Chapter 1 The History of Gastrointestinal Endoscopy
Early Efforts
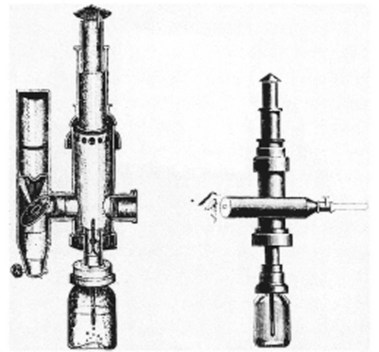
Bozini is credited with the earliest known attempt to visualize the interior of a body cavity with a primitive endoscope (Fig. 1.1). He published his work in 1805.1–3 Bozini devised a tin tube illuminated by a candle from which light was reflected by a mirror, a device he called a lichtleiter (light conductor). He used this device to examine the urethra, urinary bladder, and vagina, but it was an impractical instrument that never gained wide acceptance. Although there were multiple attempts to develop more usable instruments, all directed toward the GU tract, none were widely used. The most notable efforts were by Segalas in France in 1826 and Fisher in Boston in 1827,2 both using straight metal tubes, but the lack of a satisfactory light source remained a major impediment.

Fig. 1.1 Bozzini’s lichtleiter, 1805.
(From Edmonson JM: History of the instruments for gastrointestinal endoscopy. Gastrointest Endosc 37:S28, 1991.)
The next significant development was the instrument of Desormeaux in France.2 Desormeaux’s contribution in 1855 was a better, although still inadequate, light source using a lamp fueled with alcohol and turpentine (“gazogene”) (Fig. 1.2). His instrument was based on that of Segalas. Others continued with efforts to improve the light source and the means to deliver it, but the devices were unsatisfactory for the more inaccessible areas of the GI tract.
Rigid Gastrointestinal Endoscopes
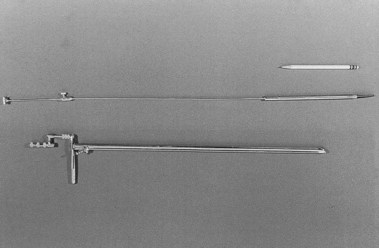
Kussmaul is credited as being the first to perform gastroscopy in 1868, using a straight rigid metal tube passed over a flexible obturator and a cooperative sword swallower (Fig. 1.3).1–4 For a light source, he used a mirror reflecting light from the Desormeaux device but found it inadequate. He also quickly discovered that gastric secretions were a problem, despite using a flexible tube he had developed earlier to empty the stomach before the procedure. The value of his efforts was the demonstration that the curves and bends of the esophagus and esophagogastric junction could be traversed with careful manipulation and that the gastric pouch could be visualized. Kussmaul apparently demonstrated his “gastroscope” several times, but the illumination was too poor to allow a clinically useful image,4 and he abandoned his efforts.

Fig. 1.3 Kussmaul’s gastroscope, 1868.
(From Edmonson JM: History of the instruments for gastrointestinal endoscopy. Gastrointest Endosc 37:S30, 1991.)
Encouraged by the efforts of Kussmaul, others switched their attention to developing esophagoscopes because the esophagus is much easier to visualize, and a less complex design than the gastroscope was required. The problems of perforation, at that time usually fatal, and of illumination remained major obstacles. Before the late 19th century, illumination of light reflected by a mirror into a straight metal tube continued to be used. As noted earlier, several light sources were developed, but the intensity left much to be desired. Several innovations were developed to solve this problem, including a burning magnesium wire, which produced a brilliant light but unacceptable heat and smoke. The most promising device seemed to be the brilliant light from a loop of platinum wire charged with direct current, introduced simultaneously by Bruck in Breslau and Milliot of Paris in 1882.2 Although the illumination was good, major difficulties were encountered with the considerable heat generated, necessitating a water cooling system, and the cumbersome batteries used for a power source. Nevertheless, the platinum wire device was an encouraging development and was used in several instruments that saw relatively wide use.
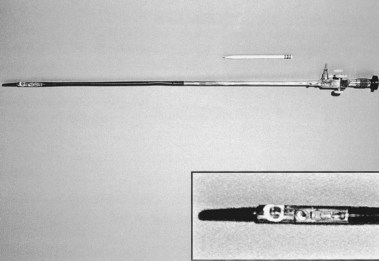
At the turn of the 20th century, Jackson, an otolaryngologist, also examined the esophagus and the stomach using a straight rigid tube and a distal electric light bulb, but few could match his talents in the GI tract. Under his influence, esophagoscopy was considered the exclusive province of ear, nose, and throat (ENT) departments in many community hospitals in the United States as late as the 1950s. The design of the esophagoscope remained a straight rigid tube, usually with a rubber finger–tipped obturator to make insertion safer. With the later addition of a 4× power lens on the proximal end and a distal incandescent bulb, various models were popular until the introduction of fiberoptics in 1961. The Eder-Hufford rigid esophagoscope (Fig. 1.4), introduced in 1949, was popular and still in use during my training in 1960–1962.
