Chapter 2
The Lungs and Chest Wall
After reading this chapter, you will be able to:
• Differentiate between the lobes and segments of the right and left lungs
• Explain why the pleural membranes normally have a subatmospheric pressure between them and the way in which this subatmospheric pressure is related to lung volume
• Describe why the systemic bronchial circulation in the lungs prevents alveolar gas and arterial blood oxygen pressures from being equal
• Explain why sympathetic stimulation, parasympathetic stimulation, and nonadrenergic noncholinergic nerve stimulation cause different effects in the lung
• List the neuronal effects a drug must have to elicit bronchodilation
• Describe the essential components of an effective cough and factors that impair cough effectiveness
• Identify which spinal cord levels correlate with diaphragmatic muscle function
• Discuss the assessment of abnormally high respiratory efforts by inspecting the chest
• Explain the functional difference between primary and accessory muscles of ventilation
The Lungs as Organs
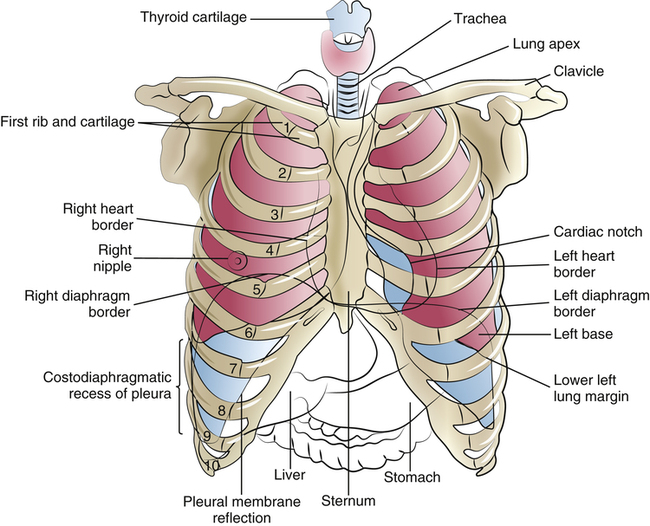
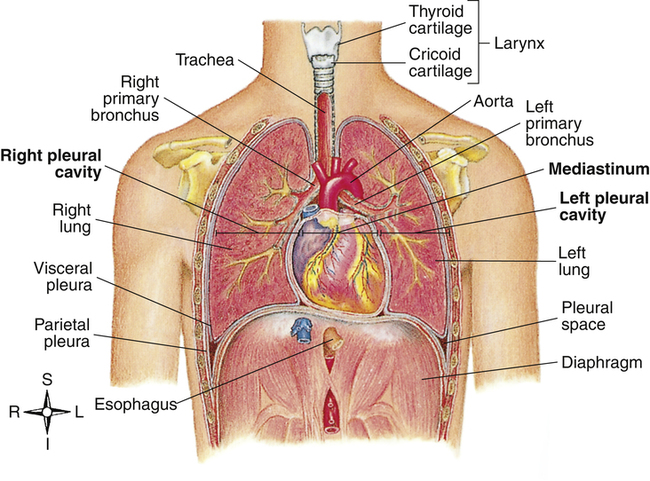
The inflated lungs are conical; the upper part is the rounded apex, and the lower concave part is the base (Figure 2-1). The costal (rib) surface of the lungs is smooth and convex and adjoins the inner chest wall. The surfaces adjoining the mediastinum are concave; the mediastinum is the central portion of the chest cavity containing the heart, aorta, esophagus, great veins, trachea, and mainstem bronchi.
Figure 2-1 shows that the apices of the lung extend above the clavicles into the base of the neck, with their top borders lying at the level of the first thoracic vertebra. The anterior border of the left lung forms the cardiac notch, which accommodates protrusion of the heart into the left half of the thoracic cavity.
The lung bases rest on the diaphragm, the major muscle of ventilation, which consists of two distinct, separately innervated muscles—the left and right hemidiaphragms. The diaphragm separates the thoracic and abdominal cavities, bowing deeply upward into the thoracic cavity (see Figures 2-1 and 2-9, A). The concave lung bases fit over the domes of the diaphragm. When the body is at rest, the outer margins of the lung’s bases lie at the level of the tenth thoracic vertebra; however, the highest points of their concave diaphragmatic surfaces bow up to the level of the eighth to ninth thoracic vertebra. The left hemidiaphragm surface is slightly lower than the right hemidiaphragm surface because (1) the heart rests on the left half of the diaphragm, pushing it downward, and (2) the liver, situated in the abdominal cavity directly below the right half of the diaphragm, props up this area (see Figure 2-1).
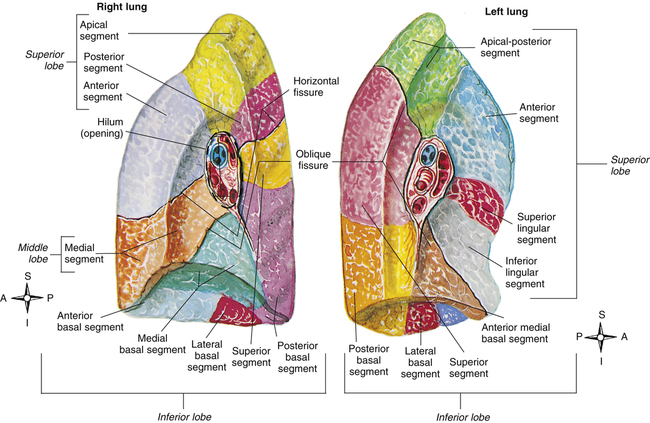
The area on the lung’s mediastinal surfaces through which arteries, veins, and the main bronchus enter is called the hilum and can be thought of as the “root” of the lung (Figure 2-2). The pulmonary ligament just below the hilum connects the membrane that covers the lung’s surface with the diaphragm below. The hilar structures and pulmonary ligament suspend and stabilize the lungs in the chest cavity. A thin anterior portion of the upper lobe of the left lung overlaps the heart and continues downward to a narrow point, forming the lingula, the tonguelike anatomical counterpart of the middle lobe of the right lung (see Figure 2-2).
