CHAPTER 22 The Cerebral Venous System in Meningioma Surgery
INTRODUCTION
Venous sacrifice has always been a key problem in neurosurgery. For many years, surgery in and around the superior longitudinal and lateral sinuses has been debated in the literature.1–6 Neurosurgeons understand the importance of Labbé, Trolard, and sylvian veins; they have learned to preserve the parasagittal bridging veins and have discovered the venous anastomotic channels, mainly with the advent of magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA). But in meningioma surgery, interest has focused more on arterial vascularization, arterial feeders, and preoperative embolization than on preoperative study of veins. However, most postoperative pitfalls in meningioma surgery, primarily in convexity and parasagittal meningiomas, have a venous origin, due to either a venous infarct or sacrifice of an anastomotic channel. Therefore, for some time we have directed much attention in preoperative angiography, MRI, and MRA toward the study of veins close to the meningioma or en route to it in a falcine location. We have also tested ourselves on the venous pathways and channels when a sinus was occluded without related neurologic signs.7 In this chapter, we consider convexity, parasagittal, and falcine meningiomas from the aspect of venous challenge.
GENERAL CONSIDERATIONS
The gold-standard treatment is complete removal of the tumor as well as the invaded dura and bone.8 But total removal should never be attempted without preservation of quality of life. Therefore, it is crucial to keep several principles in mind so as to plan the surgical opening adequately and to place the head of the patient in the best position to benefit from brain relaxation as described in the text that follows. At present, although we may rely on neuronavigation systems to avoid a wrong opening, it is also mandatory to enter in the computer program information on all the veins to preserve.
CONVEXITY MENINGIOMAS
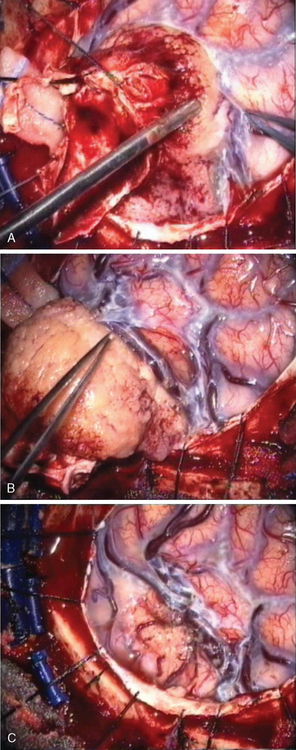
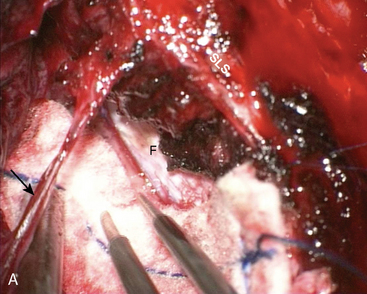
Convexity meningiomas rarely impair venous drainage but the second challenge is the dissection of the veins adhering to the tumor. The key is to stay extrapially as much as possible and to dissect the tumor in the arachnoidal plane. Many arachnoidal adhesions may be cut without coagulation (Fig. 22-1). Even bipolar coagulation is dangerous if it is too close to a vein. Meticulous dissection, never hurried, will successfully separate veins that initially seemed impossible to spare. It will keep the cortex intact in extrapial meningiomas but also preserve the integrity of the surrounding cortex in subpial tumors. Traction is applied to the tumor to lift it after progressive separation from the brain. This helps to cut arachnoid adhesions and coagulate away progressively arteries as small branches feeding the tumor, as well as veins that are more fragile and more delicate to dissect. The technique is recommended in all convexity locations, not only in the rolandic area, as brain softening from venous infarct may lead to disastrous consequences.