It was not until after 1900 that persistent efforts to develop a usable gastroscope were successful. All attempts to build a flexible instrument using a multiplicity of lenses were designed to be straightened after introduction and were fragile, easily damaged, and cumbersome. Straight tubes with simpler optics were useful, but perforations were still a problem.1 In 1911, Elsner introduced a rigid gastroscope with an outer tube through which could be passed a separate inner optical tube with a flexible rubber tip and side-viewing portal (Fig. 1.5). The rubber tip, previously used in the esophagoscope obturator, was more crucial than it might appear, for it seemed to be, along with the later addition of a flexible metal coil proximal to it, the single feature that reduced the rate of perforation. Elsner’s instrument worked as designed and was widely used, especially by Schindler, then in his native Germany, who called it the “mother of all instruments until 1932.”5
Semiflexible Gastroscopes
In 1911, Hoffman showed that an image could be transmitted through a curved line by linking several short-focus prisms. Using this principle, several instruments were constructed, but these were unsatisfactory or were not widely accepted. Schindler, working with Wolf, the renowned instrument maker, constructed a semiflexible instrument with a rigid proximal portion and a distal portion made elastic by coiled copper wire and terminating with first a rubber finger and later a small rubber ball. Illumination was with a distal incandescent light bulb. Air insufflation was made possible with a rubber bulb, expanding the stomach wall to beyond the focal length of the prisms, which were manufactured by Zeiss. In 1932, the sixth and final version was patented. This instrument, known as the Wolf-Schindler gastroscope, greatly improved the safety and efficacy of gastroscopy and was used throughout the world (Fig. 1.6).
Thanks to the published meticulous work and enthusiasm of Schindler, whose designation as the “father of gastroscopy” is well deserved, the procedure was finally widely accepted as a valuable extension of the physical examination. The era of the semiflexible gastroscope from 1932–1957 has been called “the Schindler era.” Schindler was chiefly responsible for transforming gastroscopy from a dangerous and seldom used procedure to one that was relatively safe and indispensable for evaluation of known or suspected disease of the stomach. He insisted that all clinicians who planned to use the instrument be properly trained and that “… no manipulation inside of the body is without danger; therefore no endoscopic examination should be done without reasonable indication.”6 In today’s vernacular, the risk approaches infinity if the benefit approaches zero.
Schindler was born in Berlin in 1888. He gained considerable experience as an Army physician in World War I, where he became convinced that gastritis, then an often disparaged cause of symptoms, was a bona fide disease. His interest in gastritis lasted throughout his career and undoubtedly stimulated his interest in gastroscopy. The Wolf-Schindler endoscope of 1932 and Schindler’s publications with drawings further enhanced what thereafter rapidly became a discipline. His enthusiasm for and talent in using the gastroscope led to what has been called his “gospel of gastroscopy,” which he and others spread throughout academia and to the community of practicing physicians. Because of his Jewish background, Schindler was put in “protective custody” by the Nazis, but with the help of the physicians Ortmeyer and Palmer and philanthropists in Chicago, he was able to immigrate to the United States in 1934.1–47
Chicago became the hub of GI endoscopy, and it was here, in Schindler’s home, that the first discussions were held about forming a new organization for GI endoscopy, now known, after several name changes, as the American Society of Gastrointestinal Endoscopy. In 1943, just 9 years after his arrival in the United States, Schindler left Chicago for Loma Linda University. In 1958, he accepted an appointment as Professor of Medicine at the University of Minas Gerais in Belo Horizone, Brazil. He came back to the United States in 1960 because of an eventually fatal illness of his wife and returned to his native Berlin in 1964, where he died in 1968 at the age of 80.1 Despite his acclaim in endoscopy, Schindler insisted that one must be a physician first and an endoscopist second. He was very knowledgeable in the field of general gastroenterology and published, without coauthors, a synopsis of the entire field in 1957.6
The Wolf-Schindler endoscope was introduced into the United States by Benedict, Borland, and many others. Schindler’s immigration to Chicago inspired a surge of interest in the United States, but with the outbreak of war in Europe, the German source of instruments disappeared. Several U.S. companies working with Schindler and others produced many popular gastroscopes that were significant variations on the Wolf-Schindler, including Cameron Co., which produced its first instrument in 1940.8 The Eder-Hufford semiflexible gastroscope followed in 1946,9 and American Cystoscope Makers, Inc. (ACMI) produced a gastroscope in 1950. A combination of the Eder-Hufford esophagoscope with a semiflexible gastroscope to be passed through it was the Eder-Palmer transesophagoscopic flexible gastroscope produced by the Eder Company in 1953. Each gastroscope had its proponents.
