Reference
Molecule
Number of patients
Polymorphism
Vs. normal control
F/U duration (months)
Renal progression
Biology
Lim et al. [22]
Clara cell secretory protein
N = 267
G38A
No difference
103.8 ± 52.6
AA genotype
Anti-inflammatory
Multicenter
HR 2.34, 95 % CI 1.19–4.64
Immunomodulatory
P = 0.014 (MV)
Jung et al. [20]
Chymase
N = 261
CMA rs1800875
No difference
103.2 ± 52.6
AA/AG genotype
Alternative enzyme for synthesis of angiotensin II
Single center
HR 2.351
95% CI 1.414–3.908
P = 0.001 (MV)
Jung et al. [20]
Chymase
N = 261
CMA rs1800876
No difference
103.2 ± 52.6
CC/CT genotype
Alternative enzyme for synthesis of angiotensin II
Single center
χ 2 = 4.45
P = 0.035 (UV)
Yoon et al. [23]
CD14
N = 216
159C/T
No difference
86.0 ± 51.1
CC genotype
Inflammatory responses to microorganism
Single center
HR 3.2
95 % CI 1.2–8.8
P = 0.025 (MV)
Kim et al. [18]
Angiotensinogen
N = 238
AGT M235T
No difference
102.4 ± 47.0
TT genotype
Component of renin-angiotensin system
Multicenter
HR 5.704
95% CI 1.578–20.618
P = 0.008
Lim et al. [25]
Transforming growth factor (TGF)-β1
N = 108, single center
C509T
Difference
At least 36
TT genotype
Anti-inflammatory
Poor
Profibrotic activity
Renal outcome
P = 0.042 (UV)
HR 2.202
P = 0.138 (MV)
Yoon et al. [21]
Angiotensin II type 2 receptor
N = 480
A1818T
No difference
30.3 ± 3.3
TT/AT genotype
Counter-regulatory to the vasoconstrictor action of angiotensin II type 1 receptor
Multicenter
HR 0.221
95% CI
0.052–0.940
P = 0.041
Lim et al. [24]
Megsin
N = 260
C2093T
No difference
103.0 ± 52.4
TT genotype
Serpin
HR 3.52
Superfamily
95 % CI 1.69–7.34
Progressive mesangial matrix expansion
P = 0.001 (MV)
CT genotype
HR 2.15
95 % CI 1.30–3.57
P = 0.003 (MV)
Lim et al. [24]
Megsin
N = 260
C2180T
No difference
103.0 ± 52.4
CC genotype
Serpin superfamily
HR 4.05
95 % CI 1.93–8.51
Progressive mesangial matrix expansion
P < 0.001 (MV)
CT genotype
HR 2.35
95 % CI 1.40–3.94
P = 0.001 (MV)
Lim et al. [24]
Megsin
N = 260
2093T-2180C
No difference
103.0 ± 52.4
2093 T-2180C haplotype
Serpin superfamily
Multicenter
Haplotype
HR 2.01
95 % CI 1.44–2.81
Progressive mesangial matrix expansion
P < 0.001 (MV)
GO et al. [26]
Klotho
N = 973
G395A
N/A
50.0 ± 27.8
GA + AA genotype
Related to aging
Multicenter
ESRD
Atherosclerosis
P = 0.04 (UV)
Endothelial dysfunction
Yoon et al. [19]
ACE + PAF-AH
N = 191
ACE I/D
No difference
87.3 ± 50.0
DD + DI&CT + TT
Angiotensin II-forming enzyme
Single center
C1136T
HR 4.5
Enzyme degrading PAF
95 % CI 1.6–12.7
P = 0.0039 MV)
Lee et al. [27]
EPHX2
N = 401
R287Q
No difference
74.4
GG genotype
Determines epoxyeicosatrienoic acid (EET) concentration
Single center
ESRD
HR 1.83
95 % CI 1.13–2.96
P = 0.014 (MV)
With regard to gene polymorphism of renin-angiotensin-aldosterone system, specific single nucleotide polymorphism (SNP) in angiotensin II type 1 receptor [18], aldosterone synthase, [18] and insertion/deletion polymorphism of angiotensin-converting enzyme [19] were not related to the progression of IgAN. While M235T SNP of angiotensinogen was related to renal progression only in male patients [18], specific SNP of chymase [20] and angiotensin II type 2 receptor [21] were related to renal progression in both sexes.
Gene polymorphism of molecules related to inflammation such as Clara cell secretory protein [22], CD 14 [23], and platelet-activating factor acetylhydrolase [19] and molecules related to extracellular matrix deposition such as megsin [24] and TGF-β [25] were associated with renal progression. Gene polymorphism affecting the activity of epoxide hydrolase which hydrolyze epoxyeicosatrienoic acid was associated with renal survival [26]. Interestingly, specific SNPs of klotho gene – an antiaging gene – were related to patients’ survival as well as renal survival [27].
10.1.6 Role of Complements
The activations of alternative and lectin pathway of complements by nephritogenic polymeric IgA1 molecules are known to play an important role in the pathogenesis of IgAN.
10.1.6.1 Alternative Pathway
Mesangial deposition of C3, one of the hallmarks of the activation of alternative pathway, was observed in 73.9 % of 142 Korean patients with IgAN. While C3 deposition was observed in only 53 % of patients with histologically early stage of IgAN, it was observed in 100 % of patients with advanced stage [28].
Decreased level of serum C3 lower than normal and strong mesangial C3 deposition ≥2+ degree were observed in 19.2 % and 18.8 % of 343 patients, respectively. This strong C3 mesangial deposition was associated with low serum C3 level, mesangial hypercellularity, and advanced tubulointerstitial lesions. Importantly both C3 hypocomplementemia and strong mesangial C3 deposition were independent risk factor for the doubling of serum creatinine independent of basal eGFR, proteinuria, and tubulointerstitial lesions [29].
These studies suggest that the activation of alternative pathway of complements occurs in systemic circulation as well as local mesangial area and contributes significantly to the progression of IgAN.
10.1.6.2 Other Pathways
In 23 IgAN patients, glomerular C4d staining and tubular C4d staining were observed in 56.5 % and 47.8 % of patients, respectively, whereas no C4d staining was observed in tubular basement membrane, peritubular capillary, and vascular structure. The glomerular C4d staining was related to albuminuria and tubular C4d staining to higher grade of WHO classification. Tubular deposition of C4d without any co-deposition of immunoglobulin suggested the activation of lectin pathway although clear proofs were lacking [30].
Out of 221 IgAN, 8.1 % of patients had mesangial C1q staining which is a marker of activation of classic complement pathway. The co-deposition of IgG was more frequently observed in C1q(+) patients than propensity score matched C1q(−) patients (38.9 % vs. 8.3 %). C1q(+) was an independent determinant of rate of GFR loss between C1q(+) and matched C1q(−) patients [31].
These studies suggest that the activation of lectin and classic pathway of complement contribute to renal damage in some subset of patients with IgAN.