PARASAGITTAL MENINGIOMAS
Parasagittal meningiomas are tumors arising at the convexity of the hemisphere, just off the midline adjacent to SSS and falx, which may involve one, two, or three walls of the SSS with or without occlusion of its lumen. They have a predilection to arise where arachnoidal granulation tissue is the most pronounced9 and in 15% they invade the SSS.8 Simpson8 studied the possibilities of recurrence of intracranial meningiomas and reported that the infiltration of the SSS was a major reason for tumor recurrence. It is well established that the recurrence rate correlates significantly with the quality of the resection but is somewhat tempered by the knowledge that small tumor remnants may at times remain unchanged for several years. The goal is complete removal of the tumor, but the quality of life may be compromised by the surgery. Consequently, complete removal of parasagittal meningiomas by resection of the dural attachment involving the wall(s) of the SSS, and their reconstruction, represents a real surgical challenge. In the 1970s, several neurosurgeons described their surgical techniques for reconstruction of the SSS and collateral veins in dogs4,10 or in patients with good clinical and radiologic results.1,3 At the time, only computed tomography (CT) and conventional angiography were available. For the most part, classification of meningiomas was essentially based on surgical findings.
CLASSIFICATION, DIAGNOSIS, AND PREOPERATIVE PLANNING
In 1978, we described a surgical classification with 8 subtypes of parasagittal meningiomas1 but as a result of our experience during the last 20 years, we have simplified it into 5 categories (Table 22-1) that are more in accordance with our current surgical policy.11 This classification is designed to help plan a rational surgical strategy.
| Type I: | The meningioma is attached only to the outer surface of the sinus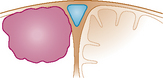 |
| Type II: | The meningioma enters the lateral recess of the SSS.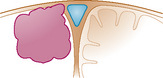 |
| Type III: | The meningioma invades one SSS wall.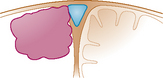 |
| Type IV: | The meningioma invades two walls of a still patent sinus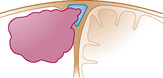 |
| Type V: | The meningioma spreads over the midline, invades the three walls with occlusion of the SSS.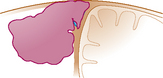 |
Reproduced with permission from Hancq S, Balériaux D, Brotchi J. Surgical treatment of parasagittal meningiomas. Semin Neurosurg 2003;14(3):203–10.
CT provides a means to see bone invasion by the meningioma, but currently MRI with and without gadolinium is the most accurate radiologic exam to determine the configuration, size, and consistency of the tumor, and the relationship among the meningioma, the adjacent brain, and the blood vessels. But the gold-standard examination today is MRI combined with MRA, as we reported in 1996.7 MRA provides all the crucial information concerning the venous system without the invasiveness of the DSA: the degree of SSS invasion, the permeability or the thrombosis of the SSS, and the major pathways of collateral circulation on both sides. MRA is even superior to DSA because MRA detects blood flow in all directions simultaneously:
OPERATIVE APPROACH
Position
The patient is placed in a supine, lateral, or prone position according to the location of the meningioma (anterior third, middle third, or posterior third of the SSS). In the anterior third, the patient is supine with the head slightly elevated. In middle third prerolandic area, the patient is placed in a lateral position with the head well elevated so that the scalp over the center of the tumor is uppermost,12 but in front of the rolando–parietal area, we prefer to put the patient in a lateral position with the tumor down, similar to the position used in the posterior third. In the posterior third, we prefer to place the patient in an adapted three-quarter prone position with the tumor below the midline. The position, which we commonly use for pineal area tumors, takes advantage of gravity by allowing the brain to fall away from the midline, which avoids unnecessary brain retraction.13,14 This is of special interest when the connection with the falx is significant, or when the attachment to the dural convexity is small.
Bone Flap
The bone flap is one of the major steps of the procedure. If not well done, surgery may be very difficult and uncontrollable bleeding may occur. A free parasagittal bone flap, centered over the tumor (2–3 cm posterior and 2–3 cm anterior to the tumor boundaries) is performed with several burr holes just over the midline in type I and beyond the midline in all other types except in special situations described later. Indeed, it is necessary to have perfect control over the SSS in most cases. However, great care should be taken to avoid injuring the contralateral dura and subsequent veins. The bone cuts across the midline are performed last, after the other bone cuts have already been made, so that if there is any suspicion of an air embolus, the bone flap can be quickly elevated. Cautious preoperative study of the diploic veins enables the surgeon to preserve those important anatomic networks in designing and cutting the bone flap to avoid injuring the vital pathways when the SSS is occluded. To avoid cortical damage, the dura mater and bone are carefully separated because of the presence of draining veins and the possible invasion of the dura or bone by the meningioma. If there is an extensive bone invasion, it may be safe to create a crown of burr holes around the involved bone and to leave it attached to the tumor.15 It is therefore possible to elevate the surrounding bone flap without any risk of cortical damage. Invaded bone is removed by rongeurs or a high-speed drill. After the bone flap is elevated, venous bleeding from the SSS may be controlled by Gelfoam and gentle pressure with cotton strips.
