The Esophagus
Embryologically, the trachea and esophagus are intimately related. The trachea and esophagus both develop from the foregut as a median ventral diverticulum. Familiarity with the embryologic development of the esophagus and trachea is important to understand the congenital abnormalities that arise from these structures. The classic description of these malformations proposes impairment in the process of septation of the trachea and esophagus.1
In humans, normal development of the foregut begins during the fourth week of gestation. At 22 days’ gestation, the foregut endoderm differentiates into a ventral respiratory part and a dorsal esophageal part. The separation of the respiratory part from the esophageal part is achieved by the formation of lateral longitudinal tracheoesophageal folds. The trachea and esophagus elongate first distally and then proximally. At 6 to 7 weeks’ gestation, the separation of the esophagus and trachea is complete. At birth, the esophagus is 8–10 cm in length.2 The length of the esophagus will double in the first few years of life.
Endoscopy
The main value of rigid esophagoscopy in current pediatric practice is for therapeutic procedures such as dilation of an esophageal stricture or removal of a foreign body. Rigid esophagoscopy requires general anesthesia with endotracheal intubation and muscle relaxation. The child is positioned supine with a roll under the shoulders to extend the neck. With care taken to protect the teeth, the esophagoscope, with its bevel up, is introduced into the oral cavity along the hard and soft palates to identify the cricopharyngeus muscle and enter the esophagus. Once the most distal aspect of evaluation is reached, it is easy to examine the esophagus fully when withdrawing the scope to identify any lesions or foreign bodies missed on insertion.
Complications related to passage of a rigid or flexible endoscope are typically at the level of the cricopharyngeus muscle. Perforation of the cricopharyngeus occurs in about 0.093% with flexible endoscopy and 0.074% in rigid esophagoscopy.3 Perforation during diagnostic esophagoscopy is exceedingly rare.
A more thorough description of pediatric esophagoscopy and emerging therapeutic endoscopic techniques can be found elsewhere.4–7
Foreign Body Esophageal Injury
Coins are the most frequently ingested foreign body.8,9 When foreign bodies become lodged in the esophagus, they may cause serious complications. Between 10–20% of foreign bodies may lodge in the esophagus and place the patient at risk for developing complications such as aortoesophageal fistula,10 esophageal perforation,11 esophageal stricture,12 tracheoesophageal fistula,13 and respiratory distress.14 There are four sites of physiologic narrowing in the esophagus: (1) the cricopharyngeus of the UES; (2) the aortic notch; (3) the left main-stem bronchus; and (4) the LES. The cricopharyngeus is the narrowest point in the gastrointestinal tract. Endoscopic removal has been the preferred approach in many referral centers and has been highly successful with low complication rates.
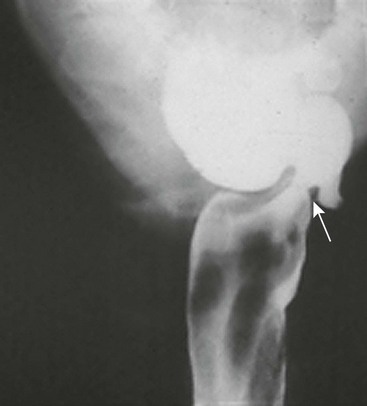
Key principles of endoscopic management of esophageal foreign bodies are to protect the airway, maintain control of the object during extraction, and avoid causing additional damage. Children are more likely than adults to be asymptomatic and have an increased frequency of respiratory symptoms. There should be a high suspicion of foreign body ingestion in infants with excessive drooling, refusal of food, and unexplained coughing or gagging. Anteroposterior and lateral chest radiographs are the best diagnostic tests for radiopaque objects. The flat surface of a coin is best seen on the anteroposterior view when it is lodged in the esophagus, whereas the lateral view will show the flat surface when it is lodged in the trachea (Fig. 26-1).
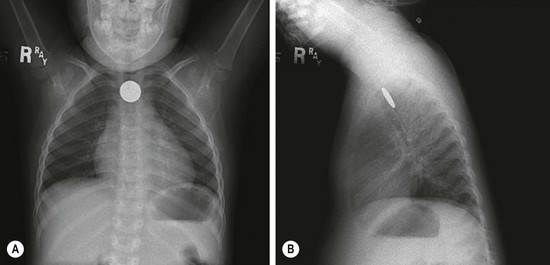
FIGURE 26-1 (A) Anteroposterior and (B) lateral radiographs demonstrate a coin lodged at the cricopharyngeus muscle. Note that coins will most often orient with the flat surface facing anteroposteriorly.
Rigid esophagoscopy has long been considered the gold standard for removal of retained foreign bodies. This procedure has been proven to be highly successful with low complication rates. Other approaches have been described to treat esophageal coins including flexible endoscopy,15 bougienage, 16, 17 Foley balloon extraction under fluoroscopy,8 and brief observation trials.17,18
There is much more concern for serious injury with the ingestion of button batteries. There are four mechanisms of injury caused by batteries: (1) the toxic effect due to absorption of substances, particularly batteries containing mercuric oxide; (2) electrical discharge and mucosal burn; (3) pressure necrosis; and (4) caustic injury from leakage. Severe esophageal damage may occur in as little as four hours after ingestion and perforation in as little as six hours after ingestion.19 Emetics should not be given, owing to their ineffectiveness and possible reflux of a battery back into the esophagus.
There were 20 reported cases of esophageal injury from ingested button batteries from 1979 to 2004.19 Undoubtedly, there are many more unreported incidents. Complications from the ingestion of button batteries include death from vascular invasion and uncontrollable hemorrhage,20 esophageal perforation,21 tracheoesophageal fistula (see Fig. 11-4),22,23 and bilateral vocal cord paralysis.24
Further information about esophageal foreign bodies can be found in Chapter 11.
Chemical Esophageal Injuries
In 2010, the American Association of Poison Control Centers reported that 50% of all human exposures occurred in children under the age of 6 years.25 Household cleaning substances are the third most frequent exposure in children, accounting for 116,000 ingestions per year. In children, ingestions are primarily accidental. The mean age for ingestions is 3.7 years, and 60% of patients are male.26
Caustic injuries are classified similar to burns (Table 26-1). The classification of injury is based on endoscopic evaluation and is used clinically to help predict subsequent clinical outcomes and course. First-degree caustic injuries are superficial and will result in edema and erythema. The esophageal mucosa will slough, but no stricture will form. Second-degree injuries involve the mucosa, submucosa, and muscle layers. They result in deep ulceration and granulation tissue after which collagen deposition and contraction occur. If there is circumferential injury, a stricture may develop. Third-degree injuries are transmural with deep ulcerations that result in a black appearance to the lining of the esophagus. These injuries can result in esophageal perforation. Patients with grade 2b or 3 will develop strictures in 70–100% of cases.27,28 Endoscopic grading of mucosal injury directly predicts risk of complications with a ninefold increase in morbidity with each incremental increase in injury grade.29 Patients who present with peritonitis, mediastinitis, disseminated intravascular coagulation, or shock may require emergency resection of the damaged esophageal tissue.
