Chapter 13. The lumbar spine
SUMMARY
This chapter outlines the relevant anatomy to enable discussion of evidence for the causes of back pain and differential diagnosis. The clinical examination procedure will be outlined and interpreted, and the models used in orthopaedic medicine will be identified to act as a guide to treatment using this approach.
ANATOMY
There are five lumbar vertebrae, each with a large vertebral body designed for weight-bearing (Fig. 13.1). Each vertebral body consists of a shell of cortical bone surrounding a cancellous cavity of supporting struts and cross-beams called trabeculae. This provides a lightweight box with the strength to support longitudinally applied loads. The intervening intervertebral disc provides a mechanism for shock absorption, distribution of forces and movement (Jensen 1980).
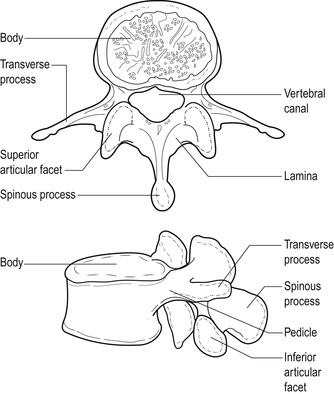 |
| Figure 13.1
Typical lumbar vertebra.
From Anatomy and Human Movement by N Palastanga, D Field and R Soames. Reprinted by permission of Elsevier Ltd.
|
The stabilizing function of the lumbar spine is achieved by the various bony processes that make up the posterior elements of the lumbar vertebrae, i.e. the pedicles and laminae, and the articular, spinous and transverse processes. The position and direction of the articular processes that form the synovial zygapophyseal joints prevent forward sliding and rotation of the vertebral bodies, while the spinous and transverse processes act as leverage and provide attachment for muscles.
The vertebral foramen is surrounded by the vertebral body in front and the posterior elements behind. It is triangular in the lumbar spine and is larger than that in the thoracic spine but smaller than that in the cervical spine. Together the vertebral foramina form the vertebral canal which contains the termination of the spinal cord opposite the L1–L2 disc, and the cauda equina. The vertebral canal can vary in shape and this may be relevant to pathology.
The lumbar lordosis, the posterior postural concavity, compensates for the inclination of the sacrum and maintains the upright posture. The wedge-shaped lumbosacral disc and vertebral body of L5 contribute to the lordosis as well as the antigravity effect of the constant activity in the erector spinae muscles that prevent the trunk from falling forwards (Oliver & Middleditch 2006).
The intervertebral disc
The intervertebral disc has special biomechanical requirements. It is strong to sustain weight and transmit loads while being able to deform to adjust to movement. The intervertebral disc has three parts: a central nucleus pulposus surrounded by a peripheral annulus fibrosus which blends above and below into vertebral end-plates.
The nucleus pulposus accommodates to movement and transmits compressive loads from one vertebral body to another. Its normal consistency has been likened to that of toothpaste. It is composed of irregularly arranged collagen fibres and cartilage cells scattered within amorphous ground substance. The collagen fibres are composed of type II collagen, suited to accept pressure and compression (see Ch. 2). The nucleus in particular has great water-binding capacity through its proteoglycan content. The fluid nature of the nucleus allows it to deform under pressure while the vertebral end-plates prevent its superior and inferior deformation. In this way the intervertebral disc supports and transmits loads.
Although it has long been recognized for its fluid properties, the nucleus also behaves as a viscoelastic solid under dynamic conditions. Iatridis et al (1996) investigated the viscoelastic properties of the healthy nucleus pulposus, showing it to be sensitive to different loading rates. The higher loading rates produced failure of the end-plate and vertebral body, while slower loading rates produced progressive failure of the annulus and disc herniation.
The annulus fibrosus consists of a geometrically organized arrangement of collagen and elastic fibres bound together by a proteoglycan gel, allowing it to support weight without buckling. Types I and II collagen exist in the annulus fibrosus, but the majority of fibres are type I, suited to withstand tensile forces. The fibres are arranged in concentric lamellae around the central nucleus. These tightly packed lamellae are arranged circumferentially at the periphery and can sustain high compressive loads. Adams et al (2002) suggest an analogy to the stiffness of a telephone directory rolled into a cylinder and stood on its end. In each lamella, the collagen fibres lie parallel to each other, inclined at an angle of approximately 65–70° to the vertical (Fig. 13.2). The direction of fibres alternates in adjacent lamellae (Bogduk 2005). Marchand & Ahmed (1990) noted a number of irregularities within the laminate structure of the annulus, particularly at the posterolateral corners, where a number of incomplete layers were seen. Increased stresses applied to the annulus in this region could produce fissuring and provide the nuclear material with an escape route.
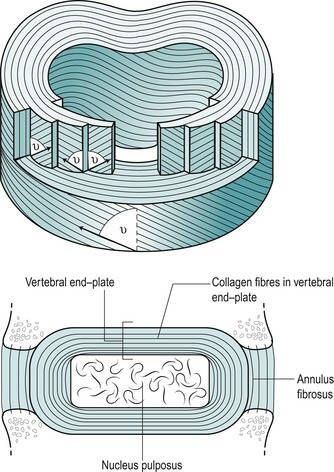 |
| Figure 13.2
Laminate structure of the disc.
Reprinted from Clinical Anatomy of the Lumbar Spine and Sacrum. N Bogduk © 1998, by permission of Elsevier.
|
The annulus fibrosus acts like a ligament, restraining excessive movement to stabilize the intervertebral joint while allowing flexibility to permit normal movement. The alternating oblique annular fibres resist horizontal and vertical forces, allowing the annulus to oppose movement in all directions (Bogduk 1991).
The outer half of the annulus at least is known to have a nerve supply (Cavanaugh et al 1995, Coppes et al 1997). The main source of the supply is believed to be the sinuvertebral nerve and branches of the sympathetic trunks and its grey rami communicantes (Adams et al 2002) (Fig. 13.3). The sinuvertebral nerve supplies the disc at one level and the disc above (Bogduk 2005).
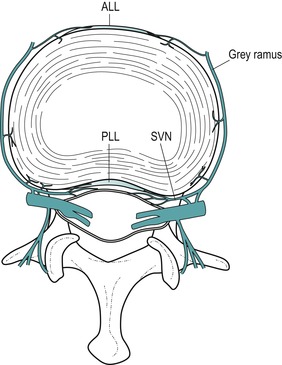 |
| Figure 13.3
The nerve supply of a lumbar intervertebral disc depicted in a transverse view of the lumbar spine. Branches of the grey rami communicantes and the sinuvertebral nerves (SNV) are shown entering the disc and the anterior and posterior longitudinal ligaments (ALL, PLL). Branches from the sinuvertebral nerves also supply the anterior aspect of the dural sac and dural sleeve.
Reprinted from Clinical Anatomy of the Lumbar Spine and Sacrum. N Bogduk © 1998, by permission of Elsevier.
|
The vertebral end-plates are thin layers of cartilage, approximately 1 mm thick, covering the superior and inferior surfaces of the discs. They form a permeable barrier for diffusion, mainly between the nucleus and the cancellous bone of the vertebral bodies. They fail relatively easily under excessive compressive loading.
Properties of the intervertebral disc
The intervertebral disc at rest possesses an intrinsic pressure due to the compressive effect of the elastic ligamentum flavum (Oliver & Middleditch 2006). This preloaded or prestressed state provides it with an intrinsic stability to resist applied forces such as body weight (Jensen 1980). The resting pressure is affected by posture and loading, being lowest in the lying position and highest in the sitting position, with a further increase if external loading is applied (Nachemson 1966). In the sitting position the spine usually rests in a degree of flexion and the activity in psoas major contributes a compressive effect on the disc as it stabilizes the spine.
The terms ‘somatic’ and ‘radicular’ are discussed within Chapter 1. Clinically, somatic back pain and radicular pain are affected by movements and posture, as well as being increased by straining, coughing or laughing. An increase in intradiscal pressure of about 50% was noted when straining was performed in standing, due to the increase in loading produced by muscle activity (Nachemson & Elfstrom 1970). The chart originating from the investigations of Nachemson (1966) has been reproduced in several publications as a useful guide to the variation in intradiscal pressure with different postures and activities (Fig. 13.4).
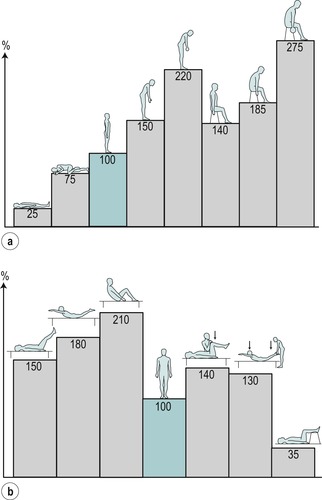 |
| Figure 13.4
Discal pressures. Relative change in pressure (or load) in the third lumbar disc: (a) in various positions; (b) in muscle-strengthening exercises.
From A Nachemson 1976 The lumbar spine: an orthopaedic challenge. Spine 1:59–71, with permission.
|
Movement of the spine involves simultaneous tension, compression and shear at different locations of the disc affecting intradiscal pressure and fluid flow. Flexion, extension and side flexion produce tension resulting in stretching of the annulus on one side, and compression on the other side through body weight (Jensen 1980). Flexion includes a component of forward translation that is stabilized by the zygapophyseal joints, while extension is limited by bony impaction of the inferior articular processes against the lamina of the vertebra below. Axial rotation produces torsion in the intervertebral discs, with tension in half of the annular fibres that are inclined towards the direction of the rotation, and impaction of the zygapophyseal joints. Side flexion is a composite movement which includes side flexion and rotation (Bogduk 2005).
As a viscoelastic material, the intervertebral disc is subjected to the phenomena of creep, hysteresis and set (Twomey & Taylor 1982, Oliver & Twomey 1995, Bogduk 2005), as discussed in Chapter 2. The creep behaviour of flexion and extension is similar, with the amount of creep increasing with load and progressing with time. Creep also increases with age when hysteresis recovery is slower. Flexion creep, in particular, has implications for occupations that require a constant flexed posture, e.g. manual workers. It may also be responsible for fatigue in the disc, making it vulnerable to a sudden applied force – the ‘straw that breaks the camel’s back’.
Nutrition of the intervertebral disc
The lumbar discs have a relatively poor blood supply since no arteries enter the disc and it is the largest avascular tissue in the human body (Adams et al 2002, Paesold et al 2007). Nutrition of the intervertebral disc occurs through two routes: the blood vessels situated around the peripheral annulus and those in the central portion of the vertebral end-plate. The outer annulus may be supplied with nutrients from blood vessels in the adjacent longitudinal ligaments but the supply to the nucleus pulposus cells is almost completely dependent on diffusion via the end-plate capillary network (Paesold et al 2007). The mechanisms involved are diffusion and fluid flow and are interrelated. Both are affected by posture and motion (Adams & Hutton 1986).
The water content of the disc varies and represents a balance between two opposing osmotic and hydrostatic pressures, i.e. a swelling pressure (imbibition) which hydrates the disc and a mechanical pressure (posture, movement, loading and creep) which dehydrates the disc. Diurnal decrease in the total length of the spine is offset by its recovery in the supine position overnight (Parke & Schiff 1971, Porter 1995). Flexion postures cause a larger fluid outflow from the disc than erect or lordotic postures, with this outflow being further reduced when the spine is unloaded by lying down. Alternating between rest and activity will enhance fluid flow (Adams & Hutton 1983, 1986). Factors that influence the nutrition of the disc are increased loading, vibration or spinal deformity. Factors that compromise the vascular supply include smoking, vascular disease and diabetes (Buckwalter 1995).
Zygapophyseal joints (facet joints)
The zygapophyseal joints are synovial joints that provide stability of the spine, control of movement and protection of the intervertebral discs (Fig. 13.5) (Taylor & Twomey 1994). The articular facets are covered with articular cartilage and the joints are surrounded by a fibrous capsule and lined with synovium. The superior articular facets face posteromedially and grasp onto the inferior articular facets of the vertebra above, which face anterolaterally. The resultant plane of the joint facilitates flexion and extension movements, but prevents rotation. It also restricts translation in healthy joints, helping to protect the lumbar disc from the shearing forces responsible for fissuring (Bogduk 1991, Taylor & Twomey 1994).
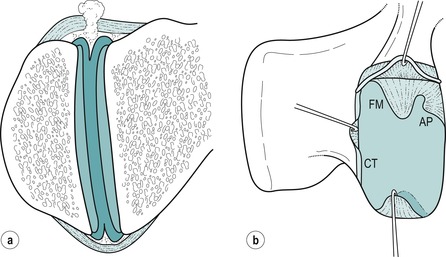 |
| Figure 13.5
Intra-articular structures of the lumbar zygapophyseal joints. (a) Coronal section of a left zygapophyseal joint showing fibroadipose meniscoids projecting into the joint cavity from the capsule over the superior and inferior poles of the joint. (b) Lateral view of a right zygapophyseal joint, in which the superior articular process has been removed to show intra-articular structures projecting into the joint cavity across the surface of the inferior articular facet. The superior capsule is retracted to reveal the base of a fibroadipose meniscoid (FM) and an adipose tissue pad (AP). Another fibroadipose meniscoid at the lower pole of the joint is lifted from the surface of the articular cartilage. A connective tissue (CT) rim has been retracted along the posterior margin of the joint.
Reprinted from Clinical Anatomy of the Lumbar Spine and Sacrum. N Bogduk © 1998, by permission of Elsevier.
|
The fibrous capsule consists of an outer layer of regularly arranged connective tissue and an inner layer of yellow elastic fibres. Anteriorly the capsule is replaced by the ligamentum flavum, while some of the deep fibres of multifidus give the capsule reinforcement medially (Yamashita et al 1996). The superior and inferior aspects of the capsules are loose and contain intra-articular structures consisting of fat and meniscoid structures (Bogduk 2005). Fine nerve fibres thought to conduct nociceptive and proprioceptive sensations have been found (Yamashita et al 1996).
The zygapophyseal joints cannot be discounted as a cause of back pain since, as synovial joints, they may be subjected to trauma or arthritis. Degenerative changes usually coexist in the intervertebral joint of the same segment.
Authors have looked at the pattern of pain referral of the zygapophyseal joints in an attempt to establish a recognized syndrome and pain referral patterns (Mooney & Robertson 1976, Bogduk 1994). Schwarzer et al (1994a) acknowledged this joint as a possible source of pain, but questioned the existence of a facet syndrome. Some authors suggest that the zygapophyseal joint is responsible for the acute locked back, as the intra-articular structures become trapped between the articular surfaces (Twomey & Taylor 1994, Bogduk 2005), while Kuslich et al (1991) demonstrated that stimulation of the zygapophyseal joint capsule very rarely generates leg pain. In a study of the relative contributions of the disc and zygapophyseal joint in chronic low back pain, pain was noted to arise more commonly from the disc than the zygapophyseal joint (Schwarzer et al 1994b).
Laslett & van Wijmen (1999) suggest that the symptomatic zygapophyseal joint presents with pain that settles well on lying down and four of the following criteria: age greater than 65; pain not increased by coughing; no pain on flexion in standing; pain not increased in rising from flexion; pain not increased by extension/rotation; or pain not increased by extension in standing.
The authors of this text accept the lack of evidence to attribute the cause of low back pain to one specific structure and that will also apply to the symptomatic zygapophyseal model presented above. Orthopaedic medicine treatments have traditionally been based on the discal model but it may be more clinically relevant to apply treatments selected on the basis of a particular set of signs and symptoms rather than to attempt to be too pedantic with regard to pathology (see below). It must be acknowledged that a lesion in any structure within a spinal segment will influence neighbouring structures and similarly treatment cannot be directed to one anatomical structure in isolation.
Ligaments
Anterior and posterior longitudinal ligaments are well developed in the lumbar region where both stabilize the vertebral bodies and control movement (Fig. 13.6). The anterior longitudinal ligament is widest in the lumbar spine where it covers most of the anterior and lateral surfaces of the vertebral bodies and intervertebral discs. The posterior longitudinal ligament is relatively weaker and has a denticulate arrangement that permits the passage of vascular structures. Superficial fibres bridge several vertebrae while deeper fibres pass over two joints and have lateral extensions intimately related to the intervertebral disc (Parke & Schiff 1971). The strong central portion of the posterior longitudinal ligament provides resistance to central disc displacement, deflecting it laterally where the lateral extensions are deficient and offer a space for posterolateral displacement.
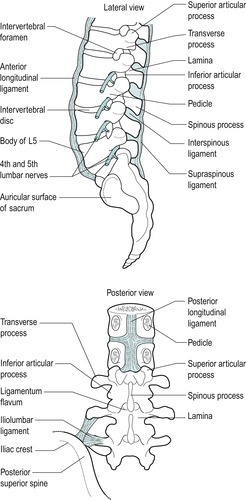 |
| Figure 13.6
The ligaments of the lumbar spine.
|
The ligamentum flavum consists of predominantly yellow elastic fibres and connects adjacent laminae. It controls lumbar flexion by ‘braking’ the separation of the laminae and assisting the return to the upright posture. The elastic fibres also restore the ligament to its normal length after stretching, to prevent buckling into the spinal canal and compression of the spinal cord or cauda equina. Such pathology may arise in the degenerate ligament and contribute to stenosis.
The iliolumbar ligament provides stability for the lumbo-sacral junction (Yamamoto et al 1990), attaching the L5 transverse process to the pelvis. Sometimes a band also passes from the transverse process of L4. This anchorage of L5 to the pelvis may restrict the amount of accommodation possible for disc herniation. Disc herniation at the L5, S1 level may produce severe pain with the patient fixed in flexion, whereas herniation above this level, usually L4–L5, may be accommodated more readily by a lateral shift that will reduce pain.
LUMBAR SPINAL NERVES
The termination of the spinal cord lies approximately level with the L1–L2 disc, and the lumbar and sacral nerve roots descend vertically in the cauda equina, surrounded by the dural sac, to exit via their appropriate lumbar or sacral intervertebral foramina.
Dorsal (sensory) and ventral (mainly motor) nerve roots join to form the relatively short spinal nerve that is situated in the intervertebral foramen, together with the dorsal root ganglion. The dorsal root ganglion is the collected cell bodies of all sensory nerve fibres related to that segment. The cell bodies of the motor axons are located in the anterior horns of the grey matter in the spinal cord. In the intervertebral foramen the spinal nerve is surrounded by the dural nerve root sleeve, which eventually blends with the epineurium of the nerve. Immediately after leaving the intervertebral foramen the spinal nerve divides into dorsal and ventral rami.
Spinal nerves do not possess the same protective connective tissue sheaths as peripheral nerves and are therefore said to be vulnerable to direct mechanical injury (Rydevik & Olmarker 1992).
There are five pairs of lumbar nerves, five pairs of sacral nerves and one pair of coccygeal nerves. Their dorsal and ventral nerve roots pass in the cauda equina in an infero-lateral direction to reach their appropriate level, before joining to emerge through the intervertebral foramina as the spinal nerves. Until the coccygeal level is reached there are several nerve roots passing vertically in the cauda equina (Fig. 13.7).
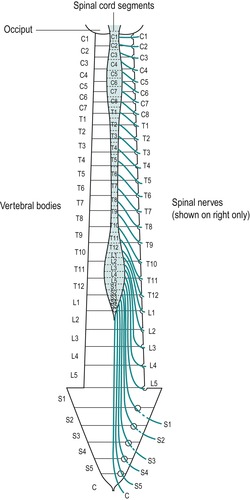 |
| Figure 13.7
The cauda equina and emerging nerve roots.
From Functional Anatomy of the Spine by J Oliver and A Middleditch. Reprinted by permission of Elsevier.
|
The clinical implications of this should be recognized as it is possible for a lumbar disc herniation to encroach on more than one nerve root. It also explains how a lumbar disc herniation could compress the S4 nerve root to affect bladder function.
DEGENERATIVE CHANGES IN THE LUMBAR SPINE
There is a suggestion that the intervertebral discs degenerate first and the subsequent reduction in the ability of the disc to distribute loads equally in all directions causes secondary degenerative changes in the zygapophyseal joints and ligaments (Acaroglu et al 1995, Prescher 1998). The degenerative process has been noted to be most predominant in the L4, L5 and L5, S1 levels but affects all levels of the lumbar spine, resulting in an overall reduction in spinal mobility (Prescher 1998) (Fig. 13.8).
