35 The Extremely Premature Infant (Micropremie)
THE PRETERM INFANT IS defined by birth before 37 weeks gestation. Preterm infants can be classified as low–birth-weight infants (less than 2500 g), very low–birth-weight infants (less than 1500 g), and extremely low–birth-weight (ELBW) infants (less than 1000 g). Morbidity and mortality in this population has decreased over the past 25 years, especially in the ELBW infant group, in which the mortality in 2011 is less than 30% in Level 3 hospitals, compared with 80% in 1980.1–3 This decrease in mortality is the result of many factors, including the use of surfactant shortly after birth, antenatal glucocorticoid administration, specialization of neonatal care units, and changes in mechanical ventilator therapy. However, many of these surviving infants develop coexisting diseases that require care by an anesthesiologist. For the purpose of this chapter, we will focus on the very low and extremely low–birth-weight infant, or “micropremie,” and discuss developmental physiology and its impact on anesthetic care; neonatal emergencies are discussed in Chapter 36.
Physiology of Prematurity Related to Anesthesia
Respiratory System
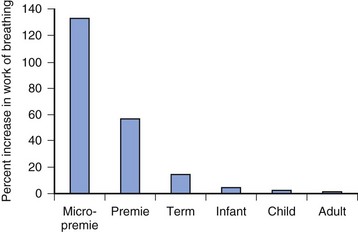
The small airways predispose the micropremie to obstruction and difficulty with ventilation. Resistance to airflow is inversely proportional to the fifth power of the radius in the upper airway and to the fourth power of the radius beyond the fifth bronchial division (see also Fig. 12-7). As a result, insertion of an endotracheal tube (ETT) increases resistance and work of breathing far greater for the micropremie (2.5 or 3 mm inside diameter [ID]) than for a larger infant (4 mm ID), child (5 mm ID), and adult (7 mm ID) (Fig. 35-1). Similarly, partial occlusion of the ETT by secretions, blood, or kinking increases the work of breathing to a much greater extent in the micropremie. Partial occlusion of the natural airway from loss of muscle tone during anesthesia and sedation also increases the work of breathing more in the micropremie. Consequently, general anesthesia often requires placement of an ETT to ensure airway patency, and assisted ventilation to overcome the increased work of breathing.
Diseases that narrow the airway, such as subglottic stenosis, tracheal stenosis, and tracheobronchomalacia, occur commonly in the micropremie, and the associated reduction in airway diameter further increases both resistance to airflow and work of breathing. Subglottic stenosis necessitates the placement of a smaller ETT than would otherwise be placed, further increasing airflow resistance. Tracheal stenosis often occurs near the carina and, although not necessitating a smaller ETT, it increases airway resistance from the stenosis distal to the ETT. With tracheobronchomalacia, the intrathoracic airways collapse during exhalation, again increasing resistance and the work of breathing (see also Fig. 12-10). Positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) helps stent open the airway. Mechanical ventilation, rather than spontaneous ventilation during anesthesia, prevents fatigue from increased work of breathing, and maintains ventilation and oxygenation. During anesthesia the use of smaller inspiratory-to-expiratory ratios prevent air trapping and hyperinflation of lung segments.
Micropremie lungs are particularly susceptible to oxygen toxicity, volutrauma, and the development of bronchopulmonary dysplasia (BPD). Mechanical lung injury is no longer thought to be caused by the use of high peak-inspiratory pressures, but rather related to increased end-inspiratory lung volumes and frequent collapse and reopening of alveoli. A ventilation strategy using small tidal volumes (4 to 6 mL/kg), greater respiratory rates, PEEP sufficient to avoid alveolar collapse, and permissive hypercapnia reduces lung injury in the premature lung.4 Randomized controlled trials of permissive hypercapnia (Paco2 45 to 55 mm Hg) showed smaller periods of assisted ventilation, reduced incidence of BPD, and no increase in adverse neurodevelopmental effects.5 The use of high inspired oxygen concentrations leads to the development of free radical species, which contribute to pulmonary epithelial cell injury.
A severity index for BPD based on the need for supplemental oxygen and/or positive-pressure ventilation or nasal CPAP has been developed and shown to identify a spectrum of risk for adverse pulmonary and neurodevelopmental outcomes in preterm infants (Table 35-1).6 Although this severity index has not been studied in the context of anesthetic risk, experience suggests that such infants requiring supplemental oxygen, positive pressure, or medications for reactive airways are at greater risk for perioperative pulmonary complications. Anesthetic goals include minimizing the inspired oxygen concentration and tidal volumes while maintaining oxygen saturation (Sao2 90% to 94%) and ventilation (Paco2 50 to 55 mm Hg). The use of smaller tidal volumes decreases the risk of pneumothoraces and interstitial emphysema.
TABLE 35-1 Severity-Based Diagnostic Criteria for Bronchopulmonary Dysplasia (BPD)
| Gestational age | <32 weeks |
| Time point of assessment | 36 weeks postmenstrual age or discharge home, whichever comes first |
| Therapy with oxygen >21% for at least 28 days plus: | |
| Mild BPD | Breathing room air |
| Moderate BPD | Need for <30% oxygen |
| Severe BPD | Need for ≥30% oxygen and/or positive-pressure ventilation or nasal continuous airway pressure |
From Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116:1353-60.
Respiratory Control
Micropremies possess a biphasic ventilatory response to hypoxia. Initially, ventilation increases in response to hypoxia, but after several minutes, ventilation decreases and apnea may ensue.7 The ventilatory response to carbon dioxide is decreased in the micropremie, and hypoxia further blunts this response.8,9 Anesthetic drugs depress the ventilatory responses to both hypoxia and hypercapnia. Hypoxia and hypercapnia occur commonly as a result of apnea and hypoventilation during emergence and recovery from anesthesia. Thus the combination of anesthetic effects and an immature respiratory control system, as well as immature intercostal and diaphragmatic muscles,10,11 increase the risk of hypoxia, hypercapnia, and apnea in the postoperative period.
Apneic episodes occur commonly in the micropremie but decrease with advancing postconceptional age.12 These apneic episodes usually involve both a failure to breathe (central apnea) and a failure to maintain a patent airway (obstructive apnea). Central apnea results from decreased respiratory center output, although it may be precipitated by abrupt changes in oxygenation, pulmonary mechanics, brain hemorrhage, hypothermia, or airway stimulation. Apnea may also occur without a precipitating event (i.e., idiopathic). Preterm infants with apnea do not increase ventilation in response to hypercapnia, compared with those without apnea, thereby delaying resumption of breathing and prolonging the apneic episode.13 During obstructive apnea, the airway becomes obstructed in the hypopharynx and larynx as a result of pharyngeal muscle incoordination. Anesthetic drugs may further decrease pharyngeal muscle tone, precipitating airway obstruction during recovery from anesthesia. The combination of anesthetic effects and immature respiratory control place the micropremie at risk for central and obstructive apnea for a prolonged period of time during recovery from anesthesia.
Not surprisingly, apnea occurs commonly after anesthesia and surgery in preterm infants.14,15 Like apnea of prematurity, postoperative apnea may be central, obstructive, or mixed in origin.16 The term postoperative apnea usually means prolonged apnea (greater than 15 seconds) or brief apnea accompanied by bradycardia (heart rate 80 beats per minute or less). Postoperative apnea typically occurs as a cluster of episodes over several minutes, with minutes of normal breathing in between the clusters. Bradycardia may occur with apnea, usually beginning at the onset of apnea and not in response to hypoxia. Arterial oxygen desaturation usually follows the apnea, although many apneic episodes may not have any associated desaturation. Arterial desaturation is worse with obstructive apnea than with central apnea.16
Several different terms have been used to describe the age of the fetus, leading to some confusion in the literature. Gestational age, menstrual age, conceptional age, and postnatal age are all used with somewhat different meanings, even in this book, so we present here the definitions of these terms according to the American Academy of Pediatrics, Committee on the Fetus and Newborn from 2004.17 The gestational or menstrual age of the neonate is the interval from the first day of the mother’s last menstrual cycle until birth of the fetus. The postmenstrual age is the sum of the menstrual age and the postnatal age. The conceptional age is defined as the interval between conception and birth, although the former is generally unknown. The postconceptional age (PCA) is the sum of the conceptional age and the postnatal age. Postconceptual age actually refers to a concept, not conception, but this term has been used interchangeably with postconceptional age in the apnea literature in anesthesia. The postnatal (or chronological) age is the age of the infant since birth. Controversy over this terminology continues, as some argue that the menstrual age overestimates the “in utero” age of the fetus because 10 to 14 days may lapse between the onset of menses and conception. On the other hand, terms such as conceptional (and postconceptional) age are imprecise because the date of conception is usually unknown, and thus these terms are not recommended.17
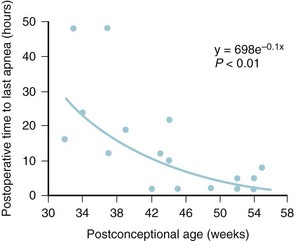
The incidence of postoperative apnea depends on PCA, hematocrit, and the type of surgical procedure (Fig. 35-2; see also Fig. 4-7 and E-Fig. 4-5).14–1618 The most significant risk factor is the PCA; the lesser the PCA, the greater the risk, with the incidence of postoperative apnea in the micropremie greater than 50%.14,15 Postoperative apnea can occur in the micropremie even without a history of apnea of prematurity.14 Anemia (hematocrit less than 30%) and younger gestation increase the risk of apnea for a given PCA.15,18
Postoperative apnea usually begins within an hour of emergence from anesthesia.14 In the micropremie, it can continue to occur up to 48 hours postoperatively, despite the elimination of anesthetic agents (see Fig. 35-2). In fact, postoperative apnea can occur after surgery with desflurane- or sevoflurane-based anesthetics, or even after surgery for which a regional anesthetic was administered and no general anesthetic drugs were used.19,20 Postoperative apnea is more common after major procedures, such as a laparotomy, compared with peripheral surgical procedures, such as inguinal hernia repair. These observations indicate that the neurohormonal response to surgery and postoperative pain may play an important role in the origins of postoperative apnea. Management of postoperative apnea includes close observation with a cardiorespiratory monitor and pulse oximeter, administration of intravenous (IV) methylxanthines (e.g., caffeine, theophylline),21 and prevention of anemia or hypovolemia. Nasal CPAP or tracheal intubation and mechanical ventilation may be required for several days postoperatively if these measures fail.