TABLE 26-1
Classification of Caustic Esophageal Injuries
| Grade | Endoscopic Findings |
| 1 | Mucosal edema and erythema |
| 2a | Friability, hemorrhage, blisters, erosions, erythema, white exudate |
| 2b | Findings of grade 2a plus deep or circumferential ulceration |
| 3a | Small and scattered area of necrosis |
| 3b | Extensive necrosis |
Following a corrosive ingestion, patients may be asymptomatic or may present with nausea, vomiting, dysphagia, odynophagia, drooling, abdominal pain, chest pain, or stridor. There are no conclusive data to correlate laboratory values or symptoms with degree of injury. The absence of symptoms or oropharyngeal lesions does not exclude esophageal injury. In fact, 12% of patients who are asymptomatic and 61% of patients without oral injury will be found to have esophageal injury at endoscopy.26,30,31 In symptomatic patients, the presence of three or more symptoms is an important predictor of severe esophageal injury.28
There is some controversy regarding whether all patients with a history of corrosive ingestion need to undergo endoscopy. Some authors advocate that all patients with suspected corrosive ingestion undergo endoscopy as part of the medical evaluation because even asymptomatic patients may have esophageal injury.27,32 However, these injuries are typically mild, require no further acute treatment, and are at low risk for long-term complications. There is some evidence that asymptomatic patients may not benefit from routine endoscopy.28,33 Certainly all patients who are symptomatic require endoscopic evaluation of the esophagus.
Patients found to have grade 1 or 2a injury on endoscopy can be allowed oral intake and are discharged once symptoms have resolved. Patients with grade 2b and 3 injuries should be observed for 24 to 48 hours and then may have their diets slowly advanced. Because of the frequency with which strictures occur in grades 2b and 3 injuries, these patients should have a barium swallow on day 21 after injury to evaluate for stricture (Fig. 26-2). Once a stricture is identified, gradual dilations are initiated. Strictures refractory to dilation may need operative resection.
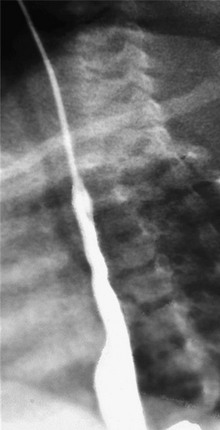
FIGURE 26-2 This barium swallow was performed three weeks after lye ingestion. Note the significant narrowing in the proximal two-thirds of the esophagus.
There is no standardized acute management protocol for caustic esophageal injuries. There are several authors who advocate the use of antibiotics, steroids, and proton pump inhibitors in the treatment of severe esophageal injury.27,31,32 Prospective studies have shown no benefit for the use of steroids in the treatment of caustic esophageal injuries in children.34 There have been no rigorous studies evaluating the use of antibiotics or proton pump inhibitors in these patients. Because of the heterogeneous nature of ingestions and the various substances, location, and degree of injury, the optimal management remains unclear.
Treatment modalities for esophageal strictures include bougienage, esophageal stent placement, intralesional corticosteroid injection, and endoscopic dilations after stricture formation. Multiple dilations may be required for strictures to resolve. Operative intervention may be necessary if these treatments fail, if malignant transformation occurs, or if lengthy or tight strictures develop. Esophageal carcinoma is a late but serious complication of severe caustic injuries. The incidence is 1000 times the expected occurrence rate of patients of a similar age.35 Long-term surveillance for development of esophageal malignancy in severe injuries is important.
Esophageal Strictures
In general, esophageal strictures in children do not involve malignancies. The causes of benign esophageal strictures in childhood include reflux esophagitis, corrosive ingestion, and anastomotic scarring. Anastomotic and corrosive strictures may be aggravated by GER. The incidence of stricture in patients with GER approaches 15%.36 Management includes relief of the obstruction as well as correction of the reflux. Anastomotic strictures tend to be discrete and short, whereas corrosive strictures are more likely to be irregular and long. Peptic esophageal strictures are short and usually located in the lower third of the esophagus (Fig. 26-3).
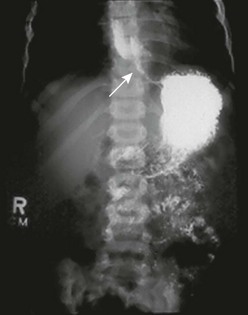
FIGURE 26-3 This barium study was performed in a child with dysphagia and pain. There is marked narrowing (arrow) of the distal esophagus secondary to gastroesophageal reflux and a peptic stricture.
Treatment of strictures includes medical therapy, bouginage, fundoplication, stricture resection, and interposition grafting. Most commonly, the initial treatment of strictures is balloon catheter dilation with aggressive medical management of the GER. The theoretical advantage of balloon dilation over bouginage is that the stricture is gradually dilated by uniform radial force whereas bouginage exerts an abrupt shearing force that may cause injury to the mucosa leading to further scarring and stricture. Patients may need several dilations over several months. The perforation rate with balloon dilation is less than 2%.37,38 Strategies to prevent perforation include choosing a balloon size that is not greater than the size of the patient’s esophagus, not overinflating the balloon, and gradually increasing the size of the balloon over several sessions for tight strictures.
GER-associated strictures are treated with a combination of preoperative dilations and antireflux procedures.39 Preoperative management includes proton pump inhibitors, maximal nutritional support, and optimization of respiratory status. Fundoplication is delayed until nutritional optimization is met, esophagitis has resolved, and the stricture has been dilated. Most patients undergo three to five dilations prior to fundoplication. Postoperative dilations are then performed until the stricture is resolved. Almost 90% of patients have complete resolution of the stricture and the associated GER with this management protocol.
Esophageal Perforation
Esophageal perforation is a rare but life-threatening event in children. Iatrogenic injuries, as a result of nasogastric tube placement, stricture dilations, and endotracheal intubation are the most common reasons found in infants and children. Due to the fact the esophagus lacks a serosal layer, a perforation allows bacteria and digestive enzymes to leak into the mediastinum. The surrounding loose areolar connective tissue is unable to contain the spread of infection and inflammation, and may lead to mediastinitis, empyema, abscess, and sepsis.40
Esophageal perforations may be iatrogenic, or result from ingestion of a foreign body, caustic substances, spontaneous, infectious, or traumatic. In the pediatric literature, nearly 80% of esophageal perforations are attributable to iatrogenic causes.41–43 In children, the most common cause of iatrogenic esophageal perforation is from stricture dilation. The most common location of perforation in children is the thoracic esophagus. Typically, a perforation in the upper thoracic esophagus will result in findings in the left thorax, whereas perforations in the distal thoracic esophagus will present with right-sided thoracic findings. This is in contrast to the neonate, where the pharyngoesophgeal junction is the most common site of perforation. This is likely due to the fact this region is the narrowest area of the esophagus.
