THE HEART AS A PUMP
VALVE FUNCTION AND VALVE DISEASE
Functional anatomy of the heart
The heart lies obliquely in the thorax within the mediastinum—the space between the lungs which also contains the major blood vessels, oesophagus and trachea. It typically weighs about 325 ± 75 g in men and 275 ± 75 g in women. The heart can be described as having three surfaces and an apex. About two thirds of the heart is to the left of the mid-line. The anterior surface of the heart is formed mainly by the right ventricle and is in contact with the ribs and sternum (Fig. 3.1). The inferior surface of the heart is formed mainly by the left ventricle and is in contact with the diaphragm. The posterior surface of the heart is formed mainly by the left atrium. This surface is also known as the base of the heart. The apex which is anterior to the rest of the heart consists only of the left ventricle and forms an important clinical landmark when assessing the size of the heart. The aorta and the pulmonary trunk arise from the left and right ventricles respectively at the superior pole of the heart (Fig. 3.2). The superior and inferior vena cavae open into the upper and lower parts of the right atrium. There are four pulmonary veins which open into the back of the left atrium. The junction between the atria and the ventricles is marked by the atrioventricular groove and the junction between the ventricles both posteriorly and anteriorly is marked by the interventricular grooves.
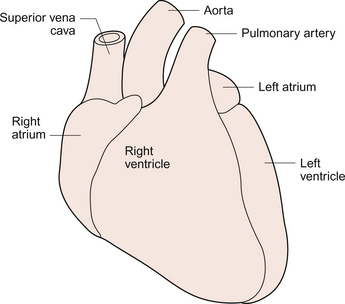
Fig. 3.1 External anatomy of the heart. Frontal (anterior) view of the external anatomy of the heart.
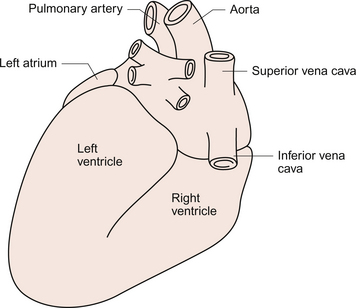
Fig. 3.2 External anatomy of the heart. Posterior-inferior view of the external anatomy of the heart.
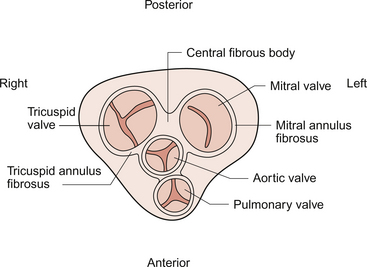
The fibrous skeleton of the heart is a framework of dense collagen around the atrioventricular junction and the arterial outflow. It provides structural support for the heart, particularly by stabilizing the heart valves, and it also prevents the heart from being overstretched (Fig. 3.3). The electrical insulation provided by this fibrous skeleton forms the barrier between the atria and the ventricles and ensures that electrical conduction normally only occurs through the bundle of His (see Chapters 2 and 7).
The heart is surrounded by the pericardium. The fibrous pericardium is a tough sac enclosing the heart and providing attachments to adjacent structures. The serous pericardium has a visceral layer which is attached to the surface of the heart and a parietal layer lining the fibrous pericardium. The smooth layers of the serous pericardium slide easily over each other and allow the heart to move within the thorax as it beats. The very narrow space between the layers of serous pericardium is filled with pericardial fluid.
The right atrium has only a thin (about 2 mm) wall. There is a rough portion belonging to the auricular appendage and a smooth portion which receives the superior and inferior vena cava and the coronary sinus, the major part of the venous drainage for the heart itself. Blood flows from the right atrium into the right ventricle through an atrio-ventricular valve which has three cusps—the tricuspid valve (Fig. 3.3). The right ventricle is more muscular (about 4–5 mm thick) than the right atrium. The tricuspid valve is attached to the papillary muscles of the ventricular wall by the chordae tendinae (heart strings) which are made of fibrous tissue and help to stabilize the valve and prevent blood from flowing back into the right atrium when the ventricle contracts.