10.2 Treatment and Prognosis
10.2.1 Role of Renal Biopsy Findings in Predicting Renal Outcome
IgAN is characterized by highly variable clinical courses and the differences in response to a specific therapy resulting in highly variable renal outcome within individual patients. Even though proteinuria is generally regarded as the best predictor of renal outcome, there are substantial numbers of the patients with no proteinuria at presentation who ultimately show renal progression. Hence, many studies about the correlation between renal biopsy findings and renal progression have been performed to define a role of renal pathology for prediction of renal outcomes beyond clinical parameters.
10.2.1.1 Pathologic Grading Systems Before the Introduction of Oxford Classification
H.S. Lee’s grading system developed in 1987 by incorporating mesangial proliferation, segmental lesion, crescent, and interstitial fibrosis showed good correlation with proteinuria, hypertension, and impaired renal function at the time of biopsy. Interestingly, episodes of macroscopic hematuria which is a favorable factor for renal progression in Korea were less frequent in patients with higher grade of this system [28].
Some modification of H.S. Lee’s grading system with particular emphasis on crescent, segmental sclerosis, and global sclerosis which implicate, respectively, active necrotizing glomerular inflammation, podocyte depletion, and resultant irreversible glomerular damage correlated well with patients’ age, CCr, 24-h urinary protein, and the prevalence of hypertension in dose-dependent manner. Moreover, this grading system predicted renal progression independent of clinical risk findings such as initial renal function and proteinuria, whereas the Hass classification did not [32].
Class IV/V lesions of WHO classification were a prognostic factor of renal progression independent of renal insufficiency (serum creatinine ≥1.4 mg/dL) and heavy proteinuria [33].
These studies suggest that specific histological features such as mesangial proliferation, crescent, segmental sclerosis, and advanced tubulointerstitial lesions rather than the whole system of histological classification were suggested to be significant prognostic factors independent of clinical features even before the introduction of Oxford classification.
10.2.1.2 Validation Studies of Oxford Classification
After the Oxford classification was introduced as a new histological classification system for IgAN in 2009, several studies were performed to validate the usefulness of this classification in Korean patients.
All studies identified T1 and T2 lesions, namely, advanced tubulointerstitial lesions, as a predictor of renal progression independent of clinical findings in multivariable analysis [34–36]. T1 and T2 were also predictors of renal progression in posttransplant IgAN [37]. These findings confirmed the well-established prognostic importance of tubulointerstitial lesion in essentially all glomerular diseases including IgAN.
E1 was associated with more frequent prescription of steroid in studies where steroid was infrequently used, i.e., 18 % and 11 % of subjects of study, respectively [35, 36]. But in one study in which 38 % of patients had received steroid, S1 but not E1 was associated with the more frequent use of steroid [34]. RAS blockade treatment was more frequent in patients with M1 [34, 36]. Thus, at least in current situation, where the prescription of immunosuppressive drugs is largely dependent on the decision of individual physician without agreed guideline for immunosuppressive treatment, the reported relationship of specific Oxford lesions with steroid treatment be viewed with the consideration for criteria by which steroid was used in a specific study.
The frequency of M1, S1, E1, T1, and T2 increased along with the increasing grade of WHO classification [34] and also showed correlation with Hass classification in patients with posttransplant IgAN [37]. All MEST variables, especially S and E, correlated with activity index in semiquantitative classification, but only S and T showed relationship with chronic index [34]. Hass and Oxford classifications were comparable in providing additive predictive value for renal progression to known clinical parameters such as proteinuria and eGFR [36], but Oxford classification was superior to Haas classification in posttransplant IgAN [37]. Thus, pre-existing pathological grading systems of IgAN such as WHO and Hass classification could provide useful prognostic information as much as Oxford classification, but Oxford classification is superior to old systems in the convenience in clinical application and consistency in interpretation of pathologic findings by different pathologists.
Similar to conclusion from Oxford group, a retrospective analysis of 430 patients in which 18.8 % of patients had crescent showed that crescent was not a prognostic factor for renal progression independent of clinical findings and T lesion although patients with crescent had higher UPCR, lower eGFR, lower serum albumin level, and more prescription of RAS blockade and glucocorticoid treatment during follow-up. Because the patients in this study had a relatively good basal eGFR of 80.5 ± 24.1 ml/min/1.73 m2, this study could not give the answer about the prognostic significance of crescent in patients having worse eGFR <30 ml/min/1.73 m2 as Oxford classification could not [38]. But in posttransplant IgAN, 4-year graft survival after biopsy was 30.0 % in patients having crescents compared with 70.8 % patients without crescents despite the enhanced immunosuppression in the former group. Moreover, IgAN was the cause of graft failure in 66.7 % of patients with crescents, whereas IgAN was the cause of graft failure in only 13.6 % of patients without crescents [39]. Thus, the prognostic significance of crescent in IgAN remained to be defined especially in patients with low basal eGFR and/or patients on the immunosuppressive therapy at the time of renal biopsy.
10.2.2 Significance of Proteinuria in Treatment and Prognosis
10.2.2.1 Normal or Low-Grade Proteinuria at Presentation
Although the excellent prognosis is taken for granted for patients presenting with normotension, normal renal function, and normal or low-grade proteinuria, this does not apply to all such patients.
IgAN constituted 33.3 % (n = 52) of 156 patients who underwent renal biopsy due to isolated hematuria without proteinuria, hypertension, and azotemia. During a follow-up of mean duration of 31.6 ± 14.1 months, five patients with IgAN developed proteinuria and/or hypertension and/or eGFR <60 ml/min/1.73 m2 [40].
In a retrospective analysis of 153 patients with IgAN presenting with benign clinical manifestation, i.e., proteinuria <0.5 g/day, normotension, and eGFR >60 ml/min at the time of renal biopsy, 31 % (n = 36) of patients achieved clinical remission defined as disappearance of microscopic hematuria, proteinuria <0/2 g/day, and normal renal function. But 11 patients developed proteinuria >1 g/day, three patients showed greater than 50 % increase in serum creatinine, and six patients developed ESRD. Interstitial fibrosis at renal biopsy and hypoalbuminemia were independent risk factors for the development significant proteinuria and renal progression [41].
10.2.2.2 Nephrotic Syndrome
Out of 1,076 patients, typical nephrotic syndrome was observed in 100 (10.2 %) patients, 93 of whom presented with generalized edema at initial presentation. Histologically only four patients showed typical minimal change disease-like features, and C3 deposition was observed in 91 % of patients. A total of 48 patients achieved complete remission, spontaneously in 24 patients and by immunosuppressive treatment in another 24 patients. Partial response and no response occurred in 32.0 % and 20.0 % of patients, respectively. During the median follow-up of 45.2 months, the doubling of serum creatinine occurred in 24 % of nephrotic patients compared with 7.1 % in non-nephrotic patients. In patients with nephrotic syndrome, the risk for reaching the doubling of serum creatinine was highest for patients with no response followed by partial response (HR 14.49, 95 % CI 1.14–183.76, p = 0.04 and HR 215, 95 % CI 15–2983, p < 0.001 respective for partial response and nonresponse compared with complete response). The prognosis of complete responder was excellent with only two patients reaching the doubling of serum creatinine during follow-up. In 24 patients having spontaneous complete remission, Haas IV or V classifications were observed in 11 patients. Female sex, initial serum creatinine <1.2 mg/dL, and more than 50 % reduction of proteinuria within 3 months after the onset of nephrotic syndrome were associated with likelihood of attaining spontaneous remission [4].
