors beyond Breslow depth on predicting sentinel lymph node positivity in melanoma. Cancer. 2007;109:100.
129. Uren RF, Howman-Giles RB, Shaw HM, et al. Lymphoscintigraphy in high risk melanoma of the trunk: predicting draining node groups, defining lymphatic channels and locating the sentinel node. J Nuc Med. 1993;34:1435.
130. Kroon HM, Lowe L, Wong S, et al. What is a sentinel node? Re-evaluating the 10% rule for sentinel lymph node biopsy in melanoma. J Surg Oncol. 2007;95:623.
131. Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;230:453.
132. Karimipour DJ, Lowe L, Su L, et al. Standard immunostains for melanoma in sentinel lymph node specimens: which ones are most useful? J Am Acad Dermatol. 2004;50:759.
133. Cohen LM, McCall MW, Hodge SJ. Successful treatment of lentigo maligna and lentigo maligna melanoma with Mohs micrographic surgery aided by rush permanents sections. Cancer. 1994;73:2964.
134. Wagner JD, Davidson D, Coleman JJIII, et al. Lymph node tumor volumes in patients undergoing sentinel lymph node biopsy for cutaneous melanoma. Ann Surg Oncol. 1999;6:398.
135. Cascinelli N, Belli F, Santinami M, et al. Sentinel lymph node biopsy in cutaneous melanoma: the WHO Melanoma Program experience. Ann Surg Oncol. 2000;7:469.
136. Su LD, Fullen DR, Lowe L, et al. Desmoplastic and neurotropic melanoma analysis of 33 patients with lymphatic mapping and sentinel lymph node biopsy. Cancer. 2004;100:598.
137. Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976.
138. O’Brien CJ, Uren RF, Thompson JF, et al. Prediction of potential metastatic sites in cutaneous head and neck melanoma using lymphoscintigraphy. Am J Surg. 1995;170:461.
139. Eicher SA, Clayman GL, Myers JN, et al. A prospective study of intraoperative lymphatic mapping for head and neck cutaneous melanoma. Arch Otolaryngol Head Neck Surg. 2002;128:241.
140. Schmalbach CE, Nussenbaum B, Rees RS, et al. Reliability of sentinel lymph node mapping with biopsy for head and neck cutaneous melanoma. Arch Otolaryngol Head Neck Surg. 2003;129:61.
141. Gershenwald JE, Colome MI, Lee JE, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol. 1998;16:2253.
142. Alex JC, Krag DN, Harlow SP, et al. Localization of regional lymph nodes in melanomas of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:135.
143. Patel SG, Coit DG, Shaha AR, et al. Sentinel lymph node biopsy for cutaneous head and neck melanomas. Arch Otolaryngol Head Neck Surg. 2002;128:285.
144. Ollila DW, Foshag LJ, Essner R, et al. Parotid region lymphatic mapping and sentinel lymphadenectomy for cutaneous melanoma. Ann Surg Oncol. 1999;6:150.
145. Wells KE, Stadelmann WK, Rapaport DP, et al. Parotid selective lymphadenectomy in malignant melanoma. Ann Plast Surg. 1999;43:1.
146. Estourgie SH, Nieweg OE, Valdes Olmos RA, et al. Review and evaluation of sentinel node procedures in 250 melanoma patients with a median follow-up of 6 years. Ann Surg Oncol. 2003;10:681.
147. Estourgie SH, Nieweg OE, Kroon BB. High incidence of in-transit metastases after sentinel node biopsy in patients with melanoma. Br J Surg. 2004;91:1370.
148. Pawlik T, Ross M, Johnson M, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol. 2005;12:587.
149. Thomas JM, Clark MA. Selective lymphadenectomy in sentinel node-positive patients may increase the risk of local/in-transit recurrence in malignant melanoma. Eur J Surg Oncol. 2004;30:686.
150. Thomas JM, Clark MA. Are there guidance issues relating to sentinel node biopsy for melanoma in the UK? Br J Plast Surg. 2004;57:689.
