I. Mucocutaneous HSV infections
A. Infections in immunosuppressed patients
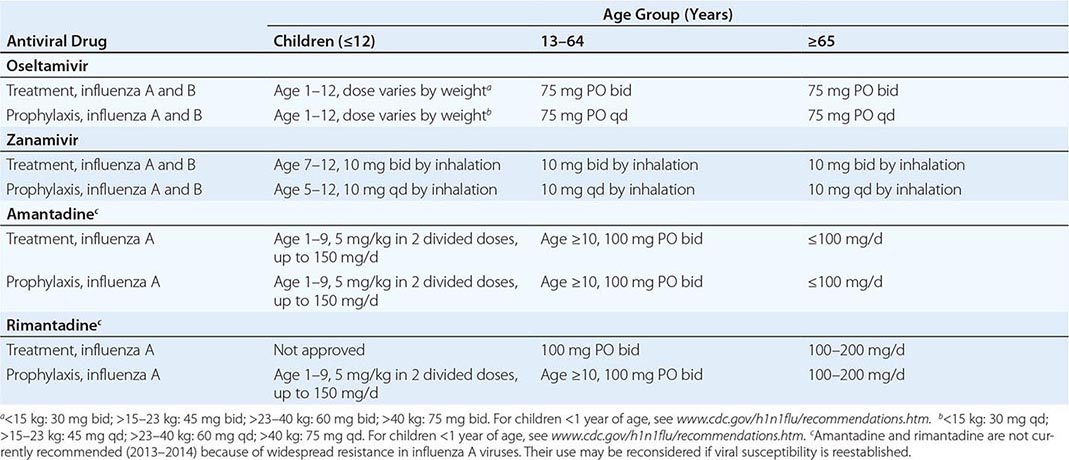
1. Acute symptomatic first or recurrent episodes: IV acyclovir (5 mg/kg q8h) or oral acyclovir (400 mg qid), famciclovir (500 mg bid or tid), or valacyclovir (500 mg bid) is effective. Treatment duration may vary from 7 to 14 days.
2. Suppression of reactivation disease (genital or oral-labial): IV acyclovir (5 mg/kg q8h) or oral valacyclovir (500 mg bid) or acyclovir (400–800 mg 3–5 times per day) prevents recurrences during the 30-day period immediately after transplantation. Longer-term HSV suppression is often used for persons with continued immunosuppression. In bone marrow and renal transplant recipients, oral valacyclovir (2 g/d) is also effective in reducing cytomegalovirus infection. Oral valacyclovir at a dose of 4 g/d has been associated with thrombotic thrombocytopenic purpura after extended use in HIV-positive persons. In HIV-infected persons, oral acyclovir (400–800 mg bid), valacyclovir (500 mg bid), or famciclovir (500 mg bid) is effective in reducing clinical and subclinical reactivations of HSV-1 and HSV-2.
B. Infections in immunocompetent patients
1. Genital herpes
a. First episodes: Oral acyclovir (200 mg 5 times per day or 400 mg tid), valacyclovir (1 g bid), or famciclovir (250 mg bid) for 7–14 days is effective. IV acyclovir (5 mg/kg q8h for 5 days) is given for severe disease or neurologic complications such as aseptic meningitis.
b. Symptomatic recurrent genital herpes: Short-course (1- to 3-day) regimens are preferred because of low cost, likelihood of adherence, and convenience. Oral acyclovir (800 mg tid for 2 days), valacyclovir (500 mg bid for 3 days), or famciclovir (750 or 1000 mg bid for 1 day, a 1500-mg single dose, or 500 mg stat followed by 250 mg q12h for 3 days) effectively shortens lesion duration. Other options include oral acyclovir (200 mg 5 times per day), valacyclovir (500 mg bid), and famciclovir (125 mg bid for 5 days).
c. Suppression of recurrent genital herpes: Oral acyclovir (400–800 mg bid) or valacyclovir (500 mg daily) is given. Patients with >9 episodes per year should take oral valacyclovir (1 g daily or 500 mg bid) or famciclovir (250 mg bid or 500 mg bid).
2. Oral-labial HSV infections
a. First episode: Oral acyclovir is given (200 mg 5 times per day or 400 mg tid); an oral acyclovir suspension can be used (600 mg/m2 qid). Oral famciclovir (250 mg bid) or valacyclovir (1 g bid) has been used clinically. The duration of therapy is 5–10 days.
b. Recurrent episodes: If initiated at the onset of the prodrome, single-dose or 1-day therapy effectively reduces pain and speeds healing. Regimens include oral famciclovir (a 1500-mg single dose or 750 mg bid for 1 day) or valacyclovir (a 2-g single dose or 2 g bid for 1 day). Self-initiated therapy with 6-times-daily topical penciclovir cream effectively speeds healing of oral-labial HSV. Topical acyclovir cream has also been shown to speed healing.
c. Suppression of reactivation of oral-labial HSV: If started before exposure and continued for the duration of exposure (usually 5–10 days), oral acyclovir (400 mg bid) prevents reactivation of recurrent oral-labial HSV infection associated with severe sun exposure.
3. Surgical prophylaxis of oral or genital HSV infection: Several surgical procedures, such as laser skin resurfacing, trigeminal nerve-root decompression, and lumbar disk surgery, have been associated with HSV reactivation. IV acyclovir (3–5 mg/kg q8h) or oral acyclovir (800 mg bid), valacyclovir (500 mg bid), or famciclovir (250 mg bid) effectively reduces reactivation. Therapy should be initiated 48 h before surgery and continued for 3–7 days.
4. Herpetic whitlow: Oral acyclovir (200 mg) is given 5 times daily (alternative: 400 mg tid) for 7–10 days.
5. HSV proctitis: Oral acyclovir (400 mg 5 times per day) is useful in shortening the course of infection. In immunosuppressed patients or in patients with severe infection, IV acyclovir (5 mg/kg q8h) may be useful.
6. Herpetic eye infections: In acute keratitis, topical trifluorothymidine, vidarabine, idoxuridine, acyclovir, penciclovir, and interferon are all beneficial. Debridement may be required. Topical steroids may worsen disease.
II. Central nervous system HSV infections
A. HSV encephalitis: IV acyclovir (10 mg/kg q8h; 30 mg/kg per day) is given for 10 days or until HSV DNA is no longer detected in cerebrospinal fluid.
B. HSV aseptic meningitis: No studies of systemic antiviral chemotherapy exist. If therapy is to be given, IV acyclovir (15–30 mg/kg per day) should be used.
C. Autonomic radiculopathy: No studies are available. Most authorities recommend a trial of IV acyclovir.
III. Neonatal HSV infections: Oral acyclovir (60 mg/kg per day, divided into 3 doses) is given. The recommended duration of IV treatment is 21 days. Monitoring for relapse should be undertaken. Continued suppression with oral acyclovir suspension should be given for 3–4 months.
IV. Visceral HSV infections
A. HSV esophagitis: IV acyclovir (15 mg/kg per day) is given. In some patients with milder forms of immunosuppression, oral therapy with valacyclovir or famciclovir is effective.
B. HSV pneumonitis: No controlled studies exist. IV acyclovir (15 mg/kg per day) should be considered.
V. Disseminated HSV infections: No controlled studies exist. IV acyclovir (5 mg/kg q8h) should be tried. Adjustments for renal insufficiency may be needed. No definite evidence indicates that therapy will decrease the risk of death.
VI. Erythema multiforme associated with HSV: Anecdotal observations suggest that oral acyclovir (400 mg bid or tid) or valacyclovir (500 mg bid) will suppress erythema multiforme.
VII. Infections due to acyclovir-resistant HSV: IV foscarnet (40 mg/kg IV q8h) should be given until lesions heal. The optimal duration of therapy and the usefulness of its continuation to suppress lesions are unclear. Some patients may benefit from cutaneous application of trifluorothymidine or 5% cidofovir gel.
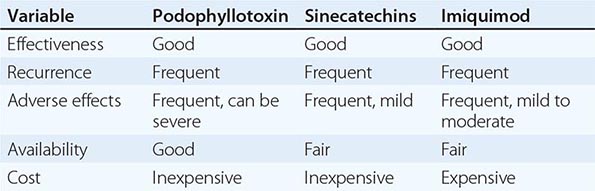
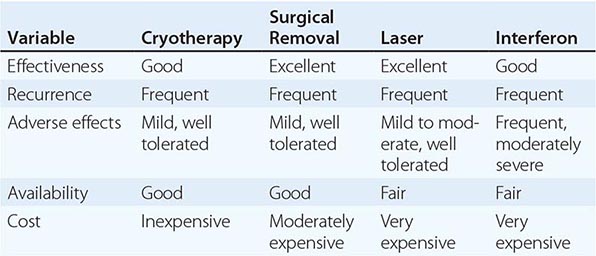
Increasingly, shorter courses of therapy are being used for recurrent mucocutaneous infection with HSV-1 or HSV-2 in immunocompetent patients. One-day courses of famciclovir and valacyclovir are clinically effective, more convenient, and generally less costly than longer courses of therapy (Table 216-1). These short-course regimens should be reserved for immunocompetent hosts.
SUPPRESSION OF MUCOCUTANEOUS HERPES
Recognition of the high frequency of subclinical reactivation provides a well-accepted rationale for the use of daily antiviral therapy to suppress reactivations of HSV, especially in persons with frequent clinical reactivations (e.g., those with recently acquired genital HSV infection). Immunosuppressed persons, including those with HIV infection, may also benefit from daily antiviral therapy. Recent studies have shown the efficacy of daily acyclovir and valacyclovir in reducing the frequency of HSV reactivations among HIV-positive persons. Regimens used include acyclovir (400–800 mg twice daily), famciclovir (500 mg twice daily), and valacyclovir (500 mg twice daily); valacyclovir at a dose of 4 g/d was associated with thrombotic thrombocytopenic purpura in one study of HIV-infected persons. In addition, daily treatment of HSV-2 reduces the titer of HIV RNA in plasma (0.5-log reduction) and in genital mucosa (0.33-log reduction).
REDUCED HSV TRANSMISSION TO SEXUAL PARTNERS
Once-daily valacyclovir (500 mg) has been shown to reduce transmission of HSV-2 between sexual partners. Transmission rates are higher from males to females and among persons with frequent HSV-2 reactivation. Serologic screening can be used to identify at-risk couples. Daily valacyclovir appears to be more effective at reducing subclinical shedding than daily famciclovir.
ACYCLOVIR RESISTANCE
Acyclovir-resistant strains of HSV have been identified. Most of these strains have an altered substrate specificity for phosphorylating acyclovir. Thus, cross-resistance to famciclovir and valacyclovir is usually found. Occasionally, an isolate with altered TK specificity arises and is sensitive to famciclovir but not to acyclovir. In some patients infected with TK-deficient virus, higher doses of acyclovir are associated with clearing of lesions. In others, clinical disease progresses despite high-dose therapy. Almost all clinically significant acyclovir resistance has been seen in immunocompromised patients, and HSV-2 isolates are more often resistant than HSV-1 strains. A study by the Centers for Disease Control and Prevention indicated that ~5% of HSV-2 isolates from HIV-positive persons exhibit some degree of in vitro resistance to acyclovir. Of HSV-2 isolates from immunocompetent patients attending sexually transmitted disease clinics, <0.5% show reduced in vitro sensitivity to acyclovir. The lack of appreciable change in the frequency of detection of such isolates in the past 20 years probably reflects the reduced transmission of TK-deficient mutants. Isolation of HSV from lesions persisting despite adequate dosages and blood levels of acyclovir should raise the suspicion of acyclovir resistance. Therapy with the antiviral drug foscarnet is useful in acyclovir-resistant cases (Chap. 215e). Because of its toxicity and cost, this drug is usually reserved for patients with extensive mucocutaneous infections. Cidofovir is a nucleotide analogue and exists as a phosphonate or monophosphate form. Most TK-deficient strains of HSV are sensitive to cidofovir. Cidofovir ointment speeds healing of acyclovir-resistant lesions. No well-controlled trials of systemic cidofovir have been reported. True TK-negative variants of HSV appear to have a reduced capacity to spread because of altered neurovirulence—a feature important in the relatively infrequent presence of such strains in immunocompetent populations, even with increasing use of antiviral drugs.
ACYCLOVIR EFFICACY IN THE DEVELOPING WORLD
![]() Early studies of acyclovir-like drugs were performed solely in the developed world. Recent studies have shown that, although acyclovir-like drugs are effective in the developing world, their clinical and virologic benefits seem reduced from those in European and U.S. populations. The mechanism of this phenomenon is uncertain. Acyclovir therapy does not reduce the rate of HIV acquisition; however, HIV load among MSM in the United States decreased by 1.3 log10 in contrast to 0.9 log10 among Peruvian MSM and 0.5 log10 among African women.
Early studies of acyclovir-like drugs were performed solely in the developed world. Recent studies have shown that, although acyclovir-like drugs are effective in the developing world, their clinical and virologic benefits seem reduced from those in European and U.S. populations. The mechanism of this phenomenon is uncertain. Acyclovir therapy does not reduce the rate of HIV acquisition; however, HIV load among MSM in the United States decreased by 1.3 log10 in contrast to 0.9 log10 among Peruvian MSM and 0.5 log10 among African women.
PREVENTION
The success of efforts to control HSV disease on a population basis through suppressive antiviral chemotherapy and/or educational programs will be limited. Barrier forms of contraception (especially condoms) decrease the likelihood of transmission of HSV infection, particularly during periods of asymptomatic viral excretion. When lesions are present, HSV infection may be transmitted by skin-to-skin contact despite the use of a condom. Nevertheless, the available data suggest that consistent condom use is an effective means of reducing the risk of genital HSV-2 transmission. Chronic daily antiviral therapy with valacyclovir can also be partially effective in reducing acquisition of HSV-2, especially among susceptible women. There are no comparative efficacy studies of valacyclovir versus condom use. Most authorities suggest both approaches. The need for a vaccine to prevent acquisition of HSV infection is great, especially in light of the role HSV-2 plays in enhancing the acquisition and transmission of HIV-1.
A substantial portion of neonatal HSV cases could be prevented by reducing the acquisition of HSV by women in the third trimester of pregnancy. Neonatal HSV infection can result from either the acquisition of maternal infection near term or the reactivation of infection at delivery in the already-infected mother. Thus strategies for reducing neonatal HSV are complex. Some authorities have recommended that antiviral therapy with acyclovir or valacyclovir be given to HSV-2-infected women in late pregnancy as a means of reducing reactivation of HSV-2 at term. Data are not available to support the efficacy of this approach. Moreover, the high treatment-to-prevention ratio makes this a dubious public health approach, even though it can reduce the frequency of HSV-associated cesarean delivery.
217 |
Varicella-Zoster Virus Infections |
DEFINITION
Varicella-zoster virus (VZV) causes two distinct clinical entities: varicella (chickenpox) and herpes zoster (shingles). Chickenpox, a ubiquitous and extremely contagious infection, is usually a benign illness of childhood characterized by an exanthematous vesicular rash. With reactivation of latent VZV (which is most common after the sixth decade of life), herpes zoster presents as a dermatomal vesicular rash, usually associated with severe pain.
ETIOLOGY
Early in the twentieth century, similarities in the histopathologic features of skin lesions resulting from varicella and herpes zoster were demonstrated. Viral isolates from patients with chickenpox and herpes zoster produced similar alterations in tissue culture—specifically, the appearance of eosinophilic intranuclear inclusions and multinucleated giant cells. These results suggested that the viruses were biologically similar. Restriction endonuclease analyses of viral DNA from a patient with chickenpox who subsequently developed herpes zoster verified the molecular identity of the two viruses responsible for these different clinical presentations.
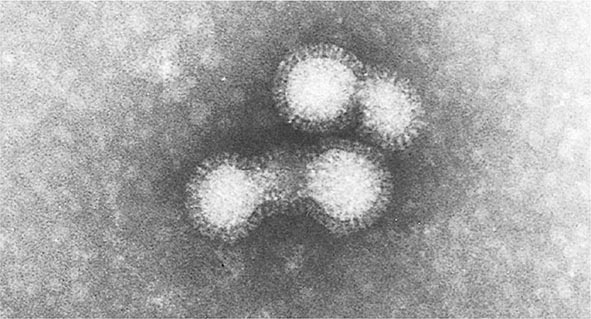
VZV is a member of the family Herpesviridae, sharing with other members such structural characteristics as a lipid envelope surrounding a nucleocapsid with icosahedral symmetry, a total diameter of ~180–200 nm, and centrally located double-stranded DNA that is ~125,000 bp in length.
PATHOGENESIS AND PATHOLOGY
Primary Infection Transmission occurs readily by the respiratory route; the subsequent localized replication of the virus at an undefined site (presumably the nasopharynx) leads to seeding of the lymphatic/reticuloendothelial system and ultimately to the development of viremia. Viremia in patients with chickenpox is reflected in the diffuse and scattered nature of the skin lesions and can be confirmed in selected cases by the recovery of VZV from the blood or routinely by the detection of viral DNA in either blood or lesions by polymerase chain reaction (PCR). Vesicles involve the corium and dermis, with degenerative changes characterized by ballooning, the presence of multinucleated giant cells, and eosinophilic intranuclear inclusions. Infection may involve localized blood vessels of the skin, resulting in necrosis and epidermal hemorrhage. With the evolution of disease, the vesicular fluid becomes cloudy because of the recruitment of polymorphonuclear leukocytes and the presence of degenerated cells and fibrin. Ultimately, the vesicles either rupture and release their fluid (which includes infectious virus) or are gradually reabsorbed.
Recurrent Infection The mechanism of reactivation of VZV that results in herpes zoster is unknown. Presumably, the virus infects dorsal root ganglia during chickenpox, where it remains latent until reactivated. Histopathologic examination of representative dorsal root ganglia during active herpes zoster demonstrates hemorrhage, edema, and lymphocytic infiltration.
Active replication of VZV in other organs, such as the lung or the brain, can occur during either chickenpox or herpes zoster but is uncommon in the immunocompetent host. Pulmonary involvement is characterized by interstitial pneumonitis, multinucleated giant cell formation, intranuclear inclusions, and pulmonary hemorrhage. Central nervous system (CNS) infection leads to histopathologic evidence of perivascular cuffing similar to that encountered in measles and other viral encephalitides. Focal hemorrhagic necrosis of the brain, characteristic of herpes simplex virus (HSV) encephalitis, develops infrequently in VZV infection.
EPIDEMIOLOGY AND CLINICAL MANIFESTATIONS
Chickenpox Humans are the only known reservoir for VZV. Chickenpox is highly contagious, with an attack rate of at least 90% among susceptible (seronegative) individuals. Persons of both sexes and all races are infected equally. The virus is endemic in the population at large; however, it becomes epidemic among susceptible individuals during seasonal peaks—namely, late winter and early spring in the temperate zone. Much of our knowledge of the disease’s natural history and incidence predates the licensure of the chickenpox vaccine in 1995. Historically, children 5–9 years old are most commonly affected and account for 50% of all cases. Most other cases involve children 1–4 and 10–14 years old. Approximately 10% of the population of the United States over the age of 15 is susceptible to infection. VZV vaccination during the second year of life has dramatically changed the epidemiology of infection, causing a significant decrease in the annualized incidence of chickenpox.
The incubation period of chickenpox ranges from 10 to 21 days but is usually 14–17 days. Secondary attack rates in susceptible siblings within a household are 70–90%. Patients are infectious ~48 h before onset of the vesicular rash, during the period of vesicle formation (which generally lasts 4–5 days), and until all vesicles are crusted.
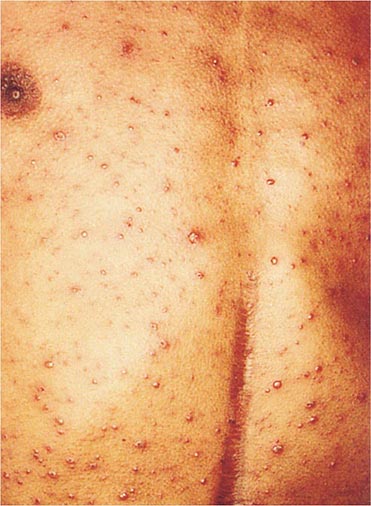
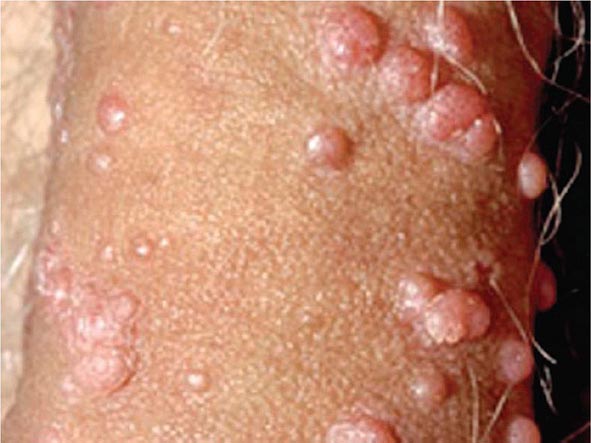
Clinically, chickenpox presents as a rash, low-grade fever, and malaise, although a few patients develop a prodrome 1–2 days before onset of the exanthem. In the immunocompetent patient, chickenpox is usually a benign illness associated with lassitude and with body temperatures of 37.8°–39.4°C (100°–103°F) of 3–5 days’ duration. The skin lesions—the hallmark of the infection—include maculopapules, vesicles, and scabs in various stages of evolution (Fig. 217-1). These lesions, which evolve from maculopapules to vesicles over hours to days, appear on the trunk and face and rapidly spread to involve other areas of the body. Most are small and have an erythematous base with a diameter of 5–10 mm. Successive crops appear over a 2- to 4-day period. Lesions can also be found on the mucosa of the pharynx and/or the vagina. Their severity varies from one person to another. Some individuals have very few lesions, while others have as many as 2000. Younger children tend to have fewer vesicles than older individuals. Secondary and tertiary cases within families are associated with a relatively large number of vesicles. Immunocompromised patients—both children and adults, particularly those with leukemia—have lesions (often with a hemorrhagic base) that are more numerous and take longer to heal than those of immunocompetent patients. Immunocompromised individuals are also at greater risk for visceral complications, which occur in 30–50% of cases and are fatal 15% of the time in the absence of antiviral therapy.
FIGURE 217-1 Varicella lesions at various stages of evolution: vesicles on an erythematous base, umbilical vesicles, and crusts.
The most common infectious complication of varicella is secondary bacterial superinfection of the skin, which is usually caused by Streptococcus pyogenes or Staphylococcus aureus, including strains that are methicillin-resistant. Skin infection results from excoriation of lesions after scratching. Gram’s staining of skin lesions should help clarify the etiology of unusually erythematous and pustulated lesions.
The most common extracutaneous site of involvement in children is the CNS. The syndrome of acute cerebellar ataxia and meningeal inflammation generally appears ~21 days after onset of the rash and rarely develops in the pre-eruptive phase. The cerebrospinal fluid (CSF) contains lymphocytes and elevated levels of protein. CNS involvement is a benign complication of VZV infection in children and generally does not require hospitalization. Aseptic meningitis, encephalitis, transverse myelitis, and Guillain-Barré syndrome can also occur. Reye’s syndrome has been reported in children concomitantly treated with aspirin. Encephalitis is reported in 0.1–0.2% of children with chickenpox. Other than supportive care, no specific therapy (e.g., acyclovir administration) has proved efficacious for patients with CNS involvement.
Varicella pneumonia, the most serious complication following chickenpox, develops more often in adults (up to 20% of cases) than in children and is particularly severe in pregnant women. Pneumonia due to VZV usually has its onset 3–5 days into the illness and is associated with tachypnea, cough, dyspnea, and fever. Cyanosis, pleuritic chest pain, and hemoptysis are frequently noted. Roentgenographic evidence of disease consists of nodular infiltrates and interstitial pneumonitis. Resolution of pneumonitis parallels improvement of the skin rash; however, patients may have persistent fever and compromised pulmonary function for weeks.
Other complications of chickenpox include myocarditis, corneal lesions, nephritis, arthritis, bleeding diatheses, acute glomerulonephritis, and hepatitis. Hepatic involvement, distinct from Reye’s syndrome and usually asymptomatic, is common in chickenpox and is generally characterized by elevated levels of liver enzymes, particularly aspartate and alanine aminotransferases.
Perinatal varicella is associated with mortality rates as high as 30% when maternal disease develops within 5 days before delivery or within 48 h thereafter. Illness in this setting is unusually severe because the newborn does not receive protective transplacental antibodies and has an immature immune system. Congenital varicella, with clinical manifestations of limb hypoplasia, cicatricial skin lesions, and microcephaly at birth, is extremely uncommon.
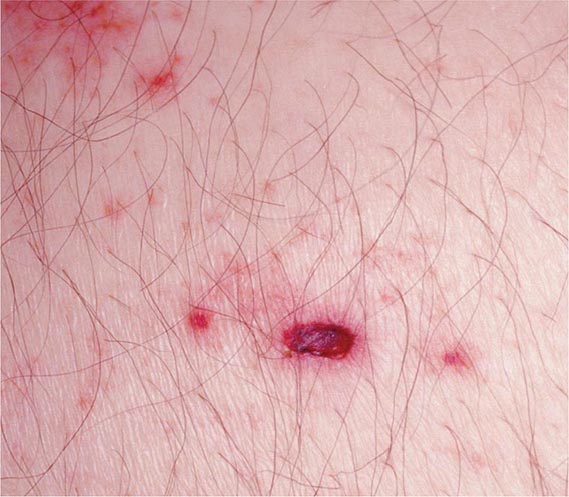
Herpes Zoster Herpes zoster (shingles) is a sporadic disease that results from reactivation of latent VZV from dorsal root ganglia. Most patients with shingles have no history of recent exposure to other individuals with VZV infection. Herpes zoster occurs at all ages, but its incidence is highest (5–10 cases per 1000 persons) among individuals in the sixth decade of life and beyond. Data suggest that 1.2 million cases occur annually in the United States. Recurrent herpes zoster is exceedingly rare except in immunocompromised hosts, especially those with AIDS.
