Chapter 8. The cervical spine
CHAPTER CONTENTS
Summary181
Anatomy 181
The joints of the cervical spine 183
Intervertebral discs 184
Cervical spinal nerves 185
Cervical arteries 186
Differential diagnosis at the cervical spine 188
Mechanical cervical lesions 188
Other causes of head and neck pain, arm pain and associated signs and symptoms 189
Cervical artery dysfunction 193
Cervical lesions – a classification system of three clinical models 203
Clinical Model 1: acute torticollis 204
Clinical Model 2 204
Clinical Model 3: presenting with referred arm symptoms 204
Treatment of cervical lesions 205
SUMMARY
Safety is of paramount importance in the application of manual techniques to the cervical spine. As a contribution to safety, this chapter begins with a summary of the key points of cervical anatomy, highlighting structures involved in the pre-treatment testing procedures and the treatment techniques themselves.
Differential diagnosis and the elements of clinical examination will be discussed. Patients with a mechanical lesion, and thus suitable for the treatments subsequently described, will be identified. The contraindications to treatment will be emphasized and guidelines for safe practice will be given, since both are of vital importance.
ANATOMY
The spinal column is a series of motion segments, each of which consists of an interbody joint and its two adjacent zygapophyseal joints. The resultant bony canal is protective, but while the structural arrangement of the lumbar spine as a whole is suited to weight-bearing, movement and stability, the cervical spine is designed principally for mobility.
The cervical spine is the most mobile area of the spine and its wide range and combinations of movement are related to changes in the direction of vision, the positioning of the upper limbs and hands, and locomotion. It is also an area of potential danger as it gives bony protection to major blood vessels that supply the brain and the spinal cord (Taylor & Twomey 1994, Nordin & Frankel 2001, Kerry & Taylor 2006).
Its mobility is at the expense of stability and it has a close neurophysiological connection to the vestibular and visual systems. It can therefore be the source of a ‘plethora of symptoms’ (Kristjansson 2005).
Anatomically and functionally, the cervical spine can be divided into two segments. The upper segment consists of the atlas and the axis (C1 and C2). Its structure is designed for mobility, with approximately one-third of cervical flexion and extension and over half of axial rotation occurring at this level (Mercer & Bogduk 2001). The lower segment consists of the remaining cervical vertebrae (C3–C7), and contributes to overall mobility.
The atlas (C1) is composed of two lateral masses, supporting articular facets, and their joining anterior and posterior arches. The superior facets articulate with the head at the atlanto-occipital joints and their condylar shape facilitates nodding movements of the head (Netter 1987, Mercer & Bogduk 2001). The inferior facets articulate with the axis at the atlantoaxial joints where rotation is the principal movement.
The axis (C2) has broad superior articular facets which support the lateral masses of the atlas and which are responsible for bearing the axial load of the head and atlas, transmitting the load to the rest of the cervical spine. The axis supports the dens or odontoid process on its superior surface, the dens providing a pivot around which the atlas rotates at the synovial median atlantoaxial joint (Mercer & Bogduk 2001). There is no intervertebral disc between the atlas and axis.
The internal ligaments of the upper cervical segment are particularly important to its stability (Fig. 8.1). The tectorial membrane is a superior extension of the posterior longitudinal ligament that covers the dens and its ligaments, acting as protection for the junction of the spinal cord and the medulla. The transverse ligament of the atlas is a strong horizontal band with extensions passing vertically and horizontally from its midpoint to form a ligamentous complex called the cruciform ligament. This, together with the apical ligament of the dens, is responsible for keeping the dens in close contact with the atlas. Any instability in the upper cervical segment, e.g. as occurs with rheumatoid arthritis, trauma or Down’s syndrome, is an absolute contraindication to orthopaedic medicine techniques.
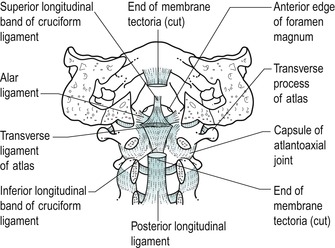 |
| Figure 8.1
Upper cervical internal ligaments.
From Functional Anatomy of the Spine by Oliver J and Middleditch A. Reprinted by permission of Elsevier Ltd.
|
The lower cervical segment consists of typical cervical vertebrae C3–C6 and the atypical C7 which is known as the vertebra prominens because of its long spinous process. A typical cervical vertebra consists of a small, broad, weight-bearing vertebral body (Fig. 8.2), the superior surface of which is raised on each side, rather like a bucket seat, to form unciform processes. The unciform processes articulate with corresponding facets on the vertebra above to form the uncovertebral joints or the joints of Luschka.
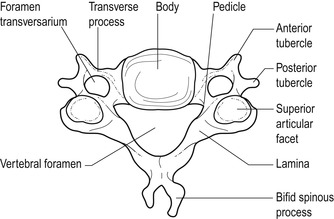 |
| Figure 8.2
Typical cervical vertebra.
From Anatomy and Human Movement by Palastanga N, Field D and Soames R. Reprinted by permission of Elsevier Ltd.
|
Posteriorly lie two short pedicles and two long, narrow laminae forming the vertebral arch which, together with the vertebral body, surround a large, triangular vertebral canal. The laminae come together to form a bifid spinous process. Superior and inferior articular processes, at the junction of the pedicles and laminae, articulate at the synovial zygapophyseal joints which form an articular pillar on either side of the spine.
Short, gutter-shaped transverse processes slope anterolaterally to transport the emerging nerve root. The foramen transversarium, a distinctive feature in the transverse processes on each side of the cervical vertebrae, houses the vertebral artery.
The ligaments of the lower cervical segment assist stability and allow mobility. The anterior longitudinal ligament protects the anterior aspect of the intervertebral joints and, with other anterior soft tissues, limits cervical extension. The ligamentum nuchae is a strong, fibroelastic sheet protecting the joints posteriorly and providing an intermuscular septum. The ligamentum flavum is a highly elastic ligament linking adjacent laminae. In the cervical spine it allows separation of the vertebrae during flexion and assists the neck’s return to the upright posture. Its elastic properties also allow it to return to its original length, so preventing buckling into the spinal canal where it can sometimes compromise the spinal cord.
The posterior longitudinal ligament passes from the axis to the sacrum and is at its broadest in the cervical spine where it covers the entire floor of the cervical vertebral canal, supporting the disc and possibly preventing its posterior displacement. It is taut in flexion and relaxed in extension. Mercer & Bogduk (1999) identify three distinct layers of the posterior longitudinal ligament. The deep layer, consisting of short fibres, spans each intervertebral joint and extends in an alar (wing-shaped) pattern as far as the posterior end of the base of the uncinate process, where it is believed to compensate for a deficient posterior annulus fibrosus.
The joints of the cervical spine
The joints of the lower cervical segment consist of the interbody joint anteriorly and the two zygapophyseal joints posteriorly. The interbody joint is made up of the intervertebral joint and the uncovertebral joints. When stacked, the joints can be considered to form three pillars in a triangular formation, the vertebral bodies and uncinate processes forming the anterior column and the two articular pillars formed by the zygapophyseal joints arranged posteriorly (Mercer & Bogduk 1999).
The intervertebral joint is a symphysis formed between the relatively avascular intervertebral disc and the adjacent vertebral bodies. The disc contributes to mobility and, as it ages, assists the uncovertebral joints in providing translatory glide to the movements of flexion and extension (see below).
The uncovertebral joints (joints of Luschka) are formed between the unciform processes and corresponding facets on the vertebral body above (Fig. 8.3), though their existence has been challenged (Mercer & Bogduk 1999). They may be true synovial joints or adventitious fibrous joints which have developed through clefts or fissures in the lateral corners of the annulus fibrosis of the intervertebral disc originally described by Hurbert von Luschka in 1858 (Prescher 1998). These fissures are absent in young children and appear to develop in conjunction with the development of the unciform process.
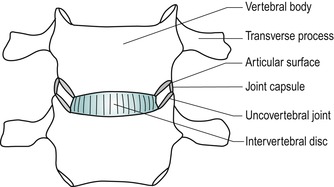 |
| Figure 8.3
Uncovertebral joints.
From Anatomy and Human Movement by Palastanga N, Field D and Soames R. Reprinted by permission of Elsevier Ltd.
|
Once formed, the fissures in the annulus develop a pseudocapsule in which vascularized synovial folds have been seen. Prescher (1998) relates the development of these fissures and the uncovertebral ‘joint’ to the development of the cervical lordosis resulting in a change in configuration and the magnitude of the loads transmitted through the cervical spine. This results in strong shear forces being transmitted through the intervertebral disc, particularly during rotation and side flexion. C3–C5 are the most loaded segments and it is at these levels that the fissures in the disc first appear.
The uncovertebral joints also contribute to mobility by providing a translatory gliding component to flexion and extension as well as stabilizing the spine by limiting the amount of side flexion. The gliding component produces shear which extends horizontal fissuring of the disc medially from the uncovertebral joints. This, together with the degenerative process, may eventually produce a bipartite disc (see below) (Taylor & Twomey 1994, Mercer & Bogduk 1999).
The position of the uncovertebral joints gives bony protection to the nerve root from posterolateral disc displacement. As synovial ‘joints’, degenerative changes can have an effect on the uncovertebral joints. Osteophyte formation on the uncinate process occurs predominantly in the lower cervical segments. Posterior osteophytes can encroach on the intervertebral canal leading to compression of the emerging spinal nerve root, whereas anterior osteophytes may compress the vertebral artery.
The zygapophyseal joints are synovial plane joints with relatively lax fibrous capsules to facilitate movement. The articular facets are angled at approximately 45° to the vertical so that side flexion and rotation of the lower cervical spine occur as a coupled movement. This angle of inclination adds to the component of translatory glide during flexion and extension (Taylor & Twomey 1994).
A number of intra-articular structures have been described, particularly vascular synovial folds, similar to the alar folds of the knee, as well as fat pads and meniscoid structures. All are highly innervated and can be a potential source of pain (Taylor & Twomey 1994, Oliver & Middleditch 2006). As synovial joints, the zygapophyseal joints are prone to degenerative changes and, being placed near the exiting nerve root, osteophyte formation may affect the size of the intervertebral foramen.
Orthopaedic medicine treatment was traditionally based on the discal model, but it should be remembered that any structure that receives a nerve supply can be a potential source of pain. Since the cervical spine is made up of individual motion segments, a lesion of one part of the segment will tend to influence the rest of that segment. Similarly, treatment directed to one part of a segment will affect the segment as a whole.
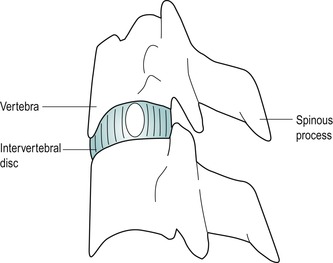
Intervertebral discs
There are six cervical discs which facilitate and restrain movement as well as transmit load from one vertebral body to the next (Fig. 8.4). Cervical discs are approximately 5 mm thick (Palastanga et al 2006) and the thinnest of all the intervertebral discs. Each forms part of the anterior wall of the intervertebral foramen and is thicker anteriorly, contributing to the cervical lordosis. The intervertebral disc consists of an annulus fibrosus, nucleus pulposus, and transitional superior and inferior vertebral end-plates.
 |
| Figure 8.4
Cervical vertebrae, interbody joint and posterior elements.
From Anatomy and Human Movement by Palastanga N, Field D and Soames R. Reprinted by permission of Elsevier Ltd.
|
The difference in function between the lumbar and cervical spine and the traditionally accepted view that cervical discs are smaller versions of lumbar discs does not hold true. Mercer & Bogduk (1999) acknowledged this in their investigation of the form of the human adult intervertebral cervical disc and its ligaments. At birth the nucleus consists of no more than 25% of the entire disc and it undergoes rapid degeneration with age so that, by the age of 30, the nucleus is undistinguishable as such.
The structure of the annulus fibrosus, described by Mercer & Bogduk (1999), is different anteriorly and posteriorly (Fig. 8.5). The anterolateral annulus forms a crescent shape when viewed from above, thicker in the median plane and thinner laterally, tapering out to the unciform processes. It forms a dense, anterior interosseous ligament. The fibres arise from the superior surface of the lower vertebra, fanning out in an alar fashion laterally, but in the midline form a tightly interwoven pattern with fibres from opposite sides, not the true laminate structure as seen in the lumbar spine. A distance of 2–3 mm from the surface of the anterior annulus, collagen fibres have been found embedded with proteoglycans and forming a fibrocartilaginous mass which has a pearly appearance and the consistency of soap.
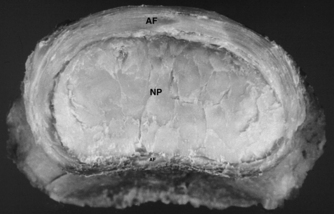 |
| Figure 8.5
Photograph showing the top view of a 39-year-old cervical intervertebral disc. The annulus fibrosus (AF) is thick and fibrous, tapering posteriorly towards the uncinate region. Posteriorly the thin annulus fibrosus (AF) is found only towards the midline. Centrally the nucleus pulposus (NP) appears as a fibrocartilaginous core.
From Grieve’s Modern Manual Therapy, 3rd edn by Boyling J D, Jull G A (eds). Reprinted by permission of Elsevier Ltd.
|
On a deeper plane, the fibrocartilaginous mass becomes more homogeneous and less laminated, forming what the authors interpret as the nucleus of the disc. Clefts, presumably the uncovertebral ‘joints’, are seen to extend into the fibrocartilaginous core laterally at the uncovertebral region, partially into the core in younger patients but totally transecting the posterior two-thirds of the disc in older specimens (bipartite disc). Covering the clefts in the uncovertebral region is thin periosteofascial tissue, the annular fibres being deficient here.
The posterior annulus demonstrates different features to the anterior annulus. It consists of a thin layer of one set of vertically oriented collagen fibres, not more than 1 mm thick, passing between adjacent vertebrae and extending out as far as the unciform process on each side where no oblique posterior annular fibres were found. Deep to this is the fibrocartilaginous core. It would appear that the deep layer of the posterior longitudinal ligament, with short fibres spanning each intervertebral joint, compensates for the lack of annular fibres in the posterior part of the disc, supporting the nucleus posteriorly. Posterolaterally the alar fibres of the posterior longitudinal ligament alone contain the nucleus and these may become torn or stretched by a bulging disc, or nuclear material may herniate under or through them.
The vertebral end-plate offers protection by preventing the disc from bulging into the vertebral body. It acts as a semipermeable membrane which, by diffusion, facilitates the exchange of nutrients between the vertebral body and the disc.
Cyriax (1982) stated that ‘from a clinical point of view nuclear protrusions form only a small minority of cervical disc displacements’. He postulated that a true nuclear disc lesion occurs only in adolescents and young adults, presenting as an acute torticollis. His hypothesis would seem to be substantiated by Taylor & Twomey (1994) who suggested that early degeneration of the cervical nucleus makes nuclear protrusion in the cervical spine unlikely, unless precipitated by severe trauma. A central bar-like protrusion of the annulus was more likely to occur in the cervical spine. This appeared to be refuted by the work of Mercer & Bogduk (1999) described above, given the relatively deficient posterior cervical annulus identified. Perhaps, like lumbar discs, herniated cervical discs may consist of degenerate nuclear material. Posterolateral herniation of the disc could be possible through the weak supporting alar fibres of the posterior longitudinal ligament in the uncovertebral region. Indeed, one specimen from the Mercer & Bogduk study (1999) illustrates a disc bulge and a herniation, both below their respective alar fibres.
The position of the uncovertebral joints and the large vertebral canal in the cervical spine may both also exert a protective influence on disc movement. Degenerate cervical discs may prolapse directly posteriorly, encroaching on the dura through the posterior longitudinal ligament, rather than laterally at the uncovertebral region, to affect the dural nerve root sleeve and underlying nerve root in the intervertebral canal. Alternatively, discal material may be reflected posterolaterally in the vertebral canal by the stronger median part of the posterior longitudinal ligament to cause unilateral pressure on the dura.
The triangular vertebral canal is at its largest in the cervical spine and, even though the cervical cord is enlarged in this region, there may be room for a prolapse to be accommodated. Posterolateral prolapse through the weaker alar portion in the uncovertebral region may encroach on the dural nerve root sleeve and underlying nerve root. However, it would seem that only a very large posterolateral prolapse would be able to compress the nerve root at the same level, unless there is canal stenosis caused by osteophyte formation, an infolding ligamentum flavum or congenital factors.
Since the extent of pain referral is thought to be related to the amount of pressure on the dural nerve root sleeve (Mooney & Robertson 1976), brachial pain is not as commonly associated with cervical disc lesions as sciatica is with lumbar disc lesions. Dural pressure due to a cervical disc tends to produce central or unilateral scapular pain.
It is assumed that cervical discs are innervated in a similar way to lumbar discs, since no studies have refuted this as yet. Bogduk (1994a) acknowledged the paucity of data on cervical discs but claimed that the few studies that had been done had been positive, demonstrating that the cervical discs do have an innervation. In that respect, the data on cervical discs are in accord with those on lumbar discs. At least the outer third and possibly the outer half of the annulus fibrosis receives a nerve supply from branches of a posterior longitudinal plexus, derived from the cervical sinuvertebral nerves, as well as from a similar plexus formed from cervical sympathetic trunks and the vertebral nerves, and from penetrating branches from the vertebral nerve (Bogduk 1994a). Mendel et al (1992) found evidence of nerve fibres and mechanoreceptors in the posterolateral region of the annulus.
The cervical disc may produce either primary disc pain, pain due to the mechanical effect of secondary compression of pain-sensitive structures, or pain associated with chemical or ischaemic effects. The mechanism of pain produced by disc displacement is covered in greater detail in Chapter 13 since much of the investigative work on pain production has been performed in the lumbar region and no studies have been identified that relate to the cervical spine. If findings can be extrapolated to the cervical spine, disc material is thought to undergo a process of degradation which contributes to its herniation. The chemical mediators of inflammation may play a role in the pathophysiology of cervical radiculopathy which renders the nerve root pain-sensitive (Kang et al 1995).
Cervical spinal nerves
There are eight cervical spinal nerves, each approximately 1 cm long. Each nerve, together with the dorsal root ganglion, occupies a large, funnel-shaped intervertebral foramen (Fig. 8.6). The spinal nerve is composed of one dorsal or posterior nerve root and one anterior or ventral nerve root, the ventral nerve root emerging more caudally from the dura mater (Tanaka et al 2000). The dorsal nerve root carries sensory fibres and the ventral nerve root, motor fibres.
