Chapter 32
The Cerebral Cortex
Histology of the Cerebral Cortex
Neurotransmitters of the Cerebral Cortex
Neuron Types in the Cerebral Cortex
Intrinsic Circuitry of the Cerebral Cortex
Synopsis of Thalamocortical Relationships
Blood Supply to the Cerebral Cortex
Dominant Hemisphere and Language
Wernicke Aphasia and Broca Aphasia
Conduction Aphasia and Global Aphasia
Parietal Association Cortex: Space and Attention
The cerebral cortex is the organ of thought. More than any other part of the nervous system, the cerebral cortex is the site of the intellectual functions that make us human and that make each of us a unique individual. These intellectual functions include the ability to use language and logic and to exercise imagination and judgment.
OVERVIEW
The cerebral cortex is a dense aggregation of neuron cell bodies that ranges from 2 to 4 mm in thickness and forms the surface of each cerebral hemisphere. The total area of the cerebral cortex is about 2500 cm2, a little larger than a single page of a newspaper. Neurons in the cortex receive input from many subcortical structures by way of the thalamus and also from other regions of the cortex via association fibers. Cortical neurons, in turn, project to a wide range of neural structures, including other areas of the cerebral cortex, the thalamus, the basal nuclei, the cerebellum via the pontine nuclei, many of the brainstem nuclei, and the spinal cord.
The cerebral cortex is divided into distinct functional areas, some of which are devoted to the processing of incoming sensory information, others to the organization of motor activity, and still others primarily to what are considered higher intellectual functions. These functions include memory, judgment, the planning of complex activities, the processing of language, mathematical calculations, and the construction of an internal image of an individual’s surroundings. In this chapter, the focus is on (1) the basic internal organization of the cerebral cortex at the cellular level, (2) the parceling of the cortex into distinct subregions on the basis of cellular organization and neural connections, and (3) the functional properties of some higher order association cortical regions.
HISTOLOGY OF THE CEREBRAL CORTEX
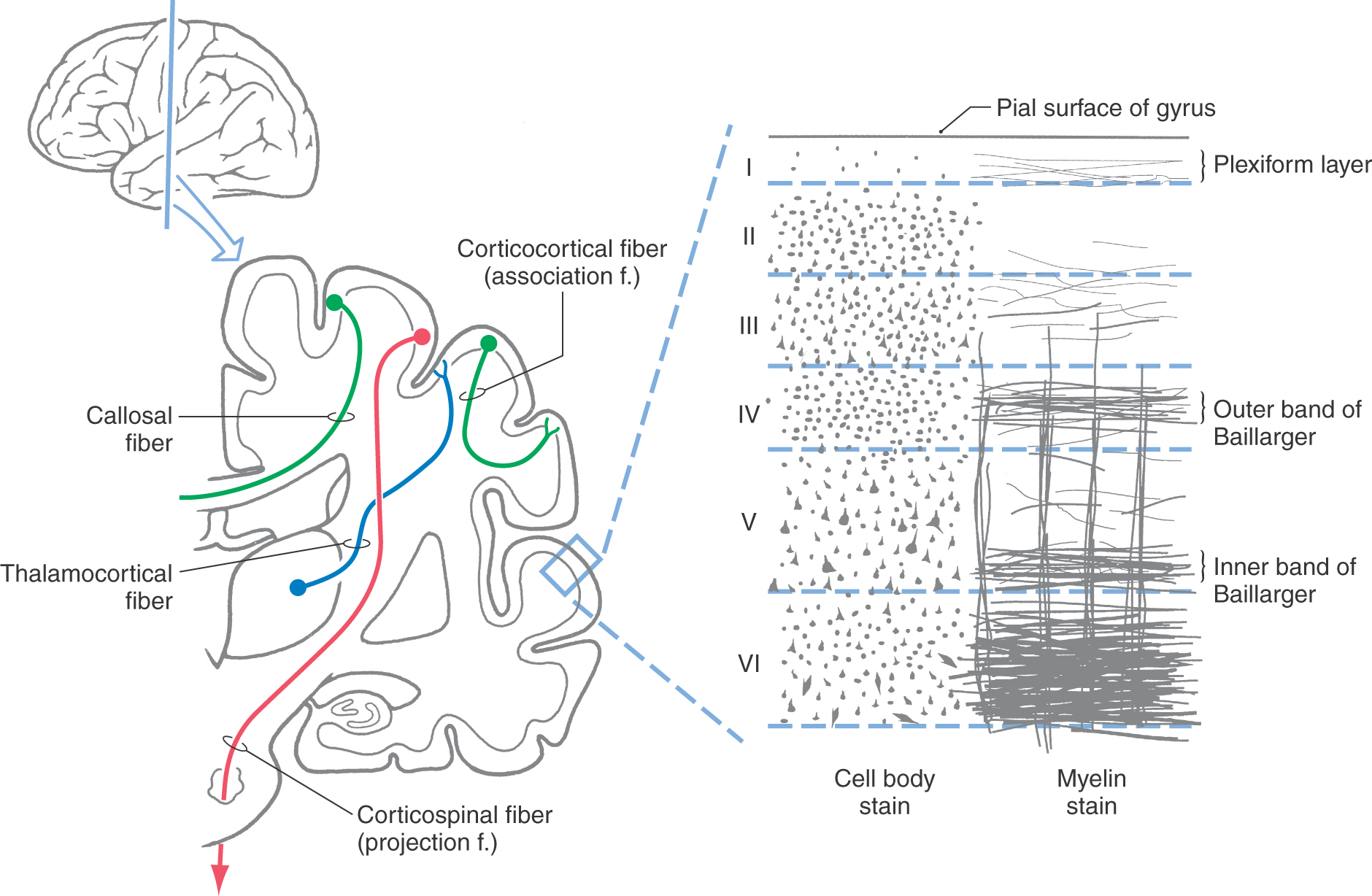
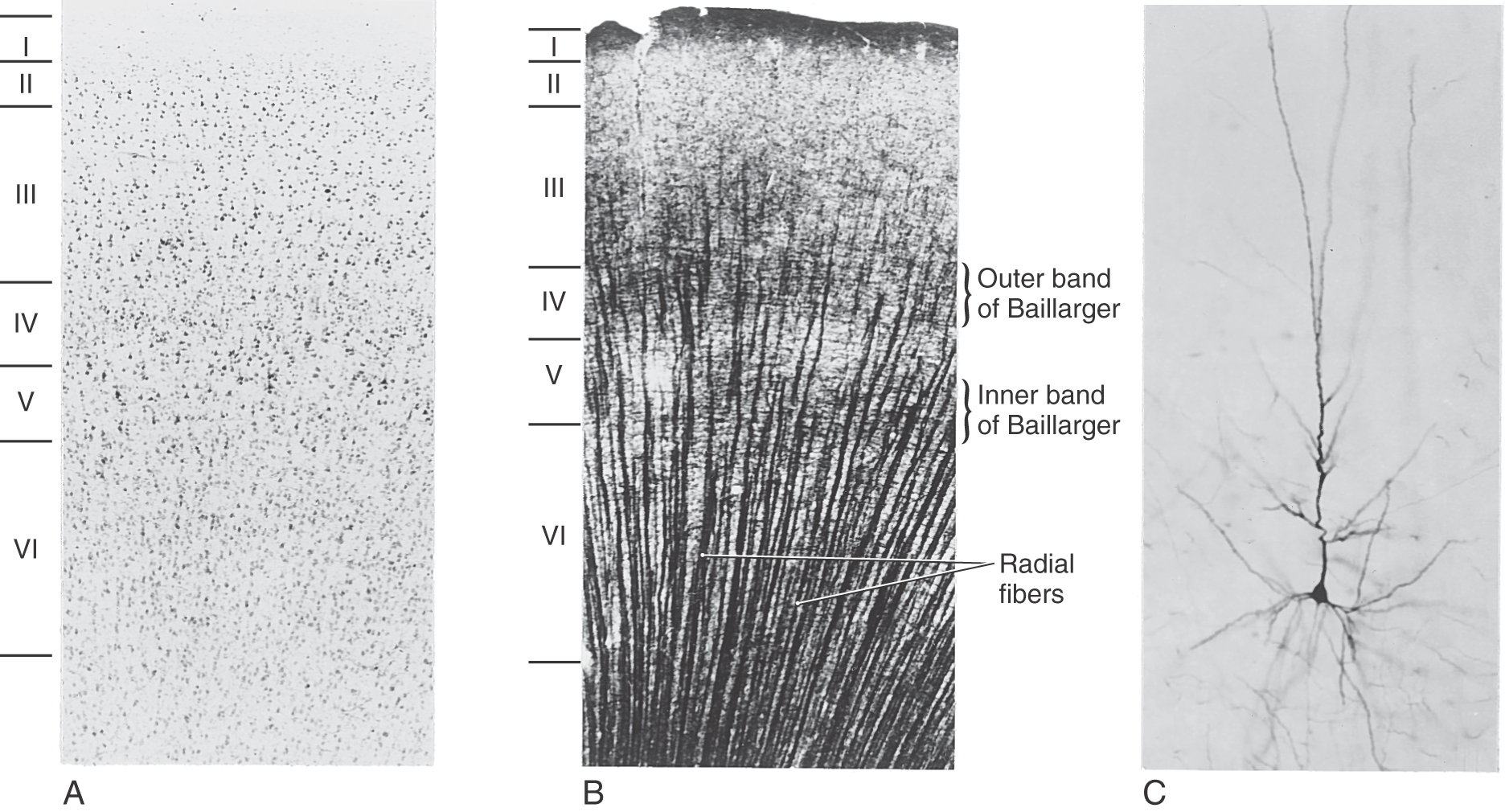
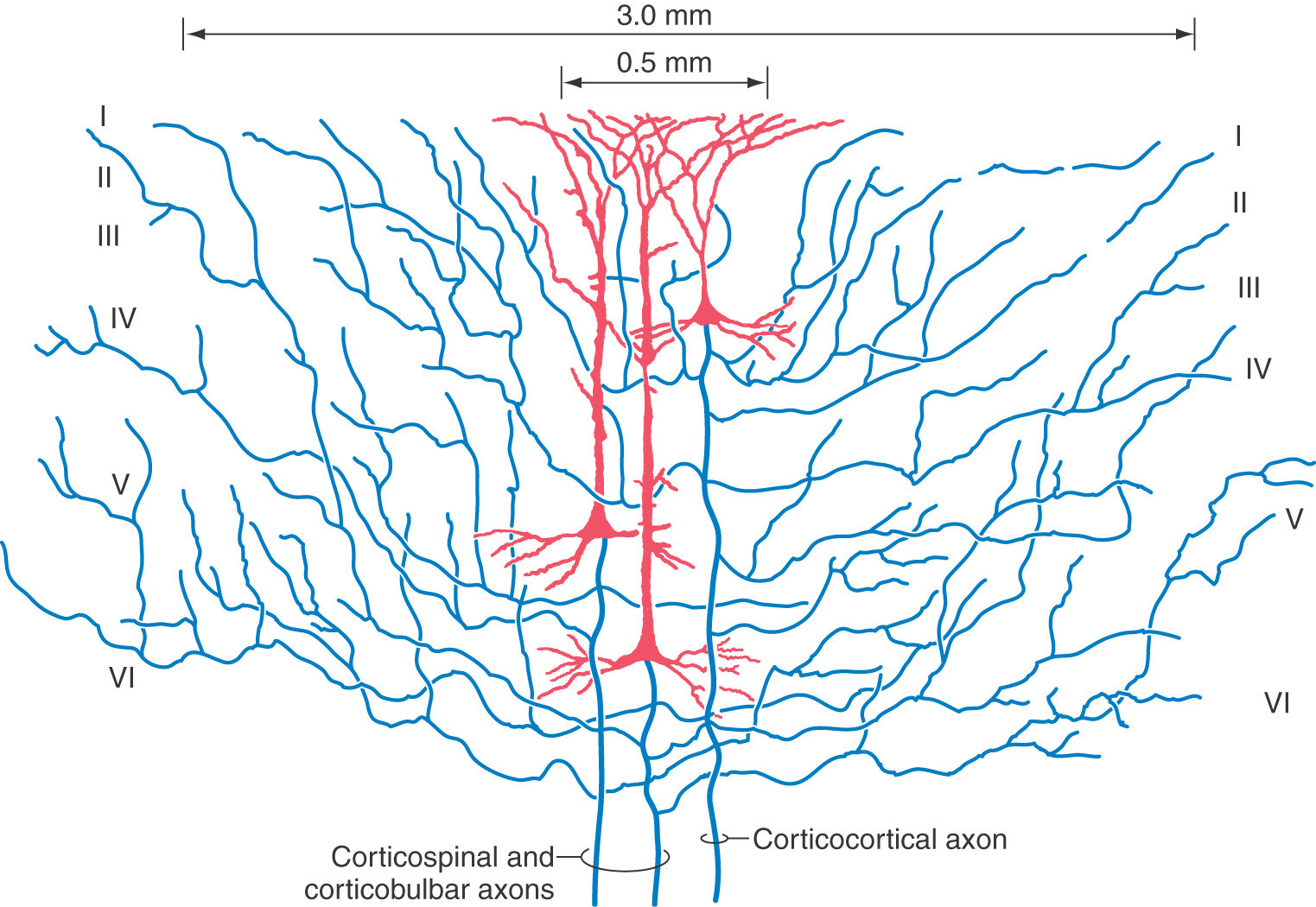
The gray matter of the cerebral cortex is composed of neuron cell bodies of variable sizes and shapes, intermixed with myelinated and unmyelinated fibers (Figs. 32-1 and 32-2A). These cell bodies may be visualized with stains that bind to the rough endoplasmic reticulum (Nissl substance). Such stains leave the axons and dendrites almost invisible. Substances that bind to the lipoprotein of the myelin sheath surrounding some axons will make the myelinated portion of the fibers visible (Figs. 32-1 and 32-2B). Yet another way of looking at cortical cells is to immerse small blocks of tissue in dilute silver salts, which precipitate on the membranes of the entire neuron. This reaction causes the cell body, its dendrites, and portions of the axon to become visible (Fig. 32-2C); this technique is called the Golgi method. The basic connections of a given region of cortex include projection fibers to subcortical structures, callosal fibers to cortex in the opposite hemisphere, association fibers to cortex in the same hemisphere, and thalamocortical fibers, which provide virtually all of the neural input to the cortex that originates in noncortical structures (Fig. 32-1).