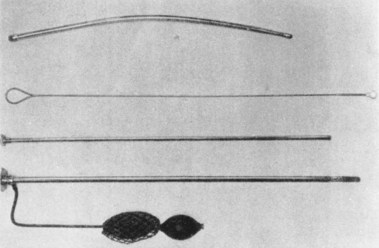
Biopsy
With the availability of instruments for visualization, it became apparent that tissue must be obtained to identify the nature of the observed abnormalities. Instruments for blind biopsies were used early on, but a device was needed that would allow the operator to obtain a biopsy specimen of abnormal tissue directly when seen at endoscopy. The Benedict Operating Gastroscope was produced in 1948 based on a 1940 model by Kenamore (Fig. 1.7).10 The Benedict instrument, on which I was trained in 1960, was a popular instrument that was widely used. In the debates about the necessity for biopsy, Benedict, originally a surgeon who switched entirely to endoscopy, stated that gastroscopy was not a routine procedure and should be reserved for difficult problems in differential diagnosis, but “gastroscopic examination is not complete unless the gastroscopist has some means of biopsy readily available.”11 It soon became clear that the correlation between histology and a diagnosis based on visualization alone was often widely discrepant, and certain diagnoses could not be reliably made without tissue examination. Efforts such as wash and brush cytology continued and have persisted in various forms to the present time.
Fiberoptics
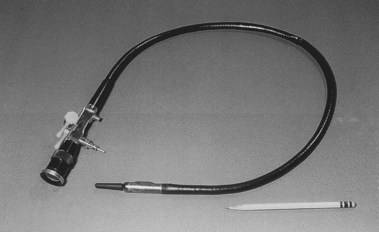
The principle of internal reflection of light along a conduction pathway was used by Lamm in October 1930.1 The image was severely degraded by light escaping from the thin fibers of quartz he used, although the potential for total flexibility was present. Lamm could not interest Schindler or others in his efforts, and the experiment was discontinued. Almost 25 years later, in 1954, Hirschowitz, in fellowship training at the University of Michigan, visited Hopkins and Kapany in London to review their work12 with glass fibers, which totally confirmed the work of Lamm and his predecessors. Hirschowitz became convinced that application of this principle could be used to develop a totally new and superior endoscope. He began work with a graduate student, Curtiss, who developed a technique of coating glass fibers with glass of a different optical density, preventing the escape of light and degradation of the image. This was the critical discovery that made the principle of internal reflection through glass fibers workable.
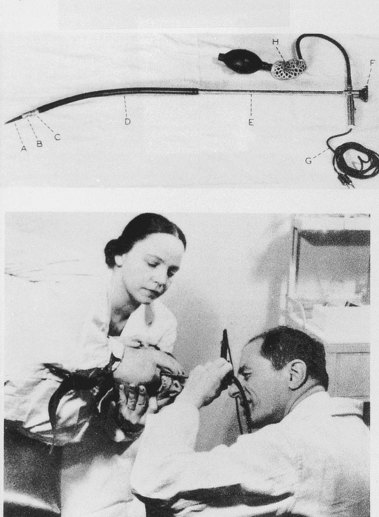
In 1957, Hirschowitz demonstrated his fiberscope, and he published his work in 1958 (Fig. 1.8).13 His audience was not impressed, and it took another 3 years, working with ACMI, to produce a marketable scope, which he called the Hirschowitz Gastroduodenal Fiberscope. This was a very flexible side-viewing instrument with an electric light on its distal end, an air channel, and an adjustable focusing lens proximally. The tip lacked what was by then the “obligatory” rubber finger, and this omission was a source of criticism; one was added on a later model. Although some individuals criticized the quality of the image, most believed the size and brightness were superior to the semiflexible scopes. This model, the ACMI 4990, was introduced to the market late in 1960 after being tested by Hirschowitz on himself and numerous patients. In 1961, I was in Gastroenterology fellowship at the Emory University Clinic with Schroder. I vividly recall his reaction when we first used our new fiberscope around March 1962 (Fig. 1.9). When he finished our first examination, he turned to me and said, “Anybody want to buy a used Benedict operating scope?” I don’t think we ever used it again. The Hirschowitz Gastroduodenal Fiberscope was clearly superior in my young view, and I finished my training with that instrument.
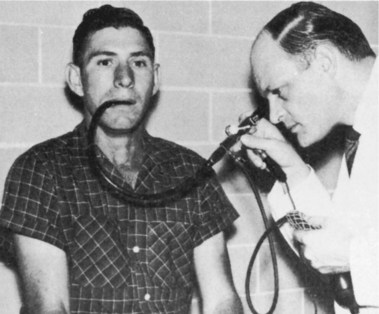
Fig. 1.8 Hirschowitz examining the stomach of an outpatient.