Patients with thoracic esophageal perforations may present with respiratory distress, dysphagia, fever, chest pain, or subcutaneous emphysema. It is important that any child who is symptomatic following an endoscopic or esophageal dilation be evaluated for esophageal perforation. The initial study is a chest radiograph in the anteroposterior and lateral views. Findings suggestive of esophageal perforation include pneumothorax, pleural effusion, subcutaneous emphysema, pneumopericardium, or pneumomediastinum. A contrast esophagram is the preferred diagnostic study to determine the presence of an esophageal perforation. Frequently, water-soluble contrast is used initially and followed by barium if no leak is initially seen. There is a 10% false-negative rate with esophogography.44 Chest CT can also suggest esophageal perforation, with findings such as mediastinal air, fluid, or esophageal thickening.
Treatment is guided by the type and extent of injury as well as the clinical status of the child. Most patients with esophageal perforation can be successfully managed with an aggressive conservative approach (Fig. 26-4). Patients are started on broad-spectrum antibiotics. If feasible, a nasogastric tube is inserted beyond the perforation into the stomach. This allows enteral alimentation during the healing process. Otherwise, parenteral nutrition is started. Drainage of thoracic air or fluid is performed to control the leak. It is likely the esophageal perforation will heal spontaneously, once drainage is established, sepsis is controlled, and adequate nutritional support is provided.
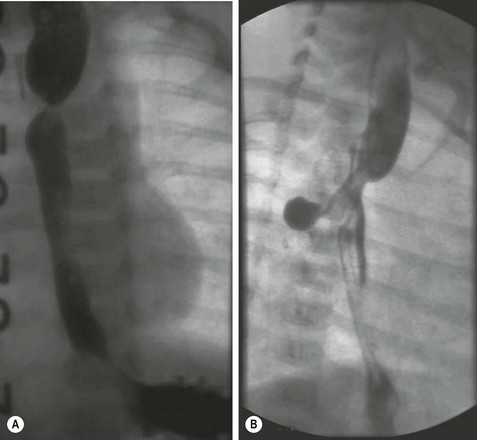
FIGURE 26-4 (A) This infant developed a stricture at the anastomosis after esophageal atresia repair. Multiple balloon dilations were required. (B) Unexpectedly, at the time of one of these dilations, an esophageal perforation developed. Note the contrast leak through the esophageal perforation. This child was managed nonoperatively, and the perforation eventually sealed without the need for operative intervention.
If the patient shows signs of clinical deterioration, has persistence of esophageal leak despite treatment, or has extensive intrapleural spillage, then operative exploration may be needed. Primary repair of the perforation may be possible if it is recognized within 24 hours. The perforation is closed in a single layer with reinforcement with pericardial fat, pleura, stomach, diaphragm, or omentum. If the perforation is large and diagnosed after 24 hours, there is typically widespread mediastinal inflammation. In this setting, the esophagus is friable and will not hold sutures. Historically, this has been managed with cervical esophagostomy and gastrostomy. However, even with extensive inflammation, conservative management with wide drainage and gastrostomy has resulted in spontaneous healing of the perforation.43
Morbidity and mortality of esophageal perforation is directly related to delay in the diagnosis and treatment. Complications of esophageal perforation are typically pulmonary in nature, such as pneumonia or atelectasis. There is a fivefold increase in complications following a delay in diagnosis of greater than 24 hours.42 The mortality of esophageal perforation in the pediatric population is 4%, which is lower than reported for adults.42
Congenital Esophageal Stenosis
Congenital esophageal stenosis (CES) is a rare childhood condition with an incidence of 1 in 25,000 to 50,000 live births.45,46 It is associated with esophageal atresia in one-third of patients. The remaining are considered to be isolated cases.47,48 Three histopathologic variants are seen: tracheobronchial remnants, membranous diaphragms or webs, and diffuse fibrosis of the muscularis and submucosa.49 Most infants have normal physical findings at birth. As a result, CES is rarely diagnosed in the neonatal period. The onset of symptoms, most commonly vomiting, dysphagia, and failure to thrive, typically develop with the introduction of solid food at ages 4 to 10 months. Respiratory symptoms are found in about 10% of patients.50 An esophagogram may show an abrupt or tapered stenosis, commonly at the junction of the middle and distal third of the esophagus (Fig. 26-5). Additional evaluation, including pH probe and endoscopy, is necessary to exclude the more common diagnosis of GER-associated stricture and esophagitis. When associated with esophageal atresia, the stenosis is usually found in the distal one-third of the esophagus.50,51
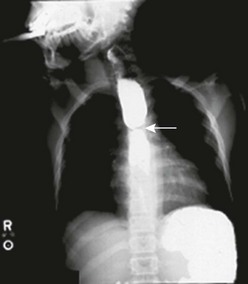
FIGURE 26-5 This child presented with significant dysphagia and weight loss. An esophagogram revealed marked narrowing of the mid esophagus (arrow) due to congenital esophageal stenosis. This child underwent resection of the stenosis and primary repair of the esophagus.
The absence of esophagitis will be confirmed on esophagoscopy. However, tracheobronchial remnants can be missed on biopsy specimens that are taken too superficially.51 Endoscopic ultrasonography has been shown to be helpful in differentiating tracheobronchial remnants as the cause of the stenosis from stricture due to reflux.52,53
Dilatation of a CES does not provide long-term benefit. Case reports have described successful treatment with endoscopic electrocauterization and balloon dilation.54 However, limited resection of the stenosis through the left chest (either open or thoracoscopically48) with primary end-to-end anastomosis is the preferred treatment for long-term relief.
Esophageal Duplication
Congenital esophageal duplication is a rare anomaly of the esophagus, with an incidence of 1 in 8000 births, accounting for 10–15% of all gastrointestinal duplications.55 Histologic criteria for diagnosis include attachment to the esophagus, enclosure of the duplication by two muscle layers, and lining of the duplication by epithelium. Patients tend to present with respiratory symptoms, vomiting, regurgitation, and a possible neck mass. Diagnosis can be made by contrast esophagography. Because the duplication may increase in size with time and compress surrounding structures, operative resection is the treatment of choice. This can be approached via posterolateral thoracotomy or thoracoscopy. Operative guidelines include preserving the vagus and phrenic nerves, and reconstructing the muscular wall of the esophagus. Air insufflation of the esophagus intraoperatively with endoscopy or with a nasogastric tube is helpful to assess the integrity of the esophageal wall after resection. See Chapter 39 for further information about esophageal duplications.
Achalasia
Achalasia is a chronic motility disorder of the esophagus characterized by absent or poor esophageal peristalsis and failure of the LES to relax during swallowing. The specific etiology is unknown. In children, this has been associated with various syndromes, including trisomy 21 and triple A syndrome (achalasia, alacrima, and adrenocorticotropic hormone [ACTH] insensitivity).
Data obtained from the Healthcare Cost and Utilization Project (HCUP) from 1997 to 2006 found an annual hospitalization rate of 0.25/100,000 for patients with achalasia under the age of 18 years.56 Symptoms of achalasia are age dependent. Infants present with frequent regurgitation, choking, pneumonia, and failure to thrive. Symptoms in older children are similar to those seen in adults and include dysphagia, vomiting, and weight loss.
