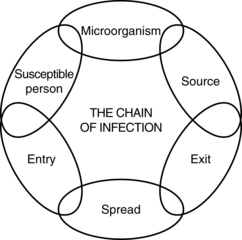
15 The endocrine system
Endocrine Gland: A group of hormone-secreting and hormone-excreting cells.
Gluconeogenesis: The conversion of amino acids into glucose.
Glycogenesis: The deposition of glycogen in the liver.
Hormone: A biochemical substance secreted by a specific endocrine gland and transported in the blood to distant points in the body for regulation of rates of physiologic processes.
Lipolysis: The mobilization of deposited fat.
Releasing Factor: A hormone of unknown chemical structure secreted by the hypothalamus.
Releasing Hormone: A hormone secreted from the hypothalamus.
Stress: A chemical or physical disturbance in the cells or tissues produced by a change either in the external environment or within the body that necessitates a response to counteract the disturbance.
Target Organ: A gland whose activities are regulated by tropic hormones.
Tropic Hormone: A hormone that regulates the blood level of a specific hormone secreted from another endocrine gland.
Arthur C. Guyton, the great professor of physiology, always began his lecture with, “The essence of physiology is regulation and control.”1 That statement is true for the endocrine system, which is regarded as one of the two physiologic regulating and control systems—the other being the nervous system. Many interrelationships exist between the endocrine and the nervous systems. Dysfunction of the endocrine system is associated with overproduction or underproduction of a single hormone or multiple hormones. This dysfunction may be the primary reason for surgery, or it may coexist in patients who need surgery on other organ systems. To ensure appropriate nursing interventions for the patient with endocrine dysfunction in the postanesthesia care unit (PACU), the perianesthesia nurse must understand the physiology and pathophysiology of the endocrine system.2,3
Mediators of the endocrine system: the hormones
A hormone is a biochemical substance synthesized in an endocrine gland and secreted into body fluids for regulation or control of physiologic processes in other cells of the body. Biochemically, hormones are either proteins (or derivatives of proteins or amino acids) or steroids.1–3
Protein hormones, such as the releasing hormones, catecholamines, and parathormone, fit the fixed-receptor model of hormone action. In this model, the stimulating hormone, called the first messenger, combines with a specific receptor for that hormone on the surface of the target cell. This hormone-receptor combination activates the enzyme adenylate cyclase in the membrane. The portion of the adenylate cyclase that is exposed to the cytoplasm causes the immediate conversion of cytoplasmic adenosine triphosphate into cyclic adenosine monophosphate (AMP). The cyclic AMP then acts as a second messenger and initiates any number of cellular functions.1–3
In the mobile receptor model, a steroid hormone, because of its lipid solubility, passes through the cell membrane into the cytoplasm, where it binds with a specific receptor protein. The combined receptor protein-hormone either diffuses or is transported through the nuclear membrane and transfers the steroid hormone to a smaller protein. In the nucleus, the hormone activates specific genes to form the messenger ribonucleic acid (RNA). The messenger RNA then passes out of the nucleus into the cytoplasm, where it promotes the translation process in the ribosomes to form new proteins. Hormones that fit the fixed-receptor model produce an almost instantaneous response on the part of the target organ. In contrast, because of their action on the genes to cause protein synthesis, when the steroid hormones are secreted a characteristic delay in the initiation of hormone response varies from minutes to days.1–3
Physiology of the endocrine glands
Pituitary gland
The pituitary gland rests in the sella turcica of the sphenoid bone at the base of the brain. This gland is divided into anterior and posterior lobes. Because of its glandular nature, the anterior lobe is called the adenohypophysis; the posterior lobe is an outgrowth of a part of the nervous system, the hypothalamus, and is called the neurohypophysis. The pituitary gland receives its arterial blood supply from two paired systems of vessels: (1) the right and left superior hypophyseal arteries from above and (2) the right and left inferior hypophyseal arteries from below. The anterior lobe receives no arterial blood supply. Instead, its entire blood supply is derived from the hypophyseal portal veins. This rich capillary system facilitates the rapid discharge of releasing hormones that have target cells in the anterior hypophysis.1–3