Pleural Membranes
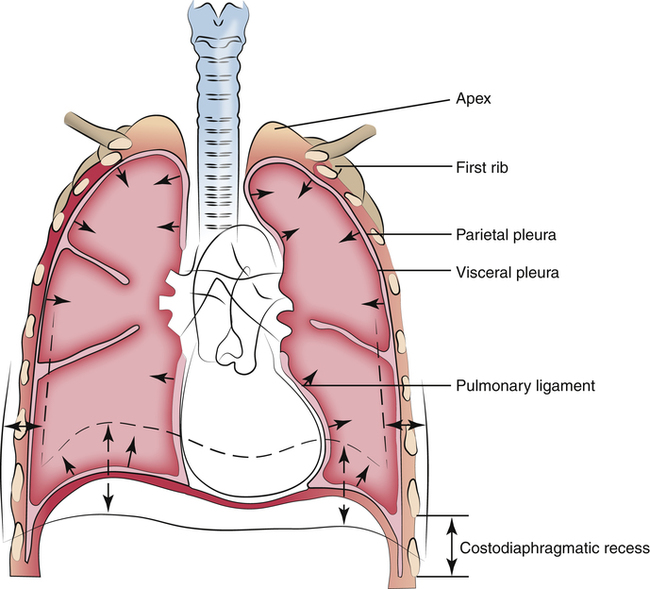
The visceral and parietal pleurae are one continuous membrane, forming sealed envelopes surrounding each lung. The visceral pleura, attached to the lung’s surface, doubles back at the hilar region to form the parietal pleura, which is attached to the inner chest wall surface (Figure 2-3). The potential space between visceral and parietal membranes is called the pleural space. Normally, the visceral and parietal pleurae are separated only by an extremely thin layer of serous pleural fluid. Pleural fluid lubricates the membranes, allowing nearly frictionless movement as they slide over one another during breathing. The cohesive forces of pleural fluid molecules prevent separation of the membranes, similar to the way a film of water prevents two glass microscope slides from being pulled apart. The lung passively follows movements of the chest wall and diaphragm. Intrapleural pressure, or pressure between the pleural membranes, is subatmospheric during normal breathing because the chest wall and lung recoil in opposite directions, creating a vacuum in the sealed pleural space.
The lowest margin of the diaphragm meets the chest wall in an area called the costophrenic recess. The lung’s borders do not extend into this recess, but the parietal pleural membrane does (see Figure 2-1). If the pleural membranes become inflamed by disease, fluid may form in the pleural space, creating what is called a pleural effusion; in such situations, fluid settles into the costophrenic recess of the pleural space, blunting the normally sharp angles of the costophrenic junctions as seen on a chest x-ray image. This fluid can be removed with a syringe and large-gauge needle (thoracentesis) or by surgically inserting a chest tube into the pleural cavity; several liters of fluid can collect in the pleural space, which compresses the lung and restricts its expansion.
Blood Supply to the Lungs
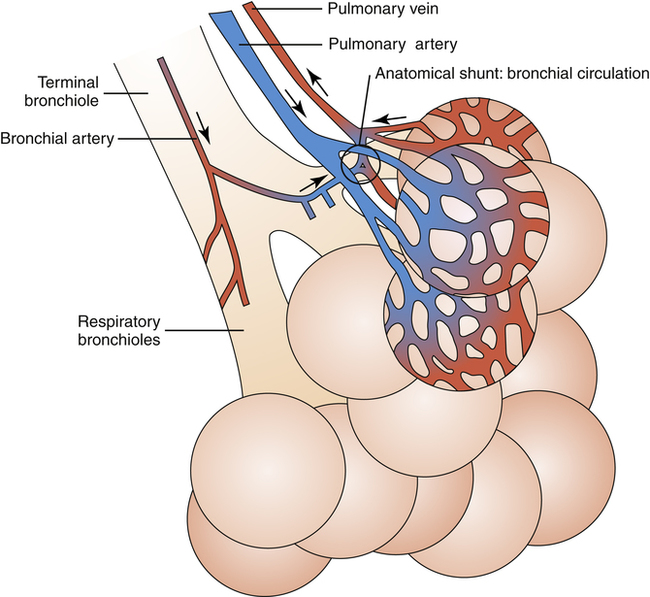
The metabolic requirements of the lung are met by two separate blood supplies—the pulmonary and systemic circulations. The pulmonary circulation originates from the right ventricle of the heart as the pulmonary artery and carries oxygen-poor blood to the lungs to be reoxygenated. Pulmonary arterioles subdivide many times to form extensive capillary beds that surround the alveoli like a fine net. Beyond the alveoli, capillaries converge to form venules and pulmonary veins, which carry oxygenated blood to the heart’s left atrium (Figure 2-4). The entire cardiac output flows through the pulmonary circulation and its fine capillary network; in this way, the pulmonary capillaries act as a kind of filter through which all blood flow must pass. The main function of this circulation is to bring blood into contact with alveolar gas so that oxygen and carbon dioxide exchange (respiration) can occur.
The lung’s systemic blood supply, the bronchial circulation, arises from the aorta as the bronchial arteries, which supply all of the airway walls from the major bronchi down to the respiratory bronchioles. In contrast to the pulmonary circulation, it is only a small fraction of the cardiac output. When blood flow passes through the capillary beds of the bronchial wall, oxygen diffuses out of the blood into airway wall tissues. On leaving the capillaries, this oxygen-poor blood takes at least two different courses: (1) one third to one fourth of it is channeled into the true bronchial veins into the azygos vein and then into the heart’s right atrium and (2) the remaining two thirds to three fourths of it drains directly into the pulmonary veins, mixing oxygen-poor bronchial venous blood directly with the freshly oxygenated blood of the pulmonary veins. This mixing reduces the overall oxygen content of the pulmonary venous blood that enters the left atrium, which the left ventricle eventually pumps into the systemic arterial circulation (see Figure 2-4).
anastomoses, which constitute part of the normal “anatomical shunt” found in the pulmonary circulation. Shunting refers to the mixing of unoxygenated blood with oxygenated blood. In this case, unoxygenated venous blood from the bronchial circulation mixes with oxygenated blood in the pulmonary veins, which is eventually pumped into the aorta and the systemic arteries. This normal anatomical shunting means that systemic arterial blood can never have the same partial pressure of oxygen as alveolar gas; this gives rise to the normal P(A-a)O2 (alveolar-to-arterial oxygen pressure difference). Vessels carrying blood to the heart are called veins, and vessels carrying blood away from the heart are called arteries. In the systemic circulation, arteries carry oxygenated blood, and veins carry deoxygenated blood; this is reversed in the pulmonary circulation. In common medical usage, the terms arterial blood and venous blood generally refer to oxygenated blood and deoxygenated blood.
