25 The future of botulinum toxin
Summary and Key Features
• The clinical use of BoNT-A continues to expand as physicians dream up new uses for it
• The use of BoNT-A topically will expand the use of the neurotoxin as well as helping us to better understand its effect on the skin and appendages
• Changes in the molecule may allow for increased duration of effect and also allow different targeting of the endoprotease, for example allowing a greater antinociceptive effect
• The question of a central effect of BoNT-A is still unresolved. There is no doubt that the toxin can move along neurons and can be demonstrated in the brain in experimental situations. Whether this will have any relevance to the clinical use of the toxin remains to be seen
• The use of glabellar BoNT-A in the treatment of depression is another exciting area for research
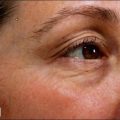
Topical versus injectable
We had long presumed that the large size of the BoNT-A molecule would prevent it from passing through intact skin. That assumption has been proven incorrect and the effectiveness of topically applied BoNT-A has now been established (see Ch. 10). This will have important implications beyond the current clinical development both in axillary hyperhidrosis and in lateral orbital wrinkles and lines (crow’s feet). We would expect that the advantage of a needleless approach will encourage many more individuals to have excessive sweating treated, especially that of the palms and soles, face, and scalp. Also, those individuals who have normal levels of sweating but who choose not to sweat in these areas can be safely and effectively treated.
Alam M, Barrett KC, Hodapp RM, et al Botulinum toxin and the facial feedback hypothesis: Can looking better make you feel happier? Journal of the American Academy of Dermatology 58: 1061–1072
Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. Journal of Neuroscience. 2008;28(14):3689–3696.
Blood AJ, Tuch DS, Makris N, et al. White matter abnormalities in dystonia normalize after botulinum toxin treatment. Neuroreport. 2006;17(12):1251–1255.
Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatologic Surgery. 2006;32:645–650.
Lawrence GW, Ovsepian SV, Wanj J, et al. Extra-vesicular intra-neuronal migration of internalised botulinum toxins without detectable inhibition of distal neurotransmission. Biochemical Journal. 2012;441:443–452.
Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. Journal of Cosmetic Dermatology. 2009;8:24–26.
Solomon S. Botulinum toxin for the treatment of chronic migraine: the placebo effect. Headache. 2011;51:980–984.
Stone HF, Zhu Z, Thai T, et al. Characterization of diffusion and duration of action of a new botulinum toxin type A formulation. Toxicon. 2011;58:159–167.