N7-methylation of theophylline (or aminophylline) to produce caffeine is well-developed in the neonate, whereas oxidative demethylation (CYP1A2) responsible for caffeine metabolism is deficient. Theophylline is effective for the management of postoperative apnea in the preterm neonate, in part because it is a prodrug of caffeine, which is effective in controlling apnea in this age group and can only be cleared slowly by the immature kidney. Consequently, the half-life of caffeine is ∼72 hours in the extreme premature neonate, which decreases to 4 to 6 hours by 6 months of age. Clearance increases from 0.004 L/kg/hr in the premature neonate to 0.119 L/kg/hr by 6 months.22–26 Although therapeutic drug monitoring is not required, target concentrations 5 to 20 mg/L are considered therapeutic.27 One study suggests a loading IV or oral dose of 10 mg/kg followed by 2.5 mg/kg by mouth, once daily.26
Cardiovascular System
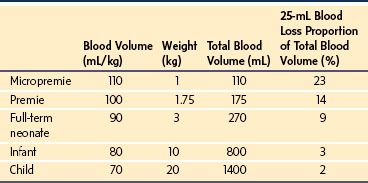
The micropremie remains at greater risk of cardiovascular collapse during anesthesia and surgery than does the full-term infant for several reasons. The fetal heart differs from the infant heart in that it has more connective tissue, less organized contractile elements, and increased dependence on extracellular calcium concentration. In addition, the less compliant fetal heart has a flatter Frank-Starling curve and is less sensitive to catecholamines because of near-maximal baseline β-adrenergic stimulation (see Chapter 16).28,29 Consequently, cardiac output depends more on heart rate in the micropremie than it does in the term neonate. The increased resting heart rate in the micropremie also does not permit cardiac output to increase to the same extent as in an infant or child. The micropremie has a small absolute blood volume (Table 35-2). Therefore, relatively little blood loss during surgery can cause hypovolemia, hypotension, and shock. Because autoregulation is not well developed in the micropremie, the heart rate may not increase with hypovolemia, and blood flow and oxygen delivery to the brain and heart may decrease with relatively little blood loss.30 Anesthesia blunts baroreceptor reflexes in the micropremie, further limiting the ability to compensate for hypovolemia.31 The combination of limited ventricular stroke volume reserve, an increased heart rate, small blood volume, and limited autoregulation predispose the micropremie to cardiovascular collapse during major surgery.
TABLE 35-2 Circulating Blood Volume in Micropremies, Premies, Full-Term Neonates, Infants, and Children
Failure of the ductus arteriosus to close in the micropremie further increases this risk. A patent ductus arteriosus (PDA) promotes pulmonary hypertension and congestive heart failure. Changes in systemic or pulmonary vascular resistance alter the direction of flow through the PDA or the foramen ovale.32 Increased pulmonary vascular resistance predisposes to right-to-left shunting that worsens with hypoxia, hypercarbia, acidosis, and hypothermia. Paradoxical embolism is another concern.33 Fluid restriction and diuretic therapy, often used to treat congestive heart failure from left-to-right shunting through a PDA, further increase the risk of hypotension during surgery. In contrast to full-term neonates, the success of inhaled nitric oxide in the micropremie with hypoxic respiratory failure and pulmonary hypertension remains unclear.34–36
Neurologic Development
Although mortality in extremely preterm infants has improved over the years, many survivors experience cognitive impairment and long-term disability.37,38 Regions of the central nervous system develop at different times during gestation; consequently, the impact of premature birth on the central nervous system (CNS) depends on gestational age at birth and the severity of cardiovascular, respiratory, and other postnatal stressors. The area of the brain most susceptible to injury in the micropremie is the periventricular white matter.38 The white matter consists of preoligodendrocytes, astrocytes, and neuronal axons. Late in the second trimester (24 to 27 weeks gestation), preoligodendrocytes and astrocytes multiply tremendously and most cortical and subcortical structures begin to develop.38 During this period, the periventricular white matter is particularly susceptible to neurologic injury. The periventricular white matter is perfused by arteries penetrating from the cortical surface and by lenticulostriate arteries from the circle of Willis. As a result, the periventricular white matter is a “watershed region” and susceptible to poor perfusion and hypoxic-ischemic injury during conditions of hypotension, reduced cardiac output, hypoxemia, and hypocarbia.
Neural pathways allowing for perception of pain develop during the first, second, and third trimesters (see Chapter 43).39 During the first trimester, peripheral sensory receptors and spinal reflex arcs develop that lead to the presence of a “withdrawal reflex” to non-noxious stimuli. Neurons that transmit nociception appear in the dorsal root ganglia at 19 weeks gestation, and afferent neurons from the thalamus reach the cortical subplate and cortical plate between 20 and 24 weeks gestation. However, it is not until early in the third trimester (29 weeks) that pathways between the thalamus and somatosensory cortex are functional. Significant controversy exists regarding the exact gestational age at which perception and memory of pain occur. Nevertheless, our approach in the micropremie is to administer anesthesia during surgery and provide pain management postoperatively.
Long-Term Neurologic Complications of Prematurity
Long-term neurologic and developmental disabilities remain common in the micropremie and include cerebral palsy, cognitive deficits, behavioral abnormalities, as well as hearing and visual impairment. In one cohort of ELBW infants, only 25% were classified as normally developed at 5 years of age, whereas 20% exhibited major disabilities.37 Brain magnetic resonance imaging (MRI) identifies a spectrum of abnormalities. The most common abnormality is diffuse high signal intensity on T2-weighted imaging in the periventricular cerebral white matter. Diffusion-weighted imaging shows increased apparent diffusion coefficient values, indicative of increased water content and delayed white matter maturation, suggesting ischemia-reperfusion injury in periventricular white matter, which has activated microglia and damaged preoligodendrocytes.38,40 Damage to preoligodendrocytes impairs myelination of cerebral white matter axons and accounts for many of the fine motor, speech, and cognitive deficits. On MRI, tissue volumes in the basal ganglia, corpus callosum, amygdala, and hippocampus are reduced and correlate with smaller full-scale, verbal, and performance IQ scores.41 Collectively, these MRI findings indicate that different regions of the brain vary in their susceptibility to injury during development and that such injuries lead to specific long-term disturbances in neurocognitive function.
Intraventricular Hemorrhage
 Grade 1: hemorrhage limited to the germinal matrix
Grade 1: hemorrhage limited to the germinal matrix
 Grade 2: hemorrhage extending into the ventricular system
Grade 2: hemorrhage extending into the ventricular system
 Grade 3: hemorrhage into the ventricular system and with ventricular dilatation
Grade 3: hemorrhage into the ventricular system and with ventricular dilatation
Although micropremie infants with grade 3 or 4 IVH are more likely to exhibit severe long-term neurocognitive sequelae, even micropremie infants with grade 1 and 2 IVH display poorer neurodevelopmental outcomes compared with those without IVH.42 Early onset of IVH appears during the first day of life. Risk factors include fetal distress, vaginal delivery, reduced Apgar scores, metabolic acidosis, severe hypercapnia, and the need for mechanical ventilation.43,44 Late onset of IVH appears days to weeks after birth. Risk factors include respiratory distress syndrome, seizures, pneumothoraces, hypoxemia, acidosis, severe hypocarbia, and the use of vasopressor infusions.43 Rapid fluctuations in cerebral blood flow, cerebral blood volume, and cerebral venous pressure appear to play a role in the development of IVH.45 Factors that may decrease the incidence and severity of IVH include administration of antenatal glucocorticoids, or indomethacin.
Retinopathy of Prematurity
Retinopathy of prematurity (ROP) occurs in approximately 50% of ELBW infants, with the incidence being inversely proportional to birth weight and gestational age (see Chapter 32).46 Although the pathogenesis of ROP is not completely understood, extremes in arterial oxygenation (hypoxia or hyperoxia)47 and exposure to bright light appear to play a role.48 One theory holds that the combination of hyperoxic vasoconstriction of retinal vessels (also known as vaso-obliteration), induction of vascular endothelial growth factor, and free oxygen radicals damage the spindle cells in the retina.49 A Cochrane review concluded that liberal oxygen delivery to a preterm infant is more harmful to the retina than restrictive oxygen delivery, although the data reviewed failed to specify the optimum blood oxygen concentrations that should be delivered.50 Evidence points to additional factors in the pathogenesis of ROP, including genetic polymorphisms51 and antenatal and neonatal exposure to inflammation.52
ROP appears to be multifactorial in origin and oxygen tension is just one of many contributory factors. During anesthesia, our goal is to deliver the minimum inspired oxygen concentration that provides oxygen saturations between 90% and 94% and to avoid significant fluctuations in oxygen saturations. It should be noted however that ROP has occurred in children with cyanotic congenital heart disease53 and that no anesthesia-associated cases have been reported over the past 25 years. Nevertheless it is reasonable to aim for saturation values in the ranges described here.
Temperature Regulation
The micropremie is susceptible to hypothermia. Heat loss in children occurs by four possible routes: radiation (39%), convection (34%), evaporation (24%) and conduction (3%). In the micropremie, evaporative heat loss and insensible fluid loss are increased because the epidermis has less keratin.54 Conductive and convective heat losses are also increased because the micropremie has little subcutaneous fat for insulation and a large surface area to mass ratio. Thermal regulation is not well developed in the micropremie. Nonshivering thermogenesis, which depends on brown fat stores, is decreased and regulation of skin blood flow is less efficient.55 During anesthesia, measures should be undertaken to minimize radiation and convective heat loss by warming the operating room (OR) to 78° F to 80° F (25.5° C to 26.6° C) before the neonate arrives, and minimize convective heat loss during transport (i.e., use a thermoneutral incubator). Using a warming pad on the operating table reduces conductive heat loss; use of overhead heat lamps reduces radiant heat loss; and keeping the skin dry reduces evaporative heat loss. The most effective means for warming is a forced-air warmer. Temperature should be carefully monitored as overheating the infant may readily occur.
Renal and Metabolic Function
In the micropremie, kidney function is decreased as a result of fewer nephrons and smaller glomerular size.56 Glomeruli continue to form postnatally until approximately 40 days.57 During this period, reduced cardiac output, hypotension, and nephrotoxic drugs may inhibit glomerular growth and development. Creatinine concentrations depend on production that is reduced in micropremies with limited muscle mass, and on excretion that is reduced because of immature renal function. Baseline plasma creatinine concentrations increase with increasing prematurity and remain increased until 3 weeks of age.58 In addition, the normal increase in creatine clearance in term infants occurs more slowly in the micropremie. Creatinine concentrations in the first few days after birth are increased and reflect maternal transplacental transfer.59 It is for this reason that antibiotic dosing must be adjusted to take renal immaturity into consideration, so as not to administer excessive doses that might result in ototoxicity.60
Very preterm infants easily become hyponatremic because of reduced proximal tubular reabsorption of sodium and water, and reduced receptors for hormones that influence tubular sodium transport. As many as one-third of ELBW neonates develop hyponatremia.61 Frequent assessment of sodium and free water requirements is important during critical illness. Increased plasma potassium concentrations occur in preterm infants during the first few days after birth. The increase results from a shift in potassium from the intracellular to extracellular space.62 These increases are greater as gestational age and birth weight decrease.63 Reduced cardiac output and urine output may further increase serum potassium concentrations and predispose to cardiac arrhythmias.64
Glucose Regulation
The micropremie is at risk for both hypoglycemia and hyperglycemia. Decreased glycogen and body fat predispose to fasting hypoglycemia, whereas decreased insulin production with infusion of dextrose predisposes to hyperglycemia.65,66 Glucose production is poorly regulated within a large range of glucose and insulin concentrations. The micropremie is also relatively insulin resistant and requires a greater infusion rate of insulin to achieve normoglycemia.67 The use of total parenteral nutrition and glucocorticoids places the micropremies at increased risk for hyperglycemia.