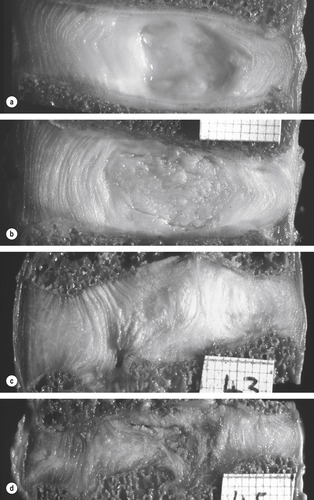 |
| Figure 13.8
Lumbar intervertebral discs sectioned in the mid-sagittal plane, anterior on left. These discs, which were not subjected to any post-mortem loading, represent the first four stages of disc degeneration. (a) Grade 1 disc, typical of ages 15–40 years. (Male 35 years.) (b) Grade 2 disc, typical of ages 35–70 years. The nucleus appears fibrous, and there is some brown pigmentation typical of ageing. However, the disc’s structure is intact and not ‘degenerated’. (Male, 47 years, L2–3.) (c) Grade 3 disc, showing moderate degenerative changes. Note the annulus bulging into the nucleus, damage to the inferior end-plate, and the lack of pigmentation in some regions of the disc. (Male, 31 years, L2–3.) (d) Grade 4 disc, showing severe degeneration. Note the brown pigmentation, the disruption to both end-plates, and internal collapse of the annulus, with corresponding reduction in disc height. (Male, 31 years, L4–5.)
From The Biomechanics of Back Pain by M Adams, N Bogduk, K Burton and P Dolan. Reprinted by permission of Elsevier Ltd.
|
The most marked ageing or degenerative changes occur in the nucleus of the intervertebral disc. There is a reduction in the water and proteoglycan content and a change in the number and nature of the collagen fibres, with the typical type II fibres of the nucleus changing to resemble the type I fibres of the annulus (Umehara et al 1996, Bogduk 2005, Paesold et al 2007). The gel-like appearance is lost as fibrocartilaginous tissue replaces the central portion of the disc and there is a resulting reduction in disc hydrostatic properties and flexibility (Paesold et al 2007). The water content of the nucleus reduces from 90% at birth to approximately 65–71% by the age of 75 and reduces the preloaded state of the disc (Jensen 1980, Taylor & Twomey 1994, Bogduk 2005). A strong familial predisposition for disc degeneration has been demonstrated through twin studies (Paesold 2007). In section the ageing nucleus pulposus develops the appearance of a dry, crumbling mass, changing in colour from the normal glassy greyish-blue towards yellow and brown – an observation that is sometimes referred to as ‘brown degeneration’ (Prescher 1998).
In losing some of its fluid properties, the nucleus is less able to exert a radial pressure on the annulus. Therefore, a greater portion of vertical load is supported by the annulus and the greater stresses contribute to circumferential tears and radial fissures. The lumbar discs become stiffer and less resilient and overall there is reduced mobility in the lumbar spine.
Cell proliferation has been observed in the degenerating nucleus, with lacunae formation (small spaces) containing multi-cell clusters, approximately half of which show signs of necrosis and others of apoptosis (programmed cell death). The outcome is cell loss from the disc (Paesold et al 2007).
Neovascularization of the inner portions of the disc has also been described, possibly accompanied by the growth of nerve fibres (Paesold et al 2007). This is an interesting development from the traditional understanding that the disc does not have its own blood supply and that only the outer third is innervated. Nerve growth factor has been identified exclusively in painful discs when compared to non-painful discs and is a possible mechanism of pain production. Direct treatment to inhibit its action could therefore be a possibility in the treatment of back pain. The need for further research is clear to be able to examine the interplay between neovascularization and neoinnervation, either or both of which could be of relevance regarding pain production and sensation in degenerate discs.
Degeneration affects the structure of the annulus, with a decrease in the number of lamellae. Individual lamellae become thicker and there is fraying, splitting and breakdown of the laminate structure with less evidence of a transitional zone between annulus and nucleus (Marchand & Ahmed 1990, Bernick et al 1991, Holm 1993, Acaroglu et al 1995). This degenerative process causes a change in the tensile properties of the annulus that affect its mechanical properties, as well as rendering it vulnerable to failure at lower stresses (Acaroglu et al 1995).
Three types of annular defect are noted (Osti & Cullum 1994):
• Rim lesions – discrete defects between the outer annulus and the vertebral body
• Circumferential tears – more common in the lateral and posterior layers
• Radial fissures – commonly seen in degenerating discs, extending from the nucleus.
Prescher (1998) explores the results of degeneration of the intervertebral disc material, suggesting that ‘dislocation’ of the annulus and/or the nucleus can occur, which may help in rationalizing the disc as a possible cause of spinal pain. The ‘dislocation’ may present as a ‘disc protrusion’ – a bulging of the disc in a posterior, lateral or anterior direction, which mainly involves nuclear material pushing outwards and stretching or ‘bulging’ the annular fibres. Since annular fibres contain nociceptive nerve endings, stimulation of these may produce primary discal symptoms.
If annular fibres tear, the pressurized nuclear tissue pushes outward through the defect, resulting in ‘disc prolapse’ where it may be responsible for compression or irritation of other pain-sensitive structures, resulting in secondary discal symptoms. The prolapsed tissue may be directed posteriorly, posterolaterally and anteriorly. If it detaches completely, it is referred to as ‘sequestrated’, when it may be directed further, either cranially or caudally. Posterolateral disc prolapse may encroach into the intervertebral canal and may compromise the emerging spinal nerve root, since the intervertebral disc is located at the same level as the emerging spinal nerve in the lumbar spine.
Bone density in the vertebral bodies reduces with age, causing weakening of the trabecular system, a loss of the horizontal trabeculae and a gradual collapse of the vertebral end-plate (Taylor & Twomey 1994, Prescher 1998). This results in the intervertebral disc bowing into the concavity of the weakened end-plate, with consequences for the nutrition of the disc via this route. A loss of overall height with ageing may not be due to a loss in disc height therefore, but to a loss in vertebral body height due to the collapse of the vertebral end-plate and subsequent migration of the discal material into the vertebral body (Taylor & Twomey 1994, Bogduk 2005). However, Prescher (1998) suggests that a pronounced decrease in disc height may result in adjacent spinous processes coming into contact with one another (‘kissing spines’) causing grinding, sclerotic changes and even pseudoarthroses.
Prescher (1998) also discusses degeneration, i.e. spondyloarthrosis of the zygapophyseal joints, suggesting that this particularly affects lumbar joints in patients over 30. Loss of disc height would appear to be the trigger, causing caudal migration of the inferior articular processes and a posterior displacement (or retrolisthesis) of the vertebral body. Advanced spondyloarthrosis may result in anterior displacement of the vertebral bodies, particularly L4, which occurs with the pars interarticularis intact. This phenomenon was noticed by Junghanns (cited in Prescher 1998) and was termed a pseudospondylolisthesis, occurring more frequently in females. This is a separate entity to a spondylolisthesis which affects L5 predominantly and occurs more commonly in males.
Lumbar lesions
Any structures in the lumbar region that receive a nerve supply can be a primary source of somatic pain. Congenital or acquired disorders of a single component of the motion segment cannot exist without affecting the functions of other components of the same segment and the functions of other segmental levels of the spine (Parke & Schiff 1971). However, Schwarzer et al (1994b) consider zygapophyseal joint pain to be uncommon, with discogenic pain a singular, independent disorder.
The zygapophyseal joints, as synovial joints, can be affected by arthritis and the presence of intra-articular structures makes derangement of the joint a possibility. The intervertebral disc is known to degenerate and since the outer annulus receives a nerve supply it can be a primary source of pain. Herniations of discal material are well recognized and are a secondary cause of pain, affecting other pain-sensitive structures.
Traction exerted on the dura and noxious stimulation of the back muscles, ligaments and lumbar zygapophyseal joints have both provoked a pain response (Bogduk 1994). Compression of normal nerves does not provoke a pain response while stimulation of swollen, stretched or compressed nerve roots has been shown to produce leg pain, with the dorsal root ganglion tending to be more tender than other parts of the nerve (Kuslich et al 1991). In the same study, stimulation of the outer annulus fibrosus and the posterior longitudinal ligament produced back pain while there was tenderness on stimulation of the capsule of the zygapophyseal joint, also associated with localized back pain. Pain may be produced in any pain-sensitive structure through chemical, mechanical or ischaemic mechanisms, although it seems more than likely that all factors coexist in disc pathology or herniation.
Chemical pain is the result of irritation of the noci-ceptive nerve endings by the products of inflammation, generally following tissue damage. The products of inflammation can either sensitize nerve endings so that they respond to a lower threshold of stimulus, or activate silent nociceptors (see p. 372) to provoke a response.
Mechanical pain occurs through stretching, compression or distortion of connective tissue structures stimulating the intervening nociceptors. Mechanical stress ultimately produces vascular changes and ischaemia, which activates nociceptors.
While acknowledging the existence of all pain-sensitive structures within the spinal joints, the discal model is central to the concepts of treatment in orthopaedic medicine. With evidence from scans more readily available, the disc would seem to be a major contributor to spinal pain, but the mechanisms of internal derangement, disc herniation and the recovery from disc pathology, often spontaneously, remain unknown. More recent work has concentrated on the chemical effects of displaced nuclear material, while the exact mechanism of pain produced by mechanical compression is unclear.
Primary disc pain
The outer part of the annulus fibrosus receives a nerve supply, some of which is thought to be nociceptive. Pressure exerted on the outer annulus and injection of contrast medium into the disc have each provoked a pain response (Bogduk 1994). Roberts et al (1995) found sensory nerve endings in the form of mechanoreceptors in intervertebral discs and the posterior longitudinal ligament. Golgi tendon organs were the most frequently seen. These may directly elicit pain or modulate muscle activity, perhaps in the form of muscle spasm that is often associated with lumbar lesions. Coppes et al (1997) established a more extensive disc innervation in severely degenerative human lumbar discs, when compared with normal discs, that invaded deeper than the outer third of the annulus. The nociceptive properties of some of the nerves were suggestive of substance P immunoreactivity.
As with all connective tissue structures, the elastic property of the collagen fibres in the annulus, enhanced by ‘crimp’, allows it to tolerate tensile forces. When placed under excessive mechanical tension the annulus deforms and may directly squeeze or distort pain-sensitive nerve endings, producing pain of mechanical origin. Once forces exceed normal limits microtrauma occurs, producing pain of chemical origin. The most vulnerable position for the annulus is when it is placed under rotational strains in flexion, ultimately resulting in circumferential splits (Bogduk 2005). Shear and tensile forces initiate damage at the peripheral portion of the disc but not at the centre, since fibre strain is always minimal at the centre and maximal at the periphery (Brinckmann 1986).
With consideration for the chemical contribution to low back pain, substance P causes the release of inflammatory mediators that affect the local environment and may sensitize nociceptors, resulting in chronic pain (Zimmermann 1992, Beaman et al 1993, Palmgren et al 1996). Substance P immunoreactive nerve fibres have been identified in the zygapophyseal joint capsule and synovial folds, the supraspinous ligament, posterior longitudinal ligament and the annulus fibrosus. Some fine unmyelinated and small myelinated fibres in the annulus are thought to serve as a type of pain fibre – termed silent nociceptor – that is not excited by mechanical stress, but responds to algesic chemicals produced at times of tissue damage or inflammation (Cavanaugh 1995).
There is no evidence as yet to support a nerve supply to the nucleus pulposus and pathological processes are thought to occur internally within the nucleus without provoking a pain response.
Secondary disc pain
Mixter & Barr (1934) suggested that a displaced fragment of the intervertebral disc into the vertebral canal causes mechanical compression of the lumbar nerve roots and sensory root ganglia. However, the mechanism by which back or leg pain is produced by this mechanical compression is still not fully understood. Kuslich et al (1991) demonstrated that stimulation of a normal nerve root did not produce a pain response, while stimulation of an already swollen, stretched or compressed nerve root produced leg pain. However, no suggestion was made for how much, or for how long, mechanical stress should be applied to a previously undamaged nerve root before changes occur to make it sensitive.
The extruded disc material in disc prolapse may consist of nuclear material, sometimes with end-plate material and occasionally elements of the annulus (Bogduk 1991, Brock et al 1992). However, normal disc material does not usually rupture and the nucleus is thought to undergo some process of deterioration or degradation, in order for it to be displaced (Bogduk 1991). Hormonal, nutritional or viral factors, or simply an acceleration of the degenerative process, have been proposed as possible reasons for the degradation of the nucleus.
Bogduk (2005) offers a plausible explanation of mechanical trauma together with an autoimmune reaction within the nucleus. Proteins in the nucleus may act as an antigen which, when exposed to the circulation for the first time, triggers an autoimmune response. Intrinsically, this can occur via contact with the circulatory plexus associated with the vertebral end-plate through microfracture due to compressive loading. The provoked autoimmune inflammatory response causes degradation of the nucleus which continues once the microfractures heal. Degrading the nuclear material in this way renders it capable of herniation. Changes must also occur within the annulus since herniation of nuclear material can only occur through a radial fissure in a weakened annular wall (Brinckmann 1986).
The posterolateral corners of the annulus are irregular, thin and potentially weak (Umehara et al 1996). Radial fissures and circumferential splits commonly develop here, providing an escape route for the degraded nuclear material, when a force is applied sufficient to expel it. Flexed postures, especially combined with rotation, trigger backwards herniation of the nuclear material through a weakened annular wall (Bogduk 2005).
As well as being a primary source of pain, the prolapsed disc can have a secondary effect on any pain-sensitive structure lying within the vertebral canal or intervertebral foramen. This effect can be mechanical through compression and distortion, chemical through the inflammatory process and ischaemic through the pressure of oedema.
Once discal material enters the vertebral canal it may again be treated as foreign and stimulate an extrinsic autoimmune inflammatory response as it comes into contact with the circulation in the vertebral canal. The resulting chemical mediators affect adjacent pain-sensitive structures. If the prolapsed fragment is small, it will be dealt with by the macrophage system; if large, the inflammatory process continues until the fragment is eventually organized into scar tissue (Hirabayashi et al 1990).
Both mechanical and inflammatory mechanisms can produce ischaemia. The inflammatory mediators produced are thought to have a role in somatic pain (McCarron et al 1987, Bogduk 1994) and in radicular pain (Doita et al 1996, Kang et al 1996, Takahashi et al 1996, Greening 2004). Peng et al (2007) use the term ‘chemical radiculo-pathy’ to describe the irritation of nerve roots by inflammatory mediators travelling into the epidural space following disruption of the annulus.
The quality of pain seems to be instrumental in distinguishing somatic and radicular pain. Somatic referred pain is produced when any sensitive structure is stimulated and is deep, aching and hard to localize. Radicular pain is produced when a nerve root is compressed or irritated and was described by Smyth & Wright, cited in Bogduk (2005), as shooting, lancinating pain felt in a relatively narrow band, approximately 4 cm wide, into the limb (see Ch. 1).
Compression of undamaged nerves produces numbness, paraesthesia and muscle weakness. Under some circumstances, which are not well understood, compression alters nerve root conduction and compromises nutritional support, causing the nerves to become pain-sensitive through inflammation, ischaemia or both (Garfin et al 1995). Studies suggest that the products of the auto-immune inflammatory process stimulated by the displaced discal material may increase the sensitivity of the nerve root to bradykinin and be involved in the pathophysiology of radiculopathy (Saal 1995, Kang et al 1996, Takahashi et al 1996).
The mechanical effects of compression of a nerve root may be direct or more probably indirect through ischaemia. A sequence of events may be induced involving impairment of nutrition and increased microvascular permeability, leading to intraneural oedema, blockage of axonal transport and altered function (Rydevik & Olmarker 1992). For referred leg pain to be radicular in origin, arising from compression of a nerve root, it must be accompanied by other signs of compression – paraesthesia and muscle weakness. If these are absent, pain referred to the limb must be somatic in origin (Bogduk 2005).
However, in clinical practice, signs and symptoms of somatic and radicular pain coexist since, for a disc protrusion to compress a nerve root, it must first compress and stimulate nerve endings in its dural nerve root sleeve. Thus the dural nerve root sleeve produces somatic referred pain, either mechanical or chemical in origin, while the nerve root may produce radicular pain and other signs and symptoms of nerve compression.
In studies on peripheral nerves, a critical level of pressure is significant for structural and functional changes to occur and longer periods of compression would seem to be responsible for more damage (Jancalek & Dubovy 2007). Prolonged compression may produce changes in axonal transport impairing the transport of proteins from the nerve cell body to the distal parts of the body and resulting in compression-induced effects in the distal axonal segment (double crush syndrome).
The trauma evoked by compression may alter the permeability of the intraneural vessels, resulting in oedema. The oedema usually persists after removal of the compression and therefore may adversely affect the nerve root for longer. The presence of intraneural oedema is thus related to intraneural fibrosis and adhesion formation.
Unfortunately, at the time of writing, there do not appear to have been many experimental studies on spinal nerves, but they are known to be more susceptible to compression than peripheral nerves since they do not have the same protective connective tissue sheaths. The critical pressure levels for compression to induce impairment of nerve nutrition or function are not known, or the length of time that compression needs to be applied before changes occur and they become pain-sensitive.
Huang et al (2007) describe that traction injuries to the dorsal nerve root can avulse the sensory axons, e.g. cauda equina which cannot regenerate through axonal regrowth from the dorsal root ganglion into the spinal cord. There is a divide between the central nervous system (CNS) and the peripheral nervous system (PNS), the transition zone. Regeneration cannot occur backwards from the PNS to the CNS and, since the nerve roots themselves are components of the PNS, this has considerable significance if disruption of the S4 nerve root is suspected.
Differential diagnosis at the lumbar spine
The mechanism by which the lumbar intervertebral disc produces pain is probably complicated, with several factors contributing to the diagnosis. It is important to understand the anatomy and the possible mechanisms for pain production, as discussed above.
Patients with non-mechanical causes of back pain can present with signs and symptoms that mimic those of disc pathology. It is important to recognize those features that allow them to be identified as ‘red flags’ (indicators of serious spinal pathology), since manual techniques are either contraindicated or not appropriate, and the patient needs to be referred to the appropriate specialist.
Mechanical lumbar lesions
A recap of the terminology used to describe disc herniation is provided below to add clarity to the following discussion. In contrast to the cervical spine, where the degenerate disc tends to displace as a central bar-like protrusion of the annulus, the degenerate lumbar disc involves degradation and herniation of nuclear material through a weakened annular wall. This herniation may occur as a protrusion into the weakened annulus where it may produce primary disc pain, or as a prolapse where nuclear material moves into the vertebral canal. Here it can have a secondary effect on any pain-sensitive structure by mechanisms involving compression, inflammation and ischaemia.
A central prolapse affects central structures, in particular the posterior longitudinal ligament and the dura mater. Pain arising from compression of the dura mater is multisegmental in nature (see Ch. 1). A posterolateral prolapse affects unilateral structures, mainly the dural nerve root sleeve and nerve root, which tend to produce segmental pain.
A disc herniation, either protrusion or prolapse, produces a pattern of signs and symptoms that are progressive, with a history of increasing, worsening episodes. Often the precipitating factor is trivial. The dural nerve root sleeve and nerve root are vulnerable in the lumbar spine to posterolateral prolapse, with pain and other associated symptoms referred into the leg. A classification of clinical models has been established to aid diagnosis and to establish treatment programmes. These are outlined in the treatment section later in this chapter, since they relate directly to treatment selection.
Disc protrusion
• Degenerate disc material bulges into the weakened laminate structure of the annulus, where it can produce primary disc pain since the outer annulus receives a nerve supply.
Disc prolapse
• Discal material passes through a radial or circumferential fissure in the annulus which provides it with an escape route into the vertebral canal where it has a secondary effect on the pain-sensitive structures in the vertebral canal. The sequela of this is sequestration of the disc.
Other causes of back pain, leg pain and associated signs and symptoms
Non-mechanical lesions, including serious pathology, have features that do not ‘fit’. Since they represent contraindications to manual orthopaedic medicine treatments, they must be recognized and the patients referred appropriately.
Arthritis, in any form, presents with the capsular pattern which is demonstrated by the lumbar spine as a whole.
• Degenerative osteoarthrosis affects the intervertebral joint and the zygapophyseal joint. The consequences of degeneration and degradation of the intervertebral disc lead to increased possibility of disc herniation. Disruption of the intervertebral joint affects the zygapophyseal joint, causing the joint surfaces to bear increased weight.
• Osteophytes may form at the peripheral margins of the disc, possibly in association with rim lesions of the annulus, as well as at the zygapophyseal joints. Overall the degenerative changes may lead to spinal stenosis.
• Spinal stenosis is a term that has become synonymous with neurogenic or spinal claudication. It should be used to define any symptomatic condition in which limited space in the vertebral canal is a significant factor (Porter 1992). Lateral stenosis affects the nerve root; central stenosis affects the spinal cord or cauda equina and may coexist with lateral stenosis.
Some patients have a developmental abnormality where the spinal canal has a trefoil shape in cross-section (Vernon-Roberts 1992) and spinal stenosis is particularly prevalent in this group. Narrowing can also occur as a result of degenerative changes through ageing, injury, disease, or as a result of surgery (Lee et al 1995). Irrespective of cause, a small vertebral canal can have clinical significance for back pain (Porter & Oakshot 1994).