Among 581 patients with IgAN, 48 patients (8.3 %) presented with nephrotic syndrome. Of 25 patients who received high-dose corticosteroid, 12 patients achieved complete remission of nephrotic syndrome. Seven episodes of relapse in five patients occurred during the tapering of corticosteroids, six of which respond well to reintroduction of high-dose steroid with complete remission. Compared with nonresponders, responders were characterized by clinical features similar to minimal change disease, i.e., rapid onset of edema, higher weight gain, higher proteinuria, lower serum albumin, and lower serum creatinine, and pathological features of lower histological grade and lower mesangial IgA deposition. While none of 12 responders did not progress to ESRD, 38 % of nonresponders to steroid and 43 % of patients who did not receive steroid treatment reached ESRD [42].
Collectively, these studies suggest that about 10 % of IgAN patients present with nephrotic syndrome in which there are considerable heterogeneity in histopathology, clinical course, response to treatment, and prognosis. Prognosis of nephrotic patients was largely determined by the reduction of proteinuria spontaneously or by steroid treatment.
10.2.2.3 Optimal Proteinuria Target for Renal Protection
Because the level of residual proteinuria attained spontaneously or by treatment was a major determinant of renal progression irrespective of risk factors at initial presentation such as eGFR, blood pressure, proteinuria, and histological findings, the optimal target for the reduction of proteinuria is always an important topic in IgAN.
In a retrospective analysis of 500 patients, the risk for developing 50 % decline in eGFR increased markedly in patients with 1.0 g/g ≤ TAP (time-averaged proteinuria) ≥2.99 g/g (HR 25.0, 95 % CI 3.17–197.0, p = 0.002) and TAP ≥3.0 (HR 244.07, 95 % CI 27.09–2198.98, p < 0.001) compared with patients with TAP <0.3 g/g. Although there was no difference in the development of 50 % decline in eGFR or ESRD between the patients with time-averaged proteinuria during clinical course (TAP) <0.3 g/g and patients with 0.3 g/g ≤ TAP ≥ 0.3–0.99 g/g, eGFR slope was lower in patients with TAP <0.3 g/g than patients with 0.3≤ TAP ≥0.99 (−0.41 ± 1.68 vs. −0.73 ± 2.82 ml/min/1.73 m2/year, p = 0.03) [43].
In another retrospective study of 125 patients, the risk for reaching to eGFR <15 ml/min/1.73 m2 increased by tenfolds in patients with residual proteinuria after treatment >1.0 g/day compared with the patients with residual proteinuria <0.3 g/day. Although statistically insignificant probably due to relatively small numbers of patients, eGFR slope in patients with residual proteinuria of 0.3–0.99 was larger than patients with residual proteinuria <0.3 g/day (−1.24 ± 1.25 vs. −1.06 ± 1.69 ml/min/1.73 m2/year, p = 0.580). The relapse of proteinuria more than 1 g/day, which occurred 5.2 ± 3.2 years after the initiation of treatment, was more frequent in patients with residual proteinuria of 0.3–0.99 g/day than patients with proteinuria <0.3/day (41.5 % vs. 26.3 %, p = 0.025) [44].
These studies suggest that the maintenance of proteinuria <1 g/day as recommended by KDIGO was a reasonable therapeutic target in renal protection, but the attainment of proteinuria <0.3 g/day might provide additional renal protection especially in young and otherwise healthy patients in whom a long life span is expected.
10.2.3 Drug Therapy
10.2.3.1 Renin-Angiotensin System Blockade (RASB)
Angiotensin-converting enzyme inhibitor (ACEI) decreased proteinuria from 3.85 ± 2.54 g/day to 1.68 ± 1.91 g/day in 24 patients in which five of whom proteinuria decreased below 0.5 g/day. ACEI also decreased the proteinuria of ten patients whose baseline proteinuria was greater than 3 g/day from 4.89 ± 1.43 g/day to 1.38 ± 1.21 g/day [45]. Insertion/deletion polymorphism of ACE gene did not influence the anti-proteinuric effect of ACEI [46].
In a trial of 4 weeks of washout period followed by 12 weeks of active treatment in which patients were randomized to once-daily losartan 50 mg/day or amlodipine 5 mg/day without any dietary restriction, all but one patient treated by losartan showed reduction in proteinuria, whereas proteinuria slightly increased in patients treated by amlodipine without no difference in blood pressure attained during the trial. Proteinuria decreased by 42.7 % from baseline after 4 weeks treatment of losartan and further reduced by 54.4 % after 12 weeks of treatment (2.3 ± 1.5 g/day, 1.4 ± 1.4 g/day, and 1.1 ± 1.2 g/day, respectively, at baseline, 4 weeks, and 12 weeks after treatment). No treatment-induced change in CCr was noted in both groups. Whereas there were no difference in serum level of TGF-ß between normal volunteer and patients with IgAN, urinary TGF-ß and reflecting renal production of TGF-ß were 20-folds higher in patients with IgAN than in normal control. Losartan but not amlodipine decreased urinary TGF-ß, but there was neither relationship between urinary TGF-ß level and proteinuria nor between decrement of urinary TGF-ß and reduction of proteinuria by losartan [47].
A crossover trial of once-daily candesartan 4 mg or placebo over 12 weeks on 19 patients who had been stably maintained on 5–7,5 mg of ramipril for at least 6 months showed the reduction of proteinuria from 4.1 ± 0.3 g/day at placebo period to 3.1 ± 0.3 g/day at combination period without change in blood pressure, serum potassium level, and renal function [48]. The addition of candesartan reduces urinary TGF-β level by 28.9 ± 6.0 %, but the reduction in proteinuria did not correlate with the reduction in urinary TGF-β level [49]. Although the combination of low dose of ACEI and angiotensin receptor blocker (ARB) reduced the proteinuria further in IgA patients having high basal proteinuria >3.0 g/day, the reduction rate in proteinuria by addition of ARB was only 12.3 ± 4.5 %, and residual proteinuria remained high in this study.
In posttransplant IgAN, more than 50 % reduction of proteinuria by enalapril was observed in 71.4 % of patients without segmental sclerosis but only 28.6 % of patients having segmental sclerosis suggestive of the inhibitory effect of segmental sclerosis on the anti-proteinuric effect of ACEI in these patients [50].
