Chapter 30
The Hypothalamus
Boundaries of the Hypothalamus
Blood Supply of the Hypothalamus
Intrinsic Hypothalamic Connections
One of the most rostral cell groups to influence visceral function, and the one that has direct input to all other visceral nuclei in the neuraxis, is the hypothalamus. In addition to its role in regulating visceromotor functions, the hypothalamus, through a variety of circuits, influences circadian rhythms, neurohormones, reproductive functions, general homeostasis, and behavior.
OVERVIEW
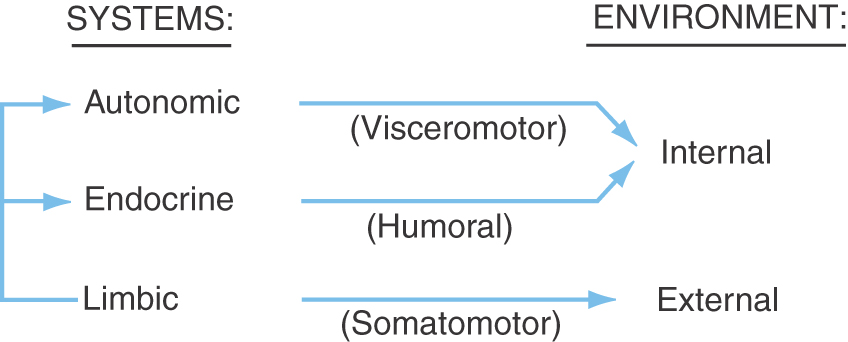
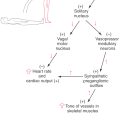
The hypothalamus is the part of the diencephalon involved in the central control of visceral functions (through the visceromotor and endocrine systems) and affective or emotional behavior (via the limbic system) (Fig. 30-1). Although its primary role is in the maintenance of homeostasis, the hypothalamus partially regulates numerous functions, including water and electrolyte balance, food intake, temperature, blood pressure, possibly the sleep-waking mechanism, circadian rhythmicity, and general body metabolism. The hypothalamus (at about 4 g) is dwarfed in size by the rest of the brain (weighing approximately 1300 to 1400 g). However, it is perhaps the most important 4 g in the entire body. In short, the hypothalamus influences our responses to both the internal and external environments (Fig. 30-1) and is necessary for life.
BOUNDARIES OF THE HYPOTHALAMUS
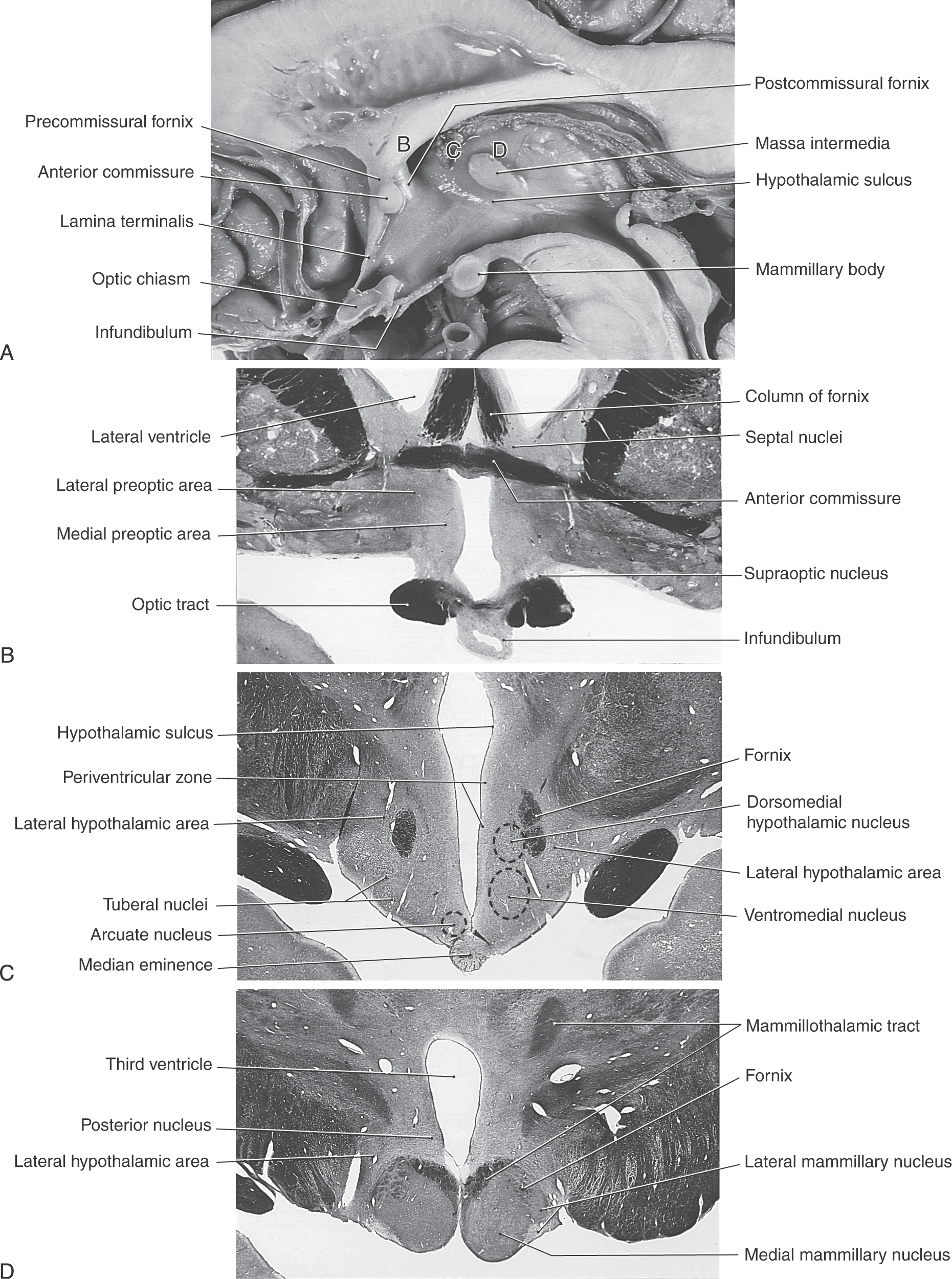
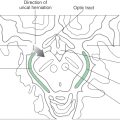
The rostral boundary of the hypothalamus is the lamina terminalis, a thin membrane that extends ventrally from the anterior commissure to the rostral edge of the optic chiasm and represents the anterior boundary of the third ventricle (Fig. 30-2A). The lamina terminalis separates the hypothalamus from the more rostrally located septal nuclei. Superiorly, the hypothalamus is bounded by the hypothalamic sulcus, a shallow groove that separates the hypothalamus from the dorsal thalamus (Fig. 30-2A, C). The lateral boundary of the hypothalamus is formed rostrally by the substantia innominata and caudally by the medial edge of the posterior limb of the internal capsule (Fig. 30-2B, C; see also Fig. 15-7). Medially, the hypothalamus is bordered by the inferior portion of the third ventricle. Caudally, the hypothalamus is not sharply demarcated, merging instead into the midbrain tegmentum and the periaqueductal gray. Externally, the boundary between the hypothalamus and the midbrain is represented by the caudal edge of the mammillary body. This is an especially good landmark to use when viewing a sagittal magnetic resonance image in the diagnosis of hypothalamic lesions.
HYPOTHALAMUS AND PITUITARY
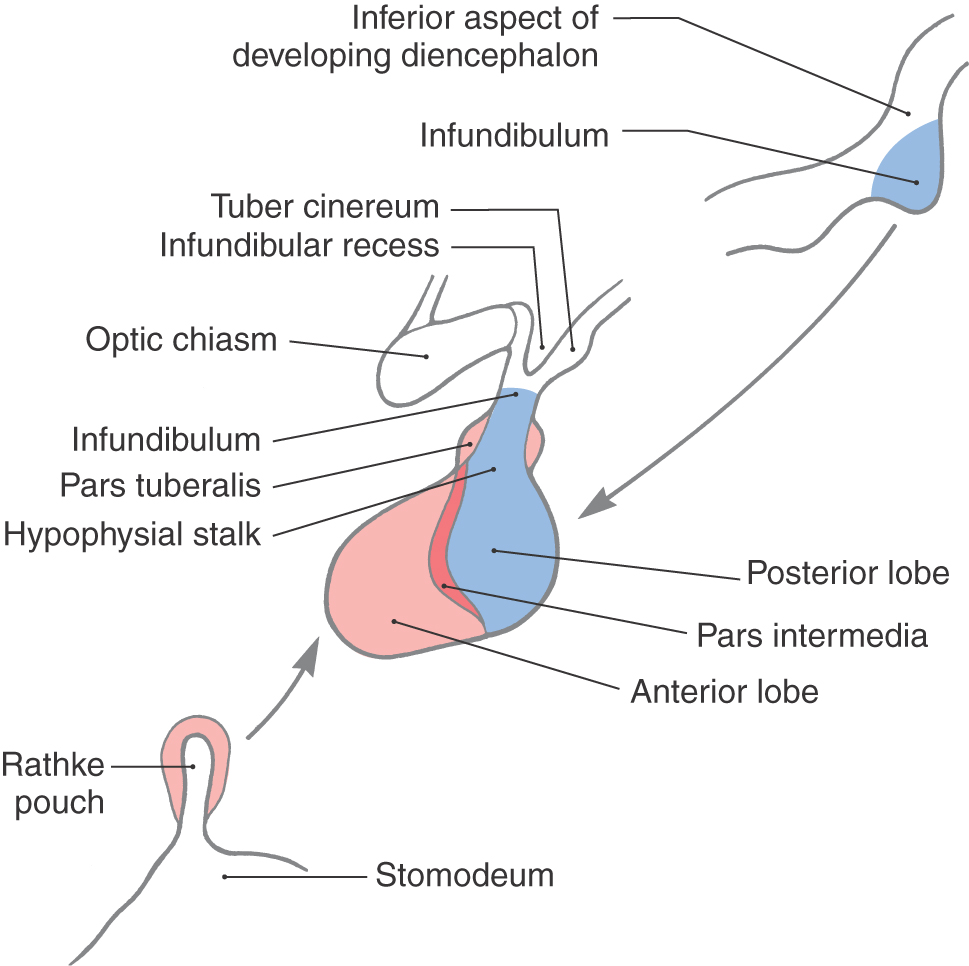
Inferiorly, the hypothalamus is continuous with the pituitary gland (located in the sella turcica and covered by the diaphragma sellae) by way of the infundibulum and the hypophysial stalk (Fig. 30-3). The infundibulum is located immediately caudal to the optic chiasm, is somewhat funnel shaped (hence its name), and contains a small portion of the third ventricle, the infundibular recess. The infundibulum continues into the pituitary by a stalk of tissue that is sometimes called the hypophysial stalk. This stalk passes through an opening in the diaphragma sellae.
The pituitary originates from two sources and directions. The posterior lobe (pars nervosa) arises as an outpocketing of the inferior surface of the developing diencephalon (Fig. 30-3). The anterior lobe (adenohypophysis) arises as an infolding of the ectodermal lining of the roof of the developing oral cavity (the stomodeum) and is commonly referred to as the Rathke pouch (Fig. 30-3). The smaller portions of the pituitary, the tuberal part (pars tuberalis) and the intermediate part (pars intermedia), also originate in association with the anterior lobe. As development progresses, these separate structures join to form the pituitary of the adult (Fig. 30-3).
Although the pituitary is well protected in the sella turcica, it is, at the same time, subject to a variety of potential insults (tumor, vascular, surgical) in this confined location. In addition, the extension of the hypophysial stalk and infundibulum through the diaphragma sellae is a vulnerable relationship. For example, trauma to the head may result in a shearing of the stalk and the eventual development of diabetes insipidus.