Degenerative spinal stenosis can be associated with osteophyte formation at the vertebral body or zygapophyseal joints, with reactive proliferation of capsular and soft tissues, and fibrous scarring around the nerve roots. The vertebral canal can be compromised by thickening of the ligamentum flavum which shows a 50% increase in thickness with ageing over a normal lifespan (Twomey & Taylor 1994). Degenerative spondylolisthesis may also narrow the canal (Osborne 1974, Rauschning 1993).
A disc prolapse may significantly reduce the size of both the vertebral and intervertebral foramina and Porter et al (1978) noted that the risk of developing disabling symptoms from disc prolapse is inversely related to the size of the spinal canal. The anterior margin of the canal can be indented to compress the cauda equina by a lax posterior longitudinal ligament overlying degenerate prolapsed discs. Cyriax (1982) termed this the ‘mushroom phenomenon’.
Spinal stenosis can produce neurogenic or spinal claudication which was recognized by Verbiest, in 1954, as due to structural narrowing of the vertebral canal compressing the cauda equina and producing claudication symptoms (Porter 1992). Men over the age of 50 with a lifestyle that has involved heavy manual work may be affected. The entire cauda equina can be compressed centrally causing bilateral symptoms, or the emerging nerve root can be affected (Osborne 1974). The patient complains of discomfort, pain, paraesthesia and heaviness in one or both legs while standing or walking. There may be night cramps and restless legs. A long history of back pain may be present and the patient may have undergone back surgery at some time. The symptoms are usually of several months’ duration. There is usually a threshold distance when the symptoms develop and a tolerance when they have to stop; the tolerance distance is about twice the threshold (Porter 1992). These symptoms are similar to those of the ischaemic pain associated with intermittent claudication of peripheral vascular disease and with the age group affected; the two conditions can coexist, making diagnosis difficult.
With neurogenic claudication, stooping or bending forwards relieves the symptoms and allows the patient to continue. Flexion increases the space in the canal and tightens the ligaments, straightening out the buckling that tends to occur with degeneration. The patient can usually walk uphill, which involves a flexed posture, easier than walking downhill, which involves an extended posture.
On examination, the patient often stands with a stooped posture, with flexed hips and knees and a flattened lumbar spine with loss of the lordosis. This posture becomes more evident on walking. The capsular pattern is present with marked loss of spinal extension. Extension may produce the pain as it decreases the calibre of the spinal canal while, conversely, flexion relieves the pain (Osborne 1974). Dynamic variations in flexion and extension are related to changes in the buckling of the ligamentum flavum and the herniation of the intervertebral discs (Rauschning 1993). The rest of the examination may be unremarkable and back pain itself may not be a feature. Neurological signs are often absent.
Management may involve spinal decompressive surgery or advice on how to live with the condition. Symptoms do not usually resolve, but they do not always get worse.
Kotil & Bilge (2007) report on two cases of haematoma in the ligamentum flavum as a rare cause of low back and leg pain in elderly patients. The signs were consistent with L5 root compression. A cautious approach is required for leg pain in the elderly, especially as a first presentation. Full consideration should be given to the entire clinical presentation and further investigation is wise if there is any suspicion that the cause is non-mechanical.
• Rheumatoid arthritis can affect the spinal joints and this has been covered in Chapter 8. Ankylosing spondylitis is discussed in Chapter 14.
Structural abnormalities can be completely asymptomatic or may produce pain, inflammation and neurological signs, or coexist with disc herniation.
• Spondylolysis is a defect in the pars interarticularis (the neural arch between the lamina and the pedicle) of L5 and sometimes L4.
• Spondylolisthesis is an anterior shift of one vertebral body on another, usually involving slippage of L5 on S1. It may be congenital, acquired through degeneration, trauma, or as a sequela to spondylolysis. It is commonly associated with over-training in such sports as gymnastics, involving hyperextension, and the rotational stresses involved in fast bowling (Bush 1994). If symptomatic, the main symptom is back pain that may be referred to the buttocks. The pain is aggravated by exercise and standing and is eased by sitting. Inspection may reveal excessive skin folds above the defect and a step defect may be felt on palpation (Norris 2004). On examination, extension is limited and painful and passive overpressure of the affected vertebra produces the pain.
Diagnosis is confirmed by oblique X-rays that show the typical ‘Scottie dog’ view. If the ‘Scottie dog’ is wearing a collar, there is a defect in the pars interarticularis and the patient has spondylolysis. If the head of the ‘Scottie dog’ is separated from the neck, the patient has spondylolisthesis.
X-ray assesses the degree of spondylolisthesis which is measured by the distance the slipped upper vertebra moves forward on its lower counterpart. Slippage is divided into four degrees, progressing from a first-degree slip, which is a forward displacement of one quarter of the anteroposterior diameter of the vertebral body, to a fourth-degree slip with a full anteroposterior diameter displacement (Corrigan & Maitland 1983).
A particular feature of serious non-mechanical conditions is an unwell patient with possible weight loss. Neoplasm of the lumbar spine, although relatively uncommon, should be considered as a possible cause of low back and leg pain. Metastases may be secondary to carcinoma of the bronchus, breast, ovary, prostate, thyroid or kidney. Metastatic invasion may involve bone or may be intradural. Primary bone tumours occasionally affect the posterior elements of the vertebrae and multiple myeloma can produce backache due to vertebral involvement.
• Neoplasm involving the lumbar spine may be clinically silent or may produce pain in isolation or cause associated neurological deficit (Findlay 1992). The pain may be due to compression or distortion of pain-sensitive structures and/or to destructive changes in the bone. Neurological deficit is usually of a lower motor neuron type and it may begin either at the same time as the pain or prior to it.
The pain of neoplastic disease has characteristic features. It is usually deep-seated, boring, relatively constant, steadily worsening and often persistent at night. If there is collapse of the vertebral body, the pain will be associated with movement and activity due to the spinal instability (Findlay 1992). Aside from night pain, symptoms of weakness, fatigue and weight loss should be considered to be serious in a patient complaining of back pain.
The signs and symptoms of a tumour can mimic a disc lesion. Palma et al (1994) reported three cases of neurinoma of the cauda equina initially misdiagnosed as a disc lesion. Pain which worsens during recumbency and improves in sitting and walking, together with bilateral, multiple root involvement, is more indicative of an expanding lesion in the cauda equina than sciatica. Unusual cases of a primary extraosseous Ewing sarcoma in a 15-year-old girl with a history of chronic back and leg ache (Allam & Sze 1994) and primary Hodgkin’s disease of the bone presenting clinically with an extradural tumour (Moridaira et al 1994) exist in the literature.
• Infection may cause osteomyelitis or discitis and epidural abscess is possible. Pyogenic organisms, e.g. Staphylococcus aureus, Myobacterium tuberculosis or, rarely, Brucella, may be responsible (Kumar & Clark 2002). The clinical presentation of spinal infection varies from a complaint of back pain only, to being very ill, emaciated and febrile and with a raised erythrocyte sedimentation rate. On examination tenderness is elicited on percussion of the affected vertebra and widespread muscle spasm may be present. X-ray may show loss of bony contour, cavitation and collapse and possibly an associated paravertebral abscess (Kemp & Worland 1974).
• Aortic aneurysms are commonly abdominal (Kumar & Clark 2002). When they rupture they may present with epigastric pain that radiates through to the back. The patient is shocked and a pulsatile mass is felt. This situation is a medical emergency. Galessiere et al (1994) presented three cases of chronic, contained rupture of aortic aneurysms associated with vertebral erosion. The patients presented with a history of chronic backache.
Non-organic back pain should be considered at the lumbar spine, although true psychogenic back pain is rare. Anxiety tends to be associated with acute back pain while depression is associated with chronic back pain; both are indicators of the patient’s distress. Back pain may begin as a physical problem and, as such, is generally addressed by mechanical or physical treatments. If low back pain becomes chronic, psychosocial factors may be more obvious than physical signs, and illness behaviour becomes a relevant component. Illness behaviour is a normal phenomenon and a physical problem exists with varying degrees of illness behaviour (Waddell 1998). As clinicians, the physical and psychosocial factors that coexist in chronic pain must therefore be understood in order to provide total care. The interested reader is referred to the work of Waddell (1998) which includes the biopsychosocial model and describes non-organic or behavioural signs, the so-called ‘yellow flags’, as indicators of pychological distress and risk of long-term disability.
COMMENTARY ON THE EXAMINATION
Observation
A general observation of the patient’s face, posture and gait will alert the examiner to the seriousness of the condition. Patients in acute pain will generally look tired. They may have adopted an antalgic posture of flexion or lumbar scoliosis which is generally indicative of an acute locked back, possibly due to a disc lesion. A lumbar lateral shift is pathognomic of a disc lesion (Porter 1995). Patients may not be able to sit during the examination due to discomfort from this particular posture. Their gait may be uneasy with steps taken cautiously, obviously wary of provoking twinges of pain by sudden movements or pain on weight-bearing. A dropped foot may be evident on walking and will lead you to consider involvement of the L4 nerve root affecting the tibialis anterior muscle and interfering with function.
The Box on page 379 lists the ‘red flags’ for the possible presence of serious pathology that should be listened for and identified throughout the subjective and objective examination. In isolation, many of the flags may have limited significance but it is for the clinician to consider the general profile of the patient and to decide whether contraindications to treatment exist and/or whether onward referral is indicated.
History (subjective examination)
The history is particularly important at the spinal joints. Vroomen et al (1999) published a systematic review of the diagnostic value of the history and examination of patients with suspected sciatica, establishing that very little attention has been paid to the history of such events. Pain distribution was the only sensitive sign of the level of disc herniation, with straight leg raising seemingly a sensitive sign and crossed straight leg raise a strong indicator of nerve root compression. Disagreement exists in the literature with regard to the value of decreased muscle strength, sensory loss and altered reflexes as signs of nerve root involvement and it is unclear whether the physical examination adds much to the diagnostic value of the history.
There is a close relationship between signs and symptoms from the lumbar spine, sacroiliac joint and hip joint. The history will help with the differential diagnosis but conditions at each of these areas can coexist. Major pathology can affect the spine, such as malignancy, infection, spondyloarthropathy or fracture, but these represent a small percentage of the problems compared with mechanical lesions of the lower back (Swezey 1993). X-ray findings can be misleading, particularly when showing the degenerative changes of osteoarthrosis which may or may not be painful. Similarly, spondylolysis progressing to spondylolisthesis occurs in approximately 5% of adults but is symptomatic in only half of them (Swezey 1993).
The age, occupation, sports, hobbies and lifestyle of the patient may give an indication of provoking mechanisms. Often the incident precipitating the episode of back pain may be relatively minor, though factors predisposing to the event may have been continuing for some time. While cure will be the initial aim of treatment for this presenting incident, ultimately the management of the condition and prevention of recurrence will become the patient’s responsibility. Patients will require advice and guidance on management of their back condition to prevent chronicity.
Many occupations involve a flexion lifestyle, e.g. sedentary office work and brick-laying apply postural stress to the intervertebral joint. These patients require advice about changing postures to minimize the stress inflicted by work. Directives on the manual handling of loads are in existence and patients should receive advice about this in their place of work. The vibration of motor vehicle driving may have an influence, as well as the sitting posture involved, which is known to increase intradiscal pressure (Osti & Cullum 1994).
Assessment of patients with chronic low back pain presents a particular challenge to the clinician. Emotional, environmental and industrial factors may influence pain perception, while monotony or dissatisfaction at work or home is relevant (Osti & Cullum 1994). Distinction will need to be made between the true physical symptoms of the presenting condition and those relating to psychosocial factors that influence the way the patient reacts to the pain (see above). Enquiry should be made about the possibilities of secondary gain factors relating to disability, or the presence of psychological or social stresses that might predispose the patient to chronic pain disorders (Swezey 1993). Standard questions on quality of sleep, tiredness levels, concentration, appetite, etc. may establish whether the patient is depressed.
The site of the pain will give an indication of its origin (see Ch. 1). Lumbar pain is generally localized to the back and buttocks or felt in the limb in a segmental pattern. Sacroiliac pain may be unilateral, felt in the buttock, or more commonly in the groin, and occasionally referred into the leg. The hip joint may produce an area of pain in the buttock consistent with the L3 segment, pain in the groin, or pain referred down the anteromedial aspect of the thigh and leg to the medial aspect of the ankle. Dural pain is multisegmental and will be central or bilateral. Pressure on the dural nerve root sleeve will be referred segmentally to the relevant dermatome. Pressure on the nerve root will refer pain to the relevant limb with accompanying symptoms of paraesthesia at the distal end of the dermatome.
Vucetic et al (1995) considered the difficulty that patients have in giving a precise verbal description of pain. They support the use of a pain drawing, which may take a few minutes to obtain, but the result can be grasped at a glance. Waddell (1992) warns that how a patient draws pain is influenced by emotional distress and that non-anatomical, widespread and magnified drawings tell of the patient’s distress rather than the physical characteristics of the pain.
The spread of pain will not only give an indication of its origin, but also the severity or irritability of the lesion. Generally, the more peripherally the pain is referred, the greater the source of irritation. A mechanical lesion due to a displaced lumbar disc produces central or unilateral back or buttock pain. If the pain shifts into the leg, it generally ceases or is reduced in the back. Pain of non-musculoskeletal origin does not follow this pattern and serious lesions produce an increasing spreading pain, with pain in the back remaining as severe as that felt peripherally.
The onset and duration of the pain can assist the choice of treatment (see below). In very general terms, a sudden onset of pain may respond to manipulation, while a gradual onset of pain may respond better to traction. The whole clinical picture will need to be reasoned through in order to make a decision about treatment, which may contradict these rules of thumb.
The nature and mode of onset are important. The patient may remember the exact time and mode of onset which may have involved a flexed and rotated posture. If lifting was involved in the precipitating episode, it may only have been a trivial weight. If the patient reports a gradual onset of pain, it is worth questioning further for details of previous activity. A minor traumatic incident some time before the onset of back pain or the maintenance of a sustained flexion posture may have been sufficient to provoke the symptoms.
The gradual onset of degenerative osteoarthrosis is common in the zygapophyseal joints and hip joints, while a subluxation of the sacroiliac joint can occur with a sudden onset. Serious pathology develops insidiously.
If trauma is involved, the exact nature of the trauma should be ascertained and any possible fracture eliminated. Direct trauma may produce soft tissue contusions, while fracture may involve the spinous process, transverse process, pars interarticularis, vertebral body or vertebral end-plate. Compression fractures of the vertebral body are common in horse riders and those falling from a height, and involve the vulnerable cancellous bone of the vertebral body (Hartley 1995). Hyperflexion injuries may cause ligamentous lesions or involve the capsule of the zygapophyseal joint, while hyperextension injuries compress the zygapophyseal joints. Both forces can injure the intervertebral disc.
The symptoms and behaviour need to be considered. The behaviour of the pain will give an indication of the irritability of the patient’s condition and provide clues to differential diagnosis. Serious pathologies of the spine, including fractures, tumours or infections are relatively rare, accounting for less than 1% of all medical cases seen for spinal assessment. Despite this, the clinician must remain alert to clinical indicators that need more extensive investigation than the basic clinical examination (Sizer et al 2007).
The pattern of all previous episodes of back pain should be ascertained, as in disc lesions a pattern of gradually worsening and increasing episodes of pain usually emerges.
The mechanisms whereby the disc herniation can cause pain have been discussed earlier in this chapter. The typical pattern of pain from disc herniation is usually one of a central pain which moves laterally. As the pain moves laterally, the central pain usually ceases or reduces. A gradually increasing central pain accompanied by an increasing leg pain is indicative of serious pathology and this pain is usually not altered by either rest or activity.
The daily pattern of pain is important and typically a disc lesion produces either a pattern of pain that is better first thing in the morning after rest, becoming worse as the day goes on, or, since the disc imbibes water overnight, the patient may experience increased pain on weight-bearing first thing in the morning due to increased pressure on sensitive tissues. Patients can sleep reasonably well at night as they are usually able to find a position of ease.
Mechanical pain can cause an on/off response through compression or distortion of pain-sensitive structures. This can involve the annulus itself or structures in the vertebral canal. The patient with a disc lesion usually complains of pain on movement easing with rest. Changing pressures in the disc affect the pain and it tends to be worse with sitting and stooping postures than when standing or lying down. In an acute locked back, small movements can create exquisite twinging pain.
Herniated disc material may produce an inflammatory response resulting in chemical pain. Chemical pain is characteristically a constant ache associated with morning stiffness. Sharper pain can also be associated with chemical irritation as the nerve endings become sensitized and respond to a lower threshold of stimulation. It is important to differentiate mechanical back pain from inflammatory arthritis and sacroiliac joint lesions through consideration of other factors, since they also produce pain associated with early-morning stiffness.
Radicular pain is generally a severe lancinating pain, often burning in nature, which is felt in the distribution of the dermatome associated with the nerve root. Sciatica is commonly associated with lumbar disc pathology and will occur if the L4–L5, S1 or S2 nerve roots are involved. If the higher levels are involved, pain will similarly be referred into the relevant segment.
The language used by patients to describe the quality of their pain will indicate the balance between the physical and emotional elements of their pain. Words such as ‘throbbing’, ‘burning’, ‘twinging’ and ‘shooting’ describe the sensory quality of the pain; emotional characteristics are expressed in such words as ‘sickening’, ‘miserable’, ‘unbearable’ and ‘exhausting’ or vocal complaints such as moans, groans and gasps (Waddell 1992, 1998).
The other symptoms described by the patient provide evidence for differential diagnosis, contraindications to treatment and the severity or irritability of the lesion. An increase in pressure through coughing, sneezing, laughing or straining can increase the back pain and this is the main dural symptom. Paraesthesia is usually felt at the distal end of the dermatome and is a symptom of nerve root compression. Confirmation of this is made through the objective compression signs of muscle weakness, altered sensation and reduced or absent reflexes.
Specific questions must be asked concerning pain or paraesthesia in the perineum and genital area as well as bladder and bowel function. The presence of any of these symptoms indicates compression of the S4 nerve root at the preganglionic extent which could produce irreversible damage, and indicates immediate referral for surgical opinion. A transition zone exists in the preganglionic region between the peripheral and central nervous systems and repair cannot occur across the transition zone if the nerve is disrupted (Huang et al 2007). This study examines the possibility of surgical repair of the nerve to encourage regeneration but the repair has been performed in rats only at this stage.
Manipulation is absolutely contraindicated in these cases. The symptoms of difficulty in passing water, inability to retain urine or lack of sensation when the bowels are opened are important. It is not unusual to find urinary frequency or difficulty in defecating associated with effort in hyperacute lumbar pain.
Bilateral sciatica with objective neurological signs and bilateral limitation of straight leg raise suggest a massive central protrusion compressing the cauda equina through the posterior longitudinal ligament, with possible rupture of the ligament (Cyriax 1982). It is an absolute contraindication to manipulation, since a worsening of the situation could lead to irreversible damage to the cauda equina, as mentioned above. The symptoms of cauda equina compression should be distinguished from multisegmental, dural reference of pain into both legs, where there may be bilateral limitation of straight leg raise but no neurological signs.
Questioning the patient about other joint involvement will indicate whether inflammatory arthritis exists or if there is a tendency towards degenerative osteoarthrosis.
The past medical history and the patient’s current general health will help to eliminate possible serious pathology, past or present. An unexplained recent weight loss may be significant in systemic disease or malignancy. Visceral lesions can refer pain to the back, e.g. kidney, aortic aneurysm or gynaecological conditions. Infections should be obvious, with an unwell patient showing a fever. Malignancy can affect the lumbar and pelvic region but the pattern of the pain behaviour does not generally fit that of musculoskeletal origin. Past history of primary tumour may indicate secondaries as a possible cause of back pain. Serious conditions produce an unrelenting pain; night pain is usually a feature and is responsible for the patient looking tired and ill. As well as past medical history, establish any ongoing conditions and treatment. Explore other previous or current musculoskeletal problems with previous episodes of the current complaint, any treatment given and the outcome of treatment.
The medications taken by the patient will indicate their current medical state as well as alerting the examiner to possible contraindications to treatment. Anticoagulant therapy and long-term oral steroids are contraindications to manipulation. It is useful to know what analgesics are being taken and how frequently. This gives an indication of the severity of the condition and can be used as an objective marker for progression of treatment, with the need for less analgesia indicating a positive improvement. If patients are currently taking antidepressant medication, this may indicate their emotional state and possibly exclude them from manipulation. Care is needed in making this decision, however, since antidepressants can be used in low doses as an adjunct to analgesics in back pain.
