The Endocrine System
SYSTEMWIDE ELEMENTS
a. Hormones are molecules that are synthesized and secreted by specialized cells and released into the blood, exerting biochemical effects on target cells away from the site of origin
b. Hormones control metabolism, transport of substances across cell membranes, fluid and electrolyte balance, growth and development, adaptation, and reproduction
2. Chemically categorized by physiologic action
a. Peptide or protein hormones: Vasopressin (antidiuretic hormone [ADH]) thyrotropin-releasing hormone (TRH), insulin, growth hormone (somatotropin [GH]), follicle-stimulating hormone (FSH), luteinizing hormone (LH), corticotropin (adrenocorticotropic hormone [ACTH]), calcitonin
b. Steroids: Glucocorticoids (cortisol), mineralocorticoids (aldosterone), estradiol, progesterone, testosterone
c. Amines and amino acid derivatives: Norepinephrine, epinephrine, triiodothyronine (T3), thyroxine (T4)
a. Specificity of hormone action is determined by the presence of a specific hormone receptor on or in the target cell
b. Receptors distinguish hormones from each other and translate the hormonal signal into a cellular response
c. The hormone-receptor complex initiates intracellular events that lead to the biologic effects of the hormone acting on the target cell
Pituitary Gland
1. Location: Base of the skull in the sphenoid bone; connected to the hypothalamus by the pituitary stalk (infundibulum), which links the nervous and endocrine systems
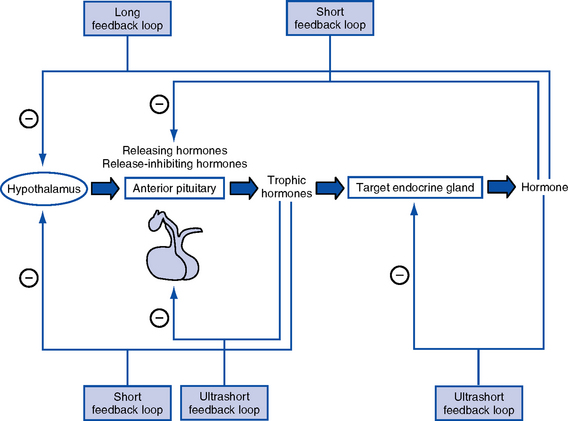
a. Anterior lobe (adenohypophysis—75% of gland): Hormones are controlled by hypothalamic releasing or inhibiting hormones in response to stimuli received in the central nervous system
3. Anterior pituitary hormones
(a) Stimulation: GH-releasing hormone (GRH) in response to physical and/or emotional stress, starvation, hypoglycemia, other protein-depleted states
(b) Inhibition: Somatostatin from the hypothalamus, postprandial hyperglycemia, and pharmacologic doses of corticosteroids
(a) Increases rate of protein synthesis
(c) Decreases protein catabolism
(d) Decreases carbohydrate use
(e) Stimulates bone and cartilage growth
(f) Works with insulin, thyroid hormones, and sex steroids to promote growth
iii. Disorders resulting from dysfunction: Not of significance in critical care
(a) Stimulation: Corticotropin-releasing hormone (CRH) in response to physical or emotional stress, trauma, hypoglycemia, hypoxia, surgery, decreased plasma cortisol levels
(b) Inhibition: Increased plasma cortisol levels exert negative feedback on CRH and thus ACTH; stress can overcome this negative feedback
ii. Physiologic activity: Production and release of adrenocortical hormones (glucocorticoids, adrenal androgens, and mineralocorticoids)
(a) Stimulation: TRH in response to low concentration of thyroid hormones
(b) Inhibition: Somatostatin from the hypothalamus, increased thyroid hormone levels
(a) Increases synthesis of thyroid hormones
(b) Releases stored thyroid hormones
(c) Stimulates iodide uptake into thyroid cells
(d) Increases size, number, and secretory activities of thyroid cells
iii. Disorders resulting from dysfunction: See Thyroid Gland
d. Other anterior pituitary hormones under hypothalamic control
4. Posterior pituitary hormones
(a) Stimulation: Increase in plasma osmolality, hypoxia, reduction in blood volume or blood pressure
Thyroid Gland
1. Location: Immediately below the larynx laterally and anterior to the trachea
2. Composition: Two lobes connected by an isthmus
3. Regulation of secretion (thyroid hormones)
a. Stimulation: TSH stimulates thyroid hormone release, which is regulated by TRH from the hypothalamus; decreased levels of thyroid hormones stimulate the release of TSH and TRH
b. Inhibition: Elevated levels of thyroid hormones inhibit TSH and TRH
a. Increases the metabolic activity of cells, which results in increased oxygen consumption, increased rate of chemical reactions, and heat production
b. Stimulates carbohydrate, fat, and protein metabolism
c. Works with insulin, GH, and sex steroids to promote growth
d. Critical for fetal neural and skeletal system development (intrauterine hypothyroidism causes cretinism)
e. Positive chronotropic and inotropic effects on the heart
f. Required for a normal hypoxic and hypercapnic drive in respiratory centers
h. Increases metabolism and clearance of steroid hormone and insulin
5. Disorders resulting from dysfunction
a. Thyroid enlargement (goiter)
b. Excess: Hyperthyroidism (chronic), thyroid storm (acute)
c. Deficiency: Hypothyroidism (chronic), myxedema coma (acute)
6. Thyrocalcitonin (calcitonin)
Parathyroid Glands
1. Location: Four glands on the posterior surface of the thyroid gland
2. Composition: Chief cells release PTH
a. Stimulation: Decrease in serum calcium level
b. Inhibition: Increase in serum levels of calcium and vitamin D metabolites, hypermagnesemia, and hypomagnesemia
Adrenal Glands
1. Location: Retroperitoneal, superior to the kidney
2. Composition: Two separate endocrine tissues that produce distinct hormones
a. Glucocorticoids (cortisol is the major hormone)
(a) Stimulation: ACTH (diurnal variation—increased 1 hour after awakening, incidence of myocardial infarction increased in the morning)
(b) Inhibition: Cortisol exerts negative feedback on the anterior pituitary and hypothalamus
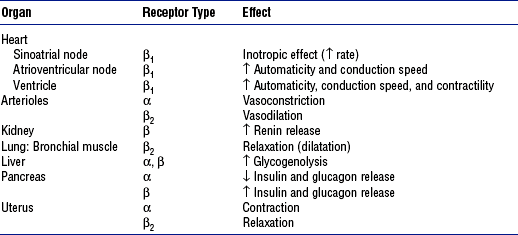
4. Medullary hormones: Epinephrine and norepinephrine
a. Regulation of secretion: Stimulated by fear, anxiety, pain, trauma, fluid loss, hemorrhage, extremes in temperature, surgery, hypoxia, hypoglycemia, hypokalemia, hypernatremia, hypotension
Pancreas
1. Location: Lies transversely behind the peritoneum and stomach
2. Composition: Exocrine and endocrine components. Endocrine functions originate from the islet cells, which constitute less than 2% of the total pancreatic volume; 65% of the islet cells are beta cells, which produce insulin. Glucagon is produced by the alpha cells; somatostatin and gastrin are produced by the delta cells.