Tumor Removal
The meningioma is carefully dissected from the adjacent brain under microscopic control. It is important to stay close to the tumor capsule. When the tumor is extrapial, it may be separated as described in the preceding text for convexity meningiomas. When the lesion is subpial, it is important to clearly define the plane with adjacent brain. The use of cottonoid is useful and safe. The key is to work circumferentially from the periphery to the midline, from the surface to the depth, with meticulous coagulation and division of all the small blood vessels between the brain and the tumor capsule. As a blood vessel on the surface of the meningioma is encountered, it must first be identified, and if the vessel supplies the tumor (and not the brain) it may be coagulated on the tumor and cut. Most of the arteries may be freed from the tumor provided the small lateral feeding branches are located, and these are coagulated and divided. Sometimes it may be useful to internally debulk very firm meningiomas with an ultrasonic aspirator or cautery loops to obtain easier access to the separation plane to dissect. As few retractions as possible of the adjacent brain should be made, as most of those tumors have close relationships with the motor area. Another key point is the absolute preservation of all the bridging veins, which should be separated from the tumor and not injured to avoid neurologic deficit. Nevertheless, sometimes it may be necessary to reconstruct a bridging vein. For example, a large rolandic vein may be totally embedded by the meningioma, and its separation without wall injury is not always possible. Smooth dissection will preserve it, but occasionally a tear may occur. The injury should be cautiously repaired. We achieved this twice with good clinical and radiologic results.7 In the case of sinus repair (see later), we take advantage of a venous graft collateral branch which is sutured end-to-end to the stump of the bridging vein. Microvascular laboratory training is strongly encouraged.
SSS SURGICAL STRATEGY
In 1978, we showed the feasibility of SSS reconstruction and described our surgical technique.1 That aggressive surgery has also been successful in the hands of others3–5,16–20 and also confirmed by us.2 It may be successful in experienced hands only and it is currently a matter of debate with the revolution of brain imaging. In the 1970s, only CT and DSA were available, and in the 1980s conventional MRI, but we could not anticipate the venous anatomy as well as we do today and we were attempting tumor removal with opening of the SSS, resection of one or two walls, grafting the SSS, sometimes completely, without knowledge of the existence of collateral bypasses. Cortical venous rerouting in parasagittal meningiomas was already described in 1974,21 as well as the role of scalp veins as collateral pathways with parasagittal meningiomas occluding SSS.22 The question of grafting in totally occluded SSS with the help of sinus pressure monitoring has also been raised.19 With the new radiologic imaging, surgery can be meticulously prepared with preservation of cortical draining veins and bypasses and adjacent brain, keeping in mind the need to minimize or prevent neurologic deficit.7 The question of sinus grafting is again a matter of debate.
What Do We Recommend?
