Chapter 31
The Limbic System
Cytoarchitectural Definitions of the Limbic Cortex
Blood Supply to the Limbic System
Many complex brain systems are organized in a way that allows their function to be readily deduced. For example, even though the connections of the somatosensory pathways with the brainstem, thalamus, and cortex are complex, each component plays a fairly clear-cut role. The processing of somatosensory information is generally well understood. In contrast, some systems are interconnected in such a way that a given function may be carried out by several components acting in cooperation, and a given component may participate in several functions. The limbic system is a case in point. This system comprises structures that receive inputs from diverse areas of the neuraxis and participate in complicated and interrelated behaviors such as memory, learning, and social interactions. Thus lesions involving the limbic system generally result in a wide range of deficits.
OVERVIEW
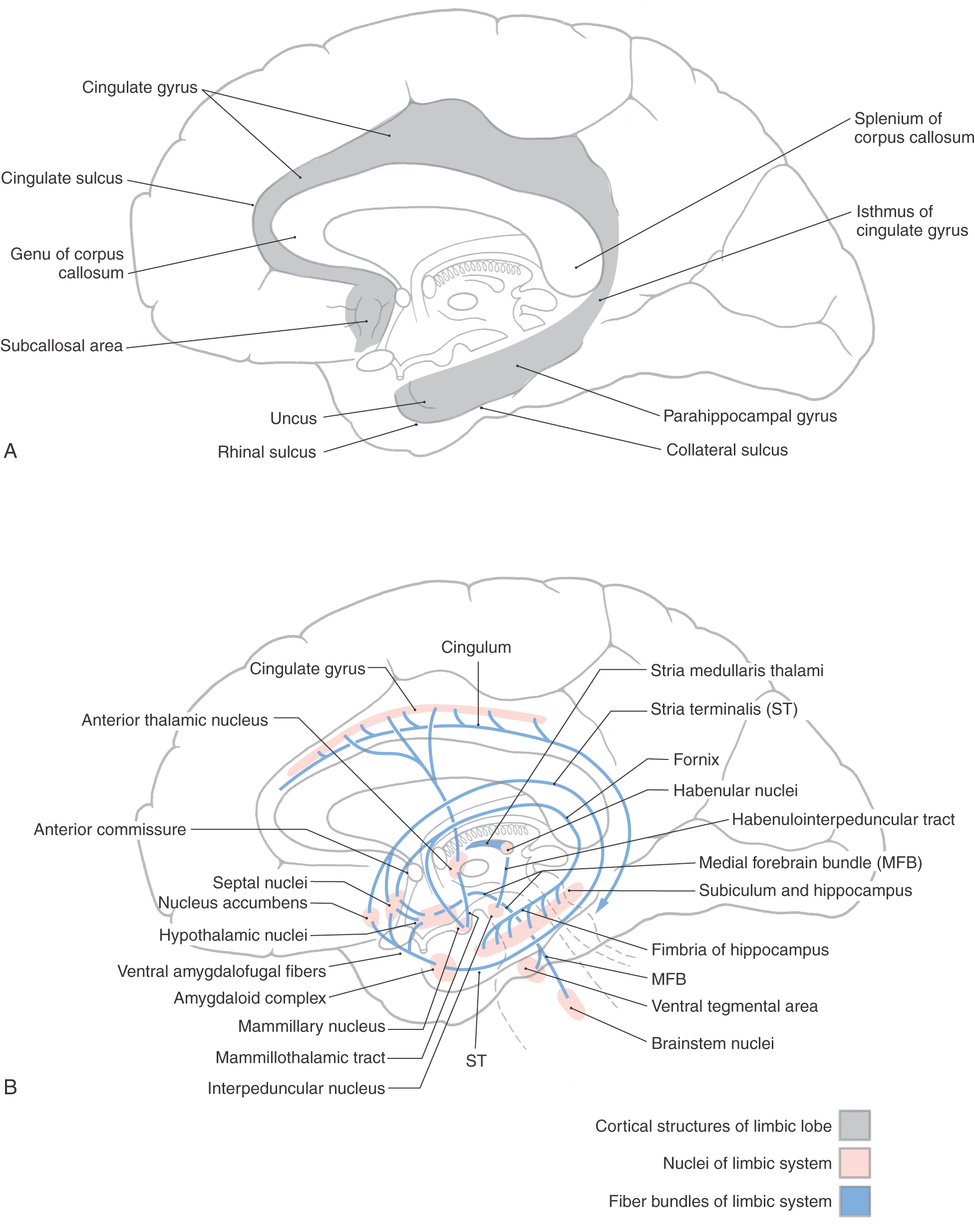
The concept of a limbic system actually encompasses two levels of structural and functional organization. The first level consists of the cortical structures on the most medial edge (the limbus) of the hemisphere; these collectively form the limbic lobe (Fig. 31-1A). Beginning just anterior to the lamina terminalis and proceeding caudally, these are the subcallosal area, containing the parolfactory and paraterminal gyri; the cingulate gyrus; the isthmus of the cingulate gyrus; the parahippocampal gyrus; and the uncus (Fig. 31-2). The limbic lobe also includes the hippocampal formation.
Figure 31-2. Cortical structures of the limbic lobe as seen on an anterior (ventral) view of the hemisphere.
In 1878, Broca (1824-1880) noted that the limbic lobe, which is present in all mammals, represents a relatively large part of the cerebral cortex in phylogenetically lower forms, and he postulated that it might be related to olfaction. Because of this latter point, the term rhinencephalon (“smell-brain”) was later coined and used interchangeably with limbic lobe. However, it is now known that the limbic lobe has little if any olfactory function in humans. Thus the term “rhinencephalon,” although historically interesting, is antiquated and has disappeared from use.
The second level includes structures of the limbic lobe plus a variety of subcortical nuclei and tracts that collectively form the limbic system (Fig. 31-1B). The subcortical nuclei of the limbic system include, among others, the septal nuclei and nucleus accumbens (nucleus accumbens septi); various nuclei of the hypothalamus, especially those associated with the mammillary body; the nuclei of the amygdaloid complex and adjacent substantia innominata; and parts of the dorsal thalamus, particularly the anterior and dorsomedial nuclei. Additional structures connected with the limbic system include the habenular nuclei, ventral tegmental area, and periaqueductal gray. Furthermore, the prefrontal cortex is considered by some investigators to be an important component of the limbic system, primarily because of its potential influence on various other cortical and subcortical parts of the limbic system. Cortical targets of the prefrontal cortex include the cingulate gyrus, whereas the hypothalamus, dorsal thalamus, amygdaloid complex, and nuclei of the midbrain represent subcortical targets.
The main efferent fiber bundles of the limbic system are the fornix (primarily efferents of the hippocampus and subiculum), the stria terminalis and ventral amygdalofugal pathway (both are mainly efferents of the amygdaloid complex), and the mammillothalamic tract (efferents of the medial mammillary nucleus; Fig. 31-1B). A few additional nuclei and smaller tracts will be introduced as connections and functions of the limbic system.
