Chapter 9 The lumbar muscles and their fasciae
1 Psoas major, which covers the anterolateral aspects of the lumbar spine.
2 Intertransversarii laterales and quadratus lumborum, which connect and cover the transverse processes anteriorly.
3 The lumbar back muscles, which lie behind and cover the posterior elements of the lumbar spine.
Psoas major
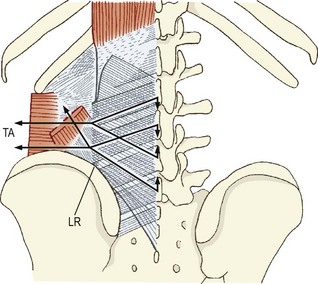
The psoas major has diverse but systematic attachments to the lumbar spine (Fig. 9.1). At each segmental level from T12–L1 to L4–5, it is attached to the medial three-quarters or so of the anterior surface of the transverse process, to the intervertebral disc, and to the margins of the vertebral bodies adjacent to the disc.1 An additional fascicle arises from the L5 vertebral body. Classically, the muscle is also said to arise from a tendinous arch that covers the lateral aspect of the vertebral body.2 Close dissection,1 however, reveals that these arches constitute no more than the medial, deep fascia of the muscle, and that the fascia affords no particular additional origin; the most medial fibres of the muscle skirt the fascia and are anchored directly to the upper margin of the vertebral body. Nonetheless, the fascia forms an arcade deep to the psoas, over the lateral surface of the vertebral body, leaving a space between the arch and the bone that transmits the lumbar arteries and veins (see Ch. 11).
The muscle fibres from the L4–5 intervertebral disc, the L5 body and the L5 transverse process form the deepest and lowest bundle of fibres within the muscle. These fibres are systematically overlapped by fibres from the disc, vertebral margins and transverse process at successively higher levels. As a result, the muscle in cross-section is layered circumferentially, with fibres from higher levels forming the outer surface of the muscle and those from lower levels buried sequentially, deeper within its substance. Within the muscle, bundles from individual lumbar segments have the same length, such that those from L1 become tendinous before those from successively lower levels. This isometric morphology indicates that the muscle is designed exclusively to act on the hip.1
Biomechanical analysis reveals that the psoas has only a feeble action on the lumbar spine with respect to flexion and extension. Its fibres are disposed so as to extend upper lumbar segments and to flex lower lumbar segments. However, the fibres act very close to the axes of rotation of the lumbar vertebrae and so can exert only very small moments, even under maximal contraction.1 This denies the psoas any substantial action on the lumbar spine. Rather, it uses the lumbar spine as a base from which to act on the hip.
However, the psoas potentially exerts massive compression loads on the lower lumbar discs. The proximity of the lines of action of the muscle to the axes of rotation minimises its capacity as a flexor but maximises the axial compression that it exerts. Upon maximum contraction, in an activity such as sit-ups, the two psoas muscles can be expected to exert a compression load on the L5–S1 disc equal to about 100 kg of weight.1
Intertransversarii laterales
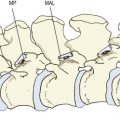
The intertransversarii laterales consist of two parts: the intertransversarii laterales ventrales and the intertransversarii laterales dorsales. The ventral intertransversarii connect the margins of consecutive transverse processes, while the dorsal intertransversarii each connect an accessory process to the transverse process below (Fig. 9.2). Both the ventral and dorsal intertransversarii are innervated by the ventral rami of the lumbar spinal nerves,3 and consequently cannot be classified among the back muscles which are all innervated by the dorsal rami (see Ch. 10). On the basis of their attachments and their nerve supply, the ventral and dorsal intertransversarii are considered to be homologous to the intercostal and levator costae muscles of the thoracic region.3
Quadratus lumborum
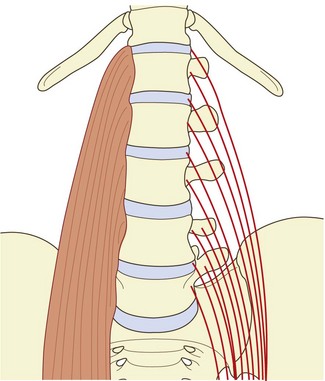
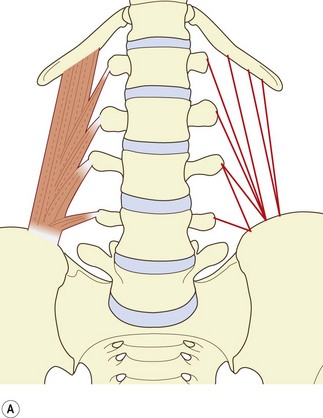
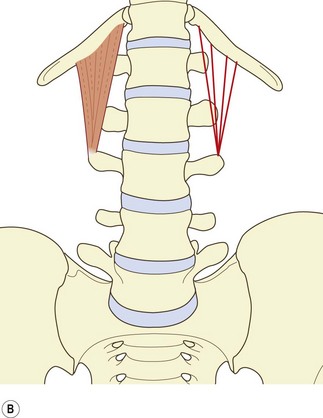
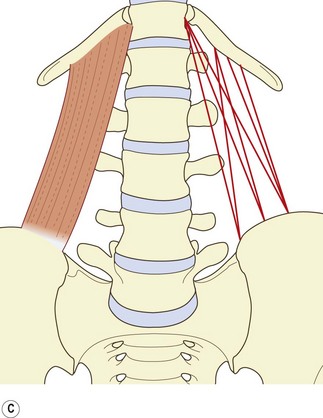
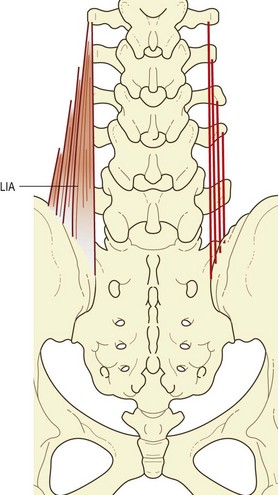
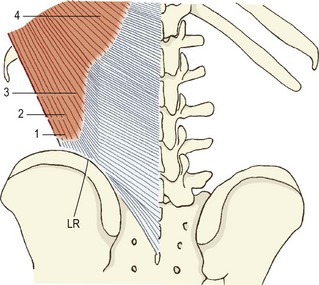
The quadratus lumborum is a wide, more or less rectangular, muscle that covers the lateral two-thirds or so of the anterior surfaces of the L1 to L4 transverse processes and extends laterally a few centimetres beyond the tips of the transverse processes. In detail, the muscle is a complex aggregation of various oblique and longitudinally running fibres that connect the lumbar transverse processes, the ilium and the 12th rib (Fig. 9.3).4
The muscle can be considered as consisting of four types of fascicle arranged in three layers.5 Iliocostal fibres connect the ilium and the 12th rib. Iliolumbar lumbar fibres connect the ilium and the lumbar transverse processes. Lumbocostal fibres connect the lumbar transverse processes and the 12th rib. A fourth type of fascicle connects the ilium and the body of the 12th thoracic vertebra. Occasionally, fascicles may connect the lumbar transverse processes to the body of the 12th thoracic vertebra.
The posterior layer (see Fig. 9.3A) consists of iliolumbar fascicles inferiorly and medially, and iliocostal fascicles laterally.5 The iliolumbar fibres arise from the iliac crest, and most consistently insert into the upper three lumbar transverse processes. Occasionally, some fascicles also insert into the L4 transverse process.
The middle layer (see Fig. 9.3B) typically arises by a common tendon from the anterior surface of the L3 transverse process. Its fascicles radiate to the inferior anterior aspect of the medial half or so of the 12th rib.5 Occasionally these fascicles are joined by ones from the L2, L4 and L5 transverse processes.
The anterior layer (see Fig. 9.3C) consists of more or less parallel fibres stemming from the iliac crest and passing upwards. The more lateral fibres insert into the lower anterior aspect of the 12th rib. More medial fibres insert into a tubercle on the lateral aspect of the body of the 12th thoracic vertebra.5 These latter fascicles may be joined at their insertion by fascicles from the lumbar transverse processes, most often from the L4 and L5 levels, when they occur.
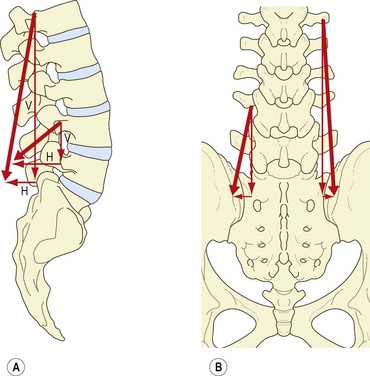
The irregular and inconstant structure of the quadratus lumborum makes it difficult to discern exactly its function. Classically one of the functions of this muscle is said to be to fix the 12th rib during respiration.2 This fits with many, although not most, of its fibres inserting into the 12th rib. The majority of fibres, and the largest, however, anchor the lumbar transverse processes and the 12th thoracic vertebra to the ilium. These attachments indicate that a major action of the muscle would be lateral flexion of the lumbar spine. However, the strength of the muscle is limited by the size of its fascicles and their moment arms. For lateral flexion, the quadratus lumborum can exert a maximum moment of about 35 Nm.5
Since the fascicles of the quadratus lumborum act behind the axes of sagittal rotation of the lumbar vertebrae, they are potentially extensors of the lumbar spine. However, in this role their capacity is limited to about 20 Nm,5 which amounts to less than 10% of the moment exerted by the posterior back muscles.