Although the pituitary gland is called the master gland, it is actually regulated by other endocrine glands and by the nervous system. The secretion of the hormones of the anterior hypophysis is primarily influenced and controlled by the higher centers in the hypothalamus. Releasing hormones are secreted by the hypothalamic nuclei through the infundibular tract to the portal venous system of the pituitary gland to their respective target cells of the adenohypophysis. Consequently, the hypothalamus brings about fine regulation of the action of the anterior pituitary, and still higher nervous centers apparently further modulate the production of the releasing factors. As a result, the many influences that enter the brain and central nervous system impinge on the anterior pituitary gland either to enhance or to dampen its activity.1–3
Hormonal control of the pituitary involves certain feedback systems. For example, corticotropin-releasing hormone stimulates the production and release of adrenocorticotropin (ACTH). The increased concentration of ACTH causes the hypothalamus to decrease its production of corticotropin-releasing hormone, which in turn reduces ACTH production and ultimately reduces the blood level of ACTH. Therefore, when exogenous corticoids are administered chronically, ACTH secretion decreases and the adrenal cortex atrophies. However, the removal of endogenous corticoids with a bilateral adrenalectomy can result in a tumor of the pituitary gland because of the absence of the feedback depression of the corticotropin-releasing hormone.1–3
The posterior lobe of the pituitary gland has an abundant nerve supply. Nerve cell bodies in the posterior lobe produce two neurosecretions (antidiuretic hormone and oxytocin), which are stored as granules at the site of the nerve cell bodies. When the hypothalamus detects a need for either neurohypophyseal hormone, nerve impulses are sent to the posterior lobe and the hormone is released by granules into the neighboring capillaries. Consequently, the hormonal function of the posterior lobe is under direct nervous system regulation. 1–3
Hormones of the adenohypophysis
Growth hormone, or somatotropin
The growth hormone is unique because it stimulates no target gland but acts on all tissues of the body. Its primary functions are maintaining blood glucose levels and regulating skeletal growth. Growth hormone conserves blood glucose by increasing fat metabolism for energy. It enhances the active transport of amino acids into cells, increases the rate of protein synthesis, and promotes cell division. In addition, growth hormone enhances the formation of somatomedin, which acts directly on cartilage and bone to promote growth. The active secretion of growth hormone is regulated in the hypothalamus via growth hormone–releasing hormone. Stimuli such as hypoglycemia, exercise, and trauma cause the hypothalamus to secrete growth hormone–releasing hormone, which is transported to the anterior lobe of the pituitary gland and released into the blood. Secretion of growth hormone can be inhibited by somatostatin, also called growth hormone–inhibiting hormone, which is secreted by the hypothalamus and the delta cells of the pancreas.1–3
Hyposecretion of the growth hormone before puberty leads to dwarfism, or failure to grow. After puberty, growth hormone hypofunction can result in the condition known as Simmonds’ disease. This disease is characterized by premature senility, weakness, emaciation, mental lethargy, and wrinkled dry skin. Giantism is the result of growth hormone hyperfunction before puberty. After puberty, when the epiphyses of the long bones have closed, growth hormone hyperfunction leads to acromegaly. In this disease, the face, hands, and feet become enlarged. Patients with acromegaly are prone to airway obstruction caused by protruding lower jaws and enlarged tongues. Therefore constant vigilance to the respiratory status of these patients is essential in the PACU.1–3
Thyroid-stimulating hormone, or thyrotropin
The follicular cells of the thyroid are the target for thyroid-stimulating hormone (TSH). This hormone promotes the growth and secretory activity of the thyroid gland. Production of TSH is regulated in a reciprocal fashion by the blood levels of thyroid hormone and the formation of thyrotropin-releasing hormone in the hypothalamus.3,4