(From Hirschowitz BI: Endoscopic examination of the stomach and duodenal cap with the fiberscope. Lancet 1:1074–1078, 1961.)
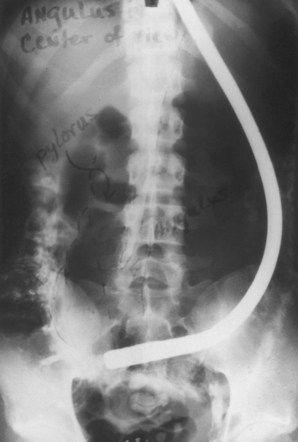
There were problems with the fiberscope noted by us and by others. The distal light source would become so heated that thermal injury to the gastric mucosa was possible unless the tip was continuously moved. In prolonged procedures, protein in gastric secretions would coagulate on the bulb and the adjacent visualizing port, totally obscuring the lens. As the number of procedures with a single instrument increased, some glass fibers would break, producing small black dots in the visual field. This was a persistent problem with fiberscopes during their entire history and especially apparent in training programs where a single scope was used by several trainees on many patients. The side-viewing lens prevented visualization of the esophagus, and the scope had to be passed blindly through the pharyngeal orifice. The previous semiflexible scopes in use shared this problem, and it was not considered a defect at the time. The flexibility itself resulted in some difficulty in advancing because attempts to push the instrument through the pylorus and into the gut resulted in more bowing in the gastric pouch (Fig. 1.10). Although one could sometimes visualize the duodenum, this was done by overinflating the stomach and looking through the pylorus without actually entering it. If one managed to introduce the tip into the duodenum, as occasionally happened, the visual field was inside the focal length of the instrument, and only a “red-out” was observed. Others had similar complaints.4
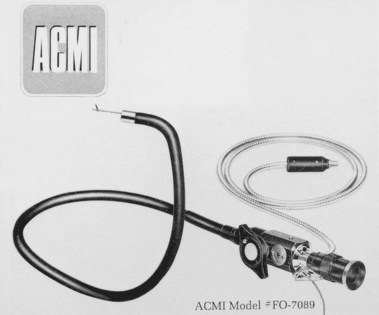
Many clinicians did not believe the additional expense of replacing the older, beloved instruments with which they had been successful for many years was warranted. Even ACMI officials did not see the fiberscope as totally replacing the instruments with lens systems.2 Despite reservations, comparison and experiential studies showed the advantages of the new fiberscopes.14–17 Following the flagship ACMI model 4990, several models of the fiberscope were introduced by ACMI and other companies, each with significant improvements, including the controllable tip in the side-viewing ACMI model 5004. Visualization of the gastric pouch, including retroflexed views of the cardia, was complete. The major objection to these instruments was the inability to pass the instrument under direct vision and examine the esophagus; also, the area beyond the pylorus could not be consistently examined.
Most clinicians were already fully trained in use of the Eder-Hufford esophagoscope, and in the absence of a forward-viewing fiberscope, use of the Eder-Hufford esophagoscope continued. A forward-viewing scope was mandatory. LoPresti modified the tip of the fiberscope to create the foroblique fiberoptic esophagoscope in 1964.18 Passing the instrument under direct vision was possible, and clinicians immediately discovered that they could examine not only the esophagus, but also a large portion of the proximal stomach. At a length of 90 cm, however, one could not reach the duodenum. Working with ACMI, LoPresti produced the longer Panview Mark “87” gastro-Esophageal Endoscope in 1970. By about 1971, the instrument had been lengthened to 105 cm with a four-way controllable tip capable of 180 degrees of deflection (Fig. 1.11).
Endoscopic Retrograde Cholangiopancreatography (ERCP)
With access to the duodenum, the ampulla of Vater became visible. It followed that one should be able to inject contrast material into the bile and pancreatic ducts and increase diagnostic capabilities. Initial attempts in 1968 by McCune and associates19 to modify an existing scope were only partially successful but did show that endoscopic visualization by injection of radiologic contrast agents into ducts was possible. In 1970, Machida and Olympus in Japan produced usable, side-viewing scopes with controllable tips and elevators to move the injection tube to the ampulla.