CYTOARCHITECTURAL DEFINITIONS OF THE LIMBIC CORTEX
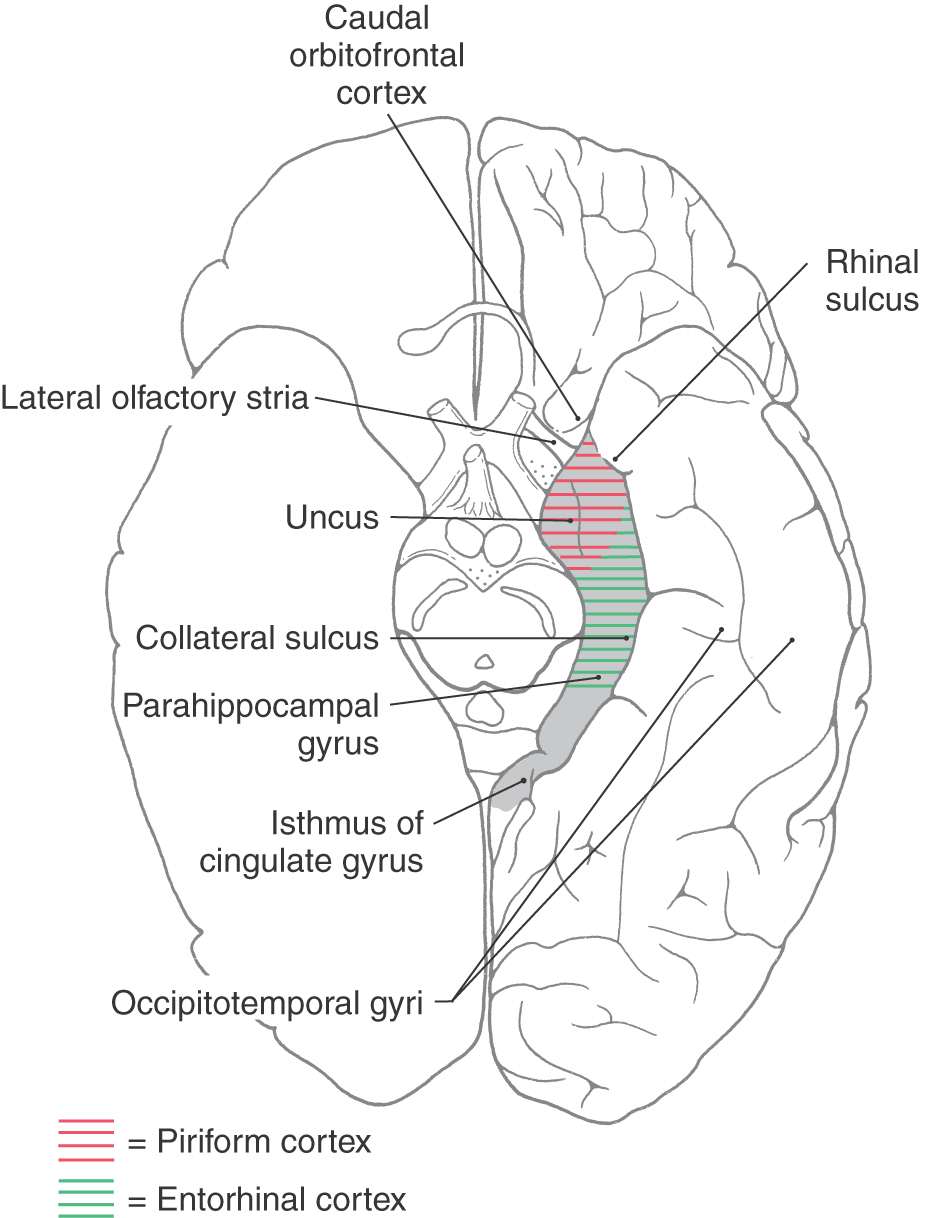
The human cerebral cortex can be divided into several areas on the basis of the number of cell layers present. Most of the cerebral cortex (more than 90%) has six cell layers and is called the neocortex or neopallium (isocortex). Examples of neocortex include the primary sensory, motor, and association cortices. The cortical regions that have fewer than six layers are structurally and functionally associated with the limbic system or with olfaction and are classified as allocortex. Those structures that comprise three to five cellular layers are called the paleocortex (paleopallium or periallocortex) and are represented by the cortex of the parahippocampal gyrus (the entorhinal cortex), the uncus (the piriform cortex), and the cortex overlying the termination of the lateral olfactory stria (lateral olfactory gyrus) (Fig. 31-2). The lateral olfactory stria is directly rostromedial to the piriform cortex. Structures having only three cellular layers are classified as archicortex (archipallium or allocortex) and are represented by the dentate gyrus and hippocampus.
The separation between neocortex and allocortex is never sharp but instead consists of transitional areas where one cortical region blends into the next. Such areas are represented by caudal parts of the orbitofrontal cortex, the temporal pole, parts of the insula, and portions of the parahippocampal and cingulate gyri. They are especially important because they funnel input from association areas of the neocortex into the allocortex.
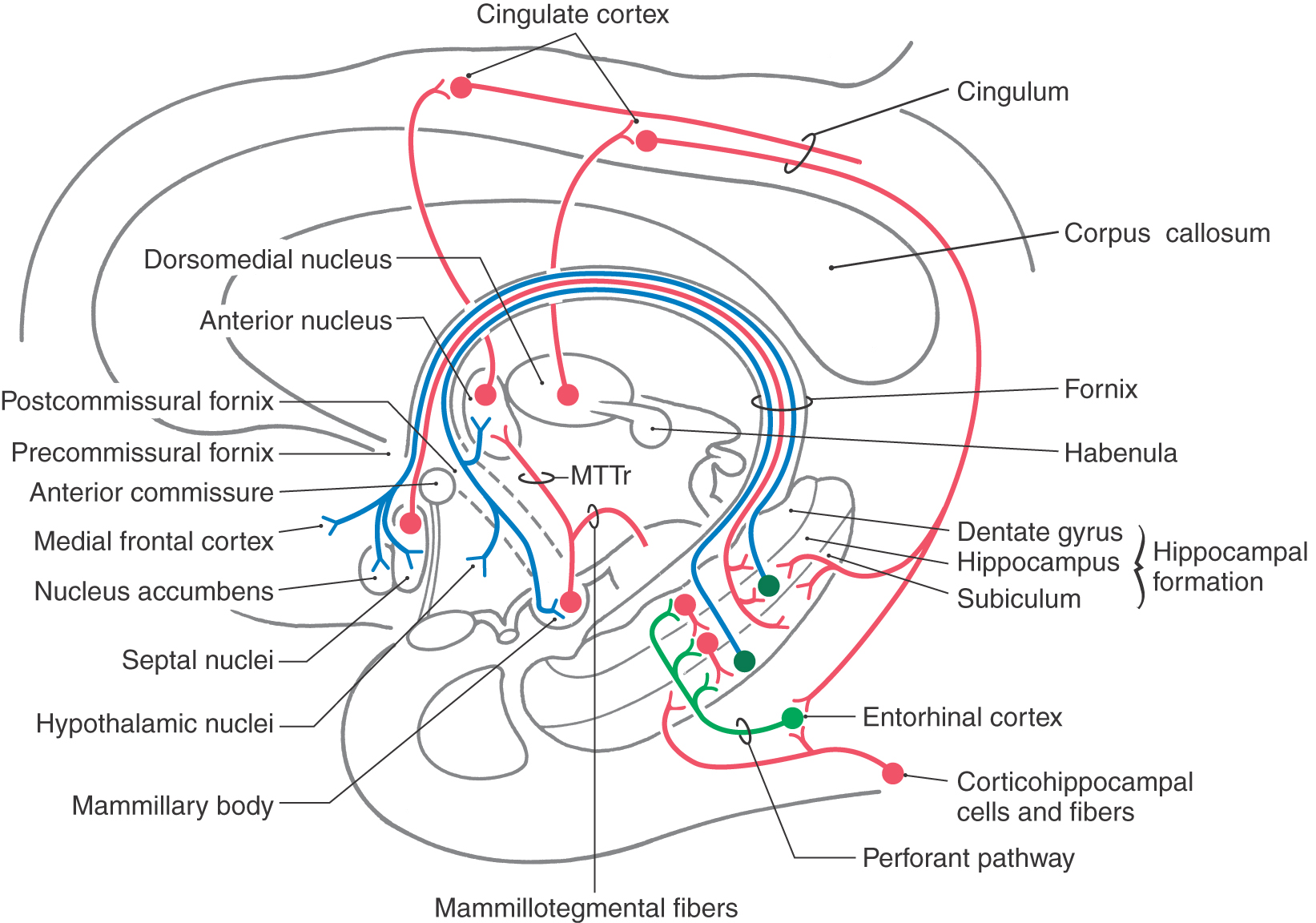
EARLY FUNCTIONAL CONCEPTS
In the late 1930s, two pivotal observations were made that formed the basis for the concept of a limbic system. First, largely on the basis of the morphology of the brain, a circuit for the elaboration of emotion was proposed. This pathway is now called the Papez circuit in recognition of the anatomist James Papez (1883-1958), who initially described its components. This model, although surprisingly simple, has proved to be quite important in understanding limbic function. The circuit suggested that emotion, mediated through the hypothalamus, is controlled and modulated by fibers from the fornix. Specifically, the cortical control of emotional activity can be viewed as a pathway originating from the cingulate gyrus (see Fig. 31-5). In brief, the Papez circuit consists of the following structures and cell groups. The cingulate gyrus projects, via the cingulum, to the hippocampal formation (mainly the subiculum and the Ammon horn) as well as to the entorhinal cortex. The hippocampus then projects, via the alveus and the various parts of the fornix, to the mammillary nuclei by coursing through the postcommissural fornix. In turn the medial mammillary nucleus projects to the anterior nucleus of the thalamus by way of the mammillothalamic tract, and the anterior nucleus projects to the cortex of the cingulate gyrus. This was the first time a specific anatomic substrate was proposed for a phenomenon as complex as emotion.
The second pivotal observation was that bilateral removal of large parts of the temporal lobe in monkeys resulted in a constellation of dysfunctions that came to be known as the Klüver-Bucy syndrome in recognition of Heinrich Klüver (1897-1979) and Paul Bucy (1904-1992). Whereas this syndrome was initially described in animal experiments, it has been repeatedly documented in humans with bilateral temporal lobe injuries that involve primarily the amygdaloid complex.