These limitations in strength leave the actual function of the quadratus lumborum still an enigma.
The lumbar back muscles
1 The short intersegmental muscles – the interspinales and the intertransversarii mediales.
2 The polysegmental muscles that attach to the lumbar vertebrae – the multifidus and the lumbar components of the longissimus and iliocostalis.
3 The long polysegmental muscles, represented by the thoracic components of the longissimus and iliocostalis lumborum, which in general do not attach to the lumbar vertebrae but cross the lumbar region from thoracic levels to find attachments on the ilium and sacrum.
The descriptions of the back muscles offered in this chapter, notably those of the multifidus and erector spinae, differ substantially from those given in standard textbooks. Traditionally, these muscles have been regarded as stemming from a common origin on the sacrum and ilium and passing upwards to assume diverse attachments to the lumbar and thoracic vertebrae and ribs. However, in the face of several studies of these muscles,6–9 it is considered more appropriate to view them in the reverse direction – from above downwards. Not only is this more consistent with the pattern of their nerve supply9,10 but it clarifies the identity of certain muscles and the identity of the erector spinae aponeurosis, and reveals the segmental biomechanical disposition of the muscles.
Interspinales
The lumbar interspinales are short paired muscles that lie on either side of the interspinous ligament and connect the spinous processes of adjacent lumbar vertebrae (see Fig. 9.2). There are four pairs in the lumbar region.
Intertransversarii mediales
The intertransversarii mediales can be considered to be true back muscles for, unlike the intertransversarii laterales, they are innervated by the lumbar dorsal rami.3,10 The intertransversarii mediales arise from an accessory process, the adjoining mamillary process and the mamillo-accessory ligament that connects these two processes.11 They insert into the superior aspect of the mamillary process of the vertebra below (see Fig. 9.2).
A tantalising alternative suggestion is that the intertransversarii (and perhaps also the interspinales) act as large proprioceptive transducers; their value lies not in the force they can exert but in the muscle spindles they contain. Placed close to the lumbar vertebral column, the intertransversarii could monitor the movements of the column and provide feedback that influences the action of the surrounding muscles. Such a role has been suggested for the cervical intertransversarii, which have been found to contain a high density of muscle spindles.12–14 Indeed, all unisegmental muscles of the vertebral column have between two and six times the density of muscles spindles found in the longer polysegmental muscles, and there is growing speculation that this underscores the proprioceptive function of all short, small muscles of the body.15–17
Multifidus
The multifidus is the largest and most medial of the lumbar back muscles. It consists of a repeating series of fascicles which stem from the laminae and spinous processes of the lumbar vertebrae and exhibit a constant pattern of attachments caudally.9
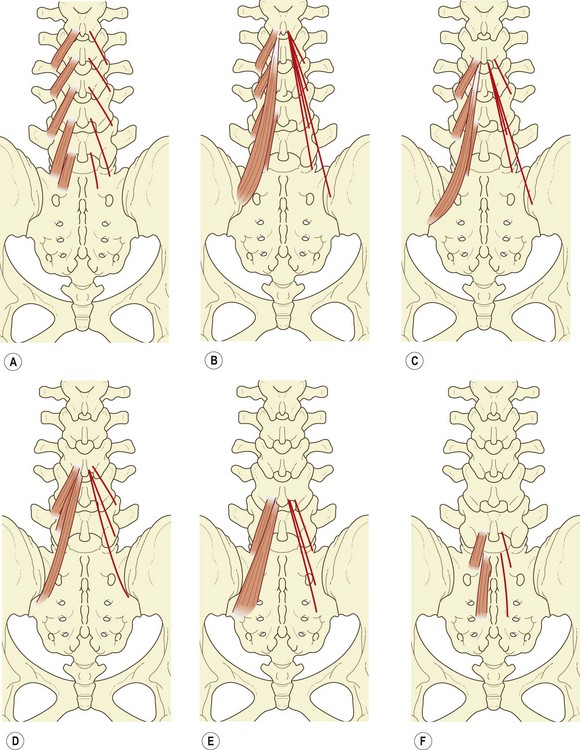
The shortest fascicles of the multifidus are the ‘laminar fibres’, which arise from the caudal end of the dorsal surface of each vertebral lamina and insert into the mamillary process of the vertebra two levels caudad (Fig. 9.4A). The L5 laminar fibres have no mamillary process into which they can insert, and insert instead into an area on the sacrum just above the first dorsal sacral foramen. Because of their attachments, the laminar fibres may be considered homologous to the thoracic rotatores.
The fascicle from the base of the L1 spinous process inserts into the L4 mamillary process, while those from the common tendon insert into the mamillary processes of L5, S1 and the posterior superior iliac spine (Fig. 9.4B).
The fascicle from the base of the spinous process of L2 inserts into the mamillary process of L5, while those from the common tendon insert into the S1 mamillary process, the posterior superior iliac spine, and an area on the iliac crest just caudoventral to the posterior superior iliac spine (Fig. 9.4C).
The fascicle from the base of the L3 spinous process inserts into the mamillary process of the sacrum, while those fascicles from the common tendon insert into a narrow area extending caudally from the caudal extent of the posterior superior iliac spine to the lateral edge of the third sacral segment (Fig. 9.4D). The L4 fascicles insert onto the sacrum in an area medial to the L3 area of insertion, but lateral to the dorsal sacral foramina (Fig. 9.4E), while those from the L5 vertebra insert onto an area medial to the dorsal sacral foramina (Fig. 9.4F).
It is noteworthy that while many of the fascicles of multifidus attach to mamillary processes, some of the deeper fibres of these fascicles attach to the capsules of the zygapophysial joints next to the mamillary processes (see Ch. 3).18 This attachment allows the multifidus to protect the joint capsule from being caught inside the joint during the movements executed by the multifidus.
The key feature of the morphology of the lumbar multifidus is that its fascicles are arranged segmentally. Each lumbar vertebra is endowed with a group of fascicles that radiate from its spinous process, anchoring it below to mamillary processes, the iliac crest and the sacrum. This disposition suggests that the fibres of multifidus are arranged in such a way that their principal action is focused on individual lumbar spinous processes.9 They are designed to act in concert on a single spinous process. This contention is supported by the pattern of innervation of the muscle. All the fascicles arising from the spinous processes of a given vertebra are innervated by the medial branch of the dorsal ramus that issues from below that vertebra (see Ch. 10).9,10 Thus, the muscles that directly act on a particular vertebral segment are innervated by the nerve of that segment.
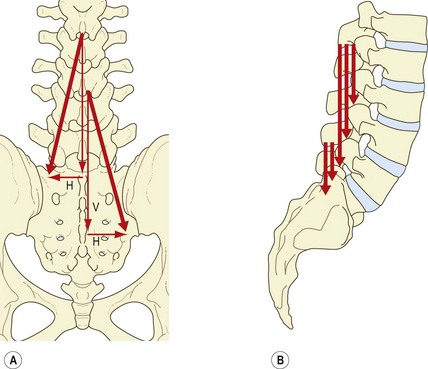
In a posterior view, the fascicles of multifidus are seen to have an oblique caudolateral orientation. Their line of action can therefore be resolved into two vectors: a large vertical vector and a considerably smaller horizontal vector (Fig. 9.5A).7
The small horizontal vector suggests that the multifidus could pull the spinous processes sideways, and therefore produce horizontal rotation. However, horizontal rotation of lumbar vertebrae is impeded by the impaction of the contralateral zygapophysial joints. Horizontal rotation occurs after impaction of the joints only if an appropriate shear force is applied to the intervertebral discs (see Ch. 7) but the horizontal vector of multifidus is so small that it is unlikely that the multifidus would be capable of exerting such a shear force on the disc by acting on the spinous process. Indeed, electromyographic studies reveal that the multifidus is inconsistently active in derotation and that, paradoxically, it is active in both ipsilateral and contralateral rotation.19 Rotation, therefore, cannot be inferred to be a primary action of the multifidus. In this context, the multifidus has been said to act only as a ‘stabiliser’ in rotation,18,19 but the aberrant movements, which it is supposed to stabilise, have not been defined (although see below).