Adrenocorticotropin
Adrenocorticotropin promotes glucocorticoid, mineralocorticoid, and androgenic steroid production and secretion by the adrenal cortex. This hormone is released in response to stimuli such as pain, hypoglycemia, hypoxia, bacterial toxins, hyperthermia, hypothermia, and physiologic stress. More specifically, the hypothalamus monitors for these various stressors and on excitation, corticotropin-releasing hormone (CRH) is secreted, which stimulates ACTH secretion from the adenohypophysis. Levels of adrenocortical hormones in the blood regulate secretion of ACTH with a hypothalamic feedback mechanism.3,4
Gonadotropic hormones
Gonadotropic hormones regulate the growth, development, and function of the ovaries and testes. The gonadotropic hormones are the follicle-stimulating hormone and the luteinizing hormone. Secretion of the gonadotropic hormones is stimulated by gonadotropin-releasing hormone; the hormones are secreted by the hypothalamus.4
Hormones of the neurohypophysis
Antidiuretic hormone, or vasopressin
During normal activities of daily living, antidiuretic hormone (ADH) is secreted in small amounts into the blood stream for promotion of reabsorption of water by the renal tubules, which leads to a decreased excretion of water by the kidneys. When ADH is secreted in large quantities, vasoconstriction of the smooth muscles occurs and ultimately elevates the blood pressure. The pressor effects of ADH are produced only with large doses that are not in the usual physiologic range. The secretion of ADH is regulated by several feedback loops, one of which involves plasma osmolality. Within the hypothalamus are osmoreceptors, whose function is secretion of ADH when plasma osmolarity is increased. Alternatively, dilution of plasma inhibits ADH secretion. The second feedback loop or major stimulus of ADH secretion is the volume or stretch receptors located in the left atrium. These receptors are activated when the extracellular fluid volume is increased; with this activation, ADH secretion is inhibited. The baroreceptors, which are located in the carotid sinus and aortic arch, are the receptors for the third feedback loop. A decrease in the arterial blood pressure stimulates the baroreceptors, which in turn stimulate a release of ADH. Both the stretch receptors and the baroreceptors transmit neuronal input to the brain via the vagus nerve.4,5
Pituitary dysfunction
Hyperfunction rarely involves more than one endocrine gland. Alternatively, hypofunction usually involves more than one endocrine gland, although instances of isolated deficiencies have been reported. A common cause of pituitary hypofunction is compression of glandular cells by the expansion of a functional or nonfunctional tumor. In this situation, an excess of one hormone may coexist with a deficiency of another.4,5
Pineal gland
The pineal gland is situated in the diencephalon just above the roof of the midbrain. This gland is considered an intricate and highly sensitive biologic clock because the secretory activity of the pineal gland is greatest at night. The pineal gland secretes melatonin, which affects the size and secretory activity of the ovaries and other organs. The production and release of melatonin are regulated by the sympathetic nervous system. In fact, the pineal gland is considered a neuroendocrine transducer because it converts nervous system input into a hormonal output.4,5
Thyroid gland
The thyroid gland is located in the anterior middle portion of the neck immediately below the larynx. The gland consists of two lobes that are attached by a strip of tissue called the isthmus. Structurally, this gland is composed of tiny sacs called follicles. Each follicle is formed by a single layer of epithelial cells that surround a cavity that contains a secretory product known as colloid. This colloid fluid consists mainly of a glycoprotein-iodine complex called thyroglobulin.4,5
