Chapter 25 The Future of Neurostimulation
Introduction
Neurostimulation has undergone significant advancements since Shealy implanted the first spinal cord column stimulator in 1967.1 Predictions about the future of neuromodulation have been made previously but often fall short of reality since it is very difficult to comprehend the direction and magnitude of technology that will be available in the future.2 Today most of the impulse generators that power spinal cord stimulation (SCS) systems approximate the size of cardiac pacemakers and are rechargeable. Many advances have been made in miniaturization, which has afforded physicians both greater flexibility and better outcomes in neurostimulation. Since many of the new technologies currently under development are proprietary, of these new neurostimulation devices are discussed in generalizations. Although at times it is difficult to comment on specific innovations, the body of work and product development in this arena is impressive and ongoing.
Expanding Applications of Neurostimulation
Traditionally neurostimulation has been used in the treatment of chronic pain syndromes such as lumbar and cervical radiculopathy, failed back surgery syndrome, arachnoiditis, complex regional pain syndromes, and other neuropathic syndromes. Today the role of neurostimulation is expanding to include treatment of vascular disease, cardiovascular disease, refractory migraine headaches, chronic pancreatitis, visceral pain, and urological syndromes such as pelvic pain, rectal pain, bladder and fecal incontinence, and erectile dysfunction.3 In addition, deep brain stimulation has been used effectively to treat central nervous system disorders such as Parkinson disease, and vagal nerve stimulation has been used to treat gastroparesis. Motor cortex stimulation is another exciting area that has shown promise in complex pain patterns of the head and neck.4
Advancements in Neurostimulation Electrodes
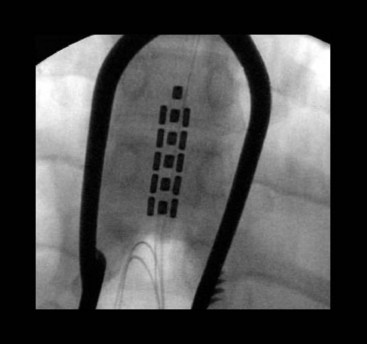
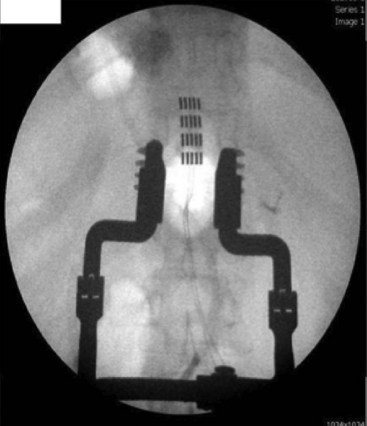
The development of multicolumn paddle laminotomy lead systems and octrode percutaneous lead systems has revolutionized the treatment of axial low back pain. Many new paddle constructs have three to five columns that allow for selective depolarization of specific spinal structures (Figs. 25-1 and 25-2). This allows for more tailored stimulation of the patient based on complex pain patterns. A new generation of percutaneous leads is currently under development that will provide physicians with even greater versatility in the treatment of neuropathic pain syndromes (Table 25-1). In addition, percutaneous multilead systems are currently under development that will offer the advantages of a laminotomy lead without the need for an invasive surgical dissection for placement. The use of a percutaneous delivery tool for a paddle construct is now approved in Europe and should be seen in the United States in the near future (Fig. 25-3). The improved efficacy and treatment options for SCS coupled with new risks identified for intrathecal drug delivery systems have led to a new interest in using stimulation earlier in the treatment algorithm.5 SCS has now moved ahead of long-term chronic opioids, reoperation for spinal disorders, and intrathecal drug delivery in patients with neuropathic and mixed pain of the low back and lower limbs.