i. Stimulation: Increases in blood glucose, gastrin, secretin, cholecystokinin, and gastrointestinal hormone levels, and β-adrenergic stimulation
ii. Inhibition: α-adrenergic effects of somatostatin, catecholamines, and drugs, including diazoxide, phenytoin, and vinblastine
iv. Works with thyroid hormones, the sex steroids, and GH to promote growth
c. Disorders resulting from dysfunction
ii. Deficiency: Diabetes mellitus
(a) Type 1: Absolute deficiency of insulin due to islet cell antibodies; genetic link, autoimmune disorder
(b) Type 2: Relative deficiency of insulin caused by decreased sensitivity of receptors to insulin, decreased production, premature destruction of insulin or receptors, and/or hyperinsulinemia; polygenetic etiologies, dietary link
i. Increases blood glucose via glycogenolysis and gluconeogenesis
iii. Increases amino acid transport to the liver and the conversion of amino acids to glucose precursors
iv. Is a major insulin-antagonistic hormone
v. Critical hormone in the recovery from insulin-induced hypoglycemia
c. Deficient glucagon production is thought to play a role in defective glucose counterregulation in insulin-induced hypoglycemia in type 1 diabetes mellitus
d. Available as a pharmacologic agent to correct insulin-induced hypoglycemia (all diabetics should have a readily available source)
PATIENT ASSESSMENT
i. Presence of pathophysiologic processes that can result in endocrine dysfunction
(a) Adrenal gland hypoperfusion
(b) Infection, inflammation, autoimmune processes
(c) Neoplasms and exposure to the chemotherapeutic agents and radiotherapy used to treat the neoplasms
ii. Pregnancy, postpartum state
iii. Presence of preexisting chronic endocrine disorder (diagnosed or undiagnosed)
iv. Poor compliance with pharmacologic therapy for a preexisting endocrine disorder
v. Presence of an unrelated critical illness in a patient with a preexisting chronic endocrine disorder
vi. Positive family history of an endocrine disorder
viii. Indicators of altered health patterns
(1) Personality changes, lethargy, emotional lability, attention span deficit, memory impairment
(3) Changes in level of consciousness
(4) Depression, paranoia, delusions, delirium
(5) Verbalizations that indicate lack of knowledge or misconceptions regarding self-care management
(i) Health perception and health management: Evidence of noncompliance with the prescribed medical regimen
b. Family history: Endocrine disorders in other family members
i. Elderly persons may be at special risk for the development of an endocrine crisis because of changes associated with aging and a diminished thirst mechanism
ii. Economically disadvantaged persons may be at risk for the development of an endocrine crisis because many of the regimens for treating chronic endocrine disorders are costly and necessitate regular medical follow-up
iii. Teenagers with poor compliance with a prescribed medical regimen, particularly diabetic patients, are at increased risk of crisis
2. Nursing examination of patient
(a) Excessive or diminutive stature
(b) Fat distribution in relation to gender and maturational level
(c) Mobility, tremor, hyperkinesis
(d) Scars, especially in the neck area
(e) Hair distribution and texture relative to gender and maturational level
(i) Presence of medical alert identification
(j) Hydration status of oral cavity
(k) Periorbital edema, ptosis, eye protrusion, stare, dry eyes
(l) Unusual pigmentation, temperature, turgor, striae, or thinning of the skin
ii. Palpation: Enlarged or nodular thyroid gland, often painful
iii. Percussion: Abnormal deep tendon reflexes (may be hyperreflexic or hyporeflexic)
(a) Neck: Bruits over the thyroid gland
(b) Heart: Distant heart sounds, third heart sound (due to pericardial effusion, heart failure)
(c) Blood pressure: Hypotension, hypertension
(d) Heart rate and rhythm disturbances
(e) Altered respiratory pattern
(g) Pericardial and/or pleural friction rub (due to effusion)
3. Appraisal of patient characteristics
i. Level 1—Minimally resilient: 88-year-old female admitted in a diabetic coma with concomitant antibiotic-resistant bacterial pneumonia
ii. Level 3—Moderately resilient: 14-year-old male admitted in diabetic ketoacidosis following an episode of the flu; no other medical conditions
iii. Level 5—Highly resilient: 57-year-old man with a blood glucose level of 50 mg/dl who reports feeling sweaty, agitated, and slightly disoriented following 3 days of vomiting with the flu
i. Level 1—Highly vulnerable: 79-year-old male with a history of myocardial infarction and subsequent congestive heart failure who develops diabetes insipidus following head trauma after a motor vehicle accident; fluid replacement causes cardiac deterioration
ii. Level 3—Moderately vulnerable: 49-year-old female who develops nephrogenic diabetes insipidus following repeated episodes of pyelonephritis with scarring
iii. Level 5—Minimally vulnerable: 44-year-old male following transsphenoidal removal of a pituitary tumor who develops diabetes insipidus but remains hemodynamically stable and responds immediately to administration of vasopressin
i. Level 1—Minimally stable: 38-year-old female who attempts suicide by ingestion of excessive amounts of thyroid hormone; arrives at the unit with severe tachycardia, hypotension, and a temperature of 105° F (40.6° C), in a coma
ii. Level 3—Moderately stable: 44-year-old female who comes for treatment with tachycardia and a blood pressure of 90/60 mm Hg after restarting thyroid hormone therapy with a new formulation