Japanese endoscopists20 developed the technique of endoscopic retrograde cholangiopancreatography (ERCP) with an 80% success rate. Vennes and Silvis21 showed the utility of ERCP in the United States and taught many physicians to use it.4 It was immediately apparent that if clinicians could visualize the biliary and pancreatic ducts endoscopically (i.e., nonsurgically), they should be able to apply by some means long-established surgical techniques for treatment of choledocholithiasis and pancreatitis, such as sphincterotomy and stone removal. In 1974, just 4 years after the demonstration of the diagnostic utility of the new ERCP scopes, Kawai and colleagues in Japan22 and Classen and Demling in Germany23 independently developed methods of endoscopic electrosurgical sphincterotomy for extraction of biliary calculi in the common duct. This procedure requires great skill; in 1976, Geenen24 reported only 62 operative procedures had been done by four endoscopists, and 7 of the procedures were failures. In 1983, Schuman4 reported that several thousands of patients had undergone ERCP, and by now, hundreds of thousands of ERCP procedures have been done. Because of advanced radiologic techniques, ERCP is now seldom used for purely diagnostic purposes.
Photography
It is one thing to describe to others what one may see through any device and another to be able to show them. The large impact of Schindler’s early publications was related, in part, to the excellent color drawings he presented. Early on, neither cameras nor photographic films were advanced enough to allow good color reproduction or sharp, accurate images in relatively poor lightening. Such documentation is essential for widespread appreciation of endoscopy by individuals who do not perform the procedure. The first clinically useful photography came with improvements in film by Kodak and the construction of an external integrated camera by Segal and Watson in 1948.25,26 Although these authors reported that approximately 61% of the images were of good quality, this was not the experience of all clinicians.4
Although an intragastric camera was developed in 1848 by Lange and Meltzung, a clinically useful device was not available until 1950, when Uji, Sugiura, and Fukami, working with Olympus Corp.,27 developed the Gastrocamera with synchronized flash that took good intragastric pictures and with a controllable distal portion. By following a prescribed pattern of rotation and flexion, a series of pictures was obtained that included the entire surface of the stomach. The big disadvantage was that the operator could not see through the instrument and had to await development of the very narrow (5-mm) film before the results could be seen. Photographs for demonstration required additional time in the photo laboratory while enlargements were made.
With the introduction of fiberoptic scopes in 1961, Olympus introduced a combination Gastrocamera fiberscope (GTF-A) in 1964, but, as Schuman4 commented, “it was just a gastroscope” and never attained popularity. Simultaneously, rapid development and physician acceptance of fiberscopes with the ability to use technically advanced 35-mm cameras with an external adapter made the Gastrocamera obsolete, and it was abandoned.
Sigmoidoscopy and Colonoscopy
The problems presented by examination of the anus and rectum were relatively easy. Straight metal tubes were used and found in the ruins of Pompeii.2 The basic design of the anoscope has not changed in the past century or more except that it is now made of disposable plastic. It remains a tapering short tube with an obturator that is removed after introduction through the anal sphincter. Examination of the rectum and sigmoid required a longer tube, but no truly satisfactory device was available until 1894, when Kelly28 at Johns Hopkins developed a 30-cm rigid tube with light reflected down the tube from a head lamp. Tuttle29 incorporated a distal light source in his proctosigmoidoscope of 25 cm in 1903. These instruments have remained the basic design for the past 100 years. Within the past 15 years or so, disposable clear plastic tubes have been widely used. These are essentially a plastic version of the Kelly and Tuttle tubes with a distal electric light source, but visualization is possible through the clear plastic. With the application of fiberoptics to sigmoidoscopy in the late 1960s, examination of the sigmoid colon became not only satisfactory, but also much more comfortable to the patient.
Overholt,30 who later went on to be the principal developer of colonoscopy using similar technology, presented his results of flexible sigmoidoscopy in 250 patients in 1968. Although early flexible sigmoidoscopes were made in variable lengths, the current length of 60 cm came to be the preferred one. Examination of the colon above the sigmoid presents obvious additional problems of multiple curves and angulations amenable only to highly flexible instruments and trained operators. Attempts, all unsuccessful, were made using semiflexible instruments, and these are reviewed by Edmonson.2 Satisfactory examination of the length of the colon was impossible until the introduction of the flexible fiberscope. Attempts to use forward-viewing gastroscopes were not technically satisfactory, although several clinicians tried, including me. Turell31 presented his attempts in 1967 using a modified gastroscope, but he concluded that the instrument was not ready for routine clinical use. By 1970, several manufacturers produced instruments specifically designed for colonoscopy, including ACMI working with Overholt in the United States and Olympus Corp. in Japan.