DIVISIONS OF THE HYPOTHALAMUS
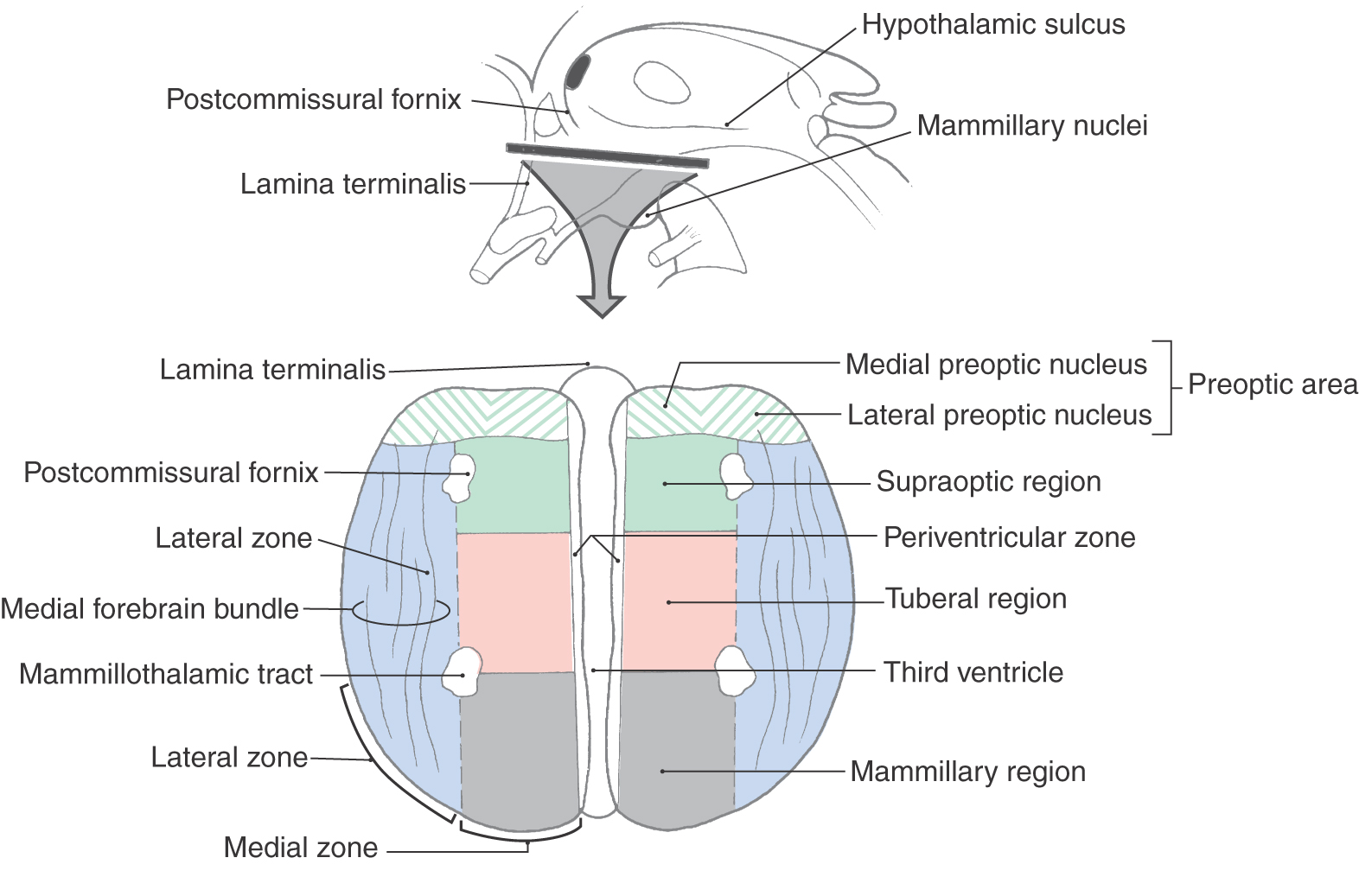
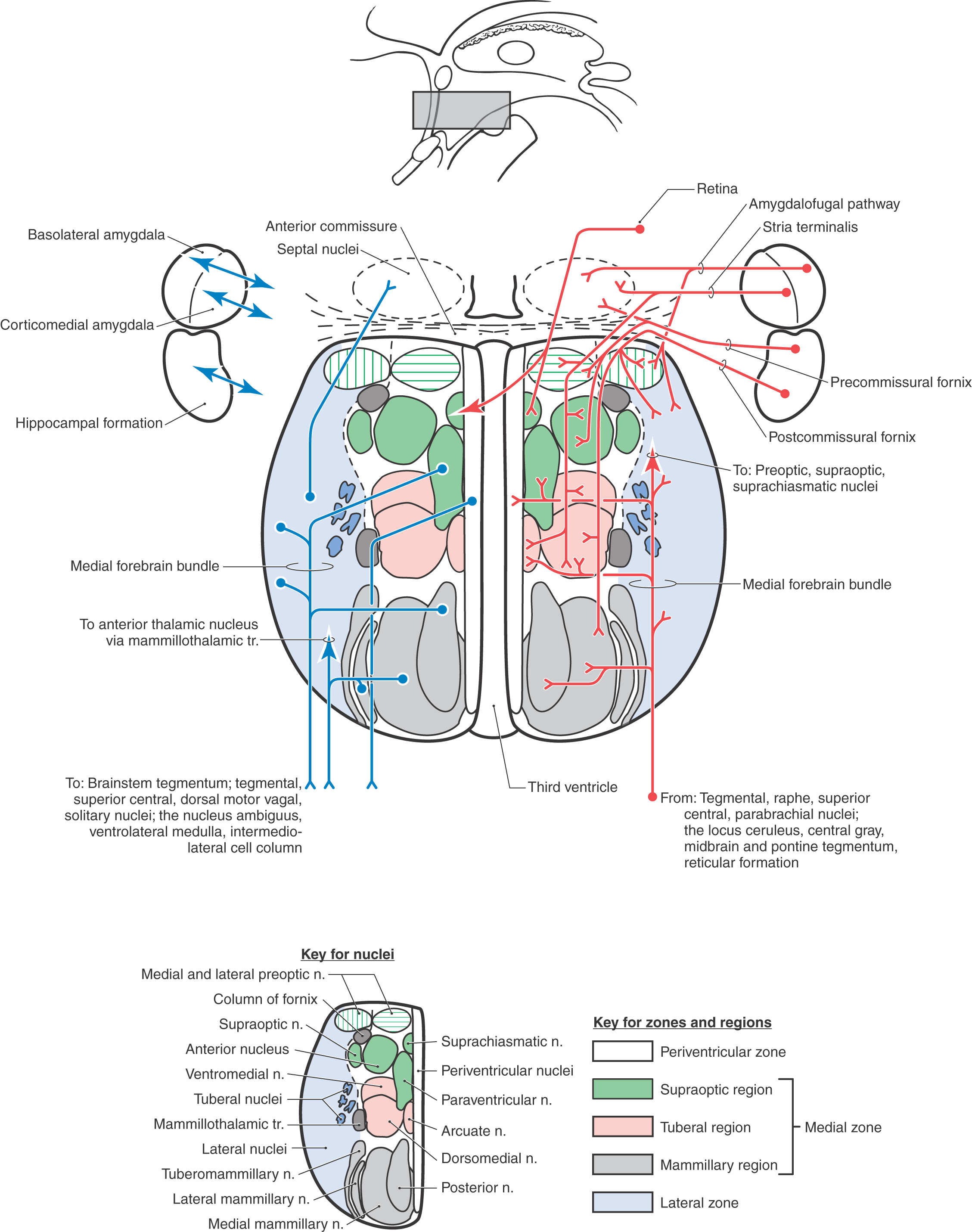
The hypothalamus can be divided into the preoptic area and the lateral, medial, and periventricular zones (Fig. 30-4). The preoptic area is a transition region that extends rostrally, by passing laterally to the lamina terminalis, to form a continuation with structures in the basal forebrain. Three zones are located caudal to the preoptic area. The thin periventricular zone is the most medial and is subjacent to the ependymal cells that line the third ventricle. The medial zone is located lateral to the periventricular zone, and a line drawn from the postcommissural fornix to the mammillothalamic tract separates it from the lateral zone (Fig. 30-4).
Figure 30-4. Diagrammatic representation of the hypothalamus in the axial (horizontal) plane showing the zones and regions.
Preoptic Area
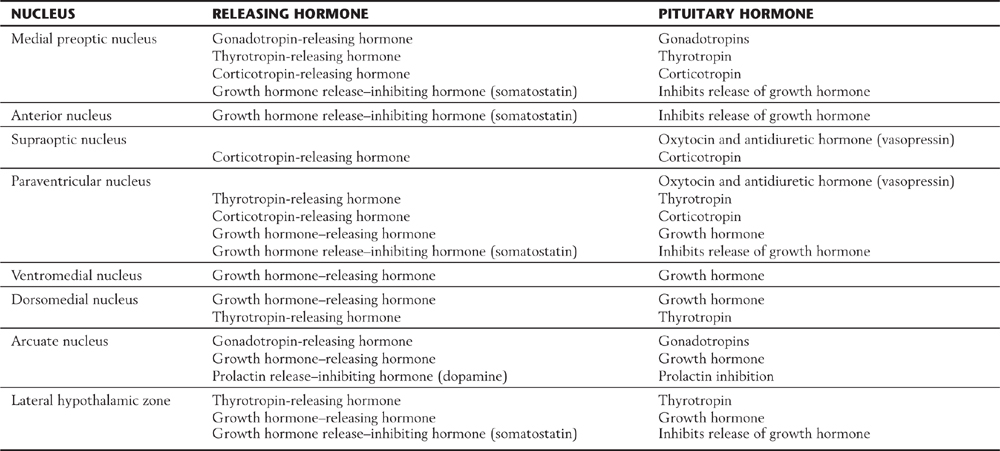
The preoptic area, although functionally a part of the hypothalamus (and diencephalon), is embryologically derived from the telencephalon. This area is composed primarily of the medial and lateral preoptic nuclei (Fig. 30-4). The medial preoptic nucleus contains neurons that manufacture gonadotropin-releasing hormone (GnRH). GnRH is transported along the tuberoinfundibular tract to capillaries of the hypophysial portal system and thence to the anterior lobe of the pituitary gland (Fig. 30-5; see Table 30-2), where it causes the release of gonadotropins (luteinizing hormone and follicle-stimulating hormone). Because gonadotropin release is continuous in males and cyclic in females, the medial preoptic nucleus of males tends to be more active and consequently larger than that of females. Accordingly, the medial preoptic nucleus is often referred to as the sexually dimorphic nucleus of the preoptic area. The medial preoptic nucleus also influences behaviors that are related to eating, reproductive activities, and locomotion. The lateral preoptic nucleus is located immediately rostral to the lateral hypothalamic zone (Fig. 30-4). The function of this nucleus is not fully established. However, through its connections with the ventral pallidum, it may function in part in locomotor regulation. Some investigators consider the nuclei of the preoptic area to be part of the supraoptic region of the medial hypothalamic zone.
Lateral Zone
The lateral zone (Fig. 30-4) contains a large bundle of axons collectively called the medial forebrain bundle (Fig. 30-4; see also Fig. 30-9). This diffuse bundle of fibers traverses the lateral hypothalamic zone and interconnects the hypothalamus with rostral areas, such as the septal nuclei, and with caudal regions, such as the brainstem reticular formation.
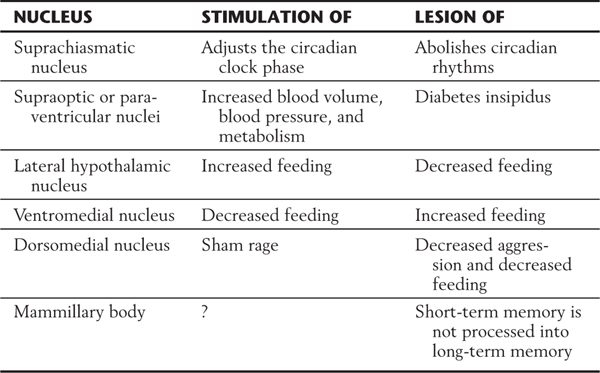
The lateral hypothalamic zone comprises a large, diffuse population of neurons commonly called the lateral hypothalamic area as well as smaller condensations of cells located in its anterior (ventral) portions. The latter cell groups are the lateral hypothalamic nucleus and the tuberal nuclei. The lateral hypothalamic nucleus is a loose aggregation of relatively large cells that extends throughout the rostrocaudal extent of the lateral hypothalamic zone. This nucleus constitutes a “feeding center.” Stimulation of this nucleus in laboratory animals promotes feeding behavior; destruction of it causes feeding behavior to attenuate, and the animal loses weight (Table 30-1). The tuberal nuclei consist of small clusters of neurons, each containing small, pale, multipolar cells. Some tuberal neurons project into the tuberoinfundibular tract and therefore may convey releasing hormones to the hypophysial portal system. Others send a histaminergic input to the cerebellum that may be involved in the regulation of motor activity.
Table 30-1 Effect of Stimulation or Lesion of the Principal Hypothalamic Nuclei
Medial Zone
The medial zone is a cell-rich region composed of many individual nuclei (Figs. 30-4 and 30-5). It is divided into three regions: the supraoptic (chiasmatic) region, the tuberal region, and the mammillary region (Fig. 30-4).
