The cerebral cortex
Basic anatomy
Hemispheres
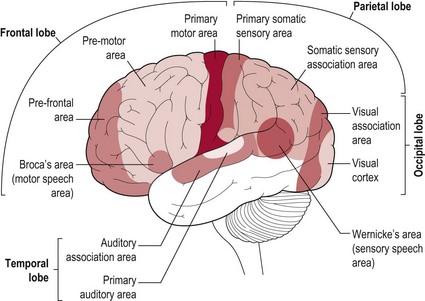
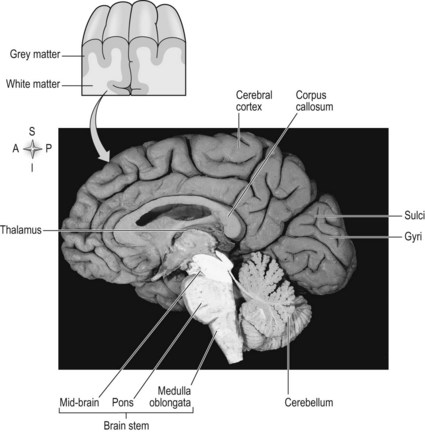
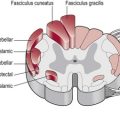
The brain is divided into two halves or hemispheres, which are divided into four lobes (frontal, parietal, temporal and occipital) (Fig. 7.1). The left and right hemispheres are connected by the corpus callosum (Fig. 7.2), which facilitates communication between the two sides of the brain. The limbic system, sometimes referred to as a 5th lobe, forms the inner border of the cortex and is made up of several different structures scattered throughout the four lobes.
Grey/white matter
The outermost layer of the cerebral cortex is termed the ‘grey matter’ (Fig. 7.2) and is primarily composed of the cell bodies of neurons (S2.6). The grey matter is an expansive sheet, approximately 2–4 mm (0.08–0.16 inches) thick, which is intricately folded to form grooves termed ‘sulci’ and raised areas termed ‘gyri’ (Fig. 7.2). The compact nature of the grey matter allows closer contact between neurons and consequently faster communication. The white matter (Fig. 7.2) below is formed predominantly by the myelinated axons of the neurons (S2.6), which connect to different regions of the central nervous system.
The internal capsule
The internal capsule lies superiorly to the thalamus (S2.9) and consists of neurons passing from the thalamus to the cerebral cortex and vice versa. Between the cortex and the internal capsule, the ascending and descending neurons form the corona radiata. All afferent and efferent information is routed through the internal capsule and most sensory information enters the cerebral cortex via the thalamus.
Connections
The left and right hemispheres of the cerebral cortex are connected by corpus callosum with each hemisphere being connected, in the main, to the contralateral side of the body. Thereby the left hemisphere receives information from the right side of the body and has motor control of the right side of the body. This is the result of the decussation or crossing of the ascending and descending tracts (S2.14, 15). However, there are a minority of neurons which remain ipsilateral, e.g. in the corticospinal tract (S2.14). That is, some neurons within the tracts remain on the same side of the body. This phenomenon is thought to have important implications during rehabilitation of neurologically impaired patients, especially those following stroke.
The hemispheres are richly connected to various subcortical structures such as the thalamus (Fig. 7.2) and basal ganglia, however the vast majority of connections (99%) are from one area of the cortex to another.
Cells of the cerebral cortex
Neurons
In the cerebral cortex there are two main forms of neuron – those which result in excitatory post-synaptic potentials (EPSP) and others producing inhibitory post-synaptic potentials (IPSP) (S2.6). The excitatory neurons may span several layers enabling them to pick up a wide range of information. They often have a high density of dendrites which are covered in post-synaptic sites called ‘spines’, further reinforcing this role. Inhibitory neurons however, tend to be in the minority (20%) and are more diverse in their characteristics. They often remain within the grey matter making short range local connections. Their role is considered to be modulation. Modulation is not necessarily a case of switching other neurons ‘on’ or ‘off’ but rather to grade or fine tune in the same way as we would use a dimmer switch for a light.
Glial cells
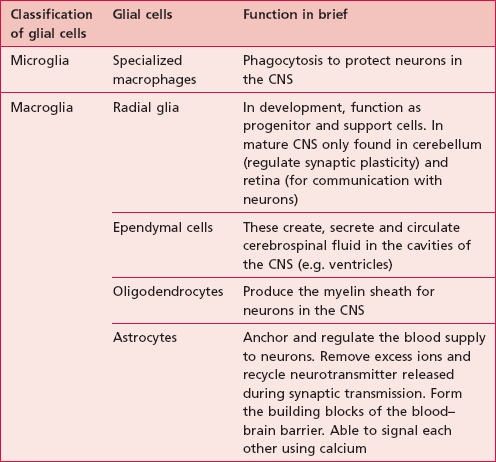
Glial cells, commonly called ‘neuroglia’ or ‘glia’ are non-neuronal cells which outnumber neurons by about 10 to 1. The four main functions of glial cells are to provide structural support for neurons, to supply neurons with nutrients and oxygen, to insulate one neuron from another and to destroy pathogens and remove dead neurons (Table 7.1).
