Chapter 2 The interbody joint and the intervertebral discs
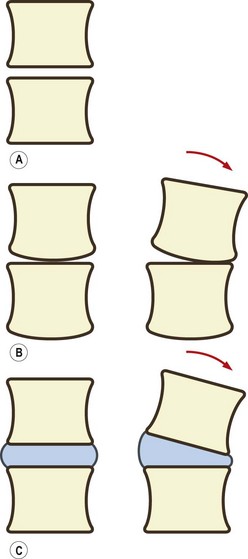
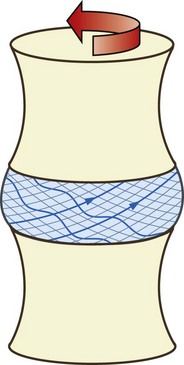
A joint could be formed simply by resting two consecutive vertebral bodies on top of one another (Fig. 2.1A). Such a joint could adequately bear weight and would allow gliding movements between the two bodies. However, because of the flatness of the vertebral surfaces, the joint would not allow the rocking movements that are necessary if flexion and extension or lateral bending are to occur at the joint. Rocking movements could occur only if one of two modifications were made. The first could be to introduce a curvature to the surfaces of the vertebral bodies. For example, the lower surface of a vertebral body could be curved (like the condyles of a femur). The upper vertebral body in an interbody joint could then roll forwards on the flat upper surface of the body below (Fig. 2.1B). However, this adaptation would compromise the weight-bearing capacity and stability of the interbody joint. The bony surface in contact with the lower vertebra would be reduced, and there would be a strong tendency for the upper vertebra to roll backwards or forwards whenever a weight was applied to it. This adaptation, therefore, would be inappropriate if the weight-bearing capacity and stability of the interbody joint are to be preserved. It is noteworthy, however, that in some species where weight-bearing is not important, for example in fish, a form of ball-and-socket joint is formed between vertebral bodies to provide mobility of the vertebral column.1
An alternative modification, and the one that occurs in humans and most mammals, is to interpose between the vertebral bodies a layer of strong but deformable soft tissue. This soft tissue is provided in the form of the intervertebral disc. The foremost effect of an intervertebral disc is to separate two vertebral bodies. The space between the vertebral bodies allows the upper vertebra to tilt forwards without its lower edge coming into contact with the lower vertebral body (Fig. 2.1C).
Structure of the intervertebral disc
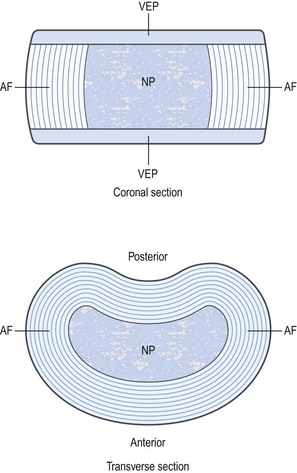
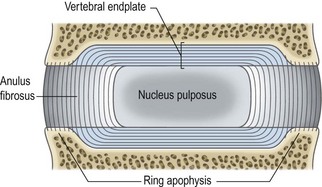
A third component of the intervertebral disc comprises two layers of cartilage which cover the top and bottom aspects of each disc. Each is called a vertebral endplate (Fig. 2.2). The vertebral endplates separate the disc from the adjacent vertebral bodies, and it is debatable whether the endplates are strictly components of the disc or whether they actually belong to the respective vertebral bodies. The interpretation used here is that the endplates are components of the intervertebral disc.
Nucleus pulposus
In typical, healthy, intervertebral discs of young adults, the nucleus pulposus is a semifluid mass of mucoid material (with the consistency, more or less, of toothpaste). Embryologically, the nucleus pulposus is a remnant of the notochord (see Ch. 12). Histologically, it consists of a few cartilage cells and some irregularly arranged collagen fibres, dispersed in a medium of semifluid ground substance (see below). Biomechanically, the fluid nature of the nucleus pulposus allows it to be deformed under pressure, but as a fluid its volume cannot be compressed. If subjected to pressure from any direction, the nucleus will attempt to deform and will thereby transmit the applied pressure in all directions. A suitable analogy is a balloon filled with water. Compression of the balloon deforms it; pressure in the balloon rises and stretches the walls of the balloon in all directions.
Anulus fibrosus
The anulus fibrosus consists of collagen fibres arranged in a highly ordered pattern. Foremost, the collagen fibres are arranged in between 10 and 20 sheets2,3 called lamellae (from the Latin lamella meaning little leaf). The lamellae are arranged in concentric rings which surround the nucleus pulposus (Figs 2.2 and 2.3). The lamellae are thicker towards the centre of the disc;4 they are thick in the anterior and lateral portions of the anulus but posteriorly they are finer and more tightly packed. Consequently the posterior portion of the anulus fibrosus is thinner than the rest of the anulus.2,5,6
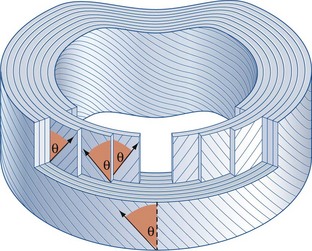
Within each lamella, the collagen fibres lie parallel to one another, passing from the vertebra above to the vertebra below. The orientation of all the fibres in any given lamella is therefore the same and measures about 65–70° from the vertical.7,8 However, while the angle is the same, the direction of this inclination alternates with each lamella. Viewed from the front, the fibres in one lamella may be orientated 65° to the right but those in the next deeper lamella will be orientated 65° to the left. The fibres in the next lamella will again lie 65° to the right, and so on (see Fig. 2.3). Every second lamella, therefore, has exactly the same orientation. These figures, however, constitute an average orientation of fibres in the mid-portion of any lamella. Near their attachments, fibres may be orientated more steeply or less steeply with respect to the sagittal plane.4
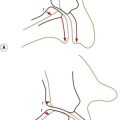
The implication of the classic description of the anulus fibrosus is that the lamellae of the anulus form complete rings around the circumference of the disc. However, this proves not to be the case. In any given quadrant of the anulus, some 40% of the lamellae are incomplete, and in the posterolateral quadrant some 50% are incomplete.4 An incomplete lamella is one that ceases to pass around the circumference of the disc. Around its terminal edge the lamellae superficial and deep to it either approximate or fuse (Fig. 2.4). Incomplete lamellae seem to be more frequent in the middle portion of the anulus.9
Vertebral endplates
Each vertebral endplate is a layer of cartilage about 0.6–1 mm thick10–12 that covers the area on the vertebral body encircled by the ring apophysis. The two endplates of each disc, therefore, cover the nucleus pulposus in its entirety, but peripherally they fail to cover the entire extent of the anulus fibrosus (Fig. 2.5). Histologically, the endplate consists of both hyaline cartilage and fibrocartilage. Hyaline cartilage occurs towards the vertebral body and is most evident in neonatal and young discs (see Ch. 12). Fibrocartilage occurs towards the nucleus pulposus; in older discs the endplates are virtually entirely fibrocartilage (see Ch. 13). The fibrocartilage is formed by the insertion into the endplate of collagen fibres of the anulus fibrosus.6
The collagen fibres of the inner lamellae of the anulus enter the endplate and swing centrally within it.3,13,14 By tracing these fibres along their entire length it can be seen that the nucleus pulposus is enclosed by a sphere of collagen fibres, more or less like a capsule. Anteriorly, posteriorly and laterally, this capsule is apparent as the innermost lamellae of the anulus fibrosus, but superiorly and inferiorly the ‘capsule’ is absorbed into the vertebral endplates (see Fig. 2.5).
