9 The cortex
Embryological development
Neuronal fate in the mammalian cortex is influenced by the timing of cell differentiation, which is dependent on both genetic and environmental factors (see Chapter 2). The cerebral cortex neurons are generated in the ventricular zone by the epithelial layer of progenitor cells that line the lateral ventricles. They migrate to the cortical plate, which eventually develops into the grey matter of the cortex. The final position assumed by these neurons depends on their ’birthmoment’, or time of last division. The migration occurs along radially organised glial cells called radial glia, which guide the migrating neurons to the cortex. The layering of the neurons in the cerebral cortex is established with an inside-first, outside-last manner so that the newest neurons must pass over and around the more mature neurons, probably gaining information from the previously established neurons as they pass.
The meninges
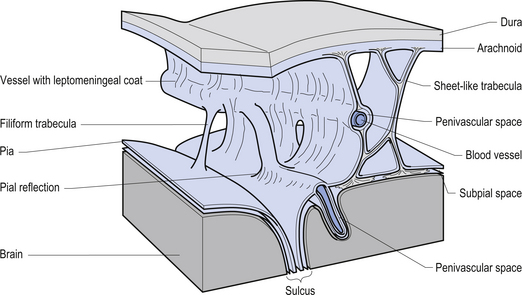
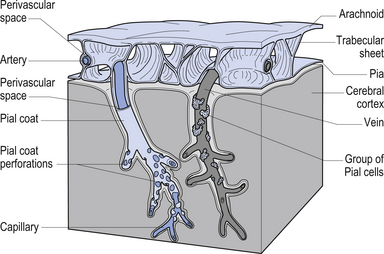
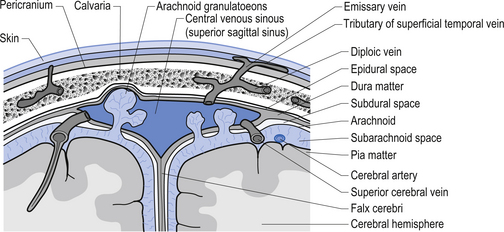
The meninges are layered structures that contain cerebrospinal fluid and give protection to the brain and spinal cord. The meninges are composed of three layers; the dura mater, the arachnoid mater, and the pia mater (Figs 9.1 and 9.2).
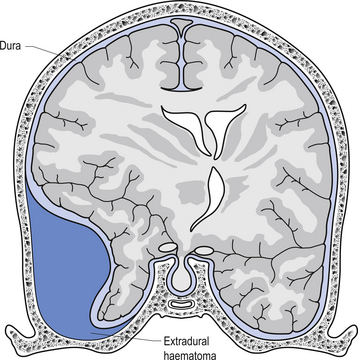
The way in which the meninges connect to each other and the structures that they attach to gives rise to three clinically important spaces or potential spaces, the epidural space, the subarachnoid space, and the subdural space. The epidural space is a potential space that can form between the dura and the bone of the skull. The meningeal arteries run in the space between the tightly adherent dura and the skull. The middle meningeal artery passes under and along the temporal bone, which is the thinnest bone of the skull and thus the most easily fractured. Trauma to the temporal bone can cause tears in the meningeal arteries and result in blood escaping into the potential epidural space. As the blood builds up, it forces the periosteal layer of dura away from the bone and bulges into the arachnoid and pia layers, eventually exerting pressure on the brain. This process is referred to as an epidural haematoma (Fig. 9.3).
Epidural haematomas are usually rapidly growing and expanding as the arterial pressure spreads the periosteal dura from the bone of the skull. The dural separation continues until it reaches a cranial suture where the dura is much more tightly joined to the skull. This results in an expansile lesion that takes the shape of a biconcave lens. Clinically, the patient may experience a lucid interval following trauma to the skull where they may not have any symptoms. Within a few hours the expanding haematoma starts to compress the brain and results in increased intracranial pressure and death if not treated.
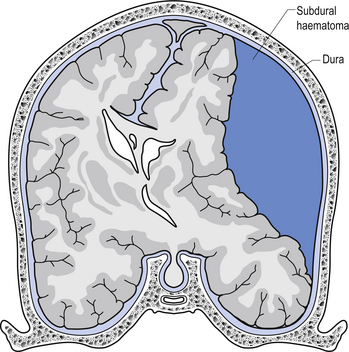
The symptoms of SDH often mimic other cerebrovascular events or space-occupying lesions. Alcohol consumption reduces clotting mechanisms and often results in head trauma from falls. Anticoagulants can also increase the risk of SDH from minor trauma in the elderly (Fig. 9.4). Chronic subdural haematomas can take weeks to months in the elderly before they start to experience symptoms. This is mainly due to the low-pressure, slow leak from the veins and the fact that brain tissue shrinks somewhat as we age and allows a greater space for the blood to occupy before interference with function occurs. Acute subdural haematomas require a considerable amount of traumatic force to occur and as such are usually associated with other serious brain injuries such as traumatic subarachnoid haemorrhage and brain contusions.
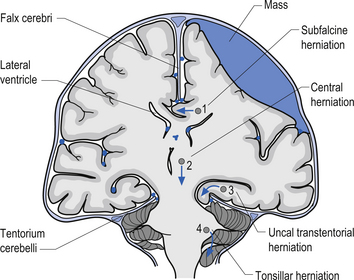
1. Transtentorial herniation involves the compression and protrusion of the medial temporal lobe, usually the uncus or periuncal areas, inferiorly through the tentorial notch (Fig. 9.5). Because of the pressure exerted on the mesencephalic area and the peduncles of the cerebrum, oculomotor dysfunction and hemiplegia can result. The oculomotor damage usually results in a dilated pupil ipsilateral to the side of the lesion due to the unopposed action of the sympathetic nerves. The hemiplegia usually occurs on the side opposite the lesion. Damage to the mesencephalic reticular system can also lead to loss of consciousness and coma.
2. Central herniation is the downward displacement of the brainstem. The action of the downward displacement may traction the abducens nerve (CN VI) and cause lateral rectus palsy (Fig. 9.5).
3. Tonsillar herniation occurs when increased intracranial pressure forces the tonsillar region of the cerebellum down through the foramen magnum of the skull. Because of the high pressure experienced in the medullary region, this condition usually results in respiratory arrest, blood pressure instability, and death (Fig. 9.5).
4. Subfalcine herniation results when the cingulate gyrus is pushed under the falx cerebri and into the other half of the brain. No specific clinical signs may be present with this condition (Fig. 9.5).
Cerebral spinal fluid (CSF)
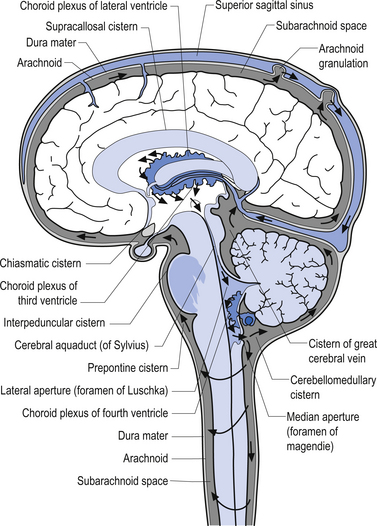
Cerebral spinal fluid is normally a clear, colourless, and odourless fluid that diffuses over the brain and spinal cord. CSF probably functions to cushion the brain and spinal cord from external jarring or shocking forces that may be transmitted through the tissues to reach these structures. CSF may also function in some capacity as a metabolic transport medium, transporting nutrients to the neuraxial cells and metabolic waste products away from the neuraxial components. CSF may also function as a pressure distributor in cases where changes in intracranial volume have occurred such as in postoperative lesions where the removed tissue area fills with CFS. The CSF is formed by the dialysis of blood across the tissues of the choroid plexuses found in the ventricle of the brain and brainstem. The circulation of CSF occurs in two systems: the internal system which includes the two lateral ventricles, the interventricular foramens, the third ventricle, the cerebral aqueduct, and the fourth ventricle; and the external system, which includes all of the external spaces surrounding the brain and spinal cord including the various cisterns. Communication between the internal and external systems occurs via two lateral apertures in the fourth ventricle referred to as the foramens of Luschka, and a medial aperture also in the fourth ventricle referred to as the foramen of Magendie. The total volume of CSF in all systems measures about 150 cm3. The CSF is formed at the rate of about 20 cm3/hr or about 480 cm3/day. This means that all of the CSF in your body is replaced about 3.2 times per day. This is accomplished via absorption of the CFS by the arachnoid granulations located along the superior longitudinal sinus which allow the CSF to enter the venous drainage system and return to the general circulation (Fig. 9.6). The CSF circulates from the lateral ventricles, through the third ventricle, to the fourth ventricle where it then enters the external system and bathes the spinal cord and the external surface of the brain (Fig. 9.7).