BLOOD SUPPLY TO THE LIMBIC SYSTEM
The blood supply to the limbic system originates from several sources. The main vessels that serve much of the limbic system are the anterior and posterior cerebral arteries, the anterior choroidal artery, and branches arising from the circle of Willis.
The subcallosal area and rostral parts of the cingulate gyrus are supplied by branches of the anterior cerebral artery as it loops around the genu of the corpus callosum (see Fig. 8-7). Most of the cingulate gyrus and its isthmus receive blood supply via the pericallosal artery, a branch of the anterior cerebral artery. Temporal branches of the posterior cerebral artery supply the parahippocampal gyrus. Although the uncus may receive some small branches from the posterior cerebral artery, it is served primarily by uncal arteries, which are branches of the M1 segment of the middle cerebral artery (see Fig. 8-6).
The anterior choroidal artery usually originates from the internal carotid artery and follows the general trajectory of the optic tract. En route it sends branches into the choroidal fissure of the temporal horn of the lateral ventricle. This vessel serves the choroid plexus of the temporal horn, the hippocampal formation, parts of the amygdaloid complex, and adjacent structures such as the tail of the caudate nucleus, the stria terminalis, and the sublenticular and retrolenticular limbs of the internal capsule.
Vessels serving hypothalamic nuclei that are functionally associated with the limbic system originate from the circle of Willis. In general, rostral areas of the hypothalamus are served by branches from the anterior communicating artery and anterior cerebral artery; posterior areas are served by branches from the posterior communicating artery and proximal posterior cerebral artery (see Fig. 8-17). The anterior nucleus of the thalamus, an important synaptic station in the limbic system, is supplied by thalamoperforating arteries that arise from the P1 segment of the posterior cerebral artery.
HIPPOCAMPAL FORMATION
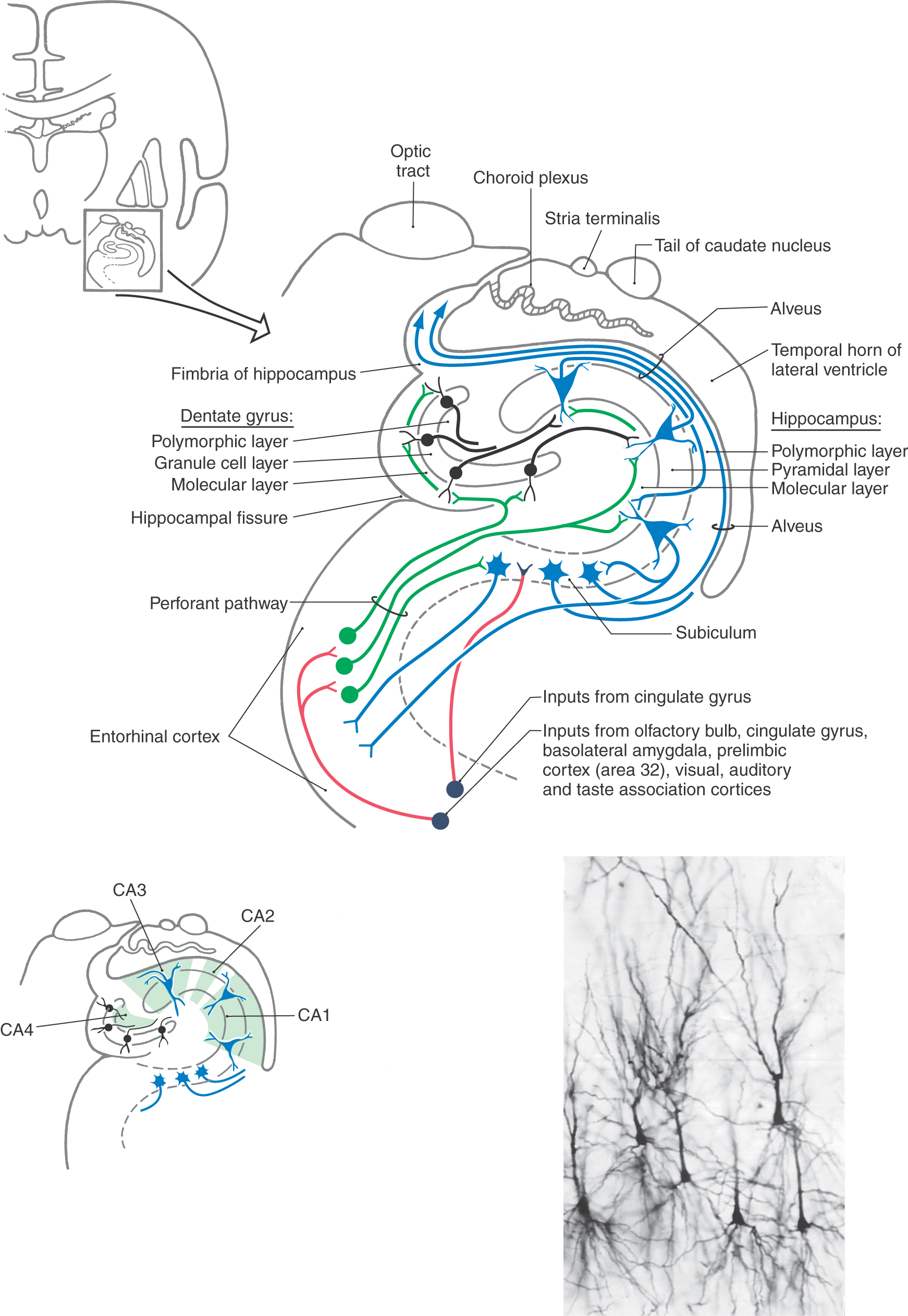
The hippocampal formation is composed of the subiculum, hippocampus (also called the hippocampus proper or horn of Ammon), and dentate gyrus (see Fig. 31-4), all of which constitute the allocortex of Brodmann. The subiculum is laterally continuous with the cortex of the parahippocampal gyrus and area of the periallocortex. Medially the edge of the hippocampal formation is formed by the dentate gyrus and the fimbria of the hippocampus.
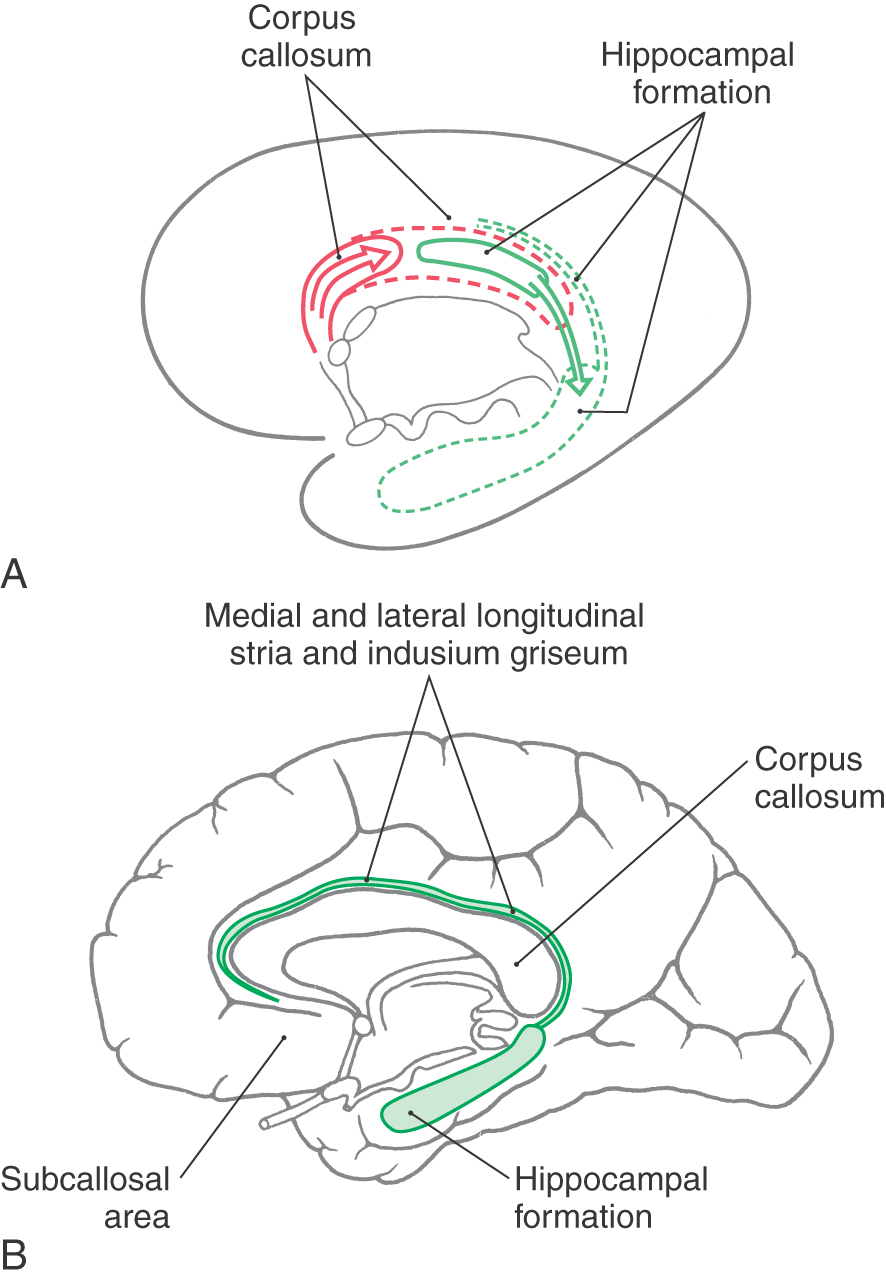
Developmentally, the hippocampal formation originates dorsally and migrates into its ventral and medial positions in the temporal lobe. During this migration, small remnants of the hippocampal formation remain behind to form the medial and lateral longitudinal striae and their associated gray matter, the indusium griseum (Fig. 31-3). These structures are small in the human brain and extend rostrally along the dorsal aspect of the corpus callosum into the subcallosal area.