The primary problem with regularly completing examinations to the cecum was not the instruments so much as it was the techniques necessary for passage of the scopes into the more proximal portions of the colon. Earlier pioneers in developing successful techniques still in use include, among others, Overholt, Wolf, Shinya, and Waye in the United States; Niwa and colleagues in Japan; Salmon and Williams in England; and Dehyle in Germany.4 Many of these early efforts were accomplished with the guidance of fluoroscopy to negotiate the more difficult turns and to identify the actual area being observed, but as experience was gained, fluoroscopy was no longer required. Learning under expert guidance and experience continues to be more necessary in colonoscopy (and ERCP) than in upper endoscopy. By 1971, the diagnostic advantage of fiberoptic colonoscopy over single-contrast barium enema was firmly established,32 and the efficacy and safety of polypectomy were established by 1973.33
Digital Endoscopy (Videoendoscopy)
In 1984, barely 20 years after introduction of the endoscopic fiberscope, Welch Allyn, Inc., replaced the coherent fiberoptic image bundle in a colonoscope with a light-sensitive computer chip or charge-coupled device on which the image was focused by a small lens (see Chapter 3).34 The digital signal was fed to a video processor, which generated an image to a television monitor. The image did not occupy the entire screen, leaving space for information to be typed in by a keyboard. The resolution of the image was at least equal to that of the fiberscope.
Advantages of the electronic instruments include an image that can be seen not only by the operator, but also by anyone with access to a connected monitor in the same or another room. This feature greatly enhances the ability to teach others about the procedure and to inform other interested physicians about the findings in the individual patient. If desired, recording of procedures can be accomplished with videotape machines, and good-quality pictures of individual frames can be made immediately with externally integrated digital equipment. Individual endoscopists found that no adjustment of techniques was necessary when videoendoscopes were used, although they had to become accustomed to looking at the monitor screen rather than through an optical system with one eye (Fig. 1.12). This feature added to the useful length of the instrument because the whole scope could be held at the waist rather that brought to eye level.
Endoscopic Ultrasonography (EUS)
In Germany in 1976, working with Siemens Co, Lutz and Rosch35 reported the use of a 1-cm ultrasonographic 4-MHz probe that could be passed through the biopsy channel of an Olympus TGF. They used it in two patients to differentiate successfully between pancreatic pseudocysts and tumors.7 In 1980, Classen’s group in Germany36 and DiMagno and colleagues37 at the Mayo Clinic reported EUS devices that were incorporated onto the tip of conventional fiberscopes, one using a 5-MHz transducer and the other using a 10-MHz transducer. These probes had good resolution at an acoustic focus depth of 3 cm. Others incorporated the transducer in the distal shaft of fiberoptic scopes and explored primarily the gut wall.33,38 By 1985, ultrasonic transducers with variable frequencies incorporated into videoendoscopes were readily available, although expensive (>$100,000 for initial setup) (Fig. 1.13). It was immediately apparent that this procedure could accurately evaluate known or suspected intramural lesions of the gut,39,40 and it was rapidly expanded to include the esophagus; problems of diagnosis and recurrence of neoplasia, especially in the pancreas; portal hypertension; the colon and rectum; and bile ducts.41 In 1991, Wiersema and colleagues42,43 showed that EUS could be used to obtain fine needle aspiration cytology of mediastinal nodes and of nodes and lesions of the upper and lower GI tract. The addition of Doppler technology has now made possible the study of the flow through various channels, including the thoracic duct and blood vessel anastomosis. The techniques of using EUS instruments differ only slightly from using videoendoscopes, but dedicated training is necessary to interpret the sonographic images obtained accurately. EUS is not amenable to self-instruction. EUS training centers have been established in academic centers, but retraining of practicing physicians is a problem.44
Capsule Endoscopy (Wireless Endoscopy)
In 2000, Iddan and colleagues45 reported the development of a capsule containing a tiny CMOS camera that could be swallowed, take video (but slowed to 2 frames per second), and transmit the video over 7 hours to a receiving digital storage unit worn by the patient as he or she goes about their normal activities. These frames are downloaded to a computer from which they are projected onto a monitor at a rate that can be controlled by the observer. Pictures can be printed of areas of interest. Gastroenterologists in Israel conducted randomized trials comparing the wireless capsule efficacy with push enteroscopy and showed superior results with the capsule.46–48
Wireless capsule endoscopy caught the imagination of gastroenterologists over the world, and capsule endoscopy has been adopted as a part of standard practice for small bowel imaging. The findings are virtually unanimous in reporting better results in identifying lesions in the small bowel compared with results from push enteroscopy.49 The capsule avoids the discomfort and need for sedation inherent in push enteroscopy. In addition to lack of biopsy capability, a major disadvantage is the reported 4 hours of review time necessary, but this would likely be overcome by training of nonphysician personnel to screen the multiple images produced. Although the major use of the capsule to date has been elucidating the cause of occult bleeding from small bowel sources, where it seems to be superior to other methods, its future in other diseases, such as those in the colon, has already been investigated in large multicenter comparative studies. The future of wireless capsule endoscopy is bright. It will be interesting to see how the principle of wireless endoscopy is united to videoendoscopes with direct wireless connection between the camera and the computer processor.