The principal action of the multifidus is expressed by its vertical vector, and further insight is gained when this vector is viewed in a lateral projection (Fig. 9.5B). Each fascicle of multifidus, at every level, acts virtually at right angles to its spinous process of origin.7 Thus, using the spinous process as a lever, every fascicle is ideally disposed to produce posterior sagittal rotation of its vertebra. The right-angle orientation, however, precludes any action as a posterior horizontal translator. Therefore, the multifidus can only exert the ‘rocking’ component of extension of the lumbar spine or control this component during flexion.
Having established that the multifidus is primarily a posterior sagittal rotator of the lumbar spine, it is possible to resolve the paradox about its activity during horizontal rotation of the trunk.7 In the first instance, it should be realised that rotation of the lumbar spine is an indirect action. Active rotation of the lumbar spine occurs only if the thorax is first rotated, and is therefore secondary to thoracic rotation. Secondly, it must be realised that a muscle with two vectors of action cannot use these vectors independently. If the muscle contracts, then both vectors are exerted. Thus, the multifidus cannot exert axial rotation without simultaneously exerting a much larger posterior sagittal rotation.
The role of the multifidus in rotation is not to produce rotation but to oppose the flexion effect of the abdominal muscles as they produce rotation. The aberrant motion ‘stabilised’ by the multifidus during rotation is, therefore, the unwanted flexion unavoidably produced by the abdominal muscles.7
Lumbar erector spinae
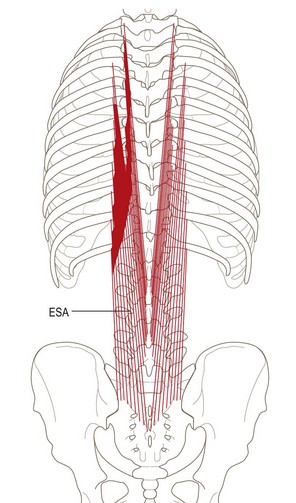
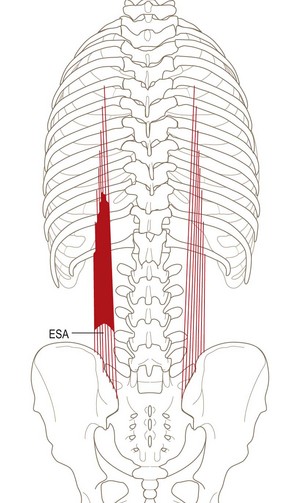
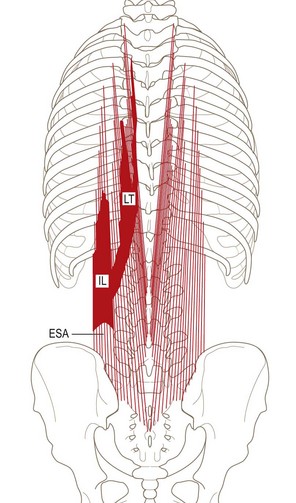
The lumbar erector spinae lies lateral to the multifidus and forms the prominent dorsolateral contour of the back muscles in the lumbar region. It consists of two muscles: the longissimus thoracis and the iliocostalis lumborum. Furthermore, each of these muscles has two components: a lumbar part, consisting of fascicles arising from lumbar vertebrae, and a thoracic part, consisting of fascicles arising from thoracic vertebrae or ribs.6,8 These four parts may be referred to, respectively, as longissimus thoracis pars lumborum, iliocostalis lumborum pars lumborum, longissimus thoracis pars thoracis and iliocostalis lumborum pars thoracis.8
In the lumbar region, the longissimus and iliocostalis are separated from each other by the lumbar intermuscular aponeurosis, an anteroposterior continuation of the erector spinae aponeurosis.6,8 It appears as a flat sheet of collagen fibres, which extend rostrally from the medial aspect of the posterior superior iliac spine for 6–8 cm. It is formed mainly by the caudal tendons of the rostral four fascicles of the lumbar component of longissimus (Fig. 9.6).
Longissimus thoracis pars lumborum
The longissimus thoracis pars lumborum is composed of five fascicles, each arising from the accessory process and the adjacent medial end of the dorsal surface of the transverse process of a lumbar vertebra (see Fig. 9.6).
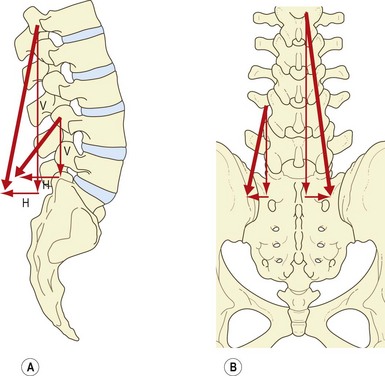
Each fascicle of the lumbar longissimus has both a dorsoventral and a rostrocaudal orientation.8 Therefore, the action of each fascicle can be resolved into a vertical vector and a horizontal vector, the relative sizes of which differ from L1 to L5 (Fig. 9.7A). Consequently, the relative actions of the longissimus differ at each segmental level. Furthermore, the action of the longissimus, as a whole, will differ according to whether the muscle contracts unilaterally or bilaterally.
The large vertical vector of each fascicle lies lateral to the axis of lateral flexion and behind the axis of sagittal rotation of each vertebra. Thus, contracting the longissimus unilaterally can laterally flex the vertebral column, but acting bilaterally the various fascicles can act, like the multifidus, to produce posterior sagittal rotation of their vertebra of origin. However, their attachments to the accessory and transverse processes lie close to the axes of sagittal rotation, and therefore their capacity to produce posterior sagittal rotation is less efficient than that of the multifidus, which acts through the long levers of the spinous processes.8
The horizontal vectors of the longissimus are directed backwards. Therefore, when contracting bilaterally the longissimus is capable of drawing the lumbar vertebrae backwards. This action of posterior translation can restore the anterior translation of the lumbar vertebrae that occurs during flexion of the lumbar column (see Ch. 7). The capacity for posterior translation is greatest at lower lumbar levels, where the fascicles of longissimus assume a greater dorsoventral orientation (Fig. 9.7B).
Iliocostalis lumborum pars lumborum
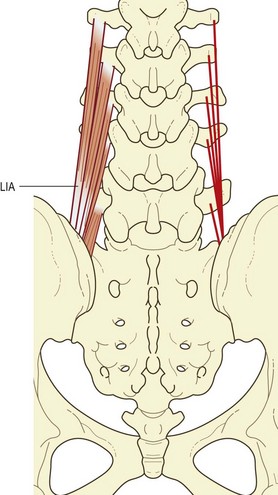
The lumbar component of the iliocostalis lumborum consists of four overlying fascicles arising from the L1 through to the L4 vertebrae. Rostrally, each fascicle attaches to the tip of the transverse process and to an area extending 2–3 cm laterally onto the middle layer of the thoracolumbar fascia (Fig. 9.8).
Although an L5 fascicle of iliocostalis lumborum is not described in the literature, it is represented in the iliolumbar ‘ligament’. In neonates and children this ‘ligament’ is said to be completely muscular in structure (see Ch. 4).20 By the third decade of life, the muscle fibres are entirely replaced by collagen, giving rise to the familiar iliolumbar ligament.20 On the basis of sites of attachment and relative orientation, the posterior band of the iliolumbar ligament would appear to be derived from the L5 fascicle of iliocostalis, while the anterior band of the ligament is a derivative of the quadratus lumborum.
The disposition of the lumbar fascicles of iliocostalis is similar to that of the lumbar longissimus, except that the fascicles are situated more laterally. Like that of the lumbar longissimus, their action can be resolved into horizontal and vertical vectors (Fig. 9.9A).
The vertical vector is still predominant, and therefore the lumbar fascicles of iliocostalis contracting bilaterally can act as posterior sagittal rotators (Fig. 9.9B), but because of the horizontal vector a posterior translation will be exerted simultaneously, principally at lower lumbar levels where the fascicles of iliocostalis have a greater forward orientation. Contracting unilaterally, the lumbar fascicles of iliocostalis can act as lateral flexors of the lumbar vertebrae, for which action the transverse processes provide very substantial levers.
Longissimus thoracis pars thoracis
The thoracic fibres of longissimus thoracis typically consist of 11 or 12 pairs of small fascicles arising from the ribs and transverse processes of T1 or T2 down to T12 (Fig. 9.10). At each level, two tendons can usually be recognised, a medial one from the tip of the transverse process and a lateral one from the rib, although in the upper three or four levels, the latter may merge medially with the fascicle from the transverse process. Each rostral tendon extends 3–4 cm before forming a small muscle belly measuring 7–8 cm in length. The muscle bellies from the higher levels overlap those from lower levels. Each muscle belly eventually forms a caudal tendon that extends into the lumbar region. The tendons run in parallel, with those from higher levels being most medial. The fascicles from the T2 level attach to the L3 spinous process, while the fascicles from the remaining levels insert into spinous processes at progressively lower levels. For example, those from T5 attach to L5, and those from T7 to S2 or S3. Those from T8 to T12 diverge from the midline to find attachment to the sacrum along a line extending from the S3 spinous process to the caudal extent of the posterior superior iliac spine.8 The lateral edge of the caudal tendon of T12 lies alongside the dorsal edge of the lumbar intermuscular aponeurosis formed by the caudal tendon of the L1 longissimus bundle.