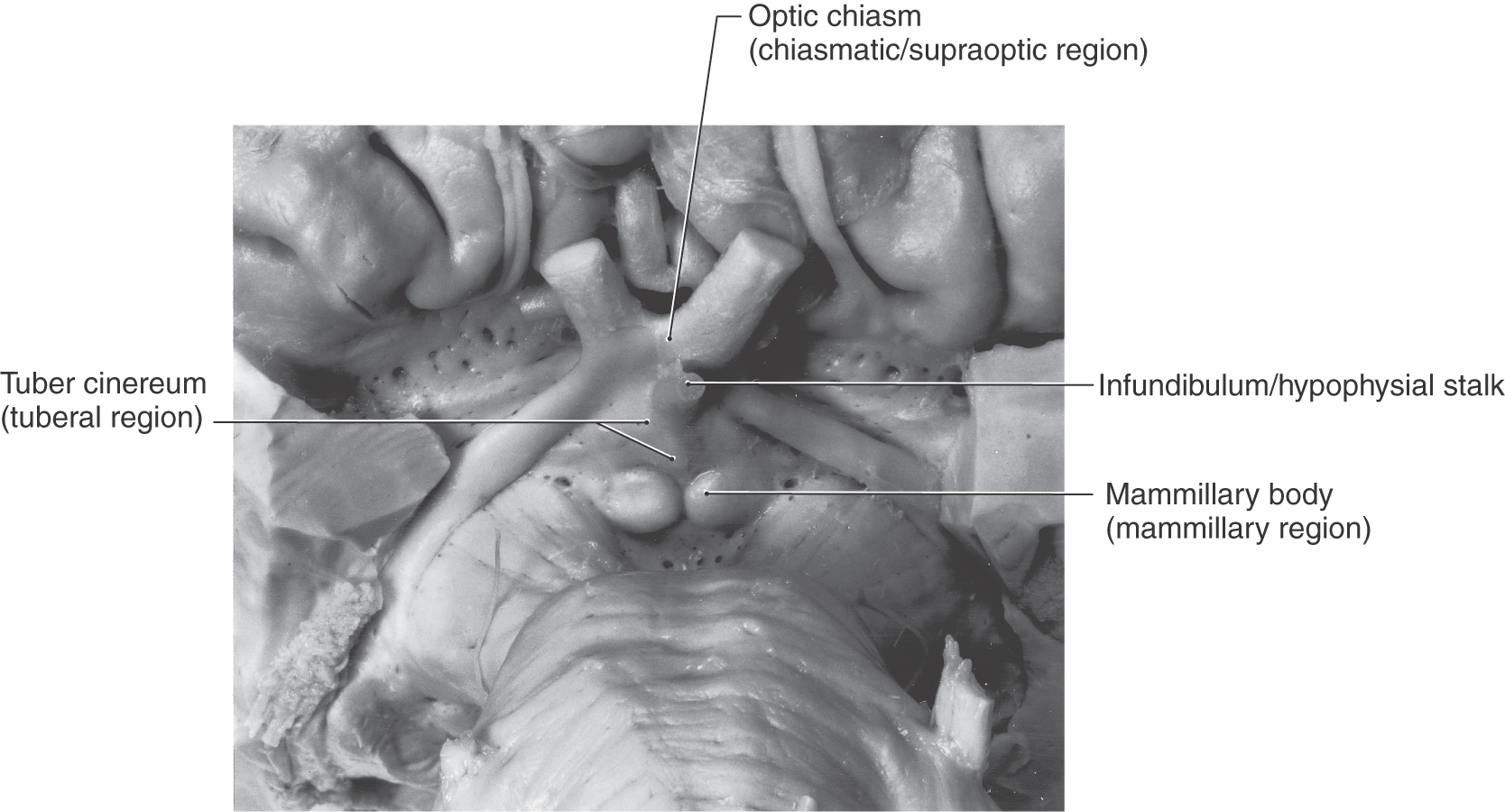
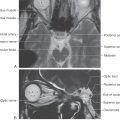
Before the major nuclei of the medial zone are considered, it should be stressed that the nuclei comprising each region of this zone are located internal to surface structures that specify the location or position of that specific region (Fig. 30-6). For example, the supraoptic region is located internal to the position of the optic chiasm (Figs. 30-5 and 30-6). The tuberal region is the widest part of the hypothalamus and in general is located internal to the position of the tuber cinereum (Figs. 30-5 and 30-6). The mammillary region is the most posterior of the three regions of the medial zone, and it is located internal to the mammillary bodies (Figs. 30-5 and 30-6).
Figure 30-6. The inferior aspect of the forebrain showing the external structures that correspond with the locations of the three internal regions that collectively comprise the medial hypothalamic zone; each region has its own respective nuclei (compare with Figs. 30-4 and 30-5). The internal region appears in parentheses under the name of the corresponding external structure.
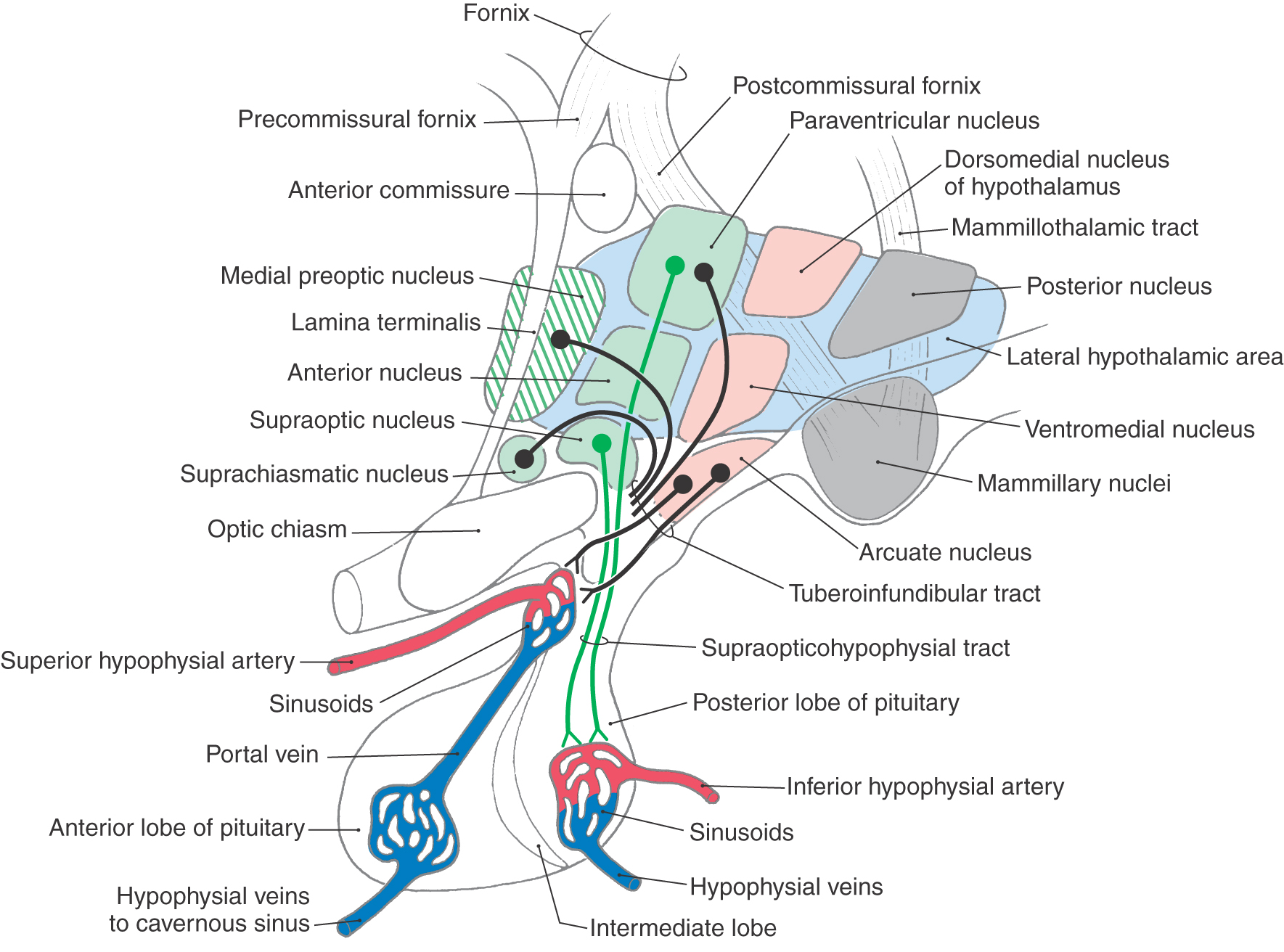
The supraoptic region contains four nuclei: the supraoptic, paraventricular, suprachiasmatic, and anterior nuclei (Fig. 30-5). Neurons of the supraoptic and paraventricular nuclei contain oxytocin and antidiuretic hormone (ADH, vasopressin) and transmit these substances to the posterior pituitary by way of the supraopticohypophysial tract for release into the circulatory system (Fig. 30-5). The functions of these hormones are discussed later in this chapter. The suprachiasmatic nucleus receives direct input from the retina and can influence other hypothalamic structures, such as the medial preoptic nucleus. It is believed that the suprachiasmatic nucleus may mediate circadian rhythms, these being the hormonal fluctuations that are secondary to light-dark cycles. The anterior nucleus is located immediately caudal to the preoptic area. Although this nucleus participates in a wide range of visceral and somatic functions, many of its neurons are involved in the maintenance of body temperature.
The tuberal region contains three nuclei: the ventromedial, dorsomedial, and arcuate nuclei (Figs. 30-2C and 30-5). The ventromedial nucleus, one of the largest and best defined of the hypothalamic nuclei, is considered to be a “satiety center.” If this nucleus is stimulated in the laboratory, the experimental animal will not engage in feeding behavior. Conversely, a lesion to this nucleus causes the animal to eat excessively and to gain weight (Table 30-1). The dorsomedial nucleus, located immediately posterior (dorsal) to the ventromedial nucleus, subserves a function relating to emotion or, at least, to emotional behavior. In laboratory animals, stimulation of the dorsomedial nucleus results in unusually aggressive behavior, which lasts only so long as the stimulation is present (Table 30-1). This phenomenon, known as sham rage, can also be elicited by the stimulation of other hypothalamic and extrahypothalamic sites. The arcuate nucleus is the primary location of neurons that contain releasing hormones. These substances are transmitted to the anterior pituitary by way of the tuberoinfundibular tract and hypophysial portal system, whereupon they influence the release of various pituitary hormones (Fig. 30-5). Lesions of the human hypothalamus frequently involve multiple nuclei. Elements of the deficits seen in animal experiments are present in various combinations or, to various degrees, in humans with lesions in, or impinging on, hypothalamic structures.
The mammillary region contains four nuclei: the medial, intermediate, and lateral mammillary and the posterior hypothalamic nuclei (Figs. 30-2A, D and 30-5). The medial mammillary nucleus is large and especially well developed in the human. It represents the primary termination point for the axons of the postcommissural fornix, which originate primarily from the subiculum of the hippocampal complex. The medial mammillary nucleus is also the source of axons that are directed to the anterior nucleus of the dorsal thalamus as the mammillothalamic tract (Fig. 30-5; see also Fig. 30-8). The latter pathway represents an important part of the limbic system. The much smaller intermediate and lateral mammillary nuclei are located lateral to the medial mammillary nucleus. The lateral mammillary nucleus receives input from the medial aspects of the midbrain reticular formation by way of the mammillary peduncle (see Fig. 30-9).
Insight into the function of the mammillary nuclei comes from experimental and clinical observations. For example, lesions of the mammillary bodies tend to impede the retention of newly acquired memory, so that an immediate memory or a short-term memory is not processed into long-term memory (Table 30-1). A patient with a mammillary lesion has no difficulty in remembering events occurring months or years before the lesion. Memory for events occurring after the lesion is, however, limited to the short term (a period of minutes), and long-term memories are not established. As a result of this anterograde amnesia, affected patients typically have severe difficulties learning new tasks and transforming these experiences into long-term memory. These specific memory deficits are characteristic of the Korsakoff syndrome, a condition that is caused by thiamine deficiency and is typically associated with chronic alcoholism. The memory deficits in this syndrome are caused by progressive degeneration in the mammillary bodies and in functionally related brain structures, such as the hippocampal complex and the dorsomedial thalamic nucleus.
Patients with the Korsakoff syndrome may have difficulty in understanding written material and in conducting meaningful conversations because they tend to forget what was just read or said. An interesting feature of this syndrome is the patient’s tendency to confabulate, that is, to string together fragmentary memories from various events into a synthesized memory of an “event” that never occurred.
The posterior hypothalamic nucleus merges almost imperceptibly with the midbrain periaqueductal gray. Accordingly, this nucleus is associated with the same myriad emotional, cardiovascular, and analgesic functions that have been attributed to the periaqueductal gray.
Periventricular Zone
The periventricular zone (Fig. 30-4), not to be confused with the paraventricular nucleus, is a very thin region composed of small cell bodies lying medial to the medial zone and immediately subjacent to the ependymal cells of the third ventricle. Many neurons of the periventricular zone synthesize releasing hormones. These neurons project by way of the tuberoinfundibular tract to the hypophysial portal system and thus influence the release of various hormones by the anterior pituitary. Consequently, many of the neurons in the periventricular zone serve a function similar to that of neurons located in the arcuate nucleus.