Structure
The subiculum of the hippocampal formation is the transitional area between the three-layered hippocampus (archicortex or allocortex) and the five-layered entorhinal cortex (paleocortex or periallocortex) of the parahippocampal gyrus (Fig. 31-4). This transitional zone, although small, can be divided into a prosubiculum, subiculum proper, presubiculum, and parasubiculum. These areas are essential for the flow of information into the hippocampal formation.
The dentate gyrus and the hippocampus are each composed of three layers, which are characteristic of archicortex (Fig. 31-4). The external layer is called the molecular layer and contains afferent axons and dendrites of cells intrinsic to each structure. The middle layer, called the granule cell layer in the dentate gyrus and the pyramidal layer in the hippocampus, contains the efferent neurons of each structure (Fig. 31-4). These layers are named according to the shape of the cell body of the principal type of neuron found therein. The dendrites of granule and pyramidal cells radiate into the molecular layer. The inner layer, called the polymorphic layer (also called the stratum oriens in the hippocampus), contains the axons of pyramidal and granule cells, a few intrinsic neurons, and many glial elements. In addition, the polymorphic layer of the hippocampus contains the elaborate basal dendrites of some larger pyramidal somata that are located in the pyramidal layer. These are called double pyramidal cells because they have dendrites extending into both molecular and polymorphic layers (Fig. 31-4). The innermost part of the hippocampus borders on the wall of the lateral ventricle and is a layer of myelinated axons arising from cell bodies located in the subiculum and hippocampus. This layer, called the alveus, is continuous with the fimbria of the hippocampus, which in turn becomes the fornix (Fig. 31-4).
The hippocampus can be divided into four regions on the basis of a variety of cytoarchitectural criteria (Fig. 31-4). These areas are designated CA1 to CA4, where CA stands for cornu ammonis (horn of Ammon). Area CA1 (a parvocellular region that can be separated into two cell layers in humans) is located at the subiculum-hippocampal interface. Area CA2 (a mixed zone) and area CA3 (a magnocellular zone) are located within the hippocampus. Area CA4 is located at the junction of the hippocampus with the dentate gyrus but within the hilus of the dentate gyrus.
Afferent Fibers
The major input to the hippocampal formation is from cells of the entorhinal cortex via a diffuse projection called the perforant pathway (Figs. 31-4 and 31-5). Most fibers of the perforant pathway terminate in the molecular layer of the dentate gyrus, although a few terminate in the subiculum and hippocampus. Granule cells in the dentate gyrus project into the molecular layer of the CA3 region of the hippocampus. CA3 neurons project into CA1 of the hippocampus, which in turn provides an input to the subiculum. In addition, the subiculum also receives a modest projection from the amygdaloid complex. Although the fornix is mainly an efferent path from the hippocampus, it also conveys cholinergic septohippocampal projections to the hippocampal formation and entorhinal cortex.
Efferent Fibers
The outflow of the hippocampal formation originates primarily from cells of the subiculum and to a lesser degree from pyramidal cells of the hippocampus (Figs. 31-4 and 31-5). In both cases, axons of these neurons enter the alveus, coalesce to form the fimbria of the hippocampus, and then continue as the fornix. These glutaminergic fibers traverse the entire extent of the fornix, although some cross the midline in the hippocampal decussation just anterior (ventral) to the splenium of the corpus callosum.
At the level of the anterior commissure, the fornix divides into postcommissural and precommissural parts (Fig. 31-5). The fibers originating in the subiculum mainly form the postcommissural fornix. Most of these fibers terminate in the medial mammillary nucleus, although some enter the ventromedial nucleus of the hypothalamus and the anterior nucleus of the dorsal thalamus (Fig. 31-5). The precommissural fornix is composed of fibers arising primarily in the hippocampus. These fibers are somewhat diffusely organized and distribute to the septal nuclei, the medial areas of the frontal cortex, the preoptic and anterior nuclei of the hypothalamus, and the nucleus accumbens (Fig. 31-5).
Complete Circuit of Papez
As noted previously, the initial segment of the Papez circuit is a projection primarily from the subiculum (a part of the hippocampal formation) to the medial mammillary nucleus via the postcommissural fornix. The circuit is completed by the following connections (Fig. 31-5): (1) a mammillothalamic tract that connects the medial mammillary nucleus to the anterior nucleus of the thalamus; (2) thalamocortical fibers from the anterior nucleus to broad expanses of the cortex of the cingulate gyrus; and (3) a projection from the cingulate cortex, via the cingulum, to the entorhinal cortex and also directly to the subiculum and hippocampus. The subiculum, via the fornix, returns information to the mammillary body.
Other areas of the cerebral cortex are recruited into the various functions associated with the Papez circuit largely through connections of the cingulate gyrus. For example, the cingulate cortex receives input from premotor and prefrontal areas and from visual, auditory, and somatosensory association cortices. In turn, the cingulate cortex not only is a major source of afferent fibers to the hippocampal formation but also projects to most cortical areas from which it receives input. The cingulate gyrus thus is not only an integral part of the Papez circuit but also an important conduit through which a wide range of information can reach the limbic system.
LIMBIC SYSTEM AND MEMORY
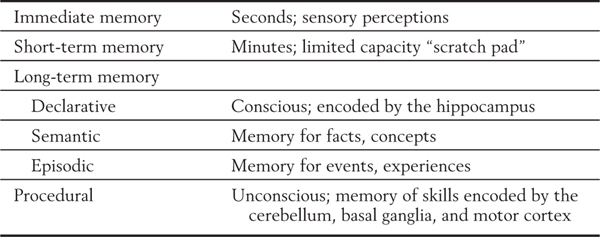
The basic function of the hippocampal formation appears to be the consolidation of long-term memories from immediate and short-term memories, a point first observed in 1900 by Bechterew (1857-1927) but essentially ignored until the 1950s. Immediate memory and short-term memory refer to types of memory that persist for seconds and minutes, respectively (Table 31-1). Normally, these memories can be incorporated into long-term memory, which can be recalled days, months, or years later. However, in persons with hippocampal lesions, this conversion is not accomplished. Although patients may be able to perform a task for seconds or minutes, they are unable to return to the task if they are distracted from it. In other words, they do not “remember” what they were doing; the short-term experience is not incorporated into long-term memory. The redundancy and feedback in the hippocampus are ideal for this imprinting of memory (Table 31-1).
Table 31-1 Categories of Memory and the Main Features of Each Category
Bilateral damage to the hippocampal formation sometimes occurs in victims of heart attack, near-drowning, or severe hypoglycemia as a result of cerebral ischemia (Fig. 31-6A). The part of the hippocampal formation most vulnerable to anoxia during an ischemic episode is the CA1 area. The CA1-subiculum interface region is referred to as the Sommer sector in pathologic conditions. Affected patients retain memories for events occurring after the ischemic episode. Consequently, they also have difficulty learning new concepts because the new information is not retained (remembered) long enough to become a long-term memory.