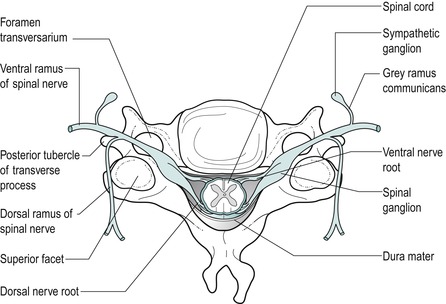 |
| Figure 8.6
Formation of a spinal nerve.
From Functional Anatomy of the Spine by Oliver J and Middleditch A. Reprinted by permission of Elsevier Ltd
|
The spinal nerve occupies one-fourth to one-third of the intervertebral foramen diameter and carries with it an investment of the dura mater, the dural nerve root sleeve. The dural nerve root sleeve is sensitive to pressure and produces pain in a segmental distribution. Cervical spinal nerves generally emerge horizontally and therefore the nerve roots are vulnerable to pressure only from the disc at that particular level, producing signs and symptoms in one segment only (Fig. 8.7). Indication of more than one nerve root involvement should be considered suspicious until proved otherwise.
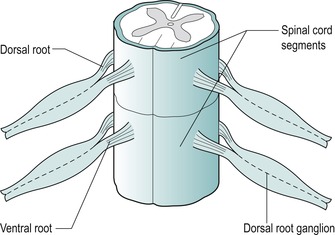 |
| Figure 8.7
Horizontal direction of emerging nerve roots.
From Anatomy and Human Movement by Palastanga N, Field D and Soames R. Reprinted by permission of Elsevier Ltd.
|
However, Tanaka et al (2000), in a cadaver study, showed that the roots below C5 reached their intervertebral foramen with increasing obliquity making compression of more than one nerve root below this level possible. At the C7–T1 disc, 78% of specimens showed that the C8 nerve roots did not have any contact with the disc at the entrance of the intervertebral foramen, which probably accounts for the low frequency of C8 radiculopathy.
Nerve root compression was found to occur at the en-trance of the intervertebral foramen and was determined to be due to herniated discs and osteophytes in the uncovertebral region anteriorly, and to the superior articular process, ligamentum flavum and periradicular fibrous tissue posteriorly (Tanaka et al 2000). Motor impairment, therefore, is suggestive of anterior compression from a disc prolapse or degenerative changes in the uncovertebral region, whereas sensory change is indicative of compression due to changes in the posterior structures. Of course a large disc prolapse or gross degenerative change, anteriorly or posteriorly, may compress both elements of the nerve root.
After it leaves the intervertebral foramen, the spinal nerve root immediately divides into ventral and dorsal rami. The sinuvertebral nerve is a mixed sensory and sympathetic nerve, receiving origin from the ventral ramus and the grey ramus communicans of the sympathetic system (Fig. 8.8). The nerve returns through the intervertebral foramen and gives off ascending, descending and transverse branches to supply structures at, above and below the segment (Oliver & Middleditch 2006, Palastanga et al 2006, Standring 2009). The structures it supplies include the dura mater, posterior longitudinal ligament and the outer part of the annulus of the intervertebral disc (Bogduk 1994b).
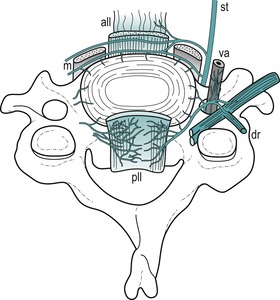 |
| Figure 8.8
A sketch of the innervation of the plexuses surrounding a cervical intervertebral disc (based on Groen et al 1990). The sinuvertebral nerves form a dense plexus accompanying the posterior longitudinal ligament (pll). Anteriorly, branches of the sympathetic trunk (st) supply the front of the disc and from a plexus accompanying the anterior longitudinal ligament (all). vr = ventral ramus, dr = dorsal ramus, va = vertebral artery, m = prevertebral muscles.
From Grieve’s Modern Manual Therapy, 2nd edn by Boyling J D and Palastanga N. Reprinted by permission of Elsevier Ltd.
|
Cervical arteries
Anatomically the vertebral artery is divided into the following four sections, which include two right-angled bends where it is vulnerable to internal and external factors which tend to compromise blood flow (Fig. 8.9) (Oliver & Middleditch 2006, Standring 2009):
• Its origin from the subclavian artery
• Its passage through each foramen transversarium except C7. In this section it gives off spinal branches which supply the spinal cord and its sheaths via the intervertebral foramen
• The first right-angled bend, turning medially to pass behind the lateral mass of the atlas
• The second right-angled bend, to turn vertically to enter the foramen magnum to unite with the other vertebral artery to form the basilar artery, which passes on to contribute to the circle of Willis.
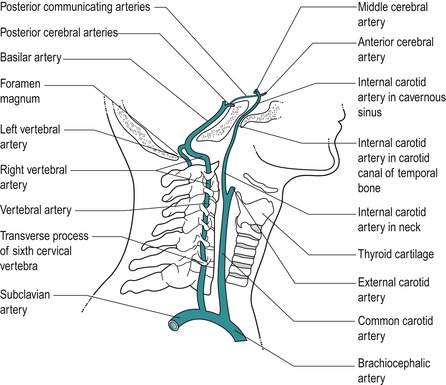 |
| Figure 8.9
Pathway of vertebrobasilar arteries.
From Anatomy and Human Movement by Palastanga N, Field D and Soames R. Reprinted by permission of Elsevier Ltd.
|
Anatomical anomalies and variations exist and commonly one vertebral artery is narrower than its partner. Clinically it is important to recognize vertebrobasilar signs and symptoms since these contraindicate certain cervical manoeuvres. The artery is elastic, particularly in its first and third sections, which allow it to accommodate to movement. Degenerative changes in the artery itself, in the intervertebral canal, uncovertebral joints and zygapophyseal joints make it vulnerable to blockage as well as possibly distorting its pathway.
The internal carotid artery arises from the bifurcation of the common carotid artery in the anterior cervical spine (Fig. 8.9). It supplies most of the ipsilateral cerebral hemisphere, the eye and accessory organs, the forehead and part of the nose. It ascends to enter the cranial cavity via the carotid canal and turns anteriorly to end by dividing into the anterior and middle cerebral arteries, which anastomose into the circle of Willis.
The internal carotid arteries together provide the most significant proportion of blood to the brain, 80%, compared with 20% passing through the posterior vertebral artery system. Blood flow is known to be influenced by neck movements, particularly extension and less so rotation (Rivett et al 1999, Kerry & Taylor 2006).
Differential diagnosis at the cervical spine
An understanding of the anatomy at the cervical spine, together with a detailed history and examination of the patient, will help with the selection of patients suitable for manual treatment and contribute to safe practice. The two areas of danger in this region are the cervical arteries and the spinal cord, and certain signs and symptoms will become evident on examination which would exclude some patients from manual treatment techniques.
Similarly, there are other causes of neck pain in which manual treatment techniques are either contra-indicated or not as appropriate. The following section will be divided into two parts. The first covers mechanical lesions, which present a set of signs and symptoms that help to establish diagnosis and to rationalize treatment programmes. The second covers the other causes of neck pain and associated signs and symptoms. On the whole, these patients are not appropriate for treatment with manual orthopaedic medicine treatment techniques and require suitable referral.
Mechanical cervical lesions
As well as being a primary source of pain, the disc, through prolapse into the vertebral or intervertebral canal, can have a secondary effect on any pain-sensitive structure lying within. This effect can be mechanical through compression and distortion, chemical through the inflammatory process and ischaemic through the pressure of oedema. For further information on these theories, the reader is referred to Chapter 13. Although the differences in the anatomy and function of the cervical and lumbar spine must be acknowledged, few studies have been unearthed to reflect patterns of radicular pain production specific to the cervical spine, although conclusions have been drawn in relation to myofascial pain patterns based on clinical observation. For the time being, cervical radicular pain patterns have been extrapolated from the lumbar model.
A central herniation of cervical disc material produces central and/or bilaterally referred symptoms. A unilateral herniation produces unilaterally referred symptoms. Reference of pain arising from compression of the central structures is characteristically multisegmental, i.e. over many segments (see Ch. 1). Involvement of the unilateral structures, dural nerve root sleeve and/or the nerve root produces segmentally referred signs and symptoms.
Since the disc degenerates early in the cervical spine, disc lesions tend to produce a pattern of symptoms which indicate a progression of the same condition. The symptoms are age-related with a pattern of increasing frequency and severity. Eventually the nerve root may become involved.
The anatomy of the zygapophyseal joint with its intra-articular structures makes it susceptible to possible mechanical derangement. It is difficult to differentiate pathology in the absence of neurological signs or symptoms, and primary disc lesions and zygapophyseal joint lesions appear to be similar in their presentation. Referral pain patterns arising from the zygapophyseal joints in symptomatic subjects have been looked at by Cooper et al (2007) who found that there was referral into the neck but minimal reference into the arm. As mentioned above, there are no similar studies for primary or secondary disc pain.
While acknowledging the zygapophyseal and other pain-sensitive somatic structures as possible causes of pain, the approach in orthopaedic medicine is traditionally based on the discal model.
Disc lesions in the cervical spine need to be reduced to prevent them contributing further to the degenerative process. A central prolapse in particular may cause osteophyte formation through ligamentous traction which may eventually threaten the spinal cord. Cyriax (1982) was emphatic in pointing out the danger of not reducing an early, minor cervical disc lesion for this reason as there comes a time when manipulation will not have an effect and a point at which it may be dangerous. Elderly patients with osteophytic cord compression often report the onset of paraesthesia associated with cervical extension and for that reason it would seem that particular attention should be paid to restoring cervical extension which would act as an indicator that reduction had been effected.
Cervical lesions produce a pattern of signs and symptoms and a set of clinical models has been established to aid diagnosis and establish treatment programmes. These are outlined later in this chapter. The models should be used as a general guide to the clinical diagnosis and treatment of cervical lesions. All show a non-capsular pattern on examination.
The following terminology will be used to describe disc lesions:
Disc protrusion
• Degenerate disc material bulges into the weakened laminate structure of the annulus, where it can produce primary disc pain since the outer annulus receives a nerve supply.
Disc prolapse
• Discal material passes through a ruptured annulus and/or posterior longitudinal ligament into the vertebral canal or intervertebral canal where it has a secondary effect on pain-sensitive structures: the posterior longitudinal ligament, dura mater, dural nerve root sleeve, nerve root and dorsal root ganglion. The sequela of this is sequestration of the disc.
Both forms of cervical disc lesion are suitable for orthopaedic medicine manual techniques, providing no contraindications exist.
Other causes of head and neck pain, arm pain and associated signs and symptoms
Generally, this group of conditions does not respond to the manual techniques described in this chapter and indeed some may be absolute contraindications. There may, however, be some overlap, particularly with the degenerative conditions, and the techniques may be attempted provided contraindications have been eliminated.
Arthritis in any joint presents with the capsular pattern. Arthritis occurs in synovial joints in the cervical spine and involves the zygapophyseal and uncovertebral joints. In the cervical spine the capsular pattern is demonstrated by the cervical spine as a whole. The limited movements have the ‘hard’ end-feel of arthritis. The pattern of symmetrical limitation of the side flexions and rotations is distinctive when compared with the asymmetrical pattern of limitation seen in disc lesions. The early degeneration of the cervical intervertebral disc occurs concurrently with degenerative changes within the other joints. The history will indicate the type of arthritis: degenerative osteoarthrosis, inflammatory or traumatic.
• Degenerative osteoarthrosis involves damage to hyaline cartilage and subchondral bone with sclerosis and osteophyte formation. Cervical movements become limited in the capsular pattern. Stiffening of the neck is particularly evident on rotation when the patient may, for example, have difficulty in reversing the car. Painful symptoms occur during acute exacerbations of the condition, precipitated by trauma or overuse.
The upper cervical segments are particularly involved in the degenerative process, with marked loss of rotation. Degenerative changes in the neck are often referred to as cervical spondylosis. However, despite gross X-ray changes, there may be little in the way of symptoms.
It is possible to have a disc lesion in an older degenerative neck, i.e. noncapsular pattern, superimposed onto a capsular pattern. The disc lesion can be treated with manual traction and the degenerative osteoarthrosis does not present a contraindication to treatment in itself. However, rotation under traction should be avoided due to the close proximity of the degenerate zygapophyseal and uncovertebral joints to the course of the vertebral artery (see below).
Degenerative changes in the uncovertebral joints lead to osteophyte formation which may encroach on the nerve root or adjacent vertebral arteries, causing problems through direct compression. Degenerative changes of the synovial joints may alter or distort the path of the vertebral artery, leading to possible compromise and symptoms.
• Matutinal headaches may be due to ligamentous contracture around the upper two cervical joints, the atlanto-occipital and atlantoaxial joints. A condition called ‘old man’s matutinal headache’ exists in which the patient, an elderly man, wakes every morning with a headache which usually eases after a few hours (Cyriax 1982). Mobilization techniques, particularly manual traction, may be appropriate for this condition.
• Tinnitus and vertigo may be associated symptoms of degenerative osteoarthrosis of the cervical spine. They can respond well to the techniques, but the vertebrobasilar system must be ruled out as a cause of the symptoms.
• Osteophytic root palsy produces a gradual onset of aching in the arm, usually with paraesthesia, as the osteophytes develop. The patient is usually elderly and will have objective neurological signs of weakness in the arm affecting one nerve root only. Since disc lesions presenting in this way are unusual in this age group, orthopaedic medicine techniques would be contraindicated.
• Cervical myelopathy may develop in association with degenerative changes in the cervical spine. Stenosis of the central canal occurs through osteophytic formation and hypertrophy and buckling of the ligamentum flavum develops. The osteophytes, a disc prolapse or a ligamentous fold may exert pressure on the spinal cord and a gradual onset of symptoms occurs with increasing disability. There may be pain, dysaesthesia of the hands consisting of numbness and tingling, clumsiness and weakness of the hands, weakness and evidence of spasticity of the lower limbs (Jenkins 1979, Connell & Wiesel 1992). The orthopaedic medicine treatment techniques described below would be contraindicated.
• Zygapophyseal joints could conceivably produce symptoms individually and in isolation to the other joints in the segment. As synovial joints they are prone to degenerative changes along with the other joints in the segment. Aprill et al (1989) suggested that distinct patterns of pain referral were associated with individual cervical zygapophyseal joints but others have refuted the existence of a facet syndrome (Schwarzer et al 1994).
• Cervical or cervicogenic headache describes a pain perceived to originate in the head but whose source is in the cervical spine (Bogduk 1992). It consists of an aching or deep pain localized to the neck, suboccipital and frontal region, precipitated or aggravated by neck movements or sustained neck postures, especially flexion. There is limitation of passive neck movements, changes in muscle contour, texture or tone and abnormal tenderness of the neck muscles (Sjaastad 1992, Beeton & Jull 1994, Jull (1994a) and Jull (1994b), Nilsson 1995, Schoensee et al 1995). The upper three cervical segments are most commonly involved and associated symptoms may consist of nausea, visual disturbances, dizziness or light-headedness (Jull (1994a) and Jull (1994b)).
Kerry & Taylor (2006) describe pain associated with carotid artery dissection that can present as ipsilateral posterior neck pain and/or frontotemporal headache. There are many forms of headache and the overlapping symptoms make it difficult to isolate headache due to primary cervical dysfunction. Orthopaedic medicine treatment techniques can be considered as an option if the carotid artery dysfunction and other contraindications have been ruled out.
• Polymyalgia rheumatica affects the middle and older age group, women more than men. It presents as pain and stiffness in the neck and shoulder girdle accompanied by fatigue, low-grade fever, depression and weight loss, and responds dramatically to small doses of oral corticosteroids (Hazelman 1995).
• Giant cell arteritis or temporal arteritis is a condition closely related to polymyalgia rheumatica. It is a vasculitis of unknown aetiology affecting the elderly. The patient presents with a severe temporal headache and scalp tenderness. The condition is treated urgently with high-dose steroids, to prevent blindness (Hazelman 1995).
• Rheumatoid arthritis is an inflammatory polyarthritis, affecting females more than males, with its onset usually between the ages of 40 and 50. It tends to follow a relapsing and remitting course. The synovial membrane becomes inflamed and thickened to become continuous with vascular tissue – a condition known as pannus. The pannus causes typical destructive changes of ligaments, cartilage and bone (Walker 1995). Rheumatoid arthritis can also involve extra-articular soft tissues, e.g. Achilles tendon, plantar fascia (Kumar & Clark 2002).
It is uncommon for rheumatoid arthritis to affect the cervical joints only, without its manifestation elsewhere, and it most commonly affects the smaller peripheral joints bilaterally. However, in patients with rheumatoid arthritis it may be silent in the cervical joints and there may be no clinical evidence of cervical spine involvement (Clark 1994).
The mechanism of the disease in the spinal joints is the same as that seen in peripheral joints, with ligament, cartilage and bone destruction. This loss of the supporting infrastructure of the spine, particularly of the upper cervical segment, is a potential hazard for significant neurological involvement of the brainstem and cervical spinal cord. Atlantoaxial subluxation is the most common manifestation, but cranial settling (vertical intrusion of the dens) and subaxial subluxation may also occur (Clark 1994, Zeidman & Ducker 1994, Mathews 1995). Rheumatoid arthritis is therefore an absolute contraindication to orthopaedic medicine techniques.
• Traumatic arthritis is produced by significant trauma causing inflammation in the cervical synovial joints and therefore a capsular pattern. This may occur following a whiplash injury. Once the capsular pattern has settled, there may be evidence of an underlying disc lesion to which mobilization can be carefully applied, providing there is no damage to the vertebrobasilar system.
• Whiplash injury occurs when a car is struck from behind, often while the occupants of the involved car are unaware. A hyperextension injury followed by a hyperflexion injury occurs. During the hyperextension phase the anterior structures, the intervertebral discs, anterior longitudinal ligament and anterior muscles can be damaged or torn and the posterior structures compressed. During the hyperflexion phase the dens may impact against the atlas, and the atlanto-occipital joint, posterior ligaments and zygapophyseal joints can be involved (Bogduk 1986). The alar and transverse ligaments and the tectorial and posterior atlanto-occipital membranes can be damaged by whiplash injury (Krakenes et al 2002, Krakenes et al 2003). Generally, a whiplash injury may produce some pain initially, but it is not until later that the ligaments stiffen and produce a secondary capsular pattern due to the trauma.
Significant bony or ligamentous damage causes immediate pain with a reluctance to move the neck. X-ray evidence of cervical instability is an absolute contraindication to orthopaedic medicine techniques. A history of recent whiplash injury has been identified as a possible risk factor of vascular accident (Kleynhans & Terrett 1985).
