Chapter 11
The Heart
For it is the heart by whose virtue and pulse the blood is moved, perfected, made apt to nourish and is preserved from corruption and coagulation. … It is indeed the fountain of life, the source of all action.
William Harvey (1578–1657)
General Considerations
The heart does not rest for more than a fraction of a second at a time. During a lifetime, it contracts more than 4 billion times. To support this active state, the coronary arteries supply more than 10 million liters of blood to the myocardium and more than 200 million liters to the systemic circulation. Cardiac output can vary under physiologic conditions from 3 to 30 L/minute, and regional blood flow can vary by 200%. This wide range occurs without any loss of efficiency in the normal state.
Diseases of the heart are common. The major disease categories are coronary heart disease (CHD), hypertension, rheumatic heart disease (RHD), bacterial endocarditis, and congenital heart disease. The clinical consequences of these conditions are usually serious.
During the past 40 years, major advances have been made in the prevention, diagnosis, and treatment of cardiovascular, lung, and blood diseases. Death rates from cardiovascular diseases (CVDs) have fortunately declined significantly, and Americans are living longer, healthier lives. Despite the tremendous progress that has been made, however, morbidity and mortality from cardiovascular, lung, and blood diseases continue to impose a major burden on patients, their families, and the national health care system. The economic cost to the nation is substantial.
Nearly 65 million Americans have one or more forms of CVD. Although the death rates have declined, CVD is still the leading cause of death, by far, in the nation. Here are the facts about the medical and financial burden of heart disease in the United States:
• Every 33 seconds someone in the United States dies from CVD.
• CHD is the most common type of heart disease, killing more than 385,000 people annually.
• By 2020, heart disease will be the leading cause of death throughout the world.
• In 2013, more than 920,000 Americans will have a heart attack; nearly half of those heart attacks will occur without prior symptoms or warning signs.
• Annually, 250,000 Americans die of sudden cardiac death: 680 every day of the year.
• One half of the victims of sudden cardiac death are younger than the age of 65.
• An estimated 80 million Americans have one or more types of heart disease.
• Approximately 8.9 million Americans have chest pain (angina).
• Currently, approximately 7.9 million Americans are alive who have had a heart attack.
The key risk factors include high blood pressure, high levels of low-density lipoprotein (LDL) cholesterol, and smoking. Approximately half of Americans (49%) have at least one of these three risk factors. Several other medical conditions and lifestyle choices can also put people at a higher risk for heart disease, including:
Heart disease accounts for approximately 38.5% of all deaths, or 1 of every 2.6 deaths. CVD kills more Americans than the next seven causes combined, including cancer. CHD, stroke, high blood pressure, and congestive heart failure have been the leading causes of death in the United States every year since 1900, with the exception of 1918, when there was a worldwide flu pandemic.
The National Institutes of Health—National Heart, Lung, and Blood Institute estimated that in 2012, there were 82,600,000 Americans with one or more forms of CVD. This included 76.4 million Americans with high blood pressure, 16.3 million with CHD, and 7 million who had suffered a stroke.
CHD is the leading cause of death in the United States, and the disease process appears to start early in life. Autopsy studies during the Korean War showed that 40% of all American soldiers who were killed in their early 20s had atheromatous involvement of one or more of their coronary arteries.
CHD is also the leading cause of mortality in women in the United States. Here are the facts:
• Of women who have heart attacks, 42% die within 1 year, compared with 24% of men.
• Women’s heart attacks experienced at younger than age 50 are twice as likely as men’s to be fatal.
Unlike other forms of cardiac disease, CHD may be severe and life threatening despite normal results on physical examination, electrocardiography, and chest radiography. The good news, however, is that from 1994 to 2004, the death rate from CHD declined 33%, perhaps because of better control of hypertension, better cholesterol management, and cigarette smoking cessation.
Systemic arterial hypertension affects approximately 20% of the American population. It is a major risk factor for coronary artery disease, as well as a prime cause of congestive heart failure and strokes. It has been well established that among patients with higher systolic or diastolic pressures, there is a greater incidence of morbidity and mortality.
Bacterial endocarditis remains a significant medical problem despite the wide use of antibiotics. The increasing number of cases is related to use of intravenous street drugs. The existence of endocarditis is often not suspected in a patient until serious sequelae develop. In addition to causing valvular damage, the persistent bacteremia can spread to the brain, myocardium, spleen, kidneys, and other sites in the body.
The incidence of congenital heart disease averages 5 per 1000 live births. If other commonly found congenital cardiovascular conditions, such as bicuspid aortic valve and mitral valve prolapse, are included, the incidence approaches 1 per 100 live births.
It is clear that the magnitude of cardiac disease is enormous, and the cost of the morbidity and mortality is directly proportional.
Structure and Physiology
The principal function of the cardiovascular system is to deliver nutrients to and remove metabolites from every cell in the body. This metabolic exchange system is produced by a high-pressure delivery system, an area of exchange, and a low-pressure return system. The high-pressure delivery system is the left side of the heart and arteries, and the low-pressure return system includes the veins and the right side of the heart. The circulation of blood through the heart is illustrated in Figure 11-1.
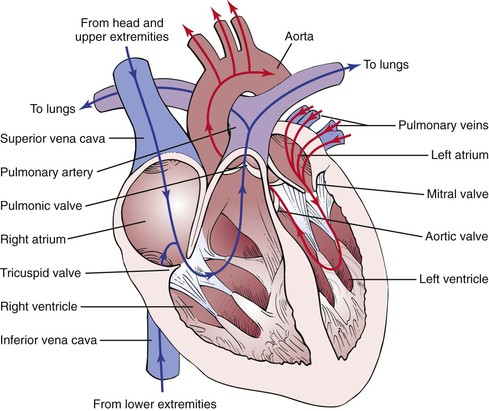
Figure 11–1 Circulation of blood through the heart.
The synchronous contraction of the heart results from the conduction of impulses generated by the sinoatrial (SA) node and propagated through the conduction system. The SA node is located at the juncture of the superior vena cava and the right atrium. The SA impulse spreads from its point of origin concentrically. When the impulse reaches the atrioventricular (AV) node, in the interatrial septum near the entrance of the coronary sinus, the impulse is slowed. It is then transmitted to the specialized conducting tissue known as the right and left bundle branches, which conduct the impulse to the specialized conducting pathways in the ventricles, Purkinje’s fibers. The impulse spreads from the endocardial to the epicardial surface of the heart. These conducting pathways are illustrated in Figure 11-2.
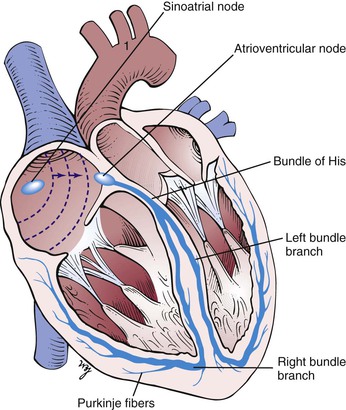
Figure 11–2 Conducting pathways of the heart.
The heart is innervated extensively by branches of the autonomic nervous system. Both sympathetic and parasympathetic fibers are present in the SA and AV nodes. The atrial muscle is also innervated by both types of fibers. The ventricular musculature is innervated predominantly by the sympathetic nervous system.
The parasympathetic fibers travel along the vagus, or tenth cranial, nerve. The sympathetic fibers descend in the spinal cord to the level of T1 to T5, where they emerge through the ventral roots to form a synapse in the thoracic and cervical sympathetic ganglia. The postganglionic fibers travel through the cervical cardiac nerves to join the parasympathetic fibers in forming the cardiac plexus, which is located near the aortic arch and the tracheal bifurcation. These neural pathways are illustrated in Figure 11-3.
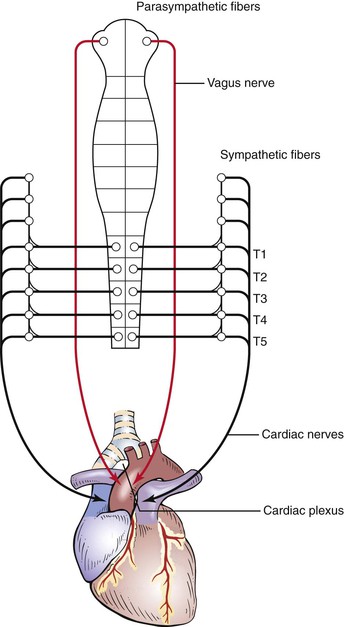
Figure 11–3 Autonomic neural pathways of the heart.
Sympathetic stimulation by norepinephrine produces marked increases in heart rate and contractility. Parasympathetic stimulation mediated by acetylcholine slows the heart rate and decreases contractility.
In addition, several receptor sites provide circulatory information to the medullary cardiovascular center in the brain. This center has cardioexcitatory and cardioinhibitory areas that regulate the neural output to the sympathetic and parasympathetic fibers. Stretch receptors in the aortic arch and in the carotid sinus monitor blood pressure. These baroreceptors respond to a decrease in blood pressure by decreasing their impulses to the medullary center. The center senses this decreased activity and increases its sympathetic efferent activity and decreases its parasympathetic efferent activity. The net result is to increase the heart rate and contractility. An increase in blood pressure causes an increase in afferent activity to the center, and the opposite changes occur.
To describe physical signs, the examiner must be able to identify the important surface topographic landmarks. Chapter 10, The Chest, describes the major areas. These areas should be reviewed at this time.
The surface projection of the heart and great vessels is illustrated in Figure 11-4. Most of the anterior cardiac surface is the right ventricle. The right atrium forms a narrow border from the third to the fifth ribs to the right of the sternum. The left ventricle lies to the left and behind the right ventricle. The left ventricular apex is normally in the fifth intercostal space at the midclavicular line. This location is commonly written as 5ICS-MCL. The apical impulse is called the point of maximum impulse (PMI). The other chambers and vessels of the heart are usually not identifiable on examination.
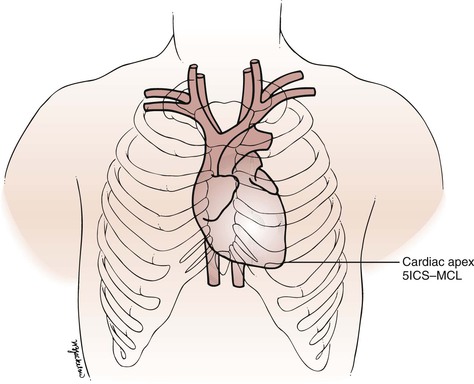
Figure 11–4 Surface topography of the heart. 5ICS-MCL, fifth intercostal space at the midclavicular line.
The four classic auscultatory areas correspond to points over the precordium, at which events originating at each valve are best heard. The areas are not necessarily related to the anatomic position of the valve, nor are all sounds heard in the area directly produced by the valve for which the area is named. The normal areas are as follows:
Aortic: Second intercostal space, right sternal border (2ICS-RSB)
Pulmonic: Second intercostal space, left sternal border (2ICS-LSB)
In addition to these four areas, the third left intercostal space, known as Erb’s point, is frequently the area at which pulmonic or aortic sounds are best heard. The five areas are illustrated in Figure 11-5. The second intercostal space to the right and left of the sternum is called the base.
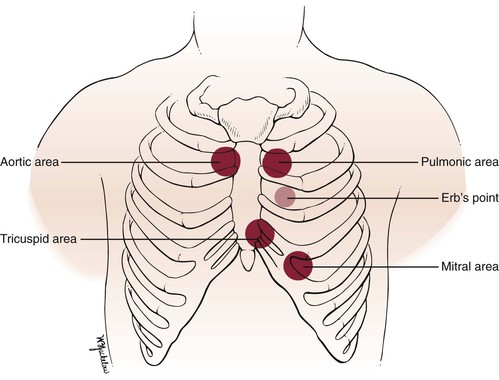
Figure 11–5 Auscultatory areas.
Remember that the left atrium is the most posterior portion of the heart. When the left atrium enlarges, it extends posteriorly and to the right.
The Cardiac Cycle
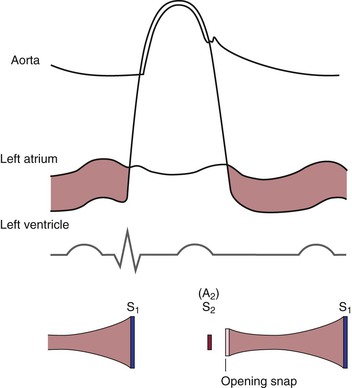
To understand the cardiac cycle, the motion of the valves and the pressures within the chambers should be reviewed. The interrelationships of valve motion are critically important and must be understood. Only with the knowledge of these cycles can the clinician fully comprehend the cardiac physical examination and heart sounds. The pressure tracings and valve motions are shown in Figure 11-6.
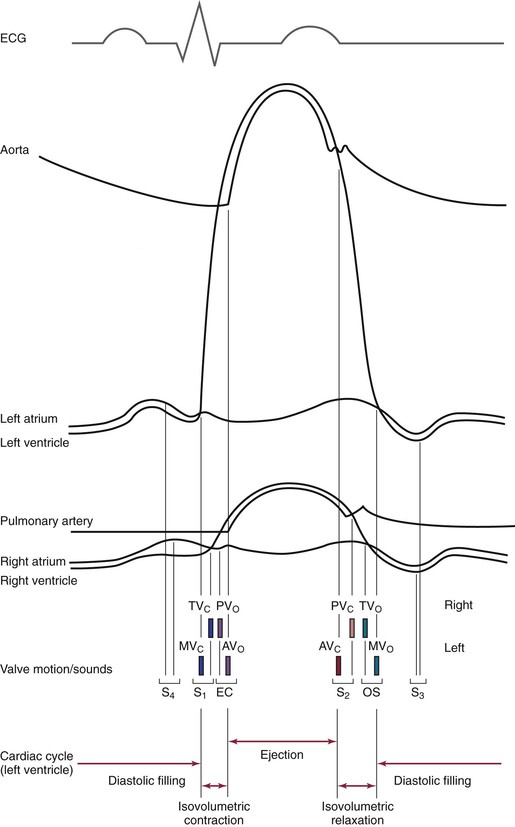
Figure 11–6 The cardiac cycle. AVo, Aortic valve opening; EC, ejection click; MVc, mitral valve closing; OS, opening snap; PVo, pulmonic valve opening; S1 to S4, first to fourth heart sounds; TVc, tricuspid valve closing.