On stimulation with TSH, thyroid hormones are produced in the following steps: (1) iodide trapping; (2) oxidation and iodination; (3) storage of the hormones in the colloid as part of the thyroglobulin molecules; and (4) proteolysis, which can be inhibited by iodide, and release of the hormones. The two hormones released from the thyroid gland are triiodothyronine (T3) and thyroxine (T4). T4 represents more than 95% of the circulating thyroid hormone and is considered to be relatively inactive physiologically in comparison with T3. Consequently, although T3 has a relatively low concentration, it passes out of the blood stream faster than T4, has a more rapid action and is probably the major biologically active thyroid hormone. After these hormones are secreted by the thyroid gland, they are transported to all parts of the body by means of plasma proteins, in the form of protein-bound iodinated compounds. As a result, the laboratory test for protein-bound iodine is useful for determining of the amount of circulating thyroid hormone in the blood.4,5
T3 and T4 regulate the metabolic activities of the body. More specifically, they regulate the rate of cellular oxidation. In addition, they are essential for the normal growth and development of the body. Other metabolic activities that are influenced by T3 and T4 are the promotion of protein synthesis and breakdown, increase of glucose absorption and utilization, facilitation of gluconeogenesis, and maintenance of fluid and electrolyte balance. The thyroid hormones are also involved in a feedback mechanism. The concentration of T3 and T4 in the blood regulates the secretion of TSH by the anterior pituitary gland. TSH regulates the growth and secretory activity of the thyroid gland.4,5
The thyroid gland also secretes thyrocalcitonin, or calcitonin, for maintenance of the proper level of calcium in the blood. More specifically, calcitonin decreases the serum concentration of calcium by counteracting the effects of parathormone and inhibiting the resorption of calcium from the bones.4,5
Parathyroid glands
The parathyroid glands are located on the posterior portion of the thyroid gland. In most instances, one parathyroid gland is present on each of the four poles of the thyroid gland. The parathyroid glands release a polypeptide hormone called parathormone. This hormone is the principal regulator of the calcium concentration in the body. Parathormone is released into the circulation by a negative feedback mechanism that depends on the serum concentration of calcium. As a result, a high serum concentration of calcium suppresses the synthesis and release of parathormone and a low serum calcium concentration stimulates the release of the hormone. Normal serum calcium concentrations depend on the regulatory mechanisms, which include parathormone, calcitonin, phosphorus, magnesium, and vitamin D. In fact, the serum calcium concentration is maintained by these regulatory mechanisms within narrow and constant limits. The normal serum calcium level is 9 to 10.3 mg/dL for men and 8.9 to 10.2 mg/dL for women. Serum levels of calcium expressed in milliequivalents per liter are half the value given in milligrams per deciliter.4,5
Parathormone influences the rate at which calcium is transported across membranes in the bone, the gastrointestinal tract, and the kidneys. More specifically, calcium release from bone is facilitated by parathormone-induced stimulation of osteoclastic activity. The absorption of calcium by the gastrointestinal tract is enhanced by the parathormone-induced synthesis of vitamin D. Parathormone activates the synthesis of vitamin D and leads to increased tubular reabsorption of calcium and enhanced renal tubular clearance of phosphorus, which results in more calcium entering the circulation.4,5
Adrenal glands
The adrenal glands are located on the apex of each kidney. Each gland consists of an outer portion called the cortex and an inner portion called the medulla. The medulla is responsible for the secretion of catecholamines (see Chapter 11). The preganglionic fibers of the sympathetic nervous system provide the stimulation that facilitates the liberation of the catecholamines by the medullary cells. The cortex composes the bulk of the adrenal gland and is responsible for the secretion of the steroids. The cortex is divided anatomically and physiologically into three zones: the zona glomerulosa, the zona fasciculata, and the inner zona reticularis. These zones are the sites of secretion of the three major steroid hormones: the mineralocorticoids, the glucocorticoids, and the androgens.1–5