Taylor & Twomey (1993) conducted an autopsy study of neck sprains and showed clefts associated with vertebral end-plate lesions in trauma victims. These were distinct from the uncovertebral clefts and central fissures associated with degeneration of cervical discs. These so-called rim lesions involved the avascular cartilage end-plates and the outer annulus and, in further experiments, showed a poor response to healing. They may be responsible for the chronic pain often associated with whiplash injuries. Posterior disc herniation through a damaged annulus and haemarthrosis of the zygapophyseal joints were also observed in the trauma victims and this is clinically significant in treating the early whiplash. The acute sprain of the joints makes this an irritable lesion which requires pain relief and reduction of inflammation. Early mobilization – in line with the principles for acute lesions laid out in Chapter 4– may be applied, providing gross bony injury and instability are not present.
Evidence exists to support early mobilization in whiplash-type injuries to avoid the chronic pain syndrome developing, with its associated psychosocial factors (Mealy et al 1986, McKinney 1989). Current literature suggests that at 3 months, approximately one-third of subjects with whiplash injury will have high levels of persisting pain and disability (Stewart et al 2007). The Chartered Society of Physiotherapy has produced guidelines for the management of whiplash associated disorder (WAD) following a review of the available evidence (Moore et al 2005). The guidelines aim to support practice and to help both physiotherapists and patients to make informed choices for continuing management following assessment.
The recommendations are summarized as follows:
• In the acute stage (0–2 weeks after injury) patients should be given education, active exercise, and advice on self-management and a return to normal activity as soon as possible.
• In the subacute stage (2–12 weeks after injury) a multimodal approach should be applied including postural training, manual techniques and psychological support. There is evidence to support that combined manipulation and mobilization, muscle retraining including deep flexor activity, acupuncture, education, advice on coping strategies, TENS (transcutaneous electrical nerve stimulation), massage and soft tissue techniques may contribute to pain reduction.
• In the chronic stage (more than 12 weeks after injury) exercise therapy, manipulation and mobilization (which may be combined) and multidisciplinary psychosocial packages may be effective. Trained health professionals (who are not necessarily psychologists) can give psychological support.
Within the orthopaedic medicine approach, gentle techniques have been devised for pain relief and return of function in the acute stage, i.e. at an earlier stage than that recommended by the guidelines. Two randomized controlled trials relating to manual mobilization techniques were scrutinized in the guidelines and both were found to have flaws.
The conclusion was that there is no evidence regarding the short- or long-term benefit of early mobilization, However, Taylor & Kerry (2005) cite Cassidy et al (1992) and Baltaci et al (2001) to support the ‘commonly held view’ that mechanical pain of acute onset responds well to early manipulation, although pain arising from whiplash injury is not specified. There does not appear to be evidence of no benefit.
Sterling & Kenardy (2008) looked at the heterogeneity of whiplash and observe that it is inappropriate to apply the same programmes of management to all individuals since they display a wide range of different characteristics and responses following the injury. They recommend that more research needs to be done to be able to identify features of the condition that may be identifiable at early assessment, particularly as predictors of poor recovery, to allow for more specific treatment directions.
The techniques suggested below include Grade A mobilization, providing there is no serious pathology. In accordance with the guidelines, the patient is given the responsibility for self-management of the condition, and is instructed about posture and exercise, advice to avoid excessive reliance on a collar, appropriate pillow support and adequate analgesia.
Serious, non-mechanical conditions may present with signs and symptoms similar to those of a mechanical presentation. For this reason, careful examination is necessary to eliminate serious disease which would be contraindicated for orthopaedic medicine treatment. Patients may present with local symptoms which are rarely provoked by movement or posture. On examination it may be difficult to reproduce the symptoms. Other more generalized features may also be present, such as increasing and unrelenting pain, night pain, weight loss, general malaise, fever, raised erythrocyte sedimentation rate or other symptoms, e.g. cough.
• Spinal infections may include osteomyelitis or epidural abscess. The organism responsible may be Staphylococcus aureus, Mycobacterium tuberculosis or, rarely, Brucella (Kumar & Clark 2002).
• Malignant disease is usually extradural with bone metastases produced most commonly from primaries in the bronchus, breast, prostate, kidney or thyroid. There may be a history of a gradual onset of pain and stiffness, the pain tends to be unrelenting and night pain is usually a feature. The pain is not relieved by different postures. On examination active movements produce pain and limitation in all directions. The passive movements are prevented by the end-feel of a twang of muscle spasm and resisted movements are painful and possibly weak. All of these signs and symp-toms are evidence of a gross lesion (Cyriax 1982). Neurological examination may reveal excessive muscle weakness involving several nerve roots, possibly bilaterally, in contrast to a disc lesion that tends to involve one nerve root only (Mathews 1995).
• Primary tumours (e.g. meningioma, neurofibroma, glioma) tend to present with a gradual onset of symptoms of cord compression and pain is not usually a major feature.
• Pancoast’s tumour is carcinoma in the apex of the lung which may erode the ribs and involve the lower brachial plexus. It accounts for only 5% of bronchial tumours. There is severe pain in the shoulder and down the medial aspect of the arm, with evidence of C8 and/or T1 palsy, possible atrophy of the ulnar aspect of the hand and a reduced triceps reflex (Pitz et al 2004). Cervical side flexion away from the painful side may be the only limited neck movement, with passive elevation of the shoulder on the symptomatic side also being painful (Cyriax 1982). Interruption of the sympathetic ganglia can produce Horner’s syndrome – constriction of the pupil and drooping of the eyelid on the side of the tumour (Kumar & Clark 2002).
The following conditions are not serious but should be considered as part of the clinical reasoning required in differential diagnosis:
• Suprascapular, long thoracic and spinal accessory neuritis usually present with pain in the scapula and upper arm of approximately 3 weeks’ duration. On examination, the neck movements are full and do not reproduce the pain. There is weakness of the appropriate muscles supplied by the affected nerve. The cause may be unknown, or it may be due to trauma or follow a viral infection. Recovery is usually spontaneous over approximately 6 weeks.
■ Suprascapular neuritis produces weakness of the supraspinatus and infraspinatus muscles
■ Long thoracic neuritis produces weakness of the serratus anterior muscle and winging of the scapula
■ Spinal accessory neuritis produces weakness of the sternocleidomastoid and trapezius muscles
• Neuralgic amyotrophy is an unusual cause of severe pain in the neck and scapular region with a bizarre pattern of muscle weakness in the infraspinatus, supraspinatus, deltoid, triceps and serratus anterior muscles. The cause of the condition is unknown, but it may follow viral infection or immunization and an allergic basis is postulated (Kumar & Clark 2002). It usually recovers spontaneously over several weeks or months, although recovery may be more prolonged in some cases.
• The pain of shingles (herpes zoster) can precede the rash and cervical pain and headache have been reported prior to the appearance of vesicles in the cervical region.
Thoracic outlet syndrome, reflex sympathetic dystrophy and work-related syndromes all present with upper limb signs and symptoms which are sometimes difficult to isolate into a simple diagnostic pattern, particularly if symptoms have been present for a long time.
■ Thoracic outlet syndrome is a term used for compression, entrapment and/or postural alterations affecting the brachial plexus and its accompanying neurovascular structures, although debate continues about its existence (Walsh 1994). Distal symptoms occur, usually due to compression of the lower trunk of the brachial plexus (C8, T1), but the upper and middle trunks can also be involved. The condition may be bilateral, with a burning, dull aching pain along the medial aspect of the forearm. Distal oedema may be associated with activity, sweating and heaviness, and circulatory changes may be seen in the hands. Paraesthesia occurs as the release phenomenon, coming on at night, some time after the pressure has been released.
■ Reflex sympathetic dystrophy describes a complex disorder of the limbs with or without obvious nerve involvement. It consists of persistent peripheral burning pain and tenderness which is termed hyperaesthesia (an abnormal response to pain) or allodynia (pain in response to stimuli that are not normally noxious). Vasomotor and sensory changes consist of sweating, colour changes and trophic skin changes together with weakness, tremor, muscle spasm and contractures (Herrick 1995).
■ Work-related syndromes of the upper limb are due to repetitive occupational overuse, producing musculoskeletal symptoms, once a certain threshold of activity is exceeded. Sometimes this may present as a simple tenosynovitis or tendinopathy but much more often it presents as a catalogue of symptoms which are non-specific. Diffuse aching, stiffness, muscle or joint tiredness are present with the chronic nature of the condition, perhaps leading to anxiety and depression (Bird 1995). The symptoms here are usually associated with factors that affect the vmobility and circulation of the nervous system. Each component needs to be recognized and a suitable treatment programme established. Neural tensioning techniques can be applied to assess neural mobility which can be addressed as part of treatment and self-management. Orthopaedic medicine techniques are not usually indicated unless specific identifiable lesions are diagnosed.
• Fibromyalgia usually presents in women, as a complex of variable symptoms including widespread musculoskeletal pain of the neck, shoulders and upper limbs. Fatigue, headache, waking unrefreshed, subjective distal swelling, poor concentration, forgetfulness and weepiness have been described (Doherty 1995). Multiple hyperalgesic tender spots and non-restorative sleep are the main diagnostic features of fibromyalgia. There are several components to management: they include the use of relaxation techniques, exercise, hydrotherapy, physiotherapy, acupuncture, muscle relaxants and drugs to improve the quality of sleep. The condition is difficult to separate from endo-genous depression and often responds to antidepressant medication (E. Huskisson, conference note 1995).
• Cervical spine instability is controversial and difficult to diagnose, and there do not appear to be valid or reliable tests to help with diagnosis (Cook et al 2005). Instability may contribute to the clinical presentation of various conditions including cervicogenic headache, chronic whiplash associated disorder, rheumatoid arthritis, osteoarthritis and segmental degeneration. Trauma, genetic predisposition (e.g Down’s syndrome), disc degeneration and surgery may compromise the stabilizing mechanisms of the spine (Cook et al 2005).
In some cases the instability can be potentially life threatening as in laxity of the transverse ligament associated with rheumatoid arthritis or Down’s syndrome where the odontoid peg may be hypoplasic or deformed. Subluxation of the atlantoaxial joint and hypermobility of the atlanto-occipital joint are associated with Down’s syndrome and may occasionally lead to compression of the spinal cord. Clinicians involved in manipulation should be aware of this risk (Pueschel et al 1992, Department of Health 1995, Matsuda et al 1995).
Upper cervical instability has been associated with localized atherosclerotic changes in the cervical vessels. The changes may be associated with repeated microtrauma as a result of increased upper cervical movement. This can be associated with connective tissue inflammatory disease, principally rheumatoid arthritis, or acute whiplash injury, as mentioned above (Kerry & Taylor 2006).
• Drop attacks are sudden episodes of weakness in the lower limbs, causing falling without loss of consciousness and complete recovery in seconds or minutes. Drop attacks are a symptom, not a diagnosis, and they can have diverse causes. They may be due to changes in tone in the lower limb originating in the brainstem, and appear to be related to transient ischaemic attacks (Kumar & Clark 2002). Any instability in the upper cervical segment – i.e. congenital ligamentous laxity of the atlanto-occipital joint, deformed odontoid process, cervical spondylosis or a spondylolisthesis – can produce drop attacks (Cyriax 1982, Hinton et al 1993).
They may also be due to the heart (a variant of syncope) or problems with both the heart and circulation to the brain. Seizures and Ménière’s disease can be associated with drop attacks and other rare causes have been reported (Hain 2009). During the subjective examination the patient must be questioned about drop attacks and the result noted. Any history of drop attacks is an absolute contraindication to orthopaedic medicine treatment techniques.
• Klippel–Feil syndrome is associated with a limited range of cervical movement, short neck and low hairline. Patients usually have developmental abnormalities, including congenital fusion of cervical vertebrae, which may predispose them to the risk of neurological sequelae (Pizzutillo et al 1994).
Cervical artery dysfunction
• Vertebrobasilar insufficiency produces symptoms through a reduced blood flow in the vertebral arteries to the hind brain. Patients often relate their symptoms to particular head positions such as looking up (Toole & Tucker 1960). Anatomical anomalies are frequently seen in the vertebral arteries and their course through the cervical foramen transversarium. Often the two arteries vary considerably in diameter (Mitchell & McKay 1995) and extrinsic or intrinsic factors may decrease the lumen of the artery permanently or temporarily. Extrinsic factors include degenerative changes in the intervertebral, zygapophyseal and uncovertebral joints, with osteophyte formation, which may compress or alter the course of the artery. Intrinsic factors include arterial disease and thrombosis.
Wallenberg’s syndrome or lateral medullary syndrome is probably the most recognized syndrome of brainstem infarction caused by vertebral artery pathology (Frumkin & Baloh 1990, Kumar & Clark 2002). The common site of injury to the vertebral artery following neck manipulation is at the level of the atlantoaxial joint. Injuries include intimal tearing, dissection or thrombus formation, intramural haematoma or vasospasm.
Dizziness and nausea are the main presenting symptoms of vertebrobasilar insufficiency. However, such symptoms are also associated with cervical dysfunction; therefore correct diagnosis is important. A full list of possible signs and symptoms of vertebrobasilar insufficiency is given with the description of the test later in this chapter.
• Carotid artery dissection can present suddenly and may arise from a sudden movement of the neck involving extension and rotation (Taylor & Kerry 2005). It typically presents with unilateral upper cervical or anterolateral neck pain with headache and/or sensitivity in the frontotemporal region (Kerry & Taylor 2006). Horner’s syndrome may be present, associated with ptosis (drooping eyelid), sunken eye, a small constricted pupil and facial dryness. Symptoms of vertebrobasilar artery insuffiency (VBI) may also be present.
• Young: Under 20
• Elderly: First episode over 55
• VBI/CAD symptoms
• Previous whiplash/trauma
• Past medical history of malignancy
• Constant progressive pain
• Unremitting night pain
• Systemically unwell
• Unexplained weight loss
• Drug abuse and HIV
• Long-term systemic steroid use
• Cough/sneeze increasing arm pain
• Pain gradually worsening over 3 months
• Neurological signs and symptoms affecting more than one nerve root
• Inflammatory arthritis
• Osteopoenic/osteoporotic
• Down’s syndrome
• Arm pain under 35
• Side flexion away being only painful cervical movement
• T1 weakness
• Horner’s syndrome
• Upper motor neurone signs
• Risk factors for atherosclerosis
COMMENTARY ON THE EXAMINATION
Observation
Before proceeding with the history, a general observation of the patient’s face, posture and gait is made, noting the posture of the neck and the carriage of the head. Neck posture will give an immediate indication of the severity and possible irritability of the condition, e.g. a wry neck associated with acute pain in which the patient has developed an antalgic posture.
The box above lists the ‘red flags’ for the possible presence of serious pathology that should be listened for and identified throughout the subjective and objective examination. In isolation, many of the flags may have limited significance but it is for the clinician to consider the general profile of the patient and to decide whether contraindications to treatment exist and/or whether onward referral is indicated.
History (subjective examination)
The history is particularly important at the spinal joints. Selection of patients for orthopaedic medicine treatment techniques relies on the discal model of diagnosis and certain aspects of the history will assist in this diagnosis as well as highlighting patients with contraindications to treatment.
The age, occupation, sports, hobbies and lifestyle of the patient may give an indication of the nature of onset and its relationship to habitual postural problems associated with a particular lifestyle.
The age of the patient is important, particularly at the cervical spine, since the type of cervical disc lesion is related to age. In the young patient, child or adolescent, neck pain may be associated with an acute torticollis, possibly a true nuclear disc lesion.
Disc lesions tend to occur in the middle-age group, with posterior or posterolateral herniation; zygaphophyseal joint lesions are also prevalent in this age group. Referred arm pain, due to a large posterolateral disc prolapse, usually occurs over the age of 35, through progressive prolapse. Arm pain presenting under the age of 35 may be indicative of serious pathology and this should be excluded. Degenerative changes in the intervertebral, uncovertebral and zygapophyseal joints occur in the older neck.
Occupation, sports, hobbies and lifestyle of the patient may contribute to the patient’s signs and symptoms. Habitual postures can contribute to postural adjustment, altered biomechanics and muscle imbalance. Examples are provided by the head-forward posture of the visual display unit operator, the side-flexed posture of holding the telephone in the crook of the neck to leave the hands free, the flexed and rotated posture of the plumber or builder, or the head-extended posture allowing the arms to be used above the head in the painter or electrician. Athletes may assume certain postures related to their sport which may precipitate or contribute to their problem.
The site and spread of the symptoms give important clues to diagnosis in the cervical spine and highlight the importance of understanding the mechanisms of referred pain in a segmental or multisegmental pattern. Pain may be localized to the neck or occur in association with symptoms felt in the scapular area, chest, upper limb or head. The sole presentation of internal carotid artery dysfunction may be unilateral upper cervical or anterolateral neck pain and headache and/or sensitivity in the frontotemporal region (Kerry & Taylor 2006).
Nerve root compression in the cervical spine can only occur at the levels at which the nerve root can be compressed. In the mid and lower cervical regions the roots are under threat from the intervertebral discs, uncovertebral joints and zygapophyseal joints which form the boundaries of the intervertebral foramen. C1 and 2 nerve roots do not run in intervertebral foramina, therefore compression of these nerve roots is not the mechanism for upper cervical pain.
It is important to establish the nature of the onset and duration of the symptoms, not just of this current episode of pain, but of all previous episodes. Cervical disc lesions tend to be a progression of the same incident, and establishing a history of increasing and worsening episodes of pain assists diagnosis.
The onset may be sudden or gradual. It may be precipitated by a single incident, such as a whiplash injury due to a motor vehicle accident, or a sudden unguarded movement, such as missing a footing. If traumatic in onset, the exact mechanism should be established: was it hyperflexion, hyperextension or excessive rotation? If the condition developed gradually, is it associated with habitual postures, or a change in posture, such as sleeping in a different bed?
The patient with whiplash injury without serious bony complications presents with pain felt at the time of the trauma which settles. Twenty-four hours later, pain and stiffness develop due to the ligamentous involvement and muscular strain. The whiplash patient who has severe pain from the time of the trauma may have more serious underlying pathology, e.g. fracture and/or dislocation.
Headache of sudden onset may be an indication of internal carotid artery dysfunction (Taylor & Kerry 2005, Kerry & Taylor 2006).
The duration of the symptoms helps to give a prognosis of the patient’s condition as well as providing an indicator for serious pathology, being always on the alert for possible contraindications to treatment. Generally, the patient who presents with a short duration of symptoms responds better to treatment. Disc pathology tends to be self-limiting and generally symptoms will resolve spontaneously. With repeated episodes, however, this tends to take longer and longer. Nerve root compression may follow a mechanism of spontaneous recovery in 3 or 4 months, providing the patient loses the central symptoms.
