Chapter 27
The Cerebellum
Cerebellar Lobes, Lobules, and Zones
Blood Supply to Cerebellar Structures
Synaptic Interactions in the Cerebellar Cortex
Vestibulocerebellar Dysfunction
Vestibular Connections of the Vermis
As indicated by its relative size (about 10% of the weight of the central nervous system), the cerebellum is important in brain function. However, it executes these responsibilities in unique ways. First, it receives extensive sensory input, but it is not involved in sensory discrimination or interpretation. Second, although it profoundly influences motor function, resection of relatively large portions of the cerebellar cortex does not result in lasting paralysis. The patient may have uncoordinated movements, but when tested for strength, the patient is not weak. Large lesions of the cerebellum may result in significant motor deficits (seen as asynergistic movements) but not in paralysis. Third, the cerebellum plays a role in motor learning and higher mental function.
OVERVIEW
The cerebellum is composed of a highly convoluted cerebellar cortex and a core of white matter containing the cerebellar nuclei. This structure is anchored to the brainstem via the cerebellar peduncles. The cerebellum is located superior to the brainstem, inferior to the tentorium cerebelli, and internal to the occipital bone. The cerebellum has a superior surface apposed to the tentorium and a convex inferior surface that abuts the inner surface of the occipital bone.
The cerebellum receives input from many areas of the neuraxis and influences motor performance through connections with the dorsal thalamus and, ultimately, the motor cortices. Lesions of these pathways result in characteristic motor dysfunctions, which may involve either proximal (axial) or distal musculature. These deficits are actually the result of altered activity in the motor cortex and its descending brainstem and spinal projections, which influence lower motor neurons of the spinal cord.
BASIC STRUCTURAL FEATURES
Cerebellar Peduncles
The cerebellum is connected to the brainstem by three pairs of cerebellar peduncles (Fig. 27-1A–C). The inferior cerebellar peduncle is composed of a larger part, the restiform body, and a smaller portion, the juxtarestiform body (Fig. 27-1B, C). The restiform body is the large ridge on the dorsolateral aspect of the medulla rostral to the level of the obex. This bundle contains mainly fibers that arise in the spinal cord or medulla. The juxtarestiform body is located in the wall of the fourth ventricle. This bundle is composed primarily of fibers that form reciprocal connections between the cerebellum and vestibular structures (Table 27-1).
Table 27-1 Synopsis of Selected Afferent and Efferent Fibers of the Cerebellum as Contained in or Associated with the Cerebellar Peduncles
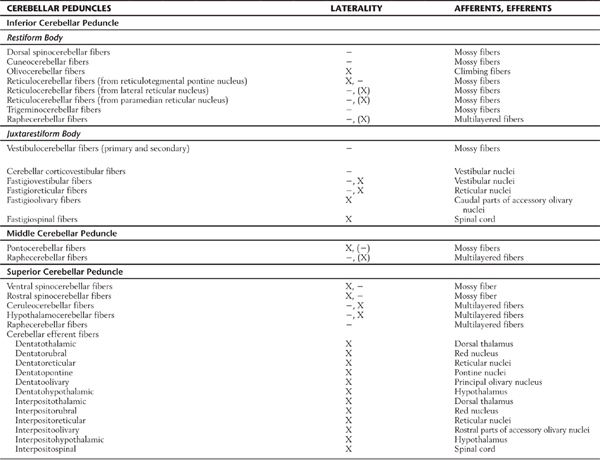
−, uncrossed; X, crossed; (−), some uncrossed; (X), some crossed.
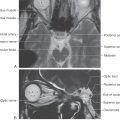
The basilar pons, which is located inferior to the exiting roots of the trigeminal nerve, is continuous into the middle cerebellar peduncle (brachium pontis), which is located superior to the exiting roots of the fifth cranial nerve (Figs. 27-1A–C and 27-2). These exiting roots represent the boundary between the basilar pons and the middle cerebellar peduncle. This large peduncle mainly conveys pontocerebellar fibers arising from the pontine nuclei of the basilar pons into the cerebellum.
Figure 27-2. Axial magnetic resonance image of the cerebellum and pons showing the relationship between the root of the trigeminal nerve and the basilar pons (inferior to the root) and the middle cerebellar peduncle (superior to the root). Compare with Figure 27-1.
The superior cerebellar peduncle (brachium conjunctivum) sweeps rostrally out of the cerebellum and penetrates into the midbrain just caudal to the exit of the trochlear nerve (Fig. 27-1A–C). Within the midbrain, these fibers cross the midline as the decussation of the superior cerebellar peduncle at the level of the inferior colliculus. This bundle contains predominantly cerebellar efferent fibers that originate from neurons of the cerebellar nuclei and distribute to the diencephalon and brainstem.
Cerebellar Lobes, Lobules, and Zones
At the most general level, it is common to divide the cerebellum into a narrow midline vermis and expansive lateral hemispheres (Figs. 27-3 and 27-4 [see pp. 374-375]). The cerebellum is further divided into anterior, posterior, and flocculonodular lobes by the primary and posterolateral fissures, respectively. The lobes of the cerebellum are composed of yet smaller divisions called lobules (Figs. 27-3 and 27-5 [see p. 375]). The lobules of the vermis are identified by Roman numerals I to X; the corresponding lateral (hemisphere) portion of each vermis lobule is identified by the same Roman numeral but with the prefix H (Figs. 27-3 and 27-5). Vermis lobules II to X have hemisphere portions HII to HX (Fig. 27-5); lobule I does not have a hemisphere part in humans. The anterior lobe comprises lobules I to V and HII to HV, and the posterior lobe, lobules VI to IX and HVI to HIX. The flocculonodular lobe consists of the nodulus (lobule X) and its hemisphere counterpart, the flocculus (lobule HX). In turn, each lobule consists of a series of individual ridges of cortex called folia (singular, folium).
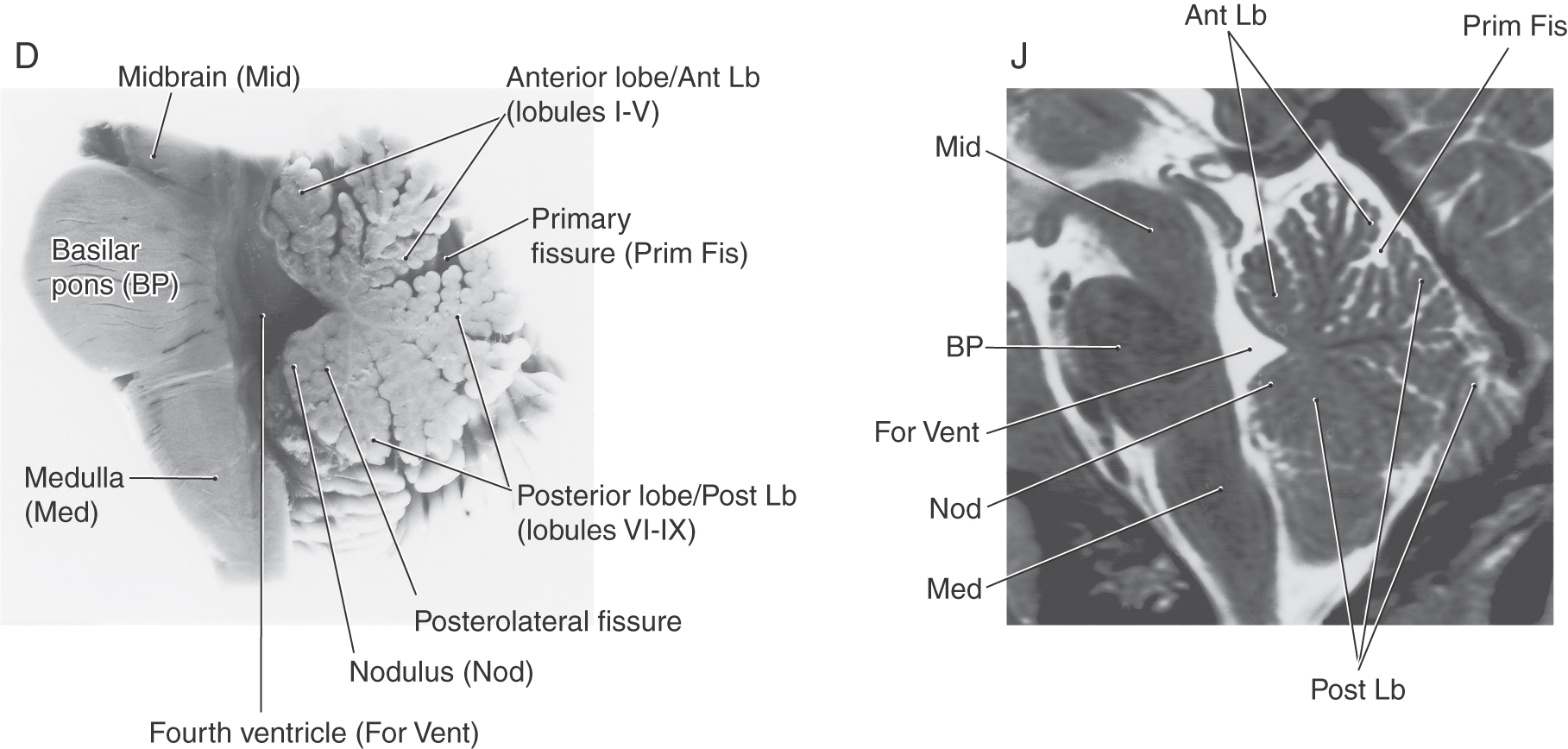
The individual folia are continuous from one hemisphere to the other, across the midline on the superior cerebellar surface (Fig. 27-4A, E, F). This pattern, obvious on the superior cerebellar surface, is disrupted on the inferior surface by the enlargement of the lateral parts of the cerebellum and consequent infolding of the midline area (Fig. 27-4B, G, I).
Superimposed on the lobes and lobules of the cerebellum are rostrocaudally oriented cortical zones that are defined on the basis of their connections. There are three principal zones on each side: the medial (vermal), intermediate (paravermal), and lateral (hemisphere) zones (Fig. 27-5). On the basis of their afferent and efferent connections, these three larger cortical zones can be subdivided further into nine smaller zones. In general, these zone patterns are the basis for the modules discussed later in this chapter. The clinical deficits that result from a cerebellar lesion depend mainly on which of the three principal zones is involved; consequently, the three-zone terminology is used in this chapter.
The medial (vermal) zone is a narrow strip of cortex adjacent to the midline that extends throughout anterior and posterior lobes and includes the nodulus (Figs. 27-4A, B and 27-5). This zone is widest in lobule VI and tapers rostrally and caudally. The intermediate (paravermal) zone lies adjacent to the medial zone and extends throughout anterior and posterior lobes but has little representation in the flocculonodular lobe (Fig. 27-5). The lateral (hemisphere) zone occupies by far the largest part of the cerebellar cortex. It includes large portions of anterior and posterior lobes and the flocculus (Figs. 27-3 and 27-5).
Cerebellar Nuclei
The four pairs of cerebellar nuclei are located within the white matter core of the cerebellum and are accessed easily by fibers traveling to and from the overlying cortex (Figs. 27-5 and 27-6). The fastigial (medial cerebellar) nucleus lies immediately adjacent to the midline and is functionally related to the overlying medial zone of the cerebellar cortex. Lateral to the fastigial nucleus are the two interposed nuclei: the globose (posterior interposed) nucleus and the emboliform (anterior interposed) nucleus. These nuclei are functionally related to the intermediate zone of the cortex. Lateral to the emboliform nucleus is the dentate (lateral cerebellar) nucleus, which appears as a large, undulating sheet of cells shaped like a partially crumpled paper bag. Its opening, the hilus, is directed anteromedially (Fig. 27-6). This nucleus is functionally related to the lateral zone of the cortex; its large size correlates with the large size of this cortical zone.
 Figure 27-6. The cerebellar nuclei in cross section, drawn from a slide. The color coding of each nucleus corresponds to its appropriate zone in Figure 27-5.
Figure 27-6. The cerebellar nuclei in cross section, drawn from a slide. The color coding of each nucleus corresponds to its appropriate zone in Figure 27-5.
Most of the signals that leave the cerebellum do so via axons that arise in the cerebellar nuclei; the remainder travel on fibers that originate in the cerebellar cortex. Collectively, axons that arise in the cerebellar nuclei constitute cerebellar efferent projections (e.g., cerebellothalamic fibers). These axons originate from cells in the cerebellar nuclei and use one of the excitatory neurotransmitters, glutamate or aspartate, and thus function to activate their targets. The juxtarestiform body contains fibers that arise from the cerebellar cortex, form the cerebellar corticovestibular projection, use the inhibitory neurotransmitter GABA, and inhibit their targets. The fastigial nuclei generally project bilaterally to the brainstem through the juxtarestiform bodies. Fibers originating in the dentate, emboliform, and globose nuclei exit the cerebellum via the superior cerebellar peduncle and cross in its decussation.
Some neurons in each cerebellar nucleus send axons or axon collaterals into the overlying cortex, where they terminate in the granular layer as mossy fibers. These axons are called nucleocortical fibers, and they exert an excitatory influence on the cerebellar cortex.
Blood Supply to Cerebellar Structures
The blood supply to the cerebellar cortex, nuclei, and peduncles is via the posterior inferior cerebellar artery (PICA), anterior inferior cerebellar artery (AICA), and superior cerebellar artery (Figs. 27-5 and 27-7 [see p. 376]). The PICA originates from the vertebral artery and supplies the posterolateral medulla (including the restiform body), the choroid plexus of the fourth ventricle, and caudomedial regions of the inferior cerebellar surface (including the vermis) (Figs. 27-5 and 27-7). Caudal parts of the middle cerebellar peduncle, the choroid plexus extending out of the foramen of Luschka, and the caudolateral portions of the inferior cerebellar surface are served by the AICA. This vessel may also supply caudal parts of the dentate nucleus (Fig. 27-5). The entire superior surface of the cerebellum, most of the cerebellar nuclei, the rostral parts of the middle cerebellar peduncle, and the superior cerebellar peduncle are served by the superior cerebellar artery (Fig. 27-7).
Figure 27-7. Origin and course of arteries serving the cerebellum as seen in lateral aspect.
CEREBELLAR CORTEX
On histologic examination, each folium of the cerebellum has a superficial cellular layer, the cerebellar cortex, and a core of myelinated fibers traveling to (afferent) or from (efferent) the overlying cortex. The cortex consists of a Purkinje cell layer insinuated between a cell-dense inner region immediately adjacent to the white matter core, the granule cell layer, and an outer pale and relatively cell sparse portion, the molecular layer (Figs. 27-8 and 27-9 [see p. 377]).
Figure 27-9. Cell types and synaptic relations in the cerebellar cortex in transverse and sagittal planes. Note the structure of the cerebellar glomerulus (lower left) and the interaction of parallel and climbing fibers (upper right) with the dendritic processes of Purkinje cells. Compare with Figure 27-10. Because of their regional specificity, the unipolar brush cells are not shown (see Fig. 27-11).
Purkinje Cell Layer
The large (40 to 65 µm in diameter) somata of Purkinje cells form a single layer at the interface of the granular and molecular layers (Figs. 27-8 to 27-10 [see p. 378]). Each Purkinje cell gives rise to an elaborate dendritic tree that radiates into the molecular layer. This dendritic tree is shaped like a fan with its wide flattened aspect oriented perpendicular to the long axis of the folium (Figs. 27-9 and 27-10). The “trunk” of the tree is a single primary dendrite, which gives rise to several secondary dendrites, which in turn branch into many tertiary dendrites. Synaptic contacts are formed mainly on short terminal branchlets that emerge primarily from the secondary and tertiary dendrites (Figs. 27-9 and 27-10). There are two types of branchlets. Smooth branchlets emerge from secondary and tertiary dendrites, whereas spiny branchlets (gemmules) arise mainly from tertiary dendrites.
Figure 27-10. Examples of cells of the cerebellar cortex (see Fig. 27-9). The Purkinje cells are shown in sagittal (A; note the beaded appearance of the dendrites) and transverse (B; note the many parallel fibers) planes. Granule cell (C and D) dendrites end as a cluster of short, claw-like processes. Dendrites of Golgi cells (D) branch into molecular and granular layers, whereas their axons (D, beaded structures) ramify in only the granular layer. Mossy fibers (E) branch profusely and have many rosettes; synaptic contacts between mossy fibers and granule cell dendrites take place at the rosette in the cerebellar glomerulus (see Fig. 27-9). At the ultrastructural level (F), the Purkinje cell dendrite is surrounded by the numerous small profiles of parallel fibers. BF, Bergmann fiber, a type of glial cell process. (A to D Courtesy of Dr. José Rafols, Wayne State University.)