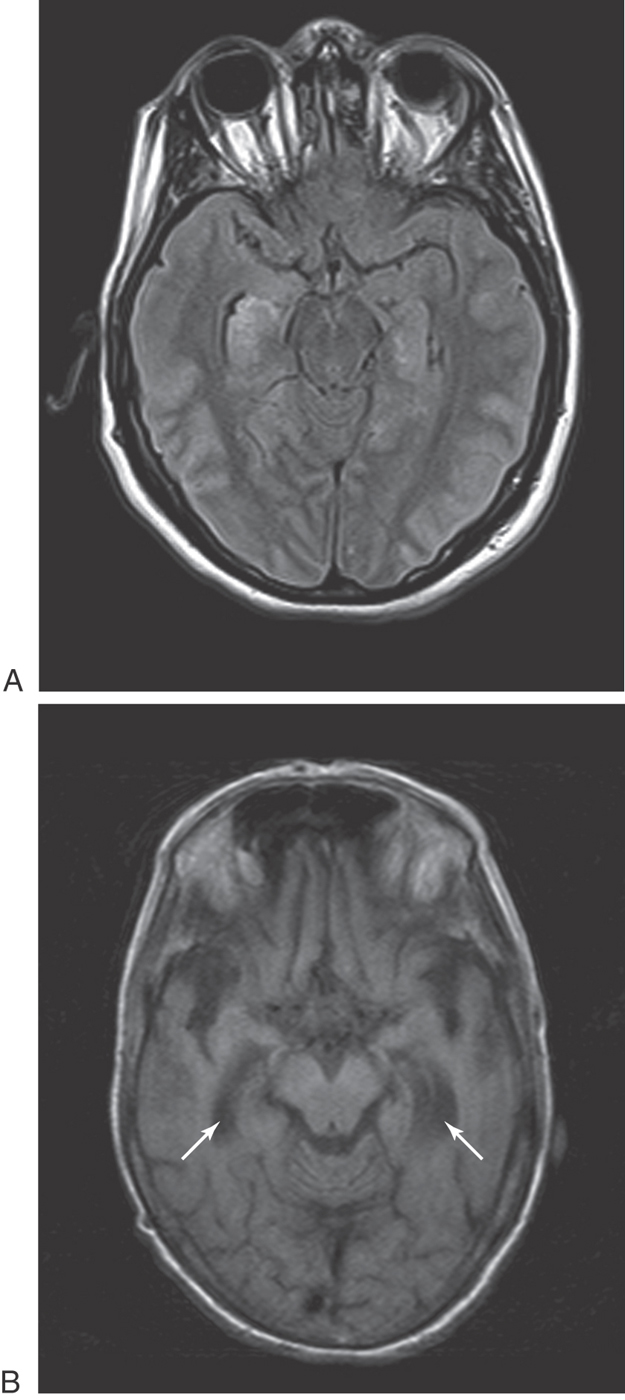
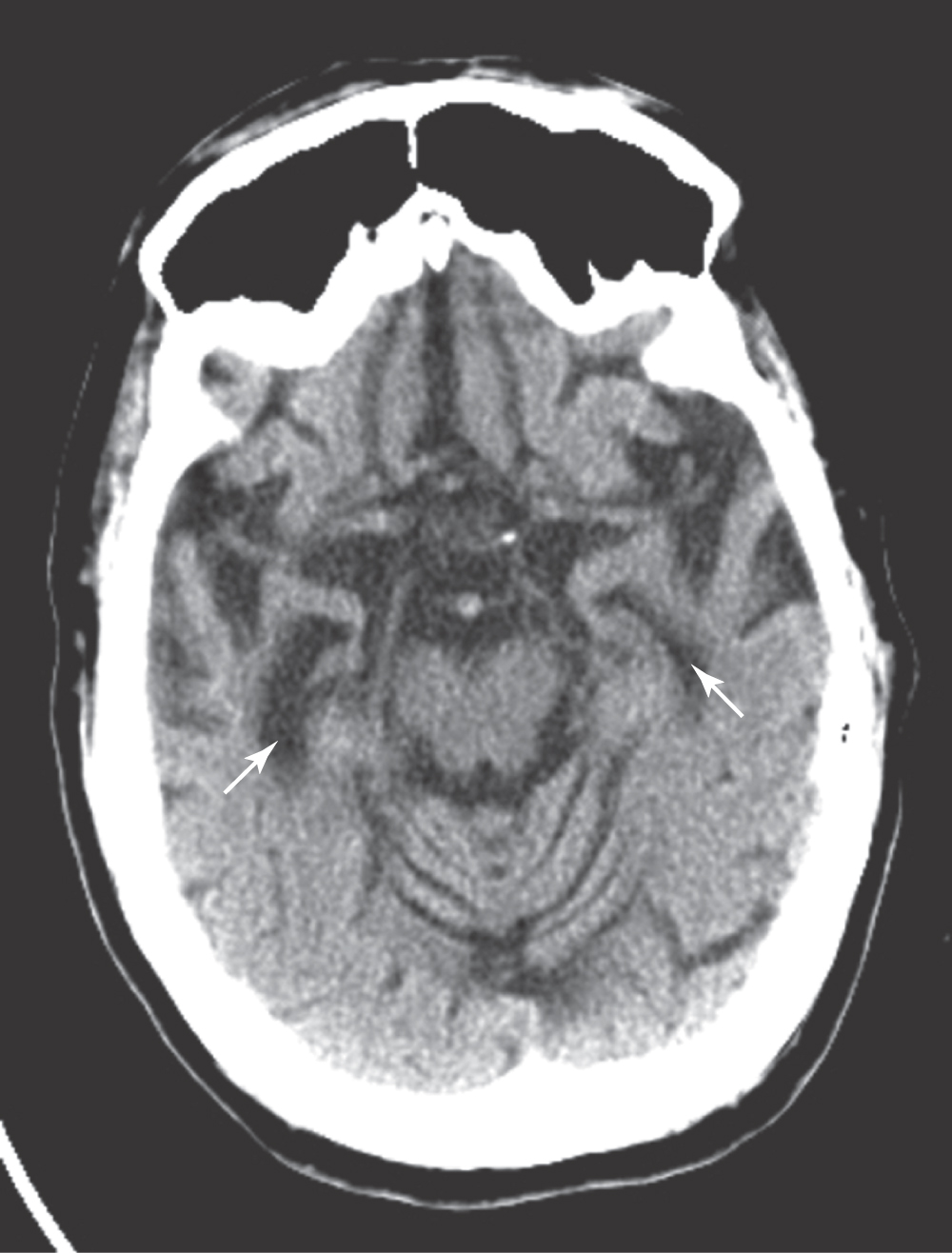
Another condition in which loss of memory and cognitive function is particularly obvious is Alzheimer disease (AD). This disease is characterized, in part, by the presence of neurofibrillary tangles, neuritic plaques, and neuronal loss in specific brain regions (Fig. 31-6B). The subiculum and entorhinal cortices are among the first sites in which these abnormalities appear. As a result, the relay of information through the hippocampal formation is impeded. It is believed that this damage is at least partially responsible for the difficulties with declarative memory characteristic of AD. Poor recall of recent events or incorporation of new facts occurs early in AD. Long-term memory impairment and behavioral changes occur in later phases. Procedural or implicit memory, the motor skills for performing tasks, is relatively spared because this type of memory is encoded by the basal ganglia and cerebellum.
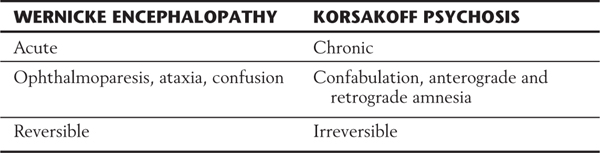
Defects in memory can also result from prolonged thiamine deficiency. This B vitamin deficiency typically seen in the context of chronic alcoholism, cancer cachexia, or any prolonged state of malnutrition causes a characteristic pattern of degeneration in the brain (Fig. 31-7). Typically, the mammillary bodies are involved, with some incursion into the dorsomedial nucleus of the thalamus and the columns of the fornix. There is also a loss of neurons in the hippocampal formation (Fig. 31-7). These patients with Korsakoff psychosis (alcoholic dementia) show a defect in short-term memory and consequently also in long-term memory for events occurring since the onset of the disease. They may appear demented, and they are prone to confabulation; that is, they tend to string together fragments of memory from several different events to form a synthetic “memory” of an event that never occurred (Table 31-2). Korsakoff psychosis is irreversible. Thiamine deficiency may also be manifested more acutely as a triad of eye movement abnormalities, ataxia, and confusion known as Wernicke encephalopathy, which is reversible with thiamine replacement. Table 31-2 contrasts the acute and chronic neurologic consequences of thiamine deficiency. In severe cases, patients may present with the Wernicke triad accompanied by profound memory loss; this condition is called Wernicke-Korsakoff syndrome.