Normally, only the closing of the heart valves can be heard. The closure of the AV valves, the tricuspid and the mitral, produces the first heart sound (S1). The closure of the semilunar valves, the aortic and the pulmonic, produces the second heart sound (S2).
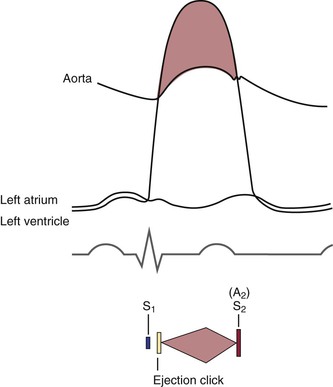
The opening of the valves can be heard only if they are damaged. When an AV valve is narrowed, or stenotic, the opening of the valve may be heard and is termed an opening snap. If a semilunar valve is stenotic, the opening may be heard and is termed an ejection click. It should be noted that in Figure 11-6, the term opening snap refers to the opening of a pathologically damaged AV valve that occurs during diastole, and the term ejection click refers to the opening of a damaged semilunar valve that occurs during systole.
The sequence of the opening and closing of the four valves is as follows:
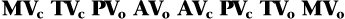
in which MV = mitral valve, TV = tricuspid valve, PV = pulmonic valve, AV = aortic valve, c = closing, and o = opening.
The mitral component of S1 occurs as a result of the closure of the mitral valve when the left ventricular pressure rises to more than the left atrial pressure; it is written M1. The tricuspid component of S1 occurs as a result of closure of the tricuspid valve when right ventricular pressure rises to more than right atrial pressure; it is written T1.
The time between the closure of the AV valves and the opening of the semilunar valves is the period of isovolumetric contraction. When the pressure in the right ventricle exceeds the diastolic pressure in the pulmonary artery, the pulmonic valve opens. A pulmonic ejection click is heard at this time if the pulmonic valve is stenotic. When the pressure in the left ventricle exceeds the diastolic pressure in the aorta, the aortic valve opens. An aortic ejection click is heard at this time if the aortic valve is stenotic.
The time between the opening and the closing of the semilunar valves is the systolic period of ejection. The point at which ejection is completed and the aortic and left ventricular curves separate is called the incisura, or dicrotic notch, and is simultaneous with the aortic component of S2, or closure of the aortic valve; this is written A2. The pulmonic valve closes at the point when the right ventricular pressure falls to less than the pulmonary diastolic pressure. This is the pulmonic component of S2 and is commonly written P2.
The time between the closure of the semilunar valves and the opening of the AV valves is called isovolumetric relaxation. The tricuspid valve opens when the pressure in the right atrium exceeds right ventricular pressure. A tricuspid opening snap may be heard if the tricuspid valve is stenotic. The mitral valve opens when the pressure in the left atrium exceeds left ventricular pressure. A mitral opening snap may occur at this time if the mitral valve is stenotic.
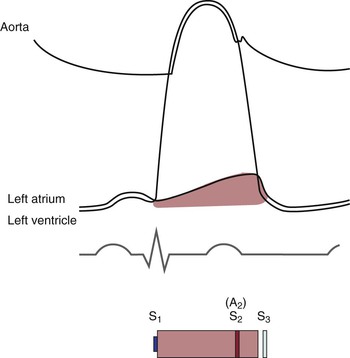
With the opening of the AV valves, the period of rapid filling of the ventricles occurs. Approximately 80% of ventricular filling occurs at this point. At the end of the rapid filling period, a third heart sound (S3) may be heard. An S3 occurs 120 to 170 msec after S2. This period is approximately the same time as it takes to say “me too.” The “me” is the S2, and the “too” is the S3. An S3 is normal in children and young adults. When present in individuals older than 30 years, it signifies a volume overload to the ventricle. Regurgitant valvular lesions and congestive heart failure may be responsible.
At the end of diastole, atrial contraction and the additional 20% of ventricular filling occur. A fourth heart sound (S4) may be heard. The interval from the S4 to the S1 is approximately the time it takes to say “middle.” The “mid-” is the S4, and the “-dle” is the S1. Note that the “mid” is much softer than the “dle,” which is quite similar to the S4-S1 cadence. An S4 is normal in children and young adults. When present in individuals older than 30 years, it is indicative of a noncompliant, or “stiff,” ventricle. Pressure overload on a ventricle causes concentric hypertrophy, which produces a noncompliant ventricle. In addition, CHD is a major cause of a stiff ventricle.
Two useful mnemonics for remembering the cadence and pathophysiologic characteristics of the third and fourth heart sounds are as follows:
The presence of an S3 or an S4 creates a cadence resembling the gallop of a horse. These sounds are therefore called gallop sounds or rhythms.
The first heart sound is loudest at the cardiac apex. Splitting of the first heart sound may be heard in the tricuspid area. The second heart sound is loudest at the base.
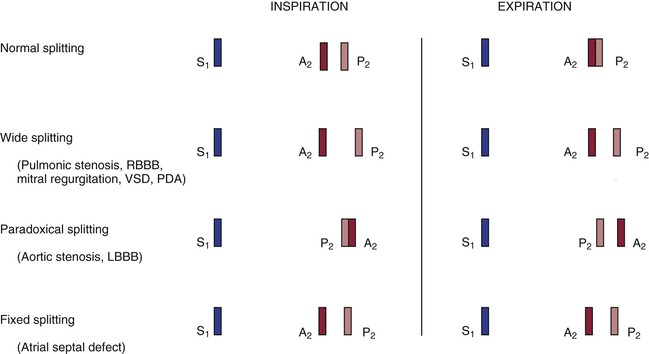
The terms A2 and P2 indicate the aortic component and the pulmonic component of S2, respectively. A2 normally precedes P2, meaning that the aortic valve closes before the pulmonic valve. With inspiration, the intrathoracic pressure lowers. This causes more blood to be drawn from the superior and inferior venae cavae into the right chambers of the heart. The right ventricle enlarges, and it takes longer for all the blood to be ejected into the pulmonary artery; thus the pulmonic valve stays open longer. P2 occurs later in inspiration, and the split between A2 and P2 is widened during inspiration in comparison with expiration. This is the cause of physiologic splitting of S2, which is diagrammed in Figure 11-7.
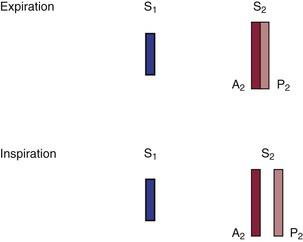
Figure 11–7 Physiologic splitting of the second heart sound.
The blood in the right ventricle is then pumped into the large-capacitance bed of the lungs. Therefore the return of blood from the lungs to the left side of the heart is decreased, and the left atrium and left ventricle become smaller. Atrial receptors trigger a reflex tachycardia that compensates for the decreased left ventricular volume. This increase in heart rate with inspiration is termed sinus arrhythmia. It is a misnomer because it is not really an arrhythmia but a normal physiologic response to a decreased left ventricular volume during inspiration.
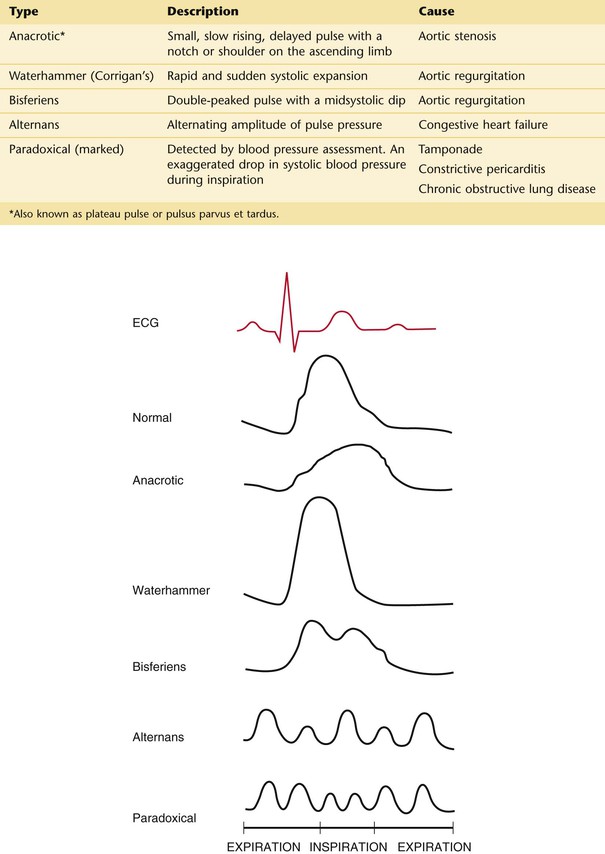
The Arterial Pulse
The arterial pulse is produced by the ejection of blood into the aorta. The normal configuration of the pulse consists of a smooth and rapid upstroke that begins approximately 80 msec after the first component of S1. There is sometimes a slight notch in the arterial pulsation toward the end of the rapid ejection period. This is called the anacrotic notch. The peak of the pulse is smooth and dome-shaped and occurs approximately 100 msec after the onset of the pulse. The descent from the peak is less steep. There is a gradual descent to the dicrotic notch, which represents the closure of the aortic valve. The contour and volume of the arterial pulse are determined by several factors, including the left ventricular stroke volume, the ejection velocity, the relative compliance and capacity of the arteries, and the pressure waves that result from the antegrade flow of blood. Figure 11-8 illustrates a characteristic arterial pulse.
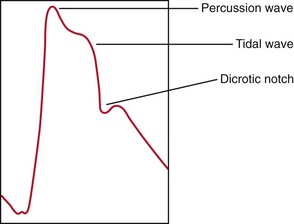
Figure 11–8 The arterial pulse.
Commonly, two waves may be present in the arterial pulse, which precedes the dicrotic notch. The percussion wave is the earlier wave and is associated with the rate of flow in the artery. The percussion wave occurs during peak velocity of flow. The tidal wave is the second wave, is related to pressure in the vessel, and occurs during peak systolic pressure. The tidal wave is usually smaller than the percussion wave, but it may be increased in hypertensive or older patients.
Blood Pressure
Arterial blood pressure is the lateral pressure exerted by a column of blood against the arterial wall. It is the result of cardiac output and peripheral vascular resistance. Blood pressure depends on the volume of blood ejected, its velocity, the distensibility of the arterial wall, the viscosity of the blood, and the pressure within the vessel after the last ejection.
Systolic blood pressure is the peak pressure in the arteries. It is regulated by the stroke volume and the compliance of the blood vessels. Diastolic blood pressure is the lowest pressure in the arteries and depends on peripheral resistance. The difference in the systolic and diastolic pressures is the pulse pressure. Systolic blood pressure in the legs is 15 to 20 mm Hg greater than in the arms, even while the individual is lying flat. This is in part related to Poiseuille’s law, according to which the total resistance of vessels connected in parallel is greater than the resistance of a single large vessel. The blood pressure in the aorta is less than the blood pressure in the branched arteries of the lower extremities.
Blood pressure varies greatly, according to the patient’s degree of excitement, degree of activity, smoking habits, pain, bladder distention, and dietary pattern. There is normally an inspiratory decline of up to 10 mm Hg in systolic blood pressure during quiet respiration.
Jugular Venous Pulse
The jugular venous pulse provides direct information about the pressures in the right side of the heart because the jugular system is in direct continuity with the right atrium. During diastole, when the tricuspid valve is open, the jugular veins are continuous with the right ventricle as well. If there is no stenotic lesion at the pulmonic or mitral valves, the right ventricle indirectly monitors the pressures in the left atrium and left ventricle. The most common cause of right-sided heart failure is left-sided heart failure. Examination of the neck veins also provides information about the cardiac rhythm.
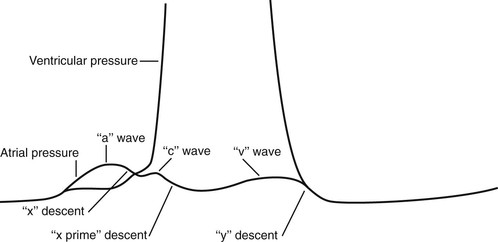
The understanding of the normal physiologic characteristics is important in the consideration of the jugular venous pulsation. Figure 11-9 is an enlargement of the atrial and ventricular pressure curves in Figure 11-6.
The “a” wave of the jugular venous pulse is produced by right atrial contraction. When the “a” wave is timed with the electrocardiogram, it is found to occur approximately 90 msec after the onset of the P wave. This time delay is related to the time from electrical stimulation of the atria to atrial contraction and to the resultant wave propagated in the neck. The “x” descent is caused by atrial relaxation, which occurs just before ventricular contraction. This drop in right atrial pressure is terminated by the “c” wave. The resulting increase in right atrial pressure is caused by tricuspid valve closure secondary to right ventricular contraction. The descent of the AV valve rings, also known as the descent of the base of the heart, produces the next change in right atrial pressure, called the “x prime” descent. As the free wall of the right ventricle approaches the septum during contraction, the AV valve rings descend toward the apex as contraction progresses. This increases the size of the atrium, causing a fall in its pressure (hence the “x prime” descent). During ventricular systole, the right atrium begins to fill with blood returning through the venae cavae. This increase in right atrial pressure as a result of its filling produces the ascending limb of the “v” wave. At the end of ventricular systole, right ventricular pressure falls rapidly. When it falls below the right atrial pressure, the tricuspid valve opens. This drop in right atrial pressure produces the “y” descent.
Normally, only the “a” and “v” waves are visible on examination. Because the “c” wave is frequently not observed, the “x” and “x prime” descents are summated into a single “x” descent. On occasion, the later portion of the “c” wave may be enlarged by a carotid artery pulsation artifact.
Evaluation of the jugular venous pulse provides information about the level of venous pressure and the type of venous wave pattern. These are described later in the section “The Jugular Venous Pulse.”
Review of Specific Symptoms
The important symptoms of cardiac disease are the following:
Chest Pain
Chest pain is probably the most important symptom of cardiac disease. It is not, however, pathognomonic for heart disease. It is well known that chest pain may result from pulmonary, intestinal, gallbladder, and musculoskeletal disorders. Ask the following questions of any patients complaining of chest pain:
“How long have you had the pain?”
“Do you have recurrent episodes of pain?”
“What is the duration of the pain?”
“How often do you get the pain?”
“What do you do to make it better?”
“What makes the pain worse? Breathing? Lying flat? Moving your arms or neck?”