Purkinje cells are the only efferent neurons of the cerebellar cortex. Axons of Purkinje cells arise from the basal aspect of its pear-shaped cell body and may give rise to recurrent collaterals. These axons traverse the granular layer and the subcortical white matter to eventually terminate in either the cerebellar or the vestibular nuclei. Purkinje cells projecting into the cerebellar nuclei (as cerebellar corticonuclear fibers) arise from all areas of the cortex, whereas those projecting into the vestibular nuclei (as cerebellar corticovestibular fibers) originate primarily from parts of the vermis and the flocculonodular lobe. Purkinje cells release γ-aminobutyric acid (GABA) at their synaptic terminals and inhibit target neurons in the cerebellar and vestibular nuclei.
Granule Cell Layer
There are three types of neuron cell bodies within the granule layer. These are granular cells, which are extraordinarily numerous and found in all areas of the cerebellar cortex; Golgi cells, which are larger and also widely distributed; and unipolar brush cells, which are small neurons that have a restricted geographic distribution within the cortex.
The most numerous neuron of the granule cell layer is the small (5 to 8 µm in diameter) granule cell (Figs. 27-8, 27-9, and 27-10C). The dendrites of these cells form claw-like endings (dendritic digits) that ramify in the vicinity of the cell body. Their axons ascend into the molecular layer, where they bifurcate to form parallel fibers. As indicated by their name, parallel fibers run parallel to the long axis of the folium. Consequently, these fibers pass through the fan-like dendritic trees of the Purkinje cell and, when doing so, make synaptic contacts with spiny branchlets (Figs. 27-9 and 27-10B, F). They also synapse with the cells intrinsic to the molecular layer, such as basket and stellate cells. The distance spanned by the parallel fibers of a granule cell ranges from 0.3 to 5.0 mm, and the number of Purkinje and other cells contacted varies accordingly. Granule cells use glutamate (or perhaps aspartate) as their neurotransmitter and thus have an excitatory effect on their target cells. In fact, the granule cells are the primary excitatory neurons of the cerebellar cortex. The unipolar brush cell is also excitatory but is found in strikingly fewer numbers and is restricted in its distribution within the cortex. All of the other neurons of the cerebellar cortex, as we shall see, are inhibitory.
The second cell type of the granular layer is the Golgi cell. The soma of this neuron is larger (at 18 to 25 µm in diameter) than that of the granule cell and is usually found in the granular layer adjacent to the Purkinje cells (Figs. 27-9 and 27-10D). Dendrites of Golgi cells branch in the granular layer but extend primarily into the molecular layer without regard to plane of orientation. Axons of Golgi neurons branch in the granular layer and form synaptic contacts on granule cell dendrites (Fig. 27-9). The Golgi cell uses GABA as a neurotransmitter and is an inhibitory interneuron in the cerebellar cortex.
The third neuronal type found within the granule layer is the unipolar brush cell. This neuron has a slightly oval cell body, measuring 9 to 14 µm in diameter at the equator, and a single stout dendritic structure arising from the soma (Fig. 27-11; see p. 379). This dendritic process ends in a brush-like configuration made up of a cluster of dendrioles. The axon of the unipolar brush cell arises from the cell body, ramifies within the granular layer, and ends as a series of two to four synaptic structures called unipolar brush cell rosettes (Fig. 27-11). In contrast to granule cells, which are distributed throughout the cerebellar cortex, the unipolar brush cells are found primarily in the flocculonodular lobe, the adjacent areas of the paraflocculus, the vermis, and (in very sparse numbers) in the hemisphere lobules HVI to HVIII. Mossy fibers arising from primary and secondary vestibulocerebellar fibers and Golgi cell axons contact the dendrioles (Fig. 27-11B). The unipolar brush cell rosette is contacted by granule cell dendrites, dendrioles of the unipolar brush cell, Golgi cell axons, and an occasional Golgi cell dendrite (Fig. 27-11B). Unipolar brush cells use glutamate as their neurotransmitter and are therefore excitatory to their targets.
The classic cerebellar glomerulus is a synaptic complex found throughout all portions of the cerebellar cortex within the neuropil of the granular layer. The glomerulus includes granule cell and Golgi cell dendrites and Golgi cell axons and a specialized synaptic segment (the mossy fiber rosette) of a mossy fiber, one type of cerebellar afferent axon (Figs. 27-9 and 27-10C–E). The mossy fiber rosette is centrally located and forms synapses with several granule cell dendrites. Golgi cell axons contact granule cell dendrites in the glomerulus, and the entire complex is encapsulated by glial cell processes.
Within the flocculonodular lobe and some adjacent cortical areas, there are many cerebellar glomeruli of the classic arrangement described before. In addition, this cortical area also contains glomeruli that are based on the unipolar brush cell rosette, in which case they may be called, in these specific cortical areas, unipolar brush cell glomeruli (Fig. 27-11B). This specific type of glomerulus is composed of the brush cell rosette, small contacts made by granular cell dendrites, larger contacts made by brush cell dendrioles, and small contacts by Golgi cell axons at the periphery of the glomerulus (Fig. 27-11B). Because unipolar brush cells and their synaptic interactions are located primarily in the flocculonodular lobe and vermis and because these cells receive vestibular inputs, their function is most likely to be related to cerebellar (and vestibular) control of eye movements, vestibuloocular reflexes, and various postural mechanisms.
Molecular Layer
The molecular layer has considerably fewer cell bodies than the granule cell layer (Fig. 27-8) but has proportionately more cell processes. These processes include parallel fibers, Purkinje cell dendrites, Golgi cell dendrites, climbing fibers, and the processes of cells intrinsic to the molecular layer (Fig. 27-9).
The intrinsic cell types of the molecular layer are stellate cells and basket cells (Fig. 27-9). Stellate cells are usually found in outer regions of the molecular layer and are frequently referred to as superficial or outer stellate cells. The somata of basket cells are located immediately above the Purkinje cell layer. The basket cell axon travels in the sagittal plane and gives rise to descending branches that form elaborate “baskets” around the Purkinje cell body. This cell derives its name from this characteristic feature.
In general, the dendritic and axonal plexuses of basket and stellate cells are oriented primarily in the sagittal plane, much like those of Purkinje cell dendrites (Fig. 27-9). Although basket and stellate cells are similar in general shape, the extent of the dendritic and axonal fields is much larger in basket cells than in stellate cells. Consequently, basket cells may influence a large number of Purkinje cells, mainly in the sagittal plane, whereas stellate cells influence a much smaller population but also in the sagittal plane. Stellate and basket cells receive excitatory (glutaminergic) inputs from parallel fibers.
Basket and stellate cells are GABAergic and inhibit their target neurons. Although these cells influence several targets in the molecular layer, for our purposes the Purkinje cell is the most important. Similarly, Purkinje and Golgi cells are also inhibitory (GABAergic), thus making the granule cell and the unipolar brush cell the only neurons in the cerebellar cortex whose outputs are excitatory to their targets. As emphasized earlier, the granule cells are numerous and found in the granular layer throughout all zones and lobules of the cortex; therefore, their excitatory output influences all cortical areas. In contrast, the unipolar brush cells are significantly fewer in number, found primarily in the granular layer of the vermis and flocculonodular lobe; therefore, their excitatory output influences a comparatively restricted area of cortex (and presumably functions in a more specific sphere).
Cerebellar Afferent Fibers
The afferent fibers to the cerebellar cortex are grouped into three types on the basis of the morphology and connections of their terminals in the cortex. These three types of cortical terminals arise as mossy fibers, climbing fibers, and multilayered (monoaminergic) fibers.
The cerebellar afferent axons that end as mossy fibers originate from cell bodies in the cerebellar nuclei (nucleocortical fibers) and from a variety of other nuclei in the spinal cord, medulla, and pons (Table 27-1). En route to the cerebellar cortex, most of these extracerebellar afferent fibers send collaterals into a cerebellar nucleus. In the granular layer, mossy fibers branch profusely, and their large terminals contact other cells at irregular intervals (the mossy fiber rosette). The rosette, which is the central element of the cerebellar glomerulus, gives the fiber a mossy appearance (Figs. 27-9 and 27-10E). Single mossy fibers may form up to 50 rosettes, and each rosette may participate in synaptic contacts with up to 10 to 15 granule cells in a cerebellar glomerulus. In addition, a mossy fiber may branch and distribute to more than one folium. Mossy fibers use glutamate as their neurotransmitter and are excitatory to granule cell and Golgi cell dendrites in the cerebellar glomerulus and to cerebellar nuclear neurons on which their collaterals terminate. Mossy fiber rosettes that end in relation to the dendrioles of the unipolar brush cells within the granular layer of the vermis and flocculonodular lobe are also excitatory to this cell type in these cortical regions.
The inferior olivary nuclei are the only source of cerebellar afferent axons that end as climbing fibers in the cerebellar cortex (Table 27-1). Olivocerebellar fibers send collaterals to the appropriate cerebellar nucleus. The climbing fibers then terminate in the molecular layer by entwining, ivy like, up the dendritic trees of Purkinje dendrites and making synaptic contacts on the more proximal parts of the Purkinje cell processes (Fig. 27-9). Each Purkinje cell is innervated by a single climbing fiber, but olivocerebellar axons may branch to serve several Purkinje cells. Climbing fibers use the neurotransmitter aspartate, and they excite Purkinje cells and the cerebellar nuclear neurons on which their collaterals terminate.
Multilayered fibers (monoaminergic or peptide containing) originate from cells of the locus ceruleus (noradrenergic), the raphe nuclei (serotoninergic), the hypothalamus (some are histaminergic), and other select locations. These fibers enter the cerebellum via the cerebellar peduncles and, in the case of some hypothalamocerebellar fibers, by passing through the periventricular gray and then into the cerebellum. En route to the cerebellar cortex, many of these fibers send collaterals to the cerebellar nuclei. In the cortex, these axons branch diffusely and terminate in molecular and granular layers, where they may influence all major cell types (Fig. 27-9). In general, these fibers modulate the output of the cerebellar cortex through two mechanisms. First, they decrease the spontaneous discharge rates of Purkinje cells. Second, both directly and via interneurons, multilayered fibers alter the responsiveness of Purkinje cells to excitation by climbing fibers and by the mossy fiber–granule cell projection.
Topographic Localization
The cerebellum receives input from a wide range of sources. Some input that originates in the periphery is conveyed via spinocerebellar and vestibulocerebellar pathways that project directly to the cerebellum. Other afferent information is indirect, having passed through multiple central pathways before entering the cerebellum. For example, responses can be recorded in selected regions of the cerebellar cortex after stimuli that activate visual, auditory, or sensorimotor areas of the cerebral cortex in primates (Fig. 27-12A). These pathways involve a cerebropontine-pontocerebellar connection. Visual and auditory cortices project to cells of the basilar pons, which in turn provide a mossy fiber input to areas of the cerebellar cortex where the eye and ear are represented (Fig. 27-12A). Similarly, the sensorimotor cortex, also via projections through the basilar pons, influences the cerebellar cortex. Taken collectively, information on these various cerebellar afferent pathways coalesces to form somatotopic representations of the body in the anterior (and extending into lobule VI) and paramedian lobules (Fig. 27-12A).
At a finer level of resolution, experimental studies in mammals have shown that body parts are not represented continuously over a large area of cerebellar cortex but instead are broken into smaller, discontinuous patches. In this pattern, a small area of cortex that receives sensory input from the arm (via mossy fiber–granule cell connections) may be located adjacent to an area that receives input from a noncontiguous region of the same upper extremity (Fig. 27-12B). In addition, each body part is represented in several locations. This pattern of spatial representation is referred to as fractured somatotopy.
Synaptic Interactions in the Cerebellar Cortex
In general, cerebellar function is regulated by modulation of the output of the cerebellar nuclei. This modulation is mediated through excitation of cerebellar nuclear cells by collaterals of afferent fibers (mossy, climbing) and subsequent inhibition of these same nuclear cells by Purkinje cell axons (corticonuclear fibers) descending from the overlying cortex (Fig. 27-13). These synaptic interactions continuously modify the efferent signals generated by cerebellar nuclear neurons; the cerebellar nuclei influence the “motor thalamus” and ultimately the efficacy of the descending motor pathways.
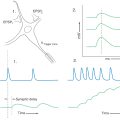
Climbing fibers synapse directly on Purkinje cells, whereas mossy fibers act through granule cells. Because a single climbing fiber makes numerous synaptic contacts on a single Purkinje cell, its influence over that cell is substantial. Consequently, the Purkinje cell response to input from one climbing fiber is represented by a complicated waveform called a complex spike (Fig. 27-14). These spikes are unique and are the result of the combined action of multiple excitatory climbing fiber synapses formed throughout the Purkinje cell dendritic tree. In contrast, each Purkinje cell receives excitatory input from many granule cells via their parallel fibers. Summation, both spatial and temporal, of parallel fiber input is responsible for modulation of the simple spike activity of Purkinje cells (Fig. 27-14). At any given moment, there is an ongoing background level of simple spike activity in the cerebellum. This level can be modulated by phasic increases or decreases in afferent inputs to the mossy fiber–granule cell–parallel fiber system. Collectively, mossy fibers have a powerful influence over Purkinje cells, and this influence may be modulated by climbing fibers through mechanisms not fully understood.
Mossy fiber–granular cell interactions within the glomerulus are one set of important circuits influencing cerebellar functions (Figs. 27-9 and 27-13). In the cerebellar glomerulus, mossy fibers excite granule cell and Golgi cell dendrites. The Golgi cell axon, in turn, synapses on and inhibits granule cell dendrites within the glomerulus. Thus the Golgi cell provides feedback inhibition to granule cells previously excited by mossy fiber activity. The granule cell axon enters the molecular layer, branches into parallel fibers, and excites Purkinje, stellate, basket, and Golgi cells (Fig. 27-13). At a basic level, mossy fiber input leads to excitation of Purkinje cells via parallel fibers, and the GABAergic Purkinje cells respond by inhibiting the cerebellar nuclei.
The synaptic interactions within the cerebellar cortex are also described in the following simplified model, which considers the cytoarchitectural and electrophysiologic properties of cerebellar cortical neurons. The inhibitory Purkinje cell outflow is modulated, in part, by the feed-forward inhibition resulting from stellate and basket cell activation (Figs. 27-13 and 27-15). Parallel fibers excite a specific population of Purkinje cells as well as stellate and basket cells located within their domain (Fig. 27-15). The last two GABAergic interneurons, in turn, inhibit Purkinje cells located adjacent to (via stellate synapses) or at some distance from (basket synapses) the row of activated parallel fibers. When a narrow bundle of parallel fibers is activated, under certain experimental conditions, basket and stellate cells can define a central row, or beam, of excited Purkinje cells. Within the beam, Purkinje cells are activated by parallel fibers and in turn inhibit cells in the cerebellar or vestibular nuclei (Fig. 27-15). Purkinje cells on either side of the activated row are inhibited by stellate and basket axons and consequently do not inhibit their target neurons in the cerebellar (or vestibular) nuclei (Fig. 27-15). These target cells are removed from their normal (background) inhibitory influence; that is, they are disinhibited.
Synaptic interactions between cells of the cerebellar cortex contribute to the activity of cerebellar nuclear neurons. The unique structural and functional properties of the cortex provide circuits for the temporal and spatial processing of information that contributes substantially to the cerebellar capacity to coordinate movement. The exact nature of these interactions has not been fully determined. However, the cytoarchitecture and synaptology within the cerebellum suggest one hypothetical model. According to this model, the excitatory outflow from each cerebellar nucleus varies dynamically in response to the combined effects of (1) the excitatory input from cerebellar afferent collaterals to cerebellar nuclear neurons and (2) the inhibitory or disinhibitory influence mediated by Purkinje cells through the various circuits of the cerebellar cortex. Temporal processing refers to timing variances that partially depend on the number of synaptic contacts within a given circuit; spatial processing refers to variances in inputs, be they from different body parts or different feedback centers within the brain.
FUNCTIONAL CEREBELLAR MODULES
It is convenient to think of the cerebellum as being arranged into compartments or modules. Each module consists of (1) an area of cortex (usually a cortical zone), (2) a white matter core that contains afferent and efferent fibers to and from that cortical area, and (3) a nucleus (or nuclei) that is functionally related to the overlying cortical area. A cerebellar cortical zone, its corresponding nucleus (or nuclei), and the afferent and efferent fibers of the white matter constitute a module.
Vestibulocerebellar Module
The flocculonodular lobe and adjacent portions of vermal lobule IX (the paraflocculus) receive afferents from the ipsilateral vestibular ganglion (primary vestibulocerebellar fibers) and vestibular nuclei (secondary vestibulocerebellar fibers). Therefore, these cortical areas are commonly called the vestibulocerebellum. Along with the fastigial nucleus, they form the vestibulocerebellar module (Fig. 27-16). Because this is phylogenetically the oldest part of the cerebellum, the vestibulocerebellum is sometimes called the archicerebellum (Greek arche, “beginning”). However, this term is not commonly used and is actually discouraged.