The presenting symptoms are often attributed to GER; however, symptoms persist following initiation of antireflux medication. A barium esophagogram will show aperistalsis of the esophagus, a dilated esophagus, and no or minimal opening of the LES, known as the ‘bird’s beak’ sign (Fig. 26-6). Esophageal manometry is the standard diagnostic test for achalasia and typical findings include an elevated LES pressure, failure of the sphincter to relax with swallowing, and low-amplitude, nonprogressive, or absent peristaltic contractions in the esophageal body.
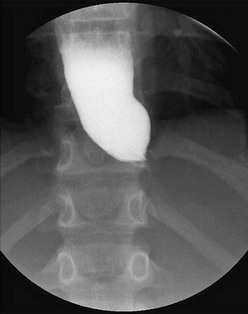
FIGURE 26-6 This barium swallow was performed in a 16-year-old patient with dysphagia secondary to achalasia. The classic ‘bird’s beak’ narrowing of the distal esophagus at the level of the spastic, contracted esophagus is seen. Also, note the dilated esophagus proximal to the lower esophageal sphincter.
Pharmacologic treatment with nitrates or calcium-channel blockers can result in a decrease in the LES pressure, but the response is temporary. The need for long-term medicine limits its usefulness in children. Intersphincteric injection of Botulinum toxin (Botox) has been used in recent years for the treatment of achalasia.57 Botox is a neurotoxin that binds to presynaptic cholinergic terminals in skeletal muscle, inhibiting the release of acetylcholine at the neuromuscular junction, which creates a chemical denervation. While initially effective, there is a high recurrence rate that also limits its applicability in children. Because of the limited success of these therapies, they not used for primary therapy but they may be considered in children unable to undergo general anesthesia.
Forceful dilation of the LES is accomplished with a balloon dilator specifically designed for the treatment of achalasia. Pneumatic dilation reduces the LES pressure by partially disrupting the sphincter muscle complex. Immediate relief of symptoms can be expected in the majority of patients following pneumatic dilation but recurrence of symptoms can be as high as 100%.58 Secondary procedures, such as repeat pneumatic dilation or surgical esophagomytomy, may be required in 80–93% of patients.58,59 Approximately half of these patients choose to undergo additional dilations and half proceed to surgical repair.58,59 The perforation rate with pneumatic dilation is about 5% 60, 61 and may be managed conservatively, or with immediate myotomy based on the patient’s clinical status.
A large, multicenter, prospective, randomized trial comparing serial pneumatic dilation to primary laparoscopic esophagomyotomy with Dor fundoplication in adults was recently conducted.62 A minimal two-year follow-up (mean 43 months) was attained in 95 patients who underwent dilation and 106 who underwent myotomy. At two years, there was no difference in lower esophageal pressure, esophageal emptying, abnormal esophageal acid exposure, or quality of life. The authors concluded that myotomy did not offer superior therapeutic success and thus dilation could be considered as first-line treatment. These data may represent a paradigm shift in the treatment for achalasia, although longer follow-up is needed as are data in children.
The goal of esophagomyotomy is to disrupt the LES enough to eliminate dysphagia without causing complications of excessive reflux. This can be accomplished with a thoracic or abdominal approach, open or minimally invasively, with or without a concomitant antireflux procedure. Excellent results have been reported in 70–93% patients at more than five years of follow-up, either with59,63 or without64,65 a fundoplication. In long-term follow-up studies, manometry has shown low LES pressures, but no significant increase in the amplitude of the esophageal contractions.66
Two areas of controversy in the operative management of achalasia include the length of the myotomy and performance of a concomitant antireflux procedure. It is believed that an extended myotomy past the LES results in postoperative GER. Surgeons who do not routinely perform an antireflux procedure advocate for a shorter distal myotomy, extending approximately 5 mm past the gastroesophageal junction to maintain a higher pressure at the LES to prevent GER.65 However, in patients treated with myotomy alone, the postoperative occurrence of GER can be as high as 47%.61,66 In addition, postoperative dysphagia occurs in 25%.63,64 These findings may indicate that not only does a limited myotomy not prevent postoperative GER, but also suggest it may not always relieve the symptoms.
In adults, there is good evidence to support a longer myotomy combined with a partial fundoplication. A prospective randomized controlled study in adults showed a fundoplication decreases the incidence of postoperative reflux from 47% to 9.1%.66 In addition to an antireflux procedure, it has been recognized in adults that a longer myotomy, extending for 2.5–3 cm onto the gastric wall, reduces postoperative dysphagia from 17% to 5%.67 There has also been good success reported in children undergoing an extended myotomy with partial fundoplication.68 As a result of these findings, the most commonly utilized operation is the laparoscopic esophagomyotomy with Dor fundoplication with extension of the esophageal myotomy 4–6 cm above and 2–3 cm distal to the gastroesophageal junction.
Persistence or recurrence of dysphagia following myotomy is thought to be the result of incomplete disruption of the muscle fibers of the distal esophagus. Adjunctive techniques to improve the adequacy of the myotomy have included intraoperative endoscopy and intraoperative mamometry.69,70 Intraoperative endoscopy correctly identifies the squamocolumnar transition at the esophagogastric junction to determine the distal extent of myotomy. Intraoperative manometery has been used to confirm reduction of LES pressures following myotomy. In one study, intraoperative manometry following myotomy showed residual high pressure in 34%, prompting extension of the myotomy.69 The use of intraoperative manometry in children has been shown to reduce recurrence of symptoms at one year to 0%.70
It is important to remember that achalasia is a chronic condition. Even following operation for achalasia, children have significantly lower quality of life (QOL) scores than children with inflammatory bowel disease and have QOL scores that are comparable to children with chronic constipation.71 Population-based studies have found a 16-fold risk of development of esophageal cancer following treatment for achalasia.72 However, the absolute risk of esophageal cancer remains relatively low, and there is no consensus regarding the need for routine surveillance in patients with achalasia.
Esophageal Replacement
The most common indications for esophageal replacement in infants and children are long-gap esophageal atresia, failed primary repair of esophageal atresia, and strictures related to reflux or corrosive injury.73–75 The colon was the first conduit used as an esophageal replacement and remains the most commonly used technique in practice today. Other alternatives that have been used with success include gastric tube, gastric transposition, and jejunal interposition graft. Which conduit is best for any given patient depends on multiple factors, including the location and length of the native remaining esophagus, the original diagnosis, the patient’s size and age, and previous procedures on the esophagus, stomach, or colon.
Regardless of the conduit used, there are several important principles. First, the esophagus is the optimal conduit, provided it functions relatively normally and has no malignant potential (e.g., Barrett esophagus). Second, a short straight tract is best because almost all conduits function as passive tubes rather than by means of intrinsic peristaltic activity. The posterior mediastinum is often the shortest and straightest route. Third, the prevention of reflux into any conduit is important. An interposition procedure that incorporates the distal normal esophagus with its gastroesophageal junction may have an advantage in preventing reflux complications. Fourth, persistence is exceedingly important. Anastomotic dilatations should not be necessary except during the healing phase. Strictures should be revised (Fig. 26-7). Complex interpositions that do not function well should be revised to provide the straightest, lowest resistance conduit possible.