Where the endplate is deficient, over the ring apophysis, the collagen fibres of the most superficial lamellae of the anulus insert directly into the bone of the vertebral body (see Fig. 2.5).14 In their original form, in younger discs, these fibres attach to the vertebral endplate which fully covers the vertebral bodies in the developing lumbar spine, but they are absorbed secondarily into bone when the ring apophysis ossifies (see Ch. 12).
Because of the attachment of the anulus fibrosus to the vertebral endplates, the endplates are strongly bound to the intervertebral disc. In contrast, the endplates are only weakly attached to the vertebral bodies13,14 and can be wholly torn from the vertebral bodies in certain forms of spinal trauma.15 It is for this and other morphological reasons that the endplates are regarded as constituents of the intervertebral disc rather than as parts of the vertebral bodies.10,12,13,16–18
Over some of the surface area of the vertebral endplate (about 10%) the subchondral bone of the vertebral body is deficient and pockets of the marrow cavity touch the surface of the endplate or penetrate a short distance into it.11,19 These pockets facilitate the diffusion of nutrients from blood vessels in the marrow space and are important for the nutrition of the endplate and intervertebral disc (see Ch. 11).
Detailed structure of the intervertebral disc
Constituents
Glycosaminoglycans
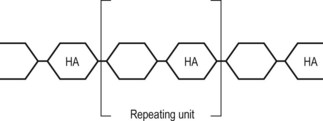
As a class of chemicals glycosaminoglycans (GAGs) are present in most forms of connective tissue. They are found in skin, bone, cartilage, tendon, heart valves, arterial walls, synovial fluid and the aqueous humour of the eye. Chemically, they are long chains of polysaccharides, each chain consisting of a repeated sequence of two molecules called the repeating unit (Fig. 2.6).20,21 These repeating units consist of a sugar molecule and a sugar molecule with an amine attached, and the nomenclature ‘glycosaminoglycan’ is designed to reflect the sequence of ‘sugar amine–sugar– …’ in their structure.
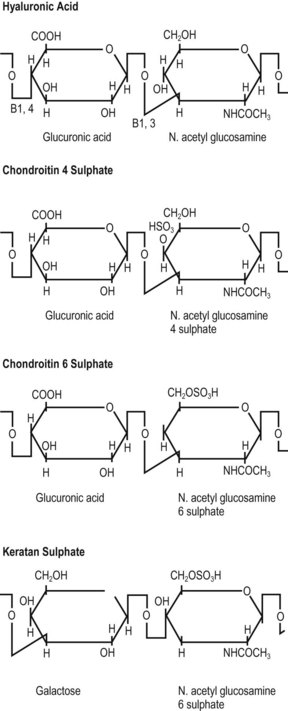
The length of individual GAGs varies but is characteristically about 20 repeating units.21 Each different GAG is characterised by the particular molecules that make up its repeating unit. The GAGs predominantly found in human intervertebral discs are chondroitin-6-sulphate, chondroitin-4-sulphate, keratan sulphate and hyaluronic acid.22,23 The structures of the repeating units of these molecules are shown in Figure 2.7.
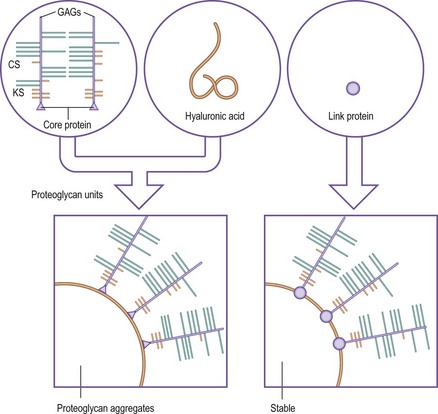
Proteoglycans
Proteoglycans are very large molecules consisting of many GAGs linked to proteins. They occur in two basic forms: proteoglycan units and proteoglycan aggregates. Proteoglycan units are formed when several GAGs are linked to a polypeptide chain known as a core protein (Fig. 2.8).22,24 A single core protein may carry as few as six or as many as 60 polysaccharide chains.21 The GAGs are joined to the core protein by covalent bonds involving special sugar molecules.22,23 Proteoglycan aggregates are formed when several proteoglycan units are linked to a chain of hyaluronic acid. A single hyaluronic chain may bind 20 to 100 proteoglycan units.22 The linkage between the proteoglycan units and the hyaluronic acid is stabilised by a relatively small mass of protein known as the link protein (see Fig. 2.8).22
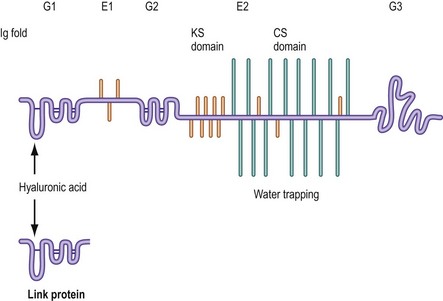
The cardinal proteoglycan of the intervertebral disc resembles that of articular cartilage and is known as aggrecan.25 Its detailed structure is shown in Figure 2.9. Its core protein exhibits three coiled regions called globular domains (G1, G2 and G3) and two relatively straight regions called extended domains (E1 and E2).26 GAGs are bound principally and most densely to the E1 domain. Chondroitin sulphate binds to the terminal three-quarters or so of the E2 domain (i.e. towards the carboxyl end, or C-terminal, of the core protein).22–2426 Keratan sulphate binds predominantly towards the N-terminal of the E2 domain but also occurs amongst the chondroitin chains.22–24,26,27 Some keratan sulphate chains also bind to the E1 domain.