Clinical examination of cerebral spinal fluid
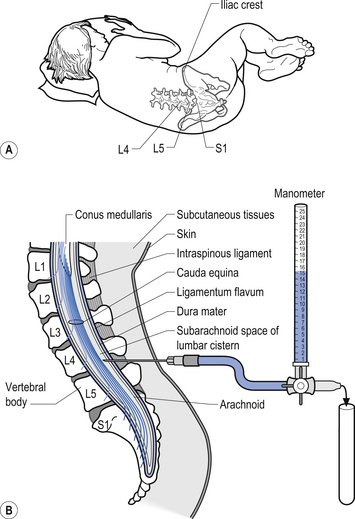
Examination of the CSF can be a valuable tool in the diagnosis of several conditions such as infection that can affect the neuraxis. A common procedure utilised to obtain CSF is the lumbar puncture. This procedure provides direct access to the subarachnoid space of the lumbar cistern which contains the CSF (Fig. 9.8). This procedure can be used to obtain samples of CSF, measure the pressure of the CSF, remove excess CSF if necessary, and act as a conduit for the administration of medication or radiographic contrast material. The CSF is examined for a variety of different elements:
1. CSF pressure—Normal value is 100–200 mmH2O (7.7–15.4 mmHg). Elevated CSF pressure may be caused by blockage of the ventricular drainage system, overproduction, or space-occupying lesions. The two most common causes are meningitis and subarachnoid haemorrhage. Brian tumours and abscesses will cause an increase after a delay of days to weeks.
2. CSF appearance—Normal CSF is clear and colourless. CSF is generally white or cloudy if significant white blood cells (WBC) are present (over 400/mm3). CSF may appear red or pink if red blood cells (RBC) are present; however, if RBC have been in the CSF for more than 4 hours the fluid may appear yellow (xanthochromia). This is due to the breakdown of haemoglobin.
3. CSF glucose—Normal is 45 mg/100 mL or higher. The most significant clinical finding is a decrease in glucose concentration. This occurs in virtually every case of bacterial meningitis. Other causes of decreased glucose include:
4. CSF protein—Normal value is considered to be 15–45 mg/100 mL in adults. In newborns it may range as high as 150 mg/100 mL. The most significant clinical finding is an elevation of protein concentration. Causes of increased protein concentration include:
5. CSF cell count—Normally the CSF contains no more than 5 cells/mm3. Under normal conditions virtually all of the cells present should be lymphocytes. Usually the highest leukocyte counts are found in acute bacterial infections such as meningitis. Usually the cell type most contributing to the leukocytosis in bacterial infections is the polymorphonuclear cell or neutrophil.
Clinical syndromes involving the meninges
Cerebral abscesses
1. Resulting from a contiguous site of focal infection, i.e. dental infection, sinusitis, otitis;
2. Resulting from a distant site, usually haematogenous spread from a lung infection; and
The classic triad of headache, fever, and focal nerve deficit is present in about 50% of cases. Other symptoms include seizures, papilloedema, nausea and vomiting, and nuchal rigidity (Vogel 1994).
Meningitis
Once a pathogen gains entrance to the CSF, the immune system’s counterattack is severely hampered, until a substantial population of the pathogen stimulates neutrophilic pleocytosis in the case of bacteria invasion or lymphocytic pleocytosis in the case of a virus invasion (Scheld 1994; Pryor 1995). Bacteria gain entrance to the CSF by one of three proposed mechanisms:
1. Chemicals released from the bacteria cause relaxation of the intercellular tight junction between the cells forming the blood–brain barrier.
2. Bacteria enter through the fenestrations of the choroid plexus.
3. Bacteria enter inside of macrophages or other cells normally circulating through the CNS.
The primary results of bacterial invasion are:
1. CSF neutrophilic pleocytosis;
2. Increased permeability of the choroid plexus leading to increased CSF pressure, which results in an increased intracranial pressure;
Normal concentration of WBC in the CSF is 0–5 cells/mm3, and almost all are normally lymphocytes.
Meningitis may be caused by a variety of organisms, including Cryptococcus neoformans, which is a yeast found in the soil with a worldwide distribution. The incidence is about 5/1 000 000 and it is most often found in people with a compromised immune system. Risk factors of immune compromise include lymphoma, diabetes, and AIDS. Symptoms of cryptococcal meningitis include:
Treponema pallidum (Syphilis)
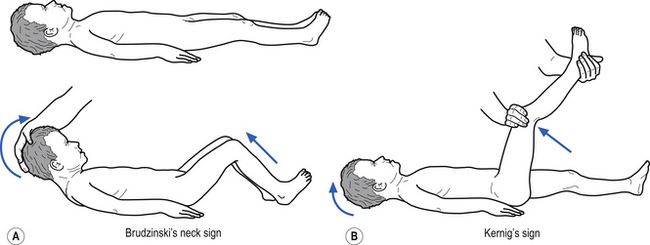
• Stiff neck (Kernig’s and Brudzinski’s signs may be positive –Fig. 9.9);
• Stiffness of shoulders and arms;
• Mental status changes including confusion, disorientation, decreased attention, irritability, sleepiness, and lethargy;
Treatment
Treatment goals are to cure the infection, reduce progression of the disorder, and reduce nerve damage as any existing nerve damage is permanent. Penicillin, tetracycline, and erythromycin are the drugs of choice. Dramatic improvement of symptoms may occur after treatment. However, a progressive disability may result.
Haemophilus influenzae
• Irritability, poor feeding in infants;
• Below normal temperature in young infants;
• Severe headache (loud screeching scream);
• Stiff neck or crying when neck flexed (Kernig’s and Brudzinski’s may be positive – Fig. 9.9);
• Pain in the back when neck is flexed or chin brought to chest;
• Bulging of the fontanelles (soft spots in the skull) of infants;
• Opisthotonos (lying with the back arched, head back, and chin up);
Meningococcus
Summary of causative agents
| Age | Agent |
|---|---|
| Less than 1 month | Group B strep. |
| 1 month to 5 years | H. Influenzae |
| 5–29 years | N. meningitidis |
| Over 29 years | S. pneumoniae |
Staphylococcus (aureus, epidermidis)
This condition is usually caused by the bacteria Staphylococcus aureus or Staphylococcus epidermidis. It may develop as a complication from surgery or from haematogenous spread from another site. Risk factors include brain surgery, CSF shunts, infections of the heart valves, and previous brain infections such as an abscess or encephalitis. The symptoms include:
CSF and serum cultures may show staph, and infections of this type often result in death.
Migraine
Some evidence suggests that pain-sensitive dura and middle meningeal artery wall may contribute to the pain of migraine headaches (see Chapter 17).