The left atrium is thin walled (about 3 mm) and, like the right atrium, has a smooth and a roughened portion. The smooth-walled part is formed by the entrance of the four pulmonary veins. The atrioventricular opening in the left side of the heart is guarded by a valve shaped like a bishop’s mitre and formed from two cusps—the mitral valve. (In Shaw’s play, St Joan declares at her trial that ‘Bishops are never in the right’—this may help you to remember that the mitral valve is in the left side of the heart!). The left ventricle has the greatest wall thickness (8–15 mm) of all the cardiac chambers with internal muscular ridges throughout except for a small area at the aortic valve opening. At the tip of the apex the wall of the left ventricle is only about 2 mm thick. The mitral valve is anchored to the left ventricle by papillary muscles and chordae tendinae. The aortic valve has three semilunar cusps. There is a dilatation, the sinus of Valsalva, in the aortic wall just above each cusp. These dilatations allow blood to flow behind the cusps at the very beginning of ventricular relaxation in diastole and thus ensure that the aortic valve shuts and that the cusps are not flattened against the side of the aorta. They also allow blood to freely enter the right and left coronary arteries (see Chapter 5).
The arterial blood supply of the heart is via the right and left coronary arteries which originate from the aorta near the aortic valve (see Chapter 5). The left coronary artery branches into the left circumflex and left anterior descending arteries. The right coronary artery branches into the marginal and right posterior descending arteries. The venous drainage of the heart is via a number of veins which drain into the coronary sinus on the inferior surface of the heart in the atrioventricular sulcus. The coronary sinus drains into the right atrium.
The cardiac cycle
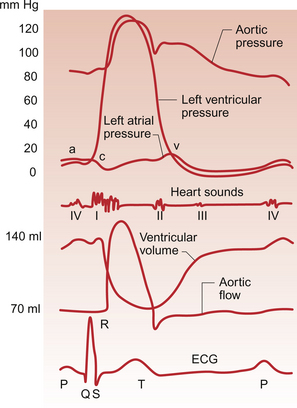
The events which comprise the cardiac cycle on the left side of the heart are shown in Figure 3.4. The equivalent values for the right side of the heart are qualitatively similar but the pressures are lower since the pulmonary circulation is a low-resistance circu lation. The onset of ventricular systole is indicated by the first heart sound which is produced by closure of the atrioventricular valves. The end of ventricular systole is indicated by the second heart sound which is produced by closure of the aortic and pulmonary valves.
On the ECG the P wave corresponds to atrial depolarization (see Chapter 7) and thus marks the onset of atrial systole. The QRS complex is associated with ventricular depolarization and thus the onset of ventricular systole. The functioning of the heart as an effective pump depends upon the valves between the atria and the ventricles (the tricuspid and mitral valves) and those between the ventricles and the main distributing arteries (the aortic and pulmonary valves) functioning correctly.
In Figure 3.4 the left ventricular pressure wave begins with a small surge of pressure when the left atrium contracts and ‘top ups’ the ventricle. In some individuals with a hypertrophied left atrium pumping blood into a stiffened left ventricle, as may occur in ischaemic heart disease or hypertension, a sound is generated which is referred to as the fourth heart sound. The increase in ventricular volume associated with atrial systole is very small as most ventricular filling is passive and occurs in the early stage of diastole when blood flows through the relaxed atrium into the relaxed ventricle. With the onset of ventricular contraction ventricular pressure rises and the mitral valve closes. This, along with the normally concurrent closure of the tricuspid valve, causes the first heart sound. If venous return is increased acutely then the closure of the tricuspid valve in the right side of the heart may be slightly delayed and the first heart sound will be ‘split’, that is closure of the two valves will be heard separately. If either valve fails to close fully then the murmur of mitral or tricuspid regurgitation, an early systolic murmur, will be heard.
The ventricle empties the stroke volume of blood into the aorta (see Chapter 4) and ventricular volume typically falls by about 70 mL (Fig. 3.4). Ventricular and aortic pressures follow a similar path as the contraction of the ventricles opposes the elastic recoil of the aorta. Blood flow in the aorta rises substantially at this time. As ventricular relaxation proceeds, ventricular pressure falls and when it is below the pressure in the aorta the aortic valve closes. This is helped by a backflow of blood created by the sinus of Valsalva as described above. Closure of the valve creates a short-lived dip in aortic pressure called the dicrotic notch. The closure of the aortic and pulmonary valves generates the second heart sound. If either the aortic or pulmonary valve fails to close fully then the murmur of aortic or pulmonary regurgitation may be heard. This is an early diastolic murmur. Ventricular pressure continues to fall and eventually the pressure in the ventricle is less than that in the atrium. At this point the mitral valve opens and blood flows into the ventricle (Fig. 3.4). If the mitral or tricuspid valve fails to open properly then the early diastolic murmur of mitral/tricuspid stenosis may be heard. Opening of these valves between the atria and ventricles heralds the period of rapid ventricular filling and may occasionally, especially in individuals with low heart rates and high stroke volumes, produce a third heart sound which is said to be analogous to a ‘sail flapping in the wind’. After closure of the aortic valve pressure in the aorta and thus blood flow is maintained by the elastic recoil of the artery wall. The oscillations in aortic pressure are therefore not as large as those seen in the ventricle and aortic flow, although it is pulsatile, is much more uniform than it would otherwise be. This is important to ensure continued perfusion of all the tissues of the body during diastole (see Chapter 10).