The N-terminal of the core protein bears the G1 domain, which is folded like an immunoglobulin; a similar structure is exhibited by the link protein. It is these coiled structures that bind hyaluronic acid and allow the aggrecan molecules to aggregate.26 The G1 domain does not assume its structure until after a newly synthesised molecule of aggrecan has left the cell that produces it.25 This ensures that aggregation occurs only in the extracellular matrix.
Large proteoglycans that aggregate with hyaluronic acid are characteristic of hyaline cartilage and they occur in immature intervertebral discs.23 They are rich in chondroitin sulphate, carrying about 100 of these chains, each with an average molecular weight of about 20 000. They carry 30–60 keratan sulphate chains, each with a molecular weight of 4000 to 8000.23 Large and moderately sized proteoglycans that do not aggregate with hyaluronic acid are the major proteoglycans that occur in the mature nucleus pulposus.23
In vivo, proteoglycan units and aggregates are convoluted to form complex, three-dimensional molecules, like large and small tangles of cotton wool (Fig. 2.10). Physicochemically, these molecules have the property of attracting and retaining water (compare this with the water-absorbing properties of a ball of cotton wool). The volume enclosed by a proteoglycan molecule, and into which it can attract water, is known as its domain.21
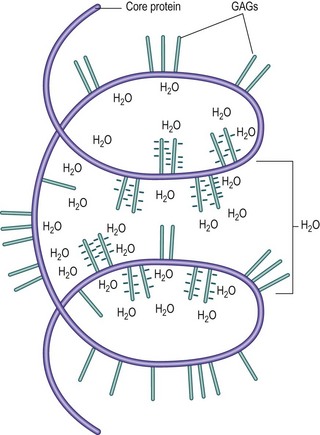
Figure 2.10 A coiled proteoglycan unit, illustrating how the ionic radicals on its GAGs attract water into its ‘domain’.
The water-binding capacity of a proteoglycan molecule is partially a property of its size and physical shape, but the main force that holds water to the molecule stems from the ionic, carboxyl (COOH) and sulphate (SO4) radicals of the GAG chains (see Fig. 2.7). These radicals attract water electrically, and the water-binding capacity of a proteoglycan can be shown to be proportional to the density of these ionic radicals in its structure. In this respect, sulphated GAGs attract water more strongly than other mucopolysaccharides of similar size that lack sulphate radicals. Furthermore, it is readily apparent that because the chondroitin sulphates have both sulphate and carboxyl radicals in their repeating units (see Fig. 2.7), they will have twice the water-binding capacity of keratan sulphate, which, although carrying a sulphate radical, lacks a carboxyl radical. The water-binding capacity of any proteoglycan will therefore be largely dependent on the concentration of chondroitin sulphate within its structure.24
Collagen
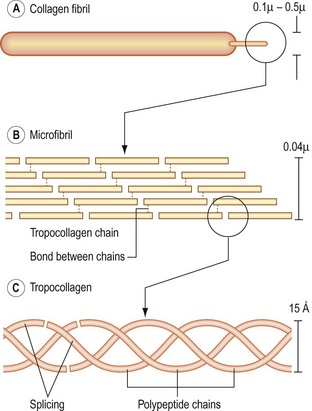
Fundamentally, collagen consists of strands of protein molecules. The fundamental unit of collagen is the tropocollagen molecule, which itself consists of three polypeptide chains wound around one another in a helical fashion and held together end to end by hydrogen bonds (Fig. 2.11). Collagen is formed when many tropocollagen molecules are arrayed end-on and side by side. When only a few tropocollagen chains are arrayed side by side, the structure formed is known as a small collagen fibril. When the structure is made thicker, by the addition of further layers of tropocollagen chains, it becomes a large fibril. The aggregation of several large fibrils forms a collagen fibre. The tropocollagen chains within a collagen fibre are held together, side by side, by covalent bonds involving a molecule of hydroxylysine (see Fig. 2.11).28–30
There are 11 types of collagen found in connective tissue.31 Each type is genetically determined and differs in the chemical nature of the polypeptide chains that form the tropocollagen molecules found in the collagen fibre and in the microstructure of the fibre. The different types of collagen are denoted by Roman numerals as types I, II, III up to type XI.
Types I, II, III, V and XI exhibit the typical triple helical structure described above. Types IV and VII are long-chain molecules that bear a globular extension at one end and whose triple helix is interrupted periodically by non-helical segments. Types VI, VIII, IX and X are much shorter molecules with interrupted or uniform helical segments that bear globular extensions at one or both ends.31
Type I, II and III molecules form most of the collagen fibres of the body; types I and II are typical of musculoskeletal tissues. Their distribution is shown in Table 2.1. Type I collagen is essentially tensile in nature and is found in tissues that are typically subjected to tension and compression. Type II collagen is more elastic in nature and is typically found in tissues habitually exposed to pressure.
