In humans the female reproductive system consists of paired ovaries and the reproductive tract comprising paired uterine tubes, a uterus and a vagina (Fig. 16.1). Prior to puberty, as in males, the reproductive system in females is quiescent. At puberty, hormones from the pituitary gland initiate developmental changes which are prerequisites for reproduction. In contrast to the male reproductive system, the female system after puberty in humans is regulated by hormones secreted by the anterior pituitary gland on a monthly, cyclical basis. The cycle of activity begins at puberty and is manifested by the onset of menstruation (the loss of blood, cells and secretions from the uterus which drain through the vagina). The time when menstruation begins is known as the menarche and it occurs between 9 and 15 years of age. During each monthly menstrual cycle female oocytes differentiate and usually a single, mature oocyte is shed from an ovary each month, a process described as ovulation. The ability to produce a haploid gamete (an ovum), ovulate, conceive, support one or more developing fetuses and give birth continues from puberty until the menopause. During the menopause, which may begin between 45 and 50 years of age, the menstrual cycles and ovulation become irregular and then cease. During and beyond the menopause the hormonal signals required to stimulate the cyclical activity of the ovaries and the uterus also cease but the factor(s) that cause this cessation are not understood.
Ovaries
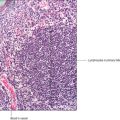
The ovaries are located in the pelvic cavity and are small (approximately 3 × 2 × 1.5 cm) and roughly ovoid in shape. Each ovary is attached by a stalk of double-layered membrane to the posterior surface of each side of the broad ligament (the peritoneal membrane surrounding the uterus) (
Fig. 16.1). Blood (and lymphatic) vessels and nerves pass to each ovary within this membrane. The ovary is divided into two main regions, an outer cortex and inner medulla, though the demarcation between the two is not well defined. A simple cuboidal epithelium, known as the germinal epithelium, covers the cortex of each ovary and is continuous with the peritoneal epithelium. This name arises from the erroneous supposition that ‘germ’ cells capable of becoming ova (the female gametes) developed from this layer. It is now known that the ‘germ’ cells which are the stem cells (oogonia) for female gametes develop in the embryonic yolk sac and migrate to the ovaries during fetal development in utero (see Mitchell B, Sharma R.
Embryology: An Illustrated Colour Text. Elsevier: 2004).
Cortex of the ovary
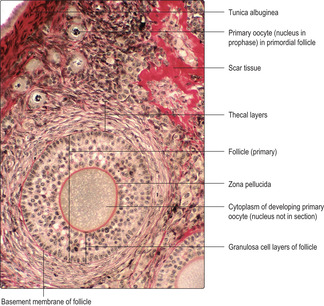
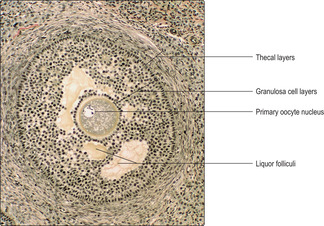
Immediately below the germinal epithelium is a thin layer of dense irregular connective tissue, the tunica albuginea. A connective tissue stroma containing some fibroblast-like cells supports structures in the cortex known as ovarian follicles (
Fig. 16.2). Ovarian follicles comprise an oogonium, or a developing oocyte, surrounded by epithelial cells. In human females, between puberty and the menopause, ovarian follicles vary widely in appearance and size as they are in various stages of development. Some stromal cells are irregularly distributed in whorls in the cortex and others lie around developing follicles as layers known as theca (
Fig. 16.2). Some of the fibroblast-like cells differentiate and are able to secrete steroid hormones.
Oogenesis and ovarian follicle development
In utero and prepubertal stages
In a developing female embryo from about the sixth week in utero, primordial, diploid oogonia migrate from the yolk sac and enter the developing ovary. From this time oogonia undergo mitosis and produce more oogonia. By about the fifth month of fetal life each ovary contains 5–7 million oogonia and migration of oogonia from the yolk sac stops. In contrast to spermatogonia, which continue to act as stem cells for spermatozoa production throughout life in males, mitosis of oogonia stops before birth and no new oogonia are formed thereafter. Indeed, the majority of oogonia die before birth.
