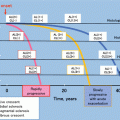
Recommendation
Long-term ACEI or ARB or ARB treatment when proteinuria is >1 g/day, with up-titration of the drug depending on blood pressure (1B)
Suggestions
Proteinuria
ACEI or ARB if proteinuria is between 0.5 and 1 g/day (in children, between 0.5 and 1 g/day per 1.73 m2) (2D)
ACEI or ARB be titrated upward as far as tolerated to achieve proteinuria <1 g/day (2C)
6-month course of corticosteroid therapy in patients with persistent proteinuria ≥1 g/day despite 3–6 months of optimized supportive care (including ACEI or ARB and blood pressure control) and GFR >50 ml/min per 1.73 m2 (2C)
Fish oil if persistent proteinuria ≥1 g/day despite 3–6 months of optimized supportive care (including ACEI or ARB and blood pressure control) (2D)
Blood pressure
Treatment goal of <130/80 mmHg in patients with proteinuria <1 g/day (not graded)
Treatment goal of <125/75 mmHg when initial proteinuria is >1 g/day (not graded)
Rapidly declining GFR
Supportive care for AKI in IgA nephropathy, with a kidney biopsy performed during an episode of macroscopic hematuria showing only ATN and intratubular erythrocyte casts (2C)
Steroids and cyclophosphamide in patients with IgA nephropathy and rapidly progressive crescentic IgA nephropathy, analogous to the treatment of ANCA vasculitis (2D)
Treatment as for MCD in nephrotic patients showing pathological findings of MCD with mesangial IgA deposits on kidney biopsy (2B)
Treatments not suggested
Immunosuppressive therapy in patients with GFR <30 ml/min per 1.73 m2 unless there is crescentic IgA nephropathy with rapidly deteriorating kidney function (2C)
Mycophenolate mofetil (2C)
Antiplatelet agents (2C)
Tonsillectomy (2C)
The rationale of the KDIGO guidelines is based on moderate-quality evidence to suggest that proteinuria more than 1 g/day is associated with an accelerated decline in kidney function, in a dose-dependent fashion and independent of other risk factors. The strongest evidence (“We recommend”) points to a proteinuria (in adults) cutoff of 1 g/day (Table 14.1), below which (and sustained) a favorable outcome is achieved. There is valid concern that an increased risk starts with proteinuria above 0.5 g/day (“We suggest”).
Noting the less controversial proteinuria cutoff of 1 g/day in adults, the KDIGO guidelines suggest additional treatment when the proteinuria is persistently more than 1 g/day despite 3–6 months of optimized supportive care (including ACEI or angiotensin II receptor blocker and blood pressure control). Choices (“we suggest”) include fish oil, a 6-month course of corticosteroid therapy, or both. The guidelines do not address how to choose between fish oil and corticosteroid, but the corticosteroid therapy should be restricted to patients with glomerular filtration rate of more than 50 ml/min per 1.73 m2 of body surface area according to the KDIGO guidelines (Table 14.1). Furthermore, the preferred dosage regimen for corticosteroids (combined pulse and oral steroids versus purely oral regimen) cannot be commented. The guidelines also draw attention to the potential of more side effects with high-dose pulse corticosteroids, as reported in non-IgA nephropathy patients [1].
It is important to take precaution and keep in mind that the KDIGO guidelines include mostly Level 2 evidence (“We suggest”) and much less Level 1 recommendation (“We recommend”). This is partly because of the considerable lack of randomized controlled trials, among which patient numbers seldom exceed 200. Another important purpose of the KDIGO guidelines is the opportunity to prioritize areas in need of research. For example, there is no randomized controlled trial of treatment in crescentic IgA nephropathy.
14.3 Published Commentaries of KDIGO Guidelines
Since the publication of KDIGO guidelines, a few reviews had been published on the topic of IgA nephropathy [6, 7] and evaluation of the guidelines [8]. In particular, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) [9] and the Canadian Society of Nephrology [10] made specific comments on the chapter of IgA nephropathy.