The pattern of distribution of neuron cell bodies is called cytoarchitecture. The cytoarchitecture of the cerebral cortex is characterized by a layered arrangement. Most of the cerebral cortex has six distinct layers of neurons and is classified as neocortex. Two regions of the cerebral cortex have fewer than six layers. The first contains only three layers, is classified as archicortex, and includes the hippocampal formation. The second contains three to five layers, is classified as paleocortex, and includes the olfactory sensory area and the nearby entorhinal and periamygdaloid cortices. The following discussion concentrates primarily on the neocortex.
LAYERS OF THE CEREBRAL CORTEX
The neuronal layers in the neocortex are designated by Roman numerals, beginning at the pial surface (Fig. 32-1). There are six layers in the neocortex, with some of these layers being further subdivided on the basis of their architectural features.
Layer I, the molecular layer, contains very few neuron cell bodies and consists primarily of axons running parallel (horizontal) to the surface of the cortex. The apical dendrites of cells located in deeper layers also ramify within layer I.
Layer II, the external granular layer, is composed of a mixture of small neurons called granule cells and slightly larger neurons that are called pyramidal cells on the basis of the shape of their cell body. The apical dendrites of these pyramidal cells extend into layer I and their axons descend into and through the deeper cortical layers.
Layer III, the external pyramidal layer, contains primarily small to medium-sized pyramidal cells along with some neurons of other types. In general, the smaller pyramidal cells are sequestered in the outer or superficial portion of layer III; the larger pyramidal cells are located in the inner or deeper portion of this layer. Their apical dendrites ascend into layer I and their axons descend into and through the deeper layers.
Layer IV, the internal granular layer, consists almost exclusively of smooth (aspiny) stellate (star-like) neurons and spiny stellate neurons, both of which have sometimes been categorized as granule cells. This layer is free of pyramid-shaped cells. It can be divided into outer (IVa) and inner (IVb) portions in many neocortical areas and into three portions (IVa, IVb, IVc) in the primary visual cortex. Layer IV is the primary target for ascending sensory information from the thalamus.
Layer V, the internal pyramidal layer, consists predominantly of medium to large pyramidal cells. Apical dendrites of the medium pyramidal cells may extend upward one or two layers, whereas those of the large pyramidal cells extend outward to layer I. The large pyramidal cells of this layer are a major source of cortical efferent fibers, including axons to the basal nuclei, brainstem, and spinal cord. Some corticocortical axons also originate in layer V. These are probably collateral branches of axons that are projecting to some subcortical target.
Layer VI, the multiform layer, contains an assortment of neuron types, including some with pyramidal and fusiform cell bodies. The dendrites of the larger cells extend into layer I; those arising from the smaller cells usually extend no farther than layer IV. The axons of the cells of this layer project to subcortical targets, such as the thalamus, and to other cortical regions as corticocortical connections.
Two features of the myelinated fibers in the neocortex are noteworthy. First, there are prominent plexuses of horizontally running myelinated fibers in layers IV and V. These are called the outer and inner bands of Baillarger, respectively (Fig. 32-1). In the primary visual cortex, bordering on the calcarine sulcus, the outer band of Baillarger is greatly expanded. This band can be seen with the naked eye in fresh and stained sections and is called the stria (line) of Gennari (see Fig. 20-20). Second, in most regions of the neocortex, there are many radially oriented bundles of axons passing between the subcortical white matter and various parts of the cortex or between inner and outer cortical layers (Fig. 32-2B).
NEUROTRANSMITTERS OF THE CEREBRAL CORTEX
A variety of neuroactive substances are associated with neurons of the cerebral cortex. Principal among these are glutamate, aspartate, and γ-aminobutyric acid (GABA). Pyramidal cells are the efferent neurons of the cerebral cortex. They are predominantly glutaminergic and are excitatory to their targets. Most interneurons within the cortex are GABAergic and are inhibitory. The pyramidal cells of the cortex and therefore the output of the cortex are modulated by a variety of cortical afferents. The influence of these afferent fibers is to act on pyramidal cells either directly or via interneurons. A variety of neuropeptides (monoamines) are also found in the cerebral cortex; they influence not only populations of neurons but also local metabolic activity and vascular smooth muscle. The most important monoamines in the cortex are (1) norepinephrine, which originates from the locus ceruleus of the pons and distributes sparsely to all cortical layers; (2) dopamine, which arises from the substantia nigra–pars compacta and the adjacent ventral tegmental area and is found in moderate amounts in layers I and VI and sparsely in layers II to V; and (3) serotonin, which arises from the raphe nuclei and distributes heavily to all cortical layers.
NEURON TYPES IN THE CEREBRAL CORTEX
Pyramidal Cells
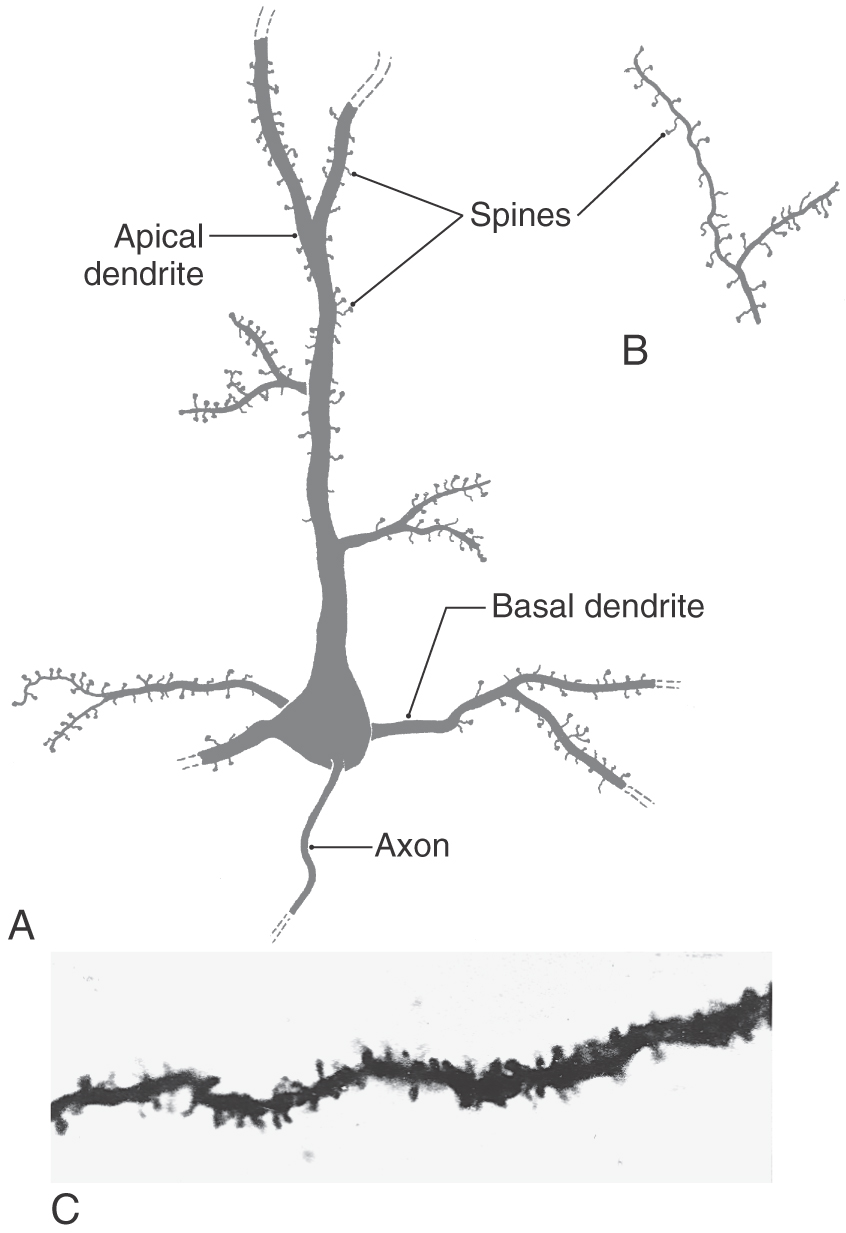
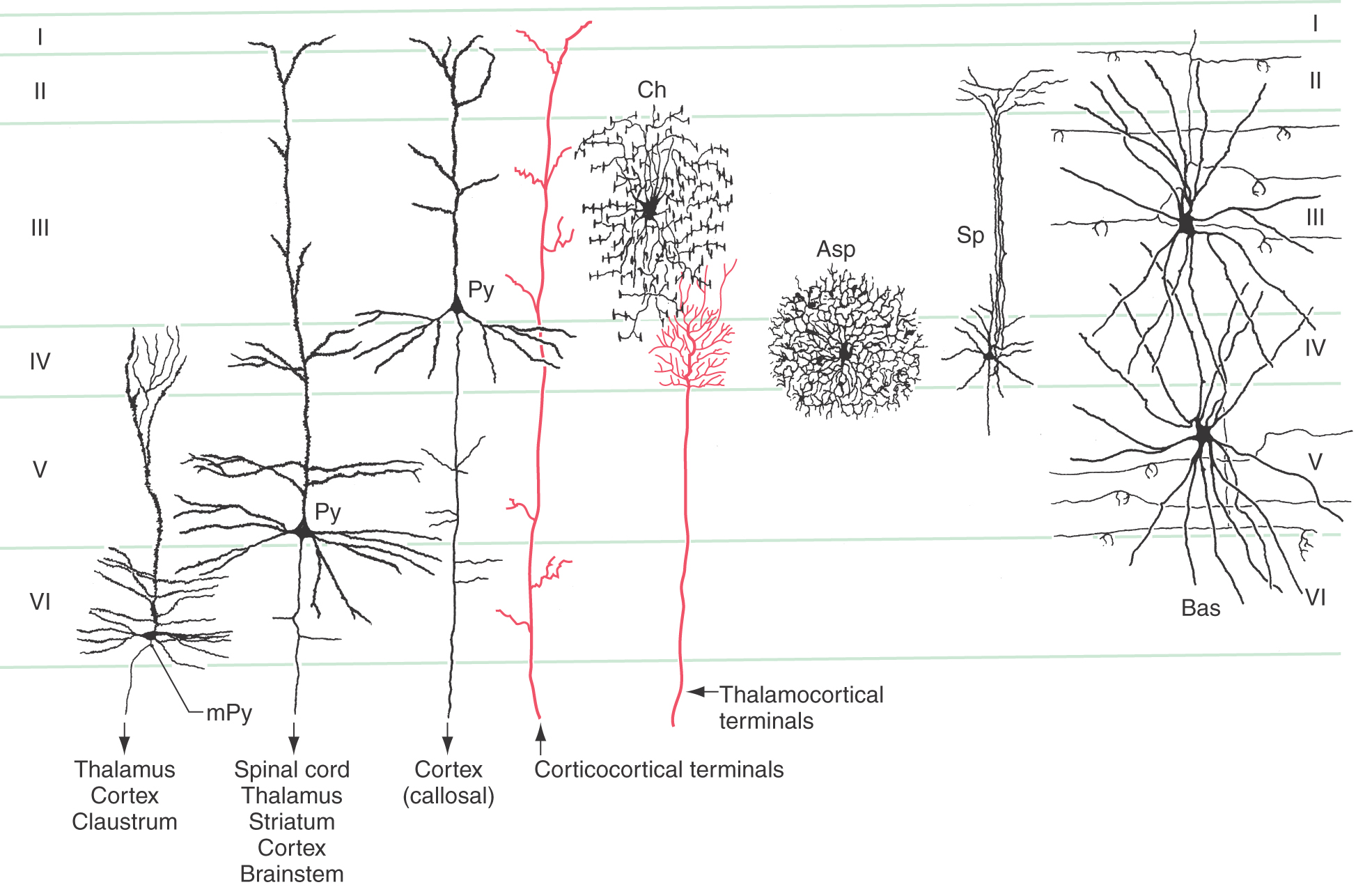