The mineralocorticoids are responsible for the maintenance of fluid and electrolyte balance. Aldosterone is, physiologically, the most important mineralocorticoid. The basic action of aldosterone is promotion of the reabsorption of sodium with stimulation of cellular sodium pumps in the target tissue. Overall, aldosterone causes increased tubular reabsorption of sodium and excretion of potassium, which decreases urinary excretion of sodium and chloride and increases urinary secretion of potassium, consequently expanding the extracellular fluid compartment. Aldosterone secretion is increased by ACTH, a depletion in sodium, and an increase in potassium. The secretion of aldosterone is also regulated by the renin-angiotensin system. When the blood supply to the kidneys is low, the juxtaglomerular cells are stimulated to release renin. Renin, which is an enzyme, enters the blood and converts the plasma protein angiotensinogen to angiotensin I. In the lungs and elsewhere, angiotensin I is converted enzymatically to the physiologically active form, angiotensin II. One of the basic actions of angiotensin II is stimulation of the adrenal cortex for secretion of aldosterone. Thus, aldosterone secretion is regulated by the blood pressure and volume; and because it causes retention of sodium and a rise in blood pressure, aldosterone also acts as a feedback mechanism to shut off the further release of renin.1,4–6
The glucocorticoids are secreted in the zona fasciculata. Cortisol (hydrocortisone) constitutes approximately 95% of the total glucocorticoid activity, with corticosterone and cortisone constituting the remaining 5%. These hormones function to preserve the carbohydrate reserves of the body with promotion of gluconeogenesis, glycogenesis, lipolysis, and oxidation of fat in the liver. Because they conserve carbohydrates, these hormones serve as functional antagonists to insulin. Finally, these hormones possess an excellent antiinflammatory action. The major regulator of their secretion is ACTH, which is secreted by cells in the anterior pituitary gland.1,4–6 ACTH is, in turn, modulated by CRH, which is secreted by the hypothalamus. Cortisol serves as a negative feedback mechanism for inhibition of both ACTH and CRH production. Physical and mental stresses stimulate the release of CRH from the hypothalamus. In addition to the catecholamines, cortisol and ACTH are considered to be the major stress hormones.1,4–7 The androgens, or sex hormones, are actively involved in the preadolescent growth spurt and the appearance of axillary and pubic hair.
Pancreas
Islet of Langerhans cells are scattered throughout the pancreas. The three islet cell types—alpha, beta, and delta—secrete glucagon, insulin, and somatostatin, respectively. Glucagon has several functions that are diametrically opposed to those of insulin. Glucagon is commonly referred to as the hyperglycemic factor, and its most important function is to increase the blood glucose level. This increased glucose level in the blood is the result of the effects of glucagon on glucose metabolism—that is, glycogenolysis (in the liver) and increased gluconeogenesis. When the blood glucose concentration decreases to less than 70 mg/dL, the alpha cells secrete glucagon to protect against hypoglycemia. In addition, amino acids enhance the secretion of glucagon. In this instance, the glucagon helps to prevent the hypoglycemia that can result because amino acids stimulate insulin release, which tends to reduce the blood glucose concentration. The secretion of glucagon appears to be inhibited by the release of somatostatin from the delta cells of the pancreas, and because it is a polypeptide, glucagon is rapidly destroyed by proteolytic enzymes.1,4–7
Insulin is a protein secreted by the beta cells of the islets of Langerhans in response to elevated levels of blood glucose. Its secretion is inhibited by low blood glucose levels and somatostatin. In addition, insulin secretion can be inhibited by epinephrine, glucocorticoids, and thyroxine. When insulin is secreted by the beta cells, a metabolic state that favors the storage of nutrients is set into action. These physiologic actions include: (1) retention of glucose by the liver; (2) slowing of hepatic glucose release; (3) increase in uptake of glucose by muscle (stored as glycogen) and adipose tissue (stored as triglycerides); (4) translocation of amino acids and neutral fats into muscle and adipose tissue; and (5) retardation of lipolysis and proteolysis. As a result, insulin seems to “open the door” of most of the cell membranes of the body to facilitate the movement of glucose, amino acids, and fatty acids into the cells. Diabetes mellitus, which is a disease that involves the synthesis, storage, and release of insulin, is discussed in detail in Chapter 48.1,4–7