A retrospective analysis of 167 patients with IgAN biopsied in author’s hospital revealed that the presence of crescents in glomeruli reduces the anti-proteinuric effects of RAS blockade administered for 1 year in the multivariable analysis adjusted for multiple factors including basal proteinuria, eGFR, and other histological features. In this study, most of reduction in proteinuria occurred within 3 months after RAS blockade, but further reduction of proteinuria was observed until 1 year after RAS blockade (Fig. 10.1) (unpublished results).
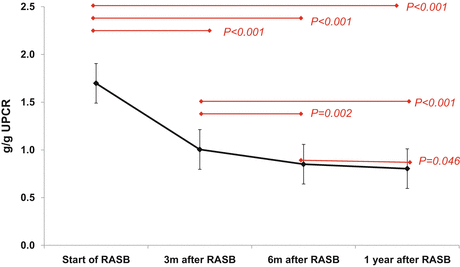

Fig. 10.1
Serial changes in UPCR after RAS blockades in patients with IgAN
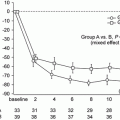
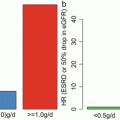
In the comparison between 914 IgAN patients without RAS blockade and 831 patients with RAS blockade, the rate of doubling of creatinine, ESRD, and death was higher in patients without RAS blockade (Fig. 10.2) despite the older age, lower basal eGFR, and higher prevalence of hypertension in the patients treated with RAS blockade. Interestingly, the protective effect of RAS blockade against death was significant in patients having DD genotype of insertion/deletion polymorphism of ACE (unpublished results).
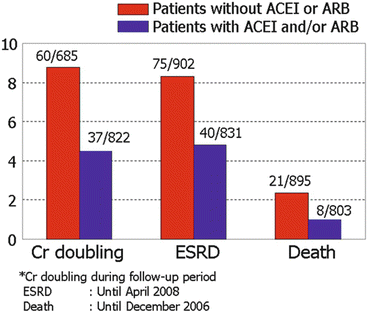

Fig. 10.2
The differences in doubling of serum creatinine, ESRD, and death according to the treatment by RAS blockades in patients with IgAN
Collectively, ACEI or ARB significantly reduced proteinuria in patients with IgAN with favorable long-term effects on the survival of patient as well as the kidney. The combination of ACEI and ARB further reduced the proteinuria with the further reduction of urinary TGF-β secretion. The most degree of anti-proteinuric effect of RASB was attained within 3 months after the administration of drug, but further lowering of proteinuria was observed until 1 year after treatment. Higher basal proteinuria before RASB especially more than 3 g/day and the presence of crescent or segmental sclerosis in renal biopsy decreased the proteinuria-lowering effect of RASB. Although DD genotype of ACE gene did not influence the anti-proteinuric effect of RASB, the patient’s survival benefit was prominent in patients with DD genotype.
10.2.3.2 Corticosteroid
In an open label trial conducted on 50 adult patients, daily administration of 1 mg/kg of oral prednisolone for 2 months followed by tapering over 4 months on the top of ARB treatment improved mean eGFR significantly from 80.2 ± 22.7 ml/min/1.73 m2 at baseline to 93.0 ± 41.2 ml/min/1.73 m2 and decreased UPCR 2.21 ± 2.0 g/g at baseline to 0.7 ± 0.7 g/g at 6 months after treatment. In addition the corticosteroid therapy also decreased the extent of hematuria including 16 patients in whom hematuria completely disappeared. The serum IgA level also decreased after treatment. Seven patients (14 %) who developed more than 20 % decrease in eGFR despite the corticosteroid therapy were characterized by lower eGFR, higher systolic blood pressure, and more frequent presence of crescent at baseline. Especially, the basal proteinuria of these patients was higher than respondent patients (UPCR 3.92 ± 2.00 g/g vs. 1.93 ± 1.88 g/g) in whom residual proteinuria after treatment was also higher (UPCR 1.63 ± 1.00 g/g vs. 0.61 ± 0.59 g/g) [51].
Similar regimen of oral prednisolone or deflazacort on the top of RAS blockade with good blood control also increased CCr by 11.5 ± 16.4 ml/min and decreased 24-h urine proteinuria by 4.4 ± 5.5 g/day and increased serum albumin level by 1.1 ± 2.3 g/dL in a retrospective analysis of 136 patients despite the relatively high basal proteinuria of 5.8 ± 5.6 g/day. Because the follow-up duration was short and too widely distributed and mean residual proteinuria after treatment was more than 1 g/day (1.4 ± 2.3 g/day), actual benefit of this treatment need more long-term follow-up to be confirmed [52].
The effect of intravenous pulse administration of 500 mg of methylprednisolone every 2 weeks for 6 months without accompanying oral corticosteroid administration improved the rate of eGFR decline in the 22 patients who had already progressed more than CKD 3 stage(−0.93 ± 0.87 vs. 0.14 ± 0.99 ml/min/1.73 m2/month, respectively, before and after treatment p = 0.007). Ten-month dialysis-free survival after treatment was 100 % in patients with basal eGFR ≥30 ml/min/1.73 m2 and 53 % in patients with eGFR <30 ml/min/1.73 m2 suggesting more prominent reno-protective effect of this intermittent steroid pulse therapy in patients having more preserved renal function before treatment. Contrary to other corticosteroid treatment, this treatment did not reduce albuminuria (UACR before and after treatment 1.71 ± 1.60 mg/g vs. 1.48 ± 1.07 mg/g) although albuminuria did not actually increase during follow-up [53].
A retrospective analysis showed that one of the main reasons for worse renal survival in IgAN than in Henöch-Schönlein purpura was earlier (durations between biopsy and steroid start: HSP vs. IgAN 9.6 ± 14.8 months vs. 25.3 ± 17.8 months) and more frequent use of steroid (HSP vs. IgAN 40.2 % vs. 14.2 %) in Henöch-Schönlein purpura. In fact, no difference in renal revival was observed between IgAN and Henöch-Schönlein purpura when matched baseline factors including steroid treatment. In fact, eGFR decreased 83.4 ml/min/1.73 m2 to 58.5 mml/min/1.73 m2, and UPCR increased from 1.1 to 2.2 g/g in IgAN patients before the initiation of steroid implicating that earlier and more liberal steroid use might improve the prognosis of IgAN patients [54].