Vascular accidents
Arteriovenous malformation (AVM)
These are congenital malformations of the arteriovenous junctions that result in large tangled areas that are often structurally delicate and can be ruptured with relatively minor trauma. The haemorrhage occurs in sinusoidal vessels that are under low pressure. These types of haemorrhage often result in focal neurological signs and headache, epilepsy, and occasionally hydrocephalus. The arachnoid villi can become blocked by blood from repeated subarachnoid haemorrhages and therefore impair CSF resorption, which leads to hydrocephalus.
The cortex
The cortex in humans is composed of several well-identified functional areas, interspersed in the cortical matter referred to as association cortex. Although we will speak of functional localisation of a variety of areas of cortex, in reality the functional systems of the neuraxis work in conjunction with each other to produce the best possible outcome for the circumstances at hand. For example, the thought processes attributed to the frontal cortex need to interact with the basal ganglion in order to flow and unfold in a meaningful way. The hippocampus and amygdala are essential functional areas for the fusion of emotions and behavioural response which are attributed to cortical functions. Movement, controlled by the motor cortex in the frontal lobe, is meaningless and random without the feedback supplied by the spinal cord and cerebellum. The cortex can be divided into the lobar areas, as outlined below.
The frontal lobes
Pyramidal cells
• Pyramid cells are named for the shape of their bodies, which resemble a pyramid.
• Most pyramidal cells are 10–50 μm in width.
• However, giant pyramidal cells (Betz cells), which may expand to 120 μm in width, are found in the precentral gyrus of the frontal lobe (motor cortex).
• The apices of the pyramidal cells are orientated towards the pial surface of the cortex.
• Extending from the apex of each pyramidal cell is a large apical dendrite that rises towards the pia, giving off multiple collateral branches as it climbs.
• Other dendrites pass laterally into the surrounding neuropil.
• From the base arises a single axon that either synapses in deeper cortical layers or exits via the white fibre tracts as a projection, association, or commissural fibre.
Fusiform cells
• These cells have their long axis orientated vertical to the cortical surface.
• They are usually concentrated only in the deepest cortical layers.
• Dendrites arise from each pole of the cell body.
• The inferior dendrite synapses within the same layer of cortex as the cell body. The superior dendrite rises up through several layers of cortex to the superficial layers.
• Axons arise from the inferior body and descend in white fibre tracts as association, commissural, or projection fibres.
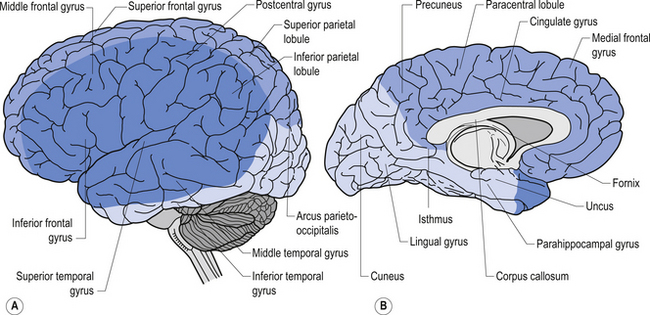
Anatomically, the frontal lobe is bounded posteriorly by the fissure of Rolando or central sulcus, and inferiorly by the fissure of Sylvius or the lateral fissure. The frontal lobe can be divided into two main areas: the precentral area and the prefrontal area. The precentral area contains areas 4 and 6 of the cortex and is composed of the precentral gyrus and the posterior portions of the superior, middle, and inferior frontal gyri. The prefrontal area is composed by the remainder of the frontal lobes and is traversed by two sulci that divide the prefrontal area into three gyri, the superior, middle, and inferior frontal gyri. The cortex varies between 1.5 and 4.5 mm in thickness and is always thicker on the exposed surface of the gyri than in the deep sulci areas.
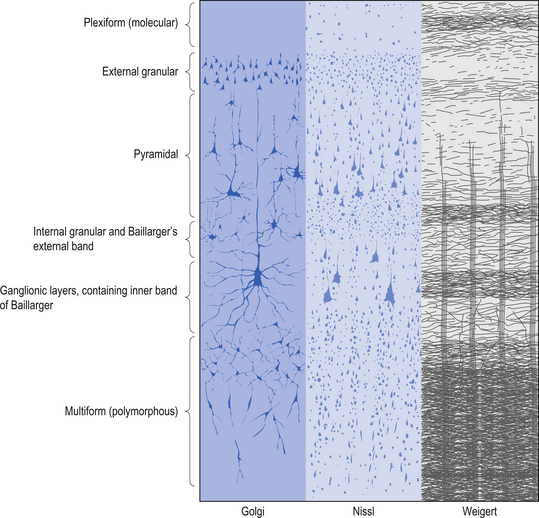
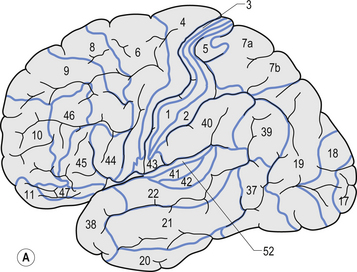
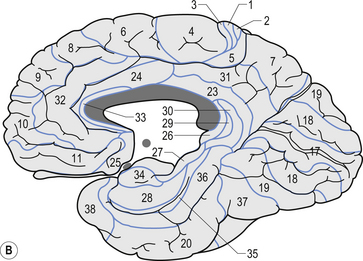
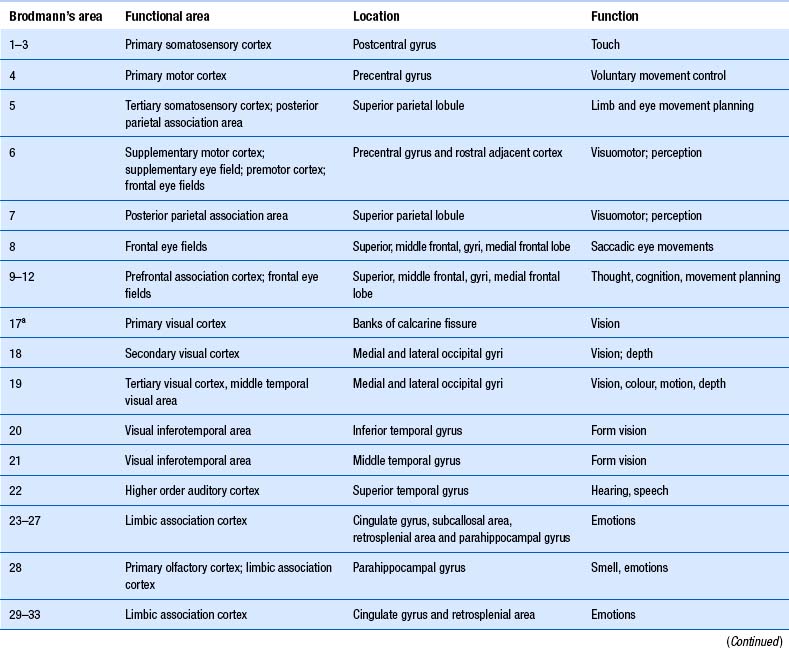
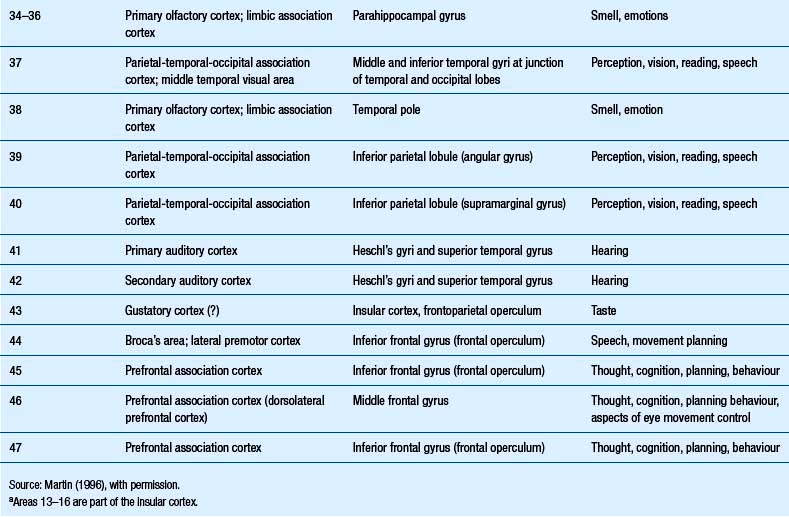
The structure of the cortex is laminar in nature, with six distinct layers present throughout most of the cortex. The thickness, number of cells, and predominant cell types in each layer vary over different areas of cortex. Listed from most superior to most inferior, these layers are the molecular layer, the external granular layer, the internal pyramidal layer, the internal granular layer, the ganglionic layer, and the fusiform or multiform layer (Fig. 9.11). The molecular layer or layer 1 is the most superior layer of the cortex. It contains the cell bodies of neuroglial cells, and axons and dendrites of neurons from deeper layers of cortex. The external granular layer or layer 2 is very dense and contains small granular cells and small pyramidal cells that project to neurons in other levels of cortex. The external or medial pyramidal layer, layer 3, contains pyramidal cells arranged in row formation. A variety of neurons projecting axons to other layers of cortex which form the association projections arise from this layer. The internal granular layer, layer 4, is thin, but its cell structure is the same as that of the external granular layer. The ganglionic layer or internal pyramidal layer, level 5, contains small granular cells, large pyramidal cells, and the cell bodies of some association fibres. The association fibres that originate here form two large tracts, the Bands of Baillarger and Kaes Bechterew. The neurons of this layer project to subcortical structures other than the thalamus including the basal ganglia, the midbrain, and the spinal cord. The fusiform layer is also known as the multiform layer, layer 6; neurons in this layer primarily project to the thalamus. All layers are present in all parts of the cortex. However, they do not have the same relative density in all areas. Depending upon the function of a particular area, some of these layers will be thicker than others in that location. The most common classification scheme used to differentiate areas of cortex based on structural and functional differences is that composed by Korbinian Brodmann in 1909. Based on microscopic evaluations of the cortex he divided the cortex into 52 different cytoarchitecturally different areas, known as Brodmann areas (Fig. 9.12). The cytoarchitectural divisions described by Brodmann have been shown to match quite closely to the functional output areas of the cortex. Some of these are outlined in Table 9.1.