• Young: Under 20
• Elderly: First episode over 55
• Violent trauma
• Past medical history of malignancy
• Constant progressive pain
• Cauda equina syndrome
• Unremitting night pain
• Systemically unwell
• Unexplained weight loss
• Drug abuse and HIV
• Long-term systemic steroid use
• Widespread neurological signs and symptoms
• Gait disturbance
• Thoracic pain
• Persisting severe restriction of lumbar flexion
• Associated abdominal pain
• Osteopenic/osteoporotic
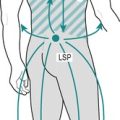
• Sign of the buttock (see p. 274)
Inspection
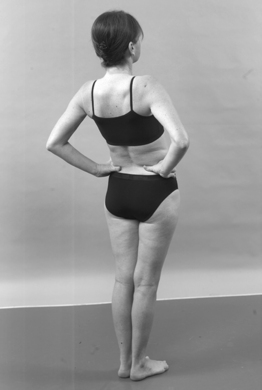
The patient should be adequately undressed down to underwear and in a good light. The difficulty in undressing, especially of socks and shoes, is associated with disc pathology and indicates the irritability of the lesion. A general inspection from behind, each side, and in front will reveal any bony deformity. The general spinal curvatures are assessed, i.e. the cervical and lumbar lordosis and the thoracic kyphosis. The level of the shoulders, inferior angles of the scapulae, buttock and popliteal creases, the position of the umbilicus and the posture of the feet can all be assessed for relevance to the patient’s present condition.
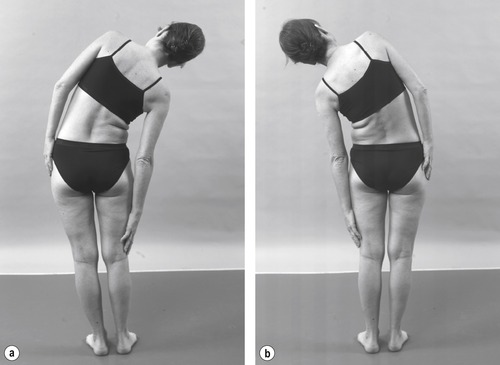
Any structural or acquired scoliosis is noted. In disc pathology, the patient may have shifted laterally to accommodate the herniation and this is evident in standing. Small deviations can be noted by assessing the distance between the waist and the elbow in the standing position. In hyperacute back pain, the patient may be fixed in a flexed posture and unable to stand upright, and any attempt to do so produces twinges of pain.
The level of the iliac crests and the posterior and anterior superior iliac spines gives an overall impression of leg length discrepancy or pelvic distortion (Fig. 13.9). If these are considered relevant, they can be investigated further. Postural asymmetry and malalignment are not necessarily indicative of symptoms. It is worth noting them, since imbalances can be explained to the patient and addressed in the final rehabilitation programme in an attempt to prevent recurrence. If structural abnormalities are considered responsible for the present condition, a full biomechanical assessment will need to be conducted.
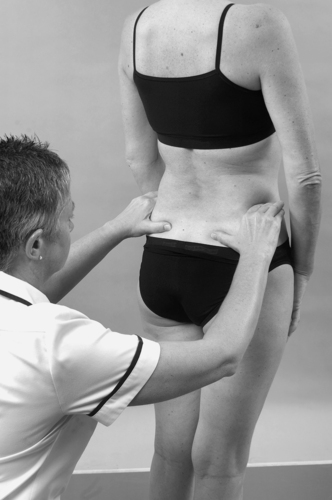 |
| Figure 13.9
Inspection for pelvic levels.
|
Colour changes and swelling are not expected in the lumbar spine unless there has been a history of direct trauma. Any marks on the skin, lipomas, ‘faun’s beards’ (tufts of hair), birthmarks or café-au-lait spots may indicate underlying spinal bony or neurological defects (Hoppenfeld 1976, Hartley 1995). An isolated ‘orange-peel’ appearance of the skin that is tough and dimpled may indicate spondylolisthesis at that level (Hartley 1995). Patients with low back pain often apply a hot water bottle to the area, which produces an erythematous skin reaction called erythema ab igne (redness from the fire). Swelling is not usually a feature but muscle spasm may give the appearance of swelling, especially to the patient.
Muscle wasting may not be obvious if the attack of low back pain is recent. Chronic or recurrent episodes of pain may show wasting in the calf muscles or possibly the quadriceps or gluteal muscles.
Palpation may be conducted to assess changes in skin temperature and sweating suggestive of autonomic involvement. Palpation for swelling is not usually necessary at the spinal joints. In standing, the lumbar spine is palpated for a ‘shelf’ that would indicate spondylolisthesis.
State at rest
Before any movements are performed, the state at rest is established to provide a baseline for subsequent comparison.
Examination by selective tension (objective examination)
The suggested sequence for the objective examination will now be given, followed by a commentary including the reasoning in performing the movements and the significance of the possible findings.
Articular signs
• Active lumbar extension (Fig. 13.10)
 |
| Figure 13.10
Active extension.
|
• Active lumbar right side flexion (Fig. 13.11a)
 |
| Figure 13.11
(a,b) Active side flexions.
|
• Active lumbar left side flexion (Fig. 13.11b)
• Active lumbar flexion (Fig. 13.12)
 |
| Figure 13.12
Active flexion.
|
• Resisted plantarflexion, gastrocnemius (Fig. 13.13): S 1, 2
 |
| Figure 13.13
Resisted plantarflexion in standing.
|
Supine lying
• Passive hip flexion (Fig. 13.14)
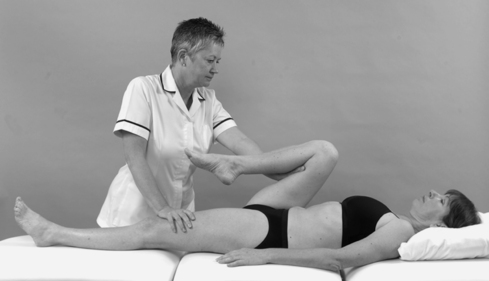 |
| Figure 13.14
Passive hip flexion.
|
• Passive hip medial rotation (Fig. 13.15)
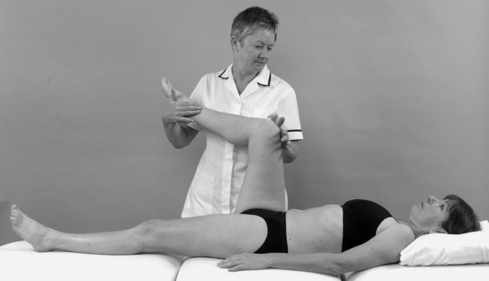 |
| Figure 13.15
Passive hip medial rotation.
|
• Passive hip lateral rotation (Fig. 13.16)
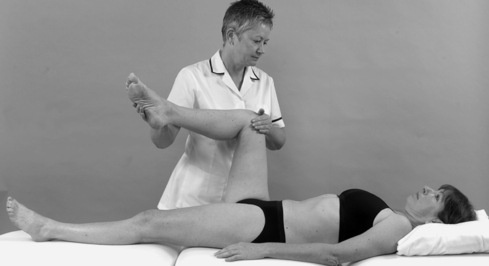 |
| Figure 13.16
Passive hip lateral rotation.
|
• Sacroiliac joint shear tests (Fig. 13.17a–c)
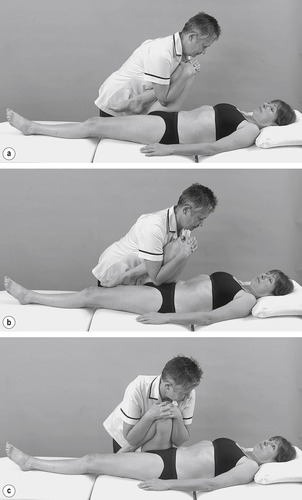 |
| Figure 13.17
(a–c) Shear tests to assess the sacroiliac joint.
|
• FABER test (Fig. 13.18)
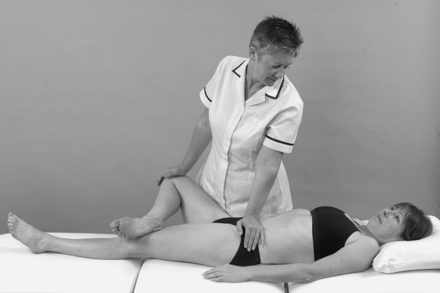 |
| Figure 13.18
FABER test to assess the sacroiliac joint.
|
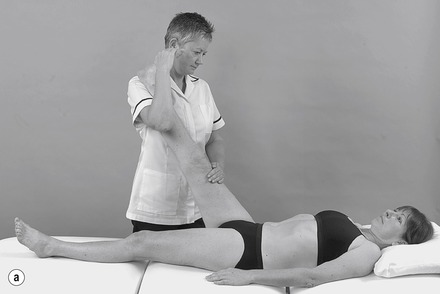
• Straight leg raise (Figure 13.19 and Figure 13.20): L4, 5, S1, 2
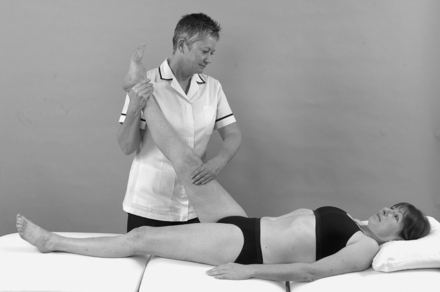 |
| Figure 13.19
Straight leg raise.
|
 |
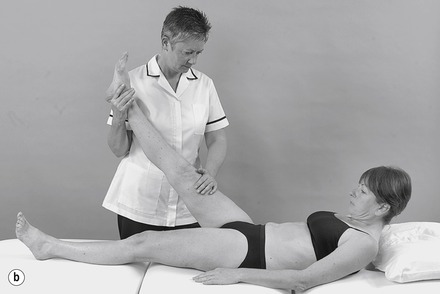 |
| Figure 13.20
(a,b) Straight leg raise with sensitizing components.
|
Resisted tests for objective neurological signs and alternative causes of leg pain; the main nerve roots involved are indicated in bold
• Resisted hip flexion, psoas (Fig. 13.21): L 2
 |
| Figure 13.21
Resisted hip flexion.
|
• Resisted ankle dorsiflexion, tibialis anterior (Fig. 13.22): L 4
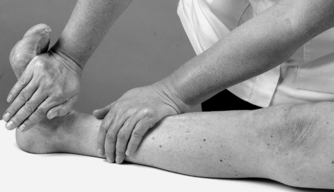 |
| Figure 13.22
Resisted ankle dorsiflexion.
|
• Resisted big toe extension, extensor hallucis longus (Fig. 13.23): L 5, S 1
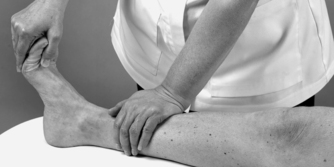 |
| Figure 13.23
Resisted extension of the big toe.
|
• Resisted eversion, peroneus longus and brevis (Fig. 13.24): L 5, S 1, 2
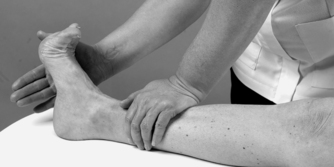 |
| Figure 13.24
Resisted ankle eversion.
|
Skin sensation (Fig. 13.25)
• Big toe only: L4 Fig. 13.25
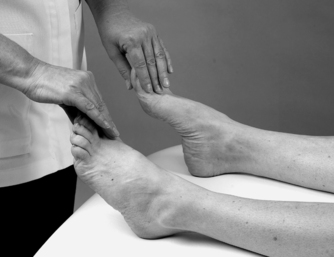 |
| Figure 13.25
Checking skin sensation.
|
• First, second and third toes: L5
• Lateral two toes: S1
• Heel: S2
Reflexes
• Knee reflex (Fig. 13.26): L2, 3, 4
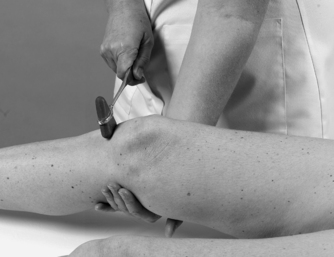 |
| Figure 13.26
Knee reflex.
|
• Ankle reflex (Fig. 13.27): S 1, 2
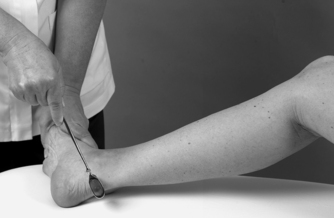 |
| Figure 13.27
Ankle reflex.
|
• Plantar response (Fig. 13.28)
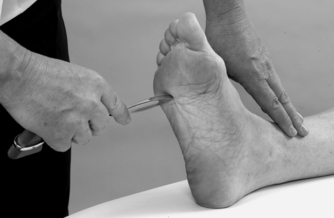 |
| Figure 13.28
Plantar response.
|
Prone lying
• Femoral stretch test (Figure 13.29 and Figure 13.30): L2, 3, 4
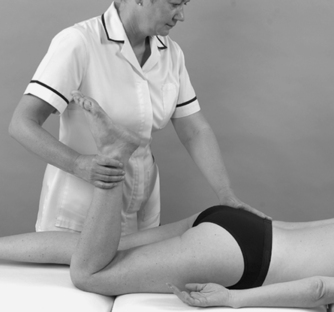 |
| Figure 13.29
Femoral stretch test.
|
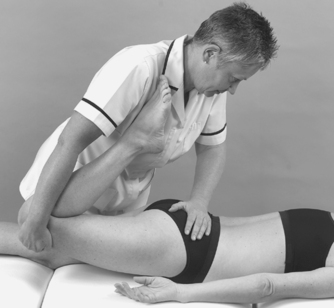 |
| Figure 13.30
Femoral stretch test with sensitizing component.
|
• Resisted knee extension, quadriceps (Fig. 13.31): L2, 3, 4
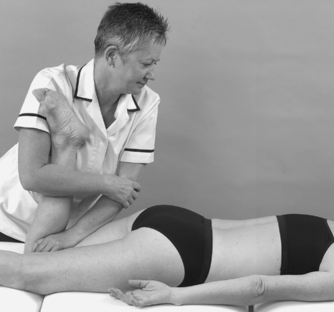 |
| Figure 13.31
Resisted knee extension.
|
• Resisted knee flexion, hamstrings (Fig. 13.32): L 5, S 1, 2
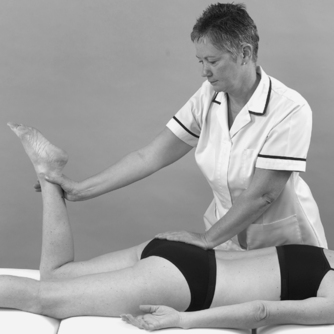 |
| Figure 13.32
Resisted knee flexion.
|
• Static contraction of the glutei (Fig. 13.33): L 5, S 1, 2
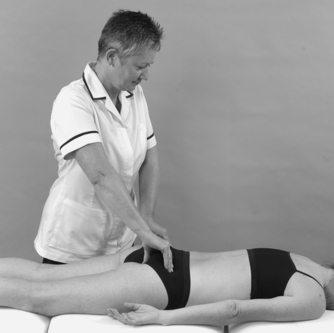 |
| Figure 13.33
Gluteal contraction, squeezing muscle bulk to assess wasting.
|
Palpation
• Spinous processes for pain, range and end-feel (Fig. 13.34)
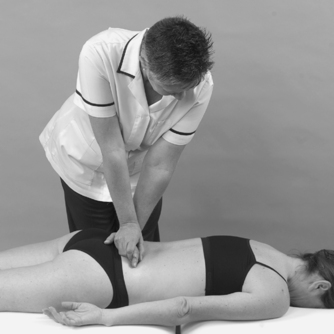 |
| Figure 13.34
Palpation.
|
Active movements are tested in the lumbar spine since, in common with the other spinal regions and shoulders, it can be a focus for ‘emotional’ symptoms. The active movements indicate the ‘willingness’ to perform the movements as well as determining the presence of the capsular or non-capsular pattern. End-feel is not routinely assessed since the information gathered from the active movements is generally sufficient.
The presence of a painful arc on any movement should be noted and Cyriax (1982) considered this to be pathognomic of disc lesion. Look for apprehension, guarding or exaggerated movements. An important finding is the non-capsular pattern, usually presenting as an asymmetrical limitation of lumbar movements and indicating a mechanical lesion. The presence of the capsular pattern indicates arthritis and it is typically found in degenerative osteoarthrosis of the more mature spine.
Gastrocnemius is assessed for objective signs of nerve root compression. Testing the muscle group against gravity in standing is convenient at this point, in terms of sequence, before lying the patient down.
The resisted tests are not part of the routine examination at the lumbar spine, but should be applied if there is a history of trauma, e.g. for a muscle lesion or suspected serious pathology, e.g. fracture, metastases or psychogenic pain (see Ch. 9).
In supine lying, other joints are eliminated from the examination to confirm that the site of the lesion is in the lumbar spine. Passive flexion, medial and lateral rotation are conducted at the hip, to assess the hip joint for the capsular pattern or other hip pathology. The sacroiliac joint is assessed by three provocative tests and the FABER test (see Ch. 14). To limit these tests to the hip and sacroiliac joint it may be necessary to place the patient’s forearm under the lumbar spine to increase the lordosis and to stabilize the spine. If the lesion in the lumbar spine is very irritable, it may not be possible to conduct these tests adequately.
The straight leg raise is applied passively to each leg in turn, keeping the knee straight. If positive, this may be interpreted as a dural sign, or as an indicator of neural tension affecting the L4, 5, S1, 2 nerve roots. It is an important clinical test for assessing nerve root tension due to a disc lesion when back or leg pain is usually produced at 30° and 40° (Supik & Broom 1994, Jönsson & Strömqvist 1995). Increased pain on the addition of neck flexion incriminates the dura mater. Further sensitizing components such as passive ankle dorsiflexion, passive ankle plantarflexion and inversion, and passive hip medial rotation and adduction can also be added to explore the mobility of the nervous system further as appropriate.
The normal range of movement for the straight leg raise is between 60 and 120°, with movement being limited by tension in the hamstrings. The range of the straight leg raise should be consistent with the range of lumbar flexion, which is also limited to a certain degree by tension in the hamstrings. A disc prolapse does not always produce a positive sign on straight leg raise. If the nerve root exits high up in the intervertebral foramen, it may escape compression from a posterolateral disc prolapse.
The limited range of the straight leg raise is dependent upon the compression on the dura mater or dural nerve root sleeves and the greater the compression the greater the limitation. A painful arc may be found which is indicative of a small disc prolapse and is a ‘useful’ finding since, empirically, it usually implies that manipulation will be beneficial.
A bilateral limitation of straight leg raise is usually due to a central disc prolapse compressing the dura mater and is accompanied by a multisegmental distribution of pain.
Bilateral sciatica from the same level producing bilateral limitation of straight leg raise and objective neurological signs is indicative of cauda equina compression. It is an absolute contraindication to manipulation and requires urgent specialist opinion.
A ‘crossed’ or ‘well leg raise’ describes the production of the pain in the back or leg on the painful side on straight leg raise of the painless limb. The intensity of the pain induced by the crossed straight leg raise is usually less than that produced on the painful side (Karbowski & Dvorak 1995). Its explanation lies in the suggestion that if a posterolateral prolapse is sitting in the ‘axilla’ of the nerve root or directly anterior to the root, straight leg raising on the painless side will pull the root against the prolapse and give a positive sign. It usually occurs at the L4 level (Cyriax 1982, Khuffash & Porter 1989). Vroomen et al (1999) identified the crossed straight leg raise test as a specific test.
The patient is assessed for root signs and alternative causes of pain by the selective application of resisted tests. Objective signs of muscle weakness, altered skin sensation and absent or reduced reflexes will indicate compression of a nerve root.
The plantar response is assessed by stroking up the lateral border of the sole of the foot and across the metatarsal heads. The normal response is flexor with flexion of the big toe. The Babinski reflex (or Babinski sign) is extension of the big toe. This reflex is normal in infants under the age of 2 years but a sign of brain or spinal cord injury in older children and adults. The extensor plantar response is therefore indicative of an upper motor neuron lesion, although this is not likely to occur with lumbar lesions since the spinal cord ends at approximately the level of the L1, 2 disc.
The femoral stretch test (prone knee-bending) is applied to assess the dura mater and the L2–L4 dural nerve root sleeves. The knee is passively flexed and, if positive, pain is usually produced at approximately 90°. A sensitizing component of hip extension can be added. Pain is usually felt in the back and the test is limited by tension in the quadriceps. If positive unilaterally it implicates the nerve roots, if positive bilaterally the dura mater is at fault. It is theoretically possible to produce a crossed or well leg femoral stretch test but this is not as commonly found in practice.
The remaining resisted tests for the quadriceps and hamstrings are conducted in prone-lying. A static contraction of the gluteal muscles is performed to assess for muscle bulk and palpation is conducted, using the ulnar border of the hand on the spinous processes, for pain, range of movement and end-feel.