Vestibulocerebellar fibers access the flocculonodular cortex and fastigial nucleus via the juxtarestiform body and convey information concerning the position of the head and body in space as well as information useful in orienting the eyes during movements. As noted earlier, the unipolar brush cell is largely unique to the granular layer of the vestibulocerebellum and is probably involved in the cerebellar and vestibular regulation of eye movement. This information is supplemented by inputs carried on olivocerebellar fibers from the contralateral olivary nuclei and on pontocerebellar fibers (only to the flocculus) from the contralateral basilar pons. These pathways convey indirect inputs from nuclei of the diencephalon and brainstem, which are concerned with a broad spectrum of information regarding visual processing and eye movements (see also Chapter 28).
The outflow of the vestibulocerebellar module consists of cerebellar corticovestibular fibers from the flocculonodular lobe, cerebellar corticonuclear fibers from the nodulus to the fastigial nucleus, and efferent fibers arising in the fastigial nucleus (Fig. 27-16). Purkinje cells in the flocculonodular cortex project, via the juxtarestiform body, directly to the ipsilateral vestibular nuclei (cerebellar corticovestibular fibers). Other Purkinje cells in the nodulus project into caudal regions of the fastigial nucleus as cerebellar corticonuclear fibers. Both of these projections are inhibitory (GABAergic) pathways. Fastigial neurons provide bilateral excitatory inputs to the vestibular and reticular nuclei (Fig. 27-16). On the ipsilateral side, these axons pass directly through the juxtarestiform body. Fibers passing to the contralateral side cross in the cerebellar white matter and form the uncinate fasciculus as they loop over the superior cerebellar peduncle. These crossed fibers enter the vestibular complex via the juxtarestiform body.
The brainstem targets of cerebellar corticovestibular fibers and of fastigial efferent fibers, the vestibular and reticular nuclei, are the sources of vestibulospinal and reticulospinal tracts, respectively. The action of cerebellar corticovestibular fibers on the vestibular nuclei is inhibitory, whereas the action of fastigial efferents on the vestibular and reticular nuclei is excitatory.
Vestibulocerebellar Dysfunction
The vestibulocerebellum influences posture, balance, and equilibrium through vestibulospinal and reticulospinal projections to extensor motor neurons that influence axial and proximal limb muscles. The vestibular nuclei also bilaterally innervate the motor nuclei of cranial nerves III, IV, and VI through fibers that ascend in the medial longitudinal fasciculus (see Chapter 28).
Damage to the flocculonodular lobe or to midline structures, such as the nodulus and fastigial nucleus, will result in an unsteady lurching gait (truncal ataxia) that resembles that seen with drunkenness. This instability is also manifested as exaggerated movements of the legs and a tendency to fall to the side, forward, or backward. The patient may stand with feet farther apart than usual (wide-based stance) in an effort to maintain balance. Patients with such lesions are unable to walk in tandem (heel-to-toe) or to walk on their heels or on their toes. Midline lesions may also result in a tremor of the axial body or head called titubation. This tremor can range in amplitude from barely noticeable to so powerful that the patient is unable to sit or stand unsupported. Nystagmus is frequently seen, and deficits in pursuit eye movements are also common. In addition, the patient’s head may tilt or turn to one side, the direction being unrelated to the laterality of the lesion.
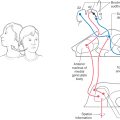
Vestibular Connections of the Vermis
In addition to the nodulus (of the flocculonodular lobe), most lobules of the vermal zone also have vestibular connections (Fig. 27-16). For example, lateral portions of the vermal cortex receive secondary vestibulocerebellar fibers and project to the ipsilateral vestibular nuclei. Like the nodular cortex of the vestibulocerebellar module, the medial portions of the vermal cortex send cerebellar corticonuclear fibers into the ipsilateral fastigial nucleus (Fig. 27-17). Consequently, the fastigial nucleus links vestibulocerebellar cortex and portions of the vermal cortex with the vestibular and reticular nuclei of the brainstem. In this respect, the vermal cortex and fastigial nucleus share the task of influencing axial musculature along with vestibulocerebellar and spinocerebellar modules.
Spinocerebellar Module
The vermal and intermediate zones receive input mainly via the posterior and anterior spinocerebellar tracts and, from the upper extremity, through cuneocerebellar fibers. Owing to this predominant input, these zones are collectively called the spinocerebellum (sometimes the paleocerebellum, although this term is not frequently used) (Figs. 27-17 and 27-18). Posterior spinocerebellar and cuneocerebellar fibers enter the cerebellum via the restiform body, whereas anterior spinocerebellar fibers course into the cerebellum in association with the superior cerebellar peduncle. Fibers that enter the vermal zone send collaterals into the fastigial nucleus, and those passing into the intermediate zone send branches into the emboliform and globose nuclei.
The output of the spinocerebellum is focused primarily on the control of axial musculature through vermal cortex and fastigial efferents and on the control of limb musculature through efferents of the globose and emboliform nuclei. Posterior spinocerebellar and cuneocerebellar fibers inform the cerebellum of limb position and movement. This information is processed in the cerebellum and, through connections with the motor cortex via the neurons of the ventral lateral thalamic nucleus, the pars caudalis (VLpc), influences movements of the extremities and muscle tone. Cells in the spinal cord that give rise to anterior spinocerebellar fibers receive primary sensory inputs and are also under the influence of descending reticulospinal and corticospinal fibers. In this respect, anterior spinocerebellar fibers provide afferent signals and feedback to the cerebellum about motor circuits in the spinal cord.
There are additional inputs to the spinocerebellar cortex. These arise in the contralateral accessory olivary nuclei (olivocerebellar fibers), the vestibular nuclei (secondary vestibulocerebellar fibers), the contralateral pontine nuclei (pontocerebellar fibers), and the reticular nuclei (reticulocerebellar fibers). These afferent axons also send collaterals into the fastigial and interposed nuclei.
The outflow of the spinocerebellar module consists of cerebellar corticonuclear fibers from vermal and intermediate cortex to fastigial, emboliform, and globose nuclei and of cerebellar efferent axons arising in these nuclei (Figs. 27-17 and 27-18). Corticonuclear fibers project in a topographic sequence into their respective nuclei on the ipsilateral side. For example, fibers from anterior parts of the vermis enter rostral portions of the fastigial nucleus, whereas those of the posterior vermis project into caudal areas of the same nucleus. In general, this pattern is repeated between the intermediate zone and the emboliform and globose nuclei.
As indicated previously, the fastigial nucleus projects bilaterally to vestibular and reticular nuclei, which, through their spinal projections, influence axial muscles. The fastigial nucleus also projects to (1) the contralateral medial accessory olivary nucleus, from which it receives input; (2) the medial areas of the anterior horn in upper levels of the spinal cord as fastigiospinal fibers; and (3) the ventral lateral nucleus of the thalamus, which in turn projects to trunk regions of the motor cortex (Fig. 27-17).
Axons from the globose and emboliform nuclei exit the cerebellum via the superior cerebellar peduncle and cross in its decussation (Fig. 27-18). From this point, some of these cerebellar efferent fibers course rostrally to terminate in the magnocellular part of the red nucleus (cerebellorubral fibers) and in the VLpc nucleus of the thalamus (cerebellothalamic fibers). These particular thalamic neurons project mainly to areas of the primary motor cortex. The red nucleus, through rubrospinal fibers, and the motor cortex, through corticospinal fibers, influence motor neurons in the contralateral spinal cord that control distal limb musculature (Fig. 27-18). Other globose and emboliform efferents travel caudally to terminate in the reticular formation (cerebelloreticular fibers) and in the inferior olivary complex (cerebelloolivary fibers). Reticular cells influence spinal motor neurons and project back to the spinocerebellum as reticulocerebellar fibers. The globose and emboliform nuclei also receive olivocerebellar fibers from the accessory olivary nuclei to which they project (Fig. 27-18).
Damage to spinocerebellar structures is frequently the result of extension from more medially or laterally located lesions. Consequently, the clinical picture is dominated by deficits characteristic of these medial or lateral regions. The lateral regions are more frequently affected.
Pontocerebellar Module
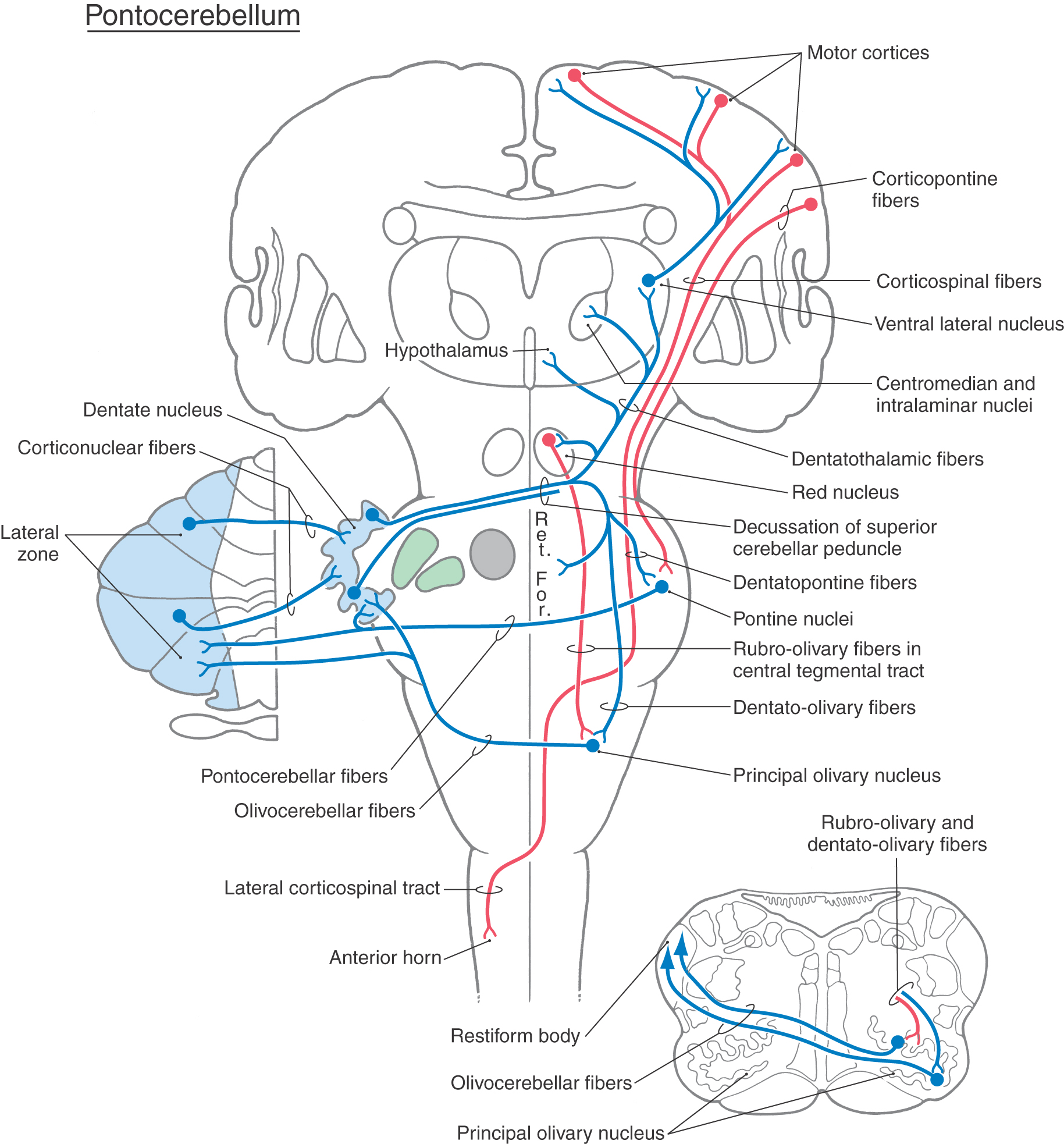
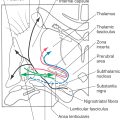
The large lateral zone receives significant input from the basilar pontine nuclei via a primarily crossed pontocerebellar fiber projection. These fibers enter the cerebellum via the middle cerebellar peduncle. Because of this predominant source of afferent fibers, the lateral zone is called the pontocerebellum (Fig. 27-18). Because the pontine nuclei receive a major projection from the ipsilateral cerebral cortex (as corticopontine fibers), this lateral zone is sometimes called the cerebrocerebellum (or neocerebellum). However, the term pontocerebellum is more appropriate and is in parallel with vestibulocerebellum and spinocerebellum. Afferent fibers that enter the lateral zone also send collaterals into the dentate nucleus (Fig. 27-19).
 Figure 27-19. Projections of the pontocerebellum (lateral zone) through the dentate nucleus. Ret. For., reticular formation.
Figure 27-19. Projections of the pontocerebellum (lateral zone) through the dentate nucleus. Ret. For., reticular formation.
The pontocerebellum functions in the planning and control of precise dexterous movements of the extremities, particularly in the arm, forearm, and hand, and in the timing of these movements. Through its connections to motor cortical areas, the dentate nucleus is capable of modulating activity in cortical neurons that project to the contralateral spinal cord.
Another important source of afferents to the pontocerebellum is the principal inferior olivary nucleus (Fig. 27-19). These olivocerebellar fibers are exclusively crossed. They enter the cerebellum via the restiform body, send collaterals to the dentate nucleus, and end in the molecular layer as climbing fibers.
The outflow of the pontocerebellar module consists of cerebellar corticonuclear fibers from the lateral zone to the dentate nucleus and cerebellar efferent fibers originating in the dentate nucleus (Fig. 27-19). As for other cerebellar regions, corticonuclear fibers of the lateral zone are topographically organized; rostral and caudal areas of the zone project to the corresponding portions of the dentate nucleus.
Neurons of the dentate nucleus send their axons out of the cerebellum via the superior cerebellar peduncle and through its decussation (Fig. 27-19). The fibers that pass rostrally project mainly to the parvocellular part of the red nucleus (dentatorubral fibers) and to the intralaminar and VLpc nuclei of the thalamus (dentatothalamic fibers). Some neurons of the parvocellular red nucleus project, as components of the central tegmental tract, to the ipsilateral inferior olivary complex (rubroolivary fibers). At the same time, cells of the VLpc nucleus project to wide areas of the motor and premotor cortices. The motor cortex, in turn, projects to the contralateral spinal cord (as corticospinal fibers) to influence motor neurons that innervate distal limb musculature (Fig. 27-19). Descending crossed projections from the dentate nucleus pass mainly to the principal olivary nucleus (dentatoolivary fibers) and, in limited numbers, to the reticular and basilar pontine nuclei. Olivocerebellar fibers arising in the principal nucleus cross the midline and distribute to the cortex of the lateral zone and to the dentate nucleus. There is also feedback to the dentate nucleus via pontocerebellar and reticulocerebellar fibers.
The relationship between the dentate nucleus and movement has been explored in experiments in monkeys. A cooling probe implanted in the cerebellar white matter adjacent to the dentate nucleus halts most of the electrical activity in dentate neurons. This significant reduction in electrical signals temporarily disconnects the dentate nucleus from its targets without permanently destroying the nucleus. Reversal of the cooling results in a resumption of normal neuronal activity. After dentate cooling, the electrical activity of neurons in the primary motor cortex (MI) that signals the initiation of a movement (in response to a visual stimulus) was delayed, as was the execution of the movement itself. This observation indicates that dentate projections, which reach the cortex via the VLpc nucleus, are essential for the initial activation of MI corticospinal neurons at the beginning of a movement.
As a result of the delay of excitatory output from the motor cortex, there are corresponding delays in muscle contraction. For example, the initial activation of an agonist muscle (biceps brachii) to a load is slowed and its overall contraction time is longer. Similarly, the activation of the antagonist muscle (triceps brachii) that occurs when the load is removed is also delayed. Furthermore, the reciprocal pattern of activation in agonists and antagonists that accompanies some movements is dramatically disrupted. Thus, as well as influencing the duration of muscle contraction, cerebellar output is involved in timing of muscle activation (and inactivation).