Recurrent symptoms may indicate a cervical disc lesion or degenerative arthrosis which tends to present with periods of exacerbation of symptoms. Inflammatory arthritis may present a similar picture, but it has usually manifested itself in other joints before involving the cervical spine.
The symptoms and behaviour need to be considered. The behaviour of the pain will give an indication of the irritability of the condition. Make a note of the daily pattern of the pain. If it is easier first thing in the morning and worse as the day goes on, easing up again with rest, this could indicate a compression problem or a postural problem. If it is uncomfortable and stiff in the morning and painful on certain movements, this is indicative of an active arthritis. It may be due to an acute episode of degenerative osteoarthrosis, or inflammatory arthritis such as rheumatoid arthritis or ankylosing spondylitis. Pain not at all relieved by rest and with unrelenting night pain indicates serious pathology such as tumour.
Of what other symptoms does the patient complain? Coughing, sneezing or straining cause an increase in the intrathecal pressure, and neck pain produced with any of these may indicate disc compression or tumour. With regard to the latter, pain produced in the arm on coughing or sneezing is considered to be a ‘red flag’, i.e. a sign of serious spinal pathology, that warrants further investigation, and would provide a contraindication to treatment.
Paraesthesia may be related to nerve root compression. Sympathetic symptoms such as hot and cold feelings, heaviness, puffiness or circulatory symptoms may be related to nerve root compression or thoracic outlet syndrome, reflex sympathetic dystrophy or work-related upper limb disorder.
Other causes of neck pain and associated symptoms should be eliminated, such as thoracic outlet syndrome, which may produce similar symptoms to upper cervical syndromes. Symptoms may include facial pain, tinnitus, auditory and visual disturbances.
From the history, specific questions must be asked to eliminate vertebrobasilar and carotid artery problems and problems of instability in the upper cervical joints, which would contraindicate treatment. A description of unilateral frontotemporal headache as ‘unlike any other’ should raise concerns of internal carotid dysfunction (Kerry & Taylor 2006). It is important to consider any risk factors for vascular disease, specifically atherosclerosis, that may help in differential diagnosis to distinguish pain associated with cervical artery dissection or to guide treatment selection to minimize the potential risk from cervical techniques.
The risk factors for atherosclerosis include: hypertension, raised blood cholesterol, lipids and free radicals, diabetes mellitus and genetic clotting disorders or propensity to thrombus formation (taking oestrogen or recent long-haul flight or surgery). Smoking, infection and direct vessel trauma also provide an increased risk of atherosclerosis (Kerry & Taylor 2006). The list is extensive and it could be argued that the risk factors apply to a large proportion of the population. The advice is to consider the patient’s profile but to maintain a wise balance between ensuring patient safety and applying appropriate treatment.
Explore any complaint of dizziness, nausea, faintness, tinnitus or visual problems. Ask specific questions about drop attacks, i.e. ‘Do you ever fall to the ground without losing consciousness?’. Establish the presence of pins and needles and numbness and note exactly where. Consider whether the distribution of these symptoms fits with segmental referral or is more a sympathetic feature. It may be pertinent to ask about headaches, fatigue and stress, or blurred or dull vision.
Other joint involvement will give an indication of previous problems which may or may not be related. Look for evidence of rheumatoid arthritis, usually in the smaller joints, remembering that the disease process may be quiet in the cervical spine. Ankylosing spondylitis usually starts in the sacroiliac joint(s) and lumbar spine or hips. Note the presence of generalized degenerative osteoarthrosis.
Ask about past medical history and consider any serious illness or operations. It is advisable to ask about previous trauma involving the neck: a history of whiplash is considered a risk factor in vascular accidents (Kleynhans & Terret 1985). Explore previous similar episodes of neck pain and any previous treatment.
Check the medications currently taken by the patient, as anticoagulants and long-term systemic steroid use may present a contraindication to the treatment techniques. Ask about any pain-relieving drugs to give an indication of how much pain control is required by the patient. As antidepressants may also be prescribed for chronic pain they may give an indication of the patient’s general pain profile. Antistatins and antihypertensive drugs should also be noted in relation to potential risk factors for diagnosis and treatment selection.
Inspection
The patient should undress down to underwear to the waist, and be in a good light. A general inspection will reveal any bony deformity. The general spinal curvatures are appreciated, assessing for any increased or decreased cervical lordosis, tilt or rotation, abnormalities in the cervicothoracic junction and upper thoracic kyphosis, scoliosis, or the presence of an antalgic posture. Abnormal fatty tissue sometimes develops over C7, in association with postural deformity at the cervicothoracic junction, and this is known as a ‘dowager’s hump’. Similar fatty tissue can often be seen in rugby players in the front row of the scrum. The head carriage is also noted, looking for excessive protraction or retraction.
Colour changes and swelling would not be expected in the cervical spine unless associated with a history of direct trauma.
A neuritis would give the appropriate wasting of the muscles supplied by the nerve involved and the neck, shoulder and scapular area should be assessed for obvious muscle wasting. Some unusual nerve pathologies produce bizarre patterns of bilateral asymmetrical wasting, e.g. neuralgic amyotrophy. In disc pathology with nerve root compression, muscle wasting may not be obvious on inspection.
State at rest
Before any movements are performed, the state at rest is established to provide a baseline for subsequent comparison.
Examination by selective tension (objective examination)
The suggested sequence for the objective examination will now be given, followed by a commentary including the reasoning in performing the movements and the significance of the possible findings.
Articular signs
• Active cervical extension (Fig. 8.10)
 |
| Figure 8.10
Active extension.
|
• Active cervical right rotation (Fig. 8.11a)
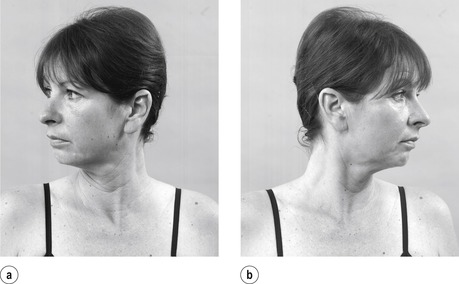 |
| Figure 8.11
Active rotations.
|
• Active cervical left rotation (Fig. 8.11b)
• Active cervical right side flexion (Fig. 8.12a)
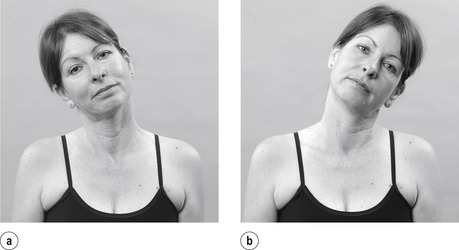 |
| Figure 8.12
Active side flexions.
|
• Active cervical left side flexion (Fig. 8.12b)
• Active cervical flexion (Fig. 8.13)
 |
| Figure 8.13
Active flexion.
|
• Passive cervical extension (Fig. 8.14)
 |
| Figure 8.14
Passive extension.
|
• Passive cervical right rotation (Fig. 8.15a)
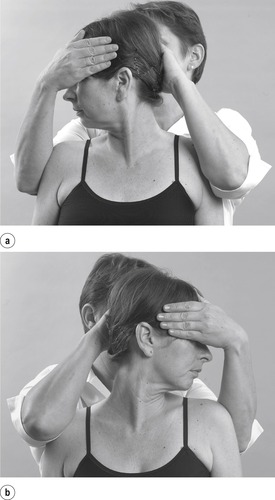 |
| Figure 8.15
Passive rotations.
|
• Passive cervical left rotation (Fig. 8.15b)
• Passive cervical right side flexion (Fig. 8.16a)
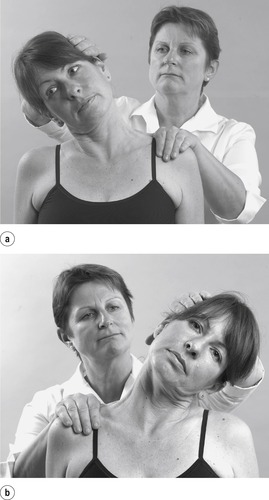 |
| Figure 8.16
Passive side flexions.
|
• Passive cervical left side flexion (Fig. 8.16b)
Resisted tests are not part of the routine examination, but may be applied here
Elimination of the shoulder joint
• Active shoulder elevation (Fig. 8.21)
 |
| Figure 8.21
Active shoulder elevation to eliminate the shoulder as a cause of pain.
|
Resisted tests for objective neurological signs and alternative causes of arm pain; the main nerve roots are indicated in bold
• Shoulder elevation, trapezius (Fig. 8.22): spinal accessory nerve XI C3, 4
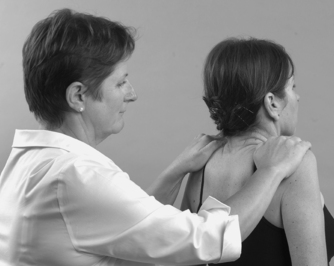 |
| Figure 8.22
Resisted shoulder elevation.
|
• Shoulder abduction, supraspinatus (Fig. 8.23): C4, 5, 6
 |
| Figure 8.23
Resisted shoulder abduction.
|
• Shoulder adduction, latissimus dorsi, pectoralis major, teres major and minor (Fig. 8.24): C5, 6, 7, 8, T1
 |
| Figure 8.24
Resisted shoulder adduction.
|
• Shoulder lateral rotation, infraspinatus (Fig. 8.25): C 5, 6
 |
| Figure 8.25
Resisted shoulder lateral rotation.
|
• Shoulder medial rotation, subscapularis (Fig. 8.26), C 5, 6
 |
| Figure 8.26
Resisted shoulder medial rotation.
|
• Elbow flexion, biceps (Fig. 8.27): C 5, 6
 |
| Figure 8.27
Resisted elbow flexion.
|
• Elbow extension, triceps (Fig. 8.28): C6, 7, 8
 |
| Figure 8.28
Resisted elbow extension.
|
• Wrist extensors (Fig. 8.29): C 6, 7, 8
 |
| Figure 8.29
Resisted wrist extension.
|
• Wrist flexors (Fig. 8.30): C6, 7, 8, T1
 |
| Figure 8.30
Resisted wrist flexion.
|
• Thumb adductors, adductor brevis (Fig. 8.31): C 8, T1
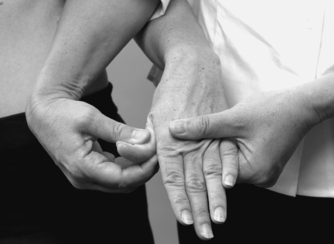 |
| Figure 8.31
Resisted thumb adduction.
|
• Finger adductors, palmar interossei (Fig. 8.32): C 8, T 1
 |
| Figure 8.32
Resisted finger adduction.
|
The routine examination of the cervical spine includes active, passive and resisted movements. Since the spinal joints are considered to be a potential focus for ‘emotional’ symptoms, six active movements are conducted assessing willingness to move, range of movement and pain. The capsular or non-capsular pattern may also emerge from these active movements.
In a non-capsular pattern of the cervical spine, some movements are limited and/or painful and others are full and pain-free. The presence of a non-capsular pattern indicates a possible disc displacement. Normally movements at the cervical spine do not occur in isolation, but the examination procedure is conducted simply by assessing the individual movements. It should, however, be remembered that flexion and extension occur with a component of translatory glide, while side flexion and rotation occur as a coupled movement.
The passive movements are assessed to determine the true limitation of range of movement and the end-feel. The pattern of limited movements should be the same as that found on active movements, although the range may be slightly more. Normally passive extension has a hard end-feel, passive rotations an elastic end-feel and passive side flexions an elastic end-feel due to tissue tension. Passive flexion is not assessed because it would tend to aggravate the symptoms of disc lesion.
The resisted tests are not part of the routine examination at the cervical spine, but should be applied if there is a history of trauma, e.g. for a muscle lesion, suspected serious pathology such as fracture or metastases, or illness behaviour. The resisted tests also assess the C1 and 2 nerve roots. Resisted flexion may produce pain in a disc lesion since it causes compression.
The shoulder joint complex is eliminated as a cause of pain by active elevation. If this is full range and pain-free, the shoulder can be eliminated from the examination.
Assessment for objective root signs is conducted by a series of resisted tests for the myotomes, looking for a pattern of muscle weakness which would indicate nerve root compression. Since it is also important to eliminate other causes of arm pain, the muscle groups are also assessed, looking for alternative causes of pain. This explains why the sequence appears to test the same nerve roots several times.
A quick check of skin sensation to light touch is made looking for differences. Paraesthesia commonly affects the distal end of the dermatomes and these are assessed, followed by the biceps, brachioradialis and triceps reflexes.
The plantar response is assessed by stroking up the lateral border of the sole of the foot and across the metatarsal heads. If the response is extensor, i.e. upgoing, it is indicative of an upper motor neuron lesion. The normal response is flexor.
The objective examination sequence provides a basic assessment framework to glean important information towards the selection of patients appropriate for the treatment techniques used in orthopaedic medicine. It also highlights possible contraindications and provides a guide for the specific treatments to be used.
Any other assessment procedures may be included throughout the sequence or explored separately afterwards, including vestibular apparatus tests for benign paroxysmal positional vertigo (BPPV), either to elicit extra information for the purposes of the application of other treatment modalities, or to confirm findings necessitating patient referral.
CERVICAL LESIONS – A CLASSIFICATION SYSTEM OF THREE CLINICAL MODELS
In the past, treatment in orthopaedic medicine has been traditionally targeted at the disc, aiming to reduce displacement, relieve pain and restore movement. However, there is a lack of confidence in these traditional pathoanatomical diagnostic labels, since the cause of pain cannot be confidently localized to one specific structure (Peake & Harte 2005). Consequently, several authors have established classifications to determine treatment programmes and to predict prognosis (McKenzie 1981, Riddle 1998, Laslett & van Wijmen 1999, Tseng et al 2006).
While not ideal, the presentation of signs and symptoms of cervical lesions has been classified here into clinical models adapted from Cyriax’s original theories. These models are judgment based and contribute to the clinical decision-making process to rationalize appropriate treatment programmes, but are not intended to be restrictive. The reader is encouraged to be inventive, to draw on other experiences and to implement the approach into their existing knowledge when putting a treatment programme together for individual patients. The treatment techniques described are by no means a cure-all for every case of neck pain. However, uncomplicated lesions of recent onset may respond well to the mobilization and manipulative techniques of orthopaedic medicine. The key is the selection of appropriate patients for treatment.
For the purposes of the following classification of lesions, a cervical lesion presents with the following features:
• An onset of neck pain which may be in a multisegmental or segmental distribution (see Ch. 1)
• Symptoms such as a cough or sneeze increasing the pain and possible paraesthesia in a segmental pattern
• No significant ‘red’ and ‘yellow flags’ present in the history
• Examination revealing a non-capsular pattern of movement
• No contraindications to treatment.
Clinical Model 1: acute torticollis
Factors from the subjective examination
• Adolescent or young adult
• Mainly central, or short bilateral or unilateral neck pain
• Patient usually wakes with severe onset of pain, not attributable to any precipitating cause.
Factors from the objective examination
• Antalgic posture
• Non-capsular pattern of painful movements
• No neurological signs.
A convenient shorthand for recording the findings of the objective examination can be found in Appendix 3 where the ‘star diagram’ is explained.
Treatment consists of reassurance to both the patient and parents and progressive positioning out of the deformity may expedite reduction. Pain-relieving modalities may be applied, e.g. analgesics, electrotherapy, massage and gentle mobilization. Gentle traction may be applied in the form of the ‘bridging’ technique and progressed to manual traction, depending on irritability. Traction is applied in line with the deformity if the neutral position cannot be assumed. The condition usually resolves spontaneously in 7–10 days.
This treatment procedure can be applied to any acute/irritable neck in the absence of contraindications, e.g. the acute whiplash patient or the patient who fits classification of Clinical Model 2, but who has adopted an antalgic ‘wry’ neck and is too irritable for the treatment regime suggested below.
Clinical Model 2
Factors from the subjective examination
• Central, or short bilateral or unilateral neck or scapular pain
• Gradual or sudden onset
• Patient may or may not recall the exact mode and time of onset.
Factors from the objective examination
• Non-capsular pattern of painful and limited movements
• No neurological signs.
Treatment of choice is to follow the cervical manual traction and mobilization routine described below, providing no contraindications to treatment exist.
Clinical Model 3: presenting with referred arm symptoms
Factors from the subjective examination
• Patient usually over the age of 35
• Initial presentation of central or unilateral neck and/or scapular pain, followed by referred arm pain (the central pain ceasing or diminishing)
• Sudden or gradual onset
• Often part of a history of increasing, worsening episodes; therefore a progression of the above scenarios
• Patient may or may not recall the exact time and mode of onset
• Patient may complain of root symptoms, i.e. paraesthesia felt in a segmental pattern.
Factors from the objective examination
• Non-capsular pattern of movements producing neck and/or arm symptoms
• Root signs may be present, i.e. muscle weakness, absent or reduced reflexes.
The hypothesis is more readily rationalized here. Due to the nerve root involvement a large posterolateral prolapse of disc material could have occurred, producing pain referred into the arm through compression of the dural nerve root sleeve or the nerve root itself. Pain, therefore, is segmental in distribution. If the nerve root is involved, there will be objective neurological signs. Since the nerve roots emerge horizontally in the cervical spine, only one nerve root should be involved in disc lesions. The quality of the pain helps to distinguish somatic and radicular pain (see Ch. 1).
Murphy (2006) presented a case report where a patient developed arm pain after cervical manipulation. It was concluded that where cases of cervical herniated disc do occur, apparently after cervical manipulation, it is impossible to be sure that the worsening symptoms actually resulted from the treatment or whether symptom progression occurred due to the natural history of the condition.
Van Zundert et al (2006) put forward the following clinical tests as useful for the diagnosis of cervical radicular pain:
• Spurling’s test: reproduction of the patient’s neck and/or arm pain by combining extension of the spine with the head rotated towards the affected shoulder whilst applying axial compression.
• Axial manual traction test: the arm pain is relieved by the application of an axial traction force of approximately 10–15 kg in supine lying.
They note that all three tests have a high specificity but low sensitivity but are valuable aids in the clinical diagnosis of a patient with neck and arm pain nonetheless.
Treatment is aimed at relieving pain. If neurological signs are present, manipulation is not strictly contra-indicated provided the neurological signs are minimal and stable, i.e. non-progressive, and that no other contraindications exist. However, the more peripheral the signs and symptoms, the less likely manipulation is to succeed, and other modalities, e.g. manual or sustained mechanical traction and mobilization, may be more effective in relieving symptoms.