Any other tests can be added to this basic routine examination of the lumbar spine, including repeated, combined and accessory movements and neural tension testing as appropriate.
If serious pathology is suspected, further tests and specialist investigations will need to be implemented. Standard X-ray investigation provides little useful information for mechanical lesions over and above that gleaned from the clinical examination. However, if the patient fails to respond in the expected way, further investigation may be indicated, including computed tomography or magnetic resonance imaging (MRI).
From the examination a working hypothesis is established and a treatment plan prepared.
MANAGEMENT OF BACK PAIN
Orthopaedic medicine aims to apply techniques of manipulation, traction and injections to lumbar lesions as appropriate. The choice of treatment will depend on the nature of the pain or the irritability of the lesion, the reference of pain and the mode of onset.
The report of the Clinical Standards Advisory Group (CSAG) (1994) gave management guidelines for back pain that have continued to be appropriate for clinical practice and the advice given to patients. The report emphasized the importance of early management of back pain, i.e. within the first 6 weeks from onset, since the establishment of chronic back pain makes any form of treatment difficult and less successful. The natural history of acute back pain is recovery, with a 90% chance of return to work within 6 weeks. However, 60% of these people will experience at least one recurrence of their pain within 1 year.
Attention to core stability in the prevention of recurrence of back pain has become an important focus in rehabilitation following an episode of back pain. Supervised exercise programmes aim to improve spinal stability and support to the spine through retraining of muscles that may be under-used or have developed abnormal patterns of recruitment. Research continues to be able to establish the most appropriate techniques and programmes to achieve lumbar stability.
Grenier & McGill (2007) set out to determine whether abdominal hollowing is more effective for lumbar stabilization than a full abdominal muscle co-contraction. Their study included a summary of the evidence to date concerning the role of transversus abdominis and the theory underpinning the development of exercise programmes to improve stability by concentrating on this muscle. The delayed onset of recruitment and activation of tranversus abdominus in some subjects with back pain is at the heart of the programmes and the recruitment of the muscle though abdominal hollowing has been a key component to improve stability. The authors note that as the demand of the exercise progression increases all muscles in the region are activated to the same degree. Although they found some evidence to support transversus abdominis as an important stabilizer, it was not possible to make claims for the stabilizing role of any specific muscle. They concluded that the drawing in of the abdomen to increase stability might be misdirected and that there is support for more general activity and a less specific approach to reduce pain and increase function.
If back pain has not settled within 6 weeks it is at risk of becoming chronic and the longer patients are off work, the lower their chances of ever returning. Patients off work for 6 months have only a 50% chance of returning to their previous job. Once they have been off work for 2 years, or have lost their job because of back pain, they will have great difficulty in returning to any kind of work and any further treatment is unlikely to avoid chronic disability.
Shaw et al (2007) evaluated the independent and shared associations of psychosocial variables on work status after the first onset of low back pain in working men, finding that in the first 2 months pain was the greater hindrance to function than the psychosocial component. From their findings they support that early intervention to reduce pain and improve function can help to prevent work disability.
Following initial assessment the CSAG report (1994) encourages selection from a diagnostic triage that forms the basis for decisions about referral, investigation and further management. This diagnostic triage consists of:
• Simple backache in which a 90% recovery is expected within 6 weeks
• Nerve root pain with objective neurological signs in which a 50% recovery is expected within 6 weeks
• Possible serious pathology, e.g. cauda equina syndrome, which requires urgent referral for specialist opinion within hours, or infection, neoplasm and inflammatory arthritis which require relatively urgent referral depending on the condition.
For more detailed information the reader is referred to the report itself.
The Chartered Society of Physiotherapy has produced ‘Clinical guidelines for the physiotherapy management of persistent Low Back Pain (LBP)’ (2006b). The guidelines are in two parts: Part 1 advises on the role of exercise and Part 2 on the role of manual therapy. Persistent LBP is defined as:
…pain associated with a primary site in a person’s low back, of musculoskeletal origin and emanating from the lumbar spine. It may include leg pain and/or pain in other areas of the spine. The pain has been present for at least six weeks and it may resolve partially or fully but may often recur.
The guidelines are extensive and based on a comprehensive evidence review. The clinical recommendations are summarized as follows:
• In both parts of the guideline it is recommended that all patients with low back pain should be given the opportunity to participate in an exercise programme, in a form appropriate and acceptable to each individual, after physiotherapy assessment.
• One or more of the following exercise regimes should be considered to reduce pain, to improve function and to improve psychological status: strengthening exercises, organized aerobic exercises, general exercises, McKenzie exercises, mobilizing exercises and hydrotherapy exercises. Core stability exercises are added to the list for improving function.
• If manual therapy is used, it should be part of a package of interventions that includes exercise and self-management to reduce pain, improve function and improve psychological status.
• Combined manipulation and manual mobilization should be considered as part of the package but not manipulation alone.
In common with the aim of all back pain management programmes, the recommendations are intended to avoid persistent back pain from becoming chronic back pain, and the aspect of self-management is an important component towards this as a development from the initial patient/therapist relationship.
A survey was conducted by Liddle et al (2009) to establish the specific use of advice and exercise by physiotherapists for the management of low back pain. Respondents did recognize the importance of advice and exercise, which were also used alongside a variety of treatments. However, exercise programme supervision and follow-up advice, both of which are considered to be important to facilitate continuing gains (Liddle et al 2007), were not widely used. Cooper et al (2009) explored patients’ perceptions of self-management of chronic low back pain and concluded that the patients could be better facilitated to self-manage their condition. They suggested that education in self-management itself should be introduced, as well as traditional patient education, with support in the form of direct access, review appointments and telephone calls.
LUMBAR LESIONS: A CLASSIFICATION SYSTEM OF FOUR CLINICAL MODELS
In the past, treatment in orthopaedic medicine has been traditionally targeted at the disc, aiming to reduce displacement, relieve pain and restore movement. However, there is a lack of confidence in traditional pathoanatomical diagnostic labels such as this, and ‘non-specific low back pain’ has been used more recently as a description of the symptoms, since the cause of pain cannot be confidently localized to one specific structure (Laslett & van Wijmen 1999). There is still no agreement on diagnosing disc lesions and the pathology is not fully understood (Lisi et al 2005). Consequently, several authors have established classifications to determine treatment programmes and to assist prognosis. The reader is referred to the work of McKenzie (1981), Riddle (1998) and Laslett & van Wijmen (1999).
Schäfer et al (2009) also promote the usefulness of developing models to guide treatment approaches by introducing a classification of low back-related leg pain, putting forward other causes of leg pain apart from those suggested by a discal model. As well as radicular pain with motor loss and musculoskeletal (somatic) causes, they propose that leg pain can also arise from central and peripheral nerve sensitization. They do acknowledge that there is likely to be overlap between the models but nonetheless they present a novel classification driven by the rationale, drawn from the literature, that accompanying leg pain is present in approximately 25–57% of all low back pain cases.
While not ideal, the presentation of signs and symptoms of lumbar lesions has been classified here into clinical models adapted from Cyriax’s original theories to acknow-ledge the current lack of clear pathoanatomical diagnosis. These models are judgment-based and contribute to the clinical decision-making process to rationalize appropriate treatment programmes; they are not intended to be restrictive. The reader is encouraged to be inventive, to draw on other experiences and to implement the approach into their existing clinical practice when putting a treatment programme together for individual patients.
The treatment techniques described are by no means a cure-all for every case of back pain. However, uncomplicated lesions of recent onset may respond well to the manipulative techniques of orthopaedic medicine. The key, as in the cervical spine, is the selection of appropriate patients for treatment.
For the purposes of the following classification of lesions, a lumbar lesion may present with the following features:
• An onset of back pain which may be in a multisegmental or segmental distribution (see Ch. 1)
• A typical mechanical history that describes pain aggravated by activity and certain postures, such as sitting, and relieved by rest and postures such as lying
• Symptoms such as a cough or sneeze increasing the pain
• Possible paraesthesia in a segmental pattern
• No significant ‘red’ or ‘yellow flags’ present in the history
• Examination revealing a non-capsular pattern of movement
• Limitation of straight leg raise and/or a positive femoral stretch test with possible objective neurological signs
• No contraindications to treatment.
Clinical Model 1: Lumbar lesion of gradual onset
Factors from the subjective examination
• Central, bilateral or unilateral pain (ideally not referred below the knee)
• Gradual onset
• Patient cannot recall the exact mode and time of onset
• May be precipitated by a period of prolonged flexion.
Factors from the objective examination
• Non-capsular pattern of pain and limitation of movement
• May have increased pain on side flexion towards the painful side
• No neurological signs.
A convenient shorthand for recording the findings of the objective examination can be found in Appendix 3 where the ‘star diagram’ is explained.
Traction is the treatment of choice for a lumbar lesion of gradual onset. However, manipulation can be applied, providing there are no contraindications, since, if successful, the response is quicker. The manipulative techniques described below may be modified and applied as mobilizing techniques using manual distraction forces (see below).
Clinical Model 2: Lumbar lesion of sudden onset
Factors from the subjective examination
• Central, bilateral or unilateral pain (ideally not referred below the knee)
• Sudden onset
• Patient can recall the exact mode and time of onset.
Factors from the objective examination
• Non-capsular pattern of pain and limitation of movement
• May have increased pain on side flexion away from the painful side
• No neurological signs.
Manipulation is the treatment of choice for lumbar lesions of sudden onset provided there are no contraindications to treatment.
Clinical Model 3: Lumbar lesion of mixed onset
Factors from the subjective examination
Factors from the objective examination
• Non-capsular pattern of pain and limitation of movement
• No neurological signs.
The treatment of choice is manipulation as it can achieve immediate results. If manipulation fails or is only partially successful, traction may be applied.
Clinical Model 4: Lumbar lesion presenting with referred leg symptoms
Factors from the subjective examination
• lnitial presentation of central or unilateral back or buttock pain, followed by referred leg pain (the central pain usually ceasing or diminishing, see below)
• Sudden or gradual onset
• Often part of a history of increasing, worsening episodes, therefore usually a progression of the above models
• Patient may or may not recall the exact time and mode of onset
• Patient may complain of root symptoms, i.e. paraesthesia felt in a segmental distribution.
Factors from the objective examination
• Non-capsular pattern of pain and limitation of movement reproducing back and/or leg symptoms
• Root signs may be present, i.e. sensory changes, muscle weakness, absent or reduced reflexes; consistent with the nerve root(s) involved.
The hypothesis is more readily rationalized here, due to the nerve root involvement. A large secondary posterolateral prolapse of disc material has occurred, initially compromising central structures and moving posterolaterally to involve the exiting nerve root in the intervertebral foramen. Pain may be referred into the leg through compression of the dural nerve root sleeve or the nerve root itself. However, the mechanism of injury to the nerve may be more complicated than expressed here and may also involve chemical and ischaemic factors, all or some of which may affect the quality of the pain response (see above).
To fall into this model as a mechanical presentation, subjectively, the pain should be worse in the leg than in the back (Koes et al 2007). The pain can radiate to the foot or toes and the numbness and paraesthesiae will tend to be in the same distribution. Straight leg raising will induce more leg pain, usually on the affected side, but the crossover straight leg raise test might be positive; this has high specificity for nerve root irritation, and is usually associated with a herniated disc. Unless there are cauda equina symptoms, the authors advise that there is no immediate need for surgery.
Jancalek & Dubovy (2007) advocate prompt intervention in cases of 2–3 months where no recovery has been detected. They looked at changes in myelinated axons during and after spinal root compression and concluded that the longer the compression, the less the recovery and the damage to the nerve root could therefore be permanent.
Pain is segmental in distribution and if the nerve root is involved there will be objective neurological signs. Since the nerve roots emerge obliquely in the lumbar spine, more than one nerve root may be involved in a disc lesion, although Koes et al (2007) suggest that it is more usual that local neurology is limited to one nerve root. Acute lumbar radiculopathy most commonly affects the L5 or S1 root and less commonly the L4 root. It is less common for the other roots to be affected (Caplan 1994).
Treatment is aimed at relieving pain. The limited evidence related to manipulation of patients with nerve root pain does not contraindicate its use but the CSAG report (1994) suggests that it should not be used in patients with severe or progressive neurological deficit to avoid the rare but serious risk of neurological complications.
Oliphant (2004) conducted a systematic review to look at the safety of spinal manipulation in the treatment of lumbar disc herniation. The risk of causing lumbar disc herniation or cauda equina syndrome with lumbar manipulation was estimated as between 1 in 1 million to 1 in over 100 million. The risk of worsening a disc herniation or cauda equina syndrome in patients presenting with lumbar disc herniation was calculated to be less than 1 in 3.7 million. Manip-ulation was calculated to be between 37 000 to 148 000 times safer than non-steroidal anti-inflammatory drugs and 55 500 to 444 000 times safer than surgery for the treatment of lumbar disc herniation. The ratios are substantial and the variation in the figures is indeed wide, but even by taking the lowest of the figures for each circumstance the risk from manipulation can be judged as low and the technique safe. Due attention to the contraindications to its use must be given.
Lisi et al (2005) reviewed the literature with regard to the safety issue that a herniated disc could be made worse by manipulation but found no evidence to substantiate that concern. Ernst (2007) also looked specifically at the adverse effects of spinal manipulation but did not report any for the lumbar spine. Oliphant (2004) suggested that even if disc herniations might have apparently been made worse, it could have been part of the progression of the disorder rather than being caused by the manipulation itself. Manipulation may therefore be carefully applied provided the neurological signs are minimal and stable, i.e. non-progressive, and that no other contraindications exist.
The more peripheral the symptoms, the less likely manipulation is to be successful. Manipulation should not be attempted if the neurological deficit is severe and progressing, but other modalities, e.g. traction and mobilization, may provide pain relief. A caudal epidural of corticosteroid and local anaesthetic may be indicated (Cyriax 1984, Cyriax & Cyriax 1993, Vroomen et al 2000, Boswell et al 2007). Alternatively spontaneous recovery is likely and may be awaited with suitable reassurance and/or analgesia being given to the patient (see below).
A relatively rare presentation of referred leg symptoms was described by Cyriax (1982) as primary posterolateral prolapse of disc material where symptoms appear in the leg without prior presentation in the back or buttock. The hypothesis is that prolapsed disc material has moved posterolaterally to compress the dural nerve root sleeve while the central pain-sensitive structures have escaped compression such that back pain is never a feature.
It is an uncommon presentation and usually affects the younger adult patient. Relatively minor symptoms of unilateral leg pain are described in the absence of low back pain and the patient may consider the pain to be due to hamstring or calf strain. A non-capsular pattern of movement is present with lumbar movements provoking the leg symptoms. Treatment of choice is traction, in the absence of contraindications, although the condition usually recovers spontaneously over several weeks.
TREATMENT OF LUMBAR LESIONS
How manipulation works is not clear and many hypotheses are proposed, including mechanical, physiological and neurophysiological mechanisms (Twomey 1992). If successful, manipulation dramatically reduces pain and produces an increased range of movement. The principles of manipulation and a general discussion on its effects are provided in Chapter 4.
Many authors have reviewed clinical manipulation trials (Abenhaim & Bergeron 1992, LaBan & Taylor 1992, Barker 1994, O’Donaghue 1994, Shekelle 1994, Koes et al 1996, van Tulder & Koes 2002). These are often part of an overview of evidence to support the wide range of available treatment approaches in, for example, the management of low back pain (Waddell et al 1996, van Tulder et al 1997). Most of the trials reviewed tend to fall short of the ideal scientific criteria necessary for good quality research (Koes et al 1995) and Hancock et al (2006) were unable to overcome the initial hurdle of establishing agreement on a placebo treatment that was deemed a fair and suitable comparison to spinal manipulation. Several studies have focused on a comparison of manipulation with other conservative approaches and from this perspective there is little evidence to support the effectiveness of manipulation over modalities such as massage, exercise, back schools etc. or the administration of analgesics (Hsieh et al 2002, Assendelft et al 2003, Cherkin et al 2003).
Harvey et al (2003) acknowledge that one of the confounders of research has been the different definitions of the therapeutic procedures involved. They describe a spinal manipulation ‘package’ that was agreed by the professional bodies representing chiropractors, osteopaths and physio-therapists in the United Kingdom. The package was used in the UK Back Pain Exercise and Manipulation (UK BEAM) Trial Team 2004a; 2004b, a national study of physical treatments in primary care.
It is generally accepted from the scientific data produced to date that spinal manipulation has shown short-term benefits of improvement in pain, movement and functional ability. Waddell et al (1996) reviewed the evidence relating to low back pain for the Royal College of General Practitioners which guided the Clinical Standards Advisory Group (CSAG) audit towards the development of guidelines for the management of acute low back pain. A system of diagnostic triage was introduced on which to base management. The guidelines have continued to be presented as a patient booklet, ‘The Back Book’ (Roland et al 1996), to promote the recommendations of the guidelines on a wider scale. For simple acute back pain, the advice is given to avoid bed rest and to stay active, and manipulation is upheld as providing short-term improvement in pain and activity levels.
Waddell (1998) reported on the evidence for manipulation which showed positive results of good short-term symptomatic relief for patients with acute back pain of less than 4–6 weeks’ duration and without nerve root pain. It was suggested that manipulation may also be effective in recurrent attacks.
A review conducted by Nadler (2004) found that the benefit from manipulation was more marked in the earlier stages of a painful episode; although the limit of the time period for ‘earlier’ is unclear.
Bogduk (2004) conducted an evidence review of prevailing approaches to the management of chronic low back pain and found manipulation to be slightly more effective than sham therapy but not more effective than other forms of care. This hints at the importance of patient selection prior to manipulation, however, and the more chronic model is not recommended as ‘ideal’ for manipulation in the orthopaedic medicine approach.
The UK BEAM Trial team (2004a, 2004b) referred to above declared that relative to ‘best care’ in general practice, manipulation followed by exercise achieved a moderate benefit at 3 months and a small benefit at 12 months; spinal manipulation achieved a small to moderate benefit at 3 months and a small benefit at 12 months; and exercise achieved a small benefit at 3 months but not 12 months. On the face of it, the trial does provide support for the benefit of manipulation but, although the trial set out with large numbers, a significant percentage of patients was lost to follow-up and the criticism was made that it was hard to establish whether it was the ‘hands on’ effect or the effect of manipulation itself that led to the improvement.
An analysis of cost-effectiveness drawn from the trial concluded that manipulation alone probably gives better value for money than manipulation followed by exercise.
In apparent contrast to the studies above, Frost et al (2004) randomized patients with mild to moderate low back pain to an advice group or a physiotherapy group. The advice group received a 1-h advice session from a physiotherapist; the advice was to remain active. Those in the therapy group were assessed by a physiotherapist and treated as judged to be appropriate by a physiotherapist for up to five further sessions of treatment. Both groups were given an advice booklet (‘The Back Book’, Roland et al 1996). In being left to the judgment of the physio-therapist, manipulation was not always applied and this does not qualify as a study on the outcomes of manipulation as such. The authors concluded that ‘routine’ physiotherapy for mild to moderate low back pain is no more effective than a session with a physiotherapist that includes advice.
‘Routine’ physiotherapy is always hard to define or standardize due to the autonomy of the physiotherapist and the general paucity of evidence to support treatment protocols. Within the study, treatments were applied by 76 physiotherapists, with different levels of training. Treatments given included mobilizing and manipulation, soft tissue techniques, stretching, strengthening exercises and the use of hot and cold treatment, and treatments could be applied in any combination. The study stimulated a response from MacAuley (2004) who highlighted the challenge of managing back pain and suggested that the study had led to the question of whether physiotherapy had a place at all as an expensive, apparently ineffective resource, and whether patients would be better to be referred for general fitness programmes along with the general advice offered in the study.
Returning to manipulation trials, Oliphant (2004) conducted a review and concluded that there was evidence that spinal manipulation has a beneficial effect on pain, straight leg raising, range of motion, size of disc herniation and neurological symptoms.
A review conducted by Williams et al (2007) concluded that that there was some evidence that manipulation also improved psychological outcomes more than mobilization. They recommend that more trials should include psychological markers in their studies and that there may be a case for comparing manipulation with cognitive behavioural therapy.
Based on empirical evidence and especially that passed on from the clinical experience of Cyriax, for selected patients, when manipulation works it works quickly, which may have positive implications for reducing the overall cost of the management of acute back pain. Until soundly disproved, the authors endorse the benefit of Cyriax manipulation techniques, based on many years of their personal clinical experience.
The long-term benefits of manipulation remain unknown although, if the aim of manipulation is to expedite recovery in the acute stage, these are of little significance. There is insufficient evidence to support or to refute the use of manipulation for chronic low back pain but manipulation is particularly appropriate for patients with uncomplicated acute back pain with symptoms of recent onset (Shekelle 1994, Waddell et al 1996).