At birth there are 1–2 million primary oocytes remaining and each is surrounded by a layer of flattened epithelial cells known as follicular cells. Each primary oocyte and the surrounding follicular cells form a spherical, primordial follicle. Such follicles are predominant in the outer regions of the cortex (
Fig. 16.2). Many primordial follicles die during childhood, leaving only about 400 000 at puberty.
Ovarian follicles in menstrual cycles
During each menstrual cycle several primordial follicles begin to develop but many become atretic (see below) and the oocyte dies. Usually, only one oocyte is released from a developed follicle every 28 days at ovulation, so that the lifetime total of female gametes shed is only around 400. In adults, ovarian follicles may be defined by the stage of their development and are categorised into four types: primordial, primary, secondary (antral) and Graafian (mature) follicles.
■
Primordial follicles (
Figs 16.2 and
16.3). These consist of a primary oocyte, in the prophase of the first meiotic division, surrounded by a single layer of flattened epithelial cells which is surrounded by a basement membrane.
■
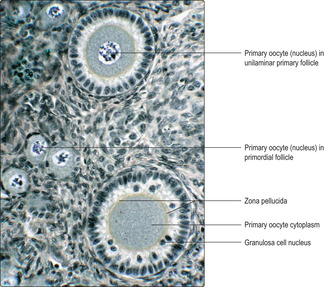
Primary follicles. Each month, several primordial follicles begin to develop and form primary follicles. This development is stimulated by follicle-stimulating hormone from the anterior pituitary gland. In each developing primary follicle the primary oocyte increases in volume and the surrounding flattened follicle cells become cuboidal or columnar in shape and are then known as granulosa cells. At this stage, the follicles are described as unilaminar primary follicles (
Fig. 16.3), and they are deeper in the ovary than primordial follicles.
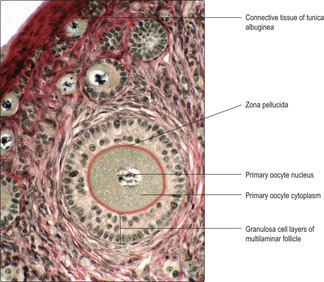
Unilaminar follicles become multilaminar follicles as mitotic activity is high in granulosa cells and the new cells form layers around each developing primary oocyte (
Fig. 16.2,
Fig. 16.3 and
Fig. 16.4). During this time, the primary oocyte enlarges considerably and a prominent deposit of condensed material known as the zona pellucida appears between the oocyte and the granulosa cells (
Fig. 16.2,
Fig. 16.3 and
Fig. 16.4). Primary oocytes, which may grow to 120 μm in diameter, may appear to lack a nucleus (
Figs 16.2 and
16.3) in routine histological sections which, at only 5–10 μm thick, can fail to include the nucleus. As primary follicles grow the basement membrane which separates the (epithelial) granulosa cells from surrounding connective tissue (
Fig. 16.2) becomes more apparent.
Stromal cells around each primary follicle form two layers during this phase: the theca interna and theca externa. The inner theca is well vascularised whereas the outer theca is composed of fibrous connective tissue, although the layers are not always distinct (Fig. 16.2). Luteinising hormone from the anterior pituitary gland binds to cells of the theca interna and this stimulates them to produce steroids. These steroids are converted by granulosa cells into oestrogens (female steroid hormones). Oestrogens are involved in stimulating the uterus each month (see below) and in the development and maintenance of the secondary sexual characteristics and of the mammary glands.
■
Secondary follicles. Some multilaminar primary follicles develop and enlarge further, under the influence of follicle-stimulating hormone. The granulosa cells produce fluid, liquor folliculi, which appears in spaces between the granulosa cells (
Fig. 16.5). These follicles are described as secondary (antral) follicles. Gradually, the fluid-filled spaces coalesce into a single large space, the antrum, and development proceeds with the formation of a Graafian (mature) follicle.