Table 2.1 Genetic types of collagen and their distribution in connective tissues
| Type | Distribution |
|---|---|
| I | Skin, bone, tendon, meniscus, dentine, anulus fibrosus |
| II | Cartilage, vitreous humour, nucleus pulposus |
| III | Dermis, heart, blood vessels, synovium |
| IV | Basement membrane |
| V | Co-distributed with type I |
| VI | Blood vessels, viscera, muscle |
| VII | Ectodermal basement membranes |
| VIII | Descemet’s membrane |
| IX | Cartilage, vitreous humour |
| X | Epiphysial plates |
| XI | Co-distributed with type II |
Type III collagen is typical of the dermis, blood vessels and synovium. Type IV collagen occurs only in basement membranes; type VII is found in basement membranes of ectodermal origin; and type VIII is found in Descemet’s membrane of the cornea; type X has been found only in epiphysial plates; type VI is characteristically found in blood vessels, viscera and muscles while type IX occurs in cartilage.31
The principal types of collagen found in the intervertebral disc are types I and II. Other types of collagen occur in much lesser amounts. Type V collagen is regularly associated with type I collagen and is co-distributed with it, but its concentration is only about 3% of that of type I. Similarly, type XI coexists with type II but at only about 3% of its concentration.31 Type IX collagen occurs in discs at about 2% of the concentration of type II; its function appears to be to link proteoglycans to collagen fibres and to control the size of type II fibrils.31 Small amounts of type VI collagen occur in both the nucleus pulposus and anulus fibrosus, and traces of type III collagen occur within the nucleus pulposus and inner anulus fibrosus; these collagens are located in the immediate pericellular regions of the matrix,32 but their functions are still unknown.31
Both type I and type II collagen are present in the anulus fibrosus but type I is the predominant form.28,29,33–37 Type II collagen predominates in the nucleus pulposus and is located between cells in the interterritorial matrix.32 Type I collagen is absent from the central portions of the nucleus or is present only in small amounts. This difference in distribution within the intervertebral disc correlates with the different biomechanical roles of the anulus fibrosus and the nucleus pulposus. From a knowledge of the biochemistry of the collagen in the intervertebral disc, it can be anticipated that the nucleus pulposus, with only type II collagen, will be involved more in processes involving pressure, while the anulus fibrosus, containing both type I and type II collagen, will be involved in both tension-related and pressure-related processes.
An important property of collagen and proteoglycans is that they can bind together. The binding involves both electrostatic and covalent bonds,20,28,37–40 and these bonds contribute to the strength of structures whose principal constituents are proteoglycans and collagen. Bonds are formed directly between proteoglycans and type I and type II collagen, or indirectly through type IX collagen.
Other proteoglycans
Like articular cartilage, the intervertebral disc contains small quantities of two small proteoglycans – decorin and biglycan41 – whose core proteins bear chains of the glycosaminoglycan dermatan sulphate, one chain in the case of decorin, two in the case of biglycan. These proteoglycans interact with collagen, fibronectin and growth factors in the matrix of the disc, and are therefore critical factors in the homeostasis and repair of the matrix.42
Enzymes
The intervertebral disc, like articular cartilage, contains proteolytic enzymes.43–45 These enzymes are known as matrix metalloproteinases (MMPs). The three main types are MMP-1 (or collagenase), MMP-2 (or gelatinase) and MMP-3 (or stromelysin). Collagenase and gelatinase have very selective substrates. Collagenase can cleave type II collagen; gelatinase cannot but it can cleave the fragments of type II collagen produced by collagenase. Stromelysin is the most destructive of the enzymes. It can cleave types II, XI and IX collagen as well as fibronectin but it also has an aggressive action on proteoglycans, cleaving aggrecan molecules between their E1 and G2 domains.
Under normal circumstances, these enzymes function to remove old components of the matrix, allowing them to be replaced with fresh components. The enzymes are secreted as inactive forms, which are subsequently activated by agents such as plasmin, and are inhibited by proteins known as tissue inhibitors of metalloproteinases, which prevent excessive enzyme activity.43,44 If the balance between activators and inhibitors is disturbed, excessive action of stromelysin may result in degradation of the matrix, at a rate that normal repair processes cannot keep up with.
Microstructure
Nucleus pulposus
The nucleus pulposus is 70–90% water17,30,46–49 although the exact proportion varies with age (see Ch. 13). Proteoglycans are the next major component, and they constitute about 65% of the dry weight of the nucleus.46,47 The water of the nucleus is contained within the domains of these proteoglycans. Only about 25% of the proteoglycans occur in an aggregated form.24 The majority are in the form of freely dispersed proteoglycan units that lack a functional binding site that would enable them to aggregate with hyaluronic acid.23
About two-thirds of the proteoglycan aggregates in the nucleus pulposus are smaller than those typically found in articular cartilage.27 Each consists of about 8 to 18 proteoglycan units closely spaced on a short chain of hyaluronic acid.27
Interspersed through the proteoglycan medium are thin fibrils of type II collagen, which serve to hold proteoglycan aggregates together.50,51 The mixture of proteoglycan units, aggregates and collagen fibres within the nucleus pulposus is referred to collectively as the matrix of the nucleus.
Collagen constitutes 15–20% of the dry weight of the nucleus22,46 and the remainder of the nucleus consists of some elastic fibres and small quantities of various other proteins known as non-collagenous proteins.30,44,46,48,52,53 These include the link proteins of the proteoglycans37,44 and other proteins involved in stabilising the structure of large collagen fibrils37 and other components of the nuclear matrix;44 however, the function of many of these non-collagenous proteins remains unknown.44
Embedded in the proteoglycan medium of the nucleus are cartilage cells (chondrocytes), and in the newborn there are also some remnant cells of the notochord (see Ch. 12).38 The cartilage cells are located predominantly in the regions of the vertebral endplates and are responsible for the synthesis of the proteoglycans and collagen of the nucleus pulposus.19,24 The type III collagen that occurs in the intervertebral disc is characteristically located around the cells of the nucleus pulposus and the inner anulus fibrosus.31
Anulus fibrosus
Water is also the principal structural component of the anulus fibrosus, amounting to 60–70% of its weight.17,30,46–49 Collagen makes up 50–60% of the dry weight of the anulus,30,33,46,52 and the tight spaces between collagen fibres and between separate lamellae are filled with a proteoglycan gel that binds the collagen fibres and lamellae together to prevent them from buckling or fraying.24 Proteoglycans make up about 20% of the dry weight of the anulus,46 and it is this gel that binds the water of the anulus. About 50–60% of the proteoglycans of the anulus fibrosus are aggregated, principally in the form of large aggregates.27 The concentration of proteoglycans and water is somewhat greater in the anterior anulus than in the posterior anulus, and in both regions increases from the outer to the inner anulus; conversely, there is progressively less collagen from the outer to the inner anulus.54
Interspersed among the collagen fibres and lamellae are chondrocytes and fibroblasts that are responsible for synthesising the collagen and the proteoglycan gel of the anulus fibrosus. The fibroblasts are located predominantly towards the periphery of the anulus while the chondrocytes occur in the deeper anulus, towards the nucleus.19,24
From a biochemical standpoint, it can be seen that the nucleus pulposus and anulus fibrosus are similar. Both consist of water, collagen and proteoglycans. The differences lie only in the relative concentrations of these components, and in the particular type of collagen that predominates in each part. The nucleus pulposus consists predominantly of proteoglycans and water, with some type II collagen. The anulus fibrosus also consists of proteoglycans and a large amount of water but is essentially ‘thickened’ by a high concentration of collagen, type II collagen being found throughout the anulus and type I concentrated largely in the outer anulus.32
The anulus fibrosus also contains a notable quantity of elastic fibres.55–58 Elastic fibres constitute about 10% of the anulus fibrosus and are arranged circularly, obliquely and vertically within the lamellae of the anulus.58 They appear to be concentrated towards the attachment sites of the anulus with the vertebral endplate.59
Vertebral endplates
The chemical structure of the vertebral endplate resembles and parallels that of the rest of the disc. It consists of proteoglycans and collagen fibres, with cartilage cells aligned along the collagen fibres.11 It resembles the rest of the disc by having a higher concentration of water and proteoglycans and a lower collagen content towards its central region, which covers the nucleus pulposus, with a reciprocal pattern over the anulus fibrosus. Across the thickness of the endplate the tissue nearer bone contains more collagen while that nearer the nucleus pulposus contains more proteoglycans and water.11 This resemblance to the rest of the disc means that at a chemical level the endplate does not constitute an additional barrier to diffusion. Small molecules pass through an essentially uniform, chemical environment to move from the vertebral body to the centre of the disc.