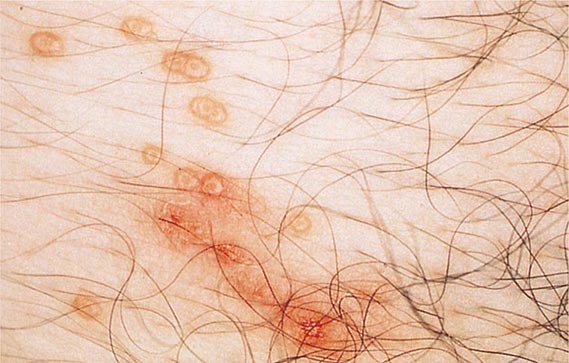
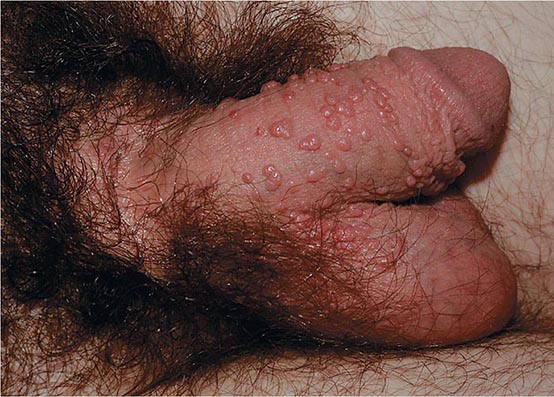
Herpes zoster is characterized by a unilateral vesicular dermatomal eruption, often associated with severe pain. The dermatomes from T3 to L3 are most frequently involved. If the ophthalmic branch of the trigeminal nerve is involved, zoster ophthalmicus results. The factors responsible for the reactivation of VZV are not known. In children, reactivation is usually benign; in adults, it can be debilitating because of pain. The onset of disease is heralded by pain within the dermatome, which may precede lesions by 48–72 h; an erythematous maculopapular rash evolves rapidly into vesicular lesions (Fig. 217-2). In the normal host, these lesions may remain few in number and continue to form for only 3–5 days. The total duration of disease is generally 7–10 days; however, it may take as long as 2–4 weeks for the skin to return to normal. Patients with herpes zoster can transmit infection to seronegative individuals, with consequent chickenpox. In a few patients, characteristic localization of pain to a dermatome with serologic evidence of herpes zoster has been reported in the absence of skin lesions, an entity known as zoster sine herpetica. When branches of the trigeminal nerve are involved, lesions may appear on the face, in the mouth, in the eye, or on the tongue. Zoster ophthalmicus is usually a debilitating condition that can result in blindness in the absence of antiviral therapy. In Ramsay Hunt syndrome, pain and vesicles appear in the external auditory canal, and patients lose their sense of taste in the anterior two-thirds of the tongue while developing ipsilateral facial palsy. The geniculate ganglion of the sensory branch of the facial nerve is involved.
FIGURE 217-2 Close-up of lesions of disseminated zoster. Note lesions at different stages of evolution, including pustules and crusting. (Photo courtesy of Lindsey Baden; with permission.)
In both normal and immunocompromised hosts, the most debilitating complication of herpes zoster is pain associated with acute neuritis and postherpetic neuralgia. Postherpetic neuralgia is uncommon in young individuals; however, at least 50% of zoster patients over age 50 report some degree of pain in the involved dermatome for months after the resolution of cutaneous disease. Changes in sensation in the dermatome, resulting in either hypo- or hyperesthesia, are common.
CNS involvement may follow localized herpes zoster. Many patients without signs of meningeal irritation have CSF pleocytosis and moderately elevated levels of CSF protein. Symptomatic meningoencephalitis is characterized by headache, fever, photophobia, meningitis, and vomiting. A rare manifestation of CNS involvement is granulomatous angiitis with contralateral hemiplegia, which can be diagnosed by cerebral arteriography. Other neurologic manifestations include transverse myelitis with or without motor paralysis.
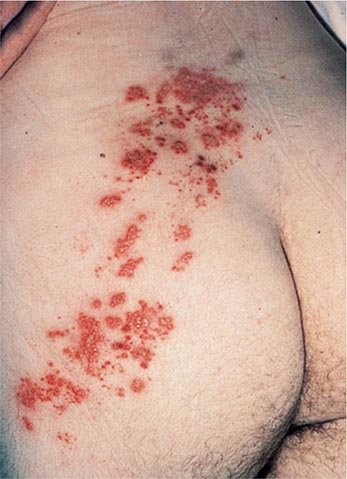
Like chickenpox, herpes zoster is more severe in immunocompromised than immunocompetent individuals. Lesions continue to form for >1 week, and scabbing is not complete in most cases until 3 weeks into the illness. Patients with Hodgkin’s disease and non-Hodgkin’s lymphoma are at greatest risk for progressive herpes zoster. Cutaneous dissemination (Fig. 217-3) develops in ~40% of these patients. Among patients with cutaneous dissemination, the risk of pneumonitis, meningoencephalitis, hepatitis, and other serious complications is increased by 5–10%. However, even in immunocompromised patients, disseminated zoster is rarely fatal.
FIGURE 217-3 Herpes zoster in an HIV-infected patient is seen as hemorrhagic vesicles and pustules on an erythematous base grouped in a dermatomal distribution.
Recipients of hematopoietic stem cell transplants are at particularly high risk of VZV infection. Of all cases of posttransplantation VZV infection, 30% occur within 1 year (50% of these within 9 months); 45% of the patients involved have cutaneous or visceral dissemination. The mortality rate in this situation is 10%. Postherpetic neuralgia, scarring, and bacterial superinfection are especially common in VZV infections occurring within 9 months of transplantation. Among infected patients, concomitant graft-versus-host disease increases the chance of dissemination and/or death.
DIFFERENTIAL DIAGNOSIS
(See also Chap. 25e) The diagnosis of chickenpox is not difficult. The characteristic rash and a history of recent exposure should lead to a prompt diagnosis. Other viral infections that can mimic chickenpox include disseminated HSV infection in patients with atopic dermatitis and the disseminated vesiculopapular lesions sometimes associated with coxsackievirus infection, echovirus infection, or atypical measles. However, these rashes are more commonly morbilliform with a hemorrhagic component rather than vesicular or vesiculopustular. Rickettsialpox (Chap. 211) is sometimes confused with chickenpox; however, rickettsialpox can be distinguished easily by detection of the “herald spot” at the site of the mite bite and the development of a more pronounced headache. Serologic testing is also useful in differentiating rickettsialpox from varicella and can confirm susceptibility in adults unsure of their chickenpox history. Concern about smallpox has recently increased because of the threat of bioterrorism (Chap. 261e). The lesions of smallpox are larger than those of chickenpox and are all at the same stage of evolution at any given time.
Unilateral vesicular lesions in a dermatomal pattern should lead rapidly to the diagnosis of herpes zoster, although the occurrence of shingles without a rash has been reported. Both HSV and coxsackie-virus infections can cause dermatomal vesicular lesions. Supportive diagnostic virology and fluorescent staining of skin scrapings with monoclonal antibodies are helpful in ensuring the proper diagnosis. In the prodromal stage of herpes zoster, the diagnosis can be exceedingly difficult and may be made only after lesions have appeared or by retrospective serologic assessment.
LABORATORY FINDINGS
Unequivocal confirmation of the diagnosis is possible only through the isolation of VZV in susceptible tissue-culture cell lines, the demonstration of either seroconversion or a fourfold or greater rise in antibody titer between acute-phase and convalescent-phase serum specimens, or the detection of VZV DNA by PCR. A rapid impression can be obtained by a Tzanck smear, with scraping of the base of the lesions in an attempt to demonstrate multinucleated giant cells; however, the sensitivity of this method is low (~60%). PCR technology for the detection of viral DNA in vesicular fluid is available in a limited number of diagnostic laboratories. Direct immunofluorescent staining of cells from the lesion base or detection of viral antigens by other assays (such as the immunoperoxidase assay) is also useful, although these tests are not commercially available. The most frequently employed serologic tools for assessing host response are the immunofluorescent detection of antibodies to VZV membrane antigens, the fluorescent antibody to membrane antigen (FAMA) test, immune adherence hemagglutination, and enzyme-linked immunosorbent assay (ELISA). The FAMA test and the ELISA appear to be most sensitive.
PREVENTION
Three methods are used for the prevention of VZV infections. First, a live attenuated varicella vaccine (Oka) is recommended for all children >1 year of age (up to 12 years of age) who have not had chickenpox and for adults known to be seronegative for VZV. Two doses are recommended for all children: the first at 12–15 months of age and the second at ~4–6 years of age. VZV-seronegative persons >13 years of age should receive two doses of vaccine at least 1 month apart. The vaccine is both safe and efficacious. Breakthrough cases are mild and may result in spread of the vaccine virus to susceptible contacts. The universal vaccination of children is resulting in a decreased incidence of chickenpox in sentinel communities. Furthermore, inactivation of the vaccine virus significantly decreases the occurrence of herpes zoster after hematopoietic stem-cell transplantation.
In individuals >50 years of age, a VZV vaccine with 18 times the viral content of the Oka vaccine decreased the incidence of shingles by 51%, the burden of illness by 61%, and the incidence of postherpetic neuralgia by 66%. The Advisory Committee on Immunization Practices has therefore recommended that persons in this age group be offered this vaccine in order to reduce the frequency of shingles and the severity of postherpetic neuralgia.
A second approach is to administer varicella-zoster immune globulin (VZIG) to individuals who are susceptible, are at high risk for developing complications of varicella, and have had a significant exposure. This product should be given within 96 h (preferably within 72 h) of the exposure. Indications for administration of VZIG appear in Table 217-1.
|
RECOMMENDATIONS FOR VZIG ADMINISTRATION |
Lastly, antiviral therapy can be given as prophylaxis to individuals at high risk who are ineligible for vaccine or who are beyond the 96-h window after direct contact. While the initial studies have used acyclovir, similar benefit can be anticipated with either valacyclovir or famciclovir. Therapy is instituted 7 days after intense exposure. At this time, the host is midway into the incubation period. This approach significantly decreases disease severity, if not totally preventing disease.
218 |
Epstein-Barr Virus Infections, Including Infectious Mononucleosis |
DEFINITION
Epstein-Barr virus (EBV) is the cause of heterophile-positive infectious mononucleosis (IM), which is characterized by fever, sore throat, lymphadenopathy, and atypical lymphocytosis. EBV is also associated with several tumors, including nasopharyngeal and gastric carcinoma, Burkitt’s lymphoma, Hodgkin’s disease, and (in patients with immunodeficiencies) B cell lymphoma. The virus is a member of the family Herpesviridae. The two types of EBV that are widely prevalent in nature are not distinguishable by conventional serologic tests.
EPIDEMIOLOGY
![]() EBV infections occur worldwide. These infections are most common in early childhood, with a second peak during late adolescence. By adulthood, more than 90% of individuals have been infected and have antibodies to the virus. IM is usually a disease of young adults. In lower socioeconomic groups and in areas of the world with deficient standards of hygiene (e.g., developing regions), EBV tends to infect children at an early age, and IM is uncommon. In areas with higher standards of hygiene, infection with EBV is often delayed until adulthood, and IM is more prevalent.
EBV infections occur worldwide. These infections are most common in early childhood, with a second peak during late adolescence. By adulthood, more than 90% of individuals have been infected and have antibodies to the virus. IM is usually a disease of young adults. In lower socioeconomic groups and in areas of the world with deficient standards of hygiene (e.g., developing regions), EBV tends to infect children at an early age, and IM is uncommon. In areas with higher standards of hygiene, infection with EBV is often delayed until adulthood, and IM is more prevalent.
EBV is spread by contact with oral secretions. The virus is frequently transmitted from asymptomatic adults to infants and among young adults by transfer of saliva during kissing. Transmission by less intimate contact is rare. EBV has been transmitted by blood transfusion and by bone marrow transplantation. More than 90% of asymptomatic seropositive individuals shed the virus in oropharyngeal secretions. Shedding is increased in immunocompromised patients and those with IM.
PATHOGENESIS
EBV is transmitted by salivary secretions. The virus infects the epithelium of the oropharynx and the salivary glands and is shed from these cells. While B cells may become infected after contact with epithelial cells, studies suggest that lymphocytes in the tonsillar crypts can be infected directly. The virus then spreads through the bloodstream. The proliferation and expansion of EBV-infected B cells along with reactive T cells during IM result in enlargement of lymphoid tissue. Polyclonal activation of B cells leads to the production of antibodies to host-cell and viral proteins. During the acute phase of IM, up to 1 in every 100 B cells in the peripheral blood is infected by EBV; after recovery, 1–50 in every 1 million B cells is infected. During IM, there is an inverted CD4+/CD8+ T cell ratio. The percentage of CD4+ T cells decreases, while there are large clonal expansions of CD8+ T cells; up to 40% of CD8+ T cells are directed against EBV antigens during acute infection. Memory B cells, not epithelial cells, are the reservoir for EBV in the body. When patients are treated with acyclovir, shedding of EBV from the oropharynx stops but the virus persists in B cells.
The EBV receptor (CD21) on the surface of B cells is also the receptor for the C3d component of complement. EBV infection of epithelial cells results in viral replication and production of virions. When B cells are infected by EBV in vitro, they become transformed and can proliferate indefinitely. During latent infection of B cells, only the EBV nuclear antigens (EBNAs), latent membrane proteins (LMPs), and small EBV RNAs (EBERs) are expressed in vitro. EBV-transformed B cells secrete immunoglobulin; only a small fraction of these cells produce virus.
Cellular immunity is more important than humoral immunity in controlling EBV infection. In the initial phase of infection, suppressor T cells, natural killer cells, and nonspecific cytotoxic T cells are important in controlling the proliferation of EBV-infected B cells. Levels of markers of T cell activation and serum interferon γ are elevated. Later in infection, human leukocyte antigen–restricted cytotoxic T cells that recognize EBNAs and LMPs and destroy EBV-infected cells are generated.
If T cell immunity is compromised, EBV-infected B cells may begin to proliferate. When EBV is associated with lymphoma in immunocompetent persons, virus-induced proliferation is but one step in a multistep process of neoplastic transformation. In many EBV-containing tumors, LMP-1 mimics members of the tumor necrosis factor receptor family (e.g., CD40), transmitting growth-proliferating signals.
CLINICAL MANIFESTATIONS
Signs and Symptoms Most EBV infections in infants and young children either are asymptomatic or present as mild pharyngitis with or without tonsillitis. In contrast, ~75% of infections in adolescents present as IM. IM in the elderly often presents with nonspecific symptoms, including prolonged fever, fatigue, myalgia, and malaise. In contrast, pharyngitis, lymphadenopathy, splenomegaly, and atypical lymphocytes are relatively rare in elderly patients.
The incubation period for IM in young adults is ~4–6 weeks. A prodrome of fatigue, malaise, and myalgia may last for 1–2 weeks before the onset of fever, sore throat, and lymphadenopathy. Fever is usually low-grade and is most common in the first 2 weeks of the illness; however, it may persist for >1 month. Common signs and symptoms are listed along with their frequencies in Table 218-1. Lymphadenopathy and pharyngitis are most prominent during the first 2 weeks of the illness, while splenomegaly is more prominent during the second and third weeks. Lymphadenopathy most often affects the posterior cervical nodes but may be generalized. Enlarged lymph nodes are frequently tender and symmetric but are not fixed in place. Pharyngitis, often the most prominent sign, can be accompanied by enlargement of the tonsils with an exudate resembling that of streptococcal pharyngitis. A morbilliform or papular rash, usually on the arms or trunk, develops in ~5% of cases (Fig. 218-1). Many patients treated with ampicillin develop a macular rash; this rash is not predictive of future adverse reactions to penicillins. Erythema nodosum and erythema multiforme also have been described (Chap. 72). The severity of the disease correlates with the levels of CD8+ T cells and EBV DNA in the blood. Most patients have symptoms for 2–4 weeks, but nearly 10% have fatigue that persists for ≥6 months.
|
SIGNS AND SYMPTOMS OF INFECTIOUS MONONUCLEOSIS |
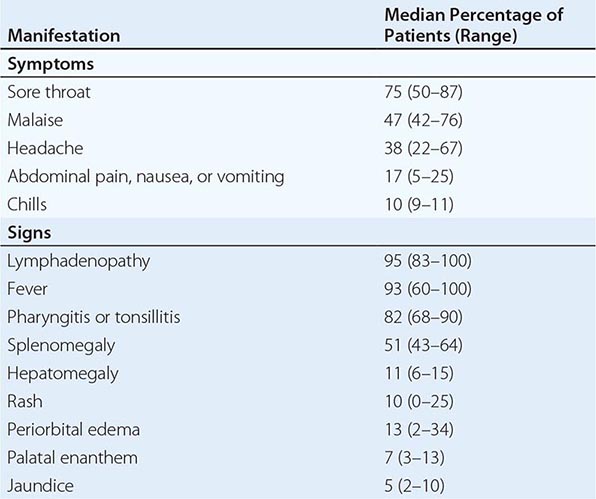
FIGURE 218-1 Rash in a patient with infectious mononucleosis due to Epstein-Barr virus. (Courtesy of Maria Turner, MD; with permission.)
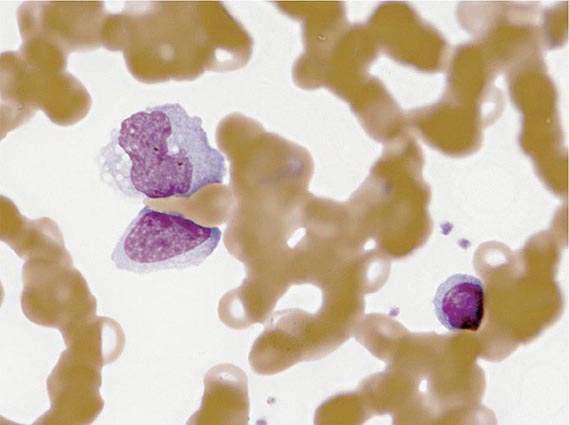
Laboratory Findings The white blood cell count is usually elevated and peaks at 10,000–20,000/μL during the second or third week of illness. Lymphocytosis is usually demonstrable, with >10% atypical lymphocytes. The latter cells are enlarged lymphocytes that have abundant cytoplasm, vacuoles, and indentations of the cell membrane (Fig. 218-2). CD8+ cells predominate among the atypical lymphocytes. Low-grade neutropenia and thrombocytopenia are common during the first month of illness. Liver function is abnormal in >90% of cases. Serum levels of aminotransferases and alkaline phosphatase are usually mildly elevated. The serum concentration of bilirubin is elevated in ~40% of cases.
FIGURE 218-2 Atypical lymphocytes from a patient with infectious mononucleosis due to Epstein-Barr virus.
Complications Most cases of IM are self-limited. Deaths are very rare and are most often due to central nervous system (CNS) complications, splenic rupture, upper airway obstruction, or bacterial superinfection.
When CNS complications develop, they usually do so during the first 2 weeks of EBV infection; in some patients, especially children, they are the only clinical manifestations of IM. Heterophile antibodies and atypical lymphocytes may be absent. Meningitis and encephalitis are the most common neurologic abnormalities, and patients may present with headache, meningismus, or cerebellar ataxia. Acute hemiplegia and psychosis also have been described. The cerebrospinal fluid contains mainly lymphocytes, with occasional atypical lymphocytes. Most cases resolve without neurologic sequelae. Acute EBV infection has also been associated with cranial nerve palsies (especially those involving cranial nerve VII), Guillain-Barré syndrome, acute transverse myelitis, and peripheral neuritis.
Autoimmune hemolytic anemia occurs in ~2% of cases during the first 2 weeks. In most cases, the anemia is Coombs-positive, with cold agglutinins directed against the red blood cell antigen. Most patients with hemolysis have mild anemia that lasts for 1–2 months, but some patients have severe disease with hemoglobinuria and jaundice. Nonspecific antibody responses may also include rheumatoid factor, antinuclear antibodies, anti–smooth muscle antibodies, antiplatelet antibodies, and cryoglobulins. IM has been associated with red-cell aplasia, severe granulocytopenia, thrombocytopenia, pancytopenia, and hemophagocytic lymphohistiocytosis. The spleen ruptures in <0.5% of cases. Splenic rupture is more common among male than female patients and may manifest as abdominal pain, referred shoulder pain, or hemodynamic compromise.
Hypertrophy of lymphoid tissue in the tonsils or adenoids can result in upper airway obstruction, as can inflammation and edema of the epiglottis, pharynx, or uvula. About 10% of patients with IM develop streptococcal pharyngitis after their initial sore throat resolves.
Other rare complications associated with acute EBV infection include hepatitis (which can be fulminant), myocarditis or pericarditis, pneumonia with pleural effusion, interstitial nephritis, genital ulcerations, and vasculitis.
EBV-Associated Diseases Other Than IM EBV-associated lymphoproliferative disease has been described in patients with congenital or acquired immunodeficiency, including those with severe combined immunodeficiency, patients with AIDS, and recipients of bone marrow or organ transplants who are receiving immunosuppressive drugs (especially cyclosporine). Proliferating EBV-infected B cells infiltrate lymph nodes and multiple organs, and patients present with fever and lymphadenopathy or gastrointestinal symptoms. Pathologic studies show B cell hyperplasia or poly- or monoclonal lymphoma.
![]() X-linked lymphoproliferative disease is a recessive disorder of young boys who have a normal response to childhood infections but develop fatal lymphoproliferative disorders after infection with EBV. The protein associated with most cases of this syndrome (SAP) binds to a protein that mediates interactions of B and T cells. Most patients with this syndrome die of acute IM. Others develop hypogammaglobulinemia, malignant B cell lymphomas, aplastic anemia, or agranulocytosis. Disease resembling X-linked lymphoproliferative disease has also been associated with mutations in XIAP. Mutations in ITK, MagT1, or CD27 are associated with inability to control EBV and lymphoma. Moreover, IM has proved fatal to some patients with no obvious preexisting immune abnormality.
X-linked lymphoproliferative disease is a recessive disorder of young boys who have a normal response to childhood infections but develop fatal lymphoproliferative disorders after infection with EBV. The protein associated with most cases of this syndrome (SAP) binds to a protein that mediates interactions of B and T cells. Most patients with this syndrome die of acute IM. Others develop hypogammaglobulinemia, malignant B cell lymphomas, aplastic anemia, or agranulocytosis. Disease resembling X-linked lymphoproliferative disease has also been associated with mutations in XIAP. Mutations in ITK, MagT1, or CD27 are associated with inability to control EBV and lymphoma. Moreover, IM has proved fatal to some patients with no obvious preexisting immune abnormality.
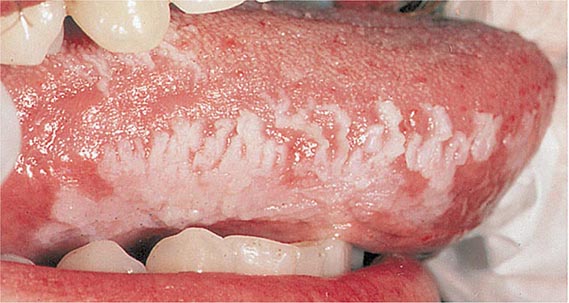
Oral hairy leukoplakia (Fig. 218-3) is an early manifestation of infection with HIV in adults (Chap. 226). Most patients present with raised, white corrugated lesions on the tongue (and occasionally on the buccal mucosa) that contain EBV DNA. Children infected with HIV can develop lymphoid interstitial pneumonitis; EBV DNA is often found in lung tissue from these patients.
FIGURE 218-3 Oral hairy leukoplakia often presents as white plaques on the lateral surface of the tongue and is associated with Epstein-Barr virus infection.
Patients with chronic fatigue syndrome may have titers of antibody to EBV that are elevated but are not significantly different from those in healthy EBV-seropositive adults. While some patients have malaise and fatigue that persist for weeks or months after IM, persistent EBV infection is not a cause of chronic fatigue syndrome. Chronic active EBV infection is very rare and is distinct from chronic fatigue syndrome. The affected patients have an illness lasting >6 months, with elevated levels of EBV DNA in the blood, high titers of antibody to EBV, and evidence of organ involvement, including hepatosplenomegaly, lymphadenopathy, and pneumonitis, uveitis, or neurologic disease.
![]() EBV is associated with several malignancies. About 15% of cases of Burkitt’s lymphoma in the United States and ~90% of those in Africa are associated with EBV (Chap. 134). African patients with Burkitt’s lymphoma have high levels of antibody to EBV, and their tumor tissue usually contains viral DNA. Malaria in African patients may impair cellular immunity to EBV and induce polyclonal B cell activation with an expansion of EBV-infected B cells. These changes may enhance the proliferation of B cells with elevated EBV DNA in the bloodstream, thereby increasing the likelihood of a c-myc translocation—the hallmark of Burkitt’s lymphoma. EBV-containing Burkitt’s lymphoma also occurs in patients with AIDS.
EBV is associated with several malignancies. About 15% of cases of Burkitt’s lymphoma in the United States and ~90% of those in Africa are associated with EBV (Chap. 134). African patients with Burkitt’s lymphoma have high levels of antibody to EBV, and their tumor tissue usually contains viral DNA. Malaria in African patients may impair cellular immunity to EBV and induce polyclonal B cell activation with an expansion of EBV-infected B cells. These changes may enhance the proliferation of B cells with elevated EBV DNA in the bloodstream, thereby increasing the likelihood of a c-myc translocation—the hallmark of Burkitt’s lymphoma. EBV-containing Burkitt’s lymphoma also occurs in patients with AIDS.
Anaplastic nasopharyngeal carcinoma is common in southern China and is uniformly associated with EBV; the affected tissues contain viral DNA and antigens. Patients with nasopharyngeal carcinoma often have elevated titers of antibody to EBV (Chap. 106). High levels of EBV plasma DNA before treatment or detectable levels of EBV DNA after radiation therapy correlate with lower rates of overall survival and relapse-free survival among patients with nasopharyngeal carcinoma.
Worldwide, the most common EBV-associated malignancy is gastric carcinoma. About 9% of these tumors are EBV-positive.
EBV has been associated with Hodgkin’s disease, especially the mixed-cellularity type (Chap. 134). Patients with Hodgkin’s disease often have elevated titers of antibody to EBV. In about half of cases in the United States, viral DNA and antigens are found in Reed-Sternberg cells. The risk of EBV-positive Hodgkin’s disease is significantly increased in young adults for several years after EBV-seropositive IM. About 50% of non-Hodgkin’s lymphomas in patients with AIDS are EBV-positive.
EBV is present in B cells of lesions from patients with lymphomatoid granulomatosis. In some cases, EBV DNA has been detected in tumors from immunocompetent patients with angiocentric nasal NK/T cell lymphoma, T cell lymphoma, and CNS lymphoma. Studies have demonstrated viral DNA in leiomyosarcomas from AIDS patients and in smooth-muscle tumors from organ transplant recipients. Virtually all CNS lymphomas in AIDS patients are associated with EBV. Studies have found that a history of IM and higher levels of antibodies to EBV before the onset of disease is more common in persons with multiple sclerosis than in the general population; additional research on a possible causal relationship is needed.
DIAGNOSIS
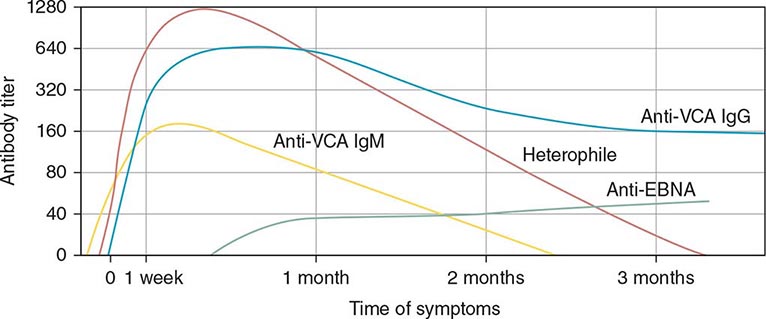
Serologic Testing (Fig. 218-4) The heterophile test is used for the diagnosis of IM in children and adults. In the test for this antibody, human serum is absorbed with guinea pig kidney, and the heterophile titer is defined as the greatest serum dilution that agglutinates sheep, horse, or cow erythrocytes. The heterophile antibody does not interact with EBV proteins. A titer of ≥40 is diagnostic of acute EBV infection in a patient who has symptoms compatible with IM and atypical lymphocytes. Tests for heterophile antibodies are positive in 40% of patients with IM during the first week of illness and in 80–90% during the third week. Therefore, repeated testing may be necessary, especially if the initial test is performed early. Tests usually remain positive for 3 months after the onset of illness, but heterophile antibodies can persist for up to 1 year. These antibodies usually are not detectable in children <5 years of age, in the elderly, or in patients presenting with symptoms not typical of IM. The commercially available monospot test for heterophile antibodies is somewhat more sensitive than the classic heterophile test. The monospot test is ~75% sensitive and ~90% specific compared with EBV-specific serologies (see below). False-positive monospot results are more common among persons with connective tissue disease, lymphoma, viral hepatitis, and malaria.