■
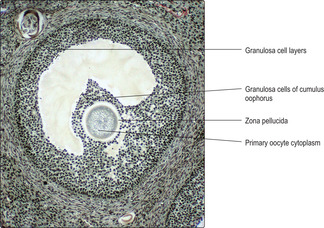
Graafian (mature) follicles. As the single antrum enlarges the granulosa cells appear as layers around the fluid and the layers become thinner as the follicle enlarges further. The oocyte, surrounded by granulosa cells, bulges into the fluid in the antrum and the whole bulge is known as the cumulus oophorus (
Fig. 16.6).
Graafian follicles may become very large (2.5 cm in diameter) and bulge from the surface of the ovary prior to ovulation. The primary oocyte (which was arrested in utero in prophase of the first reduction meiotic division) completes the first meiotic division just prior to ovulation. The result is one large secondary oocyte with half the original number of chromosomes (23 in humans) and a small cell, known as the first polar body (also with 23 chromosomes), which degenerates.
Atresia of follicles
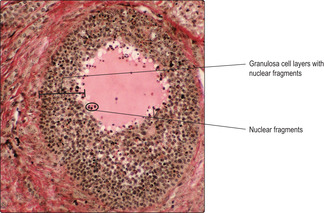
Most developing follicles fail to become mature Graafian follicles. Atresia may occur at any stage of follicle development and it involves the death of granulosa cells (
Fig. 16.7) and the oocyte. Atretic follicles are gradually replaced by scar tissue containing fibroblasts and collagen (
Fig. 16.2).
Ovulation
Formation of a corpus luteum
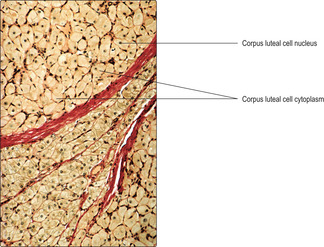
After ovulation, under the influence of luteinising hormone from the anterior pituitary gland, a corpus luteum develops. It forms from the remnants of granulosa cells from the ruptured Graafian follicle, and cells and blood vessels which grow into these remnants from the theca interna. The theca and granulosa cells forming the corpus luteum begin to secrete female steroid hormones, mainly progesterone. The secretory cells pack the corpus luteum (
Fig. 16.8), which may grow into a sphere up to about 2 cm in diameter by day 25 of the menstrual cycle. The progesterone secreted by the corpus luteum is essential in preparing the uterus for the possible arrival of a fertilised ovum and the establishment of pregnancy.
Clinical note
Ovarian cysts These are common. They are fluid-filled structures which develop from ovarian follicles or corpora lutea. Some may be follicles which have failed to rupture at ovulation. They may cause pain and/or bleeding. In the condition known as polycystic ovary syndrome a range of changes may be present, e.g. menstruation may be irregular or cease and the patient may become obese and infertile. Cysts, occasionally, become malignant. The factors causing cysts to form are not understood.
If pregnancy is established the corpus luteum doubles in size and is an essential source of progesterone during the first months of pregnancy in humans. If fertilisation and implantation do not occur, the corpus luteum degenerates, a scar forms from fibroblasts and collagen fibres, and the resultant structure, known as a corpus albicans, persists in the ovary for some time.
Medulla of the ovary
The medulla is the central core of the ovary and it is surrounded by the cortex except where vessels and nerves are connected to the ovary. Stromal cells, similar to those in the cortex, are present in the medulla but follicles are not present.