“How would you describe the pain?1 Burning? Pressing? Crushing? Dull? Aching? Throbbing? Knifelike? Sharp? Constricting? Sticking?”
“Does the pain occur at rest? With exertion? After eating? When moving your arms? With emotional strain? While sleeping? During sexual intercourse?”
Angina pectoris is the true symptom of CHD. Angina is commonly the consequence of hypoxia of the myocardium resulting from an imbalance of coronary supply and myocardial demand. Table 11-1 lists the characteristics that differentiate angina pectoris from the other types of chest pain.
Table 11–1
Characteristics of Chest Pain*
| Feature | Angina | Not Angina |
| Location | Retrosternal, diffuse | Left inframammary, localized |
| Radiation | Left arm, jaw, back | Right arm |
| Description | “Aching,” “dull,” “pressing,” “squeezing,” “viselike” | “Sharp,” “shooting,” “cutting” |
| Intensity | Mild to severe | Excruciating |
| Duration | Minutes | Seconds, hours, days |
| Precipitated by | Effort, emotion, eating, cold | Respiration, posture, motion |
| Relieved by | Rest, nitroglycerin | Nonspecific |
* Angina and other chest pain may manifest in a variety of ways. The characteristics listed here are the common manifestations. This list, however, is not exhaustive and should be used only as a guide.
Commonly, a patient may describe the angina by clenching the fist and placing it over the sternum. This is a pathognomonic sign of angina commonly referred to as Levine’s sign. Figure 11-10 demonstrates this body language.
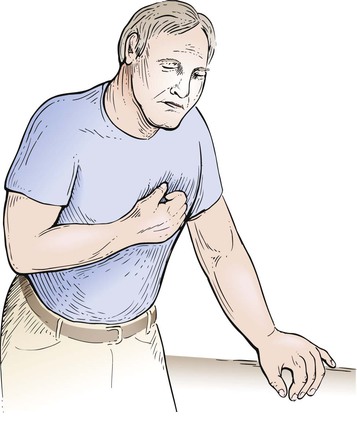
Figure 11–10 Levine’s sign.
When chest pain is related to a cardiac cause, coronary atherosclerosis and aortic valvular disease are the most common ones. Table 11-2 lists some common causes of chest pain.
Table 11–2
Common Causes of Chest Pain
| Organ System | Cause |
| Cardiac | Coronary artery disease Aortic valvular disease Pulmonary hypertension Mitral valve prolapse Pericarditis Idiopathic hypertrophic subaortic stenosis |
| Vascular | Dissection of the aorta |
| Pulmonary | Pulmonary embolism Pneumonia Pleuritis Pneumothorax |
| Musculoskeletal | Costochondritis* Arthritis Muscular spasm Bone tumor |
| Neural | Herpes zoster† |
| Gastrointestinal | Ulcer disease Bowel disease Hiatal hernia Pancreatitis Cholecystitis |
| Emotional | Anxiety Depression |
* Tietze’s syndrome, which is an inflammation of the costal cartilages.
† Shingles, which is a viral invasion of the peripheral nerves in a dermatomal distribution.
Palpitations
Palpitations are the uncomfortable sensations in the chest associated with a range of arrhythmias. Patients may describe palpitations as “fluttering,” “skipped beats,” “pounding,” “jumping,” “stopping,” or “irregularity.” Determine whether the patient has had similar episodes and what was done to extinguish them. Palpitations are common and do not necessarily indicate serious heart disease. Any condition in which there is an increased stroke volume, as in aortic regurgitation, may be associated with a sensation of “forceful contraction.” When a patient complains of palpitations, ask the following questions:
“How long have you had palpitations?”
“Do you have recurrent attacks?” If so, “How frequently do they occur?”
“When did the current attack begin?”
“Did any maneuvers or positions stop it?”
“Could you count your pulse during the attack?”
“Can you tap out on the table what the rhythm was like?”
“During the palpitations, have you ever fainted? Had chest pain?”
“Was there an associated flush, headache, or sweating associated with the palpitations?”2
“Have you noticed an intolerance to heat? Cold?”
“What kind of medications are you taking?”
“Do you take any medications for your lungs?”
“Are you taking any thyroid medications?”
“Have you ever been told that you had a problem with your thyroid?”
“How much tea, coffee, chocolate, or cola sodas do you consume a day?”
“Do you smoke?” If yes, “What do you smoke?”
“Do you drink alcoholic beverages?”
“Did you notice that after the palpitations you had to urinate?”3
In addition to primary cardiovascular causes, thyrotoxicosis, hypoglycemia, fever, anemia, pheochromocytoma, and anxiety states are commonly associated with palpitations. Hyperthyroidism is an important cause of rhythm disturbances that originate outside the cardiovascular system. Caffeine, tobacco, and drugs are also important factors in arrhythmogenicity. Sympathomimetic amines used in the treatment of bronchoconstriction are potent stimuli for arrhythmia as well. In patients with panic disorders and other anxiety states, the sensation of palpitations may occur during periods of normal rate and rhythm.
Patients who have had previous attacks of palpitations should be asked the following:
“How was your previous attack terminated?”
“How often do you get the attacks?”
“Are you able to terminate them?” If so, “How?”
“Have you ever been told that you have Wolff-Parkinson-White syndrome?”4
Table 11-3 outlines the common causes of palpitations.
Table 11–3
Common Causes of Palpitations
Extrasystoles
Atrial premature beats*
Nodal premature beats
Ventricular premature beats†
Tachyarrhythmias
Paroxysmal supraventricular tachycardia
Atrial flutter
Atrial fibrillation
Multifocal atrial tachycardia
Ventricular tachycardia
Bradyarrhythmias
Heart block
Sinus arrest
Drugs
Bronchodilators
Digitalis
Antidepressants
Smoking
Caffeine
Thyrotoxicosis
* Also known as atrial premature contractions or premature atrial contractions.
† Also known as ventricular premature contractions or premature ventricular contractions.
Dyspnea
The complaint of dyspnea is important. Patients report that they have “shortness of breath” or that they “can’t get enough air.” Dyspnea is commonly related to cardiac or pulmonary conditions. The questions relating to dyspnea are discussed in Chapter 10, The Chest. This section further delineates dyspnea as a cardiac symptom.
Paroxysmal nocturnal dyspnea (PND)5 occurs at night or when the patient is supine. This position increases the intrathoracic blood volume, and a weakened heart may be unable to handle this increased load; congestive heart failure may result. The patient is awakened about 2 hours after having fallen asleep, is markedly dyspneic, is often coughing, and may seek relief by running to a window to “get more air.” Episodes of PND are relatively specific for congestive heart failure.
The symptom of PND is often associated with the symptom of orthopnea, the need for using more pillows on which to sleep. Inquire of all patients, “How many pillows do you need to sleep?” To help quantify the orthopnea, you can state, for example, “Three-pillow orthopnea for the past 4 months.”
Dyspnea on exertion (DOE) is usually caused by chronic congestive heart failure or severe pulmonary disease. Quantify the severity of the dyspnea by asking, “How many level blocks can you walk now?” and “How many level blocks could you walk 6 months ago?” You can now attempt to quantify the dyspnea: for example, “The patient has had one-block DOE for the past 6 months. Before 6 months ago, the patient was able to walk 4 blocks without becoming short of breath. In addition, during the last 3 months, the patient has noted 4-pillow orthopnea.”
Trepopnea is a rare form of positional dyspnea in which the dyspneic patient has less dyspnea while lying on the left or right side. The pathophysiologic process of trepopnea is not well understood.
Table 11-4 lists the common causes of dyspnea.
Table 11–4
Common Causes of Dyspnea
| Organ System or Condition | Cause |
| Cardiac | Left ventricular failure Mitral stenosis |
| Pulmonary | Obstructive lung disease Asthma Restrictive lung disease Pulmonary embolism Pulmonary hypertension |
| Emotional | Anxiety |
| High-altitude exposure | Decreased oxygen pressure |
| Anemia | Decreased oxygen-carrying capacity |
Syncope
Fainting, or syncope, is the transient loss of consciousness that results from inadequate cerebral perfusion. Ask patients what they mean by “fainting” or “dizziness.” Syncope may have cardiac or noncardiac causes. When a patient describes fainting, ask the following questions:
“What were you doing just before you fainted?”
“Have you had recurrent fainting spells?” If so, “How often do you have these attacks?”
“In what position were you when you fainted?”
“Did you have any warning that you were going to faint?”
“Did you have any black, tarry bowel movements after the faint?”
The activity that preceded the syncope is important because some cardiac causes are associated with syncope during exercise (e.g., valvular aortic stenosis, idiopathic hypertrophic subaortic stenosis, and primary pulmonary hypertension). If a patient describes palpitations before the syncope, an arrhythmogenic cause may be present. Cardiac output may be reduced by arrhythmias or obstructive lesions.
The position of the patient just before fainting is important because this information may help determine the cause of the syncope. For example, if a patient fainted after rising suddenly from bed in the middle of the night (e.g., to run to answer the telephone), orthostatic hypotension may be the cause. Orthostatic hypotension is a common form of postural syncope and is the result of a peripheral autonomic limitation. There is a sudden fall in systemic blood pressure, resulting from a failure of adaptive reflexes to compensate for an erect posture. Symptoms of orthostatic hypotension include dizziness, blurring of vision, profound weakness, and syncope. Many drugs can cause orthostatic hypotension by leading to changes in intravascular volume or tone. Older patients are most prone to orthostatic hypotension. Micturition syncope usually occurs in men during straining with nocturnal urination. It may occur after considerable alcohol consumption.
Vasovagal syncope is the most common type of fainting and is one of the most difficult to manage. It has been estimated that 40% of all syncopal events are vasovagal in nature. Vasovagal syncope occurs during periods of sudden, stressful, or painful experiences, such as receiving bad news, surgical manipulation, trauma, the loss of blood, or even the sight of blood. It is often preceded by pallor, nausea, weakness, blurred vision, lightheadedness, perspiring, yawning, diaphoresis, hyperventilation, epigastric discomfort, or a “sinking feeling.” There is a sudden fall in systemic vascular resistance without a compensatory increase in cardiac output as a result of an increased vagotonia. If the patient sits or lies down promptly, frank syncope can be aborted.
Carotid sinus syncope is associated with a hypersensitive carotid sinus and is most common in the older adult population. Whenever a patient with carotid sinus syncope wears a tight shirt collar or turns the neck in a certain way, there is an increased stimulation of the carotid sinus. This causes a sudden fall in systemic pressure, and syncope results. Two types of carotid sinus hypersensitivity exist: a cardioinhibitory (bradycardia) type and a vasodepressor (hypotension without bradycardia) type. Posttussive syncope usually occurs in patients with chronic obstructive lung disease. Several mechanisms have been postulated to explain its occurrence. It is generally accepted that coughing produces an increase in intrathoracic pressure, which decreases both venous return and cardiac output. There may also be a rise in cerebrospinal fluid pressure, producing a decreased perfusion to the brain.
There are other suggested questions to ask a patient with syncope that direct attention to a neurologic cause. These are summarized in Chapter 18, The Nervous System. Table 11-5 lists the common causes of syncope.
Table 11–5
Common Causes of Syncope
| Organic System or Condition | Cause |
| Cardiac | Decreased cerebral perfusion secondary to cardiac rhythm disturbance Left ventricular output obstruction |
| Metabolic | Hypoglycemia Hyperventilation Hypoxia |
| Psychiatric | Hysteria |
| Neurologic | Epilepsy Cerebrovascular disease |
| Orthostatic hypotension | Volume depletion Antidepressant medications Antihypertensive medications |
| Vasovagal | Vasodepression |
| Micturition | Visceral reflex (vasodepressor) |
| Cough | Chronic lung disease |
| Carotid sinus | Vasodepressor response to carotid sinus sensitivity |
Fatigue
Fatigue is a common symptom of decreased cardiac output. Patients with congestive heart failure and mitral valvular disease frequently complain of fatigue. Fatigue, however, is not specific for cardiac problems. The most common causes of fatigue are anxiety and depression. Other conditions associated with fatigue include anemia and chronic diseases. You must attempt to differentiate organic from psychogenic fatigue. Ask the following questions:
“How long have you been tired?”
“Did the fatigue start suddenly?”
“Do you feel tired all day? In the morning? In the evening?”
“When do you feel least tired?”
Patients with psychogenic fatigue are tired “all the time.” They are often more tired at home than at work but occasionally describe being more tired in the morning. They may feel their best at the end of the day, which is when most patients with organic causes feel the worst.
Dependent Edema
Swelling of the legs, a form of dependent edema, is a frequent complaint of patients. Ask the following questions:
“When was the swelling first noted?”
“Are both legs swollen equally?”
“Did the swelling appear suddenly?”
“Is the swelling worse at any time of the day?”
“Does it disappear after a night’s sleep?”
“Does elevation of your feet reduce the swelling?”
“What kind of medications are you taking?”
“Is there a history of kidney, heart, or liver disease?”
“Do you have shortness of breath?” If so, “Which came first, the edema or the shortness of breath?”
If the patient is a woman, ask the following questions:
The patient with congestive heart failure has symmetric edema of the lower extremities that worsens as the day progresses. It is least in the morning after sleeping with the legs elevated in bed. If the patient also complains of dyspnea, it is helpful to determine which symptom came first. In patients with dyspnea and edema secondary to cardiac causes, the dyspnea usually precedes the edema. Bedridden patients may have dependent edema in the sacral area.
Hemoptysis
Hemoptysis is discussed in Chapter 10, The Chest. In addition to the pulmonary causes, mitral stenosis is an important cause of hemoptysis. Rupture of the bronchial veins, which are under high back pressure in mitral stenosis, produces the hemoptysis.
Cyanosis
Cyanosis is also discussed in Chapter 10, The Chest. The important questions regarding cyanosis are indicated in that chapter.
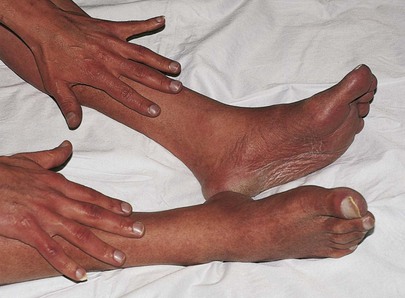
On occasion, cyanosis is noted only in the lower extremities. This is termed differential cyanosis. It is related to a right-to-left shunt through a patent ductus arteriosus (PDA). In a right-to-left shunt resulting from pulmonary hypertension, blood in the pulmonary artery crosses the PDA, which is located below the level of the carotid and left subclavian arteries; deoxygenated blood is pumped only to the lower extremity, producing cyanosis in only that location. Some blood does get to the lungs for oxygenation and is ultimately pumped out through the aorta to produce normal skin color in the upper extremity.
