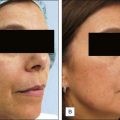
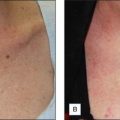
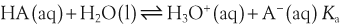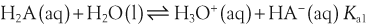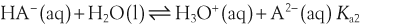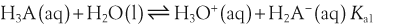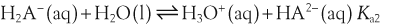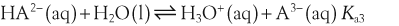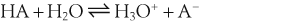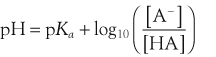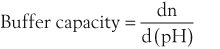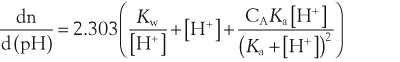1 The Chemistry of Peels
A Hypothesis of Action Mechanisms and a Proposal of a New Classification of Chemical Peelings
Useful Elements of Basic Chemistry
• Acids
Chemicals or substances having the property of an acid are said to be acidic (adjective).
• Polarity and the inductive effect
The polarity of the H—A bond is the first factor contributing to the acid strength.
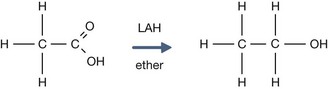
The pKa for ethanol is 16, compared to 4.76 for acetic acid.
• Atomic radius and bond strength
The size of the atom A or atomic radius is the second factor contributing to the acid strength.
• Chemical characteristics
• Buffer solution
Le Chatelier’s Principle
In a solution there is an equilibrium between a weak acid, HA, and its conjugate base, A−:
Henderson–Hasselbach Equation
The acid dissociation constant for a weak acid, HA, is defined as:
One should remember that the calculated pH may be different from measured pH.
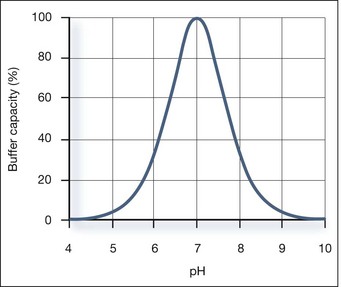
Buffer Capacity
Buffer capacity is a quantitative measure of the resistance of a buffer solution to pH change on addition of hydroxide ions. It can be defined as follows:
where dn is an infinitesimal amount of added base and d(pH) is the resulting infinitesimal change in pH. With this definition the buffer capacity can be expressed as:
where Kw is the self-ionization constant of water and CA is the analytical concentration of the acid, equal to [HA]+[A−]. The term Kw/[H+] becomes significant at pH greater than about 11.5 and the second term becomes significant at pH less than about 2. Both these terms are properties of water and are independent of the weak acid. Considering the third term, it follows that:
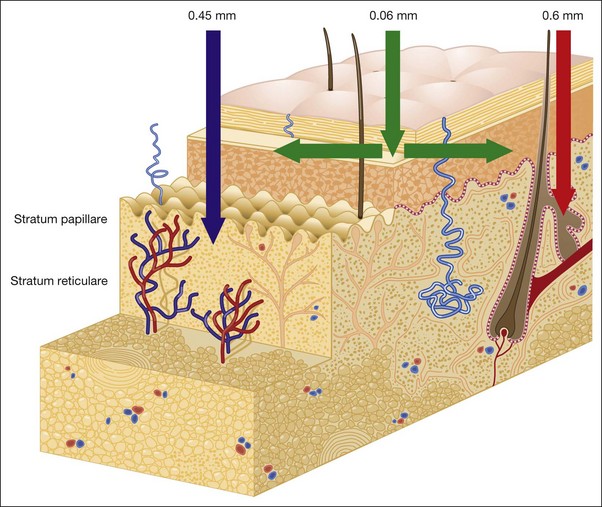
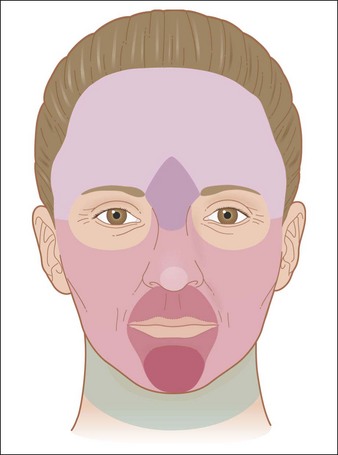
Anatomy of the Skin
• Skin basic chemistry
Basic Chemistry of the Most Used Molecules in Solutions for Chemical Peelings
Substances with Mainly Metabolic Activity
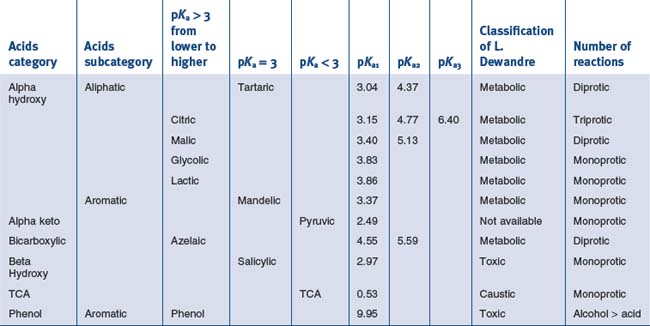
• Alpha hydroxy acids
Comparison of Their pH to Their pKa, the cosmetic actions of the ahas are interesting
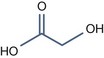
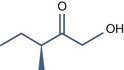
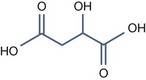
• Aliphatic alpha hydroxy acids (glycolic, lactic, malic, tartaric, citric) with pKa > 3
Tartaric Acid (pKa1 = 3.04, pKa2 = 4.37)
This is derived from grape extract and is able of delivering the same benefits as the above peels.