The most common type of neuron in the cerebral cortex is the pyramidal cell (Figs. 32-2 and 32-3A). Pyramidal cells are found in all layers of the cortex with the exception of the molecular layer (layer I), and they are the predominant cell type in layers II, III, and V (Fig. 32-4). Pyramidal cells are characterized by (1) a roughly triangular cell body; (2) a single large apical dendrite that arises from the apex of the cell body and usually extends toward the molecular layer, giving off branches along the way; (3) an array of basal dendrites that run in a predominantly horizontal direction; and (4) an axon that originates from the base of the soma, leaves the cortex, and passes through the white matter.
Figure 32-3. Examples of spines on basal and apical dendrites (A and C) and on the terminal ramifications of apical dendrites (B).
The cell bodies of most pyramidal neurons range in size from 10 to 50 µm in height. The largest, called giant pyramidal cells of Betz or Betz cells, are found almost exclusively in the primary motor cortex, which is located in the precentral and anterior paracentral gyri. Their somata may reach 100 µm in height. Betz cells are most common in the region of motor cortex that projects to the anterior horn of the lumbar spinal cord and hence are concerned with the control of leg movement. These cells are so large that they can be distinguished with the naked eye in Nissl-stained sections of the human brain.
Both apical and basal dendrites of pyramidal cells are characterized by membrane specializations called dendritic spines. These spines are small outgrowths from the dendrite that give the impression of thorns on a rosebush (Fig. 32-3). The vast majority of synaptic contacts received by a pyramidal cell are located on dendritic spines rather than directly on the dendrite shaft or on the cell body.
Pyramidal neurons represent virtually the only output pathway for the cerebral cortex. Almost all other cell types in the cortex are local circuit neurons that exert their influence within their own immediate vicinity. Axons of pyramidal cells may terminate in another region of the cortex in the same hemisphere (association fibers), decussate in the corpus callosum to terminate in the cerebral cortex of the opposite hemisphere (callosal fibers), or course through the white matter to any of the numerous subcortical targets in the forebrain, brainstem, or spinal cord (projection fibers).
Pyramidal cells display a laminar organization, with the cell bodies in a given layer projecting to specific neural targets (Fig. 32-4). In general, pyramidal neurons in layers II and III give rise to association and callosal fibers. Pyramidal cells in layer V project to many subcortical structures, including the spinal cord, as projection fibers. The neurons in layer VI send their axons to a variety of locations, including thalamic nuclei and other regions of cortex. Within the cortex, axons of pyramidal cells send off an extensive and relatively dense array of axon collaterals. These collaterals terminate in all cortical layers and extend through a horizontal area covering several millimeters around the cell body (Fig. 32-5).
Local Circuit Neurons
As mentioned previously, all the various nonpyramidal neurons of the cerebral cortex function as cortical interneurons; that is, their axons do not leave the immediate region of the cell body. These cells are often referred to as local circuit neurons or intrinsic cortical neurons.
Santiago Ramón y Cajal, working in the late 1800s and early 1900s, described a rich variety of intrinsic cortical neurons. However, by the 1950s, it had become customary to refer to virtually all intrinsic cortical neurons as stellate cells, even though many were not actually star shaped. Now the pendulum has swung in the other direction, and a number of distinct morphologic types are recognized. Some of the more important of these are illustrated in Figure 32-4: spiny and aspiny stellate cells, basket cells, and chandelier cells.
Three types of intrinsic neurons receive thalamocortical axon terminals in layer IV: the small spiny cells, the aspiny stellate cells, and dendrites of the large basket cells. Of these, the spiny cells are believed to be excitatory, whereas basket cells and aspiny stellate cells use the neurotransmitter GABA and are thus considered to be inhibitory interneurons. Most other intrinsic neurons are presumed to be inhibitory. On the other hand, pyramidal neurons are uniformly associated with excitatory neurotransmitters, glutamate and aspartate in particular.
LAMINAR ORGANIZATION
Intrinsic Circuitry of the Cerebral Cortex
The basic framework of the internal circuit diagram of small regions of the cerebral cortex is well understood. In contrast, the details of this circuitry are only partially known and are in fact so complex as to defy the construction of a detailed circuit diagram like that used to represent a computer’s electronic hardware. For example, a single axon may branch repeatedly and contact hundreds of other neurons. A single neuron may also receive synaptic contacts from thousands of other neurons. Within a small volume of cortex, there may be millions of neurons.
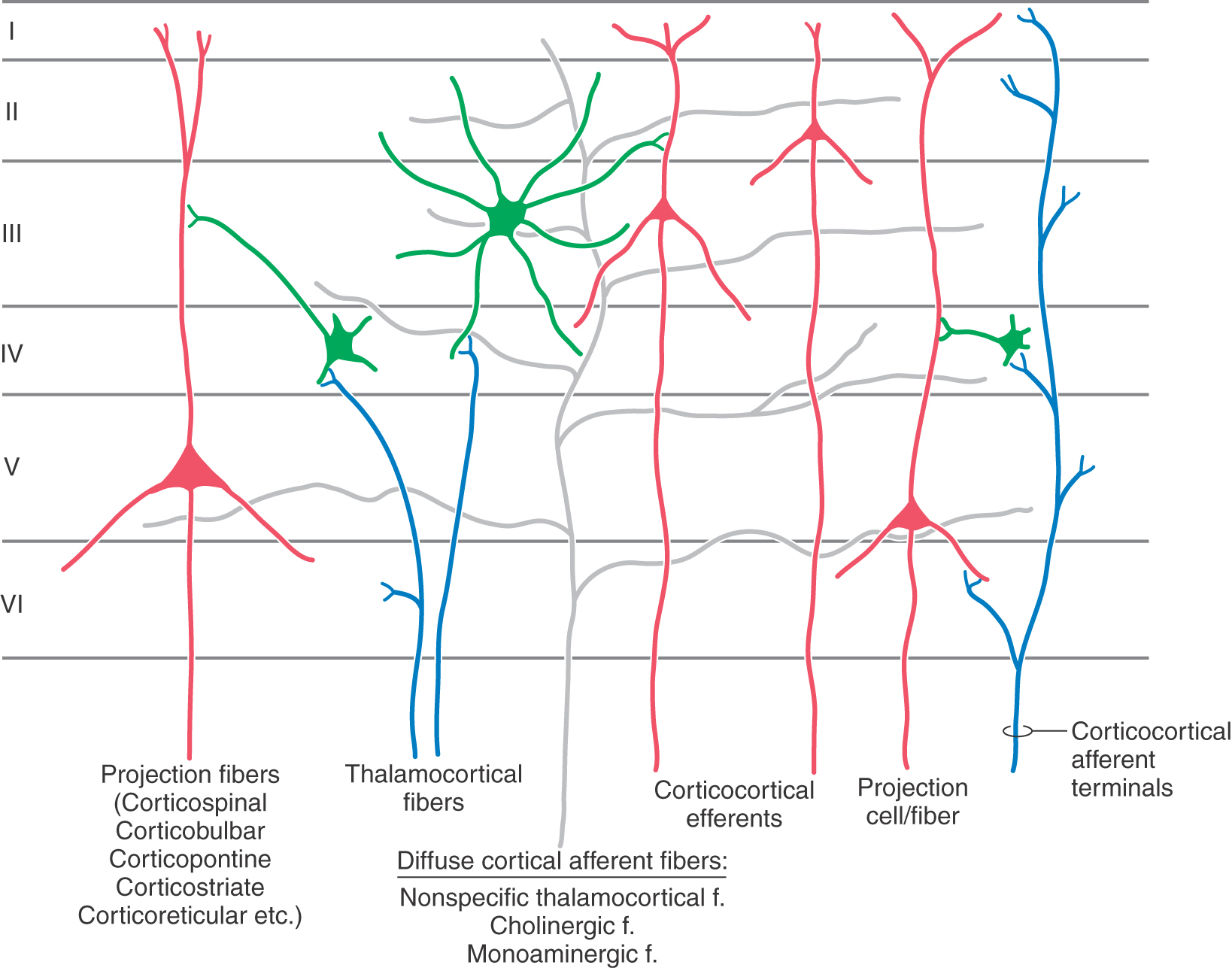
The basic framework of cortical circuitry consists of afferent fibers, local circuits for the processing of this afferent information, and efferent fibers that convey the processed information to another site (Fig. 32-6). Thalamocortical axons terminate primarily in layer IV and to a lesser extent in layers III and VI. In layer IV, they terminate on excitatory and inhibitory interneurons as well as on dendrites from neurons in other layers (Fig. 32-4). The axons of interneurons in turn may end on dendrites of pyramidal cells or of other interneurons. The local processing of information culminates in connections to pyramidal cells, which carry the information to other cortical or subcortical regions. A copy of the information also goes to neurons in the immediate vicinity via axon collaterals (Fig. 32-5).