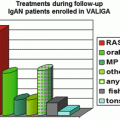
In author’s hospital, corticosteroid was used in 44 (15.7 %) out of 281 patients with IgAN. Compared with patients treated by RASB only, the patients to which corticosteroid was administered were characterized by lower eGFR (85.70 ± 31.55 vs. 75.14 ± 26.29 ml/min/1.73 m2), higher UPCR (1.65 ± 1.55 vs. 2.80 ± 1.97 g/g), lower serum albumin (4.01 ± 0.39 vs. 3.76 ± 0.46 g/dL), higher percentage of glomeruli showing segmental sclerosis (8.26 ± 8.08 vs. 13.75 ± 12.26 %), and more frequent presence of advanced tubulointerstitial lesions (17.5 vs. 38.6 %). Modified Pozzi’s regimen in which 500 mg instead of 1,000 mg of methylprednisolone was administered reduced proteinuria and the degree of hematuria and stabilized eGFR which had been decreasing before treatment (Fig. 10.3). The effects on the reduction of proteinuria and the stabilization of eGFR of modified Pozzi’s regimen were similar between the patients whose eGFR before treatment was above and below 50 ml/min/1.73 m2, but the extent of reduction of hematuria was greater in patients with pretreatment eGFR ≥50 ml/min/1.73 m2 than those with pretreatment eGFR <50 ml/min/1.73 m2. Out of 38 patients, five patients required more than two courses of corticosteroid treatment due to increased proteinuria and/or acceleration of eGFR decline after initial treatment (unpublished results).
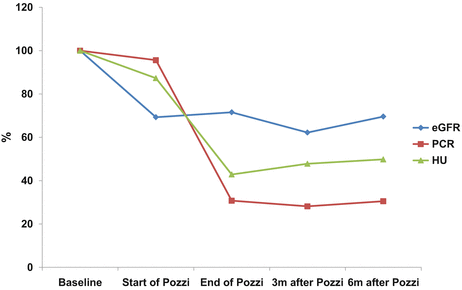

Fig. 10.3
Serial changes in eGFR, PCR, and hematuria after Pozzi’s regimen in patients with IgAN
Collectively, although there have been no settled indications for corticosteroid treatment and no agreed method of administration of corticosteroid in IgAN before the introduction of KDIGO guideline in Korea, corticosteroid treatment was effective in reducing proteinuria and inducing the improvement or stabilization of renal function even in patients with already decreased eGFR before treatment.
10.2.3.3 Calcineurin Inhibitors
A double-blind randomized trial of tacrolimus (clinicaltrial.gov identifier NCT01224028) in adult IgAN patients with UACR of 0.3−3.0 mg/g in which introduction or intensification of RAS blockade is difficult owing to further lowering of blood pressure demonstrated that the initial administration of tacrolimus of 0.1 mg/kg/day adjusted to trough level of 5−10 ng/ml for 8 weeks followed by halving the dose for remaining 8 weeks reduced albuminuria greater than placebo (reduction rate of UACR: 60.2 ± 28.2 %, 62.2 ± 33.9 %, 48.5 ± 29.8 %, and 55.5 ± 24.0 %, respectively, at 4, 8, 12, and 16 weeks after tacrolimus vs. 6.8 ± 32.2 %, 2.5 ± 35.9 %, 12.7 ± 34.2 %, and 21.9 ± 30.6 %, respectively, at 4, 8, 12, and 16 weeks after placebo). Characteristically, anti-proteinuric effect of tacrolimus was most prominent when eGFR mostly decreased from baseline, and the difference in the reduction of proteinuria between tacrolimus and placebo became unapparent after halving dose of tacrolimus in patients on ARB, suggesting hemodynamic mechanism in anti-proteinuric effects of tacrolimus [55].
The combination of cyclosporine A adjusted to trough serum level of 100−200 ng/dL for 8−12 months with prednisolone of 1−2 mg/kg/day for 4 weeks followed by gradual tapering reduced 24-h urine protein from 107.1 ± 35.1 mg/m2/h to 7.4 ± 2.4 mg/m2/h in pediatric patients. At last follow-up, proteinuria was normalized in nine patients (64.3 %) including six patients in whom hematuria also disappeared. In the follow-up biopsies after completion of cyclosporine A, histological grading of Hass classification improved in seven patients (50 %), remained the same in three patients (21 %) and aggravated in four patients (29 %), two of whom showed the clinical improvement despite the worsening of histological findings. The degree of mesangial IgA deposition in immunofluorescence examination decreased in 50 % of patients [56].
Collectively, calcineurin inhibitors could be used for the reduction of proteinuria instead of RAS blockades in patients who are intolerable to RAS blockade due to excessive lowering of blood pressure or in patients already under corticosteroid treatment for further reduction of proteinuria. The mechanism of anti-proteinuric effect of calcineurin inhibitor might be hemodynamic or non-hemodynamic depending on the clinical context where this drug is used.
10.2.3.4 Sulodexide
A retrospective study demonstrated that the daily administration of 50 mg of sulodexide, an oral formulation of mixture of heparan sulfate and dermatan sulfate, for 11.1 ± 2.7 months reduced the UPCR from 1.5 ± 0.6 g/g at baseline to 1.1 ± 0.7 g/g at the end of follow-up in 20 patients who had been maintained on RAS blockade with stable blood pressure more than 2 years. A quarter of patients showed more than 50 % reduction in proteinuria, and 40 % of patients showed UPCR less than 1 g/g at final follow-up. Interestingly, the anti-proteinuric effects of sulodexide were greater in patients with larger basal proteinuria [57].
Encouraged by anti-proteinuric effect in diabetic nephropathy of sulodexide, a multicenter randomized double-blind trial of daily administration of 50 mg and 150 mg of sulodexide for 6 months was conducted on 104 patients who had been maintained on RAS blockade with stable blood pressure. Although there were no differences in the primary outcome, i.e., more than 50 % reduction of UPCR, between treatment groups (12.5 % in placebo, 4.0 % in 75 mg of sulodexide, and 21.4 % in 150 mg of sulodexide), 150 mg of sulodexide significantly reduced the log UPCR of the patients within group from 6.38 ± 0.77 to 5.98 ± 0.94 in time-dependent manner (p = 0.045). The relatively low basal UPCR of 0.6−0.8 g/g and short duration of treatment might contribute to the negative results in this study [58].
Collectively, these studies suggested that the long-term administration of sulodexide might be effective in reducing proteinuria in patients with relatively high basal proteinuria.
10.2.3.5 Omega-3 Fatty Acid
No studies of omega-3 fatty acid in Korean patients with IgAN were published. Actually a double-blind, placebo-controlled, multicenter phase 3 trial of omega-3 fatty acid in IgAN patients with decreased renal function has been completed (clinicaltrial.gov identifier NCT 00549692), but the results are not yet published.
10.2.4 Prognosis
A retrospective analysis for 1,364 patients with IgAN showed that 10-, 20-, and 30-year renal survival rates over median period of 96 months (interquartile range (IQR) 56–187) were 82.0 %, 70.8 %, and 67.3 %, respectively. The median time to ESRD was 71 months (IQR 32–123). Initial renal function was the most important determinant of renal survival, and hypertension, segmental sclerosis ≥20 %, hypoalbuminemia were independent risk factors for renal survival. Gross hematuria was a favorable factor for renal survival.