The patient whose feet are shown in Figure 11-11 is a 30-year-old immigrant who was evaluated in the United States for cyanosis. Until the age of 20 years, he had marked “bluish discoloration” of his lower extremities and relatively normal color in his upper extremities. Over the next 10 years, there was a gradual darkening of his upper extremities. Note the marked cyanosis of the extremities and nail beds of the fingers and toes. The patient had a PDA with marked pulmonary hypertension.
Effect of Cardiac Disease on the Patient
Patients with cardiac disease are intensely fearful. Once cardiac disease has been diagnosed, a series of reactions occurs. Fear, depression, and anxiety are the outcomes. The patients, who were totally asymptomatic until their episode of “sudden death” resulting from a coronary occlusion, are scared. They were resuscitated the first time; will an episode happen again? When? During recovery in the hospital, they are afraid to leave the intensive care unit for fear that “no one will be watching.” At the time of discharge from the hospital, they are filled with anxiety. Although they desperately want to go home, they ask themselves, “What will happen if I have chest pain at home? Who will provide medical assistance?” They go through a period of depression, recognizing what they have gone through. After convalescence, they become fearful of daily situations that may provoke another attack. Can they go back to the daily “hassles” at work? Is it safe to have sexual intercourse? Despite appropriate reassurances from the clinician, their anxiety level may remain high.
Many patients with cardiac disease who have witnessed a fatal cardiac arrest in another patient in their room refuse to admit how stressful this event really was. The patients freely discuss the efficiency of the cardiac arrest team or complain that the noise kept them from sleeping. They refuse to identify with the deceased patient.
The patient with cardiac disease approaching surgery has the same fears as all surgical patients; these fears are discussed in Chapter 2, The Patient’s Responses. However, surgical procedures for the patient with cardiac disease involve the “nucleus” of the body. The conscientious clinician takes time to explain the nature of the problem and the surgical approach. Before the procedure, the clinician should allow the patient, and especially the family, to visit the intensive care unit where the patient will be for a few days after surgery. Patients should be reassured that everything possible will be done in their behalf. Their courage and determination and the clinician’s support are essential.
Physical Examination
The equipment necessary for the examination of the heart is a stethoscope, a penlight, and an applicator stick.
The physical examination of the heart includes the following:
• Assessment of blood pressure
• Assessment of the arterial pulse
The patient should be supine, and the examiner should stand on the right side of the bed. The head of the bed may be elevated slightly if the patient is more comfortable in this position.
Inspection
Evaluate General Appearance
The general inspection of the patient offers clues to cardiac diagnosis. Is the patient in acute distress? What is the patient’s breathing like? Is it labored? Are accessory muscles being used?
Inspect the Skin
The skin can reveal many changes associated with cardiac disease. Inspect the skin color. Is cyanosis present? If so, does it appear central or peripheral? Is pallor present?
The temperature of the skin may reflect cardiac disease. Severe anemia, beriberi, and thyrotoxicosis tend to make the skin warmer; intermittent claudication is associated with coolness of the lower extremity in comparison with the upper extremity.
Are xanthomata present? Tendon xanthomata are stony-hard, slightly yellowish masses that are commonly found on the extensor tendons of the fingers and are pathognomonic for familial hypercholesterolemia. The Achilles tendon and plantar tendons of the soles are also common locations for tendon xanthomata. Figure 11-12 shows tendon xanthomata on the extensor surfaces of the fingers of a patient with a total serum cholesterol concentration higher than 450 mg/dL.6
Figure 11–12 Tendon xanthomata.
Figure 11-13 shows multiple tuberous xanthomata of the hand of another patient. This patient had primary biliary cirrhosis and extremely elevated cholesterol levels. Primary biliary cirrhosis is a rare, progressive, and often fatal liver disease occurring mostly in women. Pruritus is a common symptom. Xanthomata develop in approximately 15% to 20% of affected patients and are typically found on the palms, soles, knees, elbows, and hands. The serum cholesterol, usually LDL, is often as high as 1000 to 1500 mg/dL. Antimitochondrial antibody is present in nearly 90% of patients.
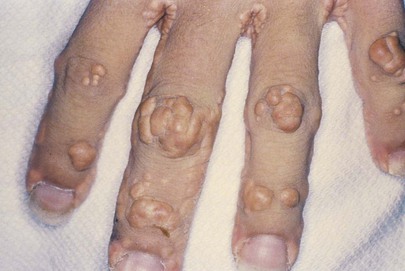
Figure 11–13 Multiple tuberous xanthomata of the hand.
Eruptive xanthomata are seen in several familial disturbances of fat metabolism, specifically hyperlipidemia types I and IV. The chest, buttocks, abdomen, back, face, and arms are most commonly affected. Figure 11-14 shows eruptive xanthomata on the abdomen of a patient with uncontrolled diabetes mellitus and hypertriglyceridemia. Eruptive xanthomata on the face of a patient are pictured in Figure 11-15. Eruptive xanthomata result from elevations in plasma triglyceride concentrations, often to levels greater than 1500 mg/dL.7 These lesions developed in this patient after excessive alcohol consumption; his serum triglyceride concentrations exceeded 2000 mg/dL. The lesions, frequently found on the abdomen, buttocks, elbows, knees, and back, are small (1 to 3 mm in diameter), yellowish papules on an erythematous base. With reduction in the level of triglycerides, the lesions may recede.
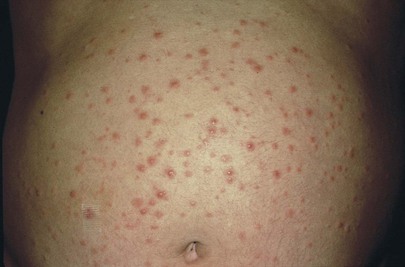
Figure 11–14 Eruptive xanthomata on the abdomen.
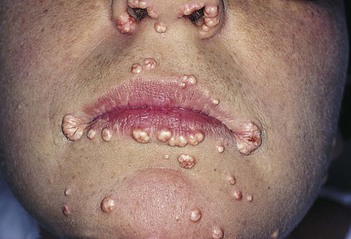
Figure 11–15 Eruptive xanthomata on the face.
Is a rash present? The presence of erythema marginatum (erythema in which the reddened areas are disc-shaped with raised edges) in a febrile patient is suggestive of acute rheumatic fever.
Are any painful lesions on the fingers or toes present? Osler’s nodes are painful lesions that occur in the tufts of the fingers and toes in patients with infective endocarditis. They are evanescent but are said to occur in 10% to 25% of patients with infective endocarditis.
Inspect the Nails
Frequently, splinter hemorrhages are visible as small, reddish-brown lines in the nail bed. These hemorrhages run from the free margin proximally and are classically associated with infective endocarditis. However, the finding is nonspecific because it is seen in many other conditions, even local trauma to the nail. A nail with splinter hemorrhages in a patient with endocarditis is shown in Figure 11-16.
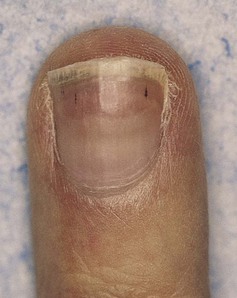
Figure 11–16 Splinter hemorrhages.
Inspect the Facies
Abnormalities of the heart may also be associated with peculiarities of the face and head. Supravalvular aortic stenosis, a congenital problem, occurs in association with widely set eyes, strabismus, low-set ears, an upturned nose, and hypoplasia of the mandible. Moon facies and widely spaced eyes are suggestive of pulmonic stenosis. Expressionless facies with puffy eyelids and loss of the outer third of the eyebrow is seen in hypothyroidism. Affected individuals may have a cardiomyopathy. The earlobe crease, or Lichtstein’s sign, is an oblique crease, often bilateral, seen frequently in patients older than 50 years with significant CHD. This sign is shown in Figure 11-17. Although it is a useful sign, there are many false-positive and false-negative findings for this sign to be very reliable.
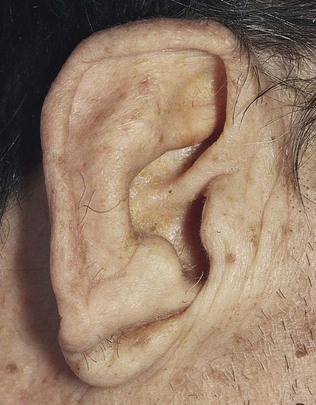
Figure 11–17 Earlobe creases.
Inspect the Eyes
The presence of yellowish plaques on the eyelids, called xanthelasma, should raise the suspicion of an underlying hyperlipoproteinemia, even though this lesion is less specific than the xanthoma. Xanthelasma in a patient with hypercholesterolemia is shown in Figure 11-18.
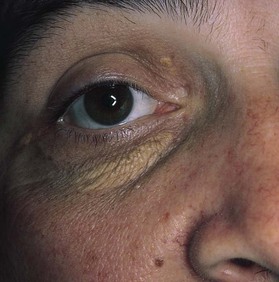
Figure 11–18 Xanthelasma.
Examination of the eyes may reveal an arcus senilis. An arcus (see Figs. 7-57 and 7-58) seen in a patient younger than 40 years should raise the suspicion of hypercholesterolemia. Opacities in the cornea may be evidence for sarcoidosis, which may be responsible for cor pulmonale or myocardial involvement. Displacement of the lens is frequently seen in patients with Marfan’s syndrome, an important cause of aortic regurgitation (see Fig. 7-74). Conjunctival hemorrhages are commonly seen in infective endocarditis. Hypertelorism, or widely set eyes, is often associated with congenital heart disease, especially pulmonic stenosis and supravalvular aortic stenosis. Retinal evaluation may furnish valuable information about diabetes (see Figs. 7-93 to 7-105), hypertension (see Figs. 7-84 and 7-106 to 7-110), and atherosclerosis. Roth’s spots may develop in patients with infective endocarditis (see Fig. 7-114).
Inspect the Mouth
Have the patient open the mouth widely. Inspect the palate. Is the palate highly arched? A high-arched palate may be associated with congenital heart problems such as mitral valve prolapse.
Are there petechiae on the palate? Infective endocarditis is often associated with palatal petechiae, as seen in Figure 11-19.
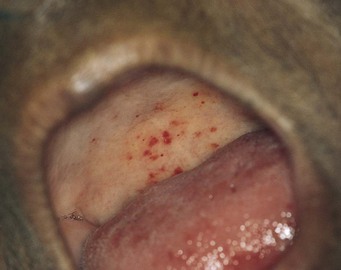
Figure 11–19 Palatal petechiae.
Inspect the Neck
Examination of the neck may reveal webbing. Webbing is seen in individuals with Turner’s syndrome,8 who may have coarctation of the aorta, and in patients with Noonan’s syndrome.9 Pulmonic stenosis is the associated cardiac abnormality in this condition.
Inspect the Chest Configuration
Inspection of the chest often reveals information about the heart. Because the chest and the heart develop at about the same time during embryogenesis, it is not surprising that anything interfering with the development of the chest may interfere with the heart. A pectus excavatum, or caved-in chest, is seen in Marfan’s syndrome and in mitral valve prolapse (see Fig. 10-7). Pectus carinatum, or pigeon breast, is also associated with Marfan’s syndrome (see Fig. 10-8).
Assess the presence of visible cardiac motions.
Inspect the Extremities
Some congenital abnormalities of the heart are associated with abnormalities of the extremities. Patients with atrial septal defects may have an extra phalanx, an extra finger, or an extra toe. Long, slender fingers may be suggestive of Marfan’s syndrome and aortic regurgitation. Short stature, cubitus valgus, and medial deviation of the extended forearm are typical of patients with Turner’s syndrome.
Blood Pressure Assessment
The Principles
Blood pressure can be measured directly with an intraarterial catheter or indirectly with a sphygmomanometer. The sphygmomanometer consists of an inflatable rubber bladder within a cloth cover, a rubber bulb to inflate the bladder, and a manometer to measure the pressure in the bladder. Indirect measurement of blood pressure involves the auscultatory detection of the appearance and disappearance of the Korotkoff sounds over the compressed artery. Korotkoff sounds are low-pitched sounds originating in the vessel that are related to turbulence produced by partially occluding an artery with a blood pressure cuff. Several phases occur in sequence as the occluding pressure drops. Phase 1 occurs when the occluding pressure falls to the systolic blood pressure. The tapping sounds are clear and gradually increase in intensity as the occluding pressure falls. Phase 2 occurs at a pressure approximately 10 to 15 mm Hg lower than that in phase 1 and consists of tapping sounds followed by murmurs.10
Phase 3 occurs when the occluding pressure falls enough to allow a large amount of volume to cross the partially occluded artery. The sounds are similar to the sounds of phase 2, except that only the tapping sounds are heard. Phase 4 is the abrupt muffling and decreased intensity of the sounds as the pressure approaches the diastolic blood pressure. Phase 5 is the complete disappearance of the sounds. The vessel is no longer compressed by the occluding cuff. Turbulent flow is no longer present.
The normal blood pressure for adults is less than 120 mm Hg systolic and less than 80 mm Hg diastolic. Borderline (prehypertensive) blood pressure is 120 to 139 systolic and 80 to 89 diastolic. When systolic and diastolic blood pressures fall into different categories, the higher category should be used to classify blood pressure level. For the diastolic blood pressure reading, the point of disappearance of the Korotkoff sounds is probably more accurate than the point of muffling. However, if the point of disappearance is more than 10 mm Hg lower than the point of muffling, the point of muffling is probably more accurate. Recording both the point of muffling and disappearance frequently helps in communication. A blood pressure might be recorded as 125/75–65: the systolic blood pressure is 125; the point of muffling is 75; the point of disappearance is 65 (the diastolic blood pressure).
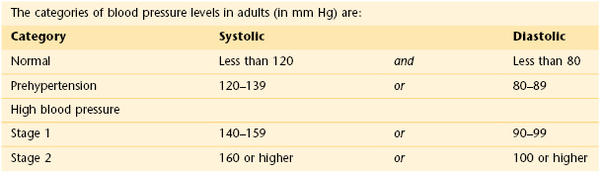
The ranges in the table apply to most adults (aged 18 and older) who don’t have short-term serious illnesses.