Pontocerebellar Dysfunction
Before the consequence of lesions affecting the pontocerebellar module is considered, two important points merit emphasis. First, damage that involves only the cerebellar cortex rarely results in permanent motor deficits. However, damage to the cortex plus nuclei or to only the nuclei results in a wide range of motor problems that may have long-term consequences. Second, lesions of the cerebellar hemisphere result in motor deficits on the ipsilateral side of the body because the motor expression of cerebellar injury is mediated primarily through corticospinal and rubrospinal pathways. In brief, the right lateral and interposed nuclei influence the left motor cortex (through the ventral lateral nucleus) and the left red nucleus, both of which in turn project to the right side of the spinal cord. Thus a lesion in the cerebellum on the right results in deficits on the right side of the body. The exceptions are a midline lesion and a lesion distal to the decussation of the superior cerebellar peduncle that may result in cerebellar signs and symptoms. The midline lesion produces bilateral deficits restricted to axial or truncal parts of the body, whereas the lesion distal to the decussation of the superior cerebellar peduncle results in deficits on the side opposite the lesion. Lesions that involve the cerebellar hemisphere frequently affect portions of the lateral and intermediate modules. It is common for these disorders to be categorized as disorders of the lateral (or hemisphere) zone or as neocerebellar disorders.
In general, lesions of the lateral cerebellum result in a deterioration of coordinated movement, which is sometimes referred to as a decomposition of movement (or dyssynergia). This deficit consists of the breakdown of movement into its individual component parts. There may also be a decrease in muscle tone (hypotonia) and in deep tendon reflexes. Ataxia involving the extremities generally is also seen in patients with lateral cerebellar lesions. Because of ataxia of the lower extremity, these patients may also have an unsteady gait and a tendency to lean or to fall to the side of the lesion.
Dysmetria (also called past-pointing) is apparent in patients when they attempt to point accurately or rapidly to moving or stationary targets. The patient may reach past the target (hypermetria) or fall short of the target (hypometria).
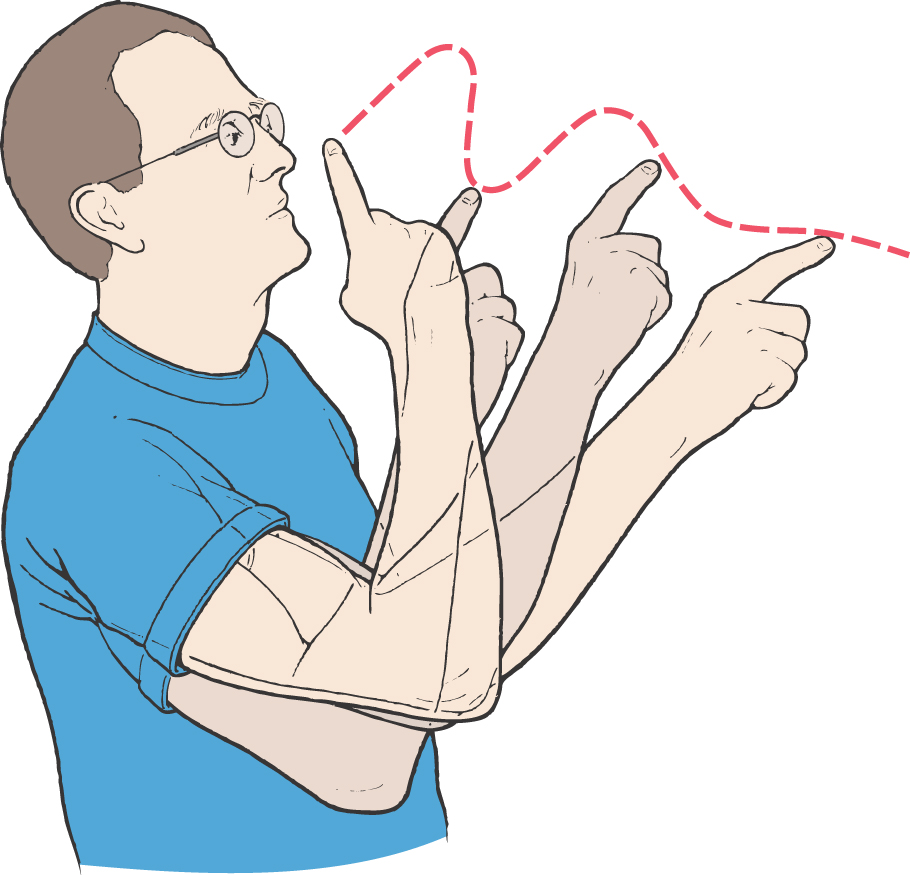
Tremor is a consistent finding in patients with lateral cerebellar lesions. A kinetic tremor, commonly called an intention tremor, is evident when the patient performs a voluntary movement and is most obvious as the endpoint, or target, is approached. This deficit is commonly seen when the patient extends the arm and then attempts to touch the index finger to the nose (Fig. 27-20). At rest there is little or no tremor, but as the finger approaches the nose, the tremor is markedly accentuated. This finding is opposite to that seen in patients with Parkinson disease, whose tremor is evident at rest (resting tremor) but largely diminishes during a voluntary movement. Patients with cerebellar lesions may also demonstrate a static tremor. This tremor is manifested when the patient stands with the arms extended (muscles contracted against gravity). There is rhythmic movement of the shoulders that also involves the upper extremities.
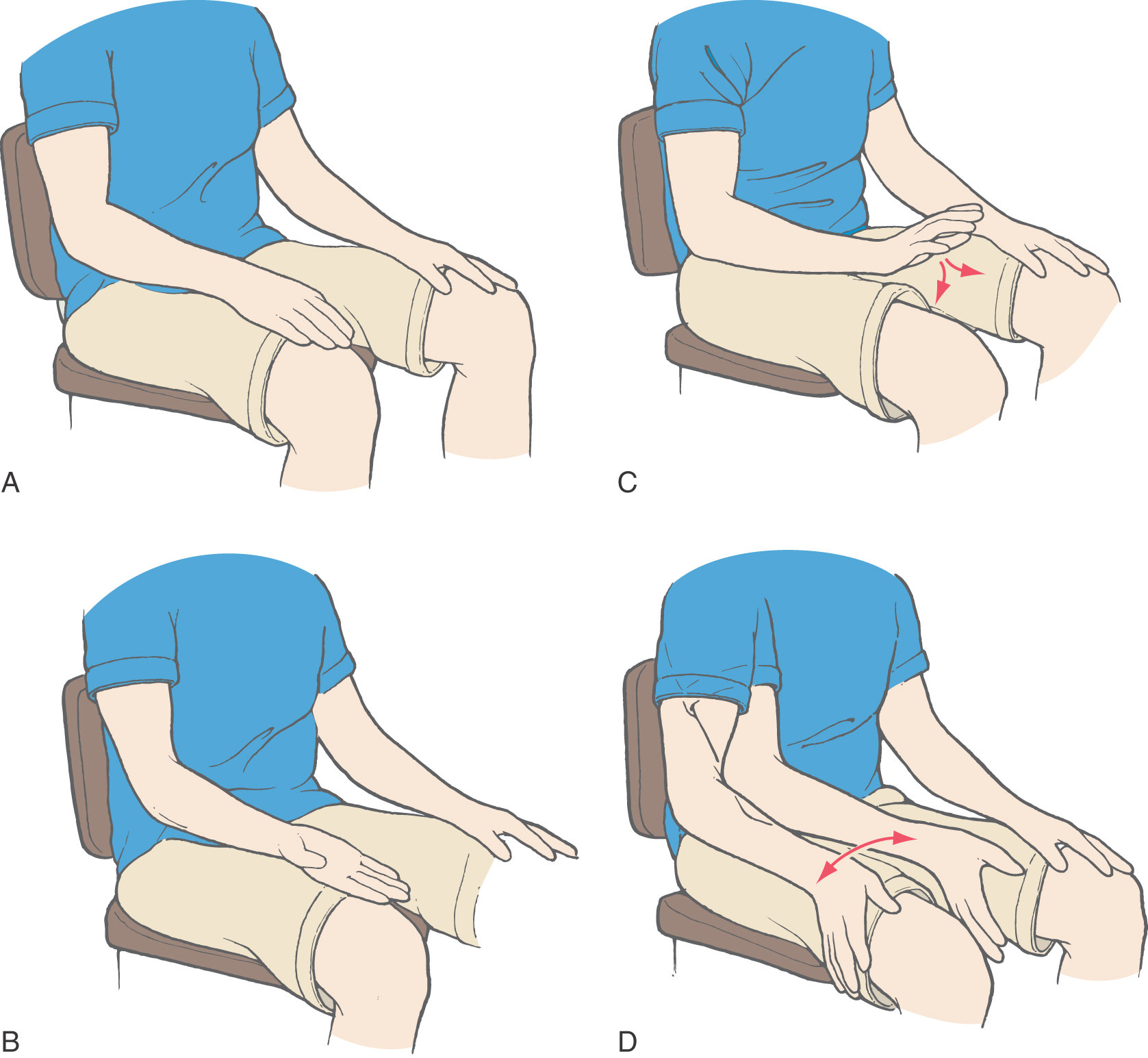
Awkward performance of rapid alternating movements, such as supinating and pronating the hand against the thigh, is called dysdiadochokinesia (Fig. 27-21). The patient may also be unable to perform rhythmic movements. This deficit is demonstrated by asking the patient to rapidly tap the table three times with the index finger, pause two seconds, tap three times, and so on. For patients with lateral cerebellar lesions, this task will be difficult or impossible.
Other lateral zone deficits include rebound phenomena, dysarthria, and ocular motor dysfunction. The rebound phenomenon (or impaired check) is an inability of agonist and antagonist muscles to adapt to rapid changes in load. For example, if the patient is asked to push against the physician’s hand and the hand is then unexpectedly removed, the patient’s arm will overshoot beyond the point where it would normally halt. Tremors or oscillations may also be evident as the arm returns to its starting point. Patients with dysarthria have slurred, garbled speech that may also be alternately slow or staccato in nature (scanning speech). This is a motor problem (not an aphasia) because the patient is still able to use words and grammar correctly. Characteristic ocular motor dysfunctions seen in patients with lateral cerebellar lesions are nystagmus and abnormalities of target-directed eye movements. Nystagmus most commonly is manifested as abnormal horizontal eye movements that consist of a slow conjugate movement away from the side of the lesion. This abnormality is opposite to that seen in lesions of the vestibular receptors, primary sensory fibers, and nuclei. In other cases, the velocity of these conjugate movements may be the same in both directions (pendular nystagmus). Abnormal target-directed eye movements may be manifested as an inability to follow a slowly moving target (disturbed pursuit movements) or difficulty in maintaining fixation on a stationary target.
CEREBELLAR INFLUENCE ON VISCEROMOTOR FUNCTIONS
The cerebellum receives input from the solitary and dorsal motor vagal nuclei and from a number of nuclei in the hypothalamus. These areas are directly involved in the control and modulation of a variety of visceromotor functions. The hypothalamus, in addition to having direct connections with the cerebellar cortex and nuclei, also projects to brainstem and spinal nuclei that are involved in the regulation of visceral functions.
Neurons in several hypothalamic areas and nuclei project to the cerebellar cortex and nuclei (hypothalamocerebellar fibers). The cerebellar nuclei, in turn, send a primarily crossed projection to the hypothalamus (cerebellohypothalamic fibers) via the superior cerebellar peduncle (Figs. 27-17 and 27-19). Through these reciprocal connections, the cerebellum may receive visceral input and influence neurons that control visceral functions.
Visceral deficits related to cerebellar lesions are rarely reported for two reasons. First, the somatomotor deficits seen in such cases are overwhelmingly diagnostic, and there is no need to look further. Second, cerebellar lesions may cause an increase in intracranial pressure with resultant pressure on the medulla. Consequently, it is difficult to separate visceral deficits that relate to the cerebellar lesion from those that relate to pressure on the medulla.
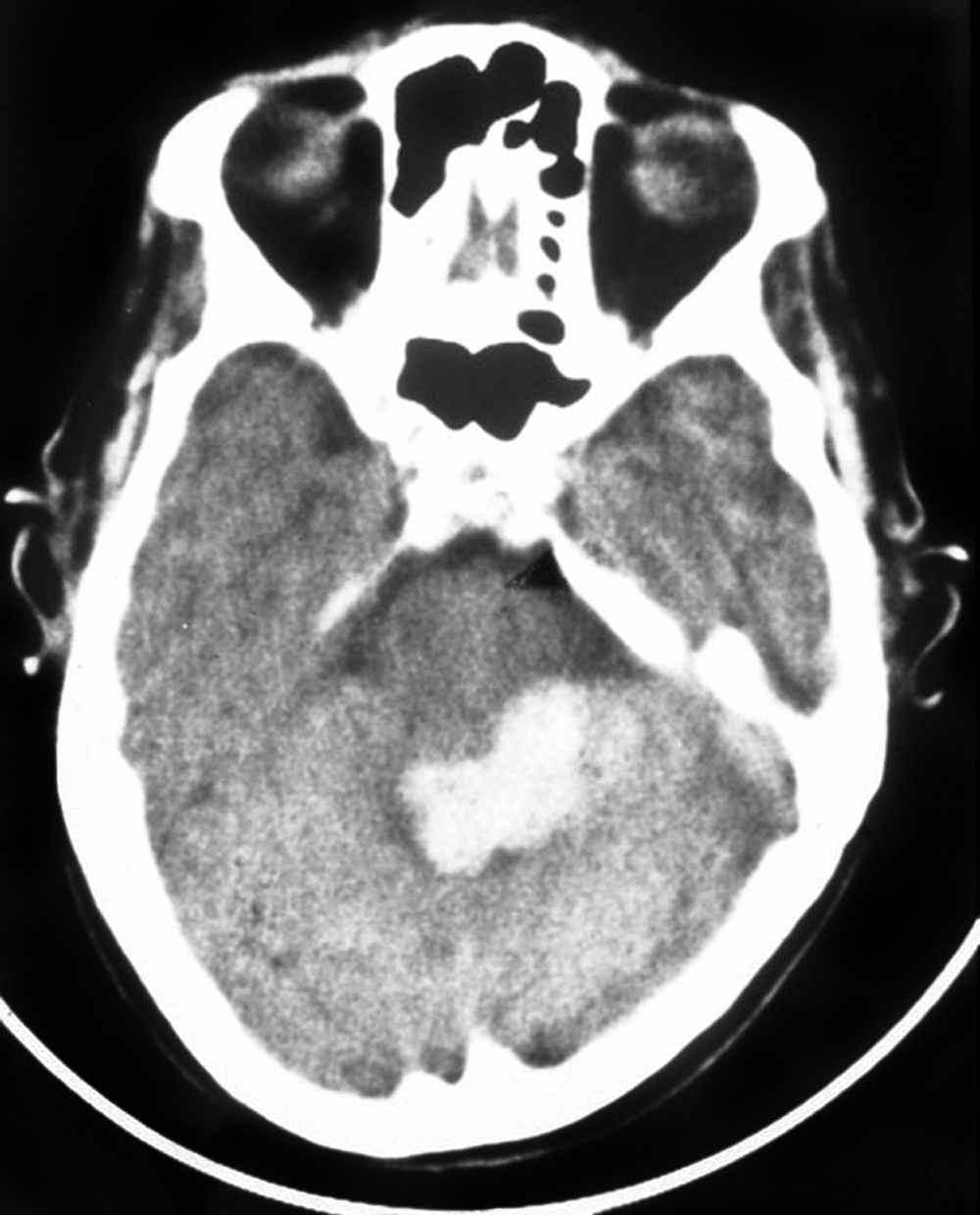
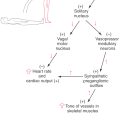
In some situations, however, visceral deficits can be related directly to the cerebellar lesion. For example, deficits of this type occurred in a patient who had an occlusion of branches of the left superior cerebellar artery with resultant damage to the cerebellar nuclei on that side (Fig. 27-22). There was no indication of increased intracranial pressure. This patient had characteristic somatomotor tremors in the left arm and leg during attempted movement on that side and showed two distinct visceral responses that occurred concurrently with the somatomotor tremor but not before or after. First, the patient’s pupils dilated during the tremor. Second, the skin on the patient’s face flushed and felt warm to the touch during the tremor. Immediately on cessation of the attempted movement (and resulting tremor) of the left hand, the patient fanned his face with his right hand and complained, “It’s hot in here.” In other cases, small lesions restricted to the fastigial nucleus have been noted to result in decreases in heart rate and blood pressure. These observations clearly suggest that the cerebellum is actively involved in the regulation of visceral function concurrent with its classically recognized influence in the somatomotor sphere.
CEREBELLUM AND MOTOR LEARNING
The involvement of the cerebellum in the learning of movements has been an interesting if not controversial topic, in part because lesions of this structure often affect the performance of the movement being learned. Consequently, it has been difficult to separate the effects of cerebellar dysfunction on motor learning from those related to performance. In addition, these effects are somewhat dependent on the type of behavior being learned. This task-dependent characteristic may reflect the fact that the cerebellum can be involved in different aspects of the learning process (i.e., acquisition, consolidation, and memory storage) when different behaviors are learned.