Metabolism
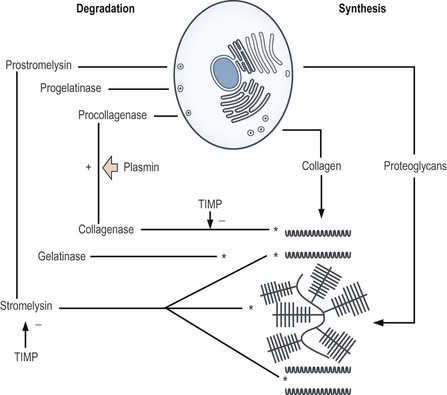
The proteoglycans and collagen of the intervertebral disc are synthesised and maintained by the chondrocytes and fibroblasts of the nucleus and anulus (Fig. 2.12). In fetal and newborn discs, cells in the nucleus exhibit far greater synthetic activity than those in the anulus, but in mature discs the greatest activity occurs in the mid-portion of the anulus, there being progressively less activity exhibited towards the outer anulus and towards the nucleus.60
Once synthesised and delivered out of the cell, the proteoglycans aggregate and bind to the collagen fibres, thereby establishing the solid phase of the matrix. Water is then retained in the domains of the proteoglycans. This matrix, however, undergoes a slow turnover. Systematically, old proteoglycans and collagen are constantly removed and replaced. Removal is achieved by the metalloproteinases. Collagenase degrades type II collagen whereas stromelysin degrades both collagen and proteoglycans (see Fig. 2.12).
All these activities require the cells to be metabolically active; they require oxygen, glucose, the substrates for the products they produce, and cofactors involved in their production. However, the disc essentially lacks a blood supply and the cells therefore rely on diffusion for their nutrition (see Ch. 11). Because of this low blood supply, the oxygen concentrations in the centre of a disc are only 2–5% of those at its periphery,61 and the cells of the disc must rely on anaerobic metabolism. As a result, the cells produce large amounts of lactic acid, which makes the environment of the disc acidic61,62 with a pH in the range of 6.9–7.1.61,62
The metabolism of cells in the nucleus is very sensitive to changes in pH. They are maximally active in pH ranges of 6.9–7.2, but below 6.8 their activity falls steeply. Below 6.3 their activity is only about 15% maximum.62
Functions of the disc
Weight-bearing
The compression stiffness of the anulus fibrosus is essentially uniform across the thickness of the anulus, although there is a tendency for the inner anulus to be less stiff than the middle and outer anuli.54 The compression stiffness of the anulus correlates inversely but weakly with its water content but not with its proteoglycan content.54
It has been shown experimentally that, under briefly applied loads, a disc with its nucleus removed maintains virtually the same axial load-bearing capacity as an intact disc.63 These observations demonstrate that the anulus fibrosus is able to act as a passive space filler and to act alone in transmitting weights from one vertebra to the next. The disc does not necessarily need a nucleus pulposus to do this – the anulus alone can be sufficient.
As a ball of fluid, the nucleus pulposus may be deformed but its volume cannot be compressed. Thus, when a weight is applied to a nucleus from above it tends to reduce the height of the nucleus, and the nucleus tries to expand radially, i.e. outwards towards the anulus fibrosus. This radial expansion exerts a pressure on the anulus that tends to stretch its collagen lamellae outwards; however, the tensile properties of the collagen resist this stretch, and the collagen lamellae of the anulus oppose the outward pressure exerted on them by the nucleus (Fig. 2.13).
For any given load applied to the disc, an equilibrium will eventually be attained in which the radial pressure exerted by the nucleus will be exactly balanced by the tension developed in the anulus. In a healthy disc with intact collagen lamellae, this equilibrium is attained with minimum radial expansion of the nucleus. The anulus fibrosus is normally so thick and strong that, during weight-bearing, it resists any tendency for the disc to bulge radially. Application of a 40 kg load to an intervertebral disc causes only 1 mm of vertical compression and only 0.5 mm of radial expansion of the disc.64
The other direction in which the nucleus exerts its pressure is towards the vertebral endplates (see Fig. 2.13) but because the endplates are applied to the vertebral bodies they too will resist deformation. The situation that arises, therefore, is that when subjected to a load, the nucleus attempts to deform but it is prevented from doing so. Radially it is constrained by the anulus fibrosus, and upwards and downwards it is constrained by the vertebral endplates and vertebral bodies. All that the nucleus can do is exert its raised pressure against the anulus and the endplates.
The advantage of the cooperative action of the nucleus and the anulus is that the disc can sustain loads that otherwise might tend to buckle an anulus fibrosus acting alone.65 The essence of the combined mechanism is the fluid property of the nucleus pulposus. The water content of the nucleus makes the disc a turgid body that resists compression, and the water content of the nucleus is therefore of critical importance to the disc. Because the water content of the nucleus is, in turn, a function of its proteoglycan content, the normal mechanics of the disc will ultimately depend on a normal proteoglycan content of the nucleus pulposus. Any change in the proteoglycan and water content of the nucleus will inevitably alter the mechanical properties of the disc (see Ch. 13).