Ten-, 20-, and 30-year patient survival rates over median period of 101 months (IQR 38–189) were 96.3 %, 91.8 %, and 82.7 %, respectively. The median time to death was 101 months (IQR 38–189). Age was predominant risk factor for death, and hypertension, hypoalbuminemia, and the occurrence of cancer were independent risk factors for patient survival. The half of patient’s death (55.7 %) occurred before reaching ESRD. The main causes of pre-ESRD death were the malignancy (30.8 %, n = 12), cardiovascular disease (12.8 %, n = 5), and infection (15.8 %, n = 6). Ten-year and 20-year survival rates after ESRD were 88.6 % and 66.3 %, respectively. The major causes of death after ESRD were renal-related causes, cardiovascular disease, and infection. Out of 209 patients who received immunosuppressive therapy, 24 (11.4 %) patients died. Half of these patients died of infection [59].
Compared to other primary GN, renal survival of the patients with IgAN was better than membranoproliferative glomerulonephritis, similar to focal segmental glomerulosclerosis, and worse than minimal change disease. Despite the similar renal survival, patient survival was better in IgAN than focal segmental glomerulosclerosis due to better patient survival after reaching ESRD. Compared to other primary glomerulonephritis, extrarenal morbidities such as diabetes mellitus, cancer, and cardiovascular complications occurred least in patients with IgAN during the follow-up. Mortality rate relative to age/sex-matched general population (SMR, standardized mortality ratio) was 1.43 (95 % CI 1.04–1.92) meaning IgAN patients have a little higher mortality than general population. But, the mortality rate of male patients mortality rate was similar to general population (SMR 1.22 CI 0.82–1.75), while the mortality rate of female patients was more than twofolds higher than general population [60].
While 5-year renal survival rate was 78.9 % in 223 patients biopsied between 1980 and 1993 [A-61], it was 88.0 % in 223 patients biopsied between 2002 and 2004, in whom RAS blockade, corticosteroid, and statin were prescribed in 87.0 %, 11.4 %, and 18.8 %, respectively [unpublished data].
Conflict of Interest
The author declares that he has no conflict of interest.
References
1.
Jae Hyun Chang, Dong Ki Kim, Hyun Wook Kim, Sun Young Park, Tae-Hyun Yoo, Beom Seok Kim, et al. Changing prevalence of glomerular diseases in Korean adults: a review of 20 years of experience. Nephrol Dial Transplant. 2009;31:2406. doi:10.1093/ndt/gfp091.
2.
Park JS, Song JH, Yang WS, Kim SB, Kim YK, Hong CD. Cytomegalovirus is not specifically associated with immunoglobulin A nephropathy. J Am Soc Nephrol. 1994;4:1623.PubMed
3.
Gyung Geun Han, Jeong Ha Pack, Sung Jin Bae, Sam Ryong Ji, Jeong Hyun Lim, Goang Yul Jang, et al. A clinical study of IgA nephropathy with serum Hepatitis B surface antigen. Korean J Nephrol. 2000;19:437.
4.
Jwa Kyung Kim, Jeong Ho Kim, Sang Choel Lee, Ea Wha Kang, Tae Ik Chang, Sung Jin Moon, et al. Clinical features and outcomes of IgA nephropathy with nephrotic syndrome. Clin J Am Soc Nephrol. 2012;7:427. doi:10.2215/CJN.04820511.
5.
In O Sun, Yu Ah Hong, Hoon Suk Park, Sun Ryoung Choi, Byung Ha Chung, Cheol Whee Park, et al. Clinical characteristics and treatment of patients with IgA nephropathy and hepatitis B surface antigen. Ren Fail. 2013;35:446. doi:10.3109/0886022X.
6.
Seung Hyeok Han, Ea Wha Kang, Jeong Hae Kie, Tae Hyun Yoo, Kyu Hun Choi, Dae-Suk Han, et al. Spontaneous remission of IgA nephropathy associated with resolution of Hepatitis A. Am J Kidney Dis. 2010;56:1163. doi:10.1053/j.ajkd.2010.08.018.
7.
Chun Soo Lim, Shouhuan Zheng, Yon Su Kim, Curie Ahn, Jin Suk Han, Suhnggwon Kim, et al. Th1/Th2 predominance and proinflammatory cytokines determine the clinicopathological severity of IgA nephropathy. Nephrol Dial Transplant. 2001;16:269. doi:10.1093/ndt/16.2.269.
8.
Chun Soo Lim, Hyung Jin Yoon, Yon Su Kim, Curie Ahn, Jin Suk Han, Suhnggwon Kim, et al. Clinicopathological correlation of intrarenal cytokines and chemokines in IgA nephropathy. Nephrology (Carlton). 2003;8:21. doi:10.1046/j.1440-1797.2003.00128.x.
9.
Mi Ra Park, Eun Hui Wang, Dong Chan Jin, Jung Ho Cha, Kweon Heang Lee, Chul Woo Yang, et al. Establishment of a 2-D human urinary proteomic map in IgA nephropathy. Proteomics. 2006;6:1066. doi:10.1002/pmic.200500023.
10.
Pyong-Gon Moon, Jeong-Eun Lee, Sungyong You, Taek-Kyun Kim, Ji-Hoon Cho, In-San Kim, et al. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics. 2011;11:2459. doi:10.1002/pmic.201000443.
11.
Noh Jin Kwak, Eun Hui Wang, Il-Young Heo, Dong-Chan Jin, Jung-Ho Cha, Kweon-Haeng Lee, et al. Proteomic analysis of alpha-1-antitrypsin in immunoglobulin A nephropathy. Proteomics Clin Appl. 2007;1:420. doi:10.1002/prca.200600288.
12.
Soon Hyo Kwon, Moo Yong Park, Jin Seok Jeon, Hyunjin Noh, Soo Jeong Choi, Jin Kuk Kim, et al. KIM-1 expression predicts renal outcomes in IgA nephropathy. Clin Exp Nephrol. 2013;17:359. doi:10.1007/s10157-012-0707-2.
13.
Ji Hye Lee, Mee Hye Oh, Jae Seok Park, Gyoung Jae Na, Hye Wook Gil, Jong Oh Yang, et al. Urokinase, urokinase receptor, and plasminogen activator inhibitor-1 expression on podocytes in immunoglobulin A glomerulonephritis. Korean J Intern Med. 2014;29:176. doi:10.3904/kjim.2014.29.2.176.
14.
Jae Ryung Shin, Seung Min Kim, Jung Sun Yoo, Ji Yoon Park, Seul Ki Kim, Joo Hee Cho, et al. Urinary excretion of β2-microglobulin as a prognostic marker in immunoglobulin A nephropathy. Korean J Intern Med. 2011;29:334–40. doi:10.3904/kjim.2014.29.3.334
15.
Ho Jun Chin, Hyun Jin Cho, Tae Woo Lee, Ki Young Na, Hyung Jin Yoon, Dong-Wan Chae, et al. The Heme Oxygenase-1 Genotype is a risk factor to renal impairment of IgA nephropathy at diagnosis, which is a strong predictor of mortality. J Korean Med Sci. 2009;24 Suppl:S30. doi:10.3346/jkms.2009.24.S1.S30.