151. Van Poll D, Thompson JF, Colman MH, et al. A sentinel node biopsy does not increase the incidence of in-transit metastasis in patients with primary cutaneous melanoma. Ann Surg Oncol. 2005;12:597.
152. McMasters KM. The sunbelt melanoma trial. Ann Surg Oncol. 2001;8:41S.
153. McMasters KM, Reintgen DS, Ross MI, et al. Sentinel lymph node biopsy for melanoma: controversy despite widespread agreement. J Clin Oncol. 2001;19:2851.
154. McMasters KM. What good is sentinel lymph node biopsy for melanoma if it does not improve survival? Ann Surg Oncol. 2004;11:810.
155. Cochran AJ, Wen DR, Huang RR, et al. Prediction of metastatic melanoma in nonsentinel nodes and clinical outcome based on the primary melanoma and the sentinel node. Mod Pathol. 2004;17:747.
156. Lee JH, Essner R, Torisu-Itakure H, et al. Factors predictive of tumor-positive nonsentinel lymph nodes after tumor-positive sentinel lymph node dissection for melanoma. J Clin Oncol. 2004;22:3677.
157. Sabel MS, Strecher VJ, Schwartz JL, et al. Predictors of nonsentinel lymph node positivity in patients with a positive sentinel node for melanoma. J Am Coll Surg. 2005;201:37.
158. Ribuffo D, Gradilone A, Vonella M, et al. Prognostic significance of reverse transcriptase-polymerase chain reaction-negative sentinel nodes in malignant melanoma. Ann Surg Oncol. 2003;10:396.
159. Shivers S, Wang X, Li W, et al. Molecular staging of malignant melanoma: correlation with clinical outcome. JAMA. 1998;280:1310.
160. McMasters KM. Molecular staging of melanoma: sensitivity, specificity, and the search for clinical significance. Ann Surg Oncol. 2003;10:336.
161. Wornom IL3rd, Smith JW, Soong SJ, et al. Surgery as palliative treatment for distant metastases of melanoma. Ann Surg. 1986;204:181.
162. Allen PJ, Coit DG. The surgical management of metastatic melanoma. Ann Surg Oncol. 2002;9:762.
163. Balch CM. Surgical treatment of advanced melanoma. In: Balch CM, Houghton AN, Sober AJ, Soong SJ, editors. Cutaneous Melanoma. St. Louis: Quality Medical Publishing, 1998.
164. Trotti A, Peters LJ. The role of radiotherapy in the primary management of cutaneous melanoma. Ann Plast Surg. 1992;28:39.
165. Creagan ET, Cupps RE, Ivins JC, et al. Adjuvant radiation therapy for regional nodal metastases from malignant melanoma, a randomized prospective study. Cancer. 1978;42:2206.
166. Ang KK, Byers RM, Peters LJ, et al. Regional radiotherapy as adjuvant treatment for head and neck malignant melanoma. Arch Otolaryngol Head Neck Surg. 1990;116:169.
167. Ang KK, Peters LJ, Weber RS, et al. Postoperative radiotherapy for cutaneous melanoma of the head and neck region. Int J Radiat Oncol. 1994;30:795.
168. Ballo MT, Bonnen MD, Garden AS, et al. Adjuvant irradiation for cervical lymph node metastases from melanoma. Cancer. 2003;97:1789.
169. Byers RM. The role of modified neck dissection in the treatment of cutaneous melanoma of the head and neck. Arch Surg. 1986;121:1338.
170. Ridge JA. Adjuvant radiation after lymph node dissection for melanoma. Ann Surg Oncol. 2000;7:550.
171. Shen P, Wanek LA, Morton DL. Is adjuvant radiotherapy necessary after positive lymph node dissection in head and neck melanoma? Ann Surg Oncol. 2000;7:554.
172. Ang KK, Geara FB, Byers RM, et al. Radiotherapy for melanoma. In: Balch CM, Houghton AN, Sober AJ, Soong SJ, editors. Cutaneous Melanoma. St. Louis: Quality Medical Publishing, 1998.
173. O’Brien CJ, Petersen-Schaefer K, Stevens GN, et al. Adjuvant radiotherapy following neck dissection and parotidectomy for metastatic malignant melanoma. Head Neck. 1997;19:589.