Uterine tube and fertilisation
Uterine tubes
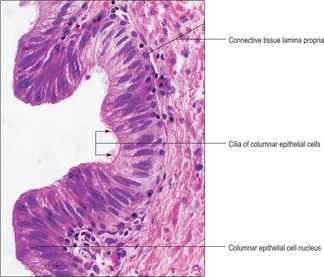
Each uterine tube in humans is about 10 cm long. The lumen at one end of each tube is open to the peritoneal cavity. This end is formed by frilly, finger-like projections (fimbriae) of the tube. Fimbriae are important in helping guide the oocyte (released at ovulation into the peritoneal cavity) into the lumen of the uterine tube. The other end of the uterine tube opens into the uterus (
Fig. 16.1). The structure of each uterine tube consists of three layers:
■ The middle layer is a muscularis. There are two, thin, ill-defined layers of smooth muscle cells, an inner circular and an outer longitudinal layer. Peristaltic contractions of the smooth muscle cells assist in moving the contents of the lumen.
■ The outer layer is a thin covering of serosa. This layer is continuous with the broad ligament covering the uterus.
Fertilisation
Once a secondary oocyte, surrounded by cells of the cumulus oophorus, enters the uterine tube it remains there for 2–3 days, during which time it is capable of being fertilised by a spermatozoon. At this stage, it has started the second (mitotic) division of meiosis but is suspended in metaphase pending possible fertilisation by a spermatozoon.
Fertilisation occurs in the uterine tube when a spermatozoon passes between cells of the cumulus oophorus and penetrates the cytoplasmic membrane of the oocyte. Once fertilised, the oocyte completes meiosis and this restores the diploid number of chromosomes. (A second polar body is released at this division and degenerates.) The fertilised oocyte undergoes rapid rounds of cell division (mitosis) and develops into a blastocyst. Further details of the subsequent development of the blastocyst are described elsewhere (see Mitchell B, Sharma R. Embryology: An Illustrated Colour Text. Elsevier: 2004).
After fertilisation, the blastocyst passes to the uterus, carried there in the fluid flowing from the uterine tube. The timing of the arrival of the blastocyst in the uterus is critical. The blastocyst has to be sufficiently developed so that it is able to attach to the uterine epithelium. In addition, it must arrive in the uterus when, and not before, the uterus is in a receptive state. This state is dependent on progesterone from the corpus luteum (see below).
Uterus
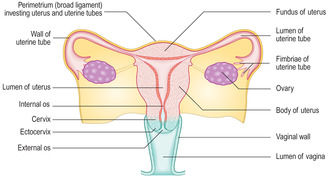
The uterus in humans is a single mid-line organ. It is able to accommodate and facilitate the development of the blastocyst and differentiation of the embryo and its further growth as a fetus. In addition, the uterus supports the development of the placenta and expels the fetus and placenta at parturition. The uterus is divided into three regions (
Fig. 16.1). The superior part is the fundus. The body is the largest part of the uterus, into which the uterine tubes open. The most inferior part of the uterus is the cervix, which projects and opens into the vagina.
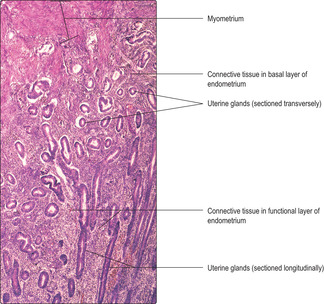
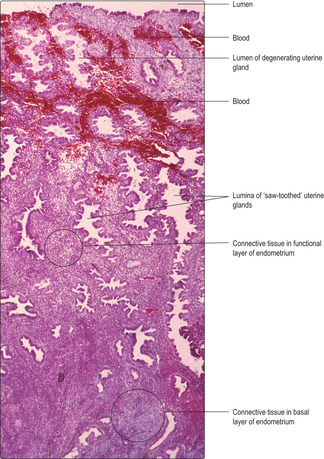
The superficial layer of the endometrium is shed at menstruation or, if pregnancy occurs, adapts and functions as a support for the blastocyst and the developing placenta and embryo (and its development into a fetus). Within the superficial layer there are helical arteries. These supply the endometrium as it increases in thickness in response to the hormonal changes occurring during the menstrual cycle (and in pregnancy). It is from the capillaries supplied by these vessels that blood is lost during menstruation. The inner basal layer of the endometrium is a relatively narrow region, though highly significant, since it is this layer that undergoes cellular proliferation and replaces the superficial layer shed at menstruation.