Table 25-1 Comparison of Available and Possible Future Neuromodulation Devices
| Available | Now | Future |
|---|---|---|
| Percutaneous leads | 8 Contacts | 64 + |
| Tripole leads | Surgical laminectomy | Percutaneous tripole |
| BION | 2 Contacts | More ± high frequency |
| Low frequency | 30 Hz-5 kHz | 40 kHz + |
| Wireless generator | Microtransponder | Wireless couple with Bluetooth devices (iPod or cell phone) |
| Rechargeable generator | 10-year life | Infinite recharging capacity |
| SNRS | Uses quad or octrodes | Intraspinal placement of Microstim devices with wireless capability |
Microstimulation devices are under development that can be placed directly over damaged peripheral nerves and used to treat neuropathic pain syndromes resulting from pathologies such as cerebral vascular accidents and carpel tunnel syndrome (Figs. 25-4 and 25-5). In addition, microstimulation devices have been used to treat migraine headaches that stem from C2 neuritis. Microstransponder is currently developing a microstimulation device that is a wireless neurostimulation system named SAINT (subcutaneous arrangement of implantable neural transponders). The system is purported to eliminate the need for an implantable impulse generator using an external controller that powers the microimplant. Several microstimulation devices (i.e., the BION [Bioness, Valencia, Calif; Boston Scientific, Natick, Mass]) (Fig. 25-6) have fully rechargeable lithium ion batteries that obviate the need for an external power source.6
Advances in Technique and Targeting of Stimulation
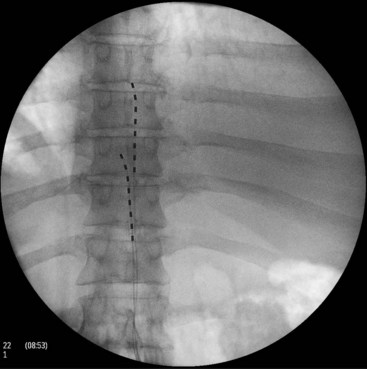
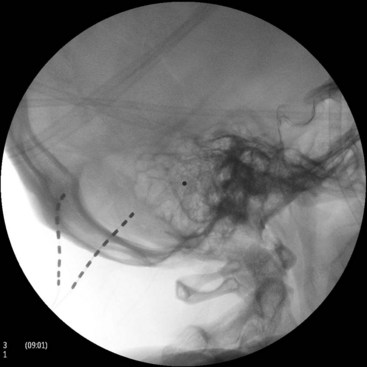
SCS of the posterior spinal column (Fig. 25-7), although effective in many chronic pain syndromes, does not provide adequate pain relief from many pain disorders that involve the peripheral nervous system. Peripheral nerve stimulation (PNS), or the selective targeting of damaged peripheral nerves, has been used successfully in the treatment of painful syndromes refractory to traditional SCS such as intercostal neuralgia and craniofacial pain. In addition, PNS allows for selective targeting of damaged nerves without stimulation of unwanted areas, which can be uncomfortable to many patients. Craniofacial pain has been especially refractory to SCS and is most commonly used to treat occipital and trigeminal nerve lesions (Figs. 25-8 and 25-9).7 With advances in microstimulation, it is likely that very selective stimulation will be achieved without many of the complications associated with traditional quadripole and octrode lead placement. Progressive novel uses of stimulation leads in craniofacial nerve lesions have shown great promises but have been complicated by erosion of the leads and anchors. In addition, tunneling of the leads to an area suitable for placement of the impulse generator requires extreme caution because of the proximity of vascular structures, which is not a concern during placement of the new microstimulation devices. New power generators will be available in the future that allow for remote supply of power to the microstimulation devices by using wireless coil generators.6 Combinations of peripheral and epidural lead placements with cross-talk between the leads can be used very effectively in the treatment of axial back pain and radiculopathy (Fig. 25-10). Alternatively, peripheral lead placement can be used alone for isolated axial back pain refractory to epidural stimulation (Fig. 25-11).
Spinal nerve root stimulation (SNRS), or the direct stimulation of the dorsal root, combines the advantages of both peripheral and dorsal column stimulation. As with dorsal column stimulation, SNRS allows for the intraspinal stabilization of the leads with the selectivity of peripheral nerve stimulation.7 There are two basic approaches to SNRS: (1) the leads are placed laterally over the selected dorsal root ganglions, or (2) the leads are placed transforaminally along the desired nerve roots. Some development is occurring in the latter form of SNRS with devices that allow more accurate delivery of transforaminal lead placement.
High-frequency stimulation represents an emerging field of neuromodulation. Neural blockade has been reported using high-frequency alternating currents (HFACs) that resulted in quick onset and reversibility and may represent another treatment alternative in treating pathological peripheral nerve injuries.7 Neuros is a company that is currently developing a device that stimulates from 5,000 Hz and up to 40,000 Hz compared to traditional SCS systems that stimulate at 30 to 100 Hz.8 This device will initially be tested in postamputation patients with residual limb pain and, in contrast to SCS, will actually block neural transmission from the severed nerves instead of masking the pain.9 High-frequency stimulation may ultimately find significant application in many peripheral nerve pain syndromes, including craniofacial pain. Combining the wireless power supply under development by Microtransponder with the high-frequency stimulation provided by Neuros may result in a significant expansion in the application of peripheral neuromodulation.