The pressure wave in the left atrium is shown in Figure 3.4. The right atrial pulse is qualitatively similar and, since there are no valves at the entrances to the atria, a similar pulse may be seen in the jugular vein if the patient’s head and thorax are tilted back at about 45 degrees. The ‘a’ wave of atrial pressure (7 mm Hg in the left atrium and 5 mm Hg in the right atrium) corresponds to atrial systole, the ‘c’ wave corresponds to the closure of the atrioventricular valves which bulge back into the atria as they close. The ‘v’ wave of the jugular or atrial pulse results from atrial filling and immediately precedes the opening of the tricuspid and mitral valves.
The case history of a young girl with a suspected cardiac valve problem is introduced in Case 3.1:1.
Valve pathology
General principles of the pathology of heart valves
Valvular heart disease can be congenital (i.e. the patient is born with the problem) or acquired (the pathology occurs as a result of illness after birth). The spectrum of valvular heart disease, particularly in the developed world, is changing as some diseases such as rheumatic heart disease become much rarer. From a pathologist’s viewpoint, disease of a valve can lead to an incompetent valve that allows blood back into the chamber from which it was expelled or a stenotic valve that impedes blood flow from one chamber to the next. A stenotic valve can of course also be incompetent.
Congenital heart disease
The common congenital valve diseases are described below.
Bicuspid aortic valve
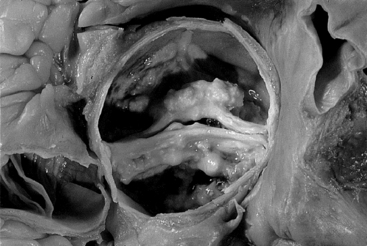
About 1% of the adult population has a congenital bicuspid aortic valve compared to the tricuspid valve found in the rest of the population. The two cusps may be of the same or different sizes and there may be a median raphe (a fibrous bar) that extends from one cusp to the wall of the aorta. The bicuspid valve is much more prone to calcification (Fig. 3.5) than a normal valve and young patients may present with the symptoms and signs of a heavily calcified valve (aortic stenosis/incompetence) that is then found to be bicuspid.
Mitral valve incompetence
In the condition ‘floppy mitral valve’ the valve cusps are larger than normal, dome- or parachute-shaped and look rather myxoid/mucoidy. The chordae tendinae often also have this appearance and are elongated. Congenital pulmonary valve stenosis (or sub- or supravalvar stenosis) also occurs.
Infective endocarditis
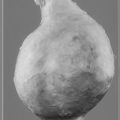
Acute IE most often occurs in the setting of previously normal valves. This form of the disease is usually very destructive and has devastating, often fatal, results. The patient may be feverish and quickly become extremely ill. The classical organism involved is Staphylococcus aureus. Intravenous drug users are particularly prone to this disease as they may inject dirty drug solutions. The water used for dissolving the drug may for example be obtained from a toilet bowl. The virulent organisms settle on the valves in the right side of the heart. Large, friable, invasive, thrombus-like masses (Fig. 3.6) occur and embolization is frequent.
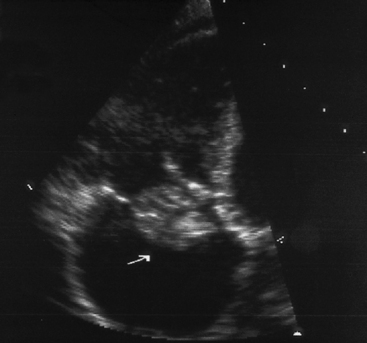
Fig. 3.6 Acute infective endocarditis vegetation on heart. Echocardiograph showing a large vegetation (arrowed) on the tricuspid valve of the heart of an intravenous drug user. Source: Haslett et al. 2003.
The common consequences of having infected, friable, vegetations on the heart valve include:
• Embolus formation—the embolus may travel down a coronary artery or enter the systemic circulation typically to the spleen, kidney or brain. At the site of impaction, infection may be set up in the wall of the vessel, leading to a weakened, dilated artery (mycotic aneurysm—see Chapter 8).