Movements
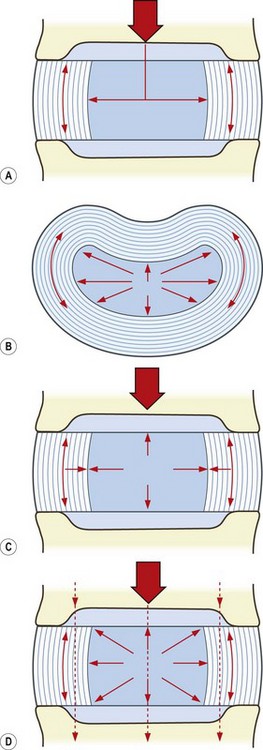
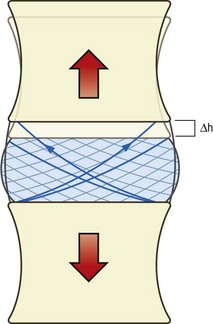
During distraction, all points on one vertebral body move an equal distance perpendicularly from the upper surface of the other vertebral body (Fig. 2.14). Consequently, the attachments of every collagen fibre in the anulus fibrosus are separated an equal distance. Every fibre is therefore strained and every fibre in the anulus resists distraction. Because of the density of collagen fibres in the anulus fibrosus, distraction is strongly resisted by the anulus. The capacity of the discs in this regard is illustrated by how well they sustain the load of the trunk and lower limbs in activities like hanging by the hands. Hanging by the hands, however, is not a common activity of daily living, and vertebral distraction is not a particularly common event. On the other hand, distraction is induced clinically, in the form of traction. A further description of the mechanics of traction, however, is deferred until Chapter 8, when it is considered in the context of the whole lumbar spine.
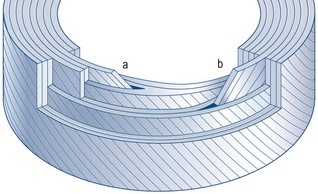
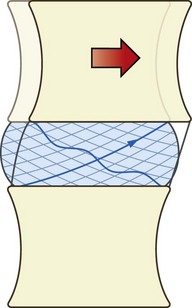
In pure sliding movements of the interbody joint, all points on one vertebra move an equal distance parallel to the upper surface of the next vertebra (Fig. 2.15). This movement is resisted by the anulus fibrosus but the fibres of the anulus act differently according to their location within the anulus and in relation to the direction of movement. In forward sliding, the fibres at the sides of the disc lie in a plane more or less parallel to the direction of movement and run obliquely between the vertebral bodies but in opposite directions in each successive lamella. Consequently, during forward sliding, only half of the fibres in the lateral anulus will be strained, for only half of the fibres have their points of attachment separated by the movement. The other half have their points approximated (see Fig. 2.15). Therefore, only half the fibres in the lateral anulus contribute to resisting forward sliding.
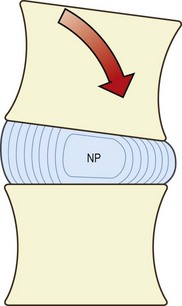
Bending or rocking movements involve the lowering of one end of the vertebral body and the raising of the opposite end. This necessarily causes distortion of the anulus fibrosus and the nucleus pulposus, and it is the fluid content of the nucleus and anulus that permits this deformation. In forward bending, the anterior end of the vertebral body lowers, while the posterior end rises. Consequently, the anterior anulus will be compressed and will tend to buckle66–69 (Fig. 2.16). The nucleus pulposus will also be compressed but mainly anteriorly. The elevation of the posterior end of the vertebral body relieves pressure on the nucleus pulposus posteriorly but at the same time stretches the posterior anulus.
Mathematical analyses indicate that if the disc is not otherwise loaded (e.g. also bearing weight), there should be no rise in nuclear pressure during bending of an interbody joint as the volume of the nucleus pulposus remains unchanged.8 Experimental studies, however, show that in cadaveric discs, 5° of bending is associated with a rise in nuclear pressure of about 0.7 kPa cm−2.70 This rise is the same regardless of the load carried by the disc, therefore the relative increase in disc pressure caused by bending decreases as greater external loads are applied. The increase in disc pressure amounts to about 22% of the total disc pressure for loads of 2 kPa cm−2 but is only 5% for loads of 10 kPa cm−2.70
The large increases in disc pressure seen in vivo during bending of the lumbar spine are not intrinsically due to the bending but are the result of the additional compressive loads applied to the discs by the action of the back muscles that control the bending (see Ch. 9).
Previous injury, or erosion as a result of disc disease, may weaken some of the lamellae of the posterior anulus, and the remaining lamellae may be insufficient to resist the tension and posterior pressure that occurs in loaded forward bending. Consequently, the pressure of the nucleus may rupture the remaining lamellae, and extrusion, or herniation, of the nucleus pulposus may result (see Ch. 15). The resistance to this type of injury is proportional to the density of collagen fibres in the posterior anulus. Thicker anuli afford more protection than thinner ones but the shape of the posterior anulus also plays a role.
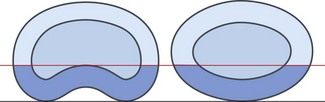
Discs that are concave posteriorly have a greater cross-sectional area of anulus posteriorly than do discs with an elliptical shape, even if the anulus is the same thickness (Fig. 2.17). Thus, concave discs are better designed than posteriorly convex discs to withstand forward bending and injury during this movement,8 and this difference has a bearing on the pattern of injuries seen in intervertebral discs (see Ch. 15).
During twisting movements of the interbody joint, all points on the lower surface of one vertebra will move circumferentially in the direction of the twist; this has a unique effect on the anulus fibrosus. Because of the alternating direction of orientation of the collagen fibres in the anulus, only those fibres inclined in the direction of movement will have their points of attachment separated. Those inclined in the opposite direction will have their points of attachment approximated (Fig. 2.18). Thus, at any time, the anulus resists twisting movements with only half of its complement of collagen fibres. Half of the number of lamellae in the anulus will be stretched, while the other half will be relaxed. This is one of the reasons why twisting movements of an interbody joint are the most likely to injure the anulus (see Chs 8 and 15).