Iliocostalis lumborum pars thoracis
The iliocostalis lumborum pars thoracis consists of fascicles from the lower seven or eight ribs that attach caudally to the ilium and sacrum (Fig. 9.11). These fascicles represent the thoracic component of iliocostalis lumborum and should not be confused with the iliocostalis thoracis, which is restricted to the thoracic region between the upper six and lower six ribs.
Erector spinae aponeurosis
One of the cardinal revelations of studies of the lumbar erector spinae6,8 is that this muscle consists of both lumbar and thoracic fibres. Modern textbook descriptions largely do not recognise the lumbar fibres, especially those of the iliocostalis.6 Moreover, they do not note that the lumbar fibres (of both longissimus and iliocostalis) have attachments quite separate to those of the thoracic fibres. The lumbar fibres of longissimus and iliocostalis pass between the lumbar vertebrae and the ilium. Thus, through these muscles, the lumbar vertebrae are anchored directly to the ilium. They do not gain any attachment to the erector spinae aponeurosis, which is the implication of all modern textbook descriptions that deal with the erector spinae.
The erector spinae aponeurosis is described as a broad sheet of tendinous fibres that is attached to the ilium, the sacrum, and the lumbar and sacral spinous processes, and which forms a common origin for the lower part of erector spinae.2 However, as described above, the erector spinae aponeurosis is formed virtually exclusively by the tendons of longissimus thoracis pars thoracis and iliocostalis pars thoracis.6,8 The medial half or so of the aponeurosis is formed by the tendons of longissimus thoracis, and the lateral half is formed by the iliocostalis lumborum (Fig. 9.12). The only additional contribution comes from the most superficial fibres of multifidus from upper lumbar levels, which contribute a small number of fibres to the aponeurosis (see Figs. 9.10, 9.11).9 Nonetheless, the erector spinae aponeurosis is essentially formed only by the caudal attachments of muscles acting from thoracic levels.
Thoracolumbar fascia
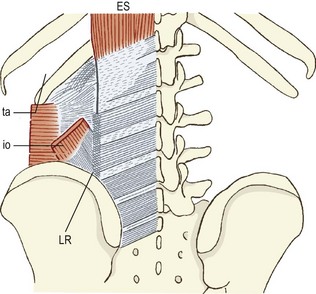
The thoracolumbar fascia consists of three layers of fascia that envelop the muscles of the lumbar spine, effectively separating them into three compartments. The anterior layer of thoracolumbar fascia is quite thin and is derived from the fascia of quadratus lumborum. It covers the anterior surface of quadratus lumborum and is attached medially to the anterior surfaces of the lumbar transverse processes. In the intertransverse spaces, it blends with the intertransverse ligaments and may be viewed as one of the lateral extensions of the intertransverse ligaments (see Ch. 4). Lateral to the quadratus lumborum, the anterior layer blends with the other layers of the thoracolumbar fascia.
The posterior layer of thoracolumbar fascia covers the back muscles. It arises from the lumbar spinous processes in the midline posteriorly and wraps around the back muscles to blend with the other layers of the thoracolumbar fascia along the lateral border of the iliocostalis lumborum. The union of the fasciae is quite dense at this site, and the middle and posterior layers, in particular, form a dense raphe which, for purposes of reference, has been called the lateral raphe.21
Traditionally, the thoracolumbar fascia has been ascribed no other function than to invest the back muscles and to provide an attachment for the transversus abdominis and the internal oblique muscles.2 However, in recent years there has been considerable interest in its biomechanical role in the stability of the lumbar spine, particularly in the flexed posture and in lifting. This has resulted in anatomical and biomechanical studies of the anatomy and function of the thoracolumbar fascia, notably its posterior layer.21–24
On close inspection, the posterior layer exhibits a cross-hatched appearance, manifest because it consists of two laminae: a superficial lamina with fibres orientated caudomedially and a deep lamina with fibres oriented caudolaterally.21,24
The superficial lamina is formed by the aponeurosis of latissimus dorsi, but the disposition and attachments of its constituent fibres differ according to the portion of latissimus dorsi from which they are derived (Fig. 9.13). Those fibres derived from the most lateral 2–3 cm of the muscle are short and insert directly into the iliac crest without contributing to the thoracolumbar fascia. Fibres from the next most lateral 2 cm of the muscle approach the iliac crest near the lateral margin of the erector spinae, but then deflect medially, bypassing the crest to attach to the L5 and sacral spinous processes. These fibres form the sacral portion of the superficial lamina. A third series of fibres becomes aponeurotic just lateral to the lumbar erector spinae. At the lateral border of the erector spinae, they blend with the other layers of thoracolumbar fascia in the lateral raphe, but then they deflect medially, continuing over the back muscles to reach the midline at the levels of the L3, L4 and L5 spinous processes. These fibres form the lumbar portion of the superficial lamina of the posterior layer of thoracolumbar fascia.
Beneath the superficial lamina, the deep lamina of the posterior layer consists of bands of collagen fibres emanating from the midline, principally from the lumbar spinous processes (Fig. 9.14). The bands from the L4, L5 and S1 spinous processes pass caudolaterally to the posterior superior iliac spine. Those from the L3 spinous process and L3–4 interspinous ligament wrap around the lateral margin of the erector spinae to fuse with the middle layer of thoracolumbar fascia in the lateral raphe. Above L3 the deep lamina progressively becomes thinner, consisting of sparse bands of collagen that dissipate laterally over the erector spinae. A deep lamina is not formed at thoracic levels.
Additionally, the deep lamina alone forms a series of distinct ligaments. When viewed bilaterally, the bands of fibres from the L4 and L5 spinous processes appear like alar ligaments anchoring these spinous processes to the ilia. The band from the L3 spinous process anchors this process indirectly to the ilium via the lateral raphe. Thirdly, the lateral raphe forms a site where the two laminae of the posterior layer fuse not only with the middle layer of thoracolumbar fascia but also with the transversus abdominis whose middle fibres arise from the lateral raphe (see Fig. 9.14). The posterior layer of thoracolumbar fascia thereby provides an indirect attachment for the transversus abdominis to the lumbar spinous processes. The mechanical significance of these three morphological features is explored in the following section.
Functions of the back muscles and their fasciae
Minor active movements
In the upright position, the lumbar back muscles play a minor, or no active, role in executing movement, for gravity provides the necessary force. During extension, the back muscles contribute to the initial tilt, drawing the line of gravity backwards25,26 but are unnecessary for further extension. Muscle activity is recruited when the movement is forced or resisted27 but is restricted to muscles acting on the thorax. The lumbar multifidus, for example, shows little or no involvement.28
The lateral flexors can bend the lumbar spine sideways, but once the centre of gravity of the trunk is displaced lateral flexion can continue under the influence of gravity. However, the ipsilateral lateral flexors are used to direct the movement, and the contralateral muscles are required to balance the action of gravity and control the rate and extent of movement. Consequently, lateral flexion is accompanied by bilateral activity of the lumbar back muscles, but the contralateral muscles are relatively more active as they are the ones that must balance the load of the laterally flexing spine.25,26,29–32 If a weight is held in the hand on the side to which the spine is laterally flexed, a greater load is applied to the spine, and the contralateral back muscles show greater activity to balance this load.29,31
Maintenance of posture
During standing at ease, the back muscles may show slight continuous activity,19,25–27,30,32–42 intermittent activity25,27,32,42,43 or no activity,36,39–42 and the amount of activity can be influenced by changing the position of the head or allowing the trunk to sway.25
The explanation for these differences probably lies in the location of the line of gravity in relation to the lumbar spine in different individuals.27,36,41,42,44 In about 75% of individuals the line of gravity passes in front of the centre of the L4 vertebra, and therefore essentially in front of the lumbar spine.36,41 Consequently, gravity will exert a constant tendency to pull the thorax and lumbar spine into flexion. To preserve an upright posture, a constant level of activity in the posterior sagittal rotators of the lumbar spine will be needed to oppose the tendency to flexion. Conversely, when the line of gravity passes behind the lumbar spine, gravity tends to extend it, and back muscle activity is not required. Instead, abdominal muscle activity is recruited to prevent the spine extending under gravity.36,41
Activities that displace the centre of gravity of the trunk sideways will tend to cause lateral flexion. To prevent undesired lateral flexion, the contralateral lateral flexors will contract. This occurs when weights are carried in one hand.25,39 Carrying equal weights in both hands does not displace the line of gravity, and back muscle activity is not increased substantially on either side of the body.25,39
During sitting, the activity of the back muscles is similar to that during standing34,35,45,46 but in supported sitting, as with the elbows resting on the knees, there is no activity in the lumbar back muscles,25,32 and with arms resting on a desk, back muscle activity is substantially decreased.34,35,45 In reclined sitting, the back rest supports the weight of the thorax, lessening the need for muscular support. Consequently, increasing the declination of the back rest of a seat decreases lumbar back muscle activity.34,35,45,47,48
Major active movements
Forward flexion and extension of the spine from the flexed position are movements during which the back muscles have their most important function. As the spine bends forwards, there is an increase in the activity of the back muscles;19,25,26,28–30,32,33,43,49–52 this increase is proportional to the angle of flexion and the size of any load carried.29,31,53,54 The movement of forward flexion is produced by gravity, but the extent and the rate at which it proceeds is controlled by the eccentric contraction of the back muscles. Movement of the thorax on the lumbar spine is controlled by the long thoracic fibres of longissimus and iliocostalis. The long tendons of insertion allow these muscles to act around the convexity of the increasing thoracic kyphosis and anchor the thorax to the ilium and sacrum. In the lumbar region, the multifidus and the lumbar fascicles of longissimus and iliocostalis act to control the anterior sagittal rotation of the lumbar vertebrae. At the same time the lumbar fascicles of longissimus and iliocostalis also act to control the associated anterior translation of the lumbar vertebrae.