FIGURE 218-4 Pattern of Epstein-Barr virus (EBV) serology during acute infection. EBNA, Epstein-Barr nuclear antigen; VCA, viral capsid antigen. (From JI Cohen, in NS Young et al [eds]: Clinical Hematology. Philadelphia, Mosby, 2006.)
EBV-specific antibody testing is used for patients with suspected acute EBV infection who lack heterophile antibodies and for patients with atypical infections. Titers of IgM and IgG antibodies to viral capsid antigen (VCA) are elevated in the serum of more than 90% of patients at the onset of disease. IgM antibody to VCA is most useful for the diagnosis of acute IM because it is present at elevated titers only during the first 2–3 months of the disease; in contrast, IgG antibody to VCA is usually not useful for diagnosis of IM but is often used to assess past exposure to EBV because it persists for life. Seroconversion to EBNA positivity also is useful for the diagnosis of acute infection with EBV. Antibodies to EBNA become detectable relatively late (3–6 weeks after the onset of symptoms) in nearly all cases of acute EBV infection and persist for the lifetime of the patient. These antibodies may be lacking in immunodeficient patients and in those with chronic active EBV infection.
Titers of other antibodies also may be elevated in IM; however, these elevations are less useful for diagnosis. Antibodies to early antigens are detectable 3–4 weeks after the onset of symptoms in patients with IM. About 70% of individuals with IM have early antigen diffuse (EA-D) antibodies during the illness; the presence of EA-D antibodies is especially likely in patients with relatively severe disease. These antibodies usually persist for only 3–6 months. Levels of EA-D antibodies are also elevated in patients with nasopharyngeal carcinoma or chronic active EBV infection. Early antigen restricted (EA-R) antibodies are only occasionally detected in patients with IM but are often found at elevated titers in patients with African Burkitt’s lymphoma or chronic active EBV infection. IgA antibodies to EBV antigens have proved useful for the identification of patients with nasopharyngeal carcinoma and of persons at high risk for the disease.
Other Studies Detection of EBV DNA, RNA, or proteins has been valuable in demonstrating the association of the virus with various malignancies. The polymerase chain reaction has been used to detect EBV DNA in the cerebrospinal fluid of some AIDS patients with lymphomas and to monitor the amount of EBV DNA in the blood of patients with lymphoproliferative disease. Detection of high levels of EBV DNA in blood for a few days to several weeks after the onset of IM may be useful if serologic studies yield equivocal results. Culture of EBV from throat washings or blood is not helpful in the diagnosis of acute infection, since EBV persists in the oropharynx and in B cells for the lifetime of the infected individual.
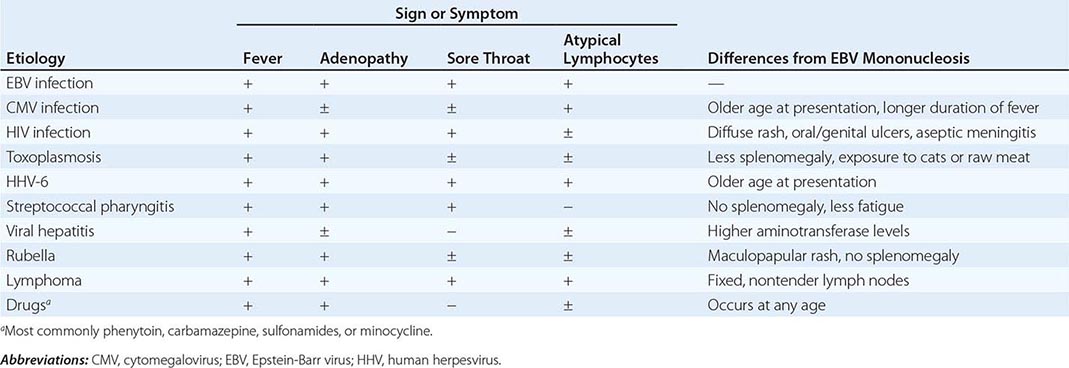
Differential Diagnosis Whereas ~90% of cases of IM are due to EBV, 5–10% of cases are due to cytomegalovirus (CMV) (Chap. 219). CMV is the most common cause of heterophile-negative mononucleosis; less common causes of IM and differences from IM due to EBV are shown in Table 218-2.
|
DIFFERENTIAL DIAGNOSIS OF INFECTIOUS MONONUCLEOSIS |
PREVENTION
The isolation of patients with IM is unnecessary. A vaccine directed against the major EBV glycoprotein reduced the frequency of IM but did not affect the rate of asymptomatic infection in a phase 2 trial.
219 |
Cytomegalovirus and Human Herpesvirus Types 6, 7, and 8 |
CYTOMEGALOVIRUS
DEFINITION
Cytomegalovirus (CMV), which was initially isolated from patients with congenital cytomegalic inclusion disease, is now recognized as an important pathogen in all age groups. In addition to inducing severe birth defects, CMV causes a wide spectrum of disorders in older children and adults, ranging from an asymptomatic subclinical infection to a mononucleosis syndrome in healthy individuals to disseminated disease in immunocompromised patients. Human CMV is one of several related species-specific viruses that cause similar diseases in various animals. All are associated with the production of characteristic enlarged cells—hence the name cytomegalovirus.
CMV, a β-herpesvirus, has double-stranded DNA, four species of mRNA, a protein capsid, and a lipoprotein envelope. Like other herpesviruses, CMV demonstrates icosahedral symmetry, replicates in the cell nucleus, and can cause either a lytic and productive or a latent infection. CMV can be distinguished from other herpesviruses by certain biologic properties, such as host range and type of cytopathology. Viral replication is associated with the production of large intranuclear inclusions and smaller cytoplasmic inclusions. CMV appears to replicate in a variety of cell types in vivo; in tissue culture it grows preferentially in fibroblasts. Although there is little evidence that CMV is oncogenic in vivo, it does transform fibroblasts in rare instances, and genomic transforming fragments have been identified.
EPIDEMIOLOGY
![]() CMV has a worldwide distribution. In many regions of the world, the vast majority of adults are seropositive for CMV, whereas only half of adults in the United States and Canada are seropositive. In regions where the prevalence of CMV antibody is high, immunocompromised adults are more likely to undergo reactivation disease rather than primary infection. Data generated in specific regions should be considered in the context of local seropositivity rates, when appropriate.
CMV has a worldwide distribution. In many regions of the world, the vast majority of adults are seropositive for CMV, whereas only half of adults in the United States and Canada are seropositive. In regions where the prevalence of CMV antibody is high, immunocompromised adults are more likely to undergo reactivation disease rather than primary infection. Data generated in specific regions should be considered in the context of local seropositivity rates, when appropriate.
Of newborns in the United States, ∼1% are infected with CMV; the percentages are higher in many less-developed countries. Communal living and poor personal hygiene facilitate spread. Perinatal and early childhood infections are common. CMV may be present in breast milk, saliva, feces, and urine. Transmission has occurred among young children in day-care centers and has been traced from infected toddler to pregnant mother to developing fetus. When an infected child introduces CMV into a household, 50% of susceptible family members seroconvert within 6 months.
CMV is not readily spread by casual contact but rather requires repeated or prolonged intimate exposure for transmission. In late adolescence and young adulthood, CMV is often transmitted sexually, and asymptomatic carriage in semen or cervical secretions is common. Antibody to CMV is present at detectable levels in a high proportion of sexually active men and women, who may harbor several strains simultaneously. Transfusion of blood products containing viable leukocytes may transmit CMV, with a frequency of 0.14–10% per unit transfused. Transfusion of leukocyte-reduced or CMV-seronegative blood significantly decreases the risk of CMV transmission.
Once infected, an individual generally carries CMV for life. The infection usually remains silent. CMV reactivation syndromes develop more frequently, however, when T lymphocyte–mediated immunity is compromised—for example, after organ transplantation, with lymphoid neoplasms and certain acquired immunodeficiencies (in particular, HIV infection; Chap. 226), or during critical illness in intensive care units. Most primary CMV infections in organ transplant recipients (Chap. 169) result from transmission via the graft. In CMV-seropositive transplant recipients, infection results from reactivation of latent virus or from infection by a new strain. CMV infection may also be associated with diseases as diverse as coronary artery stenosis and malignant gliomas, but these associations require further validation.
PATHOGENESIS
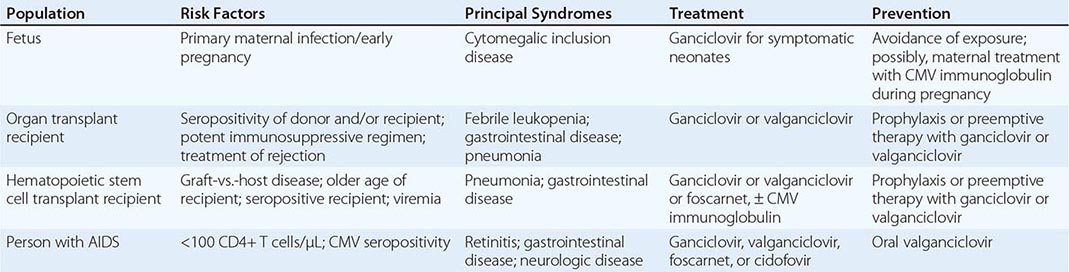
Congenital CMV infection can result from either primary or reactivation infection of the mother. However, clinical disease in the fetus or newborn is related almost exclusively to primary maternal infection (Table 219-1). The factors determining the severity of congenital infection are unknown; a deficient capacity to produce precipitating antibodies and to mount T cell responses to CMV is associated with relatively severe disease.
|
CMV DISEASE IN THE IMMUNOCOMPROMISED HOST |
Primary infection with CMV in late childhood or adulthood is often associated with a vigorous T lymphocyte response that may contribute to the development of a mononucleosis syndrome similar to that which follows infection with Epstein-Barr virus (Chap. 218). The hallmark of such infection is the appearance of atypical lymphocytes in the peripheral blood; these cells are predominantly activated CD8+ T lymphocytes. Polyclonal activation of B cells by CMV contributes to the development of rheumatoid factors and other autoantibodies during mononucleosis.
Once acquired, CMV persists indefinitely in host tissues. The sites of persistent infection probably include multiple cell types and various organs. Transmission via blood transfusion or organ transplantation is due primarily to latent infections in these tissues. If the host’s T cell responses become compromised by disease or by iatrogenic immunosuppression, latent virus can reactivate to cause a variety of syndromes. Chronic antigenic stimulation in the presence of immunosuppression (for example, after organ transplantation) appears to be an ideal setting for CMV activation and CMV disease. Certain particularly potent suppressants of T cell immunity (e.g., antithymocyte globulin, alemtuzumab) are associated with a high rate of clinical CMV syndromes. CMV may itself contribute to further T lymphocyte hyporesponsiveness, which often precedes superinfection with other opportunistic pathogens such as bacteria, molds, and Pneumocystis.
PATHOLOGY
Cytomegalic cells in vivo (presumed to be infected epithelial cells) are two to four times larger than surrounding cells and often contain an 8- to 10-μm intranuclear inclusion that is eccentrically placed and is surrounded by a clear halo, producing an “owl’s eye” appearance. Smaller granular cytoplasmic inclusions are demonstrated occasionally. Cytomegalic cells are found in a wide variety of organs, including the salivary gland, lung, liver, kidney, intestine, pancreas, adrenal gland, and central nervous system.
The cellular inflammatory response to infection consists of plasma cells, lymphocytes, and monocyte-macrophages. Granulomatous reactions occasionally develop, particularly in the liver. Immunopathologic reactions may contribute to CMV disease. Immune complexes have been detected in infected infants, sometimes in association with CMV-related glomerulopathies. Immune-complex glomerulopathy has also been observed in some CMV-infected patients after renal transplantation.
CLINICAL MANIFESTATIONS
Congenital CMV Infection Fetal infections range from subclinical to severe and disseminated. Cytomegalic inclusion disease develops in ∼5% of infected fetuses and is seen almost exclusively in infants born to mothers who develop primary infections during pregnancy. Petechiae, hepatosplenomegaly, and jaundice are the most common presenting features (60–80% of cases). Microcephaly with or without cerebral calcifications, intrauterine growth retardation, and prematurity are reported in 30–50% of cases. Inguinal hernias and chorioretinitis are less common. Laboratory abnormalities include elevated alanine aminotransferase levels in serum, thrombocytopenia, conjugated hyperbilirubinemia, hemolysis, and elevated protein levels in cerebrospinal fluid. The prognosis for severely infected infants is poor; the mortality rate is 20–30%, and few survivors escape intellectual or hearing difficulties later in childhood. The differential diagnosis of cytomegalic inclusion disease in infants includes syphilis, rubella, toxoplasmosis, infection with herpes simplex virus or enterovirus, and bacterial sepsis.
Most congenital CMV infections are clinically inapparent at birth. Of asymptomatically infected infants, 5–25% develop significant psychomotor, hearing, ocular, or dental abnormalities over the next several years.
Perinatal CMV Infection The newborn may acquire CMV at delivery by passage through an infected birth canal or by postnatal contact with infected breast milk or other maternal secretions. Of infants who are breast-fed for >1 month by seropositive mothers, 40–60% become infected. Iatrogenic transmission can result from blood transfusion; use of leukocyte-reduced or CMV-seronegative blood products for transfusion into low-birth-weight seronegative infants or seronegative pregnant women decreases risk.
The great majority of infants infected at or after delivery remain asymptomatic. However, protracted interstitial pneumonitis has been associated with perinatally acquired CMV infection, particularly in premature infants, and occasionally has been accompanied by infection with Chlamydia trachomatis, Pneumocystis, or Ureaplasma urealyticum. Poor weight gain, adenopathy, rash, hepatitis, anemia, and atypical lymphocytosis may also be found, and CMV excretion often persists for months or years.
CMV Mononucleosis The most common clinical manifestation of CMV infection in immunocompetent hosts beyond the neonatal period is a heterophile antibody–negative mononucleosis syndrome, which may develop spontaneously or follow transfusion of leukocyte-containing blood products. Although the syndrome occurs at all ages, it most often involves sexually active young adults. With incubation periods of 20–60 days, the illness generally lasts for 2–6 weeks. Prolonged high fevers, sometimes with chills, profound fatigue, and malaise, characterize this disorder. Myalgias, headache, and splenomegaly are common, but in CMV (as opposed to Epstein-Barr virus) mononucleosis, exudative pharyngitis and cervical lymphadenopathy are rare. Occasional patients develop rubelliform rashes, often after exposure to ampicillin or certain other antibiotics. Less common are interstitial or segmental pneumonia, myocarditis, pleuritis, arthritis, and encephalitis. In rare cases, Guillain-Barré syndrome complicates CMV mononucleosis. The characteristic laboratory abnormality is relative lymphocytosis in peripheral blood, with >10% atypical lymphocytes. Total leukocyte counts may be low, normal, or markedly elevated. Although significant jaundice is uncommon, serum aminotransferase and alkaline phosphatase levels are often moderately elevated. Heterophile antibodies are absent; however, transient immunologic abnormalities are common and may include the presence of cryoglobulins, rheumatoid factors, cold agglutinins, and antinuclear antibodies. Hemolytic anemia, thrombocytopenia, and granulocytopenia complicate recovery in rare instances.
Most patients recover without sequelae, although postviral asthenia may persist for months. The excretion of CMV in urine, genital secretions, and/or saliva often continues for months or years. Rarely, CMV infection is fatal in immunocompetent hosts; survivors can have recurrent episodes of fever and malaise, sometimes associated with autonomic nervous system dysfunction (e.g., attacks of sweating or flushing).
CMV Infection in the Immunocompromised Host (Table 219-1) CMV is the viral pathogen most commonly complicating organ transplantation (Chap. 169). In recipients of kidney, heart, lung, liver, pancreas, and vascularized composite (hand, face, other) transplants, CMV induces a variety of syndromes, including fever and leukopenia, hepatitis, colitis, pneumonitis, esophagitis, gastritis, and retinitis. CMV disease is an independent risk factor for both graft loss and death. Without prophylaxis, the period of maximal risk is between 1 and 4 months after transplantation. Disease likelihood and viral replication levels generally are greater after primary infection than after reactivation. Molecular studies indicate that seropositive transplant recipients are susceptible to infection with donor-derived, genotypically variant CMV, and such infection often results in disease. Reactivation infection, although common, is less likely than primary infection to be important clinically. The risk of clinical disease is related to various factors, such as degree of immunosuppression, use of antilymphocyte antibodies, lack of anti-CMV prophylaxis, and co-infection with other pathogens. The transplanted organ is particularly vulnerable as a target for CMV infection; thus there is a tendency for CMV hepatitis to follow liver transplantation and for CMV pneumonitis to follow lung transplantation.
CMV viremia occurs in roughly one-third of hematopoietic stem cell transplant recipients; the risk of severe disease may be reduced by prophylaxis or preemptive therapy with antiviral drugs. The risk is greatest 5–13 weeks after transplantation, and identified risk factors include certain types of immunosuppressive therapy, an allogeneic (rather than an autologous) graft, acute graft-versus-host disease, older age, and pretransplantation recipient seropositivity.
CMV is an important pathogen in patients with advanced HIV infection (Chap. 226), in whom it may cause retinitis or disseminated disease, particularly when peripheral-blood CD4+ T cell counts fall below 50–100/μL. As treatment for underlying HIV infection has improved, the incidence of serious CMV infections (e.g., retinitis) has decreased. However, during the first few weeks after institution of highly active antiretroviral therapy, acute flare-ups of CMV retinitis may occur secondary to an immune reconstitution inflammatory syndrome.
Syndromes produced by CMV in immunocompromised hosts often begin with prolonged fatigue, fever, malaise, anorexia, night sweats, and arthralgias or myalgias. Liver function abnormalities, leukopenia, thrombocytopenia, and atypical lymphocytosis may be observed during these episodes. The development of tachypnea, hypoxemia, and unproductive cough signals respiratory involvement. Radiologic examination of the lung often shows bilateral interstitial or reticulonodular infiltrates that begin in the periphery of the lower lobes and spread centrally and superiorly; localized segmental, nodular, or alveolar patterns are less common. The differential diagnosis includes Pneumocystis infection; other viral, bacterial, or fungal infections; pulmonary hemorrhage; and injury secondary to irradiation or to treatment with cytotoxic drugs.
Gastrointestinal CMV involvement may be localized or extensive and almost exclusively affects immunocompromised hosts. Colitis is the most common clinical manifestation in organ transplant recipients. Ulcers of the esophagus, stomach, small intestine, or colon may result in bleeding or perforation. CMV infection may lead to exacerbations of underlying ulcerative colitis. Hepatitis occurs frequently, particularly after liver transplantation. Acalculous cholecystitis and adrenalitis also have been described.
CMV rarely causes meningoencephalitis in otherwise healthy individuals. Two forms of CMV encephalitis are seen in patients with AIDS. One resembles HIV encephalitis and presents as progressive dementia; the other is a ventriculoencephalitis characterized by cranial-nerve deficits, nystagmus, disorientation, lethargy, and ventriculomegaly. In immunocompromised patients, CMV can also cause subacute progressive polyradiculopathy, which is often reversible if recognized and treated promptly.
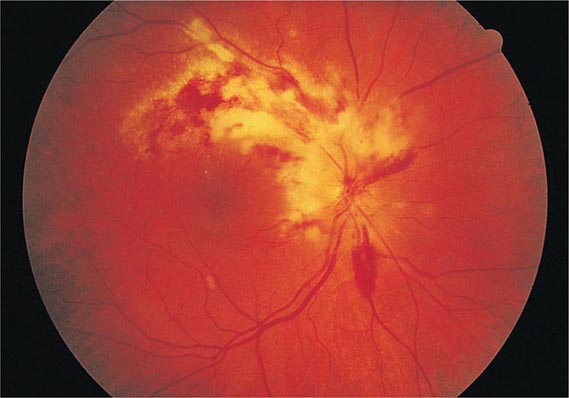
CMV retinitis is an important cause of blindness in immunocompromised patients, particularly patients with advanced AIDS (Chap. 226). Early lesions consist of small, opaque, white areas of granular retinal necrosis that spread in a centrifugal manner and are later accompanied by hemorrhages, vessel sheathing, and retinal edema (Fig. 219-1). CMV retinopathy must be distinguished from that due to other conditions, including toxoplasmosis, candidiasis, and herpes simplex virus infection.
FIGURE 219-1 Cytomegalovirus infection in a patient with AIDS may appear as an arcuate zone of retinitis with hemorrhages and optic disk swelling. Often CMV is confined to the retinal periphery, beyond view of the direct ophthalmoscope.
Fatal CMV infections are often associated with persistent viremia and the involvement of multiple organ systems. Progressive pulmonary infiltrates, pancytopenia, hyperamylasemia, and hypotension are characteristic features that are frequently found in conjunction with a terminal bacterial, fungal, or protozoan superinfection. Extensive adrenal necrosis with CMV inclusions is often documented at autopsy, as is CMV involvement of many other organs.
DIAGNOSIS
CMV infection usually cannot be diagnosed reliably on clinical grounds alone. Isolation of CMV or detection of its antigens or DNA in appropriate clinical specimens is the preferred approach. The most common method of detection is quantitative nucleic acid testing (QNAT) for CMV by polymerase chain reaction (PCR) technology, for which blood or other specimens can be used; some centers use a CMV antigenemia test, an immunofluorescence assay that detects CMV antigens (pp65) in peripheral-blood leukocytes. Such assays may yield a positive result several days earlier than culture methods. QNAT may predict the risk for disease progression, particularly in immunocompromised hosts. CMV DNA in cerebrospinal fluid is useful in the diagnosis of CMV encephalitis or polyradiculopathy. Considerable variation exists among assays and laboratories; a recently introduced international testing standard should help reduce variation in PCR test results.
Virus excretion or viremia is readily detected by culture of appropriate specimens on human fibroblast monolayers. If CMV titers are high, as is common in congenital disseminated infection and in AIDS, characteristic cytopathic effects may be detected within a few days. However, in some situations (e.g., CMV mononucleosis), viral titers are low, and cytopathic effects may take several weeks to appear. Many laboratories expedite diagnosis with an overnight tissue-culture method (shell vial assay) involving centrifugation and an immunocytochemical detection technique employing monoclonal antibodies to an immediate-early CMV antigen. Isolation of virus from urine or saliva does not, by itself, constitute proof of acute infection, since excretion from these sites may continue for months or years after illness. Detection of viremia is a better predictor of acute infection.
A variety of serologic assays detect antibody to CMV. An increased level of IgG antibody to CMV may not be detectable for up to 4 weeks after primary infection. Detection of CMV-specific IgM is sometimes useful in the diagnosis of recent or active infection; however, circulating rheumatoid factors may result in occasional false-positive IgM tests. Serology is especially helpful when used to predict risk of CMV infection and disease in transplant recipients.
PREVENTION
Prevention of CMV in organ and hematopoietic stem cell transplant recipients is usually based on one of two methods: universal prophylaxis or preemptive therapy. With universal prophylaxis, antiviral drugs are used for a defined period, often 3 or 6 months. One clinical trial demonstrated that, in CMV-seronegative recipients with seropositive donors, prophylaxis was more effective at prevention when given for 200 days rather than 100 days. With preemptive therapy, patients are monitored weekly for CMV viremia, and antiviral treatment is initiated once viremia is detected. Because of the bone marrow–suppressive effects of universal prophylaxis, preemptive therapy is more commonly employed in hematopoietic stem cell transplant recipients. For patients with advanced HIV infection (CD4+ T cell counts of <50/μL), some experts have advocated prophylaxis with valganciclovir (see below). However, side effects, lack of proven benefit, possible induction of viral resistance, and high cost have precluded the wide acceptance of this practice. Preemptive therapy is under study in HIV-infected patients.
Several additional measures are useful for the prevention of CMV transmission to CMV-naïve, high-risk patients. The use of CMV-seronegative or leukocyte-depleted blood greatly decreases the rate of transfusion-associated transmission. In a placebo-controlled trial, a CMV glycoprotein B vaccine reduced infection rates among 464 CMV-seronegative women; this outcome raises the possibility that this experimental vaccine will reduce rates of congenital infection, but further studies must validate this approach. A CMV glycoprotein B vaccine with MF59 adjuvant appeared effective in reducing the risk and duration of viremia in both seropositive and seronegative renal transplant recipients at risk for CMV infection. CMV immune globulin has been reported to prevent congenital CMV infection in infants of women with primary infection during pregnancy. Studies in hematopoietic stem cell transplant recipients have produced conflicting results.
Prophylactic acyclovir or valacyclovir may reduce rates of CMV infection and disease in renal transplant recipients, although neither drug is effective in the treatment of active CMV disease.
HUMAN HERPESVIRUS (HHV) TYPES 6, 7, AND 8
HHV-6 and HHV-7
![]() HHV-6 and -7 seropositivity rates are generally high throughout the world. HHV-6 was first isolated in 1986 from peripheral-blood leukocytes of six persons with various lymphoproliferative disorders. Two genetically distinct variants (HHV-6A and HHV-6B) are now recognized. HHV-6 appears to be transmitted by saliva and possibly by genital secretions.
HHV-6 and -7 seropositivity rates are generally high throughout the world. HHV-6 was first isolated in 1986 from peripheral-blood leukocytes of six persons with various lymphoproliferative disorders. Two genetically distinct variants (HHV-6A and HHV-6B) are now recognized. HHV-6 appears to be transmitted by saliva and possibly by genital secretions.
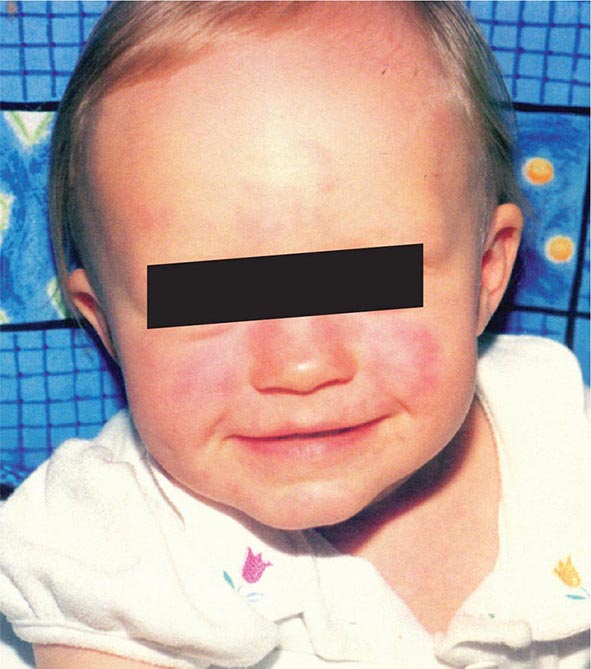
Infection with HHV-6 frequently occurs during infancy as maternal antibody wanes. The peak age of acquisition is 9–21 months; by 24 months, seropositivity rates approach 80%. Older siblings appear to serve as a source of transmission. Congenital infection also may occur, and ∼1% of newborns are infected with HHV-6; placental infection with HHV-6 has been described. Most postnatally infected children develop symptoms (fever, fussiness, and diarrhea). A minority develop exanthem subitum (roseola infantum; see Fig. 25e-5), a common illness characterized by fever with subsequent rash. In addition, ~10–20% of febrile seizures without rash during infancy are caused by HHV-6. After initial infection, HHV-6 persists in peripheral-blood mononuclear cells as well as in the central nervous system, salivary glands, and female genital tract.
