The immunosuppressed patient
An increased susceptibility to infection can arise from multiple factors (Table 43.1). Neutropenia and neutrophil dysfunction are probably the most important causes of infectious complications in patients with leukaemia. Unlike many other forms of immunosuppression, neutropenia is easy to quantify – the risk of infection rises appreciably at counts below 0.5 × 109/L and is greatest where the count is below 0.1. Lymphopenia and lymphocyte dysfunction are seen in lymphoid malignancy and after chemo- and radiotherapy. Defects in humoral immunity are particularly seen in patients with chronic lymphoid malignancies and in myeloma. The likelihood of infection is related to the severity of hypogammaglobulinaemia.
Table 43.1
Possible factors predisposing to infection in haematology patients
Cellular defects
Humoral defects
Anatomic defects
Splenectomy (see p. 10)
Types of infection
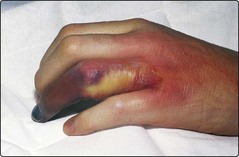
Bacterial infection in neutropenic patients may be overt – for instance a chest infection with a productive cough or the presence of infected skin lesions (Fig 43.1). However, bacterial sepsis can equally present with non-specific malaise and a pyrexia. In the latter case extensive cultures including blood, nose, throat, stool and urine are indicated.
Fungi
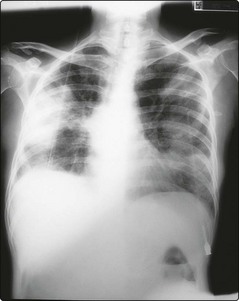
Aspergillus species, particularly Aspergillus fumigatus, are potentially deadly fungal pathogens. Infection is usually via the inhalation of airborne spores and is mainly pulmonary. A chest X-ray may show pneumonia and cavitation (Fig 43.2) but it is an insensitive diagnostic method. Other infected sites can include the paranasal sinuses, skin, central nervous system and eye. Even in disseminated disease, blood and sputum cultures are rarely positive. Thirty per cent of cases of invasive aspergillosis remain undiagnosed and untreated at death. Strategies for earlier diagnosis include regular galactomannan antigen testing, aspergillus PCR, and CT scanning of the chest.
Viruses
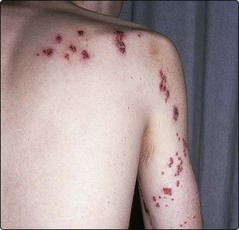
Most viral infection in immunosuppressed patients is caused by reactivation of latent organisms. Patients with deficient cell-mediated immunity (e.g. acute lymphoblastic leukaemia (ALL), stem cell transplantation, chronic lymphocytic leukaemia) are particularly susceptible. Important pathogens include herpes simplex, varicella zoster and cytomegalovirus (CMV). Clinical manifestations range from relatively trivial mouth ulcers attributable to herpes simplex through herpes zoster (shingles) (Fig 43.3) with the risk of dissemination to the potentially fatal CMV pneumonitis which complicates allogeneic stem cell transplantation. Measles can be a fatal illness in children with ALL. There may be no specific diagnostic features of viral infection and it must be considered as a possible cause of a febrile illness in the immunosuppressed patient. PCR-based diagnosis may allow earlier therapy of CMV infection after allogeneic stem cell transplantation.
Prevention of infection in the immunosuppressed patient
Treatment of infection
The pyrexial neutropenic patient
The empirical antibiotic regimens are designed to provide protection against commonly implicated organisms, particularly those causing life-threatening infection (e.g. Pseudomonas). Regimens are constantly changing – the major groups of drugs are summarised in Table 43.2.
Table 43.2
Groups of antibiotics used in the empirical treatment of infection in neutropenia
| Group | Examples |
| Antipseudomonal penicillins | Azlocillin, piperacillin |
| Aminoglycosides | Gentamicin, amikacin |
| Cephalosporins | Ceftazidime |
| Quinolones | Ciprofloxacin |
| Carbapenems | Meropenem, imipenem |
| Glycopeptides | Teicoplanin, vancomycin |