At a certain point during forward flexion, the activity in the back muscles ceases, and the vertebral column is braced by the locking of the zygapophysial joints and tension in its posterior ligaments (see Ch. 7). This phenomenon is known as ‘critical point’.26,43,44,55 However, critical point does not occur in all individuals or in all muscles.19,25,32,42 When it does occur, it does so when the spine has reached about 90% maximum flexion, even though at this stage the hip flexion that occurs in forward bending is still only 60% complete.44,55 Carrying weights during flexion causes the critical point to occur later in the range of vertebral flexion.44,55
The physiological basis for critical point is still obscure. It may be due to reflex inhibition initiated by proprioceptors in the lumbar joints and ligaments, or in muscle stretch and length receptors.55 Whatever the mechanism, the significance of critical point is that it marks the transition of spinal load-bearing from muscles to the ligamentous system.
Extension of the trunk from the flexed position is characterised by high levels of back muscle activity.19,25,26,43,52 In the thoracic region, the iliocostalis and longissimus, acting around the thoracic kyphosis, lift the thorax by rotating it backwards. The lumbar vertebrae are rotated backwards principally by the lumbar multifidus, causing their superior surfaces to be progressively tilted upwards to support the rising thorax.
Compressive loads of the back muscles
Because of the downward direction of their action, as the back muscles contract they exert a longitudinal compression of the lumbar vertebral column, and this compression raises the pressure in the lumbar intervertebral discs. Any activity that involves the back muscles, therefore, is associated with a rise in nuclear pressure. As measured in the L3–4 intervertebral disc, the nuclear pressure correlates with the degree of myoelectric activity in the back muscles.29,31,48,56,57 As muscle activity increases, disc pressure rises.
Disc pressures and myoelectric activity of the back muscles have been used extensively to quantify the stresses applied to the lumbar spine in various postures and by various activities.34,46–48,58–63 From the standing position, forward bending causes the greatest increase in disc pressure. Lifting a weight in this position raises disc pressure even further, and the pressure is greatly increased if a load is lifted with the lumbar spine both flexed and rotated. Throughout these various manoeuvres, back muscle activity increases in proportion to the disc pressure.
One of the prime revelations of combined discometric and electromyographic studies of the lumbar spine during lifting relates to the comparative stresses applied to the lumbar spine by different lifting tactics. In essence, it has been shown that, on the basis of changes in disc pressure and back muscle activity, there are no differences between using a ‘stoop’ lift or a ‘leg’ lift, i.e. lifting a weight with a bent back versus lifting with a straight back.29,47,48,64 The critical factor is the distance of the load from the body. The further the load is from the chest the greater the stresses on the lumbar spine, and the greater the disc pressure and back muscle activity.64 Performing a ‘leg’ lift with a straight back as opposed to maintaining a lordosis involves about 5% less electromyographic activity in the back muscles early in the lift but little difference thereafter.65
Strength of the back muscles
The strength of the back muscles has been determined in experiments on normal volunteers.66 Two measures of strength are available: the absolute maximum force of contraction in the upright posture and the moment generated on the lumbar spine. The absolute maximum strength of the back muscles as a whole is about 4000 N. Acting on the short moment arms provided by the spinous processes and pedicles of the lumbar vertebrae, this force converts to an extensor moment of 200 Nm. These figures apply to average males under the age of 30; young females exhibit about 60% of this strength, while individuals over the age of 30 are about 10–30% weaker.66
Easy standing involves some 2–5% of maximum isometric strength; manual handling of heavy loads involves between 75% and 100%; sitting involves between 3% and 15% of maximum activity.67
Detailed dissection studies have allowed the strength of contraction to be apportioned to individual components of the back muscles.68 Of the total extensor moment, the thoracic fibres of iliocostalis and longissimus account for some 50%. Thus, half of the extensor moment on the lumbar spine is exerted through the erector spinae aponeurosis. The other half is exerted by the muscles that act directly on the lumbar vertebrae, with the multifidus providing half of that 50% and the longissimus thoracis pars lumborum and iliocostalis lumborum pars lumborum providing the remainder. The compression loads exerted by the lumbar back muscles differ from segment to segment because of the different spans and attachments of the various muscles. However, at L5–S1 the thoracic fibres of the lumbar erector spinae exert about 42% of the total compression load, the lumbar fibres of this muscle contribute 36% and the multifidus contributes 22%.68 At higher lumbar levels, relatively more of the total compression load on the segment is exerted by the thoracic fibres of the lumbar erector spinae.
With respect to shear forces, in the upright position the various lumbar back muscles exert forces that differ in magnitude and in direction at different levels.68 This arises because of the different orientation of particular fascicles of the various muscles and because of the different orientation of particular vertebrae in the lumbar lordosis. As a result, the multifidus exerts mainly anterior shear forces at upper lumbar levels, but either anterior or posterior shear forces at lower levels; the lumbar fibres of erector spinae exert posterior shear forces on the vertebrae to which they are attached, but anterior shear forces on vertebrae below these; the thoracic fibres of lumbar erector spinae exert posterior shear forces on upper lumbar segments, but anterior shear forces on L4 and L5.68 The net effect is that the back muscles exert posterior shear forces on upper lumbar segments in the upright spine but, paradoxically, they exert a net anterior shear force on L5.
Intriguingly, flexion of the lumbar spine does not compromise the strength of the back muscles.69 The moment arms of some fascicles are reduced by flexion but those of others are increased, resulting in no significant change in the total capacity to generate moments. All fascicles, however, are elongated, but although this reduces their maximum force on active contraction, it increases the passive tension in the muscles, resulting in no reduction in total tension. Consequently, upon flexion, the total extensor moment of the back muscles and the compression load that they exert change little from those in the upright position. However, the shear forces change appreciably. The posterior shear forces on upper lumbar segments are reduced by flexion but the shear force on L5 reverses from an anterior shear force in the upright position to a posterior shear force in full flexion.69
With respect to axial rotation, although the back muscles have reasonable moment arms, they are compromised by their longitudinal orientation.70 Only their horizontal vectors can exert axial rotation but these are very small components of the action of any of the muscles. As a result, the total maximal possible torque exerted by all the back muscles is next to trivial, and that exerted by any one muscle is negligible.70 Consequently, the back muscles afford no stability to the lumbar spine in axial rotation. For that, the lumbar spine is reliant on the abdominal muscles.70
Histochemistry
Slow-twitch fibres constitute some 70% of the fibres of longissimus.67 They constitute about 55% of the iliocostalis and multifidus. Conversely, fast-twitch type A fibres constitute 20% of the fibres of multifidus, iliocostalis and longissimus, and fast-twitch type B fibres constitute 25% of the fibres of multifidus and iliocostalis but only 11% of longissimus.67
These histochemical profiles seem to correlate with the fatigue resistance and endurance times of the back muscles, which are larger than most human muscles.67 However, individuals exhibit a large variance in fatigue resistance.67 The possibilities arise that endurance may be a direct function of the density of slow-twitch fibres in the back muscles, that lack of resistance to fatigue is a risk factor for back injury, and that conditioning can change the histochemical profile of an individual to overcome this risk. These possibilities, however, remain to be explored.
A study has shown that there is no correlation between pain and either fatigue or fibre type.71 Patients with back pain my exhibit less strength than asymptomatic individuals but not because of histochemical differences in their muscles.