Summary
If the fibres of the anulus were arranged perpendicular to the vertebral bodies, they would be optimally orientated to resist distraction. However, they would afford virtually no resistance to sliding movements of the joint. The advantage of the oblique orientation is that each fibre can offer a component of resistance both vertically and horizontally, and therefore the anulus fibrosus can participate in resisting movements in all directions. The degree of obliquity governs the extent to which a fibre resists horizontal movement, versus vertical movement, and it can be shown mathematically that the orientation of 65° is optimal for the various strains that an anulus is called upon to sustain.8 A steeper orientation would enhance resistance to distraction but would compromise resistance to sliding and twisting. Conversely, a flatter orientation would enhance resistance to twisting, but would compromise resistance to distraction and bending. The alternation of the direction of fibres in alternate lamellae of the anulus fibrosus is integral to the capacity of the disc to resist twisting. Half of the lamellae are dedicated to resisting twisting to the right, the other half resist twisting to the left. For a more detailed analysis of the mechanics of the anulus fibrosus, the reader is referred to the papers of Hickey and Hukins,8 Hukins,71 Broberg72 and Farfan and Gracovetsky.73
1 Kingsley JS. The Vertebrate Skeleton. London: Murray; 1925. 22
2 Armstrong JR. Lumbar Disc Lesions, 3rd edn. Edinburgh: Churchill Livingstone; 1965. 13
3 Taylor JR. The development and adult structure of lumbar intervertebral discs. J Man Med. 1990;5:43-47.
4 Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine. 1990;15:402-410.
5 Jayson MIV, Barks JS. Structural changes in the intervertebral disc. Ann Rheum Dis. 1973;32:10-15.
6 Peacock A. Observations on the pre-natal development of the intervertebral disc in man. J Anat. 1951;85:260-274.
7 Hickey DS, Hukins DWL. X-ray diffraction studies of the arrangement of collagen fibres in human fetal intervertebral disc. J Anat. 1980;131:81-90.
8 Hickey DS, Hukins DWL. Relation between the structure of the annulus fibrosus and the function and failure of the intervertebral disc. Spine. 1980;5:100-116.
9 Tsuji H, Hirano N, Ohshima H, et al. Structural variation of the anterior and posterior anulus fibrosus in the development of human lumbar intervertebral disc: a risk factor for intervertebral disc rupture. Spine. 1993;18:204-210.
10 Eyring EJ. The biochemistry and physiology of the intervertebral disk. Clin Orthop. 1969;67:16-28.
11 Roberts S, Menage J, Urban JP. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine. 1989;14:166-174.
12 Saunders JB, de CM, Inman VT. Pathology of the intervertebral disk. Arch Surg. 1940;40:380-416.
13 Coventry MB, Ghormley RK, Kernohan JW. The intervertebral disc: its microscopic anatomy and pathology. Part I. Anatomy, development and physiology. J Bone Joint Surg. 1945;27:105-112.
14 Inoue H. Three-dimensional architecture of lumbar intervertebral discs. Spine. 1981;6:138-146.
15 MacNab I. Backache. Baltimore: Williams & Wilkins; 1977. 4–7
16 Coventry MB. Anatomy of the intervertebral disk. Clin Orthop. 1969;67:9-15.
17 Schmorl G, Junghanns H. The Human Spine in Health and Disease, 2nd American edn. New York: Grune & Stratton; 1971. 18
18 Taylor JR. Growth of the human intervertebral discs and vertebral bodies. J Anat. 1975;120:49-68.
19 Maroudas A, Nachemson A, Stockwell R, et al. Some factors involved in the nutrition of the intervertebral disc. J Anat. 1975;120:113-130.
20 Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;58:255-315.
21 White A, Handler P, Smith EL. Principles of Biochemistry, 4th edn. New York: McGraw-Hill; 1968. 871–886
22 Bushell GR, Ghosh P, Taylor TKF, et al. Proteoglycan chemistry of the intervertebral disks. Clin Orthop. 1977;129:115-123.
23 McDevitt CA. Proteoglycans of the intervertebral disc. In: Ghosh P, editor. The Biology of the Intervertebral Disc, Vol. 1. Boca Raton: CRC Press; 1988:151-170. Ch. 6
24 Urban JP, Maroudas A. The chemistry of the intervertebral disc in relation to its physiological function. Clin Rheum Dis. 1980;6:51-76.
25 Johnstone B, Bayliss MT. The large proteoglycans of the human intervertebral disc. Spine. 1995;20:674-684.
26 Hardingham T, Bayliss M. Proteoglycans of articular cartilage: changes in aging and in joint disease. Semin Arthritis Rheum. 1990;20S:12-33.
27 Buckwalter JA, Pedrini-Mille A, Pedrini V, et al. Proteoglycans of human infant intervertebral discs. J Bone Joint Surg. 1985;67A:284-294.
28 Bailey AJ, Herbert CM, Jayson MIV. Collagen of the intervertebral disc. In: Jayson MIV, editor. The Lumbar Spine and Backache. New York: Grune & Stratton; 1976:327-340. Ch. 12
29 Herbert CM, Lindberg KA, Jayson MIV, et al. Changes in the collagen of human intervertebral discs during ageing and degenerative disc disease. J Mol Med. 1975;1:79-91.
30 Naylor A, Shental R. Biochemical aspects of intervertebral discs in ageing and disease. In: Jayson MIV, editor. The Lumbar Spine and Backache. New York: Grune & Stratton; 1976:317-326. Ch. 4
31 Eyre D. Collagens of the disc. In: Ghosh P, editor. The Biology of the Intervertebral Disc, Vol. I. Boca Raton: CRC Press; 1988:171-188.
32 Roberts S, Menage J, Duance V, et al. Collagen types around the cells of the intervertebral disc and cartilage end plate: an immunolocalization study. Spine. 1991;16:1030-1038.
33 Adams P, Eyre DR, Muir H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol Rehab. 1977;16:22-29.
34 Brickley-Parsons D, Glimcher MJ. Is the chemistry of collagen in intervertebral discs an expression of Wolff’s law? A study of the human lumbar spine. Spine. 1984;9:148-163.
35 Eyre D, Muir H. Type I and Type II collagen in intervertebral disk. Interchanging radial distribution in annulus fibrosus. Biochem J. 1976;157:267-270.