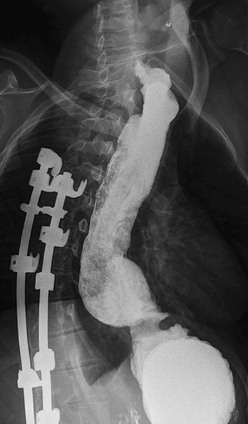
FIGURE 26-7 Barium swallow of a 28-year-old patient after successful left colon interposition for isolated esophageal atresia. The patient developed bleeding and dysphagia from peptic ulceration at the distal anastomosis, requiring revision.
Gastric Tube Esophageal Replacement
Gastric tubes have become popular because they can be created rapidly with a stapling device. The gastric tube is constructed from the greater curve of the stomach with the blood supply based on the left gastroepiploic artery. These tubes can be created from the antrum up, or from the fundus down, and can be constructed so that there is enough gastric tube to reach the neck (Fig. 26-8). The advantages of a gastric tube are its reliable blood supply, its resistance to ulceration from gastric acid reflux, and its ability to bridge long gaps. The tube is also resilient and does not become tortuous or dilated over time. Theoretical disadvantages are a long suture line, continued acid production by the tube, and reduced stomach capacity.
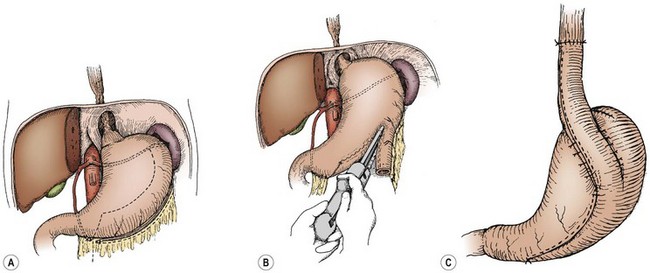
FIGURE 26-8 Graphic depiction of the technique to create a reversed gastric tube for esophageal replacement. (A) Inspection of the blood supply to the stomach and preservation of the gastroepiploic artery for creating the tube. (B) The use of a stapler to create the tube along the greater curvature of the stomach. (C) The completed reversed tube is brought up to the chest for the esophageal anastomosis.
Complications include anastomotic leak, stricture formation requiring dilation, temporary dumping syndrome, and the development of Barrett esophagus above the anastamosis.73,74 Control studies have shown normal swallowing and manometry has shown mass contractions in about two-thirds of patients.74 Overall, about 80% of patients report enjoying a normal diet.
Gastric Transposition
The gastric transposition (or pull-up procedure) is performed by mobilizing the entire stomach on a vascular pedicle, relocating the entire stomach into the mediastinum, and creating an anastomosis to the cervical esophagus in the neck. In addition, many surgeons will perform a pyloroplasty to prevent gastroparesis. The advantages of this approach include that it is technically straightforward, the stomach has an excellent blood supply, only one anastomosis is needed, and that the entire esophagus can be replaced with a low risk of necrosis, leak, or stricture. Complications following gastric transposition have included anastomotic leak, stricture, and significant swallowing problems. Mortality has been reported to be 4.6%.75 Death following gastric transposition is typically due to respiratory failure. Gastric transposition may not be the ideal substitution in patients with borderline respiratory function as a bulky stomach in the chest may contribute to further respiratory compromise. Figure 26-9 depicts a postoperative barium study after a gastric pull-up procedure for an extensive stricture following lye ingestion.
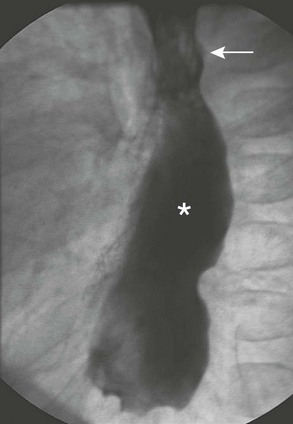
FIGURE 26-9 This infant underwent a gastric pull-up after a failed colonic interposition for isolated esophageal atresia. The native esophagus is identified by the arrow, and the gastric pull-up is marked with an asterisk.
The long-term outcome is considered good to excellent in 90%, although many patients prefer eating small frequent meals. There has been no documented evidence of deterioration in the function of the gastric transposition in 72 patients who were observed for longer than ten years.75
Colon Interposition
The right, transverse, or left colon on its vascular pedicle has been used as an esophageal replacement, either in an isoperistaltic or an antiperistaltic direction. It can be placed retrosternal or in a transhiatal posterior mediastinal location. The left colon and its vascular pedicle, based on the left colic artery, is most commonly used.76 The colon is pulled up into the neck, and an end-to-side or end-to-end esophagocolic anastomosis is performed. The gastrocolic anastomosis is then performed, followed by a fundoplication and pyloroplasty. The steps involved with a right colon interposition for esophageal atresia are seen in Figure 26-10, and the operative steps for a left or transverse colon interposition are seen in Figure 26-11.
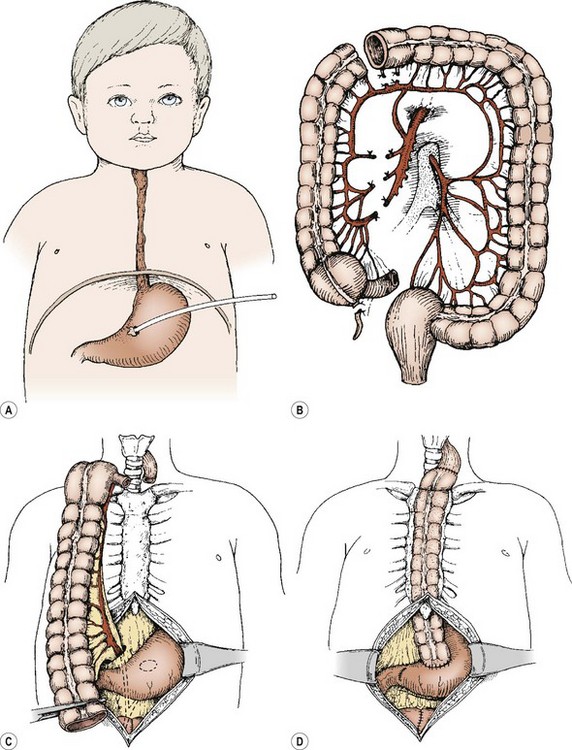
FIGURE 26-10 (A) For any esophageal lesion in which a substitution procedure may be anticipated, the gastrostomy should be placed on the lesser curve at about the level of the incisura, so that a right or left colon or gastric tube interposition can be carried out without compromising the blood supply. (B) The right colon and terminal ileum are isolated, based on blood supply from the arcades and from the middle colic artery. (C) The colon on its pedicle is brought up through the lesser sac and positioned substernally in an isoperistaltic fashion. (D) Most frequently, excision of the terminal ileum and cecum is accomplished. Careful tailoring of the distal end allows a straight conduit to be anastomosed to the antrum. Pyloroplasty may or may not be added to the procedure. The incidence of significant gastrocolic reflux is reduced by a drainage procedure.