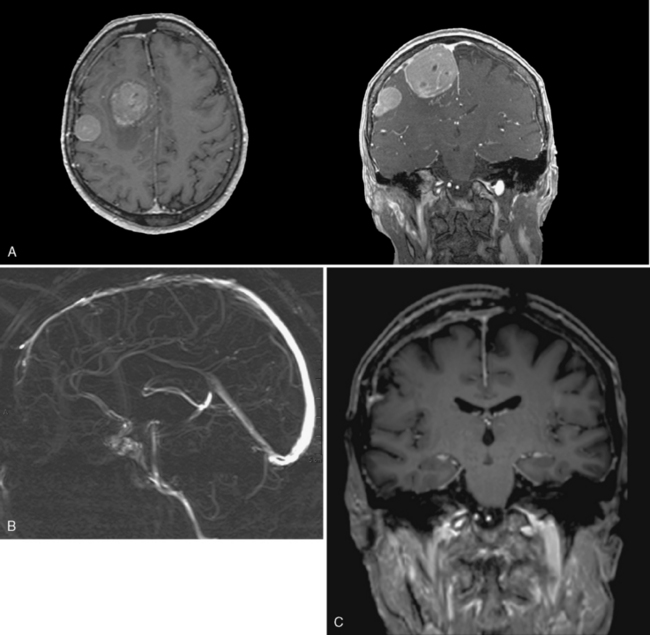
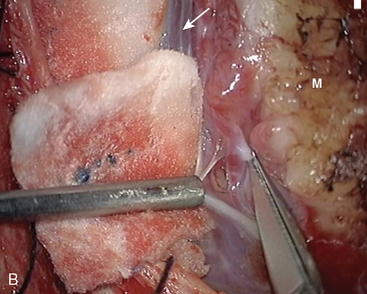
In types I and II, SSS grafting is not necessary. In type I, the attachment of the tumor to SSS can be coagulated with bipolar forceps and peeled until a clean, shiny dural surface is obtained, even by resection of the external dural sinus layer (Fig. 22-2). In type II, the lateral angle recess is progressively opened and a meningioma bud is very often removed. Then, as soon as it is cleaned, the opening is closed with a 6-0 nylon suture at the level of the angle while taking care not to narrow the SSS.
In type IV, two sinus walls are invaded. The SSS is not completely occluded. The third wall, which is sound, receives venous inflow from the opposite side, since the SSS drains both hemispheres. It would be very dangerous to excise all three walls and to interrupt the SSS. If grafting has been decided on, it should have been prepared on the table at that stage. Then, the intrasinusal portion of the meningioma may be excised as well as the two invaded sinus walls, without touching the sound third wall, to preserve the rolandic vein inflow from the contralateral cortex (for technical details, see refs. 1 and 10). The vein graft, which consists of a segment of the internal saphenous vein, is longitudinally opened and prepared at the size of the sinus walls to be replaced. The internal valves of this vein determine the direction of blood flow. Great care should be taken not to place the graft in the wrong direction! A traumatic and very regular suture maintains watertight closure. When there is a rolandic vein opening in the third wall, it should be left open and blood loss grossly controlled by digital compression by the assistant on both openings of the SSS. When there is no contralateral main vein entering the sinus, the technique described by Hakuba can be helpful in massive bleeding.3 It consists of using a shunting extracorporeal tube, ballooned at each end, and inserted into SSS beyond each extremity of the tumoral invasion. That avoids air embolism or unnecessary bleeding. But, like Sindou,7 we prefer not to place any clip on the SSS, or any tube or balloon into the SSS because it can damage the endothelium and septa, with the risk of secondary thrombosis. We prefer the interrupted gentle and soft compression by the finger of the assistant helped by small pledgets of Surgicel. Then, it is important to keep the venous graft open by attaching it by a broad suture to a piece of dura, the extremities of which are sutured under tension to the dural edges and hinged to the bone. This maintains the triangular anatomic shape of the SSS, which is mandatory for patency. Local perfusion of saline–heparin solution is maintained during the suture and platelet antiaggregates are given orally long before and after surgery.