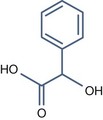
• Aromatic alpha hydroxy acid with pKa > 3
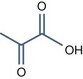
• Alpha keto acids with pKa < 3
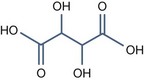
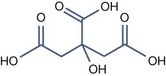
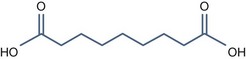
• Bi carboxylic acid with pKa > 3
Azelaic Acid (pKa1 = 4.550, pKa2 = 5.598)
It has some interesting properties:
• Beta hydroxy acid peels with pKa around 3
Substance with Mainly Caustic Activity
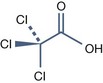
• Trichloroacetic acid peels
Trichloroacetic Acid (TCA) (pKa = 0.54)
UN 1839 is required to transport it because of its corrosive activity.
The packaging has to be unbreakable, if breakable put into closed unbreakable container.
It is preferable to keep the TCA peels solutions in opaque glass bottles.
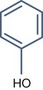
Substances with Mainly Toxic Activity
Phenol ((pKaphoh2+/phoh) – 6.4 (pKaphoh/pho−) 9.95)
How the Most Commonly Used Substances in Chemical Peels Work – A Proposal for Classification
Allinger NL, Cava MP, de Jongh DC, et al. Chimie organique [Organic chemistry]. vols 1–3. Montreal: McGraw-Hill; 1990.
Arnaud P. Chimie organique – ouvrage d’initiation à la chimie organique. [Organic Chemistry – introductory work to organic chemistry]. Paris: Dunod; 1992.
Brody HJ. Chemical peeling. St Louis: Mosby; 1997.
Carlson BM. Integumentary, skeletal, and muscular systems. Human Embryology and Developmental Biology. St Louis: Mosby; 1994. pp 153–181
Demas PN, Bridenstine JB, Braun TW. Pharmacology of agents used in the management of patients having skin resurfacing. Journal of Oral and Maxillofacial Surgery. 1997;55:1255-1258.
De Rossi Fattaccioli D. Histological comparison between deep chemical peeling (modified Litton’s formulae) and ultra pulsed CO2 laser resurfacing. Dermatología Peruana. 2005;15:1.
Ebbing DD, Gammon SD. General chemistry, 8th edn. Boston: Houghton Mifflin; 2005.
Halaas YP. Medium depth peels. Facial Plastic Surgery Clinics of North America. 2004;12:297-303.
Hasselbach KA. Die Berechnung der Wasserstoffzahl des Blutes aus der freien und gebundenen Kohlensäure desselben, und die Sauerstoffbindung des Blutes als Funktion der Wasserstoffzahl. Biochemische Zeitschrift. 1917;78:112-144.
Henderson LJ. Concerning the relationship between the strength of acids and their capacity to preserve neutrality. American Journal of Physiology. 1908;21(4):173-179.
Hermitte R. Aged skin, retinoids and alpha hydroxyl acids. Cosmetics and Toiletries. 1992;107:63-67.
Holbrook KA. Embryology of the human epidermis. In: Vincent C, editor. Kelley’s practice of pediatrics. Hagerstown, Maryland: Harper and Row, 1980.
Holbrook KA. Structure and function of the developing human skin. In: Goldsmith LA, editor. Physiology, biochemistry, and molecular biology of the skin. New York: Oxford University Press, 1991.
Hornby M, Peach JM. Foundations of organic chemistry. Oxford: Oxford University Press; 1991.
Howard P, Meylan W, editors. Handbook of physical properties of organic chemicals. Boca Raton: CRC/Lewis Publishers, 1997.
Kolbe H. Liebig’s Annals of Chemistry. pp 115, 201. 1860.
Kortum G, Vogel W, Andrussow K. Dissociation constants of organic acids in aqueous solution. London: Butterworths; 1961. [Reprint of Pure and Applied Chemistry, vol 1(2,3), 1961]
Lagowski J, editor. Macmillan encyclopedia of chemistry. New York: Macmillan, 1997.
Lespiau R. La molécule chimique [Chemical molecule]. Paris: Félix Alcan; 1920. p. 285
Montagna W, Parakkal PF. The structure and function of skin, 3rd ed. New York: Academic Press; 1974.