The cerebellum plays a critical role in the learning of relatively simple reflexive motor behaviors. These movements include the adaptation of the vestibuloocular reflex and the classic (pavlovian) conditioning of reflexes evoked by aversive stimuli, such as the eye blink and withdrawal reflexes.
Because damage to specific regions of the cerebellum will eliminate the classically conditioned eye blink reflex and impair the adapted vestibuloocular reflex, this structure is considered by some to be a possible storage site for the plastic changes established during the acquisition of these behaviors. Other researchers contend that sites outside the cerebellum also may be involved. However, it has been difficult to demonstrate which locations are most important because the cerebellum is so critical to the function of circuits required for the performance of these reflexes. For example, current studies indicate that temporary cerebellar lesions in animals often used to disrupt learning mechanisms also produce significant changes in the tonic discharge rate of cerebellar nuclear neurons projecting to these circuits. Future studies using methods that do not impair the cerebellum’s contribution to performance and its tonic action on brainstem circuits will likely be required to resolve this question.
Although the cerebellum also is believed to be involved in the learning of voluntary, complex motor skills, the extent and nature of its involvement have been more difficult to understand than its role in the learning of certain reflexes. Initial studies demonstrated that normal subjects performed better after practicing a new task than did patients with cerebellar damage. This finding was first ascribed to the presence of a learning deficit in these patients. However, subsequent experiments that examined the rate at which a new motor skill is acquired revealed that cerebellar patients can learn to perform new movements even though the quality of the performance is affected by their deficit. Furthermore, animals can learn volitional limb movements even when the cerebellar nuclei are inactivated. Thus the cerebellum is not as essential for the learning of many volitional movements as it is for the modification of the specific reflexes reviewed previously. However, cerebellar dysfunction does result in a decrease in the quality and consistency of the learned behaviors.
Together these findings suggest that even though the cerebellum is not required for learning of volitional movements, it is involved in the acquisition process. This argument is supported further by imaging experiments showing that specific regions in the cerebellum are activated during the learning of novel movements. In addition, the acquisition of complex behaviors in animals is associated with increased modulation of cells in the cerebellar nuclei when the animal first learns to perform the task correctly and consistently on successive trials.