iii. Level 5—Highly stable: 32-year-old female with hyperthyroidism as evidenced by abnormal laboratory test results who responds well to propranolol and propylthiouracil and who is scheduled for thyroidectomy
i. Level 1—Highly complex: 88-year-old female admitted in hyperosmolar, nonketotic coma. Patient has a history of malnutrition and chronic obstructive pulmonary disease. She lives alone, has mobility impairments, and has no family nearby. Has Medicare insurance only.
ii. Level 3—Moderately complex: 44-year-old male admitted in diabetic ketoacidosis following inability to obtain insulin. Patient recently became unemployed and lost health insurance and prescription coverage.
iii. Level 5—Minimally complex: 79-year-old male with newly diagnosed diabetes who has a blood glucose level of 200 mg/dl and a hemoglobin A1C fraction of 8.6%. Patient shows cardiovascular and neurologic stability. Has good family support and insurance. Is well educated.
i. Level 1—Few resources: Patient with newly diagnosed diabetes who has no insurance and no family, is unemployed, is new to the area, and is homeless
ii. Level 3—Moderate resources: Patient with newly diagnosed diabetes who has Medicare coverage and a niece who lives an hour away and who currently resides in an assisted living facility
iii. Level 5—Many resources: Patient with newly diagnosed diabetes who has insurance and prescription coverage. Patient is well educated and has strong family support. Independent in care and finances.
i. Level 1—No participation: 48-year-old female admitted in myxedema coma following thyroidectomy. Patient did not start thyroid replacement therapy after surgery. No family nearby.
ii. Level 3—Moderate level of participation: 68-year-old female admitted with bradycardia, anemia, and fatigue who confesses to having stopped thyroid hormone replacement therapy because she couldn’t afford to visit her physician for a new prescription
iii. Level 5—Full participation: A 14-year-old male who is treated successfully for diabetic ketoacidosis and who, with his family, requests assistance in learning more about his disease and its management
g. Participation in decision making
i. Level 1—No participation: Fiftyish homeless male admitted in diabetic ketoacidosis. Patient is alcoholic with a history of mental health problems requiring hospitalization. No known family.
ii. Level 3—Moderate participation: 78-year-old patient with newly diagnosed diabetes who has a history of prostate cancer. Has a sister who is also diabetic. Asks for information to access home nursing care for assistance.
iii. Level 5—Full level of participation: 44-year-old patient admitted in addi-sonian crisis. Patient has durable power of attorney for health care and living will. Patient’s family is present with the patient and fully knowledgeable about the disease. Family provides history and treatment authorization, and will be available to aid in care after discharge.
i. Level 1—Not predictable: 44-year-old patient with brittle diabetes admitted in diabetic ketoacidosis for the fourth time this year. Poorly compliant with the medical regimen, smokes; chronic obstructive pulmonary disease has been newly diagnosed.
ii. Level 3—Moderately predictable: 32-year-old diabetic patient admitted in diabetic ketoacidosis. Responds well to administration of insulin and fluids. Aware that her triggers for diabetic ketoacidosis are infection, particularly bladder infections, and the flu.
iii. Level 5—Highly predictable: 88-year-old female admitted from a nursing home in hyperosmolar, nonketotic coma following dehydration caused by the flu. Responds well to rehydration and insulin.
a. Laboratory: Blood and urine
ii. Glucose, ketoacid, blood urea nitrogen, cholesterol, creatinine, serum creatine phosphokinase levels
iii. Plasma osmolality, hematocrit, white blood cell count with differential
PATIENT CARE
1. Fluid volume deficit (hypovolemia)
2. Fluid volume excess (hypervolemia)
iv. Pulmonary congestion and dyspnea
v. Deterioration of mental status
b. Goals of care: Fluid and electrolyte balance is achieved and maintained
3. Altered carbohydrate, fat, and/or protein metabolism
i. Hyperglycemia with or without ketosis
ii. Decreased serum albumin level
i. Body weight normalizes and stabilizes
ii. No evidence of ketosis is present
4. Need for patient and family education and discharge planning
i. Lack of knowledge or skills may seriously compromise self-care
ii. Patient and/or family is unable to explain or follow instructions correctly
iii. Patient and/or family raises questions and requests information
b. Goals of care: Patient demonstrates knowledge and skills needed for providing self-care and contacting health care resources
c. Collaborating professionals on health care team
i. Assess patient and family knowledge of the health disorder and the required self-care
ii. Provide appropriate information about the health disorder and self-care
iii. Provide an opportunity for the patient and family to demonstrate needed skills
iv. Provide appropriate resources for additional information and support
e. Evaluation of patient care: Ability of the patient and family to explain and demonstrate optimal self-care management
SPECIFIC PATIENT HEALTH PROBLEMS
1. Pathophysiology: Occurs when any organic lesion or chemical substance (e.g., alcohol) affecting the hypothalamus or posterior pituitary interferes with ADH synthesis and transport or release. Deficiency results in the inability to conserve water and the excretion of large amounts of dilute urine.