Present Indications for SSS Reconstruction
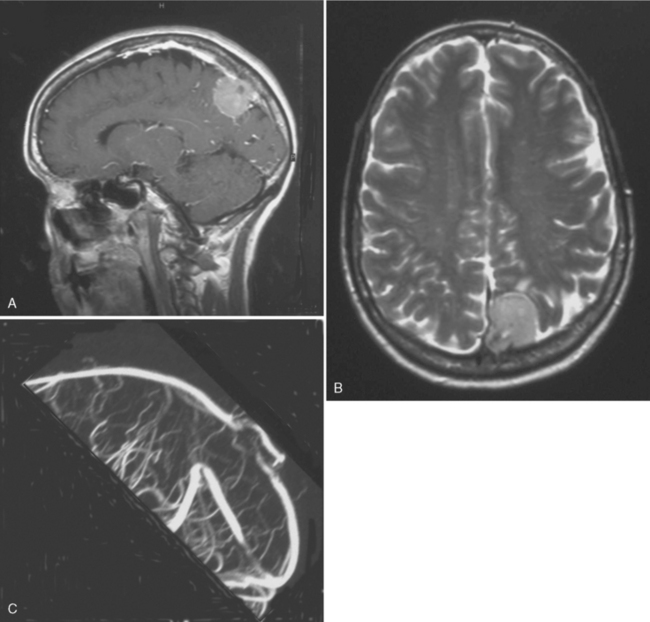
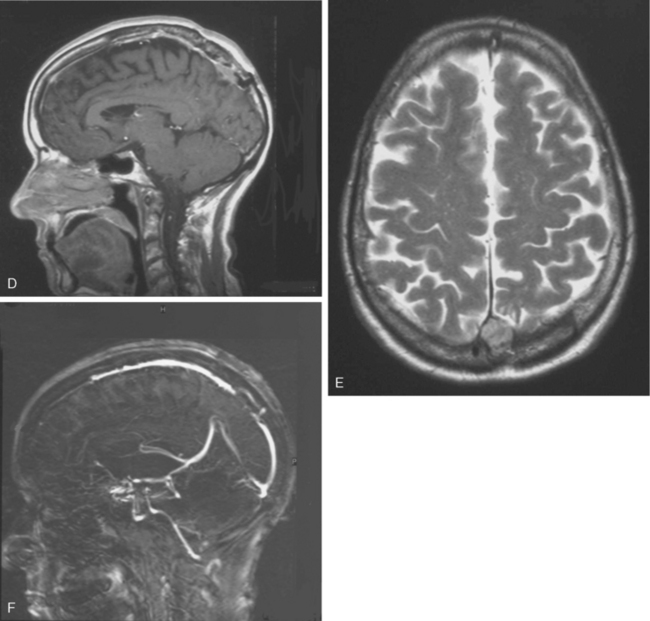
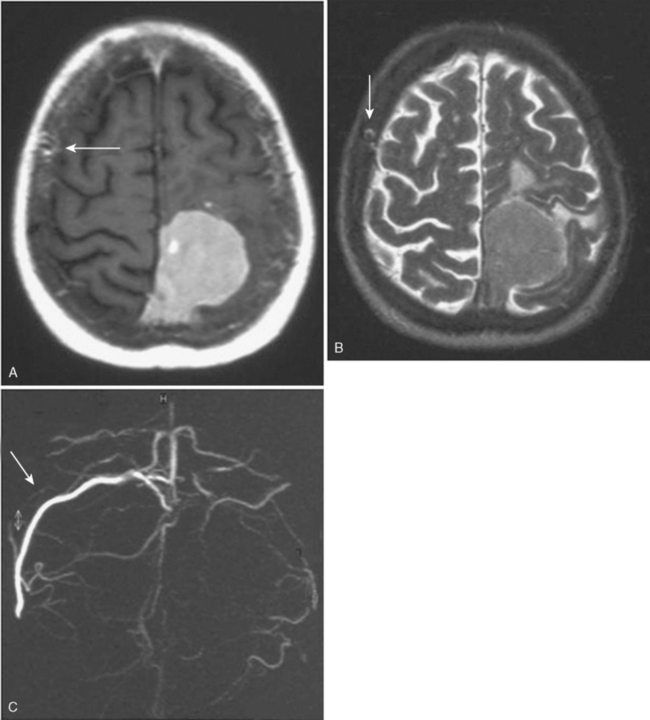
Since 1978, other diagnostic tools have been available, such as MRI, which more accurately defines and visualizes the tumor but especially follows the precise evolution of a residual meningioma. It is now well established that a meningioma is a slowly growing tumor. Even if the resection is not totally performed, the residue can remain stable for years. Even if a regrowth occurs, it can be only a few millimeters per year with a progressive collateral circulation and progressive sinus thrombosis preventing any neurologic deficit. Interest in refined stereotactic radiosurgical techniques has also been reported.23–25 With this new information, and even if we showed 30 years ago the feasibility of a sinus reconstruction, this surgery is not without risk and requires experienced neurosurgeons. Further, we observed 2 recurrences at 10 and 13 years after complete tumor removal and total SSS grafting, which raised the question of radical resection with SSS grafting. Both cases had a postoperative patent graft that came to late secondary occlusion due to tumor recurrence without any neurologic deficit. For this reason, we reviewed our position on the indication of the sinus reconstruction, which is not without risk. But avoiding the interruption of a partially occluded SSS is mandatory. The reconstruction of the sinus wall is now rarely performed in our center. If a parasagittal meningioma invades the sinus wall without occlusion of the SSS (types III and IV), we remove the extrasinusal meningioma and follow the residue via annual MRI and MRA (Fig. 22-3). If the residue regrows, a complementary gamma-knife procedure is performed to attempt to control the tumor before any second surgery. Of course, we need long follow-up to estimate the accuracy of that strategy. We now try not to touch the sinus until its complete obliteration. SSS receives blood from both hemispheres, but also from other dural, cortical, or diploic veins. Because of these anastomotic networks, clinical signs are frequently absent after SSS obstruction. Progressive gradual obstruction of the SSS may lead to a well developed collateral venous pathway26 that must be kept intact in parasagittal meningioma surgery, which is our current approach with type V meningiomas (Fig. 22-4). Preoperatively, we carefully study the venous pathway and the venous flow direction and search for all collateral venous channels. We prepare a preoperative venous chart and plan to preserve all those channels at surgery. In this way, we may