Much insight can be gleaned from the discussion from these two documents [9, 10]. Their focuses were summarized in Table 14.2. A few points deserve mentioning. First, both commentaries highlighted the corticosteroid treatment threshold for IgA nephropathy: trial of corticosteroids should only be considered in patients with preserved renal function or glomerular filtration rate above 50 ml/min per 1.73 m2. Admittedly, this is reasonable to draw such conclusion based on the inclusion criteria and subject profiles of the three major trials of corticosteroids [11–13]. Nonetheless, concern about corticosteroid side effects explains the need to emphasize the treatment criteria. The Canadian commentary advises the consideration of corticosteroids at the level of the individual patients, taking into account of their relative contraindications to steroid therapy [10]. And, as highlighted by the US commentary [9], a meta-analysis of corticosteroid treatment in IgA nephropathy suggested an increased efficacy with shorter-term high-dose therapy compared to longer-term low-dose therapy [14]. Second, the controversy of using mycophenolate mofetil can be demonstrated by the different views from the two commentaries. The US commentary generally agreed with the recommendation not to use mycophenolate mofetil in patients with IgA nephropathy, but hinted on the racial differences in the response to mycophenolate mofetil [9]. The benefit of mycophenolate mofetil in Asian patients, as shown in the reduction of proteinuria, a decrease in rate of decline in glomerular filtration rate and end-stage renal disease risk after 6 years of follow-up [15, 16], was acknowledged in the commentary. On the other hand, the Canadian commentary argued against the use of mycophenolate mofetil and reminded us the potential toxicity (including severe pneumonia such as Pneumocystis pneumonia) as observed in observational studies, in line with what were recommended by the KDIGO guidelines.
Table 14.2
Published comments on KDIGO clinical practice guideline concerning IgA nephropathy
|
Commentary source
|
Critiques, remarks, and rationale
|
Implication and suggestion
|
|---|---|---|
|
National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) US commentary [9]
|
No trials showing ACEI or ARB decrease the risk for ESRD from IgA nephropathy
|
No need to decrease proteinuria to <0.5 g/day/1.73 m2 in children
|
|
No objective evidence for superiority of proteinuria goal <0.5 g/day per 1.73 m2 to <1.0 g/day per 1.73 m2 in children
|
Target blood pressure goals in children based on gender and age norms outlined by the National Institutes of Health (NIH) Task Force on Blood Pressure Control in Children, aiming for blood pressure below the 95th percentile
|
|
|
Agree with the additional benefit of corticosteroids
|
||
|
Agree with not treating with cyclophosphamide or azathioprine
|
Trial of corticosteroids restricted to patients with preserved renal function (concept about “point of no return”)
|
|
|
Generally agree with not using mycophenolate mofetil, but there may be some benefit in patients of Asian ancestry
|
Reasonable to use mycophenolate mofetil as an alternative agent if corticosteroids fail or are poorly tolerated in patients of Asian ancestry
|
|
|
Conflicting evidence on efficacy of fish oil supplements but there is little risk
|
Suggest future research comparing racial differences in response to immunosuppressive agents
|
|
|
Low-quality evidence to the benefit of antiplatelet therapy (and expected low adherence to the three-times-daily regimen with dipyridamole)
|
Suggest using fish oil as stated Suggest not using antiplatelet agents
|
|
|
No benefit of tonsillectomy alone
|
Suggest tonsillectomy should not be routinely performed, but there may be benefit when tonsillectomy is combined with corticosteroids (and with recurrent bouts of tonsillitis and macroscopic hematuria)
|
|
|
Observational studies support the use of steroids plus cyclophosphamide for crescentic IgA nephropathy
|
Suggest the use of steroids and cyclophosphamide in rapidly progressive crescentic IgA nephropathy (as well as those with rapid deterioration in GFR without ATN and with crescents approaching 50 % of glomeruli on biopsy)
|
|
|
Sampling error affects renal biopsy interpretation
|
||
|
Canadian Society of Nephrology commentary [10]
|
Lack of RCT evidence on blood pressure targets: opinion-based target of 130/80 versus 125/75 mmHg
|
A full 6-month trial of corticosteroids is not required prior to determination if the patient is likely to “respond” and benefit from corticosteroids
|
|
Benefits of corticosteroids should be judged at individual patient level and relative contraindications
|
Suggest evaluation of histologic pattern (that predicts patient response to corticosteroids)
|
|
|
No comment on recurrent proteinuria after stopping corticosteroid therapy (and adjunctive role of azathioprine)
|
Suggest 3 g/day purified polyunsaturated fatty acid (as lowest effective dose)
|
|
|
Challenge for individual patients to meet and maintain the fish oil formulation target
|
14.4 New Literature After Publication of KDIGO Guidelines
Do we have new data on managing IgA nephropathy after the publication of KDIGO guidelines? A few clinical trials and updated publications are available.