Normant H, Normant J. Chimie organique [Organic chemistry]. Paris: Masson; 1968.
Pauwels. Les alpha-hydroxyacides en pratique dermatologique [The alpha-hydroxyacids in dermatological practice]. BEDC. 1994;2:437-453.
Pavia DL, Lampman GM, Kriz GS. Organic chemistry volume 1: Organic chemistry 351. Mason, OH: Cenage Learning; 2004.
Perrin DD. Dissociation constants of inorganic acids. London: Butterworths; 1969. [Reprint of Pure and Applied Chemistry, vol 20(2) 1969]
Po HN, Senozan NM. Henderson–Hasselbach equation: Its history and limitations. Journal of Chemistry Education. 2001;78:1499-1503.
de Levie R. The Henderson–Hasselbalch equation: Its history and limitations. Journal of Chemistry Education. 2003;80:146.
de Levie R. The Henderson Approximation and the mass action law of Guldberg and Waage. The Chemical Educator. 2002;7:132-135.
Resnick SS, Resnik BL. Complications of chemical peeling. Dermatology Clinics. 2005;13:309-312.
Rubin MG. Manual of chemical peels: superficial and medium depth. Philadelphia: Lippincott Williams & Wilkins; 1995.
Schmitt R. [no title found]. Journal fuer Praktische Chemie. 1885;31:397.
Solomons TWG. Organic chemistry, 3rd edn. New York: Wiley; 1984.
Streitwiezer A, Heathcock CH. Introduction to organic chemistry, 2nd ed. Macmillan; 1981.
Tenenbaum A 1999 Laserpeel. Longo L, Hoffstetter AG, Pascu ML (eds). Proceedings of the SPIE Laser Florence ’99: A Window on the Laser Medicine. 4166:169–179
Tenenbaum A 2009 La tecnica Endopeel-Vol: La medicina estetica- A.Redaelli- 2009 Ed SEE Firenze, pp 60–62, 71–73, 79, 227, 234
Tenenbaum A, Tiziani M (in press) The philosophy of synergy in rejuvenation’s techniques-Tecniche Endopeel-Tecniche per il lifting non chirurgico del viso e del corpo- Ed Evolution MD
Van Scott EJ, Yu RJ. Control of keratinization with alpha-hydroxy acids and related compounds. Archives of Dermatology. 1974;110:586-590.
Van Scott EJ, Yu RJ. Alpha-hydroxy-acids: procedures for use in clinical practice. Cutis. 1989;43:22-229.
Vollhardt KPC, Schore NE. Traité de chimie organique [Organic Chemistry Handbook]. Brussels: De Boeck-Wesmael; 1998. p. 1350
Zumdahl S, Zumdahl S. Chemistry, 8th edn. New York: Houghton Mifflin; 2009.

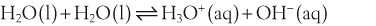
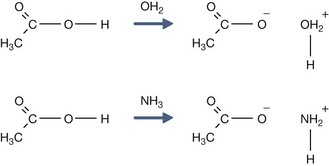
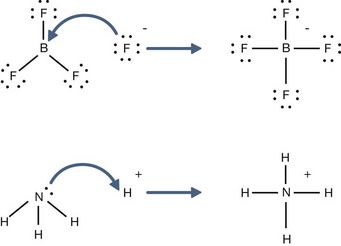
 H+ + A−, where HA represents the acid and A− is the conjugate base. Acid–base conjugate pairs differ by one proton, and can be interconverted by the addition or removal of a proton (protonation and deprotonation, respectively). Note that the acid can be the charged species and the conjugate base can be neutral in which case the generalized reaction scheme could be written as HA
H+ + A−, where HA represents the acid and A− is the conjugate base. Acid–base conjugate pairs differ by one proton, and can be interconverted by the addition or removal of a proton (protonation and deprotonation, respectively). Note that the acid can be the charged species and the conjugate base can be neutral in which case the generalized reaction scheme could be written as HA  H+ + A. In solution there exists an equilibrium between the acid and its conjugate base. The equilibrium constant K is an expression of the equilibrium concentrations of the molecules or the ions in solution. Brackets indicate concentration, such that [H2O] means the concentration of H2O. The acid dissociation constant Ka is generally used in the context of acid-base reactions. The numerical value of Ka is equal to the concentration of the products divided by the concentration of the reactants, where the reactant is the acid (HA) and the products are the conjugate base and H+.
H+ + A. In solution there exists an equilibrium between the acid and its conjugate base. The equilibrium constant K is an expression of the equilibrium concentrations of the molecules or the ions in solution. Brackets indicate concentration, such that [H2O] means the concentration of H2O. The acid dissociation constant Ka is generally used in the context of acid-base reactions. The numerical value of Ka is equal to the concentration of the products divided by the concentration of the reactants, where the reactant is the acid (HA) and the products are the conjugate base and H+.