a. Central or neurogenic diabetes insipidus (ADH sensitive)
i. Idiopathic (30%): Autoimmune (common), familial (rare)
ii. Trauma: Injury to hypothalamus or pituitary trauma (most common cause of polyuria after neurosurgery)
iii. Craniopharyngioma, pituitary tumor
iv. Infections: Meningitis, encephalitis
v. Vascular disorder: Aneurysm
vi. Infiltrative disorders (histiocytosis X, sarcoidosis)
vii. Malignancy (lung cancer, leukemia, lymphoma)
viii. History of an impaired thirst mechanism or a state in which the patient is confused, incapacitated, or otherwise unable to secure fluids
b. Nephrogenic diabetes insipidus (ADH insensitive): Most common forms are the following:
i. Renal: Polycystic kidneys, pyelonephritis, congenital disorder
c. Pharmacologic agents: Ethanol, lithium, glyburide, and phenytoin inhibit ADH secretion and action
d. Insufficient exogenous ADH in a person with diabetes insipidus
c. Decreased skin turgor, dry mucous membranes
e. Tachycardia; hypotension if the patient has become dehydrated
a. Elevated plasma osmolality (>295 mOsm/kg), decreased urine osmolality (<500 mOsm/kg; can be as low as 30 mOsm/kg)
c. Low urine specific gravity (1.001 to 1.005)
d. Water deprivation test: With adequate stimulus for ADH release (simple dehydration), the kidneys cannot concentrate urine. Differentiates psychogenic polydipsia from diabetes insipidus; no response occurs in either neurogenic or nephrogenic diabetes insipidus.
e. ADH test: To demonstrate that the kidneys can concentrate urine with exogenous ADH. Corrects central diabetes insipidus; no response in nephrogenic diabetes insipidus
f. Low plasma ADH levels in patients with central diabetes insipidus
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Patients with diabetes insipidus may experience the spontaneous resolution of symptoms or require lifetime medication. The success of pharmacologic therapy depends solely on patient compliance. Throughout their course of recovery and discharge, patients with diabetes insipidus may be expected to have needs in the following areas:
Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH)
1. Pathophysiology: Syndrome characterized by plasma hypotonicity and hyponatremia that result from aberrant secretion of ADH, which in turn is caused by the failure of the negative feedback system. Dysfunction results in water intoxication.
a. Central nervous system disorders
i. Trauma: Skull fracture, subdural hematoma, subarachnoid hemorrhage, cerebral contusion, post neurosurgery
iii. Infections: Meningitis, encephalitis, brain abscess, Guillain-Barré syndrome, AIDS
iv. Vascular disorders: Aneurysm, cerebral vascular accident
b. Stimulation of ADH release via hypoxia and/or low left atrial filling pressure
c. Pharmacologic agents: Either increase ADH secretion or potentiate its action
i. Cancer chemotherapeutic agents: Cyclophosphamide, vincristine
ii. Chlorpropamide, acetaminophen, amitriptyline, thiazide diuretics, carbamazepine, pentamidine
d. Excessive exogenous ADH therapy
e. Ectopic ADH production associated with bronchogenic, prostatic, or pancreatic cancers and with leukemia
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: If the underlying cause of SIADH is treated, the symptoms will resolve. If the precipitating cause cannot be removed or treated, the patient will require ongoing electrolyte monitoring throughout recovery and discharge. Patients with SIADH may be expected to have needs in the following areas:
Thyrotoxicosis (Thyroid Storm)
1. Pathophysiology: Life-threatening augmentation of the signs and symptoms of hyperthyroidism; rare, because hyperthyroidism in most patients is well controlled by antithyroid drug therapy
a. Surgical procedures or trauma of any kind
c. Poor compliance with antithyroid therapy (rare)
d. Past or present use of methimazole or propylthiouracil, with disruption of established medication regimen; use of antiarrhythmic agents
a. Confusion, overt psychosis, coma
b. Warm, moist, flushed, soft skin; hyperthermia (105° F [40.6° C]). Diagnosis is confirmed by high fever and altered mental status in a severely ill hyperthyroid patient.