36 Eyre D, Muir H. Quantitative analysis of types I and II collagen in human intervertebral discs at various ages. Biochimica et Biophysica Acta. 1977;492:29-42.
37 Ghosh P, Bushell GK, Taylor TFK, et al. Collagen, elastin, and non-collagenous protein of the intervertebral disk. Clin Orthop. 1977;129:123-132.
38 Meachim G, Cornah MS. Fine structure of juvenile human nucleus pulposus. J Anat. 1970;107:337-350.
39 Pearson CH, Happey F, Naylor A, et al. Collagens and associated glycoproteins in the human intervertebral disc. Ann Rheum Dis. 1972;31:45-53.
40 Stevens FS, Jackson DS, Broady K. Protein of the human intervertebral disc. The association of collagen with a protein fraction having an unusual amino acid composition. Biochim Biophys Acta. 1968;160:435-446.
41 Johnstone B, Markopoulous M, Neame P, et al. Identification and characterization of glycanated and non-glycanated forms of biglycan and decorin in the human intervertebral disc. Biochem J. 1993;292:661-666.
42 Kuettner KE. Cartilage integrity and homeostasis. In: Klippel JH, Dieppe PA, editors. Rheumatology. St Louis: Mosby; 1994:7.6.1-7.6.16.
43 Kanemoto M, Hukuda S, Komiya Y, et al. Immunohistochemical study of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 in human intervertebral discs. Spine. 1996;21:1-8.
44 Melrose J, Ghosh P. The noncollagenous proteins of the intervertebral disc. In: Ghosh P, editor. The Biology of the Intervertebral Disc, Vol. I. Boca Raton: CRC Press; 1988:189-237. Ch. 8
45 Sedowfia KA, Tomlinson IW, Weiss JB, et al. Collagenolytic enzyme systems in human intervertebral disc. Spine. 1982;7:213-222.
46 Beard HK, Stevens RL. Biochemical changes in the intervertebral disc. In: Jayson MIV, editor. The Lumbar Spine and Backache. 2nd edn. London: Pitman; 1980:407-436.
47 Gower WE, Pedrini V. Age-related variation in protein polysaccharides from human nucleus pulposus, annulus fibrosus and costal cartilage. J Bone Joint Surg. 1969;51A:1154-1162.
48 Naylor A. Intervertebral disc prolapse and degeneration. The biochemical and biophysical approach. Spine. 1976;1:108-114.
49 Puschel J. Der Wassergehalt normaler und degenerierter Zwischenwirbelscheiben. Beitr path Anat. 1930;84:123-130.
50 Inoue H, Takeda T. Three-dimensional observation of collagen framework of lumbar intervertebral discs. Acta Orthop Scand. 1975;46:949-956.
51 Sylven B, Paulson S, Hirsch C, et al. Biophysical and physiological investigations on cartilage and other mesenchymal tissues. J Bone Joint Surg. 1951;33A:333-340.
52 Dickson IR, Happey F, Pearson CH, et al. Variations in the protein components of human intervertebral disk with age. Nature. 1967;215:52-53.
53 Taylor TKF, Little K. Intercellular matrix of the intervertebral disk in ageing and in prolapse. Nature. 1965;208:384-386.
54 Best BA, Guilak F, Setton LA, et al. Compressive mechanical properties of the human anulus fibrosus and their relationship to biochemical composition. Spine. 1994;19:212-221.
55 Buckwalter JA, Cooper RR, Maynard JA. Elastic fibers in human intervertebral disks. J Bone Joint Surg. 1976;58A:73-76.
56 Hickey DS, Hukins DWL. Collagen fibril diameters and elastic fibres in the annulus fibrosus of human fetal intervertebral disc. J Anat. 1981;133:351-357.
57 Hickey DS, Hukins DWL. Aging changes in the macromolecular organization of the intervertebral disc. An X-ray diffraction and electron microscopic study. Spine. 1982;7:234-242.
58 Johnson EF, Berryman H, Mitchell R, et al. Elastic fibres in the anulus fibrosus of the human lumbar intervertebral disc. A preliminary report. J Anat. 1985;143:57-63.
59 Johnson EF, Chetty K, Moore IM, et al. The distribution and arrangement of elastic fibres in the IVD of the adult human. J Anat. 1982;135:301-309.
60 Bayliss MT, Johnstone B, O’Brien JP. Proteoglycan synthesis in the human intervertebral disc: variation with age, region and pathology. Spine. 1988;13:972-981.
61 Holm S, Maroudas A, Urban JP, et al. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101-119.
62 Ohshima H, Urban JP. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine. 1992;17:1079-1082.
63 Markolf KL, Morris JM. The structural components of the intervertebral disc. J Bone Joint Surg. 1974;56A:675-687.
64 Hirsch C, Nachemson A. New observations on mechanical behaviour of lumbar discs. Acta Orthop Scand. 1954;23:254-283.
65 Roaf R. A study of the mechanics of spinal injuries. J Bone Joint Surg. 1960;42B:810-823.
66 Brown T, Hansen RJ, Yorra AJ. Some mechanical tests on the lumbosacral spine with particular reference to the intervertebral discs. J Bone Joint Surg. 1957;39A:1135-1164.
67 Shah JS. Structure, morphology and mechanics of the lumbar spine. In: Jayson MIV, editor. The Lumbar Spine and Backache. 2nd edn. London: Pitman; 1980:359-405. Ch. 13
68 Shah JS, Hampson WGJ, Jayson MIV. The distribution of surface strain in the cadaveric lumbar spine. J Bone Joint Surg. 1978;60B:246-251.
69 White AA, Panjabi MM. Clinical Biomechanics of the Spine. Philadelphia: Lippincott; 1978.
70 Nachemson A. The influence of spinal movements on the lumbar intradiscal pressure and on the tensile stresses in the annulus fibrosus. Acta Orthop Scand. 1963;33:183-207.
71 Hukins DWL. Disc structure and function. In: Ghosh P, editor. The Biology of the Intervertebral Disc, Vol. I. Boca Raton: CRC Press; 1988:1-37. Ch. 1
72 Broberg KB. On the mechanical behaviour of intervertebral discs. Spine. 1983;8:151-165.
73 Farfan HF, Gracovetsky S. The nature of instability. Spine. 1984;9:714-719.