Chapter 65 The effect of polysomnography on pediatric adenotonsillectomy postoperative management
1 INTRODUCTION
Sleep disordered breathing (SDB) often presents in the pediatric population with loud snoring, respiratory pauses and mouth-breathing. For those with enlarged adenoids or tonsils, adenotonsillectomy can be curative. The decision for surgery is based upon the clinical history and physical examination. The polysomnogram is the gold standard for diagnosis of SDB, however, it is costly, time limiting and often not readily available and it is unclear which patient groups require or benefit from its use preoperatively.1,2 Several studies have identified populations at high risk for postoperative complications including children with craniofacial disorders, failure to thrive, neurological impairment, Down syndrome, obstructive sleep apnea and children age 3 or less.3–7 Because of the increased risk, preoperative polysomnography and postoperative overnight observation have been recommended for these patients to differentiate primary snoring from obstructive sleep apnea.8,9 In addition, several authors have concluded that polysomnographic results are predictive of postoperative complications and postoperative course.3–5,7 A recent retrospective study is summarized here which reviewed the postoperative course of children 3 years of age and younger and correlated these findings with the severity of obstruction as measured by preoperative polysomnogram.
4 RESULTS
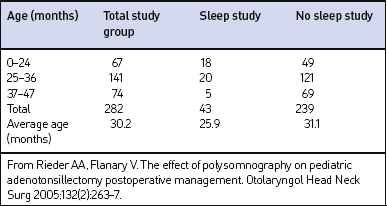
The charts for 305 children age 3 years of age or younger were reviewed. Twenty-three children were excluded from the study for the following: incomplete chart (n=8), procedure miscoded (n=2), additional procedures performed (n=5), or procedure performed during period of acute illness (n=8). The remaining 282 sets of patient data were included in the statistical analysis. The average age was 30 months. Table 65.1 summarizes the age distribution. Upper airway obstruction was identified as the primary indication for surgery in 271 of the patients.
Sleep studies, including complete 16-channel (n=38), 4-channel (n=3), or hardcopy pulse oximetry (n=2), were performed preoperatively in 43 patients. The average age of patients that had a preoperative PSG was significantly younger at 25.9 months, compared to 31.1 months in the non-PSG group (P<0.0001). Twenty-seven patients were classified to have obstructive sleep apnea (OSA) (Res-piratory Disturbance Index [RDI] ranged from 1 to 52) and 11 were classified to have upper airway resistance syndrome (UARS). UARS was defined as abnormally increased upper airway resistance during sleep that led to increased respiratory effort and sleep fragmentation without defined apneic or hypopneic episodes or notable oxygen desaturations.10 Sleep studies without a calculated RDI or UARS were classified as normal, mildly abnormal, or significantly abnormal by the pulmonologist reading the study.
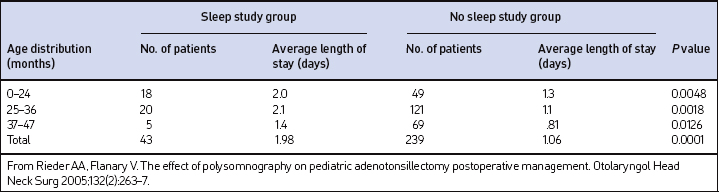
When the subjects were grouped using their RDI as a measure of OSA severity, there was no statistical difference between the average length of stay (LOS) for OSA patients. The average LOS for patients with UARS (2.2 days) was significantly longer when compared to the average LOS stay (1.57 days) for patients with OSA (P=0.0448). The average LOS, when calculated by age group in the non-PSG and PSG groups, was less in the non-PSG group at 1.06 days compared to 1.98 days in the PSG group (P<0.0001). The shortest average LOS occurred in the 37–47 month age distribution in both groups. The average LOS for both groups is listed in Table 65.2. The discharge criteria for patients typically required that the patients not require any supplemental oxygen and were able to maintain oxygen saturations above 90% without periods of sustained desaturation attributed to upper airway obstruction.
Complications were identified in 62 patients (21.9%). For this study, a complication was defined as any documented desaturation event (regardless of SaO2 nadir) or any other event/complication that required medical intervention or readmission, or prolonged the patient’s LOS. Complications occurred in 43 of 239 patients (17.9%) in the non-PSG group, and in 19 patients of 43 (44.2%) in the PSG group. Of those 62 patients, the most common complication identified was oxygen desaturation representing 65.1% of the total complications in the non-PSG group and 57.9% in the PSG group. Overall, desaturation events occurred in 13.8% (n=39) of the entire study population. Complications for each group are listed in Table 65.3.
| Group with sleep study (43) | Group without sleep study (239) | |
|---|---|---|
| Complication | No. of patients | No. of patients |
| Desaturation event | 11 (26%) | 28 (12%) |
| Poor oral intake | 6 (14%) | 10 (4%) |
| Pneumonia/atelectasis | 0 | 3 (1%) |
| Seizure | 0 | 1 (0.4%) |
| Bleeding | 1 (2%) | 1 (0.4%) |
| Death | 1 (2%) | 0 |
| Total no. of patientswith complications | 19 (44%) | 43 (18%) |
From Rieder AA, Flanary V. The effect of polysomnography on pediatric adenotonsillectomy postoperative management. Otolaryngol Head Neck Surg 2005;132(2):263–7.