An epidural of corticosteroid and local anaesthetic may be indicated, but this is a specialist procedure usually conducted under ultrasonography. Since the natural history of disc herniation is one of recovery over time without the intervention of surgery, a mechanism of spontaneous recovery may occur (Saal et al 1996, Bush et al 1997).
TREATMENT OF CERVICAL LESIONS
It is recommended that a course in orthopaedic medicine is attended before the treatment techniques below are applied in clinical practice (see Appendix 1).
Treatment of cervical lesions depends on the nature of the pain and classification of the symptoms (see above). If the pain is subacute, i.e. with low severity and irritability, a regime of mobilization can be commenced, progressing to manipulation if necessary. If the pain is acute, i.e. with high severity and irritability, a gentler approach to treatment is adopted. In chronic cervical conditions, a thorough examination may reveal several components to the diagnosis, adverse neural tension and muscle imbalance, for example, together with underlying psychological factors such as anxiety and depression. All components of the patient’s condition must be addressed and the orthopaedic medicine approach and treatment techniques should form only part of the treatment programme. Their use is not intended to be exclusive.
Vernon et al (2007) conducted a review to explore the effect of manual therapy on patients with chronic neck pain, not due to whiplash injury, and not including headache or arm pain. There was moderate- to high-quality evidence that patients with the features described show clinical improvements from a course of spinal manipulation or mobilization at 6, 12 and up to 104 weeks post-treatment. The patient with more chronic neck pain is not usually put forward for the techniques in this text but the review does stimulate thought for the wider application of the approach.
In terms of likely response to manipulation, the ideal patient will fit into the pattern of signs and symptoms that indicate Clinical Model 2. In this model there will be a history of sudden or gradual onset of central, short bilateral or unilateral neck and/or scapular pain. On examination there is a non-capsular pattern of limited movement and no objective neurological signs. To be ideal in terms of likely effectiveness of treatment, the symptoms should be of recent onset and have minimal reference of pain. The more peripheral the symptoms, the less successful the techniques described tend to be.
Provided there are no contraindications to treatment, a regime of cervical mobilization is commenced. Treatment techniques are chosen based on a continuous assessment approach. After every technique the patient’s comparable signs are reassessed.
Following each session of treatment, advice is given about neck care, general posture, sleeping postures and pillows. Maintenance exercises are given if they are appropriate and the patient is started on a self-help programme to prevent recurrence.
Mobilization and manipulation techniques in orthopaedic medicine usually produce immediate results. One, two or three sessions of treatment would be expected. Vernon & Humphreys (2008) reviewed change scores in randomized controlled trials after one session of manual therapy and found moderate- to high-quality evidence that immediate improvements were obtained after one session of manipulation. The evidence for mobilization was less substantial with fewer studies reporting smaller immediate improvements. There was no evidence for a single session of manual traction. If a patient is not responding to the approach, it is abandoned and other mobilization and treatment modalities attempted.
Contraindications
It is impossible to be absolutely definitive about all contraindications and nothing can substitute for a rigorous assessment of the presenting signs and symptoms and an accurate diagnosis of a mechanical cervical lesion.
‘Red flags’ are signs and symptoms found in the patient’s subjective and objective examination that may indicate serious pathology and provide contraindications to cervical manipulation (Greenhalgh & Selfe 2006, Sizer et al 2007) (see Red flags p. 194).
The absolute contraindications are highlighted in the discussion below but there are several relative contraindications that should be considered as well. It may be useful to use the mnemonic ‘ COINS’ (a contraction of ‘contraindications’), as an aide-mémoire to be able to create mental categories for the absolute contraindications: Circulatory, Osseous, Inflammatory, Neurological and suspicious features indicating Serious pathology. If the first and last two letters are pushed together as ‘ CONS’, the crucial need for consent is emphasized.
The treatment regime discussed in this chapter is absolutely contraindicated in the absence of informed patient consent. The patient should be given all details of their diagnosis together with the proposed treatment regime and a discussion of the risks and benefits should ensue to enable them to give their informed consent (Oppenheim et al 2005). Consent is the patient’s agreement, written or oral, for a health professional to provide care. It may range from an active request by the patient for a particular treatment regime to the passive acceptance of the health professional’s advice. The process of consent, within the context of orthopaedic medicine, is ‘fluid’ rather than one instance in time when the patient gives their consent. The patient is constantly monitored and feedback is actively requested. The treatment procedures can be progressed or stopped at the patient’s request or in response to adverse reactions. Reassessment is conducted after each technique and a judgment made about proceeding. The patient has a right to refuse consent and this should be respected and alternative treatment options discussed. For further information on consent, the reader is referred to the Department of Health website: www.dh.gov.uk/consent.
Drop attacks are an absolute contraindication to the orthopaedic medicine cervical mobilization procedure, including manipulation, since these may be indicative of upper cervical instability or related to transient ischaemic attacks. Similarly, patients with a history of cerebrovascular accident, transient ischaemic attacks or other upper motor neuron lesions would be contraindicated and other treatment modalities should be considered if neck pain is a feature in these patients. Upper cervical spine instability is also associated with Down’s syndrome and active cervical rheumatoid arthritic changes. Ligamentous laxity may be a feature of pregnancy, making pregnancy a relative contraindication to manipulation.
A gradual onset of increasing pain over 3 months may be indicative of inflammatory arthritis, which may be a relative contraindication. Ankylosing spondylitis and other spondyloarthropathies, for example, may not affect the cervical spine, in which case the cervical mobilization regime discussed below would not be contraindicated and individual judgments would need to be made for each case. Rheumatoid arthritis, which usually affects the smaller joints symmetrically, may be progressing subclinically in the cervical spine; therefore the techniques discussed below would be contraindicated.
A subjective examination will screen a patient for symptoms of vertebrobasilar insufficiency and, if present, the treatment regime discussed below would be absolutely contraindicated. However, dizziness, a major symptom of vertebrobasilar insufficiency, is more commonly a symptom of cervical dysfunction. Reid & Rivett (2005) conducted a systematic review of the effectiveness of manual therapy for cervicogenic dizziness and found that there was some evidence, though acknowledged as limited, to support the use of manual therapy in treating cervicogenic dizziness. Therefore accurate diagnosis is essential here in order to prevent a large group of patients being denied a worthwhile treatment regime. The merits of the testing procedure for vertebrobasilar insufficiency will be discussed below and currently it is advisable to conduct a recognized test before applying manipulation. A positive vertebrobasilar test would be an absolute contraindication to manipulation. If the cause of dizziness cannot be ascertained, it would be wise to err on the side of caution.
Suspicious features indicative of non-mechanical lesions would be an absolute contraindication to the orthopaedic medicine treatment regime. These symptoms should not be considered in isolation but in the general context of the whole examination procedure and may include unexplained weight loss, poor general health, pain unaffected by posture or activity, constant pain of which night pain is a feature and double root palsy and cord signs such as spastic gait and/or abnormal plantar response. The patient with a past history of primary tumour is not strictly contraindicated, but diagnosis of a mechanical lesion must be certain before proceeding with the treatment regime since the pain of bony metastases may mimic mechanical pain. Suspicious findings occur in Pancoast’s tumour (a tumour in the apex of the lung) which may affect the lower trunks of the brachial plexus resulting in medial arm pain and C8 and/or T1 palsy that may be evident as atrophy of the ulnar border of the hand and a reduced triceps reflex (Pitz et al 2004). Objective examination may reveal provocation of pain by side flexion away from the painful side as the only positive cervical sign and this would be an unusual finding in a cervical disc lesion. Consider risk factors for atherosclerosis and symptoms that could be associated with internal carotid artery dysfunction, including headache described as ‘unlike any other’ (Kerry & Taylor 2006).
Symptoms of cervical disc lesions can generally be traced back to show a history of increasing and worsening episodes. Therefore cervical radiculopathy generally affects the over 35 age group. Of course, this can also occur in the younger age group, but diagnosis is again paramount, since this can be a suspicious feature associated with tumour. As in the lumbar spine, for the arm symptoms to be indicative of a mechanical lesion, the central symptoms are expected to subside considerably at the onset of arm pain. The nerve roots emerge horizontally, certainly in the upper cervical spine (see discussion in the anatomy section); therefore multiple nerve root involvement should be considered suspicious until the screening procedures in the subjective and objective examination confirm a mechanical diagnosis. Similarly, double nerve root involvement is unusual, particularly as the vertebral canal in the cervical region is large and can more readily accommodate a disc prolapse than in the lumbar spine. Bilateral arm signs and symptoms would therefore be an unusual presentation of a mechanical lesion. If, during the application of manual traction, the patient experiences an onset of paraesthesia in a multisegmental distribution, i.e. both hands, then the procedure should be stopped. Cyriax & Cyriax (1993) suggested this phenomenon is due to adherent dura, presumably therefore having implications for the mobility of the spinal cord, and alternative treatment options should be explored.
Cord signs, which may be associated with cervical myelopathy, are an absolute contraindication to cervical manipulation. A central disc prolapse may encroach on the dura mater producing bilateral symptoms; therefore central techniques might be appropriate, but rotatory techniques under traction would not. The importance of reducing small uncomplicated disc lesions is important to prevent future degenerative changes threatening the spinal cord (Ombregt et al 2003).
The acute cervical lesion is too irritable for manipulation but may well respond to mobilization, particularly the bridging or manual traction technique described below. An antalgic posture indicates severity. In radiculopathy with minimal, stable neurological signs, manipulation is not contraindicated but may not be effective. Increasing unstable neurological signs are a contraindication as the prolapse could be increased.
A history of recent trauma has been identified as a risk factor in vertebrobasilar insufficiency due to damage to the lining of the vertebral artery; therefore manipulation is contraindicated. Past history of trauma, head injury, fracture and previous surgery are all relative contraindications and judgments will have to be made on individual cases.
The drug history will identify the contraindication of anticoagulation therapy to manipulation due to the risk of intraspinal bleed. The long-term use of systemic steroid medication predisposes the patient to osteoporosis; this and a known diagnosis of osteoporosis is a contraindication to manipulation. Patients considered susceptible to osteoporosis may be relatively contraindicated and individual judgments will need to be made. Caution should be exhibited with patients who have blood clotting disorders.
Patients with psychosocial components, the so-called ‘yellow flags’, may be unsuitable for manipulation and even the mobilization regime may present a relative contraindication; again judgments will need to be made on individual cases.
Safety recommendations for spinal manipulative techniques are included in Appendix 2.
The cervical mobilization procedure
It is important to emphasize that the treatment techniques in this section will be described carefully in a step-by-step fashion to enable their application. However, the professional judgment and existing skill of the operator will allow each technique to be adapted.
The order of the techniques as they appear here merely provides a guide towards the development of skills at each stage and it is not necessarily an order of efficacy. It is important to reassess the patient’s comparable signs constantly and to decide on the next step in the light of patient response. In this way the orthopaedic medicine approach can properly be integrated into existing practice as fresh decisions are made and the underlying hypothesis is modified. It is traditional to start each session with manual traction as this technique seems to achieve the greatest response; if progression is required then the other techniques are carried out, with a vertebrobasilar artery test conducted prior to the application of manipulation in each session of treatment.
Manipulation as such, defined as a minimal amplitude, high velocity thrust at the end of range, is only carried out if judgment deems it necessary. The mobilization procedure suggested is usually sufficient to reduce most uncomplicated cervical lesions, i.e. with no neurological signs or symptoms.
The benefit of this cervical mobilization procedure outweighs the small risk of vertebrobasilar complications but that is not to take the risk lightly. The appropriate steps to minimize this risk, suggested in the guidelines below, should always be taken, but debate continues on the true incidence of complications from cervical manipulation, and it has been ascertained that the tests themselves carry a degree of risk. The reader is recommended to check for the most recent developments before applying the techniques, as safety issues are in a state of change and the most up-to-date guidelines should be adhered to.
The techniques involve traction, slight extension of the head and sometimes rotation, and these are the positions that have been implicated in causing compromise of the vertebral arteries. However, it should be emphasized that this is not the only area of danger in this region. The spinal cord is vulnerable to central disc herniation and these same positions assist centralization of the herniated disc material and aim to prevent further central displacement that could potentially endanger the cord.
It is not possible to say how many times each technique is to be attempted since the process of reassessment is the basis for each clinical decision and the circumstances vary in every clinical encounter. Nonetheless, a balance must be found between sidestepping the question and providing a recipe for treatment. Some guidance will be given in terms of expectations, with respect to the different states and stages of the conditions presented.
Generally, the more acutely painful lesions require gentle treatment, but reassurance and advice may be more appropriate in the early stages without the application of passive treatment. For the subacute or less irritable states, if the technique is helping, it is repeated; if no change occurs after a few attempts, the next technique is chosen; if the patient’s signs and symptoms are abolished, treatment should be stopped. Once improvement plateaus in each session, or if the progress is uncertain, the treatment is either modified to an alternative modality or ceased, with the patient being reassessed at the next session when a new status quo will have emerged.
In general, lesions producing central or bilateral pain should be treated with central techniques, i.e. manual traction, traction with leverage, anteroposterior glide. Lesions producing unilateral pain are treated with manual traction, progressing to rotational mobilizations under traction and manipulation if necessary. The monitoring that takes place during this step-by-step progression of forces is most important to safety.
Bridging technique
The indication for use is the patient with a highly irritable lesion: an acute whiplash injury; the acute torticollis seen in Clinical Model 1 and an acute presentation of Clinical Model 3. Progression to manual traction may be made as the irritability of the lesion is reduced. It is a method of applying gentle traction and should not produce an increase in symptoms.
Position the patient supine on the couch a little way down from the top edge. Sit on a chair at the head of the couch and gently adjust the patient’s position so that your forearms and elbows are fully supported, with your hands resting under the patient’s neck (Fig. 8.38). Make a bridge with the fingers and rest them under the occiput. Tilt the hands into radial deviation, which tips the head slightly back, and pull, flexing your forearms by pulling yourself towards the patient, to apply minimal traction. Hold the pull for a few seconds, according to patient comfort. Gently release and repeat as required, gradually applying more traction as the tissues relax. This technique can be adjusted and applied to different spinal segmental levels.
 |
| Figure 8.38
Bridging technique.
|
The following mobilization procedure is applied to the cervical lesion that ideally falls into Clinical Model 2. Manual traction may also be appropriate in Clinical Models 1 and 3. The rotation techniques are not contraindicated for either, but the lesion is possibly too irritable and may be made worse. The patient who falls into Clinical Model 3 and displays root signs will probably not be helped by manipulation, but again it is not a strict contraindication provided the neurological signs are not severe or progressive.
Manual traction (Cyriax 1984)
This is always recommended as the first technique to be performed in each treatment session for the less irritable or subacute neck. It is generally comfortable for the patient and allows both you and the patient to become used to the handling techniques. It is often not necessary to progress beyond this stage if positive results are achieved. After each technique an increase in range and/or decrease in symptoms is to be expected.
Explain the technique and its intention to the patient. All mobilization techniques are carefully monitored by the operator for a change in symptoms. Patients are instructed to signal if they wish the manoeuvre to stop for any reason. It is important that patients appreciate that they have control over each manoeuvre – the final overpressure of the Grade C manipulation (see below) is the only exception to this – which is why feedback on discomfort should be obtained from the patient right up to the moment of its application.
Raise the couch to be approximately level with your hips. Position the patient in supine with their shoulders level with the head of the couch and supporting their head in your hands. An assistant is needed to apply counterpressure at the same time as you apply the manual traction, by restraining the movement of the patient’s shoulders, adopting a walk-standing position at the side of the couch. If no assistant is available, restraining Cyriax ‘horns’ or non-slip matting may be used.
Position one of your hands to cup the occiput or support just below the occiput, allowing the head to tip into slight extension; the cervical spine itself should remain in a neutral position. The other hand rests comfortably around the patient’s chin, avoiding the trachea, and your forearm lies along the side of the face.
Place both feet directly under the patient’s head and bend both your knees. Lean out with straight arms to apply the traction using body weight (Fig. 8.39). Perform the technique slowly, allowing the traction to establish for several seconds, then pull yourself back to the upright position and release the traction.
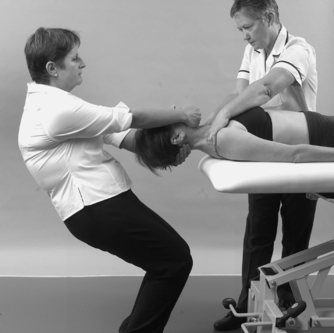 |
| Figure 8.39
Manual traction.
|
Sit the patient up slowly and re-examine the comparable signs, before deciding on the next manoeuvre.
Assess the patient’s body weight compared with your own. If you are much heavier than the patient do not apply maximum body weight. Less body weight can be achieved by positioning the feet a little further back but the arms should still be straightened.
A patient with dentures should leave them in situ with some padding placed between the teeth to help prevent discomfort or possible breakage.
This technique of manual traction is essential to most other manoeuvres and it is worthwhile practising it to gain confidence and competence.
A patient classified as Clinical Model 3 who experiences a reduction in signs and symptoms after the application of manual traction can be progressed to sustained mechanical traction as described below, since in the authors’ clinical experience manual traction provides a short temporary effect with this model.
Manual traction plus rotation (Cyriax 1984, Cyriax & Cyriax 1993)
The pain-free, or least painful, rotation is chosen first as this will be more comfortable for the patient. The hand holds are the same as above, with a right rotation requiring your right hand to be positioned around the chin (Fig. 8.40), and vice versa for a left rotation.
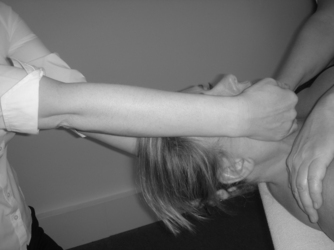 |
| Figure 8.40
Hand positioning for manual traction plus rotation to the right.
|
Apply the traction as above. Allow it to establish for a few seconds, then rotate into the least painful rotation by side-flexing your body. Return to the mid-position and release the traction as above. Three variations of this technique can be applied, and each is a progression of the other.
• Grade A: mid-range rotation (Fig. 8.41)
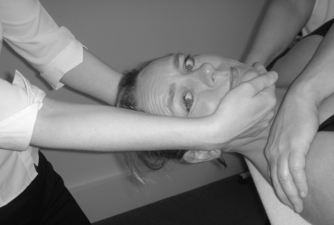 |
| Figure 8.41
Manual traction plus rotation, Grade A.
|
• Grade B: to end of available range (Fig. 8.42). Here it is so important to understand the nature of end-feel and to be sensitive to any abnormal end-feel such as muscle spasm, which suggests the technique should be abandoned.