A microstimulator, the BION (Boston Scientific, Boston Mass) has been used successfully in the treatment of migraine headaches (see Fig. 25-6).10 The device has a cathode on one end and an anode on the other end with a programmable microchip and telemetry capability.10 Trentman and associates10 recently reported the results of a clinical trial using the BION in the treatment of refractory migraine headaches. Of the nine patients enrolled in the initial clinical trial, eight completed the follow-up at 1 year and were judged to have fair or better results in reduction of pain and disability, and five out of the eight had greater than 90% reduction in pain.10 In addition, the successful treatment of hemicrania using the BION device has been reported with success rates of 80% to 90% in a small cohort. Another microstimulation company, Bioness, is developing a device that can be used in the treatment of median neuropathies and in poststroke syndromes that result in painful peripheral neuropathic syndromes.11
Another emerging field in neurostimulation is burst stimulation. Traditional dorsal column stimulation results in the perception of paresthesias in the distribution of nerves affected by the neuropathic pain syndrome and often involves recruitment of nerve fibers that are not in the dermatomal distribution of pain. Some failed dorsal column stimulation trials result from either stimulation of unwanted areas or intolerance to the paresthesia that results from traditional dorsal column stimulation. A new technique of burst stimulation has been developed that uses a 40-Hz burst mode with five spikes of 500 Hz per burst.12 In one clinical trial with a small cohort, burst stimulation resulted in the perception of paresthesias in only 17% of patients, which was stable at 1-year follow-up.13
Advances in Imaging
New methods of imaging and expanded uses of traditional techniques in the placement of both intraspinal and peripheral neurostimulation devices are being developed. Huntoon and Burgher13 have successfully used ultrasound during placement of standard neurostimulation electrodes targeted to multiple peripheral nerves. The median, ulnar, radial, peroneal, and posterior tibial nerves were all successfully targeted and stimulated with standard electrodes using ultrasonography during the study. The advantages of ultrasound used in peripheral electrode placement include the direct visualization of the nerves and surrounding vascular structures without requiring the skeletonization of the targeted nerve.13 In addition to ultrasound, several companies are developing stereotactical imaging techniques that will allow for placement of both intraspinal and peripheral neurostimulation devices without the need for continued use of fluoroscopy and the inherent risks of radiation exposure.
1 Shealy NC, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns. Anes Analg Curr Res. 1967;46(4):489-491.
2 Deer TR. Current and future trends in spinal cord stimulation for chronic pain. Curr Pain Headache Rep. 2001;5(6):503-509.
3 Bernstein AJ, Peters KM. Expanding indications for neuromodulation. Urol Clin North Am. 2005;32:59-63.
4 Levy R, Deer TR, Henderson J. Intracranial neurostimulation for pain control: a review. Pain Physician. 2010;13(2):157-165.
5 Deer TR. A critical time for practice change in the pain treatment continuum: we need to reconsider the role of pumps in the treatment algorithm. Pain Med Editorial. 2010;11(7):987-989.
6 Personal communication. Oct 2009, Bioness Co.
7 Stewart MR, Winfree CJ. Neurostimulation techniques for painful peripheral nerve disorders. Neurosurg Clin North Am. 2009;20:111-120.
8 Ackerman DM, et al. Effect of bipolar cuff electrode design on block thresholds in high-frequency electrical neural conduction block. IEEE Trans Biomed Eng. 2009;17(5):469-476.
9 Stuart M. Device investors look for gains in pain. Start Up. Feb 1, 2010.
10 Trentman T, et al. Greater occipital nerve stimulation via the BION microstimulator: implantation technique and stimulation parameters. Pain Phys. 2009;12:621-628.
11 Burns B, Watkins L, Goadsby PJ. Treatment of hemicrania continua by occipital nerve stimulation with a BION device: long-term follow-up of a crossover study. Lancet Neurol. 2008;7:1001-1012.
12 De Ridder D, et al. Burst spinal cord stimulation: toward paresthesia-free pain suppression. Neurosurgery. 2010;66:986-990.
13 Huntoon M, Burgher AH. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: original cases and outcomes. Pain Med. 2009;10(8):1369-1377.