■
Myometrium. This is the muscularis of the uterus. It is composed of three or four thick and ill-defined layers of smooth muscle. Arcuate arteries in the middle of the myometrium supply the helical arteries in the endometrium. Hypertrophy and hyperplasia of smooth muscle cells are stimulated by oestrogen and these changes are particularly important in pregnancy. Contractions of the myometrium are stimulated by oxytocin from the posterior pituitary (
Chapter 14) and by prostaglandins from the region of the uterus in pregnancy known as decidua (see Mitchell B, Sharma R.
Embryology: An Illustrated Colour Text. Elsevier: 2004). These contractions result in expulsion of the fetus and placenta at parturition.
■
Perimetrium (
Fig. 16.1). The thin, outer, serosal (peritoneal) covering of the uterus is known as the perimetrium. It consists of a single layer of squamous epithelial cells beneath which is loose connective tissue attached to the myometrium. The perimetrium invests most of the uterus as the broad ligament, encloses vessels and nerves passing to the uterus and is continuous with the peritoneum. As the uterus expands during pregnancy the fluid secreted onto the surface of the perimetrium by its epithelial cells ensures relatively friction-free movement between the uterus and adjacent structures such as gut tubes.
Clinical note
Ectopic pregnancy Sometimes a developing blastocyst becomes arrested in its passage from the uterine tube to the uterus. It may then develop and attach to the mucosal epithelium of the uterine tube. The wall of the uterine tube is not able to sustain the developing blastocyst for long and blood vessels in the wall of the tube rupture. The result is haemorrhage, possibly into the peritoneal cavity and possibly into the uterus and showing as a vaginal discharge. These events usually cause severe pain and loss of the embryo and can cause death of the woman.
Cervix of the uterus
The cervix is the lower part of the uterus, and the lower part of the cervix projects into the vagina as the ectocervix (
Fig. 16.1). In the cervix, the smooth muscle forming the myometrial layer in the rest of the uterus is mostly replaced by connective tissue containing collagen and elastin fibres. The lumen of the cervix communicates with the lumen of the body of the uterus at the internal os and opens into the vagina at the external os.
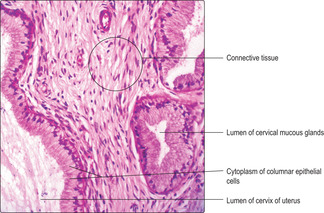
The lumen of the cervix is lined by a simple columnar epithelium. Deep slits and tubes, lined by columnar epithelial cells, extend into the connective tissue of the cervical wall. Cervical epithelial cells are palely stained in routine (H&E) histological preparations (
Fig. 16.11) in contrast to the rest of the uterus. Cervical epithelial cells synthesise and secrete mucus, which is rich in carbohydrates. This mucus does not stain strongly in routine preparations. Cervical mucus varies in viscosity during the menstrual cycle. The mucus is relatively watery at mid-cycle when ovulation occurs, but more viscous at other times and during pregnancy. The thicker mucus helps prevent the entry of microorganisms into the uterus. The watery mucus provides relatively little resistance to swimming spermatozoa during the short time after ovulation that an oocyte is available for fertilisation in the uterine tube. Cervical mucus drains to the vagina and acts as a lubricant during sexual intercourse. The surface of the cervix where it projects into the vagina is covered by stratified, squamous, non-keratinising epithelium, similar to that which lines the vagina itself (see below).
■
Proliferative (follicular) phase. After menstruation, the damaged endometrium is repaired as epithelial, stromal and endothelial cells in the basal layer proliferate and replace the shed functional layer with straight tubular glands and connective tissue stroma (
Fig. 16.10). This phase lasts from about day 5 of the cycle to about day 14. The proliferative and repair activities are regulated indirectly by follicle-stimulating hormone (FSH) from the anterior pituitary gland. FSH stimulates the growth of ovarian follicles and as the number of granulosa cells increases they secrete increasing levels of oestrogen into the blood. The timing of the secretion of these oestrogens and the effect they have on endometrial repair is important because it helps to ensure that the uterus is in a receptive state appropriate for the arrival of a blastocyst.