174. Stevens G, Thompson JF, Firth I, et al. Locally advanced melanoma, results of postoperative hypofractionated radiation therapy. Cancer. 2000;88:88.
175. Terando A, Sabel MS, Sondak VK. Melanoma: adjuvant therapy and other treatments. Curr Treat Options Oncol. 2003;4:187.
176. Harwood AR. Conventional fractionated radiotherapy for 51 patients with lentigo maligna and lentigo maligna melanoma. Int J Radiation Oncol Biol Phys. 1983;9:1019.
177. Buzaid AC, Bedikian A, Houghton AN. Systemic chemotherapy and biochemotherapy. In: Balch CM, Houghton AN, Sober AJ, Soong SJ, editors. Cutaneous Melanoma. St. Louis: Quality Medical Publishing, Inc, 1998.
178. Lui P, Cashin R, Machado M, et al. Treatments for metastatic melanoma: synthesis of evidence from randomized trials. Cancer Treat Rev. 2007;33:665.
179. Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138.
180. Biasco G, Pantaleo MA, Casadei S. Treatment of brain metastases of malignant melanoma with temozolomide. N Engl J Med. 2001;345:621.
181. Hill GJ, Moss SE, Golomb FM, et al. DTIC and combination therapy for melanoma: III. DTIC (NSC 45388) Surgical Adjuvant study COG Protocol 7040. Cancer. 1981;47:2556.
182. Pawlik TM, Sondak VK. Malignant melanoma: current state of primary and adjuvant treatment. Clin Rev Oncology Heme. 2003;45:245.
183. Shaw PM, Sivanandham M, Bernik SF, et al. Adjuvant immunotherapy for patients with melanoma: are patients with melanoma of the head and neck candidates for this therapy? Head Neck. 1997;19:595.
184. Kirkwood JM, Strawderman MH, Ernstoff MC, et al. Interferon alpha-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7.
185. Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of Intergroup Trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444.
186. Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of Intergroup Trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370.
187. Kilbridge KL, Cole BF, Kirwood JM, et al. Quality-of-life-adjusted survival analysis of high-dose adjuvant interferon alpha-2b for high-risk melanoma patients using intergroup clinical trial data. J Clin Oncol. 2002;20:1311.
188. Cascinelli N, Belli F, MacKie RM, et al. Effect of long-term adjuvant therapy with interferon alpha-2a in patients with regional node metastases from cutaneous melanoma: a randomized trial. Lancet. 2001;358:866.
189. Hancock BW, Wheatley K, Harris S, et al. Adjuvant interferon in high-risk melanoma: the AIM HIGH study—United Kingdom Coordinating Committee on Cancer Research randomized study of adjuvant low-dose extended-duration interferon alfa-2a in high-risk resected malignant melanoma. J Clin Oncol. 2004;22:53.
190. Schuchter LM. Adjuvant interferon therapy for melanoma: high-dose, low-dose, no dose, which dose? J Clin Oncol. 2004;22:7.
191. Moschos SJ, Kirkwood JM. Present status and future prospects for adjuvant therapy of melanoma: time to build upon the foundation of high-dose interferon alfa-2b. J Clin Oncol. 2004;22:11.
192. Chapman PB, Parkinson DR, Kirkwood JM. Biologic Therapy. In: Balch CM, Houghton AN, Sober AJ, Soong SJ, editors. Cutaneous Melanoma. St. Louis: Quality Medical Publishing, 1998.
193. Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA. 1994;271:907.
194. Langley RGB, Barnhill RL, Mihm MC, et al. Neoplasms: cutaneous melanoma. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz AI, editors. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw-Hill, 2003.
195. Bittner M, Meltzer P, Chen Y, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536.
196. Myers JN. Adjuvant immunotherapy for patients with melanoma. Head Neck. 1998;20:270.
197. Trask PC, Paterson AG, Hayasaka S, et al. Psychosocial characteristics of individuals with non-stage IV melanoma. J Clin Oncol. 2001;19:2844.