Glucose and the Brain
Multiple animal models and clinical studies implicate hyperglycemia as detrimental to the adult brain during global and focal ischemia.68 In contrast, hyperglycemia in neonates appears to protect the brain from ischemic damage.69–71 Studies in both neonatal rat and pig hypoxia-ischemia models observed less brain damage with greater glucose concentrations. Many mechanisms exist for this strikingly different outcome between neonates and adults.72 Relatively mild hypoglycemia is known to cause brain damage in preterm infants.73 Micropremies with critical illness are especially prone to hypoglycemia because they contain limited stores of glucose and consume glucose anaerobically. Thus the administration of dextrose-containing fluids (carefully controlled with an infusion pump so as to minimize wide fluctuations in glucose values) and close monitoring of blood glucose concentrations is vital during anesthesia. Mild or moderate hyperglycemia during surgery is best managed by reducing the rate of infusion of dextrose-containing solutions and not administering insulin, with its attendant risk of hypoglycemia.
Hepatic and Hematologic Function
Immature hepatic function leads to a reduction in many hepatic proteins important for drug metabolism. In addition, reduced albumin synthesis decreases albumin concentrations compared with term neonates (see Fig. 6-6), thus enhancing the “free” (unbound) concentration of anesthetic drugs that are highly bound to albumin (see Chapter 6). The micropremie is at particular risk for spontaneous liver hemorrhage.74,75 This occurs most commonly during laparotomy for necrotizing enterocolitis (NEC), is associated with large IV fluid resuscitation, and is difficult to control surgically. Recombinant factor VIIa has been used to stop liver hemorrhage when administration of other blood products has been unsuccessful.76
The ideal hematocrit level for the micropremie remains controversial. In the micropremie with reduced oxygen saturations and cardiac output, tissue oxygen delivery will be maximized by maintaining the hematocrit between 44% and 48%. In a randomized study of liberal versus restrictive transfusion in neonates between 500 and 1300 grams, intraparenchymal brain hemorrhage, periventricular leukomalacia, and episodes of apnea occurred more frequently in the restrictive transfusion group.77 The risks of blood transfusion in the micropremie must be balanced against the benefits of improved oxygen delivery and fewer medical complications.
Thrombocytopenia (platelet count less than 150,000/mm3) occurs in as many as 70% of micropremies.78 Although the etiology of thrombocytopenia is often unknown, pathophysiologic processes such as sepsis, disseminated intravascular coagulation, and NEC are common causes. Preoperative evaluation should include a recent platelet count and the availability of platelets for major procedures.
Anesthetic Agents and the Micropremie
Anesthetics and the Immature Brain
Research in immature animals indicates that anesthetics are both neuroprotective and neurotoxic. Inhalational anesthetics protect against hypoxic-ischemic injury in neonatal pigs and rats.79–81 The anesthetic must be administered before and during the ischemic event at a concentration of 1 MAC (minimal alveolar concentration) to be effective. Thus for surgery in which there is a risk of brain ischemia, use of an inhalational anesthetic may afford some advantage over IV agents. Cardiac surgery, ventricular shunt insertion, and vein of Galen embolization represent examples of procedures that are performed in preterm infants and that carry a risk of brain ischemia. The MAC for sevoflurane has not been established in preterm infants and many sick preterm infants cannot tolerate even relatively modest concentrations of potent anesthetic agents.
Of particular concern are the reports in immature rats and other animals, including primates, that prolonged exposure to commonly used anesthetics, such as isoflurane, ketamine, and midazolam, induces apoptosis in many regions of the brain (see Chapter 23).82,83 In rodents, exposure for at least 2 hours at 1 MAC of isoflurane produces apoptosis. A combination of isoflurane, midazolam, and nitrous oxide produces more neuronal degeneration than isoflurane or midazolam alone; nitrous oxide alone is not neurotoxic. When affected rats matured to adulthood, neurocognitive impairment was detected.82 The neurotoxicity is brain-region specific and very dependent on the developmental age of the rodent. Rats are most sensitive to the neurotoxic effects of anesthetics on postnatal day 7, more so than on postnatal day 4 or beyond postnatal day 10.84 The most susceptible age in rats, 7 days, corresponds to human brain development around mid-gestation. This suggests that if this phenomenon applies to humans, the preterm infant could potentially be more susceptible to anesthetic neurotoxicity than is the full-term infant.
The mechanism for the neurotoxicity appears to be attributable to the neurotransmitters glutamate and γ-aminobutyric acid, which act as trophic factors in the developing brain.85 In the immature brain, these trophic factors promote synaptic growth and plasticity and are obligate for neuronal survival. The inhaled anesthetics, ketamine, and midazolam exert their anesthetic effects by altering synaptic transmission through blockade of glutamate and γ-aminobutyric acid receptors. In the immature brain, this blockade also precipitates neuronal cell death by apoptosis.86 In contrast, several anesthetics and medications may protect against apoptosis (see Chapter 23). A confounding factor is that neurodegeneration and apoptosis is a normal developmental phenomenon in the maturing fetal brain. Furthermore, anesthesia-induced neuronal cell death in neonatal animals may not directly translate into long-term neurologic abnormalities. Indeed, evidence suggests that sevoflurane-induced cognitive impairment, in the form of short-term memory deficiency in neonatal rodents, is offset by delayed exercise.87 Moreover, immature animals that undergo painful procedures without anesthesia experience neuronal degeneration.88,89 Preterm infants who receive anesthesia and sedation for painful procedures experience less morbidity and mortality than those who do not.90 Curiously, the combination of surgery and anesthesia in neonatal rats produces more apoptosis than either intervention alone suggesting that in this model, anesthetics are neither neuroprotective themselves nor do they offset the apoptotic effects of surgery.91 In summary, the neurodegeneration precipitated by inhaled anesthetics, ketamine, and benzodiazepines depends on developmental age, brain region, and duration of exposure. Based on the animal models, the micropremie exposed to several hours of large concentrations of inhaled agents with nitrous oxide and midazolam is potentially at risk, as is the micropremie exposed to surgery with insufficient anesthesia. Thus our approach at the present time for emergency surgery is to use small concentrations of inhaled agent with opioids and regional anesthesia whenever possible.
Of even greater concern may be the sedatives that are administered for prolonged periods of time in the intensive care unit (ICU), although one study found “no evidence of an association between dose and duration of sedation and/or analgesia drugs given during the preoperative, intraoperative, and postoperative period and major adverse developmental outcomes” in children undergoing repair of congenital heart disease in the first 6 weeks of life.92
Inhalation Anesthetic Agents
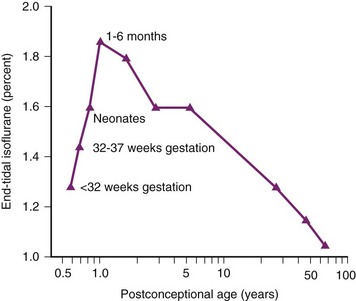
The MAC defines the anesthetic depth for inhaled agents at which 50% of patients respond to a painful stimulus with movement; this measure allows comparison of the effects of inhaled anesthetics at equipotent doses. The MAC of isoflurane in the micropremie (less than 32 weeks PCA) is approximately 20% less than that in full-term neonates (E-Fig. 35-1; see also Fig. 6-16), and that at equipotent doses of isoflurane (1 MAC), systolic arterial pressure decreased similarly in all age-groups, 20% to 30%.93 Sevoflurane affords a rapid induction and emergence from general anesthesia. Desflurane is contraindicated for induction of anesthesia but is widely used for maintenance of anesthesia administered through an ETT. However, desflurane causes more airway irritability than isoflurane or sevoflurane, and as a result, it is not recommended for infants with severe BPD. Desflurane, sevoflurane, and isoflurane decrease arterial blood pressure in a dose-dependent manner, possibly through decreasing the systemic vascular resistance or by myocardial depression. One possible mechanism to explain the myocardial depression is that the baseline ionized calcium concentrations in preterm infants, especially critically ill neonates are reduced.94,95 Because inhalational anesthetics block the calcium channels,96 and because the neonatal heart depends on the plasma ionized calcium for contractility to a greater extent than do the hearts of older children,97 preterm infants may be more susceptible to the cardio-depressant effects of inhalational anesthetics.
Nitrous oxide is not routinely used in the micropremie for several reasons. First, nitrous oxide must be delivered in inspired concentrations ranging from 50% to 75% to reduce the MAC of other agents; therefore, its role in micropremies, a group often requiring supplemental oxygen, is limited. Second, because of its blood gas solubility, nitrous oxide rapidly enters air-filled cavities; therefore it is not recommended for use in infants with bowel obstruction, NEC, pulmonary interstitial emphysema, or pneumothoraces, which are common disorders in micropremies.98 Third, in neonatal and young rats, nitrous oxide demonstrates no antinociceptive effects, which contrasts its antinociceptive effect in adolescent and adult rats.99 This observation requires validation in humans.
Intravenous Agents
IV agents include opioids, benzodiazepines, barbiturates, propofol, ketamine, and dexmedetomidine. Fentanyl possesses analgesic and sedative properties, however, it does not reliably produce unconsciousness or amnesia and, by itself, is not considered an anesthetic in children or adults. Nevertheless, the use of fentanyl as an anesthetic has been justified in preterm infants because they were deemed to be inherently amnestic by virtue of their age, even though the age at which “consciousness” and memory occurs is unknown. Preterm infants (less than 1500 g) who receive IV fentanyl (30 to 50 µg/kg) and pancuronium for ligation of a PDA exhibit remarkable hemodynamic stability, with only a 5% decrease in blood pressure.100 Another study examined the dose response of 25 neonates undergoing a variety of thoracic and abdominal procedures. A dose of 10 to 12.5 µg/kg administered together with a muscle relaxant produced hemodynamic stability for 75 minutes.101 Hypertension and tachycardia did not occur with skin incision, suggesting that analgesic concentrations necessary for surgery are achieved with this dose of fentanyl.