Two types of hypertension need to be recognized. White coat hypertension is a common phenomenon (approximately 15–20% of all stage 1 hypertensives) in which the patient’s blood pressure is higher in the doctor’s office than if measured at home or at work. These patients are at low cardiovascular risk. In contrast, masked hypertension is more serious because these individuals have normal blood pressures in the medical facility, but actually have much higher blood pressures throughout the day, indicative of a high risk of CVD. Masked hypertension has been estimated to occur in 10% of the general population.
The size of the cuff is important for the accurate determination of blood pressure. It is recommended that the cuff be snugly applied around the arm, with its lowest edge 1 inch above the antecubital fossa. The cuff should be approximately 20% wider than the diameter of the extremity. The bladder should overlie the artery. The use of a cuff that is too small for a large arm results in an erroneously high reading of blood pressure.
Another cause of falsely elevated blood pressure readings is lack of support of the patient’s arm. To obtain an accurate measurement, the cuff must be at heart level. If the arm is not supported, the patient is performing isometric exercise, which raises the recorded pressure. In contrast, excessive pressure on the diaphragm of the stethoscope produces a spuriously lower reading of the diastolic blood pressure without any significant alteration of systolic pressure. If the arm is held correctly, no skin indentations should occur.
The auscultatory gap is the silence that occurs between the disappearance of the Korotkoff sounds after the initial appearance and their reappearance at a lower pressure. The auscultatory gap is present when there is a decreased blood flow to the extremities, as is found in hypertension and in aortic stenosis. Its clinical importance lies in the fact that the systolic blood pressure may be mistaken for the lower blood pressure, the point of reappearance.
Determine Blood Pressure by Palpation
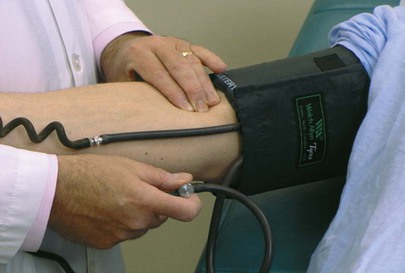
Blood pressure assessment is performed with the patient lying comfortably in the supine position. The cuff bladder is centered over the right brachial artery. If the arm is obese, a large adult or thigh cuff should be used. The arm should be slightly flexed, and it should be supported at approximately the level of the heart. To determine the systolic blood pressure adequately and to exclude an error as a result of an auscultatory gap, blood pressure is first assessed by palpation. In this procedure, the right brachial or right radial artery is palpated while the cuff is inflated above the pressure required to obliterate the pulse. The adjustable screw is opened slowly for deflation. The systolic pressure is identified by the reappearance of the brachial pulse. As soon as the pulse is felt, the adjustable screw is opened for rapid deflation. This assessment is demonstrated in Figure 11-20.
Determine Blood Pressure by Auscultation
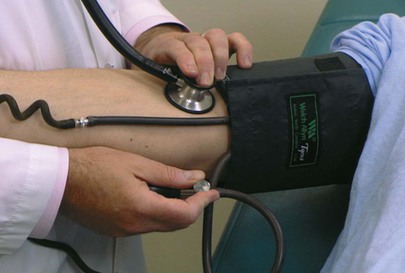
Blood pressure by auscultation is assessed in the right arm by inflating the cuff to approximately 20 mm Hg above the systolic pressure that was determined by palpation. The diaphragm of the stethoscope should be placed over the artery as close to the edge of the cuff as possible, preferably just under the edge. The cuff is deflated slowly while the Korotkoff sounds are evaluated. The systolic blood pressure, the point of muffling, and the point of disappearance are determined. The systolic blood pressure is the point at which the initial tapping sounds are heard. The technique of determining auscultatory blood pressure is shown in Figure 11-21. If the blood pressure is high, it is useful to measure the blood pressure again at the end of the examination, when the patient may be calmer.
Rule Out Orthostatic Hypotension
After the patient has been recumbent for at least 5 minutes, measure the baseline blood pressure and pulse. Then have the patient stand, and repeat the measurements immediately.
Orthostatic hypotension is defined as a drop in systolic blood pressure of 20 mm Hg or more, in association with the development of symptoms such as dizziness or syncope, when the patient assumes the standing position. In most affected patients, there is also an increase in heart rate.
Rule Out Supravalvular Aortic Stenosis
If hypertension is detected in the right arm, perform the following test: Place the cuff on the patient’s left arm, and determine the auscultatory pressure. It is not necessary to remeasure the palpatory pressure or reevaluate for orthostatic changes. In supravalvular aortic stenosis, there is a difference in the blood pressures in the arms; hypertension may be detected in the right arm, whereas hypotension may be present in the left arm.
Rule Out Coarctation of the Aorta
If the blood pressure is elevated in the arms, determination of the blood pressure in the lower extremities is important for ruling out coarctation of the aorta. The patient is asked to lie on the abdomen while the thigh cuff, which is 6 cm wider than the arm cuff, is placed around the posterior aspect of the midthigh. The stethoscope is placed over the artery in the popliteal fossa. The Korotkoff sounds are determined as in the upper extremity. If a thigh cuff is not available, the regular cuff can be applied to the lower leg with the distal border just at the malleoli. The stethoscope is placed over either the posterior tibial or the dorsalis pedis artery, and the auscultatory blood pressure is taken. A leg systolic blood pressure that is lower than that in the arm should raise suspicion of coarctation of the aorta.
Rule Out Cardiac Tamponade
In the presence of low arterial blood pressure and a rapid and feeble pulse, it is necessary to rule out the presence of cardiac tamponade. A valuable clinical sign suggestive of cardiac tamponade is the presence of a marked paradoxical pulse (also known as a pulsus paradoxus), which is characterized by an exaggeration of the normal inspiratory fall in systolic pressure. There is much confusion about the definition of a normal paradoxical pulse. A normal paradoxical pulse should be defined as the normal fall (approximately 5 mm Hg) in systolic arterial pressure during inspiration. It is the magnitude of the phenomenon that should determine whether the pulsus paradoxus is normal or abnormal.
The technique for assessing the magnitude of a paradoxical pulse is as follows: Have the patient breathe as normally as possible. Inflate the blood pressure cuff until no sounds are heard. Gradually deflate the cuff until sounds are heard in expiration only. Note this pressure. Continue to deflate the cuff slowly until sounds are heard during inspiration. Note this pressure. If the difference in these two pressures exceeds 10 mm Hg, a marked (abnormal) pulsus paradoxus is present; cardiac tamponade may be the cause. Cardiac tamponade results when there is an increase in intrapericardial pressure that interferes with normal diastolic filling. A marked paradoxical pulse is not a specific phenomenon for tamponade because it is also seen in large pericardial effusions, in constrictive pericarditis, and in conditions associated with increased ventilatory effort, such as asthma and emphysema.
The Arterial Pulse
The following information is gained from palpation of the arterial pulse:
Determine the Cardiac Rate
Cardiac rate is routinely assessed by the radial pulse. The examiner should stand in front of the patient and grasp both radial arteries. The second, third, and fourth fingers should overlie the radial artery, as shown in Figure 11-22. The examiner should count the pulse for 30 seconds and multiply the number of beats by 2 to obtain the beats per minute. This method is accurate for most regular rhythms. If the patient has an irregularly irregular rhythm, as is found in atrial fibrillation, a pulse deficit may be present. In atrial fibrillation, many impulses bombard the AV node and ventricles. Owing to the varying lengths of diastolic filling periods, some of the contractions may be very weak and unable to produce an adequate pulse wave despite ventricular contraction. A pulse deficit, which is the difference between the apical (precordial) and radial pulses, occurs. In such cases, only auscultation of the heart, not the radial pulse, provides an accurate assessment of the cardiac rate.
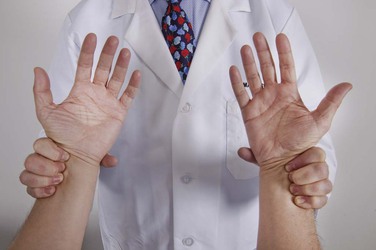
Figure 11–22 Technique for evaluating the radial artery pulses.
Determine the Cardiac Rhythm
When palpating the pulse, carefully evaluate the regularity of the rhythm. The slower the rate, the longer you should palpate. If the rhythm is irregular, is there a pattern to the irregularity?
Cardiac rhythm may be described as regular, regularly irregular, or irregularly irregular. A regularly irregular rhythm is a pulse with an irregularity that occurs in a definite pattern. An irregularly irregular pulse has no pattern.
Palpate the Carotid Artery
Assess the carotid artery pulse by standing at the patient’s right side, with the patient lying on the back. Auscultate the patient’s carotid arteries for bruits first (see Chapter 12, The Peripheral Vascular System). If a bruit is present, do not palpate the carotid artery. If a cholesterol plaque is present, palpation may produce an embolus.
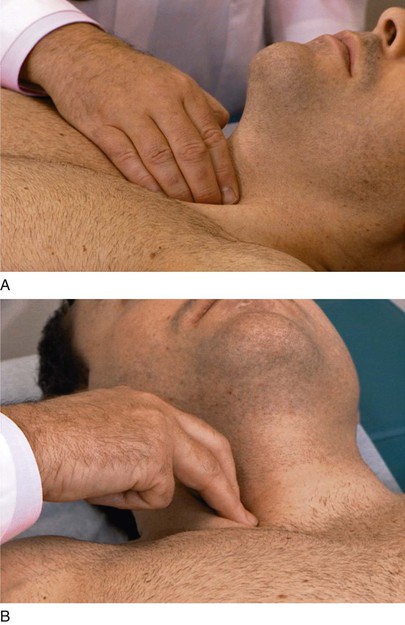
To palpate the carotid artery, place your index and third fingers on the patient’s thyroid cartilage and slip them laterally between the trachea and the sternocleidomastoid muscle. You should be able to feel the carotid pulsations just medial to the sternocleidomastoid muscle. Palpation should be performed low in the neck to avoid pressure on the carotid sinus, which would cause a reflex drop in blood pressure and heart rate. Each carotid artery is evaluated separately. Never press on both carotid arteries at the same time. After the right carotid artery is evaluated, stand in the same position, and place the same fingers back on the patient’s trachea and slip them laterally to the left to feel the left carotid artery. This technique is demonstrated in Figure 11-23.
Evaluate the Characteristics of the Pulse
The carotid artery is used for the assessment of the contour and amplitude of the pulse. Contour is the shape of the wave. It is frequently described as the speed of the upward slope, downward slope, and duration of the wave. Place a hand firmly against the carotid artery until maximal force is felt. At this moment, the wave form should be discernible. The pulse may be described as normal, diminished, increased, or double-peaked. The normal carotid pulse wave is smooth, with the upward stroke steeper and more rapid than the downward stroke. A diminished pulse is a small, weak pulse. The palpating finger feels a gentle pressure rise with a distinct peak. An increased pulse is a large, strong, hyperkinetic pulse. The palpating finger feels an increased rate of rise of the ascending limb of the pulse and a brisk tap at its peak. A double-peaked pulse has a prominent percussion and tidal wave with or without a dicrotic wave. Arterial pulse abnormalities are summarized in Figure 11-24.
The Jugular Venous Pulse
Both the external and internal jugular veins provide information about the wave forms and right atrial pressure. The external jugular vein is easier to visualize than the internal jugular vein. The pulsations of the internal jugular vein are beneath the sternocleidomastoid muscle and are visible as they are transmitted through the surrounding tissue. The vein itself is not visible. Because the right internal jugular vein is straighter than the left, only the right jugular veins are evaluated.
Determine the Jugular Wave Forms
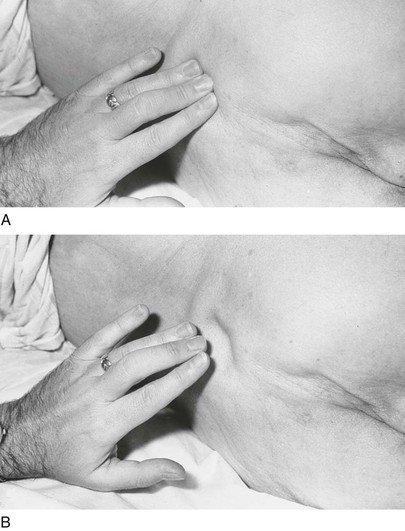
To visualize the jugular wave forms, the patient should lie flat without a pillow so that his or her neck is not flexed and does not interfere with the pulsations. The patient’s trunk should be at approximately 25 degrees to the horizontal. The higher the venous pressure, the greater the elevation required; the lower the pressure, the lower the elevation needed. The patient’s head should be turned slightly to the right and slightly down to relax the right sternocleidomastoid muscle. Standing on the patient’s right side, the examiner should place his or her right hand, holding a small pocket flashlight, on the patient’s sternum and shine the light tangentially across the right side of the patient’s neck. Shadows of the pulsations will be cast on the sheet behind the patient. The light and shadows magnify the wave forms. This technique is demonstrated in Figure 11-25. If no wave forms are seen, the angle of elevation of the head of the bed should be reduced. To help identify the wave forms, the examiner can time the cardiac cycle by palpating the cardiac impulse beneath his or her right hand or by feeling the left carotid impulse with his or her left hand. The descents, rather than the waves themselves, tend to be more obvious. If the neck veins are visible at the jaw margin while the patient is seated, the examiner should watch for the wave forms at the angle of the jaw with the patient seated upright.
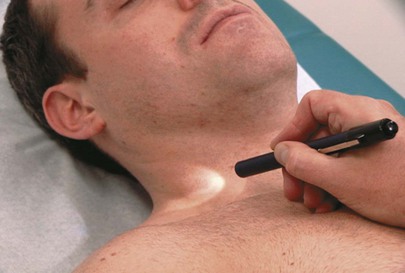
Figure 11–25 Technique for evaluating the jugular wave forms.
The jugular pulse must be differentiated from the pulsation of the carotid artery. Table 11-6 lists the most important characteristic differences of these pulses.
Table 11–6
Differentiation of Jugular and Carotid Pulses
| Feature | Internal Jugular Pulse | Carotid Pulse |
| Palpation | Not palpable | Palpable |
| Waveforms | Multiform: two or three components | Single |
| Quality | Soft, undulating | Vigorous |
| Pressure* | Wave forms obliterated | No effect |
| Inspiration | Decreased height of wave forms | No effect |
| Sitting up | Decreased height of wave forms | No effect |
| Valsalva maneuver | Increased height of wave forms | No effect |
* Light pressure on the vessel above the sternal end of clavicle.