In older age groups, HHV-6 has been associated with mononucleosis syndromes; in immunocompromised hosts, encephalitis, pneumonitis, syncytial giant-cell hepatitis, and disseminated disease are seen. In transplant recipients, HHV-6 infection may also be associated with graft dysfunction. Acute HHV-6-associated limbic encephalitis has been reported in hematopoietic stem cell transplant recipients and is characterized by memory loss, confusion, seizures, hyponatremia, and abnormal electroencephalographic and MRI results. High plasma loads of HHV-6 DNA in hematopoietic stem cell transplant recipients are associated with allelic-mismatched donors, use of glucocorticoids, delayed monocyte and platelet engraftment, development of limbic encephalitis, and increased all-cause mortality rates. Like many other viruses, HHV-6 has been implicated in the pathogenesis of multiple sclerosis, although further study is needed to distinguish between association and etiology.
HHV-7 was isolated in 1990 from T lymphocytes from the peripheral blood of a healthy 26-year-old man. The virus is frequently acquired during childhood, albeit at a later age than HHV-6. HHV-7 is commonly present in saliva, which is presumed to be the principal source of infection; breast milk also can carry the virus. Viremia can be associated with either primary or reactivation infection. The most common clinical manifestations of childhood HHV-7 infections are fever and seizures. Some children present with respiratory or gastrointestinal signs and symptoms. An association has been made between HHV-7 and pityriasis rosea, but evidence is insufficient to indicate a causal relationship.
Clustering of HHV-6, HHV-7, and CMV infections in transplant recipients can make it difficult to sort out the roles of the various agents in individual clinical syndromes. HHV-6 and HHV-7 appear to be susceptible to ganciclovir and foscarnet, although definitive evidence of clinical response is lacking.
HHV-8
Unique herpesvirus-like DNA sequences were reported during 1994 and 1995 in tissues derived from Kaposi’s sarcoma (KS) and body cavity–based lymphoma occurring in patients with AIDS. The virus from which these sequences were derived is designated HHV-8 or Kaposi’s sarcoma–associated herpesvirus (KSHV). HHV-8, which infects B lymphocytes, macrophages, and both endothelial and epithelial cells, appears to be causally related not only to KS and a subgroup of AIDS-related B cell body cavity–based lymphomas (primary effusion lymphomas) but also to multicentric Castleman’s disease, a lymphoproliferative disorder of B cells. The association of HHV-8 with several other diseases has been reported but not confirmed.
![]() HHV-8 seropositivity occurs worldwide, with areas of high endemicity influencing rates of disease. Unlike other herpesvirus infections, HHV-8 infection is much more common in some geographic areas (e.g., central and southern Africa) than in others (North America, Asia, northern Europe). In high-prevalence areas, infection occurs in childhood, seropositivity is associated with having a seropositive mother or (to a lesser extent) older sibling, and HHV-8 may be transmitted in saliva. In low-prevalence areas, infections typically occur in adults, probably with sexual transmission. Concurrent epidemics of HIV-1 and HHV-8 infections among certain populations (e.g., men who have sex with men) in the late 1970s and early 1980s appear to have resulted in the frequent association of AIDS and KS. Transmission of HHV-8 may also be associated with organ transplantation, injection drug use, and blood transfusion; however, transmission via blood transfusion in the United States appears to be quite rare.
HHV-8 seropositivity occurs worldwide, with areas of high endemicity influencing rates of disease. Unlike other herpesvirus infections, HHV-8 infection is much more common in some geographic areas (e.g., central and southern Africa) than in others (North America, Asia, northern Europe). In high-prevalence areas, infection occurs in childhood, seropositivity is associated with having a seropositive mother or (to a lesser extent) older sibling, and HHV-8 may be transmitted in saliva. In low-prevalence areas, infections typically occur in adults, probably with sexual transmission. Concurrent epidemics of HIV-1 and HHV-8 infections among certain populations (e.g., men who have sex with men) in the late 1970s and early 1980s appear to have resulted in the frequent association of AIDS and KS. Transmission of HHV-8 may also be associated with organ transplantation, injection drug use, and blood transfusion; however, transmission via blood transfusion in the United States appears to be quite rare.
Primary HHV-8 infection in immunocompetent children may manifest as fever and maculopapular rash. Among individuals with intact immunity, chronic asymptomatic infection is the rule, and neoplastic disorders generally develop only after subsequent immunocompromise. Immunocompromised persons with primary infection may present with fever, splenomegaly, lymphoid hyperplasia, pancytopenia, or rapid-onset KS. Quantitative analysis of HHV-8 DNA suggests a predominance of latently infected cells in KS lesions and frequent lytic replication in multicentric Castleman’s disease.
Effective antiretroviral therapy for HIV-infected individuals has led to a marked reduction in rates of KS among persons dually infected with HHV-8 and HIV in resource-rich areas. HHV-8 itself is susceptible in vitro to ganciclovir, foscarnet, and cidofovir. A small, randomized, double-blind, placebo-controlled, crossover trial suggested that oral valganciclovir administered once daily reduced HHV-8 replication. However, clinical benefits of valganciclovir or other drugs in HHV-8 infection have not yet been demonstrated. Sirolimus has been shown to inhibit the progression of dermal KS in kidney transplant recipients while providing effective immunosuppression.
220e |
Molluscum Contagiosum, Monkeypox, and Other Poxvirus Infections |
The poxvirus family includes a large number of related DNA viruses that infect various vertebrate hosts. The poxviruses responsible for infections in humans, the geographic locations in which these infections are found, the host reservoirs, and the main manifestations are listed in Table 220e-1. Infections with orthopoxviruses—e.g., smallpox (variola major) virus (Chap. 261e) or the zoonotic monkeypox virus—can result in systemic, potentially lethal human disease. Other poxvirus infections cause primarily localized skin disease in humans.
|
POXVIRUSES AND HUMAN INFECTIONS |
MOLLUSCUM CONTAGIOSUM
Molluscum contagiosum virus is an obligate human pathogen that causes distinctive proliferative skin lesions. These lesions measure 2–5 mm in diameter and are pearly, flesh-colored, and umbilicated, with a characteristic dimple at the center (Fig. 220e-1). A relative lack of inflammation and necrosis distinguishes these proliferative lesions from other poxvirus lesions. Lesions may be found—singly or in clusters—anywhere on the body except on the palms and soles and may be associated with an eczematous rash.
FIGURE 220e-1 Molluscum contagiosum is a cutaneous poxvirus infection characterized by multiple umbilicated flesh-colored or hypopigmented papules.
Molluscum contagiosum is highly prevalent among children and is the most common human disease resulting from poxvirus infection. Swimming pools are a common vector for transmission. Atopy and compromise of skin integrity increase the risk of infection. Genital lesions are more common in adults, to whom the virus may be transmitted by sexual contact. The incubation period ranges from 2 weeks to 6 months, with an average of 2–7 weeks. In most cases, the disease is self-limited and regresses spontaneously after 3–4 months in immunocompetent hosts. There are no systemic complications, but skin lesions may persist for 3–5 years. Molluscum contagiosum can be associated with immunosuppression and is frequently seen among HIV-infected patients (Chap. 226). The disease can be more generalized, severe, and persistent in AIDS patients than in other groups. Moreover, molluscum contagiosum can be exacerbated in the immune reconstitution inflammatory syndrome (IRIS) associated with the initiation of antiretroviral therapy.
The diagnosis of molluscum contagiosum is typically based on its clinical presentation and can be confirmed by histologic demonstration of the cytoplasmic eosinophilic inclusions (molluscum bodies) that are characteristic of poxvirus replication. Molluscum contagiosum virus cannot be propagated in vitro, but electron microscopy and molecular studies can be used for its identification.
There is no specific systemic treatment for molluscum contagiosum, but a variety of techniques for physical ablation have been used. Cidofovir displays in vitro activity against many poxviruses, and case reports suggest that parenteral or topical cidofovir may have some efficacy in the treatment of recalcitrant molluscum contagiosum in immunosuppressed hosts.
MONKEYPOX
![]() Although monkeypox virus was named after the animal from which it was originally isolated, rodents are the primary viral reservoir. Human infections with monkeypox virus typically occur in Africa when humans come into direct contact with infected animals. Human-to-human propagation of monkeypox infection is rare. Human disease is characterized by a systemic illness and vesicular rash similar to those of variola. The clinical presentation of monkeypox can be confused with that of the more common varicella-zoster virus infection (Chap. 217). Compared with the lesions of this herpesvirus infection, monkeypox lesions tend to be more uniform (i.e., in the same stage of development), diffuse, and peripheral in distribution. Lymphadenopathy is a prominent feature of monkeypox infection.
Although monkeypox virus was named after the animal from which it was originally isolated, rodents are the primary viral reservoir. Human infections with monkeypox virus typically occur in Africa when humans come into direct contact with infected animals. Human-to-human propagation of monkeypox infection is rare. Human disease is characterized by a systemic illness and vesicular rash similar to those of variola. The clinical presentation of monkeypox can be confused with that of the more common varicella-zoster virus infection (Chap. 217). Compared with the lesions of this herpesvirus infection, monkeypox lesions tend to be more uniform (i.e., in the same stage of development), diffuse, and peripheral in distribution. Lymphadenopathy is a prominent feature of monkeypox infection.
The first outbreak of human monkeypox infection in the Western Hemisphere occurred during 2003, when more than 70 cases were reported in the midwestern United States. The outbreak was linked to contact with pet prairie dogs that had become infected while being housed with rodents imported from Ghana. Patients presented most frequently with fever, rash, and lymphadenopathy ~12 days after exposure. Nine patients were hospitalized, but there were no deaths. Smallpox vaccination can provide cross-reactive immunity to monkeypox infection; studies of people exposed in the outbreak detected subclinical infection in a few vaccinated individuals—an observation suggesting the possibility of long-term vaccine protection. The risk of human disease from animal orthopoxvirus infections may increase as smallpox immunity wanes in the general population and the popularity of exotic animals as household pets grows.
OTHER ZOONOTIC POXVIRUS INFECTIONS
Cowpox and buffalopox are rare zoonotic infections characterized by cutaneous poxlike lesions and mild systemic illness. Outbreaks of similar poxlike lesions among cattle and farm workers in Brazil have been due to Cantagalo and Araçatuba viruses, which are virtually identical to vaccinia virus and may have become established in cattle during smallpox vaccination programs.
Parapoxviruses are widely scattered among animal species, but only a few are known to cause human disease via direct contact with infected animals. Parapoxviruses are antigenically distinct from orthopoxviruses and share no cross-immunity. Tanapox virus belongs to a separate, antigenically distinct genus and usually causes a single nodular lesion on the exposed area after contact with infected monkeys.
221 |
Parvovirus Infections |
Parvoviruses, members of the family Parvoviridae, are small (diameter, ~22 nm), nonenveloped, icosahedral viruses with a linear single-strand DNA genome of ~5000 nucleotides. These viruses are dependent on either rapidly dividing host cells or helper viruses for replication. At least four groups of parvoviruses infect humans: parvovirus B19 (B19V), dependoparvoviruses (adeno-associated viruses; AAVs), PARV4/5 virus, and human bocaviruses (HBoVs). Human dependoparvoviruses are nonpathogenic and will not be considered further in this chapter.
PARVOVIRUS B19
DEFINITION
![]() B19V is the type member of the genus Erythroparvovirus. On the basis of viral sequence, B19V is divided into three genotypes (designated 1, 2, and 3), but only a single B19V antigenic type has been described. Genotype 1 is predominant in most parts of the world; genotype 2 is rarely associated with active infection; and genotype 3 appears to predominate in parts of western Africa.
B19V is the type member of the genus Erythroparvovirus. On the basis of viral sequence, B19V is divided into three genotypes (designated 1, 2, and 3), but only a single B19V antigenic type has been described. Genotype 1 is predominant in most parts of the world; genotype 2 is rarely associated with active infection; and genotype 3 appears to predominate in parts of western Africa.
EPIDEMIOLOGY
![]() B19V exclusively infects humans, and infection is endemic in virtually all parts of the world. Transmission occurs predominantly via the respiratory route and is followed by the onset of rash and arthralgia. By the age of 15 years, ~ 50% of children have detectable IgG; this figure rises to >90% among the elderly. In pregnant women, the estimated annual seroconversion rate is ~1%. Within households, secondary infection rates approach 50%.
B19V exclusively infects humans, and infection is endemic in virtually all parts of the world. Transmission occurs predominantly via the respiratory route and is followed by the onset of rash and arthralgia. By the age of 15 years, ~ 50% of children have detectable IgG; this figure rises to >90% among the elderly. In pregnant women, the estimated annual seroconversion rate is ~1%. Within households, secondary infection rates approach 50%.
Detection of high-titer B19V in blood is not unusual (see “Pathogenesis,” below). Transmission can occur as a result of transfusion, most commonly of pooled components. To reduce the risk of transmission, plasma pools are screened by nucleic acid amplification technology, and high-titer pools are discarded. B19V is resistant to both heat and solvent-detergent inactivation.
PATHOGENESIS
B19V replicates primarily in erythroid progenitors. This specificity is due in part to the limited tissue distribution of the primary B19V receptor, blood group P antigen (globoside). Infection leads to high-titer viremia, with >1012 virus particles (or IU)/mL detectable in the blood at the apex (Fig. 221-1), and virus-induced cytotoxicity results in cessation of red cell production. In immunocompetent individuals, viremia and arrest of erythropoiesis are transient and resolve as the IgM and IgG antibody response is mounted. In individuals with normal erythropoiesis, there is only a minimal drop in hemoglobin levels; however, in those with increased erythropoiesis (especially with hemolytic anemia), this cessation of red cell production can induce a transient crisis with severe anemia (Fig. 221-1). Similarly, if an individual (or, after maternal infection, a fetus) does not mount a neutralizing antibody response and halt the lytic infection, erythroid production is compromised and chronic anemia develops (Fig. 221-1).
FIGURE 221-1 Schematic of the time course of parvovirus B19V infection in (A) normals (erythema infectiosum), (B) transient aplastic crisis (TAC), and (C) chronic anemia/pure red-cell aplasia (PRCA). (Reprinted with permission from NS Young, KE Brown: N Engl J Med 350:586, 2004. © 2004 Massachusetts Medical Society. All rights reserved.)
The immune-mediated phase of illness, which begins 2–3 weeks after infection as the IgM response peaks, manifests as the rash of fifth disease together with arthralgia and/or frank arthritis. Low-level B19V DNA can be detected by polymerase chain reaction (PCR) in blood and tissues for months to years after acute infection. The B19V receptor is found in a variety of other cells and tissues, including megakaryocytes, endothelial cells, placenta, myocardium, and liver. Infection of these tissues by B19V may be responsible for some of the more unusual presentations of the infection. Rare individuals who lack P antigen are naturally resistant to B19V infection.
CLINICAL MANIFESTATIONS
Erythema Infectiosum Most B19V infections are asymptomatic or are associated with only a mild nonspecific illness. The main manifestation of symptomatic B19V infection is erythema infectiosum, also known as fifth disease or slapped-cheek disease (Fig. 221-2 and Fig. 25e-1A). Infection begins with a minor febrile prodrome ~7–10 days after exposure, and the classic facial rash develops several days later; after 2–3 days, the erythematous macular rash may spread to the extremities in a lacy reticular pattern. However, its intensity and distribution vary, and B19V-induced rash is difficult to distinguish from other viral exanthems. Adults typically do not exhibit the “slapped-cheek” phenomenon but present with arthralgia, with or without the macular rash.
FIGURE 221-2 Young child with erythema infectiosum, or fifth disease, showing typical “slapped-cheek” appearance.
Polyarthropathy Syndrome Although uncommon among children, arthropathy occurs in ~50% of adults and is more common among women than among men. The distribution of the affected joints is often symmetrical, with arthralgia affecting the small joints of the hands and occasionally the ankles, knees, and wrists. Resolution usually occurs within a few weeks, but recurring symptoms can continue for months. The illness may mimic rheumatoid arthritis, and rheumatoid factor can often be detected in serum. B19V infection may trigger rheumatoid disease in some patients and has been associated with juvenile idiopathic arthritis.
Transient Aplastic Crisis Asymptomatic transient reticulocytopenia occurs in most individuals with B19V infection. However, in patients who depend on continual rapid production of red cells, infection can cause transient aplastic crisis (TAC). Affected individuals include those with hemolytic disorders, hemoglobinopathies, red cell enzymopathies, and autoimmune hemolytic anemias. Patients present with symptoms of severe anemia (sometimes life-threatening) and a low reticulocyte count, and bone marrow examination reveals an absence of erythroid precursors and characteristic giant pronormoblasts. As its name indicates, the illness is transient, and anemia resolves with the cessation of cytopathic infection in the erythroid progenitors.
Pure Red-Cell Aplasia/Chronic Anemia Chronic B19V infection has been reported in a wide range of immunosuppressed patients, including those with congenital immunodeficiency, AIDS (Chap. 226), lympho-proliferative disorders (especially acute lymphocytic leukemia), and transplantation (Chap. 169). Patients have persistent anemia with reticulocytopenia, absent or low levels of B19V IgG, high titers of B19V DNA in serum, and—in many cases—scattered giant pronormoblasts in bone marrow. Rarely, nonerythroid hematologic lineages are also affected. Transient neutropenia, lymphopenia, and thrombocytopenia (including idiopathic thrombocytopenic purpura) have been observed. B19V occasionally causes a hemophagocytic syndrome.
![]() Recent studies in Papua New Guinea and Ghana, where malaria is endemic, suggest that co-infection with Plasmodium and B19V plays a major role in the development of severe anemia in young children. Further studies must determine whether B19V infection contributes to severe anemia in other malarial regions.
Recent studies in Papua New Guinea and Ghana, where malaria is endemic, suggest that co-infection with Plasmodium and B19V plays a major role in the development of severe anemia in young children. Further studies must determine whether B19V infection contributes to severe anemia in other malarial regions.
Hydrops Fetalis B19V infection during pregnancy can lead to hydrops fetalis and/or fetal loss. The risk of transplacental fetal infection is ~30%, and the risk of fetal loss (predominantly early in the second trimester) is ~9%. The risk of congenital infection is <1%. Although B19V does not appear to be teratogenic, anecdotal cases of eye damage and central nervous system (CNS) abnormalities have been reported. Cases of congenital anemia have also been described. B19V probably causes 10–20% of all cases of nonimmune hydrops.
Unusual Manifestations B19V infection may rarely cause hepatitis, vasculitis, myocarditis, glomerulosclerosis, or meningitis. A variety of other cardiac manifestations, CNS diseases, and autoimmune infections have also been reported. However, B19V DNA can be detected by PCR for years in many tissues; this finding is of no known clinical significance, but its interpretation may cause confusion regarding B19V disease association.
DIAGNOSIS
Diagnosis of B19V infection in immunocompetent individuals is generally based on detection of B19V IgM antibodies (Table 221-1). IgM can be detected at the time of rash in erythema infectiosum and by the third day of TAC in patients with hematologic disorders; these antibodies remain detectable for ~3 months. B19V IgG is detectable by the seventh day of illness and persists throughout life. Quantitative detection of B19V DNA should be used for the diagnosis of early TAC or chronic anemia. Although B19V levels fall rapidly with the development of the immune response, DNA can be detectable by PCR for months or even years after infection, even in healthy individuals; therefore, quantitative PCR should be used. In acute infection at the height of viremia, >1012 B19V DNA IU/mL of serum can be detected; however, titers fall rapidly within 2 days. Patients with aplastic crisis or B19V-induced chronic anemia generally have >105 B19V DNA IU/mL.
|
DISEASES ASSOCIATED WITH HUMAN PARVOVIRUS B19 INFECTION AND METHODS OF DIAGNOSIS |
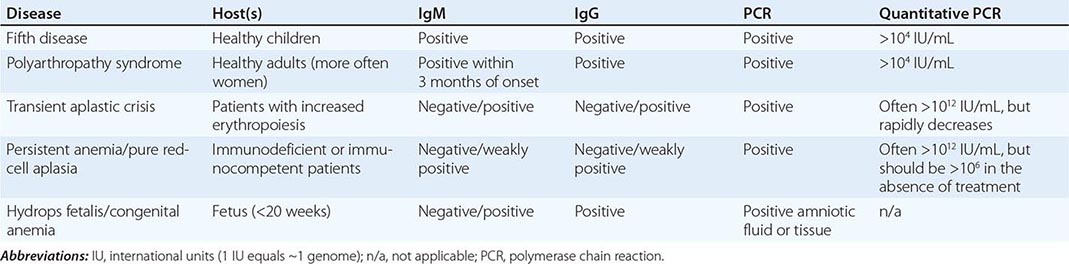
PREVENTION
No vaccine has been approved for the prevention of B19V infection, although vaccines based on B19V virus-like particles expressed in insect cells are known to be highly immunogenic. Phase 1 trials of a putative vaccine were discontinued because of adverse side effects.
PARV4/5
DEFINITION
The PARV4 viral sequence was initially detected in a patient with an acute viral syndrome. Similar sequences, including the related PARV5 sequence, have been detected in pooled plasma collections. The DNA sequence of PARV4/5 is distinctly different from that of all other parvoviruses, and this virus is now classified as a member of the newly described genus Tetraparvovirus.
EPIDEMIOLOGY
![]() PARV4 DNA is commonly found in plasma pools but at lower concentrations than levels of B19V DNA found before in plasma pools prior to screening. The higher levels of PARV4 DNA and IgG antibody in tissues (bone marrow and lymphoid tissue) and sera from IV drug users than in the corresponding specimens from control patients suggest that the virus is transmitted predominantly by parenteral means in the United States and Europe. Evidence for nonparenteral transmission in other parts of the world is limited.
PARV4 DNA is commonly found in plasma pools but at lower concentrations than levels of B19V DNA found before in plasma pools prior to screening. The higher levels of PARV4 DNA and IgG antibody in tissues (bone marrow and lymphoid tissue) and sera from IV drug users than in the corresponding specimens from control patients suggest that the virus is transmitted predominantly by parenteral means in the United States and Europe. Evidence for nonparenteral transmission in other parts of the world is limited.
CLINICAL MANIFESTATIONS
To date, PARV4/5 infection has been associated only with mild clinical disease (rash and/or transient aminotransferase elevation).
HUMAN BOCAVIRUSES
DEFINITION
Animal bocaviruses are associated with mild respiratory symptoms and enteritis in young animals. HBoV1 was originally identified in the respiratory tract of young children with lower respiratory tract infections. More recently, HBoV1 and the related viruses HBoV2, HBoV3, and HBoV4 have all been identified in human fecal samples.
EPIDEMIOLOGY
![]() Seroepidemiologic studies with HBoV virus-like particles suggest that human bocavirus infection is common. Worldwide, most individuals are infected before the age of 5 years.
Seroepidemiologic studies with HBoV virus-like particles suggest that human bocavirus infection is common. Worldwide, most individuals are infected before the age of 5 years.
CLINICAL MANIFESTATIONS
HBoV1 DNA is found in respiratory secretions from 2–20% of children with acute respiratory infection, often in the presence of other pathogens; in these circumstances, the role of HBoV1 in disease pathogenesis is unknown. Clinical disease due to HBoV1 is associated with evidence of primary infection (IgG seroconversion or the presence of IgM), HBoV1 DNA in serum, or high-titer HBoV1 DNA (>104 genome copies/mL) in respiratory secretions. Symptoms are not dissimilar from those of other viral respiratory infections, and cough and wheezing are commonly reported. There is no specific treatment for bocavirus infection. The role of human bocaviruses in childhood gastroenteritis remains to be established.
222 |
Human Papillomavirus Infections |
Investigation of human papillomavirus (HPV) infection began in earnest in the 1980s after Harold zur Hausen postulated that infection with these viruses was associated with cervical cancer. It is now recognized that HPV infection of the human genital tract is extremely common and causes clinical states ranging from asymptomatic infection to genital warts (condylomata acuminata); dysplastic lesions or invasive cancers of the anus, penis, vulva, vagina, and cervix; and a subset of oropharyngeal cancers. This chapter describes the epidemiology of HPV in general and as a pathogen, the natural history of HPV infections and associated cancers, strategies to prevent HPV infection and HPV-associated disease, and treatment modalities.
PATHOGENESIS
![]() Molecular Overview HPV is an icosahedral, nonenveloped, 8000-base-pair, double-stranded DNA virus with a diameter of 55 nm. Like those of other papillomaviruses, HPV’s genome consists of an early (E) gene region, a late (L) gene region, and a noncoding region that contains regulatory elements. The E1, E2, E5, E6, and E7 proteins are expressed early in the growth cycle and are necessary for viral replication and cellular transformation. The E6 and E7 proteins cause malignant transformation by targeting the human cell cycle–regulatory molecules p53 and Rb (retinoblastoma protein), respectively, for degradation. Translation of the L1 and L2 transcripts and splicing of an E1^E4 transcript occur later. The L1 gene encodes the 54-kDa major capsid protein that makes up the majority of the virus shell; the 77-kDa L2 minor protein constitutes a smaller percentage of the capsid mass.
Molecular Overview HPV is an icosahedral, nonenveloped, 8000-base-pair, double-stranded DNA virus with a diameter of 55 nm. Like those of other papillomaviruses, HPV’s genome consists of an early (E) gene region, a late (L) gene region, and a noncoding region that contains regulatory elements. The E1, E2, E5, E6, and E7 proteins are expressed early in the growth cycle and are necessary for viral replication and cellular transformation. The E6 and E7 proteins cause malignant transformation by targeting the human cell cycle–regulatory molecules p53 and Rb (retinoblastoma protein), respectively, for degradation. Translation of the L1 and L2 transcripts and splicing of an E1^E4 transcript occur later. The L1 gene encodes the 54-kDa major capsid protein that makes up the majority of the virus shell; the 77-kDa L2 minor protein constitutes a smaller percentage of the capsid mass.
More than 125 HPV types have been identified and are numerically designated according to a unique L1 gene sequence. Approximately 40 HPV types are regularly found in the anogenital tract and are subdivided into high-risk and low-risk categories on the basis of the associated risk of cervical cancer. For example, HPV-6 and HPV-11 cause genital warts and ~10% of low-grade cervical lesions and are thus designated low risk. HPV-16 and HPV-18 cause dysplastic lesions and invasive cancers of the cervix and are considered high risk.
HPV targets basal keratinocytes after microtrauma has exposed these cells to the virus. The HPV replication cycle is completed as keratinocytes undergo differentiation. Virions are assembled in the nuclei of differentiated keratinocytes and can be detected by electron microscopy. Infection is transmitted by contact with virus contained in these desquamated keratinocytes (or with free virus) from an infected individual.