The general pattern of termination of corticocortical axons is quite different from that of thalamocortical axons. Corticocortical axons branch repeatedly and make synaptic contacts on neurons in all layers of the cortex (Fig. 32-4).
The cerebral cortex receives a third set of inputs, called diffuse inputs, which consists of fibers that branch extensively and end diffusely over a wide area of cortex without respect for cytoarchitectural boundaries (Fig. 32-6). These inputs arise from a variety of sources, including certain nonspecific nuclei of the thalamus (e.g., the ventral anterior, central lateral, and midline nuclei), the locus ceruleus, and the basal nucleus (of Meynert). These structures are generally concerned with regulation of overall levels of cortical excitability and the associated phenomena of arousal, sleep, and wakefulness.
Cytoarchitecture
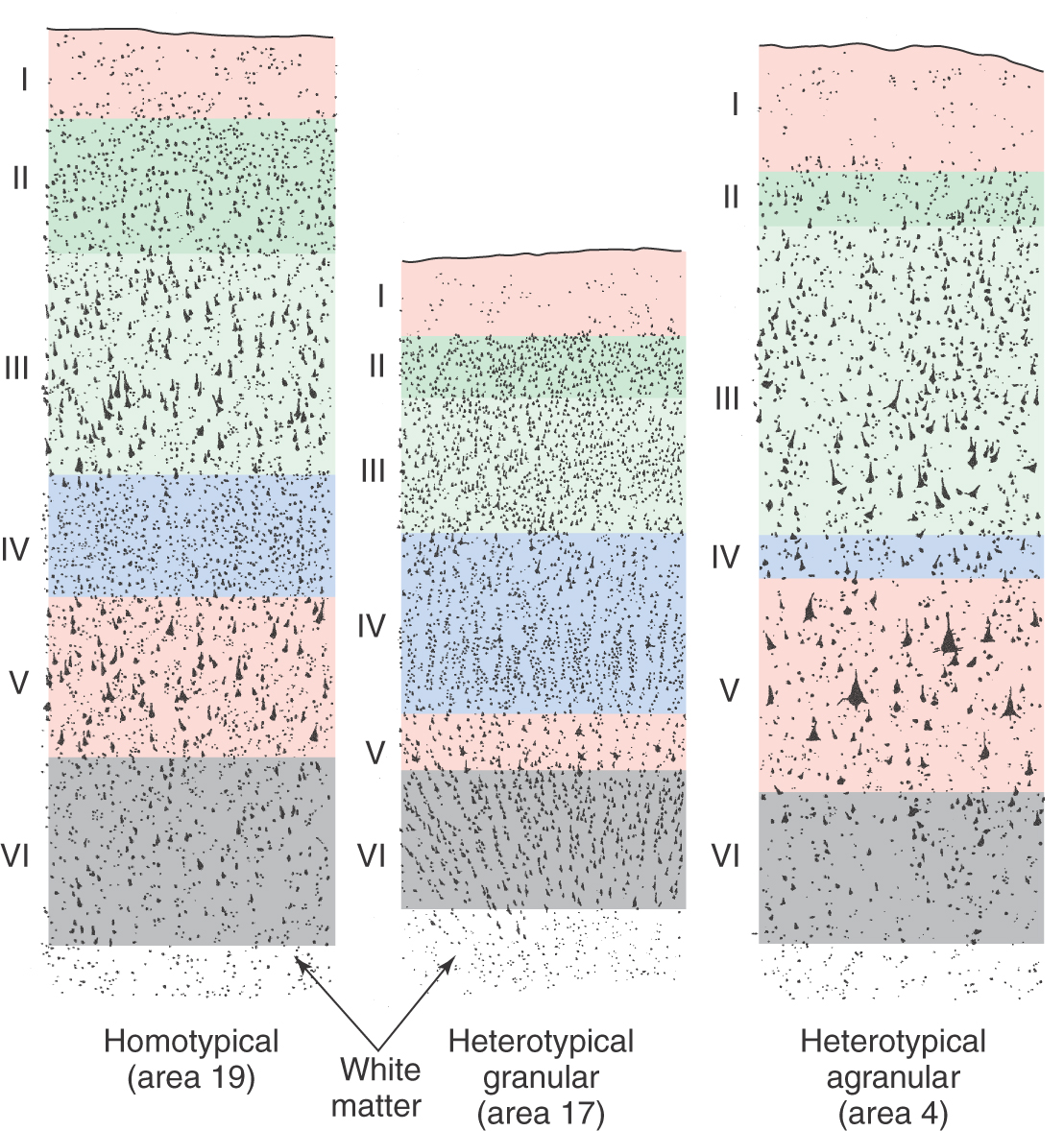
The cytoarchitecture of the cortex differs from one area to another in ways that are related to function (Fig. 32-7). In primary sensory cortex, layer IV, the major input layer of cortex, is especially thick, whereas layer V, the major projection layer, is narrow and indistinct. Cortex with this pattern is called heterotypical granular cortex. In primary motor cortex, the pattern is reversed: layer IV is almost invisible, and layer V is very thick, seeming to merge directly with layer III. Thus the projection layer is prominent and the input layer is small. Cortex of this type is called heterotypical agranular cortex. In most other areas of the neocortex, including the association cortices, the six layers are all clearly represented and are of roughly equal thickness. This type of cortex is called homotypical.
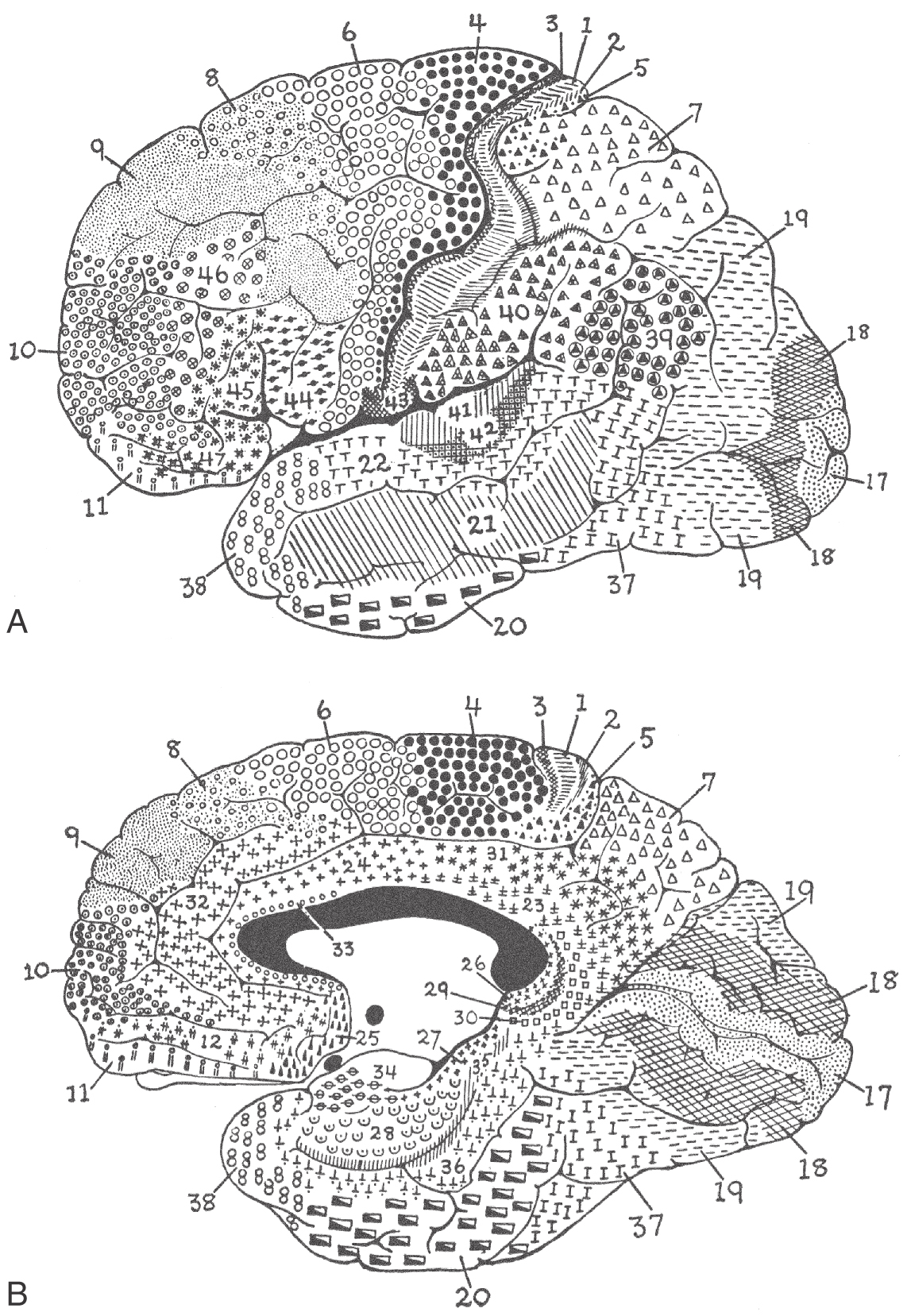
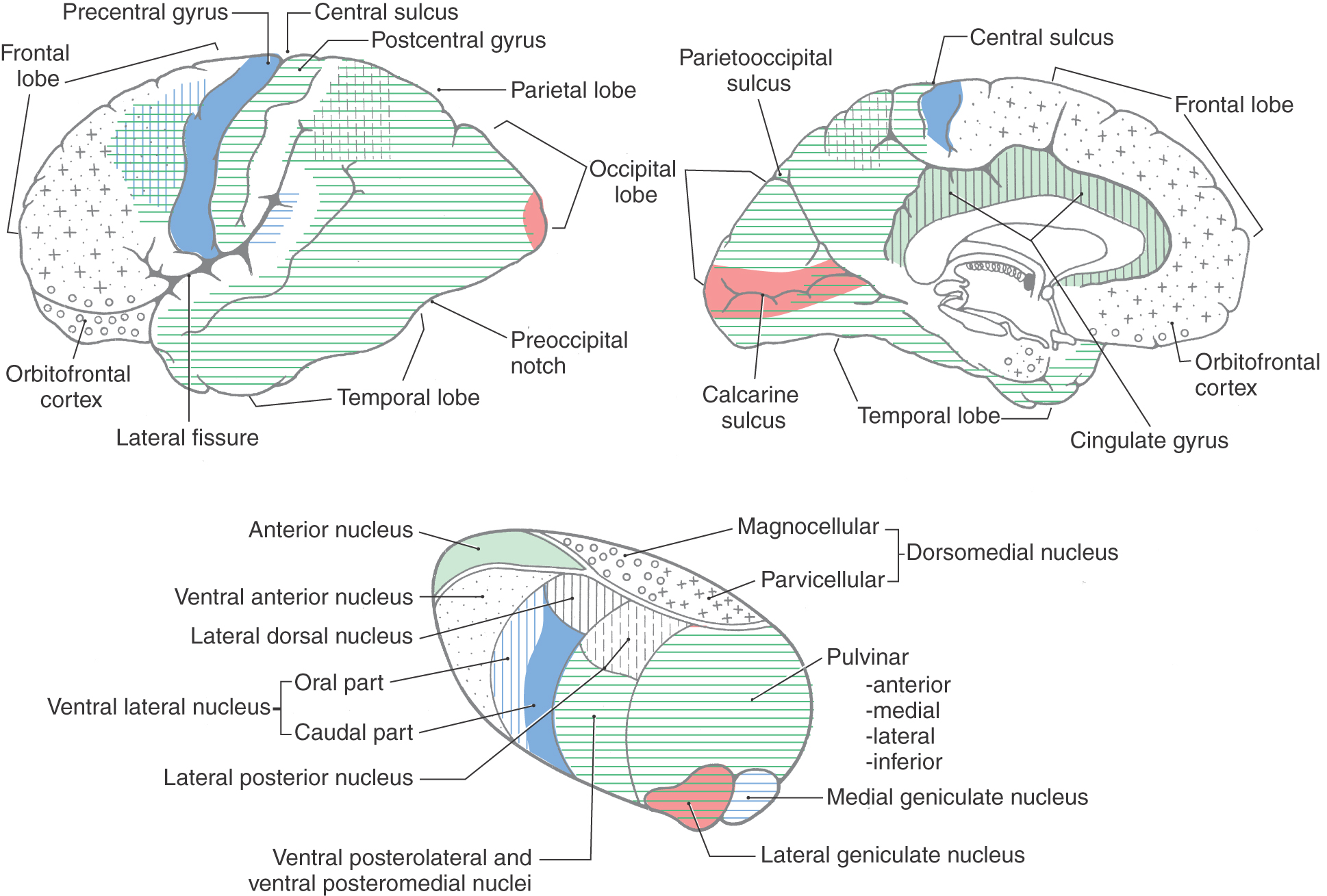
The cerebral cortex has been subdivided on the basis of cytoarchitectural differences by many different investigators. The most famous of these, Korbinian Brodmann, worked in the early part of the twentieth century. He identified 47 distinct areas (Fig. 32-8), and his numbering scheme is still in common use today in both research and clinical settings. For example, the primary visual cortex is Brodmann area 17, and the primary motor cortex is area 4. In most instances, Brodmann cytoarchitectural areas are coextensive with cortical regions that have specific functional characteristics.