Estimate the Jugular Venous Pressure
To assess the pressure in the right side of the heart, it is necessary to establish a reference level. The standard reference is the manubriosternal angle. At any degree of elevation, this position is used to measure the pressure in the jugular venous system and right heart. The patient should be positioned at whatever angle between the supine and upright positions best demonstrates the top of the neck veins. An imaginary horizontal line is then drawn from this height to above the sternal angle. The heart is approximately 5 cm below the sternal angle; thus if the height of the column is 3 cm above the sternal angle, the estimated venous pressure is 3 + 5 cm, or 8 cm water. If the top of the column is 3 cm above the sternal angle, central venous pressure is probably elevated. The specificity of this finding is estimated to be from 93% to 96% (Sankoff & Zidulka, 2008). If there is neck vein distention up to the jaw margin while the patient is seated at 90 degrees, the right atrial pressure usually exceeds 15 mm Hg.11 In Figure 11-26, the neck veins are distended to the angle of the jaw while the patient is seated upright. The patient’s right atrial pressure was 21 mm Hg.
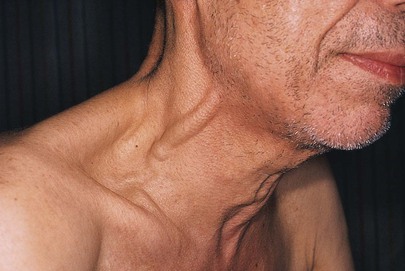
Figure 11–26 Neck vein distention.
Evaluate the Hepatojugular Reflux
A useful test in assessing high jugular venous pressure is that of the hepatojugular reflux, also known as abdominal compression. By applying pressure over the liver, the examiner can grossly assess right ventricular function. Patients with right ventricular failure have dilated sinusoids in the liver. Pressure on the liver pushes blood out of these sinusoids and into the inferior vena cava and right side of the heart, causing further distention of the neck veins. The procedure is performed with the patient lying in bed, mouth open, breathing normally; this prevents a Valsalva maneuver. The examiner places his or her right hand over the patient’s liver in the right upper quadrant and applies a firm, progressive pressure. Compression is maintained for 10 seconds. The normal response is for the internal and external jugular veins to show a transient increase in distention during the first few cardiac cycles, which is followed by a fall to baseline levels during the later part of the compression. In patients with right ventricular failure or elevated pulmonary artery wedge pressure, the neck veins remain distended during the entire period of compression; this distention diminishes rapidly (at least 4 cm) on sudden release of the compressing hand. If the examination is incorrectly performed with the patient’s mouth closed, a Valsalva maneuver results and produces inaccurate results of the hepatojugular reflux test.
Like most other clinical maneuvers, the hepatojugular reflux test must be performed in a standardized manner. If performed correctly, this test can be of considerable value in the bedside assessment of the patient. The test result correlates best with the pulmonary artery wedge pressure and, as such, is a reflection of increased central blood volume. Ewy (1988) evaluated this test and showed that in the absence of right ventricular failure, a positive test result is suggestive of a pulmonary artery wedge pressure of 15 mm Hg or greater.
Percussion
Percuss the Heart’s Borders
The technique of percussion is discussed in Chapter 10, The Chest. Percussion of the heart is performed at the third, fourth, and fifth intercostal spaces from the left anterior axillary line to the right anterior axillary line. Normally, there is a change in the percussion note from resonance to dullness approximately 6 cm lateral to the left of the sternum. This dullness is attributable to the presence of the heart.
A percussion dullness distance of greater than 10.5 cm in the left fifth intercostal space has a sensitivity of 91.3% and a specificity of 30.3% for detecting increased left ventricular end-diastolic volume (LVEDV) or left ventricular mass. Percussion dullness of more than 10.5 cm in the fifth intercostal space has a sensitivity of 94.4% and a specificity of 67.2% in detecting cardiomegaly. In patients with a palpable apical impulse of greater than 3 cm in the left decubitus position, the sensitivity of detecting increased LVEDV or left ventricular mass increases to 100% and the specificity is 40%.
Palpation
Palpation is performed to evaluate the apical impulse, the right ventricle, the pulmonary artery, and the left ventricular motions. The presence or absence of thrills13 is also determined by palpation. The PMI describes the outward motion of the cardiac apex as it rotates counterclockwise, as viewed from below, to strike the anterior chest wall during isovolumetric contraction.
Palpate the Point of Maximum Impulse
The examiner should stand on the right side of the patient, with the bed at a level comfortable for the examiner. Palpation for the PMI is most easily performed with the patient in a sitting position. Only the examiner’s fingertips should be applied to the patient’s chest in the fifth intercostal space, midclavicular line, because they are the most sensitive for assessing localized motion. The PMI should be noted. This technique is demonstrated in Figure 11-27. If the apical impulse is not felt, the examiner should move his or her fingertips in the area of the cardiac apex. The PMI is usually within 10 cm of the midsternal line and is no larger than 2 to 3 cm in diameter. A PMI that is laterally displaced or is felt in two interspaces during the same phase of respiration is suggestive of cardiomegaly.
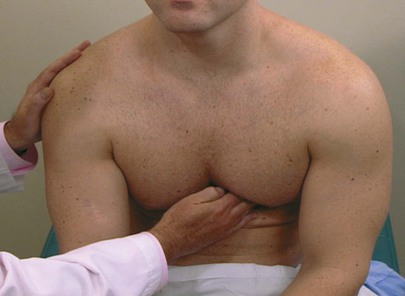
Figure 11–27 Technique for assessing point of maximum impulse.
The PMI is felt in approximately 70% of normal individuals while they are sitting. If it cannot be felt in the sitting position, the patient should be reevaluated while supine and in the left lateral decubitus position. The position of the PMI in the left lateral decubitus position must be assessed with the understanding that the normal cardiac impulse is now shifted slightly to the left. If the patient is in the left lateral decubitus position and the PMI is not laterally displaced, the examiner can suspect that cardiomegaly is not present.
In a patient without conditions predisposing to left ventricular hypertrophy, a palpable apical impulse felt in the left lateral decubitus position that is greater than 3 cm is said to be a specific (91%) and sensitive (92%) indicator of left ventricular enlargement. An apical diameter greater than 3 cm is predictive (86%) of an increased LVEDV. In patients with an apical diameter of less than 3 cm and a normal LVEDV, the negative predictive value is 95%.
The PMI usually corresponds to the left ventricular apex, but in patients with an enlarged right ventricle, the heart is rotated clockwise, as viewed from below, and the PMI may actually be produced by the right ventricle. This rotation turns the left ventricle posteriorly and makes it difficult to palpate. The apical impulse by the right ventricle is diffuse, whereas that of the left ventricle tends to be more localized.
In patients with chronic obstructive lung disease, the overinflation of the lungs displaces the PMI downward and to the right. The PMI in such patients is felt in the epigastric area, at the lower end of the sternum. In patients with chronic obstructive lung disease, a PMI in the normal location is suggestive of cardiomegaly.
Palpate for Localized Motion
The patient should now lie down so that all four main cardiac areas can be palpated. The examiner uses his or her fingertips to assess any localized motion. This technique is demonstrated in Figure 11-28.
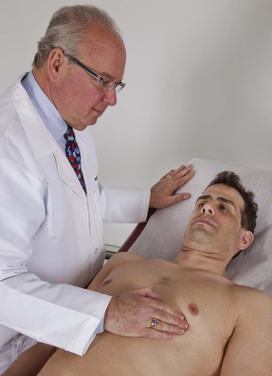
Figure 11–28 Technique for assessing localized cardiac motion.
The presence of a systolic impulse in the second intercostal space to the left of the sternum is suspect for pulmonary hypertension. This impulse is caused by the closure of the pulmonic valve under increased pressure. The presence of this impulse is suggestive of a dilated pulmonary artery, but it may also be felt in thin individuals without pulmonary hypertension.
Palpate for Generalized Motion
After the chest has been palpated with the fingertips, the examiner uses the proximal portion of his or her hand to palpate for any large area of sustained outward motion, called a heave or lift. The examiner again palpates each of the four main cardiac areas. The technique for assessing heaves is demonstrated in Figure 11-29. The presence of a right ventricular rock, which is a sustained left parasternal impulse associated with lateral retraction, is suggestive of a large right ventricle.
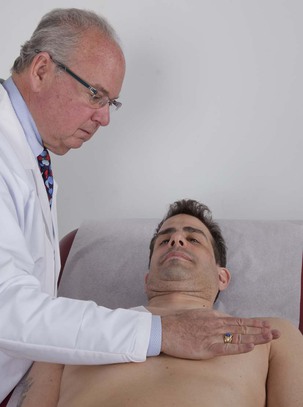
Figure 11–29 Technique for assessing generalized cardiac motion.
Any condition that increases the rate of ventricular filling during early diastole can produce a palpable impulse that occurs after the main left ventricular impulse. This second impulse in the area of the PMI is usually felt in association with an S3. Frequently, an S3 is more easily felt than heard.
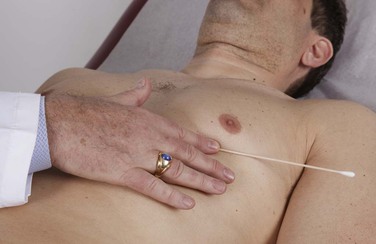
The use of a tongue blade or an applicator stick can be helpful to reinforce visually what has been palpated. The tip of the stick is placed directly over the area and held in place by the examiner’s finger. This acts as a fulcrum, and the motions tend to be magnified by the movement of the stick. The technique is demonstrated in Figure 11-30.
Palpate for Thrills
Thrills are the superficial vibratory sensations felt on the skin overlying an area of turbulence. The presence of a thrill indicates a loud murmur. Thrills are best felt by using the heads of your metacarpal bones rather than the fingertips and applying very gentle pressure on the skin. If too much pressure is applied, thrills are not felt. The palpation of thrills is generally of little importance because auscultation reveals the presence of the loud murmur that has produced the thrill. Therefore the finding of a thrill adds little to the diagnosis, but it is an interesting physical sign to alert the examiner as to what will be heard.
Auscultation
The Technique
Proper auscultation requires a quiet area. Every attempt should be made to eliminate extraneous noise from radios, televisions, and so forth. The earpieces of the stethoscope are directed anteriorly or parallel to the direction of the external auditory canal. If the earpieces are put in backward, the openings of the earpieces impinge on the wall of the external canal and lower the intensity of the sounds. The earpieces should fit properly so as to be comfortable but tight enough to exclude external noises.
It is often useful for you to close your eyes when listening to the heart. Sounds that are more difficult to hear sound louder with your eyes closed, because the brain is flooded with all types of sensory input when the eyes are open. The input from the eyes appears to be the most important. The next important sensory input is auditory, which is followed by tactile input. If you eliminate the distraction of visual stimuli, the brain concentrates more on the auditory input, and the sounds become more evident.
As indicated in Chapter 10, The Chest, the bell of the stethoscope should be applied lightly to the skin, whereas the diaphragm should be pressed tightly against the skin. High-pitched sounds, such as valve closure, systolic events, and regurgitant murmurs, are better heard with the diaphragm. Low-pitched sounds, such as gallop rhythms or the murmur of AV stenosis, are better heard with the bell.
It is common in many countries to examine patients through their clothing or a hospital gown. In the United States, however, never listen through any type of clothing.
There are several other pitfalls in auscultation. Make sure that the stethoscope is in good shape: Cracked tubing certainly interferes with good listening. Both the examiner and the patient must be comfortable for the best hearing. An examiner who is straining over the patient and is uncomfortable will want to finish the examination quickly without a proper assessment. Always inspect and palpate before auscultation. Accumulate as much information as possible before listening.
Auscultate the Cardiac Areas
The examiner should be on the right side of the patient while the patient lies flat on the back. If not already at the proper height, the bed should be adjusted so that the examiner is comfortable. The examiner should listen in the aortic, pulmonic, tricuspid, and mitral areas. However, the examiner should not limit auscultation to these areas alone. The examiner should start at any area and move the stethoscope gradually over the precordium from area to area. The areas have been established to provide some degree of standardization.
While listening at the apex and LLSB with the bell, the examiner should determine whether an S3 or an S4 is present.
Cardiac murmurs may radiate widely. The examiner should determine where the sounds are loudest or best heard. There are no acoustic walls in the chest. A murmur typically heard at the apex with radiation to the axilla may be heard in the neck, if it is loud enough. The murmur in this example is probably loudest at the apex and axilla.
The Standard Auscultation Positions
The four standard positions for auscultation are shown in Figure 11-31. They are as follows:
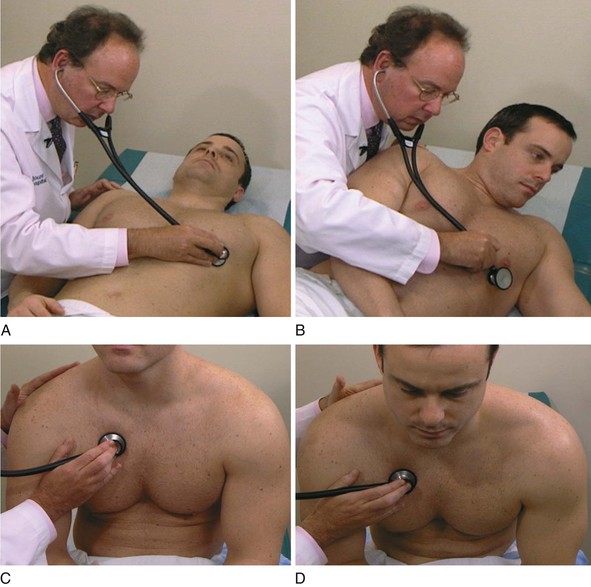
Figure 11–31 Positions for auscultation. A, The supine position, used for listening to all areas. B, The left lateral decubitus position, used for listening with the bell in the mitral area. C, The upright position, used for listening to all areas. D, The upright, leaning-forward position, used for listening with the diaphragm at the base positions.
All precordial areas are examined while the patient is supine. Using a systematic approach, the examiner starts at either the aortic area or the apex and carefully listens to the heart sounds. After all areas are examined, the patient is then instructed to turn onto the left side. The examiner should now listen at the apex for the low-pitched diastolic murmur of mitral stenosis, which is best heard with the bell of the stethoscope. Then the patient sits upright, and all areas are examined with the diaphragm of the stethoscope. Finally, the patient sits up and leans forward. The patient is asked to exhale and hold his or her breath while the examiner, using the diaphragm, listens for the high-pitched diastolic murmur of aortic regurgitation at the right and left second and third intercostal spaces.