FEEDING MOTIVATION
The lateral hypothalamic nucleus, as stated earlier, is commonly referred to as a feeding center. This is because stimulation of this nucleus will elicit feeding behaviors, whereas a lesion of this nucleus will inhibit the motivation to feed. Stimulation and lesions of the ventromedial nucleus have the opposite effects (Table 30-1). Consequently, the ventromedial nucleus is generally thought of as a satiety center. In recent years, new concepts about feeding motivation have emerged that expand what is known about this topic. These concepts, involving a variety of hormones and peptides, are only briefly explained as follows.
Fat cells secrete a hormone (leptin) that is carried to leptin receptors on various neurons of the arcuate nucleus. Some of these neurons contain the peptides α-melanocyte–stimulating hormone (αMSH) and cocaine- and amphetamine-regulated transcript (CART). Other neurons within the arcuate nucleus contain neuropeptide Y (NPY) and agouti-related peptide (AgRP).
Neurons containing αMSH and CART project to the lateral hypothalamus, paraventricular nucleus, and lateral horn of the spinal cord. These projections promote an increase of thyroid-stimulating hormone (TSH) and adrenocorticotropic hormone (ACTH). This causes an increase in metabolism, an increase in sympathetic tone, and a decrease in feeding.
Neurons containing NPY and AgRP also project to the lateral hypothalamus and paraventricular nucleus. These projections promote the decrease of TSH and ACTH and cause a decrease in metabolism, an increase in parasympathetic tone, and an increase in feeding. This increase in feeding is thought to be partly mediated by lateral hypothalamic neurons containing the peptide neurotransmitters melanin-concentrating hormone and orexin. Neurons containing these peptides project to widespread areas of cerebral cortex and are thought to be involved in the promotion of various feeding strategies.
BLOOD SUPPLY OF THE HYPOTHALAMUS
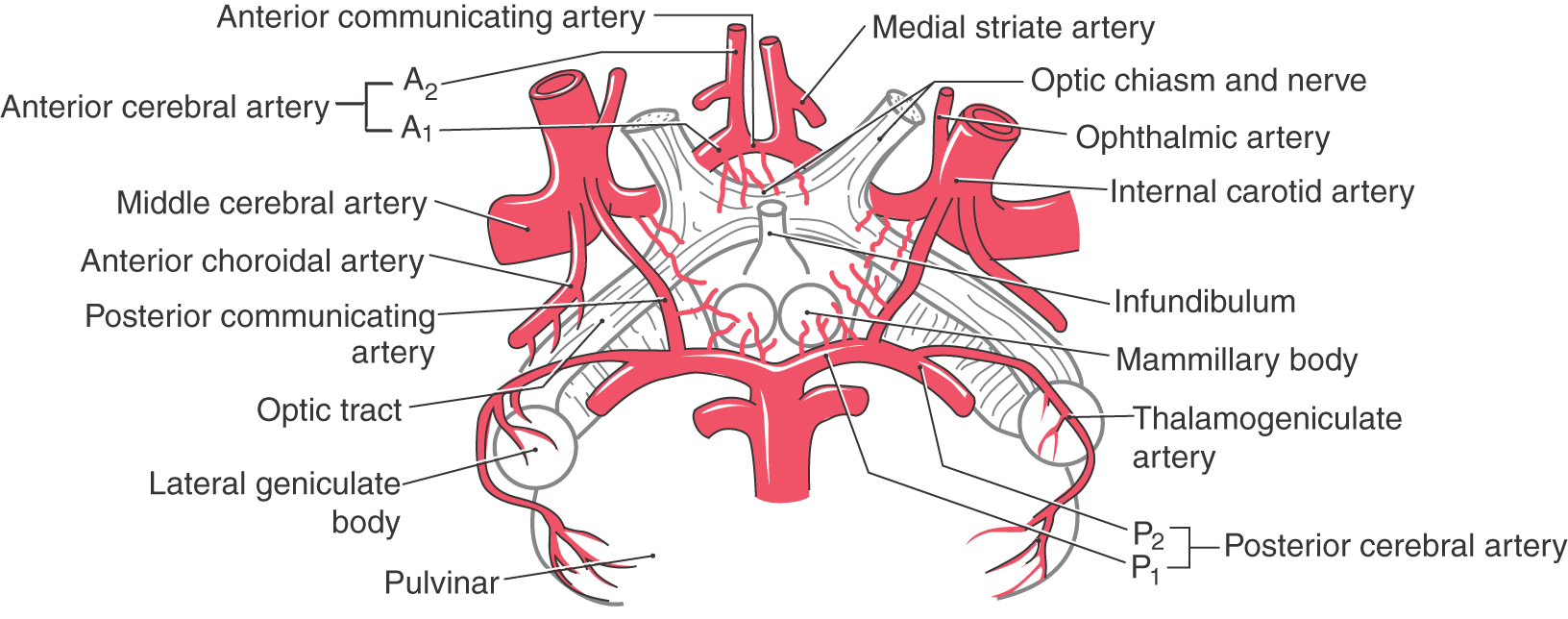
The hypothalamus and some immediately adjacent structures are served by small perforating arteries that arise from the circle of Willis (Fig. 30-7). Those branches from the anterior communicating artery and the A1 segment of the anterior cerebral artery constitute the anteromedial group of perforating arteries (also see Fig. 8-17). In general, these vessels serve the nuclei of the preoptic area and supraoptic region, the septal nuclei, and rostral portions of the lateral hypothalamic area. A few perforating arteries may also arise from the bifurcation of the internal carotid artery.
Figure 30-7. The blood supply to the hypothalamus.
The small perforating arteries that originate from the posterior communicating artery and the P1 segment of the posterior cerebral artery constitute the posteromedial group (see Fig. 30-7; also see Fig. 8-17). These vessels serve primarily the nuclei of the tuberal and mammillary regions. Branches arising from the rostral portion of the posterior communicating artery distribute to the tuberal region, whereas the mammillary region is served by branches of the caudal parts of the posterior communicating artery and of P1. In addition, the posteromedial group also sends branches into the middle and caudal parts of the lateral hypothalamic area. The large thalamoperforating arteries usually arise from P1. Although these vessels distribute mainly to rostral areas of the dorsal thalamus, they do give rise to some small branches that enter the posterior hypothalamus.
The hypophysial arteries arise from the internal carotid artery. The inferior branches (or rami) originate from the cavernous part of this large vessel, whereas the superior branches (Fig. 30-5) are from the cerebral (supraclinoid) part of the internal carotid. Although small, these are an important source of arterial blood supply to the pituitary (Fig. 30-5).
HYPOTHALAMIC AFFERENT FIBERS
The hypothalamus is connected to diverse sites, including the hippocampus, amygdala, brainstem tegmentum, various thalamic nuclei, septal nuclei, and even neocortical areas such as the infralimbic and cingulate cortex. With very few exceptions, these connections are reciprocal. The following are the most important input-output relationships of the hypothalamus.
Fornix
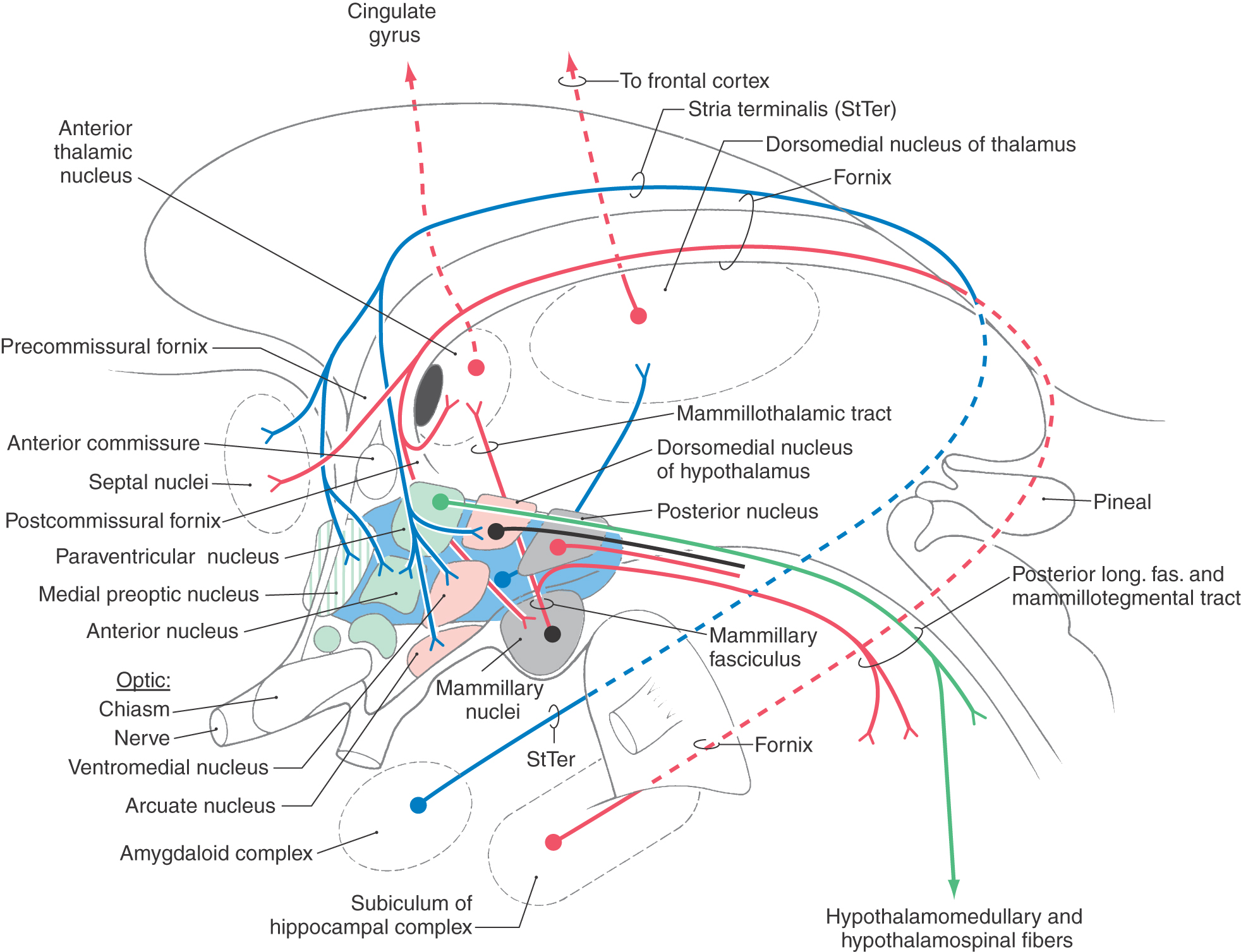
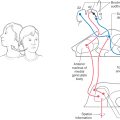
The fornix arises from neurons in the subiculum and the hippocampus (two components of the hippocampal complex) and is the largest single input to the hypothalamus (Figs. 30-5 and 30-9). As the fornix approaches the anterior commissure, it divides into a small precommissural bundle, derived largely from the hippocampus, and a large postcommissural bundle, arising mainly from the subiculum. The precommissural bundle passes to septal and preoptic nuclei and to the anterior hypothalamic region, whereas the postcommissural bundle projects primarily to the medial mammillary nucleus, with lesser inputs to the anterior thalamic nucleus and lateral hypothalamus (Figs. 30-8 and 30-9).
Medial Forebrain Bundle
The medial forebrain bundle is a diffuse, composite structure containing mainly fibers that course rostrocaudally through the lateral hypothalamic zone. It contains ascending and descending fibers that interconnect the septal nuclei (including the nucleus accumbens septi), hypothalamus, and midbrain tegmentum (Fig. 30-9).
Amygdalohypothalamic Fibers
Two major afferent fiber systems project to the hypothalamus from the amygdaloid complex: the stria terminalis, which is phylogenetically older, and a newer composite bundle called the ventral amygdalofugal pathway (Fig. 30-9). The stria terminalis originates from the corticomedial portion of the amygdala and terminates in the septal nuclei, preoptic area, and medial hypothalamic zone (Fig. 30-9). This bundle, which accompanies the terminal vein at the juncture of the caudate nucleus and the thalamus, follows the arched configuration of the caudate nucleus and thus takes a path similar to that of the fornix (Fig. 30-9). The ventral amygdalofugal pathway originates from the basolateral portion of the amygdaloid complex and passes rostromedially beneath the lentiform nucleus and through the area of the substantia innominata to enter the hypothalamus (Fig. 30-9). The axons in this pathway terminate primarily in the lateral hypothalamic zone and the septal and preoptic nuclei. This bundle also contains fibers that pass from the hypothalamus to the amygdala.