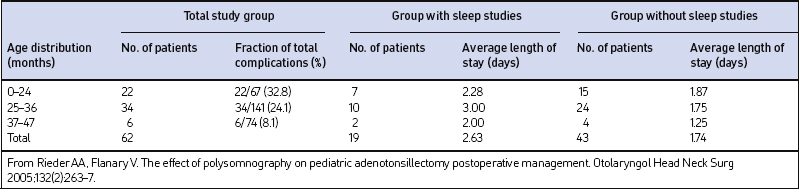
Table 65.4 lists the total complications and LOS by age group for the 62 patients with complications. The highest complication rate (32.8%) was found in children of 24 months and younger, while the lowest rate (8.1%) occurred in the group aged 37–47 months. There was no statistical difference in the mean age of the patients who had complications in the PSG group (26.5 months) compared to the non-PSG group (27.2 months) (P=0.7279). The average LOS for patients with complications in the non-PSG group was 1.74 days compared to 2.63 days in the PSG group. A trend toward a longer average LOS was identified in the PSG group but the difference did not reach statistical significance (P=0.0997).
Co-morbidities and associated medical conditions were identified in 88 patients. The most common conditions are listed in Table 65.5. Fifty-seven (23.8%) of the non-PSG patients were found to have co-morbidities, compared to 31 (72%) patients in the PSG group. Notably, 11 of 43 patients (25.5%) with complications in the non-PSG had co-morbidities compared to 14 of 19 patients (73.6%) with complications in the PSG group.
| Co-morbidity | No. of patients |
|---|---|
| Asthma/reactive airway disease | 25 |
| Neurologic impairment (Down syndrome, developmental delay, cerebral palsy) | 18 |
| Prematurity | 10 |
| Craniofacial anomaly or syndrome | 9 |
| Neurologic impaired swallow | 8 |
| Laryngomalacia | 6 |
| Platelet dysfunction | 6 |
| Seizure disorder | 4 |
| Other (recurrent croup, cardiac anomalies, recurrent pneumonia, reflux) | 10 |
From Rieder AA, Flanary V. The effect of polysomnography on pediatric adenotonsillectomy postoperative management. Otolaryngol Head Neck Surg 2005;132(2):263–7.
5 DISCUSSION
Sleep disordered breathing and habitual snoring are common in the pediatric population with a reported prevalence between 4–11% and 10–15% respectively.11,12 Upper airway obstruction (UAO) secondary to adenotonsillar hypertrophy is noted to be the primary cause of sleep disordered breathing in children, and has replaced recurrent tonsillitis as the most common indication for pediatric adenotonsillectomy.13 Adenotonsillectomy is considered the primary surgical treatment option for children with obstructive sleep disorders, having been shown to improve sleep, breathing, PSG results, enuresis and patient quality of life.14,15
Several studies have compared formal PSG to the clinical diagnosis of OSA.16,17 These studies showed that the two correlate poorly; however, they were completed before there was a clear consensus on the definition of pediatric OSA and prior to recognition of UARS, making it hard to draw any conclusions. In actual practice, the decision to perform an adenotonsillectomy is often made based upon clinical history and physical exam without the benefit of a diagnostic sleep study. This is consistent with the findings of our study in which only 15% of the patients (n=43) had a formal preoperative sleep study of any type. In children suspected of having OSA, these sleep studies have been shown to have a high predictive value.22 Sleep studies are typically ordered for patients with co-morbidities, for patients whose clinical diagnosis is unclear or are considered to be at increased surgical risk. This trend is reflected in the data from this study in which 72% of the patients with sleep studies had other co-morbidities compared to 24% of the patients without sleep studies.
PSG has also been advocated to assist with postoperative planning and monitoring and as a method to predict the likelihood of postoperative respiratory complications after adenotonsillectomy. Several studies have looked at the risk factors associated with postoperative complications.3–5,7 All of these studies identify age younger than2 or 3 years of age as a significant risk factor for postoperative respiratory compromise. Other risk factors identified include craniofacial anomalies, failure to thrive, hypotonia, morbid obesity, Apnea/Hypopnea Index greater than 5 (or 10) and low preoperative oxygen saturation nadir. These are all retrospective studies which used a number of different inclusion/exclusion criteria for patients along with a variety of definitions and complication classifications.
Pediatric post-adenotonsillectomy complication rates have ranged from 0% to 32% in published series with airway issues or compromise representing 0–16% of the overall complications.6,18–20 Those studies that were limited to younger children (3 years of age and younger) demonstrated a rate of airway complications ranging from 0% to 5%.6,18,19 Our study compares similarly with an overall complication rate of 22%, but our rate of airway complications was higher with a rate of 14% (n=39). However, it is misleading to directly compare complication rates between these studies as different airway complication definitions were used. For this study, a complication was defined as any documented desaturation event (regardless of SaO2 nadir) or any other event or complication that required medical intervention or readmission, or prolonged the patient’s length of hospital stay. Some studies have identified minor and major complications depending upon the intervention required7 while other studies have attempted to identify complications based on the degree of airway obstruction or oxygen saturation nadir.4,5 Furthermore, the significance of postoperative desaturations is controversial. Theoretically, a decrease in oxygen saturation may increase the postoperative patient risk; however, no stratification of desaturation events was used and no formal studies currently support this conclusion.