16.
Ho Jun Chin, Hyun Jin Cho, Tae Woo Lee, Ki Young Na, Kook Hwan Oh, Kwon Wook Joo, et al. The mildly elevated serum bilirubin level is negatively associated with the incidence of end stage renal disease in patients with IgA nephropathy. J Korean Med Sci. 2009;24 Suppl:S22. doi:10.3346/jkms.2009.24.S1.S22.
17.
Hye Ryoun Jang, Soo Min Park, Yu ji Lee, Jung Eun Lee, Woo Seong Huh, Dae Joong Kim, et al. The origin and the clinical significance of urinary angiotensinogen in proteinuric IgA nephropathy patients. Ann Med. 2012;44:448. doi:10.3109/07853890.2011.558518.
18.
Sun Moon Kim, Ho Jun Chin, Yun Kyu Oh, Yon Su Kim, Suhnggwon Kim, Chun Soo Lim. Blood pressure-related genes and the progression of IgA nephropathy. Nephron Clin Pract 2009;113:c301. doi:10.1159/000235948.
19.
HJ Yoon, H Kim, HL Kim, SG Lee, S H Zheng, JH Shi, et al. Interdependent effect of angiotensin-converting enzyme and platelet-activating factor acetylhydrolase gene polymorphisms on the progression of immunoglobulin A nephropathy. Clin Genet. 2002;62:128. doi:10.1034/j.1399-0004.2002.620205.x.
20.
Eun Sook Jung, Sun Moon Kim, Ho Jun Chin, Ran Hui Cha, Yun Kyu Oh, Yon Su Kim, et al. Impact of polymorphisms of the genes encoding angiotensin II-forming enzymes on the progression of IgA nephropathy. Nephron Clin Pract 2011;118:c122. doi:10.1159/000321140.
21.
Hyung Jin Yoon, Ho Jun Chin, Ki Young Na, Dong Wan Chae, Suhnggwon Kim, Un Sil Jeon, et al. Association of angiotensin II Type 2 receptor gene A1818T polymorphism with progression of Immunoglobulin A nephropathy in Korean Patients. J Korean Med Sci. 2009;24 Suppl:S38. doi:10.3346/jkms.2009.24.S1.S38.
22.
CS Lim, SM Kim, YK Oh, YS Kim, DW Chae, JS Han, et al. Association between the clara cell secretory protein (CC16) G38A polymorphism and the progression of IgA nephropathy. Clin Nephrol. 2007; 67:73.
23.
Yoon HJ, Shin JH, Yang SH, Chae DW, Kim H, Lee DS, et al. Association of the CD14 gene-159C polymorphism with progression of IgA nephropathy. J Med Genet. 2003;40:104. doi:10.1136/jmg.40.2.104.CrossRefPubMedPubMedCentral
24.
25.
26.
Jung Pyo Lee, Seung Hee Yang, Dong Ki Kim, Hajeong Lee, Bora Kim, Joo Youn Cho, et al. In vivo activity of epoxide hydrolase according to sequence variation affects the progression of human IgA nephropathy. Am J Physiol Renal Physiol. 2011;300:F1283. doi:10.1152/ajprenal.00733.2010.
27.
Gang Jae Ko, Eun Ah Lee, Un Sil Jeon, Heui Jung Pyo, Ho Jun Chin, Dong Wan Chae, et al. The association of Klotho Polymorphism with disease progression and mortality in IgA nephropathy. Kidney Blood Press Res. 2012;36:191. doi:10.1159/000343408.
28.
Lee HS, Koh HI, Lee HB, Park HC. IgA nephropathy in Korea: a morphological and clinical study. Clin Nephrol. 1987;27:131.PubMed
29.
Seung Jun Kim, Hyang Mo Koo, Beom Jin Lim, Hyung Jung Oh, Dong Eun Yoo, Dong Ho Shin, et al. Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS One. 2012;7:e40495. doi:10.1371/journal.pone.0040495.
30.
Young In Maeng, Min Kyung Kim, Jae Bok Park, Chang Ho Cho, Hoon Kyu Oh, Woo Jung Sung, et al. Glomerular and tubular C4d depositions in IgA nephropathy: relations with histopathology and with albuminuria. Int J Clin Exp Pathol. 2013;6:904.
31.
Hong Joo Lee, So Young Choi, Kyung Hwan Jeong, Ji Youn Sung, Sung Kyoung Moon, Ju Young Moon, et al. Association of C1q deposition with renal outcomes in IgA nephropathy. Clin Nephrol. 2013;80:98. doi:10.5414/CN107854.
32.
Hyun Soon Lee, Myung Suk Lee, Sa Min Lee, Sang Yun Lee, Eun Sun Lee, Eun Young Lee, et al. Histological grading of IgA nephropathy predicting renal outcome: revisiting H. S. Lee’s glomerular grading system. Nephrol Dial Transplant. 2005;20:342.
33.
Shin Wook Kang, Kyu Hun Choi, Jong Hoon Park, Seung Woo Lee, Ho Yung Lee, Dae Suk Han, et al. Prognostic factors and renal survival rates in IgA nephropathy. Yonsei Med J. 1995;36:45.
34.
Seok Hui Kang, Sun Ryoung Choi, Hoon Suk Park, Ja Young Lee, In O Sun, Hyeon Seok Hwang, et al. The Oxford classification as a predictor of prognosis in patients with IgA nephropathy. Nephrol Dial Transplant. 2012;27:252. doi:10.1093/ndt/gfr295.
35.
Ho young Lee, Sul Hee Yi, Mi Seon Seo, Jin Nam Hyun, Jin Seok Jeon, Hyunjin Noh, et al. Validation of the Oxford classification of IgA nephropathy: a single-center study in Korean adults. Korean J Intern Med. 2012;27:293.
36.
Kyoung Sook Park, Seung Hyeok Han, Jeong Hae Kie, Ki Heon Nam, Mi Jung Lee, Beom Jin Lim, et al. Comparison of the Haas and the Oxford classifications for prediction of renal outcome in patients with IgA nephropathy. Hum Pathol. 2014;45:236. doi:10.1016/j.humpath.2013.08.019.
37.
Beom Jin Lim, Dong Jin Joo, Myoung Soo Kim, Yu Seun Kim, Soon Il Kim, Yeon hee Kim, et al. Usefulness of Oxford classification in assessing immunoglobulin A nephropathy After Transplantation. Transplantation. 2013;95:1491. doi:10.1097/TP.0b013e318291de65.
38.
Mi Jung Lee, Seung Jun Kim, Hyung Jung Oh, Kwang Il Ko, Hyang Mo Koo, Chan Ho Kim, et al. Clinical implication of crescentic lesions in immunoglobulin A nephropathy. Nephrol Dial Transplant. 2014;29:356. doi:10.1093/ndt/gft398.
39.