Enteroscopy
The small intestine can be regarded as the final frontier of GI endoscopy. Although capsule endoscopy provides remarkable images of the small bowel mucosa, therapy with a capsule-based instrument is many years away. Surgically assisted small bowel enteroscopy may be performed via either the transoral or anal route or via a mid–small bowel enterotomy incision. The disadvantage of this technique is its invasive nature.50 Endoscopic examination of the small intestine has remained technically difficult. The many loops of the small intestine prevent progression of the instrument tip by simple pushing. This problem was overcome initially with the use of the Sonde enteroscope,51 which is a very fine, floppy instrument with a balloon at the tip. The Sonde enteroscope progressed through much of the small bowel under peristalsis, and then the proceduralist would slowly withdraw the instrument, assessing the mucosa while pulling back. This technique was thought to visualize 50% to 70% of the mucosal surface.52 However, the procedure was uncomfortable, was time-consuming, and did not permit therapeutics, all of which limited its use.
The concept of small bowel enteroscopy was revolutionized by Yamamoto with the introduction of the double-balloon enteroscope in 2001.53 This technique uses traction between a balloon at the tip of the enteroscope and another balloon on a flexible overtube to concertina the loops of small bowel and provide traction for forward movement. The procedure requires per oral and anal procedures to examine the entire small intestine, and even then only in a minority of Western patients is the whole small bowel visualized. Nonetheless, double balloon–assisted enteroscopy permits endoscopic therapeutics to most of the small bowel with the need for surgical assistance. A single balloon version is also available.
1 Modlin IM. A brief history of endoscopy. Milano: MultiMed; 2000.
2 Edmonson JM. History of the instruments for gastrointestinal endoscopy. Gastrointest Endosc. 1991;37:S27-S56.
3 Haubrich WS. Gastrointestinal endoscopy. In: Kirsner JB, editor. The growth of gastroenterologic knowledge during the twentieth century. Philadelphia: Lea & Febiger; 1994:474-490.
4 Schuman B. The development of the endoscope. In: DiMarino AJJr, Benjamin SB, editors. Gastrointestinal disease an endoscopic approach, Vol I. Malden, MA: Blackwell Science; 1997:9-24.
5 Schindler R. Gastroscopy. The endoscopic study of gastric pathology. Chicago: University of Chicago Press; 1950.
6 Schindler R. Synopsis of gastroenterology. Philadelphia: Grune & Stratton; 1957.
7 Kirsner JB. American gastroscopy—yesterday and today. Gastrointest Endosc. 1991;37:643-648.
8 Schindler R. An American built gastroscope. Am J Dig Dis. 1940;7:256-257.
9 Hufford AR. A new light weight, extra flexible gastroscope. Rev Gastroenterol. 1946;13:381.
10 Kenamore B. A biopsy forceps for the flexible gastroscope. Am J Dig Dis. 1940;7:539.
11 Benedict EB. Gastroscopic biopsy. Gastroenterology. 1959;37:447-448.
12 Hopkins HH, Kapany NS. A flexible fiberscope using static scanning. Nature. 1954;173:39-41.
13 Hirschowitz BI, Curtiss LE, Pollard HM. Demonstration of the new gastroscope, the “fiberscope.”. Gastroenterology. 1958;35:50-53.
14 Weisinger BB, Cramer AB, Zacharis LC. Comparative accuracy of the fiberscope and standard gastroscope in the diagnosis of gastric lesions: Preliminary report. Gastroenterology. 1963;44:858A.
15 Burnett W. An evaluation of the gastroduodenal fibrescope. Gut. 1962;3:361-365.
16 Cohen NN, Hughes RW, Manfredo HE. Experience with 1000 fibergastroscopic examinations of the stomach. Am J Dig Dis. 1966;11:943-950.
17 Paulson M, Gladsden ES. Esophagoscopy, gastroscopy, gastroenteroscopy, and proctosigmoidoscopy. In: Paulson M, editor. Gastroenterologic medicine. Philadelphia: Lea & Febiger; 1969:217-258.
18 LoPresti PA, Hilmi AM. Clinical experience with a new foroblique fiber optic esophagoscope. Am J Dig Dis. 1964;9:690-697.
19 McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of Vater: A preliminary report. Ann Surg. 1968;167:753-755.
20 Takagi K, Ikeda S, Nakagawa Y, et al. Retrograde pancreatography and cholangiography by fiber duodenoscope. Gastroenterology. 1970;59:445-452.
21 Vennes JA, Silvis SE. Endoscopic visualization of bile and pancreatic ducts. Gastrointest Endosc. 1972;18:149-152.
22 Kawai K, Akasaka Y, Murakami K, et al. Endoscopic sphincterotomy of the ampulla of Vater. Gastrointest Endosc. 1974;20:148-151.