COLUMNAR ORGANIZATION
A second, vertical pattern of organization is superimposed on the horizontal layered pattern described earlier. Unlike the cortical layers, this vertical pattern is not immediately obvious in histologic sections stained for neuron cell bodies (Nissl stains). However, when Golgi-stained material is studied, it is clear that neurons are often grouped together so that their cell bodies, axons, and apical dendrites form clusters that are oriented at right angles to the surface of the cortex.
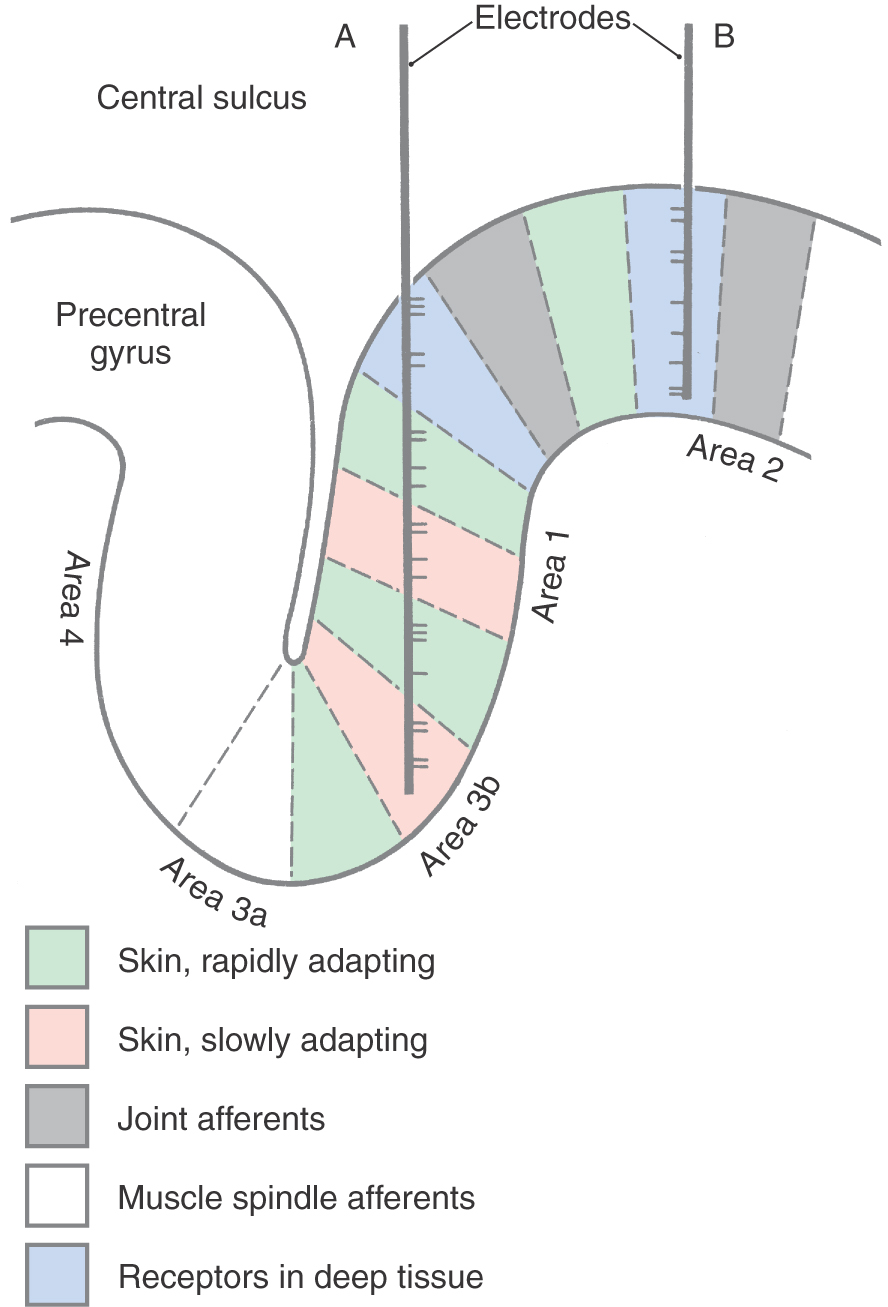
Mountcastle was the first to demonstrate physiologically the existence of a vertical (columnar) organization in the cerebral cortex by recording the activity of hundreds of individual neurons in the primary somatosensory cortex of cats and monkeys. Within an area of cortex a few millimeters in diameter, all neurons had overlapping or adjacent receptive fields. For example, in one cortical region, all neurons might have receptive fields on a finger, whereas in a nearby region, the neurons might have receptive fields on the wrist. Within a cortical region in which all neurons had about the same receptive field, the neurons responded to different sensory submodalities. Some neurons were activated by light touch on the skin, others by joint rotation, and still others by strong pressure on deep tissue. However, when a microelectrode was inserted at right angles to the surface of the cortex, all the neurons encountered were activated by only one of these submodalities (Fig. 32-9B). In contrast, when a microelectrode was moved parallel or obliquely relative to the surface of the cortex, it encountered neurons of different submodalities as it moved from one functionally related group of neurons to another (Fig. 32-9A).
The basis of columnar organization in primary sensory cortices is selective input from relay nuclei of the thalamus. Obviously, if all of the cells in one column respond to maintained pressure on the skin while the cells in an adjacent column respond to joint position, the signals from the respective sensory receptors must have been continuously segregated all the way from the periphery through the posterior column nuclei and the ventrobasal complex of the thalamus to terminate in the cortex.
The anatomic basis of the columnar organization of the cortex is understood in the greatest detail in the visual cortex. In this region, at least three types of regularly repeating features are superimposed on the laminar patterns of neurons: the stimulus orientation columns, the ocular dominance columns, and the cytochrome oxidase–rich blobs. These features are discussed in detail in Chapter 20.
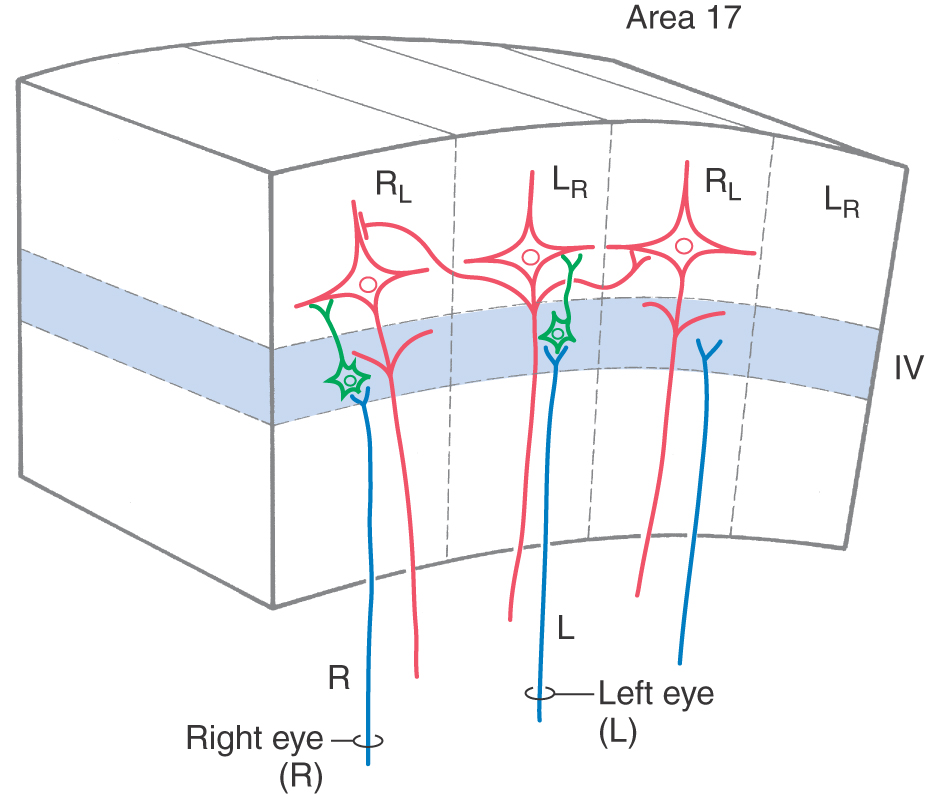
The ocular dominance columns in the visual cortex provide a clear example of the role of thalamic input in columnar organization. Neurons in the layers of the lateral geniculate nucleus that receive input from the right eye send their axons to layer IV of the right eye–dominant columns (Fig. 32-10). Here, the axons terminate predominantly on spiny and aspiny stellate cells, which in turn project to pyramidal cells. Collaterals of pyramidal cell axons provide one pathway by which neural signals can spread from one column to influence activity in adjacent columns (Fig. 32-5). This influence may be either excitatory via direct connections or inhibitory via interneurons. The right eye therefore has a direct and strong influence on neurons in right eye–dominant columns (RL in Fig. 32-10) and an indirect and weaker influence on neurons in the adjacent left eye–dominant columns (LR in Fig. 32-10).
Connections between one region of the cortex and another, through either association fibers or callosal fibers, may also be arranged in a columnar pattern. For example, axons that originate in the inferior parietal lobule terminate in multiple columns in the ipsilateral and contralateral cingulate cortices. Columns of corticocortical axon terminals that originate in different functional regions may either overlap each other or interdigitate with each other.