Table 31-2 Neurologic Consequences of Thiamine Deficiency and a Comparison of Wernicke Encephalopathy with Korsakoff Psychosis
LONG-TERM POTENTIATION AND MEMORY
The process of long-term potentiation at individual synapses is the probable mechanism that underlies the consolidation of short-term memory into long-term memory. When several synapses are present on a single cell, the input from these synapses is integrated; that is, the small potential changes (EPSP and IPSP, see Chapter 3) are added together. In long-term potentiation, one synapse fires in a particular temporal pattern (such as bursts or trains of action potentials). This synaptic activity increases the likelihood that the target cells will be activated by that synapse and other synapses. This increased likelihood may be due to an increased probability that transmitter will be released from the presynaptic cell or an increased response in the postsynaptic cell to the same amount of neurotransmitter, or both. Long-term potentiation has been demonstrated at terminals of the perforant pathway in the dentate gyrus and at the synapses of CA3 pyramidal cells on CA1 cells. These connections use the neurotransmitter glutamate.
According to one current model, the release of glutamate causes a change in the biochemistry of the N-methyl-D-aspartate (NMDA)–type glutamate receptors of the hippocampal cells, allowing an increased number of calcium ions to enter the cell. The calcium influx causes a second postsynaptic biochemical change. The gaseous neuromodulator nitric oxide is released and diffuses back to the presynaptic terminal. It acts on the presynaptic terminal to permanently increase the release of glutamate. At this type of synapse, a brief, sustained increase in current synaptic activity increases the probability that future synaptic activity will take place. That is, the more the circuit is activated, the easier it is to activate. This mechanism causes stimuli and responses to be paired in the process we call memory. The increased probability of activation lasts in isolated preparations for hours; it cannot be measured in human brains but may be permanent.
AMYGDALOID COMPLEX
Structure
The amygdaloid complex is an almond-shaped group of cells in the rostromedial part of the temporal lobe internal to the uncus (Fig. 31-1). It is immediately rostral to the hippocampal formation and the anterior end of the temporal horn of the lateral ventricle. The amygdaloid complex is composed of a number of nuclei. For our purposes, these nuclei can be grouped into a larger basolateral group and a smaller corticomedial group (including the central nucleus). The corticomedial group is more closely related to olfaction, whereas the basolateral group has extensive interconnections with cortical structures.
Afferent Fibers
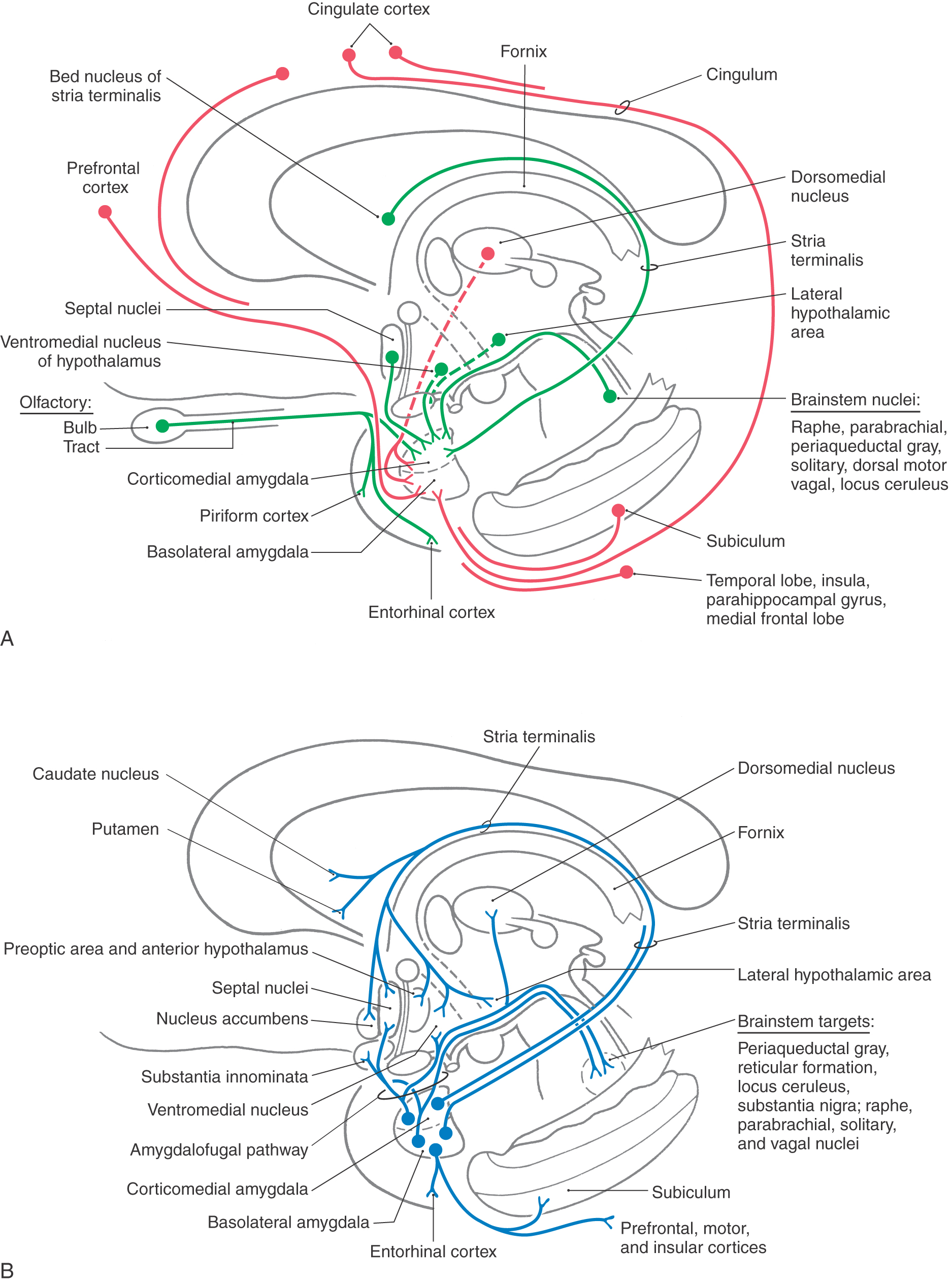
The basolateral cell groups of the amygdala receive inputs from the dorsal thalamus, the prefrontal cortex, the cingulate and parahippocampal gyri, the temporal lobe and insular cortex, and the subiculum (Fig. 31-8A). These fibers supply a wide range of somatosensory, visual, and visceral information to the amygdaloid complex.
Figure 31-8. Semidiagrammatic representation of the afferent (A) and efferent (B) connections of the amygdaloid complex.
The corticomedial cell group receives olfactory input, fibers from the hypothalamus (ventromedial nucleus, lateral hypothalamic area), and fibers from the dorsomedial and medial nuclei of the dorsal thalamus (Fig. 31-8A). In addition, this cell group, particularly its central nucleus, receives ascending input from nuclei in the brainstem known to be involved in visceral functions. Among others, these include the parabrachial nuclei, the solitary nucleus, and portions of the periaqueductal gray.
Efferent Fibers
The two major efferent pathways of the amygdaloid complex are the stria terminalis and the ventral amygdalofugal pathway (Figs. 31-1B and 31-8B). The stria terminalis is a small fiber bundle that arises primarily from cells of the corticomedial group. Through most of its course, this bundle lies in the groove between the caudate nucleus and the dorsal thalamus, where it is accompanied by the terminal vein. It is associated along its length with discontinuous aggregations of cells, which collectively are called the bed nucleus of the stria terminalis. This tract distributes to various nuclei of the hypothalamus (the preoptic nuclei, ventromedial nucleus, anterior nucleus, and lateral hypothalamic area), to the nucleus accumbens and the septal nuclei, and to the rostral areas of the caudate nucleus and putamen (Fig. 31-8B).
The ventral amygdalofugal pathway is the major efferent fiber bundle of the amygdaloid complex. These fibers arise from the basolateral cell group and the central nucleus of the corticomedial cell group and follow two general trajectories (Fig. 31-8B). Axons primarily from the basolateral cells pass medially through the substantia innominata (in which some of these fibers terminate) to eventually synapse in the hypothalamus and septal nuclei. The substantia innominata gives rise to a diffuse cholinergic projection to the cerebral cortex. It is probable that these fibers play a role in the activation of the cerebral cortex in response to behaviorally significant stimuli. In addition, cells of the basolateral group also project diffusely to frontal and prefrontal, cingulate, insular, and inferior temporal cortices (Fig. 31-8B). Other fibers, mainly from the central nucleus, turn caudally and descend diffusely in the brainstem to terminate in visceral (dorsal motor vagal) nuclei, raphe nuclei (magnus, obscurus, pallidus), and other areas such as the locus ceruleus, parabrachial nuclei, and periaqueductal gray (Fig. 31-8B). As noted previously, most of those brainstem areas that receive input from the amygdala project back to this structure.