The motor cortex
Classification of cortical neurons
1. Interneurons ○ Neurons that have axons that do not leave the cortex.
The motor cortex is located anterior to the central sulcus of Rolando and continues medially into the paracentral lobule. The primary area of motor cortex is Brodmann’s area 4 and is in the precentral gyrus. The motor cortex is somatotopically organised so that areas in the cortex correspond to areas of the body. These connections are depicted by the motor homunculus of man. The amount of tissue in the precentral gyrus dedicated to the innervation of a particular part of the body is proportional to the amount of motor control needed by that area, not just its physical size on the body. For example, much more of the motor strip is dedicated to the control of the fingers than to the legs even though the legs are much larger in physical mass of the body. Monosynaptic connections with ventral horn neurons are important for individuated finger movements. Indirect connections with interneurons are important for controlling larger groups of muscles in behaviours such as reaching and walking. Motor activity is modulated by a continuous stream of tactile, visual, and proprioceptive information, which arrives via the thalamus, needed to make voluntary movement both accurate and properly sequenced. Motor association areas are also modulated by the cerebellum and basal ganglia, which then project to the primary motor areas.
Functional projections of the motor cortex
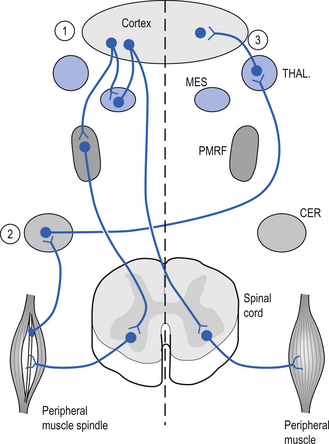
The motor cortex projects ipsilaterally to the reticular formation of the mesencephalon and the neostriatum of the basal ganglion where activation of glutaminergic neurons produces excitation. Reciprocal projections between the mesencephalon and the cortex ensure that the cortex will receive stimulation whenever the mesencephalon is excited. The cortex also projects to the ipsilateral pontomedullary reticular formation (PMRF) and the contralateral cerebellum via the pontine nuclear groups and the pontine reticular formation. Excitation of the PMRF results in a number of functional activities including an increase in activation of the ipsilateral gamma motor neurons that result in an increase in sensitivity of ipsilateral muscle spindle fibres. This results in an increased feedback to the contralateral cortex via the cerebellum and thalamus. This functional circuit can be utilised to stimulate areas of contralateral cortex clinically (Fig. 9.13). The mesencephalon and basal ganglia are sometimes referred to as areas of singularity. This means that there are fewer sources of integration than in other areas like the PMRF, and changes in frequency of firing (FOF) may have a more profound impact on the function of these areas of the nervous system. Decreased cortical activity can lead to a lack of modulation of primitive behaviour that originates in the mesencephalic motor centres and mesolimbic circuits, which is referred to as a release phenomenon. Writer’s cramp, spasmodic torticollis, and facial tics are all conditions that may be caused by defects in basal ganglionic circuits and unchecked responses originating in the mesencephalon or cerebral cortex. Another example is the impulsive behaviour of children who have been diagnosed with ADHD and the inability of their brain to inhibit irrelevant signals through corticostriatothalamic circuits. These children are functioning at a more subcortical level (Melillo & Leisman 2004).
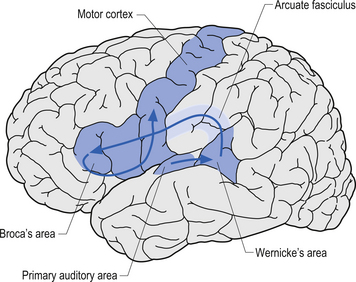
Broca’s area is found on the inferior third frontal gyrus in the hemisphere dominant for language. This area is involved in the coordination or programming of motor movements for the production of speech sounds. While it is essential for the execution of the motor movements involved in speech, it does not directly cause movement to occur. The firing of neurons here does not generate impulses for motor movement; that is the function of neurons in the motor strip. The neurons in Broca’s area generate motor programming patterns when they fire. This area is also involved in syntax, which involves the ordering of words in speech. Injuries to Broca’s area may cause apraxia or Broca’s aphasia (Fig. 9.14).
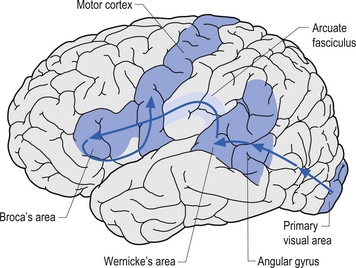
The angular gyrus lies near the superior edge of the temporal lobe, immediately posterior to the supramarginal gyrus. It is involved in the recognition of visual symbols. This area may be one of the most important cortical areas of speech and language and may act as the master integration centre for all other association cortices. The angular gyrus is also a very human portion of the brain, as it is not found in non-human species. Fibres of many different types travel through the angular gyrus, including axons associated with hearing, vision, and the meaning of these stimuli to the individual at any given moment. The arcuate fasciculus, the groups of fibres connecting Broca’s area to Wernicke’s area in the temporal lobe, also projects and receives projections from this area. The following disorders may result from damage to the angular gyrus in the hemisphere dominant for speech and language: anomia, which is difficulty with word-finding or naming; alexia with agraphia, which is difficulty with reading and writing; left–right disorientation, the inability to distinguish right from left; finger agnosia, which is the lack of sensory perceptual ability to identify by touch; and acalculia which refers to difficulties with arithmetic (Fig. 9.15).