Hyeon Joo Jeong, Yu Seun Kim, Ki Hwan Kwon, Soon Il Kim, Myoung Soo Kim, Kyu Hun Choi et al. Glomerular crescents are responsible for chronic graft dysfunction in post-transplant IgA nephropathy. Pathol Int. 2004;54:837.
40.
Byung Soo Kim, Yong Kyun Kim, Young Shin Shin, Young Ok Kim, Ho Cheol Song, Yong Soo Kim, et al. Natural history and renal pathology in patients with isolated microscopic hematuria. Korean J Intern Med. 2009;24:356. doi:10.3904/kjim.2009.24.4.356.
41.
Hajeong Lee, Jin Ho Hwang, Jin Ho Paik, Hyun Jin Ryu, Dong Ki Kim, Ho Jun Chin, et al. Long-term prognosis of clinically early IgA nephropathy is not always favorable. BMC Nephrol. 2014;15:94. doi:10.1186/1471-2369-15-94.
42.
Sun Moon Kim, Kyung Chul Moon, Kook-Hwan Oh, Kwon Wook Joo, Yon Su Kim, Curie Ahn, et al. Clinicopathologic characteristics of IgA nephropathy with steroid-responsive nephrotic syndrome. J Korean Med Sci. 2009;24 Suppl:S44. doi:10.3346/jkms.2009.24.S1.S44.
43.
Ki Heon Nam, Jeong Hae Kie, Mi Jung Lee, Tae-Ik Chang, Ea Wha Kang, Dong Wook Kim, et al. Optimal proteinuria target for renoprotection in patients with IgA nephropathy. PLoS One. 2014;9(7):e101935. doi:10.1371/journal.pone.0101935.
44.
Hyeon Seok Hwang, Byung Soo Kim, Young Shin Shin, Hye Eun Yoon, Joon Chang Song, Bum Soon Choi, et al. Predictors for progression in immunoglobulin A nephropathy with significant proteinuria. Nephrology (Carlton). 2010;15:236. doi:10.1111/j.1440-1797.2009.01196.x.
45.
You Cheol Hwang, Tae Won Lee, Myung Jae Kim, Moon Ho Yang, Chun Gyoo Ihm. Clinical course of patients with IgA nephropathy between combined treatment of immunosuppressive agents and ACE Inhibitor and ACE Inhibitor alone. Korean J Intern Med. 2001;16:105.
46.
Han SY, Kwon YJ, Jo SK, Shin JH, Cha DR, Cho WY, et al. ACE gene polymorphism and renal responsiveness to ACE inhibitors in IgA nephropathy patients. Korean J Intern Med. 2000;15:13.CrossRefPubMedPubMedCentral
47.
Hyeong Cheon Park, Zhong Gao Xu, Sorae Choi, Young Suck Goo, Shin Wook Kang, Kyu Hun Choi, et al. Effect of losartan and amlodipine on proteinuria and transforming growth factor‐β1 in patients with IgA nephropathy. Nephrol Dial Transplant. 2003;18:1115. doi:10.1093/ndt/gfg090.
48.
Moon Jae Kim, Joon Ho Song, Ju Hyun Suh, Seoung Woo Lee, Gyoung A Kim. Additive antiproteinuric effect of combination therapy with ACE inhibitor and angiotensin II receptor antagonist: differential short-term response between IgA nephropathy and diabetic nephropathy. Yonsei Med J. 2003;44:463.
49.
50.
Hyeon Joo Jeong, Yu Seun Kim, Kye Won Kwon, Myoung Soo Kim, Soon Kim, Kyu Hun Choi, et al. Segmental glomerulosclerosis in IgA nephropathy after renal transplantation: relationship with proteinuria and therapeutic response to enalapril. Clin Transplant. 2003;17:108. doi:10.1034/j.1399-0012.2003.02067.x.
51.
Choi S, Lee D, Jeong KH, Moon JY, Lee SH, Lee TW, et al. Prognostic relevance of clinical and histological features in IgA nephropathy treated with steroid and angiotensin receptor blockers. Clin Nephrol. 2009;72:353. doi:10.5414/CNP72353.CrossRefPubMed
52.
Ji Min Jeong, Dae Hun Lim, Hyung Chul Lee, Seul Hyun Oh, Joon Seok Choi, Pyung Kyun Park, et al. Renoprotective effect of deflazacort in IgA nephropathy with proteinuria. Korean J Med. 2009;7:593.
53.
Tae Young Kim, Soon Bae Kim, Su-Kil Park. The efficacy of steroid pulse therapy in patients with IgA nephropathy. Clin Nephrol. 2012;78:100. doi:10.5414/CN107418.
54.
Hyung Jung Oh, Song Vogue Ahn, Dong Eun Yoo, Seung Jun Kim, Dong Ho Shin, Mi Jung Lee, et al. Clinical outcomes, when matched at presentation, do not vary between adult-onset Henöch-Schönlein purpura nephritis and IgA nephropathy. Kidney Int. 2012;82:1304. doi:10.1038/ki.2012.302.
55.
Yong Chul Kim, Ho Jun Chin, Ho Suk Koo, Suhnggwon Kim. Tacrolimus decreases albuminuria in patients with IgA nephropathy and normal blood pressure: a double-blind randomized controlled trial of efficacy of tacrolimus on IgA nephropathy. PLoS One. 2013;8:e71545. doi:10.1371/journal.pone.0071545.
56.
Jae Il Shin, Beom Jin Lim, Pyung Kil Kim, Jae Seung Lee, Hyeon Joo Jeong, Ji Hong Kim. Effects of cyclosporin A therapy combined with steroids and angiotensin converting enzyme inhibitors on childhood IgA nephropathy. J Korean Med Sci. 2010;25:723. doi:10.3346/jkms.2010.25.5.723.
57.
Byeong Yun Yang, Hee Seon Lee, Sang Heon Song, Ihm Soo Kwak, Soo Bong Lee, Dong Won Lee, et al. Use of low-dose sulodexide in IgA nephropathy patients on renin–angiotensin system blockades. Kidney Res Clin Pract 2012;31:163.
58.
Kitae Bang, Ho Jun Chin, Dong Wan Chae, Kwon Wook Joo, Yon Su Kim, Suhnggwon Kim, et al. Anti-proteinuric effect of sulodexide in immunoglobulin A nephropathy. Yonsei Med J. 2011;52:588. doi:10.3349/ymj.2011.52.4.588.
59.
Hajeong Lee, Dong Ki Kim, Kook-Hwan Oh, Kwon Wook Joo, Yon Su Kim, Dong-Wan Chae, et al. Mortality of IgA nephropathy patients: a single center experience over 30 years. PLoS One. 2012;7:e51225. doi:10.1371/journal.pone.0051225.
60.
Hajeong Lee, Dong Ki Kim, Kook-Hwan Oh, Kwon Wook Joo, Yon Su Kim, Dong-Wan Chae, et al. Mortality and renal outcome of primary glomerulonephritis in Korea: observation in 1,943 biopsied cases. Am J Nephrol 2013;37:74. doi:10.1159/000345960.