Nervous Control of the Lungs and Thoracic Musculature
The voluntary skeletal muscles of the chest wall and diaphragm are innervated by the somatic nervous system, whereas the involuntary smooth airway muscle of the lung is innervated entirely by the autonomic nervous system. The sympathetic and parasympathetic divisions of the autonomic system control most of the body’s visceral functions. (The term “viscera” refers to the soft organs of the thoracic and abdominal cavities.) Somatic and autonomic nervous systems are compared in Table 2-1. The somatic system provides only motor innervation to the ventilatory muscles; the autonomic system supplies both motor (efferent) and sensory (afferent) nerves to the lung.
TABLE 2-1
Comparison of Autonomic and Somatic Motor Nervous Systems
| Features | Somatic Motor Nervous System | Autonomic Nervous System |
| Target tissues | Skeletal muscle | Smooth muscle, cardiac muscle, and glands |
| Regulation | Control of all conscious and unconscious movements of skeletal muscle | Unconscious regulation, although influenced by conscious mental functions |
| Response to stimulation | Skeletal muscle contracts | Target tissues are stimulated or inhibited |
| Neuron arrangement | One neuron extends from the CNS to skeletal muscle | Two neurons in series; the preganglionic neuron extends from the CNS to an autonomic ganglion, and the postganglionic neuron extends from the autonomic ganglion to the target tissue |
| Neuron cell body location | Neuron cell bodies are in motor nuclei of the cranial nerves and in the ventral horn of the spinal cord | Preganglionic neuron cell bodies are in autonomic nuclei of the cranial nerves and in the lateral horn of the spinal cord; postganglionic neuron cell bodies are in autonomic ganglia |
| Number of synapses | One synapse between the somatic motor neuron and the skeletal muscle | Two synapses—first in the autonomic ganglia, second at the target tissue |
| Axon sheaths | Myelinated | Preganglionic axons myelinated, postganglionic axons unmyelinated |
| Neurotransmitter substance | Acetylcholine | Acetylcholine released by preganglionic neurons; either acetylcholine or norepinephrine released by postganglionic neurons |
| Receptor molecules | Receptor molecules for acetylcholine are nicotinic | In autonomic ganglia, receptor molecules for acetylcholine are nicotinic; in target tissues, receptor molecules for acetylcholine are muscarinic, whereas receptor molecules for norepinephrine are either α- or β-adrenergic |
Modified from Seeley RR, Stephens TD, Tate P: Anatomy & physiology, ed 3, New York, 1995, McGraw-Hill.
Somatic Innervation
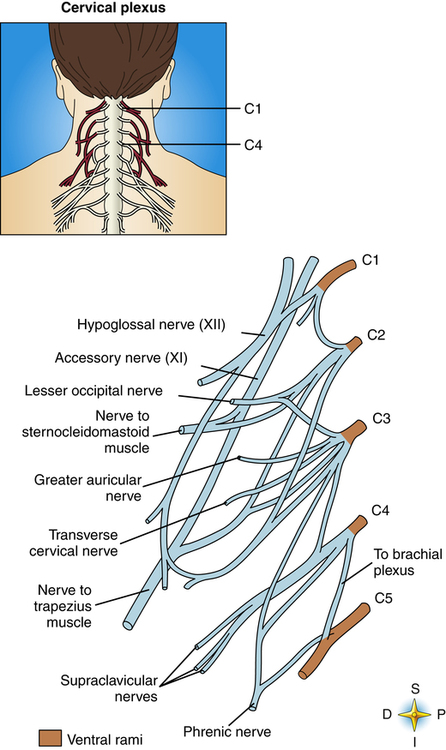
The paired phrenic nerves supply motor innervation to the hemidiaphragms. Phrenic nerves originate from the right and left cervical nerve plexuses as branches of cervical spinal nerves C3 to C5 (Figure 2-5). Phrenic nerves cross in front of the scalenus anterior muscles of the neck and enter the chest, sandwiched between subclavian arteries and veins. Thoracic surgery, neck trauma, and cancerous tumors sometimes injure or compress the phrenic nerve, causing paralysis of the diaphragm. However, breathing may still be possible even with a paralyzed diaphragm if intercostal nerves and muscles are intact.
Autonomic Innervation
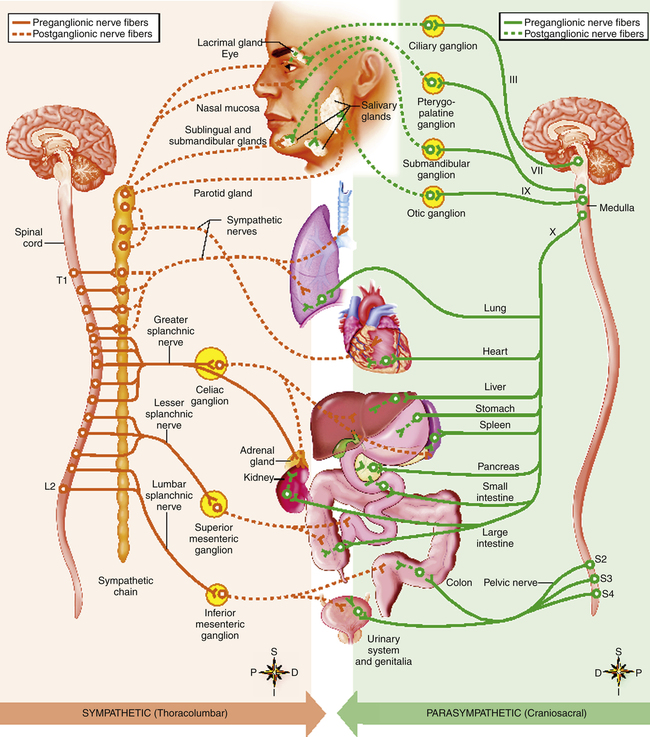
Fibers between the spinal cord and ganglia are preganglionic fibers; the fibers between the ganglia and the innervated organ are postganglionic fibers (Figure 2-6). In the parasympathetic system, the ganglia are near, or even in, the structures they innervate; preganglionic fibers are long, and postganglionic fibers are short. The opposite is true for the sympathetic system, in which the ganglionic junctions are located a short distance from the spine, forming linked ganglia resembling a chain of beads called the sympathetic chain (Table 2-2 and see Figure 2-6). To some extent, postganglionic fibers from both systems innervate the same structures, producing a balance between excitatory and inhibitory responses (Table 2-3).