Other Afferent Fibers
The mammillary peduncle is a diffuse bundle of fibers that originates from medial portions of the midbrain reticular formation and terminates primarily in the lateral mammillary nucleus. Some of these axons enter the medial forebrain bundle and project to the septal nuclei. The dorsomedial nucleus of the thalamus gives rise to thalamohypothalamic fibers that course anteriorly (ventrally) to enter the lateral hypothalamus. The only direct neocortical projection to the hypothalamus originates from the prefrontal cortex. This corticohypothalamic fiber projection is sparse and terminates primarily in the lateral hypothalamic area. As mentioned earlier, the suprachiasmatic nucleus receives a projection from the retina that is involved in behavioral rhythms and light-dark cycles. These retinohypothalamic fibers arise as direct axons from the optic chiasm or as collaterals of retinogeniculate fibers.
HYPOTHALAMIC EFFERENT FIBERS
The various subdivisions of the hypothalamus have diffuse projections to numerous sites throughout the neuraxis. Only the major projections are summarized here. A useful generalization is that most structures projecting to the hypothalamus receive a reciprocal input from the hypothalamus. For example, the hypothalamus receives an amygdalohypothalamic projection and gives rise to hypothalamoamygdaloid fibers. For ease of discussion, the efferents are divided into those to forebrain structures (ascending) and those to brainstem and spinal targets (descending).
Ascending Projections
The mammillary fasciculus originates as a well-defined bundle from the medial mammillary nucleus (Figs. 30-8 and 30-9). It passes posteriorly (dorsally) for a short distance, then bifurcates into the mammillothalamic tract and the mammillotegmental tract (Fig. 30-8). The mammillothalamic tract projects to the anterior nucleus of the thalamus and is an important part of the circuit of Papez (see Chapter 31). The mammillotegmental tract (discussed later) turns caudally and distributes to the tegmental nuclei of the midbrain reticular formation, thus reciprocating the mammillary peduncle. Hypothalamothalamic fibers arise mainly from the lateral preoptic area and project to the dorsomedial nucleus of the thalamus. The hypothalamus also projects to the amygdaloid nucleus (hypothalamoamygdaloid fibers) via the stria terminalis and the ventral amygdalofugal pathway. These fibers originate from various hypothalamic nuclei and project primarily to corticomedial nuclei of the amygdaloid complex.
Descending Projections
There are four main descending projections from the hypothalamus. These are hypothalamospinal and hypothalamomedullary fibers, the posterior (dorsal) longitudinal fasciculus, and the mammillotegmental tract (Figs. 30-8 and 30-9).
Hypothalamospinal and hypothalamomedullary fibers arise mainly from the paraventricular nucleus, although some originate from cells located in the lateral and posterior hypothalamic areas (Fig. 30-8). These fibers descend through the periaqueductal gray and adjacent reticular formation of the midbrain and rostral pons and then shift to an anterolateral (ventrolateral) position in the medulla. Hypothalamomedullary fibers terminate in the solitary nucleus, dorsal vagal motor nucleus, nucleus ambiguus, and other nuclei of the anterolateral medulla. Hypothalamospinal fibers traverse the periaqueductal gray and dorsal tegmentum of the midbrain and pons. These fibers then shift laterally, passing through the anterolateral medulla and lateral funiculus of the spinal cord to terminate on neurons of the intermediolateral cell column (general visceral efferent preganglionic cells). Hypothalamomedullary and hypothalamospinal fibers form an essential and direct link between the hypothalamus and autonomic nuclei of the medulla and spinal cord. Lesions in the anterolateral medulla may disrupt these fibers. Although the functional effect of disruption of hypothalamomedullary fibers is not fully understood, injury to hypothalamospinal fibers results in a loss of sympathetic outflow to the ipsilateral face and head (causing a Horner syndrome) and to the ipsilateral side of the body.
The posterior (dorsal) longitudinal fasciculus originates from nuclei of the medial hypothalamic zone, whereas fibers of the mammillotegmental tract arise from the medial mammillary nucleus (Figs. 30-8 and 30-9). Fibers of these pathways descend through and largely terminate in the periaqueductal gray. The mammillotegmental tract, being more anteriorly (ventrally) located, also terminates on neurons of the posterior (dorsal) and anterior (ventral) tegmental nuclei situated in the periaqueductal gray of the caudal midbrain (Fig. 30-9). Some neurons of the periaqueductal gray function, at least in part, in relaying information to visceral areas of the brainstem, such as the solitary and dorsal motor vagal nuclei. Consequently, the posterior longitudinal fasciculus and mammillotegmental tract are generally viewed as relatively short tracts that indirectly influence the autonomic nuclei of the brainstem.
INTRINSIC HYPOTHALAMIC CONNECTIONS
The pathways that interconnect the many nuclei of the hypothalamus are numerous and complex. Only two especially important ones are considered here: the supraopticohypophysial tract and the tuberoinfundibular tract. Both of these tracts link the hypothalamus to the pituitary (Fig. 30-5).
Supraopticohypophysial Tract
Two hormones are released by the posterior pituitary: oxytocin and antidiuretic hormone (ADH, vasopressin) (Table 30-2). These hormones are synthesized in large (magnocellular) neurons of the supraoptic and paraventricular nuclei. They are transported to the posterior pituitary in the axons of these neurons, which form the supraopticohypophysial tract. In the posterior pituitary, they are stored in specialized axon terminals, sometimes called Herring bodies, which release them in response to the arrival of action potentials from the nerve cell body. The activity of these hypothalamic neurons—and thus the release of the hormones—is regulated in response to appropriate stimuli. Once released, the hormones enter a capillary plexus in the posterior pituitary and are conveyed to the general circulation by hypophysial veins.
Table 30-2 Hypothalamic-Releasing Hormones and the Pituitary Hormones They Influence
Neurons containing oxytocin release this hormone during coitus, nipple suckling, and periods in which there is an increased level of estrogen. The release of oxytocin induces the contraction of smooth muscle in the uterus and of the myoepithelial cells in the mammary gland. The effect of oxytocin on the uterus is critical during and after childbirth. A synthetic form of oxytocin (Pitocin) is often administered to hasten labor and delivery. After the baby is born, oxytocin continues to be important. During nursing, for example, the baby’s suckling causes oxytocin to be released from the posterior pituitary, and oxytocin in turn causes the myoepithelial cells of the milk glands to contract, expelling milk. The oxytocin released during nursing also has beneficial effects on the postpartum uterus. Specifically, the contractions of uterine muscles caused by oxytocin help this organ to gradually regain its original form and size.
Neurons containing antidiuretic hormone (ADH, vasopressin) (Table 30-2) are influenced primarily by fluctuations in the osmolarity of the blood. The relative concentration of sodium chloride in blood plasma is normally about 300 mOsm. This osmolarity is largely a function of how much water is retained within the body. In the process of maintaining fluid balance homeostasis, small deviations from normal blood osmolarity occur throughout each day. These deviations serve as stimuli that influence the release of ADH from the posterior pituitary. Because these stimuli occur frequently, the neurons containing ADH (unlike those containing oxytocin) are tonically active. Thus, small amounts of ADH are released numerous times each day.
When the blood osmolarity is high, the release of ADH from the posterior pituitary is facilitated. On entering the systemic circulation, ADH has a primary effect on the kidneys. Specifically, ADH causes the collecting tubules to increase their resorption of water from the developing urine, thereby returning water to the circulatory system. The additional water serves to dilute the blood, causing the blood osmolarity to be decreased. Consequently, however, the urine becomes more concentrated. As a result, urine output is diminished and the urine that is produced has a darker color.
When the blood osmolarity is low, the release of ADH from the posterior pituitary is inhibited. Consequently, the amount of ADH in the systemic circulation will be diminished. In response, the collecting tubules of the kidneys decrease their resorption of water from the developing urine. Consequently, water remains in the urine and is not returned to the circulatory system. The effect of this renal conservation of water is an increase in the concentration of the blood, causing the blood osmolarity to be increased. Accordingly, there is also an increased output of pale-colored (dilute) urine.
Lesions of the supraoptic or paraventricular nucleus or of the supraopticohypophysial tract produce a syndrome known as diabetes insipidus, which is characterized by polyuria (increased urination) and polydipsia (increased consumption of water) (Table 30-1). This condition is due to a deficit of circulating ADH. It is of interest that ethanol causes a decrease in the release of ADH from the posterior pituitary. This is the reason that consumption of alcoholic beverages tends to cause copious urination and consequent dehydration and thirst.
The main function of ADH (vasopressin) is to assist in the maintenance of normal blood osmolarity and blood pressure. Normally, ADH increases blood pressure by increasing blood volume. However, ADH at high levels will cause contraction of vascular smooth muscle and may also result in increased blood pressure. In this regard, the release of ADH from the posterior pituitary often occurs in those situations in which an increase of blood pressure would be beneficial. For example, the hypotension that occurs in conjunction with hypovolemia (decreased blood volume) represents a stimulus that promotes the release of ADH. As a result, arterial constriction takes place and blood pressure is elevated. Accordingly, the hypotensive state is partially alleviated.
Tuberoinfundibular Tract
Most of the input to the pituitary through the tuberoinfundibular tract comes from small (parvicellular) neurons located in the arcuate nucleus and the periventricular zone. Neurons of the paraventricular, suprachiasmatic, tuberal, and medial preoptic nuclei also contribute to this tract (Fig. 30-5). These axons convey various releasing hormones to the median eminence (the most inferior aspect of the tuberal area) and to the infundibulum of the pituitary gland (Table 30-2). The substances are then released into a primary plexus of fenestrated capillaries (sinusoids), from which they are carried by portal veins to a secondary plexus of fenestrated capillaries in the pituitary (Fig. 30-5). The releasing hormones of the hypothalamus include thyrotropin-releasing hormone, growth hormone–releasing hormone, growth hormone release–inhibiting hormone (somatostatin), corticotropin-releasing hormone, gonadotropin-releasing hormone, and prolactin-releasing hormone (Table 30-2).
In the anterior lobe, the hypothalamic hormones regulate the functioning of hormone-producing adenohypophysial cells. The hormones of the adenohypophysis include growth hormone (primarily affecting the development of the musculoskeletal system), gonadotropins (affecting the ovary and testis), corticotropin (affecting the cortex of the adrenal gland), thyrotropin (affecting the thyroid gland), and prolactin (affecting milk production) (Table 30-2). Hormones leave the anterior pituitary via hypophysial veins and are distributed in the systemic circulation.
PITUITARY TUMORS
The pituitary gland is anatomically and functionally linked to the hypothalamus. Indeed, it is through the pituitary that many hypothalamic functions are expressed. Hormones and releasing hormones manufactured in the hypothalamus are transported via the supraopticohypophysial and tuberoinfundibular tracts and are released into the hypophysial portal system. Small lesions within the hypothalamus can block the manufacture and transportation of these substances, thereby adversely affecting pituitary functions. Similarly, tumors (adenomas) occurring within the pituitary can easily encroach on the neighboring hypothalamus and thus jeopardize its functions. It is therefore appropriate to briefly consider pituitary tumors at this point.