The pharmacokinetics of fentanyl (30 µg/kg) in preterm infants yielded plasma concentrations that remained constant for up to 120 minutes, indicating a reduced clearance.102 The elimination half-life of fentanyl ranged from 6 to 32 hours in preterm infants, significantly greater than the 2- to 3-hour half-life observed in children and adults.102 The clearance of fentanyl is 7 mL/min/kg at 25 weeks, 10 mL/min/kg at 30 weeks, and 12 mL/min/kg at 35 weeks postmenstrual age.103 These studies demonstrated that the half-life and volume of distribution of fentanyl are increased, whereas the clearance is reduced in preterm infants compared with adults.104 These changes may be explained by immature CPY450 3A4, reduced proteins, immature kidneys, and a patent ductus venosus. In a subset of infants with increased intra-abdominal pressure (after repair of a gastroschisis or omphalocele), the elimination half-life of fentanyl is 1.5- to 3-fold greater than that in other infants of the same age.104 This likely results because increased intraabdominal pressure decreases hepatic blood flow, the rate limiting step in the metabolism of drugs with a large hepatic extraction ratio, such as fentanyl.104 The increased volume of distribution decreases the initial plasma concentration of fentanyl compared with that in adults. These pharmacokinetic differences, combined with an increased propensity to apnea, serve to prolong analgesia, as well as prolong respiratory depression, increase the risk of postoperative apnea, and slow recovery of consciousness. In the micropremie, mechanical ventilation may be required for several days after large doses of fentanyl.
Similarly, the elimination half-life of morphine is markedly prolonged in preterm infants compared with that in children and adults.105–108 The elimination half-life of morphine ranges from 6 to 16 hours in the micropremie, compared with 2 to 4 hours in the adult. We prefer fentanyl instead of large-dose morphine (2 to 3 mg/kg) for anesthesia because it has fewer hemodynamic adverse effects.
Remifentanil, a relatively new synthetic, short-acting opioid, is rapidly inactivated by plasma and tissue esterases and, because of its short half-life, is administered by continuous infusion. The half-life of remifentanil in adults is 3 to 4 minutes, independent of the duration of infusion, and similar to that in infants or children.109 A multicenter study that compared halothane and remifentanil for maintenance of anesthesia in infants undergoing pyloromyotomy showed similar intraoperative hemodynamic stability intraoperatively with the two techniques, but significantly fewer “new onset apneas” with remifentanil compared with halothane.110,111 Interestingly, the most rapid clearance of remifentanil was in infants and children younger than 2 years of age, thus allowing an intense opioid effect intraoperatively that rapidly dissipates on terminating the infusion.112 Remifentanil has been used to provide anesthesia in infants weighing 400 to 580 grams with apparent good hemodynamic stability.113,114 A study examining cord blood from preterm infants found high nonspecific esterase activity, comparable to that of term infants, thus suggesting that preterm infants should be able to rapidly metabolize remifentanil.115
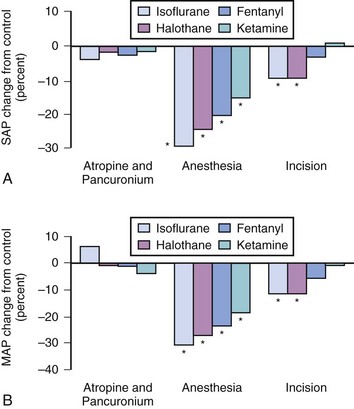
Ketamine, a phencyclidine derivative, affords several advantages compared with inhaled and other IV agents. It provides analgesia, amnesia, and unconsciousness yet minimally depresses cardiovascular function (Fig. 35-3).116 However, ketamine anesthesia depresses ventilation and airway reflexes, which predisposes to airway obstruction, apnea, and gastric aspiration. Thus we recommend the use of an ETT when ketamine is used for surgical procedures in the micropremie. In the setting of brief painful procedures, IV ketamine can be used as an anesthetic without an ETT.117
Other IV agents include thiopental, propofol, and benzodiazepines. These agents induce loss of consciousness but possess less analgesia than ketamine. Thiopental is a short-acting barbiturate primarily used for the induction of anesthesia. The micropremie requires less thiopental for induction than does the infant (2 to 3 mg/kg vs. 5 to 6 mg/kg, respectively), a relationship similar to the MAC of isoflurane.118 In the micropremie, we only use thiopental for neurosurgical procedures involving increased intracranial pressure. However, thiopental is no longer available in the USA. Propofol is primarily used to induce anesthesia and has largely replaced thiopental for this purpose. A word of caution is needed regarding the use of propofol for induction of anesthesia in neonates. Several reports highlight episodes in otherwise stable infants of protracted hypotension and low cardiac output state that were associated with hypoxia after propofol boluses (1 to 3 mg/kg IV). The mechanism underlying these responses remains unclear although acute pulmonary hypertension with reversion to persistent fetal circulation remains a strong possibility.119,120 In our experience, the micropremie can be anesthetized with a propofol infusion (50 to 200 µg/kg/min) supplemented with fentanyl as needed for analgesia. The selection of infusion pumps that allow for delivery of small volumes accurately is vital. The infusion rate of propofol in these small infants must be carefully and meticulously checked, as a 10-fold overdose of propofol has been reported, with a successful recovery.121 Recovery from propofol anesthesia is delayed in micropremies compared with term infants, because micropremies have both less fat and muscle tissue to redistribute the drug, and reduced clearance. In pediatric ICUs, propofol infusions have been implicated in unexpected deaths (propofol infusion syndrome).122 Until the safety of long-term administration of propofol has been examined in preterm infants, other alternatives for prolonged sedation should be considered.
Benzodiazepines, such as midazolam and diazepam, have been used in the neonatal intensive care unit (NICU) for sedation. As with thiopental and propofol, these drugs do not provide analgesia and are not recommended as the sole anesthetic for surgery. However, the combination of a benzodiazepine and opioid provides complete anesthesia for surgery. Midazolam clearance is markedly decreased in the micropremie when compared with the term neonate or infant, and will be further prolonged in the setting of decreased liver function.123 Midazolam can cause systemic hypotension, depress ventilation, and impair airway reflexes in preterm infants. The hypotension caused by midazolam is greater in the presence of fentanyl; thus both drugs must be titrated in small doses when administered concomitantly.124 One study noted an 8% to 23% decrease in arterial pressure after a bolus of 0.1 mg/kg of midazolam in preterm infants.125
Anesthetic Considerations for Surgical Procedures
To ensure the safe delivery of care in these micropremies, particular attention must be paid to ensuring the accurate delivery of medications and fluids. These infants require only small fractions of the medications in most vials and ampoules. As a result, either tuberculin syringes should be used to carefully measure the very small aliquots of medications or the medication in the vial should be diluted so that a measurable and accurate fraction of the content of the vial can be given. Tuberculin syringes present several challenges, including difficulty in removing air bubbles from the syringe, and the very small volume that will be administered. The volume of medication may be so small that it is no larger than the volume of the stem of the clave or stopcock, resulting in less drug than intended being administered to the infant. To prevent this problem, a saline flush should follow each medication administration. Diluting every medication introduces the risk of a drug dosing error that could lead to an overdose or underdose. In all instances, it is prudent to verify the dose and dilution of the medication with a colleague. To ensure drug is not lost in the dead space of the IV set, each clave or stopcock should be flushed with saline after the medication has been given. All medications should be administered into the IV set as close to the skin insertion as possible, to minimize the volume of fluids needed to flush the medication into the child (see Figure 51-3).
Fluid overload is always a concern in these small infants. To minimize the fluids administered, all IV infusions should be delivered through a pump. Free-flowing IVs are dangerous sources for fluid overload, which may open a ductus arteriosus and cause congestive heart failure. Finally, meticulous care must be taken to remove air bubbles from all IV administration systems, solutions, and medications that are administered. See Chapter 51 for further discussion on infusion pumps and the implications of IV administration dead space on drug delivery.126,127
Exploratory Laparotomy for Necrotizing Enterocolitis
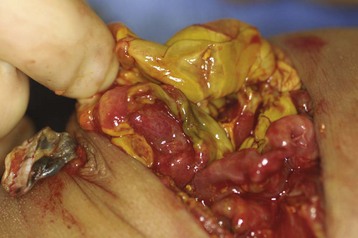
Necrotizing enterocolitis, a life-threatening condition mainly afflicting preterm neonates, occurs in about 5% of ELBW infants (see Chapter 36).128 Although NEC may be treated medically, the micropremie with NEC is more likely to require surgery; mortality ranges from 10% to 50%.129–131 The pathogenesis of NEC is incompletely understood; intestinal mucosal ischemia is thought to play a key role. Other key contributing factors include inflammation of bowel mucosa, alterations in normal intestinal flora by antibiotic therapy, gastric alkalinity, low systemic cardiac output, and red blood cell transfusion in the preceding 48 hours.132–135 Early signs of NEC include feeding intolerance, increased work of breathing, lethargy, and temperature instability; later signs include hypotension, abdominal distention, apnea, thrombocytopenia, coagulopathy, and multisystem organ failure. Classic radiographic findings include gas in the intestinal wall (pneumatosis intestinalis) and biliary tract, and free air within the abdomen. Indications for surgical exploration include the presence of perforation or continued clinical deterioration despite medical management (Fig. 35-4).
Surgical management of the micropremie with NEC involves either initial primary peritoneal drainage or a laparotomy with resection of necrotic bowel. Primary peritoneal drainage requires a small surgical incision and fewer anesthetic requirements and can be performed at the bedside. Some infants who undergo initial peritoneal drainage will subsequently require laparotomy if their condition worsens. Peritoneal drainage has generally been favored in the smaller and more unstable preterm infants for logistical reasons; thus the ability to compare the two management strategies has been difficult because of multiple confounding variables.128,136 A prospective randomized, multicenter trial comparing laparotomy and peritoneal drainage in infants weighing less than 1500 g with perforated NEC found no difference in survival, development of short-gut syndrome, or length of hospital stay between the two approaches.137
General endotracheal anesthesia with neuromuscular blockade remains the anesthesia technique for NEC surgery. NEC increases the risk for aspiration. Tracheal intubation may be achieved by “awake intubation” or by rapid-sequence anesthetic induction. We prefer an awake intubation in the micropremie because effective cricoid pressure is difficult to apply, a very small force may distort the airway in the infant rendering intubation difficult,138 and arterial desaturation occurs rapidly during apnea. After the administration of IV atropine, awake intubation is performed using an oxyscope, a laryngoscope with an oxygen port to allow for oxygen delivery during direct laryngoscopy (see E-Fig. 12-12, A and B). Awake intubation should be completed efficiently and rapidly in less than 15 seconds. To accomplish this, all equipment and monitors should be prepared, including an ETT with a “hockey stick” bend using a stylet. After preoxygenating the infant and administering 10 to 20 µg/kg IV atropine, the infant’s arms are brought up by an assistant and held against the head (hands pointing to the anesthesiologist) with the elbows adjacent to the ears. This secures the child’s arms and shoulders, preventing the infant from laterally rotating the neck, raising the shoulders, or moving the arms during laryngoscopy. Once the infant is positioned, a #1 straight blade is inserted into the mouth at the right commissure and the tip is advanced toward the glottic opening in one smooth motion. When the infant gags, the ETT, which is being held in the other hand, is poised to pass through the cords. Once the position of the tube is confirmed with the presence of carbon dioxide on the capnogram, the assistant immediately administers a predetermined dose of IV propofol to rapidly induce anesthesia. Inhalational anesthesia and muscle relaxant, as indicated, should be administered while the ETT is taped in place. Chest auscultation in the axillae will confirm a properly placed ETT.
