The Classification of Ocular Surface Transplantation
Introduction
The ocular surface of the eye is a complex functional unit consisting of several elements that all interrelate. These include the eyelids, lubrication, conjunctiva and cornea. Homeostasis of the ocular surface is vital for the maintenance of good corneal epithelium, which in turn ensures good corneal clarity and vision. Lids provide protection to the ocular surface, as well as distribution of tears through a wiper-type action picking up tears from the lower lid meniscus and distributing this across the corneal surface. Lubrication is important in terms of content, osmolality and quantity.1 Normal conjunctiva provides mucins from goblet cells along with cytokines and the corneal limbus has been recognized and accepted as the source of limbal stem cells for the replacement of normal corneal phenotype.2–3
Numerous techniques to rehabilitate the ocular surface have developed over the last two decades. Rehabilitation of the ocular surface includes improving the ocular surface environment and, in particular, ensuring control of inflammation, good lubrication, lid closure and elimination of keratinization and symblephara. Restoration of the normal corneal phenotype and appropriate corneal clarity is highly dependent on a good environment.4 A number of transplantation techniques have been employed over the years, and many have been described with various terminology, including autologous and allograft conjunctival transplantation,5–7 keratoepithelioplasty,8 homotransplantation of limbal cells,9 limbal transplantation,10 and autologous11 and allograft limbal transplantation.12–15 These terms are not always clear in terms of tissue source (auto- or allogeneic) and precise anatomic location. Limbal transplantation, for instance, can be conjunctival alone or corneoscleral.16 Additionally, in the last 15 years tissue engineered techniques have become more popular and include culture and expansion of presumed stem cells and transplantation back to the host or to another recipient.17–27
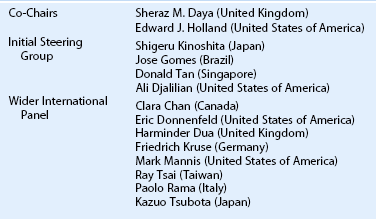
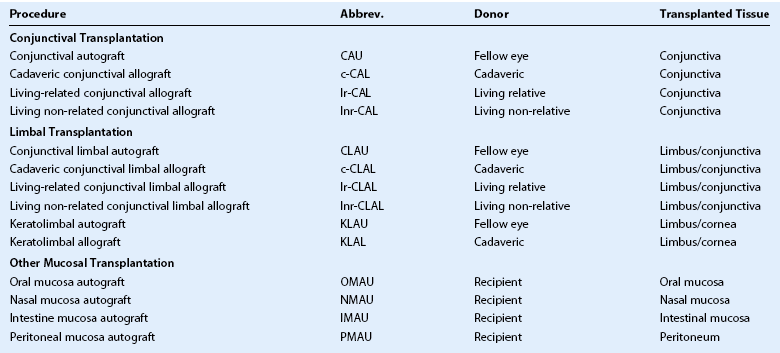
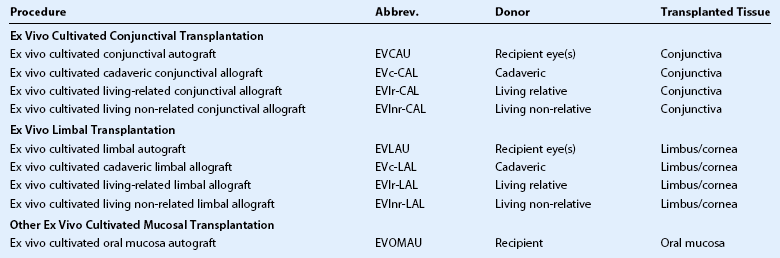
Clarity of communication is necessary as is the ability to accurately compare outcomes of these innovative procedures. Holland and Schwartz, recognizing the need for common terminology, provided a rationale for common nomenclature illustrating a variety of techniques described in the literature, often using similar terminology.16 The authors proposed a classification based on: (1) anatomic source of the tissue and (2) genetic source (autologous, allogeneic and living related). Since the publication in1996, further developments have resulted in the description of more techniques, as well as new sources of tissue28–35 along with the introduction of cell culture techniques.36–44 In order to include all current techniques and procedures, the Cornea Society felt there was a need for an internationally agreed nomenclature. This nomenclature was established by an international group of cornea surgeons involved in ocular surface transplantation through an initial steering committee (Table 39.1) and once ratified by the Board of the Cornea Society was published in Cornea.45 A literature search was performed to ascertain types of ocular surface rehabilitative procedures reported for which inclusion in the new nomenclature. The committee agreed to expand on the principles initially proposed by Holland and Schwartz in 1996.16 The nomenclature was based on the following criteria: (1) anatomic source of the tissue being transplanted, (2) genetic source – autologous or allogeneic and to reflect histocompatability in the latter group, whether living-related or not, and (3) cell culture techniques. Types of procedures were broadly categorized by the anatomic type, source, and whether it was tissue engineered (Box 39.1). Further categorization according to anatomic type of tissue, namely conjunctival, limbal and other mucosal grafts is listed in Table 39.2. Tissue engineered procedures are listed in Table 39.3 and classified according to anatomic source of tissue.