The cortex receives axons from four major transmitter-dependent projection systems
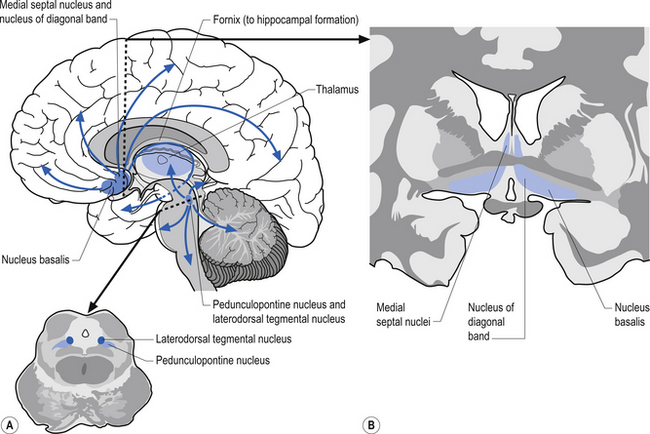
The cholinergic projection system consists of three different neuron pools that project to different functional areas. Two of the groups project axons directly to cortical areas and the third group projects to the cortex indirectly through the thalamus. The first group of neurons is located in the basal forebrain in a nuclear group referred to as the nucleus basalis of Meynert. This nuclear group contains neurons that project cholinergic axons directly to widespread areas of cortex. The second group of neurons project almost exclusively to the hippocampal formation and arise from neurons in the medial septal nuclei and the nucleus of the diagonal band of Broca. The cholinergic activity of these two groups of neurons is usually facilitory in nature. The third group of cholinergic projection axons arises from neurons located in two areas of the pontomesencephalic region of the brainstem. The first group of neurons is located in the lateral portion of the reticular formation and periaqueductal grey areas in a nuclear group of neurons referred to as the pedunculopontine tegmental nuclei. The second group of neurons is located at the junction between the midbrain and pons referred to as the laterodorsal tegmental nuclei. Projection axons from both of these nuclear groups terminate in various nuclei, including the intralaminar nuclei of the thalamus. The postsynaptic thalamic neurons then project to widespread areas of cortex (Fig. 9.16).
Clinical tests indicating decreased dopamine function
• Decreased blink rate and loss of modulation of blink reflexes during glabellar tap reflex.
• Increased withdrawal reflexes.
• Altered modulation of pupillary tone with cognitive activity.
• Poor fixation and loss of visual stability.
• Anti-saccadic and OKN testing can reveal abnormalities in frontal and basal ganglionic circuits that reflect these behaviours.
• Turning behaviour (tendency to turn in a particular direction) is dependent on asymmetries in dopaminergic transmission. It is thought that individuals (and dogs!) have a tendency to turn away from the side of greater dopaminergic transmission.
• Slow-wave cortical responses (P300s) can also more objectively reveal abnormalities in EEG responses to sensory stimuli in a range of cognitive and mood disorders.
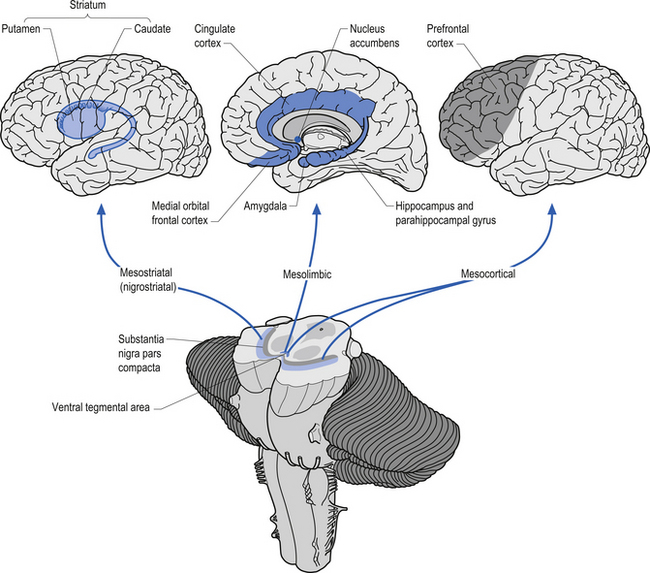
The dopaminergic projection system consists of three different neuron pools, the mesostriatal, the mesolimbic, and the mesocortical groups that project to different functional areas (Fig. 9.17). The mesostriatal group of neurons is located in the substantia nigra pars compacta of the midbrain and projects mainly to the caudate and putamen. Lesions to this pathway result in movement disorders such as Parkinson’s disease. Some evidence for the asymmetric distribution of dopamine in this projection system has been documented. The close association of dopamine and motor control has led to the speculation that dopamine should be more concentrated in the hemispheres dominating motor control. This is the left hemisphere for the majority of humans. Several studies have demonstrated that this is in fact the case (Rossor et al. 1980; Glick et al. 1982; Wagner et al. 1983). Other studies have demonstrated that factors related to dopamine metabolism and dopamine-specific activation of adenylate cyclase have also been asymmetrical with higher activity levels in the contralateral hemisphere to hand preference (Glick et al. 1983; Yamamoto & Freed 1984).
The mesolimbic projection pathway arises from neurons in the ventral tegmentum of the midbrain and projects to the medial temporal cortex, the amygdala, the cingulate gyrus, and the nucleus accumbens, all areas associated with the limbic system. Lesions or dysfunction of these projections is thought to contribute to the positive symptoms of schizophrenia, such as hallucinations.
The mesocortical projection pathway arises from neurons in the ventral tegmental and substantia nigral areas of the midbrain and terminates in widespread areas of prefrontal cortex. The projections seem to favour motor cortex and association cortical areas over sensory and primary motor areas (Fallon & Loughlin 1987). Dopaminergic neurons do not discharge in response to movement, but instead in relation to conditions involving probability and imminence of behavioural reinforcement and reward. Firing of reward neurons shifts from time of reward to presentation of the cue, or from unconditional to conditioned stimulus. This suggests that dopaminergic modulation is involved with higher integrative cortical functions and the regulation of cortical output activities (Clark et al. 1987). Damage or dysfunction in these projections may contribute to the cognitive aspects of Parkinson’s disease and the negative symptoms of schizophrenia. Clinical measures of dopamine activity can be very important in monitoring patients with disorders of dopamine function such as in movement disorders and schizophrenia.
Blink rate has been shown to be an accurate biophysical correlate of dopamine function (Gallois et al. 1985). A faster blink rate is observed in individuals who have higher dopaminergic output. A faster blink rate is also observed during visual and vestibular stimulation in individuals who have signs of vestibulocerebellar dysfunction. Decreased blink rate as demonstrated by the glabellar tap reflex and loss of modulation of blink reflexes can be an accurate sign of dopamine deficiency or dysfunction.
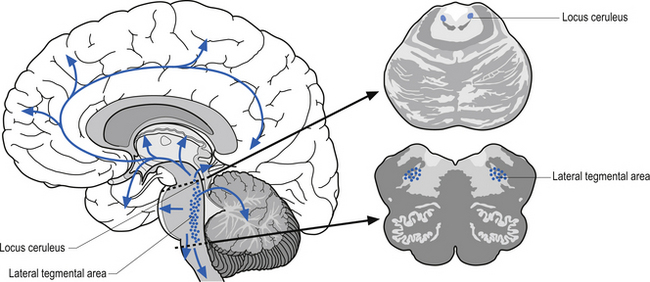
The noradrenergic projection system consists of neurons in two different locations in the rostral pons and the lateral tegmental area of the pons and medulla. The neurons in the rostral pons area are referred to as the locus ceruleus and together with the neurons in the lateral tegmental area of the pons and medulla project to all areas of the entire forebrain including the limbic areas as well as to the cerebellum, brainstem, and spinal cord (Fig. 9.18). The noradrenergic projection system seems to be involved in the cerebral regulation of arousal, attention-related functions, and adaptive responses of the individual to environmental stresses (Clark et al. 1987; Morilak et al. 1986). The noradrenergic system is also involved in the modulation of affective behaviour. Norepinephrine concentrations are decreased in some types of depression (see Chapter 16). This system is also involved in neuroimmuno regulation (see Chapter 15).