Function of the cerebral cortex
Sensory
There is a regional specialization of function within the sensory areas. In simple terms, this means that neurons performing similar roles and therefore needing to communicate efficiently will tend to be found together. This organization gives rise to a cortical map of the sensory area known as a ‘topographic map’. The somatosensory topographic map is represented by a deformed human shape and is termed the ‘homunculus’ (Fig. 7.3). In the homunculus, the sizes of the different body parts reflect their relative importance in terms of the density of innervations. For example, areas with lots of sensory receptors, such as the fingertips and the lips have a larger area of representation because the number of sensory neurons entering the sensory cortex is greater.
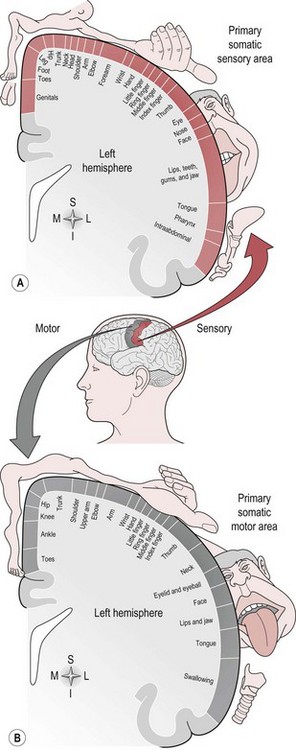
Figure 7.3 The sensory and motor homunculus.
Motor
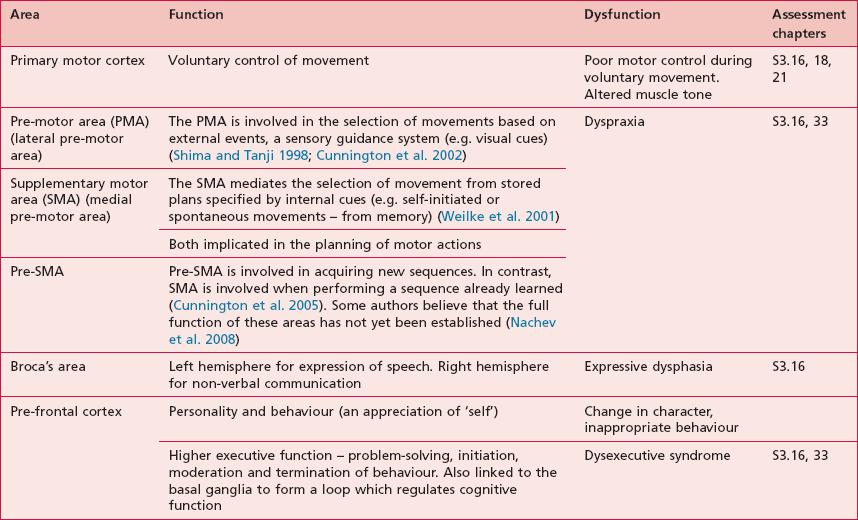
The motor areas are located in both hemispheres of the frontal lobe and control the contralateral side of the body; they are also arranged topographically forming a motor homunculus (Fig. 7.3). Three main areas of the cortex have been found to be important in voluntary movement:
Anatomical areas and their function linked to assessment
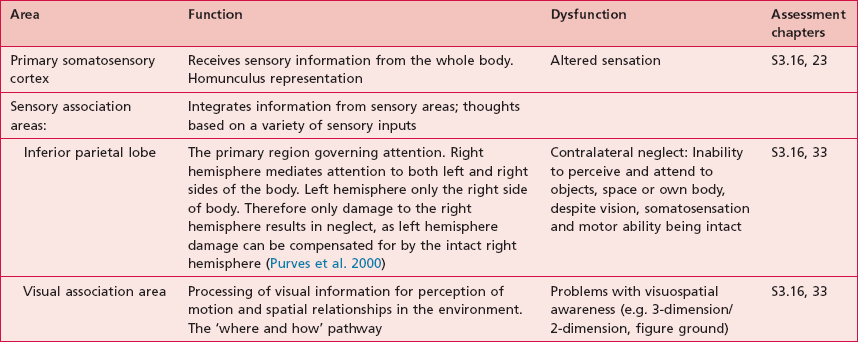
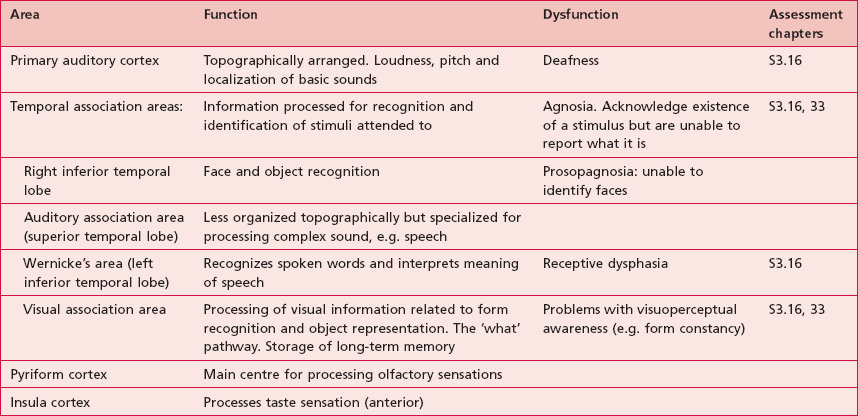
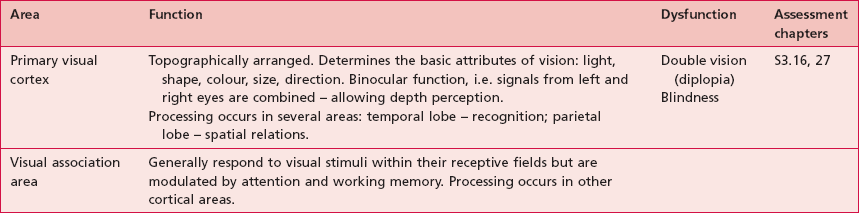
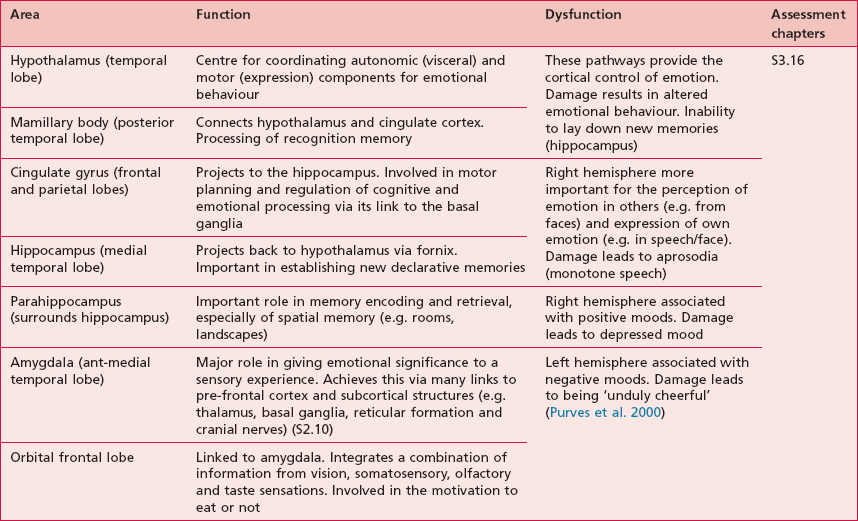
Based upon past and present research, Tables 7.2–7.6 represent a summary of the basic functions of the four main lobes and the limbic lobe. Each table also includes the terminology used to describe a dysfunction of the relevant function and the section/chapter code linking it to an assessment tool, where appropriate. It may be useful to read these tables in conjunction with Figure 7.1, which shows the anatomical position of these areas within the lobes of the cerebral cortex.
References and Further Reading