safely remove completely a type V parasagittal meningioma, with the occluded SSS, without the need of a venous graft. But great care is taken to keep intact all the collateral venous bypasses that have developed with time, shunting the occluded SSS without any neurologic warning. If any of those collateral veins is impaired, venous infarction and brain swelling almost always occur, which could explain rare severe brain swelling in patients treated by en bloc resection of a meningioma with occluded SSS without venous reconstruction.
FALCINE MENINGIOMAS
The problem is to safely arrive at the level of the falx. Patient positioning is also important and we follow exactly the procedure described in the preceding text for parasagittal meningiomas. Preoperative MRA or DSA will provide information on the location of the major bridging veins, thereby enabling us to adapt the bone opening according to those data. Further, the dura opening is delicate because of the bridging veins. Sometimes, we leave in place a piece of dura over a big vein when its separation seems too dangerous. Here, more than elsewhere, taking advantage of brain gravity by making the opening on the side down is of great importance. A problem frequently encountered is the opening of the interhemispheric fissure because of thick arachnoid adhesion between some large veins and the dura (Fig. 22-5). Under the microscope and with patience and precision, the white arachnoid sheet may be dissected, divided, and separated from the dura close to the midline, allowing us to achieve free space to control the falx above and below the meningioma. Thereafter, safe cutting the falx all around the tumor may begin, dividing also all feeders. Great care should be taken to avoid any injury on the contralateral hemisphere. Sometimes, the tumor is too large to be delivered between large bridging veins and it is therefore wise to make a piecemeal debulking or to divide the meningioma into several small pieces.
[1] Bonnal J., Brotchi J. Surgery of the superior sagittal sinus in parasagittal meningiomas. J Neurosurg. 1978;48:935-945.
[2] Bonnal J., Brotchi J. Reconstruction of the superior sagittal sinus in parasagittal meningiomas. In: Schmidek H.H., editor. Meningiomas and their surgical management. Philadelphia: WB Saunders; 1991:221-229.
[3] Hakuba A., Huh C.W., Tsujikawa S., Nishimura S. Total removal of a parasagittal meningioma of the posterior third of the sagittal sinus and its repair by autogenous vein graft. Case report. J Neurosurg. 1979;51:379-382.
[4] Sindou M., Mazoyer J.-F., Fischer G., et al. Experimental bypass for sagittal sinus repair. Experimental study. J Neurosurg. 1976;44:325-329.