As mentioned above, the principal aim of manipulation, and for all early treatment programmes, is to prevent the development of chronic back pain. Childs et al (2006) set out to determine if patients who do not receive manipulation for their low back pain are at an increased risk of worsening disability, compared to patients who receive manipulation. Seventy patients received manipulation and exercise and 61 patients were assigned to an exercise group without manipulation. The study found that those in the latter group were eight times as likely to experience a worsening in disability than those in the manipulation group. The importance of patient selection was emphasized.
Ideal patients are those that fall into Clinical Models 2 and 3 but those that fall into Clinical Model 1 may also benefit (see above). Although there is little evidence to support its use, manipulation can be attempted for chronic back pain and for referred leg pain of somatic origin but is not indicated in patients with the severe lancinating pain of radicular origin or severe or progressive neurological deficit.
The success of manipulation depends on the selection of suitable patients and indiscriminate manipulation will produce unsatisfactory results (Childs et al 2006). Orthopaedic medicine spinal manipulation techniques aim to reduce the signs and symptoms of a lumbar disc lesion. In terms of expectations of treatment outcomes, the ideal patient for manipulation has, in summary:
• Mainly central or short unilateral back or buttock pain (the more distal the pain, the less likely manipulation is to succeed)
• Recent onset of pain, preferably within the last 6 weeks
• History of sudden onset of pain; the patient recalls the exact time and mode of onset
• Non-capsular pattern on examination
• Pain increased by side flexion away from the painful side
• No objective neurological signs
• No contraindications to manipulation.
Contraindications to lumbar manipulation
It is impossible to be absolutely definitive about all contra-indications and nothing can substitute for a rigorous assessment of the presenting signs and symptoms and an accurate diagnosis of a mechanical lumbar lesion.
‘Red flags’ are signs and symptoms found in the patient’s subjective and objective examination that may indicate serious pathology and provide contraindications to lumbar manipulation (Greenhalgh & Selfe 2006, Sizer et al. 2007) (see ‘Red flags’ Box, p. 379).
The absolute contraindications are highlighted in the discussion below but there are several relative contraindications that should be considered as well. It may be useful to use the mnemonic ‘ COINS’ (a contraction of ‘ contra indication s’), as an aide-mémoire to be able to create mental categories for the absolute contraindications: Circulatory, Osseous, Inflammatory, Neurological and suspicious features indicating Serious pathology. If the first and last two letters are pushed together as ‘ CONS’, the crucial need for consent is emphasized.
The treatment regime discussed below is contraindicated in the absence of informed patient consent. The patient should be given all details of their diagnosis together with the proposed treatment regime and a discussion of the risks and benefits should ensue to enable them to give their informed consent. Consent is the patient’s agreement, written or oral, for a health professional to provide care. It may range from an active request by the patient for a particular treatment regime to the passive acceptance of the health professional’s advice. The process of consent, within the context of the orthopaedic medicine, is ‘fluid’ rather than one instance in time when the patient gives their consent. The patient is constantly monitored and feedback is actively requested. The treatment procedures can be progressed or stopped at the patient’s request or in response to adverse reactions. Reassessment is conducted after each technique and a judgment made about proceeding. The patient has a right to refuse consent and this should be respected and alternative treatment options discussed. For further information on consent, the reader is referred to the Department of Health website: www.doh.gov.uk/consent.
Signs and symptoms of cauda equina syndrome including S4 symptoms of saddle anaesthesia, sacral sensory loss, and signs of bladder or bowel dysfunction require urgent neurosurgical referral (Lehmann et al 1991, O’Flynn et al 1992, Dinning & Schaeffer 1993, Jalloh & Minhas 2007). Studies have shown that considerable improvements in sensory, motor and sphincter deficits are more likely to be achieved if surgery is performed within 48 h (Jalloh & Minhas 2007).
Bilateral sciatica from the same level with bilateral limitation of straight leg raise and bilateral objective neurological signs indicate a large, central disc prolapse threatening the cauda equina and each provides an absolute contraindication to manipulation (Dinning & Schaeffer 1993). This presentation must not be confused with multisegmental reference of bilateral pain in the absence of neurological signs, as these patients may benefit from the central treatment techniques suggested below.
Severe or progressive neurological deficit associated with Clinical Model 4 is too irritable for the treatment techniques suggested below. Radicular lesions in the young are rare and full imaging should be conducted on young patients presenting with numbness, weakness and absent reflexes (Sizer et al 2007). Similarly, hyperacute pain in which the patient has twinges and has difficulty even moving or assuming different postures is too irritable to manipulate. Manipulation may be an inappropriate technique for the patient with symptoms of neurogenic (spinal) claudication and associated spinal stenosis. The patient taking anticoagulant therapy, such as warfarin, is absolutely contraindicated due to the risk of intraspinal bleeding. The patient with blood clotting disorders should also be considered at risk.
Through a thorough subjective and objective examination it should be possible to screen the patient presenting with their first episode of backache under 20 or over 50 and the patient with a past history of primary tumour to ensure that the back pain is mechanical in origin. Long-term systemic steroid use, inflammatory arthritis, known osteoporosis or HIV are all relative contraindications to manipulation. The systemically unwell patient or the patient experiencing constant, progressive non-mechanical pain or other signs of serious spinal pathology is not appropriate for manipulative techniques and fracture may need to be excluded in patients suffering recent traumatic incidents such as a road traffic accident.
Hayes et al (2006) looked at the assessment for ‘red flags’ in an Accident & Emergency Department where a proactive approach is taken for practitioners to screen for serious pathology as a cause of acute low back pain. Abdominal examination is carried out to check for aneurysm and masses and rectal examination is performed if saddle anaesthesia is reported. Patients over 50 are at increased risk of tumour, abdominal aortic aneurysm and infection. In those over 65, degenerative spinal stenosis and compression osteoporotic fractures should be ruled out. Pre-pubescent children can have infection: osteomyelitis and discitis, and tumours of the spine or spinal cord are a possibility. Older children up to the age of 18 can have herniated disc or mechanical strain but spondylosis, spondylolisthesis and tumour should be considered.
Caution should be taken with the pregnant patient, although there is no evidence to suggest that manipulation is dangerous (Lisi 2006). Lisi conducted a case series of 17 women with low back pain in pregnancy and more work does need to be done on the safety and effectiveness of spinal manipulation in pregnancy. Discussion with the patient of the risks and benefits will allow informed consent should the clinician judge the manipulation to be appropriate.
‘Yellow flags’ from the history and examination may highlight degrees of illness behaviour which make the patient inappropriate for manipulation.
The lumbar manipulation procedure
It is recommended that a course in orthopaedic medicine is attended before the treatment techniques described are applied in clinical practice (see Appendix 1). Two types of manipulative technique are used, the first incorporating a short or long lever arm according to the effect required:
• Rotational manoeuvres for unilateral pain
• Central manoeuvres for central pain.
The techniques described below are conducted with the couch at a suitable height for the operator. Generally this should be as low as possible. The techniques may be easier to perform if the patient is asked to take in a small breath, with the technique being applied after the patient has breathed out. This encourages patient relaxation and will allow for the effective application of the overpressure with minimal tissue resistance.
Rotational manoeuvres
The following manoeuvres are described in a suggested order of progression for the novice manipulator but, once experience is gained in the application of the techniques, any may be chosen as a starting point and the order presented is not an order of efficacy. The comparable signs are assessed after every manoeuvre and the next man-oeuvre is chosen based on the outcome. As long as a technique is gaining an increase in range and/or a decrease in pain, it can be repeated. Improvement may reach a plateau or the feedback from the patient may become unclear. Only professional judgment will reliably dictate when treatment should be stopped in each treatment session. It is better to err towards the side of caution in the early stages of acquiring manipulative skill.
Distraction technique
Position the patient in side-lying with the painful side uppermost. Flex the upper hip and knee with the knee just resting over the side of the bed to assist the rotational stress. Extend the lower leg. Pull the underneath shoulder firmly through such that the uppermost shoulder is positioned backwards and the pelvis positioned forwards. Stand behind the patient at waist level and place one hand over the greater trochanter, pointing outwards. Put your other hand comfortably on the patient’s uppermost shoulder with your fingers pointing away from your other hand. Apply rotation with the hand on the greater trochanter until the pelvis lies just forwards of the midline and the patient’s waist is upwards. Apply pressure equally through both hands to impart a distraction force; you will see the patient’s waist crease stretch out as you lean through your arms (Fig. 13.35). Keep your arms straight as you apply a minimal amplitude, high velocity thrust once all of the slack has been taken up.
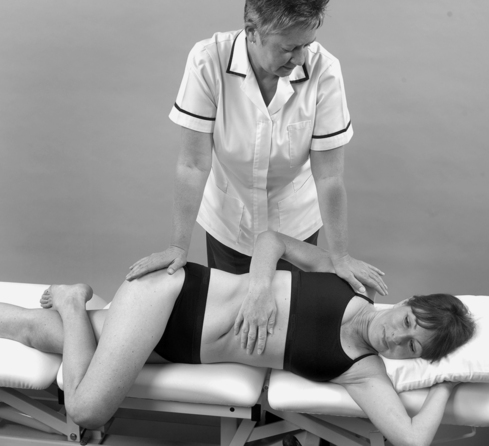 |
| Figure 13.35
Distraction technique.
|
Short-lever rotation technique – pelvis forwards
Position the patient in supine lying with the hips and knees flexed (crook lying). Ask the patient to lift and rotate the hips so that they are lying with the painful side uppermost and the shoulders relatively flat. Position the legs as for the distraction technique. Stand in front of the patient with one hand fixing the patient’s shoulder while the other is placed with the heel of the hand on the blade of the ilium, with the forearm horizontal and your fingers pointing back towards you. Apply pressure through the hand on the ilium in a horizontal direction towards you to achieve a rotational strain (Fig. 13.36). Apply a minimal amplitude, high velocity thrust once all the slack has been taken up. If you find it difficult to apply the thrust with your hand against the ilium, slide your hand towards you to place your forearm against the bone to give you improved leverage.
 |
| Figure 13.36
Short-lever rotation technique – pelvis forwards.
|
Short-lever rotation technique – pelvis backwards (Cyriax 1984, Cyriax & Cyriax 1993)
Position the patient in side-lying with the painful side uppermost. Take the lower arm behind the patient and place the upper arm into elevation, resting in front of the patient’s face. Extend the upper leg and flex the hip and knee of the lower leg. The shoulder will now be positioned forwards and the pelvis backwards. Stand behind the patient; place one hand on the scapula to give a little distraction to take up the slack. Place the other hand on the front of the pelvis with your forearm horizontal and pointing back towards you (Fig. 13.37). Apply a minimal amplitude, high velocity thrust once all of the slack is taken up.
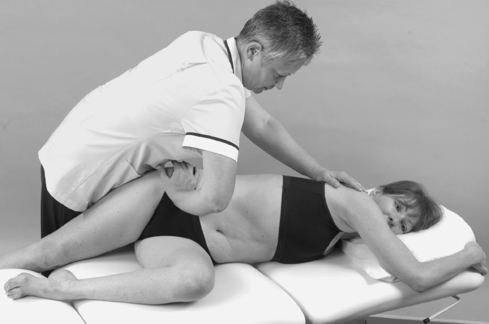 |
| Figure 13.37
Short-lever rotation technique – pelvis backwards.
|
Long-lever rotation technique (Cyriax 1984, Cyriax & Cyriax 1993)
This is a stronger rotational manoeuvre and care is recommended in its application to older patients to avoid placing undue strain on the neck of the femur.
Position the patient as for the short-lever rotation – pelvis forwards technique. Stand in front of the patient at waist level, facing the patient’s feet to allow the arms to be placed more vertically. Fix the shoulder with one hand and place the other hand behind the knee, with your thumb in the knee crease. Lean on the knee to produce a rotation strain (Fig. 13.38). Apply a minimal amplitude, high velocity thrust once all of the slack is taken up.
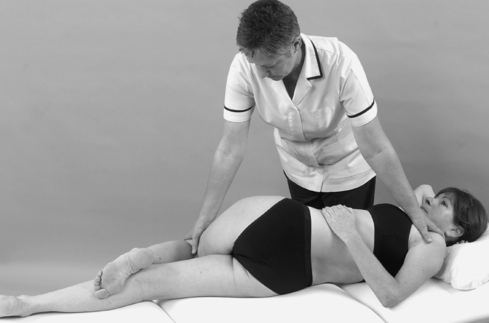 |
| Figure 13.38
Long-lever rotation technique.
|
‘Pretzel’ technique
This is a strong, long-lever rotation technique when used as a manipulation. It can be broken down into its individual stages and used as a mobilizing technique for hyperacute pain in which the lesion is too irritable for manipulation (see below). It helps in both instances to be clear on the different stages of the technique. It may be useful for correcting a lateral shift (Cyriax & Cyriax 1993).
• Stand on the patient’s painless side with the patient in supine-lying. Flex the knees and cross the good leg over the bad (Fig. 13.39).
 |
| Figure 13.39
‘Pretzel’ technique: starting position with knees flexed and ‘good’ leg placed over the ‘bad’.
|
• Flex both hips (Fig. 13.40).
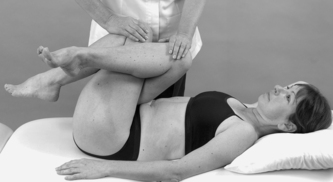 |
| Figure 13.40
Both hips flexed.
|
• Place your knee which is furthest from the patient’s head at the patient’s waist to act as a pivot point. Place your hands on the patient’s knees and side-flex the lumbar spine to gap the affected side (Fig. 13.41).
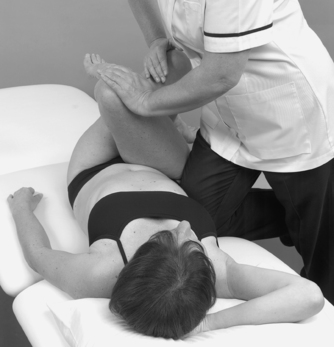 |
| Figure 13.41
Spine side-flexed around pivot of caudal knee placed in patient’s waist.
|
• Rotate the pelvis towards you until the patient’s knees are resting on your thigh (Fig. 13.42).
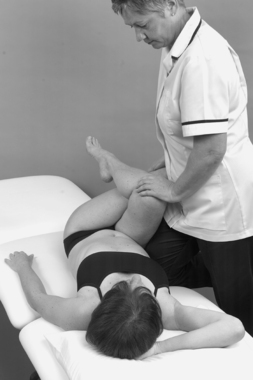 |
| Figure 13.42
Pelvis rotated forwards to rest patient’s knees against thigh.
|
• Gently lower your thigh, taking the pelvis further into rotation, ensuring that the other hand fixes the patient’s shoulder flat on the couch (Fig. 13.43). Apply a minimal amplitude, high velocity thrust once all of the slack is taken up. Help the patient back to the starting position.
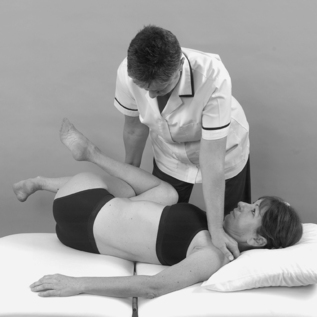 |
| Figure 13.43
Lowering the patient’s knees by removing the thigh, to take the pelvis into rotation, stabilizing the shoulder on the couch. Taking up the slack before applying the Grade C thrust.
|
For hyperacute pain, each step is conducted individually using Grade A mobilization, constantly monitoring for improvement before progressing to the next step. Progression through the steps is made cautiously and steadily and may take 5 or 10 min to achieve in the very irritable state. The end of range will not necessarily be reached before proceeding to the next step. The technique should not aggravate the pain and the patient should be firmly and comfortably supported throughout. This is not a manipulation as such and any other mobilizing modality may be applied at each stage.
Extension manoeuvres
Extension manoeuvres are used for central pain or pain that is referred unilaterally into the back only. They may be used as first-line treatment if a patient presents with central pain, or as a progression of the rotational manoeuvres as the pain centralizes. Extension manoeuvres should be avoided if hypermobility or spondylolisthesis is present.
Straight extension thrust technique (Cyriax 1984, Cyriax & Cyriax 1993)
This technique is indicated if a small central pain exists. Position the patient in prone-lying and palpate the spinous processes to locate the painful level. Place the ulnar border of your hand over the tender spinous process and reinforce it with the other hand by placing the thumb web over your fingers. Apply pressure directly down onto the spinous process through straight arms (Fig. 13.44). Apply a minimal amplitude, high velocity thrust once all the slack is taken up by lifting and dropping your head down between your shoulders. Be careful not to lose the end of range by lifting your hands as you raise your head.
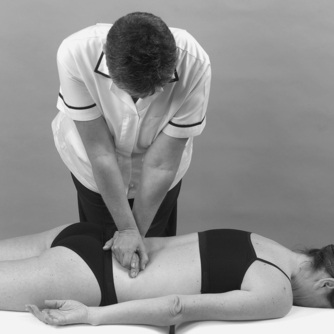 |
| Figure 13.44
Straight extension thrust technique.
|
Unilateral extension thrust technique (Cyriax 1984, Cyriax & Cyriax 1993)
If the pain centralizes to a short unilateral pain, or the patient presents with a short unilateral pain, this technique may be applied. Position the patient in prone-lying, stand on the painful side and palpate the spinous process to locate the painful level. Place the ulnar border of the hand over the transverse process at the tender level on the side furthest away from you. The pisiform should be adjacent to the spinous process and the pressure is applied through the paravertebral muscles for patient comfort. Stand close to the bed with your knees hooked onto the edge to enable you to lean over the patient. Apply pressure down onto the transverse process through arms as straight as possible, directing the pressure back towards your own knees (Fig. 13.45). Apply a minimal amplitude, high velocity thrust once all of the slack is taken up by lifting and dropping your head between your shoulders.
 |
| Figure 13.45
Unilateral extension thrust technique.
|
Extension technique with leverage (Cyriax 1984, Cyriax & Cyriax 1993)
If the above fails to clear unilateral pain, this technique is a little stronger. Position the patient in prone lying and stand on the painless side. Position one hand flat, just above the painful level and on the painful side, adjacent to the spinous processes. Wrap the other hand over and under the leg on the painful side just above the knee. Stand close to the couch, lift the leg on the painful side into full extension of the hip and lumbar spine and step backwards to apply side flexion, gapping the painful side (Fig. 13.46 a, b). Apply a minimal amplitude, high velocity thrust, continuing the direction of movement, once all of the slack is taken up.
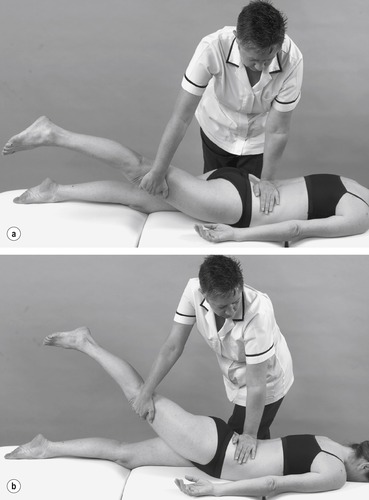 |
| Figure 13.46
(a, b) Extension technique with leverage.
|
Lumbar traction
The major indication for lumbar traction is the presence of disc displacement of gradual onset or Clinical Model 1 (see above) which may be diagnosed following thorough assessment of the patient with consideration of the history and the presenting signs and symptoms (See Chapter 4 for further discussion on effects of traction). Traction may be applied to any of the other clinical models if other treatments have been unsuccessful or only partially successful, provided that there are no contraindications.
Contraindications to lumbar traction
• Patients with acute lumbago or sciatica usually associated with twinges and antalgic postures: the application of traction may be quite comfortable but as the traction is released the patient will be far worse and the pain and twinges will be agonizing. It may take some hours for the pain to subside sufficiently to allow the patient to get up and the whole experience is awful for both patient and therapist alike. With patients with less irritable back pain, symptoms are often eased as traction is applied, but be particularly cautious if the history, signs and symptoms reveal an acute situation and the pain is completely relieved by traction.
• Patients with severe cardiac or respiratory problems may not be able to tolerate either the straps or the supine-lying position. A bad cough also contraindicates treatment since pain will be made much worse.
• Patients with claustrophobia or other psychological disorders may become panic-stricken while undergoing traction, although such patients do not usually give consent to its application in the first place.
• Inflammatory conditions affecting the spine are likely to be aggravated by traction.
• S4 symptoms only may theoretically respond to traction but it is always best to exercise caution in any situation that implies a large central protrusion, including bilateral sciatica from the same level (Cyriax 1982). Worsening of the situation could lead to increased compression on the cauda equina and possible permanent damage to bladder function.