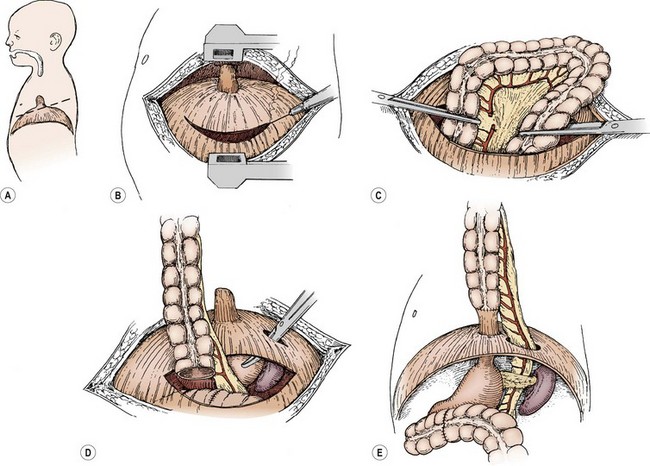
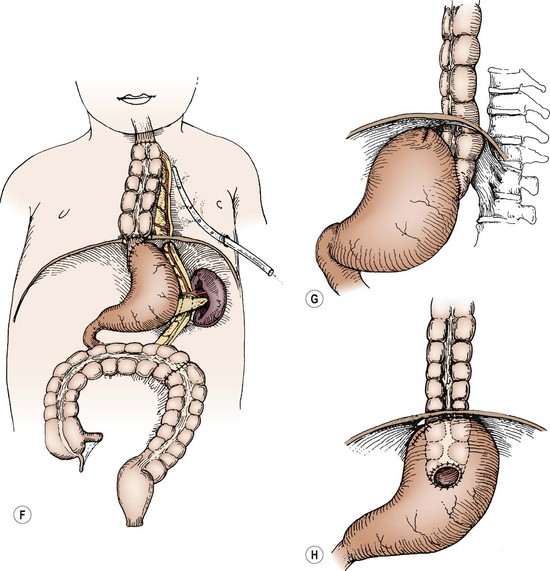
FIGURE 26-11 The left colon or transverse colon substitution described by Waterston is illustrated in this patient with isolated esophageal atresia. However, it works equally well for other lesions requiring esophageal replacement. (A) A standard posterolateral left thoracotomy at about the sixth intercostal space. (B) Incision of the diaphragm peripherally. (C) A section of colon is isolated and its vascular pedicle is developed, usually based on the left colic artery. It may be necessary to base it on the middle colic artery, in which case, this interposed colon is placed in an antiperistaltic manner. (D) The colon and its vascular pedicle are delivered behind the spleen and pancreas and through a separate posterior opening in the diaphragm, so that the abdominal viscera do not stretch or otherwise obstruct the blood supply to this colon segment. (E) The distal anastomosis may be made to the remnant of the distal esophagus (as is depicted) or to the posterior aspect of the stomach. (F) The upper anastomosis is made to the esophagus either within the mediastinum or within the neck. Adequate drainage of the pleura is necessary to prevent empyema. A fundoplication after the method of Thal also may be added to this procedure, if the distal esophagus is used. This technique reduces the amount of reflux that can interfere with the healing or that can produce ulcers in the colon. (G) A lateral view of an alternative method of cologastrostomy with Waterston’s procedure. (H) The segment of colon is shown within the abdominal cavity, which may possibly reduce the incidence of gastrocolic reflux.
A colon interposition is a relatively straightforward procedure, and the colon is readily positioned into the thorax without causing respiratory compromise. Disadvantages of this approach include the need for three anastomoses, an increased risk for anastomotic leak, strictures at the esophagocolic anastomosis, and tortuosity or redundancy of the graft over time.
Timing of Colon Interposition
In those patients in whom esophageal atresia has developed without a distal fistula, and in whom attempts at stretching are successful, the colon can be interposed in the newborn period. Several authors report performing colonic interposition once the patient is 3 months of age or 5 kg in weight.77–79 Many pediatric surgeons, however, prefer a cervical esophagoscopy and gastrostomy, and wait to perform the colon interposition when the patient is walking, usually over the age of 1 year. Both approaches have theoretical and practical advantages. Most reported experience has been in patients who are 12 to 18 months of age and are walking to allow the upright position to counteract reflux into the graft and allow for passive emptying of the neoesophagus.80 During the period from esophagostomy to colon interposition, it is important to provide sham feedings to develop oral–motor coordination. If sham oral feedings accompany gastrostomy feedings, the patient may then associate a full stomach with swallowing.
Jejunal Substitution
The jejunum has been used as an esophageal substitute much more commonly in adults than in children. Advantages to using jejunum include a diameter that is similar to the esophagus and does not occupy much space in the thoracic cavity. Also, the jejunum retains its peristaltic activity. Significant disadvantages include the precarious vascular supply of a pedicled jejunal graft. Also, a tension-free anastomosis may be difficult to achieve.
Esophageal substitution with free and pedicled jejunal grafts has resulted in significant perioperative morbidity, including intraoperative repeat interpositions for immediate graft loss, early postoperative graft loss, anastomotic leaks, late anastomotic strictures, and graft redundancy.81,82 Less than 50% of patients are able to achieve a completely oral diet without reliance on gastrostomy feeds.81 In spite of technically sound outcomes, it is known that significant complications exist, including death, graft necrosis, ischemia, and strictures.
Complications of Esophageal Substitution
Interposition of a well-vascularized graft is sometimes followed several days later by fever, increased leukocytosis, and drainage from the proximal anastomosis. An anastomotic leak from tension or mild ischemia can be managed expectantly if the conduit appears viable. A contrast study, however, may demonstrate that the mucosal pattern of the interposed segment is ischemic. The interposition must be inspected and removed if it is necrotic. In this case, a cervical esophagostomy should be established and a different form of substitution planned. Many investigators have reported using the left colon after failure of the right colon. Others may not be inclined to sacrifice additional colon and would perform a gastric tube or gastric transposition.
Proximal strictures between the esophagus and the interposition usually are the result of insufficient blood supply to the interposition (Fig. 26-12). Anastomosis to a scarred esophagus also results in stricture. After a reasonable healing period, persistent strictures should be revised rather than repeatedly dilated.
References
1. Kluth, D, Fiegel, F. The embryology of the foregut. Semin Pediatr Surg. 2003; 12:3–9.
2. Skandalakis, JE, Ellis, H. Embryologic and anatomic basis of esophageal surgery. Surg Clin North Am. 2000; 80:85–155.
3. Michel, L, Grillo, HC, Malt, RA. Esophageal perforation. Ann Thorac Surg. 1982; 33:203–210.
4. Lloyd, DA. Rigid endoscopy of the esophagus—basic technique. In: Najmaldin A, ed. Operative Endoscopy and Endoscopic Surgery in Infants and Children. London: Hodder Arnold; 2005:117–120.
5. Crabbe, DCG. Upper gastrointestinal endoscopy–basic techniques. In: Najmaldin A, ed. Operative Endoscopy and Endoscopic Surgery in Infants and Children. London: Hodder Arnold; 2005:121–126.
6. Lee, KK, Anderson, MA, Baron, TH, et al. Modifications in endoscopic practice for pediatric patients. Gastrointest Endosc. 2008; 67:1–9.
