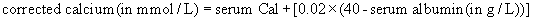Chapter 42 The immunosuppressed patient
Patients with immunosuppression present with emergencies related to their increased susceptibility to infection and to their underlying condition.
Failure of a component of the immune system leads to vulnerability to different infective agents.
B cell defects occur in haematological malignancies, myeloma, AIDS and congenital disorders such as common variable immune deficiency. Patients are predisposed to infection by encapsulated bacteria (Streptococcus pneumoniae, Haemophilus influenzae, Neisseria), staphylococci, giardia and enterovirus.
CANCER PATIENTS
Febrile neutropenia
Associated signs of infection may be present such as tachypnoea, tachycardia, altered mental state, dehydration and acidosis. Localising signs may be absent; however, a thorough examination of the lungs, oropharynx, skin, catheters, perineum and perianal area, sinuses and urinary tract is essential and may reveal the site of infection. Non-specific symptoms in the absence of fever may still indicate infection, especially if the patient is on high-dose corticosteroids.
Management
Antifungals
Antifungals are not routinely used in the initial treatment of febrile neutropenia, unless there is evidence of fungal infection such as sinusitis. They may be introduced when fever persists beyond 96 hours, especially in patients with pulmonary infiltrates.
NONINFECTIOUS COMPLICATIONS OF CANCER AND ITS TREATMENT
Spinal cord compression
Hypercalcaemia
Increased osteolysis releases calcium and phosphate into the circulation. This can be caused by primary (myeloma or leukaemia) or metastatic bone lesions (commonly breast or lung) or by the action of paraneoplastic hormone-like substances.
Hypokalaemia is common. The patient may be significantly dehydrated and have renal impairment.
Tumour lysis syndrome
Management
HIV INFECTION
Patients with HIV infection most commonly present with complications of immunosuppression and its treatment. With the improved prognosis of HIV in the era of highly active antiretroviral treatment (HAART), patients increasingly present with medical or surgical conditions unrelated to HIV and, while their treatment may be complicated by their HIV, their outcome and survival may be excellent.
Primary HIV infection
Pulmonary infections
PCP (Pneumocystis jiroveci (carinii) pneumonia)
Investigations
Arterial blood gas analysis assists in grading severity and planning treatment. Mild to moderate cases have a PaO2 > 70 mmHg on room air (A-a gradient < 35 mmHg, or O2 saturations > 94% on room air), whereas severe cases have a PaO2 < 70 mmHg on room air (A-a gradient > 35 mmHg, or O2 saturations < 94% on room air).
Management
Tuberculosis (TB)
Investigations and diagnosis
The chest X-ray shows upper lobe cavitation or hilar adenopathy with diffuse infiltrates. Pleural effusions may be present. Miliary TB is uncommon in patients with poor cell-mediated immunity. Extrapulmonary manifestations or presentations are more common in patients with HIV infection.
Central nervous system (CNS) infections
Toxoplasma gondii
Presenting features are altered mental state, focal signs, headache, fever and seizures.
Investigations
The diagnosis is made by contrast CT, which shows ring-enhancing lesions.
Toxoplasma serology is supportive, and cerebral toxoplasmosis is very unlikely in a patient with negative serology. In this case the diagnosis is likely to be primary CNS lymphoma.
Gastrointestinal tract (GIT) infections
Candidiasis
Systemic infections
Cytomegalovirus (CMV)
Opportunistic CMV infection affects patients with CD4+ counts below 50 cells/μL. CMV retinitis presents with floaters, decreasing acuity and field loss. Fundoscopy reveals peripheral flame haemorrhages and exudates. However, the retinal lesions are often very peripheral and so can be missed. Dilation of the pupil and ophthalmological review are required to exclude diagnosis. Any part of the GIT may be involved, with colitis, oesophagitis and gastritis being common presentations. CNS involvement causes encephalopathy and a polyradiculopathy. Interstitial pneumonitis is rare in HIV, in contrast to solid organ transplant recipients. CMV adrenalitis presents with symptomatic adrenal insufficiency, hyponatraemia or hyperkalaemia.
MALIGNANCY
Cervical cancer remains a common problem in HIV-infected women, with no impact on the disease from antiretrovirals. Treatment is as for the immunocompetent patient. Human papilloma virus (HPV) infection may also cause premalignant and malignant changes in the rectum of HIV-infected men who have sex with men (MSM).
Non-AIDS-defining malignancies are becoming more common with increasing longevity.
ANTIRETROVIRAL DRUGS
The introduction of HAART, combination therapy with three or four antiretroviral drugs, has led to improvements in morbidity and mortality, decreased transmission and increased quality of life. Patients often have restoration of their immune function and a fall in viral load to undetectable levels. The main classes of antiretrovirals are nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors and protease inhibitors. More recently two types of entry inhibitors (fusion inhibitors and chemokine (C-C motif) receptor 5 (CCR5)-blockers) have been licensed. In addition the first drug in a potent new class of antiretrovirals, the integrase inhibitors, has been licensed for use. Specific inhibitors include:
Drug toxicities
Hypersensitivity reactions
Abacavir is associated with a life-threatening hypersensitivity syndrome, usually beginning in the first 10–14 days of treatment, with rash, fever, nausea, vomiting and abdominal pain. Patients may develop interstitial pneumonitis, hypotension and respiratory failure. The cause of this is now known to be strongly linked to the carriage of HLA-B5701. All patients being considered for abacavir therapy should be screened for the carriage of this HLA-allele prior to commencement.