TABLE 2-2
Comparison of Sympathetic and Parasympathetic Divisions
| Feature | Sympathetic Division | Parasympathetic Division |
| Location of preganglionic cell body | Lateral horns of spinal cord gray matter (T1-L2) | Brainstem and lateral horns of spinal cord gray matter (S2-S4) |
| Outflow from central nervous system | Spinal nerves, sympathetic nerves, and splanchnic nerves | Cranial nerves and pelvic nerves |
| Ganglia | Sympathetic chain ganglia along spinal cord for spinal and sympathetic nerves; collateral ganglia for splanchnic nerves | Terminal ganglia near or on effector organ |
| Number of postganglionic neurons for each preganglionic neuron | Many | Few |
| Relative length of neurons | Short preganglionic; long postganglionic | Short postganglionic |
From Seeley RR, Stephens TD, Tate P: Anatomy & physiology, ed 3, New York, 1995, McGraw-Hill.
TABLE 2-3
Comparison of Sympathetic and Parasympathetic Divisions
| Organ | Effect of Sympathetic Stimulation | Effect of Parasympathetic Stimulation |
| Heart | ||
| Muscle | Increased rate and force (β1) | Slowed rate (c) |
| Coronary arteries | Dilated (β2), constricted (α)∗ | Dilated (c) |
| Systemic blood vessels | ||
| Abdominal | Constricted (α) | None |
| Skin | Constricted (α) | None |
| Muscle | Dilated (β2), constricted (α) | None |
| Lungs | ||
| Bronchi | Dilated (β2) | Constricted (c) |
| Liver | Glucose released into blood (β2) | None |
| Skeletal muscles | Breakdown of glycogen to glucose (β2) | None |
| Metabolism | Increased up to 100% (α, β) | None |
| Glands | ||
| Adrenal | Release of epinephrine and norepinephrine (c) | None |
| Salivary | Constriction of blood vessels and slight production of a thick, viscous secretion (α) | Dilation of blood vessels and thin, copious secretion (c) |
| Gastric | Inhibition (α) | Stimulation (c) |
| Pancreas | Decreased insulin secretion (α) | Increased insulin secretion (c) |
| Lacrimal | None | Secretion (c) |
| Sweat | ||
| Merocrine | Copious, watery secretion (c) | None |
| 1.1 Apocrine | Thick, organic secretion (c) | None |
| Gut | ||
| Wall | Decreased tone (β2) | Increased motility (c) |
| Sphincter | Increased tone (α) | Decreased tone (c) |
| Gallbladder and bile ducts | Relaxed (β2) | Contracted (c) |
| Urinary bladder | ||
| Wall | Relaxed (β2) | Contracted (c) |
| Sphincter | Contracted (α) | Relaxed (c) |
| Eye | ||
| Ciliary muscle | Relaxed for far vision (β2) | Contracted for near vision (c) |
| Pupil | Dilated (α) | Constricted (c) |
| Arrector pili muscles | Contraction (α) | None |
| Blood | Increased coagulation (α) | None |
| Sex organs | Ejaculation (α) | Erection (c) |
α, Alpha-adrenergic receptors; β, beta-adrenergic receptors; c, cholinergic receptors.
∗Sympathetic stimulation of the heart normally increases coronary artery blood flow because of increased cardiac muscle demand for oxygen. (Local control is discussed in Chapter 17.)
From Seeley RR, Stephens TD, Tate P: Anatomy & physiology, ed 3, New York, 1995, McGraw-Hill.
Sympathetic preganglionic fibers originate in the thoracic and lumbar portions of the spinal cord—hence the term “thoracolumbar” in reference to the sympathetic division. A single preganglionic neuron may synapse with many postganglionic neurons that branch out to numerous organs. Thus, sympathetic stimulation usually elicits a widespread response involving many different organs.1
Parasympathetic fibers originate from the brainstem and sacral spinal cord—hence the term “craniosacral” in reference to the parasympathetic division. About three fourths1 of all parasympathetic fibers are in the vagus nerve (see cranial nerve X in Figure 2-6), which sends out many branches to the visceral organs. Vagal stimulation is synonymous with parasympathetic stimulation. In contrast to the sympathetic division, a parasympathetic preganglionic fiber usually synapses with a single postganglionic fiber; parasympathetic stimulation often evokes the response of a single organ.1
Efferent (Motor) Responses
Sympathetic Responses
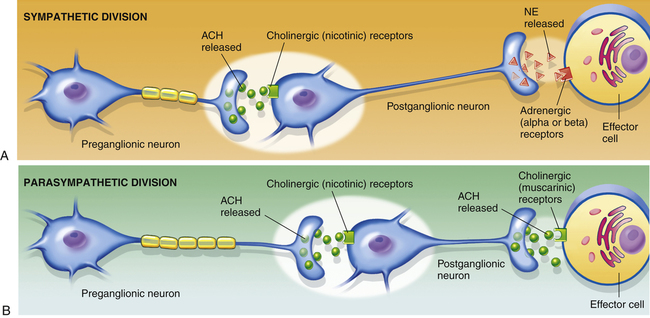
Sympathetic postganglionic fibers secrete the neurotransmitter norepinephrine, also known as noradrenaline, and these neurons are called adrenergic fibers. Sympathetic stimulation causes smooth airway muscle relaxation, which increases airway diameter (bronchodilation) and decreases resistance to airflow. Drugs that elicit sympathetic responses in the airways are called adrenergic bronchodilators; they are commonly used to reverse bronchoconstriction in patients with asthma and chronic bronchitis. Paradoxically, there is little or no anatomical evidence for sympathetic innervation of the airways, and yet sympathetic nervous system stimulation causes bronchodilation.2 Sympathetic bronchodilation occurs as follows: an extension of the sympathetic ganglionic chain, called the greater splanchnic nerve (see Figure 2-6), carries sympathetic impulses to the adrenal gland medulla, which secretes epinephrine directly into the bloodstream. Circulating epinephrine stimulates adrenergic receptors on smooth airway muscle cells, which are abundant in the lung despite the scarcity of sympathetic innervation. Circulating epinephrine is the only natural mechanism for sympathetic bronchodilation in humans.2
Parasympathetic Responses
Parasympathetic postganglionic fibers innervate lung smooth airway muscle, mucous glands, and pulmonary blood vessels. They secrete the neurotransmitter acetylcholine, and they are called cholinergic fibers. Parasympathetic impulses are the major neural bronchoconstrictor mechanism and the major determinant of airway diameter.2 These cholinergic impulses normally maintain a mild degree of continuous smooth muscle contraction, providing a normal baseline smooth muscle tone. However, excessive cholinergic stimulation can cause intense airway muscle contraction or bronchospasm, greatly increasing resistance to airflow. Drugs used to block cholinergic impulses in such circumstances are called anticholinergic bronchodilators.