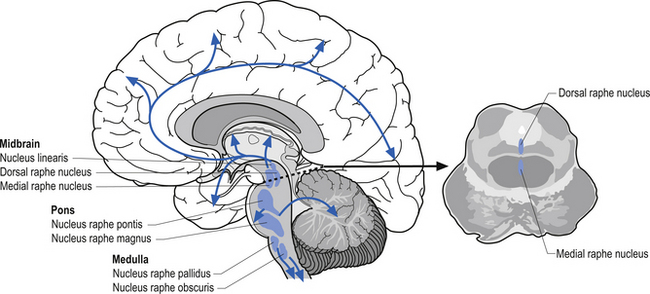
The serotinergic projection system consists of a group of nuclei in the midbrain pons and medulla referred to as the raphe nuclei and additional groups of neurons in the area postrema and caudal locus ceruleus. These nuclei can be divided into rostral and caudal groups. The rostral raphe nuclei project ipsilaterally via the median forebrain bundle to the entire forebrain where serotonin can act as either excitatory or inhibitory in nature, depending on the situation (Fallon & Loughlin 1987). The caudal raphe nuclei project to the cerebellum, medulla, and spinal cord (Fig. 9.19). Serotonin projection pathways are thought to play a role in a variety of psychological activities. Dysfunction of serotonin modulation can lead to depression, anxiety, obsessive-compulsive behaviour, aggressive behaviour, and eating disorders (Arora & Meltzer 1989; Spoont 1992). Serotonin activity has also been shown to be asymmetrical in nature with a predominance towards the right hemisphere (Arato et al. 1987, 1991; Demeter et al. 1989).
The nature of the above neurotransmitter projection systems seems to suggest that the transmitter activities follow the psychological asymmetrical distribution of cortical or hemispheric function. Neurotransmitters closely associated with up-regulation or down-regulation of autonomic or psychological arousal such as norepinephrine and serotonin are more concentrated in the right hemisphere, emphasising the well-known role of the right hemisphere in arousal. In contrast, neurotransmitters more closely associated with control of movement such as dopamine are more concentrated in the dominant movement hemisphere, which is on the left in the majority of people (Wittling 1998).
The parietal lobes
The parietal lobes provide a representation of external and intrapersonal space by integrating somatic, visual, and auditory evoked potentials from neighbouring lobes. The parietal areas are also an essential source of presynaptic inputs for frontal and limbic association areas and subcortical structures. Therefore, damage can lead to changes in cognition, mood, and behaviour just as a cerebellar or frontal lobe lesion can. The parietal lobes can be divided into superior and inferior functional areas. The superior parietal area is involved in visually guided action in the context of intact perception and awareness and the inferior parietal area is involved with visual perception and awareness. The angular gyrus and supramarginal gyrus of the inferior lobe may also be involved in the development of neglect syndromes. The somatosensory association area projects information to higher-order somatosensory association areas include parahippocampal, temporal association, cingulate cortices, and the premotor cortex where it is integrated for use in motor control, eye–hand coordination, memory-related tactile experience, and touch.
Somatic sensibility comprises a description of the nature of different types of afferent information. There are four major classes of somatic information: discriminative touch, proprioception, nociception, and temperature sense. There are two classes of somatic sensation, epicritic and protopathic, that are detected by encapsulated and unencapsulated receptors, respectively (see Chapter 5).
The temporal lobes
The temporal lobes are involved in the central processing of vision (ventral stream), hearing, smell, taste, and vestibular input and are also heavily involved in memory, behaviour, and emotion. The temporal lobe is inferior to the lateral fissure and anterior to the occipital lobe. It is separated from the occipital lobe by an imaginary line rather than by any natural boundary. The temporal lobe can be divided into three gyri, the superior, middle, and inferior, and by two sulci, the superior and inferior. It is also involved in semantics, or word meaning, as Wernicke’s area is located there. Wernicke’s area is located on the posterior portion of the superior temporal gyrus (see Fig. 9.14). In the hemisphere dominant for language, this area plays a critical role in the ability to understand and produce meaningful speech. A lesion here will result in Wernicke’s aphasia. Heschl’s gyrus, area 41, which is also known as the anterior transverse temporal gyrus, is the primary acoustic area. There are two secondary acoustic or acoustic association areas which make important contributions to the comprehension of speech. They are not completely responsible for this ability, however, as many areas, including Wernicke’s area, are involved in this process.
Kluver-Bucy syndrome
Temporal lobe lesions also produce tameness or hypo-emotionality, visual agnosia, and changes in dietary and sexual behaviour.
What does it mean?
• Anomia is a difficulty with word-finding or naming. Someone suffering from anomia can list the functions of an object and explain its meaning, but cannot recall its name.
• Alexia with agraphia refers to difficulties with reading and writing.
• Left–right disorientation is an inability to distinguish right from left.
• Finger agnosia or tactile agnosia is the lack of sensory perceptual ability to identify by touch.
The occipital lobes
The occipital lobe, which is the most posterior lobe, has no natural boundaries. It is involved in vision. The primary visual area is divided by the calcarine sulcus and receives input from the optic tract via the thalamus (see Fig. 9.15). The superior visual field is represented below the calcarine sulcus. The inferior visual field is represented above the calcarine sulcus. The visual-processing units in the visual cortex are composed of horizontal columns of neurons called hypercolumns with a variety of interneuron projections from surrounding horizontal neurons. Hypercolumns are the processing modules of all information about one part of the visual world. Columnar units are linked by horizontal connections within the same layer, particularly cells that respond to similar orientations of stimuli but belong to different receptive fields. The horizontal neuronal projections from horizontal interneurons are thought to mediate the ‘physiological fill-in effect’ and the ‘contextual effect’ whereby we evaluate objects in the context in which we see them.
Cortical asymmetry
Cerebral asymmetry (Hemisphericity)
The study of brain asymmetry, or hemisphericity, has a long history in the behavioural and biomedical sciences but is probably one of the most controversial concepts in functional neurology today. The fact that the human brain is asymmetric is fairly well established in the literature (Geschwind & Levitsky 1968; LeMay & Culebras 1972; Galaburda et al. 1978; Falk et al. 1991; Steinmetz et al. 1991). The exact relationship between this asymmetric design and the functional control exerted by each remains controversial.
Traditionally, the concepts of hemisphericity were applied to the processing of language and visuospatial stimuli. Today, the concept of hemisphericity has developed into a more elaborate theory that involves cortical asymmetric modulation of such diverse constructs as approach versus withdrawal behaviour, maintenance versus interruption of ongoing activity, tonic versus phasic aspects of behaviour, positive versus negative emotional valence, asymmetric control of the autonomic nervous system, and asymmetric modulation of sensory perception, cognitive, attentional, learning, and emotional processes (Davidson & Hugdahl, 1995).