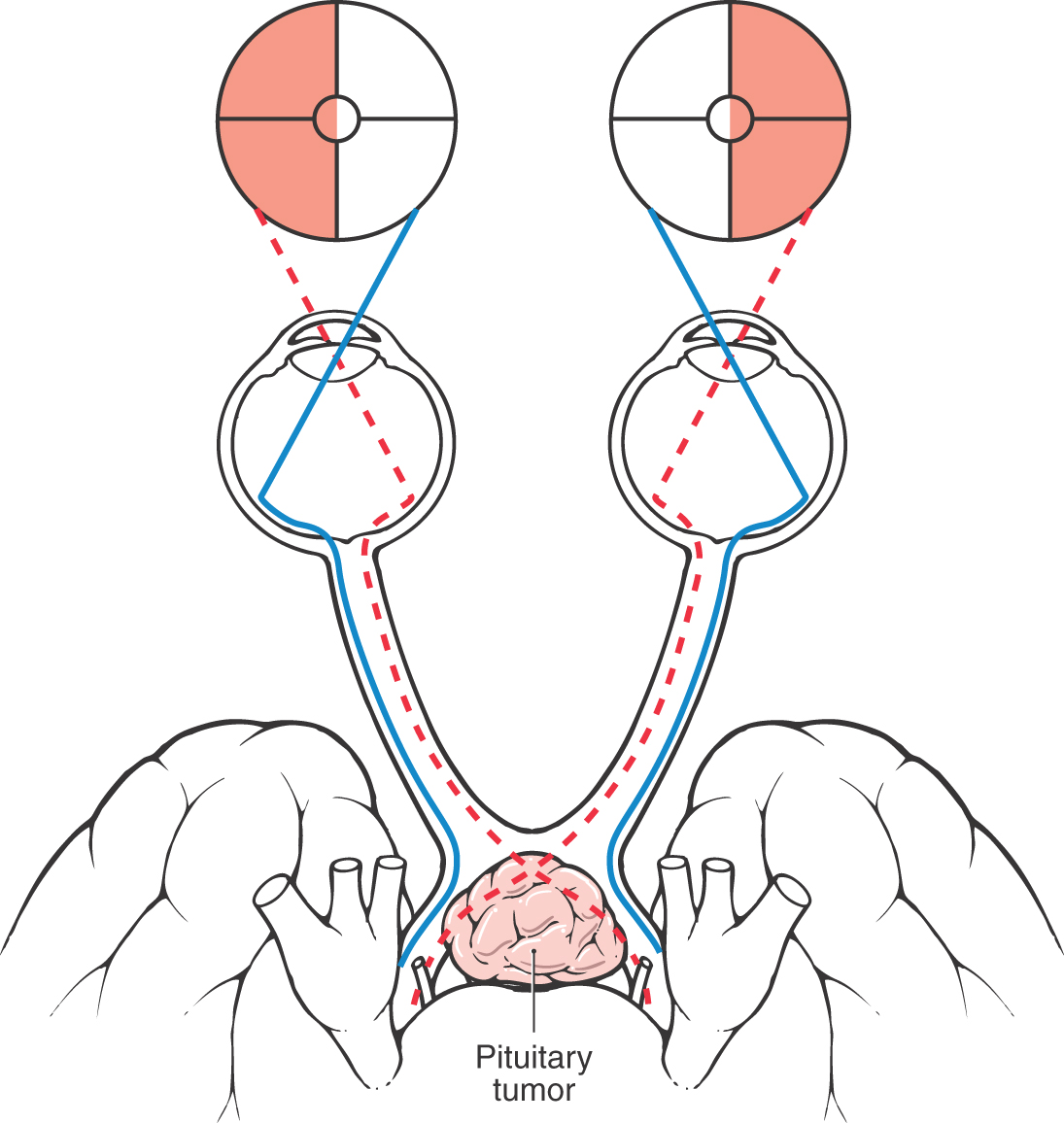
Although visual deficits are not a specific topic of this chapter (see Chapter 20), such deficits are frequently experienced by patients with pituitary tumors. These deficits may reflect damage to the optic nerve immediately rostral to the chiasm, to the chiasm itself (uncrossed fibers or decussating fibers), or to the optic tract immediately caudal to the chiasm. For example, a pituitary tumor pressing on the optic chiasm may damage axons originating from the nasal half of each retina. This may result in a loss of vision in the temporal half of the visual field for each eye (a bitemporal hemianopia), thereby reducing peripheral vision (Fig. 30-10).
Tumors occurring within the pituitary gland account for 12% of primary brain tumors. In autopsy studies, the overall occurrence of incidental (undiagnosed) pituitary adenomas has been as high as 22.5% and as low as 3.2%, depending on the thickness of sectioned pituitary glands. In young adults, pituitary tumors that occur most frequently are generally noncancerous. In contrast, adenomas that occur with increasing frequency in the fifth, sixth, and seventh decades of life are discovered as an incidental finding.
Pituitary tumors can be classified according to their secretory characteristics, size, or biologic invasiveness. Secreting tumors produce an excess of one or more pituitary hormones; prolactin is the most commonly affected of these hormones. A second classification of these tumors is by size; microadenomas are tumors less than 1 cm in greatest dimension, whereas tumors greater than 1 cm across are referred to as macroadenomas. A third classification of these tumors is according to invasiveness; invasive tumors may erode and extend into the dura mater and even the sphenoid bone.
Nonsecreting pituitary tumors do not secrete hormones and as a result are often undiagnosed until they grow to a considerable size and exert pressure on nearby structures (e.g., the optic chiasm and hypothalamus). Patients with these large pituitary tumors have symptoms that often include visual disturbances (in 60% to 70% of patients) and headaches.
Secreting Tumors
Growth Hormone–Secreting Tumors
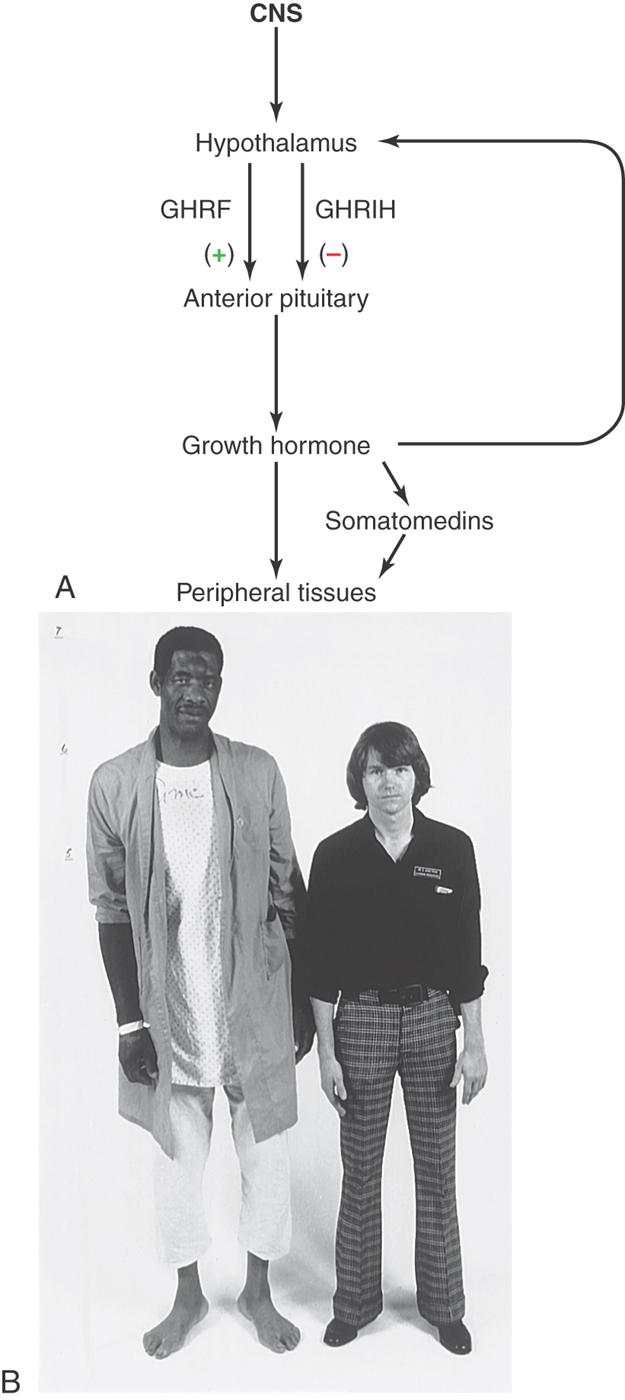
Under normal conditions, release of growth hormone is stimulated by growth hormone–releasing hormone (GHRH) and inhibited by growth hormone release–inhibiting hormone (GHRIH), both of which are controlled by the hypothalamus (Fig. 30-11A). These factors act on the pituitary somatotrophs, one of the acidophils of the anterior pituitary, to cause the release of growth hormone, which potentially may act on all tissues of the body. The effects of growth hormone, also called somatotropin, are mediated by the liver through insulin-like growth factor (IGF-1, sometimes called somatomedins) with secondary effects on peripheral tissues, especially connective tissue and bone metabolism, as well as liver metabolic activity. Whereas somatotropin may promote growth and metabolism of fat and inhibit glucose uptake, excessive levels may result in diabetes mellitus.
In the clinical setting, secreting pituitary tumors are also commonly referred to as hormonally active or hypersecreting tumors. The clinical manifestations of a secreting tumor are due to the effects of the biologic activity of the specific pituitary hormone that is overproduced.
In cases of excessive production of growth hormone, if the growth occurs before closure of the epiphyseal plates of the long bones, patients experience uncontrolled growth in height referred to as gigantism (Fig. 30-11B). These patients may have large muscles, but they are actually rather weak because their muscles contain excessive amounts of connective tissue rather than muscle fibers.
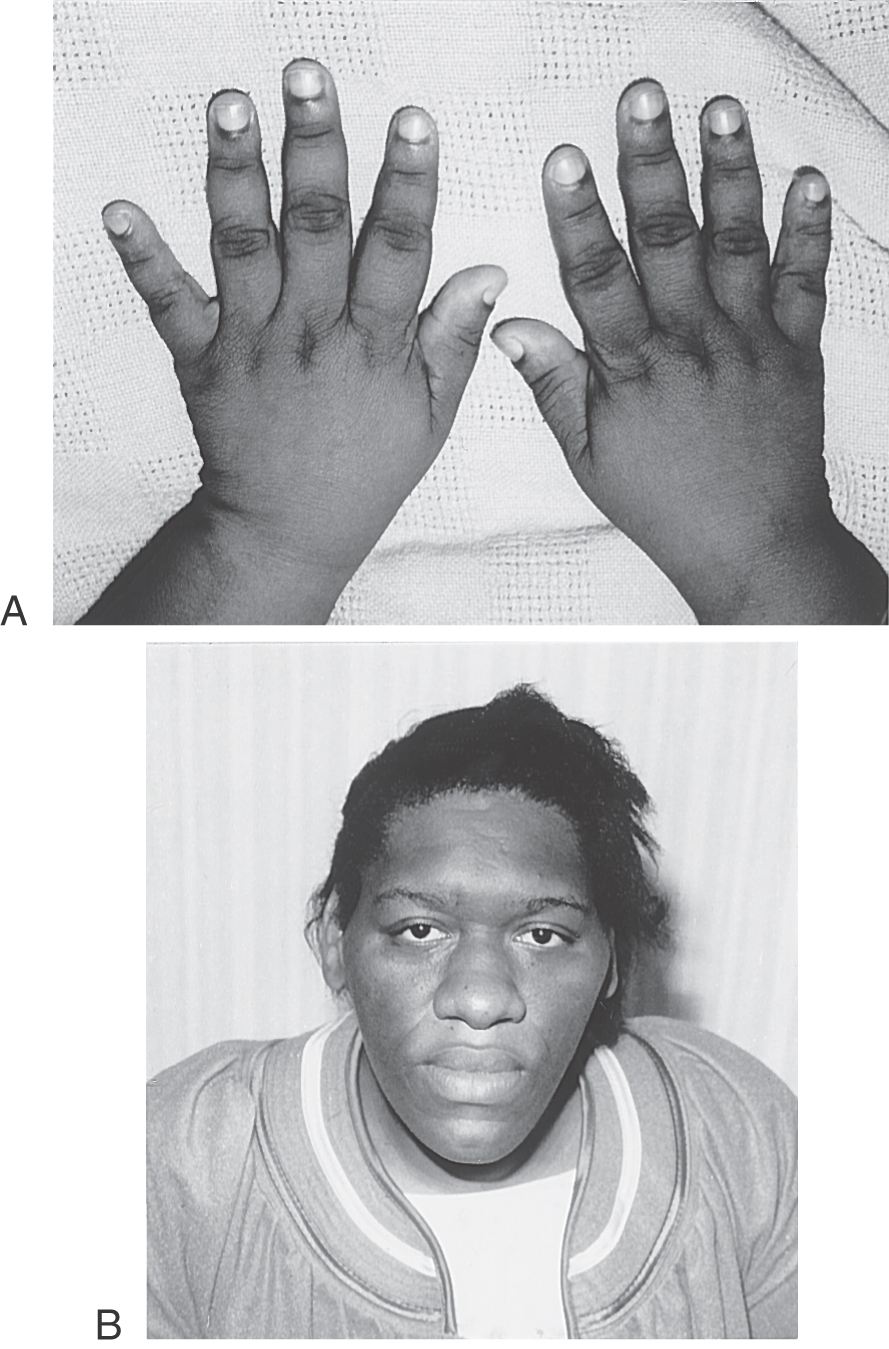
On the other hand, the overproduction of growth hormone after the growth plates have closed results in a condition termed acromegaly, referring to the enlargement of the digits of the patient (Fig. 30-12A). Patients with acromegaly have typical facial changes (Fig. 30-12B), with elongation of the face, malocclusion of the jaw, and gaps in the lower dentition. Other changes include frontal and mastoid sinus bulges, a bulbous nose, thickened lips, and very large hands and feet (Fig. 30-12B). Associated with these external manifestations are excessive cortical thickening of bone, cardiac failure secondary to heart enlargement (cardiomegaly) as well as hypertension and diabetes mellitus.
Figure 30-12. A female patient with acromegaly. Note the characteristic appearance of the enlarged hands (A) and the facial features (B).
Thyrotropin-Secreting Tumors
A much rarer form of pituitary tumor can result from the overproduction of thyrotropin hormone. The result may be either hypothyroidism or hyperthyroidism. Frequently, the patients have abnormal cardiovascular function as well as tremor. With the progression of these tumors, symptoms such as headaches, visual disturbances, and parasellar cranial nerve (i.e., cranial nerves III, IV, and VI) dysfunction may be recognized. The cranial nerve dysfunction generally involves the abducens nerves first, then the oculomotor and trochlear nerves, as the tumor enlarges in a lateral direction.