Lifting
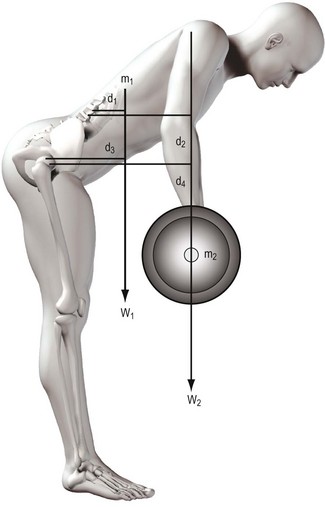
In biomechanical terms, the act of lifting constitutes a problem in balancing moments. When an individual bends forwards to execute a lift, flexion occurs at the hip joint and in the lumbar spine. Indeed, most of the forward movement seen during trunk flexion occurs at the hip joint.55 The flexion forces are generated by gravity acting on the mass of the object to be lifted and on the mass of the trunk above the level of the hip joint and lumbar spine (Fig. 9.15). These forces exert flexion moments on both the hip joint and lumbar spine. In each case, the moment will be the product of the force and its perpendicular distance from the joint in question. The total flexion moment acting on each joint will be the sum of the moments exerted by the mass to be lifted and the mass of the trunk. For a lift to be executed, these flexion moments have to be overcome by a moment acting in the opposite direction. This could be exerted by longitudinal forces acting downwards behind the hip joint and vertebral column or by forces acting upwards in front of the joints, pushing the trunk upwards.
There are no doubts as to the capacity of the hip extensors to generate large moments and overcome the flexion moments exerted on the hip joint, even by the heaviest of loads that might be lifted.72,73 However, the hip extensors are only able to rotate the pelvis backwards on the femurs; they do not act on the lumbar spine. Thus, regardless of what happens at the hip joint, the lumbar spine still remains subject to a flexion moment that must be overcome in some other way. Without an appropriate mechanism, the lumbar spine would stay flexed as the hips extended; indeed, as the pelvis rotated backwards, flexion of the lumbar spine would be accentuated as its bottom end was pulled backwards with the pelvis while its top end remained stationary under the load of the flexion moment. A mechanism is required to allow the lumbar spine to resist this deformation or to cause it to extend in unison with the hip joint.
In 1957, Bartelink74 raised the proposition that intra-abdominal pressure could aid the lumbar spine in resisting flexion by acting upwards on the diaphragm: the so-called intra-abdominal balloon mechanism. Bartelink himself was circumspect and reserved in raising this conjecture but the concept was rapidly popularised, particularly among physiotherapists. Even though it was never validated, the concept seemed to be treated as proven fact. It received early endorsement in orthopaedic circles,28 and intra-abdominal pressure was adopted by ergonomists and others as a measure of spinal stress and safe-lifting standards.75–82 In more contemporary studies, intra-abdominal pressure has been monitored during various spinal movements and lifting tasks.29,64,83
Reservations about the validity of the abdominal balloon mechanism have arisen from several quarters. Studies of lifting tasks reveal that, unlike myoelectric activity, intra-abdominal pressure does not correlate well with the size of the load being lifted or the applied stress on the vertebral column as measured by intradiscal pressure.56,57,84 Indeed, deliberately increasing intra-abdominal pressure by a Valsalva manoeuvre does not relieve the load on the lumbar spine but actually increases it.85 Clinical studies have shown that although abdominal muscles are weaker than normal in patients with back pain, intra-abdominal pressure is not different.86 Furthermore, strengthening the abdominal muscles both in normal individuals87 and in patients with back pain88 does not influence intra-abdominal pressure during lifting.
1 To generate any significant anti-flexion moment the pressure required would exceed the maximum hoop tension of the abdominal muscles89–91
2 Such a pressure would be so high as to obstruct the abdominal aorta (a reservation raised by Bartelink himself74,89)
3 Because the abdominal muscles lie in front of the lumbar spine and connect the thorax to the pelvis, whenever they contract to generate pressure they must also exert a flexion moment on the trunk, which would negate any anti-flexion value of the intra-abdominal pressure.72,73,91,92
These reservations inspired an alternative explanation of the role of the abdominal muscles during lifting. Farfan, Gracovetsky and colleagues23,72,91,93 noted the criss-cross arrangement of the fibres in the posterior layer of thoracolumbar fascia and surmised that, if lateral tension was applied to this fascia, it would result in an extension moment being exerted on the lumbar spinous processes. Such tension could be exerted by the abdominal muscles that arise from the thoracolumbar fascia, and the trigonometry of the fibres in the thoracolumbar fascia was such that they could convert lateral tension into an appreciable extension moment: the so-called ‘gain’ of the thoracolumbar fascia.91 The role of the abdominal muscles during lifting was thus to brace, if not actually extend, the lumbar spine by pulling on the thoracolumbar fascia. Any rises in intra-abdominal pressure were thereby only coincidental, occurring because of the contraction of the abdominal muscles acting on the thoracolumbar fascia.
Subsequent anatomic studies revealed several liabilities of this model.21 First, the posterior layer of thoracolumbar fascia is well developed only in the lower lumbar region, but nevertheless its fibres are appropriately orientated to enable lateral tension exerted on the fascia to produce extension moments at least on the L2–L5 spinous processes (Fig. 9.16). However, dissection reveals that of the abdominal muscles the internal oblique offers only a few fibres that irregularly attach to the thoracolumbar fascia; the transversus abdominis is the only muscle that consistently attaches to the thoracolumbar fascia, but only its very middle fibres do this. The size of these fibres is such that, even upon maximum contraction, the force they exert is very small. Calculations revealed that the extensor moment they could exert on the lumbar spine amounted to less than 6 Nm.94 Thus, the contribution that abdominal muscles might make to anti-flexion moments is trivial, a conclusion also borne out by subsequent independent modelling studies.83
A totally different model of lifting was elaborated by Farfan and Gracovetsky.23,72,91 Noting the weakness of the back muscles, these authors proposed that extension of the lumbar spine was not required to lift heavy loads or loads with long moment arms. They proposed that the lumbar spine should remain fully flexed in order to engage, i.e. maximally stretch, what they referred to as the ‘posterior ligamentous system’, namely the capsules of the zygapophysial joints, the interspinous and supraspinous ligaments, and the posterior layer of thoracolumbar fascia, the latter acting passively to transmit tension between the lumbar spinous processes and the ilium.
The attraction of this model was that it overcame the problem of the relative weakness of the back muscles by dispensing with their need to act, which in turn was consistent with the myoelectric silence of the back muscles at full flexion of the trunk and the recruitment of muscle activity only once the trunk had been elevated and the flexion moment arm had been reduced. Support for the model also came from surgical studies which reported that if the midline ligaments and thoracolumbar fascia were conscientiously reconstructed after multilevel laminectomies, the postoperative recovery and rehabilitation of patients were enhanced.95
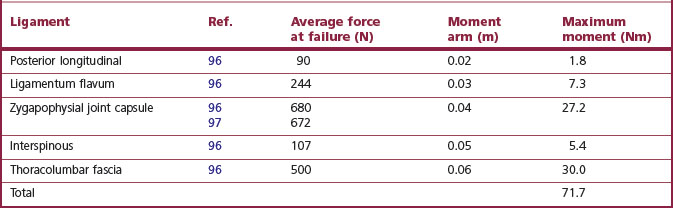
The strength of spinal ligaments varies considerably but average values can be calculated. Table 9.1 summarises some of the available data. It is evident that the strongest posterior ‘ligaments’ of the lumbar spine are the zygapophysial joint capsules and the thoracolumbar fascia forming the midline ‘supraspinous ligament’. However, when the relatively short moment arms over which these ligaments act are considered, it transpires that the maximum moment they can sustain is relatively small. Even the sum total of all their moments is considerably less than that required for heavy lifting and is some four times less than the maximum strength of the back muscles. Of course, it is possible that the data quoted may not be representative of the true mean values of the strength of these ligaments but it does not seem likely that the literature quoted underestimated their strength by a factor of four or more. Under these conditions, it is evident that the posterior ligamentous system alone is not strong enough to perform the role required of it in heavy lifting. The posterior ligamentous system is not strong enough to replace the back muscles as a mechanism to prevent flexion of the lumbar spine during lifting. Some other mechanism must operate.
Table 9.1 Strength of the posterior ligamentous system. The average force at failure has been calculated using raw data provided in the references cited. The moment arms are estimates based on inspection of a representative vertebra, measuring the perpendicular distance between the location of the axes of rotation of the lumbar spine and the sites of attachment of the various ligaments
One such mechanism is that of the hydraulic amplifier effect.93 It was originally proposed by Gracovetsky et al.93 that because the thoracolumbar fascia surrounded the back muscles as a retinaculum it could serve to brace these muscles and enhance their power. The engineering basis for this effect is complicated, and the concept remained unexplored until very recently. A mathematical proof has been published which suggests that by investing the back muscles the thoracolumbar fascia enhances the strength of the back muscles by some 30%.98 This is an appreciable increase and an attractive mechanism for enhancing the antiflexion capacity of the back muscles. However, the validity of this proof is still being questioned on the grounds that the principles used, while applicable to the behaviour of solids, may not be applicable to muscles; and the concept of the hydraulic amplifier mechanism still remains under scrutiny.