Cholinergic innervation is greatest in the large airways, diminishing peripherally as airways become smaller. This characteristic may explain why anticholinergic bronchodilator drugs are less useful than adrenergic bronchodilator drugs when bronchoconstriction involves mostly small airways. In contrast, sympathetic receptors are more uniformly distributed, and adrenergic bronchodilators are equally effective in large and small airways.2
Adrenergic and Cholinergic Receptors
Before a neurotransmitter can stimulate a postganglionic fiber or an effector organ cell, it must first bind with the cell’s highly specific transmembrane receptor known as a G protein–coupled receptor; the intracellular end of the receptor is linked to a G protein molecule (so named because it is part of a family of guanine nucleotide-binding proteins).2 When a neurotransmitter molecule binds with the extracellular end of the receptor, the receptor’s shape changes, activating the G protein inside the cell. G protein activation stimulates an intracellular enzyme, initiating a complex sequence of events that ultimately stimulates or inhibits the nerve fiber or effector organ, depending on the type of receptor and neurotransmitter molecule involved.
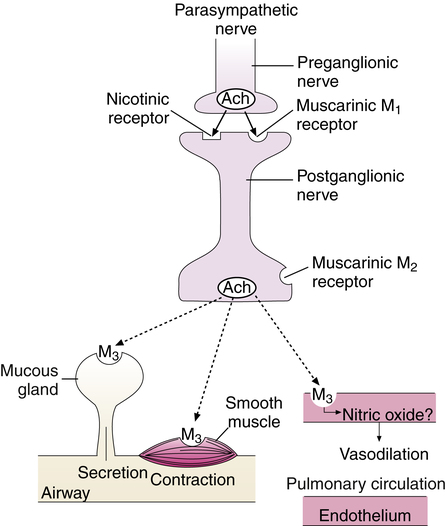
Both sympathetic and parasympathetic preganglionic fibers secrete acetylcholine at ganglionic junctions where they synapse with postganglionic fibers. At these junctions, receptors on postganglionic nerve fibers of both divisions are cholinergic; that is, they are specific for the acetylcholine. These cholinergic receptors are called nicotinic receptors because of their sensitivity to nicotine; when acetylcholine binds with nicotinic receptors, postganglionic fibers propagate the nerve impulse to the neuromuscular junction of the effector organ. At the neuromuscular junction, sympathetic (adrenergic) fibers secrete norepinephrine, and parasympathetic (cholinergic) fibers again secrete acetylcholine. The organs innervated by these fibers have adrenergic and cholinergic transmembrane receptors in their cell membranes specific for norepinephrine and acetylcholine molecules. Cholinergic receptors at the neuromuscular junction are different from receptors at the ganglionic junction and are called muscarinic receptors because of their sensitivity to muscarine, a substance derived from a mushroom. When acetylcholine molecules bind with muscarinic receptors, they produce smooth airway muscle contraction (bronchoconstriction) and increased glandular mucus secretion. In contrast, when norepinephrine molecules bind with adrenergic receptors of effector organ cells, they produce bronchodilation, which decreases airway resistance. Figure 2-7 illustrates the locations of neurotransmitters and receptors.
Parasympathetic Muscarinic Receptor Subtypes
At least five muscarinic receptor subtypes have been identified, three of which are present in the human lung. These subtypes are designated M1, M2, and M3 receptors, and all are sensitive to acetylcholine.2 M1 receptors are present along with nicotinic receptors on the preganglionic fiber at the ganglionic junction. Although nicotinic receptors are primarily responsible for transmitting neural impulses through the parasympathetic ganglion, M1 receptor stimulation enhances transmission. M2 receptors are present on the postganglionic nerve ending at the neuromuscular junction. M2 receptors have a negative feedback function; when bound by acetylcholine, they inhibit its further release from the nerve ending, limiting the effects of parasympathetic stimulation. M3 receptors are present in airway smooth muscle and mucous glands; when bound by acetylcholine, they cause smooth muscle contraction and increased mucus production (Figure 2-8).
Adrenergic Receptor Subtypes
As with cholinergic receptors, there are different types and subtypes of adrenergic receptors; the major types are designated alpha (α) and beta (β) receptors (see Figure 2-7). Beta receptors are subdivided further into beta1 (β1) and beta2 (β2) receptors. Alpha receptors are less distinctly subdivided into alpha1 and alpha2 receptors.2 Chemical substances that stimulate these receptors are called agonists, whereas substances that block their responses are called antagonists. Alpha and beta agonists generally produce opposite effects; for example, beta2 agonists cause vasodilation, whereas beta1 agonists cause vasoconstriction. Similarly, some alpha-mediated functions can be excitatory, and some can be inhibitory.