c. Severe tachycardia, third heart sound, irregular pulse (especially in an otherwise young and healthy person), hypotension, shock
d. Adventitious breath sounds caused by pulmonary edema
e. Hyperkinesis and tremor, restlessness and agitation, hyperreflexia
g. Nausea, abdominal pain, hepatomegaly
h. Eyelid lag, retracted eyelids, stare, exophthalmos, irritated eyes
j. Goiter: Diffuse or multinodular, nontender, audible bruits
a. Elevated total and free T3 and T4 levels and reduced TSH level (TSH level may remain normal if the precipitating event was a viral infection)
b. Elevated hepatic aminotransferase level; hyperbilirubinemia common
c. Elevated levels of alkaline phosphatase and creatine phosphokinase
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Once cardiovascular stability is restored and the patient is stabilized, definitive management will include thyroidectomy or pharmacologic termination of thyroid function. These treatments will render the patient hypothyroid, which requires lifelong thyroid hormone replacement. Throughout their recovery and discharge, patients with thyrotoxicosis may be expected to have needs in the following areas:
(a) Mechanism: Secondary to severe tachycardia
(b) Management: Inotropic support; IV administration of β-adrenergic antagonists
iii. Hypoxemia and hypercarbia
(a) Mechanism: Due to pulmonary edema
(b) Management: Oxygen therapy, pulse oximetry, monitoring of ABG levels, intubation, and mechanical ventilation, if needed
Myxedema Coma
1. Pathophysiology: Life-threatening emergency resulting from extreme hypothyroidism. Often occurs in the presence of concurrent illness but may manifest as the initial findings in hypothyroidism. May also be due to noncompliance with the thyroid replacement therapy regimen, especially in the elderly living alone.
a. Decompensation of a preexisting hypothyroid state after infection; trauma; exposure to cold; administration of tranquilizers, barbiturates, and narcotics; or other physical stress. Preexisting hypothyroidism may result from
i. Autoimmune or idiopathic condition (most common cause)
ii. Destruction of the thyroid gland after radioactive iodine therapy for hyperthyroidism
v. Dysfunction within the hypothalamic-pituitary axis (hypophysectomy, pituitary irradiation, pituitary infarction)
b. Insufficient provision of exogenous thyroid hormone (e.g., a hypothyroid patient who discontinues replacement therapy, a critically ill patient who has preexisting hypothyroidism but does not receive continued replacement therapy while hospitalized)
c. Family history of Graves’ disease, Hashimoto’s thyroiditis, or type 1 diabetes mellitus
d. Lithium carbonate: Blocks thyroid hormone synthesis and release; can cause hypothyroidism
a. Nonpitting edema of the feet and hands; periorbital edema
c. Loss of the eyebrows and scalp hair; cool, rough, dry skin
d. Goiter may not be palpable because of atrophy, prior radiation, or prior surgery
e. Delayed deep tendon reflexes, especially the relaxation phase
h. Blood pressure inconclusive
b. Respiratory acidosis, hypoxemia
d. Enlarged cardiac outline, pleural and pericardial effusions on radiograph
e. Electrocardiogram (ECG): Sinus bradycardia, T-wave depression, ST changes, prolonged RT and QT intervals
f. Low free T4, T3 resin uptake, increased TSH level
h. High cholesterol level, hyperlipoproteinemia
i. Because of the potential for concurrent adrenal insufficiency, a rapid ACTH stimulation test should be performed for patients with myxedema
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Once their condition is stabilized, patients will require lifelong thyroid hormone replacement and compliance with the medical regimen to prevent reoccurrence. Throughout their clinical course, patients with myxedema coma may be expected to have needs in the following areas:
Hypoparathyroidism and Hyperparathyroidism
1. Pathophysiology: Parathyroid gland dysfunction or production of a tumor-derived PTH-related peptide is associated with disturbances in calcium and phosphorus balance and bone metabolism. See Chapter 5 for further discussion of the pathophysiology of calcium and phosphorus imbalances.
a. Hyperparathyroidism (hypercalcemia)
i. Primary hyperparathyroidism: Increased secretion of PTH resulting from a benign neoplasm or adenoma (80% of cases)
ii. Secondary hyperparathyroidism: Compensatory response to hypocalcemia caused by chronic renal failure, osteomalacia, or intestinal malabsorption syndromes
iii. Humoral hypercalcemia of malignancy: Squamous cell carcinomas of the lung, head, and neck; hypernephroma; ovarian cancers secrete a PTH-like peptide
3. Signs and symptoms: See Chapter 5
4. Diagnostic study findings: Include measurement of intact PTH levels, vitamin D levels, and levels of total and ionic calcium, phosphorus, magnesium, and urinary cAMP
5. Goals of care: See Chapter 5
6. Collaborating professionals on health care team: See Chapter 5
7. Management of patient care (see also Chapter 5)
i. Acute hypercalcemia: Interventions include hydration and administration of furosemide
ii. Humoral hypercalcemia of malignancy: Treatment may include administration of calcitonin, glucocorticoids, diphosphonates, or mithramycin and diuretics
b. Hypocalcemia: Interventions include administration of calcium, vitamin D, magnesium
Acute Adrenal Insufficiency (Addisonian Crisis)
1. Pathophysiology: Deficiency of cortisol production with electrolyte and fluid abnormalities that result in life-threatening cardiovascular collapse
a. Acute injury to or infection of the adrenal glands
b. Critical illness in a patient with chronic adrenal insufficiency
c. Abrupt cessation of corticosteroid therapy
d. Current or past corticosteroid use of 20 mg of hydrocortisone or its equivalent for longer than 7 to 10 days has the potential for suppressing the hypothalamic-pituitary-adrenal axis. Recovery may take 2 to 12 months or longer.