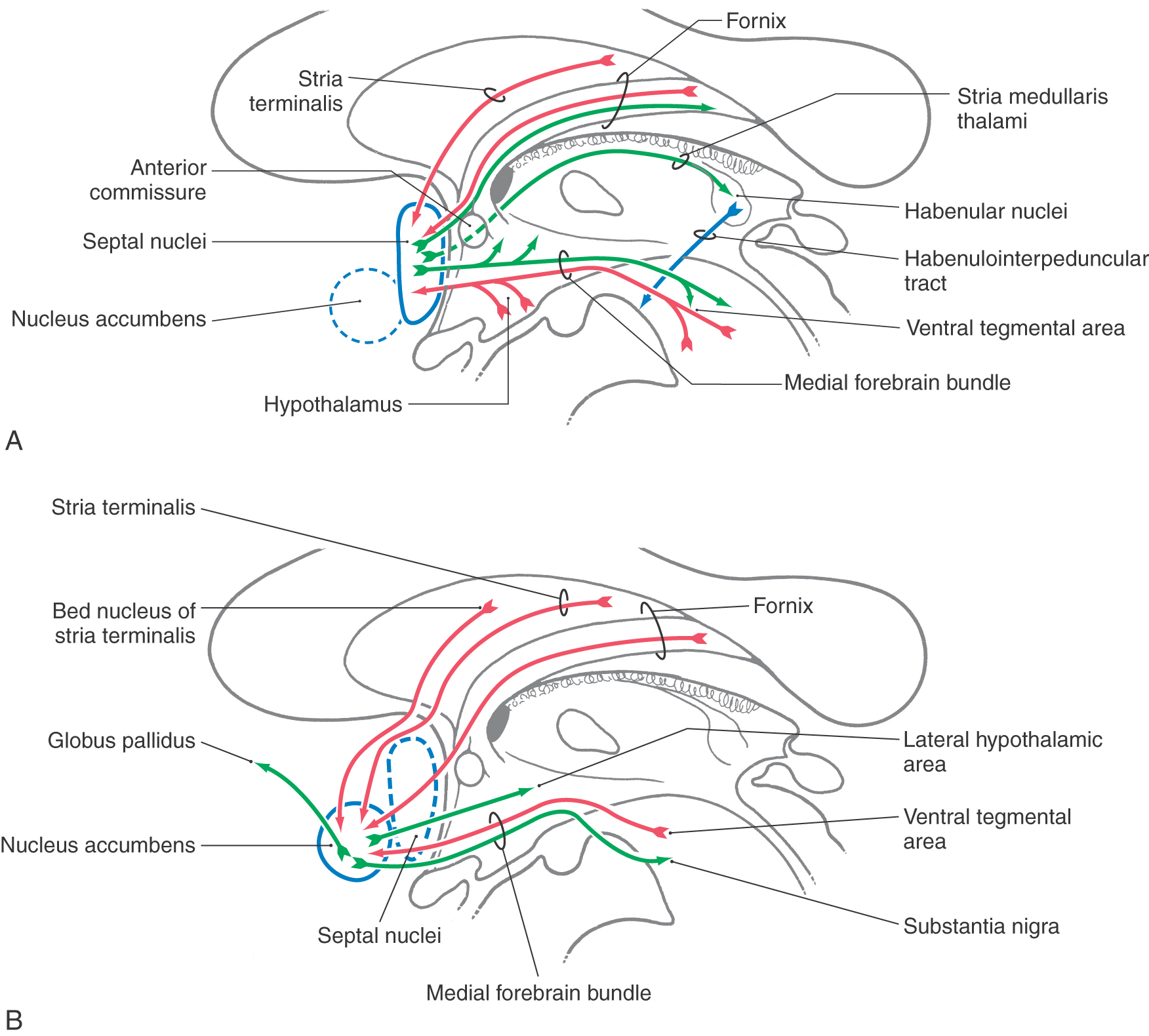
Another route by which hippocampal and amygdaloid efferents influence the brainstem is through the stria medullaris thalami. This bundle conveys fibers from the septal nuclei (targets of amygdaloid and hippocampal inputs) to the habenular nuclei (Fig. 31-9A). The latter cell groups, in turn, give rise to the habenulointerpeduncular tract, which projects to the interpeduncular nucleus and other midbrain sites, including the ventral tegmental area and periaqueductal gray.
SEPTAL REGION
The septal region (septal nuclei), excluding the nucleus accumbens, is a small area just rostral to the anterior commissure and in the medial wall of the hemisphere (Figs. 31-1B and 31-9A). These nuclei extend into the base of the septum pellucidum. Despite their relatively small size, the septal nuclei have been implicated in myriad functions in animal models on the basis of the patterns of their inputs and outputs. In contrast, there is little clinical information about their function in humans. Rage behavior has been seen in a small group of patients with midline infarcts in this area.
The principal afferent pathways to the septal nuclei include fibers from the hippocampus (via the fornix), the amygdaloid complex (via the stria terminalis and ventral amygdalofugal pathways), and the ventral tegmental area of the midbrain (Fig. 31-9A). Fibers also originate from the preoptic, anterior, and paraventricular hypothalamic nuclei and from the lateral hypothalamic area. Many of the fibers in the stria terminalis and fornix also send branches into the nucleus accumbens.
The main efferent projections from the septal nuclei (Fig. 31-9A) are septohippocampal fibers (in the fornix), projections to the habenular nuclei, the medial thalamic nuclei (via the stria medullaris thalami), and the ventral tegmental area (via the medial forebrain bundle). The preoptic, anterior, and ventromedial nuclei and the lateral hypothalamic areas also receive input from the septal nuclei.
The medial forebrain bundle is a diffuse group of fibers that courses rostrocaudally through the lateral hypothalamic area (Fig. 31-9A). This bundle is complex in that it conveys ascending inputs into the hypothalamus and through this area into the septal region. It also is a major conduit through which the septal nuclei and portions of the hypothalamus communicate with the brainstem (Fig. 30-9). The dopamine-containing fibers in this area are thought to be related to perceptions of pleasure or drive reduction.
NUCLEUS ACCUMBENS
The nucleus accumbens (nucleus accumbens septi) is located in the rostral and ventral forebrain where the head of the caudate nucleus and the putamen are continuous (see Fig. 26-4). These cells receive input from the amygdaloid complex (primarily via the ventral amygdalofugal pathway), from the hippocampal formation (through the precommissural fornix), and from cells of the bed nucleus of the stria terminalis (Fig. 31-9B). The ventral tegmental area also gives rise to ascending fibers that enter the nucleus accumbens via the medial forebrain bundle. In addition, amygdalofugal fibers traversing the stria terminalis also enter the nucleus accumbens.
Cells within the nucleus accumbens have receptors for a variety of neurotransmitters, including endogenous opiates. The nucleus accumbens may play an important role in behaviors related to addiction. Recent observations in addicted humans likewise reinforce the concept that the nucleus accumbens is a gratification site. These patients show marked binding of substances to cells in nucleus accumbens.
Efferent projections of the nucleus accumbens include fibers to the hypothalamus, nuclei of the brainstem, and the globus pallidus (Fig. 31-9B). Nucleus accumbens fibers to the last target represent an important route through which the limbic system may access the motor system.
LIMBIC SYSTEM AND BEHAVIOR
In recent years, the term limbic system has been used mainly in reference to emotion-related areas of the brain and the pathways that interconnect them. These areas are generally composed of sites that function to alter the emotions. These sites, which are often interspersed in a given region of the brain, are frequently called either aversion centers or gratification centers. If an aversion center is stimulated, the person will experience fear and sorrow. On the other hand, stimulation of a gratification center will result in pleasure. Functional interconnections between aversion and gratification centers probably contribute to emotional stability (Table 31-3).