23 Classen M, Demling L. Endoskopische sphinckterotomie der papilla Vateri und steinextraktion aus dem ductus choledochus. Dtsch Med Wochenschr. 1974;99:496-497.
24 Geenen JE. Endoscopic papillotomy. In: Demling L, Classen M, editors. Endoscopic sphincterotomy of the papilla of vater. Stuttgart: Georg Thieme, 1978.
25 Segal HL, Watson JS. Color photography through the flexible gastroscope. Gastroenterology. 1948;10:575-585.
26 Segal HL. The history of gastroscopic color photography. Bull Gastrosc Esophagosc. 1960;7:7.
27 Ashizawa S, Sakai Y. Gastrocamera: Its past and future. In: Berry HL, editor. Gastrointestinal panendoscopy. Springfield, IL: Charles C. Thomas; 1974:223-229.
28 Kelly HA. A new method of examination and treatment of diseases of the rectum and sigmoid flexure. Ann Surg. 1895;21:468-478.
29 Tuttle JP. A treatise on diseases of the anus, rectum, and pelvic colon. New York: S. Appleton & Co; 1903.
30 Overholt B. Flexible fiberoptic sigmoidoscopes. CA Cancer J Clin. 1969;19:80-84.
31 Turell R. Fiber optic sigmoidoscopes: Up to date developments. Am J Surg. 1967;113:305-307.
32 Wolff WI, Shinya H. Colonofiberoscopy. JAMA. 1971;217:1509-1512.
33 Wolff WI, Shinya H. Polypectomy via the fiberoptic colonoscope: Removal of neoplasms beyond the reach of the sigmoidoscope. N Engl J Med. 1973;288:329-332.
34 Sivak MVJr, Fleischer DE. Colonoscopy with a VideoEndoscope: Preliminary experience. Gastrointest Endosc. 1984;30:1-5.
35 Lutz H, Rosch W. Transgastroscopic ultrasonography. Endoscopy. 1976;8:203-205.
36 Strohm WD, Phillip J, Hagenmuller F, et al. Ultrasonic tomography by means of an ultrasonic fiberendoscope. Endoscopy. 1980;12:241-244.
37 DiMagno EP, Buxton JL, Regan PT, et al. Ultrasonic endoscope. Lancet. 1980;1:629-631.
38 Gordon SJ, Rifkin B, Goldberg RB. Endoscopic evaluation of mural abnormalities of the upper gastrointestinal tract. Gastrointest Endosc. 1986;32:193-198.
39 Kawai K, Tanaka Y, Yasuda K. Clinical evaluation of endoscopic ultrasonography (EUS). Gastrointest Endosc. 1983;29:183A.
40 Sivak MV, George C. Endoscopic ultrasonography: Preliminary experience. Gastrointest Endosc. 1983;29:187A.
41 Symposium. Endoscopic ultrasonography. Gastrointest Endosc. 1990;36:S1-S46.
42 Wiersema MJ, Hawes RH, Wiersema LM, et al. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc. 1992;38:35-39.
43 Rex RK, Tarver RD, Wiersema M, et al. Endoscopic transesophageal fine needle aspiration of mediastinal masses. Gastrointest Endosc. 1991;37:465-468.
44 Hoffman BJ, Hawes RH. Endoscopic ultrasound and clinical competence. Gastrointest Endosc Clin N Am. 1995;5:879-884.
45 Iddan G, Meron G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature. 2000;405:417.
46 Appleyard M, Fireman Z, Glukhovsky A, et al. A randomized trial comparing wireless-capsule endoscopy with push enteroscopy for detection of small bowel lesions. Gastroenterology. 2000;119:1431-1438.
47 Appleyard M, Glukhovsky A, Swain P, et al. Wireless-capsule diagnostic endoscopy for recurrent small-bowel bleeding. N Engl J Med. 2001;344:232-233.
48 Scapa E, Jacob H, Lewkowicz S, et al. Initial experience of wireless-capsule endoscopy for evaluating occult gastrointestinal bleeding and suspected small bowel pathology. Am J Gastroenterol. 2002;97:2776-2779.
49 Ell C, Remke S, May A, et al. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy. 2002;34:685-689.
50 Greenberg G, Phillips M, Tovee E, et al. Fibreoptic endoscopy during laparotomy in the diagnosis of small intestinal bleeding. Gastroenterology. 1976;71:133-135.
51 Tada M, Kawai K. Small bowel endoscopy. Scand J Gastroenterol. 1984;19(Suppl 102):39-52.
52 Lewis BS, Waye JD. Total small bowel enteroscopy. Gastrointest Endosc. 1987;33:435-438.
53 Yamamoto H, Sekine Y, Saito Y. Total enteroscopy with a non-surgical, steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220.