e. Adrenal hemorrhage may occur with anticoagulant therapy
f. Ketoconazole and etomidate can interfere with steroid biosynthesis
g. Rifampin increases the metabolic clearance rate of corticosteroids
h. Often seen in patients with human immunodeficiency virus infection
a. Hyponatremia, hyperkalemia, hypercalcemia
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: If the precipitating event is avoidable (e.g., abrupt withdrawal of steroid use), symptoms will not recur. Any patient requiring continued steroid use will need close monitoring; physiologic stress (illness, surgery) may require increased dosage or cause inadvertent discontinuation of steroid use. Abrupt withdrawal or unmet increased demand will increase symptoms throughout the course of recovery and discharge. Patients with acute adrenal insufficiency may be expected to have needs in the following areas:
Diabetic Ketoacidosis
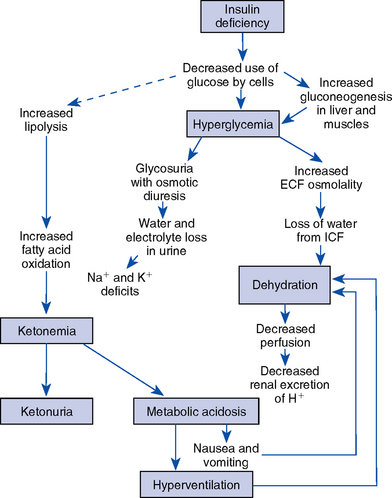
1. Pathophysiology: Diabetic ketoacidosis (DKA) is the most serious metabolic complication of insulin-dependent, or type 1, diabetes mellitus. DKA is a state of insulin deficiency combined with an increase in the level of insulin-antagonistic hormones (glucagons, cortisol, catecholamines, and GH). The result is altered metabolism of carbohydrate, fat, and protein (Figure 6-2) and hyperglycemia.
a. Decreased insulin level with gluconeogenesis and increased insulin resistance result in exaggerated hepatic glucose production
b. Ketosis and metabolic acidosis result from increased synthesis of ketones and lactic acidosis
c. Fluid and electrolyte imbalance and osmotic diuresis are caused by glycosuria; accompanied by loss of sodium, potassium, and chloride
d. Altered mental status results from hyperosmolality, cellular dehydration, acidosis, and possibly impaired oxygen dissociation, because glycosylated hemoglobin binds oxygen more tightly
a. Diagnosed diabetes mellitus
i. Insufficient exogenous insulin: Dose missed or insufficient for needs
b. Undiagnosed diabetes mellitus: Positive family history of diabetes
a. Blurred vision, diminished level of consciousness
b. Nausea, abdominal cramping, vomiting
c. Polyphagia, polyuria, polydipsia
f. Decreased skin turgor, dry mucous membranes
g. Fruity odor to breath (ketosis)
a. Elevated plasma and urine glucose levels: Plasma glucose level above 250 mg/dl; presence of glucose in urine (normal = no trace)
b. Metabolic acidosis: Arterial pH less than 7.3; serum HCO3− less than 18 mEq/dl
c. Positive results for serum and urine ketones
e. Anion gap: Na+ − (Cl− + HCO3−) > 10 (other formulas may be used)
f. ECG: May reflect hypokalemia, although serum potassium level may be normal or elevated; flat T waves
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: DKA can reoccur easily in diabetic patients if medication compliance, diet, and sick-day management are not well understood. Reoccurrence is most common in teens and patients with newly diagnosed diabetes. Patients with DKA may have needs in the following areas:
(a) For patients with newly diagnosed diabetes: Education about the disease, pathophysiology, and self-care management
(b) For patients with previously diagnosed diabetes: Education about the self-care regimen, compliance, and sick-day management
(a) Administer IV fluids to correct dehydration based on corrected sodium
(1) Corrected Na+ = Measured serum Na+ + {[(Serum glucose in mg/dl − 100)/100] × 1.6}
(2) 0.9% NaCl is recommended if the corrected Na+ level is low
(3) 0.45% NaCl is recommended if the corrected Na+ level is normal or high
(4) 5% dextrose added to prevent hypoglycemia when the glucose level is less than 250 mg/dl
(b) Administer regular insulin via IV bolus then continuous drip
(1) Change to SQ insulin 1 to 2 hours before stopping the drip to prevent the recurrence of ketosis and accelerated hyperglycemia
(2) Monitor the serum glucose level hourly
(3) Measure urine ketone levels (insulin infusion may be stopped if the patient stops excreting ketones)
(4) Insulin infusion is usually stopped when the serum glucose level is less than 250 mg/dl
(c) Administer sodium bicarbonate if the pH is less than 7.0
(a) Mechanism: Ketosis secondary to insulin deficiency and stress hormone excess
(b) Management: Administration of insulin and sodium bicarbonate as needed
(a) Mechanism: Secondary to insulin deficiency, ketosis, stress hormone excess, infection
(b) Management: Insulin administration with reduction of serum glucose levels at rates not to exceed 100 mg/dl/hr
(a) Mechanism: Due to osmotic diuresis induced by hyperglycemia; deficit worsened by vomiting and/or inadequate oral intake
(a) Mechanism: Secondary to insulin therapy and a decrease in levels of circulating insulin-antagonist hormones (blood glucose level <50 mg/dl); hypoglycemia can precipitate dysrhythmias, extend infarcts
(b) Management: Requires administration of a rapid-acting carbohydrate, 50% dextrose IV or glucose-containing solution orally if consciousness is not depressed
a. Acid-base and potassium levels within normal limits
c. Blood glucose level stabilized at 150 to 200 mg/dl with no episodes of hypoglycemia
e. Restoration of fluid balance as evidenced by I&O, laboratory values, and cardiovascular stability
f. Resolution of any infection
g. If the diabetes is newly diagnosed, referral of the patient for diabetic management teaching
h. If the diabetes is preexisting, ability of the patient to identify precipitating factors and to modify self-care management as needed
Hyperglycemic, Hyperosmolar Nonketotic Coma (Hyperglycemic, Nonacidotic Diabetic Coma)
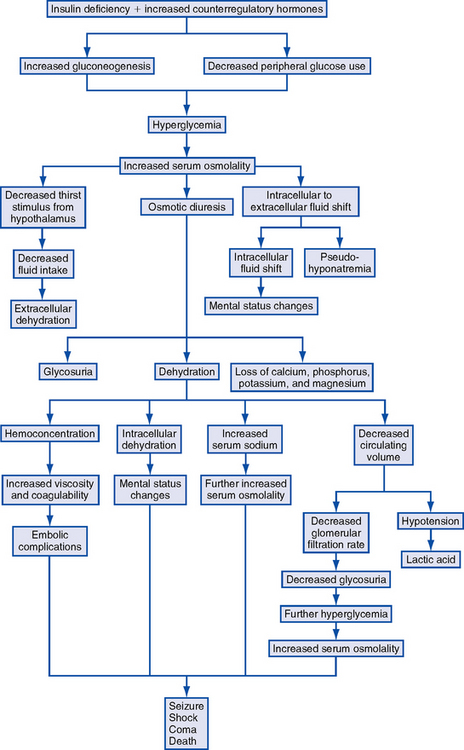
1. Pathophysiology: Life-threatening hyperglycemic emergency accompanied by hyperosmolality, severe dehydration, and alterations in neurologic status without ketosis. Pathophysiologic processes (Figure 6-3) include the following:
a. Relative insulin deficiency that impairs glucose transport across the cell membrane. There may be sufficient insulin present to inhibit lipolysis or ketogenesis in the liver but not enough to control hyperglycemia. Not uncommon for some ketosis to be present, but pH is rarely lower than 7.3.