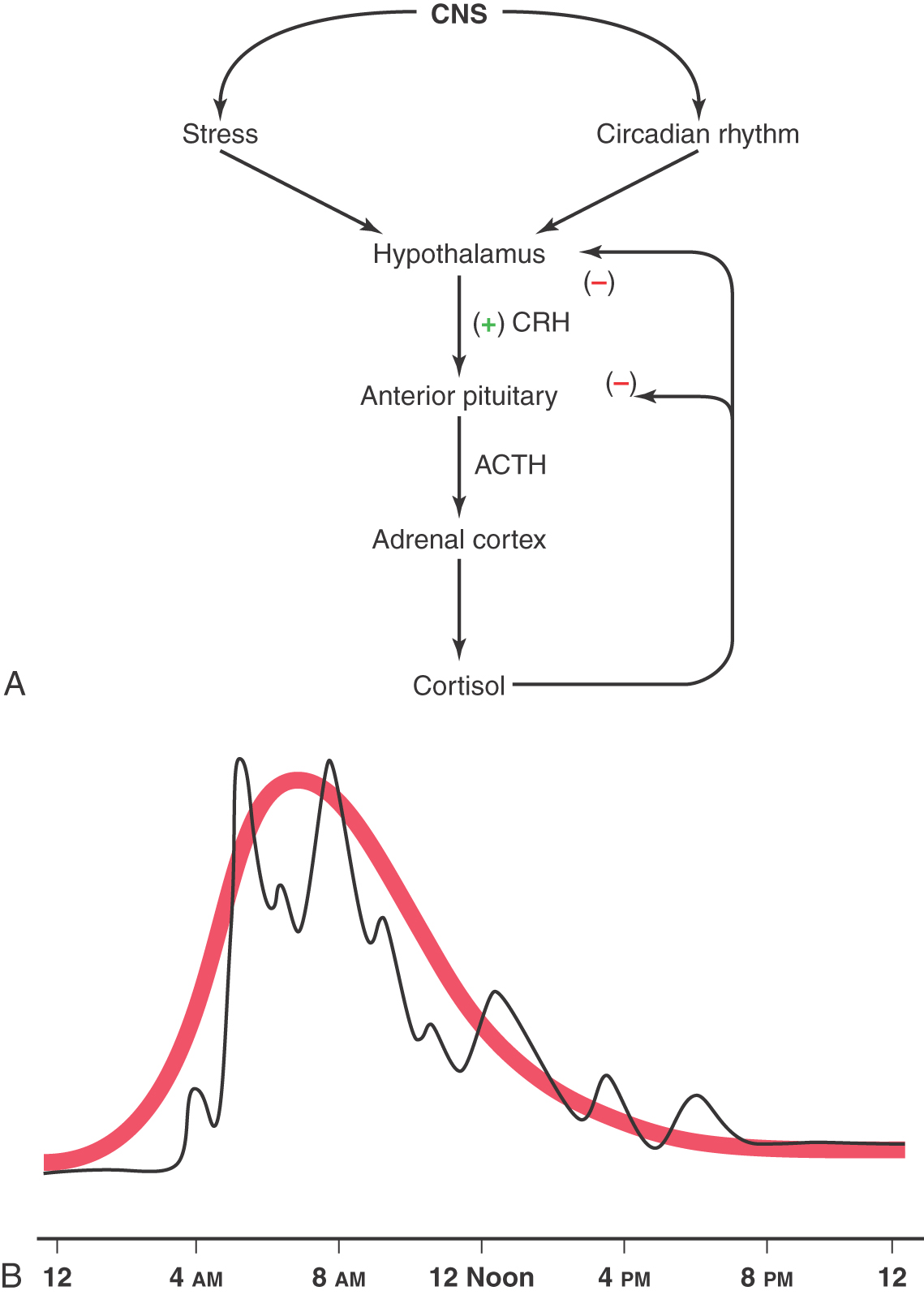
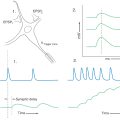
Adrenocorticotropic Hormone–Secreting Tumors (Cushing Disease)
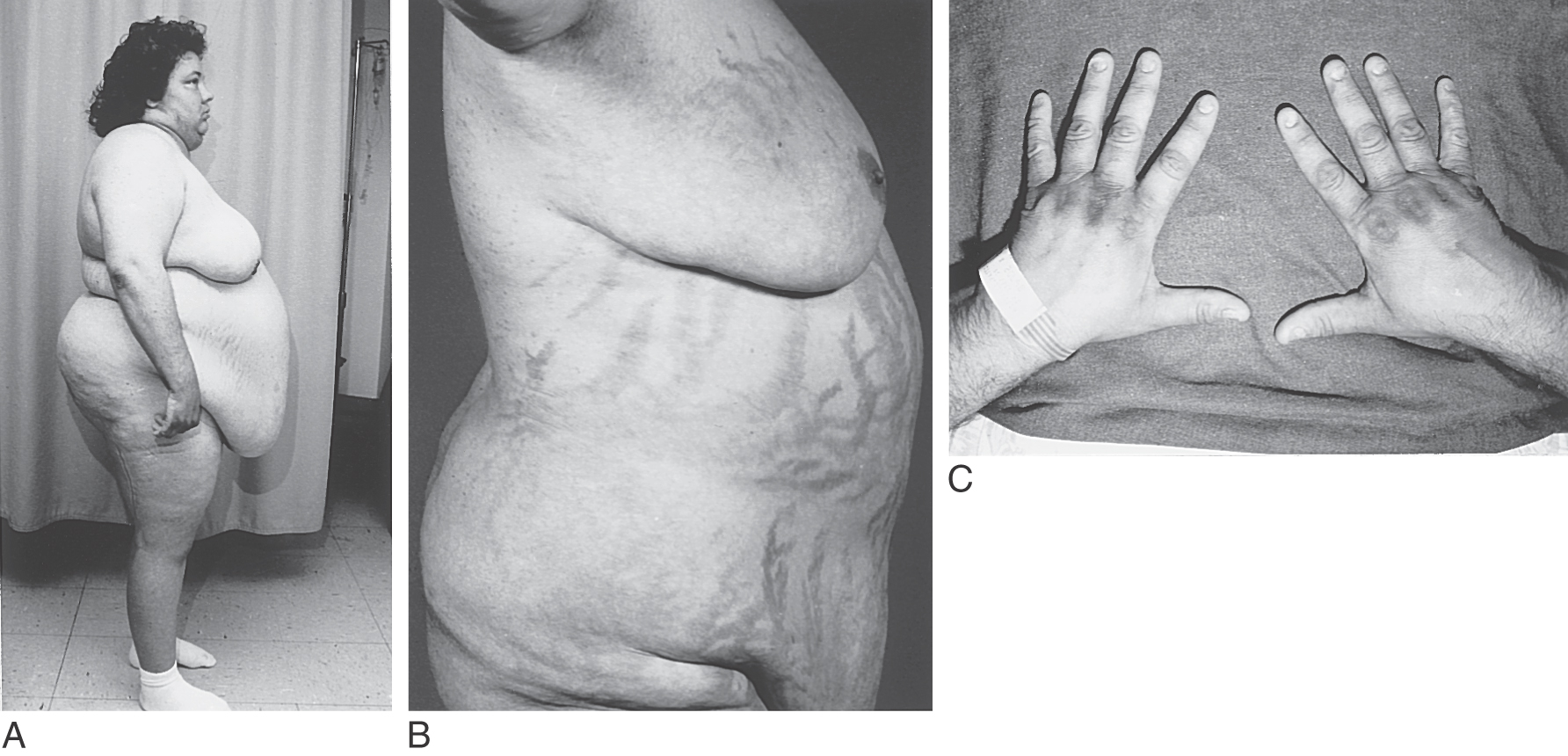
During times of stress, the central nervous system acts through a “biologic clock” to cause the hypothalamus to initiate corticotropin-releasing hormone (CRH) to stimulate the corticotroph cells of the anterior pituitary. In turn, these pituitary cells release adrenocorticotropic hormone (ACTH). This hormone acts on the adrenal cortex to release cortisol, which has a negative feedback on both the hypothalamic release of CRH and the pituitary release of ACTH (Fig. 30-13). The release of cortisol oscillates throughout a 24-hour period, reflecting the alternating signals from the hypothalamus. Plasma levels may be relatively high at some times during this 24-hour cycle or relatively low (Fig. 30-13B). Consequently, in determination of the level of cortisol in the blood, the time and circumstances under which samples are taken must be considered. An overproduction of corticotropin leads to a form of hyperadrenalism known as Cushing disease (Fig. 30-14). In this instance, the excessive ACTH from the pituitary gland results in excessive adrenal cortisol secretion with the resultant classic clinical features of Cushing disease. In general, affected patients have central truncal obesity, moon-like facies, facial hirsutism, and a dorsal cervical hump, commonly called a buffalo hump, which results from enlargement of the fat pad in this area (Fig. 30-14A). The centropedal obesity has a hallmark finding of violaceous striae (stretch marks). These striae are clearly different from those seen in pregnancy or in excessive weight gain. Striae in Cushing disease (Fig. 30-14B) are purple to violet, whereas striae in other situations are usually pale white. These patients also have hyperpigmentation (Fig. 30-14C), easy bruising, hypertension, osteopenia, and emotional lability.
Prolactin-Secreting Tumors
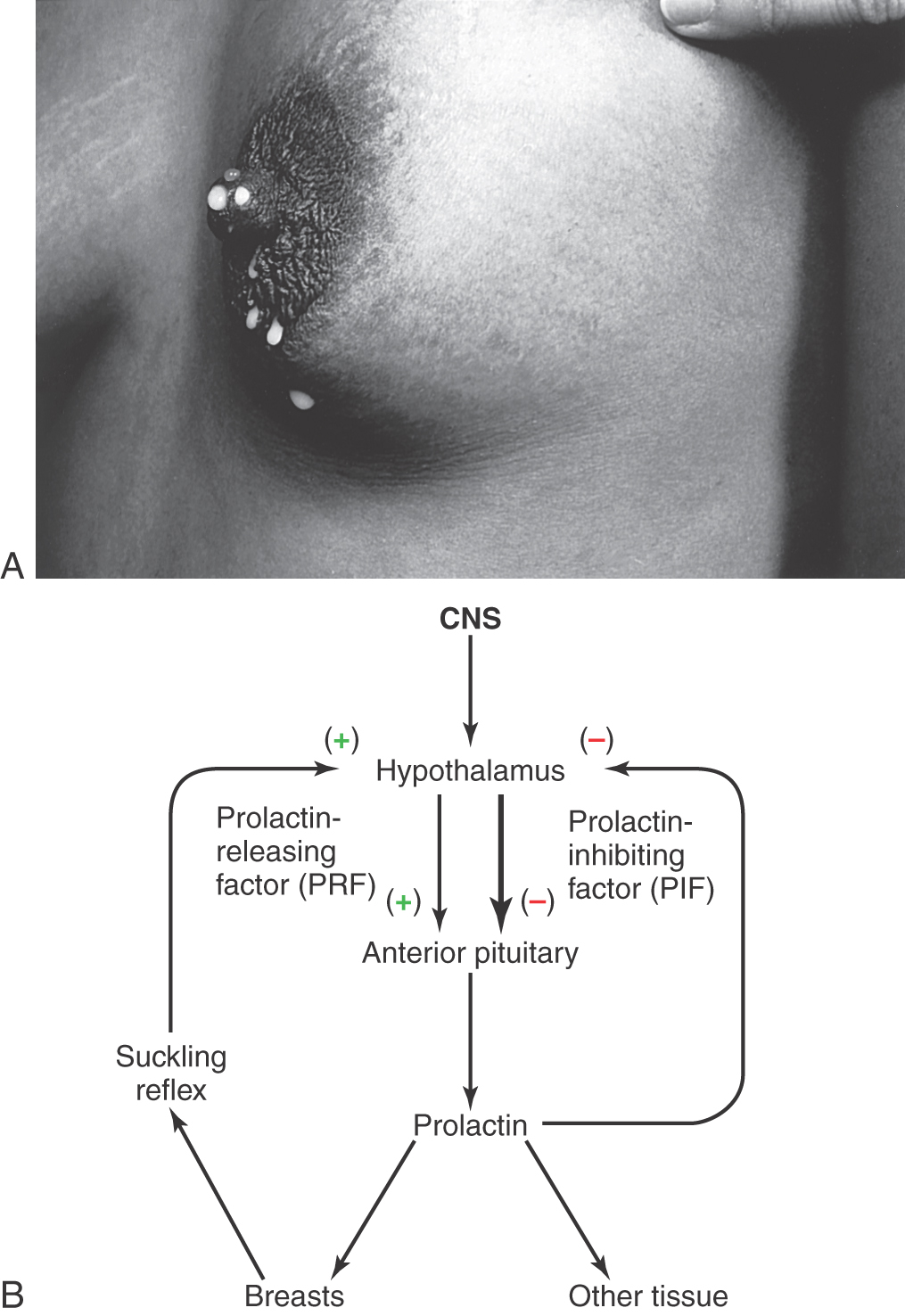
An overproduction of prolactin in women results in the syndrome of galactorrhea (milk production) (Fig. 30-15A) and amenorrhea (absence of menstrual periods). Hyperprolactinemia is seen physiologically as part of a normal pregnancy. However, amenorrhea, without galactorrhea, may be seen in women who are aggressively athletic and have little body fat; this is mediated through a different physiologic mechanism and not related to a pathologic cause.