SYNOPSIS OF THALAMOCORTICAL RELATIONSHIPS
The details of thalamocortical projections are described in the chapters devoted to specific systems. At this juncture, however, it is appropriate to briefly review what areas of the cortex are functionally related to which of the thalamic nuclei (Fig. 32-11).
The cortex of the frontal lobe encompasses Brodmann areas 4, 6, 8 to 12, 32, and 44 to 47 (Fig. 32-8). The primary somatomotor cortex (area 4) and the premotor and supplementary motor cortices (area 6) receive input mainly from the ventral lateral nucleus of the thalamus and subserve important motor functions. Lateral, medial, and orbital aspects of the frontal lobe receive thalamocortical fibers mainly from the dorsomedial and anterior nuclei of the thalamus (Fig. 32-11); these cortical areas, through a variety of direct and indirect connections, relate primarily to higher cortical functions (see under the heading Prefrontal Cortex and Plans for Future Operation). Of particular note are the pars orbitalis and pars triangularis of the inferior frontal gyrus, damage to which results in Broca aphasia (discussed later).
Areas 3, 1, 2, 5, 7, 39, 40, and 43 are located in the parietal lobe (Fig. 32-8). The primary somatosensory cortex (areas 3, 1, and 2) receives inputs from the ventral posterolateral and ventral posteromedial nuclei. These thalamic nuclei receive a full range of somatosensory input through synaptic relays in the spinal cord and brainstem and transmit this information to the cerebral cortex. The inferior parietal lobule comprises, in general, areas 39 and 40. Along with area 22, these areas are the cortical regions associated with Wernicke aphasia (discussed later).
The occipital and temporal lobes encompass areas 17 to 22, 36 to 38, and 41 and 42 (Fig. 32-8). These areas of the cortex plus portions of the parietal lobe have extensive connections with the pulvinar nucleus of the thalamus and are involved in the processing of visual and auditory information at several different functional levels (Fig. 32-11). Located in this geographic area are the primary sensory cortices for vision and hearing. Area 17, on the banks of the calcarine sulcus, is the primary visual cortex; areas 41 and 42, in the depth of the lateral fissure in the transverse temporal gyri, constitute the primary auditory cortex (Fig. 32-8). These cortical areas receive input from the lateral and medial geniculate nuclei of the thalamus, respectively.
The limbic lobe, which forms the most medial edge of the hemisphere, contains areas 23 to 31 and 33 to 35. The cingulate cortex receives fibers primarily from the anterior nucleus of the thalamus but also from the lateral dorsal nucleus (Fig. 32-11). Other regions of the limbic lobe have some connections with the dorsomedial nucleus. Many of the subcortical targets of the parahippocampal and uncal cortices are structures such as the hippocampal formation. This area in turn projects to a variety of thalamic and basal forebrain targets.
BLOOD SUPPLY TO THE CEREBRAL CORTEX
The blood supply to the cerebral cortex and to subcortical structures of the telencephalon, including the internal capsule, is discussed in Chapters 8 and 16. In this section the general nature of these patterns is summarized.
The cerebral cortex is served, in toto, by the anterior, middle, and posterior cerebral arteries. The anterior and middle cerebral arteries are the terminal branches of the internal carotid artery, and the posterior cerebral artery is formed by the bifurcation of the basilar artery (see Fig. 8-2, Fig. 8-6 and Fig. 8-8).
The anterior cerebral artery is joined to its counterpart just anterior to the optic chiasm by the anterior communicating artery. Proximal to the anterior communicating artery, the A1 segment of the anterior cerebral artery gives rise to small branches that serve rostral portions of the hypothalamus and immediately adjacent optic structures. Segments of the anterior cerebral artery distal to the anterior communicating artery are A2 (infracallosal), A3 (precallosal), and A4 and A5 (supracallosal and postcallosal). Cortical branches of the anterior cerebral artery (A2 to A5) distribute to the medial surface of the hemisphere caudally to about the position of the parietooccipital fissure. The distal portions of these branches arch over the edge of the hemisphere (from its medial to its lateral surface) for a short distance (see Fig. 8-4, Fig. 8-8 and Fig. 16-11). Located in the domain of the branches of this major vessel (especially segments A4 and A5) are the foot, lower extremity, and hip areas of the primary somatomotor and primary somatosensory cortices.
The middle cerebral artery passes laterally from its origin from the internal carotid artery and branches, in general, into superior and inferior trunks (these are M2 branches) over the insular cortex. These proceed as the M3 segment over the inner surface of the operculum and exit the lateral sulcus to fan out as the cortical branches that collectively comprise the M4 segment. Terminal branches of the superior and inferior trunks (as the M4 segment) serve the cortex on the lateral surface of the hemisphere above and below the lateral fissure, respectively (see Fig. 8-9, Fig. 8-10 and Fig. 16-11). In addition to lateral portions of the frontal cortex, parietal and temporal association cortices are served by branches of the middle cerebral artery. Also located in the distribution area of this vessel are the trunk, upper extremity, and head regions of the primary somatomotor and primary somatosensory cortices (via branches of the superior trunk) and the primary auditory cortex (inferior trunk).
The cortex forming the inferior surface of the temporal lobe and the medial aspect of the occipital lobe is served by branches of the posterior cerebral artery (see Fig. 8-8, Fig. 8-14 and Fig. 16-11). As with the anterior cerebral artery, the terminal rami of the posterior cerebral artery loop over the edge of the inferior and medial surface of the hemisphere to serve small portions of the lateral aspect of the hemisphere. The posterior cerebral artery serves large expanses of the visual association cortex and some of the cortical structures associated with the limbic system. In addition, the calcarine artery supplies the primary visual cortex located on and in the banks of the calcarine sulcus.
The wedge-shaped areas of overlap between the distal branches of the anterior and middle cerebral arteries and the posterior and middle cerebral arteries form what are called border zones (see Fig. 8-15). These areas are particularly susceptible to hypoperfusion during episodes of systemic hypotension. Such events may result in watershed infarcts.
HIGHER CORTICAL FUNCTIONS
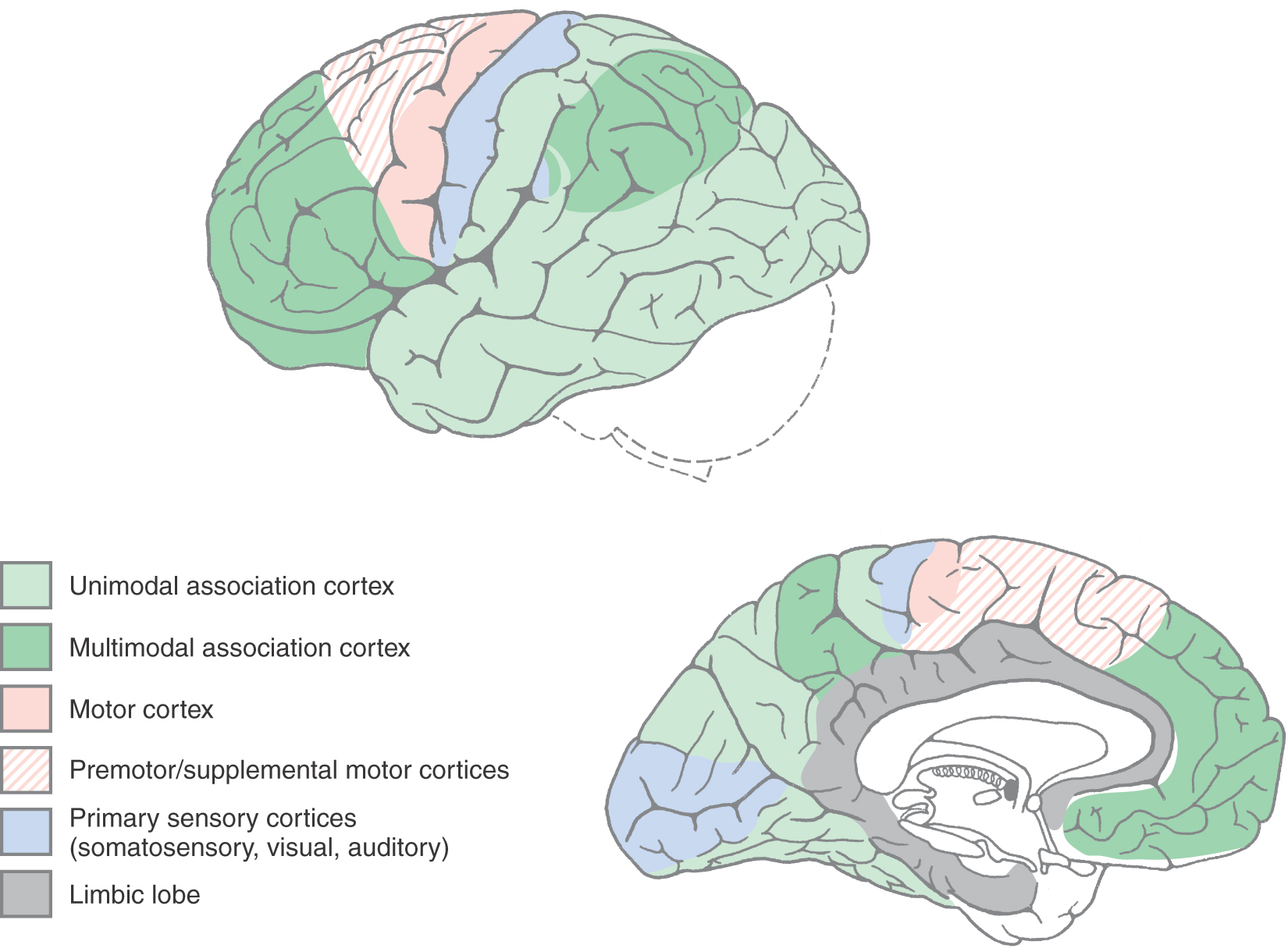
The cerebral cortex is generally considered to be the seat of higher intellectual functions, those faculties of thought that have reached their most complex levels in humans. Although other brain structures, including the thalamus, corpus striatum, claustrum, and cerebellum, contribute to these functions, the multimodal association cortex is closely linked to the most complex intellectual functions, such as logical analysis, judgment, language, and imagination.
The cerebral cortex can be divided into four general functional categories: sensory, motor, unimodal association cortex, and multimodal association cortex (Fig. 32-12). The primary sensory areas, except that for olfaction, receive thalamocortical fibers from diencephalic relay nuclei that are functionally related to each modality. For example, the ventral posterior complex of the thalamus projects to the primary somatosensory cortex (Brodmann areas 3, 1, and 2) in the postcentral gyrus. Similarly, the lateral geniculate nucleus projects to the primary visual cortex (area 17) in the banks of the calcarine sulcus, and the medial geniculate nucleus projects to the primary auditory cortex in the transverse temporal gyri (areas 41 and 42).
Adjacent to each primary sensory area is a region of cortex that is devoted to a higher level of information processing relevant to that specific sensory modality. These areas are called unimodal association cortices (Fig. 32-12). For example, visual unimodal association cortex (areas 18, 19, 20, 21, and 37) occupies all of the occipital lobe outside area 17 (the primary visual sensory area) as well as much of the inferior gyrus of the temporal lobe. Within these visual association areas, the basic elements of visual sensation are molded into an overall perception of the visual world. Similarly, the somatosensory association cortex lies just posterior to the postcentral gyrus in area 5, and the auditory association cortex is in the superior temporal gyrus (area 22) next to the primary auditory cortex. In all of these examples, the primary sensory cortices (i.e., areas 3, 1, 2; 17; and 41 and 42) receive input from their respective thalamic relay nuclei. In turn, the primary sensory areas project, via corticocortical fibers, to their corresponding association cortices (i.e., areas 3, 1, 2 to area 5; area 17 to areas 18 and 19; and areas 41 and 42 to area 22).