7. Isaza, N, Garcia, P, Dutta, S. Advances in pediatric minimal access therapy: A cautious journey from therapeutic endoscopy to transluminal surgery based on the adult experience. JPGN. 2008; 46:359–369.
8. Little, DC, Shah, SR, St Peter, SD, et al. Esophageal foreign bodies in the pediatric population: Our first 500 cases. J Pediatr Surg. 2006; 41:914–918.
9. Tokar, B, Cevik, AA, Ilhan, H. Ingested gastrointestinal foreign bodies: Predisposing factors for complications in children having surgical or endoscopic removal. Pediatr Surg Int. 2007; 23:135–139.
10. McComas, BC, van Miles, P, Katz, BE. Successful salvage of an 8-month-old child with and aortoesophageal fistula. J Pediatr Surg. 1991; 26:1394–1395.
11. Raval, MV, Campbell, BT, Phillips, JD. Case of the missing penny: Thoracoscopic removal of a mediastinal coin. J Pediatr Surg. 2004; 39:1758–1760.
12. Uchida, K, Inoue, M, Konishi, N, et al. Esophageal stricture with a pseudodiverticulum caused by the unrecognized ingestion of a small foreign body in a child: Report of a case. Surg Today. 2005; 35:774–777.
13. Hammond, P, Jaffray, B, Hamilton, L. Tracheoesophageal fistula secondary to disk battery ingestion: A case report of gastric interposition and tracheal patch. J Pediatr Surg. 2007; 42:E39–E41.
14. Miller, RS, Willging, JP, Rutter, MJ, et al. Chronic esophageal foreign bodies in pediatric patients: A retrospective review. Int J Pediatr Otorhinolaryngol. 2004; 68:265–272.
15. Popel, J, El-Hakim, H, El-Matary, W. Esophageal foreign body extraction in children: Flexible versus rigid endoscopy. Surg Endosc. 2011; 25:919–922.
16. Dahshan, AH, Donovan, GK. Bougienage versus endoscopy for esophageal coin removal in children. J Clin Gastroenterol. 2007; 41:454–456.
17. Soprano, JV, Mandl, KD. Four strategies for the management of esophageal coins in children. Pediatrics. 2000; 105:e5.
18. Waltzman, ML, Baskin, M, Wypij, D, et al. A randomized clinical trial of the management of esophageal coins in children. Pediatrics. 2005; 116:614–619.
19. Yardeni, D, Yardeni, H, Coran, AG, et al. Severe esophageal damage due to button battery ingestion: Can it be prevented? Pediatr Surg Int. 2004; 20:496–501.
20. Stuth, EAE, Stucke, AG, Cohen, RD, et al. Successful resuscitation of a child after exsanguination due to aortoesophageal fistula from undiagnosed foreign body. Anesthesiology. 2001; 95:1025–1026.
21. Samad, L, Ali, M, Ramzi, H. Button battery ingestion: Hazards of esophageal impaction. J Pediatr Surg. 1999; 34:1527–1531.
22. Slamon, NB, Hertzog, JH, Penfil, SH, et al. An unusual case of button battery-induced traumatic tracheoesophageal fistula. Pediatr Emerg Care. 2008; 24:313–316.
23. Imamoglu, M, Cay, A, Kosucu, P, et al. Acquired tracheo-esophageal fistulas caused by button battery lodged in the esophagus. Pediatr Surg Int. 2004; 20:292–294.
24. Bernstein, JM, Burrows, SA, Saunders, MW. Lodged oesophageal button battery masquerading as a coin: An unusual case of bilateral vocal cord paralysis. Emerg Med J. 2007; 24:e15.
25. Bronstein, AC, Spyker, DA, Cantilena, LR, et al. 2010 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th Annual Report. Clinical Toxicology. 2011; 49:910–941.
26. Dogan, Y, Erkan, T, Cokugras, FC, et al. Caustic gastroesophageal lesions in childhood: An analysis of 473 cases. Clin Pediatr. 2006; 45:435–438.
27. Baskin, D, Urganci, N, Abbasoglu, L, et al. A standardized protocol for the acute management of corrosive ingestion in children. Pediatr Surg Int. 2004; 20:824–828.
28. BetallI, P, Falchetti, D, Giuliani, S, et al. Caustic ingestion in children: is endoscopy always indicated? The results of an Italian multicenter observational study. Gastrointest Endosc. 2008; 68:434–439.
29. Poley, JW, Steyerberg, EW, Kuipers, EJ, et al. Ingestions of acid and alkaline agents: Outcome and prognostic value of early upper endoscopy. Gastrointest Endosc. 2004; 60:372–373.
30. Gaudreault, P, Parent, M, McGuigan, MA, et al. Predictability of esophageal injury from signs and symptoms: A study of caustic ingestion in 378 children. Pediatrics. 1983; 71:767–770.
31. Riffat, F, Cheng, A. Pediatric caustic ingestion: 50 consecutive cases and a review of the literature. Dis Esophagus. 2009; 22:89–94.
32. Broto, J, Asensio, M, Soler, C, et al. Conservative treatment of caustic esophageal injuries in children: 20 years of experience. Pediatr Surg Int. 1999; 15:323–325.
33. Gupta, SK, Croffie, JM, Fitzgerald, JF. Is esophagogastroduodenoscopy necessary in all caustic ingestions? JPGN. 2001; 32:50–53.
34. Anderson, KD, Rouse, TM, Randolph, KG. A controlled trial of corticosteroids in children with corrosive injury of the esophagus. N Engl J Med. 1990; 323:637–640.
35. Kay, M, Wyllie, R. Caustic ingestions in children. Curr Opin Pediatr. 2009; 21:651–654.
36. O’Neill, AJ, Betts, J, Ziegler, MM, et al. Surgical management of reflux strictures of the esophagus in childhood. Ann Surg. 1982; 196:453–460.
37. Lan, LC, Wong, KK, Lin, SC, et al. Endoscopic balloon dilation of esophageal strictures in infants and children: 17 years’ experience and a literature review. J Pediatr Surg. 2003; 38:1712–1715.
38. Said, M, Mekki, M, Golli, M, et al. Balloon dilatation of anastomotic structures secondary to surgical repair of oesophageal atresia. Br J Radiol. 2003; 76:26–31.
39. Numanoglu, A, Millar, AJ, Brown, RA, et al. Gastroesophageal reflux strictures in children, management and outcome. Pediatr Surg Int. 2005; 21:631–634.
40. Panieri, E, Millar, AJ, Rode, RA, et al. Iatrogenic esophageal perforations in children: Patterns of injury, presentation, management and outcome. J Pediatr Surg. 1996; 31:890–895.
41. Peng, L, Quan, X, Zongzheng, J, et al. Videothoracoscopic drainage for esophageal perforation with mediastinitis in children. J Pediatr Surg. 2006; 41:514–517.
42. Engum, SA, Gosfeld, JL, West, KW, et al. Improved survival in children with esophageal perforation. Arch Surg. 1996; 31:604–611.