The hypothalamus influences the lactotroph cells of the anterior pituitary to increase prolactin production by the action of prolactin-releasing factor (PRF) or to inhibit prolactin production through the action of prolactin-inhibiting factor (PIF) (Fig. 30-15B). However, prolactin is the pituitary hormone that is tonically inhibited; in this sense, the inhibitory pathway predominates (Fig. 30-15B, bold arrow). The dominance of the inhibitory pathway is interrupted during pregnancy, birth, and nursing. After birth, the suckling reflex stimulates further PRF and promotes prolactin production, which ensures further milk production. At the cessation of breast-feeding, the PIF pathway again dominates, the suckling reflex is absent, and milk production stops; this is usually accompanied by a decrease in breast size.
There are, however, other causes of hyperprolactinemia that require differentiation from either tumor or pregnancy (e.g., hypothyroidism, drug use). In men, hyperprolactinemia may be indicated by decreased libido, impotency, or infertility. This type of hypersecretory tumor is one of the most common pathologic causes of infertility in men.
Gonadotropin-Secreting Tumors
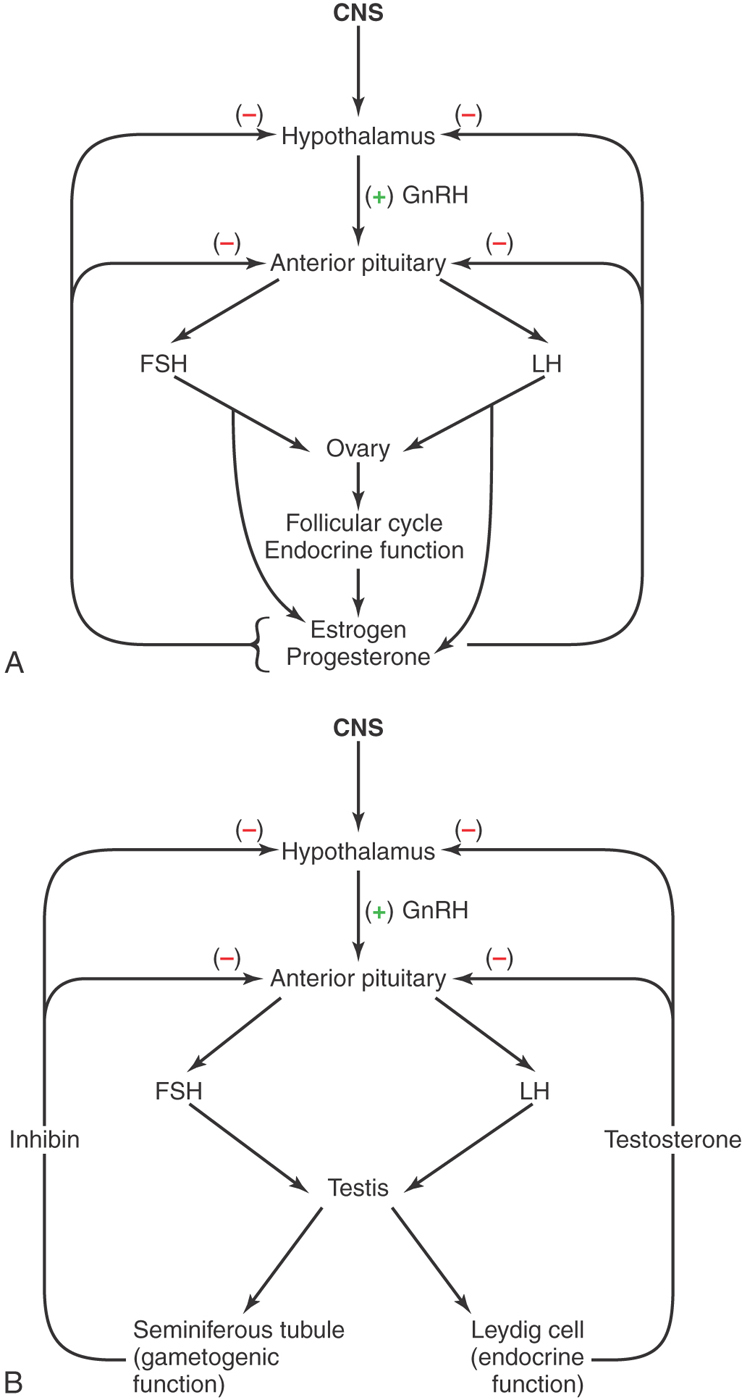
The cascade of events that result in the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) arises from a release of gonadotropin-releasing hormone (GnRH) from the hypothalamus to the anterior pituitary (Fig. 30-16). Gonadotropic cells of the pituitary in turn produce LH and FSH, which influence the ovary in the female and the testis in the male (Fig. 30-16A, B).
Gonadotropin-secreting tumors produce either excess LH or excess FSH. Excessive secretion of FSH causes no known symptoms in either men or postmenopausal women. However, in menopausal women, excessive FSH release may completely shut down the menstrual cycle. Excessive LH secretion has been reported to cause premature pubertal changes (precocious puberty) in males and possibly disruption of the ovarian cyclicity in females. In most instances, however, gonadotroph adenomas come to clinical attention because of their mass effect, with visual impairment, headaches, and occasionally diplopia (double vision) caused by compression of the oculomotor nerve due to lateral extension of the tumor.
In males, the hypothalamus releases GnRH that acts on the gonadotrophs to release both FSH and LH, which act dominantly on the testes for both gametogenic function (sperm production) and endocrine function (testosterone release). Both of these hormonal activities can, through negative feedback, inhibit the release of GnRH, FSH, and LH (Fig. 30-16B). In females, the menstrual cycle is regulated by the hypothalamic release of GnRH, which causes a sequential release of FSH and LH acting on the ovaries. The interaction of the cyclic release of estrogen and progesterone with a possible role for inhibin is intrinsically responsible for the regulated menstrual cycle (Fig. 30-16A).
REGIONAL FUNCTIONS OF THE HYPOTHALAMUS
Because of the interrelationships existing among the autonomic, endocrine, and limbic systems (Fig. 30-1) and because of the small size of the hypothalamus, it is difficult to assign a specific function to each of its individual nuclei. Some hypothalamic nuclei participate in functions that are shared with other nuclei within the same vicinity. Consequently, these latter nuclei may participate in functions that are not uniquely their own. Instead, they may act in concert to affect functions that are attributable to hypothalamic regions rather than specific nuclei.
Although the functions of some hypothalamic nuclei are largely known, the functions of others are not fully understood. Because of this and the fact that many hypothalamic nuclei participate in regional functions, some investigators believe that the hypothalamus should not be described as containing functional “centers,” such as a feeding center or a satiety center (Table 30-1). Furthermore, because of the functional interrelationships that exist among certain nuclei within given hypothalamic regions, there is merit in thinking of the hypothalamus in terms of regional functions. Clinical observations are consistent with this view.
On the basis of clinical observations and experimental data, it is appropriate to divide the hypothalamus into two areas that share similar but opposing functions. These regions are the caudolateral and rostromedial areas of the hypothalamus (Fig. 30-4). In general, the caudolateral area consists of the lateral hypothalamic zone and the mammillary region, and the rostromedial area consists of the supraoptic region and much of the tuberal regions (Figs. 30-8 and 30-9).
Caudolateral Hypothalamus
Activation (stimulation) of the caudolateral hypothalamus produces behavioral manifestations that are generally associated with anxiety. These include (1) increased activity of the sympathetic division of the visceromotor system, (2) increased aggressive behavior, (3) increased hunger, and (4) increased body temperature (resulting from cutaneous vasoconstriction and shivering).
A lesion in the caudolateral hypothalamus typically results in manifestations opposite to those caused by stimulation. For example, damage in this area results in the inhibition of sympathetic activities and the reduction of body temperature.
Rostromedial Hypothalamus
Activation (stimulation) of the rostromedial hypothalamus produces behavioral manifestations that are generally associated with contentment. These include (1) increased activity of the parasympathetic division of the visceromotor system, (2) increased passive behavior, (3) increased satiety, and (4) decreased body temperature (due to cutaneous vasodilation and sweating).
The phenomenon of sweating is something of an oddity. Even though sweat glands are innervated by sympathetic nerve fibers, sweating is compatible with parasympathetic function in that it helps keep us cool. That the sympathetic terminals innervating most sweat glands are cholinergic, like the parasympathetic terminals innervating viscera, and that sweating can be elicited from the rostromedial hypothalamus support this observation.
Lesions in the rostromedial hypothalamus usually elicit behaviors opposite to those described for stimulation. For example, a lesion in this area results in the inhibition of parasympathetic activities and an increase in body temperature.
HYPOTHALAMIC REFLEXES
All the vital functions of the hypothalamus, including the maintenance of blood pressure, body temperature, and water balance, are controlled through reflexes and are typically not subject to conscious control. However, through meditation or other means, some people can learn to alter certain hypothalamic responses. For example, biofeedback training enables some people to alter blood pressure and body temperature, which are generally under hypothalamic control. The neural mechanisms underlying these feats are largely unknown.
The internal environment is controlled partly through hypothalamic reflexes, which are typically mediated by the autonomic or endocrine systems (Fig. 30-1). Three examples are described in the following sections.
Baroreceptor Reflex
The baroreceptor reflex (which is discussed in more detail in Chapter 19 and is illustrated in Fig. 19-8 and Fig. 19-9) regulates blood pressure in response to input from baroreceptors in the aortic arch and carotid sinus. These receptors are called extrinsic because they are outside the central nervous system. They sense variations in blood pressure and transmit the information to neurons in the solitary nucleus of the medulla. Neurons of the solitary nucleus project to and activate cells in the dorsal vagal nucleus, which in turn project to the terminal ganglia of the heart and influence heart rate. A blood pressure level above normal activates the solitary nucleus, leading to a decrease in heart rate and force of cardiac contraction and consequently a decrease in blood pressure; a blood pressure level below normal has the opposite effect.
The hypothalamus is capable of influencing the baroreceptor reflex via a somewhat more complex pathway. The solitary nucleus contains cells that transmit baroreceptor information to the paraventricular, dorsomedial, and lateral hypothalamic nuclei. In turn, neurons in these hypothalamic areas project to the dorsal vagal nucleus of the medulla. By this route, the hypothalamus can powerfully modulate the responsiveness of the baroreceptor reflex.
Temperature Regulation Reflex
The reflex that maintains a constant body temperature depends on input from specialized temperature-sensing neurons in the hypothalamus (called intrinsic receptors because they are inside the central nervous system). When temperature of the blood reaching the hypothalamus rises above normal, these neurons stimulate regions in the rostral hypothalamus that are responsible for activation of physiologic mechanisms for heat dissipation—sweating and cutaneous vasodilation. These effects are mediated by autonomic pathways. Conversely, when the blood temperature is below normal, the temperature sensors stimulate regions in the caudal hypothalamus that activate mechanisms for heat conservation (cutaneous vasoconstriction, mediated by autonomic pathways) and for heat production (shivering, mediated by reticulospinal pathways).
Water Balance Reflex
As noted previously, the volume and osmolarity of the blood are kept constant by reflex mechanisms. Unlike the baroreceptor and temperature regulation reflexes, which are entirely neural, the water balance reflex is neurohumoral; its efferent limb consists of a hormonal signal carried by ADH. In brief, the reflex works as follows. The osmolarity of the blood is monitored by specialized osmolarity-sensitive neurons located in the anterior hypothalamus near the preoptic and paraventricular nuclei. The output from these receptors influences the release of ADH by ADH-producing neurons in the supraoptic and paraventricular nuclei. When blood osmolarity is too high, more ADH is released and water resorption from the collecting tubules of the kidney is increased. When blood osmolarity is too low, the release of ADH is inhibited and renal water resorption is reduced. Accordingly, water is not resorbed into the blood but stays in the urine.
Sources and Additional Reading
Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770.
Haymaker W, Anderson E, Nauta WJH. The Hypothalamus. Springfield, Ill: Charles C Thomas; 1969.