Norepinephrine and epinephrine, both secreted into the bloodstream by the adrenal glands, excite alpha and beta receptors differently. Norepinephrine excites alpha receptors much more than beta receptors, whereas epinephrine excites them both about equally.1 Table 2-3 lists some of the differences between alpha and beta receptor functions. About two thirds of the beta receptors in the lung are of the beta2 type.2 Pulmonary beta2 receptors are concentrated in airway smooth muscle, airway epithelium, vascular smooth muscle, and submucosal glands, whereas the smaller number of beta1 receptors are restricted to alveolar walls and submucosal glands.2 There is no evidence of alpha receptors on airway smooth muscle, although they are present on the smooth muscle associated with blood vessels that supply the airways.2
From the foregoing discussion, one can see that both cholinergic blocking drugs and beta2 agonist drugs cause bronchodilation. Beta2 agonists and cholinergic antagonists are the two major drug types used to reverse bronchospasm in diseases such as asthma and chronic obstructive pulmonary disease (COPD). COPD includes diseases that narrow the airways for purely structural reasons (e.g., because of loss of elastic recoil or airway mucosal edema), which means that normal cholinergic smooth muscle tone decreases airway diameter more than it does in normal airways. This characteristic of COPD may explain why anticholinergic drugs are often more effective than beta2 agonists as bronchodilators in patients with COPD.2
The preceding characterizations of neural airway control have been simplified; it is now known that the autonomic nervous system comprises more than just adrenergic and cholinergic neurons and that all neurons interact in complex ways.2 One autonomic division can modify the function of the other. Every nerve secretes multiple transmitters, although each division is generally associated with one primary neurotransmitter.2
Nonadrenergic Noncholinergic Responses
Bronchodilator nerves exist in human airways that are not blocked by drugs that block adrenergic or cholinergic receptors. These nerves are called nonadrenergic noncholinergic (NANC) nerves; other neurotransmitters besides norepinephrine and acetylcholine bind with autonomic receptors.2 It was previously thought that each autonomic nerve type had its own unique neurotransmitter, but a newer theory of cotransmission holds that in addition to secreting norepinephrine or acetylcholine, all postganglionic fibers release or cotransmit a host of NANC transmitters that modify autonomic receptor responses. These additional transmitters can either facilitate or antagonize the effects of the primary neurotransmitter. NANC neurotransmitters play an important role in modulating not only airway smooth muscle tone but also immune system homeostasis.
Similar to sympathetic and parasympathetic autonomic divisions, NANC neurons can be either inhibitory (i-NANC) or excitatory (e-NANC). i-NANC neurons are efferent fibers that cause bronchodilation, whereas e-NANC neurons are sensory fibers (also known as C-fibers) that lead to bronchoconstriction. The main i-NANC neurotransmitters in human airways are nitric oxide (NO) and vasoactive intestinal peptide (VIP); VIP is the most potent bronchodilator of the two. NO and VIP are coreleased with acetylcholine in cholinergic fibers, where they are believed to exert an important limiting effect on cholinergic bronchoconstriction.2 (i-NANC fibers share a common ganglion with parasympathetic fibers.2) VIP also exists in mast cell granules where it is thought to limit the bronchoconstriction caused by mast cell histamine release (see Chapter 1). Superoxide ions generated by inflammatory cells in asthma may deplete neuronally generated NO, possibly reducing the limiting effect that NO has on cholinergic bronchoconstriction.2
Exhaled Nitric Oxide in Asthma
i-NANC neurons contain the enzyme nitric oxide synthase (NOS), which is responsible for the synthesis of NO. In contrast to acetylcholine and norepinephrine, NO is not stored in vesicles in the neuron fiber ending but is synthesized in response to neural stimuli; it then diffuses out of the neuron into extracellular spaces and nearby smooth muscle. There are three forms of NOS: constitutive NOS (cNOS), which is constituted in the neuron; inducible NOS (iNOS), which is induced in airway epithelial cells by inflammation; and endothelial NOS (eNOS), which is another constitutive form of NOS that is generated in vascular endothelial cells.2 Because asthma is an inflammatory disease of the airways, it stimulates the production of iNOS, which would be expected to elevate the concentration of NO in the exhaled gas of asthmatics. Asthmatics have higher concentrations of exhaled
NO than normal individuals, especially during exacerbations of the disease.2 Monitoring exhaled NO is one way to identify early stages of asthma exacerbations.
Afferent (Sensory) Responses
Most sensory afferent nerves from the lung are parasympathetic (vagal) in origin. At least three kinds of afferent receptors have been identified:2 (1) slowly adapting stretch receptors; (2) rapidly adapting irritant receptors; and (3) C-fibers, also classified as NANC excitatory nerves.3 These nerves play a critical role in defense of the airways; their stimulation evokes powerful reflexes that limit the penetration of harmful substances into the respiratory tract that could injure the delicate alveolar gas-exchange surface.
Slowly Adapting (Stretch) Receptors
Slowly adapting receptors (SARs) continue to fire as long as the stimulus persists. These stretch receptors are located in the smooth muscle of conducting airways. They are stimulated by a deep inspiration, which is part of the Hering-Breuer reflex. A deep inspiration inhibits vagal discharge and causes smooth muscle relaxation and bronchodilation.2
Deep inspirations have bronchodilating and bronchoprotective effects in normal healthy people, and these effects are not present in patients with asthma; the airway smooth muscle fibers in asthmatics are not stretched by deep inspiration as much as they are in subjects without asthma.4 In one study, subjects with and without asthma performed several deep inspirations before inhaling methacholine, a provocative substance that elicits bronchospasm. Asthmatic subjects had a much greater bronchospastic response to methacholine than subjects without asthma. The investigators concluded that deep inspiration is a normal physiological protective mechanism that is absent in patients with asthma, even if asthma is mild.4 Apparently, deep inspiration does not stretch asthmatic airways as much as it stretches healthy airways; in addition, asthmatic airways reconstrict more rapidly after deep inspiration compared to healthy airways.4
Rapidly Adapting (Irritant) Receptors
Rapidly adapting receptors (RARs) are activated by various irritants, including inhaled gases (e.g., cigarette smoke, ozone, sulfur dioxide), water, hypertonic solutions, acidic substances, mechanical stimulation (including distortion from bronchoconstriction), and histamine-induced bronchoconstriction.2 RARs are widespread in the larynx, trachea, carina, and mainstem bronchi, firing vigorously on stimulation and then quickly adapting to a slower firing rate.