While not absolute contraindications, there are instances where traction is ill-advised or unlikely to be successful:
• The pain of disc protrusions with neurological deficit is unlikely to be relieved by traction since the protrusion is too large to be reduced. Manipulation is less likely still to be effective and the treatment of choice is epidural anaesthesia (see below).
• Sciatica which has lasted for more than 6 months is unlikely to respond to traction. The patient may be advised to await spontaneous recovery, to try epidural anaesthesia or to seek a surgical opinion, according to the severity of the symptoms and the patient’s choice.
• Care is needed with hypermobility.
Technique
Friction-free electrically operated traction beds have been designed which may be used in conjunction with electronically operated units that supply options for either rhythmical or static traction (Fig. 13.47). Neither is an absolute necessity for the application of static traction as suggested by Cyriax & Cyriax (1993). The debate of sustained versus intermittent traction is considered in Chapter 4. Far less sophisticated apparatus may be used which is just as effective, but adjustments may then be needed to calculate the distracting force applied, on the basis of overcoming the frictional forces created between the patient and the couch (see below).
 |
| Figure 13.47
Lumbar traction couch.
|
A thoracic and a pelvic harness are affixed to either end of a couch and applied to the patient (Fig. 13.48). Harnesses of modern design are usually comfortable and easy to readjust since they have Velcro™ fastenings. A simple device is required for taking up the slack and applying a continuous pull-down through the pelvic belt, so providing traction to the intervening lumbar spine.
 |
| Figure 13.48
Lumbar traction being applied.
|
The principle of application is that the pull should be ‘as strong as is comfortable’. In the early stages this was the only guideline provided and there was no way of knowing the exact poundage being applied. A spring balance was then introduced in series with the pelvic rope, which gave some indication of poundage. However, Judovitch & Nobel (1957) calculated the coefficient of friction to be 0.5 and, since half the body weight is distributed below the level of L3, then a force of ½ × 0.5=¼ of the patient’s body weight is required to overcome the friction between the body and the couch before the distracting force is applied to the spine. This is an important factor in calculating the actual poundages being applied when using a standard couch. When using a friction-free couch, the poundages registering on the accompanying machine are relatively faithful to the actual poundages being applied.
Feedback from the patient is just as important as in the days before a means of measuring poundages was devised and ‘as strong as is comfortable’ should still be the rule. An approximate estimate of the appropriate poundage may be made by assessing the size and weight of the patient, in the light of experience with other patients.
Before the commencement of treatment, a thorough explanation should be given to the patient of the reasons for applying the technique and the likely outcome, including any possible adverse effects such as stiffness or increased proximal pain. Consent should then be gained.
Before applying traction a selected comparable sign should be tested, such as the straight leg raise or particular lumbar movements in standing.
The patient does not need to be completely undressed for traction. Light clothing may be worn, being careful to remove belts, buckles, car keys, etc., and ensuring that clothing can separate in the middle. The patient is instructed to lie down sideways on the couch before rolling into supine lying.
The usual starting position is in supine-lying but the number of pillows under head and knees may be adjusted for comfort. As the knees are lifted so the lumbar lordosis will flatten and a stool may be used to achieve Fowler’s position (hips and knees flexed to 90°) if the patient is more comfortable with the spine completely flat.
Other positions such as prone-lying may be tried if the patient is more comfortable in lumbar extension. Theoretically there are eight combinations of straps varying the patient’s position between supine and prone, and choosing to place each of the thoracic and pelvic straps either underneath or on top of the patient. In practice, however, lying supine or prone, with the straps underneath the patient in both instances, are the two most popular and comfortable positions.
The thoracic belt should be tight enough to grip the chest to prevent it from sliding up towards the axillae, which is very uncomfortable and a little undignified for the well-endowed female. However, the grip should not be so tight as to restrict breathing, although patients will tend to find that they employ apical breathing more than diaphragmatic breathing while the traction is being applied.
The pelvic harness should sit comfortably above or around the iliac crests where it can pull down on the pelvis as the traction is applied but without slipping. The pelvic harness should not be so tight as to compress the abdomen uncomfortably and patients should be warned not to have a heavy meal before treatment.
If using a friction-free couch, ensure that the sliding lumbar section of the table is locked in its fixed position while the harnesses are being applied. The lumbar spine segment being treated should initially be placed over the division in the table.
The slack is taken up in the pelvic rope to apply tension to the system. This may be done by hand or through pulleys in the more rudimentary units, or by machine in an automatic unit. Traction is then applied steadily, either manually by releasing ropes, turning wheels, pumping down on handles, etc., or automatically.
At the first treatment, 20 min of traction is usually sufficient and provides enough time to be effective but not enough to overtreat, causing severe after-treatment stiffness or soreness. Feedback is encouraged from patients throughout treatment and it is essential to know if they become uncomfortable or if there is a marked increase in their pain. Simple adjustments can be made to lessen discomfort from the straps or to reduce the traction as necessary.
The patient should always be supplied with a means of summoning help such as a bell or buzzer. In subsequent treatments the time may be increased to 30 min. These timings are suggested on an empirical basis and little research has been done on this. Often the time allocated for treatment sessions in the appointment system is the limiting factor.
After treatment the traction should be released steadily and slowly, gaining feedback from the patient throughout, to be guided on the appropriate rate. The electrical units do not usually allow for this flexibility but do release steadily.
The traction belts are then released and patients are encouraged to wriggle for a minute or two. They are allowed to roll over but should not sit up until the residual stiffness following the application of traction has eased.
When ready, patients should be asked to turn onto their side, if they have not already done so, and to push themselves up sideways to avoid straining the back. Some patients need to sit for a moment or two until any dizziness or light-headedness arising from postural hypotension has subsided.
When dressing, patients should be advised to avoid bending, and should put tights or socks on by bringing the foot up towards them and doing shoes up by bringing the foot up onto a chair rather than by bending to the floor.
Patients should be encouraged to continue with their everyday activities within the limits of pain, but prolonged sitting or lifting should be avoided while the treatment is continuing.
In terms of treatment frequency and duration, ideally the patient should be seen daily, but the ideal is rarely attained due to the various time and financial constraints in both the National Health Service and the private sector. Traction is often perceived as an expensive use of resources that is not easily justified on the basis of its poor evidence base. However, evidence to demonstrate conclusively that it is ineffective is also wanting. In the authors’ clinical experience it is still worth applying traction as frequently as possible and it will not necessarily fail if the ideal conditions are not available. Improvement should become apparent after three or four treatments and, if no improvement is evident, modification of the traction position may be made before abandoning the modality altogether.
The physical treatment techniques of manipulation and traction have some use in the treatment of back pain but, as has already been emphasized, the selection of suitable patients for such techniques is essential. The treatment programme for all patients should include a positive approach to the management of back pain with an emphasis on balanced activity and rest. Patients should be involved in the management of their condition and a home treatment regime should be implemented, including postural and ergonomic advice where appropriate. The reader is recommended to the McKenzie approach for the management of lumbar disc lesions, which particularly complements the orthopaedic medicine approach, although the hypotheses on pathology and explanation of effect differ.
Lumbar injections
Caudal epidural injections
In 1901, Sicard introduced local anaesthetic into the epidural space and this technique has continued, with the addition of corticosteroid in the 1950s, to be efficacious in the management of discogenic sciatica (Dilke et al 1973, Bush & Hillier 1991, Bush 1994). It is a well-tolerated procedure with a high patient satisfaction rate and relatively few side-effects. Recent adverse publicity of arachnoiditis is associated with the intrathecal rather than the epidural route (Bowman et al 1993).
Indications for epidural injection
• Hyperacute pain which fails to settle with adequate analgesia and up to 3 days’ bed rest, and is too irritable for manipulation or traction
• Radicular pain which has failed to respond to conservative management during the first 6 weeks from onset
• Chronic back or leg pain, with or without neurological deficit, which has failed to respond to conservative measures
• As a trial to treat pain before surgical intervention is considered.
Bush & Hillier (1991) indicated that active intervention with caudal epidural injection of triamcinolone acetonide plus procaine improves signs and symptoms at the 4-week follow-up, with improvement maintained at 1-year follow-up. Boswell et al (2007) looked at the evidence for interventional techniques in the management of chronic low back pain and concluded that the evidence is strong for the role of epidural injection in achieving short-term relief for chronic low back pain and radicular pain. Dincer et al (2007) also support the use of epidural injection in the management of subacute and chronic low back pain with radicular pain, in comparison to non-steroidal anti-inflammatory drugs, provided that the injections are performed by experienced specialists.
Manchikanti, Cash, & McManus et al. (2008a), Manchikanti, Singh, & Cash et al. (2008b), Manchikanti, Singh, & Cash et al. (2008c) and Manchikanti, Cash, & McManus et al. (2008d) set out to evaluate the effectiveness of caudal epidural injections, with or without steroids, by conducting a series of four studies on chronic low back pain associated with: discogenic pain without disc herniation or radiculitis; disc herniation and radiculitis; post-surgery syndrome; and spinal stenosis. All groups demonstrated significant improvement of approximately 70%. Conn et al (2009) confirmed the evidence provided by Manchikanti et al as Level 1 for both discogenic pain without disc herniation or radiculitis, and chronic pain secondary to disc herniation or radiculitis. The evidence for the effectiveness of caudal epidural injection for post-surgery syndrome and spinal stenosis ranked only slightly below.
However, the advantage of adding steroid was not demonstrated, although the amount of steroid used may be less than that suggested by other experts in the field, and within this text. On the basis of the findings of the studies, Huntoon & Burgher (2008) support the use of epidural anaesthesia prior to surgery, as the option with less risk, but they also challenge the addition of steroid, urging a return to an ‘earlier time’ when local anaesthetic was used alone (Cyriax 1984, Cyriax & Cyriax 1993).
The mechanism by which caudal epidural produces the improvement in pain relief is still debated and several hypotheses exist (Bush & Hillier 1991, Dincer et al 2007). Disc material can exert a mechanical effect through compression, a chemical effect through inflammation and an ischaemic effect through oedema. Introducing cortico-steroid into the epidural space may directly affect the chemical and indirectly the ischaemic effects of pain. The introduction of fluid into a fluid-filled space can mechanically affect the relationship between the disc and the nerve root, possibly breaking down scars and adhesions, while the introduction of local anaesthetic may have sufficient short-term effects to break the pain cycle. Huntoon & Burgher (2008) recommend further study into the mechanisms involved in the persisting pain relief achieved with epidural injection of local anaesthetic alone, to be able both to explain and to guide practice.
Epidural injection via the caudal route can be carried out as an outpatient procedure. It is recommended that the procedure should only be conducted by an experienced medical practitioner, after appropriate training. Once through the sacrococcygeal ligament, the sacral hiatus connects directly to the epidural space (Dincer et al 2007). The injection involves the introduction of 40–80 mg triamcinolone acetonide in 20–30 mL of 0.5% procaine hydrochloride via the sacral hiatus using a no-touch technique, with observation of blood or cerebrospinal fluid backflow (Figure 13.49 and Figure 13.50) (Bush 1994).
 |
| Figure 13.49
Caudal epidural injection.
|
 |
| Figure 13.50
Caudal epidural injection, showing direction of approach and needle position.
|
Sclerosant therapy
Sclerosant or prolotherapy is used to treat chronic spinal instability and chronic pain (Refer also to chapter 4). It involves the injection of a chemical irritant into the ligaments surrounding an unstable spinal or sacroiliac segment. The chemical irritant produces an inflammatory response, causing fibroblast hyperplasia and subsequent increase in strength of the supporting ligaments (Ongley et al 1987).
The patient undergoes manipulation first of all to ensure that a full range of movement is achievable and to reduce the disc displacement. An injection used to be given of a solution called P2G comprising phenol, dextrose and glycerine but these days most practioners are using hypertonie dextrose. Each osseoligamentous junction is infiltrated using a peppering technique (Fig. 13.51(a–c)). Injections are given at weekly intervals with a maximum of three or four injections. The patient is instructed to avoid flexion to allow the ligamentous tissue to contract sufficiently to stabilize the joints.
 |
| Figure 13.51
(a–c) Sclerosant injection, indicating needle placement to pepper the lumbosacral and sacroiliac ligaments.
|
Orthopaedic medicine courses include the principles of sclerosant injections for medical practitioners but a period of supervised clinical practice is recommended.
Mechanism of spontaneous recovery
Irrespective of treatment, the natural history of a disc prolapse is one of resolution (Bogduk 1991). Koes et al (2007) propose that, in patients with sciatica, 60% have recovered by 3 months, 70% by a year and 30% have pain that persists for a year or longer.
The possible mechanisms for spontaneous recovery will now be discussed.
Bush et al (1992) demonstrated that a high proportion of patients with discogenic sciatica made a good recovery together with resolution of the disc herniation in a significant number. They concluded that, with good pain control, nature can be allowed to run its course. Ellenberg et al (1993) prospectively studied 14 patients with definite radiculopathy and disc herniation on computed tomographic scan. They showed that the natural history of disc herniation with radiculopathy is improvement or complete recovery in 78% in 6–18 months, with non-surgical management.
Pople & Griffith (1994) suggested that a disc protrusion stretches the posterior longitudinal ligament, producing predominantly back pain, while a disc prolapse exiting through a tear in the posterior longitudinal ligament produces reduced tension. This explains why patients with an extruded fragment often experience a decrease or complete resolution of back pain as the root symptoms begin. According to Cyriax (1982), nerve root pain is expected to recover spontaneously provided the backache ceases when the pain shifts into the leg. This may take 8–12 months but the older the patient, the longer or less likely the spontaneous recovery. Cyriax (1982) considered recovery to occur through mechanisms of shrinkage of the prolapsed disc material or through its accommodation by vertebral erosion. Nerve root atrophy may occur with the patient losing pain through ischaemia of the nerve root, but the neurological signs tending to take longer to recover.
With advanced technology involving magnetic resonance imaging the position of the prolapse can be seen and monitored. It may be seen to disappear completely, reduce or remain evident and is not necessarily indicative of symptomatology. In some cases the improvement in clinical findings is seen before recessive changes are observed on magnetic resonance imaging (Teplick & Haskin 1985, Delauche-Cavallier et al 1992, Komori et al 1996), or the prolapse can persist with no symptoms at all (Bush et al 1992). A prolapsed fragment of nuclear material may be reduced through a process of disc absorption that involves neovascularization and macrophage phagocytosis (Saal et al 1990, Doita et al 1996, Ito et al 1996).
REFERENCES
Abenhaim, L.; Bergeron, A.M., Twenty years of randomized trials of manipulative therapy for back pain: A review, Clin. Invest. Med. 15 (1992) 527–535.
Acaroglu, E.R.; Iatridis, J.C.; Setton, L.A.; et al., Degeneration and ageing affect the tensile behaviour of human lumbar annulus fibrosus., Spine 20 (1995) 2690–2701.
Adams, M.A.; Hutton, W.C., The effect of posture on the fluid content of lumbar intervertebral discs, Spine 8 (1983) 665–671.
Adams, M.A.; Hutton, W.C., The effect of posture on diffusion into lumbar intervertebral discs, J. Anat. 147 (1986) 121–134.
Adams, M.; Bogduk, N.; Burton, K.; et al., The Biomechanics of Back Pain. ( 2002)Churchill Livingstone, Edinburgh.
Allam, K.; Sze, G., MR of primary extraosseous Ewing sarcoma, Am. J. Neuroradiol. 15 (1994) 305–307.
Assendelft, W.J.J.; Morton, S.C.; Yu, E.I.; et al., Spinal manipulative therapy for low back pain: a meta-analysis of effectiveness relative to other therapies, Ann. Intern. Med. 138 (11) ( 2003) 871–881.
Barker, M.E., Spinal manipulation: a general practice study, J. Orthop. Med. 16 (1994) 42–44.
Beaman, D.N.; Graziano, G.P.; Glover, R.A.; et al., Substance P innervation of lumbar spine facet joints, Spine 18 (1993) 1044–1049.
Bernick, S.; Walker, J.M.; Paule, W.J., Age changes to the annulus fibrosus in human intervertebral discs, Spine 16 (1991) 520–524.
Bogduk, N., The lumbar disc and low back pain, Neurosurg. Clin. N. Am. 2 (1991) 791–806.
Bogduk, N., Innervation, pain patterns and mechanisms of pain production, In: (Editor: Twomey, L.T.) Clinics in Physical Therapy, Physical Therapy of the Low Back2nd edn ( 1994)Churchill Livingstone, Edinburgh, pp. 93–109.
Bogduk, N., Management of chronic low back pain, Med. J. Aust. 180 (2) ( 2004) 79–83.
Bogduk, N., Clinical Anatomy of the Lumbar Spine and Sacrum. 4th edn ( 2005)Churchill Livingstone, Edinburgh.
Bogduk, N.; Twomey, L.T., Clinical Anatomy of the Lumbar Spine. ( 1991)Churchill Livingstone, Edinburgh.
Boswell, M.; Trescot, A.; Datta, S.; et al., Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain, Pain Physician 10 (2007) 7–111.
Bowman, S.J.; Wedderburn, L.; Whaley, A.; et al., Outcome assessment after epidural corticosteroid injection for low back pain and sciatica, Spine 18 (1993) 1345–1350.
Brinckmann, P., Injury of the annulus fibrosus and disc protrusions – an in vitro investigation on human lumbar discs, Spine 11 (1986) 149–153.
Brock, M.; Patt, S.; Mayer, H., The form and structure of the extruded disc, Spine 17 (1992) 1457–1461.
Buckwalter, J.A., Aging and degeneration of the human intervertebral disc, Spine 20 (1995) 1307–1314.
Bush, K., Lower back pain and sciatica – how best to manage them, Br. J. Hosp. Med. 51 (1994) 216–222.
Bush, K.; Hillier, S., A controlled study of caudal epidural injections of triamcinolone plus procaine for the management of intractable sciatica, Spine 16 (1991) 572–575.
Bush, K.; Cowan, N.; Katz, D.E.; et al., The natural history of sciatica associated with disc pathology, Spine 17 (1992) 1205–1212.
Caplan, L.R., Management of patients with lumbar disc herniations with radiculopathy, Eur. Neurol. 34 (1994) 114–119.
Cavanaugh, J.M., Neural mechanisms of lumbar pain, Spine 20 (1995) 1804–1809.
Cavanaugh, J.M.; Kallakuri, S.; Ozaktay, A.C., Innervation of the rabbit lumbar intervertebral disc and posterior longitudinal ligament, Spine 20 (1995) 2080–2085.
Chartered Society of Physiotherapy, Clinical Guidelines for the Physiotherapy Management of Persistent Low Back Pain (LBP): Part 1 Exercise. ( 2006)CSP, London.
Chartered Society of Physiotherapy, Clinical Guidelines for the Physiotherapy Management of Persistent Low Back Pain (LBP): Part 2 Manual Therapy. ( 2006)CSP, London.
Cherkin, D.C.; Sherman, K.J.; Deyo, R.A.; et al., A review of the evidence for the effectiveness, safety and cost of acupuncture, massage therapy and spinal manipulation for back pain, Ann. Intern. Med. 138 (11) ( 2003) 898–907.
Childs, J.D.; Flynn, T.W.; Fritz, JM., A perspective for considering the risks and benefits of spinal manipulation in patients with low back pain, Man. Ther. 11 (2006) 316–320.
Clinical Standards Advisory Group, Back Pain. ( 1994)HMSO, London.
Conn, A.; Buenaventura, R.M.; Datta, S.; et al., Systematic review of caudal epidural injections in the management of chronic low back pain, Pain Physician 12 (2009) 109–135.
Cooper, K.; Smith, B.H.; Hancock, E., Patients’ perceptions of self-management of chronic low back pain: evidence for enhancing patient education and support, Physiotherapy 95 (2009) 43–50.
Coppes, M.; Enrico, M.; Ralph, T.; et al., Innervation of ‘painful’ lumbar discs, Spine 22 (20) ( 1997) 2342–2349.
Corrigan, B.; Maitland, G.D., Practical Orthopaedic Medicine. ( 1983)Butterworths, London.
Cyriax, J., 8th ednTextbook of Orthopaedic Medicine. Vol. 1 ( 1982)Baillière Tindall, London.
Cyriax, J., 11th ednTextbook of Orthopaedic Medicine. Vol. 2 ( 1984)Baillière Tindall, London.
Cyriax, J.; Cyriax, P., Cyriax’s Illustrated Manual of Orthopaedic Medicine. ( 1993)Butterworth Heinemann, Oxford.
Delauche-Cavallier, M.-C.; Budet, C.; Laredo, J.-D.; et al., Lumbar disc herniation, Spine 17 (1992) 927–933.
Dilke, T.W.F.; Burry, H.C.; Grahame, R., Extradural corticosteroid injection in management of lumbar nerve root compression, Br. Med. J. 2 (1973) 635–637.