Burns, GA, Young, MP. Analysis of the connectional organization of neural systems associated with the hippocampus in rats. Philosophical Transactions of the Royal Society, Series B. Biological Sciences. 2000; 355:55–70.
Cunnington, R, Windischberger, C, Deecke, L, et al. The preparation and execution of self-initiated and externally-triggered movement: a study of event related fMRI. Neuroimage. 2002; 15:373–385.
Cunnington, R, Windischberger, C, Moser, E. Premovement activity of the presupplementary motor area and the readiness for action: studies of time-resolved event-related functional MRI. Human Movement Science. 2005; 24:644–656.
Herrup, K, Yang, Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nature Reviews Neuroscience. 2007; 8:368–378.
Nachev, P, Kennard, C, Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008; 9:856–869.
Passingham, RE, Stephan, KE, Kotter, R. The anatomical basis of functional localization. Nature Reviews and Neuroscience. 2002; 38:606–616.
Purves, D, Augustine, GJ, Fitzpatrick, D, et al. Neuroscience. Sunderland: Sinauer Associates; 2000.
Shima, K, Tanji, J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. Journal of Neurophysiology. 1998; 80:3247–3260.
Shipp, S. Structure and function of the cerebral cortex. Current Biology 17. 2007; 12:R443–R449.
Weilke, F, Spiegel, S, Boecker, H, et al. Time-resolved fMRI of activation patterns in M1 and SMA during complex voluntary movement. Journal of Neurophysiology. 2001; 85:1858–1863.
Welker, W. Why does the cerebral cortex fissure and fold? A review of determinants of gyri and sulci. In: Jones EG, Peters A, eds. Cerebral Cortex, Vol 8b: Comparative structure and evolution of cerebral cortex, Part 2. New York: Plenum Press, 1991.
Wolosker, H, Dumin, E, Balan, L, et al. Amino acids in the brain: d-serine in neurotransmission and neurodegeneration. The FEBS Journal. 2008; 275(14):3514–3526.
Young, MP, Hilgetag, CC, Scannell, JW. On imputing function to structure from the behavioural effects of brain lesions. Philosophical Transactions of the Royal Society, Series B. Biological Sciences. 2000; 355:147–161.