b. Hyperosmolality resulting from hyperglycemia and hypernatremia may impair insulin secretion, promote insulin resistance, and inhibit free fatty acid release from adipose tissue
c. Fluid shifts from intracellular to extracellular space to offset hyperosmolality
d. Osmotic diuresis caused by hyperglycemia results in extracellular fluid volume depletion; fluid deficits usually are greater than those seen in DKA
e. Severe electrolyte losses (sodium, chloride, phosphate, magnesium, potassium) occur with osmotic diuresis
f. Volume depletion compromises glomerular filtration, diminishing urinary escape of glucose
a. Inadequate insulin secretion and/or action (newly diagnosed type 2, or non–insulin-dependent, diabetes)
b. Advanced age and severe dehydration (majority of patients)
c. Concomitant illness that increases glucose production or contributes to dehydration, including sepsis, pancreatitis, stroke, uremia, burns, myocardial infarction, and gastrointestinal hemorrhage
d. Lack of ready access to fluids or inability to recognize or express the need for fluids
e. Use of insulin or oral hypoglycemic, disruption of an established medication regimen
f. Use of medications known to elevate glucose levels and/or resist insulin action, including corticosteroids, thiazide diuretics, phenytoin, sympathomimetics
g. Preadmission medication regimen that suggests cardiovascular or renal disease; crisis is more common in late-middle-aged patients and in elderly patients with preexisting renal or cardiovascular disease
a. Severely elevated glucose levels (>1000 mg/dl)
c. Sodium and potassium levels vary with the state of hydration; often severely depleted as a result of osmotic diuresis; hypokalemia necessitates potassium replacement
d. Plasma hyperosmolality (>330 mOsm/kg)
e. Acidosis, if present, usually caused by lactic acid or renal dysfunction
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Hyperglycemic, hyperosmolar nonketotic coma (HHNKC) can develop rapidly in an elderly patient with type 2 diabetes who becomes ill and then dehydrated. These patients will require aggressive sick-day management to prevent recurrence. Throughout their course of recovery and discharge, patients with HHNKC may be expected to have needs in the following areas:
(a) Mechanism: Many of these patients are elderly or have preexisting heart disease. Rapid fluid replacement to treat severe fluid deficits may result in heart failure. The patient may require placement of a central venous catheter or pulmonary artery catheter.
(b) Management: Provide inotropic support, administer diuretics, oxygen
(a) Mechanism: As hyperglycemia resolves, the serum glucose level must be closely monitored to avoid hypoglycemia
(b) Management: Stop insulin administration; give IV glucose if the patient is conscious or PO glucose (orange juice) if tolerated
Hypoglycemic Episode
1. Pathophysiology: Decrease in serum glucose level to 50 mg/dl or below. Glucose production (feeding and/or liver gluconeogenesis) lags behind glucose use. May be caused by decreased clearance of insulin or oral hypoglycemia agents or by drug interactions.
i. Insulin dose greater than the body’s current needs
ii. Sudden rotation of injection sites from a hypertrophied area to one with unimpaired absorption
b. Oral hypoglycemic therapy, especially with sulfonylurea agents
c. Insufficient caloric consumption—a meal or snack missed or delayed or intake compromised due to nausea, vomiting, or anorexia
d. Strenuous physical exercise that is not compensated by increased food intake or decreased dose of insulin
e. Potentiation of hypoglycemic medications
i. Renal insufficiency (decreased creatinine clearance)
ii. Use of medications that potentiate the action of the sulfonylureas (phenylbutazone, large doses of salicylates, sulfonamides)
f. Excessive alcohol intake, which inhibits gluconeogenesis
g. Decreased requirements for exogenous insulin resulting from
i. Recovery from physiologic stress, which decreases the levels of insulin-antagonistic hormones and thus decreases the need for insulin
ii. Weight loss, which decreases insulin resistance
iii. Immediate postpartum period: Sudden reduction in antiinsulin effects of placental hormones
h. Use of pentamidine to treat Pneumocystis carinii infection, which is associated with pancreatic islet cell necrosis with resultant acute increase in insulin release
i. Presence of other health problems (e.g., severe liver disease, pancreatic islet cell tumor)
j. Use of regular insulin can be associated with a rapid fall in glucose levels and may prompt more adrenomedullary symptoms. Use of intermediate-acting insulins or continuous insulin infusion devices may result in a more gradual drop in plasma glucose level and thus may produce central nervous system symptoms (neuroglycopenia).