Gonads
The hormone testosterone is produced in the interstitial cells of the testes. The synthesis and secretion of this hormone are regulated by luteinizing hormone, which is secreted by the anterior pituitary gland. Testosterone regulates the development and maintenance of the male secondary sexual characteristics and produces some metabolic effects on bone and skeletal muscle. Another action of this hormone is the modulation of male behavior with limbic system stimulation. Estrogen, another gonadal hormone, is secreted by the ovarian follicles in response to the follicle-stimulating hormone and the luteinizing hormone of the anterior pituitary gland and is responsible for the development and maintenance of the secondary sexual characteristics in the female. Estrogen, along with progesterone, which is produced by the cells of the corpus luteum, plays an important role in the menstrual cycle.1,4–7
Selected syndromes and diseases associated with the endocrine system
Hypoadrenocorticism
A reduction in function of the hormones associated with the pituitary-adrenal axis can develop as a result of: (1) the destruction of the adrenal cortex by degenerative disease, neoplastic growth, or hemorrhage; (2) a deficiency of ACTH; or (3) prolonged administration of corticosteroid drugs. Primary adrenal insufficiency (Addison disease) results from destruction of the adrenal cortex. Although it was previously believed that Addison disease is mainly caused by idiopathic atrophy resulting in an autoimmune dysfunction, new studies suggest that specific targeting of T and B lymphocytes towards steroidogenic organs could also be a likely cause of this disease. Other causes of Addison disease include tuberculosis, histoplasmosis, bilateral hemorrhage from anticoagulation therapy, surgical removal of the adrenal glands, tumor chemotherapy, metastasis to the adrenal glands, and sepsis.7–14
A deficiency of ACTH is associated with panhypopituitarism. Patients who have been administered frequent “bursts” of exogenous steroid preparations such as prednisone can have a suppression of output of endogenous corticosteroids because of augmentation of the feedback mechanism to the anterior pituitary gland. Concern about the development of hypoadrenocorticism should be shown in the case of any patient who has received 20 mg of prednisone per day for more than 2 weeks in the preceding 12 months (although author opinions vary on dosage and length of time). The recovery of the normal function of the pituitary-adrenal axis may be as long as 12 months after the discontinuation of steroid therapy. Patients who are even remotely suspected to have hypoadrenocorticism are usually administered steroids before, during, and after surgery.7–14
This perioperative steroid coverage is needed because infection, injury, operation, or other stressors activate the pituitary-adrenal axis. If this axis is suppressed (i.e., hypoadrenocorticism), acute adrenal insufficiency (i.e., addisonian crisis) can develop, which is a life-threatening situation that requires prompt action by the PACU nurse. Clinical manifestations of the addisonian crisis include dehydration, nausea, vomiting, muscular weakness, and hypotension, followed by fever, marked flaccidity of the extremities, hyponatremia, hyperkalemia, azotemia, and shock. Therefore the PACU nurse should monitor patients who are even remotely likely to have the addisonian crisis develop. If some of the signs and symptoms appear, the attending physician should be notified immediately. The severely ill patient must be treated while the diagnosis is being confirmed. Dexamethasone 2 to 4 mg is usually administered intravenously along with IV therapy of 5% dextrose in normal saline solution. Dexamethasone is the drug of choice because it does not interfere with the diagnostic tests and yet does provide the needed glucocorticoid. If dexamethasone is not available, administration of a single 100-mg intravenous dose of hydrocortisone is advantageous to obtain both the glucocorticoid and the mineralocorticoid activity. This dose can be followed by 50 to 100 mg of hydrocortisone administered parenterally every 6 hours. During the administration of the treatment, the PACU nurse should continuously monitor the patient’s cardiorespiratory status.7–14