[5] Sindou M. Meningiomas invading the sagittal or transverse sinuses, resection with venous reconstruction. J Clin Neurosci. 2001;8(Suppl. 1):8-11.
[6] Sindou M.P., Alvernia J.E. Results of attempting radical tumor removal and venous repair in 100 consecutive méningiomas involving the major dural sinuses. J Neurosurg. 2007;105:514-525.
[7] Brotchi J., Patay Z., Baleriaux D. Surgery of the superior sagittal sinus and neighboring veins. In: Hakuba A., editor. Surgery of the intracranial venous system. Tokyo: Springer-Verlag; 1996:207-219.
[8] Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
[9] Maxwell R.E., Chou S.N. Parasagittal and falx meningiomas. In: Schmidek H.H., editor. Meningiomas and their surgical management. Philadelphia: WB Saunders; 1991:211-221.
[10] Bonnal J., Buduba C. Surgery of the central third of the superior sagittal sinus. Experimental study. Acta Neurochir. 1974;30:207-215.
[11] Hancq S., Balériaux D., Brotchi J. Surgical treatment of parasagittal meningiomas. Semin Neurosurg. 2003;14(3):203-210.
[12] Ojeman R.G., Ogilvy C.S. Convexity, parasagittal and parafalcine meningiomas. In: Appuzo M.L.J., editor. Brain surgery. Complication avoidance and management. New York: Churchill Livingstone; 1993:187-202.
[13] Brotchi J., Raftopoulos C., Levivier M., et al. Lésions de la région pinéale et falco-tentorielle. Abord occipito-pariétal en trois-quart ventral avec volet infrasagittal. Neurochirurgie. 1991;37:410-415.
[14] Schevach I., Cohen M., Rappaport Z.H. Patient positioning for the operative approach to midline intracerebral lesions: technical note. Neurosurgery. 1992;31:154-155.
[15] Al-Mefty O., editor. Meningiomas. New York: Raven Press, 1991.
[16] Steiger H.J., Reulen H.J., Huber P., Boll J. Radical resection of the superior sagittal sinus meningioma with venous interposition graft and reimplantation of the rolandic veins. Case report. Acta Neurochir (Wien). 1989;100:108-111.
[17] Bederson J.B., Eisenberg M.B. Resection and replacement of the superior sagittal sinus for treatment of parasagittal meningioma: technical case report. Neurosurgery. 1995;37:1015-1019.
[18] Murata J., Sawamura Y., Saito H., Abe H. Resection of a recurrent parasagittal meningioma with cortical vein anastomosis: technical note. Surg Neurol. 1997;48:592-597.
[19] Schmid-Elsaesser R., Steiger H.J., Yoursy T., et al. Radical resection of meningiomas and arteriovenous fistulas involving critical dural sinus segments: experience with intraoperative sinus pressure monitoring and elective sinus reconstruction in 10 patients. Neurosurgery. 1997;41:1005-1018.
[20] Hakuba A. Reconstruction of dural sinus involved in meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:371-382.
[21] Marc J.A., Schechter M.M. Cortical venous rerouting in parasagittal meningiomas. Radiology. 1974;112:85-92.
[22] Waga S., Handa H. Scalp veins as collateral pathway with parasagittal meningiomas occluding superior sagittal sinus. Neuroradiology. 1976;11:199-204.
[23] Kondziolka D., Flickinger J.C., Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma study group. Neurosurgery. 1998;43:405-414.
[24] Kondziolka D., Nathoo N., Flickinger J.C., et al. Long-term results after radiosurgery for beningn intracranial tumors. Neurosurgery. 2003;53:815-822.
[25] Pollock B.E., Stafford S.L., Utter A., et al. Stereotactic radiosurgery provides equivalent tumor control to Simpson grade I resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003;55:1000-1005.
[26] Oka K., Go Y., Kimura H., Tomonaga M. Obstruction of the superior sagittal sinus caused by parasagittal meningiomas: the role of collateral venous pathways. J Neurosurg. 1994;81:520-524.