Immune Response to HPV Infection A cell-mediated immune response plays an important role in controlling the progression of natural HPV infection. Histologic examination of lesions in individuals who experience regression of genital warts demonstrates infiltration of T cells and macrophages. CD4+ T cell regulation is particularly important in controlling HPV infections, as evidenced by the higher rates of infection and disease among immunosuppressed individuals, particularly those who are infected with HIV. Specific T-cell responses may be measured against HPV proteins, the most important of which appear to be the E2 and E6 proteins. In women with HPV-16 cervical infection, a strong T-cell response to HPV-16-derived E2 protein is associated with a lack of progression of cervical disease.
Natural HPV infection of the genital tract gives rise to a serum antibody response in only 60–70% of individuals because there is no viremic phase during infection. Significant, although incomplete, protection against type-specific reinfection is associated with the presence of neutralizing antibodies. Serum antibodies likely reach the cervical epithelium and secretions by transudation and exudation. Therefore, protection against infection is related to the amount of neutralizing antibody at the site of infection and lasts as long as levels of neutralizing antibodies are sufficient.
EPIDEMIOLOGY AND NATURAL HISTORY OF HPV-ASSOCIATED MALIGNANCY
General Population HPV is transmitted by sexual intercourse, by oral sex, and possibly by touching of a partner’s genitalia. In cross-sectional and longitudinal studies, ~40% of young women have evidence of HPV infection, with peaks during the teens and early twenties—soon after first coitus. The number of lifetime sexual partners correlates with the likelihood of HPV infection and the subsequent risk of HPV-associated malignancy. HPV infection may develop in a monogamous person whose partner is infected. Most HPV infections become undetectable after 6–9 months. However, with prolonged follow-up and frequent sampling, the same HPV types may again be detected weeks or months after becoming undetectable. Whether such episodic detection indicates viral latency followed by reactivation or reinfection with an identical HPV type is still debated.
![]() Although HPV is the causative agent of several cancers, most attention has focused on cervical cancer—the second most common cancer among women worldwide, which affects more than 500,000 women and kills more than 275,000 women annually. More than 85% of all cervical cancer cases and deaths occur in women living in low-income countries, especially in sub-Saharan Africa, Asia, and South and Central America. A quarter-century of evidence shows that HPV causes nearly 100% of cervical cancers. HPV infection is the most significant risk factor for cervical cancer; relative risks range from 10 to 20 and exceed 100 in prospective and case-control studies, respectively. The time from HPV infection to cervical cancer diagnosis may exceed 20 years. Cervical cancer peaks in the fifth and sixth decades of life among women living in developed countries but as much as a decade earlier among women living in resource-poor countries. Persistent carriers of oncogenic HPV types are at greatest risk for high-grade cervical dysplasia and cancer. Why only certain HPV infections eventually lead to malignancy is not clear. Biomarkers that can predict which women will develop cervical cancer are not available. Immunosuppression in general plays a significant role in re-detection/reactivation of HPV infections, while other factors such as smoking, hormonal changes, Chlamydia infection, and nutritional deficits promote viral persistence and cancer.
Although HPV is the causative agent of several cancers, most attention has focused on cervical cancer—the second most common cancer among women worldwide, which affects more than 500,000 women and kills more than 275,000 women annually. More than 85% of all cervical cancer cases and deaths occur in women living in low-income countries, especially in sub-Saharan Africa, Asia, and South and Central America. A quarter-century of evidence shows that HPV causes nearly 100% of cervical cancers. HPV infection is the most significant risk factor for cervical cancer; relative risks range from 10 to 20 and exceed 100 in prospective and case-control studies, respectively. The time from HPV infection to cervical cancer diagnosis may exceed 20 years. Cervical cancer peaks in the fifth and sixth decades of life among women living in developed countries but as much as a decade earlier among women living in resource-poor countries. Persistent carriers of oncogenic HPV types are at greatest risk for high-grade cervical dysplasia and cancer. Why only certain HPV infections eventually lead to malignancy is not clear. Biomarkers that can predict which women will develop cervical cancer are not available. Immunosuppression in general plays a significant role in re-detection/reactivation of HPV infections, while other factors such as smoking, hormonal changes, Chlamydia infection, and nutritional deficits promote viral persistence and cancer.
The International Agency for Research on Cancer concludes that HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 are carcinogenic in the uterine cervix. HPV-16 is particularly virulent and causes 50% of cervical cancers. Worldwide, HPV-16 and HPV-18 cause 70% of cervical squamous cell carcinomas and 85% of cervical adenocarcinomas. Oncogenic types other than HPV-16 and HPV-18 cause the remaining 30% of cervical cancers. HPV-16 and HPV-18 also cause nearly 90% of anal cancers worldwide. Although oncogenic HPV infection is necessary for the development of cervical malignancy, only ~3–5% of infected women will ever develop this cancer, even in the absence of cytologic screening.
In addition to cervical and anal cancer, other HPV-associated cancers include vulvar and vaginal cancer, which are associated with HPV in 50–70% of cases; penile cancer, which is caused by HPV in 50% of cases; and oropharyngeal squamous cell carcinoma (OPSCC). Over the past two decades, an epidemic of OPSCC related to oncogenic infection with HPV (primarily HPV-16) has developed. Annual rates of OPSCC among men in the United States have been increasing from a low of 0.27 case/100,000 in 1973 to 0.57 case/100,000 as of 2004; rates in women have remained relatively stable at ~0.17 case/100,000 per year. The increase in the incidence of OPSCC is greatest among white men 40–50 years of age. Nearly 14,000 new cases were diagnosed in the United States in 2013. Annual rates of OPSCCs of the base of the tongue and the tonsil have increased dramatically—i.e., by 1.3% and 0.6%, respectively. Fewer data are available from developing countries about OPSCCs.
Effects of HIV on HPV-Associated Disease HIV infection accelerates the natural progression of HPV infections. HIV-infected persons are more likely than other individuals to develop genital warts and to have lesions that are more recalcitrant to treatment. HIV infection has been consistently associated with precancerous cervical lesions, including low-grade cervical intraepithelial neoplasia (CIN) and CIN 3, the immediate precursor to cervical cancer. Women with HIV/AIDS have significantly higher rates of cervical cancer and of subsets of some vulvar, vaginal, and oropharyngeal tumors than women in the general population. Studies indicate a direct relation between low CD4+ T lymphocyte counts and the risk of cervical cancer. Some studies show a reduced likelihood of HPV infection and precancerous lesions of the cervix in HIV-infected women receiving antiretroviral therapy (ART). The incidence of cervical cancer among HIV-infected women has not changed significantly since ART was introduced, possibly because of preexisting oncogenic HPV infections.
![]() The burden of HPV-related cancers is expected to increase in HIV-infected patients, given the prolonged life expectancies made possible by ART. For women living in developing countries where cervical cancer screening is not widely available, this situation may have significant consequences. Thus, elucidating the interactions of HIV infection and cervical cancer with cofactors such as diet, other sexually transmitted infections, and environmental exposures is a research focus with potentially enormous implications for women living in low- and middle-income countries.
The burden of HPV-related cancers is expected to increase in HIV-infected patients, given the prolonged life expectancies made possible by ART. For women living in developing countries where cervical cancer screening is not widely available, this situation may have significant consequences. Thus, elucidating the interactions of HIV infection and cervical cancer with cofactors such as diet, other sexually transmitted infections, and environmental exposures is a research focus with potentially enormous implications for women living in low- and middle-income countries.
Similar to that of cervical cancer, the incidence of anal cancer is strongly influenced by HIV infection. HIV-infected men who have sex with men (MSM) and HIV-infected women have much higher rates of anal cancer than HIV-uninfected populations. Specifically, the incidence has been found to be as high as 130 cases/100,000 among HIV-positive MSM as opposed to only 5 cases/100,000 among HIV-negative MSM. The advent of ART has not impacted the incidence of anal cancer and high-grade anal intraepithelial neoplasia in the HIV-infected population.
More information on screening, prevention, and treatment in the HIV-infected population can be found at the Department of Health and Human Services website (aidsinfo.nih.gov/guidelines).
CLINICAL MANIFESTATIONS OF HPV INFECTION
HPV infects the female vulva, vagina, and cervix and the male urethra, penis, and scrotum. Perianal, anal, and oropharyngeal infections occur in both genders. Figures 222-1, 222-2, and 222-3 show vulvar, penile, and perianal warts, respectively. Genital warts are caused primarily by HPV-6 or HPV-11; their surface is either smooth or rough. Penile genital warts are usually 2–5 mm in diameter and often occur in groups. A second type of penile lesion, keratotic plaques, is slightly raised above the normal epithelium and has a rough, often pigmented surface. Vulvar warts are soft, whitish papules that either are sessile or have multiple fine, finger-like projections. These lesions are most often located in the introitus and on the labia. In nonmucosal areas, lesions are similar in appearance to those in men: dry and keratotic. Vulvar lesions can appear as smooth, sometimes pigmented papules that may coalesce. Vaginal lesions appear as multiple areas of elongated papillae. Biopsy of vulvar or vaginal lesions may reveal malignancy, which is not always reliably identified by clinical examination.
FIGURE 222-1 Vulvar warts. (Downloaded from http://www2a.cdc.gov/stdtraining/ready-to-use/Manuals/HPV/hpv-slides-2013.pdf.)
FIGURE 222-2 Condyloma acuminata of the shaft of the penis.
FIGURE 222-3 Perianal warts. (Reprinted from K Wolff, RA Johnson, AP Saavedra: Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology, 7th ed. New York, McGraw-Hill, 2013.)
Subclinical cervical HPV infections are common, and the cervix may appear normal on examination. Cervical lesions often appear as papillary proliferations near the transformation zone. Irregular vascular loops are present beneath the surface epithelium. Patients who develop cervical cancer arising from HPV infection may present with a variety of symptoms. Early carcinomas appear eroded and bleed easily. More advanced carcinomas present as ulcerated lesions or as an exophytic cervical mass. Some cervical carcinomas are located in the cervical canal and may be difficult to see. Bleeding, symptoms of a mass lesion in late stages, and metastatic disease that may manifest as bowel or bladder obstruction due to direct extension of the tumor have also been described.
Patients with squamous cell cancer of the anus have more variable presentations. The most common presentations include rectal bleeding and pain or a mass sensation. Of patients who are diagnosed with anal cancer, 20% may present with no specific symptoms at the time of diagnosis; rather, the lesion is found fortuitously.
PREVENTION OF HPV INFECTION: HPV VACCINES
Vaccines effective in preventing HPV infection and HPV-associated disease represent a major development in the last decade. The vaccines use virus-like particles (VLPs) that consist of the HPV L1 major capsid protein. The L1 protein self-assembles into VLPs when expressed in eukaryotic cells (i.e., yeast for the Merck vaccine or insect cells for the GlaxoSmithKline vaccine; see below). These VLPs contain the same epitopes as the HPV virion. However, they do not contain genetic material and cannot transmit infection. The immunogenicity of HPV vaccines relies on the development of conformational neutralizing antibodies to epitopes displayed on viral capsids.
Several large trials have demonstrated the high degree of safety and efficacy of HPV vaccines. The evidence to date has shown high and sustained efficacy against disease caused by those HPV types represented in the vaccines (HPV-6, -11, -16, and -18 in the Merck vaccine and HPV-16 and -18 in the GlaxoSmithKline vaccine). However, no therapeutic effect against active infection or disease has been found for either vaccine.
Bivalent Vaccine (Cervarix) A bivalent L1 VLP vaccine (HPV-16 and -18), marketed under the name Cervarix (GlaxoSmithKline), is administered by IM injection at months 0, 1, and 6. This vaccine was tested in 18,644 women 15–25 years of age who were residing in the United States, South America, Europe, and Asia. The primary endpoints of the study included vaccine efficacy against persistent infections with HPV-16 and -18. Investigators also assessed the vaccine’s efficacy against CIN of grade 2 or higher due to HPV-16 and -18 in women who had no evidence of infection with these HPV types at baseline; in these women, vaccine efficacy was 94.9% (95% confidence interval [CI], 87.7 to 98.4) against CIN ≥2 related to HPV-16 or HPV-18, 91.7% (95% CI, 66.6 to 99.1) against CIN ≥3, and 100% (95% CI, –8.6 to 100) against adenocarcinoma in situ.
Adverse events were evaluated in phase 3 trials in a subset of 3077 women who received the bivalent vaccine and 3080 women (controls) who received hepatitis A vaccine. Injection-site adverse events (pain, redness, and swelling) and systemic adverse events (fatigue, headache, and myalgia) were reported more frequently in the HPV vaccine group than in the control group. Serious adverse events (mainly injection-site reactions), new-onset chronic disease, or medically significant conditions occurred in 3.5% of HPV vaccine recipients and in 3.5% of women receiving the control vaccine.
The bivalent vaccine is approved in the United States for prevention of cervical cancer, CIN ≥2, adenocarcinoma in situ, and CIN 1 caused by HPV-16 and -18. This vaccine is approved for administration to girls and women 9–25 years of age.
Quadrivalent Vaccine (Gardasil) A quadrivalent L1 VLP (HPV-6, -11, -16, and -18) vaccine, marketed under the name Gardasil (Merck), is administered IM at months 0, 2, and 6. A combined efficacy analysis based on data from four randomized double-blind clinical studies including more than 20,000 participants demonstrated that the vaccine’s efficacy against external genital warts was 98.9% (95% CI, 93.7 to 100). Its efficacy was 95.2% (95% CI, 87.2 to 98.7) in protecting against CIN, 100% (95% CI, 92.9 to 100) against HPV-16- or HPV-18-related CIN 2/3 or adenocarcinoma in situ, and 100% (95% CI, 55.5 to 100.0) against HPV-16- or HPV-18-related vulvar intraepithelial neoplasia grades 2 and 3 (VIN 2/3) and vaginal intraepithelial neoplasia grades 2 and 3 (VaIN 2/3).
Safety data on the quadrivalent HPV vaccine are available from seven clinical trials including nearly 12,000 girls and women 9–26 years of age who received the vaccine and ~10,000 who received placebo. A larger proportion of young women reported injection-site adverse events in the vaccine groups than in the aluminum-containing or saline placebo groups. Systemic adverse events were reported by similar proportions of vaccine and placebo recipients and were described as mild or moderate by most participants. The types of serious adverse events reported were similar for the two groups. Ten persons who received the quadrivalent vaccine and seven persons who received placebo died during the course of the trials; no deaths were considered to be vaccine related.
During the course of the quadrivalent vaccine trials, surveillance data on the development of new medical conditions were collected for up to 4 years after vaccination. No statistically significant differences in the incidence of any medical conditions between vaccine and placebo recipients were found; this result indicated a very good safety profile. A recent safety review by the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention (CDC) examined events related to Gardasil that had been reported to the Vaccine Adverse Events Reporting System. Adverse events were consistent with those seen in previous safety studies of the vaccine. It is noteworthy that rates of syncope and venous thrombotic events were higher with Gardasil than those that have usually been documented for other vaccines.
The quadrivalent vaccine is approved for (1) vaccination of girls and women 9–26 years of age to prevent genital warts and cervical cancer caused by HPV-6, -11, -16, and -18; (2) vaccination of the same population to prevent precancerous or dysplastic lesions, including cervical adenocarcinoma in situ, CIN 2/3, VIN 2/3, VaIN 2/3, and CIN 1; (3) vaccination of boys and men 9–26 years of age to prevent genital warts caused by HPV-6 and -11; and (4) vaccination of individuals 9–26 years of age to prevent anal cancer and associated precancerous lesions due to HPV-6, -11, -16, and -18.
Cross-Protection of HPV Vaccines Women vaccinated with either of the available vaccines produce neutralizing antibodies against types related to HPV-16 or -18. Analyses of data from clinical trials suggest that both vaccines may offer cross-protection against nonvaccine types. The bivalent vaccine appears more efficacious against HPV-31, -33, and -45 than the quadrivalent vaccine, but differences in study design make direct comparisons difficult. In addition, vaccine efficacy against persistent infections with HPV-31 and -45 appeared to wane over time in the bivalent vaccine trials, whereas efficacy against persistent infection with HPV-16 or -18 remained stable.
![]() Second-Generation Vaccines While HPV-16 and -18 cause the majority of cervical cancers worldwide, global data have shown that HPV-31, -33, -35, -45, -52, and -58 are the next most frequently detected types in invasive cervical cancers. Second-generation vaccines that are in development incorporate VLPs of additional oncogenic HPV types (other than HPV-16 and -18), including HPV-31, -33, -45, -52, and -58; efficacy studies are ongoing. If vaccines with these five additional oncogenic types prove to be effective, mathematical models estimate that the level of protection could be raised to 90% of all squamous cell cervical cancers worldwide.
Second-Generation Vaccines While HPV-16 and -18 cause the majority of cervical cancers worldwide, global data have shown that HPV-31, -33, -35, -45, -52, and -58 are the next most frequently detected types in invasive cervical cancers. Second-generation vaccines that are in development incorporate VLPs of additional oncogenic HPV types (other than HPV-16 and -18), including HPV-31, -33, -45, -52, and -58; efficacy studies are ongoing. If vaccines with these five additional oncogenic types prove to be effective, mathematical models estimate that the level of protection could be raised to 90% of all squamous cell cervical cancers worldwide.
Recommendations for Vaccination The CDC’s Advisory Committee for Immunization Practice recommends administration of the quadrivalent HPV vaccine—with the schedule used in the vaccine trials—to all boys and girls 11–12 years of age as well as to boys/men and girls/women 13–26 years of age who have not previously been vaccinated or who have not completed the full series. For women, Papanicolaou (Pap) smear testing and screening for HPV DNA are not recommended before vaccination. After vaccination, Pap testing is recommended to detect disease caused by other oncogenic HPV types.
PREVENTION OF HPV-ASSOCIATED DISEASE
![]() After HPV infection occurs, prevention of HPV-associated disease relies on screening. Women residing in developing countries who lack access to cervical screening programs have a higher rate of cervical cancer and a lower rate of cancer-specific survival. Approximately 75% of women living in developed countries have been screened in the past 5 years, whereas the figure is only ~5% among women living in developing countries. Economic and logistic obstacles likely impede routine screening of these populations for cervical cancer.
After HPV infection occurs, prevention of HPV-associated disease relies on screening. Women residing in developing countries who lack access to cervical screening programs have a higher rate of cervical cancer and a lower rate of cancer-specific survival. Approximately 75% of women living in developed countries have been screened in the past 5 years, whereas the figure is only ~5% among women living in developing countries. Economic and logistic obstacles likely impede routine screening of these populations for cervical cancer.
The primary method used for cancer screening is cervical cytology via Pap smear. The guidelines of the American Society of Colposcopy and Cervical Pathology recommend initiation of cervical cancer screening at age 21, regardless of the age of sexual debut. Women 21–29 years old with a normal Pap smear should have the test repeated every 3 years. Although adolescent and young women often test positive for HPV DNA, they are at very low risk of cervical cancer. Co-testing, or testing for HPV DNA at the time of the Pap smear, is not recommended for women in this age group because the presence of HPV DNA does not correlate with the presence of high-grade squamous intraepithelial neoplasia. Women 30–65 years of age should have a Pap smear every 3 years if testing for HPV DNA is not performed. The screening interval for women in this age group can be extended to every 5 years if co-testing results are negative. HPV testing is not recommended for partners of women with HPV or for screening for conditions other than cervical cancer.
Currently, there is no clear consensus regarding screening for anal cancer and its precursors, including high-grade anal intraepithelial lesions. This lack of clarity is due to an inadequate understanding of optimal treatment for low- or high-grade anal dysplasia found during cytologic screening. The current HIV treatment guidelines suggest that there may be a benefit to screening, but an effect on the associated morbidity and mortality of anal squamous cell cancer has not been consistently demonstrated.
COUNSELING
Most sexually active adults will be infected with HPV during their lives. For all patients (vaccinated or unvaccinated), certain behavioral interventions can reduce the risk of acquiring HPV. Physicians can provide their patients with measures that can reduce this risk. The only way to avoid acquiring an HPV infection is to abstain from sexual activity, including intimate touching and oral sex. Practicing safe sex (partner reduction, condom use) may lower the likelihood of HPV transmission. Most HPV infections are controlled by the immune system and cause no symptoms or disease. Some infections lead to genital warts and cervical precancers. Genital warts can be treated for cosmetic reasons and to prevent spread of infection to others. Even after resolution of genital warts, latent virus can persist in normal-appearing skin or mucosa and thus theoretically can be transmitted to uninfected partners. Precancerous cervical lesions should be treated to prevent progression to cancer.
SECTION 13 |
INFECTIONS DUE TO DNA AND RNA RESPIRATORY VIRUSES |
223 |
Common Viral Respiratory Infections |
GENERAL CONSIDERATIONS
Acute viral respiratory illnesses are among the most common of human diseases, accounting for one-half or more of all acute illnesses. The incidence of acute respiratory disease in the United States is 3–5.6 cases per person per year. The rates are highest among children <1 year old (6.1–8.3 cases per year) and remain high until age 6, when a progressive decrease begins. Adults have 3–4 cases per person per year. Morbidity from acute respiratory illnesses accounts for 30–50% of time lost from work by adults and for 60–80% of time lost from school by children. The use of antibacterial agents to treat viral respiratory infections represents a major source of abuse of that category of drugs.
It has been estimated that two-thirds to three-fourths of cases of acute respiratory illness are caused by viruses. More than 200 antigenically distinct viruses from 10 genera have been reported to cause acute respiratory illness, and it is likely that additional agents will be described in the future. The vast majority of these viral infections involve the upper respiratory tract, but lower respiratory tract disease can also develop, particularly in younger age groups, in the elderly, and in certain epidemiologic settings.
The illnesses caused by respiratory viruses traditionally have been divided into multiple distinct syndromes, such as the “common cold,” pharyngitis, croup (laryngotracheobronchitis), tracheitis, bronchiolitis, bronchitis, and pneumonia. Each of these general categories of illness has a certain epidemiologic and clinical profile; for example, croup occurs exclusively in very young children and has a characteristic clinical course. Some types of respiratory illness are more likely to be associated with certain viruses (e.g., the common cold with rhinoviruses), whereas others occupy characteristic epidemiologic niches (e.g., adenovirus infections in military recruits). The syndromes most commonly associated with infections with the major respiratory virus groups are summarized in Table 223-1. Most respiratory viruses clearly have the potential to cause more than one type of respiratory illness, and features of several types of illness may be found in the same patient. Moreover, the clinical illnesses induced by these viruses are rarely sufficiently distinctive to permit an etiologic diagnosis on clinical grounds alone, although the epidemiologic setting increases the likelihood that one group of viruses rather than another is involved. In general, laboratory methods must be relied on to establish a specific viral diagnosis.
|
ILLNESSES ASSOCIATED WITH RESPIRATORY VIRUSES |
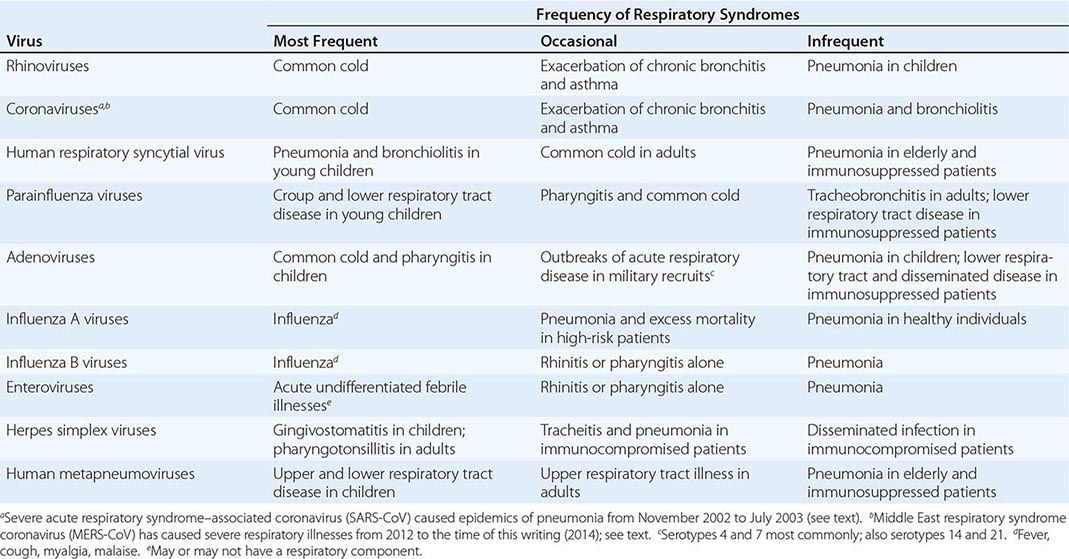
This chapter reviews viral infections caused by six of the major groups of respiratory viruses: rhinoviruses, coronaviruses, respiratory syncytial viruses, metapneumoviruses, parainfluenza viruses, and adenoviruses. The extraordinary outbreaks of lower respiratory tract disease associated with coronaviruses (severe acute respiratory syndrome [SARS] in 2002–2003 and Middle East respiratory syndrome [MERS] in 2012–2013) are also discussed. Influenza viruses, which are a major cause of death as well as morbidity, are reviewed in Chap. 224. Herpesviruses, which occasionally cause pharyngitis and which also cause lower respiratory tract disease in immunosuppressed patients, are reviewed in Chap. 216. Enteroviruses, which account for occasional respiratory illnesses during the summer months, are reviewed in Chap. 228.
RHINOVIRUS INFECTIONS
ETIOLOGIC AGENT
Rhinoviruses are members of the Picornaviridae family—small (15- to 30-nm) nonenveloped viruses that contain a single-stranded RNA genome. Human rhinoviruses were first classified by immunologic serotype and are now divided into three genetic species: HRV-A, HRV-B, and HRV-C. The 102 serotypes described initially are encompassed by HRV-A and HRV-B species, whereas HRV-C encompasses more than 60 previously unrecognized serotypes. In contrast to other members of the picornavirus family, such as enteroviruses, rhinoviruses are acid-labile and are almost completely inactivated at pH ≤3. HRV-A and HRV-B species grow preferentially at 33°–34°C (the temperature of the human nasal passages) rather than at 37°C (the temperature of the lower respiratory tract), whereas HRV-C viruses replicate well at either temperature. Of the 101 initially recognized serotypes of rhinovirus, 88 use intercellular adhesion molecule 1 (ICAM-1) as a cellular receptor and constitute the “major” receptor group, 12 use the low-density lipoprotein receptor (LDLR) and constitute the “minor” receptor group, and 1 uses decay-accelerating factor.
EPIDEMIOLOGY
![]() Rhinovirus infections are worldwide in distribution. They are a prominent cause of the common cold and have been detected in up to 50% of common cold–like illnesses by tissue culture and polymerase chain reaction (PCR) techniques. Overall rates of rhinovirus infection are higher among infants and young children and decrease with increasing age. Rhinovirus infections occur throughout the year, with seasonal peaks in early fall and spring in temperate climates. These infections are most often introduced into families by preschool or grade-school children <6 years old. Of initial illnesses in family settings, 25–70% are followed by secondary cases, with the highest attack rates among the youngest siblings at home. Attack rates also increase with family size.