Quite a contrasting model has been proposed to explain the mechanics of the lumbar spine in lifting. It is based on arch theory and maintains that the behaviour, stability and strength of the lumbar spine during lifting can be explained by viewing the lumbar spine as an arch braced by intra-abdominal pressure.99,100 This intriguing concept, however, has not met with any degree of acceptance and indeed, has been challenged from some quarters.101
With regard to loads in the sagittal plane, the passive strength of the back muscles has been neglected in discussions of lifting. From the behaviour of isolate muscle fibres, it is known that as a muscle elongates, its maximum contractile force diminishes but its passive elastic tension rises, so much so that in an elongated muscle the total passive and active tension generated is at least equal to the maximum contractile capacity of the muscle at resting length. Thus, although they become electrically silent at full flexion, the back muscles are still capable of providing passive tension equal to their maximum contractile strength. This would allow the silent muscles to supplement the engaged posterior ligamentous system. With the back muscles providing some 200 Nm and the ligaments some 50 Nm or more, the total antiflexion capacity of the lumbar spine rises to about 250 Nm which would allow some 30 kg to be safely lifted at 90° trunk flexion. Larger loads could be sustained by proportionally shortening the moment arm. Consequently, the mechanism of lifting may well be essentially as proposed by Farfan and Gracovetsky22,72,93 except that the passive tension in the back muscles constitutes the major component of the ‘posterior ligamentous system’.
1 Bogduk N, Pearcy M, Hadfield G. Anatomy and biomechanics of psoas major. Clin Biomech. 1992;7:109-119.
2 Williams PL, editor. Gray’s Anatomy, 38th ed, Edinburgh: Churchill Livingstone, 1995.
3 Cave AJE. The innervation and morphology of the cervical intertransverse muscles. J Anat. 1937;71:497-515.
4 Poirier P. Myologie. 3rd ed. vol. 2, Fasc. 1. Poirier P, Charpy A, editors. Traité d’Anatomie Humaine. Paris: Masson. 1912:139-140.
5 Phillips S, Mercer S, Bogduk N. Anatomy and Biomechanics of Quadratus Lumborum. J Engineering in Medicine. 2008;222:151-159.
6 Bogduk N. A reappraisal of the anatomy of the human lumbar erector spinae. J Anat. 1980;131:525-540.
7 Macintosh JE, Bogduk N. The biomechanics of the lumbar multifidus. Clin Biomech. 1986;1:205-213.
8 Macintosh JE, Bogduk N. The morphology of the lumbar erector spinae. Spine. 1986;12:658-668.
9 Macintosh JE, Valencia F, Bogduk N, et al. The morphology of the lumbar multifidus muscles. Clin Biomech. 1986;1:196-204.
10 Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat. 1982;134:383-397.
11 Bogduk N. The lumbar mamillo-accessory ligament. Its anatomical and neurosurgical significance. Spine. 1981;6:162-167.
12 Abrahams VC. The physiology of neck muscles; their role in head movement and maintenance of posture. Can J Physiol Pharmacol. 1977;55:332-338.
13 Abrahams VC. Sensory and motor specialization in some muscles of the neck. TINS. 1981;4:24-27.
14 Cooper S, Danial PM. Muscle spindles in man, their morphology in the lumbricals and the deep muscles of the neck. Brain. 1963;86:563-594.
15 Bastide G, Zadeh J, Lefebvre D. Are the ‘little muscles’ what we think they are? Surg Radiol Anat. 1989;11:255-256.
16 Nitz AJ, Peck D. Comparison of muscle spindle concentrations in large and small human epaxial muscles acting in parallel combinations. Am Surg. 1986;52:273-277.
17 Peck D, Buxton DF, Nitz A. A comparison of spindle concentrations in large and small muscles acting in parallel combinations. J Morphol. 1984;180:243-252.
18 Lewin T, Moffet B, Viidik A. The morphology of the lumbar synovial intervertebral joints. Acta Morphol Neerlando-Scand. 1962;4:299-319.
19 Donisch EW, Basmajian JV. Electromyography of deep back muscles in man. Am J Anat. 1972;133:25-36.
20 Luk KDK, Ho HC, Leong JCY. The iliolumbar ligament. A study of its anatomy, development and clinical significance. J Bone Joint Surg. 1986;68B:197-200.
21 Bogduk N, Macintosh J. The applied anatomy of the thoracolumbar fascia. Spine. 1984;9:164-170.
22 Fairbank JCT, O’Brien JP. The abdominal cavity and thoracolumbar fascia as stabilisers of the lumbar spine in patients with low back pain. Vol 2. Engineering Aspects of the Spine. London: Mechanical Engineering Publications; 1980. 83–88
23 Gracovetsky S, Farfan HF, Lamy C. The mechanism of the lumbar spine. Spine. 1981;6:249-262.
24 Vleeming A, Pool-Goudzwaard AL, Stoeckart R, et al. The posterior layer of the thoracolumbar fascia: its function in load transfer from spine to legs. Spine. 1995;20:753-758.
25 Floyd WF, Silver PHS. The function of the erectores spinae muscles in certain movements and postures in man. J Physiol. 1955;129:184-203.
26 Morris JM, Benner G, Lucas DB. An electromyographic study of the intrinsic muscles of the back in man. J Anat. 1962;96:509-520.
27 Ortengren R, Andersson GBJ. Electromyographic studies of trunk muscles with special reference to the functional anatomy of the lumbar spine. Spine. 1977;2:44-52.
28 Morris JM, Lucas DB, Bresler B. Role of the trunk in stability of the spine. J Bone Joint Surg. 1961;43A:327-351.
29 Andersson GBJ, Ortengren R, Nachemson A. Intradiscal pressure, intra-abdominal pressure and myoelectric back muscle activity related to posture and loading. Clin Orthop. 1977;129:156-164.
30 Carlsoo S. The static muscle load in different work positions: an electromyographic study. Ergonomics. 1961;4:193-211.
31 Ortengren R, Andersson G, Nachemson A. Lumbar loads in fixed working postures during flexion and rotation. In: Asmussen E, Jorgensen K, editors. Biomechanics VI-B, International Series on Biomechanics, Vol 2B. Baltimore: University Park Press; 1978:159-166.
32 Portnoy H, Morin F. Electromyographic study of the postural muscles in various positions and movements. Am J Physiol. 1956;186:122-126.
33 Allen CEL. Muscle action potentials used in the study of dynamic anatomy. Br J Phys Med. 1948;11:66-73.
34 Andersson BJ, Ortengren R. Myoelectric back muscle activity during sitting. Scand J Rehabil Med Suppl. 1974;3:73-90.
35 Andersson BJ, Jonsson B, Ortengren R. Myoelectric activity in individual lumbar erector spinae muscles in sitting. A study with surface and wire electrodes. Scand J Rehabil Med Suppl. 1974;3:91-108.
36 Asmussen E, Klausen K. Form and function of the erect human spine. Clin Orthop. 1962;25:55-63.
37 Carlsoo S. Influence of frontal and dorsal loads on muscle activity and on the weight distribution in the feet. Acta Orthop Scand. 1964;34:299-309.
38 De Vries HA. Muscle tonus in postural muscles. Am J Phys Med. 1965;44:275-291.
39 Jonsson B. The functions of the individual muscles in the lumbar part of the spinae muscle. Electromyography. 1970;10:5-21.
40 Joseph J, McColl I. Electromyography of muscles of posture: posterior vertebral muscles in males. J Physiol. 1961;157:33-37.
41 Klausen K. The form and function of the loaded human spine. Acta Physiol Scand. 1965;65:176-190.
42 Valencia FP, Munro RR. An electromyographic study of the lumbar multifidus in man. Electromyogr Clin Neurophysiol. 1985;25:205-221.
43 Floyd WF, Silver PHS. Function of erectores spinae in flexion of the trunk. Lancet. 1951;1:133-134.
44 Kippers V, Parker AW. Electromyographic studies of erectores spinae: symmetrical postures and sagittal trunk motion. Aust J Physiother. 1985;31:95-105.
45 Andersson BJ, Ortengren R. Lumbar disc pressure and myoelectric back muscle activity during sitting. II. Studies of an office chair. Scand J Rehabil Med. 1974;6:115-121.
46 Andersson BJ, Ortengren R, Nachemson AL, et al. The sitting posture: an electromyographic and discometric study. Orthop Clin North Am. 1975;6:105-120.
47 Nachemson AL. The lumbar spine. An orthopaedic challenge. Spine. 1976;1:59-71.
48 Nachemson A. Lumbar intradiscal pressure. In: Jayson MIV, editor. The Lumbar Spine and Backache. 2nd ed. London: Pitman; 1980:341-358. Ch. 12
49 Golding JSR. Electromyography of the erector spinae in low back pain. Postgrad Med J. 1952;28:401-406.
50 Koreska J, Robertson D, Mills RH. Biomechanics of the lumbar spine and its clinical significance. Orthop Clin North Am. 1977;8:121-123.