The anesthetic regimens that we prefer for maintenance include (1) IV fentanyl (5 to 10 µg/kg), an inhaled anesthetic (such as isoflurane, sevoflurane, or desflurane), and neuromuscular blocking drug (balanced technique), or (2) IV fentanyl (20 to 50 µg/kg), IV midazolam (0.1 mg/kg), and a neuromuscular blocking drug (high-dose opioid technique). We select the high-dose opioid technique for hemodynamically unstable infants. Pancuronium is an ideal neuromuscular blocking drug when combined with large doses of fentanyl because of its anticholinergic properties, but should be avoided in infants with renal dysfunction. If hypotension persists despite a trial of 10 to 20 mL/kg of IV fluid, we begin an infusion of epinephrine (0.02 to 0.1 µg/kg/min) or dopamine (5 to 20 µg/kg/min). Hypocalcemia during administration of citrated blood products may contribute to hypotension, and requires replacement with either calcium chloride or calcium gluconate (see Chapter 10). When severe shock persists despite IV fluid resuscitation, calcium, and inotropic support, rescue treatment with “stress dose” glucocorticoids may be beneficial. Treatment with hydrocortisone and dexamethasone has been effective in improving arterial pressure in low–birth-weight infants with refractory hypotension.139,140
Intraoperative fluid management in micropremies should begin with continuing the solution that arrives with the infant from the NICU; usually this is a calcium- and/or glucose-containing solution. Alternately, some infants may arrive with a hyperosmolar glucose or dextrose (10%) parenteral nutrition solution. In both cases, these solutions should not be discontinued, but rather continued at the same rate (by infusion pump) or slightly less throughout the surgery to avoid reactive hypoglycemia from increased circulating insulin concentrations. There is no evidence regarding the optimal infusion rates for these solutions during anesthesia. If no solution is being infused, a balanced salt solution (e.g., lactated Ringer’s solution) could be initiated at 4 mL/kg/hr, supplemented with the same solution for third space loss (at least 10 mL/kg/hr), and replacement of blood loss. If no glucose solution is being administered, then a balanced salt solution containing glucose may be administered through a pump. Serum glucose concentrations should be monitored regularly to avoid hypoglycemia. Third space losses include evaporation and vascular leak and are replaced with an isotonic salt solution. Blood losses are replaced with packed red blood cells and fresh frozen plasma to maintain the hemoglobin greater than 10 g/dL and the PT/PTT within normal range. Platelets are administered to keep the platelet count greater than 100,000/mm3. Continuous measurement of arterial pressure and serial measurement of urine output, blood gases, CBC, platelet counts, and PT/PTT aid in the fluid replacement process. Arterial blood gas analysis helps guide ventilation and inspired oxygen concentration. Warming of the operating suite to 80° F (26.7° C), a forced air warmer underneath the infant, and warmed fluids help maintain normothermia during surgery. In preterm infants with NEC, postoperative mechanical ventilation remains the rule. Postoperative analgesia can be provided with a continuous infusion of fentanyl (1 to 3 µg/kg/hr) or intermittent doses of morphine (0.1 mg/kg every 4 to 6 hours). The time it takes for the micropremie to emerge from anesthesia depends on the anesthetic technique (balanced vs. high-dose opioid) and the need for postoperative analgesics. After a high-dose opioid technique, the micropremie may take 12 to 24 hours to emerge, as compared with several hours for a balanced technique. Remifentanil may provide an alternative, which allows an intense opioid effect that rapidly dissipates on discontinuation of the infusion113,114,141–143; an alternate form of analgesia is required for postoperative pain management.
Ligation of Patent Ductus Arteriosus
Failure of the ductus arteriosus to close after birth is common in the micropremie.144 A PDA may incur significant left-to-right shunting of blood, causing excess pulmonary blood flow, congestive heart failure, and respiratory failure. In fact, the diameter of the PDA may be greater than the aorta. In the micropremie with respiratory distress syndrome or persistent pulmonary hypertension, right-to-left shunting across the PDA may occur, producing cyanosis. Significant controversy exists regarding whether a PDA should be aggressively treated, the timing of therapy, and merits of medical versus surgical therapy.145 Medical therapy involves the administration of a cyclooxygenase inhibitor, such as indomethacin or ibuprofen. Indomethacin therapy is less likely to close the PDA in micropremies compared with preterm infants, and more likely to produce complications, including thrombocytopenia, renal failure, hyponatremia, and intestinal perforation.146 Ibuprofen is equally effective for PDA closure in the micropremie, with a reduced frequency of renal failure.147 When surgery is performed by experienced teams, the incidence of major intraoperative complications is small.148 However, as many as one-third of preterm infants develop severe cardiovascular instability following PDA ligation, as evidenced by systemic hypotension, pulmonary hypertension, and myocardial dysfunction.149
The anesthetic technique of choice for PDA ligation remains fentanyl (20 to 50 µg/kg) and pancuronium (0.2 mg/kg).100 Although this technique usually does not cause hypotension or bradycardia, reduction in arterial pressure after anesthetic induction may occur because of loss of sympathetic tone, especially in the setting of hypovolemia from diuretic therapy. Thus we commonly administer 5% albumin or balanced salt solution (10 mL/kg) before induction. During mechanical ventilation, mild hypoventilation and a reduced inspired oxygen concentration help to reduce pulmonary over-circulation from the PDA. However, during surgical retraction of the lung it is usually necessary to increase the ventilator inspiratory pressure setting as well as the inspired oxygen concentration. Surgical complications of PDA ligation include inadvertent ligation or laceration of the aorta or pulmonary artery. The lower extremity oxygen saturation provides a monitor of perfusion to the legs, and loss of this signal immediately after PDA ligation may indicate aortic ligation. With ligation of the pulmonary artery, oxygen saturation in both extremities and end-tidal CO2 decrease. With successful PDA ligation, arterial diastolic and mean pressure increase, and the PDA murmur disappears.
Inguinal Hernia Repair
Inguinal hernias are common in preterm infants. In ELBW infants, an inguinal hernia occurs in approximately one-third of patients, whereas in full-term neonates, it occurs in 1%.150 Complications related to inguinal hernias and their surgical repair include incarcerated bowel, intestinal obstruction, gonadal infarction, infection, hematoma, and recurrent hernias.151 Because of the risk of incarceration and bowel infarction, the hernia should be repaired as soon as the infant is medically stable.
General anesthesia is maintained with an inhalational anesthetic agent. We avoid nitrous oxide when intestinal obstruction is present, or when the infant has complex hernias that may take several hours to repair. Preterm infants with BPD often exhibit a compensated respiratory acidosis and will have increased end-tidal CO2 during anesthesia. In these infants the ventilation parameters are set to allow permissive hypercapnia. Mechanical ventilation with small tidal volumes (4 to 6 mL/kg), increased respiratory rates, and PEEP minimizes atelectasis and reduces the risk for lung injury. If extubation is planned after surgery, caffeine (10 mg/kg) is administered IV to reduce the risk of postoperative apnea.21,152 IV fluids consist of lactated Ringer’s solution (4 mL/kg/hr) with dextrose (5 mg/kg/min administered by infusion pump) to maintain normoglycemia. A warm OR and forced-air warming blanket should be used to prevent hypothermia during the procedure.
Postoperative analgesia may be provided by regional analgesia or systemic analgesics; the choice depends on whether extubation of the trachea occurs immediately after surgery. Ilioinguinal and iliohypogastric nerve blocks or caudal epidural blocks may be used (see Chapter 41). We do not advocate percutaneous blockade because the local anesthetic injectate may distort the local tissues, making an already difficult surgery more difficult. Ilioinguinal and iliohypogastric nerve blocks consist of injecting 0.25% bupivacaine with epinephrine (1: 200,000) (1 mg/kg/side) around the nerve by the surgeon under direct vision. This block provides 6 to 8 hours of analgesia. Caudal epidural blockade consists of injecting 0.125% bupivacaine with epinephrine (1 : 200,000) (1 mL/kg) through the sacral hiatus, providing several hours of analgesia. Orally administered acetaminophen (10 to 15 mg/kg) can also be given, providing analgesia for several hours. We do not administer opioids to the micropremie whose trachea will be extubated immediately after surgery because of the risk of postoperative apnea.
The micropremie with BPD who requires supplemental oxygen, but who is not intubated, is an excellent candidate for regional anesthesia.153–156 Regional anesthesia circumvents the need to intubate the trachea and administer a general anesthetic, which may exacerbate the BPD and create a situation in which extubation after surgery may be difficult to achieve. Either a spinal or epidural anesthetic may be used to provide surgical conditions with a regional block. For spinal anesthesia, an intrathecal injection of a hyperbaric solution of tetracaine (1 mg/kg) or isobaric bupivacaine (1 mg/kg) provides 1 to 2 hours of surgical anesthesia (see Chapter 41) The addition of epinephrine (10 µg) to the tetracaine solution increases the duration of anesthesia by 30 to 60 minutes.154 Hypotension rarely occurs after spinal anesthesia in an infant.157 For epidural anesthesia, 0.75 mL/kg of 0.375% bupivacaine with epinephrine is injected into the epidural space through the sacral hiatus.155,156 Ultrasound can be used to map out the anatomy of the spinal cord and cauda equina, dura mater, and intrathecal space. During injection one can visualize the local anesthetic tracking up the epidural space to confirm its proper location (see also Chapters 41 and 42). Preterm infants are more sensitive to local anesthetic blockade than are children and adults; therefore surgical anesthesia may be achieved with reduced concentrations of local anesthetic. Another route to the epidural space is by the lumbar approach (L3-4 interspace).19 The use of the lumbar route allows a smaller dose of local anesthetic compared with the caudal route, thus decreasing the risk of toxicity, however, this route is technically more challenging.