Anatomic Type
The principle anatomic sources of tissue for ocular surface rehabilitative procedures are either conjunctiva or limbus. The use of peritonea46–48 and rectal mucosa28 as sources of cells have been described, requiring the inclusion of a third category of ‘other mucosal’ tissue. Conjunctival tissue is increasingly recognized as an important contributor to the welfare of the ocular surface and felt to be necessary in cases where there is concomitant conjunctival deficiency.49 The provision of mucins from goblet cells, as well as cytokines, contributes to the milieu and equilibrium of the ocular surface. Conjunctival tissue is not to be confused with limbal conjunctival tissue and is confined to bulbar and forniceal conjunctiva only. There have been reports suggesting the fornix as a source of conjunctival stem cells and there may be some theoretical advantage of using fornix over bulbar conjunctiva; however, as this has not been demonstrated scientifically, the committee chose not to further classify the source of conjunctiva.50
Other mucosal tissue, including buccal,29,30 nasal,33,34 rectal28 and peritoneal46–48 have been used to populate the palpebral conjunctiva and recreate the fornix. Autologous buccal mucosa has also been used with good success in tissue engineering techniques.31,32,35
Source
Histocompatibility is an important parameter that influences graft success.7 Autologous tissue, if such a source is available, is the best source; however, it is not possible in bilateral disease. The next best source of tissue is a best-matched living relative or, at a minimum, a parent or offspring, in which case half the haplotype will be in common.51,52 Non-related tissue can be either living or cadaveric and viability of the latter often depends on the preservation technique used.53 Attempts to ensure better histocompatibility by tissue marching can be performed and a non-related living donor can be used. The source of tissue that is used (autologous, living-related or cadaveric) is included in the nomenclature for both the tissue transplantation and tissue engineered sections (see Tables 39.2 and 39.3).
Tissue Engineered Grafts
Tissue engineered grafts are a more recent and exciting addition to the armamentarium of ocular surface procedures. A number of reports have demonstrated the value of ‘ex vivo’ cell cultivation techniques.17–27,36–44 Theoretical advantages include a large volume of cells without the inclusion of highly antigenic tissue as a carrier. Additionally, the loss of antigen-presenting cells further decreases the chance of acute and chronic immune rejection.26 Similar anatomic origins of tissue, as well as genetic sources (autologous, living related, non-related and cadaveric) are used in the nomenclature.
Human amniotic membrane has been used as a substrate for epithelial growth36,54 or tissue filler,55,56 as well as a biologic dressing.57–62 Although discussed by the steering committee, amniotic membrane was not included in the nomenclature. The importance of the use of amniotic membrane as an adjuvant in ocular surface rehabilitation is acknowledged; however, it is not included in the nomenclature, as no donor cells are contributed. In tissue engineering techniques, there have been reports of use of amniotic membrane as a substrate for growth,21–23,25–27,38 and others have used other substrates24,39–43 or none at all.35,44 The influence of the carrier or growth substrate on outcomes has yet to be determined and in time this may require inclusion in the nomenclature.
References
1. Tsubota, K, Tseng, SCG, Nordlund, ML. Anatomy and physiology of the ocular surface. In: Holland EJ, Mannis MJ, eds. Ocular surface disease medical and surgical management. New York: Springer-Verlag; 2002:3–15.
2. Davanger, M, Evensen, A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561.
3. Schermer, A, Galvin, S, Sun, TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62.
4. Dilly, PN. Structure and function of the tear film. Adv Exp Med Biol. 1994;350:239–247.
5. Thoft, RA. Conjunctival transplantation. Arch Ophthalmol. 1977;95:1425–1427.
6. Vastine, DW, Stewart, WB, Schwab, IR. Reconstruction of the periocular mucous membrane by autologous conjunctival transplantation. Ophthalmology. 1982;89:1072–1081.