Clinical notes
Fibroids These are benign tumours which develop from the smooth muscle cells of the myometrium of the uterus. As these tumours enlarge they disrupt the normal repair of the endometrium, and heavy bleeding may occur. In many women, after the menopause with the associated fall in steroid secretions from the ovaries, fibroids shrink and do not need to be excised.
Cervical carcinoma Most cervical cancers develop from the stratified squamous epithelium in the region of the ectocervix. A papillomavirus causes many cervical carcinomata. Routine screening of smears of cells sampled from the ectocervix may detect early changes in the appearance of the epithelial cells which could become cancerous. Further histological examination of a biopsy sample from the region may provide information on whether the basement membrane has been disrupted and epithelial cells have become malignant and spread.
The vagina
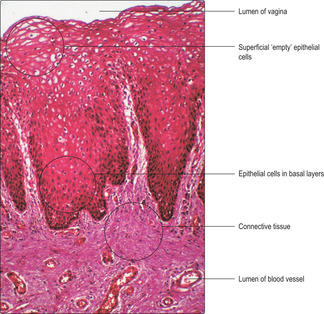
The vagina is a fibromuscular tube that leads from the uterus to the exterior. The wall of the vagina consists of three layers: an inner mucosa, a fibromuscular layer and an outer connective tissue covering which attaches the vagina to adjacent structures, e.g. the urethra.
The vaginal mucosa, in humans, comprises a non-keratinising, stratified, squamous epithelium and a connective tissue lamina propria. Cells in the upper layers of the vaginal epithelium are characterised by their content of glycogen, which is extracted during routine histological processing and so the cells appear empty (
Fig. 16.13). The surface epithelial cells are gradually shed and replaced by cells arising from stem cells in the basal layer. Glycogen, released from shed cells, is metabolised by non-pathogenic bacteria in the vaginal lumen and lactic acid is released. This symbiosis is important as the lactic acid maintains a low pH in the vaginal lumen and this provides a hostile environment for many types of pathogenic bacteria which could gain access to the upper parts of the tract (and the peritoneal cavity). The wall of the vagina mostly comprises fibrous connective tissue, which includes mainly collagen and elastin fibres. Some smooth muscle cells are also present. During sexual stimulation, cervical mucus and a watery transudate from the vaginal wall facilitate the entry of the penis during sexual intercourse.
Mammary glands
Mammary glands are paired structures lying on the anterior chest wall. After puberty in human females the glands develop under the influence of oestrogen and progesterone (mainly from the ovaries). During pregnancy and lactation oestrogen, progesterone and prolactin (from the anterior pituitary gland) further stimulate the glands. In females, mammary glands secrete milk, the defining substance for nourishing the offspring of mammals. Mammary glands are also present in males, though considerably less well developed. It is important to recognise their presence in males since, like their counterparts in females, they too may develop cancer.
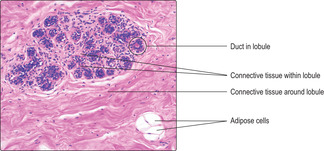
Mammary glands are classified as compound tubuloalveolar glands (Chapter 3). Each gland contains about 20 lobes separated by connective tissue, and each lobe drains through a lactiferous duct to a nipple. Connective tissue comprising collagen, fibroblasts and adipose cells separates each lobe and supports the tubuloalveolar glands within the lobes. The number of adipose cells present varies from individual to individual.