Drug interactions
Many drug interactions occur due to inhibition of induction of the hepatic cytochrome P450 system.
POST-EXPOSURE PROPHYLAXIS
In non-occupational exposures, the HIV status of the source is often unknown. Assessment of the risk of HIV transmission requires knowledge of the risk of transmission from the method of exposure and the risk of the source being HIV positive. High-risk exposures include receptive anal intercourse (1/120), sharing contaminated injecting equipment (1/50), occupational needle stick (1/333), receptive vaginal intercourse (1/1000) and insertive anal or vaginal intercourse (1/1000). Although case reports of transmission exist, the risk of transmission by oral intercourse, bites, exposure to intact mucous membranes or skin and community-acquired needle stick is so low it is not measurable.
SOLID ORGAN TRANSPLANTS
The immunosuppression required for the survival of transplanted organs leaves the recipient prone to infections, which are a leading cause of mortality. Infections are caused by a broad spectrum of pathogens and there may be minimal signs and symptoms at presentation, followed by rapid deterioration. Noninfectious causes of fever (rejection, malignancy and drugs) are common in the transplant population.
The unwell transplant patient may present with non-specific symptoms or fever alone.
Intermediate period infections
Pneumonia may be caused by a wide range of pathogens. Most cases are bacterial, but Pneumocystis, Legionella, Aspergillus, Nocardia and viruses also occur. Reactivation of latent tuberculosis can occur, often leading to disseminated disease.
The gastrointestinal tract may be infected with CMV or Clostridium difficile.
It is important to note that graft rejection also presents with fever, as do some drug reactions.
Graft-specific problems
ASPLENIA
Pneumococcal vaccine is routinely given; however response may be diminished in patients with severe underlying disease. Vaccinations for Haemophilus influenzae type B, influenza and meningococcus are also given, and patients take penicillin prophylaxis for 2–5 years.
Abbas A.K. Diseases of immunity. In Kumar K., Abbas A.K., Fausto N., editors: Robin’s & Cotran: Pathologic basis of disease, 7th edn, Elsevier, 2005.
Azoulay E., et al. Improved survival in cancer patients requiring mechanical ventilatory support: impact of noninvasive mechanical ventilatory support. Crit Care Med. 2001;29:519-525.
Bower M., Palmieri C., Dhillon T. AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Curr Opin Infect Dis. 2006;19:14-19.
Burns M.J., Langdorf M.I.. The immunocompromised patient. Mark Rosen’s Emergency medicine: concepts and clinical practice, 6th edn. Mosby, 2006.
Davis J.L., Fei M., Huang L. Respiratory infection complicating HIV infection. Curr Op Infect Dis. 2008;21:184-190.
Doar E.S., Pilcher C.D., Hecht F.M. Clinical presentation and diagnosis of primary HIV-1 infection. Curr Opin in HIV and AIDS. 2008;3:10-15.
Fidler S., et al. Primary HIV infection: to treat or not to treat? Curr Opin Infect Dis. 2008;21:4-10.
Fishman J.A. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601-2614.
Hilbert G., et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever and acute respiratory failure. N Engl J Med. 2001;344:481-487.
Huang L., et al. An official ATS Workshop summary: recent advances and future directions in Pneumocystis pneumonia (PCP). Proc Am Thorac Soc. 2006;3:655-664.
Kalkut G. Antiretroviral therapy: an update for the non-AIDS specialist. Curr Opin Oncol. 2005;17:479-484.
Keady M.T., Lowery D.W.. The solid-organ transplant patient. Mark Rosen’s Emergency medicine: concepts and clinical practice, 6th edn. Mosby, 2006.
Larche J., et al. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 2003;29:1688-1695.
Lipman M., Breen R. Immune reconstitution inflammatory syndrome in HIV. Curr Opin Infect Dis. 2006;19:20-25.
Morris A., Masur H., Huang L. Current issues in critical care of the human immunodeficiency virus-infected patient. Crit Care Med. 2006;34:42-49.
Nkwuo N., Schamban N., Borenstein M.. Selected oncologic emergencies. Mark Rosen’s Emergency medicine: concepts and clinical practice, 6th edn. Mosby, 2006.
NPEP Reference Group. Australasian Society for HIV Medicine. National guidelines for post-exposure prophylaxis after non-occupational exposure to HIV. Sexual Health. 2007;4:277-283.
Roland M.E. Postexposure prophylaxis after sexual exposure to HIV. Curr Opin Infec Dis. 2007;20:39-46.
Scott D.L., Kingsley G.H. Tumor necrosis factor inhibitors for rheumatoid arthritis. New Engl J Med. 2006;355:704-712.
Therapeutic Guidelines Ltd. Therapeutic guideline: antibiotics. Version 13.
Triant V.A., Grinspoon S.K. Vascular dysfunction and cardiovascular risk. Curr Op in HIV and AIDS. 2007;2:299-304.