Rhinovirus infections are worldwide in distribution. They are a prominent cause of the common cold and have been detected in up to 50% of common cold–like illnesses by tissue culture and polymerase chain reaction (PCR) techniques. Overall rates of rhinovirus infection are higher among infants and young children and decrease with increasing age. Rhinovirus infections occur throughout the year, with seasonal peaks in early fall and spring in temperate climates. These infections are most often introduced into families by preschool or grade-school children <6 years old. Of initial illnesses in family settings, 25–70% are followed by secondary cases, with the highest attack rates among the youngest siblings at home. Attack rates also increase with family size.
Rhinoviruses appear to spread through direct contact with infected secretions, usually respiratory droplets. In some studies of volunteers, transmission was most efficient by hand-to-hand contact, with subsequent self-inoculation of the conjunctival or nasal mucosa. Other studies demonstrated transmission by large- or small-particle aerosol. Virus can be recovered from plastic surfaces inoculated 1–3 h previously; this observation suggests that environmental surfaces contribute to transmission. In studies of married couples in which neither partner had detectable serum antibody, transmission was associated with prolonged contact (≥122 h) during a 7-day period. Transmission was infrequent unless (1) virus was recoverable from the donor’s hands and nasal mucosa, (2) at least 1000 TCID50 (50% tissue culture infectious dose) of virus was present in nasal washes from the donor, and (3) the donor was at least moderately symptomatic with the “cold.” Despite anecdotal observations, exposure to cold temperatures, fatigue, and sleep deprivation have not been associated with increased rates of rhinovirus-induced illness in volunteers, although some studies have suggested that psychologically defined “stress” may contribute to development of symptoms.
By adulthood, nearly all individuals have neutralizing antibodies to multiple rhinovirus serotypes, although the prevalence of antibody to any one serotype varies widely. Multiple serotypes circulate simultaneously, and generally no single serotype or group of serotypes has been more prevalent than the others.
PATHOGENESIS
Rhinoviruses infect cells through attachment to specific cellular receptors; as mentioned above, most serotypes attach to ICAM-1, while a few use LDLR. Relatively limited information is available on the histopathology and pathogenesis of acute rhinovirus infections in humans. Examination of biopsy specimens obtained during experimentally induced and naturally occurring illness indicates that the nasal mucosa is edematous, is often hyperemic, and—during acute illness—is covered by a mucoid discharge. There is a mild infiltrate with inflammatory cells, including neutrophils, lymphocytes, plasma cells, and eosinophils. Mucus-secreting glands in the submucosa appear hyperactive; the nasal turbinates are engorged, a condition that may lead to obstruction of nearby openings of sinus cavities. Several mediators—e.g., bradykinin; lysylbradykinin; prostaglandins; histamine; interleukins 1β, 6, and 8; interferon γ–induced protein 10; and tumor necrosis factor α—have been linked to the development of signs and symptoms in rhinovirus-induced colds.
The incubation period for rhinovirus illness is short, generally 1–2 days. Virus shedding coincides with the onset of illness or may begin shortly before symptoms develop. The mechanisms of immunity to rhinovirus infection are not well worked out. In some studies, the presence of homotypic antibody has been associated with significantly reduced rates of subsequent infection and illness, but data conflict regarding the relative importance of serum and local antibody in protection from rhinovirus infection.
CLINICAL MANIFESTATIONS
The most common clinical manifestations of rhinovirus infections are those of the common cold. Illness usually begins with rhinorrhea and sneezing accompanied by nasal congestion. The throat is frequently sore, and in some cases sore throat is the initial complaint. Systemic signs and symptoms, such as malaise and headache, are mild or absent, and fever is unusual in adults but may occur in up to one-third of children. Illness generally lasts for 4–9 days and resolves spontaneously without sequelae. In children, bronchitis, bronchiolitis, and bronchopneumonia have been reported; nevertheless, it appears that rhinoviruses are not major causes of lower respiratory tract disease in children. Rhinoviruses may cause exacerbations of asthma and chronic pulmonary disease in adults. The vast majority of rhinovirus infections resolve without sequelae, but complications related to obstruction of the eustachian tubes or sinus ostia, including otitis media or acute sinusitis, can develop. In immunosuppressed patients, particularly bone marrow transplant recipients, severe and even fatal pneumonias have been associated with rhinovirus infections.
DIAGNOSIS
Although rhinoviruses are the most frequently recognized cause of the common cold, similar illnesses are caused by a variety of other viruses, and a specific viral etiologic diagnosis cannot be made on clinical grounds alone. Rather, rhinovirus infection is diagnosed by isolation of the virus from nasal washes or nasal secretions in tissue culture. In practice, this procedure is rarely undertaken because of the benign, self-limited nature of the illness. In most settings, detection of rhinovirus RNA is more sensitive and easier by PCR than by tissue culture. Accordingly, PCR has generally become the standard for detection of rhinoviruses in clinical specimens. Given the many serotypes of rhinovirus, diagnosis by serum antibody tests is currently impractical. Likewise, common laboratory tests, such as white blood cell count and erythrocyte sedimentation rate, are not helpful.
PREVENTION
Intranasal application of interferon sprays has been effective in the prophylaxis of rhinovirus infections but is also associated with local irritation of the nasal mucosa. Studies of prevention of rhinovirus infection by blocking of ICAM-1 or by binding of drug (pleconaril) to parts of the viral capsid have yielded mixed results. Experimental vaccines to certain rhinovirus serotypes have been generated, but their usefulness is questionable because of the myriad serotypes involved and the uncertainty about mechanisms of immunity. Thorough hand washing, environmental decontamination, and protection against autoinoculation may help to reduce rates of transmission of infection.
CORONAVIRUS INFECTIONS
ETIOLOGIC AGENT
Coronaviruses are pleomorphic, single-stranded RNA viruses that measure 100–160 nm in diameter. The name derives from the crownlike appearance produced by the club-shaped projections that stud the viral envelope. Coronaviruses infect a wide variety of animal species and have been divided into four genera. Coronaviruses that infect humans (HCoVs) fall into two genera: Alphacoronavirus and Betacoronavirus. Severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) are betacoronaviruses.
In general, human coronaviruses have been difficult to cultivate in vitro, and some strains grow only in human tracheal organ cultures rather than in tissue culture. SARS-CoV and MERS-CoV are exceptions whose ready growth in African green monkey kidney (Vero E6) cells greatly facilitates their study.
EPIDEMIOLOGY
![]() Human coronavirus infections are present throughout the world. Seroprevalence studies of strains HCoV-229E and HCoV-OC43 have demonstrated that serum antibodies are acquired early in life and increase in prevalence with advancing age, so that >80% of adult populations have antibodies detectable by enzyme-linked immunosorbent assay (ELISA). Overall, coronaviruses account for 10–35% of common colds, depending on the season. Coronavirus infections appear to be particularly prevalent in late fall, winter, and early spring—times when rhinovirus infections are less common.
Human coronavirus infections are present throughout the world. Seroprevalence studies of strains HCoV-229E and HCoV-OC43 have demonstrated that serum antibodies are acquired early in life and increase in prevalence with advancing age, so that >80% of adult populations have antibodies detectable by enzyme-linked immunosorbent assay (ELISA). Overall, coronaviruses account for 10–35% of common colds, depending on the season. Coronavirus infections appear to be particularly prevalent in late fall, winter, and early spring—times when rhinovirus infections are less common.
An extraordinary outbreak of the coronavirus-associated illness known as SARS occurred in 2002–2003. The outbreak apparently began in southern China and eventually resulted in 8096 recognized cases in 28 countries in Asia, Europe, and North and South America; ~90% of cases occurred in China and Hong Kong. The natural reservoir of SARS-CoV appeared to be the horseshoe bat, and the outbreak may have originated from human contact with infected semidomesticated animals such as the palm civet. In most cases, however, the infection was transmitted from human to human. Case–fatality rates varied among outbreaks, with an overall figure of ~9.5%. The disease appeared to be somewhat milder in cases in the United States and was clearly less severe among children. The outbreak ceased in 2003; 17 cases were detected in 2004, mostly in laboratory-associated settings, and no cases have been reported subsequently.
The mechanisms of transmission of SARS are incompletely understood. Clusters of cases suggest that spread may occur via both large- and small-droplet aerosols and perhaps via the fecal–oral route as well. The outbreak of illness in a large apartment complex in Hong Kong suggested that environmental sources, such as sewage or water, may also play a role in transmission. Some ill individuals (“super-spreaders”) appeared to be hyperinfectious and were capable of transmitting infection to 10–40 contacts, although most infections resulted in spread either to no one or to three or fewer individuals.
Since it began in June 2012, another extraordinary outbreak of serious respiratory illness, MERS, has been linked with a coronavirus (MERS-CoV). Through May 2014, a total of 536 cases and 145 deaths (27%) have been reported. All cases have been associated with contact or travel to six countries in or near the Arabian Peninsula: Jordan, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates. Cases have also been reported in France, Italy, Tunisia, Germany, Spain, and the United Kingdom. Person-to-person transmission has been documented, but sustained spread in communities has not. The source of MERS-CoV has not been established, but it is suspected that bats may be the animal reservoir and that camels serve as an intermediate host.
PATHOGENESIS
Coronaviruses that cause the common cold (e.g., strains HCoV-229E and HCoV-OC43) infect ciliated epithelial cells in the nasopharynx via the aminopeptidase N receptor (group 1) or a sialic acid receptor (group 2). Viral replication leads to damage of ciliated cells and induction of chemokines and interleukins, with consequent common-cold symptoms similar to those induced by rhinoviruses.
SARS-CoV infects cells of the respiratory tract via the angiotensin-converting enzyme 2 receptor. The result is a systemic illness in which virus is also found in the bloodstream, in the urine, and (for up to 2 months) in the stool. Virus persists in the respiratory tract for 2–3 weeks, and titers peak ~10 days after the onset of systemic illness. Pulmonary pathology consists of hyaline membrane formation, desquamation of pneumocytes in alveolar spaces, and an interstitial infiltrate made up of lymphocytes and mononuclear cells. Giant cells are frequently seen, and coronavirus particles have been detected in type II pneumocytes. Elevated levels of proinflammatory cytokines and chemokines have been detected in sera from patients with SARS.
Because MERS-CoV was so recently detected, little is known at present about its pathogenesis. However, it may well be similar to that of SARS-CoV.
CLINICAL MANIFESTATIONS
After an incubation period that generally lasts 2–7 days (range, 1–14 days), SARS usually begins as a systemic illness marked by the onset of fever, which is often accompanied by malaise, headache, and myalgias and is followed in 1–2 days by a nonproductive cough and dyspnea. Approximately 25% of patients have diarrhea. Chest x-rays can show a variety of infiltrates, including patchy areas of consolidation—most frequently in peripheral and lower lung fields—or interstitial infiltrates, which can progress to diffuse involvement. In severe cases, respiratory function may worsen during the second week of illness and progress to frank adult respiratory distress syndrome accompanied by multiorgan dysfunction. Risk factors for severe disease include an age of >50 years and comorbidities such as cardiovascular disease, diabetes, and hepatitis. Illness in pregnant women may be particularly severe, but SARS-CoV infection appears to be milder in children than in adults.
Information regarding the clinical manifestations of MERS-CoV is limited. The case–fatality rate has been high in the initial cases, but this may represent an ascertainment bias, and it is clear that mild cases occur as well. The median incubation period has been estimated to be 5.2 days, and a secondary case was estimated to have an incubation period of 9–12 days. Cases have been reported that begin with cough and fever and progress to acute respiratory distress and respiratory failure within a week. Other cases have manifested as mild upper respiratory symptoms only. Renal failure has been noted, and DPP-4, the host cell receptor for MERS-CoV, is expressed at high levels in the kidney; these findings suggest that direct viral infection of the kidney may lead to renal dysfunction. Diarrhea and vomiting are also common in MERS, and pericarditis has been reported.
The clinical features of common colds caused by human coronaviruses are similar to those of illness caused by rhinoviruses. In studies of volunteers, the mean incubation period of colds induced by corona-viruses (3 days) is somewhat longer than that of illness caused by rhinoviruses, and the duration of illness is somewhat shorter (mean, 6–7 days). In some studies, the amount of nasal discharge was greater in colds induced by coronaviruses than in those induced by rhinoviruses. Coronaviruses other than SARS-CoV have been recovered occasionally from infants with pneumonia and from military recruits with lower respiratory tract disease and have been associated with worsening of chronic bronchitis. Two novel coronaviruses, HCoV-NL63 and HCoV-HKU1, have been isolated from patients hospitalized with acute respiratory illness. Their overall role as causes of human respiratory disease remains to be determined.
LABORATORY FINDINGS AND DIAGNOSIS
Laboratory abnormalities in SARS include lymphopenia, which is documented in ~50% of cases and which mostly affects CD4+ T cells but also involves CD8+ T cells and natural killer cells. Total white blood cell counts are normal or slightly low, and thrombocytopenia may develop as the illness progresses. Elevated serum levels of aminotransferases, creatine kinase, and lactate dehydrogenase have been reported.
A rapid diagnosis of SARS-CoV infection can be made by reverse-transcription PCR (RT-PCR) of respiratory tract samples and plasma early in the illness and of urine and stool later on. SARS-CoV can also be grown from respiratory tract samples by inoculation into Vero E6 tissue culture cells, in which a cytopathic effect is seen within days. RT-PCR appears to be more sensitive than tissue culture, but only around one-third of cases are positive by PCR at initial presentation. Serum antibodies can be detected by ELISA or immunofluorescence, and nearly all patients develop detectable serum antibodies within 28 days after the onset of illness.
Laboratory abnormalities in MERS-CoV infection include lymphopenia with or without neutropenia, thrombocytopenia, and elevated levels of lactate dehydrogenase. MERS-CoV can be isolated in tissue culture in Vero and LLC-MK2 cells, but PCR techniques are more sensitive and rapid and are the standard for laboratory diagnosis. Serologic tests using ELISA and immunofluorescence techniques have also been developed.
Laboratory diagnosis of coronavirus-induced colds is rarely required. Coronaviruses that cause those illnesses are frequently difficult to cultivate in vitro but can be detected in clinical samples by ELISA or immunofluorescence assays or by RT-PCR for viral RNA. These research procedures can be used to detect coronaviruses in unusual clinical settings.
PREVENTION
![]() The recognition of SARS led to a worldwide mobilization of public health resources to apply infection control practices to contain the disease. Case definitions were established, travel advisories were proposed, and quarantines were imposed in certain locales. As of this writing, no additional cases of SARS have been reported since 2004. However, it remains unknown whether the disappearance of cases is a result of control measures, whether it is part of a seasonal or otherwise unexplained epidemiologic pattern of SARS, or when or whether SARS might reemerge. The U.S. Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) maintain recommendations for surveillance and assessment of potential cases of SARS (www.cdc.gov/sars/). The frequent transmission of the disease to health care workers makes it mandatory that strict infection-control practices be employed by health care facilities to prevent airborne, droplet, and contact transmission from any suspected cases of SARS. Health care workers who enter areas in which patients with SARS may be present should don gowns, gloves, and eye and respiratory protective equipment (e.g., an N95 filtering facepiece respirator certified by the National Institute for Occupational Safety and Health).
The recognition of SARS led to a worldwide mobilization of public health resources to apply infection control practices to contain the disease. Case definitions were established, travel advisories were proposed, and quarantines were imposed in certain locales. As of this writing, no additional cases of SARS have been reported since 2004. However, it remains unknown whether the disappearance of cases is a result of control measures, whether it is part of a seasonal or otherwise unexplained epidemiologic pattern of SARS, or when or whether SARS might reemerge. The U.S. Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) maintain recommendations for surveillance and assessment of potential cases of SARS (www.cdc.gov/sars/). The frequent transmission of the disease to health care workers makes it mandatory that strict infection-control practices be employed by health care facilities to prevent airborne, droplet, and contact transmission from any suspected cases of SARS. Health care workers who enter areas in which patients with SARS may be present should don gowns, gloves, and eye and respiratory protective equipment (e.g., an N95 filtering facepiece respirator certified by the National Institute for Occupational Safety and Health).
Similarly, the WHO and the CDC have issued recommendations for identification, prevention, and control of MERS-CoV infections (www.cdc.gov/coronavirus/mers/index.html). Isolation precautions against airborne spread of infection should be instituted for patients hospitalized for suspected MERS, as described above for SARS.
Vaccines have been developed against several animal coronaviruses but not against known human coronaviruses. The emergence of SARS-CoV and MERS-CoV has stimulated interest in the development of vaccines against such agents.
HUMAN RESPIRATORY SYNCYTIAL VIRUS INFECTIONS
ETIOLOGIC AGENT
Human respiratory syncytial virus (HRSV) is a member of the Paramyxoviridae family (genus Pneumovirus). It is an enveloped virus ~150–350 nm in diameter and is so named because its replication in vitro leads to the fusion of neighboring cells into large multinucleated syncytia. The single-stranded RNA genome codes for 11 virus-specific proteins. Viral RNA is contained in a helical nucleocapsid surrounded by a lipid envelope bearing two glycoproteins: the G protein, by which the virus attaches to cells, and the F (fusion) protein, which facilitates entry of the virus into the cell by fusing host and viral membranes. HRSV is considered to be of a single antigenic type, but two distinct subgroups (A and B) and multiple subtypes within each subgroup have now been described. Antigenic diversity is reflected by differences in the G protein, whereas the F protein is relatively conserved. Both antigenic groups can circulate simultaneously in outbreaks, although there are typically alternating patterns in which one subgroup predominates over 1- to 2-year periods.
EPIDEMIOLOGY
![]() HRSV is a major respiratory pathogen of young children and the foremost cause of lower respiratory disease in infants. Infection with HRSV is seen throughout the world in annual epidemics that occur in late fall, winter, or spring and last up to 5 months. The virus is rarely encountered during the summer. Rates of illness are highest among infants 1–6 months of age, peaking at 2–3 months of age. The attack rates among susceptible infants and children are extraordinarily high, approaching 100% in settings such as day-care centers where large numbers of susceptible infants are present. By age 2, virtually all children will have been infected with HRSV. HRSV accounts for 20–25% of hospital admissions of young infants and children for pneumonia and for up to 75% of cases of bronchiolitis in this age group. It has been estimated that more than half of infants who are at risk will become infected during an HRSV epidemic.
HRSV is a major respiratory pathogen of young children and the foremost cause of lower respiratory disease in infants. Infection with HRSV is seen throughout the world in annual epidemics that occur in late fall, winter, or spring and last up to 5 months. The virus is rarely encountered during the summer. Rates of illness are highest among infants 1–6 months of age, peaking at 2–3 months of age. The attack rates among susceptible infants and children are extraordinarily high, approaching 100% in settings such as day-care centers where large numbers of susceptible infants are present. By age 2, virtually all children will have been infected with HRSV. HRSV accounts for 20–25% of hospital admissions of young infants and children for pneumonia and for up to 75% of cases of bronchiolitis in this age group. It has been estimated that more than half of infants who are at risk will become infected during an HRSV epidemic.
In older children and adults, reinfection with HRSV is frequent, but disease is milder than in infancy. A common cold–like syndrome is the illness most commonly associated with HRSV infection in adults. It has been increasingly appreciated that severe lower respiratory tract disease with pneumonitis can occur in elderly (often institutionalized) adults, in individuals with cardiopulmonary disease, and in patients with immunocompromising disorders or treatment, including recipients of hematopoietic stem cell transplants (HSCTs) and solid-organ transplants (SOTs). HRSV is also an important nosocomial pathogen; during an outbreak, it can infect pediatric patients and up to 25–50% of the staff on pediatric wards. The spread of HRSV among families is efficient: up to 40% of siblings may become infected when the virus is introduced into the family setting.
HRSV is transmitted primarily by close contact with contaminated fingers or fomites and by self-inoculation of the conjunctiva or anterior nares. Virus may also be spread by coarse aerosols produced by coughing or sneezing, but it is inefficiently spread by fine-particle aerosols. The incubation period is ~4–6 days, and virus shedding may last for ≥2 weeks in children and for shorter periods in adults. In immunosuppressed patients, shedding can continue for weeks.
PATHOGENESIS
Little is known about the histopathology of minor HRSV infection. Severe bronchiolitis or pneumonia is characterized by necrosis of the bronchiolar epithelium and a peribronchiolar infiltrate of lymphocytes and mononuclear cells. Interalveolar thickening and filling of alveolar spaces with fluid can also be found. The correlates of protective immunity to HRSV are incompletely understood. Because reinfection occurs frequently and is often associated with illness, the immunity that develops after single episodes of infection clearly is not complete or long-lasting. However, the cumulative effect of multiple reinfections is to temper subsequent disease and to provide some temporary measure of protection against infection. Studies of experimentally induced disease in healthy volunteers indicate that the presence of nasal IgA neutralizing antibody correlates more closely with protection than does the presence of serum antibody. Studies in infants, however, suggest that maternally acquired antibody provides some protection from lower respiratory tract disease, although illness can be severe even in infants who have moderate levels of maternally derived serum antibody. The relatively severe disease observed in immunosuppressed patients and experimental animal models indicates that cell-mediated immunity is an important mechanism of host defense against HRSV. Evidence suggests that major histocompatibility class I–restricted cytotoxic T cells may be particularly important in this regard.
CLINICAL MANIFESTATIONS
HRSV infection leads to a wide spectrum of respiratory illnesses. In infants, 25–40% of infections result in lower respiratory tract involvement, including pneumonia, bronchiolitis, and tracheobronchitis. In this age group, illness begins most frequently with rhinorrhea, low-grade fever, and mild systemic symptoms, often accompanied by cough and wheezing. Most patients recover gradually over 1–2 weeks. In more severe illness, tachypnea and dyspnea develop, and eventually frank hypoxia, cyanosis, and apnea can ensue. Physical examination may reveal diffuse wheezing, rhonchi, and rales. Chest radiography shows hyperexpansion, peribronchial thickening, and variable infiltrates ranging from diffuse interstitial infiltrates to segmental or lobar consolidation. Illness may be particularly severe in children born prematurely and in those with congenital cardiac disease, bronchopulmonary dysplasia, nephrotic syndrome, or immunosuppression. One study documented a 37% mortality rate among infants with HRSV pneumonia and congenital cardiac disease.
In adults, the most common symptoms of HRSV infection are those of the common cold, with rhinorrhea, sore throat, and cough. Illness is occasionally associated with moderate systemic symptoms such as malaise, headache, and fever. HRSV has also been reported to cause lower respiratory tract disease with fever in adults, including severe pneumonia in the elderly—particularly in nursing-home residents, among whom its impact can rival that of influenza. HRSV pneumonia can be a significant cause of morbidity and death among patients undergoing stem cell and solid organ transplantation, in whom case–fatality rates of 20–80% have been reported. Sinusitis, otitis media, and worsening of chronic obstructive and reactive airway disease have also been associated with HRSV infection.
LABORATORY FINDINGS AND DIAGNOSIS
The diagnosis of HRSV infection can be suspected on the basis of a suggestive epidemiologic setting—that is, severe illness among infants during an outbreak of HRSV in the community. Infections in older children and adults cannot be differentiated with certainty from those caused by other respiratory viruses. The specific diagnosis is established by detection of HRSV in respiratory secretions, such as sputum, throat swabs, or nasopharyngeal washes. Virus can be isolated in tissue culture, but this method has been largely supplanted by rapid viral diagnostic techniques consisting of immunofluorescence or ELISA of nasopharyngeal washes, aspirates, and (less satisfactorily) nasopharyngeal swabs. With specimens from children, these techniques have sensitivities and specificities of 80–95%; they are somewhat less sensitive with specimens from adults. RT-PCR detection techniques have shown even higher rates of sensitivity and specificity, particularly in adults. Serologic diagnosis may be made by comparison of acute- and convalescent-phase serum specimens by ELISA or by neutralization or complement-fixation tests. These tests may be useful in older children and adults but are less sensitive in children <4 months of age.
PREVENTION
Monthly administration of RSVIg (no longer available) or palivizumab has been approved as prophylaxis against HRSV for children <2 years of age who have bronchopulmonary dysplasia or cyanotic heart disease or who were born prematurely. Considerable interest exists in the development of vaccines against HRSV. Inactivated whole-virus vaccines have been ineffective; in one study, they actually potentiated disease in infants. Other approaches include immunization with purified F and G surface glycoproteins of HRSV or generation of stable live attenuated virus vaccines. In settings where rates of transmission are high (e.g., pediatric wards), barrier methods for the protection of hands and conjunctivae may be useful in reducing the spread of virus.
HUMAN METAPNEUMOVIRUS INFECTIONS
ETIOLOGIC AGENT
Human metapneumovirus (HMPV) is a viral respiratory pathogen that has been assigned to the Paramyxoviridae family (genus Metapneumovirus). Its morphology and genomic organization are similar to those of avian metapneumoviruses, which are recognized respiratory pathogens of turkeys. HMPV particles may be spherical, filamentous, or pleomorphic in shape and measure 150–600 nm in diameter. Particles contain 15-nm projections from the surface that are similar in appearance to those of other Paramyxoviridae. The single-stranded RNA genome codes for nine proteins that, except for the absence of nonstructural proteins, generally correspond to those of HRSV. HMPV is of only one antigenic type; two closely related genotypes (A and B), four subgroups, and two sublineages have been described.
EPIDEMIOLOGY
![]() HMPV infections are worldwide in distribution, are most frequent during the winter in temperate climates, and occur early in life, so that serum antibodies to the virus are present in 50% of children by age 2 and in nearly all children by age 5. HMPV infections have been detected in older age groups, including elderly adults, and in both immunocompetent and immunosuppressed hosts. This virus accounts for 1–5% of childhood upper respiratory tract infections and for 10–15% of respiratory tract illnesses requiring hospitalization of children. In addition, HMPV causes 2–4% of acute respiratory illnesses in ambulatory adults and elderly patients. HMPV has been detected in a few cases of SARS, but its role (if any) in these illnesses has not been established.
HMPV infections are worldwide in distribution, are most frequent during the winter in temperate climates, and occur early in life, so that serum antibodies to the virus are present in 50% of children by age 2 and in nearly all children by age 5. HMPV infections have been detected in older age groups, including elderly adults, and in both immunocompetent and immunosuppressed hosts. This virus accounts for 1–5% of childhood upper respiratory tract infections and for 10–15% of respiratory tract illnesses requiring hospitalization of children. In addition, HMPV causes 2–4% of acute respiratory illnesses in ambulatory adults and elderly patients. HMPV has been detected in a few cases of SARS, but its role (if any) in these illnesses has not been established.
CLINICAL MANIFESTATIONS
The spectrum of clinical illnesses associated with HMPV is similar to that associated with HRSV and includes both upper and lower respiratory tract illnesses, such as bronchiolitis, croup, and pneumonia. Reinfection with HMPV is common among older children and adults and has manifestations ranging from subclinical infections to common cold syndromes and occasionally pneumonia, which is seen primarily in elderly patients and those with cardiopulmonary diseases. Serious HMPV infections occur in immunocompromised patients, including those with neoplasia, recipients of HSCTs, and children with HIV infection.
DIAGNOSIS
HMPV can be detected in nasal aspirates and respiratory secretions by immunofluorescence, by PCR (the most sensitive technique), or by growth in rhesus monkey kidney (LLC-MK2) tissue cultures. A serologic diagnosis can be made by ELISA, which uses HMPV-infected tissue culture lysates as sources of antigens.