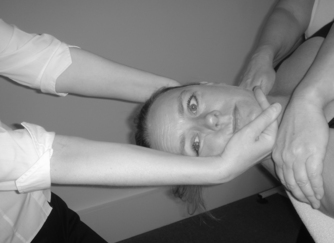 |
| Figure 8.42
Manual traction plus rotation, Grade B, and position at which a Grade C manipulation is applied.
|
If these two stages have proved unsuccessful, the procedure is now repeated using the same routine through Grades A and B into the more painful range of rotation. Under traction, this should not produce an increase in symptoms and any increase is an indication to stop. Again, progression is only made to the next step if necessary.
If this procedure has been followed, but the patient’s pain is not resolved, a return can be made to the least painful rotation to apply a Grade C manipulation, and then to the more painful rotation if necessary. It is important to note that it would be unusual for the full routine to be followed within one treatment session.
• Grade C: manipulation, the final high velocity, minimal amplitude thrust is applied at end of range. (See the ‘Guidance for pre-manipulative testing of the cervical spine’, based on Barker et al 2001, p. 212, before proceeding to manipulation.)
CERVICAL ARTERIAL DYSFUNCTION AND ASSESSMENT
Since the early 1980s, the potential risk to the cerebral blood flow from cervical manipulation techniques has received growing publicity in relation to incidents and accidents following cervical manipulation (Krueger & Okazaki 1980, Weinstein & Cantu 1991, Sinel & Smith 1993, Rivett 1994, Carey 1995, Sternbach et al 1995, Harvey et al 2003). Trauma to the vertebral or internal carotid arteries leading to stroke is a rare but devastating complication of cervical manipulation. Potential risk and precipitating factors have been suggested by various authors that are discussed here.
Haldeman et al (1999) assessed the English language literature from 1966 to 1993 to determine potential precipitating events and risk factors, classifying vertebrobasilar dissection and occlusions as traumatic, spontaneous or caused by spinal manipulation. Spinal manipulation was implicated in 115 cases and most of these were extrapolated or generalized from retrospective case reports. There was minimal detailed information on the magnitude of forces applied or the type of manipulative procedure applied.
Ernst (2007) conducted a systematic review of the literature to explore the adverse effects of spinal manipulation. Most problems were reported for the cervical spine with vertebral artery dissections caused by rotational forces occurring most commonly at the atlantoaxial joint. Vascular accidents were also reported. It was noted that chiropractors had the most incidents whilst acknowledging that they performed the greater number of manipulations. In context, however, a risk of 0.000008% was put forward which chiropractors argue is less of a risk than gastric bleeding associated with the ingestion of non-steroidal anti-inflammatory drugs.
Kerry et al (2008) argue that valid data are not available to be able to estimate the risk from cervical manual therapy, including manipulation, and that the comparison with the risks associated with medications, such as non-steroidal anti-inflammatory drugs, should be viewed with caution.
Magaray et al (2004) analysed the results from a survey of 480 members of the Australian Physiotherapy Association and presented the risk of adverse effects from manipulation in a novel way, reporting one adverse effect per 177.5 therapist weeks for high velocity thrust techniques, which were mostly associated with symptoms of VBI. The total figure for adverse effects from all manual therapy techniques applied to the cervical spine was 1.38 adverse effects per therapist over a 2-year period, i.e. 0.01 per week.
Initiation for the vertebral artery testing procedures seems to stem from cadaver studies conducted by Brown & Tatlow (1963) which showed the contralateral vertebral artery to be sometimes occluded on rotation, extension or combined rotation and extension. More recent studies have demonstrated conflicting results on the effect of position of the cervical spine on blood flow in the vertebral arteries, questioning the validity and clinical value of the pre-manipulative tests (Kerry & Taylor 2006, Kerry et al 2008).
The vertebral artery is most vulnerable in its third section, the atlanto-occipital region, where it takes two right-angled turns, one to pass behind the lateral mass of the atlas and the second to enter the foramen magnum. Here the artery is relatively fixed and immobile and manipulation involving rotation and/or extension has been implicated as a cause of vascular accident. The resultant shearing force on the arterial wall could lead to dissection, intramural haematoma and/or thrombus formation. The testing procedures commonly consist of rotation and extension which could be as hazardous as the manipulative techniques themselves; therefore the reliability and validity of the testing procedures has been questioned.
Rivett (1997) highlighted a case of a negative vertebral artery test despite complete occlusion. In contrast, Licht et al (2000) conducted a prospective study to assess vertebral artery blood flow in patients with positive pre-manipulative testing to investigate whether, despite the positive test, chiropractors would consider treating such patients if the subsequent Doppler ultrasound was normal. The majority of chiropractors surveyed agreed that they would proceed with the manipulation if the Doppler ultrasound was normal, despite the positive test. The study therefore concluded that a positive pre-manipulative test is not an absolute contra-indication to cervical manipulation.
Rivett et al (1999) conducted a two-group experimental pilot study with a small sample of 20 subjects, to determine the effect of rotation or extension on vertebral artery and internal carotid artery blood flow (the sustained positioning was held for up to 60, which is not comparable with the short duration that the position is assumed for the manipulative procedure). Some support for the reliability of pre-manipulative testing was demonstrated as significant changes occurred in blood flow in end-range positions involving contralateral rotation and extension. It was concluded that the screening procedures may be useful tests of the adequacy of the collateral circulation in preventing ischaemia if the blood flow through one of the vertebral arteries was critically reduced, for instance by cervical manipulation.
Mitchell et al (2004) set out to see if the rotation used in a standard vertebrobasilar artery insufficiency (VBI) test was associated with a measurable change in intracranial vertebral artery blood flow in young, healthy adults. The validity, specificity and sensitivity of the VBI test had been questioned since the results of previous blood flow studies had not been in agreement. Sustained, end-of-range, contralateral rotation of the cervical spine was shown to be associated with a significant decrease in mean intracranial vertebral blood flow velocity, irrespective of side. This supported other reports of decreased blood flow in both the intracranial and extracranial artery. The authors recommended that mobilization or manipulation should not be carried out immediately after applying the test to ensure there is sufficient time for the latent effect of the test to subside. The rotation was held for 30 s, which is a standard time for the test but not for the application of end-range rotation techniques (as applied in orthopaedic medicine). This does highlight the risk of the test itself and whether it is justified prior to mobilization as well as manipulation.
Kerry et al (2008) stressed the point that studies providing information on the effects on blood flow during cervical movements in healthy normals were a step towards establishing the effect of blood flow on cervical artery symptoms but emphasized that there was little evidence of correlation between blood flow changes and symptoms of VBI. Reduced blood flow to the brain can be recorded but without signs of cerebral ischaemia. The issue of false positives and negatives within the pre-manipulative testing procedures also affects the reliability of the tests. The applicability of hand-held Doppler ultrasound units to assist in identifying flow dysfunction is a focus of ongoing research (Kerry & Taylor 2006).
The Australian Physiotherapy Association (APA) was probably the first group to introduce a formal protocol for pre-manipulative testing of the cervical spine; the protocol was published in the Australian Journal of Physiotherapy (1988). A ‘Guidance for pre-manipulative testing of the cervical spine’ (Barker et al 2001) was devised as a joint venture between the Society of Orthopaedic Medicine and the Manipulation Association of Chartered Physiotherapists and was published in Manual Therapy, the Journal of Orthopaedic Medicine and subsequently in Physiotherapy. The guidance aimed to guide the decision-making process towards safe application of cervical manipulation, to heighten awareness of the risks and complications, and to improve quality of care by informing of current best practice.
A flowchart was produced on the basis of the guidance, which has been adapted in Figure 8.43 to guide clinical reasoning and pre-treatment screening. However, the reader is strongly advised to ascertain the most up-to-date guidelines in place at the time of reading this text (see MACP website –http://www.macpweb.org).
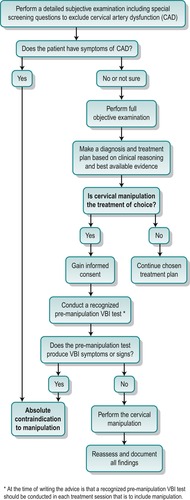 |
| Figure 8.43
Guidance for pre-manipulative testing of the cervical spine – flowchart.
|
The original APA protocol has now been updated and the results of the survey conducted by Magaray et al (2004) (as mentioned above) fed into the review. The survey found that of the 480 members who responded, two-thirds found the protocol valuable in their practice but just under a fifth used it only to satisfy legal requirements. The issue of consent was looked at as part of the protocol. Approximately one-third of respondents always informed the patient about potential dangers of cervical manipulation and a third (not necessarily the same respondents) sought consent on every occasion at which manipulation was used. The time taken to apply the protocol and gain consent was cited as the main criticism of the protocol with the doubts concerning the validity of the pre-manipulative tests and the possible deterrent to patients having the technique if too much was made of the risks – especially as the risks appear to be low. The survey drew out some honest insights from those who are expected to apply the protocol, which may resonate with other practising manual therapists.
The Manipulation Association of Chartered Physiotherapists (MACP) commissioned a review to begin in 2004 to satisfy an uncertainty in those performing manual therapy relating to the nature of cervical spine arterial insufficiency, the risks of manual therapy with respect to arterial complications, and to clarify the MACP’s stance on pre-cervical spine treatment screening (Kerry et al 2007). The review was conducted by an international body of reviewers and a variety of evidence sources were considered in categories relating to blood flow studies, case reports, surveys and reviews, and haemodynamic principles. The initial document created was published as a consultation document and input from all stakeholders was encouraged. As an outcome, an evidence-based information document on ‘Cervical Arterial Dysfunction and Manipulative Therapy’ has been published on the MACP website (http://www.macpweb.org) to guide clinical reasoning in the assessment of neck pain and headache, especially with regard to the possibility of the existence of vascular dysfunction. The review itself has been published in Manual Therapy (Kerry et al 2008).
Kerry & Taylor (2006) widened the focus of arterial dysfunction to include the internal carotid arteries. Their paper considered pathologies in both the vertebrobasilar arterial system (posterior system) and the internal carotid arteries (anterior system) and described the likely clinical presentations of both. If there is reduction of blood flow through the vertebral arteries, vertebrobasilar artery insufficiency symptoms may be evident that they summarize as the ‘5 Ds’ and ‘3 Ns’: Dizziness; drop attacks; diplopia; dysarthria; dysphagia: Ataxia: Nausea; numbness, nystagmus. Principal symptoms and signs of internal carotid artery dysfunction include pain in the neck, frontotemporal region or face; Horner’s syndrome; tinnitus; cranial nerve palsies; loss of vision and retinal infarction. Transient ischaemic attack and stroke could be serious outcomes of both vertebral and internal carotid artery dissection.
The benefit gained in pain relief and restoration of movement by an expertly applied manipulative technique should not be sacrificed in an attempt to be too cautious. Conflicting evidence of the risk versus the benefit can be found in the literature. Refshauge et al (2002) postulated that the risks outweigh the benefits while Jull et al (2002), in response, suggested that many of their arguments were biased or flawed and did not support their recommendations. Kerry et al (2008) doubt the accuracy of any risk–benefit analysis saying that the data are not available to support the analysis from either side.
The risk does nevertheless exist and techniques involving traction and rotation have received criticism for being the most hazardous (Grant 1988). However, as discussed above, what is often not taken into account is that there are two potential danger areas within the cervical spine: the cervical arteries and the spinal cord. There can be risks in not treating cervical disc lesions as potential osteophyte formation arising from traction on the vertebral end-plate could compromise the spinal cord.
Kerry & Taylor (2006) appeal to manual therapists to be suspicious of cervical vascular pathology, especially in cases of trauma or in acute onset and where a headache may be described as ‘unlike any other’. They recommend the expansion of manual therapy theory to include haemodynamic principles: their relationship to movement, anatomy and biomechanics and the implications for testing procedures; looking for vascular risk factors and including cranial nerve tests where internal carotid dissection is suspected.
The non-systematic review conducted by Kerry et al (2008) was the most up-to-date review at the time of writing. The term ‘cervical arterial dysfunction’ is now firmly embedded in the consciousness of musculoskeletal practitioners and the review examines several levels of evidence to support a comprehensive discussion on the association between cervical spine manual therapy and cervical artery dysfunction leading to cerebral ischaemic events. The review also searched for evidence relating to haemodynamics, arterial trauma and vascular pathology, and blood flow studies.
The review concluded that there was little support for the value of pre-manipulative screening tests in indicating vascular patency or predicting injury. This view had previously been put forward by Thiel & Rix (2005). There is evidence to support the relationship between vascular disease risk factors and cervical artery dysfunction (CAD) and a broader view of the patient’s health profile is recommended. Headache and neck pain are common presenting signs of vascular dissection and must at least be considered as part of differential diagnosis, especially in the acute neck. If there is a strong likelihood of cervical artery dissection, provocative pre-manipulation tests should not be performed and the patient should be referred appropriately (Thiel & Rix 2005).
Since cervical artery haemodynamics are influenced by movement as a whole, and not just by manipulative thrust techniques, a form of vascular risk assessment should be considered prior to all manual therapy procedures. Clinical reasoning may be limited if only the vertebral arteries are considered and there should be a wider risk assessment pertaining to the whole cervical arterial system and the range of possible vascular pathologies.
Before embarking on any cervical technique the clinician is recommended to follow the guidance for safe practice as suggested below. The guidance has been devised to minimize the risk of complications following cervical mobilization techniques. Several points need to be considered before embarking on the testing regime.
Dizziness is one of the first symptoms of vertebrobasilar insufficiency, but it is also a symptom associated with cervical dysfunction, cervicogenic headaches, benign positional vertigo and inner ear problems. The vertebrobasilar artery system supplies the vestibular nuclei in the brainstem and the labyrinth of the inner ear (Grant 1988). A careful history will assist diagnosis. If benign postural vertigo is suspected, diagnosis can be confirmed by performing Hallpike–Dix positional testing which, if positive, demonstrates a characteristic torsional nystagmus when the head is reclined and turned to the affected side (Lempert et al 1995, Magarey et al 2004, Johnson et al 2008). The test should not be considered in isolation, but in the context of the whole assessment procedure.
Cervical traction is used as a technique by itself, or in conjunction with mobilization and manipulation. It is used to decompress the cervical joints, protecting the spinal cord from further posterior displacement of the disc during the technique. Traction is recommended as a first manoeuvre; it is relatively gentle and can be stopped immediately if adverse effects are noted or reported. It often achieves an increase in range and decrease in pain, making the addition of further techniques unnecessary. Manipulation is only applied when a regime of manual traction and traction with mobilization has failed to be effective. By progressing the treatment in this way, adverse vertebrobasilar artery symptoms should be picked up before a manipulation is applied.
As a footnote to this section there is some evidence that manipulation to the thoracic spine may be useful in the management of patients with neck pain due to the biomechanical link between the cervical and thoracic spines (Cleland et al 2005, Cleland, Childs, & Fritz et al. (2007a) and Cleland, Glynn, & Whitman et al. (2007b)). As risk of complication is lower with a thoracic spine manipulation, thoracic manipulation might be a suitable, safer alternative to cervical manipulation.
Guidance for pre-manipulative testing of the cervical spine
The guidance for pre-manipulative testing (Barker et al 2001) aims to identify patients with a potential risk of vascular accident if the vertebral artery is compromised. Rather than testing for vertebrobasilar insufficiency, it aims to be a predictor of the ability of the collateral circulation to maintain perfusion of the brain should the cervical arteries be occluded. A detailed history, together with the objective examination, establishes a diagnosis. If manipulation is the treatment of choice, the clinician should discuss the risks and benefits of the proposed technique with the patient to gain informed consent. The flowchart (see Fig. 8.43) is self-explanatory, and it is currently advised that a recognized pre-manipulative test is conducted in each treatment session that is to include cervical manipulation. Some clinicians choose to apply the testing procedure prior to other cervical mobilization techniques, but this is not currently mandatory. Local policy sometimes drives the decision and justification should be available if the policy is challenged. The decision should be based on clinical judgment in the light of best available evidence and the reader is advised to keep abreast of developments occurring in the intervening time.
Any recognized pre-manipulative testing procedure can be applied. Positions can include extension, combined extension and rotation, the pre-manipulative position or rotation alone (Kerry & Taylor 2006). Kerry et al (2008) observe that the extension element of testing has been removed from the new Australian Physiotherapy Association protocol (2006) and appears to have taken into account that extension has been reported as the most influential movement for internal carotid artery flow. The test described below places the patient’s cervical spine in the simulated position for the manipulative procedures. However, this is a combined position, which is not exactly the same as the position for the individual techniques, nor can the manipulative thrust be simulated.
Position the patient in supine lying with the shoulders level with the end of the couch. Support the head comfortably. Place the head into a degree of extension (not full extension), with side flexion and rotation of the cervical spine (Fig. 8.44). Keep the patient talking and the eyes open while maintaining the position for 30 s unless symptoms are evoked before this time. Allow the patient to rest in neutral before repeating the test to the other side.
 |
| Figure 8.44
Vertebrobasilar artery test.
|
The presence of dizziness, nausea, vomiting, sweating, pallor, blurred vision, dysarthria, fainting, tinnitus or paraesthesia in the head or arm or nystagmus constitutes a positive test.
The main symptoms and signs of a positive vertebrobasilar artery test can be remembered using the five Ds: dizziness, diplopia, drop attacks, dysarthria and dysphagia – a useful aide-mémoire attributed to Coman (Grant 1988, Kerry & Taylor 2006).
If the test is found to be positive, slide the patient back onto the couch to remain lying in supine to recover. Help the patient to sit up steadily and check by questioning and observation that the symptoms have subsided before terminating the session, or continuing with a suitable alternative treatment modality.
Following the recommended guidance is the current basis for ensuring maximum safety and preparation for the unexpected. The clinician must take all due care while applying the test and the treatment techniques.
Anteroposterior glide under traction (Cyriax 1984, Cyriax & Cyriax 1993)
This technique is applied if the symptoms have centralized and the range of movement has increased, but extension remains slightly limited. The importance of fully reducing a disc prolapse is based on the hypothesis that a small central bulge, if left in situ, will gradually cause ligamentous traction and osteophyte formation, both of which have the potential to threaten the cord in later life. Therefore it aims to clear extension.
Place the couch a little lower than hip height. Position the patient supine on the couch as before, with an assistant ready to apply counterpressure. Stand sideways-on to the patient with both feet parallel and close to the couch, under the patient’s head. One hand cups below the occiput and the forearm supports the weight of the head while cradling it against your abdomen. The other hand is positioned on the chin to be able to apply both traction and retraction (Fig. 8.45). Make a bridge by spreading your index finger and thumb. Apply the web to the chin and curl the remaining fingers so that they tuck around and under the chin (Fig. 8.46).