Syndrome of inappropriate antidiuretic hormone hypersecretion
The syndrome of inappropriate antidiuretic hormone hypersecretion (SIADH) occurs in the event of continued secretion of ADH in the presence of serum hypoosmolality. More specifically, the feedback loops that regulate ADH secretion and inhibition fail. Usually, both dilution and expansion of the blood volume serve to stimulate a suppression of the release of ADH. However, in SIADH, the feedback loops do not respond appropriately to the osmolar or volume change, and a pathologic positive feedback loop continues, thus resulting in continued production of ADH.7–14
When hemorrhage and trauma occur during a surgical procedure, ADH secretion is appropriately elevated. In this situation, SIADH can be induced as a result of overzealous fluid administration. Because of the urinary sodium loss that occurs along with the water retention, the syndrome of acute water intoxication may be seen in the PACU. The symptoms of water intoxication derive from increased brain water, inoperative sodium pump, and hyponatremia. The symptoms begin with headache, muscular weakness, anorexia, nausea, and vomiting and lead to confusion, hostility, disorientation, uncooperativeness, drowsiness, and terminal convulsions or coma. These symptoms usually do not occur if the serum sodium level is higher than 120 mEq/L. Therefore, in patients who have had major vascular surgery, trauma, or hemorrhage, the perianesthesia nurse should assess frequently for the symptoms of SIADH and notify the attending physician if the symptoms become evident. The focus of treatment for SIADH is fluid restriction, diuresis with mannitol or furosemide, and administration of sodium chloride. In addition, the perianesthesia nurse should frequently assess the neurologic signs and cardiorespiratory status of the patient with SIADH and measure and record accurately the intake and output of all fluids.7–14
Summary
The hormones secreted by the endocrine system along with the nervous system provide regulation and control of the body. Dysfunction of the production of any one of the hormones can have disastrous effects on the body. Surgery is performed on many of the glands or organs where the secreting endocrine gland is located. Of particular significance to the perianesthesia nurse are the parathyroid glands. One of the adverse outcomes of thyroid surgery is the accidental removal of one or more of the parathyroid glands. This complication is one of many that perianesthesia nurses need to assess when the site of surgery is located near an endocrine gland. The hormone insulin was briefly described, with a more detailed discussion of insulin and diabetes in Chapter 48.15–18
1. Hall J. Guyton and Hall textbook of medical physiology, ed 12. Philadelphia: Saunders; 2011.
2. Melmed M, et al. Williams textbook of endocrinology, ed 12. Philadelphia: Saunders; 2011.
3. Drake R, et al. Gray’s anatomy for students, ed 2. Philadelphia: Churchill Livingstone; 2009.
4. Barrett KE, et al. Ganong’s review of medical physiology, ed 23. New York: McGraw-Hill Medical; 2009.
5. Coursin DB, et al. Endocrine complications in intensive care unit patients,. Semin Anesth Perioperative Med Pain. 2002;21(1):59–74.
6. Degroot LJ, Jameson L. Endocrinology, ed 5. Philadelphia: Saunders; 2006.
7. Aitkenhead A, et al. Textbook of anaesthesia, ed 5. Philadelphia: Churchill Livingstone; 2007.
8. Mazzaferri E. Year book of endocrinology. St. Louis: Mosby; 2007.
9. Atlee J. Complications in anesthesia, ed 2. Philadelphia: Saunders; 2007.
10. Benumof J, Saidman L. Anesthesia and perioperative complications, ed 2. St. Louis: Mosby; 1999.
11. Fleisher LA. Anesthesia and uncommon diseases, ed 5. Philadelphia: Saunders; 2007.
12. Longnecker D, et al. Principles and practice of anesthesiology, ed 2. St. Louis: Mosby; 1998.
13. Miller R, et al. Miller’s anesthesia, ed 7. Philadelphia: Churchill Livingstone; 2009.
14. Nagelhout J, Plaus K. Nurse anesthesia, ed 4. St. Louis: Saunders; 2010.
15. Stoelting R, Miller R. Basics of anesthesia, ed 6. Philadelphia: Churchill Livingstone; 2011.
16. Shorten G, et al. Postoperative pain management: an evidence-based guide to practice. Philadelphia: Saunders; 2006.
17. Noble K. Thyroid storm. J Perianesth Nurs. 2006;21(2):119–425.
18. Townsend CM, et al. Sabiston textbook of surgery: the biological basis of modern surgical practice. ed 19. Philadelphia: Saunders; 2012.