Mammary glands in non-pregnant women are in a resting state. Within each lobe there are lobules comprising clusters of epithelial-lined ducts (
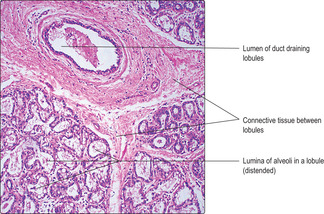
Fig. 16.14) separated by loose connective tissue, but there are no secretory cells. During pregnancy, under the influence of hormones (oestrogen, progesterone and prolactin) the epithelial cells of the ducts proliferate, the duct system extends, and spherical clusters of secretory cells form as alveoli at the ends of the ducts. Ducts and alveoli swell and fill the lobules. The secretory alveoli are lined by cuboidal epithelial cells and are surrounded by myoepithelial cells. The epithelial cells synthesise and secrete milk, which is released from the nipple post partum. During lactation, alveoli may appear distended with secretion (
Fig. 16.15). The expulsion of milk is stimulated by suckling. It involves the secretion of oxytocin (from the posterior pituitary) and the contraction of myoepithelial cells surrounding the alveolar epithelium.
Milk contains proteins, lipids, lactose (milk sugar), minerals and vitamins, which together constitute the nutrition required by infants. Milk also contains antibodies and various immune cells. At this early stage, the infant has little immunity of its own and the passage of antibodies in milk from the mother is the principal means by which a newborn is afforded some immune protection from potential microbiological invaders from their new world.
Clinical note
Breast cancer The most common swellings and disorders occurring in breasts are not cancerous. However, the epithelial cells lining the ducts in the breast may become cancerous in women (and men) and produce lumps or distortions. Spread of cancerous cells from the breast occurs via the rich network of blood and lymphatic vessels in the breast. Early diagnosis of the cell types present in breast lumps and appropriate treatment improves survival rates.
The female reproductive system
■ This consists of paired ovaries and the reproductive tract (paired uterine tubes, a single uterus (in humans) and a vagina).
Ovaries
■ Ovaries contain ovarian follicles, which contain oocytes.
■ Each month, after puberty, several follicles begin to grow:
■ follicle cells increase in number and are known as granulosa cells; they surround a developing oocyte and secrete mainly oestrogens.
■ Usually, only one follicle grows to maturity each month and one oocyte is shed at ovulation (other developing follicles become atretic and die).
■ After ovulation, a corpus luteum develops from the remnants of the follicle and a surrounding layer (theca) of connective tissue cells and blood vessels.
■ The pituitary hormones follicle-stimulating hormone and luteinising hormone, respectively, control the development of follicles and corpora lutea.
■ If fertilisation does not occur the corpus luteum degenerates, progesterone levels fall and menstruation occurs.
■ If fertilisation occurs, the corpus luteum grows and secretes progesterone, which prepares the uterus to support the developing embryo until the placenta forms.
Uterine tubes
■ These are lined by a simple epithelium composed of secretory and ciliated cells.
■ They have sparse connective tissue lamina propria connecting the epithelium to a muscularis of two layers of smooth muscle.
■ They are covered by a serosal (peritoneal) membrane.
■ They receive the oocyte after ovulation, are the site of fertilisation and transfer the blastocyst to the uterus.
Uterus
■ A simple epithelium lines most of the uterus and glands dip into the mucosa as far as the muscularis externa:
■ most of the mucosa is shed at menstruation and bleeding occurs
■ after menstruation, the remaining cells in the basal mucosal layer proliferate (under the influence of oestrogens) and repair the superficial layer
■ after ovulation (under the influence of progesterone) the mucosa thickens, the epithelial cells begin to store glycogen and secrete glycoproteins. The glands become saw-toothed in shape.
■ The muscularis externae is formed of several layers of smooth muscle which undergoes hyperplasia and hypertrophy during pregnancy.
■ Part of the uterus, the cervix, is lined by columnar epithelial cells which secrete mucus. The outer wall of the cervix is formed mostly of connective tissue.
Vagina
■ A stratified squamous epithelium lines the vagina and the upper cells contain glycogen.
■ The vaginal wall is formed largely by connective tissue.