1. The diaphragm contracts, causing a deep inspiration.
2. A slight inspiratory pause occurs.
3. The muscles in the larynx close the glottis, sealing the upper airway.
4. The abdominal expiratory muscles contract forcefully, generating a high intrapulmonary pressure against the closed glottis (100 to 200 cm H2O in healthy adults).
5. The glottis suddenly opens, explosively releasing compressed gas.
Irritant receptors in the more distal bronchi cause bronchoconstriction rather than cough.7 Bronchoconstriction increases cough receptor sensitivity resulting from mechanical distortion, whereas bronchodilation decreases their sensitivity.2 For this reason, persistent cough in patients with asthma can often be diminished by treatment with bronchodilator drugs.
C-Fibers
C-fibers (e-NANC fibers) are located in the lung parenchyma, conducting airways, and pulmonary blood vessels; they play an important role in defending the lower respiratory tract and respond to various chemical and physical stimuli by causing bronchoconstriction.2 Bronchial C-fibers are apparently involved in the bronchoconstriction caused by cold air breathing in exercise-induced asthma.5 C-fiber endings are located deep in the lung parenchyma near pulmonary capillaries and alveoli and are often called J-receptors (juxtacapillary receptors).2 Stimulation of J-receptors causes rapid, shallow breathing; severe laryngeal constriction on expiration; slowed heart rate (bradycardia); and mucous secretion. J-receptors are stimulated by alveolar inflammatory processes such as pneumonia and by increased pulmonary capillary pressure and pulmonary edema as seen in patients with congestive heart failure. J-receptors probably contribute to the sensation of dyspnea (shortness of breath) that accompanies such conditions.2
Thoracic Anatomy
The thorax is a cavity formed by the rib cage and its muscles (intercostal muscles), the vertebrae, sternum, and diaphragm. It can be subdivided into three cavities: the left and right pleural cavities and the mediastinum (Figure 2-9).
Thoracic Cage
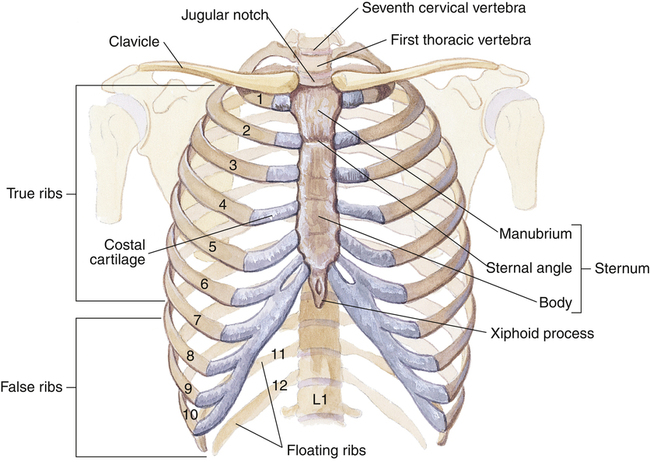
The ribs are situated such that their vertebral attachments are higher than their sternal attachments, making them slant downward anteriorly (Figure 2-10). The 12 thoracic vertebrae articulate with all 12 ribs, but not all ribs connect directly with the sternum anteriorly. Ribs 1 to 7 connect directly with the sternum via cartilages and are called vertebrosternal ribs; ribs 8 to 10 are connected to the lower sternum by a common cartilage and are called vertebrochondral ribs. The last two ribs, 11 and 12, do not connect with the sternum and are called floating ribs.
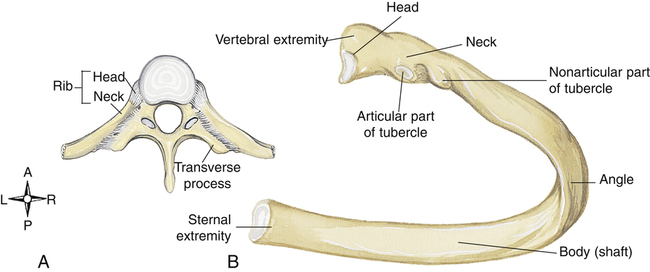
The head of a typical rib articulates with the bodies of two adjacent vertebrae, and the rib tubercle articulates with the transverse process of one vertebra (Figure 2-11). A costal groove on the underside of each rib provides a protective channel for nerves, arteries, and veins; needles or tubes inserted between the ribs into the chest should be placed immediately above the rib to prevent damage to nerves or vessels. The first two or three ribs are extremely short and have limited movement. Lower ribs are more likely to be fractured or separated from their sternal attachments in traumatic accidents. Fractured first and second ribs indicate an extremely severe blow to the thorax.
The sternum, sometimes called the breast bone, consists of the manubrium, body, and xiphoid process (see Figure 2-10). At the junction of the manubrium, the body, and the second rib is the sternal angle, also called the angle of Louis. The angle of Louis marks the level of the carina in the lung and is adjacent to the second rib; it is a reference point for counting ribs. Counting ribs is necessary for locating intrathoracic structures and inserting chest tubes.
Rib Movements
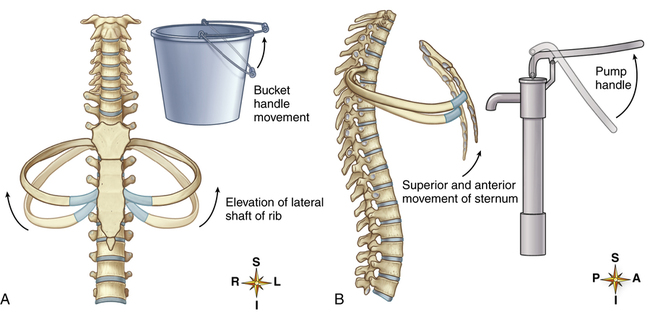
sternal ends of the ribs rise and fall, whereas the vertebral ends are fixed. Muscular contraction elevates only the sternal ends of the ribs, causing a pump-handle motion that moves the sternal ends of the ribs up and away from the vertebral column. This motion increases the anterior-posterior distance between the sternum and vertebra, enlarging the thoracic cavity volume (Figure 2-12).