43. Martinez, L, Rivas, S, Hernandez, F, et al. Aggressive conservative treatment of esophageal perforations in children. J Pediatr Surg. 2003; 38:685–689.
44. Gander, JW, Berdon, WE, Cowls, RA. Iatrogenic esophageal perforation in children. Pediatr Surg Int. 2009; 25:395–401.
45. Amae, S, Nio, M, Kamiyama, T, et al. Clinical characteristics and management of congenital esophageal stenosis: A report on 14 cases. J Pediatr Surg. 2003; 38:565–570.
46. Vasudevan, SA, Kerendi, F, Lee, H, et al. Management of congenital esophageal stenosis. J Pediatr Surg. 2002; 37:1024–1026.
47. Ibrahim, AHM, Al Malki, TA, Hamaza, AF, et al. Congenital esophageal stenosis associated with esophageal atresia: New concepts. Pediatr Surg Int. 2007; 23:533–537.
48. Martinez-Ferro, M, Rubio, M, Piaggio, L, et al. Thoracoscopic approach for congenital esophageal stenosis. J Pediatr Surg. 2006; 41:E5–E7.
49. Murphy, SG, Yazbeck, S, Russo, P. Isolated congenital esophageal stenosis. J Pediatr Surg. 1995; 30:1238–1241.
50. Takamizawa, S, Tsugawa, C, Mouri, N, et al. Congenital esophageal stenosis: Therapeutic strategy based on etiology. J Pediatr Surg. 2002; 37:197–201.
51. Sneed, WF, LaGarde, DC, Kogutt, MS, et al. Esophageal stenosis due to cartilaginous tracheobronchial remnants. J Pediatr Surg. 1979; 14:786–788.
52. Kouchi, K, Yoshida, H, Matsunaga, T, et al. Endosonographic evaluation in two children with esophageal stenosis. J Pediatr Surg. 2002; 37:934–936.
53. Usui, N, Kamata, S, Kawahara, H, et al. Usefulness of endoscopic ultrasonography in the diagnosis of congenital esophageal stenosis. J Pediatr Surg. 2002; 37:1744–1746.
54. Chao, H, Chen, S, Kong, M. Successful treatment of congenital esophageal web by endoscopic electrocauterization and balloon dilation. J Pediatr Surg. 2008; 43:E13–E15.
55. Achildi, O, Grewal, H. Congenital anomalies of the esophagus. Otolaryngol Clin North Am. 2007; 40:219–244.
56. Sonnenberg, A. Hospitalization for achalasia in the United States 1997–2006. Dig Dis Sci. 2009; 54:1680–1685.
57. Walton, JM, Tougas, G. Botulinum toxin use in pediatric achalasia: A case report. J Pediatr Surg. 1997; 32:916–917.
58. Lee, CW, Kays, DW, Chen, MK, et al. Outcomes of treatment of childhood achalasia. J Pediatr Surg. 2010; 45:1173–1177.
59. Pastor, AC, Mills, J, Marcon, MA, et al. A single center 26-year experience with treatment of esophageal achalasia: Is there an optimal method? J Pediatr Surg. 2009; 44:1349–1354.
60. Speiss, AE, Kahrilas, PJ. Treating achalasia. From whalebone to laparoscope. JAMA. 1998; 280:638–642.
61. Csendes, A, Braghetto, I, Henriques, A, et al. Late results of a prospective randomized study comparing forceful dilatation and oesophagomyotomy in patients with achalasia. Gut. 1989; 30:299–304.
62. Boeckxstaens, GE, Annese, V, Bruley des Varannes, S, et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med. 2011; 364:1807–1816.
63. Morris-Stiff, G, Foster, ME, Khan, R, et al. Long-term results of surgery for childhood achalasia. Ann R Coll Surg Engl. 1997; 79:432–434.
64. Karnak, J, Senocak, ME, Tanyet, FC, et al. Achalasia in childhood: Surgical treatment and outcome. Eur J Pediatr Surg. 2001; 11:223–229.
65. Vaos, G, Demetriou, L, Velaoras, C, et al. Evaluating long-term results of modified Heller limited esophagomyotomy in children with esophageal achalasia. J Pediatr Surg. 2008; 43:1262–1269.
66. Richards, WO, Torquati, A, Holzman, MD, et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia. A prospective randomized double-blind clinical trial. Ann Surg. 2004; 240:405–415.
67. Wright, AS, Williams, CW, Pellegrini, CA, et al. Long-term outcomes confirm the superior efficacy of extended Heller myotomy with Toupet fundoplication for achalasia. Surg Endosc. 2007; 21:713–718.
68. Patti, MG, Albanese, C, Holcomb, GW, III., et al. Laparoscopic Heller myotomy and Dor fundoplication for esophageal achalasia in children. J Pediatr Surg. 2001; 36:1248–1251.
69. Chapman, JR, Joehl, RJ, Murayama, KM, et al. Achalasia treatment. Improved outcome of laparoscopic myotomy with operative manometry. Arch Surg. 2004; 139:508–513.
70. Jafri, M, Alonso, M, Kaul, A, et al. Intraoperative manometry during laparoscopic Heller myotomy improves outcome in pediatric achalasia. J Pediatr Surg. 2008; 43:66–70.
71. Marlais, M, Fishman, JR, Fell, JM, et al. Health-related quality of life in children with achalasia. J Paediatr Child Health. 2011; 47:18–21.
72. Sandler, RS, Nyren, O, Ekbom, A, et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA. 1995; 274:1359–1362.
73. Borgnon, J, Tounian, P, Auber, F, et al. Esophageal replacement in children by an isoperistaltic gastric tube: A 12-year experience. Pediatr Surg Int. 2004; 20:829–833.
74. Gupta, L, Bhatnagar, V, Gupta, AK, et al. Long-term follow-up of patients with esophageal replacement by reversed gastric tube. Eur J Pediatr Surg. 2011; 21:88–93.
75. Spitz, L. Gastric transposition in children. Semin Pediatr Surg. 2009; 18:30–33.
76. Burgos, L, Barrena, S, Andres, AM, et al. Colonic interposition for esophageal replacement in children remains a good choice: 33-year median follow-up of 65 patients. J Pediatr Surg. 2010; 45:341–345.
77. Arul, GS, Parikh, D. Oesophageal replacement in children. Ann R Coll Surg Engl. 2008; 90:7–12.
78. Cowles, RA, Coran, AG. Gastric transposition in infants and children. Pediatr Surg Int. 2010; 26:1129–1134.
79. Hamza, AF. Colonic replacement in cases of esophageal atresia. Semin Pediatr Surg. 2009; 18:40–43.
80. Tannuri, U, Tannuri, CA. Should patients with esophageal atresia be submitted to esophageal substitution before they start walking? Dis Esophagus. 2011; 24:25–29.
81. Cauchi, JA, Buick, RG, Gornall, P, et al. Oesophageal substitution with free and pedicled jejunum: Short- and long-term outcomes. Pediatr Surg Int. 2007; 23:11–19.
82. Bax, KM. Jejunum for bridging long-gap esophageal atresia. Semin Pediatr Surg. 2009; 18:34–39.