PREVENTION
Vaccines against HMPV are in the early stages of development.
PARAINFLUENZA VIRUS INFECTIONS
ETIOLOGIC AGENT
Parainfluenza viruses belong to the Paramyxoviridae family (genera Respirovirus and Rubulavirus). They are 150–200 nm in diameter, are enveloped, and contain a single-stranded RNA genome. The envelope is studded with two glycoproteins: one possesses both hemagglutinin and neuraminidase activity, and the other contains fusion activity. The viral RNA genome is enclosed in a helical nucleocapsid and codes for six structural and several accessory proteins. All types of parainfluenza virus (1, 2, 3, 4A, and 4B) share certain antigens with other members of the Paramyxoviridae family, including mumps and Newcastle disease viruses.
EPIDEMIOLOGY
![]() Parainfluenza viruses are distributed throughout the world; infection with serotypes 4A and 4B has been reported less widely, probably because these types are more difficult than the other three to grow in tissue culture. Infection is acquired in early childhood; by 5 years of age, most children have antibodies to serotypes 1, 2, and 3. Types 1 and 2 cause epidemics during the fall, often occurring in an alternate-year pattern. Type 3 infection has been detected during all seasons, but epidemics have occurred annually in the spring.
Parainfluenza viruses are distributed throughout the world; infection with serotypes 4A and 4B has been reported less widely, probably because these types are more difficult than the other three to grow in tissue culture. Infection is acquired in early childhood; by 5 years of age, most children have antibodies to serotypes 1, 2, and 3. Types 1 and 2 cause epidemics during the fall, often occurring in an alternate-year pattern. Type 3 infection has been detected during all seasons, but epidemics have occurred annually in the spring.
The contribution of parainfluenza infections to respiratory disease varies with both the location and the year. In studies conducted in the United States, parainfluenza virus infections have accounted for 4.3–22% of respiratory illnesses in children. The major importance of these viruses is as a cause of lower respiratory illness in young children, in whom they rank second only to HRSV in that regard. Parainfluenza virus type 1 is the most common cause of croup (laryngotracheobronchitis) in children, whereas serotype 2 causes similar, although generally less severe, disease. Type 3 is an important cause of bronchiolitis and pneumonia in infants, whereas illnesses associated with types 4A and 4B have generally been mild. Unlike types 1 and 2, type 3 frequently causes illness during the first month of life, when passively acquired maternal antibody is still present. Parainfluenza viruses are spread through infected respiratory secretions, primarily by person-to-person contact and/or by large droplets, and by contact with fomites contaminated with respiratory secretions. The incubation period has varied from 3 to 6 days in experimental infections but may be somewhat shorter for naturally occurring disease in children.
In adults, parainfluenza virus infections are generally mild and account for fewer than 10% of respiratory illnesses. The advent of contemporary laboratory methods for diagnosis has increased awareness of the impact of parainfluenza infections in adults. In a recent study, parainfluenza virus was the third most common viral isolate from patients 16–64 years old who required hospitalization (0.7 isolate/1000 population). In the 2009 influenza pandemic, parainfluenza virus type 3 was the second most common cause of illness after influenza virus.
PATHOGENESIS
Immunity to parainfluenza viruses is incompletely understood, but evidence suggests that immunity to infections with serotypes 1 and 2 is mediated by local IgA antibodies in the respiratory tract. Passively acquired serum neutralizing antibodies also confer some protection against infection with types 1, 2, and (to a lesser degree) 3. Studies in experimental animal models and in immunosuppressed patients suggest that T cell–mediated immunity may also be important in parainfluenza virus infections. Lack of cellular immune responses is associated with an increased risk of progressive and fatal disease in HSCT recipients.
CLINICAL MANIFESTATIONS
Parainfluenza virus infections occur most frequently among children, in whom initial infection with serotype 1, 2, or 3 is associated with an acute febrile illness in 50–80% of cases. Children may present with coryza, sore throat, hoarseness, and cough that may or may not be croupy. In severe croup, fever persists, with worsening coryza and sore throat. A brassy or barking cough may progress to frank stridor. Most children recover over the next 1 or 2 days, although progressive airway obstruction and hypoxia ensue occasionally. If bronchiolitis or pneumonia develops, progressive cough accompanied by wheezing, tachypnea, and intercostal retractions may occur. In this setting, sputum production increases modestly. Physical examination documents nasopharyngeal discharge and oropharyngeal injection, along with rhonchi, wheezes, or coarse breath sounds. Chest x-rays can show air trapping and occasionally interstitial infiltrates.
In older children and adults, parainfluenza infections tend to be milder, presenting most frequently as a common cold or as hoarseness, with or without cough. Lower respiratory tract involvement in older children and adults is uncommon, although tracheobronchitis and community-acquired pneumonia have been reported in adults.
Parainfluenza viruses, most frequently type 3, are important pathogens in immunosuppressed patients—particularly in HSCT recipients but also in SOT recipients (especially recipients of lung transplants). Patients receiving cancer chemotherapy are also at risk for severe parainfluenza infection. Severe, prolonged, and even fatal parainfluenza-associated respiratory illnesses have been reported in children and adults with severe immunosuppression.
LABORATORY FINDINGS AND DIAGNOSIS
The clinical syndromes caused by parainfluenza viruses (with the possible exception of croup in young children) are not sufficiently distinctive to be diagnosed on clinical grounds alone. A specific diagnosis is established by detection of virus in respiratory tract secretions, throat swabs, or nasopharyngeal washings. Growth of the virus in tissue culture is detected either by hemagglutination or by a cytopathic effect. A rapid diagnosis may be made by identification of parainfluenza antigens in exfoliated cells from the respiratory tract with immunofluorescence or ELISA, although these techniques appear to be less sensitive than tissue culture. Highly specific and sensitive PCR assays have also been developed and have now become the standard for viral diagnosis. Serologic diagnosis can be established by hemagglutination-inhibition, complement-fixation, or neutralization testing of acute- and convalescent-phase specimens. However, because frequent heterotypic responses occur among the parainfluenza serotypes, the serotype causing illness often cannot be identified by serologic techniques alone.
Acute epiglottitis caused by Haemophilus influenzae type b must be differentiated from viral croup. Influenza A virus is also a common cause of croup during epidemic periods.
PREVENTION
Vaccines against parainfluenza viruses are under development.
ADENOVIRUS INFECTIONS
ETIOLOGIC AGENT
Adenoviruses are complex DNA viruses that measure 70–80 nm in diameter. Human adenoviruses belong to the genus Mastadenovirus, which includes 51 serotypes. Adenoviruses have a characteristic morphology consisting of an icosahedral shell composed of 20 equilateral triangular faces and 12 vertices. The protein coat (capsid) consists of hexon subunits with group-specific and type-specific antigenic determinants and penton subunits at each vertex primarily containing group-specific antigens. A fiber with a knob at the end projects from each penton; this fiber contains type-specific and some group-specific antigens. Human adenoviruses have been divided into seven subgroups (A through G) on the basis of the homology of DNA genomes and other properties. Revised criteria for classifying human adenoviruses have been proposed; reflecting recent approaches to the characterization of novel adenoviruses, the revised criteria include genome sequence and computational analysis in addition to traditional serologic criteria. The adenovirus genome is a linear double-stranded DNA that codes for structural and nonstructural polypeptides. The replicative cycle of adenovirus may result either in lytic infection of cells or in the establishment of a latent infection (primarily involving lymphoid cells). Some adenovirus types can induce oncogenic transformation, and tumor formation has been observed in rodents; however, despite intensive investigation, adenoviruses have not been associated with tumors in humans.
EPIDEMIOLOGY
Adenovirus infections most frequently affect infants and children. Infections occur throughout the year but are most common from fall to spring. In the United States, adenoviruses account for ~10% of acute respiratory infections in children but for <2% of respiratory illnesses in civilian adults. Nearly 100% of adults have serum antibody to multiple serotypes—a finding indicating that infection is common in childhood. Types 1, 2, 3, and 5 are the most common isolates from children. Certain adenovirus serotypes—particularly 4 and 7 but also 3, 14, and 21—are associated with outbreaks of acute respiratory disease in military recruits. Clusters of particularly severe disease have been seen with adenovirus 14.
Adenovirus infection can be transmitted by inhalation of aerosolized virus, by inoculation of virus into conjunctival sacs, and probably by the fecal-oral route as well. Type-specific antibody generally develops after infection and is associated with protection—albeit incomplete—against infection with the same serotype.
CLINICAL MANIFESTATIONS
In children, adenoviruses cause a variety of clinical syndromes. The most common is an acute upper respiratory tract infection, with prominent rhinitis. On occasion, lower respiratory tract disease, including bronchiolitis and pneumonia, also develops. Adenoviruses, particularly types 3 and 7, cause pharyngoconjunctival fever, a characteristic acute febrile illness of children that occurs in outbreaks, most often in summer camps. The syndrome is marked by bilateral conjunctivitis in which the bulbar and palpebral conjunctivae have a granular appearance. Low-grade fever is frequently present for the first 3–5 days, and rhinitis, sore throat, and cervical adenopathy develop. The illness generally lasts for 1–2 weeks and resolves spontaneously. Febrile pharyngitis without conjunctivitis has also been associated with adenovirus infection. Adenoviruses have been isolated from cases of whooping cough with or without Bordetella pertussis; the significance of adenovirus in that disease is unknown.
In adults, the most frequently reported illness has been acute respiratory disease caused by adenovirus types 4 and 7 in military recruits. This illness is marked by a prominent sore throat and the gradual onset of fever, which often reaches 39°C (102.2°F) on the second or third day of illness. Cough is almost always present, and coryza and regional lymphadenopathy are frequently seen. Physical examination may show pharyngeal edema, injection, and tonsillar enlargement with little or no exudate. If pneumonia has developed, auscultation and x-ray of the chest may indicate areas of patchy infiltration.
Adenoviruses have been associated with a number of non–respiratory tract diseases, including acute diarrheal illness caused by types 40 and 41 in young children and hemorrhagic cystitis caused by types 11 and 21. Epidemic keratoconjunctivitis, caused most frequently by types 8, 19, and 37, has been associated with contaminated common sources such as ophthalmic solutions and roller towels. Adenoviruses have also been implicated in disseminated disease and pneumonia in immunosuppressed patients, including recipients of SOTs or HSCTs. In HSCT recipients, adenovirus infections have manifested as pneumonia, hepatitis, nephritis, colitis, encephalitis, and hemorrhagic cystitis. In SOT recipients, adenovirus infection may involve the organ transplanted (e.g., hepatitis in liver transplants, nephritis in renal transplants) but can disseminate to other organs as well. In patients with AIDS, high-numbered and intermediate adenovirus serotypes have been isolated, usually in the setting of low CD4+ T cell counts, but their isolation often has not been clearly linked to disease manifestations. Adenovirus nucleic acids have been detected in myocardial cells from patients with “idiopathic” myocardiopathies, and adenoviruses have been suggested as causative agents in some cases.
LABORATORY FINDINGS AND DIAGNOSIS
Adenovirus infection should be suspected in the epidemiologic setting of acute respiratory disease in military recruits and in certain clinical syndromes (such as pharyngoconjunctival fever or epidemic keratoconjunctivitis) in which outbreaks of characteristic illnesses occur. In most cases, however, illnesses caused by adenovirus infection cannot be differentiated from those caused by a number of other viral respiratory agents and Mycoplasma pneumoniae. A definitive diagnosis of adenovirus infection is established by detection of the virus in tissue culture (as evidenced by cytopathic changes) and by specific identification with immunofluorescence or other immunologic techniques. Rapid viral diagnosis can be established by immunofluorescence or ELISA of nasopharyngeal aspirates, conjunctival or respiratory secretions, urine, or stool. Highly sensitive and specific PCR assays and nucleic acid hybridization are available and have become the standard for diagnosis based on clinical specimens. Adenovirus types 40 and 41, which have been associated with diarrheal disease in children, require special tissue-culture cells for isolation, and these serotypes are most commonly detected by direct ELISA of stool or by PCR. Serum antibody rises can be demonstrated by complement-fixation or neutralization tests, ELISA, radioimmunoassay, or (for those adenoviruses that hemagglutinate red cells) hemagglutination-inhibition tests.
PREVENTION
Live vaccines have been developed against adenovirus types 4 and 7 and have been highly efficacious in control of acute respiratory disease among military recruits. These vaccines consist of live, unattenuated virus administered in enteric-coated capsules. Infection of the gastrointestinal tract with types 4 and 7 does not cause disease but stimulates local and systemic antibodies that are protective against subsequent acute respiratory disease due to those serotypes. These vaccines were not produced from 1999 to 2011 but are now available again and are being used effectively in military recruits. Adenoviruses are also being studied as live-virus vectors for the delivery of vaccine antigens and for gene therapy.
224 |
Influenza |
DEFINITION
Influenza is an acute respiratory illness caused by infection with influenza viruses. The illness affects the upper and/or lower respiratory tract and is often accompanied by systemic signs and symptoms such as fever, headache, myalgia, and weakness. Outbreaks of illness of variable extent and severity occur nearly every year. Such outbreaks result in significant morbidity rates in the general population and in increased mortality rates among certain high-risk patients, mainly as a result of pulmonary complications.
ETIOLOGIC AGENT
Influenza viruses are members of the Orthomyxoviridae family, of which influenza A, B, and C viruses constitute three separate genera. The designation of influenza viruses as type A, B, or C is based on antigenic characteristics of the nucleoprotein (NP) and matrix (M) protein antigens. Influenza A viruses are further subdivided (subtyped) on the basis of the surface hemagglutinin (H) and neuraminidase (N) antigens; individual strains are designated according to the site of origin, isolate number, year of isolation, and subtype—for example, influenza A/California/07/2009 (H1N1). Influenza A has 18 distinct H subtypes and 11 distinct N subtypes, of which only H1, H2, H3, N1, and N2 have been associated with epidemics of disease in humans. Avian influenza A viruses have been associated with small outbreaks and sporadic cases in humans (see below). Influenza B and C viruses are designated similarly to influenza A viruses, but H and N antigens from these viruses do not receive subtype designations because intratypic variations in influenza B antigens are less extensive than those in influenza A viruses and may not occur with influenza C virus.
Influenza A and B viruses are major human pathogens and the most extensively studied of the Orthomyxoviridae. Type A and type B viruses are morphologically similar. The virions are irregularly shaped spherical particles, measure 80–120 nm in diameter, and have a lipid envelope from the surface of which the H and N glycoproteins project (Fig. 224-1). The hemagglutinin is the site by which the virus binds to sialic acid cell receptors, whereas the neuraminidase degrades the receptor and plays a role in the release of the virus from infected cells after replication has taken place. Influenza viruses enter cells by receptor-mediated endocytosis, forming a virus-containing endosome. The viral hemagglutinin mediates fusion of the endosomal membrane with the virus envelope, and viral nucleocapsids are subsequently released into the cytoplasm. Immune responses to the H antigen are the major determinants of protection against infection with influenza virus, whereas those to the N antigen limit viral spread and contribute to reduction of the infection. The lipid envelope of influenza A virus also contains the M proteins M1 and M2, which are involved in stabilization of the lipid envelope and in virus assembly. The virion also contains the NP antigen, which is associated with the viral genome, as well as three polymerase (P) proteins that are essential for transcription and synthesis of viral RNA. Two nonstructural proteins function as an interferon antagonist and posttranscriptional regulator (NS1) and a nuclear export factor (NS2 or NEP).
FIGURE 224-1 An electron micrograph of influenza A virus (×40,000).
The genomes of influenza A and B viruses consist of eight single-strand RNA segments, which code for the structural and nonstructural proteins. Because the genome is segmented, the opportunity for gene reassortment during infection is high; reassortment often takes place during infection of cells with more than one influenza A virus.
EPIDEMIOLOGY
![]() Influenza outbreaks occur virtually every year, although their extent and severity vary widely. Localized outbreaks take place at variable intervals, usually every 1–3 years. Global pandemics have occurred at variable intervals, but much less frequently than interpandemic outbreaks (Table 224-1). The most recent pandemic emerged in March of 2009 and was caused by an influenza A/H1N1 virus that rapidly spread worldwide over the next several months.
Influenza outbreaks occur virtually every year, although their extent and severity vary widely. Localized outbreaks take place at variable intervals, usually every 1–3 years. Global pandemics have occurred at variable intervals, but much less frequently than interpandemic outbreaks (Table 224-1). The most recent pandemic emerged in March of 2009 and was caused by an influenza A/H1N1 virus that rapidly spread worldwide over the next several months.
|
EMERGENCE OF ANTIGENIC SUBTYPES OF INFLUENZA A VIRUS ASSOCIATED WITH PANDEMIC OR EPIDEMIC DISEASE |
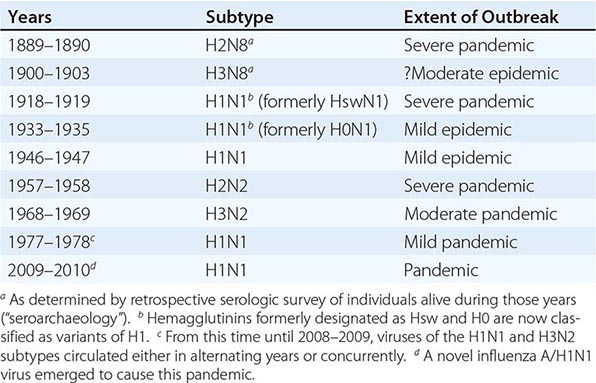
Influenza A Virus • ANTIGENIC VARIATION AND INFLUENZA OUTBREAKS AND PAN DEMICS The most extensive and severe outbreaks of influenza are caused by influenza A viruses, in part because of the remarkable propensity of the H and N antigens of these viruses to undergo periodic antigenic variation. Major antigenic variations, called antigenic shifts, are seen only with influenza A viruses and may be associated with pandemics. Minor variations are called antigenic drifts. Antigenic variation may involve the hemagglutinin alone or both the hemagglutinin and the neuraminidase. An example of an antigenic shift involving both the hemagglutinin and the neuraminidase is that of 1957, when the predominant influenza A virus subtype shifted from H1N1 to H2N2; this shift resulted in a severe pandemic, with an estimated 70,000 excess deaths (i.e., deaths in excess of the number expected without an influenza epidemic) in the United States alone. This excess mortality was significantly greater than that during interpandemic influenza seasons. In 1968, an antigenic shift involving only the hemagglutinin occurred (H2N2 to H3N2); the subsequent pandemic was less severe than that of 1957. In 1977, an H1N1 virus emerged and caused a pandemic that primarily affected younger individuals (i.e., those born after 1957). As shown in Table 224-1, H1N1 viruses circulated from 1918 to 1956; thus, individuals born prior to 1957 would be expected to have some degree of immunity to H1N1 viruses. The pandemic of 2009–2010 was caused by an A/H1N1 virus against which little immunity was present in the general population, although approximately one-third of individuals born before 1950 had some apparent immunity to related H1N1 strains.
During most outbreaks of influenza A, a single subtype has circulated at a time. However, since 1977, H1N1 and H3N2 viruses have circulated simultaneously, resulting in outbreaks of varying severity. In some outbreaks, influenza B viruses have also circulated simultaneously with influenza A viruses. In 2009–2010, the pandemic A/H1N1 virus appeared to circulate nearly exclusively.
FEATURES OF PANDEMIC AND INTERPANDEMIC INFLUENZA A Pandemics provide the most dramatic evidence of the impact of influenza A. However, illnesses occurring between pandemics (interpandemic disease) also account for extensive mortality and morbidity, albeit over a longer period. In the United States, influenza was associated with an average of 23,000 excess deaths per season in 1976–2007 and with a maximum of 48,600 excess deaths during the 2003–2004 season.
Influenza A viruses that circulate between pandemics demonstrate antigenic drifts in the H antigen. These antigenic drifts result from point mutations in the RNA segment that codes for the hemagglutinin and occur most frequently in five hypervariable regions. Epidemiologically significant strains—that is, those with the potential to cause widespread outbreaks—exhibit changes in amino acids in at least two of the major antigenic sites in the hemagglutinin molecule. Because two point mutations are unlikely to occur simultaneously, it is believed that antigenic drifts result from point mutations occurring sequentially during the spread of virus from person to person. Antigenic drifts have been reported nearly annually since 1977 for H1N1 viruses and since 1968 for H3N2 viruses.
Interpandemic influenza A outbreaks usually begin abruptly, peak over a 2- to 3-week period, generally last for 2–3 months, and often subside almost as rapidly as they began. In contrast, pandemic influenza may begin with rapid transmission at multiple locations, have high attack rates, and extend beyond the usual seasonality, with multiple waves of attack before or after the main outbreak. In interpandemic outbreaks, the first indication of influenza activity is an increase in the number of children with febrile respiratory illnesses who present for medical attention. This increase is followed by increases in rates of influenza-like illnesses among adults and eventually by an increase in hospital admissions for patients with pneumonia, worsening of congestive heart failure, and exacerbations of chronic pulmonary disease. Rates of absence from work and school also rise at this time. An increase in the number of deaths caused by pneumonia and influenza is generally a late observation in an outbreak. Attack rates have been highly variable from outbreak to outbreak in interpandemic influenza but most commonly are in the range of 10–20% of the general population.
![]() Although pandemic influenza may occur throughout the year, interpandemic influenza occurs almost exclusively during the winter months in the temperate zones of the Northern and Southern hemispheres. In those locations, it is highly unusual to detect influenza A virus at other times, although rises in serum antibody titer or even outbreaks have been noted rarely during warm-weather months. In contrast, influenza virus infections occur throughout the year in the tropics. Where or how influenza A viruses persist between outbreaks in temperate zones is unknown. It is possible that the viruses are maintained in the human population on a worldwide basis by person-to-person transmission and that large population clusters support a low level of interepidemic transmission. Alternatively, human strains may persist in animal reservoirs. Convincing evidence to support either explanation is not available. In the modern era, rapid transportation may contribute to the transmission of viruses among widespread geographic locales.
Although pandemic influenza may occur throughout the year, interpandemic influenza occurs almost exclusively during the winter months in the temperate zones of the Northern and Southern hemispheres. In those locations, it is highly unusual to detect influenza A virus at other times, although rises in serum antibody titer or even outbreaks have been noted rarely during warm-weather months. In contrast, influenza virus infections occur throughout the year in the tropics. Where or how influenza A viruses persist between outbreaks in temperate zones is unknown. It is possible that the viruses are maintained in the human population on a worldwide basis by person-to-person transmission and that large population clusters support a low level of interepidemic transmission. Alternatively, human strains may persist in animal reservoirs. Convincing evidence to support either explanation is not available. In the modern era, rapid transportation may contribute to the transmission of viruses among widespread geographic locales.
The factors that result in the inception and termination of outbreaks of influenza A are incompletely understood. A major determinant of the extent and severity of an outbreak is the level of immunity in the population at risk. With the emergence of an antigenically novel influenza virus to which little or no immunity is present in a community, extensive outbreaks may occur. When the absence of immunity is worldwide, epidemic disease may spread around the globe, resulting in a pandemic. Such pandemic waves can continue for several years, until immunity in the population reaches a high level. In the years following pandemic influenza, antigenic drifts among influenza viruses result in outbreaks of variable severity in populations with high levels of immunity to the pandemic strain that circulated earlier. This situation persists until another antigenically novel pandemic strain emerges. On the other hand, outbreaks sometimes end despite the persistence of a large pool of susceptible individuals in the population. It has been suggested that certain influenza A viruses may be intrinsically less virulent and cause less severe disease than other variants, even in immunologically virgin subjects. If so, then other (undefined) factors besides the level of preexisting immunity must play a role in the epidemiology of influenza.
Avian and Swine Influenza Viruses Aquatic birds are the largest reservoir of influenza A viruses, harboring 16 hemagglutinin (H1–H16) and nine neuraminidase (N1–N9) subtypes. (In addition, H17N10 and H18N11 viruses are found in bats.) Influenza A pandemic strains in 1957 (A/H2N2) and in 1968 (A/H3N2) resulted from reassortment of gene segments between human and avian viruses. The influenza A/H1N1 virus that caused the most severe pandemic of modern times (1918–1919) appears to have been an adaptation of an avian virus to human infection. Thus, there is concern that avian influenza viruses with novel hemagglutinin and neuraminidase antigens have the potential to emerge as pandemic strains.
![]() Avian influenza A viruses have been reported to cause sporadic cases and small outbreaks in humans, usually after direct contact with birds (most commonly poultry). Sustained person-to-person transmission in the community has not been observed. Avian influenza A/H5N1 virus has been noted to cause illness in humans since 1997, with 648 cases reported to the World Health Organization as of January 2014. It is not clear whether the high observed case–fatality rate (59%) reflects preferential detection of severe cases. A/H7N7 infections have been noted in poultry industry workers; conjunctivitis was the most prominent feature, although a minority of individuals also had respiratory illness. More than 333 cases of avian A/H7N9 infection have been reported in China, with case–fatality rates of 36% among the infected patients admitted to the hospital. Most H7N9 isolates are sensitive to neuraminidase inhibitors, but a few isolates have exhibited high-level resistance to oseltamivir and diminished sensitivity to zanamivir. Infections with avian H9N2 viruses have been reported primarily among children in Hong Kong and have consisted largely of mild respiratory illnesses. Mild cases of illness due to influenza H10N7 virus in Egypt and Australia have also been reported. In 2013, the first cases of human infection with avian A/H10N8 and H6N1 viruses were described.
Avian influenza A viruses have been reported to cause sporadic cases and small outbreaks in humans, usually after direct contact with birds (most commonly poultry). Sustained person-to-person transmission in the community has not been observed. Avian influenza A/H5N1 virus has been noted to cause illness in humans since 1997, with 648 cases reported to the World Health Organization as of January 2014. It is not clear whether the high observed case–fatality rate (59%) reflects preferential detection of severe cases. A/H7N7 infections have been noted in poultry industry workers; conjunctivitis was the most prominent feature, although a minority of individuals also had respiratory illness. More than 333 cases of avian A/H7N9 infection have been reported in China, with case–fatality rates of 36% among the infected patients admitted to the hospital. Most H7N9 isolates are sensitive to neuraminidase inhibitors, but a few isolates have exhibited high-level resistance to oseltamivir and diminished sensitivity to zanamivir. Infections with avian H9N2 viruses have been reported primarily among children in Hong Kong and have consisted largely of mild respiratory illnesses. Mild cases of illness due to influenza H10N7 virus in Egypt and Australia have also been reported. In 2013, the first cases of human infection with avian A/H10N8 and H6N1 viruses were described.
Influenza A viruses also circulate in swine but rarely infect humans. Whereas humans primarily have α-2,6-galactose receptors for hemagglutinins and birds primarily have α-2,3-galactose receptors, swine have both types of receptors. Thus, swine hosts efficiently sustain simultaneous infection with both human and avian viruses, thereby facilitating reassortment of genetic segments between viruses of both species. The pandemic A/H1N1 strain of 2009–2010 was a quadruple reassortant among swine, avian, and human influenza viruses. The influenza A virus subtypes that circulate most commonly in swine are H1N1, H1N2, and H3N2. When a predominantly swine virus causes infections in humans, it is designated a variant virus by the addition of “v” after the subtype. For example, influenza A/H3N2v virus was responsible for 321 cases of human infection reported in the United States in 2011 and 2012 and for 18 cases in 2013. Almost all of the affected patients had had close contact with swine. Only limited person-to-person transmission of swine influenza virus has been noted. Since 2005, 16 human cases caused by A/H1N1v virus and 5 caused by A/H1N2v virus have been detected in the United States.