51 Okada M. Electromyographic assessment of the muscular load in forward bending postures. J Fac Sci (Tokyo). 1970;8:311-336.
52 Pauly JE. An electromyographic analysis of certain movements and exercises. I. Some deep muscles of the back. Anat Rec. 1966;155:223-234.
53 Andersson GBJ, Ortengren R, Herberts P. Quantitative electromyographic studies of back muscle activity related to posture and loading. Orthop Clin North Am. 1977;8:85-96.
54 Schulz A, Andersson GBJ, Ortengren R, et al. Analysis and quantitative myoelectric measurements of loads on the lumbar spine when holding weights in standing postures. Spine. 1982;7:390-397.
55 Kippers V, Parker AW. Posture related to myoelectric silence of erectores spinae during trunk flexion. Spine. 1984;7:740-745.
56 Andersson G. Loads on the lumbar spine: in vivo measurements and biomechanical analyses. In: Winter DA, Norman RW, Wells RP, et al, editors. Biomechanics IX-B, International Series on Biomechanics. Champaign: Human Kinetics; 1983:32-37.
57 Ortengren R, Andersson GBJ, Nachemson AL. Studies of relationships between lumbar disc pressure, myoelectric back muscle activity, and intra-abdominal (intragastric) pressure. Spine. 1981;6:98-103.
58 Andersson G, Ortengren R, Nachemson A. Quantitative studies of the load on the back in different working postures. Scand J Rehabil Med Suppl. 1978;6:173-181.
59 Andersson BJ, Ortengren R, Nachemson A, et al. Lumbar disc pressure and myoelectric activity during sitting. I. Studies on an experimental chair. Scand J Rehabil Med. 1974;6:104-114.
60 Andersson BJ, Ortengren R, Nachemson A, et al. Lumbar disc pressure and myoelectric back muscle activity during sitting. IV. Studies on a car driver’s seat. Scand J Rehabil Med. 1974;6:128-133.
61 Nachemson A. The load on lumbar disks in different positions of the body. Clin Orthop. 1966;45:107-122.
62 Nachemson AL, Elfstrom G. Intravital dynamic pressure measurements in lumbar discs. A study of common movements, manoeuvers and exercises. Scand J Rehabil Med. 1970;2(suppl 1):1-40.
63 Nachemson A, Morris JM. In vivo measurements of intradiscal pressure. J Bone Joint Surg. 1964;46:1077-1092.
64 Andersson GBJ, Ortengren R, Nachemson A. Quantitative studies of back loads in lifting. Spine. 1976;1:178-184.
65 Vakos JP, Nitz AJ, Threlkeld AJ, et al. Electromyographic activity of selected trunk and hip muscles during a squat lift: effect of varying the lumbar posture. Spine. 1994;19:687-695.
66 McNeill T, Warwick D, Andersson G, et al. Trunk strengths in attempted flexion, extension, and lateral bending in healthy subjects and patients with low-back disorders. Spine. 1980;5:529-538.
67 Jorgensen K, Nicholaisen T, Kato M. Muscle fiber distribution, capillary density, and enzymatic activities in the lumbar paravertebral muscles of young men: significance for isometric endurance. Spine. 1993;18:1439-1450.
68 Bogduk N, Macintosh JE, Pearcy MJ. A universal model of the lumbar back muscles in the upright position. Spine. 1992;17:897-913.
69 Macintosh JE, Bogduk N, Pearcy MJ. The effects of flexion on the geometry and actions of the lumbar erector spinae. Spine. 1993;18:884-893.
70 Macintosh JE, Pearcy MJ, Bogduk N. The axial torque of the lumbar back muscles: torsion strength of the back muscles. Aust NZ J Surg. 1993;63:205-212.
71 Crossman K, Mahon M, Watson PJ, et al. Chronic low back pain – associated paraspinal muscle dysfunction is not the result of a constitutionally determined ‘adverse’ fibre-type composition. Spine. 2004;29:628-634.
72 Farfan HF. Muscular mechanism of the lumbar spine and the position of power and efficiency. Orthop Clin North Am. 1975;6:135-144.
73 Farfan HF. The biomechanical advantage of lordosis and hip extension for upright activity. Man as compared with other anthropoids. Spine. 1978;3:336-342.
74 Bartelink DL. The role of abdominal pressure in relieving the pressure on the lumbar intervertebral discs. J Bone Joint Surg. 1957;39B:718-725.
75 Davis PR. Posture of the trunk during the lifting of weights. BMJ. 1959;1:87-89.
76 Davis PR. The use of intra-abdominal pressure in evaluating stresses on the lumbar spine. Spine. 1981;6:90-92.
77 Davis PR, Stubbs DA. Safe levels of manual forces for young males (1). Appl Ergon. 1977;8:141-150.
78 Davis PR, Troup JDG. Pressures in the trunk cavities when pulling, pushing and lifting. Ergonomics. 1964;7:465-474.
79 Stubbs DA. Trunk stresses in construction and other industrial workers. Spine. 1981;6:83-89.
80 Troup JDG. Relation of lumbar spine disorders to heavy manual work and lifting. Lancet. 1965;1:857-861.
81 Troup JDG. Dynamic factors in the analysis of stoop and crouch lifting methods: a methodological approach to the development of safe materials handling standards. Orthop Clin North Am. 1977;8:201-209.
82 Troup JDG. Biomechanics of the vertebral column. Physiotherapy. 1979;65:238-244.
83 McGill SM, Norman RW. Potential of lumbodorsal fascia forces to generate back extension moments during squat lifts. J Biomed Eng. 1988;10:312-318.
84 Leskinen TPJ, Stalhammar HR, Kuorinka IA, et al. Hip torque, lumbosacral compression, and intra-abdominal pressure in lifting and lowering tasks. In: Winter DA, Norman RW, Wells RP, et al, editors. Biomechanics IXB, International Series on Biomechanics. Champaign: Human Kinetics; 1983:55-59.
85 Nachemson AL, Andersson GBJ, Schultz AB. Valsalva maneuver biomechanics. Effects on trunk load of elevated intra-abdominal pressure. Spine. 1986;11:476-479.
86 Hemborg B, Moritz U. Intra-abdominal pressure and trunk muscle activity during lifting. II. Chronic low-back patients. Scand J Rehabil Med. 1985;17:5-13.
87 Hemborg B, Moritz U, Hamberg J, et al. Intra-abdominal pressure and trunk muscle activity during lifting – effect of abdominal muscle training in healthy subjects. Scand J Rehabil Med. 1983;15:183-196.
88 Hemborg B, Moritz U, Hamberg J, et al. Intra-abdominal pressure and trunk muscle activity during lifting. III. Effects of abdominal muscle training in chronic low-back patients. Scand J Rehabil Med. 1985;17:15-24.
89 Farfan HF, Gracovetsky S. The abdominal mechanism. Paper presented at the International Society for the Study of the Lumbar Spine Meeting, Paris; 1981.
90 Farfan HF, Gracovetsky S, Helleur C. The role of mathematical models in the assessment of task in the workplace. In: Winter DA, Norman RW, Wells RP, et al, editors. Biomechanics IXB, International Series on Biomechanics. Champaign: Human Kinetics; 1983:38-43.
91 Gracovetsky S, Farfan HF, Helleur C. The abdominal mechanism. Spine. 1985;10:317-324.
92 Bearn JG. The significance of the activity of the abdominal muscles in weight lifting. Acta Anat. 1961;45:83-89.
93 Gracovetsky S, Farfan HF, Lamy C. A mathematical model of the lumbar spine using an optimal system to control muscles and ligaments. Orthop Clin North Am. 1977;8:135-153.
94 Macintosh JE, Bogduk N, Gracovetsky S. The biomechanics of the thoracolumbar fascia. Clin Biomech. 1987;2:78-83.
95 Crock HV, Crock MC. A technique for decompression of the lumbar spinal canal. Neuro-Orthopaedics. 1988;5:96-99.
96 Mykelbust JB, Pintar F, Yoganandan N, et al. Tensile strength of spinal ligaments. Spine. 1988;13:526-531.
97 Cyron BM, Hutton WC. The tensile strength of the capsular ligaments of the apophyseal joints. J Anat. 1981;132:145-150.
98 Hukins DWL, Aspden RM, Hickey DS. Thoracolumbar fascia can increase the efficiency of the erector spinae muscles. Clin Biomech. 1990;5:30-34.
99 Aspden RM. Intra-abdominal pressure and its role in spinal mechanics. Clin Biomech. 1987;2:168-174.
100 Aspden RM. The spine as an arch. A new mathematical model. Spine. 1989;14:266-274.