Table 31-3 Limbic Structures and Behavior: Main Behavioral and Clinical Characteristics of the Amygdala Contrasted with Those of the Nucleus Accumbens
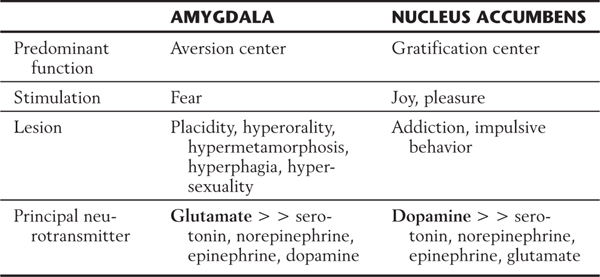
Although most limbic structures contain both gratification and aversion centers, one or the other type of center seems to predominate in some structures (Table 31-3). For example, the hippocampus and amygdala have an abundance of aversion centers, whereas the nucleus accumbens contains an abundance of gratification centers. Consequently, stimulation of the amygdala may elicit fear, whereas stimulation of the nucleus accumbens results in feelings of joy and pleasure.
The emotion-related deficits resulting from small lesions in the limbic system are difficult to predict. However, the effects of relatively large lesions are more stereotypic. They typically result in the flattening of emotions, as reflected by the fact that emotional extremes (joy and anxiety) are reduced. This phenomenon, presumably due to the loss of both aversion and gratification centers, commonly results from large lesions in the amygdala, hippocampus, fornix, or cingulate or prefrontal cortex.
Bilateral lesions of the anterior part of the cingulate gyrus greatly diminish the emotional responses of the patient and may result in akinetic mutism. This is a state in which the patient is immobile, mute, and unresponsive but not in a coma. Other patients with cingulate damage may be alert but have no idea of who they are. Patients may also be unable to recall the order in which past events occurred.
Klüver-Bucy Syndrome
As mentioned previously, bilateral temporal lobe lesions that largely abolish the amygdaloid complex cause a set of behavioral changes called the Klüver-Bucy syndrome. These deficits were initially described in a series of animal experiments, but they have also been seen in patients as a result of either trauma to the temporal lobe or temporal lobe surgery for epilepsy. Damage to the amygdaloid complex frequently involves portions of adjacent structures and of the surrounding white matter, and these incursions into other structures may contribute to the clinical picture. Damage to the amygdala and the hippocampus results in a greater memory deficit than the deficit noted with damage to either one alone.
The Klüver-Bucy syndrome is characterized by the following deficits. First, the patient is no longer able to recognize objects by sight (visual agnosia) and may also exhibit tactile and auditory agnosia. Second, there is the tendency to examine objects excessively by mouth (hyperorality) or to smell them. Even a harmful object such as a lit match may be examined by being brought to the lips or touched with the tongue. Third, the patient may have a compulsion to intensively explore the immediate environment (hypermetamorphosis) and to overreact to visual stimuli. Fourth, placidity is characteristically seen. The animal or the patient may no longer show fear or anger, even when such a reaction is appropriate. Fifth, the subject may eat in excessive amounts (hyperphagia), even when not hungry, or may eat objects that are not food. For example, a patient may eat leaves or swallow marbles. Sixth, there is a striking augmentation in sexual behavior (hypersexuality). In humans, this takes the form of suggestive behavior and talk and vague, ill-conceived attempts at sexual contact. In addition to these predictable deficits, these patients may also experience amnesia, dementia, or aphasia, depending on the extent of the lesion of the temporal lobe.
TEMPORAL LOBE SEIZURES
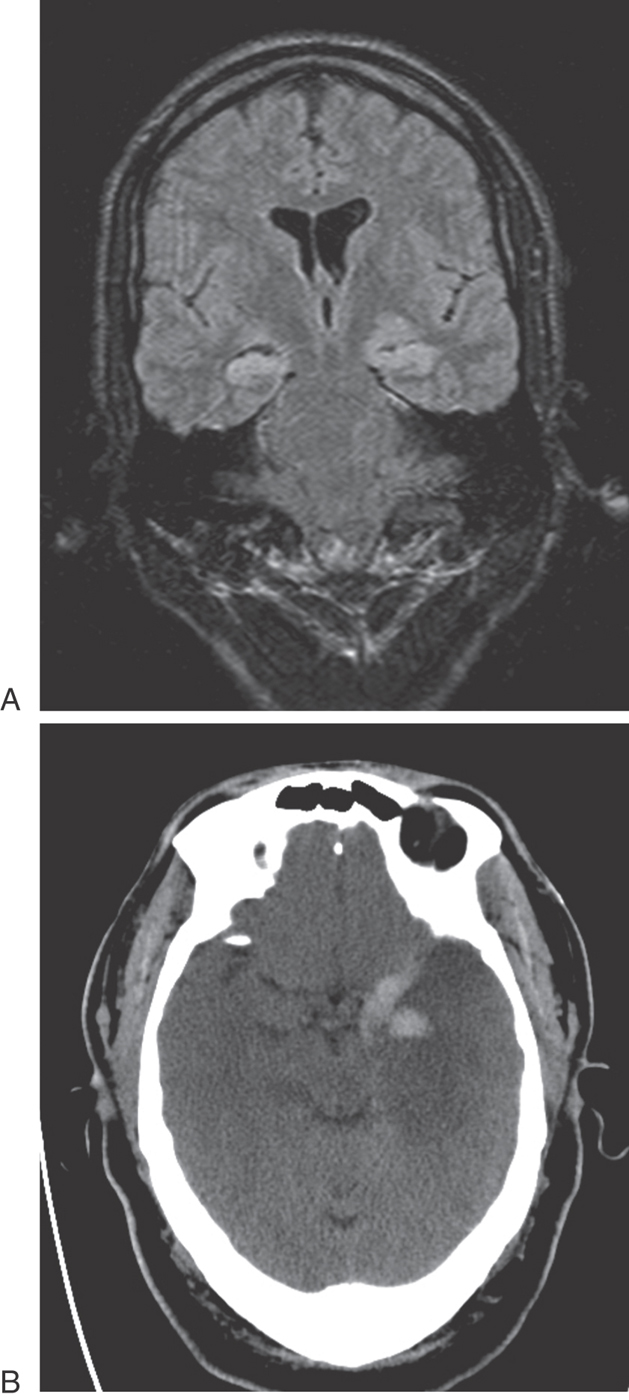
Limbic structures are very sensitive to seizure activity. Damage to the mesial temporal lobes, mesial temporal sclerosis (Fig. 31-10A), is the most common cause of complex partial seizures. This type of seizure starts in a specific area of the brain, resulting in a wide range of physical and emotional behaviors with alteration of consciousness. Partial seizures are often associated with a warning or an aura. For example, seizures that start in the area of the uncus may be associated with olfactory or gustatory hallucinations referred to as uncinate fits. These sensations are explainable given the functions of the amygdala and the destinations of the olfactory-gustatory fiber systems. Seizures starting in other areas of the limbic system may be associated with visual illusions, sensations of impending doom, déjà vu (unfamiliar seems familiar), jamais vu (familiar seems unfamiliar), or even autonomic disturbances. The spectrum of physical and emotional behaviors seen with seizures originating from this part of the brain led to the term psychomotor seizure, which is seen in older literature.
The temporal lobes may be damaged in a variety of ways, including trauma, hypoxia, and infection. The herpes simplex virus has a predilection for the temporal lobes (Fig. 31-10B). Patients with herpes encephalitis typically have high fever, confusion, personality changes, and seizures. The viral infection causes hemorrhage and necrosis of the temporal lobes, leaving most survivors with permanent difficulty in forming new memories.
Epilepsy (recurrent seizure activity) is also related to the malfunction of the hippocampus. It is well established that most areas of the hippocampus are involved in the pathogenesis of epilepsy and sustain damage as a result of recurrent seizures. Surprisingly, area CA2 is the most resistant to sustained seizure activity, whereas the Sommer sector and CA4 are the most vulnerable. Poorly controlled seizures may result in permanent and progressive memory impairment and emotional problems. The organization of the hippocampal subfields and the function of the amygdala are clinically apparent in the field of epilepsy.
LIMBIC SYSTEM AND COGNITIVE FUNCTION
There is a trend to look at the limbic system as a set of structures that influence not only emotion but also cognitive functions. The area on which there is the most agreement in this regard is memory. Other influences, however, are mediated through the nucleus basalis and are undoubtedly related to the control of cortical excitability. The full contribution of this upstream control is related to the transfer of information from limbic structures to the cerebral cortex. The manner in which limbic structures and the cortex interact is undergoing extensive revision. We know that visual information enters the entorhinal cortex by first synapsing in the perirhinal cortex (area 35) of the proisocortex. Thus visual place memories are formed in the hippocampus by specific pathways only recently elaborated. Undoubtedly, other sensory modalities enter the hippocampus through similar transitional regions. Such inputs can perhaps account for the cognitive deficits that follow selective limbic system damage.
Sources and Additional Reading
Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer’s disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33:441–459.
Isaacson RL. The Limbic System. New York: Plenum Press; 1974.
Squire LR. Memory and Brain. New York: Oxford University Press; 1987.