The remaining portions of the cerebral cortex that are not motor in function are classified as multimodal association cortex (Fig. 32-12). These areas receive information from several different sensory modalities and create for us a complete experience of our surroundings. Multimodal association areas are critical to our ability to communicate with use of language, to use reason to extrapolate future events on the basis of present experience, to make complex and long-range plans, and to imagine and create things that have never existed. An example of long-range planning is going to college so you can go to medical school so you can do a residency and become a physician. This section concentrates on the cortical areas responsible for three of these higher functions: language, the appreciation of space, and the planning of behavior.
Dominant Hemisphere and Language
The cerebral hemisphere that controls language is called the dominant hemisphere. In most people, language functions are processed in the left hemisphere. As evidence of this left brain dominance, brain lesions that adversely affect language are found in the left hemisphere in about 95% of cases. Almost all right-handed individuals and about half of left-handed individuals are left cerebral dominant. It follows that the right cerebral hemisphere, in most of the general population, is the nondominant hemisphere.
Language is the faculty of communication using symbols organized by a system of grammar to describe things and events and to express ideas. In humans, the senses of vision and audition are closely linked to language, but language itself transcends any particular sensory system. Helen Keller was blind and deaf but used language eloquently to communicate very complex and subtle ideas. Language ability can be impaired selectively, with little or no change in the senses of vision or hearing, by brain damage in either the parietal-temporal junction or the frontal lobe. This impairment, termed aphasia, is a disturbance of the comprehension and formulation of language, not a disorder of hearing, vision, or motor control.
Wernicke Aphasia and Broca Aphasia
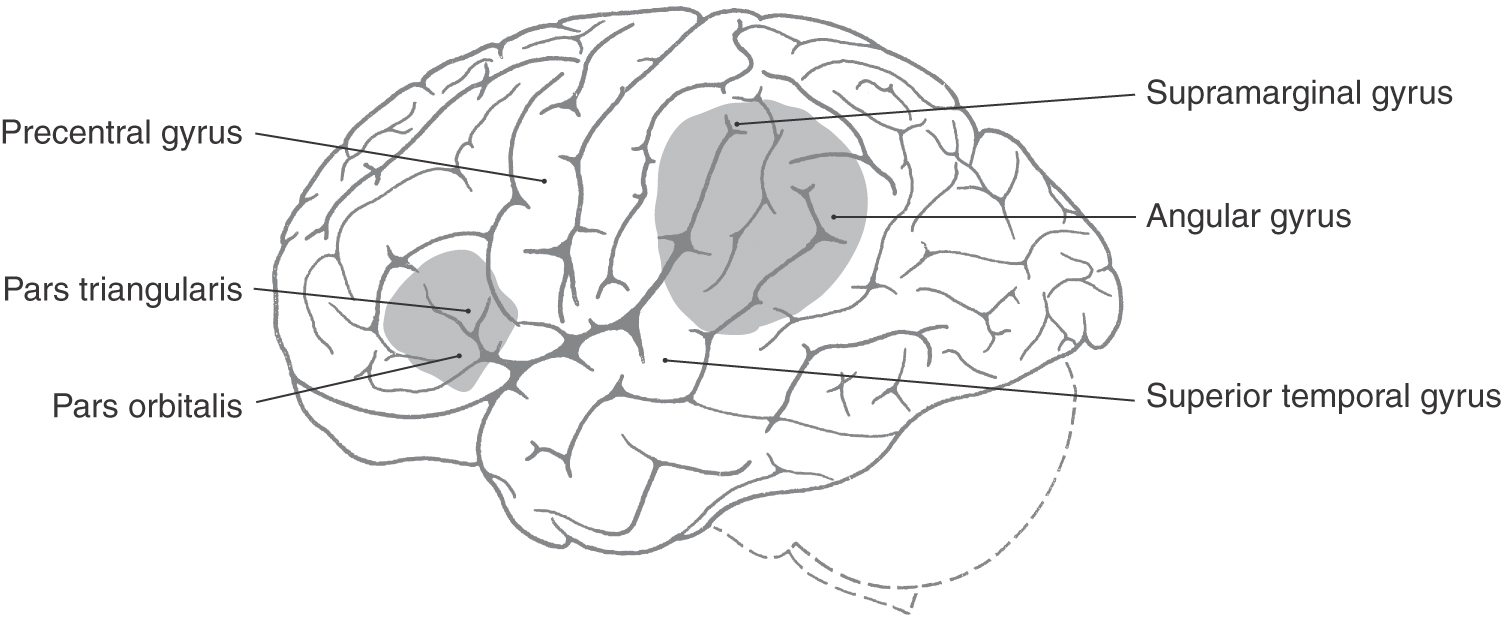
The two classic types of aphasia are Broca aphasia and Wernicke aphasia. Broca aphasia, also termed expressive aphasia or nonfluent aphasia, consists of a loss of the ability to speak fluently. Lesions that produce this deficit are located in the inferior frontal gyrus of the left hemisphere, primarily in Brodmann areas 44 and 45 (Fig. 32-13). Wernicke aphasia is primarily a defect of the comprehension rather than the expression of language. This deficit is seen after injury to the supramarginal and angular gyri (areas 37, 39, and 40) and the posterior part of the superior temporal gyrus (area 22) in the left hemisphere (Fig. 32-13).
Patients with the most severe form of Broca aphasia are unable to speak (mutism), although they are able to swallow and to breathe normally and to make guttural sounds. Their problem is not one of paralysis of the vocal apparatus. Rather, it is a difficulty in turning a concept or thought into a sequence of meaningful sounds. In less severe cases or in patients in the recovery process, limited speech is possible. Short, habitual phrases such as “hi,” “fine, thank you,” and “yes” and “no” are the first to come back. However, speech is slow and labored, enunciation is poor, and nonessential words are commonly omitted (telegraphic speech). Affected persons typically have as much difficulty with writing (agraphia) as with speaking. Although the patient is able to understand spoken or written language and can communicate verbally to some degree, the extremely laborious nature of the process of communication causes considerable frustration. Under particular emotional stress, patients may use inappropriate or vulgar words or phrases to express their distress.
The most common causes of Broca aphasia are tumors and occlusions of frontal M4 branches of the middle cerebral artery. Mild aphasia without other deficits indicates that the damage affects only cortical areas. However, full-blown Broca aphasia indicates that the damage extends beyond the Broca area of the cortex to include insular cortex and subjacent white matter. Patients typically have contralateral motor signs and symptoms, such as weakness (paresis) of the lower part of the face, lateral deviation of the tongue when it is protruded, and weakness of the arm. Aphasia plus these motor problems suggests an occlusion of branches from the proximal parts of the middle cerebral artery (M1), including the lenticulostriate arteries, which serve the internal capsule (see Fig. 16-12).
The second major type of aphasia is Wernicke aphasia (also described as receptive or fluent aphasia). Patients with severe Wernicke aphasia are unable to understand what is said to them, are unable to read (alexia), are unable to write comprehensible language (agraphia), and display fluent paraphasic speech. Paraphasic speech refers to the ability of patients to produce clear, fluent, melodic speech at a normal or even faster than normal rate. The content of the speech, however, may be unintelligible because of frequent errors of word choice, inappropriate use of words, or use of made-up nonsense words. An example of this type of speech is “we went to drive in the bridge for red pymarids [sic] were crooking the lawn browsers.” Such speech is sometimes called word salad. In less severe cases, paraphasias frequently occur. For example, in trying to say “the cat has claws,” the patient may use an incorrect but similar-sounding word (“the cat has clads”—a literal paraphasia) or a word that seems appropriate to the patient but is incorrect (“the cat has tires”—a verbal paraphasia). A surprising finding is that patients with Wernicke aphasia are much less aware of the extent of their disability than are patients with Broca aphasia, and they are usually less frustrated and depressed about it. In contrast, patients with Broca aphasia are completely conscious of their communication problems and are often exceedingly frustrated or despairing.
Wernicke aphasia may result from occlusion of temporal and parietal M4 branches of the middle cerebral artery. In addition, hemorrhage into the thalamus (or tumors in the thalamus) may produce Wernicke aphasia by extending laterally and caudally to invade the subcortical white matter. If this damage impinges on the optic radiations (see Chapter 20), a contralateral homonymous hemianopia may accompany the patient’s other disabilities.
Conduction Aphasia and Global Aphasia
The severity and duration of the aphasia depend on the severity of the associated brain damage. In mild cases, only one or two symptoms may be discernible, and those may resolve quickly. A major stroke or severe traumatic injury, however, may produce a full-blown set of signs and symptoms that will never completely disappear.
Other, less common types of aphasia have also been described. These include conduction aphasia, which results from interruption of the connections linking the Broca and Wernicke areas. In this disorder, comprehension is normal and expression is fluent but the patient has difficulty translating what someone has said to him or her into an appropriate reply. A more profound disorder is global aphasia, which occurs when occlusion of the left internal carotid or the most proximal portion of the middle cerebral artery (M1segment) produces damage that encroaches on both the Broca and the Wernicke areas, and the loss of language is virtually complete.
Several additional points should be mentioned. First, damage to the basal nuclei, particularly to the head of the caudate on the left side, has been associated with language disorders similar to Wernicke aphasia. Second, although we have referred so far to spoken and written language (i.e., verbal language), aphasia can also affect nonverbal language. A deaf person who uses American Sign Language can lose the ability to use or to understand sign language after focal brain damage in the left hemisphere. Third, although most aspects of language are processed in the left hemisphere, some features are influenced by lesions in the nondominant parietal lobe. In particular, a patient with a right parietal lesion may have difficulty appreciating the prosody of speech. This term refers to the variations in vocal inflections, emotional content, and melody that may alter the meaning of a spoken sentence, as in “George is here.” versus “George is here!” versus “George is here?” versus “George is here!”
Parietal Association Cortex: Space and Attention
A completely different set of intellectual functions is mediated in the parietal association cortex of the nondominant hemisphere. Although the segregation of functions between the two parietal lobes is not complete, the parietal association cortex is nevertheless the most highly lateralized in the brain, with language functions concentrated in the left hemisphere and spatial relationships and related selective attention concentrated in the right hemisphere.