The 2014 blood pressure guidelines from the Eighth Joint National Committee (JNC 8) deserve discussion. One of their recommendations (with a weak expert opinion graded E) advises blood pressure goal of 140/90 mmHg in patients aged 18 years or older with chronic kidney disease [17]. This appears to deviate from the more stringent blood pressure goal listed in KDIGO guidelines on managing IgA nephropathy (Table 14.1). However, it should be emphasized that, in clinical practice, the up-titration of ACEI or angiotensin II receptor blocker in patients with IgA nephropathy is more often driven by the proteinuria reduction need than the blood pressure lowering need. The pertinent question, in other words, is whether a stringent blood pressure might confer risk in patients with IgA nephropathy.
New clinical trial is also available on management strategies of early IgA nephropathy. The KDIGO guidelines did not address the treatment of IgA nephropathy patients with a proteinuria lower than 0.5 g/day, although these patients are often not treated (and are monitored periodically). The best data now come from a randomized trial of 60 Chinese patients with IgA nephropathy, protein excretion less than 0.5 g/day (mean 0.11 g/day), normal blood pressure, and renal function [18]. They were randomized to ramipril 2.5 mg daily or no treatment for 5 years. No treatment benefit was reported, in support of a watchful waiting strategy in this group of early IgA nephropathy patients [18].
Some patients have proteinuria at least 0.75 g/day after renin-angiotensin system blockade. The Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy (STOP-IgAN) trial [19] randomized these patients to supportive care (including a target blood pressure of 125/75 mmHg) and to immunosuppression. The addition of glucocorticoid (with or without cyclophosphamide and azathioprine) did not significantly improve the renal outcomes [19]. There are subsets of patients who cannot achieve urinary protein excretion below 1 g/day despite proper supportive therapy. One approach is focused on further drug treatment aiming at additional antiproteinuric strategies. The results of two open-label trials of oral vitamin D calcitriol have now provided signals of benefit in addition to best supportive care (including renin-angiotensin-aldosterone system blockade) [20, 21].
Another approach, particularly in Japan, is to target tonsils as a source of abnormal IgA that forms immune complexes and deposits in the glomeruli. The KDIGO guidelines explicitly advise against the use of tonsillectomy (Table 14.1). Although randomized trials are lacking, a recent multicenter randomized trial recruited 80 Japanese patients, who were randomized to receive tonsillectomy with steroid pulses or steroid pulses alone. They showed nonsignificantly higher remission rates, defined as proteinuria reduction to below 0.3 g/g creatinine and hematuria to less than five cells per high-power field, in the tonsillectomy plus steroid pulse group [22]. This study was limited by the small sample size, limited statistical power, short duration of follow-up (12 months), patient crossover to alternative treatment group, addition of ACEI or angiotensin II receptor blocker during follow-up course, and patient loss (nearly 20 %) [23]. Given the invasive nature of surgery, an accompanied commentary of the study concluded that data do not support the contention that tonsillectomy should be used in the absence of clear otorhinolaryngological indication [23]. In a recent meta-analysis of tonsillectomy for IgA nephropathy, it is also suggested that data should be interpreted with caution, apart from its limited generalizability (when most studies involved Japanese population) [24].
14.5 Conclusion
Before the availability of new approaches to IgA nephropathy, the current KDIGO guidelines provide the highest-quality evidence for managing patients with primary IgA nephropathy. Additional information on management of recurrent IgA nephropathy (after renal transplantation) and relevance of the guidelines to the recent Oxford Classification of IgA nephropathy [25] should be sought.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
1.
KDIGO clinical practice guidelines for glomerulonephritis – chapter 10: immunoglobulin A nephropathy. Kidney Int Suppl. 2012;2:S209–17
2.
3.
KDIGO clinical practice guidelines for glomerulonephritis – chapter 2: general principles in the management of glomerular disease. Kidney Int Suppl. 2012;2:S156–62
4.
5.
AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23.CrossRef
7.
8.
9.
10.
11.
12.
13.
14.
Lv J, Xu D, Perkovic V, Ma X, Johnson DW, Woodward M, et al. Corticosteroid therapy in IgA nephropathy. J Am Soc Nephrol. 2012;23:1108–16.CrossRefPubMedPubMedCentral
15.
16.
17.
18.
19.
20.
21.
22.
Kawamura T, Yoshimura M, Miyazaki Y, Okamoto H, Kimura K, Hirano K, et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol Dial Transplant. 2014;29:1546–53.CrossRefPubMedPubMedCentral
23.
24.
25.
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–45.CrossRef