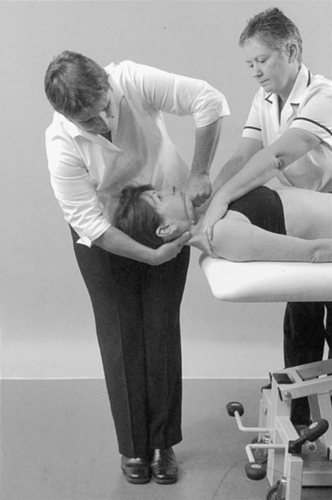 |
| Figure 8.45
Anteroposterior glide under traction, starting position.
|
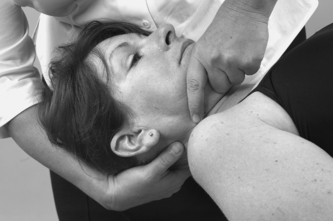 |
| Figure 8.46
Anteroposterior glide under traction, hand position.
|
Lean out sideways as far as possible to apply traction. Once traction is established, bend your knees and apply pressure over the chin, taking the chin into maximum retraction to produce the anteroposterior glide while avoiding pressure from the knuckles against the larynx (Fig. 8.47). Allow the chin to return to neutral, avoiding protraction, and release the traction. Sit the patient up and reassess. If the manoeuvre has helped it can be repeated, this time using several retractions.
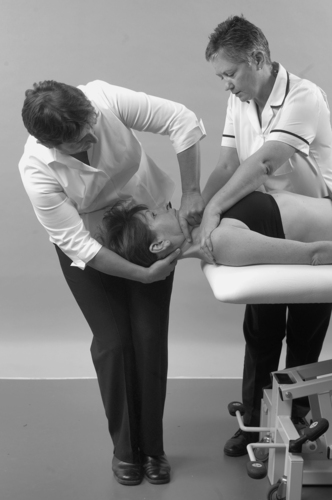 |
| Figure 8.47
Anteroposterior glide under traction, finishing position.
|
Lateral glide
This manoeuvre is used to relieve post-treatment soreness and to mobilize any residual tightness in the neck. It may be useful in mobilizing restricted side flexion in particular.
The couch is again slightly lower than hip height, as for the position for the anteroposterior glide under traction technique described above. No traction is applied with the lateral glide. An assistant, when available, stands parallel to the couch and the patient lies supine as above, but close to the assistant. The assistant grasps the patient’s opposite arm with both hands and holds the patient close to prevent lateral movement of the thorax.
Cup the patient’s head in both hands, fingers around the occiput and thumbs parallel with the mandible. Your thenar eminences should support just in front of the ears, resting comfortably over the temporomandibular joint (Fig. 8.49). Bend your knees and position your abdomen against the patient’s head but not enough to compress it. Apply the lateral glide by rocking from foot to foot, gradually increasing the pressure of your thenar eminences against the patient’s head as the patient relaxes and more movement becomes available (Fig. 8.48). To ensure that this is a rhythmical lateral gliding movement without side flexion, keep the patient’s nose straight and apply gentle pressure to the face as you move towards each side.
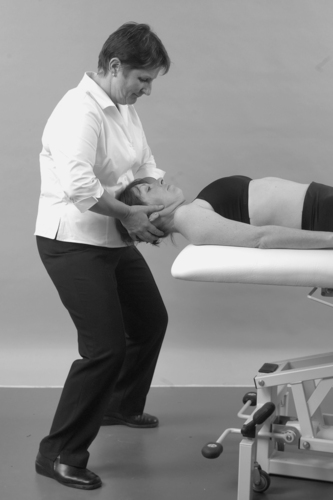 |
| Figure 8.48
Lateral glide.
|
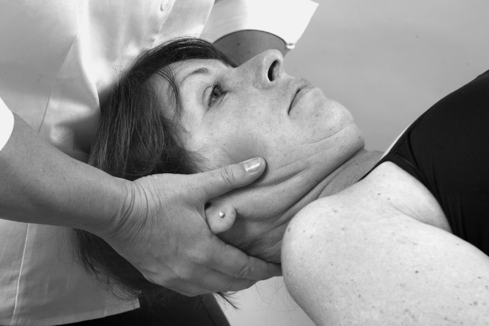 |
| Figure 8.49
Lateral glide, hand position.
|
Advanced manoeuvres
These are stronger manoeuvres and it is definitely recommended that they should be applied only after attending an orthopaedic medicine course.
Traction with leverage (Cyriax 1984, Cyriax & Cyriax 1993)
The indication for this technique is that stronger traction is required for central or bilateral symptoms.
Position the patient in supine lying with the occiput level with the end of the couch. Cup the occiput and rest the back of your hand on the couch. Apply manual traction as described previously. At the end of the technique, bend your knees smartly to apply more traction, using your hand underneath as a pivot (Fig. 8.50). Maintain the traction as you straighten your knees.
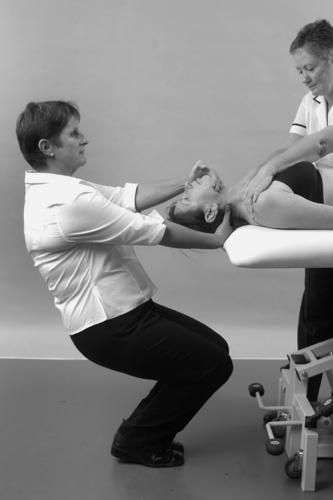 |
| Figure 8.50
Traction with leverage.
|
Manual traction plus rotation
This is the same technique as the rotation technique described previously, but it applies a different hand-hold with the neck starting in a degree of rotation, which gives a stronger rotation overall.
Start with the patient’s head turned into a small degree of rotation before applying the technique in order to secure a good grip. Cup your hand, pronate your forearm and place it comfortably on the side of the patient’s face with the fingers under the chin, such that they face the direction of the rotation you are aiming towards (Fig 8.51). Stand with your feet rotated a little in that same direction. Apply the manual traction and rotation in the progression recommended for the rotation techniques described above. (See Guidance for pre-manipulative testing, Fig. 8.43, before proceeding to manipulation.)
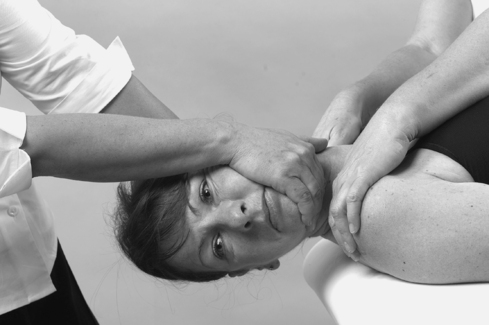 |
| Figure 8.51
Manual traction plus rotation. The alternative hand position produces stronger rotation (Grade B shown).
|
Manual traction plus side flexion (Cyriax 1984, Cyriax & Cyriax 1993)
If the rotations have not cleared the symptoms, a side flexion technique can be applied. The technique can be progressed through the Grade A (mid-range), Grade B (full range) and Grade C (manipulation at the end of range) as described for the rotations above. (See Guidance for pre-manipulative testing, Fig. 8.43, before proceeding to manipulation.)
Always start by side-flexing the patient away from the painful side.
Position the patient as for manual traction. An assistant stands on the side to which the neck will be side-flexed and moves the patient a little closer so that the patient’s shoulder fits into the corner of the couch. The assistant then reaches over to place a hand over the patient’s opposite shoulder to fix it by holding onto the top edge of the couch, so resisting the side flexion movement (Fig. 8.52).
 |
| Figure 8.52
Manual traction plus side flexion, hand position.
|
If you will be moving towards the patient’s left side flexion, place your left foot against the side of the couch, or against the assistant’s extended right foot. Lean out sideways to give traction, taking your right foot off the floor (Fig. 8.53). After a short pause to allow the traction to take effect, swing your body backwards, pivoting on your left foot and applying side flexion to the patient’s neck. The technique can be performed as a Grade A (mid-range) or Grade B (to the end of range). For the Grade C, whilst maintaining control, apply a smart thrust at the end of range and maintain traction as you swing your body back to the straight pull position, when you can steadily put your right foot to the floor and release the traction.
 |
| Figure 8.53
Manual traction plus side flexion.
|
As you practise this technique you will find that the momentum of the movement will help achieve a smooth transition through the various stages. Take care not to perform the technique too quickly or strongly whilst your skills improve.
Reverse the assistant’s position and the instructions if side flexion towards the right side is indicated.
Mechanical cervical traction
Cyriax did not advocate the use of cervical traction to the same extent as lumbar and was of the opinion that it was used ‘far too often’ (Cyriax 1982). A systematic review of the literature on the efficacy of traction for neck and back pain was presented by van der Heijden et al (1995), but most of the selected studies proved to be of poor quality. No clear indications of the effectiveness or ineffectiveness of traction could be ascertained from this study. Peake & Harte (2005) reviewed trials conducted between 1966 and 2004, to update the review of van der Heijden et al (1995) but remarked that the evidence had not changed substantially since the earlier review. They were unable to establish evidence either for or against the application of cervical traction. In spite of the lack of evidence to support the technique, the authors suggest that apparent benefits might be due to mobilization of the muscle and connective tissue, increased venous and lymphatic flow or psychological improvement, irrespective of any effect on the spinal joints.
Hickling (1972) described the application of sustained cervical traction in either sitting (cervical suspension) or lying, and various studies have been published to explore the merits or disadvantages of each. Cervical suspension, the application of vertical traction with the patient sitting, appeared to be the choice of Cyriax, but it may also be applied in supine lying (long traction) or inclined half-lying. Colachis & Strohm (1965) quoted the paper of Gartland which mentioned Krusen’s list of advantages of cervical suspension over long or horizontal traction:
• Convenience of application
• Elimination of friction
• Accuracy of measurement
• Facilitation of manipulation.
Stoddard (1954) was critical of cervical suspension since he observed that his patients found it difficult to relax in this position. He also believed that sustained traction impaired blood flow, and favoured intermittent traction. Deets et al (1977) mention that Maitland had the opposite view and preferred traction in sitting since his patients found it more comfortable. They also report that Crue found that greater foraminal separation was observed in the supine position than in sitting.
Colachis & Strohm (1965), in a study of 10 normal medical students, found that the separation of the cervical vertebrae under traction increased with the angle of flexion to the horizontal of the rope applying the pull. They found that the poundage applied had a comparatively greater effect on the joint separation and the maximum of 30 lb (14 kg) used in their investigation had the greatest effect. Judovitch (1952) studied radiographs of the cervical spine under different poundages of traction and found that 25 lb (11 kg) of vertical traction straightened the cervical lordosis, while at 45 lb (20.5 kg) a mean stretch of 5 mm was achieved in the cervical spine. Deets et al (1977) cite a further study of Colachis & Strohm which demonstrated that the greatest elongation of the posterior portion of the disc was observed with the angle of rope pull at 35° to the horizontal.
Hickling (1972) recounted Cyriax’s suggestion that cervical suspension should be just sufficient to lift the patient’s buttocks from the chair, implying that forces of near body weight were being applied. However, this was for a short duration of 1–5 min. In lying, average treatments ranged from 15 to 25 lb (7–11 kg), according to the size and overall response of the patient. The traction was applied horizontally or in slight flexion at an unspecified angle but with consideration for patient comfort and preference.
Readers must weigh up for themselves the pros and cons of applying traction in the sitting versus supine position. The characteristics of the individual patients encountered will form part of the decision-making process.
Another factor in the application of cervical traction is the length of time for which it should be applied. Colachis & Strohm (1965) noted that no further increase of intervertebral separation occurred after 7 s of sustained traction and Judovitch (1952) stated that the time of application should be decreased as greater forces were applied. Hickling (1972) suggested that traction of 15–25 lb (7–11 kg) should be continued for between 15 and 25 min. This recommendation is based on empirical findings and more work needs to be done to establish the optimum duration of treatment.
The literature is unclear in its support of either sustained or intermittent traction. Moeti & Marchetti (2001) used intermittent traction for patients with radicular symptoms and demonstrated that patients with symptoms of more than 12 weeks’ duration had minimal improvement, and those with symptoms for less than 12 weeks had a better outcome. Improved circulation, reduction of adhesions and reduction of pain via presynaptic inhibition at spinal cord level have been suggested as possible mechanisms for improvement due to intermittent traction (Wong et al 1997). Chung et al (2002) used MRI to evaluate the reducibility of cervical disc herniation under traction on 29 patients and seven healthy volunteers. Using a traction device which essentially consisted of a portable inflatable ‘accordion’-shaped neck collar to produce 30 lb of traction force over 10 min, an increase in length of the vertebral column was demonstrated in both groups, and 21 patients had complete or partial reduction of the disc herniation.
Contraindications to mechanical cervical traction
These are largely the same as for cervical manipulation and the reader is referred to the discussion on contraindications above.
Care should be taken in applying traction to the elderly and they must be thoroughly questioned for the presence of any contraindications.
Most traction beds and mobile traction equipment of modern design have the facility to apply cervical and lumbar traction.
A cervical harness is required to rest under the occiput and chin, with straps or cord passing to a spreader bar above the head. There are several types of harness which nowadays are made of washable materials, which also have the advantage of being pliable to give comfortable support. The simplest uses two padded rectangles which mould to the head as the traction is applied, but others are made of more substantial materials and have adjustable clasps to accommodate the differing shapes of patients.
The straps to the spreader bar should be long enough to give safe clearance above the head. A rope passes from the central point of the spreader bar via a pulley or pulleys to a fixing cleat in manually applied units, or directly into the housing of the automatic device.
Technique
Explain the technique carefully to the patient, mentioning possible after-effects such as stiffness or temporary increase in discomfort, and ask for consent to proceed.
If applying traction in the sitting position, use a firm chair with comfortable back support. Patients often like to have their arms resting on two or three pillows on their lap, both for support and to encourage relaxation. If applying traction in supine ensure that the patient is comfortable with one or two pillows under the head and knees as desired.
Put the cervical harness in place under the jaw and occiput, providing extra padding if necessary, and place tissue between the patient and the harness, for reasons of hygiene. Attach the harness to the traction unit and place on the appropriate setting, considering the patient’s size and general demeanour.
Discuss the treatment time with patients, explaining that the treatment aims to be as ‘long and strong’ as is comfortable, but they can call a halt to the treatment at any time. In practice, patients are usually comfortable with about 15 min traction at the first treatment and even on subsequent attendances 20 min is usually the maximum required.
Give patients sight of a clock and an alarm bell or buzzer and apply the traction steadily. Keep in close contact with the patient while the traction is being applied, since occasionally patients can become light-headed.
At the end of the treatment time release the traction slowly and observe the patient’s response as you do so. Let patients sit for a moment or two while they roll the shoulders and relax the tissues, and allow them to get up when they feel ready.
Apply the cervical harness in the same way as for sitting traction. Adjust the angle of pull and select the appropriate setting. Feedback from the patient will ensure that the traction is strong and comfortable. There is a convention that lesser weights are applied in traction in supine than in the sitting position, but for a longer time (Hickling 1972). On this basis, weights of 5–7 kg might be applied on the first treatment for 20 min, for example, increasing to 10 kg for 25–30 min at subsequent attendances.
Traction should be applied, preferably, on a daily basis but weekends can be excluded to allow any soft tissue discomfort from the application of the harness to subside. Patients may not be able to attend daily due to time or financial constraints and the pressures on outpatient departments may also deny this frequency. However, it is still appropriate to try the technique as frequently as possible since satisfactory results can still be achieved.
Improvement usually occurs after two to four treatments and the whole treatment episode may be continued over a 2- or 3-week period if necessary. If there is no change in the symptoms after four treatments the position, the weight, angle of pull or the time of application may be adjusted, but if there is still no change the technique should be abandoned and another modality or course of action selected.
Advice on neck care and posture should be given to patients while they are undergoing treatment and an appropriate gentle exercise programme should be devised.
REFERENCES
Aprill, C.; Dwyer, A.; Bogduk, N., Cervical zygapophyseal joint pain patterns II: a clinical evaluation, Spine 15 (1989) 458–461.
Australian Physiotherapy Association (APA), Protocol for premanipulative testing of the cervical spine, Aust. J. Physiother. 34 (1988) 97–100.
Australian Physiotherapy Association. 2006. Clinical guidelines for assessing vertebrobasilar insufficiency in the management of cervical spine disorders. Online. Available: http://www.Physiotherapy.asn.au
Barker, S.; Kesson, M.; Ashmore, J.; et al., Guidance for pre-manipulative testing of the cervical spine, Physiotherapy 87 (6) ( 2001) 318–322.
Beeton, K.; Jull, G., Effectiveness of manipulative physiotherapy in the management of cervicogenic headache: a single case study, Physiotherapy 80 (1994) 417–423.
Bird, H.A., 1995. Work related syndromes. In: Collected Reports on the Rheumatic Diseases. Arthritis and Rheumatism Council for Research, London, pp. 162-164.
Bogduk, N., The anatomy and pathophysiology of whiplash, Clin. Biomech. 1 (1986) 92–101.
Bogduk, N., The anatomical basis for cervicogenic headache, J. Manipulative Physiol. Ther. 15 (1992) 67–70.
Bogduk, N., The innervation of the intervertebral discs, In: (Editors: Boyling, J.D.; Palastanga, N.) Grieve’s Modern Manual Therapy2nd edn. ( 1994)Churchill Livingstone, Edinburgh, pp. 149–161.
Bogduk, N., Innervation and pain patterns of the cervical spine, In: (Editor: Grant, R.) Physical Therapy of the Cervical and Thoracic Spine2nd edn. ( 1994)Churchill Livingstone, Edinburgh, pp. 65–76.
Brown, B.St.J.; Tatlow, W.T., Radiographic studies of the vertebral arteries in cadavers – effects of position and traction on the head, Radiology 81 (1963) 80–88.
Bush, K.; Chaudhuri, R.; Hillier, S.; et al., The pathomorphologic changes that accompany the resolution of cervical radiculopathy, Spine 22 (2) ( 1997) 183–186.
Carey, P.F., Suggested protocol for the examination and treatment of the cervical spine – managing the risk, J. Can. Chiropr. Assoc. 39 (1995) 35–40.
Chung, T.-S.; Lee, Y.-J.; Kang, S.-W.; et al., Reducibility of cervical disk herniation: evaluation at MR imaging during cervical traction with a nonmagnetic traction device., Radiology 225 (2002) 895–900.