The Influence of Breathing
The examiner should pay special attention to the influence of breathing on the intensity of heart sounds. Most murmurs or sounds originating in the right side of the heart are accentuated with inspiration. This is related to the increased return of blood that occurs with inspiration and the resultant increased right ventricular output. In addition, an S3 or an S4 originating in the right side of the heart is accentuated during inspiration.
Time the Cardiac Events
To interpret heart sounds accurately, the examiner must be able to time the events of the cardiac cycle. The most reliable way of identifying S1 and S2 is to time the sounds by palpating the carotid artery. While the examiner’s right hand is positioning the stethoscope, the left hand is placed on the patient’s carotid artery. This technique is demonstrated in Figure 11-32. The sound that precedes the carotid pulse is the S1. The S2 follows the pulse. The carotid, not the radial pulse, must be used. The time delay from S2 to the radial pulse is significant, and errors in timing will result.
Figure 11–32 Technique for timing the heart sounds.
Approach to Careful Auscultation
Until the examiner gains expertise in cardiac examination, heart sounds should be evaluated in the manner suggested in Table 11-7. The examiner should take time in each area before continuing on to the next area. The examiner should listen to several cardiac cycles at each position to be certain of the observations made, which include respiratory effects.
Table 11–7
Approach to Cardiac Auscultation
| Position | Evaluate |
| Supine | S1 in all areas S2 in all areas Systolic murmurs or sounds in all areas |
| Left lateral decubitus | Diastolic events at apex with bell of stethoscope |
| Upright | S1 in all areas S2 in all areas Systolic murmurs or sounds in all areas Diastolic murmurs or sounds in all areas |
| Upright, leaning forward | Diastolic events at base with diaphragm of stethoscope |
Describe Any Murmurs Present
If a murmur is present, attention should be directed to the following features:
Timing of murmurs as to systole and diastole is paramount. Does the systolic murmur begin with, or after, S1? Does it end before, with, or after S2? Does the murmur occupy the entire systolic period? Murmurs occurring throughout systole are termed holosystolic or pansystolic. These murmurs begin with S1 and end after S2. A systolic ejection murmur begins after S1 and ends before S2. Does the murmur occur only in early systole, midsystole, or late systole? Does the murmur persist throughout the entire diastolic period? Such murmurs are termed holodiastolic.
In which area is the murmur best heard?
The radiation of the murmur can provide a clue as to its cause. Does it radiate to the axilla? The neck? The back?
The intensity of a murmur is graded from I to VI, based on increasing loudness. The following grading system, although antiquated, serves as a means of communicating the intensity of the murmur:
I: Lowest intensity, often not heard by inexperienced listeners
II: Low intensity, usually audible to inexperienced listeners
III: Medium intensity without a thrill
IV: Medium intensity with a thrill
V: Loudest murmur that is audible when the stethoscope is placed on the chest; associated with a thrill
VI: Loudest intensity: audible when stethoscope is removed from chest; associated with a thrill
Murmurs can be described, for example, as “grade II/VI,” “grade IV/VI,” or “grade II–III/VI.” Any murmur associated with a thrill must be at least a grade IV/VI. A grade IV/VI murmur is louder than a grade II/VI murmur only because there is more turbulence; both or neither may have clinical significance. The “/VI” is used because there is another, less popular, grading system involving only four categories. An important axiom to remember is the following:
Describe Any Pericardial Rubs
Friction rubs are extracardiac sounds of short duration that have a unique quality similar to the sound of scratching on sandpaper. Rubs may result from irritation of the pleura (i.e., a pleural rub) or of the pericardium (i.e., a pericardial rub). Pericardial rubs typically have three components: one systolic and two diastolic. The systolic component occurs during ejection; the two diastolic components occur during rapid filling and atrial contraction. Pericardial rubs are best heard with the patient sitting while holding breath in expiration. Patients with pericardial rubs commonly have chest pain that is lessened by sitting forward. A rub that disappears while the patient holds the breath originates from the pleura.
The Goals of Auscultation
The goals at the end of auscultation are to be able to describe the following:
• The intensity of S1 in all areas
• The intensity of S2 in all areas
With experience, the examiner is able to listen to all parts of the cardiac cycle in one area and compare the sounds and events with those of other areas. Normally, S1 is loudest at the apex, and S2 is loudest at the base. Splitting of S2 into A2 and P2 during inspiration is best heard at the pulmonic area with the patient lying on his or her back. This increases venous return and widens the A2–P2 split.
Examination for Edema
When peripheral venous pressure is high, as in congestive heart failure, pressure in the veins is distributed in a retrograde manner to the smaller vessels. Transudation of fluid occurs, and edema of dependent areas results. This increase in tissue fluid produces edema that “pits.” Dependent edema occurs most commonly in patients with heart, kidney, or liver failure, as well as in patients who have received excessive intravenous fluids.
Test for Edema
To test for pitting edema, the examiner presses his or her fingers into a dependent area, such as the patient’s shin, for 2 to 3 seconds. If pitting edema is present, the fingers sink into the tissue, and when the fingers are removed, the impression of the fingers remains. This technique is demonstrated in Figure 11-33.
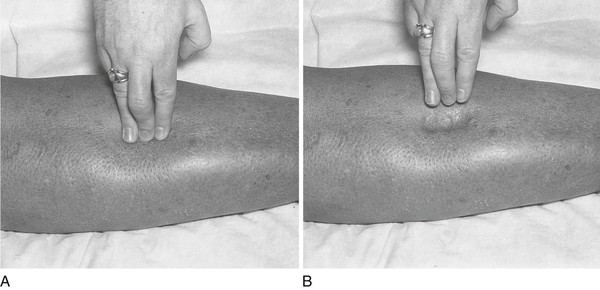
Figure 11–33 Technique for testing for pitting edema. A, The examiner presses into the patient’s shin area. B, When pitting edema is present, indentation occurs after the fingers are lifted.
Pitting edema is usually quantified from 1+ to 4+, depending on the depth and how long the indentation persists. The grading for pitting edema is:
• 1+ roughly 2 mm in depth; disappears rapidly,
• 2+ roughly 4 mm in depth; disappears in 10 to 15 sec
• 3+ roughly 6 mm in depth; may last more than 1 minute; dependent extremity swollen
• 4+ 8 mm pit or deeper, may last 2 to 5 min; dependent extremity grossly distorted
In patients who are bedridden, the dependent area is usually the sacrum and not the shins. The examiner should evaluate for edema over the sacrum in these patients. Figure 11-34 illustrates a 4+ sacral edema in a bedridden patient.
Clinicopathologic Correlations
The metabolic syndrome is a combination of medical risk factors that creates an increased risk for developing both CVD and diabetes (see also Chapter 29, Assessment of Nutritional Status). According to the American Heart Association and the National Heart, Lung, and Blood Institute, five risk factors make up metabolic syndrome. They are increased blood pressure, a high blood sugar level, excess body fat around the waist (central obesity), and abnormal cholesterol and triglyceride levels. The metabolic syndrome is present if the patient has three or more of the following signs:
• Blood pressure equal to, or greater than, 130/85 mm Hg
• Fasting blood sugar equal to, or higher than, 100 mg/dL
• Large waist circumference (length around the waist):14
▪ Men: 40 inches or more (102 cm or more)
▪ Women: 35 inches or more (88 cm or more)
According to the American Heart Association, 47 million Americans have the metabolic syndrome: that’s approximately one out of every six people. The syndrome runs in families and is more common among African Americans, Hispanics, Asians, and Native Americans. The risks of developing metabolic syndrome increases as one ages. The prevalence of this syndrome in the United States is approximately 34% in adults 20 years or older.
The discussion now focuses on the pathologic changes that result in the following auscultatory events:
Abnormalities of the First Heart Sound
The factors that are responsible for the intensity of S1 are as follows:
When the AV valve stiffens as a result of fibrosis or calcification, its closure is louder. The pathologically deformed valve of mitral stenosis produces an accentuated or louder S1. After many years, as the valve becomes increasingly calcified, it becomes unable to move, causing S1 to soften.
The position of the valve at the time of ventricular contraction affects the intensity of S1. The arc of coaptation is the angle through which the valve closes. If the valve is in a midposition, it travels less than when it closes from a widely opened position. The more it is opened, the wider the arc of coaptation, and the louder is S1. This situation is directly related to the pressure in the left atrium at the moment that the left ventricular pressure exceeds it and closes the valve. This can occur in conditions in which there is a shortened PR interval on the electrocardiogram. The mitral valve is opened normally during diastole for ventricular filling. The P wave of the electrocardiogram corresponds to atrial contraction, which elevates left atrial pressure (the “a” wave of the left atrial tracing), further opening the mitral valve in late diastole. If the PR interval is short, ventricular contraction occurs so quickly after atrial contraction that the atrial pressure is still high when the left ventricular pressure exceeds it. The mitral valve stays open longer and closes later than normal, during the rapid rate of rise of pressure of the ventricle, which accentuates S1.
In general, the longer the PR interval, the softer the S1. Lengthening of the PR intervals, as is seen in Wenckebach’s phenomenon,15 produces an S1 that softens until the dropped beat occurs.
Whenever the heart is farther from the chest wall, the S1 is softer than normal. In patients who are very obese or who have chronic obstructive lung disease, the intensity of the S1 is softer than normal. In patients with a large pericardial effusion, the S1 is likewise soft.
Abnormalities of the Second Heart Sound
Abnormalities of the Intensity of the Second Heart Sound
The conditions that change the intensity of S2 are the following:
Any condition that produces an increase in the systolic pressure increases the intensity of the S2. Conversely, conditions that lower the systolic pressure soften the S2. Hypertension raises aortic systolic pressure and produces a loud A2 component of S2.
Calcification or fibrosis of the semilunar valves produces a softening of their closure, S2. Because the semilunar valves are a morphologically different type of valve, fibrosis does not cause an increased intensity, as in closure of a fibrotic AV valve.
Abnormalities of Splitting of the Second Heart Sound
Normal physiologic splitting of S2 was discussed in the section, “The Cardiac Cycle.” This section deals with abnormalities of splitting.
Any condition that delays right ventricular systole, either electrically or mechanically, delays P2 and produces a widened splitting of S2. Right ventricular emptying is delayed by a right bundle branch block or pulmonic stenosis. The pulmonic component of S2 is delayed during both inspiration and expiration, and wide splitting of S2 occurs.
Any condition that shortens left ventricular systole allows A2 to occur earlier than normal, and wide splitting likewise occurs. Conditions such as mitral regurgitation, ventricular septal defect, and PDA shorten left ventricular systole, and the S1-A2 interval is shorter than normal. In these conditions, there is a “double outlet” to the left ventricle, and systole therefore is shorter. In a ventricular septal defect, with a left-to-right shunt, not only is left ventricular systole shorter but also right ventricular systole is prolonged. Both factors are crucial in producing the wide splitting of S2.
Any condition, either electrical or mechanical, that delays left ventricular emptying produces paradoxical splitting of S2. Left bundle branch block or aortic stenosis delays left ventricular emptying. These conditions delay the closure of the aortic valve after right ventricular systole and P2 have occurred. The normal sequence of A2-P2 is reversed. During inspiration, P2 moves normally away from S1 toward A2. The split is said to be narrowed. With expiration, P2 moves normally and approaches S1; the P2-A2 split widens. This widening during expiration is paradoxical. Other conditions, such as left ventricular failure and severe hypertension, delay left ventricular ejection and cause paradoxical splitting of S2.
Fixed splitting of S2 is the auscultatory hallmark of an atrial septal defect. In this situation, the split is wide and does not change with respiration. This is because inspiratory increases in venous return to the right atrium normally raise its pressure. During expiration, the right atrial pressure is lower, but the left-to-right atrial shunt keeps the volume in the right atrium constant during respiration; therefore, normal splitting does not occur.
Normal physiologic splitting of the second heart sound and abnormalities of splitting are illustrated in Figure 11-35.
Systolic Clicks
Ejection clicks are high-pitched sounds that occur early in systole at the onset of ejection and are produced by the opening of pathologically deformed semilunar valves. Pulmonic or aortic stenosis may produce ejection clicks. The sounds are short and have the quality of a “click.” Pulmonic ejection clicks are best heard at the pulmonic area, and aortic ejection clicks are heard at the aortic area. As calcification progresses, the mobility of the valve decreases, and the ejection click disappears.
Midsystolic clicks are not ejection clicks. They occur in the middle of systole. They may be single or multiple, and they may change in position during the cardiac cycle with various maneuvers that change ventricular geometry. The most common condition associated with a midsystolic click is prolapse of the mitral or tricuspid valve.
Diastolic Opening Snaps
The opening of an AV valve is normally silent and occurs approximately 100 msec after S2. This is about as long as it takes to say “ma-ma” quickly. An opening snap is a diastolic event that is the sound of the opening of a pathologically deformed AV valve. The sound is sharp and high-pitched. The mitral opening snap of mitral stenosis occurs after A2; the tricuspid opening snap of tricuspid stenosis occurs after P2.
The interval between S2 and the opening snap is termed the S2-OS interval and has specific significance as to the severity of the stenosis. As mitral stenosis worsens, the resistance and obstruction to flow increase. Pressure in the left atrium increases, and the gradient across the mitral valve increases. The mitral valve, therefore, opens earlier than normal when the left ventricular pressure falls below left atrial pressure. The S2-OS time shortens as the severity increases. Try saying “ma-da” as quickly as possible. This interval is about 50 to 60 msec and approximates a very short S2-OS interval, as heard in severe mitral stenosis.
Murmurs
Murmurs are produced when there is turbulent energy in the walls of the heart and blood vessels. Obstruction to flow or flow from a narrow vessel to a large-diameter vessel produces turbulence. Turbulence sets up eddies that strike the walls to produce vibrations that the examiner recognizes as a murmur. Murmurs can also be produced when there is a large volume of blood through a normal opening. In this circumstance, the normal opening is relatively stenotic for the increased volume. “Blowing” murmurs are produced by large gradients with variable flow volumes. “Rumbling” murmurs result from areas of small gradients that depend on flow. “Harsh” murmurs result from large gradients and high flow.