• Valve perforation or destruction and/or local spread of infection and inflammation into the myocardium or aorta. This may lead to, or exacerbate, cardiac failure.
• Immune complex tissue injury—immune complexes are generated as a response to continued infection and these may set up local reactions if they settle in the kidneys (glomerulonephritis), skin (vasculitis) or joints (arthralgia).
History taking for cardiac disease
The classical symptoms of cardiac disease are:
• chest pain particularly with exertion
• dyspnoea (awareness of breathing)
A careful note of risk factors for cardiac disease should be made:
The above factors will all be useful in identifying heart failure (see Chapter 6) which in the majority of cases will be preceded by some form of chronic illness. Exercise intolerance and peripheral oedema will point to heart failure as will orthopnoea. This is an inability to breath comfortably when lying flat which causes the individual to prop themselves up with several pillows in order to sleep. Orthopnoea is predominantly a symptom of left heart failure. Sitting up reduces the preload (venous return) on the right side of the heart and hence reduces the output into the pulmonary circuit. Pulmonary engorgement (increased lung stiffness) which generates the sensation of dyspnoea is therefore reduced.
The degree of exercise intolerance as evidenced by the onset of angina may be a useful guide to the level of coronary insufficiency. Chest pain on vigorous exertion such as walking up stairs implies a lower level of insufficiency than chest pain walking on the level. Recording these parameters will allow the progress of disease to be monitored and give a yardstick for benefit from intervention (see Chapters 5 and 13).
Palpitations, simply the awareness of one’s own heartbeat, is a common presentation but a rarer symptom of actual cardiac disease. Awareness of an abnormal heart rhythm may precede a collapse, which is usually strong evidence of an arrhythmia compromising cardiac output. However collapse may be the first ‘symptom’ of a serious arrhythmia. The description by witnesses to a collapse may be crucial, with the major differential diagnosis being neurological events such as seizures. If the individual is aware of the palpitations the key points to elicit in history taking are onset/offset and regularity. The heart cannot be partly in an abnormal rhythm so paroxysmal arrhythmias are typified by sudden onset and offset. Inappropriate sinus tachycardia, the most common cause of palpitations, generally starts and stops gradually. Regularity of palpitations is another important characteristic. Ectopic beats tend to give an occasional irregularity with most beats coming at a normal interval. Bigeminy and trigeminy (couplets and triplets) tend to be irregular in a consistent manner. The commonest arrhythmia in clinical practice is atrial fibrillation (see Chapter 7). The chaotic nature of the heart rate means that it is very distinctly ‘irregularly irregular’.
Ankle swelling and other signs of peripheral oedema represent evidence of increased right atrial pressure and a resultant increase in hydrostatic pressure in the capillary bed (see Chapter 11). It is particularly a result of right ventricular failure as opposed to the pulmonary oedema of left heart failure (see Chapter 6). Frequently both ventricles will fail together, especially as the commonest cause of right heart failure is left heart failure.
Clinical examination of the cardiovascular system
• examination of the hands for clubbing (Fig. 3.7) and splinter haemorrhages (Fig. 3.8)
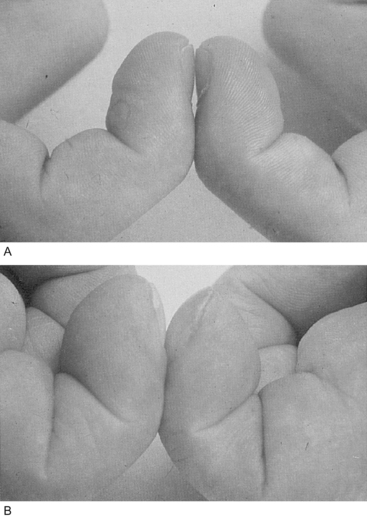
Fig. 3.7 Finger clubbing. Comparison of (A) normal nail beds and (B) finger clubbing in a patient with infective endocarditis. Source: Underwood 2004.
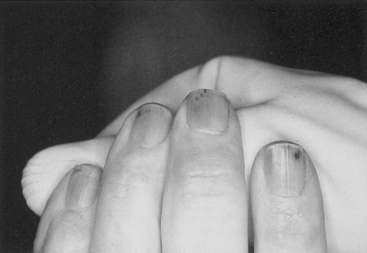
Fig. 3.8 Splinter haemorrhages. Splinter haemorrhages in the nail bed of a patient with infective endocarditis. Source: Underwood 2004.