Clark, C., Rheumatoid involvement of the cervical spine., Spine 19 (1994) 2257–2258.
Cleland, J.A.; Childs, J.D.; McRae, M.; et al., Immediate effects of thoracic manipulation in patients with neck pain: a randomized clinical trial, Man. Ther. 10 (2005) 127–135.
Cleland, J.A.; Childs, J.D.; Fritz, J.M.; et al., Development of a clinical predication rule for guiding treatment of a subgroup of patients with neck pain: use of thoracic spine manipulation, exercise and patient education, Phys. Ther. 87 (1) ( 2007) 9–23.
Cleland, J.A.; Glynn, P.; Whitman, J.; et al., Short term effects of thrust versus no thrust mobilization/manipulation directed at the thoracic spine in patients with neck pain: a randomised controlled trial, Phys. Ther. 87 (4) ( 2007) 431–440.
Colachis, S.C.; Strohm, B.R., A study of tractive forces and angle of pull on vertebral interspaces in the cervical spine., Arch. Phys. Med. Rheumatol. 46 (1965) 820–830.
Connell, M.D.; Wiesel, S.W., Natural history and pathogenesis of cervical disc disease, Orthop. Clin. N. Am. 23 (1992) 369–380.
Cook, C.; Brismée, J.-M.; Fleming, R.; et al., Identifiers suggestive of clinical cervical spine instability: a Delphi study of physical therapists, Phys. Ther. 85 (9) ( 2005) 895–906.
Cooper, G.; Bailey, B.; Bogduk, N., Cervical zygapophyseal joint pain maps., Am. Acad. Pain Med. 8 (2007) 344–352.
Cyriax, J., 8th edn.Textbook of Orthopaedic Medicine. vol. 1 ( 1982)Baillière Tindall, London.
Cyriax, J., 11th edn.Textbook of Orthopaedic Medicine. vol. 2 ( 1984)Baillière Tindall, London.
Cyriax, J.H.; Cyriax, P.J., Cyriax’s Illustrated Manual of Orthopaedic Medicine. ( 1993)Butterworth Heinemann, Oxford.
Deets, D.; Hands, K.L.; Hopp, S.S., Cervical traction, Phys. Ther. 57 (1977) 255–261.
Department of Health, Cervical spine instability in people with Down syndrome. ( 1995)Chief Medical Officer’s Update, 7. HMSO, London.
Doherty, M., Fibromyalgia syndrome, In: (Editors: Lewin, I.G.; Seymour, C.A.) Collected Reports on the Rheumatic Diseases ( 1995)Arthritis and Rheumatism Council for Research, London, pp. 83–86.
Ernst, E., Adverse effects of spinal manipulation: a systematic review, J. R. Soc. Med. 100 (2007) 330–338.
Frumkin, L.R.; Baloh, R.W., Wallenberg’s syndrome following neck manipulation., Neurology 40 (1990) 611–615.
Grant, R., Dizziness testing and manipulation of the cervical spine, In: (Editor: Grant, R.) Physical Therapy of the Cervical and Thoracic Spines ( 1988)Churchill Livingstone, Edinburgh, pp. 111–124.
Greenhalgh, S.; Selfe, J., Red Flags. ( 2006)Elsevier, Edinburgh.
Hain, T., 16 March, 2009. Drop attacks. Online. Available: http://dizziness-and-balance.com
Haldeman, S.; Kohlbeck, F.; McGregor, M., Risk factors and precipitating neck movements causing vertebrobasilar artery dissection, Spine 24 (8) ( 1999) 785–794.
Harvey, E.; Burton, A.K.; Moffett, J.K.; et al., Spinal manipulation for low back pain: a treatment package agreed to by the UK chiropractic, osteopathy and physiotherapy professional associations, Man. Ther. 8 (2003) 46–51.
Hazelman, B.L., Polymyalgia rheumatica and giant cell arteritis, In: (Editors: Lewin, I.G.; Seymour, C.A.) Collected Reports on the Rheumatic Diseases ( 1995)Arthritis and Rheumatism Council for Research, London, pp. 97–100.
Herrick, A.L., Reflex sympathetic dystrophy, In: (Editors: Lewin, I.G.; Seymour, C.A.) Collected Reports on the Rheumatic Diseases ( 1995)Arthritis and Rheumatism Council for Research, London, pp. 132–137.
Hickling, J., Spinal traction technique, Physiotherapy 58 (1972) 58–63.
Hinton, M.A.; Harris, M.B.; King, A.G., Cervical spondylolysis, Spine 18 (1993) 1369–1372.
Jenkins, D.G., Clinical features of arm and neck pain., Physiotherapy 65 (1979) 102–105.
Johnson, E.; Landel, R.; Kusunose, R.; et al., Positive patient outcome after manual cervical spine manage-ment despite a positive vertebral artery test, Man. Ther. 13 (2008) 367–371.
Judovitch, B., Herniated cervical disc, Am. J. Surg. 84 (1952) 646–650.
Jull, G.A., Headaches of cervical origin, In: (Editor: Grant, R.) Physical Therapy of the Cervical and Thoracic Spine2nd edn. ( 1994)Churchill Livingstone, Edinburgh, pp. 261–285.
Jull, G., Cervical headache – a review, In: (Editors: Boyling, J.D.; Palastanga, N.) Grieve’s Modern Manual Therapy2nd edn. ( 1994)Churchill Livingstone, Edinburgh, pp. 333–347.
Jull, G.; Magarey, M.; Niere, K.; et al., Physiotherapy, a responsible profession to use cervical manipulation: response to Refshauge et al, Aust. J. Physiother. 48 (2002) 180–183.
Kang, J.D.; Georgescu, H.I.; McIntyre-Larkin, L.; et al., Herniated cervical intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2, Spine 20 (1995) 2373–2378.
Kerry, R.; Taylor, A., Cervical arterial dysfunction assessment and manual therapy, Man. Ther. 11 (2006) 243–253.
Kerry, R.; Taylor, A.; Mitchell, J.; et al., Cervical Arterial Dysfunction and Manipulative Therapy: Information Document., MACP, Westbourne. ( 2007).
Kerry, R.; Taylor, A.; Mitchell, J.; et al., Cervical arterial dysfunction and manual therapy: a critical literature review to inform professional practice, Man. Ther. 13 (2008) 278–288.
Kleynhans, A.M.; Terrett, A.G.J., The prevention of complications from spinal manipulative therapy, In: (Editors: Glasgow, E.F.; Twomey, L.T.; Scull, E.R.; et al.) Aspects of Manipulative Therapy ( 1985)Churchill Livingstone, Edinburgh, pp. 161–175.
Krakenes, J.; Kaale, B.R.; Moen, G.; et al., MRI assessment of the alar ligaments in the late stage of whiplash injury – a study of structural abnormalities and observer agreement, Neuroradiology 44 (2002) 617–624.
Krakenes, J.; Kaale, B.R.; Moen, G.; et al., MRI assessment of the alar ligaments in the late stage of whiplash injury, Neuroradiology 46 (2) ( 2003) 165–166.
Kristjansson, E., The cervical spine and proprioception, In: (Editors: Boyling, J.D.; Jull, G.A.) Grieve’s Modern Manual Therapy ( 2005)Churchill Livingstone, Edinburgh, pp. 243–256.
Krueger, B.R.; Okazaki, H., Vertebral-basilar distribution infarction following chiropractic cervical manipulation, Mayo Clin. Proc. 55 (1980) 322–331.
Kumar, P.; Clark, M., Clinical Medicine. 5th edn. ( 2002)Baillière Tindall, London.
Laslett, M.; van Wijmen, P., Low back and referred pain: diagnosis and a proposed new system of classification., NZ J. Physiother. 27 (2) ( 1999) 5–14.
Lempert, T.; Gresty, M.A.; Bronstein, A.M., Benign positional vertigo – recognition and treatment, Br. Med. J. 311 (1995) 489–491.
Licht, P.; Christensen, H.; Hoilund-Carlsen, P., Is there a role for pre-manipulative testing before cervical manipulation?J. Manipulative Physiol. Ther. 23 (3) ( 2000) 175–179.
McKenzie, R.A., The lumbar spine: mechanical diagnosis and therapy.. ( 1981)Spinal Publications, Waikanae, New Zealand.
McKinney, L.A., Early mobilization and outcome in acute sprains of the neck, Br. Med. J. 299 (1989) 1006–1008.
Magaray, M.E.; Rebbeck, T.; Coughlan, B.; et al., Pre-manipulative testing of the cervical spine review, revision and new guidelines, Man. Ther. 9 (2004) 95–108.
Mathews, J.A., Acute neck pain – differential diagnosis and management, In: (Editors: Lewin, I.G.; Seymour, C.A.) Collected Reports on the Rheumatic Diseases ( 1995)Arthritis and Rheumatism Council for Research, Chesterfield, pp. 142–144.
Matsuda, Y.; Sano, N.; Watanabe, S.; et al., Atlanto-occipital hypermobility in subjects with Down’s syndrome, Spine 20 (1995) 2283–2286.
Mealy, K.; Brennan, H.; Fenelon, G.C.C., Early mobilization of acute whip-lash injuries, Br. Med. J. 292 (1986) 656–657.
Mendel, T.; Wink, C.S.; Zimny, M., Neural elements in human cervical intervertebral discs, Spine 17 (1992) 132–135.
Mercer, S.; Bogduk, N., The ligaments and annulus fibrosus of the human adult cervical intervertebral discs, Spine 24 (7) ( 1999) 619–628.
Mercer, S.; Bogduk, N., Joints of the cervical vertebral column, Phys. Ther. 31 (4) ( 2001) 174–182.
Mitchell, J.; McKay, A., Comparison of left and right vertebral artery intracranial diameters, Anat. Rec. 242 (1995) 350–354.
Mitchell, J.; Keene, D.; Dyson, C.; et al., Is cervical spine rotation, as used in the standard vertebrobasilar insufficiency test, associated with a measurable change in intracranial vertebral artery blood flow?Man. Ther. 9 (2004) 220–227.
Moeti, P.; Marchetti, G., Clinical outcome from mechanical intermittent cervical traction for the treatment of cervical radiculopathy: a case series, J. Orthop. Sports Phys. Ther. 31 (4) ( 2001) 207–213.
Mooney, V.; Robertson, J., The facet syndrome, Clin. Orthop. Relat. Res. 115 (1976) 149–156.
Moore, A.; Jackson, A.; Jordan, J.; et al., Clinical guidelines for the physiotherapy management of whiplash associated disorder. ( 2005)Chartered Society of Physiotherapy, London.
Murphy, D.R., Herniated disc with radiculopathy following cervical manipulation: nonsurgical management, Spine J. 6 (4) ( 2006) 459–463.
Netter, F.H., Musculoskeletal System, Ciba Collection of Medical Illustrations. vol. 8 ( 1987)Ciba-Giegy, New Jersey.
Nilsson, N., The prevalence of cervicogenic headache in a random population sample of 20–59 year olds., Spine 20 (1995) 1884–1888.
Nordin, M.; Frankel, V.H., Basic Biomechanics of the Musculoskeletal System. 3rd edn. ( 2001)Lippincott, Williams & Wilkins, Philadelphia.
Oliver, J.; Middleditch, A., Func-tional Anatomy of the Spine. 2nd edn. ( 2006)Butterworth-Heinemann, Edinburgh.
Ombregt, L.; Bisschop, P.; tar Veer, H., A System of Orthopaedic Medicine. 2nd edn. ( 2003)Churchill Livingstone, Edinburgh.
Oppenheim, J.; Spitzer, D.; Segal, D., Nonvascular complications following spinal manipulation, Spine J. 5 (6) ( 2005) 660–666.
Palastanga, N.; Field, D.; Soames, R., Anatomy and Human Movement. 5th edn. ( 2006)Butterworth-Heinemann, Edinburgh.
Peake, N.; Harte, A., The effectiveness of cervical traction., Phys. Ther. Rev. 10 (2005) 217–229.
Pitz, C.; de la Rivière, A.B.; van Swieten, H.A.; et al., Surgical treatment of Pancoast tumours, Eur. J. Cardiothorac. Surg. 26 (2004) 202–208.
Pizzutillo, P.D.; Woods, M.; Nicholson, L.; et al., Risk factors in Klippel–Feil syndrome, Spine 19 (1994) 2110–2116.
Prescher, A., Anatomy and pathology of the ageing spine, Eur. J. Radiol. 27 (1998) 181–195.
Pueschel, S.M.; Moon, A.; Scola, F.H., Computerised tomography in persons with Down syndrome and atlantoaxial instability, Spine 17 (1992) 735–737.
Refshauge, K.M.; Parry, S.; Shirley, D.; et al., Professional responsibility in relation to cervical spine manipulation, Aust. J. Physiother. 48 (2002) 171–178.
Reid, S.A.; Rivett, D.A., Manual therapy treatment of cervicogenic dizziness: a systematic review, Man. Ther. 10 (2005) 4–13.
Riddle, D.L., Classification and low back pain: a review of the literature and critical analysis of selected systems, Phys. Ther. 78 (7) ( 1998) 708–737.
Rivett, D., Preventing neurovascular complications of cervical manipulation., Phys. Ther. Rev. 2 (1997) 29–37.
Rivett, D.; Sharples, K.J.; Milburn, P.D., Effects of pre-manipulative tests on vertebral artery and internal carotid artery blood flow – a pilot study, J. Manipulative Physiol. Ther. 22 (6) ( 1999) 368–375.
Rivett, H.M., Cervical manipulation: confronting the spectre of the vertebral artery syndrome., J. Orthop. Med. 16 (1994) 12–16.
Saal, J.S.; Saal, J.A.; Yurth, E.F., Nonoperative management of herniated cervical intervertebral disc with radiculopathy, Spine 21 (16) ( 1996) 1877–1883.
Schoensee, S.K.; Jensen, G.; Nicholson, G.; et al., The effect of mobilisation on cervical headaches, J. Orthop. Sports Phys. Ther. 21 (1995) 184–196.
Schwarzer, A.C.; Aprill, C.N.; Derby, R.; et al., Clinical features of patients with pain stemming from the lumbar zygapophyseal joints, Spine 19 (1994) 1132–1137.
Sinel, M.; Smith, D., Thalamic infarction secondary to cervical manipulation, Arch. Phys. Med. Rehabil. 74 (1993) 543–546.
Sizer, P.; Brismée, J.; Cook, C., Medical screening for red flags in the diagnosis and management of musculoskeletal spine pain, Pain Pract. 7 (1) ( 2007) 53–71.
Sjaastad, O., Cervicogenic headache – the controversial headache, Clin. Neurol. Neurosurg. 94 (Suppl.) ( 1992) 147S–149S.
Standring, S., Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 40th edn. ( 2009)Churchill Livingstone, Edinburgh.
Sterling, M.; Kenardy, J., Physical and psychological aspects of whiplash: important considerations for primary care assessment, Man. Ther. 13 (2008) 93–102.
Sternbach, G.; Cohen, M.; Goldschmid, D., Vertebral artery injury presenting with signs of middle cerebral artery occlusion, J. Vasc. Dis. 46 (1995) 843–846.
Stewart, M.; Maher, C.; Refshauge, K.; et al., Randomised controlled trial of exercise for chronic whiplash-associated disorders, Pain 128 (1-2) ( 2007) 59–68.
Stoddard, A., Traction for cervical nerve root irritation, Physiotherapy 40 (1954) 48–49.
Tanaka, N.; Fujimoto, Y.; Howard, S.; et al., The anatomic relation among the nerve roots, intervertebral foramina and intervertebral discs of the cervical spine, Spine 25 (3) ( 2000) 286–291.
Taylor, A.; Kerry, R., Neck pain and headache as a result of internal carotid artery dissection: implications for manual therapists, Man. Ther. 10 (1) ( 2005) 73–77.
Taylor, J.R.; Twomey, L.T., Acute injuries to cervical joints: an autopsy study of neck sprain, Spine 18 (1993) 1115–1122.
Taylor, J.R.; Twomey, L.T., Functional and applied anatomy of the cervical spine, In: (Editor: Grant, R.) Clinics in Physical Therapy of the Cervical and Thoracic Spine2nd edn. ( 1994)Churchill Livingstone, Edinburgh, pp. 1–25.
Thiel, H.; Rix, G., Is it time to stop pre-manipulation testing of the cervical spine?Man. Ther. 10 (2005) 154–158.
Toole, J.F.; Tucker, S.H., Influence of head position on cerebral circulation, Arch. Neurol. 2 (1960) 616–622.
Tseng, Y.-L.; Wang, W.T.J.; Chen, W.-Y.; et al., Predictors for the immediate responders to cervical manipulation in patients with neck pain, Man. Ther. 11 (2006) 306–315.
Van der Heijden, G.; Beurskens, A.; Assendelft, W.; et al., The efficacy of traction for back and neck pain: a systematic, blinded review of randomised clinical trial method, Phys. Ther. 75 (2) ( 1995) 93–104.
Van Zundert, J.; Harney, D.; Joosten, A.J.; Duriweux, M.; et al., The role of the dorsal root ganglion in cervical radicular pain: diagnosis, pathophysiology, and rationale for treatment, Reg. Anesth. Pain Med. 31 (2) ( 2006) 152–167.
Vernon, H.; Humphreys, B.K., Chronic mechanical neck pain in adults treated by manual therapy: a systematic review of change scores in randomized controlled trials of a single session, J. Man. Manip. Ther. 16 (2) ( 2008) E42.
Vernon, H.; Humphreys, B.K.; Hagino, C., Chronic mechanical neck pain in adults treated by manual therapy: a systematic review of change scores in randomized controlled trials, J. Manipulative Physiol. Ther. 30 (2007) 215–227.
Walker, D.J., Rheumatoid arthritis, In: (Editors: Lewin, I.G.; Seymour, C.A.) Collected Reports on the Rheumatic Diseases ( 1995)Arthritis and Rheumatism Council for Research, Chesterfield, pp. 39–44.
Walsh, M.T., Therapist management of thoracic outlet syndrome, J. Hand Ther. Apr/May ( 1994) 131–144.
Weinstein, S.M.; Cantu, R.C., Cerebral stroke in a semi-pro football player – a case report, Med. Sci. Sports Exerc. 22 (1991) 1119–1121.
Wong, A.; Lee, M.-Y.; Chang, W.; et al., Clinical trial of cervical traction modality with electromyographic biofeedback., Am. J. Phys. Med. Rehabil. 76 (1) ( 1997) 19–25.
Zeidman, S.M.; Ducker, T.B., Rheumatoid arthritis: neuroanatomy, compression, and grading of deficits, Spine 19 (1994) 2259–2266.