k. Patients taking β-adrenergic blocking agents (e.g., propranolol) may not exhibit adrenomedullary symptoms; the use of β-adrenergic blocking agents can also impair recovery from hypoglycemia by inhibiting glycogenolysis
4. Diagnostic study findings: Serum glucose levels lower than 50 mg/dl
5. Goals of care: Hypoglycemia and its sequelae are corrected
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Hypoglycemia is a potential complication with a high likelihood of recurrence in patients in whom diabetes has been newly diagnosed or in whom an insulin-food-activity balance either has not been achieved or has been disrupted. Throughout their recovery and discharge, patients with hypoglycemia may be expected to have needs in the following areas:
Berne, R, Levy, M, Koeppen, B, Stanton, B. Physiology, ed 5. St Louis: Mosby, 2003. chaps 45–51.
Larsen, P, Kronenberg, H, Melmed, S, Polonsky, K. Williams textbook of endocrinology, ed 10. Philadelphia: Saunders, 2003.
LiVolsi, V, Asa, S. Endocrine pathology. Edinburgh: Churchill Livingstone, 2002.
Dirksen, S, Lewis, S, Collier, I. Clinical companion to medical-surgical nursing, ed 3. St Louis: Mosby, 2004.
Bichet, DG. Nephrogenic diabetes insipidus. Am J Med. 1998;105:431–442.
Diabetes Insipidus Foundation. Welcome to the water world of diabetes insipidus: “a different diabetes!”. retrieved May 17, 2005, from http://www.diabetesinsipidus.org, 2003.
Knoers, N, Monnens, LL. Nephrogenic diabetes insipidus. Semin Nephrol. 1999;19:344–352.
Morello, J, Bichet, DG. Nephrogenic diabetes insipidus. Annu Rev Physiol. 2001;63:607–630.
Syndrome of Inappropriate Antidiuretic Hormone Secretion
Adroque, HJ, Madias, NE. Hyponatremia. N Engl J Med. 2000;342:1581–1589.
Martin, AJ. Hyponatremia. N Engl J Med. 2000;343(12):886.
Terpstra, TL. Syndrome of inappropriate antidiuretic hormone secretion: recognition and management. Medsurg Nurs. 2000;9(2):61–70.
Cooper, DS. Hyperthyroidism. Lancet. 2003;362(9382):459–468.
Diez, JJ. Hyperthyroidism in patients older than 55 years: an analysis of the etiology and management. Gerontology. 2003;49(5):316–323.
Fliers, E, Wiersinga, W. Myxedema coma. Rev Endocr Metab Disord. 2003;4(2):137–141.
Franklyn, J. Thyrotoxicosis. Clin Med. 2003;3(1):11–15.
Holcomb, SS. Thyroid diseases: a primer for the critical care nurse. Dimens Crit Care Nurs. 2002;21(4):127–133.
Roffi, M, Cattaneo, F, Topol, EJ. Thyrotoxicosis and the cardiovascular system: subtle but serious effects. Cleve Clin J Med. 2003;70(1):57–63.
Sarlis, NJ, Gourgiotis, L. Thyroid emergencies. Rev Endocr Metab Disord. 2003;4(2):129–136.
Hypoparathyroidism and Hyperparathyroidism
Levine, MA, Germain-Lee, E, Jan DeBeur, S. Genetic basis for resistance to parathyroid hormone. Horm Res. 2003;60(suppl 3):87–95.
Sosa, JA, Udelsman, R. New directions in the treatment of patients with primary hyperparathyroidism. Curr Probl Surg. 2003;40(12):812–849.
Thakker, RV. Genetic development in hypoparathyroidism. Lancet. 2001;357(9261):974–976.
Arlt, W, Allolio, B. Adrenal insufficiency. Lancet. 2003;361(19372):1881–1893.
Bloomfield, R, MacMillan, M, Noble, DW. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348(21):2157–2159.
Nieman, LK. Dynamic evaluation of adrenal hypofunction. J Endocrinol Invest. 2003;26(7 suppl):74–82.
Marik, PE, Zalooga, GP. Adrenal insufficiency in the critically ill: a new look at an old problem. Chest. 2002;122(5):1784–1796.
Diabetic Ketoacidosis; Hyperglycemic, Hyperosmolar Nonketotic Coma; Hypoglycemia
American Diabetes Association. Position statement: hyperglycemic crisis in patients with diabetes mellitus. Diabetes Care. 2002;25(suppl 1):S100–S108.
Buse, J, Evolution in the American Diabetes Association Standards of Care. Clin Diabetes 2003;(21):24–26.
Herbel, G, Boyle, PJ. Hypoglycemia: pathophysiology and treatment. Endocrinol Metab Clin North Am. 2000;29(4):725–743.
Kaufman, FR. Type I diabetes mellitus. Pediatr Rev. 2003;24(9):291–300.
Neu, A, Willasch, A, Ehehalt, S, et al. Ketoacidosis at onset of type 1 diabetes mellitus in children: frequency and clinical presentation. Pediatr Diabetes. 2003;4(2):77–81.
White, NH. Management of diabetic ketoacidosis. Rev Endocr Metab Disord. 2003;4(4):343–353.