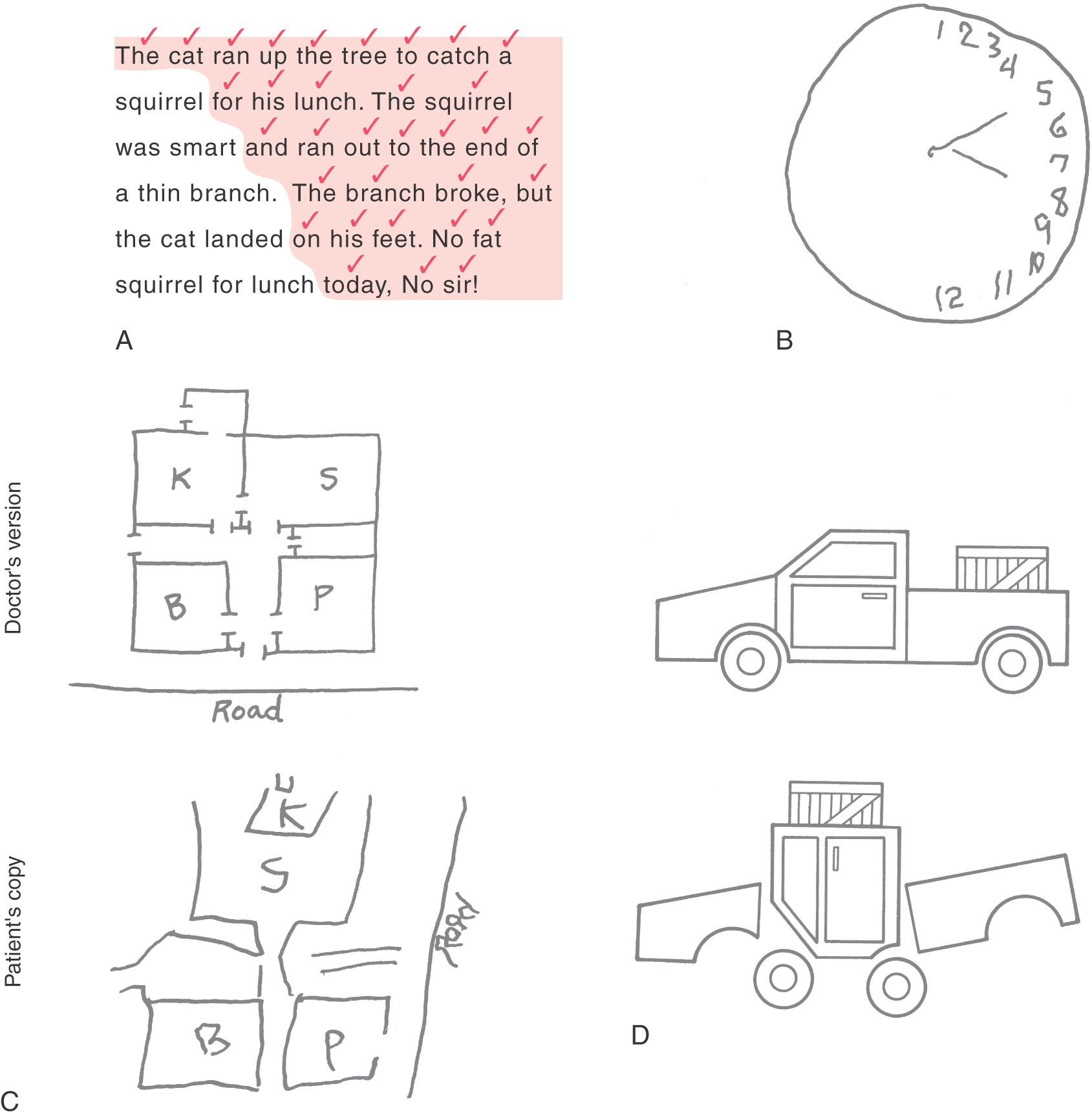
Much of our knowledge of the functional properties of different regions of the cerebral cortex has been gained from neurologic case studies of patients with cortical damage produced by stroke or head trauma. In this respect, the two great wars of the first half of the twentieth century led, inadvertently, to great progress in our understanding of the effects of brain injuries. One of the most striking symptoms of damage to the right parietal association cortex (nondominant) is a defect of attention, in which the patient seems to be completely unaware of objects and events in the left half of his or her surrounding space. This symptom is termed contralateral neglect (Fig. 32-14A, B).
Figure 32-14. Signs (A to D) of damage to the nondominant parietal association cortex. See text for details.
Contralateral Neglect and Related Symptoms
In its milder forms, contralateral neglect may simply be a tendency to ignore things on the left side of the patient’s surroundings. For example, the patient may be asked to read a short passage and check off each word in the process. As the patient reads, words on the left side of the passage are progressively ignored, and only those on the right part are perceived (Fig. 32-14A). Another way to demonstrate contralateral neglect is to draw a circle and ask the patient to draw in the numbers of a clock face. Typically, the patient with right parietal damage will put all of the numbers (1 to 12) on the right side of the circle (the side ipsilateral to the lesion), completely ignoring the left (contralateral) side of the circle (Fig. 32-14B). A patient with contralateral neglect may not be aware of people standing to the left, may bump into large stationary objects on the left, and may not respond to sounds or words coming from the left. In extreme cases, the patient may not even recognize the left side of his or her own body (asomatognosia). For example, the patient may ignore the left side when dressing or grooming (dressing apraxia) or, if in a hospital, may even demand that the staff get this “other person” (the left side of his or her own body) out of the bed.
Another characteristic group of symptoms of right parietal lobe lesions concerns the ability to function successfully within the spatial surroundings. For example, the affected person may be unable to describe the route between home and work, to draw a floor plan of his or her house (Fig. 32-14C), or to find a soft drink machine that is just down the hall. In extreme cases, the patient may not be able to navigate successfully from the bed to a chair that is just across the room and in full view.
Yet another difficulty is an inability to successfully manipulate objects in space. The patient may be unable to duplicate a simple block construction while looking at a model (Fig. 32-14D). This difficulty is termed constructional apraxia; it is related not to visual acuity or to fine motor control but rather to an inability to internalize and duplicate the spatial relationships of the individual parts of the model. In addition, disorders of affect are common, including a reduced ability to understand and appreciate humor, a loss of the ability to appreciate the prosody of speech, and often an inappropriate cheerfulness and lack of concern for or even awareness of the implications of the illness. This lack of concern may be noted even when the deficit is as serious as total left hemiplegia.
Apraxia and Agnosia
Damage in many areas of association cortex can produce higher level disorders of behavior. Apraxia is a disorder of motor control that may occur after damage in parietal association cortex, premotor cortex, or supplementary motor cortex. In this disorder, there is no paralysis of individual muscles or limbs, and muscle strength may be undiminished. Nevertheless, the affected individual is unable to coordinate his or her muscles to execute complex behavior. For example, a patient who can visually recognize a hammer, can name it, can explain what it is used for, and has the strength to pick it up will be unable to demonstrate how it is used to drive a nail into a board. Apraxia may affect the muscles of speech and thus make speech difficult. Apraxia of speech is a separate disorder from aphasia (described earlier), in which the internal processing of the symbols of language is impaired, thus affecting the understanding and production of spoken language, written language, and sign languages.
Agnosia is a general term used to describe a large group of higher level disorders of sensory perception. The term “agnosia” is derived from two Greek terms that mean a “lack of knowledge.” Agnosias are typically confined to a single sensory modality and are characterized by a patient’s difficulty in recognizing complex sensory stimuli, for example, faces or letters in the visual modality or tunes or spoken words in the auditory modality. In the clinical setting, these deficits are commonly named according to the sensory perception that is disrupted. For example, an inability to recognize a familiar object by sight is visual agnosia, whereas the inability to recognize noises or sounds is auditory agnosia. A somatosensory agnosia might involve a patient’s inability to recognize a common object such as a coin, pencil, or key by the sense of touch alone (tactile agnosia, astereognosis) or to recognize a letter or number drawn on the palm of the patient’s hand while the patient had his or her eyes closed. There are a number of variations on this general theme. This type of deficit also extends to the sense of smell (olfactory agnosia), the sense of taste (gustatory agnosia), and even the inability to identify colors (color agnosia). Agnosias are not a loss of the primary sensation (touch, vision, hearing) but are a loss of the ability to interpret the sensation. They are commonly produced by damage in modality-specific areas of the sensory association cortex.
Prefrontal Cortex and Plans for Future Operation
The other major region of multimodal association cortex is the large expanse anterior to the primary motor and premotor cortices, the prefrontal association cortex. This region has historically been connected with some of the most distinctly human intellectual traits, such as judgment, foresight, a sense of purpose, a sense of responsibility, and a sense of social propriety.
One of the earliest accounts of the effect of brain injury on higher intellectual functions described a series of events that began on September 13, 1848. A crew of railroad construction workers was blasting a right-of-way through the rugged granite mountains of Vermont. The well-liked young foreman of the crew, Phineas Gage, was in charge of placing a black powder charge in a deep hole drilled in the rock, adding a fuse, covering the powder with sand, and finally tamping the sand and powder down firmly with an iron rod before lighting the fuse and running for cover. On this day, something apparently distracted Gage, and he began to tamp down a charge before the sand had been added. The iron rod struck the granite wall of the hole and a spark ignited the powder. The 3½-foot-long, 13-pound rod was propelled out of the hole like a giant bullet.
The rod struck Gage just beneath the left eye and exited through the top of his head, destroying most of his prefrontal cortex. Amazingly, Gage was not killed instantly, and even more incredibly, he survived the inevitable serious wound infection that followed. Eventually he recovered his health, or at least the physical portion of it. Mentally, however, he was changed forever. Although he did not have paralysis, language disorders, or memory loss, his personality was radically altered. John Harlow, one of the physicians who attended Gage, perceived the importance of this case with respect to the localization of intellectual functions in the brain. In an article describing the injury and Gage’s persisting intellectual symptoms, Harlow said:
This passage, written almost 160 years ago, provides an accurate and insightful description of the major symptoms associated with destruction of the prefrontal cortex. Patients with significant bilateral damage to the prefrontal cortex have a constellation of deficits that can be summarized as follows. First, they are highly distractible, turning from one activity to another according to the novelty of a new stimulus rather than according to a plan. This deficit is sometimes described as a lack of consistency of purpose. Second, these persons have a lack of foresight. They are not able to anticipate or to predict future events on the basis of past events or present conditions. Third, they may be unusually stubborn in the face of advice with which they do not agree, and they may also perseverate in the performance of a task. Fourth, the patient with prefrontal damage displays a profound lack of ambition, a loss of the sense of responsibility, and a loss of a sense of social propriety. The first and third symptoms (distractibility versus perseveration) are obviously in conflict. It is impossible to predict which will dominate at a given moment, but both exemplify the affected person’s loss of the ability to govern his or her own actions and life according to a plan. The person is instead imprisoned in a chaotic world, with his or her actions governed by randomly changing whims.
It was this set of symptoms that prompted the Portuguese neurosurgeon Egas Moniz to develop the prefrontal lobotomy procedure in the late 1930s to treat a range of severe, intractable mental problems. At that time, mental hospitals (“insane asylums”) all over the world contained many patients who were so immobilized by anxiety that they could not even take care of their own bodily needs. They were warehoused under reprehensible conditions. The discovery that a neurosurgical procedure could alleviate the anxiety to the extent that the patients could lead a somewhat more normal existence (albeit still within the confines of a mental institution) was hailed as a great breakthrough. In these desperate patients, the symptoms as described before seemed a justifiable price to pay for freedom from the crushing anxiety that had immobilized them. Unfortunately, by the late 1940s and early 1950s, the procedure had acquired a popularity out of all proportion to its actual benefits, and it was widely misapplied (as in the movie One Flew Over the Cuckoo’s Nest). The discovery of tranquilizers in the late 1950s provided a more effective method of treatment, having fewer undesirable side effects, and prefrontal lobotomy was rapidly abandoned as a method of treatment.
Sources and Additional Reading
Blakemore C. Mechanics of the Mind. Cambridge: Cambridge University Press; 1977.
Damasio AR. Aphasia. N Engl J Med. 1992;326:531–538.
Mountcastle VB. The cortical organization of the neocortex. Brain. 1997;120:701–722.
Peters A, Jones EG. Cerebral Cortex. vols 1–14. New York: Plenum Press; 1984-1999.
Rowland LP. Merritt’s Neurology. ed 10 Baltimore: Lippincott Williams & Wilkins; 2000.
Valenstein ES. Great and Desperate Cures. New York: Basic Books; 1986.
Victor M, Ropper AH. Adams and Victor’s Principles of Neurology. ed 7 New York: McGraw-Hill; 2001.