Influenza B and C Viruses Influenza B virus causes outbreaks that are generally less extensive and are associated with less severe disease than those caused by influenza A virus, although the disease may occasionally be severe. The hemagglutinin and neuraminidase of influenza B viruses undergo less frequent and less extensive variation than those of influenza A viruses; this characteristic may account, in part, for the lesser severity of influenza B. Outbreaks of influenza B occur most frequently in schools and military camps, although outbreaks in institutions in which elderly individuals reside have also been noted on occasion. Since the 1980s, two antigenically distinct “lineages” of influenza B virus have circulated: Victoria and Yamagata.
In contrast to influenza A and B viruses, influenza C virus appears to be a relatively minor cause of disease in humans. It has been associated with common cold–like symptoms and occasionally with lower respiratory tract illness. The widespread prevalence of serum antibody to this virus indicates that asymptomatic infection may be common.
Influenza-Associated Morbidity and Mortality Rates Rates of morbidity and mortality caused by influenza outbreaks continue to be substantial. Most individuals who die in this setting have underlying diseases that place them at high risk for complications of influenza (Table 224-2). On average, there were 226,000 influenza-associated hospitalizations per year in the United States in 1979–2001. Recently, the moderately severe influenza season in 2012–2013 was associated with 381,500 hospitalizations (42 per 100,000 persons). Excess annual hospitalizations for groups of adults and children with high-risk medical conditions ranged from 40 to 1900 per 100,000 during outbreaks of influenza in 1973–2004. The most prominent high-risk conditions are chronic cardiac and pulmonary diseases and old age. Mortality rates among individuals with chronic metabolic or renal diseases or certain immunosuppressive diseases have also been elevated, although they remain lower than mortality rates among patients with chronic cardiopulmonary diseases. In the pandemic of 2009–2010, increased risk of severe disease was noted in children from birth to 4 years of age and in pregnant women. The morbidity rate attributable to influenza in the general population is considerable. It is estimated that interpandemic outbreaks of influenza currently incur annual economic costs of more than $87 billion in the United States. For pandemics, it is estimated that annual economic costs would range from $89.7 to $209.4 billion for attack rates of 15–35%.
|
PERSONS AT HIGHER RISK FOR COMPLICATIONS OF INFLUENZA OR FOR INFLUENZA-RELATED VISITS TO HEALTH CARE FACILITIES |
PATHOGENESIS AND IMMUNITY
The initial event in influenza is infection of the respiratory epithelium with influenza virus acquired from respiratory secretions of acutely infected individuals. In all likelihood, the virus is transmitted via aerosols generated by coughs and sneezes, although transmission through hand-to-hand contact, other personal contact, and even fomites may take place. Experimental evidence suggests that infection by a small-particle aerosol (particle diameter <10 μm) is more efficient than that by larger droplets. Initially, viral infection involves the ciliated columnar epithelial cells, but it may also involve other respiratory tract cells, including alveolar cells, mucous gland cells, and macrophages. In infected cells, virus replicates within 4–6 h, after which infectious virus is released to infect adjacent or nearby cells. In this way, infection spreads from a few foci to a large number of respiratory cells over several hours. In experimentally induced infection, the incubation period of illness has ranged from 18 to 72 h, depending on the size of the viral inoculum. Histopathologic study reveals degenerative changes, including granulation, vacuolization, swelling, and pyknotic nuclei in infected ciliated cells. The cells eventually become necrotic and desquamate; in some areas, previously columnar epithelium is replaced by flattened and metaplastic epithelial cells. The severity of illness is correlated with the quantity of virus shed in secretions; thus, the degree of viral replication itself may be an important factor in pathogenesis. Despite the frequent development of systemic signs and symptoms such as fever, headache, and myalgias, influenza virus has only rarely been detected in extrapulmonary sites (including the bloodstream). Evidence suggests that the pathogenesis of systemic symptoms in influenza may be related to the induction of certain cytokines, particularly tumor necrosis factor α, interferon α, interleukin 6, and interleukin 8, in respiratory secretions and in the bloodstream.
The host response to influenza infections involves a complex interplay of humoral antibody, local antibody, cell-mediated immunity, interferon, and other host defenses. Serum antibody responses, which can be detected by the second week after primary infection, are measured by a variety of techniques: hemagglutination inhibition (HI), complement fixation (CF), neutralization, enzyme-linked immunosorbent assay (ELISA), and antineuraminidase antibody assay. Antibodies to the hemagglutinin appear to be the most important mediators of immunity; in several studies, HI titers of ≥40 have been associated with protection from infection. Secretory antibodies produced in the respiratory tract are predominantly of the IgA class, and secretory antibody neutralization titers of ≥4 have also been associated with protection. A variety of cell-mediated immune responses, both antigen-specific and antigen-nonspecific, can be detected early after infection and depend on the prior immune status of the host. These responses include T cell proliferative, T cell cytotoxic, and natural killer cell activity. In humans, CD8+ as well as CD4+ T lymphocytes are directed at conserved regions of internal proteins (NP, M, and P) as well as at the surface proteins H and N. Interferons can be detected in respiratory secretions shortly after the shedding of virus has begun, and rises in interferon titers coincide with decreases in virus shedding.
The host defense factors responsible for cessation of virus shedding and resolution of illness have not been defined specifically. Virus shedding generally stops within 2–5 days after symptoms first appear, at a time when serum and local antibody responses often are not detectable by conventional techniques, although antibody rises may be detected earlier by use of highly sensitive techniques, particularly in individuals with previous immunity to the virus. It has been suggested that interferon, cell-mediated immune responses, and/or nonspecific inflammatory responses all contribute to the resolution of illness. CD8+ cytotoxic T lymphocyte responses may be particularly important in this regard.
CLINICAL MANIFESTATIONS
Influenza is most frequently described as a respiratory illness characterized by systemic symptoms, such as headache, feverishness, chills, myalgia, and malaise, as well as accompanying respiratory tract signs and symptoms, particularly cough and sore throat. In some cases, the onset is so abrupt that patients can recall the precise time they became ill. However, the spectrum of clinical presentations is wide, ranging from a mild, afebrile respiratory illness similar to the common cold (with either a gradual or an abrupt onset) to severe prostration with relatively few respiratory signs and symptoms. In most of the cases that come to a physician’s attention, the patient has a fever, with temperatures of 38°–41°C (100.4°–105.8°F). A rapid temperature rise within the first 24 h of illness is generally followed by gradual defervescence over 2–3 days, although, on occasion, fever may last as long as 1 week. Patients report a feverish feeling and chilliness, but true rigors are rare. Headache, either generalized or frontal, is often particularly troublesome. Myalgias may involve any part of the body but are most common in the legs and lumbosacral area. Arthralgias may also develop.
Respiratory symptoms often become more prominent as systemic symptoms subside. Many patients have a sore throat or persistent cough, which may last for ≥1 week and which is often accompanied by substernal discomfort. Ocular signs and symptoms include pain on motion of the eyes, photophobia, and burning of the eyes.
In the elderly, influenza may have a relatively subtle presentation. Typical features such as sore throat, myalgia, and even fever may be absent, and general symptoms such as anorexia, malaise, weakness, and dizziness may predominate.
Physical findings are usually minimal in uncomplicated influenza. Early in the illness, the patient appears flushed, and the skin is hot and dry, although diaphoresis and mottled extremities are sometimes evident, particularly in older patients. Examination of the pharynx may yield surprisingly unremarkable results despite a severe sore throat, but injection of the mucous membranes and postnasal discharge are apparent in some cases. Mild cervical lymphadenopathy may be noted, especially in younger individuals. The results of chest examination are largely negative in uncomplicated influenza, although rhonchi, wheezes, and scattered rales have been reported with variable frequency in different outbreaks. Frank dyspnea, hyperpnea, cyanosis, diffuse rales, and signs of consolidation are indicative of pulmonary complications. Patients with apparently uncomplicated influenza have been reported to have a variety of mild ventilatory defects and increased alveolar-capillary diffusion gradients; thus, subclinical pulmonary involvement may be more common than is appreciated.
In uncomplicated influenza, the acute illness generally resolves over 2–5 days, and most patients have largely recovered in 1 week, although cough may persist 1–2 weeks longer. In a significant minority (particularly the elderly), however, symptoms of weakness or lassitude (postinfluenza asthenia) may persist for several weeks and may prove troublesome for persons who wish to resume their full level of activity promptly. The pathogenetic basis for this asthenia is unknown, although pulmonary function abnormalities may persist for several weeks after uncomplicated influenza.
COMPLICATIONS
Complications of influenza occur most frequently in patients >65 years old and in those with certain chronic disorders, including cardiac or pulmonary diseases, diabetes mellitus, hemoglobinopathies, renal dysfunction, and immunosuppression. Pregnancy in the second or third trimester predisposes to complications with influenza. Children <5 years old (especially infants) are also at high risk for complications (Table 224-2).
Pulmonary Complications • PNEUMONIA The most significant complication of influenza is pneumonia: “primary” influenza viral pneumonia, secondary bacterial pneumonia, or mixed viral and bacterial pneumonia (discussed below).
Primary influenza viral pneumonia Primary influenza viral pneumonia is the least common but most severe of the pneumonic complications. It presents as acute influenza that does not resolve but instead progresses relentlessly, with persistent fever, dyspnea, and eventual cyanosis. Sputum production is generally scanty, but the sputum can contain blood. Few physical signs may be evident early in the illness. In more advanced cases, diffuse rales may be noted, and imaging findings consistent with diffuse interstitial infiltrates and/or acute respiratory distress syndrome may be present. In such cases, arterial blood-gas determinations show marked hypoxia. Viral cultures of respiratory secretions and lung parenchyma, especially if samples are taken early in illness, yield high titers of virus. In fatal cases of primary viral pneumonia, histopathologic examination reveals a marked inflammatory reaction in the alveolar septa, with edema and infiltration by lymphocytes, macrophages, occasional plasma cells, and variable numbers of neutrophils. Fibrin thrombi in alveolar capillaries, along with necrosis and hemorrhage, have also been noted. Eosinophilic hyaline membranes can be found lining alveoli and alveolar ducts.
Primary influenza viral pneumonia has a predilection for individuals with cardiac disease, particularly those with mitral stenosis, but has also been reported in otherwise-healthy young adults as well as in older individuals with chronic pulmonary disorders. In some pandemics of influenza (notably those of 1918 and 1957), pregnancy increased the risk of primary influenza pneumonia. Subsequent epidemics of influenza have been associated with increased rates of hospitalization among pregnant women, which were also noted in the pandemic of 2009–2010.
Secondary bacterial pneumonia Secondary bacterial pneumonia follows acute influenza. Improvement of the patient’s condition over 2–3 days is followed by a reappearance of fever along with clinical signs and symptoms of bacterial pneumonia, including cough, production of purulent sputum, and physical and x-ray signs of consolidation. The most common bacterial pathogens in this setting are Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae—organisms that can colonize the nasopharynx and that cause infection in the wake of changes in bronchopulmonary defenses. Secondary bacterial pneumonia occurs most frequently in high-risk individuals with chronic pulmonary and cardiac disease and in elderly individuals. Patients with secondary bacterial pneumonia often respond to appropriate antibiotic therapy when it is instituted promptly.
Mixed viral and bacterial pneumonia Perhaps the most common pneumonic complications during outbreaks of influenza have mixed features of viral and bacterial pneumonia. Patients may experience a gradual progression of their acute illness or may show transient improvement followed by clinical exacerbation, with eventual manifestation of the clinical features of bacterial pneumonia. Sputum cultures may contain both influenza A virus and one of the bacterial pathogens described above. Patchy infiltrates or areas of consolidation may be detected by physical examination and chest x-ray. Patients with mixed viral and bacterial pneumonia generally have less widespread involvement of the lung than those with primary viral pneumonia, and their bacterial infections may respond to appropriate antibacterial drugs. Mixed viral and bacterial pneumonia occurs primarily in patients with chronic cardiovascular and pulmonary diseases.
OTHER PULMONARY COMPLICATIONS Other pulmonary complications associated with influenza include worsening of chronic obstructive pulmonary disease and exacerbation of chronic bronchitis and asthma. In children, influenza infection may present as croup. Sinusitis and otitis media (the latter occurring particularly often in children) may also be associated with influenza.
Extrapulmonary Complications Myositis, rhabdomyolysis, and myoglobinuria are occasional complications of influenza infection. Although myalgias are exceedingly common in influenza, true myositis is rare. Patients with acute myositis have exquisite tenderness of the affected muscles, most commonly in the legs, and may not be able to tolerate even the slightest pressure, such as the touch of bedsheets. In the most severe cases, there is frank swelling and bogginess of muscles. Serum levels of creatine phosphokinase and aldolase are markedly elevated, and an occasional patient develops renal failure from myoglobinuria. The pathogenesis of influenza-associated myositis is also unclear, although the presence of influenza virus in affected muscles has been reported.
Myocarditis and pericarditis were reported in association with influenza virus infection during the 1918–1919 pandemic; these reports were based largely on histopathologic findings, and these complications have been reported only infrequently since that time. Electrocardiographic changes during acute influenza are common among patients who have cardiac disease but have been ascribed most often to exacerbations of the underlying cardiac disease rather than to direct involvement of the myocardium with influenza virus. Epidemiologic data have shown an association between influenza outbreaks and increased cardiovascular-associated hospitalizations.
Central nervous system (CNS) complications such as encephalitis and transverse myelitis have been associated with influenza. Encephalitis is a rare but potentially serious complication that has been reported with influenza A and B virus infections. Children <5 years of age appear to be at greatest risk. The pathogenetic mechanisms by which influenza causes CNS disease are unclear. Guillain-Barré syndrome has been reported following influenza infection and, uncommonly, after influenza vaccination (see “Prophylaxis,” below).
Toxic shock syndrome associated with S. aureus or group A streptococcal infection following acute influenza infection has been described (Chaps. 172 and 173).
Reye’s syndrome is a serious complication in children that is associated with influenza B and—to a lesser extent—influenza A virus infection as well as with varicella-zoster virus and other viral infections. An epidemiologic association between Reye’s syndrome and aspirin therapy for the antecedent viral infection has been noted; the syndrome’s incidence has decreased markedly with widespread warnings regarding aspirin use by children with acute viral respiratory infections.
In addition to complications involving the specific organ systems described above, influenza outbreaks include cases in which elderly and other high-risk individuals develop influenza and subsequently experience a gradual deterioration of underlying cardiovascular, pulmonary, or renal function—changes that occasionally are irreversible and lead to death. These deaths contribute to the overall excess mortality associated with influenza outbreaks.
LABORATORY FINDINGS AND DIAGNOSIS
During acute influenza, virus may be detected in throat swabs, nasopharyngeal swabs or washes, or sputum. Reverse-transcriptase polymerase chain reaction (RT-PCR) is the most sensitive and specific technique for detection of influenza viruses. RT-PCR can differentiate among influenza subtypes and is used for detection of avian influenza viruses. Rapid influenza diagnostic tests (RIDTs) detect influenza virus antigens by immunologic or enzymatic techniques. RIDTs yield results quickly, and some tests can distinguish between influenza A and B viruses. Although relatively specific, RIDTs vary in sensitivity with the technique and the virus to be detected.
Influenza virus may be isolated from tissue culture or chick embryos, but these labor-intensive procedures generally are no longer used for diagnostic purposes. Serologic methods for diagnosis require comparison of antibody titers in sera obtained during the acute illness with those in sera obtained 10–14 days after the onset of illness and are useful primarily in retrospect and for epidemiologic studies.
Other laboratory tests generally are not helpful in the specific diagnosis of influenza virus infection. Leukocyte counts are variable, frequently being low early in illness and normal or slightly elevated later. Severe leukopenia has been described in overwhelming viral or bacterial infection, whereas leukocytosis with >15,000 cells/μL raises the suspicion of secondary bacterial infection.
DIFFERENTIAL DIAGNOSIS
During a community-wide outbreak, a clinical diagnosis of influenza can be made with a high degree of certainty in patients who present to a physician’s office with the typical febrile respiratory illness described above. In the absence of an outbreak (i.e., in sporadic or isolated cases), influenza may be difficult to differentiate on clinical grounds alone from an acute respiratory illness caused by any of a variety of respiratory viruses or by Mycoplasma pneumoniae. Severe streptococcal pharyngitis or early bacterial pneumonia may mimic acute influenza, although bacterial pneumonias generally do not run a self-limited course. Purulent sputum in which a bacterial pathogen can be detected by Gram’s staining is an important diagnostic feature in bacterial pneumonia.
PROPHYLAXIS
The major public health measure for prevention of influenza is vaccination. Both inactivated (killed) and live attenuated vaccines are available and are generated from isolates of influenza A and B viruses that circulated in the previous influenza seasons and are anticipated to circulate in the upcoming season. For inactivated vaccines, 50–80% protection against influenza is expected if the vaccine virus and the currently circulating viruses are closely related. Available inactivated vaccines have been highly purified and are associated with few reactions. Up to 5% of individuals experience low-grade fever and mild systemic symptoms 8–24 h after vaccination, and up to one-third develop mild redness or tenderness at the vaccination site. Although the 1976 swine influenza vaccine appears to have been associated with an increased frequency of Guillain-Barré syndrome, influenza vaccines administered since 1976 generally have not been. Possible exceptions were noted during the 1992–1993 and 1993–1994 influenza seasons, when there may have been an excess risk of this syndrome (slightly more than 1 case per 1 million vaccine recipients). Large-scale studies of vaccination with the 2009 pandemic H1N1 vaccine also suggested a possible increased risk of Guillain-Barré syndrome (1 case per 1 million vaccinees). However, the overall health risk following influenza substantially outweighs the potential risk associated with vaccination.
A live attenuated influenza vaccine administered by intranasal spray is available. The vaccine is generated by reassortment between currently circulating strains of influenza A and B viruses and a cold-adapted, attenuated master strain. The cold-adapted vaccine is well tolerated and highly efficacious (>90% protective) in young children; in one study, it provided protection against a circulating influenza virus that had drifted antigenically away from the vaccine strain. Live attenuated vaccine is approved for use in healthy nonpregnant persons 2–49 years of age.
Since 1975, influenza vaccines have been trivalent—i.e., they have contained two influenza A subtypes (H3N2 and H1N1) and one influenza B component. However, two antigenically distinct lineages of influenza B virus have circulated since the 1980s, and a quadrivalent vaccine that includes both B lineages is now available (2013–2014) as well. Quadrivalent vaccines are available in both inactivated and live-attenuated vaccine formulations.
Inactivated influenza vaccines have been noted to be less immunogenic in the elderly. A higher-dose trivalent vaccine containing 60 μg of each antigen and a lower-dose, intradermally administered trivalent vaccine containing 9 μg of each antigen have been approved for use in individuals ≥65 years of age and individuals 18–64 years of age, respectively.
The influenza vaccines discussed above are manufactured in eggs and should not be administered to persons with true hypersensitivity to eggs. For use in this situation, an egg-free vaccine manufactured in cells through recombinant DNA techniques (Flublok®; Protein Sciences Corporation, Meriden, CT) has been approved. Active research is under way to develop vaccines with broad activity against antigenically distinct subtypes (“universal influenza vaccines”).
Historically, the U.S. Public Health Service has recommended influenza vaccination for certain groups at high risk for complications of influenza on the basis of age or underlying disease (Table 224-2) or for their close contacts. Although such individuals will continue to be the focus of vaccination programs, the recommendations have been progressively expanded, and immunization of the entire population above the age of 6 months has been recommended since 2010–2011. (Approved influenza vaccines are not available for infants <6 months of age.) This expanded recommendation reflects increased recognition of previously unappreciated risk factors (e.g., obesity, postpartum conditions, and racial or ethnic influences) as well as an appreciation that more widespread use of vaccine is required for influenza control. Inactivated vaccines may be administered safely to immunocompromised patients. Influenza vaccination is not associated with exacerbations of chronic nervous system diseases such as multiple sclerosis. Vaccine should be administered early in the autumn before influenza outbreaks occur and should then be given annually to maintain immunity against the most current influenza virus strains.
Although antiviral drugs provide chemoprophylaxis against influenza, their use for that purpose has been limited because of concern about current and future development of resistance. Chemoprophylaxis with oseltamivir or zanamivir has been 84–89% efficacious against influenza A and B (Table 224-3). Chemoprophylaxis with amantadine or rimantadine is no longer recommended because of widespread resistance to these drugs. In earlier studies with sensitive viruses, prophylaxis with amantadine or rimantadine was 70–100% effective against illness associated with influenza A virus.
Chemoprophylaxis for healthy persons after community exposure generally is not recommended but may be considered for individuals at high risk of complications who have had close contact with an acutely ill person with influenza. During an outbreak, antiviral chemoprophylaxis can be administered simultaneously with inactivated vaccine because the drugs do not interfere with an immune response to the vaccine. However, concurrent administration of chemoprophylaxis and live attenuated vaccine may interfere with the immune response to the latter. Antiviral drugs should not be administered until at least 2 weeks after administration of live vaccine, and administration of live vaccine should not begin until at least 48 h after antiviral drug administration has been stopped. Chemoprophylaxis may also be considered to control nosocomial outbreaks of influenza. For that purpose, prophylaxis should be instituted promptly when influenza activity is detected and must be continued daily for the duration of the outbreak.
SECTION 14 |
INFECTIONS DUE TO HUMAN IMMUNODEFICIENCY VIRUS AND OTHER HUMAN RETROVIRUSES |
225e |
The Human Retroviruses |
The retroviruses, which make up a large family (Retroviridae), infect mainly vertebrates. These viruses have a unique replication cycle whereby their genetic information is encoded by RNA rather than DNA. Retroviruses contain an RNA-dependent DNA polymerase (a reverse transcriptase) that directs the synthesis of a DNA form of the viral genome after infection of a host cell. The designation retrovirus denotes that information in the form of RNA is transcribed into DNA in the host cell—a sequence that overturned a central dogma of molecular biology: that information passes unidirectionally from DNA to RNA to protein. The observation that RNA was the source of genetic information in the causative agents of certain animal tumors led to a number of paradigm-shifting biologic insights regarding not only the direction of genetic information passage but also the viral etiology of certain cancers and the concept of oncogenes as normal host genes scavenged and altered by a viral vector.
The family Retroviridae includes seven subfamilies (Table 225e-1). Members of two of the families infect humans with pathologic consequences: the deltaretroviruses, of which human T cell lymphotropic virus (HTLV) type 1 is the most important in humans; and lentiviruses, of which HIV is the most important in humans.
|
CLASSIFICATION OF RETROVIRUSES: THE FAMILY RETROVIRIDAE |
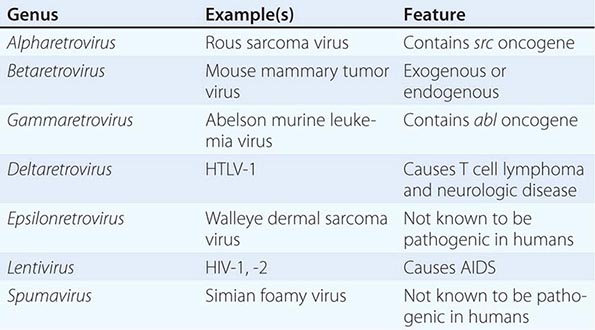
The wide variety of interactions of a retrovirus with its host range from completely benign events (e.g., silent carriage of endogenous retroviral sequences in the germline genome of many animal species) to rapidly fatal infections (e.g., exogenous infection with an oncogenic virus such as Rous sarcoma virus in chickens). The ability of retroviruses to acquire and alter the structure and function of host cell sequences has revolutionized our understanding of molecular carcinogenesis. The viruses can insert into the germline genome of the host cell and behave as a transposable or movable genetic element. They can activate or inactivate genes near the site of integration into the genome. They can rapidly alter their own genome by recombination and mutation under selective environmental stimuli.
Most human viral diseases occur as a consequence of tissue destruction either directly by the virus itself or indirectly by the host’s response to the virus. Although these mechanisms are operative in retroviral infections, retroviruses have additional mechanisms of inducing disease, including the malignant transformation of an infected cell and the induction of an immunodeficiency state that renders the host susceptible to opportunistic diseases (infections and neoplasms; Chap. 226).
STRUCTURE AND LIFE CYCLE
All retroviruses are similar in structure, genome organization, and mode of replication. Retroviruses are 70–130 nm in diameter and have a lipid-containing envelope surrounding an icosahedral capsid with a dense inner core. The core contains two identical copies of the single-strand RNA genome. The RNA molecules are 8–10 kb long and are complexed with reverse transcriptase and tRNA. Other viral proteins, such as integrase, are also components of the virion particle. The RNA has features usually found in mRNA: a cap site at the 5′ end of the molecule, which is important in the initiation of mRNA translation, and a polyadenylation site at the 3′ end, which influences mRNA turnover (i.e., messages with shorter polyA tails turn over faster than messages with longer polyA tails). However, the retroviral RNA is not translated; instead it is transcribed into DNA. The DNA form of the retroviral genome is called a provirus.
The replication cycle of retroviruses proceeds in two phases (Fig. 225e-1). In the first phase, the virus enters the cytoplasm after binding to one or more specific cell-surface receptors; the viral RNA and reverse transcriptase synthesize a double-strand DNA version of the RNA template; and the provirus moves into the nucleus and integrates into the host cell genome. This proviral integration is permanent. Although some animal retroviruses integrate into a single specific site of the host genome in every infected cell, the human retroviruses integrate randomly. This first phase of replication depends entirely on gene products in the virus. The second phase includes the synthesis and processing of viral genomes, mRNAs, and proteins using host cell machinery, often under the influence of viral gene products. Virions are assembled and released from the cell by budding from the membrane; host cell membrane proteins are frequently incorporated into the envelope of the virus. Proviral integration occurs during the S-phase of the cell cycle; thus, in general, nondividing cells are resistant to retroviral infection. Only the lentiviruses are able to infect nondividing cells. Once a host cell is infected, it is infected for the life of the cell.
FIGURE 225e-1 The life cycle of retroviruses. A. Overview of virus replication. The retrovirus enters a target cell by binding to a specific cell-surface receptor; once the virus is internalized, its RNA is released from the nucleocapsid and is reverse-transcribed into proviral DNA. The provirus is inserted into the genome and then transcribed into RNA; the RNA is translated; and virions assemble and are extruded from the cell membrane by budding. B. Overview of retroviral gene expression. The provirus is transcribed, capped, and polyadenylated. Viral RNA molecules then have one of three fates: they are exported to the cytoplasm, where they are packaged as the viral RNA in infectious viral particles; they are spliced to form the message for the envelope polyprotein; or they are translated into Gag and Pol proteins. Most of the messages for the Pol protein fail to initiate Pol translation because of a stop codon before its initiation; however, in a fraction of the messages, the stop codon is missed and the Pol proteins are translated. (Modified from JM Coffin, in BN Fields, DM Knipe [eds]: Fields Virology. New York, Raven, 1990; with permission.)