Hemisphericity does not relate strictly to the handedness of the patient and there is poor correlation between handedness and eyedness – another measure of hemisphere-specific dominance. Classic symptoms of decreased left hemisphericity include depression and dyslexia, while decreased right hemisphericity can present with attention deficits and behavioural disorders. A variety of brain functions have been attributed to the right or left hemisphere; see Table 9.2. Autonomic asymmetries are an important indicator of cortical asymmetry as this reflects fuel delivery to the brain and the integrity of excitatory and inhibitory influences on sympathetic and parasympathetic function. Large projections from each hemisphere project to the ipsilateral PMRF with smaller projections to the mesencephalic RF. Therefore, other signs of altered PMRF or mesencephalic CIS may indicate hemisphericity. During tests of cerebellar function, slowness of movement in a limb (rather than breakdown of reciprocal actions) will often represent a decrease in cortical function – rather than a cerebellar cause. Of course, the two problems may coexist because of diaschisis occurring in hemisphericity. Therefore, cortical hemisphericity is often dependent on the presence of a series of findings related to subcortical output, fuel delivery, cognition, mood, and behaviour.
| Left hemisphere | Right hemisphere |
|---|---|
| Analytical—Assesses detail | Global—Assesses the big picture |
| Processes information in sequential or linear order | Processes information randomly or in variable order |
| Verbal processing | Visuospatial processing |
| Comprehension of words | Comprehension of tone, gestures, and body language |
| Motor and cognitive control of speech | Tone of voice and gestures |
| Plans an ordered response and reacts logically | Responds impulsively or emotionally |
| Mediates thought patterns based on fact and knowledge | Mediates thought patterns based on instinct and feelings |
| Mediates creativity | |
| Fine motor control and sensory processing | Gross motor control and spatial orientation |
| Prefers familiar environment | Responds to novel environments |
| Prefers processing high temporal and spatial frequency information (e.g. higher speed and detail) | Prefers processing low temporal and spatial frequency information (e.g. lower speed and detail) |
Asymmetrical autonomic functional considerations
Cardiovascular function
With respect to cortical control of cardiovascular function, several studies have demonstrated that asymmetries in brain function influence the heart through ipsilateral pathways. These studies have shown that stimulation or inhibition at various levels on the right side of the neuraxis results in greater changes in heart rate, while increased sympathetic tone on the left side results in a lowered ventricular fibrillation threshold (Lane et al. 1992). These finding have been explained by the fact that parasympathetic mechanisms appear to be dominant in the atria, while sympathetic mechanisms are dominant in the ventricles. Direct connections were traced between the sensorimotor cortex and the nucleus of tractus solitarius (NTS), dorsal motor nucleus of the vagus (DMV), and the rostral ventrolateral medulla (RVLM). These direct cortical projections to the NTS/DMV provide the anatomical basis for cortical influences on the baroreceptor reflex and cardiac parasympathetic control. These connections were also noted to have an ipsilateral predominance.
The preferential innervation of the sinoatrial node by the right vagus and the AV node by the left vagus might predict that parasympathetic effects of left hemisphere lesions would be expressed less strongly at the sinoatrial node than those of right hemisphere lesions (Barron et al. 1994). These alterations in heart rate may be due in part to an imbalance in relative descending influences of the right and left brain on autonomic outflow (Zamrini et al. 1990).
Measuring cortical hemisphericity
Best-hand test (Bilateral line-bisection test)
The determined midpoint of a horizontal line depends on the hemisphere that is dominantly activated. Which brain side estimate is delivered depends upon which hand is chosen as the messenger. The hand giving the most accurate estimate is driven by the most behaviourally predominant side of the brain. Thus, properly utilised, two-hand line-bisection can be another biophysical window on hemisphericity (Morton 2003a).
Conducting the line-bisection test
Type 20 staggered horizontal lines 1 cm apart on two vertical 215 × 280-mm pages. Line lengths differ by 10 mm from 70 to 160 mm (top to middle of page), and then in reverse order to the bottom of the page. Start by marking the midpoint of each line with your right hand and then with your left hand on the second page. Measure the distance (to 0.5 mm precision) from the true midpoint (include − (left) or + (right)) and tally each page independently. Divide totals by 20.
Spontaneous lateral eye movements
A certain level of cortical activity is necessary to prevent random or meaningless lateral saccadic activity of the eyes. When this movement dysfunction is present it can indicate a decreased cortical function (see Chapter 13).
Remembered saccades
Remembered saccades are controlled by the contralateral frontal cortical areas. Decreased functionality in these areas results in inaccurate saccades (see Chapter 13).
Tympanic temperature
Many studies have tried to link tympanic temperature to brain activation. The exact relationship is still controversial; however, it appears that as cortical activation levels increase the tympanic temperature decreases (Cherbuin & Brinkman 2004). This is due to the counter-current cooling mechanisms that exist in this system so that as carotid flow and thus activation increases, the temperature decreases.
Asymmetric autonomic responses
Blind spot sizes
Blind spot measurements can be an indication of asymmetrical cortical or thalamic activation.
Speech patterns
Speech is a complex process that involves a variety of cortical areas that must function in a cohesive manner for successful speech production. Alterations in these patterns in conjunction with other findings can suggest decreased hemispheric function.
Cognitive and behavioural testing
Various human functions have been attributed to certain hemispheric areas. Dysfunction in these areas can often be clinically observed and act as a guide to activation states of each hemisphere. (See Table 9.3 and questionnaire in Appendix 9.1.)
| Left hemisphere behaviour | Right hemisphere behaviour | |
|---|---|---|
| Names | Recognises | Faces |
| Verbal | Instructions | Visual or Kinesthetic |
| Inhibited | Emotions | Strong |
| Words | Meaning | Body Language |
| Logical | Thoughts/ideas | Humorous |
| Sequentially | Process informationProblem solving | Subjectively, patterns |
| Serious | Appeals | Playful |
| Details and facts | Reading/listening | Main idea, big picture |
| Systematic plans | Learning | Exploration |
| Well-structured | Assignments | Open-ended |
| Outline | Remembers | Summarise |
Appendix 9.1 Right or left brain-oriented? The asymmetry questionnaire
1. I tend to avoid talking about emotional feelings.
2. I am a strong starter of projects.
3. I break the whole into parts (reproductive– reductionistic).
4. I methodically solve problems by process of elimination.
5. I think and listen quietly, keep my talk to a minimum.
6. I am very good at knowing what others are thinking.
7. I am analytical (stay within the limits of the data).
8. I tend to be interdependent, open, public, and direct.
9. I dress for success and wear high-status clothing.
10. I need closeness and to talk things out when upset.
11. I do not praise others, nor need the praise of others.
12. I tend to be more interested in people and feelings.
13. I avoid seeking evaluation by others.
14. I feel my partner (or closest friend(s)) doesn’t talk or listen to me enough.
Source: Morton BE (2003b). Asymmetry questionnaire outcomes correlate with several hemisphericity measures. Brain and Cognition 51: 372–374, with permission.
Diffuse neuronal and axonal injuries involving the cortex
Severe human traumatic brain injury (TBI) may result in widespread damage to axons, termed diffuse axonal injury (DAI) (Olsson et al. 2004). DAI is one of the most common and important pathologic features of traumatic brain injury and can be caused from almost any type of head trauma ranging from direct blunt force trauma to the head, to the impact of coup/contra-coup injuries resulting from whiplash-type trauma. The result of these injuries to the head may develop into closed head injuries ranging from mild to severe.
DAI caused by mild closed head injury (CHI) is likely to affect the neural networks concerned with the planning and execution of a variety of cortical functions, one of which includes the sequences of memory-guided saccades. This dysfunction of saccadic activity is very sensitive and can be used to identify the presence of diffuse axonal and neuronal injury following head trauma. CHI subjects show more directional errors, larger position errors, and hypermetria of primary saccades and final eye position. No deficits are usually seen in temporal accuracy including timing and rhythm of the saccades (Heitger et al. 2002). Using saccadic testing combined with the history of head injury and any of the following symptoms which have been shown to often present with traumatic brain injury can give a fairly accurate estimation of the degree of axonal injury suffered by the patient:
• Central auditory pathway dysfunction involving tinnitus, hearing deficits, and hyperacusis;
• Deficits in facial emotion perception;
• Abnormal saccadic sequence control of remembered saccades; and
Brain trauma is accompanied by regional alterations of brain metabolism, reduction in metabolic rates, and possible energy crisis. Positron emission tomography (PET) for metabolism of glucose and oxygen reveals that traumatic brain injury leads to a state of persistent metabolic crisis as defined by an elevated lactate/pyruvate ratio that is not related to ischaemia (Vespa et al. 2005). These increases in lactate are typically more pronounced in patients with a poor outcome (Clausen et al. 2005).