• pulse—rate, rhythm, character
• mucous membranes in the mouth inspected for signs of anaemia
• cardiac impulse; apex beat and right or left ventricular heave
• auscultation of heart sounds listening for added sounds, murmurs and their radiation
• auscultation of the lungs especially for signs of pulmonary oedema
Investigations will lead on from history taking and examination. An ECG (see Chapter 7) is extremely useful and as it is cheap and non-invasive is nearly always appropriate; even a baseline ECG is worth having for future reference. Chest X-ray, blood count and electrolyte assessment are all commonly performed at least once. Only the timing of the test is of significant debate since out of hours tests are generally more expensive than those performed during the normal working day. Blood cultures should always be taken if sepsis is a possibility; traditionally at least three sets of samples from different sites in the case of endocarditis and antibiotic treatment should be withheld if at all possible until this has been done so that a causative organism can be identified.
Investigations of heart disease: imaging the heart
Chest X-ray
A static chest X-ray does not give detailed anatomical or functional information about the heart. However it gives some information about the great vessels; the size of cardiac chambers and gross information about the function of the heart. The shape of the cardiac silhouette is determined by the contributions of the various chambers (Fig. 3.9). Where one or more chamber is grossly enlarged the silhouette will be larger than normal. The cardiothoracic ratio (CTR) is defined as the ratio of the widest total lateral dimension of the heart to the widest lateral dimension of the chest. The maximum normal CTR is 0.65 in children falling to 0.5 in adults. However cardiac enlargement may also be reflected in vertical dilatation with the heart shadow taking on a more globular outline. The secondary impact of poor cardiac function can be seen in the lungs with the signs of pulmonary oedema.
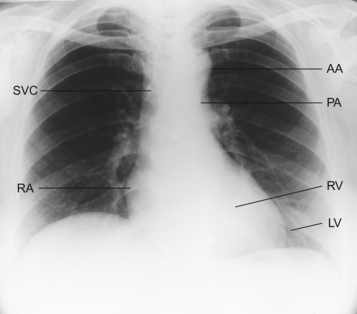
Fig. 3.9 Cardiothoracic ratio. Normal chest X-ray (posterior anterior projection). The heart is normal size with a cardiothoracic ratio (CTR) less than 50%. AA = aortic arch; PA = pulmonary artery; RV = right ventricle; LV = left ventricle; RA = right atrium; SVC = superior vena cava. Source: Swash 2001.
Cardiac catheterization and cine-angiography
The development of echocardiography has diminished the role of cardiac catheterization particularly in diagnostic terms. Although coronary angiography has the disadvantages of invasiveness, radiation exposure and reactions to the dye which may cause serious haemodynamic instability or renal impairment, it remains the gold standard assessment for coronary arteries in ischaemic heart disease.
Sudden cardiac death
Medical management of these conditions involves screening families for asymptomatic but affected individuals using ECG, echocardiography and exercise testing. Significant reductions in risk may be achieved using β-blockade pointing to the adrenergically mediated initiation of the arrhythmia in these conditions. Standard advice is to refrain from vigorous exercise. For refractory or high-risk individuals, implantable cardioversion defibrillators can be used. These devices are developments of the pacemaker technology designed to detect severe arrhythmias and deliver a cardioverting DC electric shock to the myocardium. They have recently entered the National Institute for Health and Clinical Excellence Guidelines in the UK. Unfortunately though effectively lifesaving the pain and discomfort of automatic defibrillation can be extremely debilitating and may lead to morbidity of its own, particularly if the patient does not lose consciousness prior to the delivery of the life-saving shock.
Grubb, N. R., Newby, D. E. Cardiology, second ed. Edinburgh: Elsevier; 2006.
Haslett, C., et al. Davidson’s Principles and Practice of Medicine, nineteenth ed. Edinburgh: Churchill Livingstone; 2003.
Levick, J. R. An Introduction to Cardiovascular Physiology, fifth ed. London: Arnold; 2009.
Lilly, L. S. Pathophysiology of Heart Disease, third ed. Baltimore: Williams and Wilkins; 2006.
Stevens, A., Lowe, J. Pathology, second ed. Edinburgh: Mosby; 2000.
Swash, M. Hutchison’s Clinical Methods, twenty-first ed. Edinburgh: WB Saunders; 2001.
Underwood, J. C. E. General and Systematic Pathology, fourth ed. Edinburgh: Churchill Livingstone; 2004.