Epidural anesthesia offers several advantages over spinal anesthesia in the awake infant, in that it is often easier to perform, and a catheter can be placed to repeatedly administer local anesthetic to extend the duration of the block (see Chapter 41). General anesthesia offers several advantages over regional anesthesia, including better operating conditions and greater ease in titrating anesthetic duration. An often cited advantage of regional anesthesia is the decreased incidence of postoperative apnea. However, the evidence in support of this notion is conflicting. When the incidence of postoperative apnea after spinal anesthesia, spinal anesthesia with IV ketamine, and general endotracheal inhalational anesthesia was compared,158 no postoperative apnea was reported in the spinal anesthesia group, whereas an 89% incidence was noted in the spinal/ketamine group and a 31% incidence was noted in the general anesthesia group. Thus, if there is a benefit to regional anesthesia over general anesthesia, spinal anesthesia must be administered without supplemental sedatives, such as ketamine.
In contrast, Krane and colleagues compared general endotracheal inhalational anesthesia with spinal anesthesia and found no difference in the incidence of postoperative apnea.159 In a study of more than 250 preterm infants receiving spinal anesthesia for inguinal hernia repair at a single institution experienced with this technique, spinal block was successfully placed on the first attempt in more than 90% of infants.160 Despite a high rate of successful block placement, more than 20% of the infants required supplemental anesthesia at some time during the surgery. Postoperative apnea occurred in 4.9% of infants, and all infants who developed this complication had a preoperative history of apnea. Given the technical challenges of spinal block placement for practitioners who do not perform it routinely, failure rate even with successful block placement, and persistent risk of postoperative apnea, we commonly administer general anesthesia for hernia repair in the micropremie, except for those with severe BPD, in whom we use spinal anesthesia.
Eye Surgery for Retinopathy of Prematurity
ROP may be treated with cryotherapy, laser photocoagulation, or scleral buckling surgery and/or vitrectomy. Diode laser photocoagulation is typically performed at the bedside in the NICU for moderate ROP, whereas cryotherapy and scleral buckling surgery are performed in the OR. Cryotherapy involves applying a freezing probe under direct visualization to the avascular retina anterior to the fibrovascular ridge. Scleral buckling surgery and vitrectomy, performed for severe ROP with retinal detachment, are less frequently employed because early detection and treatment with laser photocoagulation prevents ROP progression to severe disease. Laser surgery has been shown to be as effective as cryotherapy for moderate ROP, and is most commonly used because the systemic side effects are significantly less, the ocular tissues are less traumatized, and this technique has a smaller incidence of late complications (see Chapter 32).
Laser photocoagulation may be performed under topical anesthesia alone, with IV sedation, or under general anesthesia. Cryotherapy and scleral buckling surgery require general anesthesia. For laser photocoagulation, the incidence of cardiorespiratory complications is greater with topical anesthesia alone than with topical anesthesia with sedation or general anesthesia.161 Factors that influence the selection of anesthetic technique include the infant’s medical condition, gestational age, and availability of pediatric anesthesia services. The vast majority (95%) of preterm infants who require laser photocoagulation or cryotherapy develop threshold disease between 32 and 42 weeks PCA. The usual age for scleral buckling or vitrectomy is much older, between 6 months and 1 year.
Anesthesia or Sedation for Radiologic Imaging
Although not painful, MRI requires absolute immobility for the duration of the scan, typically 45 to 60 minutes. Immobility may be achieved through simulated feeding, sedation, or anesthesia. In very preterm infants (less than 30 weeks PCA) and in good-tempered older preterm infants, swaddling the infant in warm blankets and applying sugar water (“sweet-ez”) to a pacifier often promotes natural sleep, enabling the scan to be obtained. In healthy preterm infants (“premie growers”) aged 30 to 70 weeks PCA, orally administered chloral hydrate (75 mg/kg) 30 to 60 minutes before the procedure provides sedation lasting 2 hours. We perform a history and physical examination and prescribe the sedation regimen, and specially trained sedation nurses in our department administer the regimen and monitor the infant during the scan, under our supervision. It should be noted that chloral hydrate has an extremely long half-life in this population, placing the infants at risk for late re-sedation and/or apnea.162–164
In preterm infants with medical problems, we administer propofol anesthesia for the scan, titrated to effect similarly to that with laser photocoagulation for ROP. We monitor expired CO2 through a nasal cannula, sometimes insert an oral airway, and less often use a laryngeal mask airway or an ETT. In critically ill preterm infants with an ETT and mechanical ventilation, we administer IV midazolam and fentanyl or inhaled sevoflurane for the scan. In anesthesia cases, monitors include electrocardiogram, blood pressure, pulse oximetry, and expired CO2. IV pumps being used for administration of fluids or drugs must remain outside the MRI room, thus requiring extra-long tubing. All anesthesia equipment, the infant, and personnel must not contain ferrous materials. As with general anesthesia, monitoring for postoperative apnea for 24 hours after sedation is recommended (see Chapters 45 and 47).
Baum VC, Palmisano BW. The immature heart and anesthesia. Anesthesiology. 1997;87:1529–1548.
Coté CJ, Zaslavsky A, Downes JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy. Anesthesiology. 1995;82:809–822.
Friesen RH, Henry DB. Cardiovascular changes in preterm neonates receiving isoflurane, halothane, fentanyl, and ketamine. Anesthesiology. 1986;64:238–242.
Mikkola K, Ritari N, Tommiska V, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996-1997. Pediatrics. 2005;116:1391–1400.
Thome UH, Ambalavanan N. Permissive hypercapnia to decrease lung injury in ventilated preterm neonates. Semin Fetal Neonatal Med. 2009;14:21–27.
1 Perrott S, Dodds L, Vincer M. A population-based study of prognostic factors related to major disability in very preterm survivors. J Perinatol. 2003;23:111–116.
2 O’Shea TM, Klinepeter KL, Goldstein DJ, Jackson BW, Dillard RG. Survival and developmental disability in infants with birth weights of 501 to 800 grams, born between 1979 and 1994. Pediatrics. 1997;100:982–986.
3 Lasswell SM, Barfield WD, Rochat RW, Blackmon L. Perinatal regionalization for very low-birth-weight and very preterm infants: a meta-analysis. JAMA. 2010;304:992–1000.
4 Thome UH, Ambalavanan N. Permissive hypercapnia to decrease lung injury in ventilated preterm neonates. Semin Fetal Neonatal Med. 2009;14:21–27.
5 Miller JD, Carlo WA. Safety and effectiveness of permissive hypercapnia in the preterm infant. Curr Opin Pediatr. 2007;19:142–144.
6 Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360.
7 Rigatto H, Brady JP. Periodic breathing and apnea in preterm infants. I. Evidence for hypoventilation possibly due to central respiratory depression. Pediatrics. 1972;50:202–218.
8 Rigatto H, Brady JP, De La Torre Verduzco R. Chemoreceptor reflexes in preterm infants: I. The effect of gestational and postnatal age on the ventilatory response to inhalation of 100% and 15% oxygen. Pediatrics. 1975;55:604–613.
9 Rigatto H, Brady JP, De La Torre Verduzco R. Chemoreceptor reflexes in preterm infants: II. The effect of gestational and postnatal age on the ventilatory response to inhaled carbon dioxide. Pediatrics. 1975;55:614–620.
10 Keens TG, Bryan AC, Levison H, Ianuzzo CD. Developmental pattern of muscle fiber types in human ventilatory muscles. J Appl Physiol Resp Env Ex Physiol. 1978;44:909–913.
11 Keens TG, Chen V, Patel P, et al. Cellular adaptations of the ventilatory muscles to a chronic increased respiratory load. J Appl Physiol. 1978;44:905–908.
12 Daily WJ, Klaus M, Meyer HB. Apnea in premature infants: monitoring, incidence, heart rate changes, and an effect of environmental temperature. Pediatrics. 1969;43:510–518.
13 Gerhardt T, Bancalari E. Apnea of prematurity: I. Lung function and regulation of breathing. Pediatrics. 1984;74:58–62.
14 Kurth CD, Spitzer AR, Broennle AM, Downes JJ. Postoperative apnea in preterm infants. Anesthesiology. 1987;66:483–488.
15 Coté CJ, Zaslavsky A, Downes JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy. A combined analysis. Anesthesiology. 1995;82:809–822.
16 Kurth CD, LeBard SE. Association of postoperative apnea, airway obstruction, and hypoxemia in former premature infants. Anesthesiology. 1991;75:22–26.
17 Engle WA. Age terminology during the perinatal period. Pediatrics. 2004;114:1362–1364.
18 Welborn LG, Hannallah RS, Luban NLC, Fink R, Ruttimann UE. Anemia and postoperative apnea in former preterm infants. Anesthesiology. 1991;74:1003–1006.
19 Webster AC, McKishnie JD, Watson JT, Reid WD. Lumbar epidural anaesthesia for inguinal hernia repair in low birth weight infants. Can J Anaesth. 1993;40:670–675.
20 Shenkman Z, Hoppenstein D, Litmanowitz I, et al. Spinal anesthesia in 62 premature, former-premature or young infants–technical aspects and pitfalls. Can J Anaesth. 2002;49:262–269.
21 Welborn LG, Hannallah RS, Fink R, Ruttimann UE, Hicks JM. High-dose caffeine suppresses postoperative apnea in former preterm infants. Anesthesiology. 1989;71:347–349.
22 Anderson BJ, Gunn TR, Holford NH, Johnson R. Caffeine overdose in a premature infant: clinical course and pharmacokinetics. Anaesth Intensive Care. 1999;27:307–311.
23 Lee TC, Charles B, Steer P, Flenady V, Shearman A. Population pharmacokinetics of intravenous caffeine in neonates with apnea of prematurity. Clin Pharmacol Ther. 1997;61:628–640.
24 Pons G, Carrier O, Richard MO, et al. Developmental changes of caffeine elimination in infancy. Dev Pharmacol Ther. 1988;11:258–264.
25 Aldridge A, Aranda JV, Neims AH. Caffeine metabolism in the newborn. Clin Pharmacol Ther. 1979;25:447–453.
26 Aranda JV, Cook CE, Gorman W, et al. Pharmacokinetic profile of caffeine in the premature newborn infant with apnea. J Pediatr. 1979;94:663–668.
27 Natarajan G, Botica ML, Thomas R, Aranda JV. Therapeutic drug monitoring for caffeine in preterm neonates: an unnecessary exercise? Pediatrics. 2007;119:936–940.
28 Friedman WF. The intrinsic physiologic properties of the developing heart. In: Friedman WF, Lesch M, Sonnenblick EH, eds. Neonatal heart disease. Philadelphia: Grune & Stratton; 1973:21–49.