Brain tissue acidosis is known to mediate neuronal death (Marion et al. 2002).
TBI profoundly disturbs cerebral acid–base homeostasis. The observed pH changes persist for the first 24 hours after the trauma. Brain tissue acidosis is associated with increased tissue PCO2 and lactate concentration. These pathobiochemical changes are more severe in patients who remain in a persistent vegetative state or die. In brain tissue adjacent to cerebral contusions or underlying subdural haematomas, even brief periods of hyperventilation, which decreases pH and increases CO2 and lactate concentrations, can significantly increase extracellular concentrations of mediators of secondary brain injury. These hyperventilation-induced changes are much more common during the first 24–36 hours after injury than at 3–4 days (Marion et al. 2002).
Over one million whiplash injuries occur in the USA every year. Neuropsychological disturbances are often reported in whiplash patients but are largely ignored because of their borderline nature. Patients often complain of headache, vertigo, auditory disturbances, tinnitus, disturbances in concentration and memory, difficulties in swallowing, impaired vision, and temporomandibular dysfunction (Spitzer et al. 1995). This syndrome has become known as ‘whiplash brain’.
Patients who had received a whiplash injury to the neck consistently showed evidence of hypoperfusion and hypometabolism in parieto-occipital regions of the brain (Otte et al. 1997). This was hypothesised to be due to DAI from acceleration forces or increases in spinotrigeminal nociceptive inputs from the cervical spine. Spinotrigeminal and vestibular afferents are capable of altering cerebral homodynamics and frequency of firing of monoaminergic neurons in the brainstem reticular formation.
The susceptibility of axons to mechanical injury appears to be due to both their viscoelastic properties and their high organisation in white matter tracts. Although axons are supple under normal conditions, they become brittle when exposed to rapid deformations associated with brain trauma. Accordingly, rapid stretch of axons can damage the axonal cytoskeleton, resulting in a loss of elasticity and impairment of axoplasmic transport. Subsequent swelling of the axon occurs in discrete bulb formations or in elongated varicosities that accumulate transported proteins (Smith et al. 2003).
Ultimately, swollen axons may become disconnected and contribute to additional neuropathologic changes in brain tissue. DAI may largely account for the clinical manifestations of brain trauma. However, DAI is extremely difficult to detect noninvasively and is poorly defined as a clinical syndrome. Future advancements in the diagnosis and treatment of DAI will be dependent on our collective understanding of injury biomechanics, temporal axonal pathophysiology, and its role in patient outcome (Henderson et al. 2005).
A growing body of evidence indicates that spondylotic narrowing of the spinal canal and abnormal or excessive motion of the cervical spine results in increased strain and shear forces that cause localised axonal injury within the spinal cord. During normal motion, significant axial strains occur in the cervical spinal cord. At the cervicothoracic junction, where flexion is greatest, the spinal cord stretches 24% of its length. This causes local spinal cord strain. In the presence of pathological displacement, strain can exceed the material properties of the spinal cord and cause transient or permanent neurological injury. Stretch-associated injury is now widely accepted as the principal aetiological factor of myelopathy in experimental models of neural injury, tethered cord syndrome, and DAI (Henderson et al. 2005).
Alzheimer’s disease (AD) is characterised by synaptic and axonal degeneration together with senile plaques (SP). SP are mainly composed of aggregated beta-amyloid, which are peptides derived from the amyloid precursor protein (APP). Apart from TBI in itself being considered a risk factor for AD, severe head injury seems to initiate a cascade of molecular events also associated with AD (Olsson et al. 2004).
Seizures and epilepsy
Simple partial seizures can occur in any area of the brain and the symptoms generated will depend on the area of the brain involved. The one common characteristic of a simple partial seizure is that consciousness is spared. The individual can recall the events before, during, and after the seizure and may be aware of the seizure activity itself. For example, a simple partial seizure involving the right motor cortex may produce a slight twitching of the left hand. This twitching is referred to as a positive symptom because it increases activity. However, a simple partial seizure in the left frontal lobe in the area of Broca may result in impaired speech. This is referred to as a negative symptom because activity is impaired. The time that the seizure is actually taking place is referred to as the ictal period and the time period immediately following the seizure is the postictal period (Wyllie 1993).
Complex partial seizures result in a disruption in consciousness, most probably because of interference with the reticular activation system in the brainstem or because of widespread areas of cortical involvement. Complex partial seizures may occur in any area of the brain but most commonly occur in the temporal lobe. Although there are multiple causes for temporal lobe epilepsy, the most common form is referred to as mesial temporal lobe epilepsy syndrome (MTLE) or limbic epilepsy (Engel 1993). It is common for people with MTLE to experience an aura prior to the complete onset of seizure activity. The aura may manifest as an order or mental sensation such as déjà vu or as repetitive motor tasks such as lip smacking or petting motions of the hands. These types of repetitive tasks are referred to as automatisms. With MTLE, the ipsilateral basal ganglia are commonly also involved and can result in contralateral dystonias or immobility. Postictal recovery may take from minutes to hours and may include confusion, amnesia, agitation, tiredness, aggression, or depression.
Alzheimer’s disease
• Alzheimer’s disease is the most common progressive degenerative brain disease in the elderly.
• Alzheimer’s disease is characterised by synaptic and axonal degeneration together with senile plaques (SP). SP are mainly composed of aggregated beta-amyloid (Aβ), which are peptides derived from the amyloid precursor protein (APP).
• Apart from TBI in itself being considered a risk factor for AD, severe head injury seems to initiate a cascade of molecular events also associated with Alzheimer’s disease.
Alzheimer’s disease
Alzheimer’s disease is a progressive degenerative brain disease. It is the most common cause of dementia in the elderly. The prevalence of Alzheimer’s disease increases rapidly over the age of 65 when the prevalence is about 1% to the age of 85 were the prevalence is about 40% (Blumenfeld 2002). The clinical symptoms of Alzheimer’s include:
Initially, only the recent memory is affected and long-term memory is spared. Individuals can actually perform quite well even as the disease has advanced considerably by maintaining a consistent and non-variable routine, or as quite often occurs a family member will cover up the progressively more frequent lapses of memory and cognition. Inevitably, the symptoms progress to the point where the individual starts to experience difficulty with the tasks of daily living even in their routine and with family support (McKhann et al. 1984).
The neuropathology of the disease is the formation of neuritic plaques and neurofibrillary tangles. The neuritic plaques are composed of an insoluble protein called beta-amyloid and apolipoprotein E, which is enveloped in a cluster of abnormal axons and dendrites called dystrophic neurites. The neurofibrillary tangles are composed of intracellular accumulations of hyperphosphorylated microtubule associated proteins or paired helical proteins referred to as tau proteins (Blumenfeld 2002). Severe head injury seems to initiate a cascade of molecular events also associated with the development of neurofibrillary tangles and neuritic plaques and thus Alzheimer’s disease.
References
Davidson R.J., Hugdahl K. Brain Asymmetry. Cambridge, MA/London: MIT Press, 1995.
Engel J., editor. Surgical Treatment of the Epilepsies. New York: Raven, 1993.
Martin J.H. Neuroanatomy Text and Atlas. New York: McGraw-Hill, 1996.
Pryor D. Common CNS infections. New Ethicals. 32(11), 1995. pp. 32
Wyllie E. The Treatment of Epilepsy; Principles and Practice. Philadelphia: Lea & Febiger, 1993.