The so-called bucket-handle movement occurs simultaneously with the pump-handle movement. In the bucket-handle movement, the sternum and vertebral column act as fixed attachment points for the semicircular rib, which is shaped like a bucket handle. At the end of an expiration, the rib is positioned such that its curved midpoint loops downward (similar to the handle of a pail) below its attached sternal and vertebral ends. During inspiration, an upward swing of the rib causes its midpoint to move out laterally, away from the thoracic midline, increasing the side-to-side (transverse) distance between the left and right sides of the rib cage (see Figure 2-12). Combined pump-handle and bucket-handle rib movements create a two-dimensional enlargement of the thoracic cavity as the ribs elevate during inspiration. Return of the ribs to their resting positions causes thoracic volume to decrease. This changing thoracic volume provides the basis for generating pressure gradients necessary for air movement into and out of the lungs.
Ventilatory Muscles
Diaphragm
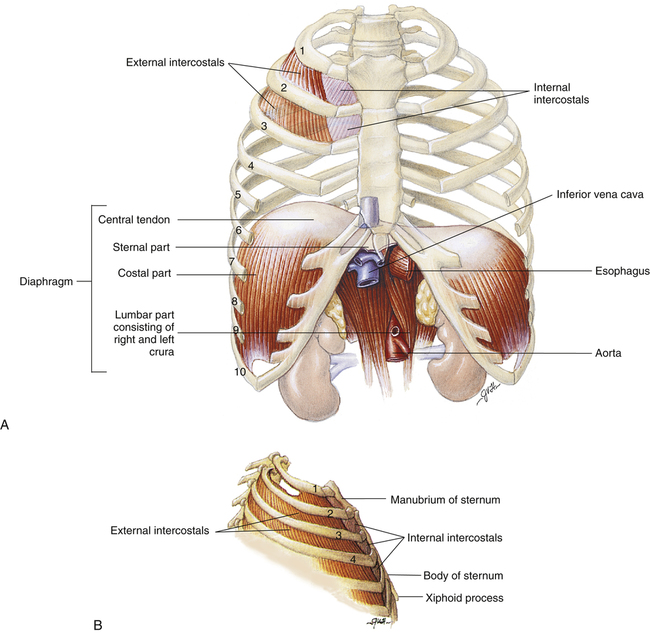
At rest, the diaphragm is bowed deeply into the thoracic cavity (Figure 2-13, A). It consists of two functionally distinct components: costal parts and crural parts. Costal diaphragm fibers originate from the lower inner rib borders (costal margins) and the lower end of the sternum. Crural fibers originate from the first three lumbar vertebrae, forming two muscle bands, the left and right crura(see Figure 2-13, A). These crural portions of the diaphragm have no direct rib cage attachments. Costal and crural fibers converge and insert into a common central tendon. The central tendon separates the diaphragm into two leaflets, the right and left hemidiaphragms. Each hemidiaphragm is innervated by its own phrenic nerve, allowing it to function independently. Normally, the hemidiaphragms function synchronously.
Intercostal Muscles
The intercostal muscles and the ribs form the semiflexible chest wall. The internal intercostal muscles are actually two separate muscles, the parasternal and the interosseous intercostal muscles. Both muscles are located between the ribs, but only the parasternals have an upper insertion into the sternum. Parasternal muscle contraction tends to elevate the ribs, assisting inspiration. Interosseous fibers are situated between the ribs such that their contraction depresses the ribs, causing expiration. The external intercostal muscles along with the parasternal intercostals elevate the ribs, resulting in inspiration (see Figure 2-13).
Scalene Muscles
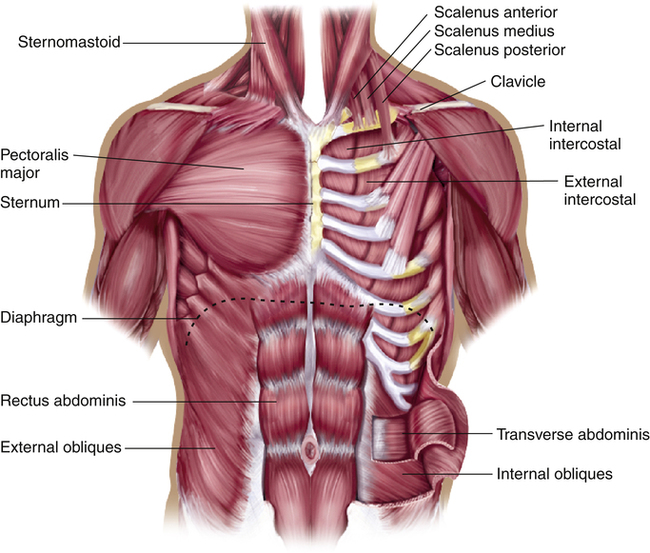
Contraction of the scalene muscles on either side of the neck elevates and fixes the first and second ribs (Figure 2-14). Traditionally, the scalenes were considered accessory inspiratory muscles that remained inactive during quiet breathing. Clinical studies have shown evidence to the contrary. Electromyographic recordings revealed low discharge frequencies from some scalene muscle units during quiet breathing in normal subjects.5 Extremely active scalene muscles at rest indicates an abnormally high work of breathing and implies abnormal lung mechanics. Scalene muscle activity is easily detected by observation and palpation. Assessment of scalene muscle participation in ventilation at rest is an important part of physical examination of the chest and yields important information about the severity of underlying lung disease.
Sternomastoid Muscles
The sternomastoids originate from the manubrium of the sternum and insert into the mastoid process of the skull on either side of the neck (see Figure 2-14). When the head is held in a fixed position, contraction of the sternomastoids elevates the sternum, slightly elevating the anterior ends of the ribs. If the sternomastoids actively assist each inspiration at rest, work of breathing and inspiratory effort are great. Use of these accessory muscles at rest implies abnormal lung mechanics—that is, high resistance to airflow or noncompliant lungs.
Pectoralis Major Muscle
The pectoralis major muscle is a fan-shaped, powerful muscle arising from the lateral borders of the sternum and inserting at the narrow end into the humerus of the arm (see Figure 2-14). If the arms and shoulders are fixed by grasping a wide stationary object, the pectoralis major can elevate the sternum and ribs. The typical posture of a person with advanced COPD is sitting, leaning forward over a table or the back of a chair, and grasping the widest portion of the object. All accessory inspiratory muscles may come into play at rest in patients with severe lung disorders characterized by high airway resistance or stiff, noncompliant lungs.