29 Baum VC, Palmisano BW. The immature heart and anesthesia. Anesthesiology. 1997;87:1529–1548.
30 Lou HC, Lassen NA, Friis-Hansen B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. J Pediatr. 1979;94:118–121.
31 Gregory GA. The baroresponses of preterm infants during halothane anaesthesia. Can Anaesth Soc J. 1982;29:105–107.
32 Evans N, Iyer P. Incompetence of the foramen ovale in preterm infants supported by mechanical ventilation. J Pediatr. 1994;125:786–792.
33 Filippi L, Palermo L, Pezzati M, et al. Paradoxical embolism in a preterm infant. Dev Med Child Neurol. 2004;46:713–716.
34 Barrington KJ, Finer NN. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev. 2006. CD000509
35 Barrington KJ, Finer N. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev. 2010. CD000509
36 Hoehn T, Krause MF, Buhrer C. Meta-analysis of inhaled nitric oxide in premature infants: an update. Klin Padiatr. 2006;218:57–61.
37 Mikkola K, Ritari N, Tommiska V, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996-1997. Pediatrics. 2005;116:1391–1400.
38 Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112:176–180.
39 Lee SJ, Ralston HJ, Drey EA, Partridge JC, Rosen MA. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294:947–954.
40 Counsell SJ, Allsop JM, Harrison MC, et al. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112:1–7.
41 Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947.
42 Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M. Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr. 2006;149:169–173.
43 Wells JT, Ment LR. Prevention of intraventricular hemorrhage in preterm infants. Early Hum Dev. 1995;42:209–233.
44 Kaiser JR, Gauss CH, Pont MM, Williams DK. Hypercapnia during the first 3 days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. J Perinatol. 2006;26:279–285.
45 Mullaart RA, Hopman JC, Rotteveel JJ, et al. Cerebral blood flow fluctuation in neonatal respiratory distress and periventricular haemorrhage. Early Hum Dev. 37, 1994. 179–178
46 Lermann VL, Fortes Filho JB, Procianoy RS. The prevalence of retinopathy of prematurity in very low birth weight newborn infants. J Pediatr (Rio J). 2006;82:27–32.
47 Sapieha P, Joyal JS, Rivera JC, et al. Retinopathy of prematurity: understanding ischemic retinal vasculopathies at an extreme of life. J Clin Invest. 2010;120:3022–3032.
48 Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–731.
49 Cunningham S, Fleck BW, Elton RA, McIntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–1465.
50 Askie LM, Henderson-Smart DJ, Ko H. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database Syst Rev. 2009. CD001077
51 Mohamed S, Schaa K, Cooper ME, et al. Genetic contributions to the development of retinopathy of prematurity. Pediatr Res. 2009;65:193–197.
52 Dammann O, Brinkhaus MJ, Bartels DB, et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: a multi-hit hypothesis. Early Hum Dev. 2009;85:325–329.
53 Kalina RE, Hodson WA, Morgan BC. Retrolental fibroplasia in a cyanotic infant. Pediatrics. 1972;50:765–768.
54 Hammarlund K, Sedin G, Stromberg B. Transepidermal water loss in newborn infants. VIII. Relation to gestational age and post-natal age in appropriate and small for gestational age infants. Acta Paediatr Scand. 1983;72:721–728.
55 Jahnukainen T, van Ravenswaaij-Arts C, Jalonen J, Valimaki I. Dynamics of vasomotor thermoregulation of the skin in term and preterm neonates. Early Hum Dev. 1993;33:133–143.
56 Rodriguez MM, Gomez A, Abitbol C, et al. Comparative renal histomorphometry: a case study of oligonephropathy of prematurity. Pediatr Nephrol. 2005;20:945–949.
57 Rodriguez MM, Gomez AH, Abitbol CL, et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol. 2004;7:17–25.
58 Bueva A, Guignard JP. Renal function in preterm neonates. Pediatr Res. 1994;36:572–577.
59 Guignard JP, Drukker A. Why do newborn infants have a high plasma creatinine? Pediatrics. 1999;103:e49.
60 de HM, Mouton JW, van den Anker JN. New dosing strategies for antibacterial agents in the neonate. Semin Fetal Neonatal Med. 2005;10:185–194.
61 Takahashi N, Hoshi J, Nishida H. Water balance, electrolytes and acid-base balance in extremely premature infants. Acta Paediatr Jpn. 1994;36:250–255.
62 Sato K, Kondo T, Iwao H, Honda S, Ueda K. Internal potassium shift in premature infants: cause of nonoliguric hyperkalemia. J Pediatr. 1995;126:109–113.
63 Yuan HC, Jeng MJ, Soong WJ, Chen SJ, Hwang BT. Hyperkalemia during the early postnatal days in premature infants. Acta Paediatr Taiwan. 2003;44:208–214.
64 Kluckow M, Evans N. Low systemic blood flow and hyperkalemia in preterm infants. J Pediatr. 2001;139:227–232.
65 Mericq V. Prematurity and insulin sensitivity. Horm Res. 2006;65:131–136.
66 Hawdon JM, Aynsley-Green A, Alberti KG, Ward Platt MP. The role of pancreatic insulin secretion in neonatal glucoregulation. I. Healthy term and preterm infants. Arch Dis Child. 1993;68:274–279.
67 Ng SM, May JE, Emmerson AJ. Continuous insulin infusion in hyperglycaemic extremely-low-birth-weight neonates. Biol Neonate. 2005;87:269–272.
68 Sieber FE, Traystman RJ. Special issues: glucose and the brain. Crit Care Med. 1992;20:104–114.
69 Vannucci RC, Mujsce DJ. Effect of glucose on perinatal hypoxic-ischemic brain damage. Biol Neonate. 1992;62:215–224.
70 Kurth CD, Priestley M, Golden J, McCann J, Raghupathi R. Regional patterns of neuronal death after deep hypothermic circulatory arrest in newborn pigs. J Thorac Cardiovasc Surg. 1999;118:1068–1077.
71 de Ferranti S, Gauvreau K, Hickey PR, et al. Intraoperative hyperglycemia during infant cardiac surgery is not associated with adverse neurodevelopmental outcomes at 1, 4, and 8 years. Anesthesiology. 2004;100:1345–1352.
72 Loepke AW, Spaeth JP. Glucose and heart surgery: neonates are not just small adults. Anesthesiology. 2004;100:1339–1341.
73 Kinnala A, Rikalainen H, Lapinleimu H, et al. Cerebral magnetic resonance imaging and ultrasonography findings after neonatal hypoglycemia. Pediatrics. 1999;103:724–729.
74 Chen CM, Hsu YH. Subcapsular hemorrhage of the liver in a neonate with extremely low birthweight. J Formos Med Assoc. 1993;92:1104–1106.
75 Emma F, Smith J, Moerman PH, et al. Subcapsular hemorrhage of the liver and hemoperitoneum in premature infants: report of 4 cases. Eur J Obstet Gynecol Reprod Biol. 1992;44:161–164.
76 Filan PM, Mills JF, Clarnette TD, Ekert H, Ekert P. Spontaneous liver hemorrhage during laparotomy for necrotizing enterocolitis: a potential role for recombinant factor VIIa. J Pediatr. 2005;147:857–859.
77 Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–1691.
78 Christensen RD, Henry E, Wiedmeier SE, et al. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. J Perinatol. 2006;26:348–353.
79 Kurth CD, Priestley M, Watzman HM, McCann J, Golden J. Desflurane confers neurologic protection for deep hypothermic circulatory arrest in newborn pigs. Anesthesiology. 2001;95:959–964.
80 Loepke AW, Priestley MA, Schultz SE, et al. Desflurane improves neurologic outcome after low-flow cardiopulmonary bypass in newborn pigs. Anesthesiology. 2002;97:1521–1527.
81 Zhan X, Fahlman CS, Bickler PE. Isoflurane neuroprotection in rat hippocampal slices decreases with aging: changes in intracellular Ca2+ regulation and N-methyl-d-aspartate receptor-mediated Ca2+ influx. Anesthesiology. 2006;104:995–1003.
82 Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882.
83 Hayashi H, Dikkes P, Soriano SG. Repeated administration of ketamine may lead to neuronal degeneration in the developing rat brain. Paediatr Anaesth. 2002;12:770–774.
84 Yon JH, niel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827.
85 Ikonomidou C, Bittigau P, Koch C, et al. Neurotransmitters and apoptosis in the developing brain. Biochem Pharmacol. 2001;62:401–405.
86 Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74.
87 Shih J, May LD, Gonzalez HE, et al. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116:586–602.
88 Bhutta AT, Anand KJ. Vulnerability of the developing brain. Neuronal mechanisms. Clin Perinatol. 2002;29:357–372.
89 Anand KJ. Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med. 2000;6:971–973.
90 Anand KJ, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326:1–9.
91 Shu Y, Zhou Z, Wan Y, et al. Nociceptive stimuli enhance anesthetic-induced neuroapoptosis in the rat developing brain. Neurobiol Dis. 2012;45:743–750.
92 Guerra GG, Robertson CM, Alton GY, et al. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy. Paediatr Anaesth. 2011;21:932–941.
93 LeDez KM, Lerman J. The minimum alveolar concentration (MAC) of isoflurane in preterm neonates. Anesthesiology. 1987;67:301–307.
94 Lynch RE. Ionized calcium: pediatric perspective. Pediatr Clin North Am. 1990;37:373–389.
95 Cardenas-Rivero N, Chernow B, Stoiko MA, Nussbaum SR, Todres ID. Hypocalcemia in critically ill children. J Pediatr. 1989;114:946–951.
96 Kanaya N, Kawana S, Tsuchida H, et al. Comparative myocardial depression of sevoflurane, isoflurane, and halothane in cultured neonatal rat ventricular myocytes. Anesth Analg. 1998;87:1041–1047.
97 Chin TK, Friedman WF, Klitzner TS. Developmental changes in cardiac myocyte calcium regulation. Circ Res. 1990;67:574–579.
98 Eger EI, II., Saidman LJ. Hazards of nitrous oxide anesthesia in bowel obstruction and pneumothorax. Anesthesiology. 1965;26:61–66.
99 Ohashi Y, Stowell JM, Nelson LE, et al. Nitrous oxide exerts age-dependent antinociceptive effects in Fischer rats. Pain. 2002;100:7–18.
100 Robinson S, Gregory GA. Fentanyl-air-oxygen anesthesia for ligation of patent ductus arteriosus in preterm infants. Anesth Analg. 1981;60:331–334.
101 Yaster M. The dose response of fentanyl in neonatal anesthesia. Anesthesiology. 1987;66:433–435.
102 Collins C, Koren G, Crean P, et al. Fentanyl pharmacokinetics and hemodynamic effects in preterm infants during ligation of patent ductus arteriosus. Anesth Analg. 1985;64:1078–1080.
103 Saarenmaa E, Neuvonen PJ, Fellman V. Gestational age and birth weight effects on plasma clearance of fentanyl in newborn infants. J Pediatr. 2000;136:767–770.