Dincer, U.; Kirap, M.; Caker, E.; et al., Caudal epidural injection versus non-steroidal anti-inflammatory drugs in the treatment of low back pain accompanied by radicular pain, Joint Bone Spine 74 (2007) 467–471.
Dinning, T.A.R.; Schaeffer, H.R., Discogenic compression of the cauda equina – a surgical emergency, Aust. N.Z. J. Surg. 63 (1993) 927–934.
Doita, M.; Kanatani, T.; Harada, T.; et al., Immunohistologic study of the ruptured intervertebral disc of the lumbar spine, Spine 21 (1996) 235–241.
Ellenberg, M.R.; Ross, M.L.; Honet, J.C.; et al., Prospective evaluation of the course of disc herniations in patients with proven radiculopathy, Arch. Phys. Med. Rehabil. 74 (1993) 3–8.
Ernst, E., Adverse effects of spinal manipulation: a systematic review, J. R. Soc. Med. 100 (2007) 330–338.
Findlay, G.F.G., Tumours of the lumbar spine, In: (Editor: Jayson, M.) The Lumbar Spine and Back Pain4th edn ( 1992)Churchill Livingstone, Edinburgh, pp. 355–369.
Frost, H.; Lamb, S.E.; Doll, H.A.; et al., Randomised controlled trial of physiotherapy compared with advice for low back pain, Br. Med. J. 329 (2004) 708–711.
Galessiere, P.F.; Downs, A.R.; Greenberg, H.M., Chronic, contained rupture of aortic aneurysms associated with vertebral erosion, Can. J. Surg. 37 (1994) 23–28.
Garfin, S.R.; Rydevik, B.; Lind, B.; et al., Spinal nerve root compression, Spine 20 (1995) 1810–1820.
Greenhalgh, S.; Selfe, J., Red Flags. ( 2006)Elsevier, Edinburgh.
Greening, J., How inflammation and minor nerve injury contribute to pain in nerve root and peripheral neuropathies, In: (Editors: Boyling, J.D.; Jull, G.A.) Grieve’s Modern Manual Therapy: the Vertebral Column ( 2004)Elsevier, Edinburgh, pp. 205–214.
Grenier, S.G.; McGill, S.M., Quantification of lumbar stability by using 2 different abdominal activation strategies, Arch. Phys. Med. Rehabil. 88 (1) ( 2007) 54–62.
Hancock, M.; Maher, C.; Latimer, J.; et al., Selecting an appropriate placebo for a trial of spinal manipulative therapy, Aust. J. Physiother. 52 (2006) 135–138.
Hartley, A., Practical Joint Assessment: Lower Quadrant. 2nd edn ( 1995)Mosby, London; pp 1. 88.
Harvey, E.; Burton, A.K.; Moffett, J.K.; et al., Spinal manipulation for low-back pain: a treatment package agreed by the UK chiropractic, osteopathy and physiotherapy professional associations, Man. Ther. 8 (1) ( 2003) 46–51.
Hayes, K.; Huckstadt, A.; Daggett, D., Acute low back pain in the emergency department., Adv. Emerg. Nurs. J. 28 (3) ( 2006) 234–247.
Hirabayashi, S.; Kumano, K.; Tsuiki, T.; et al., A dorsally displaced free fragment of lumbar disc herniation and its interesting histologic findings., Spine 15 (1990) 1231–1233.
Holm, S., Pathophysiology of disc degeneration, Acta Orthop. Scand. 64 (Suppl. 251) ( 1993) 13–15.
Hoppenfeld, S., Physical Examination of the Spine and Extremities. ( 1976)Appleton Century Crofts, Philadelphia.
Hsieh, C.-Y.J.; Adams, A.H.; Tobis, J.; et al., Effectiveness of four conservative treatments for subacute low back pain; a randomized clinical trial., Spine 27 (11) ( 2002) 1142–1148.
Huang, M.-C.; Chang, P.-T.; Tsai, M.-J.; et al., Sensory and motor recovery after repairing transacted nerve roots, Surg. Neurol. 68 (2007) 17–24.
Huntoon, M.A.; Burgher, A.H., Back to the future: the end of the steroid century?Pain Physician 11 (2008) 713–716.
Iatridis, J.C.; Weidenbaum, M.; Setton, L.A.; et al., Is the nucleus pulposus a solid or a fluid? Mechanical behaviour of the nucleus pulposus of the human intervertebral disc, Spine 21 (1996) 1174–1184.
Ito, T.; Yamada, M.; Ikuta, F.; et al., Histologic evidence of absorption of sequestration-type herniated disc, Spine 21 (1996) 230–234.
Jalloh, I.; Minhas, P., Delays in the treatment of cauda equine syndrome due to its variable clinical features in patients presenting to the emergency department, Emerg. Med. J. 24 (1) ( 2007) 33–34.
Jancalek, R.; Dubovny, P., An experimental animal model of spinal root compression syndrome: an analysis of morphological changes of myelinated axons during compression radiculopathy and after decompression, Exp. Brain Res. 179 (2007) 111–119.
Jensen, G.M., Biomechanics of the lumbar intervertebral disc: a review, Phys. Ther. 60 (1980) 765–773.
Jönsson, B.; Strömqvist, B., The straight leg raising test and the severity of symptoms in lumbar disc herniation., Spine 20 (1995) 27–30.
Judovitch, B.; Nobel, G.R., Traction therapy, a study of resistance forces, Am. J. Surg. 93 (1957) 108–114.
Kang, J.D.; Georgescu, H.I.; McIntyre-Larkin, L.; et al., Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2., Spine 21 (1996) 271–277.
Karbowski, K.; Dvorak, J., Historical perspective: description of variations of the sciatic stretch phenomenon., Spine 20 (1995) 1525–1527.
Kemp, H.B.S.; Worland, J., Infections of the spine, Physiotherapy 60 (1974) 2–6.
Khuffash, B.; Porter, R.W., Cross leg pain and trunk list, Spine 14 (1989) 602–603.
Koes, B.W.; Bouter, L.M.; Geert, J.M.G.; et al., Methodological quality of randomized clinical trials on treatment efficacy in low back pain, Spine 20 (2) ( 1995) 228–235.
Koes, B.W.; Assendelft, W.J.J.; Geert, J.M.G.; et al., Spinal manipulation for low back pain: an updated systematic review of randomized clinical trials, Spine 21 (24) ( 1996) 2860–2873.
Koes, B.; Van Tulder, M.; Peul, W., Diagnosis and treatment of sciatica, Br. Med. J. 334 (2007) 1313–1317.
Komori, H.; Shinomiya, K.; Nakai, O.; et al., The natural history of herniated nucleus pulposus with radiculopathy, Spine 21 (1996) 225–229.
Kotil, K.; Bilge, T., A ligamentum flavum hematoma presenting as an L5 radiculopathy, J. Clin. Neurosci. 14 (2007) 994–997.
Kumar, P.; Clark, M., Clinical Medicine. 5th edn ( 2002)Baillière Tindall, London.
Kuslich, S.D.; Ulstrom, C.L.; Michael, C.J., The tissue origin of low back pain and sciatica, Orthop. Clin. N. Am. 22 (1991) 181–187.
LaBan, M.M.; Taylor, R.S., Manipulation: an objective analysis of the literature., Orthop. Clin. N. Am. 23 (1992) 451–459.
Laslett, M.; van Wijmen, P., Low back and referred pain: diagnosis and a proposed new system of classification., N.Z. J. Physiother. 27 (1999) 5–14.
Lee, H.-M.; Kim, N.-H.; Kim, H.-J.; et al., Morphometric study of the lumbar spinal canal in the Korean population, Spine 20 (1995) 1679–1684.
Lehmann, O.J.; Mendoza, N.D.; Bradford, R., Beware the prolapsed disc, Br. J. Hosp. Med. 46 (1991) 52.
Liddle, S.D.; Gracey, J.H.; Baxter, G.D., Advice for the management of low back pain: a systematic review of randomised controlled trials, Man. Ther. 12 (2007) 310–327.
Liddle, S.D.; Baxter, G.D.; Gracey, J.H., Physiotherapists’ use of advice and exercise for the management of chronic low back pain: a national survey, Man. Ther. 14 (2009) 189–196.
Lisi, A., Chiropractic spinal manipulation for low back pain of pregnancy: a retrospective case series, J. Midwifery Womens Health 51 (910) ( 2006) 7–10.
Lisi, A.; Holmes, E.; Ammendolia, C., High-velocity low-amplitude spinal manipulation for symptomatic disk disease, a systematic review of the literature. J. Manipulative Physiol. Ther. 28 (2005) 429–442.
MacAuley, D., Back pain and physiotherapy: NHS treatment is of little value, Br. Med. J. 329 (2004) 694–695.
McCarron, R.F.; Wimpee, M.W.; Hudkins, P.G.; et al., The inflammatory effect of the nucleus pulposus – a possible element in the pathogenesis of low back pain, Spine 12 (1987) 760–764.
McKenzie, R.A., The Lumbar Spine: Mechanical Diagnosis and Therapy. ( 1981)Spinal Publications, Waikanae, New Zealand.
Manchikanti, L.; Cash, K.A.; McManus, C.D.; et al., Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 1 – discogenic pain without disc herniation or radiculitis, Pain Physician 11 (2008) 785–800.
Manchikanti, L.; Singh, V.; Cash, K.A.; et al., Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 2 – disc herniation and radiculitis, Pain Physician 11 (2008) 801–815.
Manchikanti, L.; Singh, V.; Cash, K.A.; et al., Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 3 – post surgery syndrome, Pain Physician 11 (2008) 817–831.
Manchikanti, L.; Cash, K.A.; McManus, C.D.; et al., Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 4 – spinal stenosis, Pain Physician 11 (2008) 833–848.
Marchand, F.; Ahmed, A.M., Investigation of the laminate structure of the lumbar disc annulus fibrosus, Spine 15 (1990) 402–410.
Mixter, W.J.; Barr, J.S., Rupture of the intervertebral disc with involvement of the spinal canal, N. Engl. J. Med. 211 (1934) 210–215.
Mooney, V.; Robertson, J., The facet syndrome, Clin. Orthop. Relat. Res. 115 (1976) 149–156.
Moridaira, K.; Handa, H.; Murakami, H.; et al., Primary Hodgkin’s disease of the bone presenting with an extradural tumour, Acta Haematol. 92 (1994) 148–149.
Nachemson, A., The load on lumbar discs in different positions of the body, Clin. Orthop. 45 (1966) 107–122.
Nachemson, A.; Elfstrom, G., Intravital dynamic pressure measurements in lumbar discs, a study of common movements, manoeuvres and exercises, Scand. J. Rehabil. Med. 1 (Suppl.) ( 1970) 1–38.
Nadler, S.F., Non pharmacologic management of pain, J. Am. Osteopath. Assoc. 104 (11 Suppl. 8) ( 2004) 6–12.
Norris, C.M., Sports Injuries: Diagnosis and Management for Physiotherapists. 3rd edn ( 2004)Butterworth Heinemann, Oxford.
O’Donaghue, C., Manipulation trials, In: (Editors: Boyling, J.; Palastanga, N.) Grieve’s Modern Manual Therapy2nd edn ( 1994)Churchill Livingstone, Edinburgh, pp. 661–672.
O’Flynn, K.J.; Murphy, R.; Thomas, D.G., Neurogenic bladder dysfunction in lumbar intervertebral disc prolapse, Br. J. Urol. 69 (1992) 38–40.
Oliphant, D., Safety of spinal manipulation in the treatment of lumbar disc herniation: a systematic review and risk assessment, J. Manipu-lative Physiol. Ther. 27 (3) ( 2004) 197–210.
Oliver, J.; Middleditch, A., Functional Anatomy of the Spine. 2nd edn ( 2006)Butterworth-Heinemann, Edinburgh.
Oliver, M.J.; Twomey, L.T., Extension creep in the lumbar spine, Clin. Biomech. 10 (1995) 363–368.
Ongley, M.J.; Klein, R.G.; Dorman, T.A.; et al., A new approach to the treatment of chronic low back pain Lancet 2, (1987) 143–146.
Osborne, G., Spinal stenosis, Physiotherapy 60 (1974) 7–9.
Osti, O.L.; Cullum, D.E., Occupational low back pain and intervertebral disc degeneration – epidemiology, imaging and pathology, Clin. J. Pain 10 (1994) 331–334.
Paesold, G.; Nerlich, A.; Boos, N., Biological treatment strategies for disc degeneration: potentials and shortcomings, Eur. Spine J. 16 (4) ( 2007) 447–468.
Palastanga, N.; Field, D.; Soames, R., Anatomy and Human Movement. 5th edn ( 2006)Butterworth-Heinemann, Edinburgh.
Palma, L.; Mariottini, A.; Muzii, V.F.; et al., Neurinoma of the cauda equina misdiagnosed as prolapsed lumbar disc, J. Neurosurg. Sci. 38 (1994) 181–185.
Palmgren, T.; Gronblad, M.; Virri, J.; et al., Immunohistochemical demonstration of sensory and autonomic nerve terminals in herniated lumbar disc tissue, Spine 21 (1996) 1301–1306.
Parke, W.W.; Schiff, D.C.M., The applied anatomy of the intervertebral disc, Orthop. Clin. N. Am. 2 (1971) 309–324.
Peng, B.; Wu, W.; Guo, J.; et al., Chemical radiculitis, Pain 127 (2007) 11–16.
Pople, I.K.; Griffith, H.B., Prediction of an extruded fragment in lumbar disc patients from clinical presentations, Spine 19 (1994) 156–158.
Porter, R.W., Spinal stenosis of the central and root canal, In: (Editor: Jayson, M.) The Lumbar Spine and Back Pain4th edn ( 1992)Churchill Livingstone, Edinburgh, pp. 313–332.
Porter, R.W., Pathology of symptomatic lumbar disc protrusion, J. R. Coll. Surg. Edinb. 40 (1995) 200–202.
Porter, R.W.; Oakshot, G., Spinal stenosis and health status, Spine 19 (1994) 901–903.
Porter, R.W.; Hibbert, C.S.; Wicks, M., The spinal canal in symptomatic lumbar disc lesions, J. Bone Joint Surg. 60B (1978) 485–487.
Prescher, A., Anatomy and pathology of the ageing spine, Eur. J. Radiol. 27 (1998) 181–195.
Rauschning, W., Pathoanatomy of lumbar disc degeneration and stenosis, Acta Orthop. Scand. 64 (Suppl. 251) ( 1993) 3–12.
Riddle, D.L., Classification and low back pain: a review of the literature and critical analysis of selected systems, Phys. Ther. 78 (7) ( 1998) 708–737.
Roberts, S.; Eisenstein, S.M.; Menage, J.; et al., Mechanoreceptors in the intervertebral discs, morphology, distribution and neuropeptides., Spine 20 (1995) 2645–2651.
Roland, M.; Waddell, G.; Klaber Moffett, J.; et al., The Back Book. ( 1996)Stationery Office, Norwich.
Rydevik, B.; Olmarker, K., Pathogenesis of nerve root damage, In: (Editor: Jayson, M.) The Lumbar Spine and Back Pain4th edn ( 1992)Churchill Livingstone, Edinburgh, pp. 89–90.
Saal, J.S., The role of inflammation in lumbar pain, Spine 20 (1995) 1821–1827.
Saal, J.A.; Saal, J.S.; Herzog, J., The natural history of lumbar intervertebral disc extrusions treated non-operatively, Spine 15 (1990) 683–686.
Schäfer, A.; Hall, T.; Briffa, K., Classification of low-back related leg pain – a proposed patho-mechanism-based approach, Man. Ther. 14 (2009) 222–230.
Schwarzer, A.C.; Aprill, C.N.; Derby, R.; et al., The relative contributions of the disc and zygapophyseal joint in chronic low back pain., Spine 19 (1994) 801–806.
Schwarzer, A.C.; Aprill, C.N.; Derby, R.; et al., Clinical features of patients with pain stemming from the lumbar zygapophyseal joints – is the lumbar facet syndrome a clinical entity?Spine 19 (1994) 1132–1137.
Shaw, W.S.; Means-Christensen, A.; Slater, M.A.; et al., Shared and independent associations of psychosocial factors on work status among men with subacute low back pain, Clin. J. Pain 23 (5) ( 2007) 409–416.
Shekelle, P.G., Spine update: spinal manipulation, Spine 19 (1994) 858–861.
Sizer, P.; Brismée, J.; Cook, C., Medical screening for red flags in the diagnosis and management of musculoskeletal spine pain., Pain Pract. 7 (1) ( 2007) 53–71.
Supik, L.F.; Broom, M.J., Sciatic tension signs and lumbar disc herniations, Spine 19 (1994) 1066–1068.
Swezey, R., Pathophysiology and treatment of intervertebral disk disease, Rheum. Dis. Clin. N. Am. 19 (1993) 741–757.
Takahashi, H.; Suguro, T.; Okazima, Y.; et al., Inflammatory cytokines in the herniated disc of the lumbar spine, Spine 21 (1996) 218–224.
Taylor, J.; Twomey, L., The lumbar spine from infancy to old age, In: (Editors: Twomey, L.; Taylor, J.) Clinics in Physical Therapy: Physical Therapy of the Low Back2nd edn ( 1994)Churchill Livingstone, Edinburgh, pp. 1–56.
Teplick, J.G.; Haskin, M.E., Spontaneous regression of herniated nucleus pulposus, Am. J. Roentgenol. 145 (1985) 371–375.
Twomey, L.T., A rationale for the treatment of back pain and joint pain by manual therapy, Phys. Ther. 72 (1992) 885–892.
Twomey, L.; Taylor, J., Flexion creep deformation and hysteresis in the lumbar vertebral column, Spine 7 (1982) 116–122.
Twomey, L.T.; Taylor, J., The lumbar spine: structure, function, age changes and physiotherapy, Aust. Physiother. J. 40th Jubilee Issue 72 (1994) 19–30.
UK BEAM Trial Team, United Kingdom back pain exercise and manipulation randomised trial: effectiveness of physical treatments for back pain in primary care, Br. Med. J. 329 (2004) 1377.
UK BEAM Trial Team Randomised trial: cost effectiveness of physical treatments for back pain in primary care Br. Med. J.329 1381
Umehara, S.; Tadano, S.; Abumi, K.; et al., Effects of degeneration on the elastic modulus distribution in the lumbar intervertebral discs, Spine 21 (1996) 811–820.
Review – Low back pain and sciatica, In: Clinical Evidence Concise ( 2002)BMJ Publishing, London.
Van Tulder, M.W.; Koes, B.W.; Bouter, L.M., Conservative treatment of acute and chronic nonspecific low back pain: a systematic review of randomized controlled trials of the most common interventions., Spine 22 (18) ( 1997) 2128–2156.
Vernon-Roberts, B., Age-related and degenerative pathology of intervertebral discs and apophyseal joints, In: (Editor: Jayson, M.) the Lumbar Spine and Back Pain4th edn ( 1992)Churchill Livingstone, Edinburgh, pp. 17–41.
Vroomen, P.; de Krom, M.; Knotternus, J., Diagnostic value of history and physical examination in patients suspected of sciatica due to disc herniation: a systematic review, J. Neurol. 246 (1999) 899–906.
Vroomen, P.; de Krom, M.; Slofstra, P.; et al., Conservative treatment of sciatica: a systematic review, J. Spinal Disord. 13 (6) ( 2000) 463–469.
Vucetic, N.; Määttänen, H.; Svensson, O., Pain and pathology in lumbar disc hernia, Clin. Orthop. Relat. Res. 320 (1995) 65–72.
Waddell, G., Understanding the patient with backache, In: (Editor: Jayson, M.) The Lumbar Spine and Back Painfourth edn ( 1992)Churchill Livingstone, Edinburgh, pp. 469–485.
Waddell, G., The Back Pain Revolution. ( 1998)Churchill Livingstone, Edinburgh.
Waddell, G.; Feder, G.; McIntosh, A.; et al., Low Back Pain Evidence Review. ( 1996)Royal College of General Practitioners, London.
Williams, N.; Hendry, M.; Lewis, R.; et al., Psychological response in spinal manipulation (PRISM): a systematic review of psychological outcomes in randomised controlled trials, Complement. Ther. Med. 15 (2007) 271–283.
Yamamoto, I.; Panjabi, M.M.; Oxland, T.R.; et al., The role of the iliolumbar ligament in the lumbosacral junction., Spine 15 (1990) 1138–1141.
Yamashita, T.; Minaki, Y.; Ozaktay, A.C.; et al., A morphological study of the fibrous capsule of the human lumbar facet joint., Spine 21 (1996) 538–543.
Zimmermann, M., Basic neurophysiological mechanisms of pain and pain therapy, In: (Editor: Jayson, M.) The Lumbar Spine and Back Pain4th edn ( 1992)Churchill Livingstone, Edinburgh, pp. 43–59.