7. Kwitko, S, Raminho, D, Barcaro, S, et al. Allograft conjunctival transplantation for 8 bilateral ocular surface disorders. Ophthalmology. 1995;102:1020–1025.
8. Thoft, RA. Keratoepithelioplasty. Am J Ophthalmol. 1984;97:1–6.
9. Pfister, RR. Corneal stem cell disease; concepts, categorization, and treatment by auto- and homotransplantation of limbal stem cells. CLAO J. 1994;20:64–72.
10. Jenkins, C, Tuft, S, Liu, C, et al. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye. 1993;7:629–633.
11. Kenyon, KR, Tseng, SCG. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722. [discussion 722–3].
12. Tsai, RJ, Tseng, SCG. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13:389–400.
13. Tsubota, K, Toda, I, Saito, H, et al. Reconstruction of the corneal epithelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995;102:1486–1496.
14. Holland, EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996;19:677–743.
15. Croasdale, CR, Schwartz, GS, Malling, JV, et al. Keratolimbal allograft: recommendations for tissue procurement and preparation by eye banks, and standard surgical technique. Cornea. 1999;18:52–58.
16. Holland, EJ, Schwartz, GS. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea. 1996;15:549–556.
17. Schrader, S, Notara, M, Beaconsfield, M, et al. Tissue engineering for conjunctival reconstruction: established methods and future outlooks. Curr Eye Res. 2009;34:913–924.
18. Vemuganti, GK, Kashyap, S, Sangwan, VS, et al. Ex-vivo potential of cadaveric and fresh limbal tissues to regenerate cultured epithelium. Ind J Ophthalmol. 2004;52:113–120.
19. Shortt, AJ, Secker, GA, Notara, MD, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52:483–502.
20. Daya, SM, Watson, A, Sharpe, JR, et al. Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology. 2005;112:470–477.
21. Grueterich, M, Espana, EM, Touhami, A, et al. Phenotypic study of a case with successful transplantation of ex vivo expanded human limbal epithelium for unilateral total limbal stem cell deficiency. Ophthalmology. 2002;109:1547–1552.
22. Koizumi, N, Rigby, H, Fullwood, NJ, et al. Comparison of intact and denuded amniotic membrane as a substrate for cell-suspension culture of human limbal epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2007;245:123–134.
23. Pellegrini, G, Traverso, CE, Franzi, AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993.
24. Rama, P, Bonini, S, Lambiase, A, et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72:1478–1485.
25. Sangwan, VS, Vemuganti, GK, Singh, S, et al. Successful reconstruction of damaged ocular outer surface in humans using limbal and conjunctival stem cell culture methods. Biosci Rep. 2003;23:169–174.
26. Schwab, IR, Reyes, M, Isseroff, RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–426.
27. Shimazaki, J, Aiba, M, Goto, E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1285–1290.
28. Mahatme, VH. Rectal mucous membrane graft for dry eye syndrome. Ind J Ophthalmol. 1999;47:129–131.
29. Hosni, FA. Repair of trachomatous cicatricial entropion using mucous membrane graft. Arch Ophthalmol. 1974;91:49–51.
30. Shore, JW, Foster, CS, Westfall, CT, et al. Results of buccal mucosal grafting for patients with medically controlled ocular cicatricial pemphigoid. Ophthalmology. 1992;99:383–395.
31. Nakamura, T, Inatomi, T, Sotozono, C, et al. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88:1280–1284.
32. Inatomi, T, Nakamura, T, Koizumi, N, et al. Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol. 2006;141:267–275.
33. Kuckelkorn, R, Schrage, N, Redbrake, C, et al. Autologous transplantation of nasal mucosa after severe chemical and thermal eye burns. Acta Ophthalmol Scand. 1996;74:442–448.
34. Wenkel, H, Rummelt, V, Naumann, GO. Long-term results after autologous nasal mucosal transplantation in severe mucus deficiency syndromes. Br J Ophthalmol. 2000;84:279–284.
35. Nishida, K, Yamato, M, Hayashida, Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196.
36. Meller, D, Dabul, V, Tseng, SCG. Expansion of conjunctival epithelial progenitor cells on amniotic membrane. Exp Eye Res. 2002;74:537–545.