The cerebellum is not likely to be a critical storage site for the memory established during the learning of volitional movements. Experiments in animals focused on this issue demonstrated that the patterns of movements required to perform previously learned complex volitional motor tasks could be recalled during the inactivation of the ipsilateral dentate and interposed nuclei. For those learned motor behaviors that may require intracerebellar memory storage sites, the mechanism responsible for establishment of the storage and even the location of these changes have not yet been resolved despite extensive experimentation in this area. It has been proposed that the climbing fiber input to the dendrites of Purkinje cells can induce plastic changes that modify the responsiveness of these neurons to specific inputs mediated by parallel fibers. However, the relevance of this mechanism to normal physiologic conditions continues to be debated. There also is evidence that the cerebellar nuclei may be a storage site in certain types of motor learning, such as the classically conditioned eye blink reflex, through processes such as long-term depression.
In summary, certain reflexes cannot be modified in the absence of the cerebellum, and lesions of this structure impair the performance of certain previously learned behaviors. This structure plays an important role in the acquisition of several motor behaviors including skilled volitional movements, although the nature of its contribution to this process has not been characterized fully. The cerebellum may be a storage site or one of multiple sites required for the learning of certain behaviors. However, it is not considered to be essential for storing engrams established during the learning of complex volitional movements. Current research also has demonstrated that this structure is involved in cognitive processes, some of which may be related to important aspects of motor learning.
Sources and Additional Reading
Bloedel JR, Dichgans J, Precht W. Cerebellar Functions. New York: Springer-Verlag; 1985.
Ito M. The Cerebellum and Neural Control. New York: Raven Press; 1984.

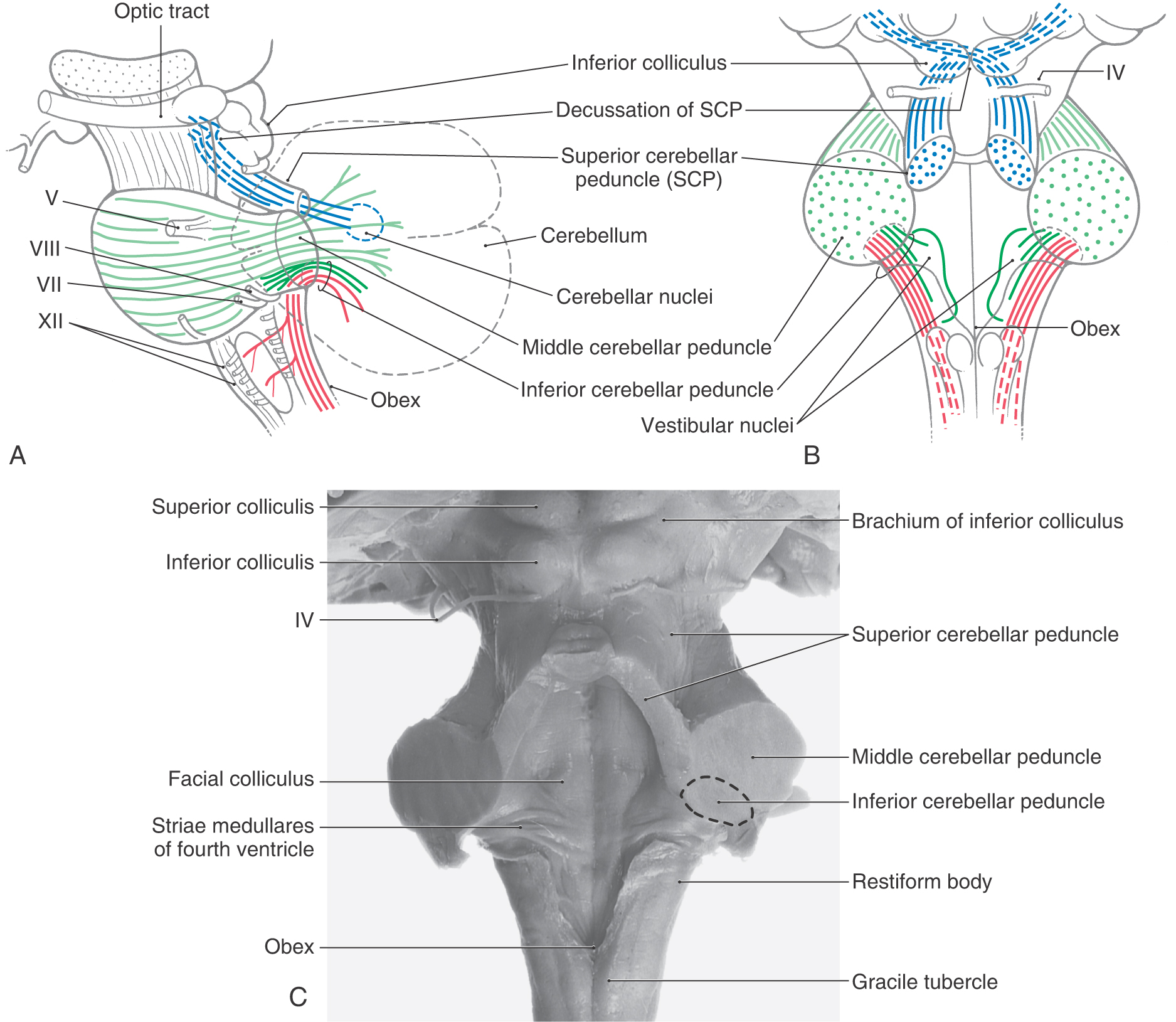
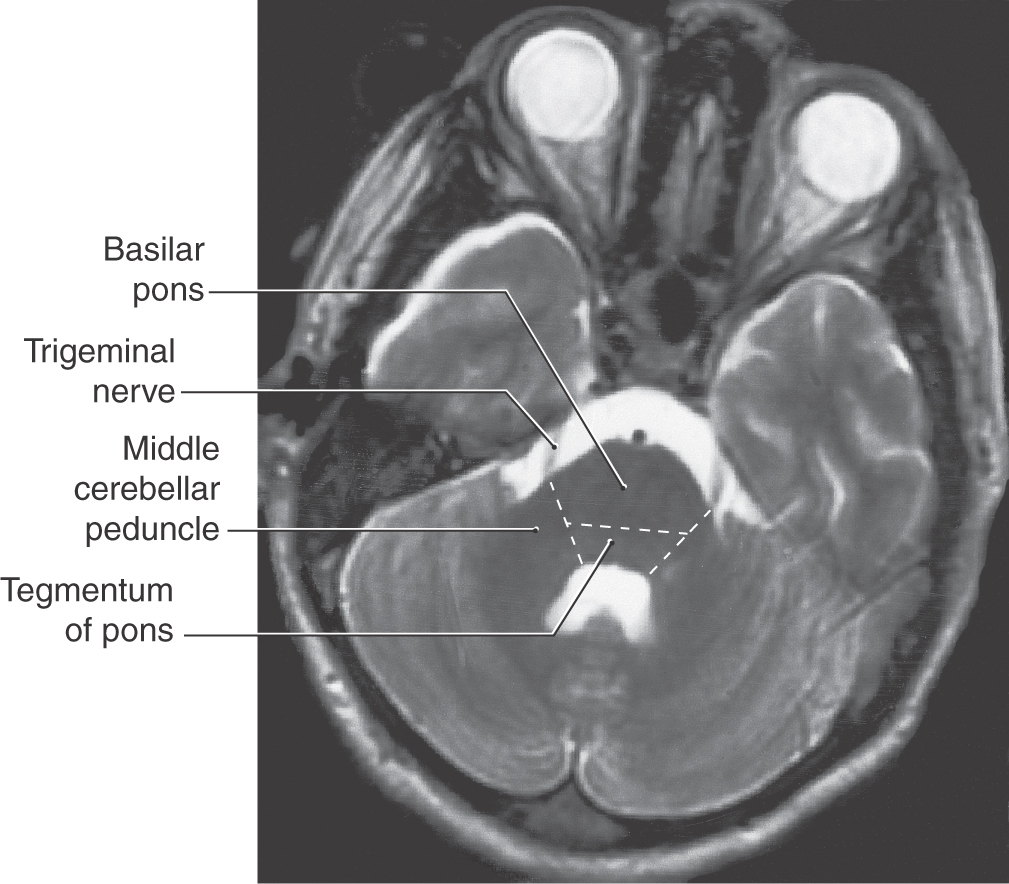
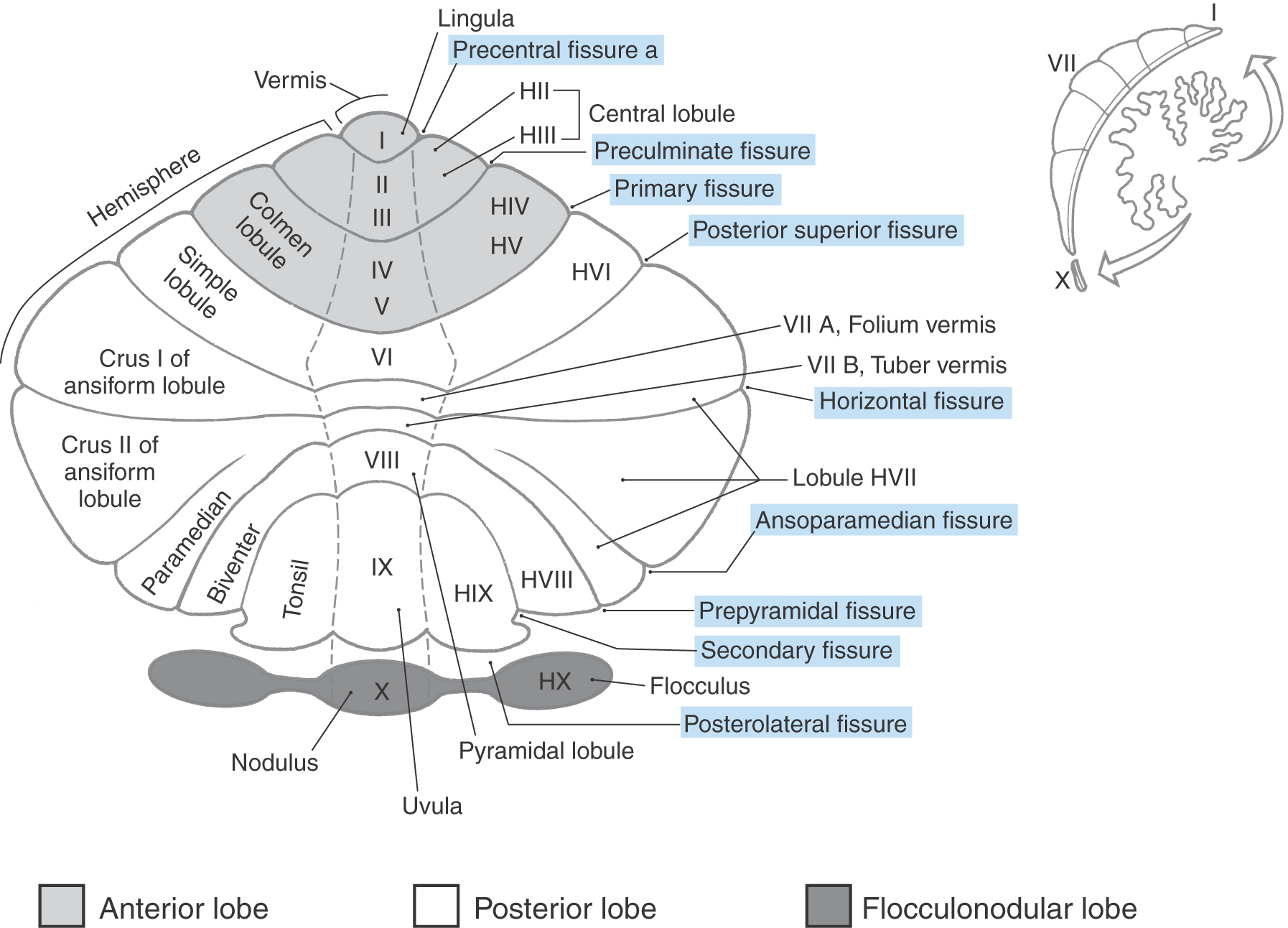
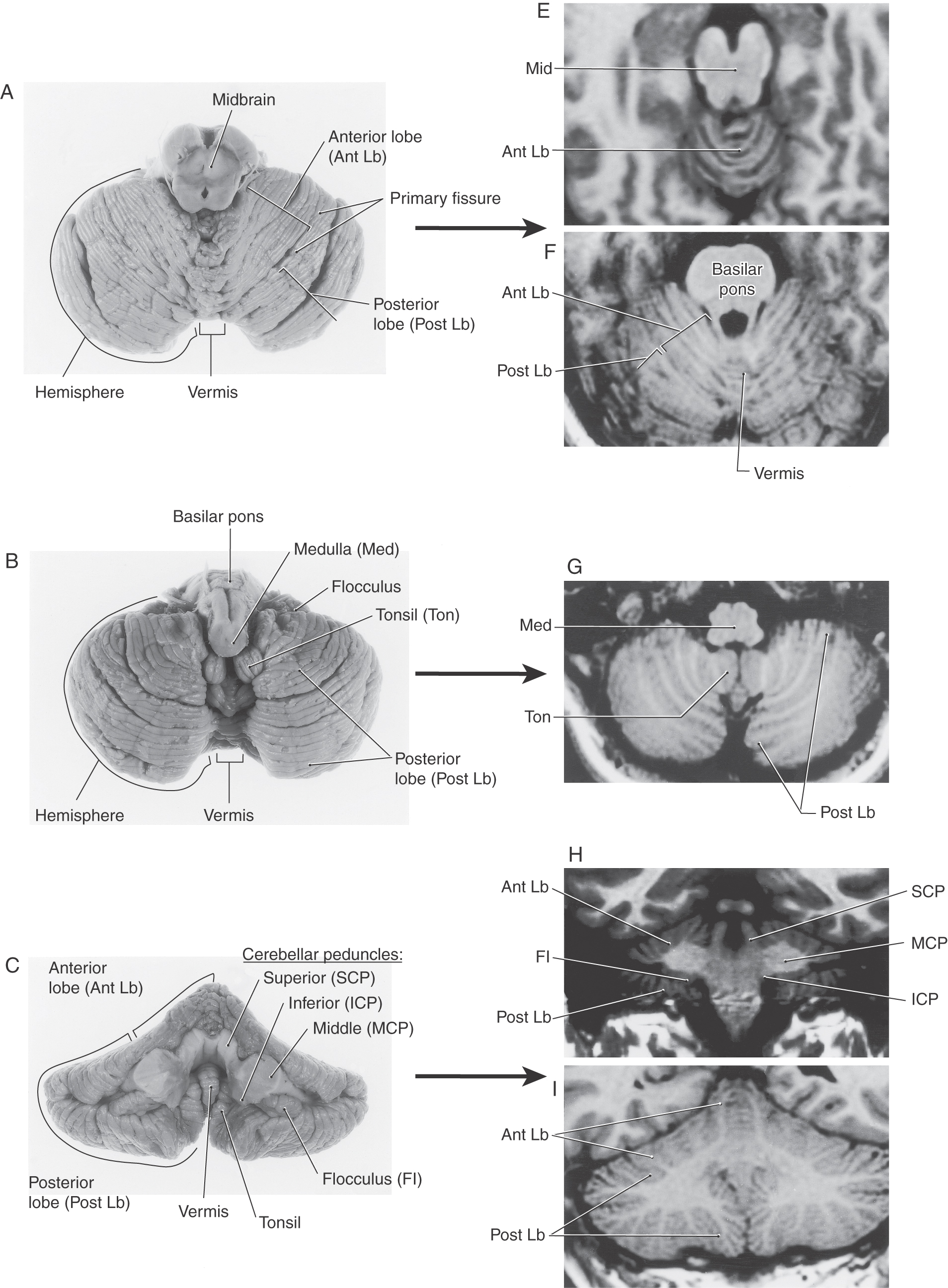
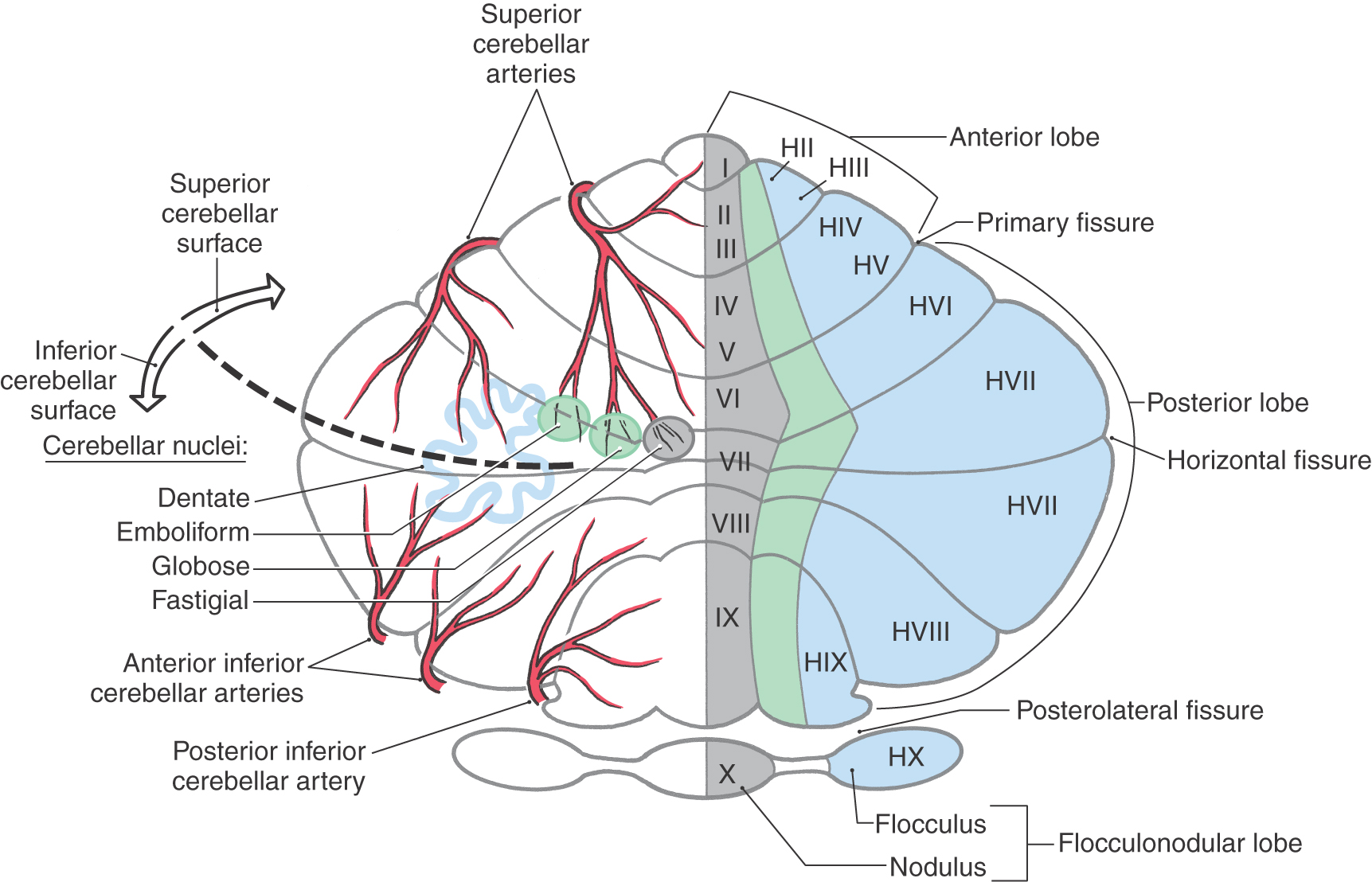
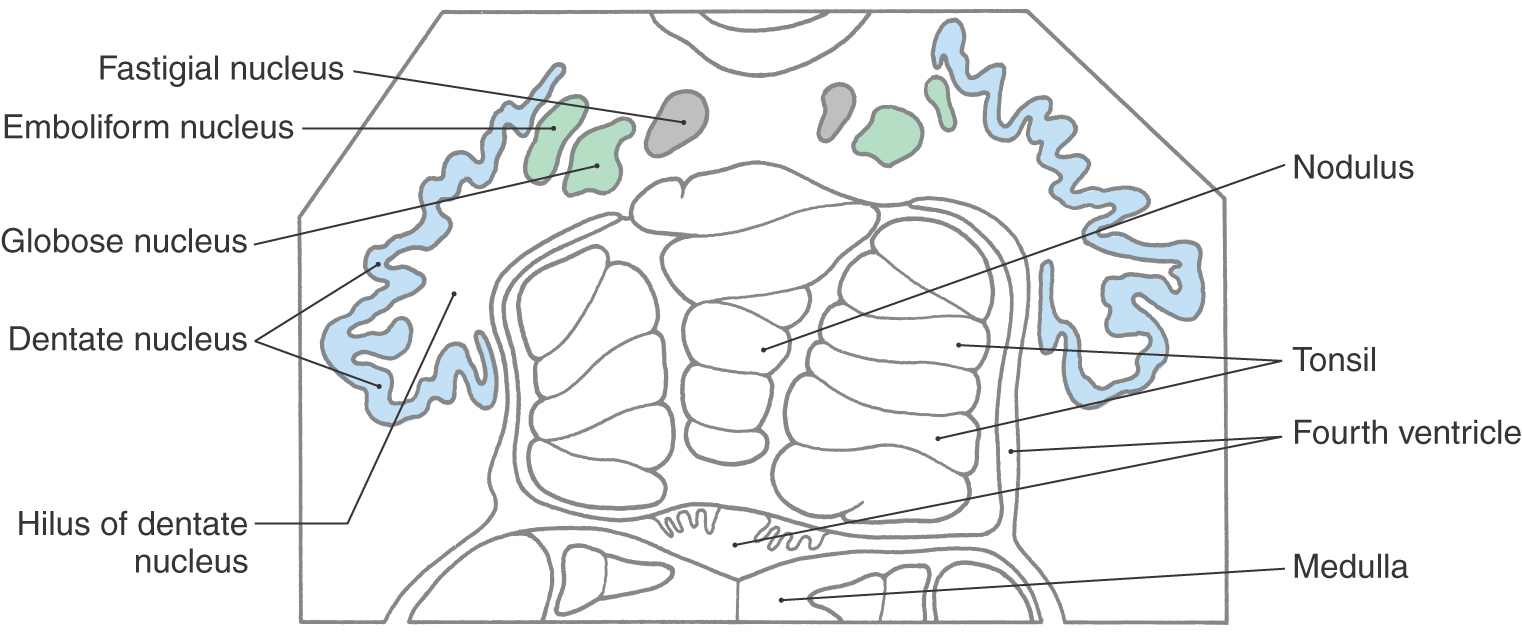
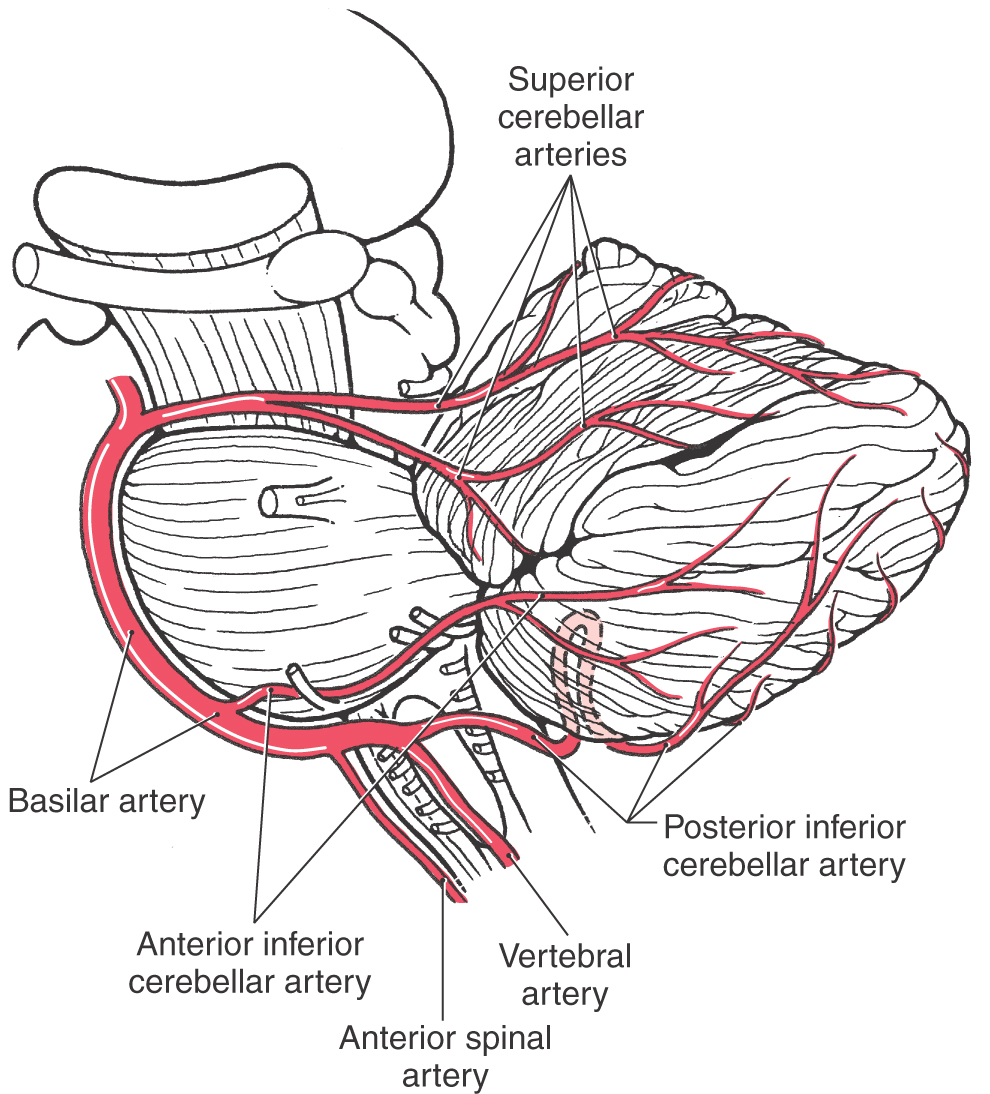
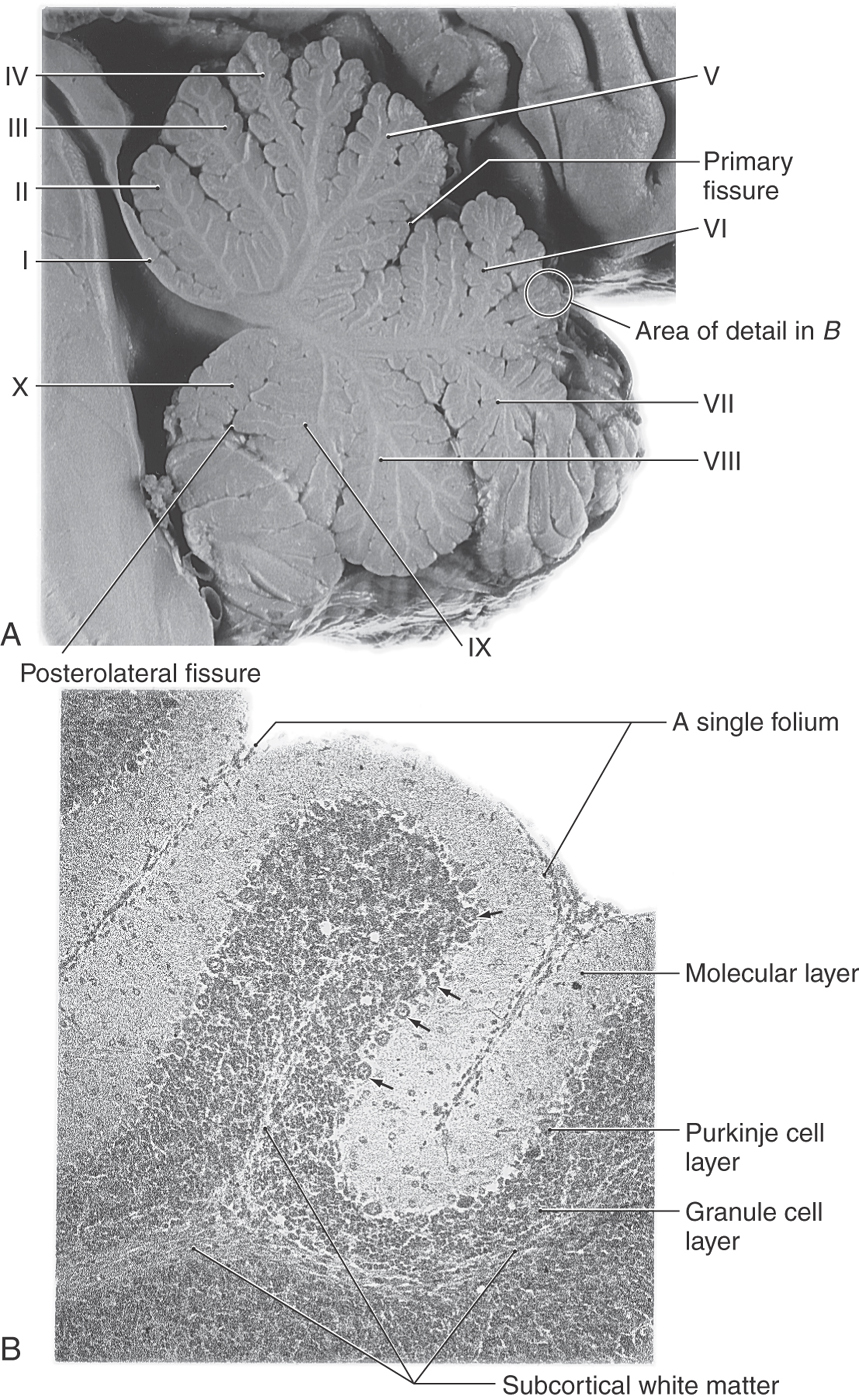
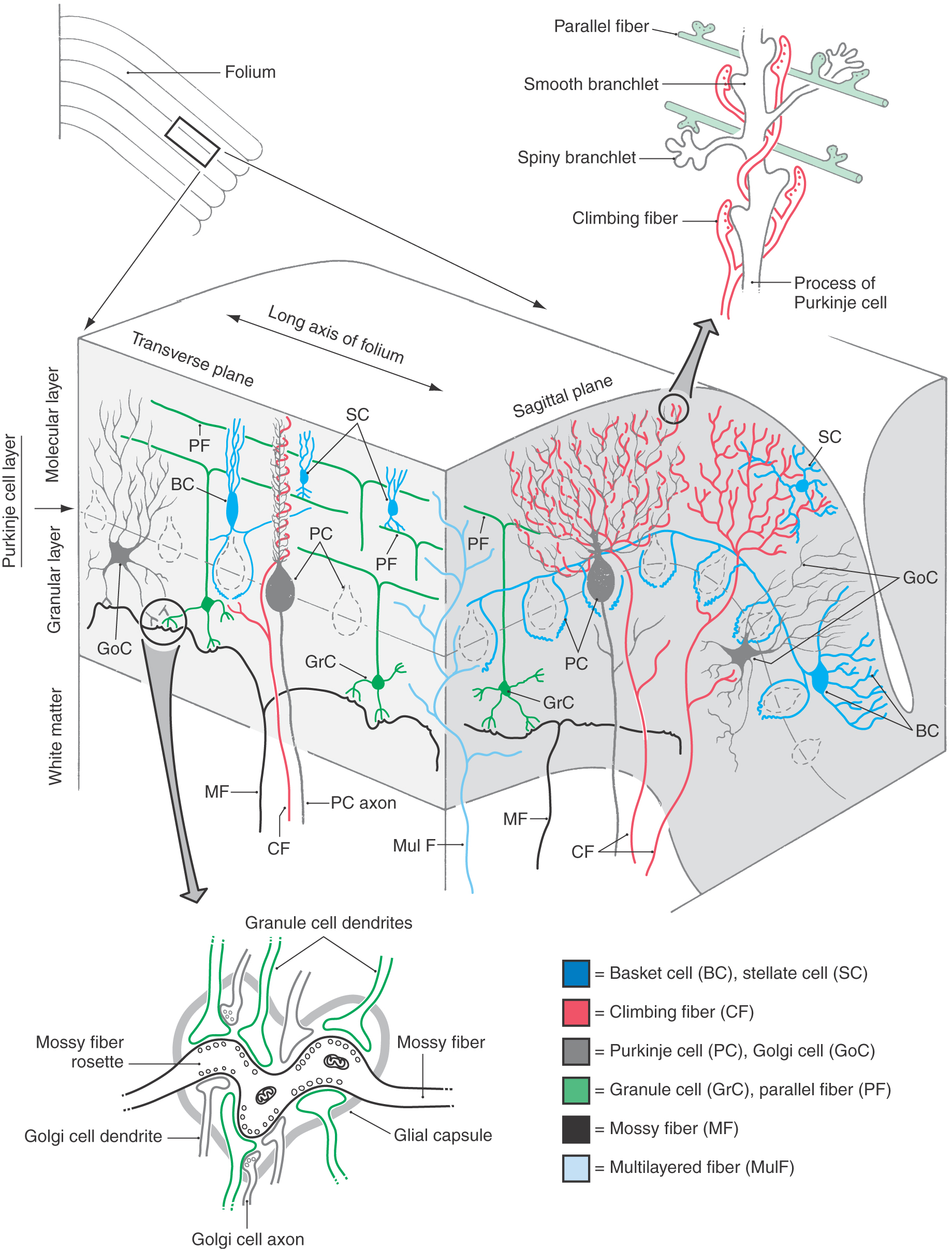
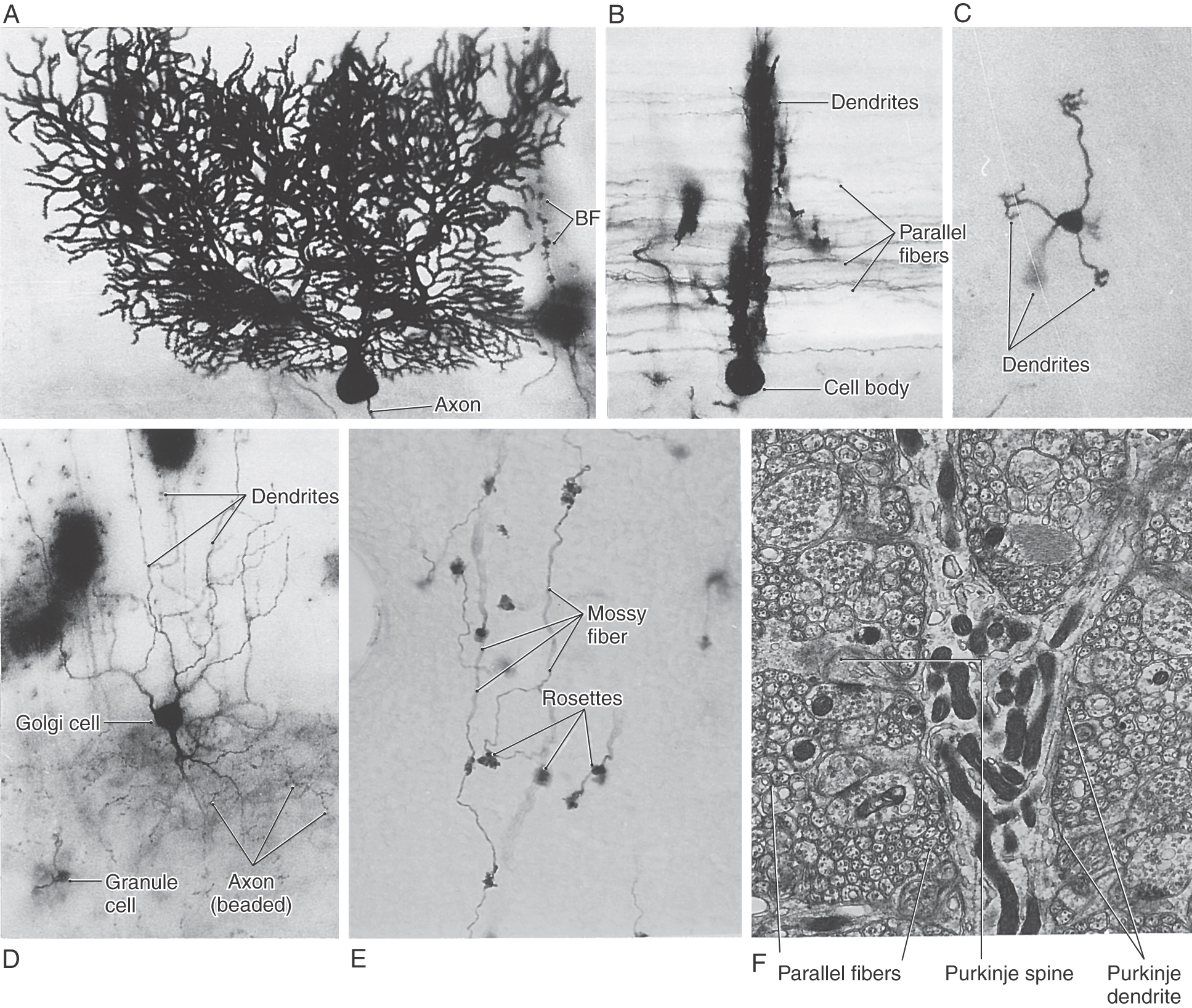
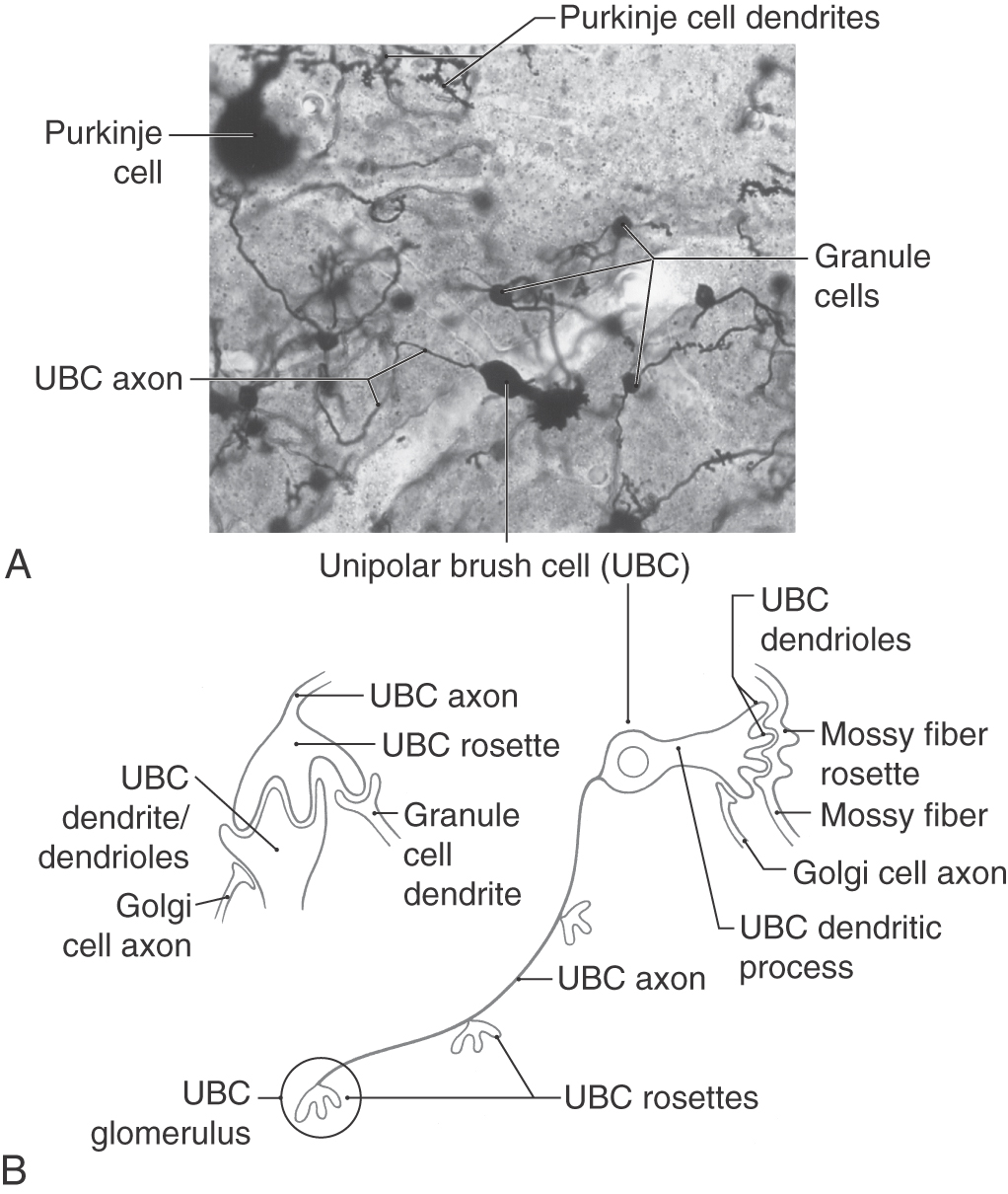
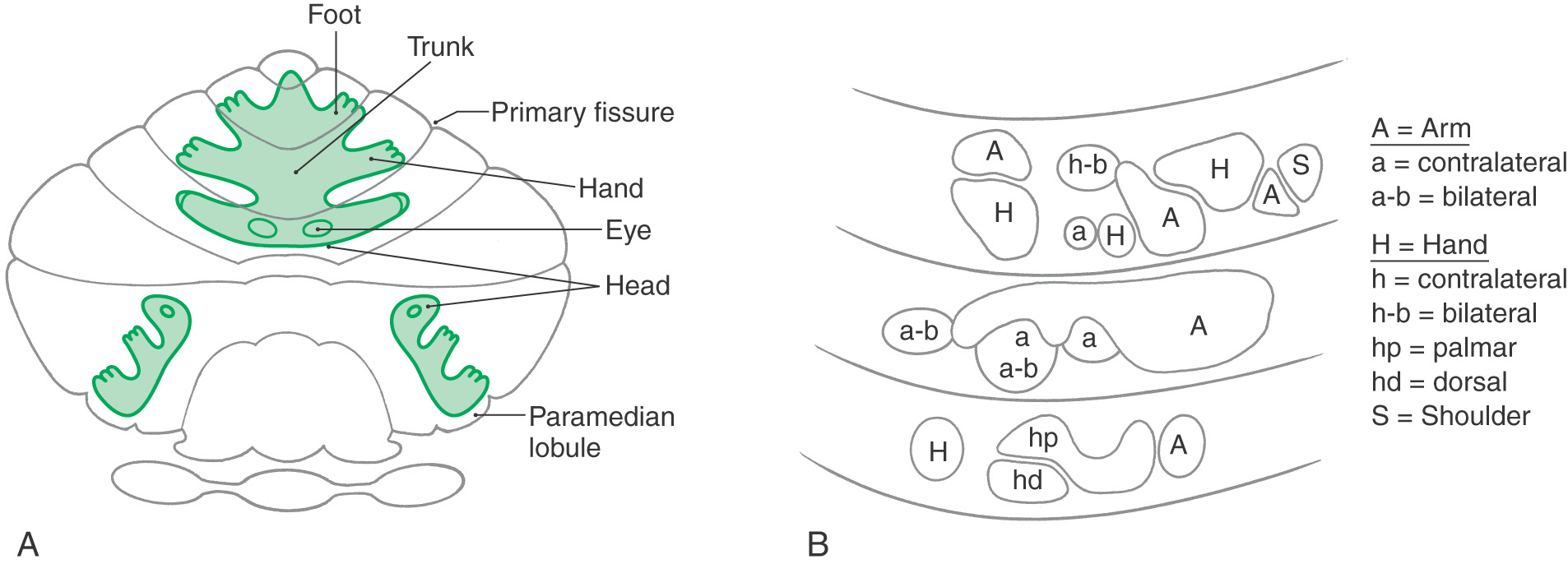
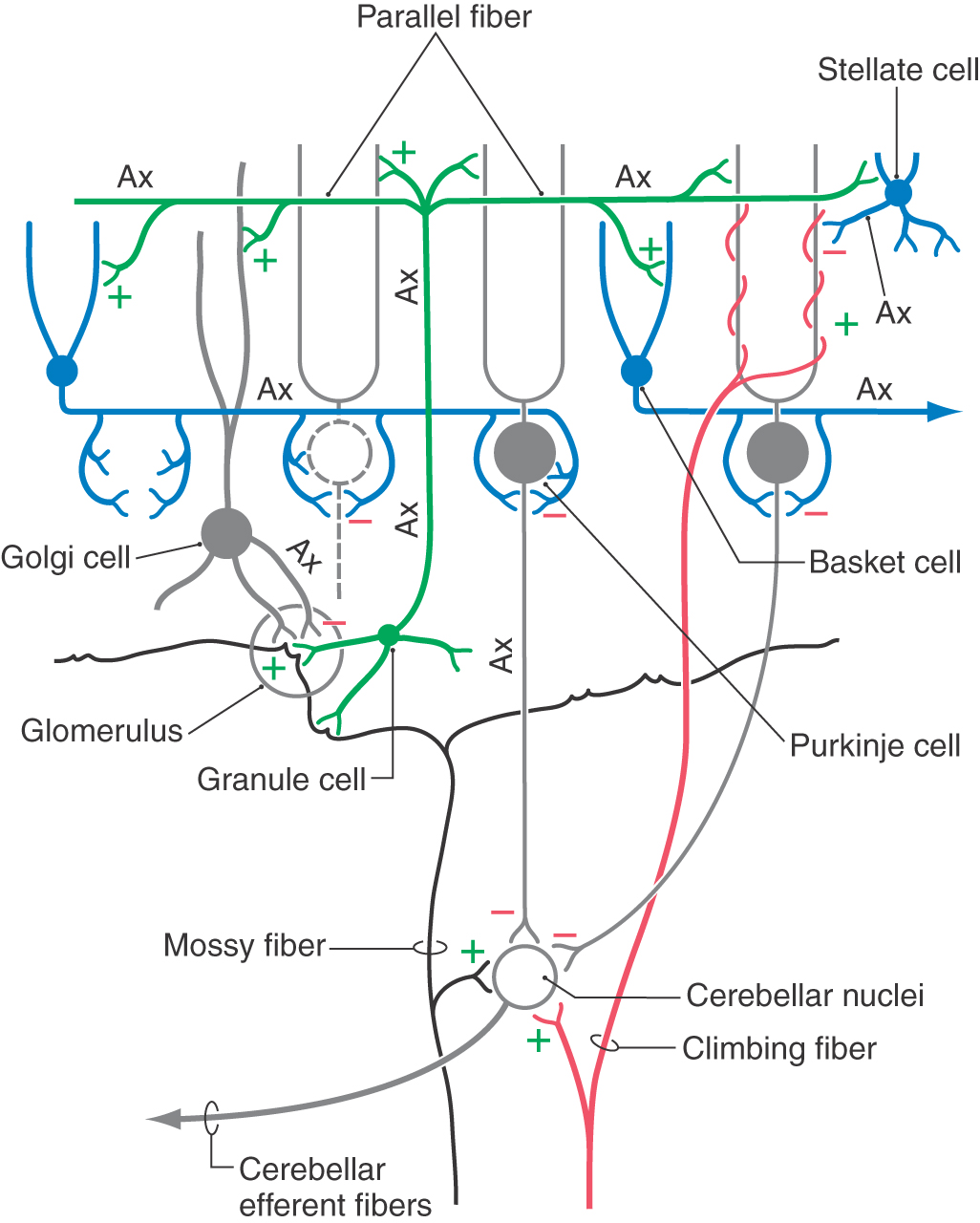
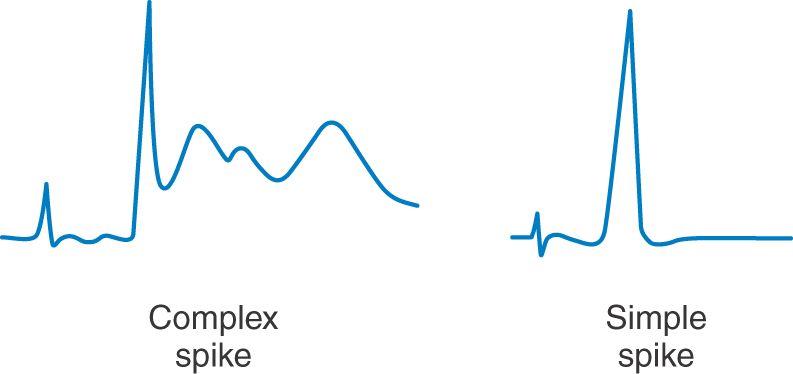
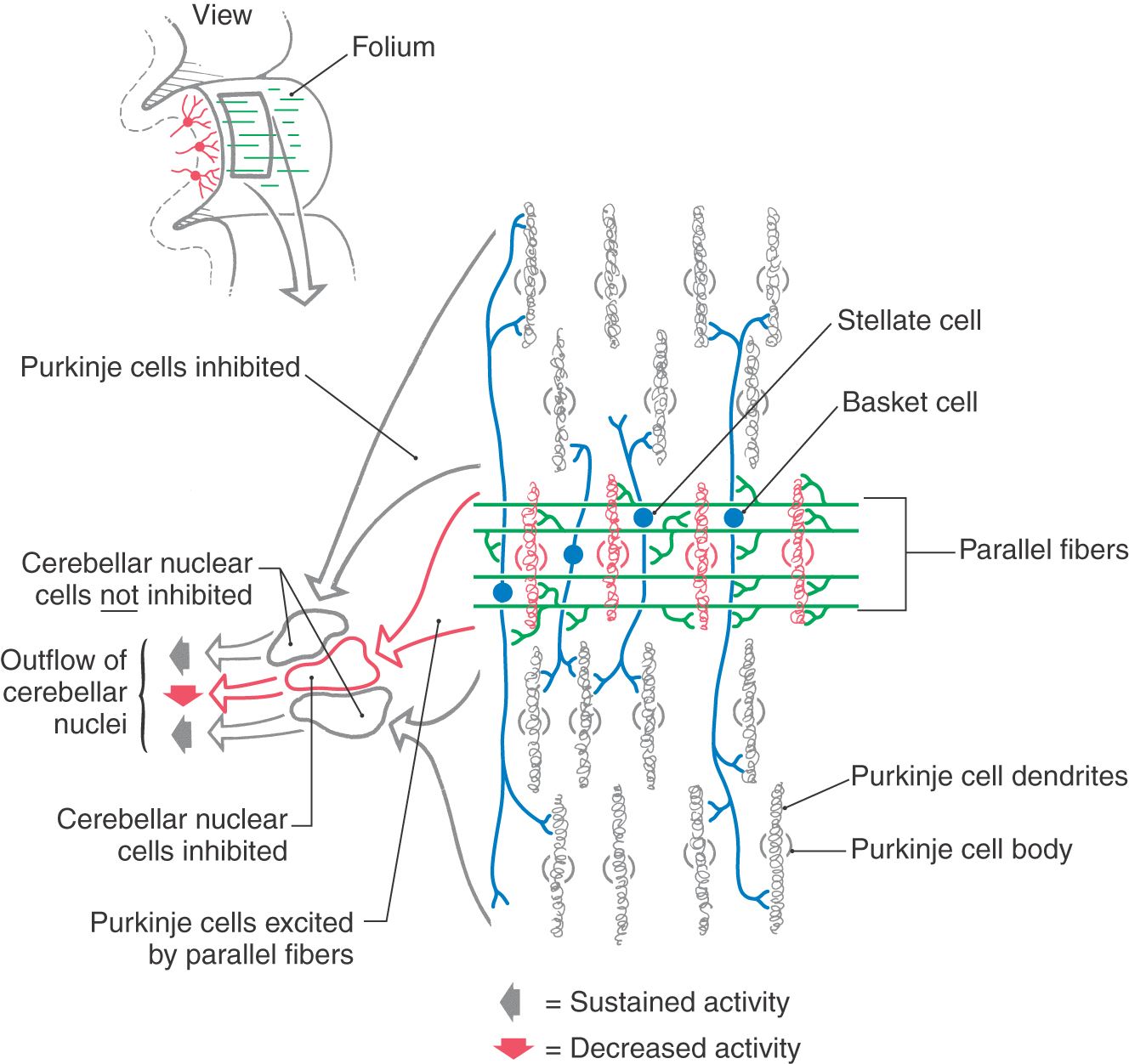
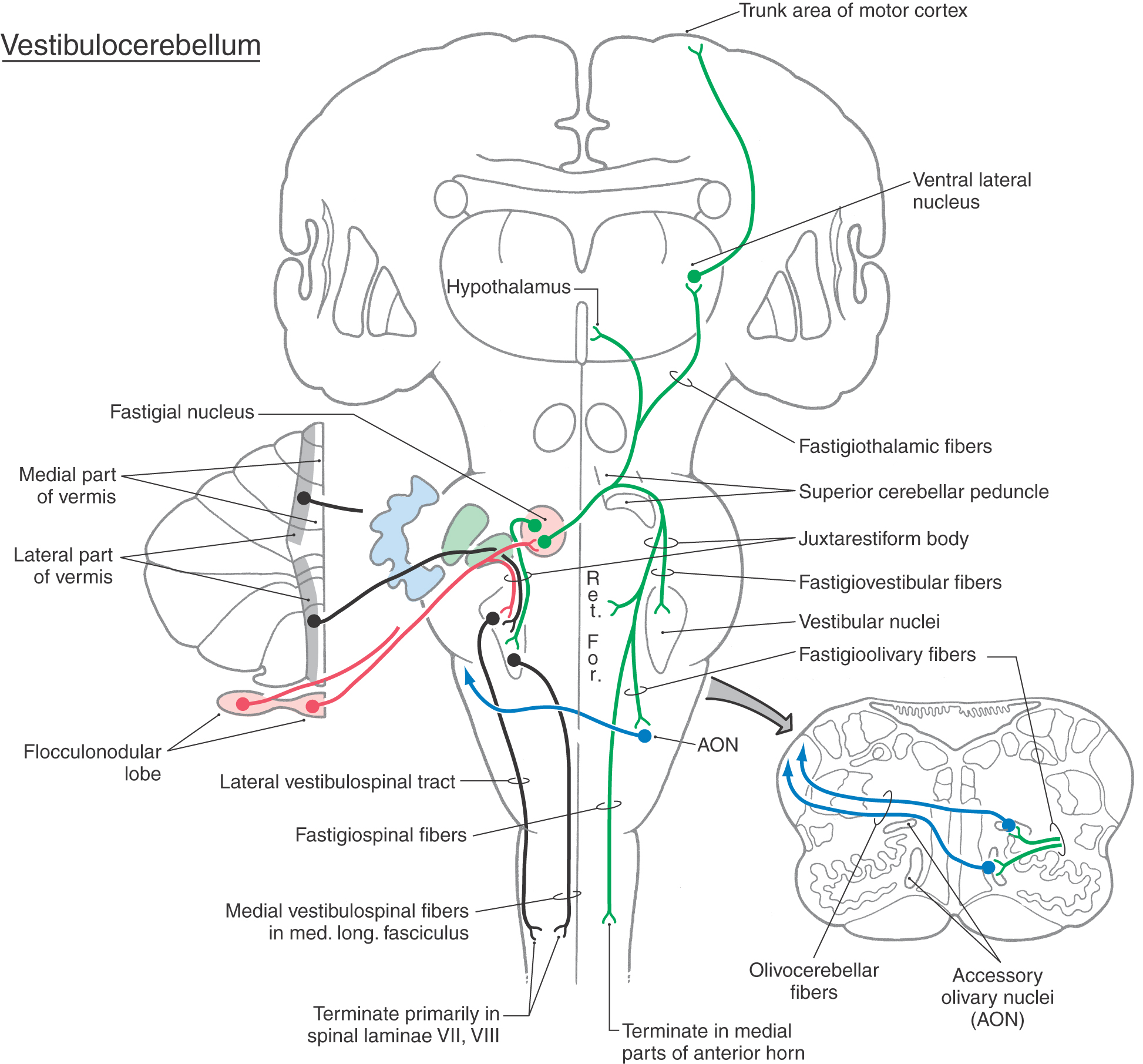
 Figure 27-16.
Figure 27-16. 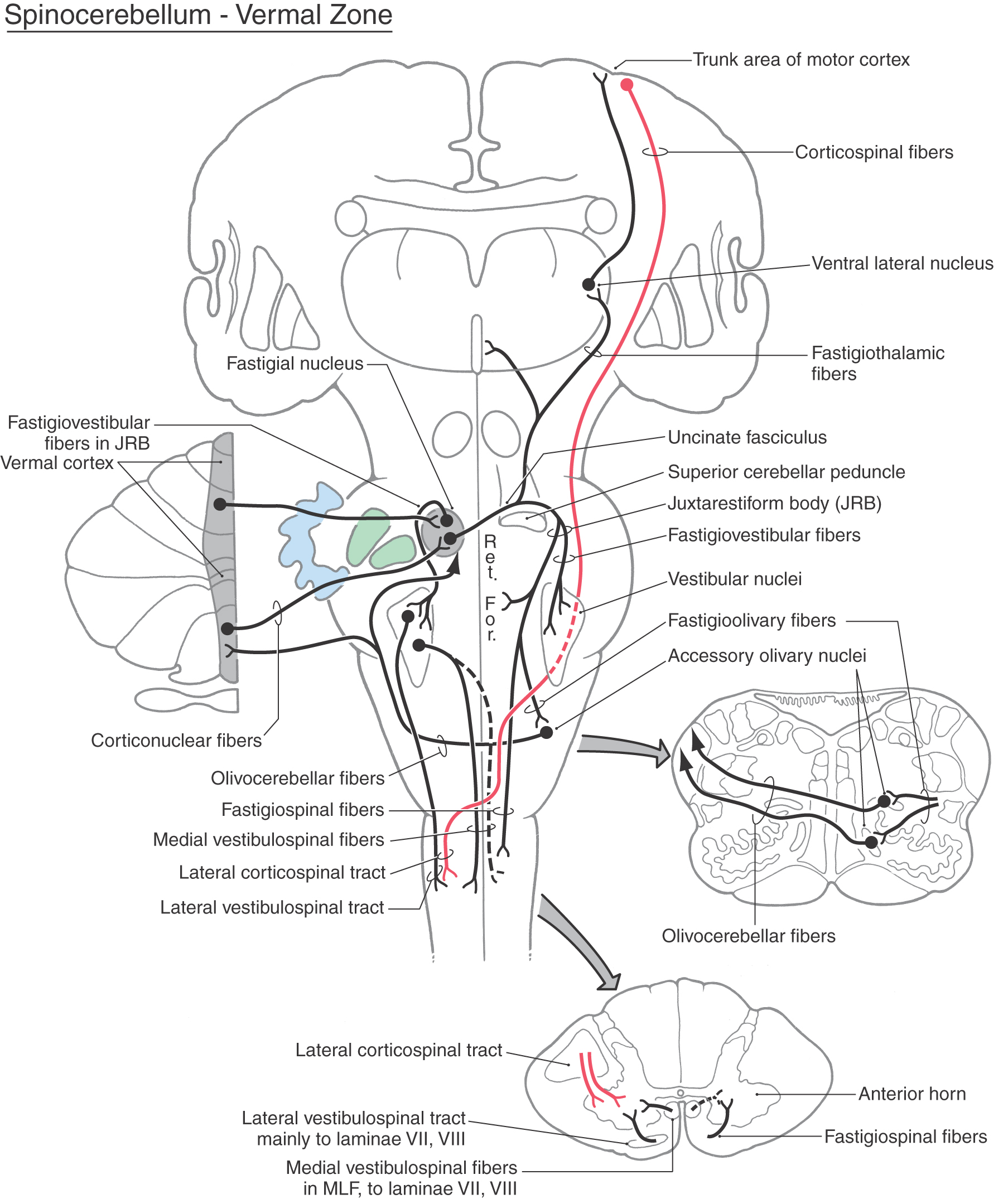
 Figure 27-17.
Figure 27-17. 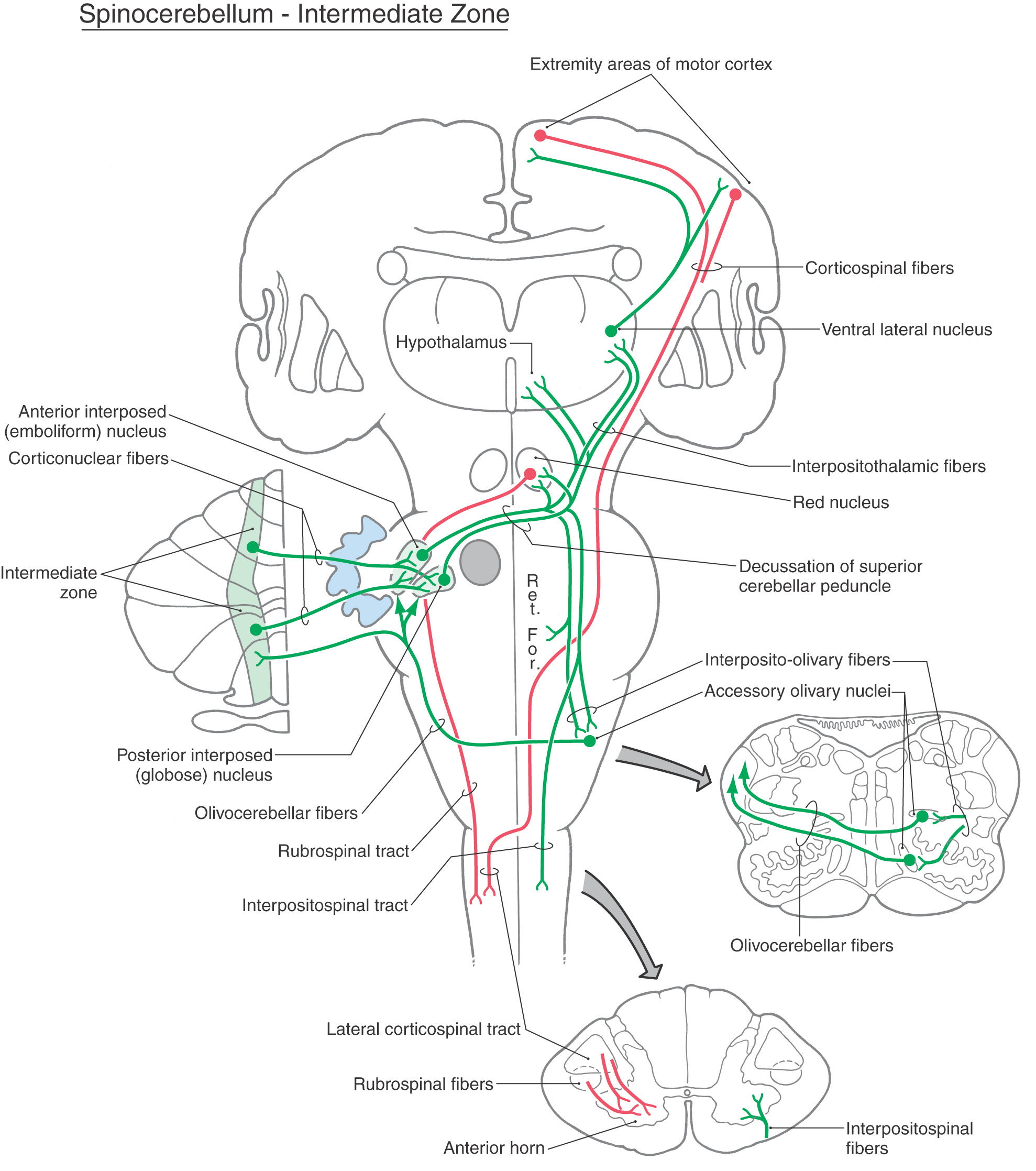
 Figure 27-18.
Figure 27-18.