An ejection murmur is a murmur produced by turbulence across a semilunar valve during systole, such as in aortic stenosis or pulmonic stenosis. Ejection murmurs appear diamond-shaped and are described as “crescendo-decrescendo.” They begin slightly after S1 and end before S2. An ejection click from stenosis of the semilunar valve may precede the murmur. These murmurs are medium-pitched and are best heard with the diaphragm of the stethoscope. Because they are based on flow, the intensity of these murmurs does not indicate the degree of severity. Increased flow across a minimally narrowed aortic valve produces a loud murmur; decreased flow across a severely stenotic aortic valve may produce a barely audible murmur. Any increase in flow or volume may produce an ejection murmur even in the presence of a normal valve. An ejection murmur as a sign of aortic stenosis is a finding of high sensitivity but low specificity. Figure 11-36 illustrates an ejection murmur.
Regurgitant systolic murmurs are produced by retrograde flow from a higher pressure area to a lower pressure area during systole, such as in mitral or tricuspid regurgitation. These murmurs are holosystolic or pansystolic. They begin with S1 and end after S2. They extend past S2 because ventricular pressure is higher than atrial pressure, even after the closure of the semilunar valve. An S3 indicative of volume overload to the ventricle is often heard. These murmurs are high-pitched and are best heard with the diaphragm of the stethoscope. The terms regurgitation, incompetence, and insufficiency are often used synonymously for this type of murmur. The preferred term is regurgitation because it implies the retrograde direction of flow. The holosystolic murmur of AV valve regurgitation is a finding of high sensitivity. Figure 11-37 illustrates a regurgitant murmur.
Diastolic AV murmurs begin after S2 with the opening of the AV valve. Mitral stenosis and tricuspid stenosis are examples of this type of murmur. There is a pause between S2 and the beginning of the murmur. Isovolumetric relaxation is occurring during this period. The murmur is decrescendo in shape, beginning with an opening snap, if the valve is mobile. These murmurs are low-pitched and are best heard with the bell of the stethoscope, with the patient lying in the left lateral decubitus position. Because the AV valve is stenotic, rapid filling does not occur, and a gradient persists throughout diastole. If the patient is in normal sinus rhythm, atrial contraction increases the gradient at the end of diastole, or presystole, and there is an increase in the murmur at this time. The diastolic AV murmur is a sensitive and specific sign of AV valve stenosis.
The first heart sound is the loudest sound. The cadence and emphasis of the sounds are best heard by saying the following mnemonic:
in which OS is the mitral opening snap, and DM is the diastolic murmur. The “aaaalve” is the presystolic accentuation heard when a patient with mitral stenosis is in normal sinus rhythm. Figure 11-38 illustrates a diastolic AV murmur.
Diastolic semilunar murmurs begin immediately after S2, as heard in aortic or pulmonic regurgitation. In contrast to the diastolic murmurs, there is no delay after S2 to the beginning of the murmur. The high-pitched murmur is decrescendo in shape and is best heard with the diaphragm of the stethoscope while the patient is sitting up and leaning forward. A diastolic semilunar murmur is a sign of low sensitivity but high specificity. Figure 11-39 illustrates the pressure curves responsible for the generation of a diastolic semilunar murmur.
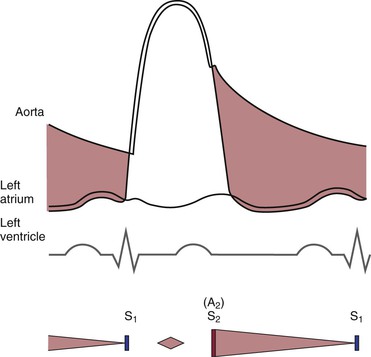
Figure 11–39 A diastolic semilunar murmur such as that occurring in aortic regurgitation. Note the systolic ejection murmur, which is related to the increased volume and flow.
Table 11-8 lists important cardiac sounds according to the cardiac cycle. Figure 11-40 lists important characteristics of the systolic murmurs of aortic stenosis and mitral regurgitation. Table 11-9 summarizes the differentiation of some additional systolic murmurs. Figure 11-41 lists important characteristics of the diastolic murmurs of mitral stenosis and aortic regurgitation.
Table 11–8
Cardiac Sounds
| Cardiac Cycle | Sound |
| Early systolic | Ejection click Aortic prosthetic valve opening sound* |
| Midsystolic to late systolic | Midsystolic click Rub |
| Early diastolic | Opening snap S3 Mitral prosthetic valve opening sound† Tumor plop‡ |
| Mid-diastolic | S3 Summation gallop§ |
| Late diastolic (sometimes called presystolic) | S4 Pacemaker sound |
* Opening and closure of the prosthetic aortic valve are heard with many prosthetic valves. The opening is comparable to an ejection click; the closing is a “prosthetic” S2.
† Opening and closure of the prosthetic mitral valve are heard with many prosthetic valves. The opening is comparable to an opening snap; the closing is a “prosthetic” S1.
‡ A left atrial myxoma that is pedunculated may “plop” in and out of the mitral annulus, simulating the auscultatory signs of mitral stenosis.
§ At fast heart rates, the diastolic period shortens. If an S3 and S4 are present, the sounds may be summated into a single sound called a summation gallop.
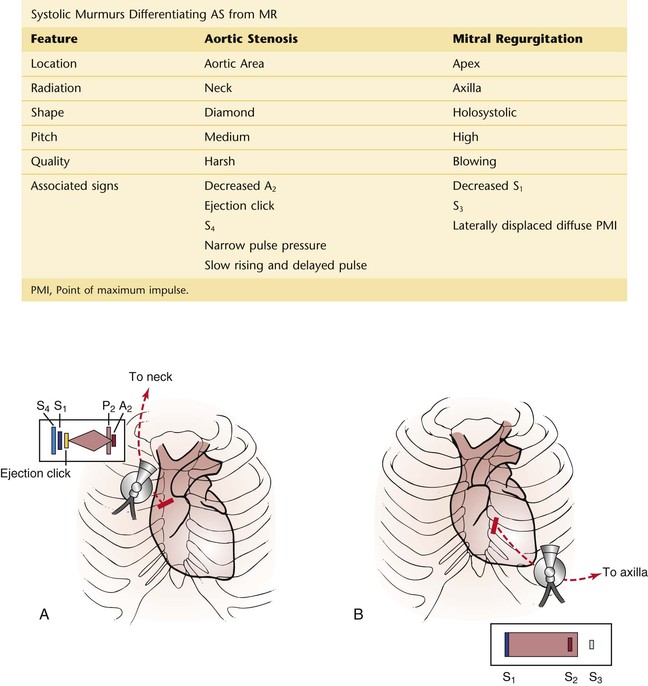
Figure 11–40 Systolic murmurs. A, Pathophysiology of aortic stenosis. Note the paradoxical splitting of the second heart sound (S2), the S4, and the ejection click. B, Mitral regurgitation. Note that the murmur ends after S2, and note the presence of the S3.
Table 11–9
Differentiation of Other Systolic Murmurs
* Usually between the apex and the left lower sternal border.
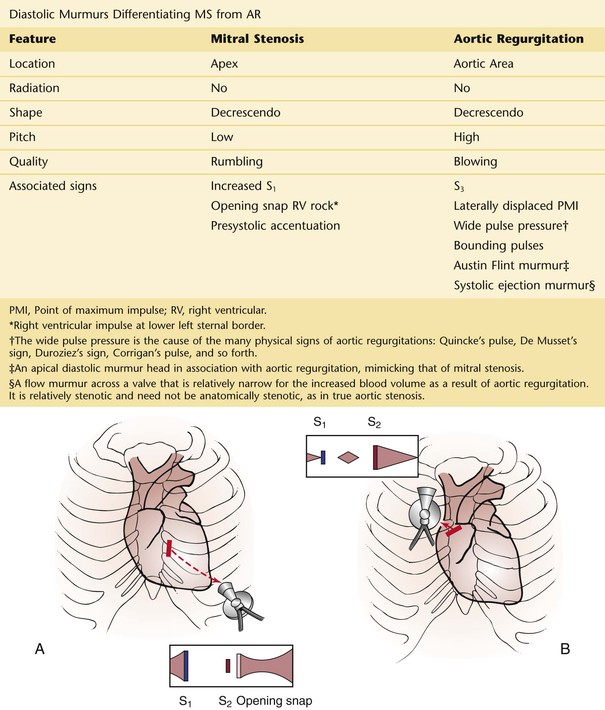
Figure 11–41 Diastolic murmurs. A, Pathophysiology of mitral stenosis. Note the intensity of S1 and the accentuation of the diastolic murmur in late diastole. B, Aortic regurgitation. Note the systolic flow murmur.
The bibliography for this chapter is available at studentconsult.com.
Bibliography
Alberti KG, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention: National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circ. 2009;120(16):1640.
Bonow RO, et al. Braunwald’s heart disease: a textbook of cardiovascular medicine. ed 9. Saunders: Philadelphia; 2012.
Centers for Disease Control and Prevention. Million hearts: strategies to reduce the prevalence of leading cardiovascular disease risk factors: United States, 2011. MMWR. 2011;60(36):1248.
Chiuve SE, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306:62.
Cook DJ, Simel DL. Does this patient have abnormal central venous pressure? JAMA. 1996;275:630.
Ducas J, Magder S, McGregor M. Validity of the hepatojugular reflux as a clinical test for congestive heart failure. Am J Cardiol. 1983;52:1299.
Dworkin LD, Cooper CJ. Renal-artery stenosis. N Engl J Med. 2009;361:1972.
Eilen SD, Crawford MH, O’Rourke RA. Accuracy of precordial palpation for detecting increased left ventricular volume. Ann Intern Med. 1983;99:628.
Elliott WJ. Ear lobe crease and coronary artery disease. Am J Med. 1983;75:1024.
Ewy G. The abdominojugular test: technique and hemodynamic correlates. Ann Intern Med. 1988;109:456.
Heckerling PS, et al. Accuracy of precordial percussion in detecting cardiomegaly. Am J Med. 1991;91:328.
Heckerling PS, et al. Accuracy and reproducibility of precordial percussion and palpation for detecting increased left ventricular end-diastolic volume and mass. JAMA. 1993;270:1943.
Henkind SJ, Benis AM, Teichholz LE. The paradox of pulsus paradoxus. Am Heart J. 1987;114:198.
Hurst JW, et al. Hurst’s the heart. ed 13. McGraw Hill: New York; 2011.
Johnson R, Swartz MH. A simplified approach to electrocardiography. WB Saunders: Philadelphia; 1986.
Kochanek KD, et al. Deaths: final data for 2009 [PDF-2M]. Natl Vital Stat Rep. 2011;60(3).
Koh H. A 2020 vision for healthy people. N Engl J Med. 2010;362:1653.
Kroenke MK. Sphygmomanometry: the correct arm position. West J Med. 1984;140:459.
Lin JS, et al. Behavioral counseling to promote physical activity and a healthful diet to prevent cardiovascular disease in adults: a systematic review of the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:736.
Maisel AS, Atwood JE, Goldberger AL. Hepatojugular reflux: useful in the bedside diagnosis of tricuspid regurgitation. Ann Intern Med. 1984;101:781.
Markel H. The stethoscope and the art of listening. N Engl J Med. 2006;354:551.
McGee S. Etiology and diagnosis of systolic murmurs in adults. Am J Med. 2010;123:913.
Myers MG, et al. Measurement of blood pressure in the office-recognizing the problem and proposing the solution. Hypertension. 2010;55:195.
Parikh NI, et al. A risk score for predicting near-term incidence of hypertension: the Framingham Heart Study. Ann Intern Med. 2008;148:102.
Pickering TG, et al. Franz Volhard Lecture: should doctors still measure blood pressure? The missing patients with masked hypertension. J Hypertension. 2008;26:2259.
Pryor DB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118:81.
Roger VL, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220.
Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emer Med. 2008;9:201.
Schouten VW, et al. Erectile dysfunction prospectively associated with cardiovascular disease in the Dutch general population: results from the Krimpen Study. Int J Impotence Res. 2008;20:92.
Silverberg DS, Shemesh E, Iaina A. The unsupported arm: a cause of falsely raised blood pressure readings. BMJ. 1977;2:1331.
Simel DS. Time, now, to recover the fun in the physical examination rather than abandon it. Arch Intern Med. 2006;166(6):603.
Sinisalo J, et al. Simplifying the estimation of jugular venous pressure. Am J Cardiol. 2007;100:1779.
U.S. Preventative Services Task Force. Aspirin for the primary prevention of cardiovascular disease. A U.S. Preventative Services Task Force recommendation statement. Ann Intern Med. 2009;150:396.
U.S. Preventative Services Task Force. Screening for high blood pressure: U.S. Preventative Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2007;147:783.
U.S. Preventative Services Task Force. Screening for lipid disorders in adults. June 2008. [Available at] http://www.uspreventiveservicestaskforce.org/uspstf/uspschol.htm [Accessed January 30, 2013] .
1 In general, it is best to allow the patient to describe the character of the pain. These descriptions are provided for the interviewer to use only when the patient is unable to characterize the pain.
2 Symptoms associated with a pheochromocytoma.
3 After an attack of paroxysmal atrial tachycardia, patients often have an urge to urinate. The pathophysiologic process is not well understood, but the association is present.
4 The use of this technical term is appropriate because a patient having this form of preexcitation may have been told of this condition and may recognize the name.
5 Be careful about the use of abbreviations. Although PND is used often to abbreviate paroxysmal nocturnal dyspnea, the abbreviation can be used also to indicate postnasal drip or postnasal discharge.
6 The desirable total cholesterol concentration for an adult is normally less than 200 mg/dL. For patients with coronary artery disease, the desirable level is less than 170 mg/dL.
7 The desirable total triglyceride concentration for an adult is normally less than 150 mg/dL.
8 Short stature, retarded sexual development, and webbed neck in a female patient, associated with an abnormality of the sex chromosomes (45, XO).
9 Male Turner’s syndrome (46, XY).
10 A murmur is a blowing auscultatory sign produced by turbulence in blood flow. These vibrations can originate in the heart or in blood vessels as a result of hemodynamic changes.
11 The normal end-expiratory right atrial pressure is approximately 3 to 7 mm Hg.
13 Low-frequency cutaneous vibrations associated with loud heart murmurs.
14 For Asian Americans, the cutoff values are 35 inches (equal or greater than 90 cm) in men or 32 inches (equal or greater than 80 cm) in women
15 Gradually increasing PR intervals until a dropped beat occurs.