6 CONCLUSION
This study reinforces previous data suggesting that the complication rate in children 3 years of age and younger may be higher than that in older children. However, the nature of the complications does not appear to have a large impact on the LOS of these children. This study also suggests that the 3 year old and younger group, in the absence of other co-morbidities, can safely undergo adenotonsillectomy without undergoing preoperative PSG provided these patients have close postoperative monitoring. Furthermore, the relative utility of PSG in the pediatric population is closely related to issues of cost, timing, and interpretation expertise. The average cost of overnight PSG at a qualified institution is $600–2800.21 In addition, while home studies are valuable in the evaluation of adults, these studies have not been validated in young children and thus they continue to require overnight stays for PSG. Given this information, it may be impractical to consider performing PSG on all pediatric patients prior to adenotonsillectomy. Higher risk, as indicated by the increased LOS, for patients with co-morbidities does appear to exist and we would reserve the use of preoperative PSG for these patients and for patients with ambiguous clinical history.
1. Leach J, Olson J, Hermann J, Manning S. Polysomnographic and clinical findings in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992;118:741-744.
2. Messner AH. Evaluation of obstructive sleep apnea by polysomnography prior to pediatric adenotonsillectomy. Arch Otolaryngol Head Neck Surg. 1999;125:353-356.
3. Biavati MJ, Manning SC, Phillips DL. Predictive factors for respiratory complications after tonsillectomy and adenoidectomy in children. Arch Otolaryngol Head Neck Surg. 1997;123:517-521.
4. McColley SA, April MM, Carroll JL, et al. Respiratory compromise after adenotonsillectomy in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992;118:940-943.
5. Rosen GM, Muckle RP, Mahowald MW, et al. Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics. 1994;93(5):784-788.
6. Slovik Y, Tal A, Tarasiuk A, et al. Complications of adenotonsillectomy in children with OSAS younger than 2 years of age. Int J Pediatr Otorhinolaryngol. 2003;67:847-851.
7. Wilson K, Lakheeram I, Morielli A, et al. Can assessment for obstructive sleep apnea predict post adenotonsillectomy respiratory complications. Anesthesiology. 2002;96:313-322.
8. Section on Pediatric Pulmonology/Subcommittee on obstructive sleep apnea syndrome. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea. Pediatrics. 2002;109:704-712.
9. American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866-878.
10. Guilleminault C, Chowdhuri S. Upper airway resistance syndrome is a distinct syndrome. Am J Respir Crit Care Med. 2000;161:1412-1416.
11. Messner A, Pelayo R. Pediatric sleep-related breathing disorders. Am J Otolaryngol. 2000;21:98-107.
12. Guilleminault C, Pelayo R. Sleep-disordered breathing in children. Ann Med. 1998;30:350-356.
13. Ross AT, Kazahaya K, Lawrence WC. Revisiting outpatient tonsillectomy in young children. Otolaryngol Head Neck Surg. 2003;128(3):326-331.
14. Goldstein NA, Fatima M, Campbell TF, et al. Child behavior and quality of life before and after tonsillectomy and adenoidectomy. Arch Otolaryngol Head Neck Surg. 2002;128:770-775.
15. Serres LM, Derkay C, Sie K, et al. Impact of adenotonsillectomy on quality of life in children with obstructive sleep disorders. Arch Otolaryngol Head Neck Surg. 2002;128:489-496.
16. Goldstein NA, Sculerati N, Walsleben JA, et al. Clinical diagnosis of pediatric obstructive sleep apnea validated by polysomnography. Otolaryngol Head Neck Surg. 1994;111(5):611-617.
17. Wang RC, Elkins TP, Keech D, Wauquier A, Hubbard D. Accuracy of clinical evaluation in pediatric obstructive sleep apnea. Otolaryngol Head Neck Surg. 1998;118:69-73.
18. Berkowitz RG, Zalzal GH. Tonsillectomy in children under 3 years of age. Arch Otolaryngol Head Neck Surg. 1990;116:685-686.
19. Helmus C. Tonsillectomy and adenoidectomy in the one and two-year-old child. Laryngoscope. 1979;89:1764-1771.
20. Postma DS, Folsom F. The case for an outpatient ‘approach’ for all pediatric tonsillectomies and/or adenoidectomies: a 4-year review of 1419 cases at a community hospital. Otolaryngol Head Neck Surg. 2002;127:101-108.
21. Leach J, Olson J, Hermann J, Manning S. Polysomnographic and clinical findings in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992;118:741-744.
22. Brouillette RT, Morielli A, Leimanis A, et al. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics. 2000;105:405-412.