104 Koehntop DE, Rodman JH, Brundage DM, Hegland MG, Buckley JJ. Pharmacokinetics of fentanyl in neonates. Anesth Analg. 1986;65:227–232.
105 Koren G, Butt W, Chinyanga H, et al. Postoperative morphine infusion in newborn infants: assessment of disposition characteristics and safety. J Pediatr. 1985;107:963–967.
106 Anand KJ, Anderson BJ, Holford NH, et al. Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial. Br J Anaesth. 2008;101:680–689.
107 Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92:208–217.
108 Saarenmaa E, Neuvonen PJ, Rosenberg P, Fellman V. Morphine clearance and effects in newborn infants in relation to gestational age. Clin Pharmacol Ther. 2000;68:160–166.
109 Michelsen LG, Hug CC, Jr. The pharmacokinetics of remifentanil. J Clin Anesth. 1996;8:679–682.
110 Davis PJ, Galinkin J, McGowan FX, et al. A randomized multicenter study of remifentanil compared with halothane in neonates and infants undergoing pyloromyotomy. I. Emergence and recovery profiles. Anesth Analg. 2001;93:1380–1386.
111 Galinkin JL, Davis PJ, McGowan FX, et al. A randomized multicenter study of remifentanil compared with halothane in neonates and infants undergoing pyloromyotomy. II. Perioperative breathing patterns in neonates and infants with pyloric stenosis. Anesth Analg. 2001;93:1387–1392.
112 Ross AK, Davis PJ, Dear Gd GL, et al. Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery or diagnostic procedures. Anesth Analg. 2001;93:1393–1401.
113 Sammartino M, Garra R, Sbaraglia F, et al. Experience of remifentanil in extremely low-birth-weight babies undergoing laparotomy. Pediatr Neonatol. 2011;52:176–179.
114 Sommer M, Riedel J, Fusch C, Fetsge OA, Hachenberg T. Intravenous anaesthesia with remifentanil in a preterm infant. Paediatr Anaesth. 2001;11:252–254.
115 Welzing L, Ebenfeld S, Dlugay V, et al. Remifentanil degradation in umbilical cord blood of preterm infants. Anesthesiology. 2011;114:570–577.
116 Friesen RH, Henry DB. Cardiovascular changes in preterm neonates receiving isoflurane, halothane, fentanyl, and ketamine. Anesthesiology. 1986;64:238–242.
117 Anand KJ. Pharmacological approaches to the management of pain in the neonatal intensive care unit. J Perinatol. 2007;27:S4–11.
118 Westrin P, Jonmarker C, Werner O. Thiopental requirements for induction of anesthesia in neonates and in infants one to six months of age. Anesthesiology. 1989;71:344–346.
119 Veyckemans F. Propofol for intubation of the newborn? Paediatr Anaesth. 2001;11:630–631.
120 Lerman J, Heard C, Steward DJ. Neonatal tracheal intubation: an imbroglio unresolved. Paediatr Anaesth. 2010;20:585–590.
121 Sammartino M, Garra R, Sbaraglia F, Papacci P. Propofol overdose in a preterm baby: may propofol infusion syndrome arise in two hours? Paediatr Anaesth. 2010;20:973–974.
122 Parke TJ, Stevens JE, Rice AS, et al. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. BMJ.. 1992;305:613–616.
123 Lee TC, Charles BG, Harte GJ, et al. Population pharmacokinetic modeling in very premature infants receiving midazolam during mechanical ventilation: midazolam neonatal pharmacokinetics. Anesthesiology. 1999;90:451–457.
124 Jacqz-Aigrain E, Daoud P, Burtin P, Maherzi S, Beaufils F. Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. Eur J Clin Pharmacol. 1992;42:329–332.
125 van Straaten HL, Rademaker CM, de Vries LS. Comparison of the effect of midazolam or vecuronium on blood pressure and cerebral blood flow velocity in the premature newborn. Dev Pharmacol Ther. 1992;19:191–195.
126 Lovich MA, Kinnealley ME, Sims NM, Peterfreund RA. The delivery of drugs to patients by continuous intravenous infusion: modeling predicts potential dose fluctuations depending on flow rates and infusion system dead volume. Anesth Analg. 2006;102:1147–1153.
127 Lovich MA, Doles J, Peterfreund RA. The impact of carrier flow rate and infusion set dead-volume on the dynamics of intravenous drug delivery. Anesth Analg. 2005;100:1048–1055.
128 Blakely ML, Tyson JE, Lally KP, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics. 2006;117:e680–e687.
129 Snyder CL, Gittes GK, Murphy JP, et al. Survival after necrotizing enterocolitis in infants weighing less than 1,000 g: 25 years’ experience at a single institution. J Pediatr Surg. 1997;32:434–437.
130 Rowe MI, Reblock KK, Kurkchubasche AG, Healey PJ. Necrotizing enterocolitis in the extremely low birth weight infant. J Pediatr Surg. 1994;29:987–990.
131 Blakely ML, Lally KP, McDonald S, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg. 2005;241:984–989.
132 Markel TA, Crisostomo PR, Wairiuko GM, et al. Cytokines in necrotizing enterocolitis. Shock. 2006;25:329–337.
133 Bin-Nun A, Bromiker R, Wilschanski M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147:192–196.
134 Guillet R, Stoll BJ, Cotten CM, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117:e137–e142.
135 Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129:529–540.
136 Petty JK, Ziegler MM. Operative strategies for necrotizing enterocolitis: The prevention and treatment of short-bowel syndrome. Semin Pediatr Surg. 2005;14:191–198.
137 Moss RL, Dimmitt RA, Barnhart DC, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med. 2006;354:2225–2234.
138 Walker RW, Ravi R, Haylett K. Effect of cricoid force on airway calibre in children: a bronchoscopic assessment. Br J Anaesth. 2010;104:71–74.
139 Ng PC, Lee CH, Bnur FL, et al. A double-blind, randomized, controlled study of a “stress dose” of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics. 2006;117:367–375.
140 Noori S, Siassi B, Durand M, et al. Cardiovascular effects of low-dose dexamethasone in very low birth weight neonates with refractory hypotension. Biol Neonate. 2006;89:82–87.
141 Penido MG, Garra R, Sammartino M, Pereira e Silva Y. Remifentanil in neonatal intensive care and anaesthesia practice. Acta Paediatr. 2010;99:1454–1463.
142 Eck JB, Lynn AM. Use of remifentanil in infants. Paediatr Anaesth. 1998;8:437–439.
143 Lynn AM. Remifentanil: the paediatric anaesthetist’s opiate? Pediatr Anaesth. 1996;6:433–435.
144 Trus T, Winthrop AL, Pipe S, et al. Optimal management of patent ductus arteriosus in the neonate weighing less than 800 g. J Pediatr Surg. 1993;28:1137–1139.
145 Noori S. Patent ductus arteriosus in the preterm infant: to treat or not to treat? J Perinatol. 2010;30(Suppl):S31–S37.
146 Little DC, Pratt TC, Blalock SE, et al. Patent ductus arteriosus in micropreemies and full-term infants: the relative merits of surgical ligation versus indomethacin treatment. J Pediatr Surg. 2003;38:492–496.
147 Linder N, Bello R, Hernandez A, et al. Treatment of patent ductus arteriosus: indomethacin or ibuprofen? Am J Perinatol. 2010;27:399–404.
148 Gould DS, Montenegro LM, Gaynor JW, et al. A comparison of on-site and off-site patent ductus arteriosus ligation in premature infants. Pediatrics. 2003;112:1298–1301.
149 Natarajan G, Chawla S, Aggarwal S. Short-term outcomes of patent ductus arteriosus ligation in preterm neonates: reason for concern? Am J Perinatol. 2010;27:431–437.
150 Craven PD, Badawi N, Henderson-Smart DJ, O’Brien M. Regional (spinal, epidural, caudal) versus general anaesthesia in preterm infants undergoing inguinal herniorrhaphy in early infancy. Cochrane Database Syst Rev. 2003. CD003669
151 Rescorla FJ, Grosfeld JL. Inguinal hernia repair in the perinatal period and early infancy: clinical considerations. J Pediatr Surg. 1984;19:832–837.
152 Welborn LG, De Soto H, Hannallah RS, et al. The use of caffeine in the control of post-anesthetic apnea in former premature infants. Anesthesiology. 1988;68:796–1006.
153 Webster AC, McKishnie JD, Kenyon CF, Marshall DG. Spinal anaesthesia for inguinal hernia repair in high-risk neonates. Can J Anaesth. 1991;38:281–286.
154 Abajian JC, Mellish RW, Browne AF, et al. Spinal anesthesia for surgery in the high-risk infant. Anesth Analg. 1984;63:359–362.
155 Gunter JB, Forestner JE, Manley CB. Caudal epidural anesthesia reduces blood loss during hypospadias repair. J Urol. 1990;144:517–519. discussion
156 Spear RM, Deshpande JK, Maxwell LG. Caudal anesthesia in the awake, high-risk infant. Anesthesiology. 1988;69:407–409.
157 Oberlander TF, Berde CB, Lam KH, Rappaport LA, Saul JP. Infants tolerate spinal anesthesia with minimal overall autonomic changes: analysis of heart rate variability in former premature infants undergoing hernia repair. Anesth Analg. 1995;80:20–27.
158 Welborn LG, Rice LJ, Hannallah RS, et al. Postoperative apnea in former preterm infants: prospective comparison of spinal and general anesthesia. Anesthesiology. 1990;72:838–842.
159 Krane EJ, Haberkern CM, Jacobson LE. Postoperative apnea, bradycardia, and oxygen desaturation in formerly premature infants: prospective comparison of spinal and general anesthesia. Anesth Analg. 1995;80:7–13.
160 Frumiento C, Abajian JC, Vane DW. Spinal anesthesia for preterm infants undergoing inguinal hernia repair. Arch Surg. 2000;135:445–451.
161 Haigh PM, Chiswick ML, O’Donoghue EP. Retinopathy of prematurity: systemic complications associated with different anaesthetic techniques at treatment. Br J Ophthalmol. 1997;81:283–287.
162 Mayers DJ, Hindmarsh KW, Sankaran K, Gorecki DK, Kasian GF. Chloral hydrate disposition following single-dose administration to critically ill neonates and children. Dev Pharm & Ther. 1991;16:71–77.
163 Malviya S, Voepel-Lewis T, Tait AR, et al. Pentobarbital vs chloral hydrate for sedation of children undergoing MRI: efficacy and recovery characteristics. Paediatr Anaesth. 2004;14:589–595.
164 Malviya S, Voepel-Lewis T, Ludomirsky A, Marshall J, Tait AR. Can we improve the assessment of discharge readiness? A comparative study of observational and objective measures of depth of sedation in children. Anesthesiology. 2004;100:218–224.