37. Ang, LP, Sotozono, C, Koizumi, N, et al. A comparison between cultivated and conventional limbal stem cell transplantation for Stevens–Johnson Syndrome. Am J Ophthalmol. 2007;143:178–180.
38. Tsai, RJ, Li, LM, Chen, JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93.
39. Rama, P, Matuska, S, Paganoni, G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:174–175.
40. Nishida, K, Yamato, M, Hayashida, Y, et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77:379–385.
41. Benhabbour, SR, Sheardown, H, Adronov, A. Cell adhesion and proliferation on hydrophilic dendritically modified surfaces. Biomaterials. 2008;29:4177–4186.
42. Deshpande, P, Notara, M, Bullett, N, et al. Development of a surface modified contact lens for transfer of cultured limbal epithelial cells for ocular surface diseases. Tissue Eng Part A. 2009;15:2889–2902.
43. Notara, M, Bullet, NA, Deshpande, P, et al. Plasma polymer coated surfaces for serum-free culture of limbal epithelium for ocular surface disease. J Mater Sci Mater Med. 2007;18:329–338.
44. Ang, LP, Tan, DT, Cajucom-Uy, H, et al. Autologous cultivated conjunctival transplantation for pterygium surgery. Am J Ophthalmol. 2005;139:611–619.
45. Daya, SM, Chan, CC, Holland, EJ. Cornea Society Nomenclature for Ocular Surface Rehabilitative Procedures. Cornea. 2011;30:1115–1119.
46. Nath, K, Shukla, BR, Nema, HV, et al. Auto-peritoneum as a conjunctival substitute. Ind J Ophthalmol. 1964;12:75–81.
47. Allen, JH. The use of peritoneum as a substitute for conjunctiva in plastic surgery: a preliminary report. Am J Ophthalmol. 1953;36:1249–1252.
48. Malhotra, M. Plastic repair of conjunctiva with peritoneum transplantation. Br J Ophthalmol. 1957;41:616–621.
49. Kruse, FE. Classification of ocular surface disease. In: Holland EJ, Mannis MJ, eds. Ocular surface disease medical and surgical management. New York: Springer-Verlag; 2002:16–36.
50. Wei, ZG, Cotsarelis, G, Sun, TT, et al. Label-retaining cells are preferentially located in fornical epithelium: implications on conjunctival epithelial homeostasis. Invest Ophthalmol Vis Sci. 1995;36:236–246.
51. Scocco, S, Kwitko, S, Rymer, S, et al. HLA-matched living-related conjunctival limbal allograft for bilateral ocular surface disorders: long-term results. Arq Bras Oftalmol. 2008;71:781–787.
52. Daya, SM, Ilari, FA. Living related conjunctiva limbal allograft for the treatment of stem cell deficiency. Ophthalmology. 2001;108:126–133. [discussion 133–4].
53. Croasdale, CR, Schwartz, GS, Malling, JV, et al. Keratolimbal allograft: recommendations for tissue procurement and preparation by eye banks, and standard surgical technique. Cornea. 1999;18:52–58.
54. Tseng, SCG, Prabhasawat, P, Barton, K, et al. Amniotic membrane transplantation with or without limbal allografts for cornea surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–441.
55. Lee, SH, Tseng, SCG. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123:303–312.
56. Paridaens, D, Beekhuis, H, van Den Bosch, W, et al. Amniotic membrane transplantation in the management of conjunctival malignant melanoma and primary acquired melanosis with atypia. Br J Ophthalmol. 2001;85:658–661.
57. Shimazaki, J, Yang, HY, Tsubota, K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997;104:2068–2076.
58. Azuara-Blanco, A, Pillai, CT, Dua, HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol. 1999;83:399–402.
59. Honovar, SG, Bansal, AK, Sangwan, VS, et al. Amniotic membrane transplantation for ocular surface reconstruction in Stevens-Johnson syndrome. Ophthalmology. 2000;107:975–979.
60. Gomes, JA, dos Santos, MS, Cunha, MC, et al. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology. 2003;110:466–473.
61. Solomon, A, Espana, EM, Tseng, SCG. Amniotic membrane transplantation for reconstruction of the conjunctival fornices. Ophthalmology. 2003;110:93–100.
62. Barabino, S, Rolando, M, Bentivoglio, G, et al. Role of amniotic membrane transplantation for conjunctival reconstruction in ocular cicatricial pemphigoid. Ophthalmology. 2003;110:474–480.